Susceptibility of Caribbean Brain Coral Recruits to Stony Coral Tissue Loss Disease (SCTLD)
- 1Department of Marine Biology and Ecology, Rosenstiel School of Marine and Atmospheric Science, University of Miami, Miami, FL, United States
- 2Center for Conservation, The Florida Aquarium, Apollo Beach, FL, United States
Stony coral tissue loss disease (SCTLD) has devastated coral populations along Florida’s Coral Reef and beyond. Although widespread infection and mortality of adult colonies have been documented, no studies have yet investigated the susceptibility of recruits to this disease. Here, we subjected eight-month-old Diploria labyrinthiformis recruits and four-month-old Colpophyllia natans recruits to two sequential exposures to SCTLD in the laboratory to track infection and assess potential resilience. Both species began to develop lesions as early as 48 h after exposure began. During the first exposure, 59.6% of C. natans recruits lost all tissue (died) within two to eight days of developing lesions, whereas D. labyrinthiformis recruits experienced slower tissue loss and minimal eventual mortality. In C. natans, larger recruits and those fused into groups of multiple genets (chimeras) exhibited the highest survivorship. In contrast, smaller and/or single (ungrouped) recruits had the lowest survivorship (9.9 - 24.8%). After 20 days, a second SCTLD exposure was delivered to further test resistance in remaining recruits, and all recruits of both species succumbed within six days. Although no recruits showed absolute resistance to SCTLD following repeated exposures, our results provide evidence that interactions between size and chimerism can impact relative resistance in C. natans. This study represents the first report of SCTLD in Caribbean coral recruits and carries implications for natural species recovery and reef restoration efforts. Additional research on the susceptibility of coral juveniles to SCTLD is urgently needed, to include different species, locations, parents, and algal symbionts, with the goals of assessing relative susceptibility and identifying potential sources of resilience for this critical life history stage.
Introduction
Stony coral tissue loss disease (SCTLD) has devastated coral reefs in Florida since its first outbreak near Miami in 2014. This disease has been found to infect at least 22 species of scleractinian corals, making it one of the most lethal coral disease outbreaks ever recorded (Precht et al., 2016). It has spread rapidly throughout Florida’s Coral Reef and beyond, resulting in extremely high rates of infection and mortality at affected sites (Precht et al., 2016; FKNMS, 2018; Walton et al., 2018; Alvarez-Filip et al., 2019; Sharp et al., 2020; Dahlgren et al., 2021; Brandt et al., 2021; Kolodziej et al., 2021). Localized outbreaks have precipitated significant declines in live coral tissue area (>60%) over short periods of time (Walton et al., 2018; Sharp et al., 2020; Heres et al., 2021; Brandt et al., 2021), with some species being more severely impacted than others (Gintert et al., 2019; Costa et al., 2021; Neely et al., 2021a; Spadafore et al., 2021). In surveys in Miami-Dade county in 2015 and 2016, Precht et al. (2016) documented 83% infection in Diploria labyrinthiformis colonies, and estimated that these populations were reduced to <25% of their original numbers. Such high rates of infection and mortality may jeopardize the long-term persistence of susceptible species (Precht et al., 2016; Neely et al., 2021a). Furthermore, these dramatic losses have altered the composition of coral communities in affected areas, threatening biodiversity and ecosystem function (Alvarez-Filip et al., 2013; Walton et al., 2018; Gilliam et al., 2019; Estrada-Saldívar et al., 2020; Sharp et al., 2020; Heres et al., 2021).
The impacts of SCTLD have been well documented in many locations experiencing outbreaks, but details on the cause(s) and dynamics of this disease remain unknown. Although the causative pathogen(s) for SCTLD remain unidentified, enrichment of disease-associated bacteria in lesions, along with the efficacy of antibiotics, suggests that bacteria play a role in disease progression (Meyer et al., 2019; Neely et al., 2020; Rosales et al., 2020; Clark et al., 2021; Neely et al., 2021b). Transmission has been shown to occur through direct contact as well as through the water column in neutrally buoyant particles (Aeby et al., 2019; Dobbelaere et al., 2020; Eaton et al., 2021). Williams et al. (2021) found that in the Florida Keys, SCTLD disproportionately affected large corals and areas with high species diversity and colony density. In an experimental exposure Dennison et al. (2021) found that corals manipulated to contain algal symbionts in the genus Durusdinium were less susceptible to SCTLD compared to their counterparts hosting Breviolum and Cladocopium, and Rubin et al. (2021) reported that inshore brain corals hosting Durusdinium largely escaped infection compared to offshore corals hosting Breviolum. Beyond these reports, however, little is known about the factors contributing to infection and/or resilience in coral colonies.
Reef surveys tracking disease prevalence in SCTLD-susceptible species have only documented the health of colonies ≥4 cm in diameter, excluding recruits (Precht et al., 2016; Gilliam et al., 2019; Kramer et al., 2019). Consequently, we have no knowledge of the extent to which Caribbean coral juveniles may have been impacted by SCTLD, nor the degree of risk they face from future outbreaks. If populations of susceptible species are to recover, it will be important to understand whether new generations of corals (i.e., sexually-produced juveniles) that recruit to the reef are susceptible to disease. In addition, as scientists and managers test active reef restoration techniques to restore depleted populations, they will need to consider the danger that SCTLD poses to outplanted corals (both asexually-produced fragments and sexually-produced recruits).
Even in the absence of environmental stressors such as disease, the early life stages of many corals are characterized by high mortality and population “bottlenecks” that severely reduce the number of living recruits over time (Miller et al., 2000; Wilson and Harrison, 2005; Vermeij and Sandin, 2008; Vermeij et al., 2011; van Woesik et al., 2014). A recruit’s tiny size leaves it vulnerable to many organisms on the reef capable of preying on, grazing over, outcompeting, or simply smothering it (Doropoulos et al., 2012; Doropoulos et al., 2016). As such, recruits benefit from growing as quickly as possible, becoming large enough to be less susceptible to predation and competition (Doropoulos et al., 2012; Doropoulos et al., 2016). In addition, larvae often settle in groups of multiple individuals, and these aggregations may have implications for survival during early ontogeny (Duerden, 1902; Frank et al., 1997; Raymundo and Maypa, 2004; Puill-Stephan et al., 2011; Puill-Stephan et al., 2012). In particular, the fusion of multiple primary polyps into a chimeric colony may allow vulnerable coral recruits to rapidly surpass size-escape thresholds (Raymundo and Maypa, 2004; Amar et al., 2008; Vermeij and Sandin, 2008; Christiansen et al., 2009; Puill-Stephan et al., 2011; Doropoulos et al., 2016; Sampayo et al., 2020). Moreover, the inherent genetic diversity of chimeric colonies is also thought to offer advantages for coral resilience (Rinkevich and Yankelevich, 2004; Rinkevich et al., 2016; Rinkevich, 2019; Huffmyer et al., 2021). With high mortality already a hallmark of early ontogeny in corals, it is likely that SCTLD outbreaks may exacerbate the bottlenecks that impact populations of juvenile corals.
To date, there are no reports on whether Caribbean coral recruits can become infected with SCTLD or how their pathology may compare to what has been observed in adult colonies. Here, we exposed four-month-old Colpophyllia natans and eight-month-old Diploria labyrinthiformis recruits to SCTLD by rearing them for four weeks in semi-recirculating laboratory systems shared with infected adult colonies of four different coral species and monitoring patterns of survival and disease progression. We tracked the fate of each recruit throughout exposure in relation to its size and recruit grouping status (chimera vs. non-chimera), and characterized susceptibility by lesion prevalence, lesion progression rates, and mortality.
Materials and Methods
Gamete Collection
This study utilized two cohorts of coral recruits collected as gametes from parent colonies in ex-situ induced spawning facilities at the Florida Aquarium’s Center for Conservation. In both cases, parent colonies had been collected by the Florida Fish and Wildlife Conservation Commission as part of the Florida Coral Rescue (under FKNMS Superintendent permit FKNMS-2017-100), after which they were loaned to The Florida Aquarium to serve as a living genetic archive. On May 20, 2020, eight Diploria labyrinthiformis colonies from the Marquesas and Key West spawned, and their gametes were pooled to make a cohort of offspring. On September 9, 2020, three Colpophyllia natans colonies spawned, and their gametes were pooled to make a cohort of offspring.
Two days post-fertilization, developing larvae were transported to an indoor laboratory at the University of Miami’s Rosenstiel School of Marine and Atmospheric Science and reared in UV-sterilized, one-micron filtered seawater (FSW), with temperature maintained at the ambient level of incoming water from Biscayne Bay (~26-30°C in spring-summer). Each batch of larvae was settled on unconditioned 2.5-cm ceramic plugs (Boston AquaFarms, USA). After five days, settlers were counted under a microscope.
Recruit Development
After settlement, recruits were provisioned with Symbiodiniaceae. Fragments of Orbicella faveolata (originally collected from Miami-Dade in 2017, having been maintained in ex-situ seawater tables since then) hosting Durusdinium trenchii were placed in aquaria with D. labyrinthiformis recruits to serve as symbiont sources (Williamson et al., 2021). The C. natans recruits were inoculated with approximately one million cells per liter each of cultured Breviolum and D. trenchii twice per week, until all recruits were visibly infected (approximately three weeks). For the next several months, no further Symbiodiniaceae sources were provided, and recruits were fed twice per week with Reef Roids (PolypLab). Temperature tracked local seasonal fluctuations of incoming seawater in Bear Cut, FL, eventually reaching 22°C in January 2021.
At this point, a subset of recruits from each cohort were sampled to characterize their Symbiodiniaceae communities. Briefly, small tissue biopsies (<0.25 cm2) were taken from each recruit using a razor blade, genomic DNA was extracted following modified organic extraction methods (Rowan and Powers, 1991; Baker and Cunning, 2016), and quantitative PCR (qPCR) assays were used to identify Symbiodiniaceae to genus level following reactions described in Cunning and Baker (2013) using a QuantStudio 3 Real-Time PCR Instrument (Applied Biosystems, USA). All D. labyrinthiformis recruits and all but two C. natans recruits hosted primarily (>90%) D. trenchii. One C. natans recruit hosted 10.4% Breviolum and 89.6% D. trenchii, and one hosted Breviolum only.
In total, 42 D. labyrinthiformis recruits (24 plugs) and 453 C. natans recruits (41 plugs) were selected for use in the following experiment. There were far fewer D. labyrinthiformis recruits available because (1) as offspring of colonies “rescued” ahead of the SCTLD front through the Florida Coral Rescue, these recruits are highly valuable and needed for a various research and restoration projects, so we had a limited number to work with; and (2) after eight months of rearing in a laboratory, many more had died [consistent with high mortality common in early life stages (Wilson and Harrison, 2005; Vermeij and Sandin, 2008)] compared with the four-month-old C. natans cohort. Although this order of magnitude difference in sample sizes created an unbalanced experimental design, we nonetheless felt it was important to include multiple species of recruits in the experiment, since so many Caribbean species are susceptible to SCTLD but exhibit different levels of disease prevalence and progression (FKNMS, 2018; Meiling et al., 2021).
SCTLD Exposure
The plugs on which each species had settled were then distributed in a randomized block design into four new 2.5-gallon aquaria, so that each aquarium contained ten plugs with C. natans and six plugs with D. labyrinthiformis (Table 1; Figure 1). After distribution, recruits from each aquarium were counted, photographed, and measured under a microscope. Because many recruits had settled in clusters, we recorded whether each recruit was part of a group when assessing its susceptibility to SCTLD. If a recruit grew to contact another to form a multi-genotype entity, it was deemed a member of a “chimera” (Figures 2A, D). In all instances of tissue contact, fusion occurred to form stable chimeras; no evidence of non-fusion or incompatible fusion were observed (Hidaka, 1985; Frank et al., 1997; Nozawa and Loya, 2005). If a recruit was isolated from other recruits with no physical contact, it was deemed “single”. ImageJ was used to measure maximum diameter and recruit skeletal area in mm2 for each recruit (including each individual member of a chimera). Each recruit was also categorized by size, based on its maximum diameter [C. natans: “small” = <1 mm, “medium” = 1-5 mm, “large” = >5 mm (Figures 2A–C); D. labyrinthiformis: “small” = <5 mm, “medium” = 5-10 mm, “large” = >10 mm (Figures 2D–F)]. These size and grouping categories were then tracked throughout the subsequent experiment.

Table 1 Experimental design showing number of plugs and recruits of each species allocated to each experimental aquarium.
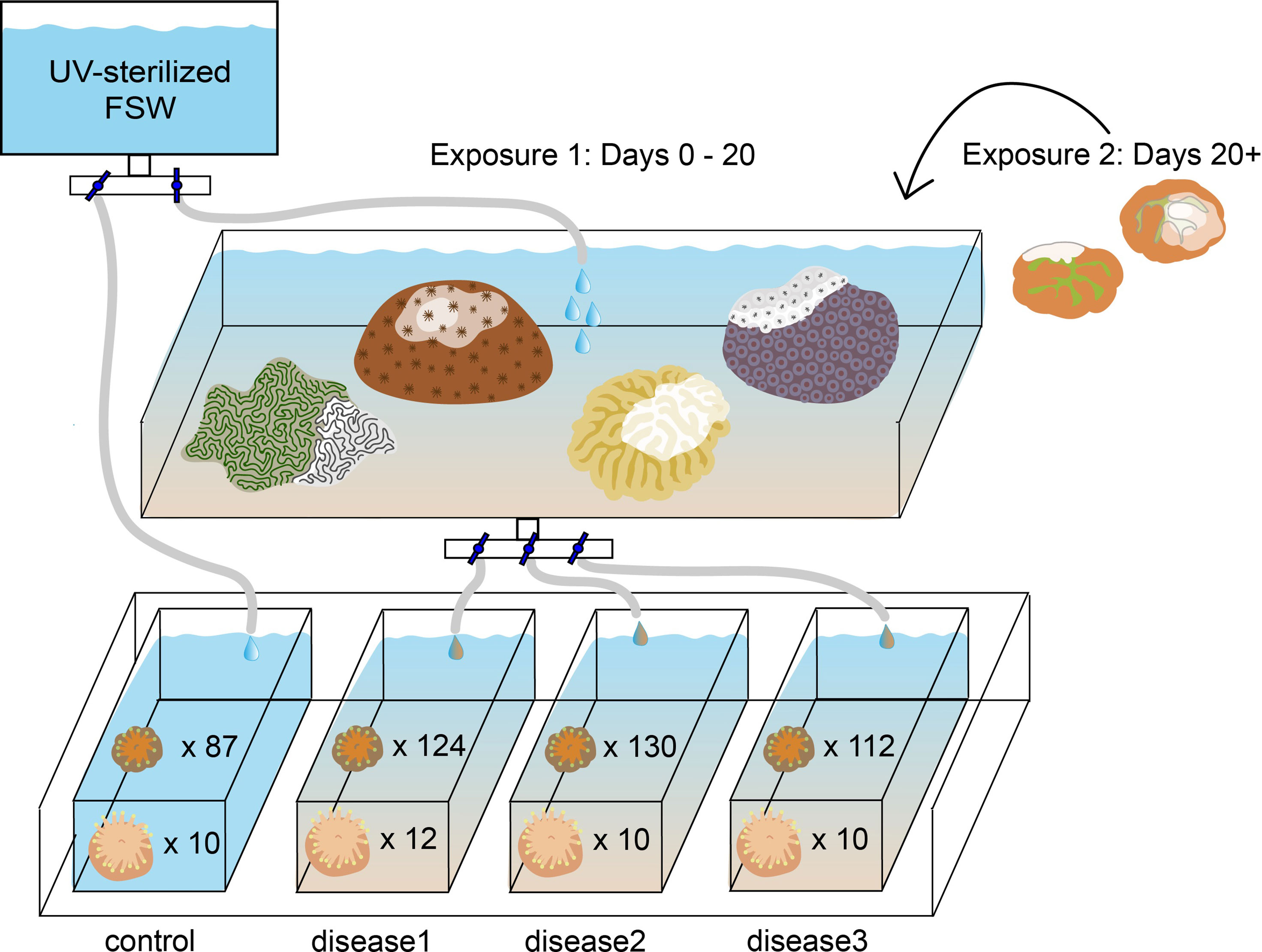
Figure 1 Experimental setup exposing eight-month-old Diploria labyrinthiformis recruits (n=42) and four-month-old Colpophyllia natans recruits (n=453) to SCTLD. Each experimental tank contained ten C. natans plugs and six D. labyrinthiformis plugs.
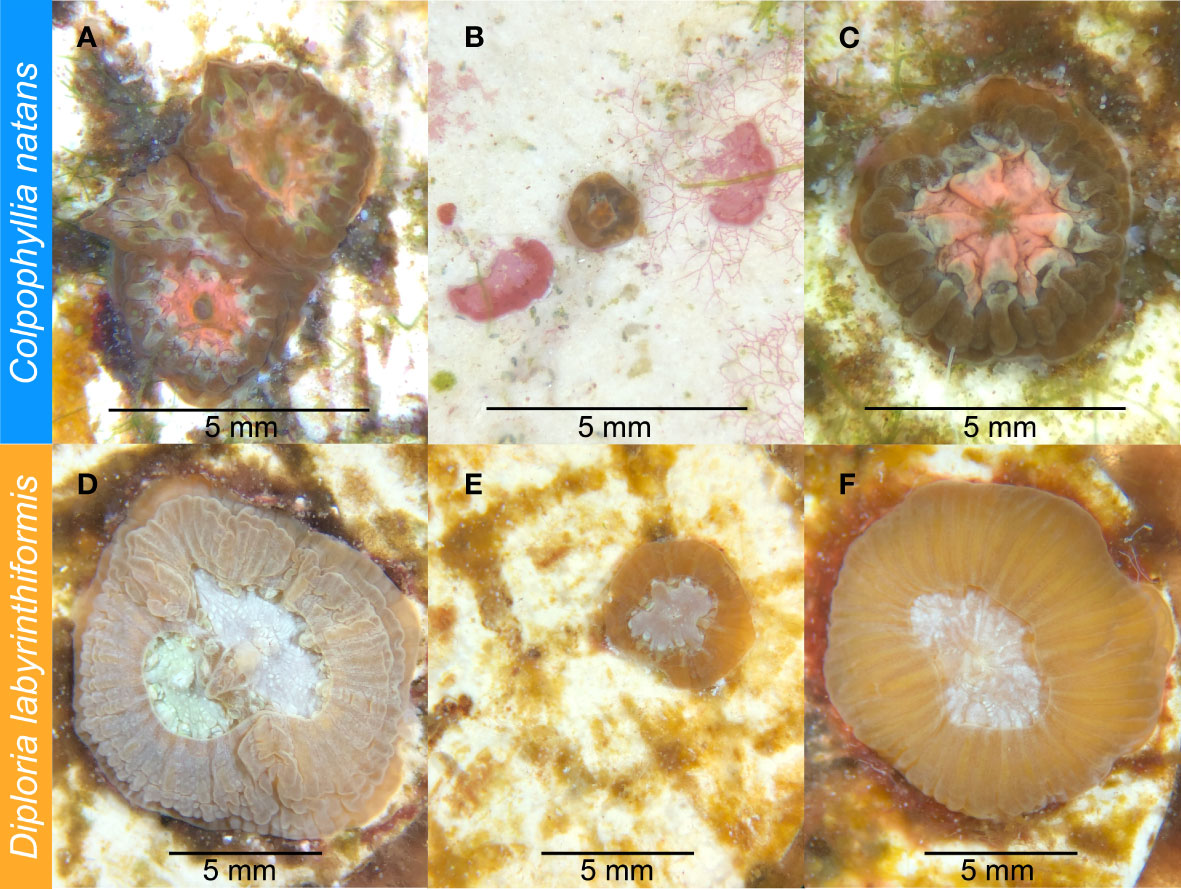
Figure 2 Four-month-old Colpophyllia natans (A-C) and eight-month-old Diploria labyrinthiformis (D-F) recruits prior to SCTLD exposure. (A) C. natans “chimera” (multi-genotype entity), made up of individual recruits ranked as “medium” in size. (B) C. natans “solitary” recruit (single-genotype entity), ranked as “small” in size. (C) C. natans solitary recruit, ranked as “large” in size. (D) D. labyrinthiformis chimera, made up of two individual recruits ranked as “medium” and “large” in size. (E) Small, solitary D. labyrinthiformis solitary recruit. (F) Large, solitary D. labyrinthiformis recruit.
As before, all aquaria were immersed in a larger 50-gallon seawater tank to maintain ambient temperature of incoming water from Biscayne Bay (~22°C in January) to mimic what recruits would experience on the reef. Light (50–70 μmol quanta m−2 s−1, measured by an Apogee Underwater Quantum PAR Meter MQ‐210) was maintained on a 12h:12h light:dark cycle using AI Hydra lights (AI Inc., USA). Irradiance and temperature were recorded with a HOBO Pendant® data logger (Onset Computer Corporation MX2202).
Three aquaria were supplied with seawater from another tank containing colonies of multiple species exhibiting acute lesions attributed to SCTLD (approximately ~30 cm2 of tissue in total), which were sloughing off tissue and mucus presumed to contain the disease causative agent (Dobbelaere et al., 2020). This treatment was designed to replicate the waterborne nature of SCTLD transmission (Precht et al., 2016; Aeby et al., 2019; Muller et al., 2020), the most probable way by which recruits would become infected. Species present in this “disease bath” included Pseudodiploria clivosa, Orbicella faveolata, Meandrina meandrites, and Siderastrea siderea, each of which presented with varying lesion sizes and rates of progression (FKNMS, 2018; Landsberg et al., 2020; Meiling et al., 2021). Water was dripped from the disease bath at 5L per hour into three of the aquaria, while the fourth (control) aquarium was supplied with UV-sterilized FSW at the same rate (Figure 1). One 4W submersible pump (VicTsing CAAA3-HG16) was placed into each aquarium to circulate water evenly.
While recruits were maintained in their respective treatments, excess turf algae was manually removed from plugs once per week to reduce overgrowth and competition. However, due to the small size and dense clustering of some recruits (particularly C. natans), it was not possible to remove all algae without risking injuring recruits. Plugs and diseased colonies were randomly moved within their respective tanks every other day to avoid proximity bias. During the exposure, a Zeiss Stemi 305 LAB microscope (ZEISS International, Jena, Germany) was used to count the number of apparently healthy, infected, and dead recruits on each plug in each aquarium every other day. Pictures of each recruit and plug were taken under the microscope to document condition and lesion progression. In order to avoid influencing lesion progression and maximize the number of recruits surviving through the first exposure, we did not sample any recruits for histological analysis.
After 20 days, several small colonies (~5 cm in diameter) of C. natans with active lesions were added to the disease bath to deliver a second exposure of SCTLD, with the intention of testing whether any remaining recruits could withstand another pulse of pathogen. This species has been reported to exhibit high rates of tissue loss (~10% per day, Meiling et al., 2021), and colonies showed areas of bright white skeleton, indicating rapid disease progression. During this second exposure (hereafter referred to as “Exposure 2”), recruits in each aquarium continued to be counted and photographed every other day.
At each time point, photographs were compared with initial and previous time points to estimate the proportion of living tissue remaining to the nearest 5%. Rates of tissue loss (% per day) were calculated by dividing the percent of tissue a recruit had lost at each time point by the number of days since it was last observed to be apparently healthy, then log transformed to meet statistical test assumptions. Because recruits were checked every other day, the maximum rate of tissue loss per day before log transformation was 50% (100% of tissue lost over two days), which is likely a conservative estimate in many cases where >50% of tissue may have been lost in one day.
We analyzed recruit responses separately for each exposure. R was used to run mixed effect regression models comparing recruit survival (binomial regression) and disease progression (Poisson mixed effect regression) using the lme4 and lmerTest R packages (Kuznetsova et al., 2017; Bates et al., 2022) with species, recruit size/area, and grouping (single vs. chimera) as fixed effects. Since recruits on a given plug were not spatially independent of one another, and each plug was only found in combination with one aquarium, we nested “plug” within “aquarium” as random effects. However, no models indicated that “aquarium” was a significant effect, so results for disease aquaria reported here are pooled. To account for unbalanced sample sizes between cohorts, and the small sample size of the D. labyrinthiformis cohort, models were also fitted with restricted maximum likelihood estimation (REML) with a Kenward-Roger correction, and the Satterthwaite approximation. Models with interactions between fixed effects were tested with Aikake’s Informarion Criteria (AIC) and Bayesian Information Criteria (BIC) to select final model parameters. In some cases, interactions did not improve model fit, and were excluded from final models. Games-Howell post-hoc analyses were used to test differences among groups and treatments, as this method does not assume equal variances or sample sizes. We tested normality of residuals with quantile-quantile plots and checked for overdispersion with the blmeco package (Korner-Nievergelt et al., 2015).
Results
Overall, SCTLD gross morphology resembled descriptions provided by FKNMS (2018) and Landsberg et al. (2020). Starting 48 h after exposure began, some recruits of both species began to exhibit lesions. These typically began basally, sloughing tissue to expose denuded skeleton as they advanced across the recruit (Figure 3). In C. natans, lesions were primarily focal, advancing from one area of tissue loss in a single front (Figure 3). In some D. labyrinthiformis recruits, tissue loss presented multifocally, progressing from more than one initial lesion (Figure 3).
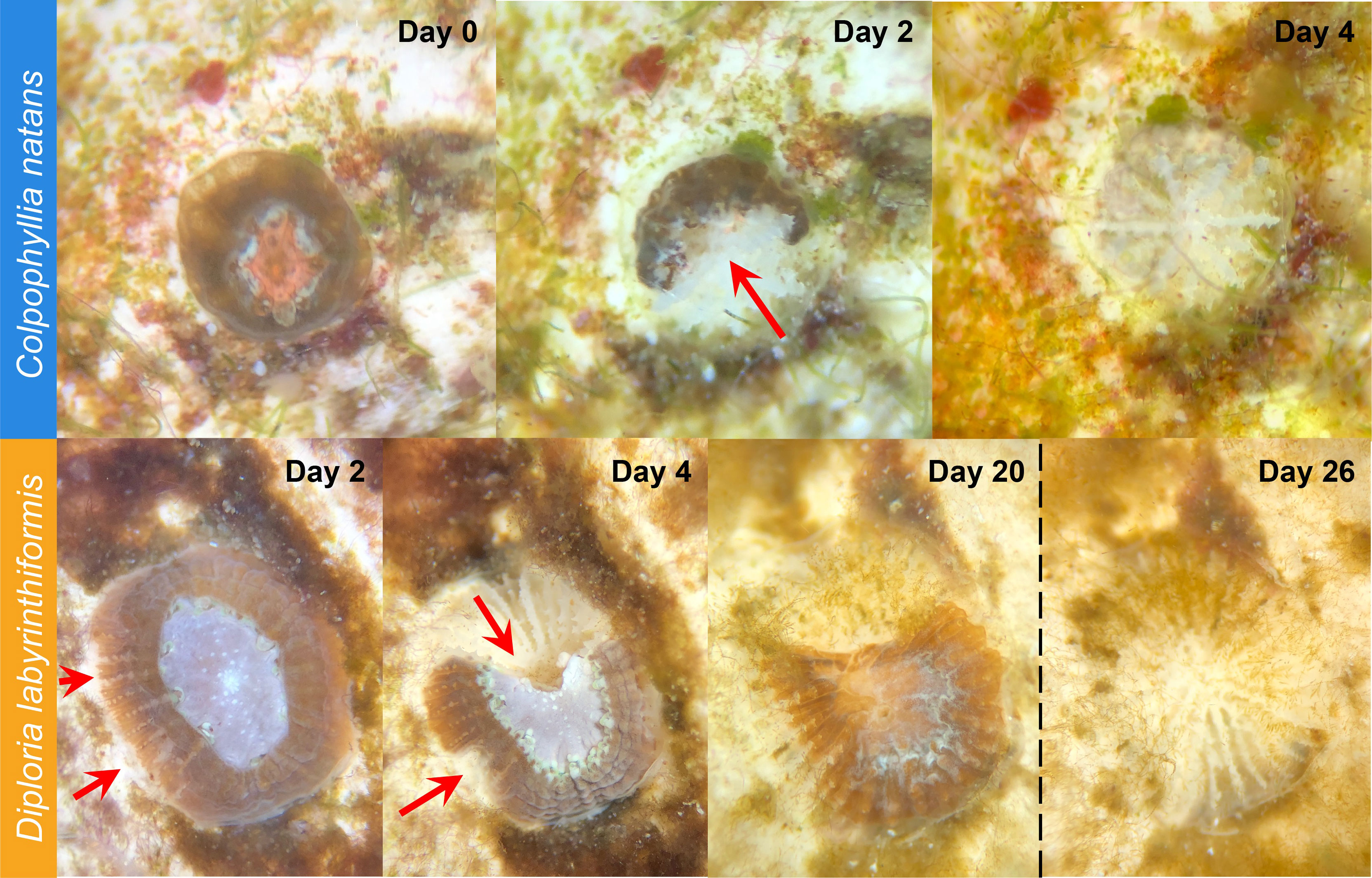
Figure 3 SCTLD progression in brain coral recruits. Top: C. natans recruit showing progression from apparently healthy to 100% tissue loss within four days (~25% tissue loss per day). Red arrow indicates active disease margins. Bottom: D. labyrinthiformis recruit showing progression from apparently healthy to ~50% tissue loss over four days, then halting lesion progression with no further major tissue loss until Exposure 2 (dashed vertical line). Red arrows indicate active disease margins.
In the first 20 days (Exposure 1), 62.8% of C. natans (n=230 recruits) in the disease treatments developed lesions and 59.6% (n=218) died (Figure 4). All C. natans recruits that developed lesions lost 100% of their tissue (died) within two to ten days after initial lesions (mean = 4.8 ± 2.1 days, Figure 5). On average, C. natans recruits with visible lesions lost 24.9 ± 16.9% of initial tissue per day (Figures 5, 6). Five small C. natans recruits in the control treatment (5.8%) died during the experiment without exhibiting signs of tissue loss, likely from algal interaction or accidental injury during algae removal.
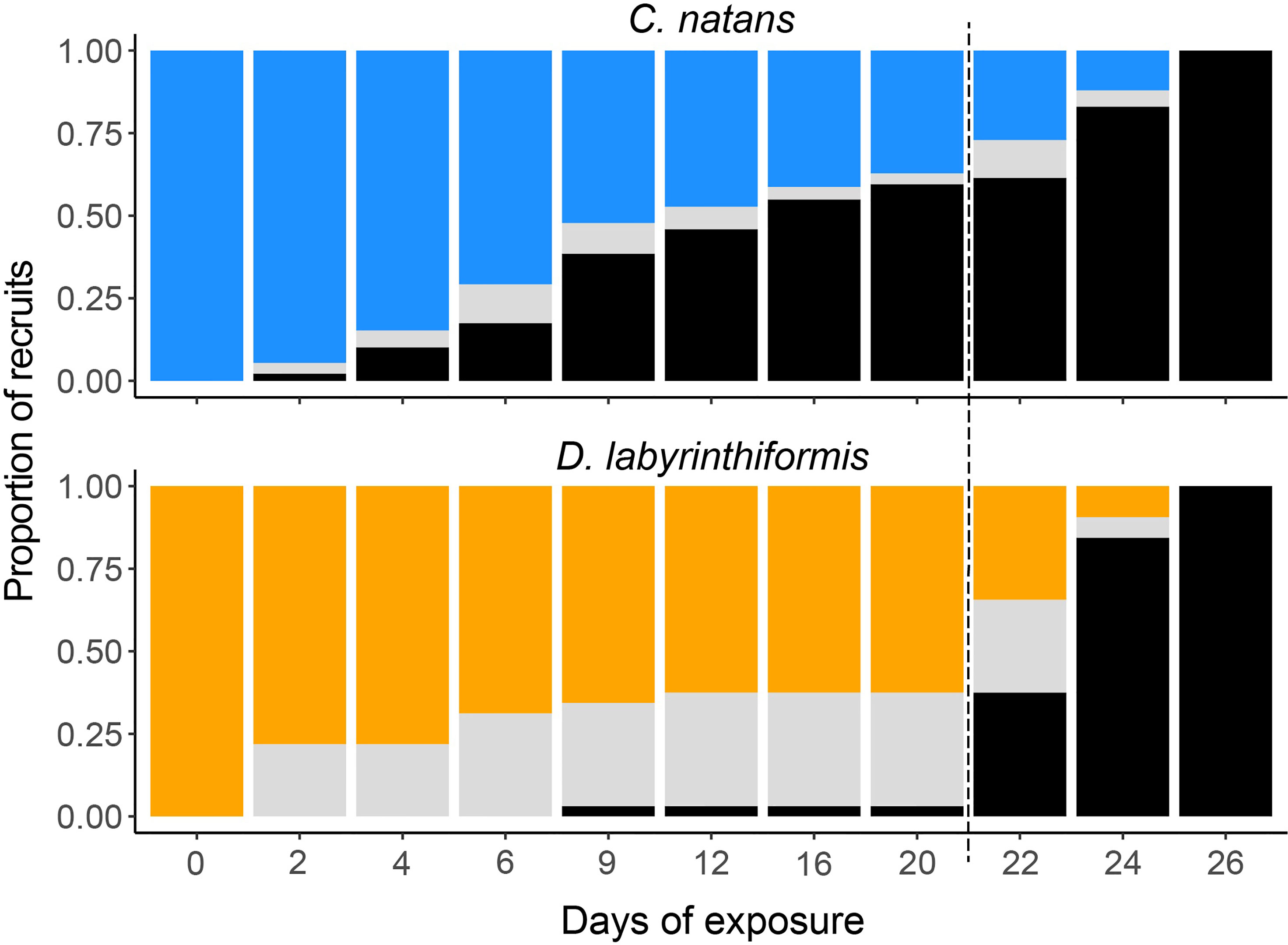
Figure 4 Condition of C. natans and D. labyrinthiformis recruits throughout SCTLD exposure. Blue (C. natans) and orange (D. labyrinthiformis) represent apparently healthy recruits, gray represents recruits exhibiting active disease lesions, and black represents recruits that have died. Dashed line indicates start of Exposure 2.
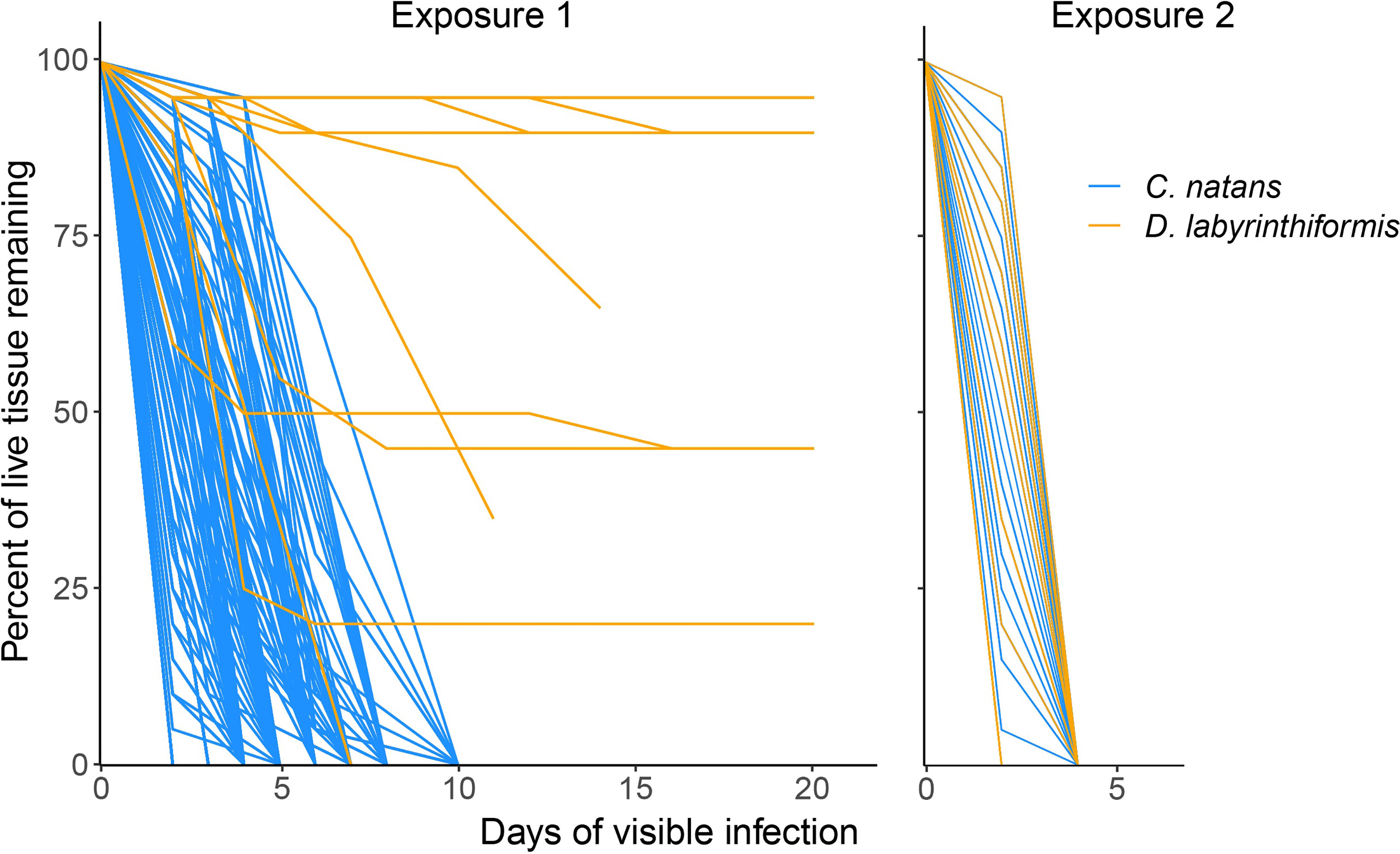
Figure 5 Loss of tissue over time in brain coral recruits exposed to SCTLD. Only those recruits that developed lesions are shown. Lines represent individual recruits after developing lesions (although many overlap with one another due to similar patterns of tissue loss). Day 0 represents the last day that a given recruit was observed to be apparently healthy and have 100% of its tissue. Exposure 1 represents 60.8% of recruits in disease aquaria, while panel Exposure 2 represents the remaining 39.2%. The lines representing some D. labyrinthiformis recruits in Exposure 1 end before reaching 0% tissue because the remaining tissue loss occurred after the second exposure was delivered.
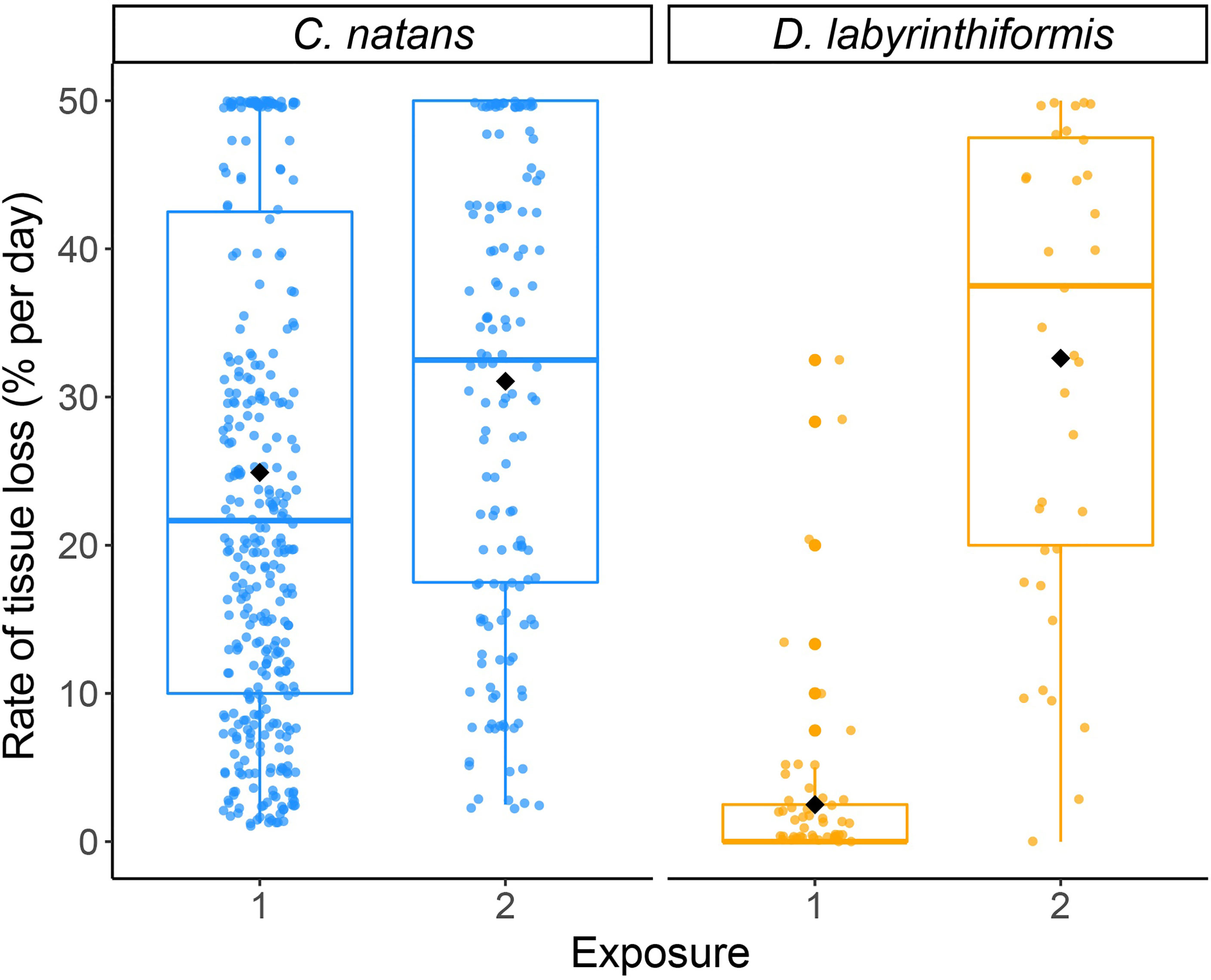
Figure 6 Proportional lesion progression rates (log-transformed) as a result of SCTLD exposure in brain coral recruits. Points show measurements of tissue loss (% of initial area per day) for individual recruits, which were jittered to reduce overlap. Black diamonds show mean rates for each species during each exposure.
During Exposure 1, 37.5% of disease-exposed D. labyrinthiformis (n=12 recruits) exhibited active disease lesions (Figure 4), and only one recruit (3.1%) died (Figure 4). D. labyrinthiformis with lesions lost tissue at an average rate of 2.5 ± 5.9% per day (Figures 5, 6). Most D. labyrinthiformis recruits experienced chronic necrosis (described by Landsberg et al. [2020] as “almost unnoticeable areas of recent necrosis apposed to skeleton overgrown with turf algae”) during the first SCTLD exposure (Figure 5). In several recruits, however, lesions progressed rapidly during the first four days, resulting in 50%-80% tissue loss, after which progression slowed or stalled entirely (Figures 3–5). No D. labyrinthiformis recruits in the control treatment exhibited tissue loss or died during the experiment.
In C. natans, Exposure 1 revealed significant, interacting effects of recruit size and grouping on SCTLD susceptibility. Survivorship in C. natans recruits exposed to SCTLD increased significantly with polyp area (p < 0.001, Table S1; Figure 7A, S1), and in grouped individuals compared with single recruits (p < 0.001, Table S1; Figures 7A, S1). For instance, a recruit that was 2 mm2 in area and grouped with others was >50% more likely to survive than a single one of the same size (Figure 7A, S1). In addition, we found a positive correlation between the number of C. natans genets (polyps) grouped together and the probability of survival during disease exposure, although this relationship did not meet the significance level of 0.05 (Table S2; Figures 7B, S2). Finally, when treated as a collective unit rather than individual recruits grouped together, chimeric entities were significantly more likely to have at least one polyp surviving than single recruits of similar size (p < 0.001; Table S3; Figures 8, S3). Within the disease aquaria, small, solitary recruits exhibited the lowest survivorship (9.9 ± 3.2%; n=91), while large chimeric groups had the highest survivorship (100%, n=11; Figures 8, S3). We were unable to detect differences in D. labyrinthiformis survivorship by size and grouping, likely due to the small sample size which provided insufficient data.
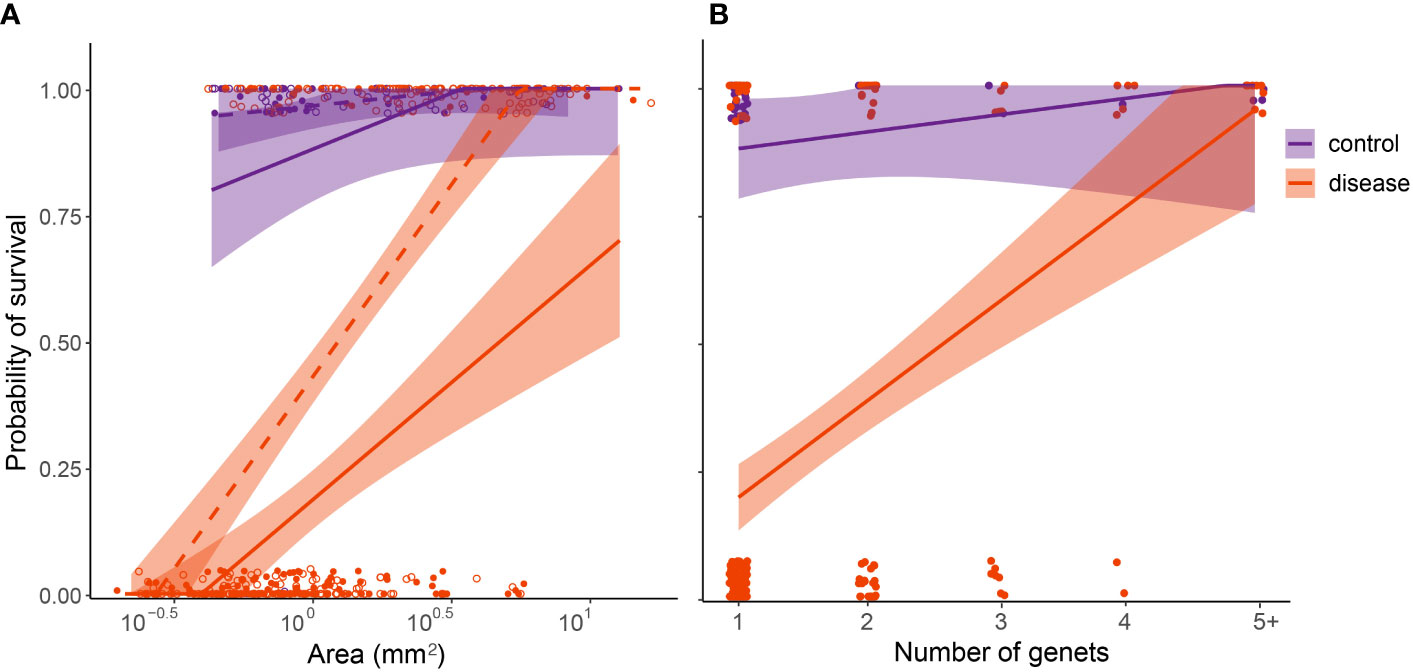
Figure 7 Relative susceptibility of four-month-old C. natans recruits to SCTLD during a 20-day experimental exposure (Exposure 1). Recruits in the control treatment were not exposed to SCTLD, and thus represent the “background mortality” level expected for these juvenile corals. (A); Survival probability is positively correlated with polyp area. Areal data were log(10) transformed to fit statistical assumptions. Solid lines and points represent single recruits, while dashed lines and unfilled points represent members of chimeric groups. (B); Survival probability is positively correlated with number of genets/polyps grouped together. Points were jittered to prevent overlap. For both (A, B), GLMs corrected for binomial survival data were used to fit the data, and shaded regions show 95% confidence intervals.
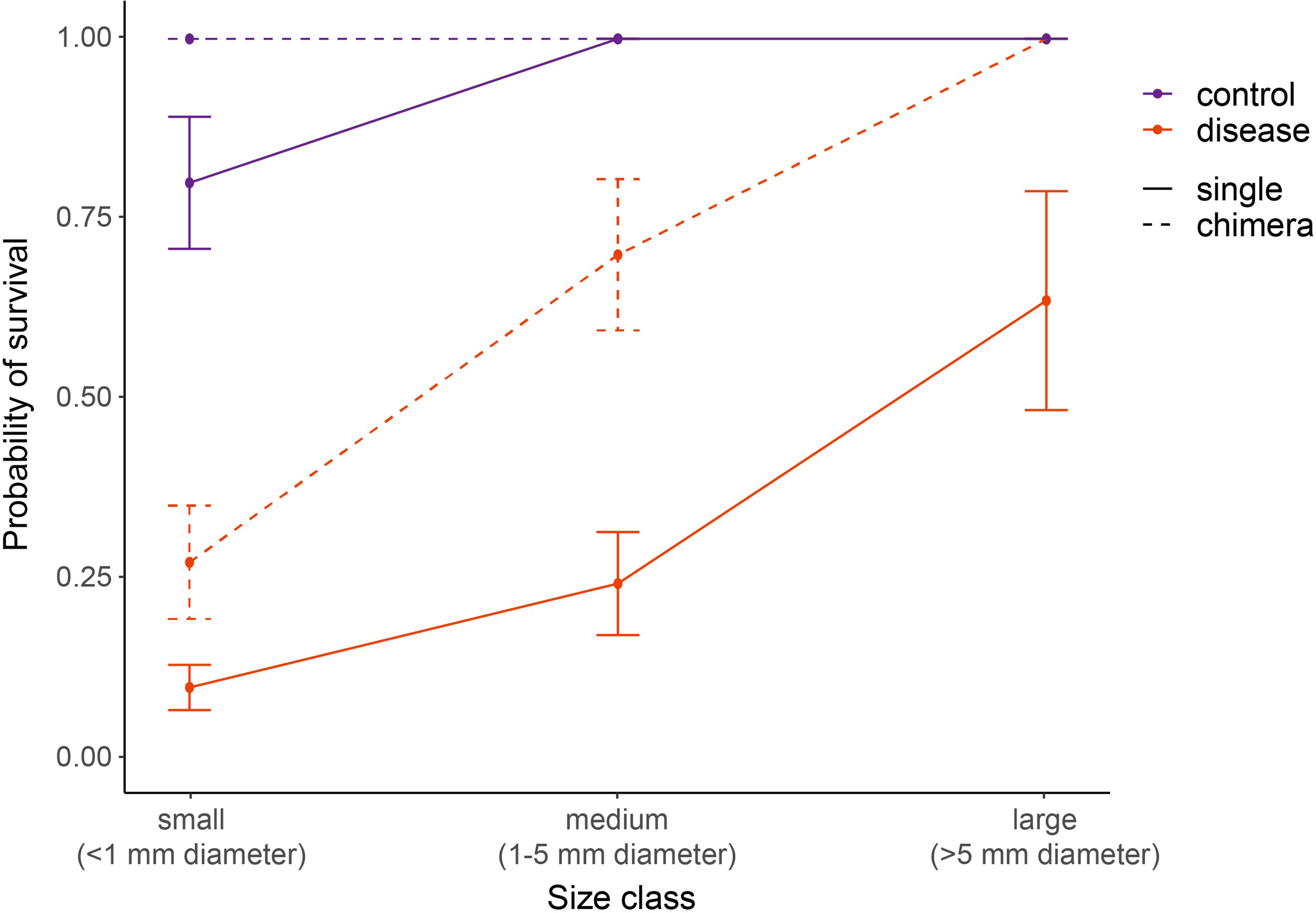
Figure 8 Relative SCTLD susceptibility of four-month-old C. natans varies by interactions of recruit size and grouping (single vs. chimera). Dashed lines indicate chimeric entities, here treated as a collective unit rather than individual recruits grouped together. “Size class” in chimeric entities refers to the total size of the collective unit, with the area of all constituent recruits added together. Recruits in the control treatment were not exposed to SCTLD, and thus represent the “background mortality” level expected for these juvenile corals. A GLM with correction for binomial survivorship data was used to fit the data. Error bars indicate standard error.
Following the addition of new active SCTLD from several small C. natans colonies (Exposure 2), all recruits of both species developed lesions and died within six days. This represented a significant increase in the rate of tissue loss in both species, from 2.5 ± 5.9% during Exposure 1 to 32.6% ± 16.7% during Exposure 2 in D. labyrinthiformis (p < 0.001; Table S4; Figures 6, S4) and from 24.9 ± 16.9% during Exposure 1 to 31.1% ± 16.1% during Exposure 2 in C. natans (p < 0.001; Table S4; Figures 6, S4).
Discussion
This study provides the first description of SCTLD pathology in Caribbean coral recruits. To date, recruits have not been reported in field surveys of SCTLD, likely due to their small size, cryptic appearance, and fast progression of disease (making divers less likely to record active lesions). This study demonstrates that, like their larger adult counterparts, recruits are vulnerable to infection and can succumb relatively quickly (days to a few weeks), reducing the likelihood that their infection would be captured by surveys. In addition, protocols commonly used to identify disease on Caribbean reefs are not designed to examine juvenile corals. The first timed diver surveys of SCTLD prevalence in southeast Florida conducted by Precht et al. (2016) omitted colonies < 5 cm in diameter. Similarly, the Florida Reef Resilience Program’s Disturbance Response Monitoring survey, intended to collect detailed monitoring data on diseased corals (FWC, 2021), only tallies corals ≤4 cm in diameter without documenting their condition (disease, recent mortality, etc.), and excludes corals <1 cm in diameter, therefore missing many recruits <1 yr in age. Finally, the Atlantic and Gulf Rapid Reef Assessment coral monitoring protocol only reports colonies ≥4 cm in diameter (AGRRA, 2021). Consequently, without accounting for the smallest colonies, we have likely underestimated the true extent of SCTLD infection and mortality on Caribbean reefs.
Lesion appearance and progression in C. natans and D. labyrinthiformis recruits largely matched similar descriptions for adult colonies (FKNMS, 2018; Landsberg et al., 2020; Eaton et al., 2021). Beginning in some recruits of both species after just 48 h of exposure, lesions continued to present in new, previously healthy recruits over time. Lesions typically began at the periphery of a recruit before advancing across it, characterized by both focal and multifocal tissue loss that left behind exposed skeleton.
As is often the case in coral settlement experiments, many plugs contained multiple recruits, particularly in C. natans, indicating that recruits were not spatially independent of one another. However, we found no evidence that this spatial relationship impacted patterns of SCTLD infection. By the end of Exposure 1, all but one C. natans plug had at least one recruit that presented with signs of SCTLD. The one unaffected plug contained a single, large recruit. These findings are consistent with those of Guzmán-Urieta and Jordán-Dahlgren (2021), who found a lack of spatial dependence on SCTLD incidence in adult Pseudodiploria strigosa, with disease mostly occurring via random distribution patterns rather than clustering, despite the clustered distribution of some colonies.
As adults, C. natans and D. labyrinthiformis are among the species that are most susceptible to SCTLD (Precht et al., 2016; Lunz et al., 2017; Alvarez-Filip et al., 2019), exhibiting high disease prevalence and rapid tissue loss (Meyer et al., 2019; Estrada-Saldívar et al., 2020; Meiling et al., 2021; Williams et al., 2021). We observed rapid rates of tissue loss in C. natans recruits throughout exposure (26.3 ± 13.6% per day), with many dying within just two days of developing initial lesions, surpassing the average rate of ~10% reported for conspecific adults (Meiling et al., 2021). Lesions progressed much more slowly, on average, in diseased D. labyrinthiformis during Exposure 1 (2.9 ± 3.7% per day), similar to the rates that Meiling et al. (2021) reported for adult colonies of several species. However, it may be inaccurate to directly compare rates of tissue loss between large, old, established adult colonies and small, single- or few-polyp recruits. Partial mortality of a single polyp may not be comparable to the partial mortality of a large coral colony, due to threshold effects arising from the anatomical constraints of individual polyps. Moreover, adults have thicker tissues and orders of magnitude more polyps than recruits, presumably giving them greater energy reserves and resources to draw upon for defense (Barnes and Lough, 1992; Loya et al., 2001).
Five small C. natans recruits in the control treatment also died during the experiment without exhibiting sloughing tissue or denuded skeleton, likely reflecting the generally low survivorship that characterizes the early life stages of corals (Wilson and Harrison, 2005; Vermeij and Sandin, 2008). None of these recruits showed signs of disease and instead appeared to be negatively affected by turf algae that grew at their bases, which could not be completely removed without damaging their skeleton or tissue. In this case, their small size seemed to be a disadvantage, as there was no mortality recorded in larger recruits in the control treatment despite similar algal growth around their bases and identical cleaning protocols during the experiment.
In addition to elucidating how Caribbean coral recruits may present with SCTLD, this study allowed us to begin investigating drivers of relative disease resistance within – but not between – cohorts. While no recruits withstood infection during a second exposure to SCTLD, 37.2% of C. natans (n=136) and 62.5% of D. labyrinthiformis (n=20) remained “apparently healthy” during the first exposure. Given our experimental design, we cannot distinguish whether the variable patterns of disease progression and mortality between them were caused by differences in age, size, species, or a combination of these factors. For example, the largest C. natans recruits were similar in area to the smallest D. labyrinthiformis recruits (~10 mm2), confounding comparison between species. In addition, since coral immunity matures throughout early ontogeny (Frank et al., 1997; Nozawa and Loya, 2005), the four-month age difference between the two cohorts may also have contributed to observed differences. The unbalanced sizes of the two cohorts in this study further prevents interpretation of differences between them. Instead, we intend to report our observations, limit analysis to within-cohort patterns, and lay the groundwork for more detailed comparative studies.
In C. natans, survivorship was significantly positively correlated with recruit area. Size has been shown to influence recruit survival in the face of other stressors, including ocean acidification, predation, and competition (Doropoulos et al., 2012). However, Williams et al. (2021) found that SCTLD disproportionately affected the largest adult colonies in the field. This suggests that despite the benefits of larger size for recruits, there may come a point later in ontogeny when size becomes a disadvantage for corals exposed to SCTLD, perhaps because greater surface area results in higher chances of infection by waterborne pathogens as they encounter coral surfaces. Since coral immunity matures throughout early ontogeny (Frank et al., 1997; Nozawa and Loya, 2005), the four-month age difference between the C. natans and D. labyrinthiformis cohorts may also have contributed to observed differences. To inform restoration efforts using sexually-produced recruits, future studies should investigate whether SCTLD vulnerability varies as recruits age and grow. Such information may help managers minimize mortality downstream by optimizing the duration of recruit grow-out in protected nurseries or ex-situ facilities prior to outplanting on reefs in the disease-endemic zone (Randall et al., 2020).
Our results also provide evidence, in C. natans, for relatively higher resistance of grouped recruits to SCTLD. This appears to be true both from the perspective of individual recruits (i.e., recruits benefit on an individual basis when grouped together) and from the perspective of the larger “chimeric” colony that results from the grouping of these recruits (i.e., the emergent entity that results from the fusion of multiple individual recruits). In C. natans, being part of a group increased the probability of survival by up to 50% compared with single recruits of the same size (Figure 7A). In addition, small and medium-sized chimeras (grouped entities) were significantly more likely to survive SCTLD exposure (i.e., at least some part of the chimeric colony remaining apparently healthy) compared with single recruits of a similar size as the grouped chimera (Figure 8). This suggests that chimerism may be functionally important for disease resistance beyond the benefits of increasing size. In some cases, one part of a chimera would become infected while other parts remained apparently healthy, suggesting that being part of a group is not universally protective. However, rarely did all members of a chimera die following the infection of one member, particularly as the number of genets forming the chimera increased (Figure 7B).
It has long been observed that coral larvae behave gregariously, resulting in aggregations of settlers that contain multiple genets and that are often spawned from different parents (Duerden, 1902; Frank et al., 1997; Hidaka et al., 1997; Raymundo and Maypa, 2004; Puill-Stephan et al., 2011; Puill-Stephan et al., 2012). The formation and fusion of multi-polyp chimeras during early ontogeny confer numerous benefits that seemingly outweigh the potential costs of conflict among partners (Amar et al., 2008). Chimeras have been shown to exhibit significantly higher survival and growth than individual recruits, and the immediate increase in size achieved through fusion helps vulnerable coral recruits rapidly surpass size-escape thresholds (Raymundo and Maypa, 2004; Amar et al., 2008; Christiansen et al., 2009; Vermeij and Sandin, 2008; Puill-Stephan et al., 2011; Doropoulos et al., 2016; Sampayo et al., 2020; Huffmyer et al., 2021). In addition, chimerism may be a powerful mechanism of “evolutionary rescue”, because the genetic variation present in multi-partner aggregations may enhance plasticity and stress tolerance compared with single-genet individuals (Rinkevich and Weissman, 1992; Rinkevich and Yankelevich, 2004; Puill-Stephan et al., 2009; Rinkevich et al., 2016; Rinkevich, 2019; Huffmyer et al., 2021). Huffmyer et al. (2021) reported that fused Pocillopora acuta recruits survived five days longer than individuals under high temperature (+2.5°C), and even longer when multiple parental genotypes were represented. In this study, the relative resistance to SCTLD observed in chimeric C. natans entities may be derived in part from their ability to draw upon a wider repertoire of resources and traits, including those involved in resisting disease. If this is the case, restoration efforts designed to promote recruit resilience in the face of SCTLD might encourage the formation of chimeras as part of ex-situ growout.
Recruit size and grouping did not explain patterns of susceptibility in D. labyrinthiformis, perhaps due to the much smaller sample size of this cohort. Over half of the D. labyrinthiformis recruits exposed to SCTLD remained visually healthy during Exposure 1, and the majority that became infected showed a stall in lesion progression after an initial wave of tissue loss (Figure 4). All but one recruit survived Exposure 1 (Figure 4). These results are consistent with field observations in which some colonies show arrested tissue loss (or escape infection altogether) while adjacent colonies exhibit high SCTLD prevalence and rapid tissue loss (Sharp et al., 2020; Guzmán-Urieta and Jordán-Dahlgren, 2021). Such patterns suggest the existence of differentially resilient colonies or communities that fail to succumb despite severe disease among many of their neighbors, sometimes leading to mortality (Sharp et al., 2020). Given that all D. labyrinthiformis recruits in our experiment were reared under identical conditions, spawned from the same cohort of eight parents, and hosted D. trenchii, fine-scale genetic variation may be responsible for variable proliferation of disease.
In this experiment, a second disease exposure revealed that although recruits showed relative differences in SCTLD susceptibility during the first exposure, no recruits of either cohort were absolutely resistant. Demonstrating a lack of absolute resistance, at least in this population of recruits, may be important in understanding the limits of fine-scale genetic variation in driving differential susceptibility. An ongoing outbreak might involve multiple exposure events in which a contagious pathogen initially infects a few colonies but then spreads over time, resulting in subsequent waves of exposure as more of the population becomes infected (Williams et al., 2021). During such events, corals may be repeatedly subjected to increasing concentrations of pathogens that they cannot withstand.
The lack of a known SCTLD pathogen means that we cannot conclude that our recruits died from SCTLD in a clinical sense, especially given the lack of histological evidence in support of our gross observations. However, the alternative hypothesis (that our recruits succumbed to a SCTLD-like syndrome following exposure to water from adult corals showing signs of the disease, but the SCTLD-like signs in the recruits were unrelated to the diseased adults) appears unlikely. We might have attempted to control for such effects by using water from a tank containing (apparently) healthy corals instead of FSW for the control treatment, and this would have allowed us to test for the potential effect of tissue, mucus, or microorganisms that corals release naturally as the cause of the disease signs we observed. However, we routinely rear many thousands of coral recruits in our systems together with fragments of adult colonies, usually to provide them with sources of Symbiodiniaceae (Williamson et al., 2021) and we have not previously seen such disease signs in our recruits before. Furthermore, such a control would not account for the additional tissue sloughed off by corals affected by SCTLD, and indeed this sloughed tissue might be a critical component in the epidemiology of the disease (perhaps by providing a source of organic carbon and/or transport mechanism for pathogens). It is thus impossible to fully control for such effects without making additional untested assumptions regarding the pathogen and its spread. Because the observations we report here in our recruits closely resemble the gross pathology observed in our adults, which also matched those described for SCTLD (FKNMS, 2018; Landsberg et al., 2020; Eaton et al., 2021), the most parsimonious explanation for our findings is that recruits in the disease treatment did indeed become infected with SCTLD. A fully crossed, balanced experiment using multiple control aquaria with such (apparently) healthy colonies should be conducted to follow up on the present study.
Other SCTLD-susceptible species, such as Orbicella faveolata and Montastraea cavernosa, are major reef-builders throughout Florida and the Caribbean and are the target of reef restoration efforts using sexually-produced recruits. As such, juveniles of these species should be tested for rates of infection and tissue loss to assess the risk of SCTLD when outplanted in areas where it is endemic or emergent (Aeby et al., 2021). Since adult colonies of different species exhibit variation in their immunocompetency (Palmer et al., 2011) and rates of lesion progression (Meiling et al., 2021), they may also show varied responses to SCTLD as juveniles. In addition, understanding how SCTLD interacts with other stressors, such as elevated temperature, sedimentation, and nutrient pollution, may be important because they can increase disease susceptibility (Ward et al., 2007; Rodriguez-Lanetty et al., 2009; Pollock et al., 2014; Vega Thurber et al., 2014; Maynard et al., 2015; Rädecker et al., 2015; Zaneveld et al., 2016; Walton et al., 2018; Aeby et al., 2020; Howells et al., 2020).
Despite increasing attention to SCTLD over the past several years, relatively little effort has been made to create standardized protocols to test transmission and susceptibility (i.e., methods for exposing corals, how to establish or titrate a standardized dose, etc.; Shore and Caldwell, 2019). It is likely that the doses delivered in many laboratory experiments, including this one, may be higher (perhaps by orders of magnitude) than those typically experienced by corals in the field, due to the spread and dilution of a waterborne pathogen. In addition, many studies have used contact assays, which may not reflect mechanisms of SCTLD transmission on the reef. As such, researchers should collaborate to identify appropriate, ecologically relevant exposure and delivery techniques to make SCTLD studies as replicable and relevant as possible (Shore and Caldwell, 2019).
Having demonstrated that both C. natans and D. labyrinthiformis recruits are indeed susceptible to SCTLD, we suggest that restoration practitioners begin to test strategies for reducing disease risk in restored coral recruits. Although antibiotic treatment has been shown to be highly effective in halting lesion progression in adult colonies (Aeby et al., 2019; National Academies, 2019; Neely et al., 2020; Neely et al., 2021b; Shilling et al., 2021; Walker et al., 2021), it would be extremely challenging for managers to detect lesions and apply antibiotics to halt disease progression in wild recruits or juveniles showing active signs of disease. Therefore, managers should consider preemptive approaches to prevent disease from infecting recruits including: (1) holobiome manipulations of juveniles with the goal of decreasing SCTLD susceptibility (Dennison et al., 2021), and/or (2) selective breeding of new generations that are more resistant to SCTLD, using parents that have survived a disease outbreak or which have been shown experimentally to be more resistant.
Holobiome manipulations include modifying the communities of algal endosymbionts (Family Symbiodiniaceae) in coral tissue (van Oppen et al., 2015). Histopathological examination of lesions has revealed that SCTLD damages Symbiodiniaceae cells and disrupts their association with host corals (Landsberg et al., 2020; Work et al., 2021), suggesting that the coral-algal relationship plays a vital role in SCTLD. Dennison et al. (2021) found that corals hosting Breviolum, both as their dominant symbionts and at background levels, were more likely to become infected with SCTLD than corals hosting other genera. In addition, inshore corals hosting Durusdinium trenchii have largely escaped the impacts of SCTLD relative to their offshore counterparts hosting members of the genus Breviolum (Rubin et al., 2021). For other coral diseases, studies have reported negative correlations between Durusdinium and disease incidence (Correa et al., 2009; Rouzé et al., 2016). Although we were not able to assess the influence of Symbiodiniaceae identity in this study because almost all recruits hosted primarily D. trenchii, future studies should test the role of different endosymbiont taxa in recruit susceptibility. If a specific taxon (e.g., Breviolum, Dennison et al., 2021) is implicated in SCTLD incidence and/or severity, managers may choose to provide recruits with sources of alternative taxa during initial symbiosis establishment (Williamson et al., 2021).
In addition to Symbiodiniaceae, some prokaryotic members of the coral microbiome have been shown to promote holobiont health and aid in stress tolerance (Nissimov et al., 2009; Shnit-Orland and Kushmaro, 2009; Krediet et al., 2013; Glasl et al., 2016; Peixoto et al., 2021). For example, Raina et al. (2016) isolated large quantities of the antimicrobial compound tropodithietic acid (TDA) produced by coral-associated Pseudovibrio bacteria, which prevented the growth of pathogens. Active delivery and/or facilitation of such beneficial microorganisms for corals (BMCs) in the form of probiotics have the potential to minimize impacts of various stressors on adult corals (Peixoto et al., 2017; Rosado et al., 2019; Peixoto et al., 2021). Given the apparent success of these treatments in adult corals, managers should consider dosing juveniles with probiotics or BMCs as an adaptive intervention prior to outplanting to reduce their risk of contracting SCTLD. However, associations with both prokaryotic and eukaryotic microbes can be transient, shifting with ontogeny and environmental conditions and thus potentially offering only a short-term buffer against disease (Little et al., 2004; Abrego et al., 2009; Sharp et al., 2012; Hernández-Agreda et al., 2016; Damjanovic et al., 2017; Cumbo et al., 2018).
In order to promote longer-term protection, future studies should investigate the heritability of SCTLD susceptibility and resistance. The recruits in this study were not sequenced, so we could not evaluate genetic effects. They were spawned from parents that were “rescued” from the Dry Tortugas ahead of the disease front as part of the Florida Coral Rescue. As such, they had never been exposed to SCTLD, and can be considered “naïve” to the disease and therefore likely susceptible. In contrast, colonies that have survived in the endemic zone for several years may harbor some degree of resistance, and managers should consider utilizing them for both asexual propagation and managed breeding (assisted gene flow) (Baums et al., 2019). Since other traits, such as heat tolerance, can be passed on to the next generation in corals (Quigley et al., 2020; Howells et al., 2021; Weeriyanun et al., 2022), it may be assumed that there are also heritable components to disease resistance. Selectively breeding corals that have persisted in the endemic zone may increase the frequency of SCTLD-resistant alleles in offspring, decreasing susceptibility while enhancing genetic diversity in dwindling populations (Baums et al., 2019; National Academies, 2019; Voolstra et al., 2021).
Since its initial outbreak in Florida, SCTLD has spread rapidly throughout the Caribbean and caused extensive mortality among susceptible species (Alvarez-Filip et al., 2019; Kramer et al., 2019; Estrada-Saldívar et al., 2020; Brandt et al., 2021; Costa et al., 2021; Dahlgren et al., 2021; FDEP, 2021a; Heres et al., 2021). In light of the considerable infection and mortality of recruits reported here, we can infer that untold numbers of coral recruits have likely been lost as a result of SCTLD outbreaks. Although these casualties have gone undocumented, they nevertheless impact the potential for species and reef recovery. As of early 2022, entire subregions of the tropical western Atlantic have become affected by SCTLD, with outbreaks spanning up to 140 km in scale (Muller et al., 2020). Such a large disturbance footprint threatens the persistence of susceptible species, with larvae from unaffected areas needing to disperse over greater distances to reach and replenish depleted reefs (Dietzel et al., 2021). The recovery of dwindling populations will require both the survival of mature colonies as broodstock and the recruitment of juveniles (Hughes and Tanner, 2000). If SCTLD outbreaks continue to reduce the number of spawning adults and infect recruited offspring early on, the potential for natural population recovery may be severely limited. For this reason, multiple, active intervention strategies will be critical to avoid extirpation of susceptible species and stem further ecosystem decline on Caribbean reefs.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://github.com/livwilliamson/Frontiers-manuscript-2022.
Author Contributions
OW, CD, and AB conceived and designed the experiment. KO’N provided coral larvae for use in experiments. OW collected data, performed statistical analyses, and wrote the manuscript. OW, CD, KO’N, and AB edited the manuscript. All authors contributed to the article and approved the submitted version.
Funding
OW was supported by a Fellowship from NOAA’s Cooperative Institute for Marine and Atmospheric Studies(CIMAS) at the University of Miami. Additional support was provided to AB by the Florida Department of Environmental Protection, and NOAA’s Coral Reef Conservation Program.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank the Florida Fish and Wildlife Commission for providing the parent colonies that spawned at The Florida Aquarium through the Coral Rescue Project. Parent corals were collected under FKNMS Superintendent’s permit FKNMS-2017-100, and larvae were transferred to the University of Miami under an Animal Transaction Agreement (ATTN#: 21A57) with the Florida Aquarium (USDA LIC# 58-C-0977, AZA# 2305000).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.821165/full#supplementary-material
References
Abrego D., van Oppen M. J. H., Willis B. L. (2009). Onset of Algal Endosymbiont Specificity Varies Among Closely Related Species of Acropora Corals During Early Ontogeny. Mol. Ecol. 18, 3532–3543. doi: 10.1111/J.1365-294X.2009.04276.X
Aeby G. S., Howells E., Work T., Abrego D., Williams G. J., Wedding L. M., et al. (2020). Localized Outbreaks of Coral Disease on Arabian Reefs Are Linked to Extreme Temperatures and Environmental Stressors. Coral Reefs. 39, 829–846. doi: 10.1007/s00338-020-01928-4
Aeby G., Ushijima B., Bartels E., Walter C., Kuehl J., Jones S., et al. (2021). Changing Stony Coral Tissue Loss Disease Dynamics Through Time in Montastraea Cavernosa. Front. Mar. Sci. 8, 699075. doi: 10.3389/FMARS.2021.699075
Aeby G. S., Ushijima B., Campbell J. E., Jones S., Williams G. J., Meyer J. L., et al. (2019). Pathogenesis of a Tissue Loss Disease Affecting Multiple Species of Corals Along the Florida Reef Tract. Front. Mar. Sci. 6, 678. doi: 10.3389/fmars.2019.00678
AGRRA. (2021) Stony Coral Tissue Loss Disease. Available at: https://www.agrra.org/coral-disease-outbreak/ (Accessed November 20, 2021).
Alvarez-Filip L., Carricart-Ganivet J. P., Horta-Puga G., Iglesias-Prieto R. (2013). Shifts in Coral-Assemblage Composition do Not Ensure Persistence of Reef Functionality. Sci. Rep. 3, 1–5. doi: 10.1038/srep03486
Alvarez-Filip L., Estrada-Saldívar N., Pérez-Cervantes E., Molina-Hernández A., González-Barrios F. J. (2019). A Rapid Spread of the Stony Coral Tissue Loss Disease Outbreak in the Mexican Caribbean. PeerJ. 11, e8069. doi: 10.7717/peerj.8069
Amar K. O., Chadwick N. E., Rinkevich B. (2008). Coral Kin Aggregations Exhibit Mixed Allogeneic Reactions and Enhanced Fitness During Early Ontogeny. BMC Evol. Bio. 8, 1. doi: 10.1186/1471-2148-8-126
Barnes D. J., Lough J. M. (1992). Systematic Variations in the Depth of Skeleton Occupied by Coral Tissue in Massive Colonies of Porites From the Great Barrier Reef. J. Exp. Mar. Biol. Ecol. 159 (1), 113–128. doi: 10.1016/0022-0981(92)90261-8
Bates D., Maechler M., Bolker B., Walker S. (2022). Lme4: Linear Mixed-Effects Models Using 'Eigen' and S4. Available at: https://cran.r-project.org/web/packages/lme4/lme4.pdf (Accessed February 1, 2022).
Baums I. B., Baker A. C., Davies S. W., Grottoli A. G., Kenkel C. D., Kitchen S. A., et al. (2019). Considerations for Maximizing the Adaptive Potential of Restored Coral Populations in the Western Atlantic. Ecol. Appl. 29 (8), e01978. doi: 10.1002/eap.1978
Bourne D., Iida Y., Uthicke S., Smith-Keune C. (2008). Changes in Coral-Associated Microbial Communities During a Bleaching Event. ISME J. 2 (4), 350–363. doi: 10.1038/ISMEJ.2007.112
Brandt M. E., Ennis R. S., Meiling S. S., Townsend J., Cobleigh K., Glahn A., et al. (2021). The Emergence and Initial Impact of Stony Coral Tissue Loss Disease (SCTLD) in the United States Virgin Islands. T. B. Front. Mar. Sci. 8,715329. doi: 10.3389/FMARS.2021.715329
Christiansen N. A., Ward S., Harii S., Tibbetts I. R. (2009). Grazing by a Small Fish Affects the Early Stages of a Post-Settlement Stony Coral. Coral Reefs 28, 47–51. doi: 10.1007/S00338-008-0429-9
Clark A. S., Williams S. D., Maxwell K., Rosales S. M., Huebner L. K., Landsberg J. H., et al. (2021). Characterization of the Microbiome of Corals With Stony Coral Tissue Loss Disease Along Florida’s Coral Reef. Microorganisms 9, 11. doi: 10.3390/microorganisms9112181
Correa A. M. S., Brandt M. E., Smith T. B., Thornhill D. J., Baker A. C. (2009). Symbiodinium Associations With Diseased and Healthy Scleractinian Corals. Coral Reefs 28 (2), 437–448. doi: 10.1007/s00338-008-0464-6
Costa S. V., Hibberts S. J., Olive D. A., Budd K. A., Long A. E., Meiling S. S., et al. (2021). Diversity and Disease: The Effects of Coral Diversity on Prevalence and Impacts of Stony Coral Tissue Loss Disease in Saint Thomas, U.S Virgin Islands. Front. Mar. Sci. 8,682688. doi: 10.3389/FMARS.2021.682688
Cumbo V., van Oppen M., Baird A. (2018). Temperature and Symbiodinium Physiology Affect the Establishment and Development of Symbiosis in Corals. Mar. Ecol. Prog. Ser. 587, 117–127. doi: 10.3354/meps12441
Cunning R., Baker A. C. (2013). Excess Algal Symbionts Increase the Susceptibility of Reef Corals to Bleaching. Nat. Clim. Change 3 (3), 259–262. doi: 10.1038/nclimate1711
Dahlgren C. P., Pizarro V., Sherman K., Greene W., Oliver J. (2021). Spatial and Temporal Patterns of Stony Coral Tissue Loss Disease Outbreaks in The Bahamas. Front. Mar. Sci. 8, 682114. doi: 10.3389/fmars.2021.682114
Damjanovic K., Blackall L. L., Webster N. S., Oppen M.J.H.v. (2017). The Contribution of Microbial Biotechnology to Mitigating Coral Reef Degradation. Microb. Biotechnol. 10 (5), 1236–1243. doi: 10.1111/1751-7915.12769
Dennison C. E., Karp R. F., Weiler B. A., Goncalves A., del Campo J., Rosales S. M., et al. (2021). "The Role of Algal Symbionts (Genus Breviolum) in the Susceptibility of Corals to Stony Coral Tissue Loss Disease in South Florida" in ((Miami FL): Florida DEP, 1–23.
Dietzel A., Connolly S. R., Hughes T. P., Bode M. (2021). The Spatial Footprint and Patchiness of Large-Scale Disturbances on Coral Reefs. Glob. Change Biol. 27 (19), 4825–4838. doi: 10.1111/GCB.15805
Dobbelaere T., Muller E. M., Gramer L. J., Holstein D. M., Hanert E. (2020). Coupled Epidemio-Hydrodynamic Modeling to Understand the Spread of a Deadly Coral Disease in Florida. Front. Mar. Sci. 7, 591881. doi: 10.3389/fmars.2020.591881
Doropoulos C., Roff G., Bozec Y. M., Zupan M., Werminghausen J., Mumby P. J. (2016). Characterizing the Ecological Trade-Offs Throughout the Early Ontogeny of Coral Recruitment. Ecol. Monogr. 86 (1), 20–44. doi: 10.1890/15-0668.1
Doropoulos C., Ward S., Marshell A., Diaz-Pulido G., Mumby P. J. (2012). Interactions Among Chronic and Acute Impacts on Coral Recruits: The Importance of Size-Escape Thresholds. Ecology 93 (10), 2131–2138. doi: 10.1890/12-0495.1
Duerden J. E. (1902). Aggregated Colonies in Madreporarian Corals. Am. Nat. 36, 461–471. doi: 10.1086/278152
Eaton K. R., Landsberg J. H., Kiryu Y., Peters E. C., Muller E. M. (2021). Measuring Stony Coral Tissue Loss Disease Induction and Lesion Progression Within Two Intermediately Susceptible Species, Montastraea Cavernosa and Orbicella Faveolata. Front. Mar. Sci. 8, 717265. doi: 10.3389/FMARS.2021.717265
Estrada-Saldívar N., Molina-Hernández A., Pérez-Cervantes E., Medellín-Maldonado F., González-Barrios F. J., Alvarez-Filip L. (2020). Reef-Scale Impacts of the Stony Coral Tissue Loss Disease Outbreak. Coral Reefs 39 (4), 861–866. doi: 10.1007/s00338-020-01949-z
FDEP (Florida Department Environmental Protection) (2021a). Florida Reef Tract Coral Disease Outbrea-Present). Available at: https://floridadep.gov/fco/coral/content/florida-reef-tract-coral-disease-outbreak (Accessed 12 Jul 2021).
FDEP (Florida Department of Environmental Protection) (2021b). Coral Rescue Team. Available at: https://floridadep.gov/rcp/coral/content/coral-rescue-team (Accessed July 12, 2021).
FKNMS (Florida Keys National Marine Sanctuary) (2018). Case Definition: Stony Coral Tissue Loss Disease (SCTLD). (Silver Spring, MD.:National Ocean Service) 1–29
Frank U., Oren U., Loya Y., Rinkevich B. (1997). Alloimmune Maturation in the Coral Stylophora Pistillata is Achieved Through Three Distinctive Stages, 4 Months Post-Metamorphosis. Proc. R. Soc. B. Biol. Sci. 264 (1378), 99. doi: 10.1098/RSPB.1997.0015
FWC (Florida Fish and Wildlife Conservation Commission) (2021). Disturbance Response Monitoring. Available at: https://ocean.floridamarine.org/FRRP/ (Accessed November 20, 2021).
Gilliam D. S., Hayes N. K., Ruzicka R., Colella M. A. (2019) Southeast Florida Coral Reef Evaluation and Monitoring Project. Available at: https://floridadep.gov/sites/default/files/SECREMP Year 17 Executive Summary_Final_0.pdf (Accessed October 13, 2021).
Gintert B. E., Precht W. F., Fura R., Rogers K., Rice M., Precht L. L., et al. (2019). Regional Coral Disease Outbreak Overwhelms Impacts From a Local Dredge Project. Environ. Monit. Assess. 191 (10), 1–39. doi: 10.1007/S10661-019-7767-7
Glasl B., Herndl G. J., Frade P. R. (2016). The Microbiome of Coral Surface Mucus has a Key Role in Mediating Holobiont Health and Survival Upon Disturbance. ISME J. 10 (9), 2280–2292. doi: 10.1038/ismej.2016.9
Guzmán-Urieta E. O., Jordán-Dahlgren E. (2021). Spatial Patterns of a Lethal White Syndrome Outbreak in Pseudodiploria Strigosa. Front. Mar. Sci. 8, 669171. doi: 10.3389/fmars.2021.669171
Heres M. M., Farmer B. H., Elmer F., Hertler H. (2021). Ecological Consequences of Stony Coral Tissue Loss Disease in the Turks and Caicos Islands. Coral Reefs 40 (2), 609–624. doi: 10.1007/S00338-021-02071-4
Hernández-Agreda A., Leggat W., Bongaerts P., Ainsworth T. D. (2016). The Microbial Signature Provides Insight Into the Mechanistic Basis of Coral Success Across Reef Habitats. MBio 7, 4. doi: 10.1128/MBIO.00560-16
Hidaka M. (1985). Tissue Compatibility Between Colonies and Between Newly Settled Larvae of Pocillopora Damicornis. Coral Reefs. 4, 111–116. doi: 10.1007/BF00300869
Hidaka M., Yurugi K., Sunagawa S., Kinzie R. III. (1997). Contact Reactions Between Young Colonies of the Coral Pocillopora Damicornis. Coral Reefs. 16, 13–20. doi: 10.1007/s003380050054
Howells E. J., Abrego D., Liew Y. J., Burt J. A., Meyer E., Aranda M. (2021). Enhancing the Heat Tolerance of Reef-Building Corals to Future Warming. Sci. Adv. 7, 34. doi: 10.1126/SCIADV.ABG6070
Howells E. J., Vaughan G. O., Work T. M., Burt J. A., Abrego D. (2020). Annual Outbreaks of Coral Disease Coincide With Extreme Seasonal Warming. Coral Reefs 39 (3), 771–781. doi: 10.1007/S00338-020-01946-2
Huffmyer A. S., Drury C., Majerová E., Lemus J. D., Gates R. D. (2021). Tissue Fusion and Enhanced Genotypic Diversity Support the Survival of Pocillopora Acuta Coral Recruits Under Thermal Stress. Coral Reefs 40 (2), 447–458. doi: 10.1007/S00338-021-02074-1
Hughes T. P., Tanner J. E. (2000). Recruitment Failure, Life Histories, and Long-Term Decline of Caribbean Corals. Ecology 81 (8), 2250. doi: 10.2307/177112
Kolodziej G., Studivan M. S., Gleason A. C. R., Langdon C., Enochs I. C., Manzello D. P. (2021). Impacts of Stony Coral Tissue Loss Disease (SCTLD) on Coral Community Structure at an Inshore Patch Reef of the Upper Florida Keys Using Photomosaics. Front. Mar. Sci. 8, 682163. doi: 10.3389/FMARS.2021.682163
Korner-Nievergelt F., Roth T., von Felten S., Guélat J., Almasi B., Korner-Nievergelt P. (2015). Bayesian Data Analysis in Ecology Using R, BUGS and Stan. Els. Sci. Technol, 75–94. doi: 10.1016/C2013-0-23227-X
Kramer P. R., Roth L., Lang J. (2019). Map of Stony Coral Tissue Loss Disease Outbreak in the Caribbean. Available at: www.agrra.org (Accessed August 26, 2021).
Krediet C. J., Ritchie K. B., Paul V. J., Teplitski M. (2013). Coral-Associated Micro-Organisms and Their Roles in Promoting Coral Health and Thwarting Diseases. Proc. R. Soc. B.: Biol. Sci. 280 (1755), 20122328. doi: 10.1098/RSPB.2012.2328
Kuznetsova A., Brockhoff B., Christensen H. B. (2017). Lmertest: Tests in Linear Mixed Effects Models. J. Stat. Softw. 82, 1–26. doi: 10.18637/jss.v082.i13
Landsberg J. H., Kiryu Y., Peters E. C., Wilson P. W., Perry N., Waters Y., et al. (2020). Stony Coral Tissue Loss Disease in Florida Is Associated With Disruption of Host–Zooxanthellae Physiology. Front. Mar. Sci. 7, 576013. doi: 10.3389/FMARS.2020.576013
Little A. F., Oppen M. J. H., Willis B. L. (2004). Flexibility in Algal Endosymbioses Shapes Growth in Reef Corals. Science 304 (5676), 1492–1494. doi: 10.1126/SCIENCE.1095733
Loya Y., Sakai K., Yamazato K., Nakano Y., Sambali H., Woesik R.v. (2001). Coral Bleaching: The Winners and the Losers. Ecol. Lett. 4 (2), 122–131. doi: 10.1046/J.1461-0248.2001.00203.X
Lunz K., Landsberg J., Kiryu Y., Brinkhuis V. (2017) Investigation of the Coral Disease Outbreak Affecting Scleractinian Coral Species Along the Florida Reef Tract. Available at: https://floridadep.gov/sites/default/files/FWRI-Diseases-Report.pdf.
Maynard J., van Hooidonk R., Eakin C. M., Puotinen M., Garren M., Williams G., et al. (2015). Projections of Climate Conditions That Increase Coral Disease Susceptibility and Pathogen Abundance and Virulence. Nat. Clim. Change 5 (7), 688–694. doi: 10.1038/nclimate2625
Meiling S. S., Muller E. M., Lasseigne D., Rossin A., Veglia A. J., MacKnight N., et al. (2021). Variable Species Responses to Experimental Stony Coral Tissue Loss Disease (SCTLD) Exposure. Front. Mar. Sci. 8, 670829. doi: 10.3389/FMARS.2021.670829
Meyer J. L., Castellanos-Gell J., Aeby G. S., Häse C. C., Ushijima B., Paul V. J. (2019). Microbial Community Shifts Associated With the Ongoing Stony Coral Tissue Loss Disease Outbreak on the Florida Reef Tract. Front. Microbiol. 10. doi: 10.3389/fmicb.2019.02244
Miller M. W., Weil E., Szmant A. M. (2000). Coral Recruitment and Juvenile Mortality as Structuring Factors for Reef Benthic Communities in Biscayne National Park, USA. Coral Reefs 19 (2), 115–123. doi: 10.1007/S003380000079
Muller E. M., Sartor C., Alcaraz N. I., van Woesik R. (2020). Spatial Epidemiology of the Stony-Coral-Tissue-Loss Disease in Florida. Front. Mar. Sci. 7, 163. doi: 10.3389/fmars.2020.00163
National Academies of Sciences, Engineering, and Medicine (2019). A Research Review of Interventions to Increase the Persistence and Resilience of Coral Reefs (Washington, DC: The National Academies Press).
Neely K. L., Lewis C. L., Lunz K. S., Kabay L. (2021a). Rapid Population Decline of the Pillar Coral Dendrogyra Cylindrus Along the Florida Reef Tract. Front. Mar. Sci. 8, 656515. doi: 10.3389/FMARS.2021.656515
Neely K. L., Macaulay K. A., Hower E. K., Dobler M. A. (2020). Effectiveness of Topical Antibiotics in Treating Corals Affected by Stony Coral Tissue Loss Disease. PeerJ. 8, 6. doi: 10.7717/PEERJ.9289
Neely K. L., Shea C. P., Macaulay K. A., Hower E. K., Dobler M. A. (2021b). Short- and Long-Term Effectiveness of Coral Disease Treatments. Front. Mar. Sci. 8, 675349. doi: 10.3389/FMARS.2021.675349
Nissimov J., Rosenberg E., Munn C. B. (2009). Antimicrobial Properties of Resident Coral Mucus Bacteria of Oculina Patagonica. FEMS Microbiol. Lett. 292 (2), 210–215. doi: 10.1111/J.1574-6968.2009.01490.X
Nozawa Y., Loya Y. (2005). Genetic Relationship and Maturity State of the Allorecognition System Affect Contact Reactions in Juvenile Seriatopora Corals. Mar. Ecol. Prog. Ser. 286, 115–123. doi: 10.3354/MEPS286115
Palmer C. V., McGinty E. S., Cummings D. J., Smith S. M., Bartels E., Mylardz L. D. (2011). Patterns of Coral Ecological Immunology: Variation in the Responses of Caribbean Corals to Elevated Temperature and a Pathogen Elicitor. J. Exp. Biol. 214 (24), 4240–4249. doi: 10.1242/JEB.061267
Peixoto R. S., Rosado P. M., Leite D. C., de A., Rosado A. S, Bourne D. G. (2017). Beneficial Microorganisms for Corals (BMC): Proposed Mechanisms for Coral Health and Resilience. Front. Microbiol. 8, 341. doi: 10.3389/fmicb.2017.00341
Peixoto R. S., Sweet M., Villela H. D. M., Cardoso P., Thomas T., Voolstra C. R., et al. (2021). Coral Probiotics: Premise, Promise, Prospects. Annu. Rev. Anim. Biosci. 9, 265–288. doi: 10.1146/ANNUREV-ANIMAL-090120-115444
Pollock F. J., Lamb J. B., Field S. N., Heron S. F., Schaffelke B., Shedrawi G., et al. (2014). Sediment and Turbidity Associated With Offshore Dredging Increase Coral Disease Prevalence on Nearby Reefs. PloS One 9 (7), e102498. doi: 10.1371/JOURNAL.PONE.0102498
Precht W. F., Gintert B. E., Robbart M. L., Fura R., Woesik R.v. (2016). Unprecedented Disease-Related Coral Mortality in Southeastern Florida. Sci. Rep. 6, 1–11. doi: 10.1038/srep31374
Puill-Stephan E., Oppen M. J. H., Pichavant-Rafini K., Willis B. L. (2011). High Potential for Formation and Persistence of Chimeras Following Aggregated Larval Settlement in the Broadcast Spawning Coral, Acropora Millepora. Proc. R. Soc. B.: Biol. Sci. 279 (1729), 699–708. doi: 10.1098/RSPB.2011.1035
Puill-Stephan E., Willis B. L., Abrego D., Raina J.-B., van Oppen M. J. H. (2012). Allorecognition Maturation in the Broadcast-Spawning Coral Acropora Millepora. Coral Reefs 31 (4), 1019–1028. doi: 10.1007/S00338-012-0912-1
Puill-Stephan E., Willis B. L., van Herwerden L., van Oppen M. J. H. (2009). Chimerism in Wild Adult Populations of the Broadcast Spawning Coral Acropora Millepora on the Great Barrier Reef. PloS One 4 (11), e7751. doi: 10.1371/JOURNAL.PONE.0007751
Quigley K. M., Bay L. K., Oppen M. J. H. (2020). Genome-Wide SNP Analysis Reveals an Increase in Adaptive Genetic Variation Through Selective Breeding of Coral. Mol. Ecol. 29 (12), 2176–2188. doi: 10.1111/mec.15482
Rädecker N., Pogoreutz C., Voolstra C. R., Wiedenmann J., Wild C. (2015). Nitrogen Cycling in Corals: The Key to Understanding Holobiont Functioning? Trends Microbiol. 23 (8), 490–497. doi: 10.1016/J.TIM.2015.03.008
Raina J., Tapiolas D., Motti C. A., Foret S., Seemann T., Tebben J., et al. (2016). Isolation of an Antimicrobial Compound Produced by Bacteria Associated With Reef-Building Corals. PeerJ 4, 8. doi: 10.7717/PEERJ.2275
Randall C., Negri A., Quigley K., Foster T., Ricardo G., Webster N., et al. (2020). Sexual Production of Corals for Reef Restoration in the Anthropocene. Mar. Ecol. Prog. Ser. 635, 203–232. doi: 10.3354/meps13206
Raymundo L. J., Maypa A. P.. (2004). Getting Bigger Faster: Mediation of Size-Specific Mortality via Fusion in Juvenile Coral Transplants. Ecol. Appl. 14, 281–295.
Rinkevich B. (2019). Coral Chimerism as an Evolutionary Rescue Mechanism to Mitigate Global Climate Change Impacts. Glob. Change Biol. 25 (4), 1198–1206. doi: 10.1111/GCB.14576
Rinkevich B., Shaish L., Douek J., Ben-Shlomo R. (2016). Venturing in Coral Larval Chimerism: A Compact Functional Domain With Fostered Genotypic Diversity. Sci. Rep. 6, 19493. doi: 10.1038/srep19493
Rinkevich B., Weissman I. L. (1992). Chimeras vs Genetically Homogeneous Individuals: Potential Fitness Costs and Benefits. Oikos 63, 119–124. doi: 10.2307/3545520
Rinkevich B., Yankelevich I. (2004). Environmental Split Between Germ Cell Parasitism and Somatic Cell Synergism in Chimeras of a Colonial Urochordate. J. Exp. Biol. 27 (20), 3531–3536. doi: 10.1242/JEB.01184
Rodriguez-Lanetty M., Harii S., Hoegh-Guldberg O. (2009). Early Molecular Responses of Coral Larvae to Hyperthermal Stress. Mol. Ecol. 18 (24), 5101–5114. doi: 10.1111/J.1365-294X.2009.04419.X
Rosado P. M., Leite D. C. A., Duarte G. A. S., Chaloub R. M., Jospin G., Rocha U. N., et al. (2019). Marine Probiotics: Increasing Coral Resistance to Bleaching Through Microbiome Manipulation. ISME J. 13 (4), 921–936. doi: 10.1038/s41396-018-0323-6
Rosales S. M., Clark A. S., Huebner L. K., Ruzicka R. R., Muller E. M. (2020). Rhodobacterales and Rhizobiales Are Associated With Stony Coral Tissue Loss Disease and its Suspected Sources of Transmission. Front. Microbiol. 11, 681. doi: 10.3389/fmicb.2020.00681
Rouzé H., Lecellier G., Saulnier D., Berteaux-Lecellier V. (2016). Symbiodinium Clades A and D Differentially Predispose Acropora Cytherea to Disease and Vibrio Spp. Colonization. Ecol. Evol. 6, 2. doi: 10.1002/ECE3.1895
Rowan R., Powers D. A.. (1991). Molecular Genetic Identification of Symbiotic Dinoflagellates (zooxanthellae). Mar. Ecol. Prog. Ser. 71, 65–73.
Rubin E. T., Enochs I. C., Foord C., Mayfield A. B., Kolodziej G., Basden I., et al. (2021). Molecular Mechanisms of Coral Persistence Within Highly Urbanized Locations in the Port of Miami, Florida. Front. Mar. Sci. 8, 695236. doi: 10.3389/FMARS.2021.695236
Sampayo E. M., Roff G., Sims C. A., Rachello-Dolmen P. G., Pandolfi J. M. (2020). Patch Size Drives Settlement Success and Spatial Distribution of Coral Larvae Under Space Limitation. Coral Reefs 39 (2), 387–396. doi: 10.1007/S00338-020-01901-1
Sharp K. H., Distel D., Paul V. J. (2012). Diversity and Dynamics of Bacterial Communities in Early Life Stages of the Caribbean Coral Porites Astreoides. ISME J. 6 (4), 790. doi: 10.1038/ISMEJ.2011.144
Sharp W. C., Shea C. P., Maxwell K. E., Muller E. M., Hunt J. H. (2020). Evaluating the Small-Scale Epidemiology of the Stony-Coral -Tissue-Loss-Disease in the Middle Florida Keys. PloS One 15 (11), e0241871. doi: 10.1371/JOURNAL.PONE.0241871
Shilling E. N., Combs I. R., Voss J. D. (2021). Assessing the Effectiveness of Two Intervention Methods for Stony Coral Tissue Loss Disease on Montastraea Cavernosa. Sci. Rep. 11, 8566. doi: 10.1038/s41598-021-86926-4
Shnit-Orland M., Kushmaro A. (2009). Coral Mucus-Associated Bacteria: A Possible First Line of Defense. FEMS Microbiol. Ecol. 67 (3), 371–380. doi: 10.1111/J.1574-6941.2008.00644.X
Shore A., Caldwell J. M. (2019). Modes of Coral Disease Transmission: How do Diseases Spread Between Individuals and Among Populations? Mar. Biol. 166, 45. doi: 10.1007/s00227-019-3490-8
Spadafore R., Fura R., Precht W. F., Vollmer S. V. (2021). Multi-Variate Analyses of Coral Mortality From the 2014–2015 Stony Coral Tissue Loss Disease Outbreak Off Miami-Dade County, Florida. Front. Mar. Sci. 8, 723998. doi: 10.3389/FMARS.2021.723998
van Oppen M. J. H., Oliver J. K., Putnam H. M., Gates R. D. (2015). Building Coral Reef Resilience Through Assisted Evolution. Proc. Nat. Acad. Sci. 112 (8), 2307–2313. doi: 10.1073/PNAS.1422301112
van Woesik R., Scott W. J., Aronson R. B. (2014). Lost Opportunities: Coral Recruitment Does Not Translate to Reef Recovery in the Florida Keys. Mar. Poll. Bull. 88, 110–117. doi: 10.1016/J.MARPOLBUL.2014.09.017
Vega Thurber R. L., Burkepile D. E., Fuchs C., Shantz A. A., McMinds R., Zaneveld J. R. (2014). Chronic Nutrient Enrichment Increases Prevalence and Severity of Coral Disease and Bleaching. Glob. Change Biol. 20 (2), 544–554. doi: 10.1111/GCB.12450
Vermeij M. J. A., Bakker J., van der Hal N., Bak R. P. M.. (2011). Juvenile Coral Abundance has Decreased by More Than 50% in Only Three Decades on a Small Caribbean Island Diversity 3 (3), 296–307. doi: 10.3390/d3030296
Vermeij M. J. A., Sandin S. A. (2008). Density-Dependent Settlement and Mortality Structure the Earliest Life Phases of a Coral Population. Ecology 89 (7), 1994–2004. doi: 10.1890/07-1296.1
Voolstra C. R., Suggett D. J., Peixoto R. S., Parkinson J. E., Quigley K. M., Silveira C. B., et al. (2021). Extending the Natural Adaptive Capacity of Coral Holobionts. Diversity Nat. Rev. Earth Environ. 2, 747–762. doi: 10.1038/s43017-021-00214-3
Walker B. K., Turner N. R., Noren H. K. G., Buckley S. F., Pitts K. A. (2021). Optimizing Stony Coral Tissue Loss Disease (SCTLD) Intervention Treatments on Montastraea Cavernosa in an Endemic Zone. Front. Mar. Sci. 8, 666224. doi: 10.3389/FMARS.2021.666224
Walton C. J., Hayes N. K., Gilliam D. S. (2018). Impacts of a Regional, Multi-Year, Multi-Species Coral Disease Outbreak in Southeast Florida. Front. Mar. Sci. 5. doi: 10.3389/fmars.2018.00323
Ward J. R., Kim K., Harvell C. D. (2007). Temperature Affects Coral Disease Resistance and Pathogen Growth. Mar. Ecol. Prog. Ser. 329, 115–121. doi: 10.3354/MEPS329115
Weeriyanun P., Collins R. B., Macadam A., Kiff H., Randle J. L., Quigley K. M. (2022). Predicting Selection–Response Gradients of Heat Tolerance in a Widespread Reef-Building Coral. J. Exp. Biol. 225,Suppl_1. doi: 10.1242/JEB.243344
Williamson O. M., Allen C. E., Williams D. E., Johnson M. W., Miller M. W., Baker A. C. (2021). Neighboring Colonies Influence Uptake of Thermotolerant Endosymbionts in Threatened Caribbean Coral Recruits. Coral Reefs. 40, 867–879. doi: 10.1007/s00338-021-02090-1
Williams S. D., Walter C. S., Muller E. M. (2021). Fine Scale Temporal and Spatial Dynamics of the Stony Coral Tissue Loss Disease Outbreak Within the Lower Florida Keys. Front. Mar. Sci. 8, 631776. doi: 10.3389/FMARS.2021.631776
Wilson J., Harrison P. (2005). Post-Settlement Mortality and Growth of Newly Settled Reef Corals in a Subtropical Environment. Coral Reefs 24 (3), 418–421. doi: 10.1007/S00338-005-0033-1
Work T. M., Weatherby T. M., Landsberg J. H., Kiryu Y., Cook S. M., Peters E. C. (2021). Viral-Like Particles Are Associated With Endosymbiont Pathology in Florida Corals Affected by Stony Coral Tissue Loss Disease. Front. Mar. Sci. 8, 750658. doi: 10.3389/FMARS.2021.750658
Keywords: coral, recruits, stony coral tissue loss disease, Diploria labyrinthiformis, Colpophyllia natans, chimera, resilience
Citation: Williamson OM, Dennison CE, O’Neil KL and Baker AC (2022) Susceptibility of Caribbean Brain Coral Recruits to Stony Coral Tissue Loss Disease (SCTLD). Front. Mar. Sci. 9:821165. doi: 10.3389/fmars.2022.821165
Received: 23 November 2021; Accepted: 31 March 2022;
Published: 03 May 2022.
Edited by:
William F. Precht, Dial Cordy and Associates, Inc., United StatesReviewed by:
Eric Jordán-Dahlgren, National Autonomous University of Mexico, MexicoSarah Annalise Gignoux-Wolfsohn, Smithsonian Environmental Research Center (SI), United States
Copyright © 2022 Williamson, Dennison, O’Neil and Baker. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Olivia M. Williamson, omw10@miami.edu
 Olivia M. Williamson
Olivia M. Williamson Caroline E. Dennison
Caroline E. Dennison Keri L. O’Neil
Keri L. O’Neil Andrew C. Baker
Andrew C. Baker