Contribution of Intermediate and High Trophic Level Species to Benthic-Pelagic Coupling: Insights From Modelling Analysis
- 1Department of Biology, University of Bari, Bari, Italy
- 2Department of Oceanography, National Institute of Oceanography and Applied Geophysics - OGS, Trieste, Italy
Benthic-pelagic coupling (BPC) is a combination of downward (from pelagic to benthic) and upward (from benthic to pelagic) flows of organic matter and nutrients mediated by trophic interactions in the food web. Hydrological changes in marine ecosystems affect BPC patterns at several temporal and spatial scales. Thus, a food-web perspective help to to quantify and disentangle the role of ecosystem components and high trophic levels species in the BPC. This study investigated the spatio-temporal variability of energy and matter flows between the benthic and pelagic domains in two areas (Salento and Calabria) of the Northern Ionian Sea (Central Mediterranean Sea) during two different periods. The region is subject to large-scale oceanographic changes, e.g., the Adriatic-Ionian Bimodal Oscillating Systems (BiOS), that might result in relevant spatial and temporal BPC changes. Four food-web models describe the trophic structure, the role of ecosystem components and energy flows in the Salento and Calabrian areas, during two BiOS periods, the anticyclonic (1995-1997) and the cyclonic phases (2003-2005). The food webs are described by 58 functional groups obtained by aggregating species into ecological domains, depth gradients and biological traits. The role of species in the BPC has been quantified using a new Benthic-Pelagic Coupling Index calculated on the basis of food web flows estimated by models. The results highlight the pivotal role of deep faunal communities, in which demersal and benthopelagic species sustain upward energy flows towards the pelagic domain and shelf faunal communities. Temporal changes driven by BiOS affect the trophic state of the deep communities resulting in considerable variations in their amount of consumption flows. In addition, the presence of submarine canyons seems to better support the stability of the Calabrian food web in both investigated periods, whereas geomorphological traits of the Salento area seem to support greater pelagic production during the cyclonic period than the anticyclonic one. Benthopelagic species show an important role as couplers. In particular, Aristaemorpha foliacea, Hoplostetus mediterraneus, Macrourids and Plesionika martia are important couplers of bathyal communities in both areas.
Introduction
The functioning of marine ecosystems is mainly driven by the primary production in the pelagic domain, where the energy moves from phytoplanktonic organisms to large pelagic predators. The pelagic food chain is not isolated, but a part of the overall pelagic matter that sinks to the seabed, such as faecal material and the remains of dead individuals, while another part is consumed by organisms that take food from the water column, including benthic organisms (Kiljunen et al., 2020). Once pelagic matter reaches the bottom, it becomes available to benthic organisms and can be recycled back into the pelagic domain through physical mechanisms, such as resuspension processes, or through a “biological transport”, this latter mediated by demersal and benthopelagic organisms (Griffiths et al., 2017). Therefore, benthic-pelagic coupling (BPC) results from the combination of downward (from pelagic to benthic) and upward (from benthic to pelagic) energy pathways. Trophic interactions connect the benthic domain to the pelagic domain in both direct (such as direct predation) and indirect ways (as trophic mediation by benthopelagic and demersal species). The term BPC is used to indicate all processes able to influence the pelagic and benthic domains without an effective distinction of the flow direction (Baustian et al., 2014).
BPC patterns in marine ecosystems concern the impacts of hydrological changes on the food web and the energy pathway mediated trophic interactions at several temporal and spatial scales (Coll et al., 2013; Cresson et al., 2020). In fact, temporal and spatial modifications to the water column structure influence the biological components with effects on the ecological community structure and trophic interactions patterns. Several studies have focused on seasonal variations in BPC mechanisms involving the pelagic or benthic domain (Pitt et al., 2008; Kiljunen et al., 2020), or on a mid-term scale exploring the relationships between nutrients, phytoplankton, and suspension feeders (Chauvaud et al., 2000). However, investigations aiming to explore the effects of these changes in the long-term (e.g., decadal scale) on the role of demersal and species in the ecosystems are very scarce, especially in the Mediterranean Sea (Agnetta et al., 2019). Yet, deepening the understanding of BPC could be very important from a fisheries management perspective, being able to provide information to address Ecosystem-Based Fishery Management (EBFM, Pikitch et al., 2004). One area highly influenced by mesoscale oceanographic features and where BPC patterns are supposed to importantly affect ecosystem dynamics in the Mediterranean basin is the Northern Ionian Sea (Menna et al., 2019).
The Northern Ionian Sea is the deepest basin in the Mediterranean Sea, where hydrography, geomorphology, temporal changes of environmental conditions and fishing impacts have shaped the structure of the demersal and benthopelagic assemblages (D’Onghia et al., 1998; Capezzuto et al., 2010; Civitarese et al., 2010; Maiorano et al., 2010; D’Onghia et al., 2012; Carlucci et al., 2018). In particular, the chemical and physical traits of the water column are affected by peculiar temporal events, which result in the reversal of the upper layer circulation in the Northern Ionian Gyre (NIG) over a decadal time scale, indicated with the term Bimodal Oscillating System (BiOS, Gačić et al., 2010). Little information exists, and few investigations have been carried out on the role of demersal and benthopelagic species in the BPC mechanisms and their relationships with hydrographic changes occurring during BiOS oscillations.
BPC patterns are mainly studied by means of stable isotope analysis (SIA) both in freshwater (Wang et al., 2020) and marine ecosystems (Duffill Telsnig et al., 2019; Kiljunen et al., 2020). Most BPC analyses focus on biogeochemical cycles, involving plankton and lower trophic level organisms (Mussap and Zavatarelli, 2017; Rodil et al., 2020). A few SIA studies in shallow seas (Kiljunen et al., 2020), and in deep habitats (Boyle et al., 2012; Trueman et al., 2014) have explored the role of intermediate and high trophic level species in BPC mechanisms. However, the use of the SIA technique is not properly suited for investigating the long-term dynamics of BPC mechanisms in complex ecosystem contexts with high numbers of species (Shiffman et al., 2012). In these contexts, the food web modelling approach could be a more efficient and less expensive way than the application of SIA protocols. In fact, food web modelling allows the quantification of energy flows and the BPC pattern in marine exploited ecosystems (Lassalle et al., 2011; Banaru et al., 2013; Agnetta et al., 2019; Carlucci et al., 2021) and it is possible to disentangle the role of species in the BPC.
The goal of this study is to identify the role of different species/groups from plankton to top predators in BPC mechanisms in the Salento and Calabria food webs previous investigated in (Ricci et al. 2019). Insights into BPC and its variability over space and time are analysed by looking at the properties and changes which occurred in the Northern Ionian Sea in two periods (the 1995-1997 anticyclonic phase, and the 2003-2005 cyclonic phase). Specifically, four mass-balance models have been realized to describe the trophic structure, species roles and energy flows in the Salento (north-eastern zone along the Apulian coast) and Calabrian (south-western zone). The role of species in coupling processes between the pelagic and benthic domains has been assessed using a new Benthic-Pelagic Coupling Index (BPCI) calculated thorough the consumption flows in the food webs estimated by a mass-balance model.
Materials and Methods
Study Area
The Northern Ionian Sea lies between Cape Otranto and Cape Passero (Sicily) along a coastline of about 1000 km. This area is characterized by very deep zones (up to 4000 m in depth) with a complex geomorphology that results in different features between the western and eastern sector. In the western area, the Calabrian shelf platform is very narrow and shaped by active canyons transporting materials from the shelf break to the deep bottoms (Capezzuto et al., 2010). Conversely, in the eastern sector, corresponding to the Apulian region, the continental shelf is wider and abrasion terraces and bioclastic calcareous deposits with several coral rocks are distributed from the shallowest to the deepest grounds (D’Onghia et al., 2016). These two sectors are divided by the Taranto Valley, a large NW-SE oriented submarine canyon with depths of over 2200 meters (Rossi and Gabbianelli, 1978). The geomorphological diversity of the basin is reflected in different habitat distribution along the coastline and in the deep grounds affecting the abundances of megafauna in benthic and pelagic domains (D’Onghia et al., 1998; Maiorano et al., 2010; D’Onghia et al., 2011; Capezzuto et al., 2018; Capezzuto et al., 2019; Carlucci et al., 2021b).
The entire basin is characterized by a complex system of water circulation both in the upper and deeper layers, showing reversals of the NIG direction affected by BiOS. The NIG inversion from anticyclonic to cyclonic, and vice versa, is influenced by the inlet of Atlantic Water (AW) eastward and salty Levantine waters westward (Civitarese et al., 2010; Liu et al., 2021). In addition, these oscillations are also influenced by the cold dense deep-water masses of the Adriatic Sea flowing in through the Otranto Channel (Figures 1A, B). BiOS induce strong impacts on biogeochemical cycles and transport of particulate organic matter (Klein et al., 1999; Boldrin et al., 2002), primary productivity (D’Ortenzio et al., 2003; Lavigne et al., 2018), the zooplankton community (Mazzocchi et al., 2003), the adaptation of allochthonous species and biodiversity (Civitarese et al., 2010), as well as the population dynamics of species at higher trophic levels (Capezzuto et al., 2010; Maiorano et al., 2010; D’Onghia et al., 2012; Carlucci et al., 2018; Ricci et al., 2021). Therefore, the complexity of these hydrological changes could be reflected in abundance changes of several species in the food web with modification to the trophic interactions and the BPC pattern. Furthermore, changes in the water column structure are not spatially homogeneous in the Ionian basin, but they show differences between the coastal areas in the western and eastern sectors (De Lazzari et al., 1999; Boldrin et al., 2002; Mazzocchi et al., 2003).
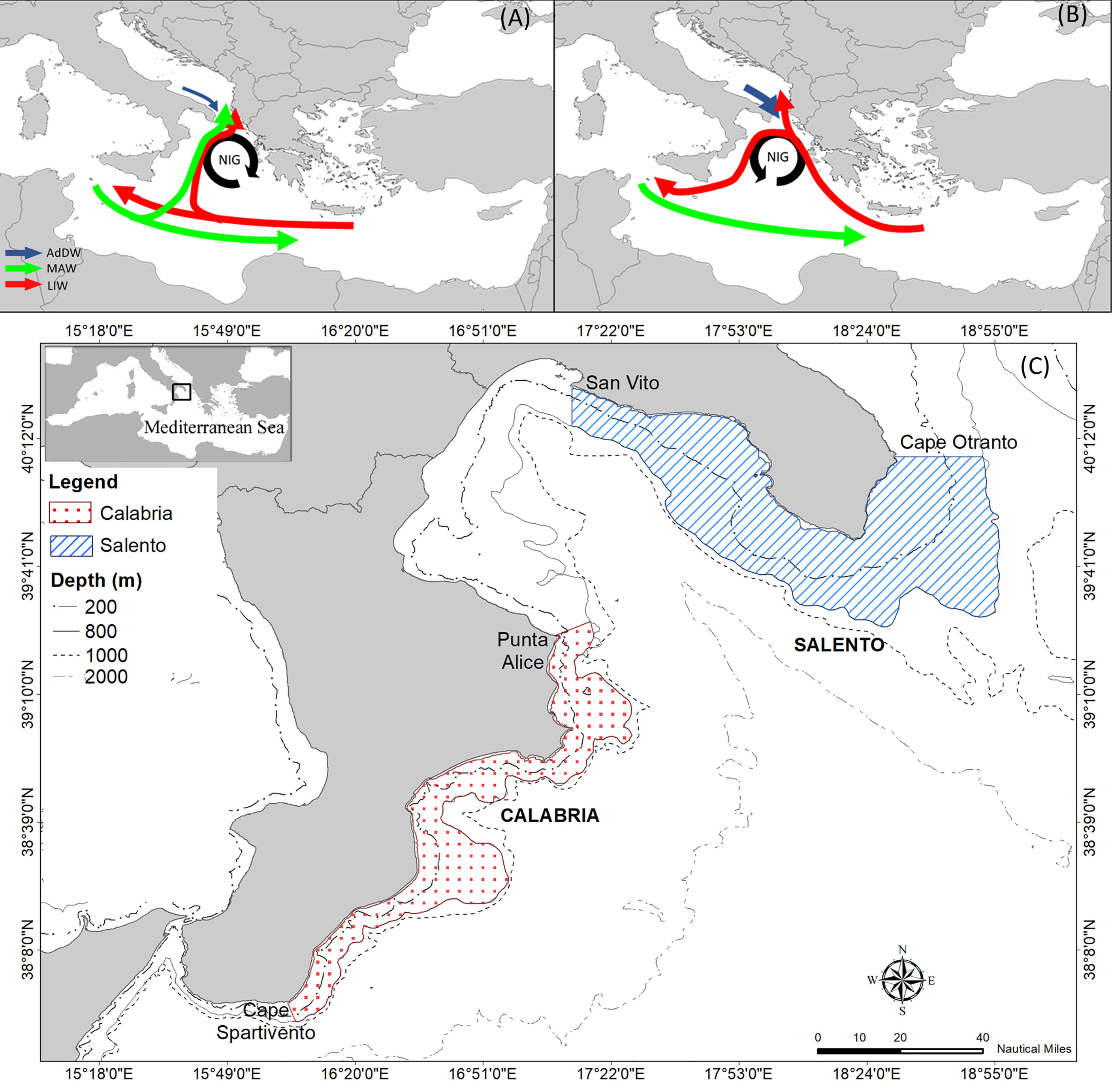
Figure 1 Scheme of the Bimodal Oscillating System (BiOS) with the representation of hydrographic circulation during (A) anticyclonic and (B) cyclonic periods in the Ionian and Adriatic Seas (modified by Civitarese et al., 2010). Water current acronyms: MAW is Modified Atlantic Water, LIW is Levantine Intermediate Water, AdDW is Adriatic Dense Water and NIG is Northern Ionian Gyre. (C) Map of modelled study areas in the Northern Ionian Sea.
In this study, the food webs of two distinct areas, extending between 10–800 m in depth, were modelled (Ricci et al., 2019). Specifically, the Salento food‐web model (SAL) represents the domain delimited by Capo Otranto and Capo San Vito (Taranto) and covers an area of approximately 6660 km2. Whereas the domain of the Calabrian food-web model (CAL) extends from Punta Alice to Capo Spartivento for approximately 3469 km2 (Figure 1C).
Modelling Approach
The mass-balance models were built by means the Ecopath with Ecosim approach (EwE, Christensen & Walters, 2004), which for over 30-years has been the most used modelling tool to study marine food web dynamics and fishing impacts (Coll and Libralato, 2012). The trophic structure is represented by Functional Groups (FGs) and trophic flows. The former, is represented by a single species, a life stage of a species, or a group of species with similar trophic, ecological, and physiological features. Trophic flows between FGs are formally described by a set of two linear equations for each FG (Christensen et al., 2008). The first equation represents the fate of production:
where (P/B) is the production to biomass ratio for a certain functional group (i), Bi is the biomass of a group (i), Yi the fishery catch of group (i), (Q/B)j is the consumption to biomass ratio for each predator (j), DCji is the proportion of the group (i) in the diet of predator (j), Ei is the net migration rate of a group from the modelled area, BAi is the biomass accumulation rate for the group (i), EEi is the ecotrophic efficiency, and the term (1−EEi) represents other mortality different from predation and fishing. The second equation represents the consumption of a group:
Equation (1) and (2) for all functional groups make a system of equations that is solved by providing EwE routine information on three out of the four basic parameters Bi, (P/Bi), (Q/Bi) and EEi. Further details on the EwE modeling approach can be found in review literature (Christensen and Walters, 2004; Christensen et al., 2008; Heymans et al., 2016).
Model Structure and Data
The food web model in both investigated areas was described by means of the same functional groups obtained from the model realized by (Ricci et al. 2019). In particular, the nomenclature adopted for demersal and benthopelagic FGs have three components: the former indicates the bathymetric domain, the second the faunal category of the main taxa, and the last describes the feeding behaviour of the group (Table 1). Therefore, FGs were mainly classified according to their belonging to ecological domains and their depth layer distribution made explicit in Table 1. The former classification was based on 5 general domains: Pelagic (PEL, including the planktonic groups and particulate organic matter), Benthopelagic (BP), Demersal (DEM), Benthic (BENT, including the bottom detritus and discards). This classification was carried out using the information on the single species obtained from Fishbase (Froese and Pauly, 2021; www.fishbase.org, version 08/2021) and Sealifebase (Palomares and Pauly, 2021; www.sealifebase.org, version 12/2021), as well as information from video surveys (e.g., Lorance and Trenkel, 2006; D’Onghia et al., 2011; D’Onghia et al., 2015). The depth layer classification aggregates FGs into 5 categories: groups of shelf grounds (SH) and neritic zone (NER) distributed at depths < 200 m; groups of open waters (or off-shore, OFS); groups on sloping grounds (SL) distributed at depths > 200 m; ubiquitous groups (U) distributed throughout the entire depth gradient and in neritic and off-shore zones (e.g., macrobenthic invertebrates, suprabenthic crustaceans, planktonic groups). The classification based on the bathymetric gradient was driven by the Centre of Gravity (COG) calculated for the species of benthopelagic, demersal and benthic domains (see Ricci et al., 2019). Finally, the classifications for domain and depth layer were combined in a unique classification named Domain-depth (Table 1).
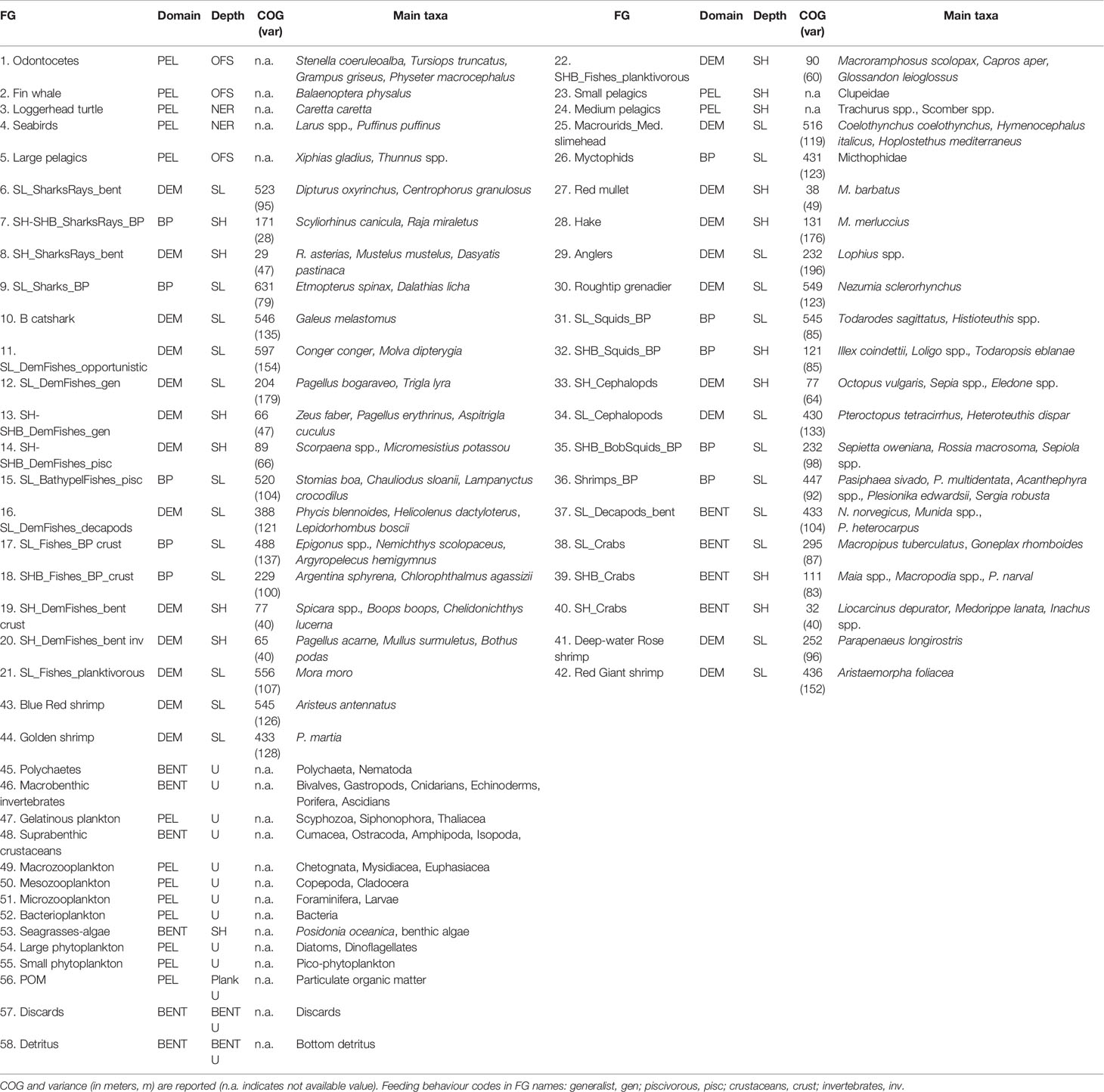
Table 1 Functional groups (FG) used in both Ecopath models classified by domains (Pelagic, PEL; Benthopelagic, BP, Demersal, DEM; Benthic, BENT) and depth layers (Neritic, NER; Off-shore, OFS; Shelf, SH; Slope, SL, Shelf-Break, SHB only for FGs; Ubiquitarian, U).
Input data (Biomass, P/B and Q/B rates, Landings and Discards) of the balancing models are reported in Tables S1, S2 for the Salento and Calabrian food webs, respectively. Biomasses (kg/km2) for a total of 276 benthopelagic and demersal species that were obtained from the experimental sampling of the “MEDiterranean International Trawl Survey” (MEDITS) research programme for all investigated periods (Spedicato et al., 2019). The biomass input of each FG was calculated as an average value for the 3-year period investigated in each model (Heymans et al., 2016). Biomass data of cetacean groups and Loggerhead turtle (1, 2, 3) were estimated by abundance data (N/km2) obtained from the OBIS SeaMap (Halpin et al., 2009) and the local mean individual weight for the Ionian Sea (Ricci et al., 2020; Carlucci et al., 2021).
Biomasses of zooplankton and benthic groups were estimated fixing EE at a value of 0.90 for the polychaetes, macrobenthic invertebrate groups and gelatinous plankton, at 0.95 for the suprabenthic crustacean groups, and at a value of 0.99 for the macro and mesozooplankton (Heymans et al., 2016) FGs. Biomasses of phytoplankton and bacterioplankton groups for each modelled area were estimated using biogeochemical data of models available from 1998 (Lazzari et al., 2012). The lack of available data for the period 1995-1997 led to the choice of using an average value for the period 1998-1999, as adopted in the previous models realized for the area (Ricci et al., 2019).
P/B and Q/B rates were obtained from the previous model in (Ricci et al. 2019) collecting data from the literature or empirical relationships based on the total mortality (Z) as an equivalent of P/B rate (Allen, 1971).
Diet information was acquired from previous models realized for the same study areas (Ricci et al., 2019; Ricci et al., 2021), with some update inherent to Engraulis encrasicolus and Sardina pilchardus diet information in the Small pelagic group obtained from the literature (Zorica et al., 2017; Hure and Mustać, 2020). Further updates concern local diets of Helicolenus dactylopterus and Pagellus bogaraveo (Capezzuto et al., 2020; Capezzuto et al., 2021).
Official annual landings by species for GSA 19 were provided by the Fisheries and Aquaculture Economic Research for the Italian Ministry of Agricultural Food and Forestry Policies (MIPAAF) with data separated for the Calabria and Apulia regions by otter trawls (OTB), long lines (LL), passive nets (GND), purse seines (PS) and other gears (MIX, mainly small-scale fisheries) in the period 1995–2005. Landings and discards by FG, species, and fishing gears were estimated for the periods 1995-1997 and 2003-2005 as reported in Ricci et al. (2019).
Four mass-balance models were developed using an average of 3 years of data describing the CAL and SAL food webs in two distinct periods, which represent the anticyclonic and cyclonic phases of the BiOS. In particular, the anticyclonic phase referred to the years 1995–1997, whereas the cyclonic phase was represented in the years 2003–2005. Therefore, the final models adopted in the analysis were: 1) Salento 1995-1997 (named model SAL_1995 or anticyclonic); 2) Salento 2003-2005 (named model SAL_2005 or cyclonic); 3) Calabrian 1995-1997 (named model CAL_1995 or anticyclonic); 4) Calabrian 2003-2005 (named model CAL_2005 or cyclonic).
Balancing Steps
Both models belonging to the period 1995-1997 were balanced according to the standard pre-balancing analysis (PREBAL, Link, 2010) and a top-down strategy described in (Ricci et al. 2019). These models were manually balanced by means of modifications to the less reliable values identified in the DC matrix and P/B and Q/B rates through the Pedigree Index (Pauly et al., 2000). In addition, it was checked that Net food conversion efficiencies (P/Q [0.05–0.3]), respiration/assimilation (R/A [<1]), and production/respiration (P/R [<1]) ratios were within expected limits (Christensen et al., 2008). Cannibalism in the diet was decreased for hake, sharks, the demersal fish groups, squids, shrimps, and the decapod crustaceans’ groups (Heymans et al., 2016).
Differently, the Salento and Calabrian models realized for the period 2003-2005 were balanced by adopting PREBAL analysis and a successive balancing step based on the Monte-Carlo routine (Ecoranger, developed in EwE, version 5, Christensen, 2008), in order to change the parameters in a progressive way following an automated procedure to improve model balancing and avoiding further manipulation of the input data. Specifically, the procedure was adopted exclusively for the diet matrix (10% changes for all elements in the diet matrix) given the higher uncertainty in the data, while the most important parameters (biomasses and catches) were kept fixed for each group during the automatic routine (Ricci et al., 2021). Therefore, the Ecoranger routine provided two balanced models (SAL_2005 and CAL_2005) by exploring the range of trophic uncertainty through a more objective procedure and adjusting the diet matrix to the estimated biomass at sea and the observed fishery catch in each period.
Analysis of BPC Patterns and Ecosytem Traits
The whole ecosystem traits selected from the Ecopath output were Total System Throughput (TST), Consumption (Q), Exports (E), Fluxes to Detritus (FD), Respiration (R), Production (P) and Net Primary Production of the system (NPP) (for details on these indicators see Heymans et al., 2014). All ecosystem traits are expressed as t km-2 y-1. These synthetic properties of the ecosystem permit a quick comparison and the detection of relevant overall differences.
BPC patterns were explored by Domain and Domain-depth classifications (Table 1). In particular, the latter classification was performed by merging the following categories between them: groups of neritic and shelf zones (Loggerhead Turtle, Seabirds, Small pelagics and Medium pelagics) were joined and named pelagic-shelf (PEL-SH); Odontocetes, Large pelagics and Fin whale represent the off-shore pelagic domain (PEL-OFS); the demersal and benthopelagic groups were in two different domains in the shelf zone (DEM_BP SH) and in the slope zone (DEM_BP SL), respectively. Seagrasses-algae, SH_Crabs and SHB_Crabs were combined in the shelf-benthic domain (BENT-SH), while SL_Crabs and SL_Decapods_bent were aggregated in the slope-benthic domain (BENT-SL). Polychaetes, Macrobenthic invertebrates and Suprabenthic crustaceans are ubiquitous groups representing a third benthic domain (BENT U). Finally, the zooplankton groups (representing the planktonic domain (PLANK), which were exclusively considered, together with the BENT U domain, as prey in the consumption flows system.
Analysis of Functional Groups’ Role
Comparison between the four models (two areas in two investigated periods) was focusing on biomass changes, keystone species ranks and trophic impacts of FGs, as well as consumption flows in the investigated models.
Biomasses (t km-2 y-1) of FGs with TL>3.0 (1-44 in Table 1) were aggregated and compared by faunistic categories, shelf and slope zones and domains. Moreover, Fishes/Invertebrates and Predator/Prey (Predators TL> 4.0) biomass ratios were calculated.
The importance of FGs as a keystone group/species in the investigated food webs was estimated by means of the Keystoness index (KSi) and Mixed Trophic Impact analysis (MTI, Ulanowicz and Puccia, 1990). MTI quantifies the relative impact of biomass change within a component (impacting group) on each of the other components (impacted groups) in the food web, including the fishing gears. Thus, positive/negative MTI values indicate an increase/decrease in biomass of group j due to a slight increase in biomass of the impacting group i. Therefore, negative impacts can be associated to prevailing top-down effects and positive ones to bottom-up effects (Libralato et al., 2006). The relative overall effect (OE) of an impacting group i represents all the direct or indirect MTI values of group i on all the other groups in the food web:
where the impact on the group itself (mij with i = j) is not considered, and OE is calculated as a relative value with respect to the maximum (Libralato et al., 2006). Therefore, the keystone groups/species are ranked using the following equation:
where pi is the relative biomass of the group, excluding detritus biomass. Changes in the KSi rank composition between two investigated time periods were analysed in both the SAL and CAL food web models. Specifically, the most important KS groups in each food web model were identified by calculating the 3rd quartile on each KS rank. In addition, changes in rel OE of each group between the first and second periods were analysed in both the SAL and CAL models.
The analysis of consumption flows was carried out to classify the role of FGs as couplers of energy flows between pelagic and benthic domains (downward flows, dQf), or vice versa, between benthic and pelagic domains (upward flows, -uQf). A pelagic FG plays the role of direct coupler, for example, if it is effected by a trophic flow (as predator or prey) involving any benthic FG. Groups belonging to the pelagic or benthic domains having direct consumption on benthic or pelagic groups, respectively, contribute directly to the BPC. On the other hand, FGs can be mediating couplers (or two-step couplers) represented by the species of demersal and benthopelagic domains. Species/groups belonging to these domains can drive downward consumption flows by consuming pelagic prey and becoming prey for benthic consumers, or in the upward direction, exploiting benthic prey and becoming prey for pelagic predators. In the latter, some groups could perform partial energy transfers, consuming pelagic or benthic preys with the consequence of transferring part of the energy within the benthopelagic or demersal domains. Successively, these partial couplers could be consumed by other benthopelagic or demersal couplers following other energy transfer pathways towards the pelagic or benthic domains. The downward (dQf) and upward (uQf) consumption flows are identified and quantified through the detailed food webs. Thus, the dQf, indicated with a positive sign, and uQf, expressed with a negative sign can be used to quantify the importance of each FG in BPC mechanisms. To summarise the contribution of each group to the BPC, the Benthic-Pelagic Coupling Index (BPCI, t km-2 y-1) was calculated as:
Therefore, dQf and uQf were calculated for each FG considered as both predators and prey (excluding non-living detritus groups 56-58 from the calculation, Table 1). To identify more important couplers, FGs with a BPCI value higher than 0.100 t km-2 y-1 are classified as direct (D), mediating (M) or partial (P) couplers, and successively, they were analysed using dQf and uQf (expressed as percentage values). High dQf values indicate the prevalence of downward flows (pelagic-benthic coupling), while high values of uQf indicate the predominance of upward flows (benthic-pelagic coupling). Lastly, the COG and variance of each demersal and benthopelagic couplers were analysed in function of BPCI.
Results
Ecosystem Traits and Consumption Flow Patterns Between Domains
Ecosystem traits of the SAL food web models showed relevant temporal differences (Figure 2 and Table S3). All indicators of flow showed values higher in the SAL 2005 models than the SAL 1995, with an increase of 22 and 21% for NPP and TST, respectively. In contrast, no relevant changes were observed in the CAL model between the two investigated periods, with the estimated NPP in 1995 (1137 t km-2 y-1) being slightly lower than that in 2005 (1132 t km-2 y-1). In addition, the CAL TST value in 2005 (4408 t km-2 y-1) was lower than the SAL value in 2005 (6547 t km-2 y-1).

Figure 2 Total System Throughput (TST), Fluxes to Detritus (FD), Sum of Production (P), Sum of Consumption (Q), Sum of Exports (E), Sum of Respiration (R) and Net Primary Production (NPP) estimated for SAL (white circle) and CAL (black triangle) models in 1995-1997 (x-axis) and in 2003-2005 (y-axis). All indicators are expressed as t km-2 y-1.
The patterns of all consumption flows (including POM and Detritus in the pelagic and benthic domains, respectively) characterizing both food webs are shown in Figure 3. The highest consumptions were estimated in the pelagic domains of each model, with a higher increase observed in the SAL food web (3225 t km-2 y-1) than the CAL one (2415 t km-2 y-1) in the 2005 period. Similarly, consumption flows from the pelagic domain to the benthic, benthopelagic and demersal domains increased in 2005 in the SAL food web. In particular, the highest increase was estimated for the Pelagic-Benthic flow, which was equal to 729 t km-2 y-1 in 1995 and 1210 t km-2 y-1 in 2005. However, a slight increase in the consumption of pelagic groups by demersal groups was detected in the CAL model from 1995 (8 t km-2 y-1) to 2005 (11 t km-2 y-1), while the flows from the pelagic domain towards the benthic domain showed a decrease from 663 t km-2 y-1 in 1995 to 616 t km-2 y-1 in 2005. Considering exchanges between the BP and DEM domains, an increase in consumption of BP prey by DEM consumers was observed in the 2005 period for both SAL (4 t km-2 y-1) and CAL (3 t km-2 y-1). In the SAL model, consumptions in the BP domain increased in 2005, as well as consumption flows between BP and the Benthic domain, while in the CAL model this condition was observed only for the flows between the DEM and Benthic domains and an increase in the consumptions in the DEM domains was estimated.
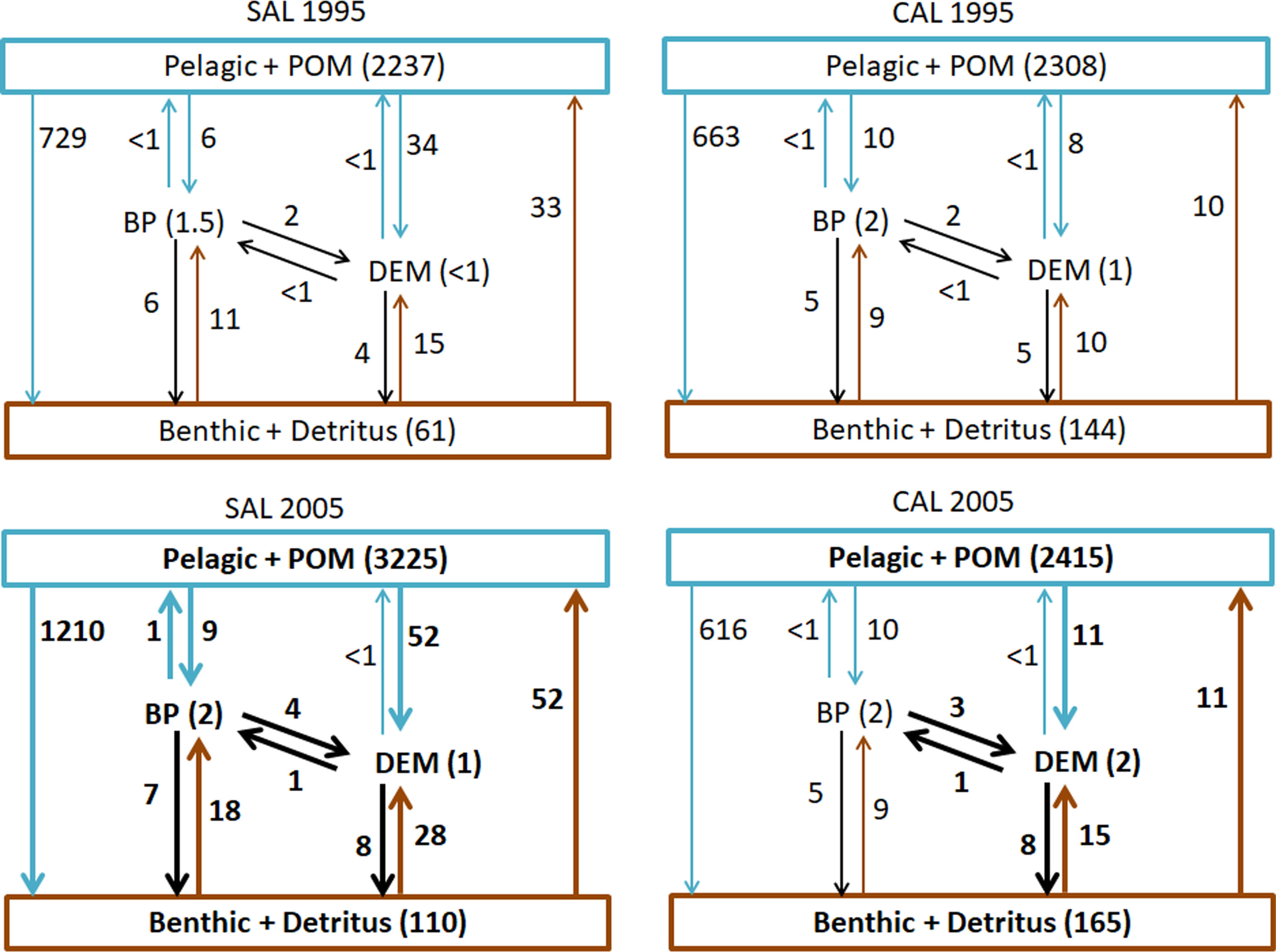
Figure 3 Consumption flows (t km-2 y-1) of groups aggregated into Pelagic+POM, Benthopelagic (BP), Demersal (DEM) and Benthic+Detritus domains analysed in the Salento (SAL) and Calabria (CAL) models for both investigated periods. Bold numbers and arrows indicate increased flows in 2005 and <1 indicate consumption flows lower than 1 t km-2 y-1.
The analysis of the flows according to the domains and depth layers distribution of FGs highlighted the pattern of energy and matter exchanges between shelf and deep zones (Figure 4 and Table S4). In the SAL model, planktonic resources were mostly consumed by demersal and benthopelagic groups of the slope (12.7 t km-2 y-1) and, secondarily, by pelagic neritic consumers (10.2 t km-2 y-1) in 1995. In addition, the ubiquitous benthic groups were mainly exploited by bathyal demersal-benthopelagic groups (8.3 t km-2 y-1), the shallowest benthic decapods (4.7 t km-2 y-1) and the shelf demersal-benthopelagic groups (3.3 t km-2 y-1). This pattern changed in the 2005 period, with the highest increases estimated for the bathyal demersal-benthopelagic groups by consuming planktonic prey (17.6 t km-2 y-1) and the ubiquitous benthic groups (14.1 t km-2 y-1). A very relevant increase in consumptions was estimated for the ubiquitous benthic groups exploited by shallowest benthic decapods (11.4 t km-2 y-1). In addition, shelf demersal-benthopelagic groups showed an increase in all consumptions, with the most relevant values estimated for planktonic prey (6.8 t km-2 y-1) and ubiquitous benthic groups (4.7 t km-2 y-1). Moreover, an increase in consumption values of up to 1.5 t km-2 y-1 was detected for bathyal demersal-benthopelagic groups towards shelf demersal-benthopelagic groups.
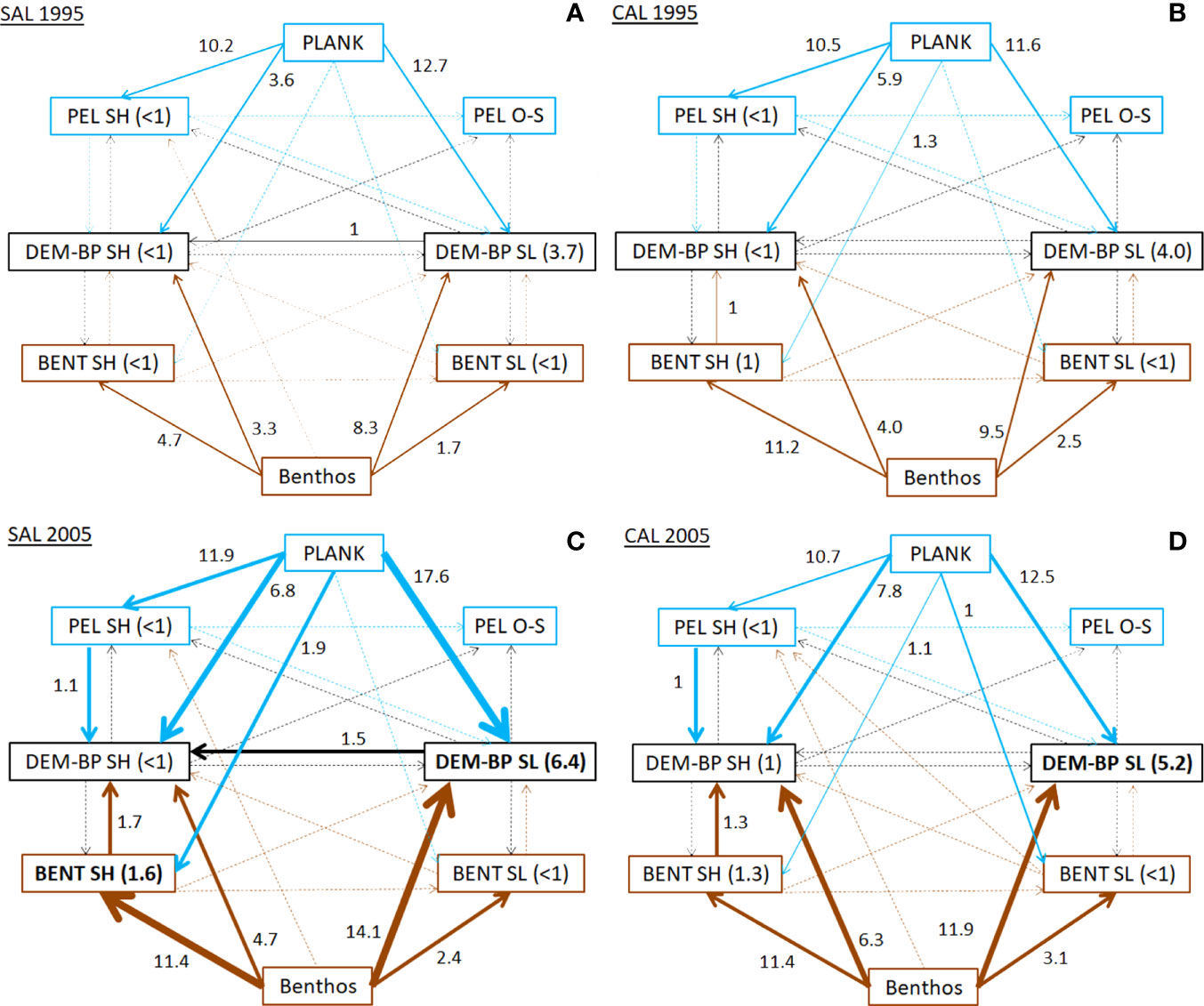
Figure 4 Consumption flows (Q, t km-2 y-1) between FGs aggregated in domains and depth layers for (A) SAL_1995, (B) CAL_1995, (C) SAL_2005 and (D) CAL_2005. Values in brackets indicate the consumption within the same domain. Lines indicate flows from pelagic (blue), benthic (brown) and other domains (black). Bold lines show Q values increases over time higher than 0.5, 2 and 4 t km-2 y-1.
In the CAL model during 1995, the consumption flows from ubiquitous benthic organisms towards the shallowest benthic decapods (11.2 t km-2 y-1), and from planktonic prey to bathyal demersal-benthopelagic groups (11.6 t km-2 y-1) and shelf pelagic consumers (10.5 t km-2 y-1) showed the highest values. In addition, the consumption of ubiquitous benthic organisms by bathyal demersal-benthopelagic groups was equal to 9.5 t km-2 y-1. In 2005, similar increases in the consumption values on planktonic and benthic groups were estimated. The most relevant rises were estimated for the consumption of ubiquitous benthic organisms by both demersal-benthopelagic groups on shelf and slope bottoms (6.3 and 11.9 t km-2 y-1, respectively). Similarly, the consumption of planktonic resources increased for both demersal-benthopelagic groups. These increases were lower than those estimated in the SAL model for the same period.
Finally, an increase in the consumption flows was observed from the shallowest benthic decapods and shelf pelagic organisms towards the shelf demersal-benthopelagic groups in both models during the cyclonic period.
Functional Groups Roles in the Investigated Food Web Models
Outputs estimated by Ecopath models for each FG are reported in Table S1. A general biomass increase was observed from 1995 to 2005 in both food webs, with the highest percentages observed in the SAL food web (Figures 5A, B and Table S5). Considering the faunistic categories, the highest biomass was estimated for bony fishes in both modelled areas. The largest percentage increases were found for decapod crustaceans, cephalopods, and elasmobranchs in the Salento model, whereas in Calabria they were for cephalopods, elasmobranchs, and bony fishes. Changes in shelf and slope grounds showed higher percentage increases in the SAL food web than in the CAL one. In addition, the estimated biomass on the shelf of the CAL area was slightly higher than that estimated on the slope. An inverse condition between biomass in the shelf and slope zones was observed in the SAL food web. The highest percentage increases were observed for groups of the demersal domain in both investigated food webs, with values higher in the SAL model than in the CAL one. In addition, a very slight decrease in the biomass of the pelagic groups was observed in the SAL model, whereas in the CAL model, decreases were observed for the groups in the benthopelagic and benthic domains. The Fish/Inv ratios showed a different condition between the investigated areas, with a decrease of 13% in the SAL food web, and an increase of 4% in the CAL food web (Figure 5C). In addition, the Predator/prey ratios showed similar increases in the SAL and CAL models (3% and 6%, respectively).
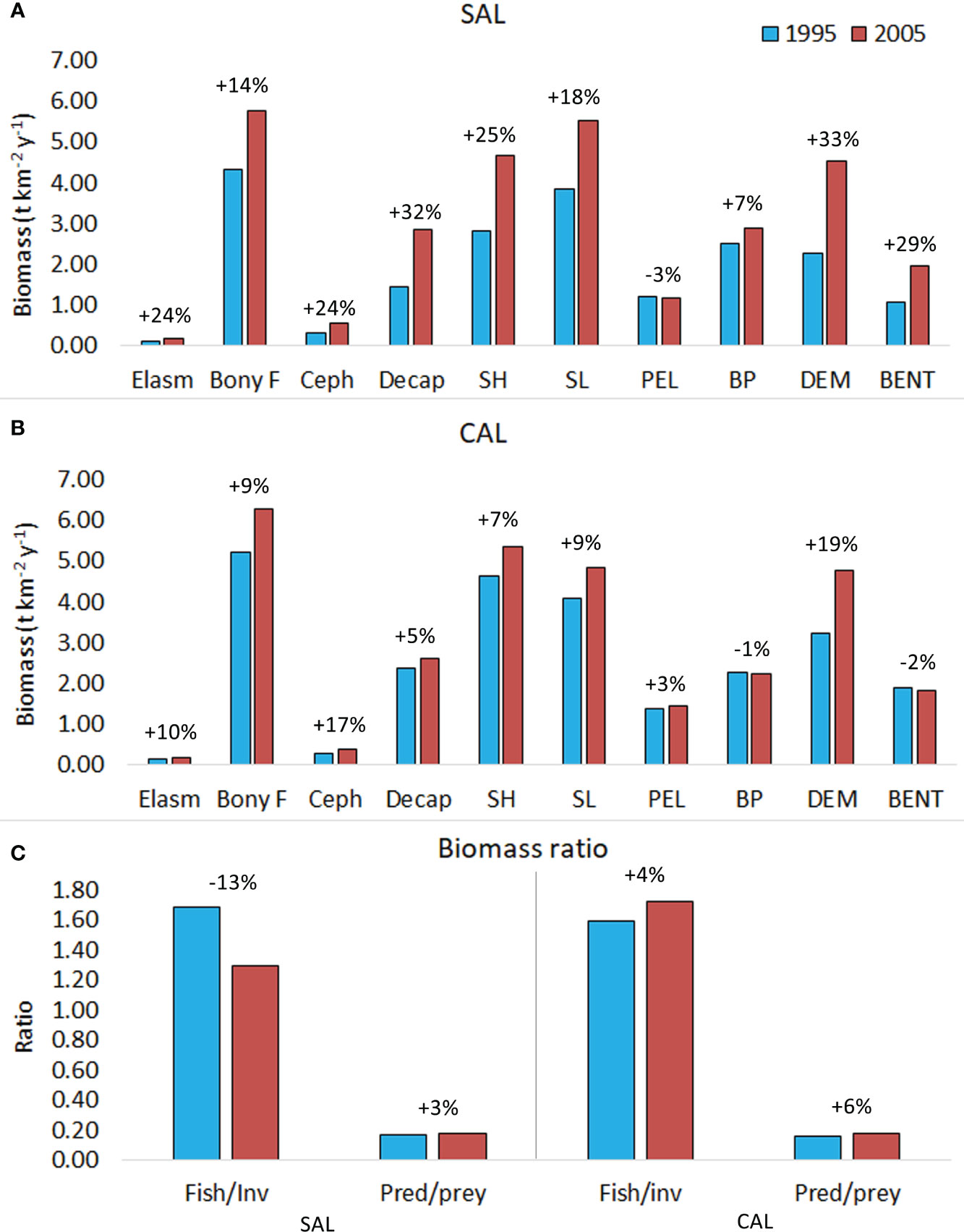
Figure 5 Biomass estimated in all investigated periods for (A) SAL (B) CAL models for faunistic categories (Elasmobranchs, Elasm; bony fishes, Bony F; cephalopods, Ceph; Decapods, Decap); shelf (SH) and slope (SL) and domains. (C) Fish/invertebrates and Predator/prey biomass ratio. Percentages indicated increase (+) or decrease (-) from 1995 to 2005 for each indicator. FGs from 1 to 44 were considered.
Macrozooplankton, Mesozoplankton and Macrobenthic invertebrates were the most impacting groups, showing the highest Overall Effect (OE) in both food webs during all investigated periods (Table S6 and Figures 6A, B). In the SAL model, Odontocetes, Medium pelagics, Parapenaeus longirostris and SL_BathypelFishes_pisc showed the highest OE values in 1995-1997 (Figure 6A). Whereas, SH_Cephalopods, SL_Squids_BP, Aristaemorpha foliacea, and SHB_Squids_BP showed the highest values in the period 2003-2005. In the CAL model, Anglers, Odontocetes, Sl_Sharks_BP and SL_BathypelFishes_pisc showed the highest OE in the period 1995-1997, whileOE values increased in SL_Squids_BP, SH_Cephalopods, SL_Crabs and A. foliacea in the period 2005-2007 (Figure 6B). The main KS groups identified in the SAL model during both investigated periods were zooplankton groups (49-50-51), Macrobenthic invertebrates, Polychaetes (46-45), Shrimps BP (36), Small pelagics (23), the cephalopods groups SH_Cephalopods. SL_Squids_BP, SHB_Squids_BP, SHB_Crabs and Small phytoplankton (Table S4 and Figures 6C–E). In addition, Medium pelagics, Odontocetes, and SL_BathypelFishes_pisc showed high KS values in 1995, but were later replaced by A. foliacea, and Suprabenthic crustaceans in 2005, and the importance of cephalopods increased as keystone groups. In the CAL model, all groups of zooplankton, SL_Squids_BP, Anglers, Medium pelagics, Macrobenthic invertebrates and Polychaetes, Shrimps BP, Small pelagics and SHB_Fishes_BP crust represented the most important keystone groups in both investigated periods (Figures 6D–F). Relevant changes in the KS rank were observed for SL_Sharks_BP, SL_BathypelFishes_pisc and Odontocetes, which showed high values in 1995. However, these groups were replaced by SH_Cephalopods, SHB_Squids_BP and Suprabenthic crustaceans during 2005.
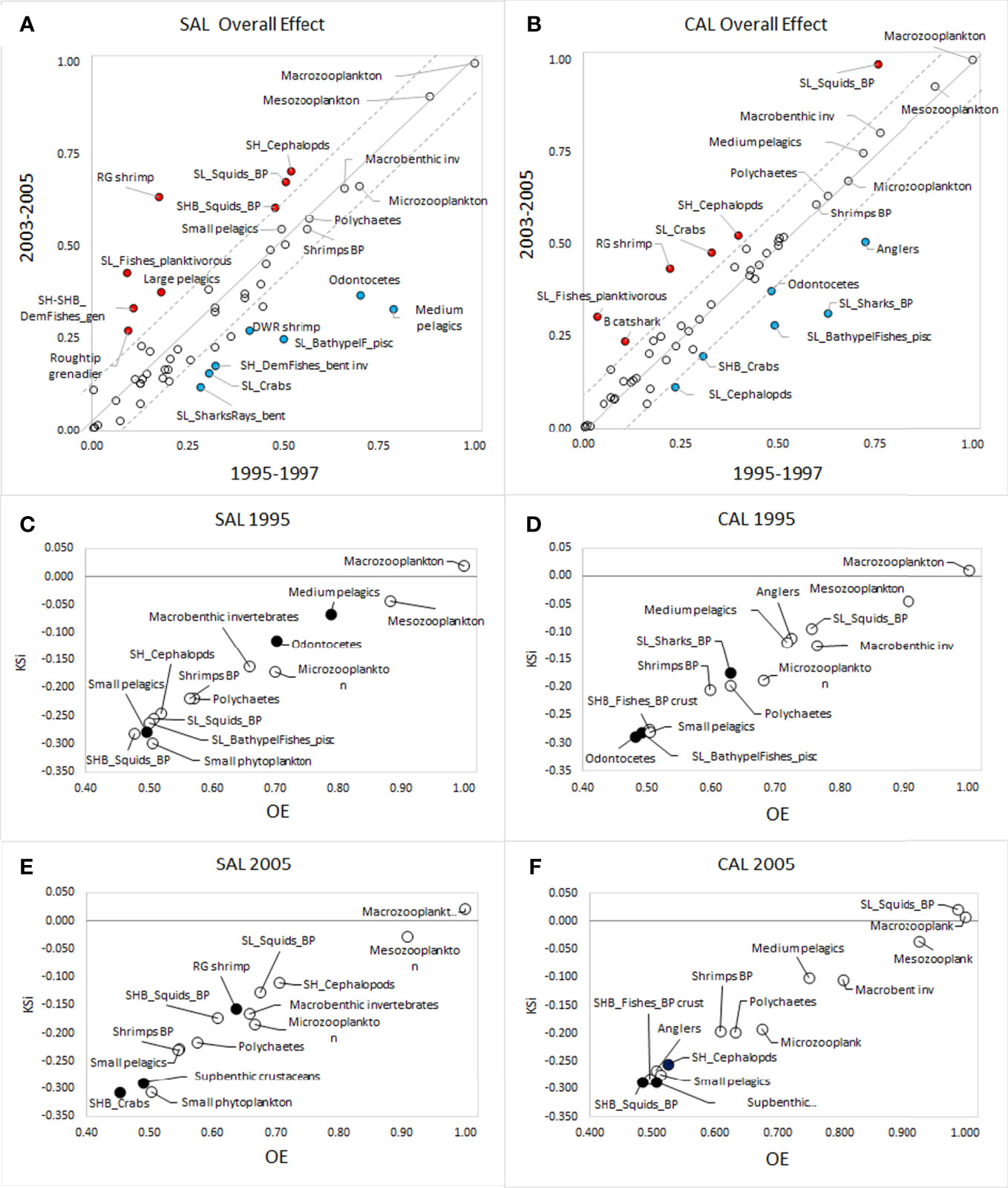
Figure 6 Overall Effect estimated for all living FGs in the (A) SAL and (B) CAL models. Values higher in the period 1995-1997 (x-axis) are blue-coloured, while values higher in the period 2003-2005 (y-axis) are red-coloured. FGs ranked by KSI and OE in models (C) SAL 1995; (D) CAL 1995, (E) SAL 2005 and (F) CAL 2005. Black circles indicate FGs in 1995 replaced by others in 2005.
Direct couplers supported the energy transfer from the pelagic to the benthic domain, such as Suprabenthic crustaceans, Macrobenthic invertebrates, Small phytoplankton, Macrozooplankton, Mesozooplankton, Polychaetes and Bacterioplankton, with dQf (downward) percentage values greater than 6%, while uQf (upward) were lacking for all these groups (Tables 2, 3 and Figures 7A–D). Differently, the upward flows from the benthic to the pelagic domain were supported by trophic interactions of demersal and benthopelagic FGs. In the deep benthopelagic domain, Shrimps BP (maximum BPCI= 11.69 t km-2 y-1, in the SAL 2005 model) and Myctophids (BPCI= 6.85 t km-2 y-1, in the SAL 2005 model) were identified as the most important mediating couplers of all the food webs models investigated. The former more predominantly supported upward flows (19-29%) than downward ones (1-3%), while the latter showed more similar percentage values between dQf (2-4%) and uQf (4-7%). Other minor benthopelagic couplers detected in both food webs are SHB_BobSquids_BP, SL_BathypelFishes_pisc, SHB_Squids_BP.

Table 2 FGs classified as direct (D), mediating (M) and partial (P) couplers (Coup.) in the SAL food web with their respective estimated downward flow (dQf), upward flow (uQf) (expressed in t km-2 y-1 and %) and Benthic-Pelagic Coupling Index (BPCI, t km-2 y-1).
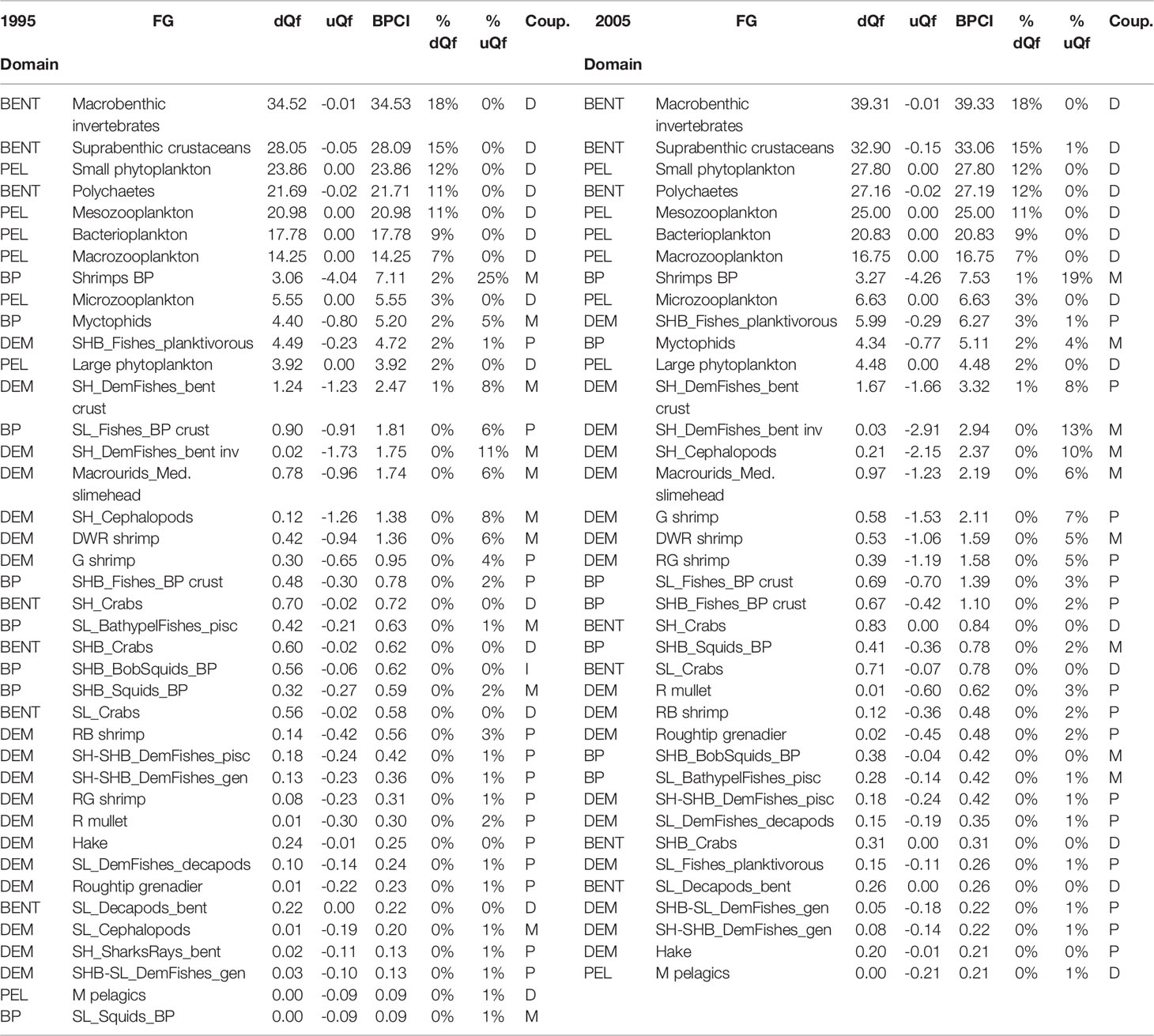
Table 3 FGs classified as direct (D), mediating (M) and partial (P) couplers (Coup.) in the CAL food web with their respective estimated downward flow (dQf), upward flow (uQf) (expressed in t km-2 y-1 and %) and Benthic-Pelagic Coupling Index (BPCI, t km-2 y-1).
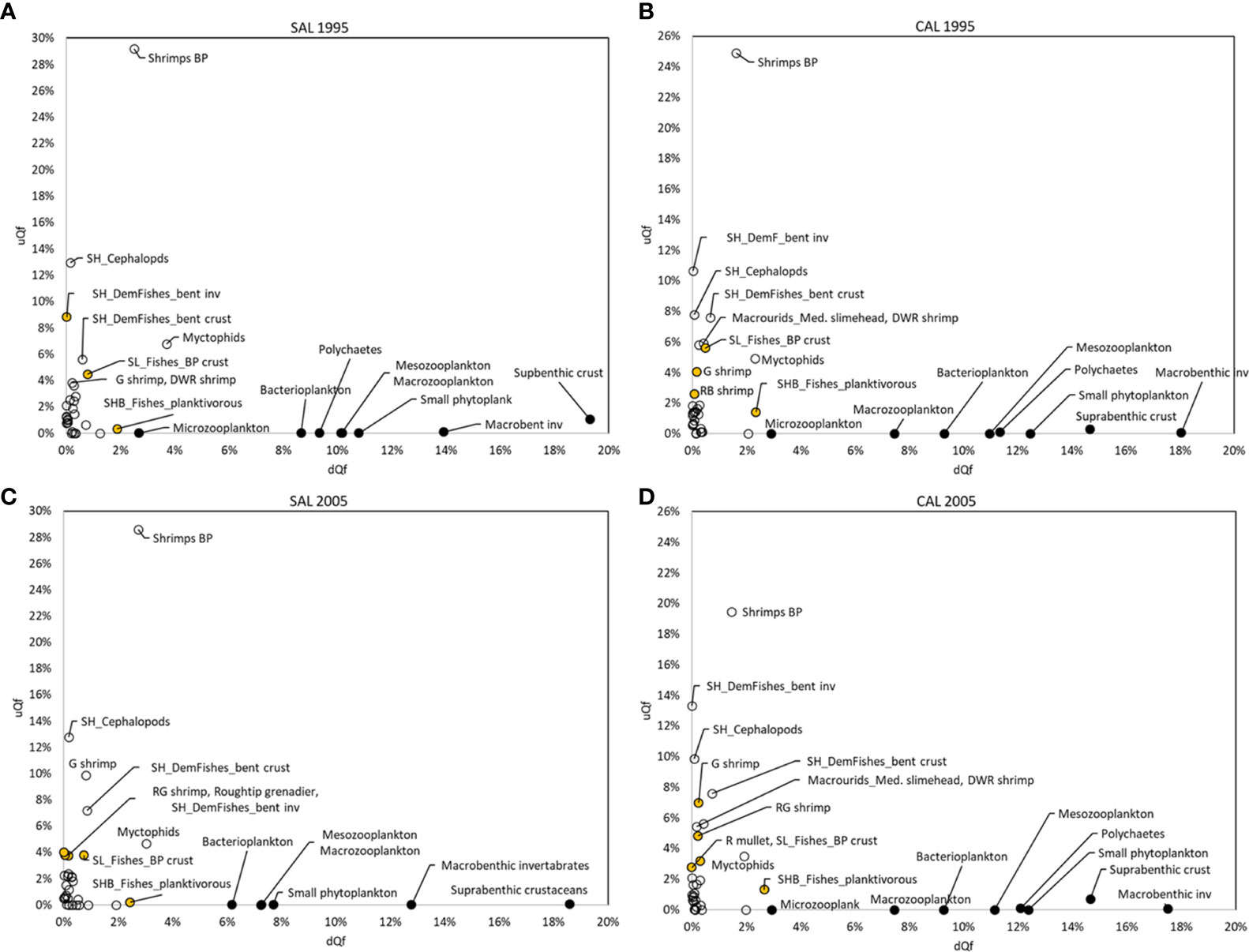
Figure 7 Direct (black circle), mediating (white) and partial (orange) couplers by downward (dQf, x-axis) and upward (uQf, y-axis) flows (%), estimated for all investigated (A–C) SAL and (B–D) CAL models.
In all investigated models, demersal FGs were mediating or partial couplers characterized by uQf values that ranged between 1-13%. SHB_F_planktivorous (Macroramphosus scolopax, Capros aper) was the only group with dQf percentage values around 2%.
In the SAL food web, SH_Cephalopods were the most important demersal mediating coupler in 1995 and 2005 (BPCI=2.07 and 3.21t km-2 y-1, respectively), with uQf values of 13% (Table 2 and Figures 7A, C) In addition, SH_DemFishes_bent inv (uQf 9%), SH_DemFishes_bent crust (uQf 6%), SL_Fishes_BP crust, Plesionika martia and P. longirostris (all with uQf values of 4%) were relevant couplers in 1995. This rank changed in 2005, when the most important couplers were P. martia (uQf 10%), SH_DemFishes_bent crust (uQf 7%), A. foliacea, Nezumia sclerorhynchus, SH_DemFishes_bent inv and SL_Fishes_BP crust (all with a uQf value of 4%).
In the CAL food web, SH_DemFishes_bent inv, SH_Cephalopods and SH_DemFishes_bent crust were the most important mediating couplers of the demersal domain in both investigated periods, with the highest uQf values in 2005 (13%, 10% and 8%, respectively) (Table 3 and Figures 7B, D). Moreover, P. longirostris, Macrourids_Med. slimehead and SL_Fishes_BP crust (all uQf values of 6%), P. martia (uQf 4%) and Aristeus antennatus (uQf 3%) were relevant couplers in 1995. Conversely, the rank changed in 2005, with P. martia (uQf 7%), Macrourids (uQf 6%), P. longirostris and A. foliacea (uQf 5%), Mullus barbatus and SL_Fishes_BP crust (uQf 3%) as the most relevant couplers.
The analysis of BPCI and COG highlights that the bathymetric position and its variance (indication of wide vertical movement of organisms) played an important role for benthopelagic and demersal couplers in the SAL and CAL models (Figures 8A–D, 9A–D). In the benthopelagic domain, Myctophids and Shrimps support the highest energy flows in the upper and middle slope. From the shelf to the upper slope, SHB_Squids_BP, SHB_Fishes_BP crust and SHB_BSquids_BP supports the main energy exchanges. This pattern showed a temporal stability in both areas. On the contrary, the demersal domain showed spatial and temporal changes in the pattern of the couplers. SHB_Fishes_planktivorous showed a greater importance in the shelf of Calabrian area. In addition, a temporal increase in the number shelf and deep couplers and their bathymetric overlap in the SAL food web was observed in 2005. SH_Crabs, SHB_Crabs contributed to this overlap on the shelf, whereas SL_Decapods_bent, A. foliacea, N. sclerorhynchus and SL_Fishes_planktivorous contributed to that on the bathyal bottoms. In the Calabrian area, this overlap pattern was more evident in 1995, with the highest contribution to the flows coupling along the bathymetric gradient by SH_Crabs, SHB_Crabs, SL_Crabs, P. longirostris, P. martia, Macrourids_Med. slimehead and A. antennatus. In 2005, the pattern was the same, except for SHB_Crabs and A. antennatus being replaced by M. barbatus and A. foliacea.
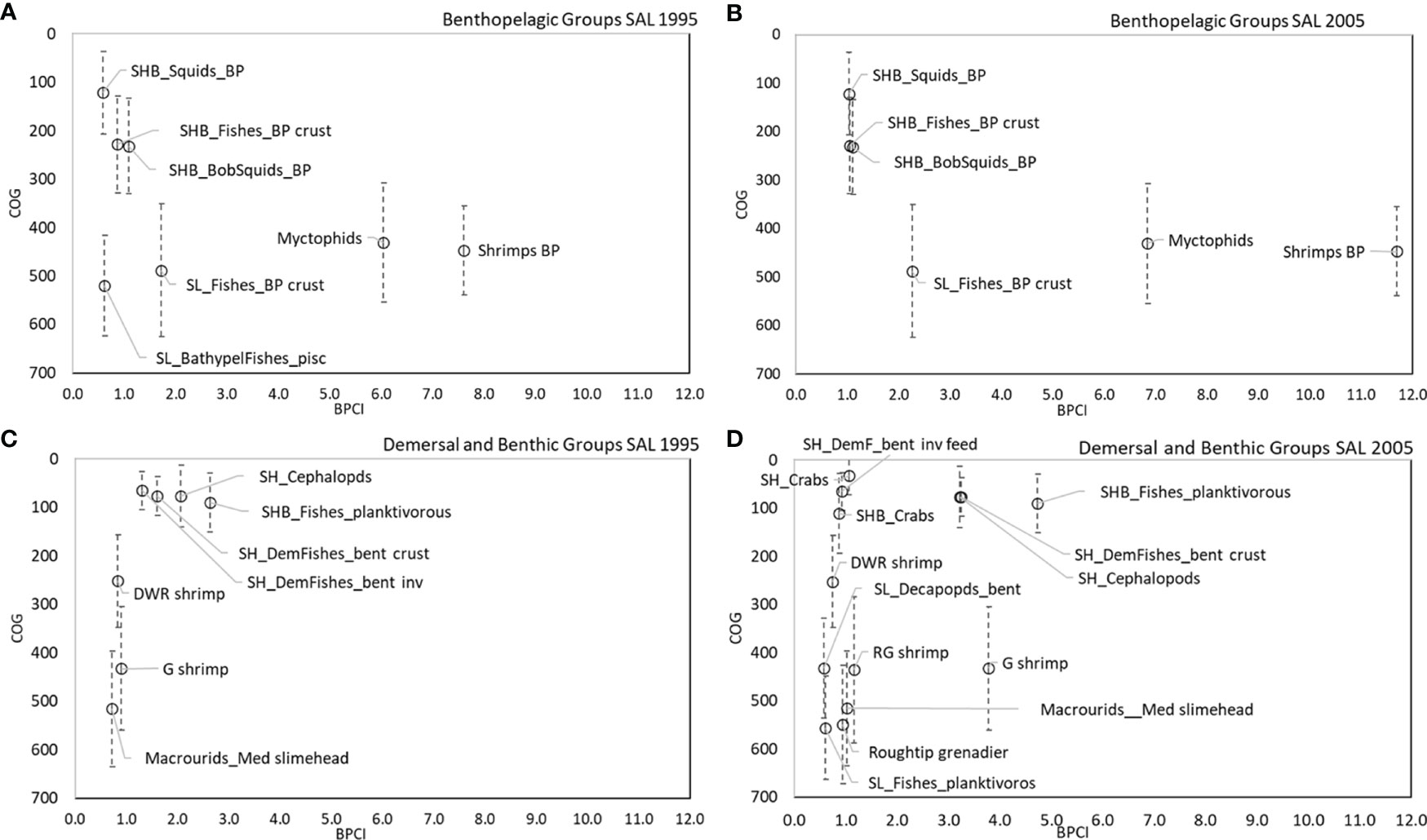
Figure 8 COG (m) of main couplers by BPCI (>0.5 t km-2 y-1) in all SAL models for (A, B) benthopelagic and (C, D) demersal domains.
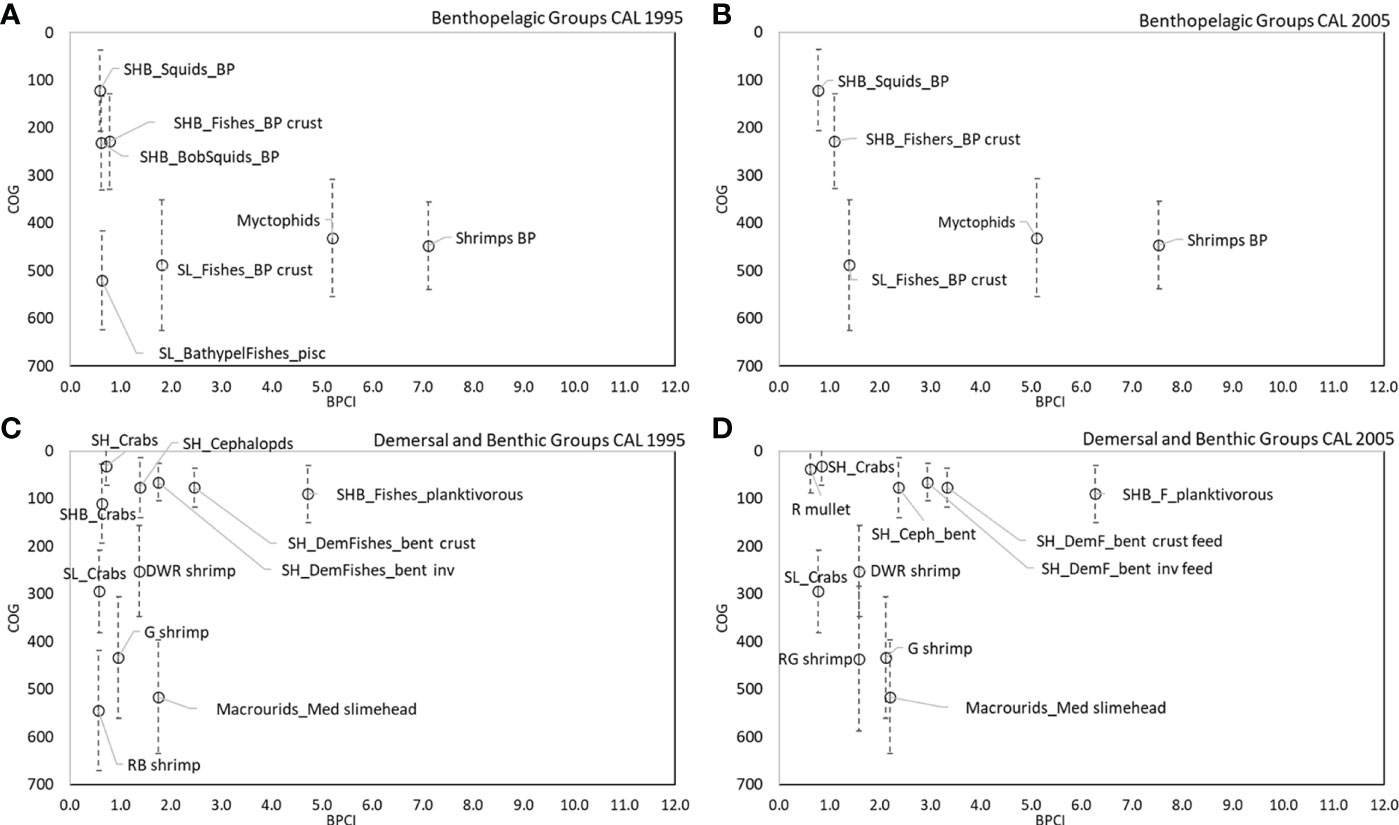
Figure 9 COG (m) of main couplers by BPCI (>0.5 t km-2 y-1) in all CAL models for (A, B) benthopelagic and (C, D) demersal domains.
Discussions
A pivotal role in the southwestern and north-eastern Ionian areas is played by deep faunal communities (D’Onghia et al., 1998; Capezzuto et al., 2010; Carlucci et al., 2018), which are able to intercept the particulate organic matter through benthic organisms that are then exploited by demersal and benthopelagic species of higher trophic levels.
The differences between the Salento and Calabrian models related to the net primary production were estimated during the cyclonic period 2003-2005. The magnitude of the consumption and production flows, as well as the complexity of ecosystem indicate a higher pelagic production in the Salento food web than the Calabrian one. These differences could be explained by the NIG direction, which favours the inflow of Adriatic Dense Waters (flowing at depths between 200-800 m), as well as this area also being impacted by the Po River nutrient load transported along eastern Italian coasts in the Ionian Sea (Gačić et al., 2010; Taricco et al., 2015). Furthermore, Adriatic Dense Waters are considered to sustain upwelling currents able to increase primary productivity in the North-eastern Ionian region because they are enriched in nutrients (Lavigne et al., 2018). These cascading oceanographic effects promote higher productivity in the cyclonic period, and increased deep particulate fluxes, which can be evinced from the results showing higher consumption fluxes of deep benthic and demersal groups in both investigated areas. These conditions are explained by the hydrographic circulation in the basin, where the long-distance particle transport is supported by deep currents from east to west up to the Sicily channel (Berline et al., 2021). In this framework, a fundamental role could be played by submarine canyons along the Calabrian sectors, where upwelling currents and water cascading processes allow movement of the deep organic matter towards the upper slope (Canals et al., 2009). Furthermore, cold-water coral habitats are found on the Apulian slope (D’Onghia et al., 2016; Vassallo et al., 2017) and in the Sicily channel (Taviani et al., 2005; Freiwald et al., 2009) and this distribution supports the idea of an ecological connectivity between the eastern and western Ionian areas regulated by deep currents carrying nutrients and organic matter exploited by these organisms (Carlier et al., 2009). A further aspect that could explain the differences in pelagic production and downward particulate fluxes between the two areas is represented by the wider shelf platform in the Apulian area, which can positively affect the particulate matter sinks to the bottom, vertical mixing and the resuspension of large amount of nutrients in the upper layer of the water column (De Lazzari et al., 1999; Boldrin et al., 2002). Conversely, in the Calabrian sector, downward flows of particulate matter linked to surface production occurring in the photic layer (D’Ortenzio et al., 2003), sink to a depth that makes nutrients resulting from organic matter degradation largely inaccessible for the phytoplankton in the area itself. Rather, particulate sinking appears to supply the bottom detritus food chain in the Calabrian canyons, as observed from results on consumption fluxes between benthic and demersal domains.
If the cyclonic period stressed spatial differences in the pelagic production and consumption between the two food webs, these characteristics were not observed during the anticyclonic phase (1995-1997). This could be due to the peculiar water circulation in this phase, when the Calabrian and Salento areas were impacted by Modified Atlantic Waters coming from the western Mediterranean Sea and they move in the upper layer of the water column (Klein et al., 1999). Thus, the absence of effects on the deep faunal communities is reasonable. Furthermore, a lower input of Adriatic Dense Waters occurred in the basin and the geostrophic circulation of the water masses was directed from the Calabrian area towards the Apulian one.
Functional Group’s Role in the BPC Process
The differences in oceanographic conditions and their cascading food web effects are confirmed in our models, stressing an increase in biomass of the groups during 2003-2005. This estimated increase was higher on the north-eastern (Salento) than on the south-western slope (Calabria), especially for deep faunal assemblages. In both food webs, the contribution to the biomass increase is given by the demersal assemblage, with the highest value observed for shelf demersal assemblage in the Salento area. Moreover, benthopelagic groups also showed a greater biomass increase in the Salento food web. However, increases estimated in the Calabrian food web were smaller than the Salento one because, during the anticyclonic period, biomasses were higher in the former food web than the latter. These observations suggest maintenance of the biomass structure of the ecosystem in the Calabrian area in both periods investigated, probably due to a refuge effect of submarine canyons (Fernandez‐Arcaya et al., 2017; Capezzuto et al., 2018; Sion et al., 2019) Although comparative studies on the temporal dynamics of species abundances between the two areas are very limited (D’Onghia et al., 1998), changes estimated by the models are consistent with fluctuations in abundance of demersal resources studied at the scale of the entire Northern Ionian basin (Capezzuto et al., 2010; Maiorano et al., 2010; Carlucci et al., 2018) and for the sharks and rays in the Calabrian area (Ricci et al., 2021).
Changes in biomass influence the trophic interactions between predators and prey, which affect trophic impacts detected by the models, as well as in the rank of keystone groups. In general, zooplankton groups assume a keystone role in the Ionian food web, being an important resource for several consumers in oligotrophic systems (Mazzocchi et al., 2003; Ricci et al., 2019). This role was maintained during the investigated periods, as were those of the Macrobenthic invertebrates and Suprabenthic crustaceans, which represent dominant structuring groups of the benthic domain. On the contrary, relevant temporal changes in the trophic impacts were mainly estimated for the groups of intermediate and high trophic levels (TL>3). Cephalopods increased in their importance as keystone groups in the cyclonic period. In particular, Shelf Cephalopods (Octopus vulgaris, Sepia spp., Eledone spp.) showed more importance in the Salento area, whereas Slope Benthopelagic Squids (Todarodes sagittatus, Histioteuthis spp.) was the most important keystone group in the CAL food web. This difference may be explained by the habitat distribution of the two groups: Shelf Cephalopods inhabit the shallowest grounds (Jereb et al., 2015), which widely extend in the Salento areas while Slope Benthopelagic Squids are distributed in the bathyal grounds exploiting several species at different levels in the water column (Rosas-Luis et al., 2014) and they can find suitable habitats in the submarine canyons. In addition, the biological traits of cephalopods, such as rapid growth, short lifespans, and plasticity, explain the faster response of these species to changes in productivity of the food web becoming a very highly impacting group in the trophic structure (Doubleday et al., 2016).
In both Northern Ionian Sea food webs, pelagic and benthic groups substantially drive energy transfer from pelagic to benthic communities in a direct manner as direct couplers. In the benthopelagic domain, flux coupling is mainly performed by Benthopelagic Shrimps (Pasiphaea spp., Acanthephyra spp., Plesionika edwardsii) and Myctophids, which show a temporal stability in their role. It is worth noting that both Benthopelagic Shrimps and Myctophids appear to support downward and upward flows in a different way. Benthopelagic Shrimps mainly plays a role in sustaining flows towards the pelagic, improving to be an effective benthic-pelagic coupler, while Myctophids supports energy transfers in both directions. The COG analysis showed that this feature is connected to the vertical movement of these species along the upper slope (300-400 m) and middle slope (500-700 m), migrations that have been widely observed (Aguzzi et al., 2007; Simão et al., 2015; Drazen and Sutton, 2017). The different trophic strategies could explain the difference between Myctophids and Benthopelagic Shrimps in the BPC patterns. Indeed, Myctophids and other mesopelagic fishes perform extensive daily migrations along the water column, eating plankton and micronekton at multiple depths in the epipelagic layers at night (Bernal et al., 2015). Whereas, the group of benthopelagic shrimps is also characterized by several deep-water crustaceans decapods, which feed on benthic prey (such as polychaetas exploited by P. edwardsii) and their opportunistic behavior is affected by the availability of planktonic resources (Cartes, 1993; Cartes, 1998). The importance of these groups in the energy exchanges has been observed in previous local models (Ricci et al., 2019; Carlucci et al., 2021), and in models realized for nearby areas, such as the Strait of Sicily model (Agnetta et al., 2019).
Demersal groups mostly contribute to BPC mechanisms in an almost exclusive way, acting as an elevator in the sustaining of upwelling flows. Thus, these groups play a critical role in the transfer of energy towards the surface, supporting recycling of the matter available for pelagic organisms (Raffaelli et al., 2003; Baustian et al., 2014). Only Shelf-break Planktivorous Fishes (M. scolopax, C. aper) showed a higher BPCI values with a relevant contribution to downward flows in the Calabrian food web, indicating an exploitation of zooplanktonic prey. This result seems to be consistent with the structural importance of these species in the Calabrian demersal assemblage (D’Onghia et al., 1998) and their feeding strategy and rapid life cycles (Carpentieri et al., 2016).
In the shelf grounds of the Salento food web, Shelf Cephalopods and Shelf Demersal Fishes benthic crustacean feeders occupy an important position as mediating couplers of ascending consumption flows during both investigated periods. According to BPCI outputs, the former supports exclusively upward consumption flows, while the latter is also involved in the downward and upward energy transfer. Indeed, Shelf Cephalopods are characterized by species mainly linked to benthic prey and exploited by different predators, such as large pelagics and odontocetes (Clarke, 1996). However, Shelf Demersal Fishes benthic crustaceans feeders (Spicara spp., Boops boops) consist of species involved in the exploitation of zooplankton resources and benthopelagic crustaceans, as well as being prey of several fish predators and the common bottlenose dolphin (Pipitone and Andaloro, 1995; Riccioni et al., 2018; Ricci et al., 2020).
In bathyal grounds of the Salento area, P. martia and P. longirostris represent the main important couplers of energy flows from deep bottoms up to the upper slope in the anticyclonic period. Successively, the golden shrimp increased its consumption becoming the most important mediating coupler in the cyclonic periods, followed by A. foliacea, N. sclerorhynchus as partial couplers. P. martia represents an important species in the bathyal assemblage of the Northern Ionian Sea (Maiorano et al., 2002.; Capezzuto et al., 2010; D’Onghia et al., 2011), which is an important opportunistic predator of planktonic and benthic resources (Cartes, 1993). Its role as coupler became more relevant in the period 2003-2005 and this could be explained by its trophic strategy, favoured by a greater productivity during the cyclonic period (Mazzocchi et al., 2003; Lavigne et al., 2018). Similarly, N. sclerorhynchus and A. foliacea are characterized by a trophic strategy that could have been supported by the increase in mesopelagic prey during the cyclonic period (Madurell and Cartes, 2006; Kapiris et al., 2010).
In the Calabrian shelf area, energy flows in the BPC pattern are mainly supported by Shelf Demersal Fishes benthic invertebrates feeders (Pagellus acarne, Mullus surmuletus) in both investigated periods, followed by Shelf Cephalopods and Shelf Demersal Fishes benthic crustaceans feeders. In addition, the role of M. barbatus as a mediating coupler of the demersal domain emerged in 2005. These observations highlight the importance of benthic invertebrates (annelids, crustaceans, and molluscs) as prey in the Calabrian food web, where crabs also play a role in the BPC mechanisms in shelf and upper slope grounds.
In bathyal grounds, Macrourids and Mediterranean Slimehead (Coelothynchus coelothynchus, Hymenocephalus italicus, Hoplostethus mediterraneus), P. longirostris, P. martia and A. antennatus showed the main contribution to the energy flows coupling in the demersal domain. This condition was confirmed in 2005, but with A. antennatus replaced by A. foliacea. In addition, the role of P. martia emerged in the cyclonic period, as observed in the Salento area. Thanks to their ecological traits and trophic positions, Macrourids (C. coelorhynchus, H. italicus) and H. mediterraneus seem to be more able to respond to the trophic changes due to the BiOS. Indeed, these species feed on suprabenthic and mesopelagic prey (Madurell and Cartes, 2005; Madurell and Cartes, 2006), which are highly sensitive to water column changes. Concerning P. longirostris, its importance in the BPC pattern seems to be due its wide displacement in the shelf-break and upper slope grounds, its exploitation of prey on benthic and deposit feeders (Benallal et al., 2020), as well as its movements during the life cycle from nursery to spawning areas, as observed in the Strait of Sicily (Fortibuoni et al., 2010). The replacement of A. antennatus by A. foliacea with an inverted ratio between the two red shrimps during the cyclonic phase has been reported in several local studies (Capezzuto et al., 2010; Carlucci et al., 2018). This could be linked to changes in the deep-water direction which occurred in the cyclonic period by affecting the water cascading into the Calabrian canyons. Indeed, A. antennatus shows a great capability in its vertical movement up and down canyons (Relini et al., 2000), and dense waters cascading facilitate its displacement in deep waters, where the species performs its important recruitment process (Company et al., 2008; D’Onghia et al., 2009). Moreover, changes in the thermohaline circulation seemed to favour A. foliacea, but not A. antennatus, which is more linked to colder and less saline waters. The best hydrodynamic conditions for A. antennatus appear to be a combination of relatively cold temperatures and high salinity, associated with moderate energy variability (Sardà et al., 2009).
Insights for Future Improvements and Analysis
The approach adopted has the potential to provide a holist view for the BPC. One of the main limitations of the approach is related to the poor biological resolution of processes involving for macrobenthic invertebrates. For the macrobenthic invertebrates, in fact, information on biomasses and diet are generally scant and also in these models the biomass of some of these groups was estimated by means the model (Heymans et al., 2016).
Another limitation is represented by the lack of data for phytoplankton biomasses before 1998-1999 (Lazzari et al, 2012) and the assumption used that the primary production condition of the period 1995-1997 was similar to the period 1998-1999 (Di Biagio et al., 2019; Cossarini et al., 2021). Nevertheless, the focus on the higher trophic level and by the integrated use of several independent information sources in the modelling approach, including details on benthopelagic and demersal species data obtained from the MEDITS surveys, partially mitigate the limitation.
The results stimulate future works on the hydrographic features and bioecological traits of deep species, which can provide some insights for future analysis. In particular, a quantification of the deep transport of particulate organic material from the north-western side to the south-western side of the Ionian Basin during the cyclonic period compared to the anticyclonic, with cascading effects on the energetic input to deep benthic and demersal communities, should be useful to shed light on spatially distributed BPC processes (Berline et al., 2021). The role of the deep and intermediate currents in this process could represent an interesting field of study to investigate particulate matter transport between these two areas of the Ionian basin.
Environmental changes driven by the water circulation inversion have relevant effects on the physical variables and as reported in several studies (Civitarese et al., 2010; Liu et al., 2021) are affecting the faunal community distributed in the slope grounds (Carlucci et al., 2018). Therefore, an important area of investigation is the effects of changes in environmental features on the growth and recruitment of several deep-sea species, such as the case of A. foliacea, which has shown large fluctuations in abundance and biomass (Capezzuto et al., 2010). In addition, the wide bathymetric displacement of several demersal and benthopelagic species, as observed for A. antennatus (D’Onghia et al., 2009), indicates population movements at depths greater than 1000 m, which are performed in response to environmental changes (Sardà et al., 2004; D’Onghia et al., 2005; Company et al., 2008; Maiorano et al., 2010; Capezzuto et al., 2010). Therefore, further modelling analysis could be addressed to include these vertical migrations, although this will not be easy given the scarcity of temporal information on deep faunal communities.
In conclusion, BPC flows analysed by means of a food web modelling approach and a new BPCI index allowed the quantification of the flow between the benthic and pelagic domains and to disentangle the contribution of species to the BPC mechanisms. In the Northern Ionian Sea, the BPC is affected by temporal changes during the BiOS phases, as well as by spatial differences between the two investigated areas, which are characterized by peculiar environmental conditions. These differences are mainly reflected in the role of some groups of benthopelagic and demersal species, which support the upward flows towards pelagic systems through their feeding behaviours and their wide variation along the bathymetric gradient in the shelf break and upper slope. Temporal changes driven by BiOS seem to have relevant influences on the trophic state of the deep communities, which showed important variations in the amount of consumption flows estimated by means of BPCI.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author Contributions
Conceptualization, PR and SL; methodology, PR, SL, RC; formal analysis, PR, SL, GC; data investigation and sampling design, GD’O, PM, AC, FC, RC, LS, AT; writing—original draft preparation, PR, SL, RC; writing—review and editing, GD’O, PM, LS, FC; AT GC, AC, SL, PR; supervision, RC, SL. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.887464/full#supplementary-material
References
Agnetta D., Badalamenti F., Colloca F., D’Anna G., Di Lorenzo M., Fiorentino F., et al. (2019). Benthic-Pelagic Coupling Mediates Interactions in Mediterranean Mixed Fisheries: An Ecosystem Modeling Approach. PloS One 14 (1), e0210659. doi: 10.1371/journal.pone.0210659
Aguzzi J., Company J. B., Abelló P., García J. A. (2007). Ontogenetic Changes in Vertical Migratory Rhythms of Benthopelagic Shrimps Pasiphaea Multidentata and P. Sivado. Mar. Ecol. Progr. Ser. 335, 167–174. doi: 10.3354/meps335167
Allen R. R. (1971). Relation Between Production and Biomass. J. Fish. Res. Board Can. 28, 1573–1581. doi: 10.1139/f71-236
Banaru D., Mellon-Duval C., Roos D., Bigot J. L., Souplet A., Jadaud A., et al. (2013). Trophic Structure in the Gulf of Lions Marine Ecosystem (North-Western Mediterranean Sea) and Fishing Impacts. J. Mar. Syst. 111, 45–68. doi: 10.1016/j.jmarsys.2012.09.010
Baustian M. M., Hansen G. J. A., de Kluijver A., Robinson K., Henry E. N., Knoll L. B., et al. (2014). “Linking the Bottom to the Top in Aquatic Ecosystems: Mechanisms and Stressors of Benthic-Pelagic Coupling,” in Eco-DAS X Symposium Proceedings Chapter: Chapter 3. Ed. Kemp P. F. (Waco, Texas, USA:Association for the Sciences of Limnology and Oceanography), P.38–P.60. doi: 10.4319/ecodas.2014.978-0-9845591-4-5.38
Benallal A. M., Baaloudj A., Kerfouf A., Bouzidi M. A., Belhadj Tahar K. (2020). Natural Diet of Deep-Water Rose Shrimp in the Beni-Saf Bay (Western Algeria). Ukr. J. Ecol. 10 (4), 109–115. doi: 10.15421/2020_176
Berline L., Doglioli A. M., Petrenko A., Barrillon S., Espinasse B., Le Moigne F. A. C., et al. (2021). Long-Distance Particle Transport to the Central Ionian Sea. Biogeosciences 18, 6377–6392. doi: 10.5194/bg-18-6377-2021
Bernal A., Pilar Olivar M., Maynou F., Luz Fernández de Puelles M. (2015). Diet and Feeding Strategies of Mesopelagic Fishes in the Western Mediterranean. Prog. Oceanogr. 135, 1–17. doi: 10.1016/j.pocean.2015.03.005
Boldrin A., Miserocchi S., Rabitti S., Turchetto M. M., Balboni V., Socal G. (2002). Particulate Matter in the Southern Adriatic and Ionian Sea: Characterisation and Downward Fluxes. J. Mar. Syst. 33–34, 389–410. doi: 10.1016/S0924-7963(02)00068-4
Boyle M. D., Ebert D. A., Cailliet G. M. (2012). Stable-Isotope Analysis of a Deep-Sea Benthic-Fish Assemblage: Evidence of an Enriched Benthic Food Web. J. Fish Biol. 80, 1485–1507. doi: 10.1111/j.1095-8649.2012.03243.x
Canals M., Danovaro R., Heussner S., Lykousis V., Puig P., Trincardi F., et al. (2009). Cascades in Mediterranean Submarine Grand Canyons. Oceanography 22 (1), 26–43. doi: 10.5670/oceanog.2009.03
Capezzuto F., Ancona F., Calculli C., Carlucci R., Sion L., Maiorano P., et al. (2021). Comparison of Trophic Spectrum in the Blackspot Seabream, Pagellus Bogaraveo (Brünnich 1768), Between Cold-Water Coral Habitats and Muddy Bottoms in the Central Mediterranean. Deep Sea Res. I 169, 103474. doi: 10.1016/j.dsr.2021.103474
Capezzuto F., Ancona F., Calculli C., Sion L., Maiorano P., D’Onghia G. (2020). Feeding of the Deep-Water Fish Helicolenus Dactylopterus (Delaroche 1809) in Different Habitats: From Muddy Bottoms to Cold-Water Coral Habitats. Deep-Sea Res. I 159, 103252. doi: 10.1016/j.dsr.2020.103252
Capezzuto F., Ancona F., Carlucci R., Carluccio A., Cornacchia L., Maiorano P., et al. (2018). Cold-Water Coral Communities in the Central Mediterranean: Aspects on Megafauna Diversity, Fishery Resources and Conservation Perspectives. Rend. Lincei Sci. Fish. Nat. 29, 589–597. doi: 10.1007/s12210-018-0724-5
Capezzuto F., Calculli C., Carlucci R., Carluccio A., Maiorano P., Pollice A., et al. (2019). Revealing the Coral Habitat Effect on Benthopelagic Fauna Diversity in the Santa Maria Di Leuca Cold-Water Coral Province Using Different Devices and Bayesian Hierarchical Modelling. Aquat. Conserv: Mar Freshw Ecosyst. 29, 1608–1622. doi: 10.1002/aqc.3144
Capezzuto F., Carlucci R., Maiorano P., Sion L., Battista D., Giove A., et al. (2010). The Bathyal Benthopelagic Fauna in the NW Ionian Sea: Structure, Patterns and Interactions. Chem. Ecol. 26, 199–217. doi: 10.1080/02757541003639188
Carlier A., Le Guilloux E., Olu K., Sarrazin J., Mastrototaro F., Taviani M., et al. (2009). Trophic Relationships in a Deep Mediterranean Cold-Water Coral Bank (Santa Maria Di Leuca, Ionian Sea). Mar Ecol. Prog. Ser. 397, 125–137. doi: 10.3354/meps08361
Carlucci R., Bandelj V., Ricci P., Capezzuto F., Sion L., Maiorano P., et al. (2018). Exploring Spatio-Temporal Changes of the Demersal and Benthopelagic Assemblages of the North-Western Ionian Sea (Central Mediterranean Sea). Mar. Ecol. Prog. Ser. 598, 1–19. doi: 10.3354/meps12613
Carlucci R., Capezzuto F., Cipriano G., D’Onghia G., Fanizza C., Libralato S., et al. (2021a). Assessment of Cetacean-Fishery Interactions in the Marine Food Web of the Gulf of Taranto (Northern Ionian Sea, Central Mediterranean Sea). Rev. Fish Biol. Fish. 31, 135–156. doi: 10.1007/s11160-020-09623-x
Carlucci R., Manea E., Ricci P., Cipriano G., Fanizza C., Maglietta R., et al. (2021b). Managing Multiple Pressures for Cetaceans’ Conservation With an Ecosystem-Based Marine Spatial Planning Approach. J. Environ. Manage. 287, 112240. doi: 10.1016/j.jenvman.2021.112240
Carpentieri P., Serpetti N., Colloca F., Criscoli A., Ardizzone G. (2016) Food Preferences and Rhythms of Feeding Activity of Two Co-Existing Demersal Fish, the Longspine Snipefish, Macroramphosus Scolopax (Linnaeus 1758), and the Boarfish Capros Aper (Linnaeus 1758), on the Mediterranean Deep Shelf. Mar. Ecol. 37, 106–118. doi: 10.1111/maec.12265
Cartes J. E. (1993). Diets of Deep-Water Pandalid Shrimps on the Western Mediterranean Slope. Mar. Ecol. Prog. Ser. 96, 49–61. doi: 10.3354/meps096049
Cartes J. (1998). Feeding Strategies and Partition of Food Resources in Deep-Water Decapod Crustaceans (400–2300 M). J. Mar. Biol. Ass. UK 78 (2), 509–524. doi: 10.1017/S002531540004159X
Chauvaud L., Jean F., Ragueneau O., Thouzeau G. (2000). Long-Term Variation of the Bay of Brest Ecosystem: Benthic-Pelagic Coupling Revisited. Mar. Ecol. Prog. Ser. 200, 35–48. doi: 10.3354/meps200035
Christensen V., Walters R. (2004). Ecopath With Ecosim: Methods, Capabilities and Limitations. Ecol. Modell. 172 (2–4), 109–139. doi: 10.1016/j.ecolmodel.2003.09.003
Christensen V., Walters C., Pauly D., Forrest R. (2008). Ecopath With Ecosim 6: A User’s Guide (Vancouver, BC: Fisheries Centre. University of British Columbia).
Civitarese G., Gačić M., Lipizer M., Eusebi Borzelli G. L. (2010). On the Impact of the Bimodal Oscillating System (BiOS) on the Biogeochemistry and Biology of the Adriatic and Ionian Seas (Eastern Mediterranean). Biogeosciences 7 (12), 3987–3997. doi: 10.5194/bg-7-3987-2010
Clarke M. R. (1996). Cephalopods as Prey. III. Cetaceans Phil. Trans. R. Soc. Lond. B351, 1053–1065. doi: 10.1098/rstb.1996.0093
Coll M., Libralato S. (2012). Contributions of Food-Web Modelling for an Ecosystem Approach of Marine Resource Management in the Mediterranean Sea. Fish. Fish. 13, 60–88. doi: 10.1111/j.1467-2979.2011.00420.x
Coll M., Navarro J., Olson R. J., Christensen V. (2013). Assessing the Trophic Position and Ecological Role of Squids in Marine Ecosystems by Means of Food-Web Models. Deep Sea Res. II 95, 21–36. doi: 10.1016/j.dsr2.2012.08.020
Company J. B., Puig P., Sardà F., Palanques A., Latasa M., Scharek R. (2008). Climate Influence on Deep Sea Populations. PloS One 3 (1), e1431. doi: 10.1371/journal.pone.0001431
Cossarini G., Feudale L., Teruzzi A., Bolzon G., Coidessa G., Solidoro C., et al. (2021). High-Resolution Reanalysis of the Mediterranean Sea Biogeochemistry, (1999–2019). Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.741486
Cresson P., Chouvelon T., Bustamante P., Bănaru D., Baudrier J., Le Loc’h F., et al. (2020). Primary Production and Depth Drive Different Trophic Structure and Functioning of Fish Assemblages in French Marine Ecosystems. Prog. Oceanogr. 186, 102343. doi: 10.1016/j.pocean.2020.102343
De Lazzari A., Boldrin A., Rabitti S., Turchetto M. M. (1999). Variability and Downward Fluxes of Particulate Matter in the Otranto Strait Area. J. Mar. Syst. 20, 399–413. doi: 10.1016/S0924-7963(98)00076-1
Di Biagio V., Cossarini G., Salon S., Lazzari P., Querin S., Sannino G., et al. (2019). Temporal Scales of Variability in the Mediterranean Sea Ecosystem: Insight From a Coupled Model. J. Mar. Syst. 197, 103176. doi: 10.1016/j.jmarsys.2019.05.002
D’Onghia G., Calculli E., Capezzuto F., Carlucci R., Carluccio A., Maiorano P., et al. (2016). New Records of Cold-Water Coral Sites and Fish Fauna Characterization of a Potential Network Existing in the Mediterranean Sea. Mar. Ecol. 37, 1398–1422. doi: 10.1111/maec.12356
D’Onghia G., Capezzuto F., Cardone F., Carlucci R., Carluccio A., Chimienti G., et al. (2015). Macro- and Megafauna Recorded in the Submarine Bari Canyon (Southern Adriatic, Mediterranean Sea) Using Different Tools. Mediterr. Mar. Sci. 16 (1), 180–196. doi: 10.12681/mms.1082
D’Onghia G., Capezzuto F., Mytilineou Ch., Maiorano P., Kapiris K., Carlucci R., et al. (2005). Comparison of the Population Structure and Dynamics of Aristeus Antennatus (Risso 1816) Between Exploited and Unexploited Areas in the Mediterranean Sea. Fish Res. 76 (1), 22–38. doi: 10.1016/j.fishres.2005.05.007
D’Onghia G., Giove A., Maiorano P., Carlucci R., Minerva M., Capezzuto F., et al. (2012). Exploring Relationships Between Demersal Resources and Environmental Factors in the Ionian Sea (Central Mediterranean). J. Mar. Biol. 2012, 279406. doi: 10.1155/2012/279406
D’Onghia G., Indennidate A., Giove A., Savini A., Capezzuto F., Sion L., et al. (2011). Distribution and Behaviour of the Deep-Sea Benthopelagic Fauna Observed Using Towed Cameras in the Santa Maria Di Leuca Cold Water Coral Province. Mar. Ecol. Prog. Ser. 443, 95–110. doi: 10.3354/meps09432
D’Onghia G., Maiorano P., Capezzuto F., Carlucci R., Battista D., Giove A., et al. (2009). Further Evidences of Deep-Sea Recruitment of Aristeus Antennatus (Crustacea: Decapoda) and its Role in the Population Renewal on the Exploited Bottoms of the Mediterranean. Fish. Res. 95, 236–245. doi: 10.1016/j.fishres.2008.09.025
D’Onghia G., Tursi A., Maiorano P., Matarrese A., Panza M. (1998). Demersal Fish Assemblages From the Bathyal Grounds of the Ionian Sea (Middle-Eastern Mediterranean). Ital. J. Zool. 65, 287–292. doi: 10.1080/11250009809386834
D’Ortenzio F., Ragni M., Marullo S., Ribera d’Alcalà M. (2003). Did Biological Activity in the Ionian Sea Change After the Eastern Mediterranean Transient? Results From the Analysis of Remote Sensing Observations. J. Geophys Res. 108 (9), 20. doi: 10.1029/2002JC001556
Doubleday Z. A., Prowse T. A. A., Arkhipkin A., Pierce G. J., Semmens J., Steer M., et al. (2016). Global Proliferation of Cephalopods. Curr. Biol. 26, R406–R407. doi: 10.1016/j.cub.2016.04.002
Drazen J. C., Sutton T. T. (2017). Dining in the Deep: The Feeding Ecology of Deep-Sea Fishes. Annu. Rev. Mar. Sci. 9,1, 337–366. doi: 10.1146/annurev-marine-010816-060543
Duffill Telsnig J. I., Jennings S., Mill A. C., Walker N. D., Parnell A. C., Polunin N. V. C. (2019). Estimating Contributions of Pelagic and Benthic Pathways to Consumer Production in Coupled Marine Food Webs. J. Anim. Ecol. 88, 405–415. doi: 10.1111/1365-2656.12929
Fernandez–Arcaya U., Ramirez-Llodra E., Aguzzi J., Allcock A. L., Davies J. S., Dissanayake A., et al. (2017). Ecological Role of Submarine Canyons and Need for Canyon Conservation: A Review. Front. Mar. Sci. 4. doi: 10.3389/fmars.2017.00005
Fortibuoni T., Bahri T., Camilleri M., Garofalo G., Gristina M., Fiorentino F. (2010). Nursery and Spawning Areas of Deep-Water Rose Shrimp, Parapenaeus Longirostris (Decapoda: Penaeidae), in the Strait of Sicily (Central Mediterranean Sea). J. Crustac. Biol. 30, 167–174. doi: 10.1651/09-3167.1
Freiwald A., Beuck L., Rüggeberg A., Taviani M., Hebbeln D. (2009). The White Coral Community in the Central Mediterranean Sea Revealed by ROV Surveys. Oceanography 22, 58–74. doi: 10.5670/oceanog.2009.06
Froese R., Pauly D. (2021). FishBase. World Wide Web Electronic Publication. Available at: www.fishbase.org.
Gačić M., Borzelli G. L. E., Civitarese G., Cardin V., Yari S. (2010). Can Internal Processes Sustain Reversals of the Ocean Upper Circulation? The Ionian Sea Example. Geophys. Res. Lett. 37, L09608. doi: 10.1029/2010GL043216
Griffiths J. R., Kadin M., Nascimento F. J. A., Tamelander T., Törnroos A., Bonaglia S., et al. (2017). The Importance of Benthic–Pelagic Coupling for Marine Ecosystem Functioning in a Changing World. Glob Change Biol. 23, 2179–2196. doi: 10.1111/gcb.13642
Halpin P. N., Read A. J., Fujioka E., Best B. D., Donnelly B., Hazen L. J., et al. (2009). OBIS-SEAMAP: The World Data Center for Marine Mammal, Sea Bird and Sea Turtle Distributions. Oceanography 22 (2), 104–115. doi: 10.5670/oceanog.2009.42
Heymans J. J., Coll M., Libralato S., Morissette L., Christensen V. (2014). Global Patterns in Ecological Indicators of Marine Food Webs: A Modelling Approach. PloS One 9 (4), e95845. doi: 10.1371/journal.pone.0095845
Heymans J. J., Coll M., Link J. S., Mackinson S., Steenbeek J., Walters C., et al. (2016). Best Practice in Ecopath With Ecosim Food-Web Models for Ecosystem-Based Management. Ecol. Modell. 331, 173–184. doi: 10.1016/j.ecolmodel.2015.12.007
Hure M., Mustać B. (2020). Feeding Ecology of Sardina Pilchardus Considering Co-Occurring Small Pelagic Fish in the Eastern Adriatic Sea. Mar. Biodivers. 50, 40. doi: 10.1007/s12526-020-01067-7
Jereb P., Allcock A. L., Lefkaditou E., Piatkowski U., Hastie L. C., Pierce G. J. (Eds.) (2015). "Cephalopod Biology and Fisheries in Europe: II. Species Accounts," in ICES Cooperative Research Report No. 325, (Copenaghen, Denmark), 360 pp. doi: 10.17895/ices.pub.5493
Kapiris K., Thessalou-Legaki M., Petrakis G., Conides A. (2010). Ontogenetic Shifts and Temporal Changes the Trophic Patterns of Deep-Sea Red Shrimp A. Foliacea (Decapods, Aristeidae) in the E. Ionian Sea (E. Mediterranean). Mar. Ecol. 31 (2), 341–354. doi: 10.1111/j.1439-0485.2009.00344.x
Kiljunen M., Peltonen H., Lehtiniemi M., Uusitalo L., Sinisalo T., Norkko J., et al. (2020). Benthic-Pelagic Coupling and Trophic Relationships in Northern Baltic Sea Food Webs. Limnol. Oceanogr. 65, 1706–1722. doi: 10.1002/lno.11413
Klein B., Roether W., Manca B. B., Bregant D., Beitzel V., Kovacevic V., et al. (1999). The Large Deep Water Transient in the Eastern Mediterranean. Deep Sea Res. I 46 (3), 371–414. doi: 10.1016/S0967-0637(98)00075-2
Lassalle G., Lobry J., Le Loc’h F., Bustamante P., Certain G., Delmas D., et al. (2011). Lower Trophic Levels and Detrital Biomass Control the Bay of Biscay Continental Shelf Food Web: Implications for Ecosystem Management. Progr. Oceanogr. 91, 561–575. doi: 10.1016/j.pocean.2011.09.002
Lavigne H., Civitarese G., Gačić M., D’Ortenzio F. (2018). Impact of Decadal Reversals of the North Ionian Circulation on Phytoplankton Phenology. Biogeosciences 15, 4431–4445. doi: 10.5194/bg-15-4431
Lazzari P., Solidoro C., Ibello V., Salon S., Teruzzi A., Béranger K., et al. (2012). Seasonal and Inter-Annual Variability of Plankton Chlorophyll and Primary Production in the Mediterranean Sea: A Modelling Approach. Biogeosciences 9, 217–233. doi: 10.5194/bg-9-217-2012
Libralato S., Christensen V., Pauly D. (2006). A Method for Identifying Keystone Species in Food Web Models. Ecol. Modell. 195 (3–4), 153–171. doi: 10.1016/j.ecolmodel.2005.11.029
Link J. S. (2010). Adding Rigor to Ecological Network Models by Evaluating a Set of Pre-Balance Diagnostics: A Plea for PREBAL. Ecol. Model. 221, 1582–1593. doi: 10.1016/j.ecolmodel.2010.03.012
Liu F., Mikolajewicz U., Six K. D. (2021). Drivers of the Decadal Variability of the North Ionian Gyre Upper Layer Circulation During 1910–2010: A Regional Modelling Study. Clim Dyn. 58, 2065–2077. doi: 10.1007/s00382-021-05714-y
Lorance P., Trenkel V. M. (2006). Variability in Natural Behaviour, and Observed Reactions to an ROV, by Mid-Slope Fish Species. J. Exp. Mar. Biol. Ecol. 332 (1), 106–119. doi: 10.1016/j.jembe.2005.11.007
Madurell T., Cartes J. E. (2005). Trophodynamics of a Deep-Sea Demersal Fish Assemblage From the Bathyal Eastern Ionian Sea (Mediterranean Sea). Deep Sea Res. I 52, 2049–2064. doi: 10.1016/j.dsr.2005.06.013
Madurell T., Cartes J. E. (2006). Trophic Relationships and Food Consumption of Slope Dwelling Macrourids From the Bathyal Ionian Sea (Eastern Mediterranean). Mar. Biol. 148 (6), 1325–1338. doi: 10.1007/s00227-005-0158-3
Maiorano P., D’Onghia G., Capezzuto F., Sion L. (2002). Life History Traits of Plesionika Martia (Decapoda: Caridea) From the Eastern-Central Mediterranean Sea. Mar Biol. 141, 527–539. doi: 10.1007/s00227-002-0851-4
Maiorano P., Sion L., Carlucci R., Capezzuto F., Giove A., Costantino G., et al. (2010). The Demersal Faunal Assemblage of the North-Western Ionian Sea (Central Mediterranean): Current Knowledge and Perspectives. Chem. Ecol. 26, 219–240. doi: 10.1080/02757541003693987
Mazzocchi M. G., Nervegna D., D’Elia G., Di Capua I., Aguzzi L., Boldrin A. (2003). Spring Mesozooplankton Communities in the Epipelagic Ionian Sea in Relation to the Eastern Mediterranean Transient. J. Geophys. Res. 108, 8114. doi: 10.1029/2002JC001640
Menna M., Suarez N. R., Civitarese G., Gačić M., Rubino A., Poulain P. M. (2019). Decadal Variations of Circulation in the Central Mediterranean and its Interactions With Mesoscale Gyres. Deep Sea Res. II 164, 14–24. doi: 10.1016/j.dsr2.2019.02.004
Mussap G., Zavatarelli M. (2017). A Numerical Study of the Benthic–Pelagic Coupling in a Shallow Shelf Sea (Gulf of Trieste). Reg. Stud. Mar. Sci. 9, 24–34. doi: 10.1016/j.rsma.2016.11.002
Palomares M. L. D., Pauly D. (2021) SeaLifeBase. World Wide Web Electronic Publication. Available at: www.sealifebase.org.
Pauly D., Christensen V., Walters C. (2000). Ecopath, Ecosim, and Ecospace as Tools for Evaluating Ecosystem Impact of Fisheries. ICES J. Mar. Sci. 57, 697–706. doi: 10.1006/jmsc.2000.0726
Pikitch E. K., Santora C., Babcock E. A., Bakun A., Bonfil R., Conover D. O., et al. (2004). Ecology. Ecosystem-Based Fishery Management. Science 305, 346–347. doi: 10.1126/science.1098222
Pipitone C., Andaloro F. (1995). Food and Feeding Habits of Juvenile Greater Amberjack, Seriola Dumerili Osteichthyes, Carangidae in Inshore Waters of the Central Mediterranean Sea. Cybium 193, 305–310.
Pitt K. A., Clement A.-L., Connolly R. M., Thibault-Botha D. (2008). Predation by Jellyfish on Large and Emergent Zooplankton: Implications for Benthic-Pelagic Coupling. Estuar. Coast. Shelf Sci. 76, 827–833. doi: 10.1016/j.ecss.2007.08.011
Raffaelli D., Bell E., Weithoff G., Matsumoto A., Cruz-Motta J. J., Kershaw P., et al. (2003). The Ups and Downs of Benthic Ecology: Considerations of Scale, Heterogeneity, and Surveillance for Benthic–Pelagic Coupling. J. Exp. Mar. Biol. Ecol. 285–286, 191–203. doi: 10.1016/S0022-0981(02)00527-0
Relini M., Maiorano P., D’Onghia G., Orsi Relini L., Tursi A., Panza M. (2000). A Pilot Experiment of Tagging the Deep Shrimp Aristeus Antennatus (Risso 1816). Sci. Mar. 64 (3), 357–361. doi: 10.3989/scimar.2000.64n3357
Ricci P., Ingrosso M., Carlucci R., Fanizza C., Maglietta R., Santacesaria F. C., et al. (2020). “Quantifying the Dolphins-Fishery Competition in the Gulf of Taranto (Northern Ionian Sea, Central Mediterranean Sea),” in MetroSea 2020 - TC19 International Workshop on Metrology for the Sea (Naples, Italy), pp.94–99.
Ricci P., Libralato S., Capezzuto F., D’Onghia G., Maiorano P., Sion L., et al. (2019). Ecosystem Functioning of Two Marine Food Webs in the North-Western Ionian Sea (Central Mediterranean Sea). Ecol. Evol. 9, 10198–10212. doi: 10.1002/ece3.552
Riccioni G., Stagioni M., Piccinetti C., Libralato S. (2018). A Metabarcoding Approach for the Feeding Habits of European Hake in the Adriatic Sea. Ecol. Evol. 8, 10435–10447. doi: 10.1002/ece3.4500
Ricci P., Sion L., Capezzuto F., Cipriano G., D’Onghia G., Libralato S. (2021). Modelling the Trophic Roles of the Demersal Chondrichthyes in the Northern Ionian Sea (Central Mediterranean Sea). Ecol. Modell. 444, 109468. doi: 10.1016/j.ecolmodel.2021.109468
Rodil I. F., Lucena-Moya P., Tamelander T., Norkko J., Norkko A. (2020). Seasonal Variability in Benthic–Pelagic Coupling: Quantifying Organic Matter Inputs to the Seafloor and Benthic Macrofauna Using a Multi-Marker Approach. Front. Mar. Sci. 7, 404. doi: 10.3389/fmars.2020.00404
Rosas-Luis R., Villanueva R., Sánchez P. (2014). Trophic Habits of the Ommastrephid Squid Illex Coindetii and Todarodes Sagittatus in the Northwestern Mediterranean Sea. Fish. Res. 152, 21–28. doi: 10.1016/j.fishres.2013.10.009
Rossi S., Gabbianelli G. (1978). Geomorfologia Del Golfo Di Taranto. Boll. Soc Geol. It. 97, 423–437.
Sardà F., Company J. B., Bahamón N., Rotllant G., Flexas M. M., Sánchez J. D., et al. (2009). Relationship Between Environment and the Occurrence of the Deep-Water Rose Shrimp Aristeus Antennatus (Risso 1816) in the Blanes Submarine Canyon (NW Mediterranean). Prog. Oceanogr. 824, 227–238. doi: 10.1016/j.pocean.2009.07.001
Sardà F., D’Onghia G., Politou C.-Y., Company J. B., Maiorano P., Kapiris K. (2004). Maximum Deep-Sea Distribution and Ecological Aspects of Aristeus Antennatus in the Western and Central Mediterranean Sea. Sci. Mar. 68 (3), 117–127. doi: 10.3989/scimar.2004.68s3117
Shiffman D. S., Gallagher A. J., Boyle M. D., Hammerschlag-Peyer C. M., Hammerschlag N. (2012). Stable Isotope Analysis as a Tool for Elasmobranch Conservation Research: A Primer for non-Specialists. Mar. Freshw. Res. 63, 635–643. doi: 10.1071/MF11235
Simão D. S., Zas E., Carbonell A., Abelló P. (2015). Pasiphaeid Shrimps in the Western Mediterranean: Geographical Variability in Distribution and Population Patterns. Sci. Mar. 79 (2), 199–209. doi: 10.3989/scimar.04147.07A
Sion L., Calculli C., Capezzuto F., Carlucci R., Carluccio A., Cornacchia L., et al. (2019). Does the Bari Canyon (Central Mediterranean) Influence the Fish Distribution and Abundance? Progr. Oceanogr. 170, 81–92. doi: 10.1016/j.pocean.2018.10.015
Spedicato M. T., Massutí E., Mérigot B., Tserpes G., Jadaud A., Relini G. (2019). The MEDITS Trawl Survey Specifications in an Ecosystem Approach to Fishery Management. Sci. Mar. 83 (S1), 9–20. doi: 10.3989/scimar.04915.11X
Taricco C., Alessio S., Rubinetti S., Zanchettin D., Cosoli S., Gačić M., et al. (2015). Marine Sediments Remotely Unveil Long-Term Climatic Variability Over Northern Italy. Sci. Rep. 5, 12111. doi: 10.1038/srep12111
Taviani M., Freiwald A., Zibrowius H. (2005). “Deep Coral Growth in the Mediterranean Sea: An Overview,” in Erlangen Earth Conference Series (Berlin, Heidelberg: Springer). doi: 10.1007/3-540-27673-4_7
Trueman C. N., Johnston G., O’Hea B., MacKenzie K. M. (2014). Trophic Interactions of Fish Communities at Midwater Depths Enhance Long-Term Carbon Storage and Benthic Production on Continental Slopes. Proc. R. Soc B 281, 20140669. doi: 10.1098/rspb.2014.0669
Vassallo P., D’Onghia G., Fabiano M., Maiorano P., Lionetti A., Paoli C., et al. (2017). A Trophic Model of the Benthopelagic Fauna Distributed in the Santa Maria Di Leuca Cold-Water Coral Province (Mediterranean Sea). Energy Ecol. Environ. 2, 114–124. doi: 10.1007/s40974-016-0047-2
Wang S. C., Liu X., Liu Y., Wang H. Z. (2020). Benthic-Pelagic Coupling in Lake Energetic Food Webs. Ecol. Model. 417, 108928. doi: 10.1016/j.ecolmodel.2019.108928
Keywords: ecosystem functioning, consumption flows, food web modelling, Northern Ionian Sea, BiOS
Citation: Ricci P, Carlucci R, Capezzuto F, Carluccio A, Cipriano G, D’Onghia G, Maiorano P, Sion L, Tursi A and Libralato S (2022) Contribution of Intermediate and High Trophic Level Species to Benthic-Pelagic Coupling: Insights From Modelling Analysis. Front. Mar. Sci. 9:887464. doi: 10.3389/fmars.2022.887464
Received: 01 March 2022; Accepted: 31 March 2022;
Published: 04 May 2022.
Edited by:
Martina Orlando-Bonaca, National Institute of Biology (Slovenia), SloveniaReviewed by:
Francesco Cozzoli, National Research Council (CNR), ItalyMaria Flavia Gravina, University of Rome “Tor Vergata”, Italy
Copyright © 2022 Ricci, Carlucci, Capezzuto, Carluccio, Cipriano, D’Onghia, Maiorano, Sion, Tursi and Libralato. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Roberto Carlucci, roberto.carlucci@uniba.it
 Pasquale Ricci
Pasquale Ricci Roberto Carlucci
Roberto Carlucci Francesca Capezzuto1
Francesca Capezzuto1  Giulia Cipriano
Giulia Cipriano Gianfranco D’Onghia
Gianfranco D’Onghia Simone Libralato
Simone Libralato