A live attenuated strain of HY9901ΔdctP provides protection against Vibrio alginolyticus to the pearl gentian grouper (♀Epinephelus fuscoguttatus × ♂Epinephelus lanceolatus)
- 1Guangdong Provincial Key Laboratory of Aquatic Animal Disease Control and Healthy Culture, Fisheries College of Guangdong Ocean University, Zhanjiang, China
- 2Key Laboratory of Control for Diseases of Aquatic Economic Animals of Guangdong Higher Education Institutes, Southern Marine Science and Engineering Guangdong Laboratory (Zhanjiang), Zhanjiang, China
- 3Guangdong Provincial Engineering Research Center for Aquatic Animal Health Assessment, Shenzhen Institute of Guangdong Ocean University, Shenzhen, China
In recent decades, vibriosis caused by Vibrio alginolyticus has become a severe threat to the global mariculture industry. There is an urgent need for an effective vaccine to alleviate this unoptimistic situation. In this study, we evaluated the safety, immunoprotection, and specific and non-specific immune response effect of ΔdctP strain as a live-attenuated vaccine to pearl gentian grouper (♀Epinephelus fuscoguttatus × ♂Epinephelus lanceolatu). The results demonstrate that the safe dose of ΔdctP was ≤1.0 ×106 CFU in pearl gentian grouper. The relative percent survival of the pearl gentian grouper challenged with the ΔdctP mutant strain by intraperitoneal injection reached 74.4%, which was significantly higher than that of the control group. Meanwhile, the expression level of immune-relative genes, including IgM, IL-1β, IL-8, IL-10, MHC-Iα, MHC2, TNF-α, TLR3, and CD4, were upregulated in liver, spleen, and head kidney within 28 d post-vaccination. Moreover, specific antibody IgM, total serum protein as well as activities of catalase, superoxide dismutase, and lysozyme in serum were significantly up-regulated in vaccinated groupers compared with those in control. Collectively, ΔdctP could be used as a live-attenuated vaccine candidate against V. alginolyticus infection in pearl gentian grouper.
Introduction
Vibrio alginolyticus, a gram-negative opportunistic pathogen, mainly distributed in the coastal and estuarine environment of coastal countries, has caused serious economic losses to the aquaculture industry in recent decades (Balcázar et al., 2010; Jacobs Slifka et al., 2017). As one of the important pathogens, V. alginolyticus extremely caused diseases to many aquatic animals in China, such as Litopenaeus vannamei (Liu and Chen, 2004), Epinephelus coioides (Wang et al., 2014), E. bruneus (Harikrishnan et al., 2012), Rachycentron canadum (Rameshkumar et al., 2017), and especially pearl gentian grouper (♀Epinephelus fuscoguttatus × ♂Epinephelus lanceolatus), which caused severe threat to the development of grouper farming industry (Chen et al., 2019; Pang et al., 2022).
Pearl gentian grouper, a new hybrid species, is hybridized between ♀E. fuscoguttatus × ♂E. lanceolatus. Pearl gentian grouper is widely cultured because of its high economic value. However, the development of the grouper farming industry was severely restricted by the outbreak of vibriosis in recent years. Vibriosis is a common bacterial disease that affects all stages of grouper growth and accounts for about 66.7% of all grouper diseases, and can result in up to 50% mortality in pearl gentian grouper. Several species, including V. alginolyticus, V. harveyi, and V. vulnificus are pathogenic to pearl gentian grouper (Wei et al., 2020). After being infected with V. alginolyticus, pearl gentian groupers have symptoms of pelvic and pectoral fin hyperemia, anal redness, and even death in serious cases (Zhang et al., 2021). Therefore, it was urgent to find effective ways to solve the current unfavorable situation of pearl gentian grouper farming.
In order to mitigate this situation, antibiotics are commonly used to control vibriosis, which leads to antibiotic-resistant bacteria and drug residue problems emerging (Xiong et al., 2010). Therefore, healthy, effective, and sustainable treatments to combat the risk of vibriosis were urgently needed, such as vaccinatopm. Vaccines could induce immune responses in the host, keeping the host sensitive enough to respond to pathogen infection, such as live attenuated vaccines, inactivated vaccines, subunit vaccines, and DNA vaccines (Mohd-Aris et al., 2019). Among them, live attenuated vaccines have greater and longer adaptive immune protection in fish: they could not only carry natural antigenic structures expressed by pathogens and mimic real infection after natural contact but also provides long-term and unaltered antigen presentation to better stimulate humoral and cell-mediated immune responses (J. Wang et al., 2014; Mohd-Aris et al., 2019; Ji et al., 2020). Therefore, live attenuated vaccines had attracted more and more attention in aquatic animal disease prevention and control (Liu et al., 2018; Chen et al., 2019a; Zhang et al., 2021; Pang et al., 2022).
Some studies had shown that V. alginolyticus HY9901 inframe-deletion strains as live attenuated vaccines surely could improve specific and non-specific immunity and reduce mortality of pearl gentian groupers, such as ΔvscB, ΔacfA, and ΔclpP (Chen et al., 2019a; Chen et al., 2020; Pang et al., 2022). VscB as a T3SS chaperone protein, could assist the secretion or transport of the T3SS effector proteins and transported into host cells and participate in host intracellular functions (Pang et al., 2022). ClpP is an ATP-dependent serine protease, it can degrade abnormal proteins in cells, participate in protein quality control and affect the physiological and biochemical metabolism of cells (Chen et al., 2020). AcfA is one of the accessory colonization factors and plays a significant role in the efficient colonization of Vibrio in the intestine (Chen et al., 2019a). DctP as C4-dicarboxylate binding protein, is part of the TRAP transporter system that is capable of collaborating with DctQ and DctM to transport 4-carboxylate dicarboxylates into the cytoplasm and be used as carbon and energy in bacteria (Kelly and Thomas, 2001; Rhie et al., 2018). dctP was firstly proved to be one of virulence factors in Vibrio cholerae (Sharma et al., 2011). In V. aginolyticus, vscB, acfA, clpP, and dctP have performed different functions, and play different roles in stabilizing the internal environment of the bacterium as well as improving the invasive ability of specific species. Although some live attenuated vaccines such as ΔvscB, ΔacfA, and ΔclpP were constructed in V. aginolyticus, more live attenuated vaccine candidates should be studied and developed to finally screen the most suitable vaccine through clinical application evaluation.
In our previous study, a HY9901ΔdctP strain was also constructed and confirmed that the virulence against pearl gentian grouper was significantly reduced (Zhang et al., 2021). However, until now, there have been no studies of a vaccine that is related to dctP. Therefore, we wondered whether ΔdctP also had potential as a live attenuated vaccine candidate.
Based on the former studies, in this study we analyzed and evaluated the immune protection effect of ΔdctP strain as live attenuated vaccines against pearl gentian groupers. Firstly, we evaluated the safety of ΔdctP strain. Moreover, we not only detected the expression pattern of immune-related genes in the liver, spleen, head kidney, and thymus but also detected enzyme activity catalase (CAT), superoxide dismutase (SOD), lysozyme (LZM), total serum protein and specific antibody IgM in serum. Finally, mortality rates were measured. The results provided a more comprehensive understanding of ΔdctP strain as an attenuated vaccine protecting pearl gentian grouper against Vibriosis caused by V. alginolyticus.
Materials and methods
Bacterial strains and fish
V. alginolyticus HY9901 was isolated from disease groupers and stored in our laboratory. V. alginolyticus HY9901 ΔdctP was constructed from our former study (Zhang et al., 2021) and stored in our laboratory. V. alginolyticus HY9901, ΔdctP was cultured in Tryptic Soy Broth (TSB, Huankai, Guangzhou, China) containing 2% NaCl at 28°C for the following study.
Healthy pearl gentian groupers with a body weight 40.0 ± 4.0 g were purchased from East-island fish farm (Zhanjiang, China). Groupers were cultured in an aerated tank containing seawater (filtered by a sea water filter bag) at 26.0 ± 1.0°C, and fed with commercial grouper feed twice a day for 2 weeks, to adapt them to a different environment. Before the experiment, six fish were randomly chosen for bacterial recovery from the spleen, liver, and head kidney on TSA plates (Tryptic soy broth with 1% agar) to ensure the fish were not infected with V. alginolyticus or other bacterium. Fish were anesthetized with 100 ng/ml tricaine methanesulfonate (MS-222) before vaccination and sample collection. All animal experiments were strictly executed according to ethical standards and approved by Guangdong Provincial Key Laboratory of Pathogenic Biology and Epidemiology for Aquatic Economic Animals Ethics Committee.
Preparation of live-attenuated vaccine
ΔdctP strain was cultured in TSB with 2% NaCl at 28°C for 12h, then collected and centrifugated at 5000 rpm for 15 min. Bacterium were washed twice with phosphate-buffered saline (PBS, pH 7.2). After that, the concentration of ΔdctP was adjusted with PBS to 1.0×109, 1.0×108, 1.0×107, 1.0×106, and 1.0×105 CFU/ml using UV-Visible Spectrophotometer Evolution 220 (Thermo Fisher Scientific, USA).
Vaccine safety evaluation
A total of 150 fish were randomly divided into 5 groups (30 fish/group), each group was intraperitoneally injected with 0.1 ml 109, 108, 107, 106, and 105 CFU/ml V. alginolyticus HY9901 ΔdctP, respectively. V. alginolyticus were re-isolated from the liver, spleen, and head kidney of dead fish. All dead fish were counted in 14 days in order to calculate the safety concentration. The experiment was performed three times.
Pearl gentian grouper vaccination
360 fish were randomly divided into PBS and ΔdctP group (180 fish/group), each group were intraperitoneally injected with 0.1 ml PBS (control group) and 1×105 CFU/ml V. alginolyticus HY9901 ΔdctP (experimented group), respectively. Liver, spleen, head kidney and thymus (n=3) were collected from 7 d to 28 d and blood (n=3) from 7 d to 56 d post-vaccination, once a week. The serum was collected by centrifugated the blood at 3500 rpm for 20 min at 4°C. Serum, liver, spleen, head kidney and thymus were stored at -80°C until used.
Pathogen load of the live-attenuated vaccine in tissues
Pathogen load of ΔdctP in tissues (liver, spleen, and head kidney) from three randomly selected fish of the ΔdctP group was calculated, from 1 d to 9 d post-vaccination, once a day. Liver, spleen, and head kidney were obtained and weighed 1.0 ± 0.05 g, respectively. All 3 tissues were added in 3 ml PBS and homogenized together using a motor-driven tissue grinder (Sangon Biotech, Guangzhou, China) at 8000 rpm. Homogenates were 10-fold gradient diluted, with 100 μl on a TCBS plate (Sangon Biotech, Guangzhou, China) at 28°C for 24 h. Colonies with yellow centers were counted. This experiment was performed three times.
Relative percent survival (RPS) of pearl gentian grouper after V. alginolyticus HY9901 challenge
After 28 d post-vaccination, 180 fish of PBS and ΔdctP groups (90 fish/group) were intraperitoneally injected with 0.1 ml V. alginolyticus HY9901 with a concentration of 7.42 × 108 CFU/ml (Zhang et al, 2021) The mortality of each group was monitored for 14 d after bacterium challenge. RPS was calculated using the formula: RPS (%) = [1-(Mortality of vaccinated fish/Mortality of control fish)] × 100%. Bacterium were re-isolated from the liver, spleen, and head kidney of dead fish and incubated on TSA plates. The 16s rDNA (Table 1) was used to identify whether the fish died of V. alginolyticus HY9901 infection. The experiment was performed three times.
Quantitative real-time quantitative PCR (qRT-qPCR) analysis
The qRT-qPCR was used to analyze the gene expression of IgM (immunoglobulinM), IL-1β (interleukin 1β), IL-8 (interleukin 8), IL-10 (interleukin 10), MHC-Iα (major histocompatibility complex Iα), MHC2 (major histocompatibility complex II), TNF-α (tumor necrosis factor α), TLR3 (Toll-like receptors 3), and CD4 (cluster of differentiation 4), with β-actin as internal reference gene. Total RNA was extracted from all samples (liver, spleen, head kidney, and thymus) using TransZol Up Plus RNA Kit (TransGen Biotech, Beijing, China). cDNA was amplified using EasyScript® One-Step gDNA Removal and cDNA Synthesis SuperMix (Takara, Beijing, China), with 1000ng RNA as a template. The qRT-PCR amplification was performed using PerfectStart® Green qPCR SuperMix (TransGen Biotech, Beijing, China) for gene expression analysis. The primers are listed in Table 1. qRT-PCR reaction system (total volume of 10 μl) was as follows: Template (cDNA) 1 μl, Forward primer (10 μM) 1 μl, Reverse primer (10 μM) 1 μl, 2×perfectStart® green qPCR superMix 5 μl and Nuclease-free water 2 μl. The reaction condition was as follows: preincubation at 94°C for 300s, 3-step amplification including 40 cycles of 94°C for 10s, 60°C for 15s, and 72°C for 20s, with melting and cooling performed finally. All data were analyzed by the 2-△△CT method (Livak and Schmittgen, 2001).
Indirect ELISA for detection of antigen-specific serum antibody IgM
Antigen-specific serum antibody IgM was detected using a slightly modified ELISA method (Wei et al., 2020). V. alginolyticus HY9901 was incubated at 28°C overnight, then added to 0.5% methanol and shook at 28°C for 48 h. V. alginolyticus was centrifuged and washed using PBS, following adjusting the concentration to 1.0 × 108 CFU/ml using 0.05 M pH 9.6 carbonate buffer solution. 100 μl V. alginolyticus was added to each well of the 96-well microplate and incubated at 4°C overnight. Wells were washed with PBS added with 0.05% Tween-20 (PBST) three times, then blocked in 100 μl 5% non-fat powdered milk (Sangon Biotech, Guangzhou, China) for 2 h. After that, wells were washed with PBST three times, then added to diluted serum (2-fold continuous gradient dilution, from 21 to 212 fold dilution) and incubated at 37°C for 1 h. Then, wells were washed with PBST three times, following adding 100 μl 1:1000 rabbit-anti-pearl gentian grouper IgM polyclonal antibody and incubated at 37°C for 1 h. After that, wells were washed with PBST three times, wells were incubated 100 μl 1:2000 mouse-anti-rabbit IgG conjugated to HRP (Boster, Wuhan, China) at 37°C for 1 h. Wells were washed with PBST three times and added to 100 μl TMB substrate solution (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) for 15 min at room temperature. Finally, 50 μl 2 M H2SO4 was quickly added to wells to stop the reaction, then the absorbance of OD450 of wells was detected using a microplate reader (Thermo Fisher scientific, USA). The formula for antibody titer: Antibody titer (experimental group T/control group C) = (OD450 of serum in experimental group - OD450 of serum in blank control). The final antibody titer was the dilution ratio of antiserum when T/C > 2.1. The experiment was performed three times.
Enzyme activity and total serum protein in fish serum
The activities of CAT, SOD, LZM, and total serum protein of fish serum samples were detected using a catalase assay kit (Visible light), superoxide Dismutase assay kit (WST-1 method), lysozyme assay kit and total protein quantitative assay kit, respectively. All kits were purchased from Nanjing Jiancheng Bioengineering Institute in China.
Histopathological analysis post-vaccination
To assess the safety of live-attenuated vaccine ΔdctP, livers and spleens of PBS and ΔdctP group 28 days after vaccination were collected for histological examination. The tissues of dying fish (V. alginolyticus HY9901 group, fish challenged by V. alginolyticus in experiment 2.6) were used as positive pathogen control. All tissues were fixed using 10% paraformaldehyde fixative for 48h, then dehydrated in ethanol with increasing concentrations (50%→70%→80%→90%→95%→100%). After that, tissues were equilibrated with xylene, following embedding in paraffin. Finally, paraffin blocks were cut to 4 μm thickness, stained by Haematoxylin–Eosine (Sangon Biotech, Shanghai, China), and the pathological alterations were observed using an optical microscope.
Statistical analysis
Data were statistics by one-way analysis of variance (ANOVA), followed by using SPSS 26.0 software with Duncan’s new multiple range test. Data were shown as mean ± SE, and the difference between data were marked as * (p < 0.05), ** (p < 0.01) and *** (p < 0.001).
Results
Safety situation of the live-attenuated vaccine
The safety situation of live attenuated vaccine ΔdctP was shown in Table 2. Simply, there were no fish dead after injection with 0.1 ml 107, 106, and 105 CFU/ml ΔdctP. Therefore, ≤1.0 ×106 CFU ΔdctP was the safe vaccine dose for these experimented groupers.
Pathogen load of the live-attenuated vaccine in tissues
ΔdctP rapidly spread but only temporally survived in tissues and gradually vanished from the host’s body (Figure 1). The results indicated that the highest bacterial number was detected in the liver, spleen, and head kidney (tissues mixture) at 24 h post-vaccination, and the bacteria had totally disappeared from all tissues at 9 d post-vaccination.
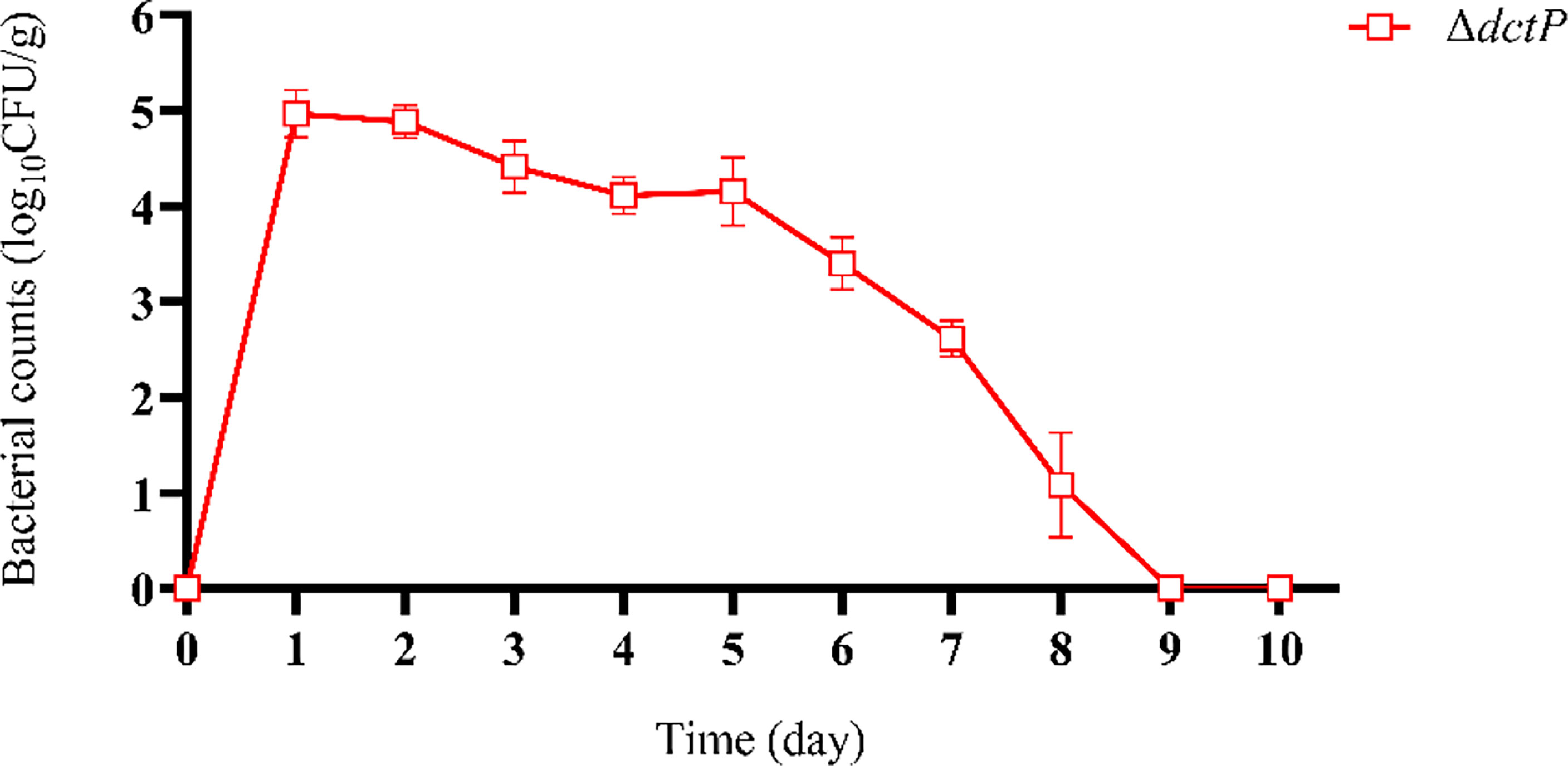
Figure 1 Pathogen load of liver, spleen, and head kidney mixture by ΔdctP strain. Values are means ± SEM (n = 3).
Vaccine efficacy
The efficacy of the live-attenuated vaccine was evaluated by fish challenged with HY9901 28 d post-vaccination. Grouper began to die starting on the second day and ending by the ninth. The RPS of the ΔdctP group was 74.4%, which was a significant increase compared with the PBS group, which was 23.3% (Figure 2). The results showed that ΔdctP only had negligible virulence and significant immunoprotection to groupers, suggesting it is a better candidate as a live-attenuated vaccine.
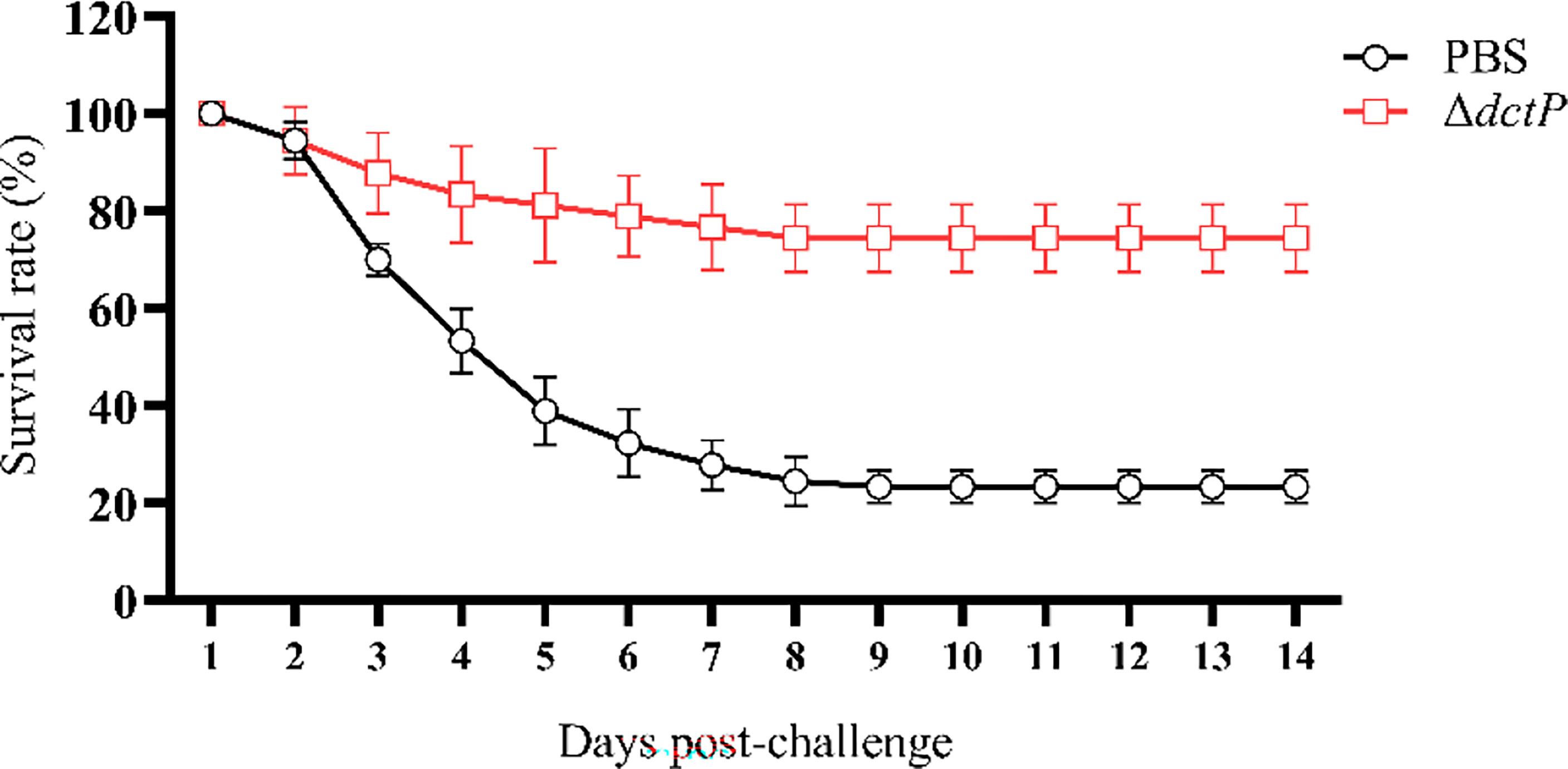
Figure 2 Survival rate of vaccinated groupers challenged with V. alginolyticus for 14 days. Values are means ± SD (n = 3).
Immune gene expression of grouper during immune protection of the vaccine
To evaluate the immune response of the pearl gentian grouper immunized with ΔdctP live-attenuated vaccine, the transcriptional levels of nine immune-related genes in the liver, spleen, head kidney, and thymus of groupers were measured over 7 d, 14 d, 21 d, and 28 d post-vaccination (Figures 3–6). The expression level of IgM was highly expressed in 4 tissues at all time points, except for the liver at 28 d, with the maximum expression levels (10.5-folds) appearing in the thymus at 14 d (Figures 3A, 4A, 5A, 6A). The expression level of MHC-Iα (Figures 3E, 4E, 5E, 6E) and TLR3 (Figures 3H, 4H, 5H, 6H) up-regulated to the highest in 4 tissues at 14 d, with the highest expression levels of 7.1-folds appeared in liver and 11.3-folds in the thymus. IL-8 (Figures 3C, 4C, 5C, 6C) expressed to the highest level in 4 tissues at 21 d, with 10.2-folds in the thymus expressed to the highest compared with the other three tissues. The expression level of MHC2 rose to the top in the spleen, head kidney, and thymus at 28 d (Figures 4F, 5F, 6F) and in the liver at 21 d (Figure 3F), with the results of 9.2-folds, 4.6-folds, 17.8-folds, and 6.8-folds, respectively. The expression level of CD4 rose to the peak in the spleen, head kidney, and thymus at 21 d (Figures 4I, 5I, 6I), and in the liver at 7 d (Figure 3I), with the results of 4.7-folds, 5.4-folds, 3.0-folds, and 3.7-folds, respectively. Moreover, the expression levels of IL-1β (Figures 3B, 4B, 5B, 6B), IL-10 (Figures 3D, 4D, 5D, 6D), and TNF-α (Figures 3G, 4G, 5G, 6G) have different expression patterns in different tissues, but significantly increased compared to those in PBS group at most of sampling time points. The highest expression levels of IL-1β, IL-10, and TNF-α were 8.8-folds in the spleen at 21 d, 6.0-folds in the spleen at 21 d, and 9.8-folds in the liver at 7 d, respectively.

Figure 3 The expression levels of immune-related genes in the liver of pearl gentian groupers by qRT-PCR post-immunization. The mRNA level of each immune-related gene was normalized to that of β-actin and relative expression was calculated as the values of the vaccinated tissues by those of the controls. (A) IgM, (B) IL-1β, (C) IL-8, (D) IL-10, (E) MHC-Iα, (F) MHC2, (G) TNF-α, (H) TLR3, and (I) CD4. Bars represented the mean relative expression (n=3) and error bars represent standard errors. The asterisks indicated significant differences: *(p < 0.05), **(p < 0.01) and ***(p < 0.001), between PBS group and ΔdctP group.
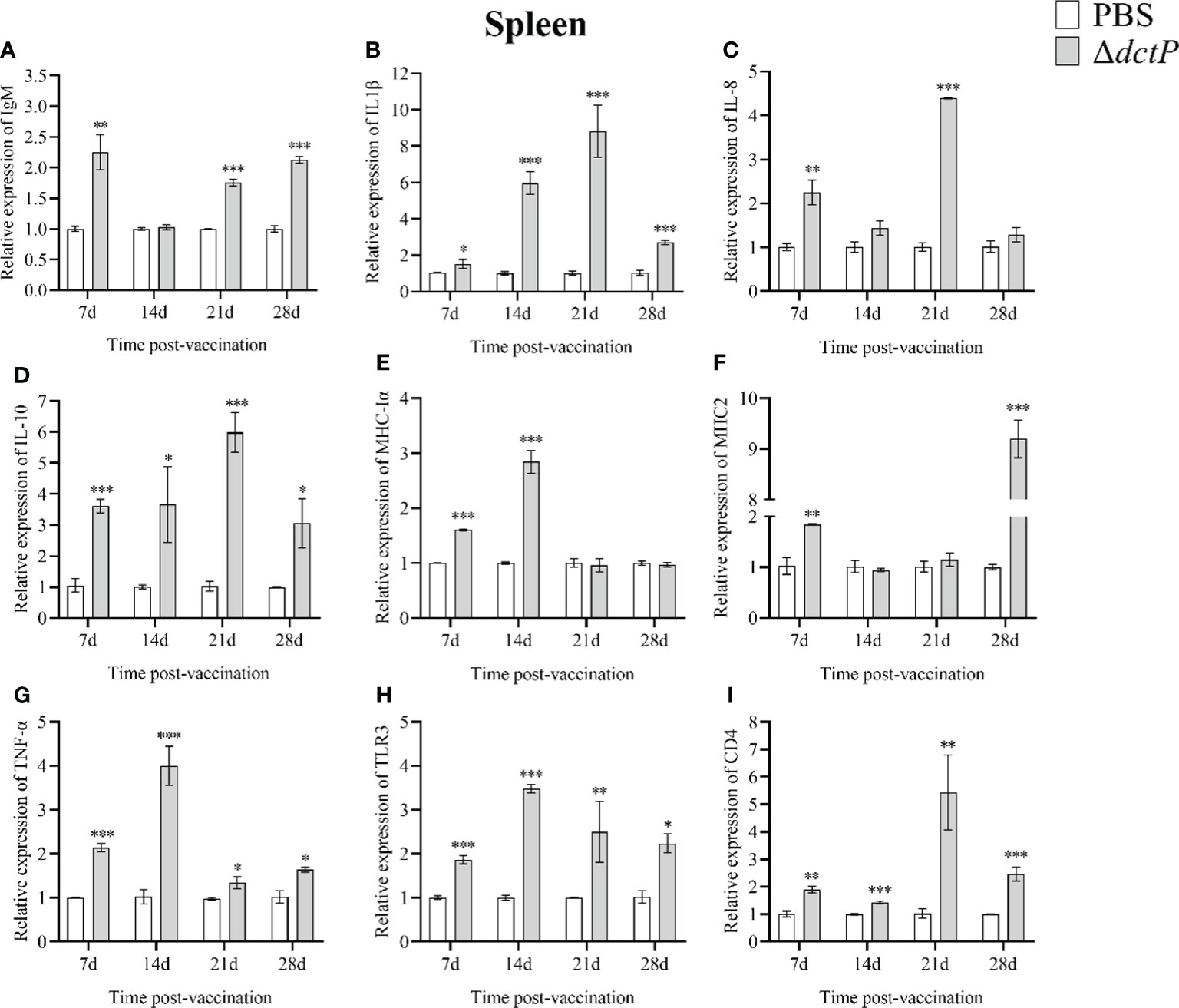
Figure 4 The expression levels of immune-related genes in the spleen of pearl gentian groupers by qRT-PCR post-immunization. The mRNA level of each immune-related gene was normalized to that of β-actin and relative expression was calculated as the values of the vaccinated tissues by those of the controls. (A) IgM, (B) IL-1β, (C) IL-8, (D) IL-10, (E) MHC-Iα, (F) MHC2, (G) TNF-α, (H) TLR3, and (I) CD4. Bars represented the mean relative expression (n=3) and error bars represent standard errors. The asterisks indicated significant differences: *(p < 0.05), **(p < 0.01) and ***(p < 0.001), between PBS group and ΔdctP group.
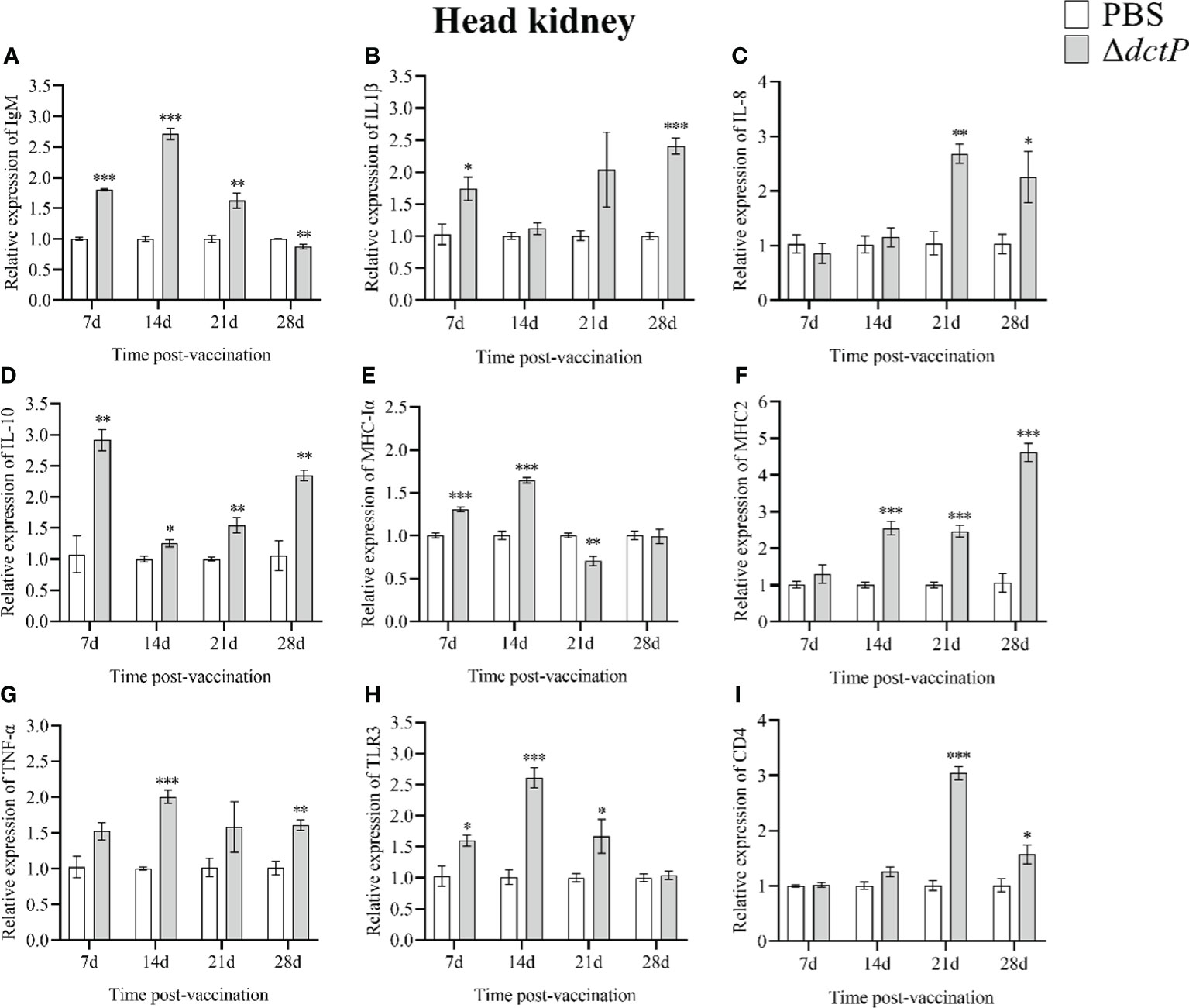
Figure 5 The expression levels of immune-related genes in the head kidney of pearl gentian groupers by qRT-PCR post-immunization. The mRNA level of each immune-related gene was normalized to that of β-actin and relative expression was calculated as the values of the vaccinated tissues by those of the controls. (A) IgM, (B) IL-1β, (C) IL-8, (D) IL-10, (E) MHC-Iα, (F) MHC2, (G) TNF-α, (H) TLR3, and (i) CD4. Bars represented the mean relative expression (n=3) and error bars represent standard errors. The asterisks indicated significant differences: *(p < 0.05), **(p < 0.01) and ***(p < 0.001), between PBS group and ΔdctP group.
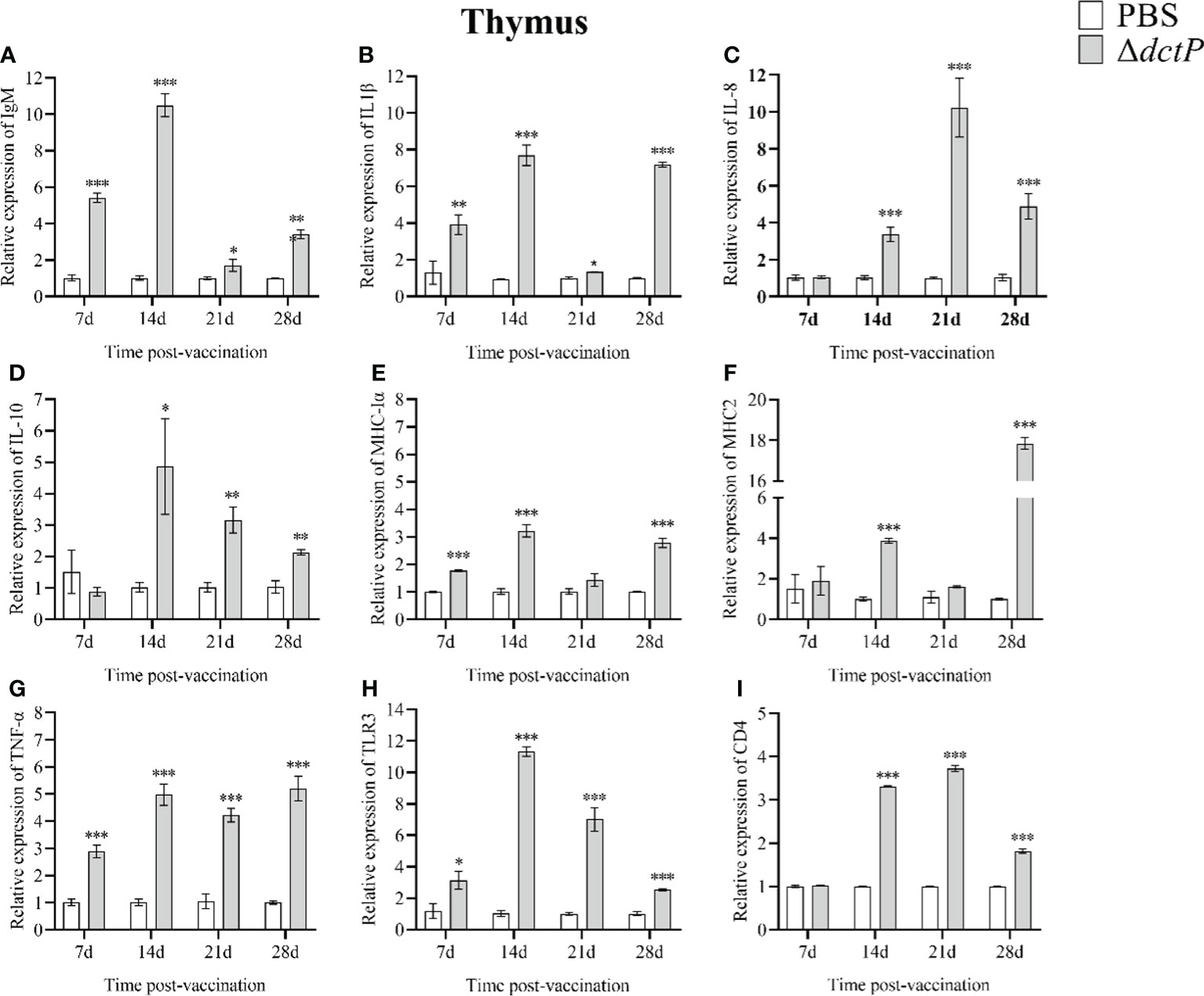
Figure 6 The expression levels of immune-related genes in the thymus of pearl gentian groupers by qRT-PCR post-immunization. The mRNA level of each immune-related gene was normalized to that of β-actin and relative expression was calculated as the values of the vaccinated tissues by those of the controls. (A) IgM, (B) IL-1β, (C) IL-8, (D) IL-10, (E) MHC-Iα, (F) MHC2, (G) TNF-α, (H) TLR3, and (I) CD4. Bars represented the mean relative expression (n=3) and error bars represent standard errors. The asterisks indicated significant differences: *(p < 0.05), **(p < 0.01) and ***(p < 0.001), between PBS group and ΔdctP group.
Antibody titers in serum of vaccinated fish
The antibody level of IgM was evaluated by ELISA from 1 to 8 weeks post-vaccination (Figure 7). Antibody titers of ΔdctP group displayed the tendency of increasing from 1 to 4 weeks and reached the highest level, then it began to drop from 5 weeks. The specific antibody titers in the ΔdctP group were significantly increased (p < 0.05) than those in the PBS group at all time points.
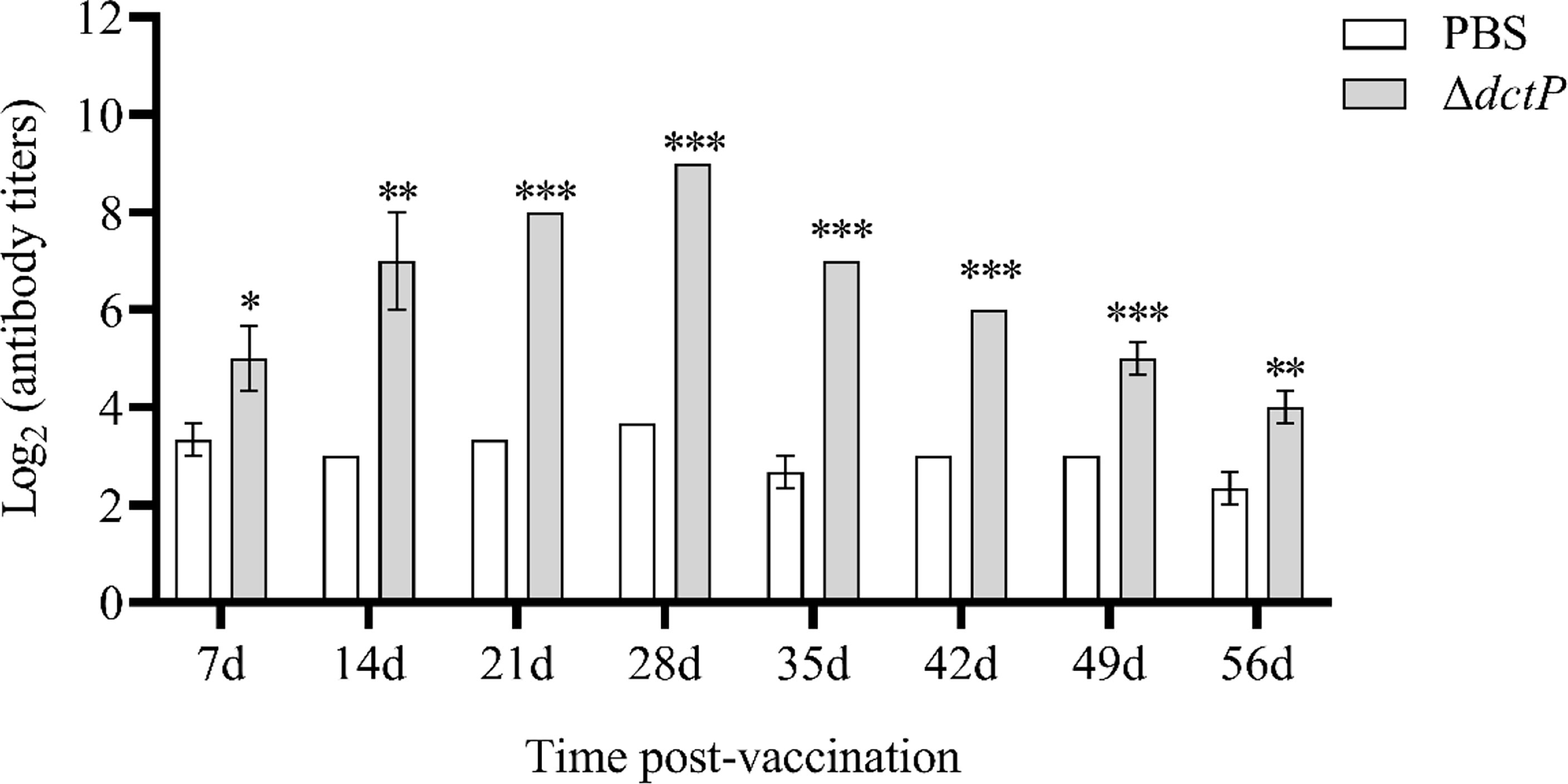
Figure 7 Analysis of V. alginolyticus-specific serum antibody titers of IgM at 7d, 14d, 21d, 28d, 35d, 42d, 49d, and 56d post-vaccination. Specific antibody titers were detected by ELISA. Bars represented the mean relative expression (n = 3) and error bars represent standard errors. The asterisks indicated significant differences: *(p < 0.05), **(p < 0.01) and ***(p < 0.001), between PBS group and ΔdctP group.
Enzyme activity of (CAT, SOD, and LZM) and total serum protein
The enzyme activity and total serum protein in fish serum from 7 d to 42 d were thoroughly detected. CAT activity rose to the highest level at 14 d post-vaccination and gradually dropped to a normal level at 28 d in the ΔdctP group (Figure 8A). SOD activity significantly increased from 7 d to 28 d (rising to the highest levels). After that, it decreased to the normal level at 35 d (Figure 8B). LZM activity began to rise at 7 d and reached its peak at 14 d, then gradually decreased until it returned to normal levels at 42 d (Figure 8C). Total serum protein gradually increased and reached the peak at 28 d, then decreased to the normal level at 42 d (Figure 8D). The activities of CAT and LZM reached the top at 14 d, and the SOD activity and total serum protein reached the peak at 28 d.
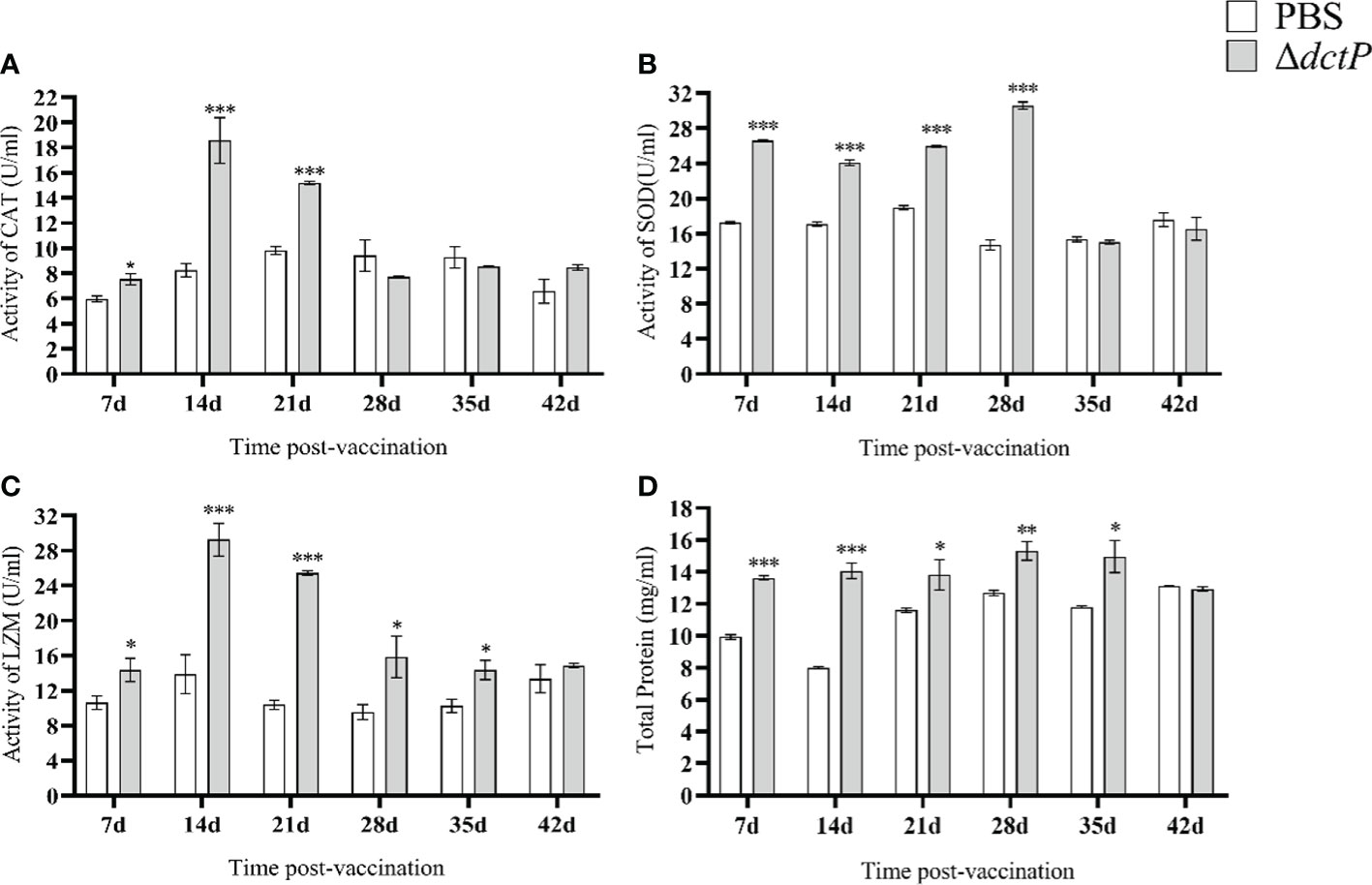
Figure 8 CAT, SOD, LZM activity and total serum protein in serum of pearl gentian groupers at 7d, 14d, 21d, 28d, 35d and 42d post-vaccination. (A) CAT activity; (B) SOD activity; (C) LZM activity; (D) total serum protein. Bars represented the mean relative expression (n = 3) and error bars represented standard errors. The asterisks indicated significant differences: *(p < 0.05), **(p < 0.01) and ***(p < 0.001), between PBS group and ΔdctP group.
Histological analysis of livers and spleens post-immunization
There were no tissue lesions in the ΔdctP group (Figures 9C, F) compared with the control group (Figures 9A, D). However, histopathological changes were seen in the liver and spleen of fish that were infected with V. alginolyticus. Hemorrhage, enlargement, and the cytoplasmic ratio were reduced in the liver (Figure 9B). Necrotic foci and splenic cord were unclear in the spleen (Figure 9E).
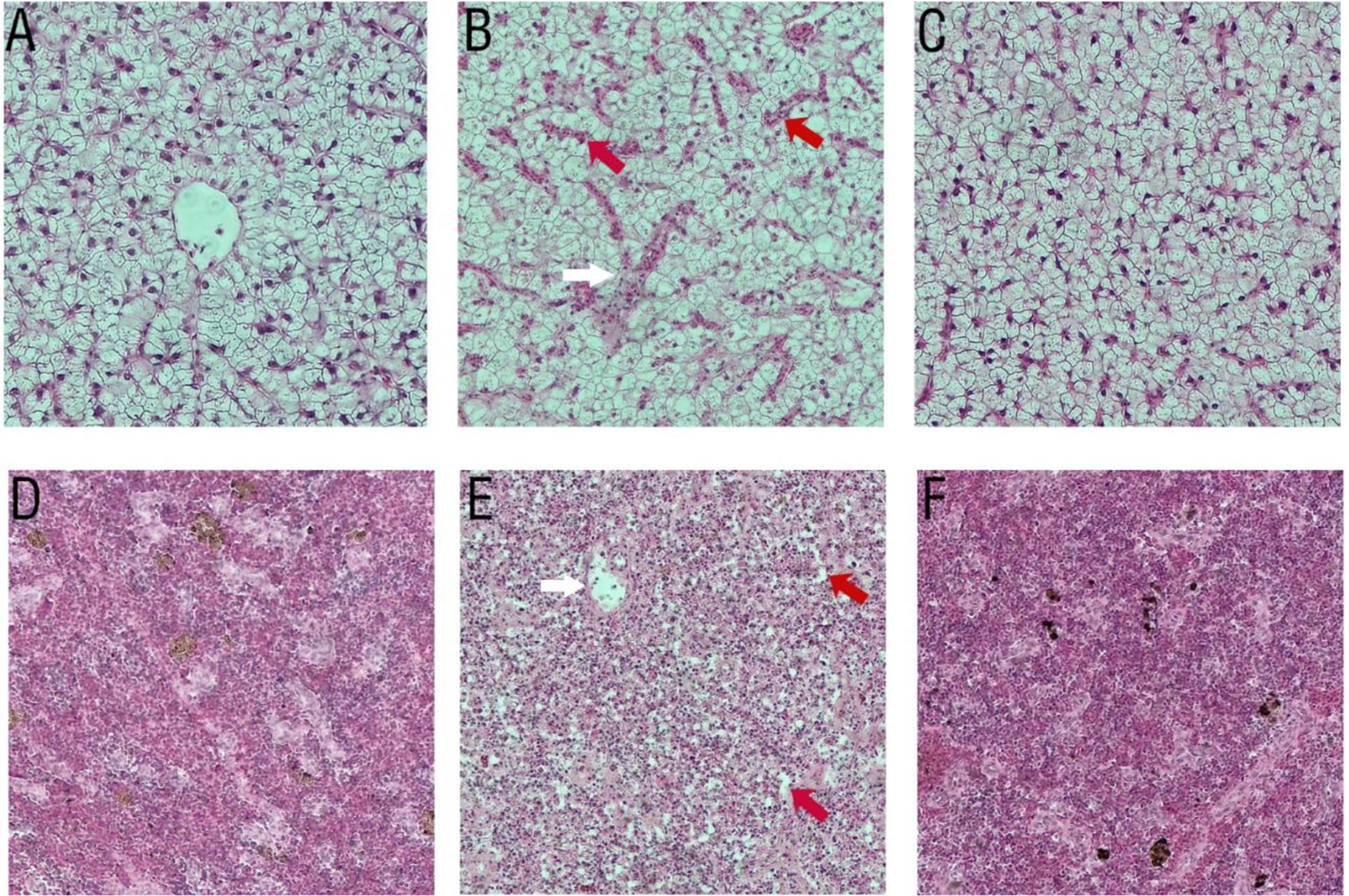
Figure 9 Histopathological changes in liver (100 × magnification) and spleen (100 × magnification) of pearl gentian grouper after infection with V. alginolyticus. (A, D) PBS group, (B, E) PBS group infected with V. alginolyticus HY9901, (C, F) ΔdctP group infected with V. alginolyticus HY9901. (A, C) Liver of group and ΔdctP group show normal hepatocytes structure and organization. (B) Liver of V. alginolyticus HY9901 group show pronounced liver sinus congestion (red arrow) and hemorrhage (white arrow). (D, F) Spleen of PBS group and ΔdctP group show normal hepatocytes structure and organization. (E) Spleen of V. alginolyticus HY9901 group shows splenic cord-unclear (red arrow) and necrotic foci (white arrow).
Discussion
In recent decades, the outbreak of vibriosis hindered the development of aquatic industry, which also stimulated the development of aquatic vaccines. Currently, many live-attenuated vaccines have been applied and achieved positive effects in preventing vibriosis infection in experimental aquatic animals (Zhang et al., 2012; Gao et al., 2014; Liu et al., 2018; Chen et al., 2019a; Pang et al., 2022). To prevent vibriosis caused by V. alginolyticus, our study firstly used dctP mutant strain as a live-attenuated vaccine to immunize pearl gentian groupers, notwithstanding ΔdctP has not been applied to any aquatic animals as a live-attenuated vaccine so far. The results indicated that ΔdctP injection could guarantee safety, prolong immune protection effect, improve specific and non-specific immunity and reduce mortality of pearl gentian groupers, which opened up the possibility of ΔdctP to be an effective live-attenuated vaccine in the future.
Live attenuated vaccines not only can proliferate in the body for a few days but also do not cause diseases. Therefore, it more effectively activates the immune response and provides longer-lasting protection in animals (Baxter, 2007). Nevertheless, for fish, live-attenuated vaccines have side effects of the possibility of virulence recovery or retention and persistence in the organism (Tlaxca et al., 2015). In this study, we tested ΔdctP to ensure its safety before being used as a live attenuated vaccine candidate. In our previous study, the genetic stability and LD50 of ΔdctP has tested, and the result showed that ΔdctP was stable to continue 30 generations and the virulence was almost 40-folds lower than that of the HY9901 (Zhang et al., 2021). In this study, we tested the safety dose situation of ΔdctP in fish and concluded that ≤1.0 ×106 CFU was safe injection dose for our experimental groupers (40.0 ± 4.0g). Moreover, we evaluated the pathogen persistence in the spleen and head kidney (tissues mixture) and indicated that ΔdctP could be disappeared totally at 9 d post-vaccination. The above results indicated that ΔdctP was safe enough to be applied as a live-attenuated vaccine candidate.
RPS is an important and relatively straightforward indicator of vaccine effectiveness. Several studies have developed V. alginolyticus mutant strains as live-attenuated vaccines to protect groupers and Danio rerio, with RPS ranging from 71% to 87% (Pang et al., 2018; Chen et al., 2019a; Chen et al., 2019; Zhou et al., 2020). In our study, the RPS of groupers injected with ΔdctP was 74.4%, indicating that ΔdctP could provide better effective protection against challenge by V. alginolyticus.
Multiple studies have concluded that live-attenuated vaccines induce stronger humoral and cellular immune responses (Killeen and DiRita, 2001). As immune organs, the liver, spleen, head kidney, and thymus play an important role in humoral and cellular immune processes. The liver plays an important role in innate immunity, metabolism, and growth, supporting host defense and promoting metabolic regulation of effective immune function (Causey et al., 2018). The spleen is the hematopoietic organ of fish with the function of purifying blood (macrophages in the spleen can capture and phagocytose foreign substances in the blood) (Fänge and Nilsson, 1985). The head kidney is the hematopoietic organ of fish and the origin of immune cells, especially B lymphocytes, which play an important role in humoral immune response (Zapata et al., 2006). The thymus is the central immune organ as well as the site of proliferation and differentiation of lymphocytes (Burnet, 1962). Therefore, the expression levels of some immune-related genes in these immune organs were analyzed in this study. IL-1 and IL-8 could activate and regulate immune cells, mediate T and B cell activation, proliferation, and differentiation, or play an important role in inflammatory response (Dinarello, 1997; Remick, 2005). IL-10 is an anti-inflammatory cytokine that plays a role in limiting the immune response to pathogens and therefore preventing damage to the host (Saraiva and O'Garra, 2010). TNF mainly participates in the body’s defense response and is also an important pro-inflammatory cytokine and immunoregulatory molecule (Nascimento et al., 2007). TLR3 plays an important role in non-specific immunity (innate immunity) in fish that involves in the defense process against bacteria invasion (Wang et al., 2018). IL-1β, IL-8, IL-10, TNF-α, and TLR3 were upregulated in tissues at different time points post-vaccination, suggesting that the innate immune response of pearl gentian groupers could be activated by ΔdctP. IgM is the earliest immunoglobulin in the initial humoral immune response of the animal body that can kill bacteria and activate complements to promote phagocytosis (Castro et al., 2013). CD4 is the co-receptor of TCR on helper T cell (TH cell) that could bind with MHC class II and MHC class I on antigen-presenting cells (APC) to participate in the TCR recognition antigen process and the transduction of T cell activation signal, respectively (Chaplin, 2010). In the present study, specific immune-associated genes: IgM, MHC-Iα, MHC2, and CD4 were elevated to varying degrees in tissues at different time points post-vaccination, suggesting that specific immunity including the cellular and humoral immune response of pearl gentian groupers could be also induced by ΔdctP.
The primary response is the process in which the antigen first enters the body, stimulates B cells to activate, proliferate and differentiate into plasma cells, and finally secrete antibodies (LeFor and Bauer, 1970). IgM is the earliest antibody produced in serum in the primary response. In our study, the expression level of specific antibody IgM reached the peak at 28 d post-immunization, and it was still upregulated even 56 d post-vaccination. The result was consistent with previous studies (Pang et al., 2018; Chen et al., 2019a; Chen et al., 2019), indicating that the effective and long-lasting humoral immune response was induced by ΔdctP.
Antioxidants in serum could defend against free radicals produced in the body and maintain internal stability. According to their response to general free radical invasion, they are classified as first, second, third, and fourth line defense antioxidants (Ighodaro and Akinloye, 2019). SOD and CAT, as antioxidants in the first line of defense, are important and indispensable in the whole defense strategy of antioxidants. SOD catalyzes the decomposition of superoxide anions (*O2) into hydrogen peroxide (H2O2) and molecular oxygen (O2), thereby reducing potentially harmful superoxide anions (Ighodaro and Akinloye, 2019). CAT catalyzes the degradation or reduction of H2O2 to H2O and O2, thus completing the detoxification process simulated by SOD (Marklund, 1984; Chelikani et al., 2004). Lysozyme could cleave the β-1,4-glycosidic bond between N-acetylmuramic acid and N-acetylglucosamine in cell walls, dissolving bacteria by rupturing the cell wall and releasing the contents (Bera et al., 2005). Blood lysozyme concentration is also an indicator of monocyte and granulocyte proliferation (Bera et al., 2005). Total serum proteins contain albumin and total globulin, which is the indicator of enhanced immunity (Peters, 1985; Busher, 1990). In our study, CAT, SOD, LZM and total serum protein were significantly up-regulated post-vaccination, which demonstrated non-specific immunity of grouper could be widely induced by ΔdctP.
In addition to injection vaccination, immersion and oral vaccination are more convenient for clinical application. Immersion vaccination has several advantages including reduced handling and stress to fish, lower cost, avoidance of damage to fillets, and the potential to effectively stimulate the teleost immune response by delivery through a route utilized by aquatic pathogens. After immersion live-attenuated vaccination, antigens are taken up by the skin, gills, or gut, and thus adherence and invasion of epithelial cells stimulates the immune system, resulting in a response and the protection of the fish (Locke et al., 2010; Bøgwald and Dalmo, 2019). Live-attenuated oral vaccination has the advantage of intestinal colonization and rapid immune responses, which could result in improved immunological protection against specific bacterial infections (Ryan et al., 2006). Therefore, ΔdctP as a live-attenuated vaccine may be also suitable for immersion and oral vaccination.
In conclusion, ΔdctP as a live-attenuated vaccine is not only safe for grouper, but effective in protecting fish from vibriosis caused by V. alginolyticus by enhancing specific and non-specific immune responses. Therefore, the current study provides an insight into the protection of grouper with live attenuated vaccines in the future.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was reviewed and approved by Guangdong Provincial Key Laboratory of Pathogenic Biology and Epidemiology for Aquatic Economic Animals Ethics Committee.
Author contributions
Experimental design, SC, JJ, and YH. Sampling, YZ, ZZ, and JZ. Analyses, YZ, ZZ, and JZ. Supervision, SC and JJ. Writing, original draft, YZ. Writing, review, and editing, SC, JJ, and YH. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (No. U20A2065), Guangdong Natural Science Foundation (No. 2021A1515010532), and the Fund of Southern Marine Science and Engineering Guangdong Laboratory (Zhanjiang) (No. ZJW-2019-06).
Acknowledgments
We would like to thank all teachers and schoolfellow for the skillful organization of sampling and technical assistance.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bøgwald J., Dalmo R. A. (2019). Review on immersion vaccines for fish: An update 2019. Microorganisms 7 (12), 627. doi: 10.3390/microorganisms7120627
Balcázar J. L., Gallo-Bueno A., Planas M., Pintado J. (2010). Isolation of Vibrio alginolyticus and Vibrio splendidus from captive-bred seahorses with disease symptoms. Antonie Van Leeuwenhoek 97 (2), 207–210. doi: 10.1007/s10482-009-9398-4
Baxter D. (2007). Active and passive immunity, vaccine types, excipients and licensing. Occup. Med. (Lond) 57 (8), 552–556. doi: 10.1093/occmed/kqm110
Bera A., Herbert S., Jakob A., Vollmer W., Gotz F. (2005). Why are pathogenic staphylococci so lysozyme resistant? the peptidoglycan O-acetyltransferase OatA is the major determinant for lysozyme resistance of Staphylococcus aureus. Mol. Microbiol. 55 (3), 778–787. doi: 10.1111/j.1365-2958.2004.04446.x
Burnet M. (1962). Role of the thymus and related organs in immunity. Br. Med. J. 2 (5308), 807–811. doi: 10.1136/bmj.2.5308.807
Busher J. T. (1990). “Serum albumin and globulin,” in Clinical methods: The history, physical, and laboratory examinations, vol. 3. , 497–499.
Castro R., Jouneau L., Pham H. P., Bouchez O., Giudicelli V., Lefranc M. P., et al. (2013). Teleost fish mount complex clonal IgM and IgT responses in spleen upon systemic viral infection. PloS Pathog. 9 (1), e1003098. doi: 10.1371/journal.ppat.1003098
Causey D. R., Pohl M. A. N., Stead D. A., Martin S. A. M., Secombes C. J., Macqueen D. J. (2018). High-throughput proteomic profiling of the fish liver following bacterial infection. BMC Genomics 19 (1), 719. doi: 10.1186/s12864-018-5092-0
Chaplin D. D. (2010). Overview of the immune response. J. Allergy Clin. Immunol. 125Suppl 2), S3–23. doi: 10.1016/j.jaci.2009.12.980
Chelikani P., Fita I., Loewen P. C. (2004). Diversity of structures and properties among catalases. Cell Mol. Life Sci. 61 (2), 192–208. doi: 10.1007/s00018-003-3206-5
Chen Y., Cai S., Jian J. (2019a). Protection against Vibrio alginolyticus in pearl gentian grouper (♀Epinephelus fuscoguttatus × ♂Epinephelus lanceolatu) immunized with an acfA-deletion live attenuated vaccine. Fish Shellfish Immunol. 86, 875–881. doi: 10.1016/j.fsi.2018.12.030
Chen Y., Wu F., Pang H., Tang J., Cai S., Jian J. (2019). Superoxide dismutase b (sodB), an important virulence factor of vibrio alginolyticus, contributes to antioxidative stress and its potential application for live attenuated vaccine. Fish Shellfish Immunol. 89, 354–360. doi: 10.1016/j.fsi.2019.03.061
Chen Y., Wu F., Wang Z., Tang J., Cai S., Jian J. (2020). Construction and evaluation of Vibrio alginolyticus ΔclpP mutant, as a safe live attenuated vibriosis vaccine. Fish Shellfish Immunol. 98, 917–922. doi: 10.1016/j.fsi.2019.11.054
Dinarello C. A. (1997). Interleukin-1. Cytokine Growth factor Rev. 8 (4), 253–265. doi: 10.1016/s1359-6101(97)00023-3
Fänge R., Nilsson S. (1985). The fish spleen: structure and function. Experientia 41 (2), 152–158. doi: 10.1007/bf02002607
Gao Y., Wu H., Wang Q., Qu J., Liu Q., Xiao J., et al. (2014). A live attenuated combination vaccine evokes effective immune-mediated protection against Edwardsiella tarda and Vibrio anguillarum. Vaccine 32 (45), 5937–5944. doi: 10.1016/j.vaccine.2014.08.074
Harikrishnan R., Kim J.-S., Balasundaram C., Heo M.-S. (2012). Immunomodulatory effects of chitin and chitosan enriched diets in Epinephelus bruneus against Vibrio alginolyticus infection. Aquaculture 326-329, 46–52. doi: 10.1016/j.aquaculture.2011.11.034
Ighodaro O. M., Akinloye O. A. (2019). First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria J. Med. 54 (4), 287–293. doi: 10.1016/j.ajme.2017.09.001
Jacobs Slifka K. M., Newton A. E., Mahon B. E. (2017). Vibrio alginolyticus infections in the USA 1988-2012. Epidemiol. Infect. 145 (7), 1491–1499. doi: 10.1017/s0950268817000140
Ji Q., Wang S., Ma J., Liu Q. (2020). A review: Progress in the development of fish vibrio spp. vaccines. Immunol. Lett. 226, 46–54. doi: 10.1016/j.imlet.2020.07.002
Kelly D. J., Thomas G. H. (2001). The tripartite ATP-independent periplasmic (TRAP) transporters of bacteria and archaea. FEMS Microbiol. Rev. 25 (4), 405–424. doi: 10.1111/j.1574-6976.2001.tb00584.x
Killeen K. P., DiRita V. J. (2001). Live attenuated bacterial vaccines. New Vaccine Technol. 151, 151–163.
LeFor W., Bauer D. (1970). Relative concentrations of IgM and IgG antibodies during the primary response. J. Immunol. 104 (5), 1276–1286.
Liu C. H., Chen J. C. (2004). Effect of ammonia on the immune response of white shrimp Litopenaeus vannamei and its susceptibility to Vibrio alginolyticus. Fish Shellfish Immunol. 16 (3), 321–334. doi: 10.1016/s1050-4648(03)00113-x
Liu X., Jiao C., Ma Y., Wang Q., Zhang Y. (2018). A live attenuated Vibrio anguillarum vaccine induces efficient immunoprotection in tiger puffer (Takifugu rubripes). Vaccine 36 (11), 1460–1466. doi: 10.1016/j.vaccine.2018.01.067
Livak K. J., Schmittgen T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods (San Diego Calif.) 25 (4), 402–408. doi: 10.1006/meth.2001.1262
Locke J. B., Vicknair M. R., Ostland V. E., Nizet V., Buchanan J. T. (2010). Evaluation of Streptococcus iniae killed bacterin and live attenuated vaccines in hybrid striped bass through injection and bath immersion. Dis. Aquat. organisms 89 (2), 117–123. doi: 10.3354/dao02192
Marklund S. L. (1984). Extracellular superoxide dismutase and other superoxide dismutase isoenzymes in tissues from nine mammalian species. Biochem. J. 222 (3), 649–655. doi: 10.1042/bj2220649
Mohd-Aris A., Muhamad-Sofie M. H. N., Zamri-Saad M., Daud H. M., Ina-Salwany M. Y. (2019). Live vaccines against bacterial fish diseases: A review. Vet. World 12 (11), 1806–1815. doi: 10.14202/vetworld.2019.1806-1815
Nascimento D. S., Pereira P. J., Reis M. I., do Vale A., Zou J., Silva M. T., et al. (2007). Molecular cloning and expression analysis of sea bass (Dicentrarchus labrax l.) tumor necrosis factor-α (TNF-α). Fish Shellfish Immunol. 23 (3), 701–710. doi: 10.1016/j.fsi.2007.02.003
Pang H., Chang Y., Zheng H., Tan H., Zhou S., Zeng F., et al. (2022). A live attenuated strain of HY9901ΔvscB provides protection against Vibrio alginolyticus in pearl gentian grouper (♀Epinephelus fuscoguttatus × ♂Epinephelus lanceolatu). Aquaculture 546, 737353. doi: 10.1016/j.aquaculture.2021.737353
Pang H., Qiu M., Zhao J., Hoare R., Monaghan S. J., Song D., et al. (2018). Construction of a Vibrio alginolyticus hopPmaJ (hop) mutant and evaluation of its potential as a live attenuated vaccine in orange-spotted grouper (Epinephelus coioides). Fish Shellfish Immunol. 76, 93–100. doi: 10.1016/j.fsi.2018.02.012
Rameshkumar P., Nazar A. K. A., Pradeep M. A., Kalidas C., Jayakumar R., Tamilmani G., et al. (2017). Isolation and characterization of pathogenic Vibrio alginolyticus from sea cage cultured cobia (Rachycentron canadum (Linnaeus 1766)) in India. Lett. Appl. Microbiol. 65 (5), 423–430. doi: 10.1111/lam.12800
Remick D. G. (2005). Interleukin-8. Crit. Care Med. 33Suppl), S466–S467. doi: 10.1097/01.ccm.0000186783.34908.18
Rhie M. N., Park B., Ko H. J., Choi I. G., Kim O. B. (2018). Transcriptome analysis and anaerobic C4 -dicarboxylate transport in Actinobacillus succinogenes. Microbiologyopen 7 (3), e00565. doi: 10.1002/mbo3.565
Ryan E. T., Calderwood S. B., Qadri F. (2006). Live attenuated oral cholera vaccines. Expert Rev. Vaccines 5 (4), 483–494. doi: 10.1586/14760584.5.4.483
Saraiva M., O'Garra A. (2010). The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 10 (3), 170–181. doi: 10.1038/nri2711
Sharma S. K., Moe T. S., Srivastava R., Chandra D., Srivastava B. S. (2011). Functional characterization of VC1929 of Vibrio cholerae El tor: role in mannose-sensitive haemagglutination, virulence and utilization of sialic acid. Microbiol. (Reading) 157Pt. 11), 3180–3186. doi: 10.1099/mic.0.050245-0
Tlaxca J. L., Ellis S., Remmele R. L. Jr. (2015). Live attenuated and inactivated viral vaccine formulation and nasal delivery: potential and challenges. Adv. Drug Delivery Rev. 93, 56–78. doi: 10.1016/j.addr.2014.10.002
Wang Y. D., Huang S. J., Chou H. N., Liao W. L., Gong H. Y., Chen J. Y. (2014). Transcriptome analysis of the effect of Vibrio alginolyticus infection on the innate immunity-related complement pathway in Epinephelus coioides. BMC Genomics 15 (1), 1102. doi: 10.1186/1471-2164-15-1102
Wang P., Zhao C., Wang C., Fan S., Yan L., Qiu L. (2018). TLR3 gene in Japanese sea perch (Lateolabrax japonicus): Molecular cloning, characterization and expression analysis after bacterial infection. Fish Shellfish Immunol. 76, 347–354. doi: 10.1016/j.fsi.2018.01.013
Wang J., Zou L. L., Li A. X. (2014). Construction of a streptococcus iniae sortase a mutant and evaluation of its potential as an attenuated modified live vaccine in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 40 (2), 392–398. doi: 10.1016/j.fsi.2014.07.028
Wei G., Cai S., Wu Y., Ma S., Huang Y. (2020). Immune effect of Vibrio harveyi formalin-killed cells vaccine combined with chitosan oligosaccharide and astragalus polysaccharides in ♀Epinephelus fuscoguttatus×♂Epinephelus lanceolatus. Fish Shellfish Immunol. 98, 186–192. doi: 10.1016/j.fsi.2020.01.015
Xiong X. P., Wang C., Ye M. Z., Yang T. C., Peng X. X., Li H. (2010). Differentially expressed outer membrane proteins of Vibrio alginolyticus in response to six types of antibiotics. Mar. Biotechnol. (NY) 12 (6), 686–695. doi: 10.1007/s10126-009-9256-4
Zapata A., Diez B., Cejalvo T., Gutierrez-de Frias C., Cortes A. (2006). Ontogeny of the immune system of fish. Fish Shellfish Immunol. 20 (2), 126–136. doi: 10.1016/j.fsi.2004.09.005
Zhang Y., Deng Y., Feng J., Guo Z., Mao C., Chen H., et al. (2021). CqsA inhibits the virulence of Vibrio harveyi to the pearl gentian grouper (♀Epinephelus fuscoguttatus × ♂Epinephelus lanceolatus). Aquaculture 535, 736346. doi: 10.1016/j.aquaculture.2021.736346
Zhang Y., Tan H., Yang S., Huang Y., Cai S., Jian J., et al. (2021). The role of dctP gene in regulating colonization, adhesion and pathogenicity of Vibrio alginolyticus strain HY9901. J. Fish Dis 45 (3), 421–434. doi: 10.1111/jfd.13571
Zhang Z., Wu H., Xiao J., Wang Q., Liu Q., Zhang Y. (2012). Immune responses of zebrafish (Danio rerio) induced by bath-vaccination with a live attenuated Vibrio anguillarum vaccine candidate. Fish Shellfish Immunol. 33 (1), 36–41. doi: 10.1016/j.fsi.2012.03.031
Zhou S., Tu X., Pang H., Hoare R., Monaghan S. J., Luo J., et al. (2020). A T3SS regulator mutant of Vibrio alginolyticus affects antibiotic susceptibilities and provides significant protection to Danio rerio as a live attenuated vaccine. Front. Cell Infect. Microbiol. 10. doi: 10.3389/fcimb.2020.00183
Keywords: vibrio alginolyticus, dctP gene, pearl gentian grouper, live-attenuated vaccine, immune response
Citation: Zhang Y, Zhang Z, Zhang J, Huang Y, Jian J and Cai S (2022) A live attenuated strain of HY9901ΔdctP provides protection against Vibrio alginolyticus to the pearl gentian grouper (♀Epinephelus fuscoguttatus × ♂Epinephelus lanceolatus). Front. Mar. Sci. 9:907407. doi: 10.3389/fmars.2022.907407
Received: 29 March 2022; Accepted: 06 June 2022;
Published: 08 August 2022.
Edited by:
Seyed Hossein Hoseinifar, Gorgan University of Agricultural Sciences and Natural Resources, IranReviewed by:
Christopher Marlowe Arandela Caipang, University of San Agustin, PhilippinesHa Thanh Dong, Asian Institute of Technology, Thailand
Copyright © 2022 Zhang, Zhang, Zhang, Huang, Jian and Cai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuanghu Cai, caish@gdou.edu.cn
 Yilin Zhang
Yilin Zhang Ziyu Zhang1,2
Ziyu Zhang1,2  Jichang Jian
Jichang Jian Shuanghu Cai
Shuanghu Cai