Nanostructure and Nanomechanics of Prorocentrum donghaiense and Their Changes Under Nitrogen Limitation by Atomic Force Microscopy
- 1CAS Key Laboratory of Marine Ecology and Environmental Sciences, Institute of Oceanology, Chinese Academy of Sciences, Qingdao, China
- 2Functional Laboratory of Marine Ecology and Environmental Science, Qingdao National Laboratory for Marine Science and Technology, Qingdao, China
- 3Center for Ocean Mega-Science, Chinese Academy of Sciences, Qingdao, China
- 4University of Chinese Academy of Sciences, Beijing, China
In this paper, atomic force microscopy was employed to study the harmful algal bloom species Prorocentrum donghaiense for the first time. Cells were immobilized in pores of polycarbonate membrane to keep moist and to acquire images of P. donghaiense at different scales. Typical ultrastructures, such as knob-like spines and valve pores, were observed on cell surfaces. These structures had similar characteristics to those observed on scanning electron microscopy images. Moreover, the height and spacing of typical nanostructure, and nanomechanical parameters such as adhesion and elasticity, were also quantified by AFM. Additionally, the changes in cell surface nanostructure and nanomechanical characteristics under nitrogen limitation were further studied. Compared with the cells under normal culture conditions, the cell surface roughness and adhesion decreased, and the elastic modulus increased for cells under nitrogen limitation. Potential changes in the ability of P. donghaiense cells to perform normal physiological functions are reflected by changes in cell surface parameters, including cell surface roughness, cell surface adhesion, and cell surface elasticity. The results of this study reveal how P. donghaiense responds to changes in the external environment under approximately physiological conditions from the perspective of changes in cell surface nanostructures and nanomechanical characteristics and provide a new understanding of its cell biology.
1 Introduction
Prorocentrum donghaiense is a common harmful algal bloom species in the coastal waters of China, especially in the East China Sea (Lu et al., 2005; Wang et al., 2021; Gu et al., 2022). Since 1990, P. donghaiense has formed blooms almost every year along the Yangtze River Estuary and offshore of the Zhejiang Province (Lu et al., 2005). Algal blooms can cover an area of hundreds to thousands of square kilometers and the cell density can be as high as 3.7×108cells/L, which seriously affects the health of the ecosystem (Lu et al., 2005; Lin et al., 2014). Moreover, in recent years, algal blooms caused by P. donghaiense have gradually expanded to Japan, South Korea, and other sea areas, and the scope of influence has further expanded (Shin et al., 2019). P. donghaiense blooms can be regulated by nitrogen (Wang et al., 2020). Thus, studying the response of P. donghaiense cells under nitrogen limitation is of great significance to thoroughly understanding the mechanism of their environmental adaptation. At present, many studies have been carried out on the physiological, biochemical, and molecular biological response of P. donghaiense cells under nitrogen limitation (Lai et al., 2011; Zhang et al., 2015; Ou et al., 2019; Zhang et al., 2019), and these studies revealed its adaptation mechanism from many points of view. However, there is no relevant research on mechanical properties of P. donghaiense cells response to nitrogen limitation under approximately physiological conditions.
Surface morphology is an important feature of biological cells. At present, the common high-resolution structure characterization method is scanning electron microscopy, but the sample preparation process is complex, and the method cannot directly observe living cells, so it is difficult to observe the physiological state of cells (Zewail, 2010). In recent years, an increasing number of studies have used atomic force microscopy to uncover the surface structure and properties of biological cells (Li et al., 2014; Pasquina-Lemonche et al., 2020). However, compared with its widespread use and applications in animal cell research, only a few microalgae related studies use atomic force microscopy technology. Atomic force microscopy is more widely used in diatom cell research, including the morphology of silica nanostructures, adsorption characteristics of the mucus layer, physical and mechanical properties of diatom cells (Higgins et al., 2000; Higgins et al., 2002; Gebeshuber et al., 2003; Higgins and Wetherbee, 2019), and the response of diatoms to environmental changes (Ma et al., 2019; Ma et al., 2020; Ma et al., 2021). Unlike diatoms, flagellated microalgae are rarely studied, which is partly due to the difficulty of cell immobilization. At present, there are also a few reports on the study of green algae by atomic force microscopy regarding understanding its cell wall structure and adsorption characteristics (Eslick et al., 2014; Pillet et al., 2019). In addition to the above applications, high-resolution atomic force microscopy is also used to study organelles. With the cyanobacteria model strain Synechococcus elongatus PCC 7942 as the research material, high-resolution imaging was performed and its photosynthetic membrane thylakoid membrane, the natural structure and mutual binding mode of photosynthetic complexes on thylakoid membrane were displayed at the nano level, and the light adaptation mechanism of thylakoid membrane structure and function was explained (Zhao et al., 2020). Atomic force microscopy can be used to characterize the response of cells to the environment under approximately physiological conditions from the ultrastructural and mechanical characteristics of the cell surface to provide a new understanding of cell biology (Lu et al., 2020; Venturelli et al., 2020).
Previous studies have shown that nitrogen limitation inhibited the growth of P. donghaiense, maintained its cell density, chlorophyll a content and particulate organic nitrogen content at a low level, and caused the downregulation of proteins involved in photosynthesis, carbon fixation, and protein and lipid synthesis (Zhang et al., 2015; Ou et al., 2019). On the basis of previous studies, this paper intends to characterize the surface nanostructure and nanomechanics of P. donghaiense cells by atomic force microscopy and to further study their changes under nitrogen limitation to reveal the response of P. donghaiense cells to nitrogen limitation from the perspective of cell surface characteristics under approximately physiological conditions, which would provide a basis for further explaining the environmental adaptation mechanism of P. donghaiense.
2 Materials and Methods
2.1 Culture of P. donghaiense
P. donghaiense cells in the later stage of exponential growth were centrifuged at 4000 g, the excess nutrients on the cell surface were washed off with sterilized seawater three times, and the cells were inoculated into normal f/2 medium with a nitrogen concentration of 883 μM and f/2 medium without a nitrogen source. The container was a 25 cm2 breathable cell culture flask (Nest), and the initial density was 1×104 cells/mL. The culture temperature was 20°C in a light:dark cycle of 12h:12h with a light intensity of 4000 lux. When the cells grew to the later stage of exponential growth in normal f/2 medium, the samples of the two groups were taken for sample determination.
2.2 SEM Image Analysis
For each sample, 2 ml algal solution was added to the same volume of 4% osmic acid for fixation for 50 min, and then algal cells were collected, dehydrated in acetone solutions of different concentrations (10%, 30%, 50%, 70%, 90%, 100%, each concentration for 15 min), dried by a CO2 critical point dryer (EM cpd300, Leica), and sprayed with gold by a coating instrument (sputter/carbon thread, EM ace200, Leica). The morphology of P. donghaiense was observed by scanning electron microscopy (Hitachi S-4800, Japan) and measured by electron microscopy software (Veltkamp et al., 1994).
2.3 Samples for AFM and Measurement Parameters
Cells were immobilized with the method used for Staphylococcus aureus cells (Pasquina-Lemonche et al., 2020), while details were modified, and polycarbonate membrane was used instead of NuNano silicon grids. P. donghaiense cells have flagella and different degrees of activity, so it is difficult to fix them when they are completely submerged in the liquid medium. In this study, a polycarbonate membrane (Millipore Isopore, with a thickness of 16 µm to trap the moisture) with a pore size similar to cell size was considered the matrix for fixation so that the cells could be properly “stuck” in the pores and kept moist. A total of 10 µL algal liquid without any treatment was directly dropped onto a 10 µm pore diameter polycarbonate membrane and scanned with atomic force microscopy (Bruker Bioscope Resolve) at room temperature 20°C with air humidity ranging from 50% to 70%. The images should be obtained while the cells are kept moist in approximately physiological conditions. When the cells are dry, salt separates out on the surface, which is also a way to judge the state of the cells. Both automatic mode and contact mode were employed in this study. The probes used are SCANASYST-AIR and DNP. The scanning frequency was 0.5 Hz, and the resolution was 512×512. The mechanical parameters were measured using the ramp mode, and cell surface areas of different sizes such as 5 µm×5 µm were selected for testing.
2.4 Image and Data Processing
The image analysis software used was NanoScope Analysis 1.8. Adhesion and elasticity were calculated by this software. After the images were scanned by atomic force microscopy, they were uniformly processed by first-order flatten, and the cell surface roughness was analyzed by a rough module. The height and spacing of the cell surface ultrastructure were analyzed by the section module, and the original data were redrawn in Excel. Similarly, after the original mechanical analysis data were exported, they were replotted and analyzed in Excel. The roughness (Ra) is the arithmetic mean of the absolute height of the cell surface in the selected area. Roughness of cells under nitrogen limitation and under normal culture conditions were counted by five cells, respectively. The roughness calculation formula is as follows:
where Z is the absolute height of the cell surface and N is the number of calculation points.
3 Results
3.1 Morphology of P. donghaiense Revealed by AFM
In this study, images of P. donghaiense cells from different angles and at different scales were obtained by atomic force microscopy (Figures 1A–F). Figure 1B shows the overall appearance from the left valve view. Figure 1C shows depression structure in the flagellum area at the top of the cell, and the flagellum of P. donghaiense cells can be observed in more detail in Figure 1D. Figure 1E shows the collar structure around the flagellum pores at the top of the cell. Figure 1F is the megacytic zone of the cell, and Figures 1G, H show the knob-like spines and valve pores on the shell surface of the cell. These morphological characteristics of the cell surface of P. donghaiense revealed in this study are similar to those obtained by Lu et al. through SEM (Lu et al., 2005), which indicates that AFM can be used to characterize the cell surface ultrastructure of P. donghaiense.

Figure 1 Images of P. donghaiense by AFM. (A) Valve view. (B) The left valve view. (C) Lateral view of cell which shows the flagellum pore. (D) Lateral view of cell which shows the flagellate. (E) The local magnification of cell which shows the collar structure. (F) The local magnification of cell which shows the megacytic zone. (G, H) The ultrastructure of the cell surface, which displays typical structures, such as knob-like spines and valve pores.
Further high-resolution imaging of the unique structure on the cell surface of P. donghaiense showed clear knob-like spines and valve pores (Figure 2A). The height measurement results show that the height of knob-like spines relative to the cell surface is approximately 100 nm, the spacing between them is approximately 400 nm, the diameter of valve pores is approximately 200 nm, and the height is 120 nm (Figures 2B, C). Similarly, by measuring the height of the indirect zone of P. donghaiense cells (Figure 2D), it can be seen that the height of the bulge on the surface of the megacytic zone is not the same, and the height ranges from approximately 100-200 nm (Figures 2E, F).

Figure 2 Nanostructure of P. donghaiense. (A) The ultrastructure of the cell surface. (B) The height and spacing value of knob-like spines in Panel A. (C) The height and spacing of valve pores on the shell surface in Panel A. (D) The megacytic zone structure of the cell. (E, F) The surface concave convex value in Panel D.
3.2 Nanostructure of P. donghaiense Under Nitrogen Limitation
Nitrogen limitation can affect the physiological activities of P. donghaiense cells, which include photosynthesis, carbon fixation, and protein and lipid synthesis. Compared with cells under normal culture conditions (Figures 3A, C), the cell surface under nitrogen limitation seemed to lack some “reticular substances” (Figures 3B, D).

Figure 3 Morphology of P. donghaiense by SEM. (A, C) The normal culture conditions. (B, D) The nitrogen limiting conditions.
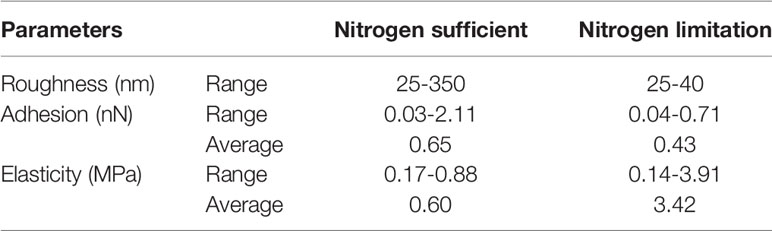
Using AFM to observe moist P. donghaiense cells under two culture conditions, clear knob-like spines and valve pores were observed on the cell surface under normal culture conditions (Figure 4A), while the pores on the cell surface of P. donghaiense under nitrogen limitation were not sufficiently clear (Figure 4B). Further analysis of the height of knob-like spines showed that there were differences in the height of knob-like spines on the surface of the two cells. The height of knob-like spines on the cell surface under normal conditions was approximately 99 nm, while that under nitrogen limited conditions was approximately 75 nm. The cell surface protrusion angle under normal culture conditions was “sharp”, while the cell surface protrusion angle under nitrogen limitation was “blunt”.
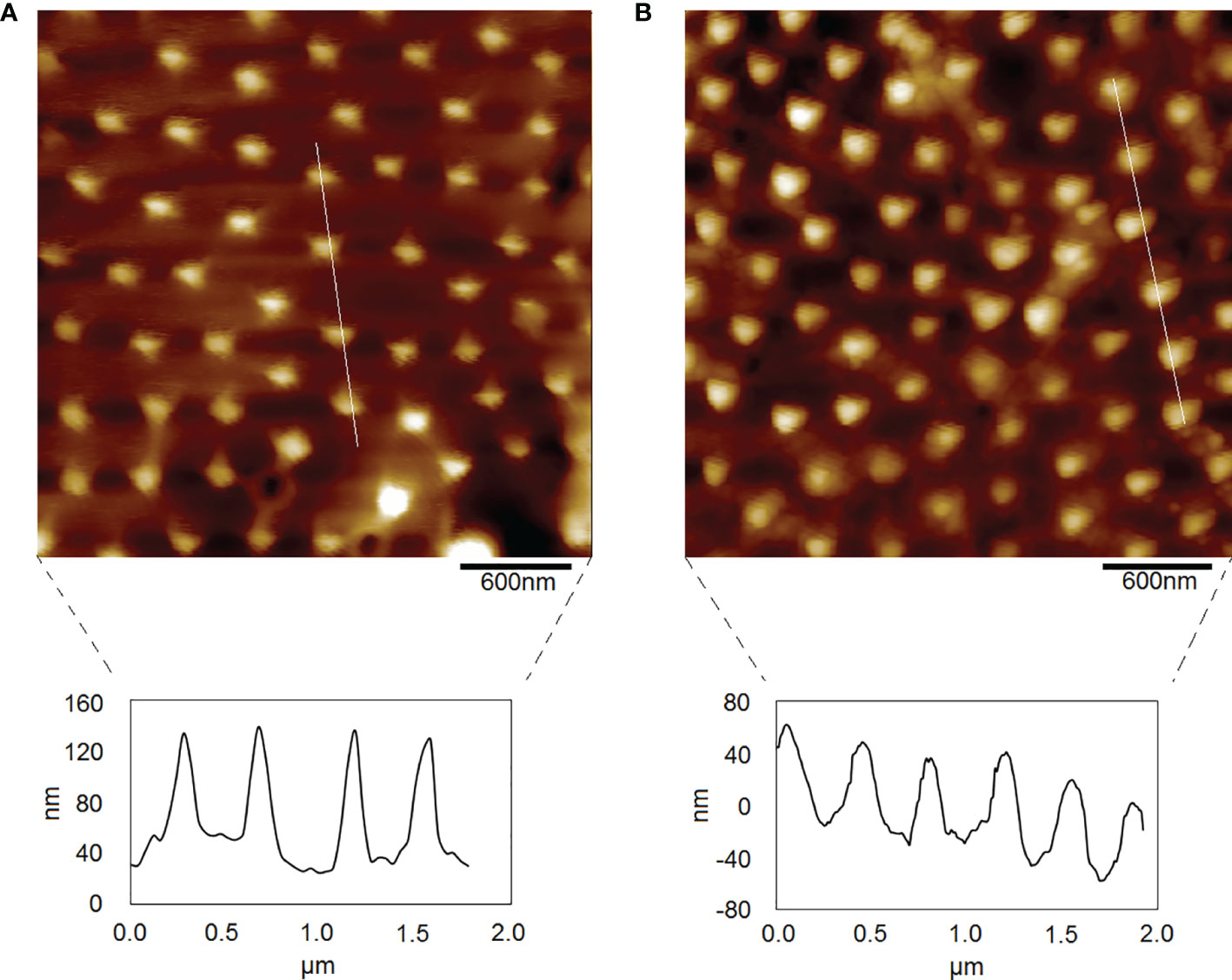
Figure 4 Morphology of P. donghaiense by AFM. (A) The normal culture condition. (B) The nitrogen limiting condition.
Further roughness analysis was carried out on 30 areas of different sizes ranging from 0.3-43.7 µm2 on the cell surface of P. donghaiense. The results showed that the variation range of cell surface roughness of P. donghaiense under normal culture conditions was 25-350 nm, while the value and range of cell surface roughness of P. donghaiense under nitrogen limitation were 25-40 nm (Figures 5A, B). Both the value and range of cell surface roughness of P. donghaiense under normal culture conditions were larger than those under nitrogen limitation. The difference was that the cell surface roughness under normal culture conditions was positively correlated with the surface area (R2= 0.858), and the cell surface roughness under nitrogen restriction was negatively correlated with the surface area (R2= 0.564) (Figures 5C, D).
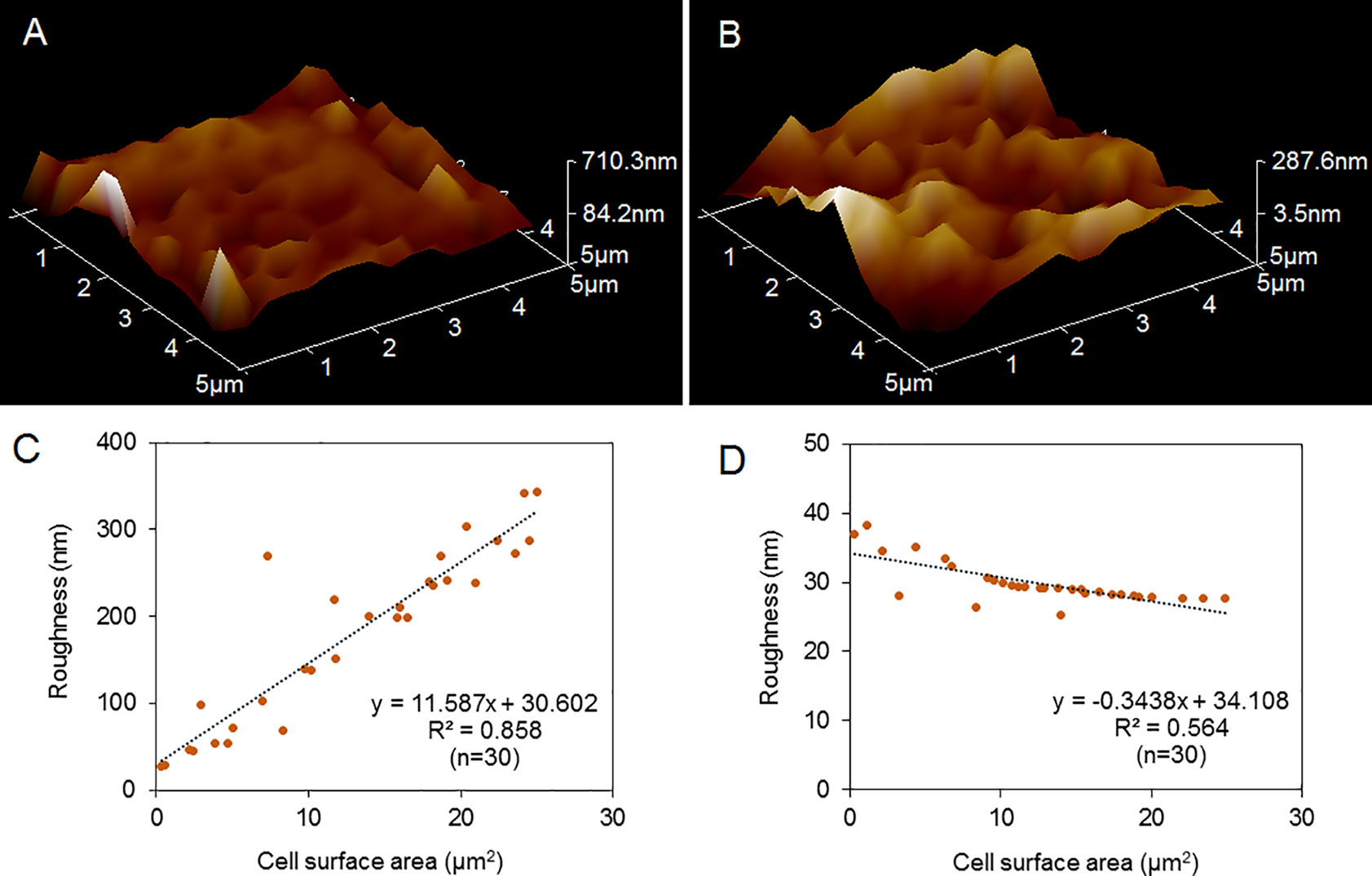
Figure 5 Comparison of the cell surface roughness of P. donghaiense. (A) 3D morphology of P. donghaiense under normal culture conditions. (B) 3D morphology of P. donghaiense under nitrogen limiting conditions. (C) Statistical analysis of roughness under normal culture conditions. (D) Statistical analysis of roughness under nitrogen limiting conditions.
3.3 Nanomechanical Characteristics of P. donghaiense Under Nitrogen Limitation
Many important physiological functions of cells depend on their mechanical properties, such as adhesion and elastic modulus. This study found that the cell surface adhesion of P. donghaiense under normal culture conditions ranged from 0.03 to 2.11 nN, with an average of 0.65 nN (Figures 6A, C). Under nitrogen limitation, the cell surface adhesion of P. donghaiense ranged from 0.04 to 0.71 nN, with an average of 0.43 nN (Figures 6B, D). The surface adhesion of P. donghaiense cells under normal culture conditions is generally greater than that under nitrogen limitation, and the distribution range is wider.

Figure 6 Comparison of the cell surface adhesion of P. donghaiense. (A, C) The normal culture conditions. (B, D) The nitrogen limiting conditions.
The elastic modulus can be regarded as an index to measure the difficulty of elastic deformation to occur in materials. The greater its value is, the greater the stress causing certain elastic deformations in materials; that is, the greater the material stiffness is, the smaller the elastic deformation under the action of certain stress. This study found that the surface elastic modulus of P. donghaiense cells under normal culture conditions ranged from 0.17 to 0.88 MPa, with an average value of 0.60 MPa (Figures 7A, C). Under nitrogen limitation, the cell surface elastic modulus of P. donghaiense ranged from 0.14 to 3.91 MPa, with an average of 3.42 MPa (Figures 7B, D). The surface elastic modulus of P. donghaiense cells under normal culture conditions is less than that under nitrogen limitation, and the whole entity is “softer”.
Figure 7 Comparison of the cell surface elastic modulus of P. donghaiense. (A, C) The normal culture conditions. (B, D) The nitrogen limiting conditions.
4 Discussion
4.1 Using Atomic Force Microscopy to Study Harmful Algal Bloom Species
For a long time, there has been a dispute about the species identification of Prorocentrum in the East China Sea. Lu et al. described the taxonomic characteristics of this species in detail, identified it as P. donghaiense, and found evenly distributed knob-like spines and valve pores on its surface by using scanning electron microscopy (Lu et al., 2005). In this paper, atomic force microscopy was employed to study moist cells in the harmful algal bloom species P. donghaiense for the first time. Typical ultrastructures such as knob-like spines and valve pores on the cell surface had similar characteristics compared with those observed on scanning electron microscopy images. What is more, the height and spacing of a typical nanostructure were quantified by AFM to reflect the microenvironment on the cell surface. Besides morphology characters, adhesion and elasticity were also revealed in this study, which is the first mechanical research of P. donghaiense.
One major advantage of AFM is that it can characterize cells under physiological conditions, which can more accurately reflect the original characteristics of cells (Demir-Yilmaz et al., 2021). Due to the lack of movement ability, diatoms can more easily adhere to the substrate to grow and fix themselves compared with other microalgae equipped with flagella, which avoids the requirements of atomic force microscopy for sample fixation (Gebeshuber et al., 2003). At present, atomic force microscopy in microalgae is mainly used in diatoms (Luis et al., 2017; Demir-Yilmaz et al., 2021). However, there are few reports on atomic force microscopy in the field of red tide algae.
For red tide algae, cell immobilization is an important challenge for the application of atomic force microscopy in the study of living cells, partly due to their strong movement ability. At present, the main methods for flagellated cell immobilization include fixed by glutaraldehyde and then adhered to the substrate (Gunther et al., 2014; Pillet et al., 2019). Unlike diatom cells that can adhere to the substrate for growth, red tide algae such as dinoflagellates have flagella and motion ability, so it is difficult to fix them. In this study, several fixation methods were compared. For dinoflagellates with different particle sizes, a polycarbonate membrane with a pore size similar to its cell size was considered the matrix for fixation so that the cells could be properly “stuck” in the pores and kept moist, and this method is similar to that used for S. aureus cells (Pasquina-Lemonche et al., 2020) and Emiliania huxleyi cells (Evans et al., 2021). In the 10 µm pore size polycarbonate membrane selected in this study, there are several types of “stuck slots”: a single 10 µm hole (Figure 8A) and two 10 µm connected holes (Figure 8B) which can immobilize P. donghaiense cells. Atomic force microscopy is an important method to study the biological mechanism of cells. Studies based on animal cells show that the elastic modulus E of cells is closely related to their life activities and health status. For example, the elastic modulus E of human cancerous cells is much lower than that of normal cells, and by directly measuring the elastic modulus E of biological cells, the cancerous state can be reflected (Cross et al., 2007). Cell differentiation is usually accompanied by changes in shape to realize special functions. To produce different shapes, cells need to change the mechanical properties of their surface (Bergert et al., 2021). By using a cell magnetic twisting instrument, Wang et al. found that integrin, a cell adhesion protein, is a cell mechanical receptor (Wang et al., 1993); moreover, mechanical stimulation can be directly transmitted to the nucleus through the cytoskeleton and nuclear membrane and directly activate gene expression. Directly stretching chromatin with mechanical force can upregulate transcription (Tajik et al., 2016).

Figure 8 Different types of “slots” in the 10-µm pore size polycarbonate membrane. (A) A single 10 μm hole. (B) Two 10 μm connected holes.
The instrument can image microalgae cells with nanoscale resolution and probe the nanomechanical properties and nanoadhesive properties of microalgae cells (Demir-Yilmaz et al., 2021). Mechanical factors can play a key role in the response and adaptation of different levels of functions, such as mechanical regulation and transportation, tissue deformation, cell growth and movement, intermolecular interactions, and signal pathways (Cross et al., 2007; Tajik et al., 2016; Bergert et al., 2021). Through studying the surface ultrastructure and biomechanics, AFM is expected to make a new breakthrough in the environmental adaptation mechanism of microalgae.
4.2 Effects of Nitrogen Limitation on P. donghaiense
Previous studies have shown that nitrogen limitation inhibited the growth of P. donghaiense, maintained its cell density, chlorophyll a content and particulate organic nitrogen content at a low level, and caused the downregulation of proteins involved in photosynthesis, carbon fixation, and protein and lipid synthesis (Zhang et al., 2015; Ou et al., 2019). In this study, the effect of nitrogen limitation on the surface morphology and mechanical characteristics of P. donghaiense cells under physiological conditions was further uncovered. Details of morphology parameters are shown in Table 1. Compared with cells under normal culture conditions, the value and range of cell surface roughness under nitrogen limitation decreased, the value and range of cell surface adhesion decreased, and the value and range of elastic modulus increased. The function of cell surface is very complex. In addition to supporting and protecting cells, the cell surface is closely related to the behavior, physiological activities, mutual recognition, adhesion, material transportation, signal transduction, cell movement, growth, differentiation, aging, and pathological process of the whole cell (Ludwig et al., 2021). The cell surface is responsible for the material exchange and energy exchange inside and outside the cell, and carries out cell recognition, information reception and transmission, cell movement, and maintenance of various cell forms through the surface structure.
4.2.1 Physiological Significance of Cell Surface Roughness
Roughness is a comprehensive reflection of cell microsurface structure, composition, and content (Hou et al., 2020). This study revealed that the value and range of P. donghaiense cell surface roughness under nitrogen limitation decreased compared with that of cells under normal culture conditions. The cell surface roughness under normal culture conditions was positively correlated with the surface area, with the cell surface roughness under nitrogen restriction negatively correlated with the surface area, which reflects the microenvironment decreased under nitrogen limitation. In terms of function, the cell surface expands function of the plasma membrane, which plays a role in supporting and protecting the cell, so that the cell has a relatively stable internal environment. The cell wall of dinoflagellates is mainly composed of cellulose and protein (Lin, 2011). These adhesion substances are an important part of the extracellular microenvironment, and sugary substances, such as sialic acid and hyaluronic acid, are charged and can adsorb ions to maintain a constant charge and pH in the microenvironment, which is beneficial to the activity of enzymes on the plasma membrane and the activity of cells. For example, there are extracellular phosphatases such as alkaline phosphatase on the cell surface of Prorocentrum minimum, which can utilize organic phosphorus, and the suitable pH condition for its function is 8 (Dyhrman and Palenik, 1997). Changes in the cell surface microenvironment are certain to affect the activity of extracellular enzymes to a certain extent.
As the roughness value and range decrease, the potential microenvironment on the cell surface decreases, which may affect various normal physiological activities. For example, research on diatom Nitzchia closterium shows that the cell surface roughness decreases with increasing salinity, which affects the components of the cell surface and further affects the adsorption of heavy metals (Ma et al., 2019). For another diatom Phaeodactylum tricornutum, the surface of the oval form has an outer extracellular polymer, and its surface roughness is greater than that of the fusiform and triradiate forms, which is also a potential mechanism for this form to adapt to environmental change (Francius et al., 2008).
4.2.2 Physiological Significance of Cell Surface Adhesion
Cell adhesion is a basic phenomenon in biology. Cells can exchange materials with the surrounding environment through adhesion. Understanding the mechanical and biological mechanism of cell adhesion and debonding is of great significance to understanding cell migration, hardness perception, cell differentiation, and other life phenomena (Zhang et al., 2017). In this study, the cell surface adhesion of P. donghaiense under normal culture conditions ranged from 0.03 to 2.11 nN, with an average of 0.65 nN. The cell surface adhesion of P. donghaiense under nitrogen limitation ranged from 0.04 to 0.71 nN, with an average of 0.43 nN. The surface adhesion of P. donghaiense cells under normal culture conditions is generally greater than that under nitrogen limitation, and the distribution range is wider.
Adhesion is of great significance to cellular roles. The reduction in cell surface adhesion under nitrogen limitation is certain to affect the normal function of cells. Algae cells can achieve the phototaxis functions through adhesion, and the phenomenon of microalgae attaching to the wall can be controlled by light. By regulating the control switch in Chlamydomonas flagella, Chlamydomonas can switch between a floating state and an attached state and adapt to environmental changes by maximizing their photosynthetic efficiency. This transformation can be realized by adjusting the adhesion of the cell surface (Kreis et al., 2018). Higgins et al. studied the adhesion of diatoms and found that EPS can be secreted in many places on the surface of diatoms, including pores on the surface of girdle bands and valves. There are active substances such as sugars, lipids, and proteins on the cell surface of diatoms (Higgins et al., 2000; Higgins et al., 2002). Microalgae EPS contains carboxyl, hydroxyl, amino, sulfhydryl, and other functional groups with adhesion, which has good adsorption performance for heavy metals, and the number of these groups can increase greatly under heavy metal stress, which plays a significant role in the bioremediation of heavy metals (Wotton, 2004).
4.2.3 Physiological Significance of Cell Surface Elasticity
The cell elastic modulus can reflect the state of biological cells. For example, the elastic modulus on the surface of cancer cells is much lower than that of normal cells (Cross et al., 2007). In this study, the surface elastic modulus of P. donghaiense cells under normal culture conditions ranged from 0.17 to 0.88 MPa, with an average of 0.60 MPa. Under nitrogen limitation, the cell surface elastic modulus of P. donghaiense ranged from 0.14 to 3.91 MPa, with an average of 3.42 MPa. The surface elastic modulus of P. donghaiense cells under nitrogen limitation was higher than that under normal culture conditions.
Increasing evidence shows that mechanical signals, such as chemical small molecules and protein signal transduction, play a decisive role in the function and fate of cells. Compared with chemical signals, mechanical signals have the characteristics of fast occurrence, short action time, and variable action effect (Mitchell and Rosenblatt, 2021). Cells respond in time through integrin receptors on their surface, and selectively convert mechanical signals to different structural components of cells in the form of tension integration. After cells are stimulated by force, the stimulation is transformed into corresponding signals into cells, causing a series of response reactions (Lin et al., 2019). The change in cell surface elasticity affects the signal conversion of the response to a certain extent, thus changing the downstream transcriptional expression.
Directly stretching chromatin with mechanical force can upregulate transcription (Tajik et al., 2016), which indicates that mechanical stimulation can be directly transmitted to the nucleus through the cytoskeleton and nuclear membrane and directly activate gene expression. From this perspective, under the condition of nitrogen limitation, the cell surface elastic modulus increases, the cell flexibility decreases, and the difficulty of directly regulating transcription and expression by external mechanical signals will certainly increase. Cell elasticity is an important parameter to maintain the structure of the cell outer wall (Zhao et al., 2005), and the cell wall of dinoflagellates is mainly composed of cellulose and protein (Lin, 2011). Beta-glucosidase can degrade cell wall polysaccharides in P. donghaiense (Shi et al., 2018). The study on green algae Chlorococcum sp. showed that the Young’s modulus under nitrogen limitation was 30% higher than that under normal culture conditions, which was related to the content of triglycerides. The cell wall thickness increased from 387 nm to 503 nm under nitrogen limitation (Yap et al., 2016). Because the surface of oval cells has a siliceous outer wall but the surface of fusiform and triradiate forms cells is mainly composed of organic substances such as sulfated glucuromannan, the Young’s modulus on the surface of oval P. tricornutum cells is five times that of the other two forms. The differential mechanical characteristics of the cell surface are conducive to the adaptation of different forms of cells (Higgins et al., 2002) to environmental changes (Francius et al., 2008).
5 Conclusions
In this study, a feasible immobilization method was used to fix P. donghaiense cells by clamping them in the pores of a polycarbonate membrane to further measure the surface morphology and mechanical parameters of moist cells. Typical ultrastructures, such as knob-like spines and valve pores, on the cell surface show similar characteristics to those observed with SEM images, which indicates that AFM can be used to characterize the cell surface ultrastructure of P. donghaiense. Moreover, the height and spacing of typical ultrastructures were also quantified. This study further found that compared with the cells under normal culture conditions, the cell surface roughness and adhesion decreased, and elastic modulus increased for cells under nitrogen limitation. This study demonstrates the first AFM image of the harmful algal species P. donghaiense with a typical nanostructure with high resolution and reveals the surface nanomechanical properties of P. donghaiense under nitrogen limitation for the first time. Changes in cell surface roughness, adhesion, and elastic modulus reflect potential changes in the ability of cells to perform normal physiological functions. The change in intracellular transcriptional expression is directly regulated by mechanical stimulation caused by the change in cell surface elasticity. This study reflects the way P. donghaiense responds to changes in the external environment from the perspective of cell surface ultrastructure and mechanical parameters, provides a new understanding of its cell biology, and demonstrates AFM as a novel and powerful technique for studying harmful algal bloom species.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author Contributions
LH and ZY designed the study. LH and JZ performed the experiments. LH, ZY, and XS conducted the analyses. LH wrote the initial manuscript. All authors contributed to the improvement of the manuscript.
Funding
This study was financially supported by the Taishan Program of Shandong Province of 2019, the Youth Talent Support Program of the Laboratory for Marine Ecology and Environmental Science, Pilot National Laboratory for Marine Science and Technology (Qingdao, LMEES-YTSP-2018-01-03), and the National Natural Science Foundation of China (No. 42006120).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bergert M., Lembo S., Sharma S., Russo L., Milovanovic D., Gretarsson K. H., et al. (2021). Cell Surface Mechanics Gate Embryonic Stem Cell Differentiation. Cell Stem Cell 28 (2), 209–216. doi: 10.1016/j.stem.2020.10.017
Cross S. E., Jin Y. S., Rao J., Gimzewski J. K. (2007). Nanomechanical Analysis of Cells From Cancer Patients. Nat. Nanotech. 2 (12), 780–783. doi: 10.1038/nnano.2007.388
Demir-Yilmaz I., Guiraud P., Formosa-Dague C. (2021). The Contribution of Atomic Force Microscopy (AFM) in Microalgae Studies: A Review. Algal. Res. 60, 1–9. doi: 10.1016/J.Algal.2021.102506
Dyhrman S. T., Palenik B. P. (1997). The Identification and Purification of a Cell-Surface Alkaline Phosphatase From the Dinoflagellate Prorocentrum Minimum (Dinophyceae). J. Phycol. 33 (4), 602–612. doi: 10.1111/j.0022-3646.1997.00602.x
Eslick E. M., Beilby M. J., Moon A. R. (2014). A Study of the Native Cell Wall Structures of the Marine Alga Ventricaria Ventricosa (Siphonocladales, Chlorophyceae) Using Atomic Force Microscopy. Microscopy-Jpn 63 (2), 131–140. doi: 10.1093/jmicro/dft083
Evans C. T., Baldock S. J., Hardy J. G., Payton O., Picco L., Allen M. J. (2021). A Non-Destructive, Tuneable Method to Isolate Live Cells for High-Speed AFM Analysis. Microorganisms 9 (4), 1–11. doi: 10.3390/microorganisms9040680
Francius G., Tesson B., Dague E., Martin-Jezequel V., Dufrene Y. F. (2008). Nanostructure and Nanomechanics of Live Phaeodactylum Tricornutum Morphotypes. Environ. Microbiol. 10 (5), 1344–1356. doi: 10.1111/j.1462-2920.2007.01551.x
Gebeshuber I. C., Kindt J. H., Thompson J. B., Del Amo Y., Stachelberger H., Brzezinski M. A., et al. (2003). Atomic Force Microscopy Study of Living Diatoms in Ambient Conditions. J. Microsc-Oxford. 212, 292–299. doi: 10.1111/j.1365-2818.2003.01275.x
Gunther T. J., Suhr M., Raff J., Pollmann K. (2014). Immobilization of Microorganisms for AFM Studies in Liquids. Rsc. Adv. 4 (93), 51156–51164. doi: 10.1039/c4ra03874f
Gu H. F., Wu Y. R., Lv S. H., Lu D. D., Tang Y. Z., Qi Y. Z. (2022). Emerging Harmful Algal Bloom Species Over the Last Four Decades in China. Harm. Algae., 111, 1–10. doi: 10.1016/j.hal.2021.102059
Higgins M. J., Crawford S. A., Mulvaney P., Wetherbee R. (2000). The Topography of Soft, Adhesive Diatom ‘Trails’ as Observed by Atomic Force Microscopy. Biofouling 16 (2-4), 133–139. doi: 10.1080/08927010009378438
Higgins M. J., Crawford S. A., Mulvaney P., Wetherbee R. (2002). Characterization of the Adhesive Mucilages Secreted by Live Diatom Cells Using Atomic Force Microscopy. Protist 153 (1), 25–38. doi: 10.1078/1434-4610-00080
Higgins M. J., Wetherbee R. (2019). The Role of Atomic Force Microscopy in Advancing Diatom Research Into the Nanotechnology Era (New York: Jenny Stanford Publishing). doi: 10.1201/9780429066597-19
Hou Y., Xie W. Y., Yu L. X., Camacho L. C., Nie C. X., Zhang M., et al. (2020). Surface Roughness Gradients Reveal Topography-Specific Mechanosensitive Responses in Human Mesenchymal Stem Cells. Small 16 (10), 1-10. doi: 10.1002/Smll.201905422
Kreis C. T., Le Blay M., Linne C., Makowski M. M., Baumchen O. (2018). Adhesion of Chlamydomonas Microalgae to Surfaces is Switchable by Light. Nat. Phys. 14 (1), 45–49. doi: 10.1038/Nphys4258
Lai J. X., Yu Z. M., Song X. X., Cao X. H., Han X. T. (2011). Responses of the Growth and Biochemical Composition of Prorocentrum Donghaiense to Different Nitrogen and Phosphorus Concentrations. J. Exp. Mar. Biol. Ecol. 405 (1-2), 6–17. doi: 10.1016/j.jembe.2011.05.010
Li M., Liu L. Q., Xi N., Wang Y. C. (2014). Research Progress in Quantifying the Mechanical Properties of Single Living Cells Using Atomic Force Microscopy. Chin. Sci. Bull. 59 (31), 4020–4029. doi: 10.1007/s11434-014-0581-2
Lin S. J. (2011). Genomic Understanding of Dinoflagellates. Res. Microbiol. 162 (6), 551–569. doi: 10.1016/j.resmic.2011.04.006
Lin Y. C., Guo Y. R., Miyagi A., Levring J., MacKinnon R., Scheuring S. (2019). Force-Induced Conformational Changes in PIEZO1. Nature 573 (7773), 230–234. doi: 10.1038/s41586-019-1499-2
Lin J. N., Yan T., Zhang Q. C., Wang Y. F., Liu Q., Zhou M. J. (2014). In Situ Detrimental Impacts of Prorocentrum Donghaiense Blooms on Zooplankton in the East China Sea. Mar. Pollut. Bull. 88 (1-2), 302–310. doi: 10.1016/j.marpolbul.2014.08.026
Ludwig N., Reissmann S., Schipper K., Gonzalez C., Assmann D., Glatter T., et al. (2021). A Cell Surface-Exposed Protein Complex With an Essential Virulence Function in Ustilago Maydis. Nat. Microbiol. 6 (6), 722–730. doi: 10.1038/s41564-021-00896-x
Lu D. D., Goebel J., Qi Y. Z., Zou J. Z., Han X. T., Gao Y. H., et al. (2005). Morphological and Genetic Study of Prorocentrum Donghaiense Lu From the East China Sea, and Comparison With Some Related Prorocentrum Species. Harm. Algae. 4 (3), 493–505. doi: 10.1016/j.hal.2004.08.015
Luis A. T., Hlubikova D., Vache V., Choquet P., Hoffmann L., Ector L. (2017). Atomic Force Microscopy (AFM) Application to Diatom Study: Review and Perspectives. J. Appl. Phycol. 29 (6), 2989–3001. doi: 10.1007/s10811-017-1177-4
Lu Z. C., Wang Z. B., Li D. Y. (2020). Application of Atomic Force Microscope in Diagnosis of Single Cancer Cells. Biomicrofluidics 14 (5), 1–12. doi: 10.1063/5.0021592
Ma J., Zhou B. B., Chen F. Y., Pan K. (2021). How Marine Diatoms Cope With Metal Challenge: Insights From the Morphotype-Dependent Metal Tolerance in Phaeodactylum Tricornutum. Ecotox. Environ. Safe. 208, 1–9. doi: 10.1016/J.Ecoenv.2020.111715
Ma J., Zhou B. B., Duan D. D., Pan K. (2019). Salinity-Dependent Nanostructures and Composition of Cell Surface and its Relation to Cd Toxicity in an Estuarine Diatom. Chemosphere 215, 807–814. doi: 10.1016/j.chemosphere.2018.10.128
Ma J., Zhou B. B., Tan Q. G., Zhang L., Pan K. (2020). The Roles of Silicon in Combating Cadmium Challenge in the Marine Diatom Phaeodactylum Tricornutum. J. Haz. Mater. 389, 1–10. doi: 10.1016/j.jhazmat.2019.121903
Mitchell S. J., Rosenblatt J. (2021). Early Mechanical Selection of Cell Extrusion and Extrusion Signaling in Cancer. Curr. Opin. Cell Biol. 72, 36–40. doi: 10.1016/j.ceb.2021.04.005
Ou L. J., Huang K. X., Li J. J., Jing W. Y., Dong H. P. (2019). Transcriptomic Responses of Harmful Dinoflagellate Prorocentrum Donghaiense to Nitrogen and Light. Mar. Pollut. Bull. 149, 1–10. doi: 10.1016/j.marpolbul.2019.110617
Pasquina-Lemonche L., Burns J., Turner R. D., Kumar S., Tank R., Mullin N., et al. (2020). The Architecture of the Gram-Positive Bacterial Cell Wall. Nature 582, 294–297. doi: 10.1038/s41586-020-2236-6
Pillet F., Dague E., Ilic J. P., Ruzic I., Rols M. P., DeNardis N. I. (2019). Changes in Nanomechanical Properties and Adhesion Dynamics of Algal for Cells During Their Growth Updates. Bioelectrochemistry 127, 154–162. doi: 10.1016/j.bioelechem.2019.02.011
Shi X. G., Liu L. M., Li Y., Xiao Y. C., Ding G. M., Lin S. J., et al. (2018). Isolation of an Algicidal Bacterium and its Effects Against the Harmful-Algal-Bloom Dinoflagellate Prorocentrum Donghaiense (Dinophyceae). Harm. Algae. 80, 72–79. doi: 10.1016/j.hal.2018.09.003
Shin H. H., Li Z., Seo M. H., Soh H. Y., Lim W. A., Park J. W. (2019). Harmful Dinoflagellate Prorocentrum Donghaiense Lu is Widely Distributed Along the East China Sea and Korean Coastal Area. Ocean. Sci. J. 54 (4), 685–691. doi: 10.1007/s12601-019-0028-4
Tajik A., Zhang Y. J., Wei F. X., Sun J., Jia Q., Zhou W. W., et al. (2016). Transcription Upregulation via Force-Induced Direct Stretching of Chromatin. Nat. Mater. 15 (12), 1287–1296. doi: 10.1038/NMAT4729
Veltkamp C. J., Chubb J. C., Birch S. P., Eaton J. W. (1994). A Simple Freeze Dehydration Method for Studying Epiphytic And Epizoic Communities Using the Scanning Electron-Microscope. Hydrobiologia 288 (1), 33–38. doi: 10.1007/Bf00006803
Venturelli L., Kohler A. C., Stupar P., Villalba M. I., Kalauzi A., Radotic K., et al. (2020). A Perspective View on the Nanomotion Detection of Living Organisms and its Features. J. Mol. Recog. 33 (12), 1–14. doi: 10.1002/Jmr.2849
Wang N., Butler J. P., Ingber D. E. (1993). Mechanotransduction Across the Cell-Surface And Through the Cytoskeleton. Science 260 (5111), 1124–1127. doi: 10.1126/science.7684161
Wang H., Hu Z. X., Chai Z. Y., Deng Y. Y., Zhan Z. F., Tang Y. Z. (2020). Blooms of Prorocentrum Donghaiense Reduced the Species Diversity of Dinoflagellate Community. Acta Oceanolog. Sin. 39 (4), 110–119. doi: 10.1007/s13131-020-1585-1
Wang D. Z., Zhang S. F., Zhang H., Lin S. J. (2021). Omics Study of Harmful Algal Blooms in China: Current Status, Challenges, and Future Perspectives. Harm. Algae. 107, 1–9. doi: 10.1016/J.Hal.2021.102079
Wotton R. S. (2004). The Essential Role of Exopolymers (EPS) in Aquatic Systems. Oceanog. And. Mar. Biol. Annu. Rev. 42, 57–94. doi: 10.1201/9780203507810
Yap B. H. J., Crawford S. A., Dagastine R. R., Scales P. J., Martin G. J. O. (2016). Nitrogen Deprivation of Microalgae: Effect on Cell Size, Cell Wall Thickness, Cell Strength, and Resistance to Mechanical Disruption. J. Ind. Microbiol. Biot. 43 (12), 1671–1680. doi: 10.1007/s10295-016-1848-1
Zewail A. H. (2010). Four-Dimensional Electron Microscopy. Science 328 (5975), 187–193. doi: 10.1126/science.1166135
Zhang C. Y., Chen G. F., Wang Y. Y., Zhang X. D., Li C. H. (2019). Molecular Characterization of Heat Shock Protein 90 From the Dinoflagellate Prorocentrum Donghaiense and its Transcriptional Response to Thermal, Copper and Nutrient Stresses. Mar. Biol. Res. 15 (4-6), 343–356. doi: 10.1080/17451000.2019.1662445
Zhang Y. J., Wei F. X., Poh Y. C., Jia Q., Chen J. J., Chen J. W., et al. (2017). Interfacing 3D Magnetic Twisting Cytometry With Confocal Fluorescence Microscopy to Image Force Responses in Living Cells. Nat. Protoc. 12 (7), 1437–1450. doi: 10.1038/nprot.2017.042
Zhang Y. J., Zhang S. F., He Z. P., Lin L., Wang D. Z. (2015). Proteomic Analysis Provides New Insights Into the Adaptive Response of a Dinoflagellate Prorocentrum Donghaiense to Changing Ambient Nitrogen. Plant Cell Environ. 38 (10), 2128–2142. doi: 10.1111/pce.12538
Zhao L. S., Huokko T., Wilson S., Simpson D. M., Wang Q., Ruban A. V., et al. (2020). Structural Variability, Coordination and Adaptation of a Native Photosynthetic Machinery. Nat. Plants 6 (7), 869–882. doi: 10.1038/s41477-020-0694-3
Keywords: Prorocentrum donghaiense, atomic force microscopy, nanostructure, nanomechanics, nitrogen limitation
Citation: He L, Yu Z, Zhu J, Cao X and Song X (2022) Nanostructure and Nanomechanics of Prorocentrum donghaiense and Their Changes Under Nitrogen Limitation by Atomic Force Microscopy. Front. Mar. Sci. 9:874888. doi: 10.3389/fmars.2022.874888
Received: 14 February 2022; Accepted: 29 March 2022;
Published: 28 April 2022.
Edited by:
Gordon T. Taylor, Stony Brook University, United StatesReviewed by:
Angela Sardo, Stazione Zoologica Anton Dohrn Napoli, ItalyKe Pan, Shenzhen University, China
Copyright © 2022 He, Yu, Zhu, Cao and Song. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhiming Yu, zyu@qdio.ac.cn
 Liyan He
Liyan He Zhiming Yu
Zhiming Yu Jianan Zhu1,2,3
Jianan Zhu1,2,3  Xihua Cao
Xihua Cao