A New Classification Tool and a Systematic Review of Macroalgal Studies Disentangle the Complex Interactive Effects of Warming and Nutrient Enrichment on Primary Production
- 1Faculty of Fisheries, Kagoshima University, Kagoshima, Japan
- 2United Graduate School of Agricultural Sciences, Kagoshima University, Kagoshima, Japan
- 3Key Laboratory of Mariculture, Ministry of Education, Ocean University of China, Qingdao, China
In order to understand how global warming effects on ecosystem primary production may change depending on nutrient enrichment, a new classification is proposed to disentangle and recognize the combination of interactions among several factors, based on the effect direction (positive, negative, or neutral) and its changes induced in it by the other factor (synergizing, antagonizing, inducing no change, or changing it in some other way). Marine macroalgae were chosen (as primary producers for which there is the most experimental information available) to review the relevant studies on which this new classification can be tested. It was observed the positive effects of elevated temperature and nutrient enrichment often synergized each other within the temperature range between relatively low and optimal growth levels. However, the negative effect of further temperature elevation from optimal to higher levels was antagonized by nutrient enrichment in some studies but was synergized in others, depending on the range of temperature elevation. The positive effect of nutrient enrichment was antagonized (but still positive) by temperature increase above the optimum in many cases, although the effect sometimes switched to a negative effect depending on the magnitude of nutrient enrichment. These results predict that global warming will enhance bottom-up effects on primary production in cold seasons and areas, and there will be a negative warming effect on production in hot seasons and areas, but it may be possible to mitigate this effect by appropriate levels of nutrient enrichment.
Introduction
The combined effects of two different factors can be classified into additive or non-additive (interactive) effects, based on whether interaction of the factors is significant or not, as a result of analysis of variance (ANOVA) (Folt et al., 1999; Crain et al., 2008; Brown et al., 2014; Piggott et al., 2015; Côté et al., 2016; Gunderson et al., 2016). An additive effect is calculated as the sum of the individual effects of the two factors, whereas non-additive effects are not simply summed because the effect of one factor varies depending on changes in the other factor (Folt et al., 1999; Crain et al., 2008; Brown et al., 2014; Piggott et al., 2015; Côté et al., 2016; Gunderson et al., 2016). Historically, this non-additive effect has been classified as either synergism or antagonism, depending on whether the cumulative effect of the factors is greater or less than the additive sum of the individual effects, respectively (Folt et al., 1999; Crain et al., 2008; Brown et al., 2014; Gunderson et al., 2016). Synergism and antagonism can be distinguished easily when both factors produce their effect in the same direction (double positive or double negative). However, this classification is problematic when factors have opposing effects (i.e., one positive, and the other negative), because a synergism of one factor and an antagonism of another can occur at the same time (Piggott et al., 2015). To resolve this problem, Piggott et al. (2015) classified these interactions into 4 types: positive synergism (+S, where the cumulative effect is more positive than the additive sum); negative antagonism (–A, where the cumulative effect is less negative than the additive sum); positive antagonism (+A, where the cumulative effect is less positive than the additive sum); and negative synergism (–S, where the cumulative effect is more negative than the additive sum). This seemed to succeed in classifying all possible combinations of cumulative effects of two factors.
In further considering the study of combined effects of two factors, it is of interest to understand how the effect of one factor varies due to changes in the other factor. However, this is difficult based on the classification proposed by Piggott et al. (2015) because the meanings of the four types can vary depending on whether the two factors combine their effects in the same or opposing direction. For example, positive antagonism (+A) means that the effects of both factors are antagonized when these factors are double positive, but when their effects work in opposing directions, one factor may be antagonized while the other is synergized. Therefore, a deeper classification scheme is needed to directly describe the changes of one factor with respect to the changes in the other.
Global warming and climatic associated changes are considered to be caused by increasing concentration of greenhouse gasses such as CO2 in the atmosphere (Zandalinas et al., 2021). To mitigate climate change, the conservation and restoration of ecosystem primary producers, such as terrestrial plants, seagrass, and marine macroalgae, are necessary because they contribute to fix and sequester atmospheric CO2 (Nunes et al., 2020; Watanabe et al., 2020). The biomass and productivity of primary producers are generally regulated by bottom-up (nutrient supply) and top-down (herbivory) effects (Gruner et al., 2008; Sellers et al., 2021), but have also been negatively affected by global warming in recent years (Lobell et al., 2012; Bita and Gerats, 2013; Smale, 2020). Meanwhile, recent studies have shown that the effect of increased temperature on the growth of primary producers can be synergized or antagonized by nutrient enrichment (Strain et al., 2014; Ordóñez et al., 2015; Thomas et al., 2017; Egea et al., 2018; Ostrowski et al., 2021), implying that the management of local nutrient environments might help to mitigate the negative effects of warming. Although the number of such studies is still limited for plant species (Ordóñez et al., 2015; Egea et al., 2018), data have accumulated for marine macroalgae, which can be easily cultured (i.e., they do not have below-ground system) on a small scale (i.e., few incubators) and for shorter experimental periods (a few weeks or months). Hence, a literature review of macroalgal studies to date has the potential to provide data to understand how warming effects vary with nutrient enrichment and inform the management of local nutrient environments to mitigate the negative effects of warming.
Here, a new classification is proposed to disentangle specific effects among two interacting factors, based on the direction of one factor (positive, negative, or neutral) and its change due to the other factor (synergized, antagonized, not changed, or changed in some other way). Based on this classification, a literature review was used to investigate retrospectively variations in the effects of increased temperature on the growth of marine macroalgae due to the influence of nutrient enrichment, and vice versa.
A New Classification of Interaction Type
A new classification to describe how the effect of a certain factor A, varies with some other factor B is proposed, yielding in 11 types of different interactions (Figure 1). In this classification, the initial direction of the effect of factor A was classified as positive (P), neutral (O), or negative (N). The changes of the effect of factor A by factor B were divided into five categories: synergism (S), neutral (O), antagonism (A), inversion to negative (N), and inversion to positive (P). As a result, each of these types of interaction is given a two-letter code, as follows. PS: the positive effect of A is synergized by B. PO: the positive effect of A is not changed by B. PA: the positive effect of A is antagonized by B. PN: the positive effect of A is reversed to a negative effect by B. OP: the effect of A is neutral but becomes positive through the effect of B. OO: the effect of A is neutral and this is not changed by B. ON: the effect of A is neutral but becomes negative through the effect of B. NP: the negative effect of A is reversed to positive by B. NA: the negative effect of A is antagonized by B. NO: the negative effect of A is not changed by B. NS: the negative effect of A is synergized by B.
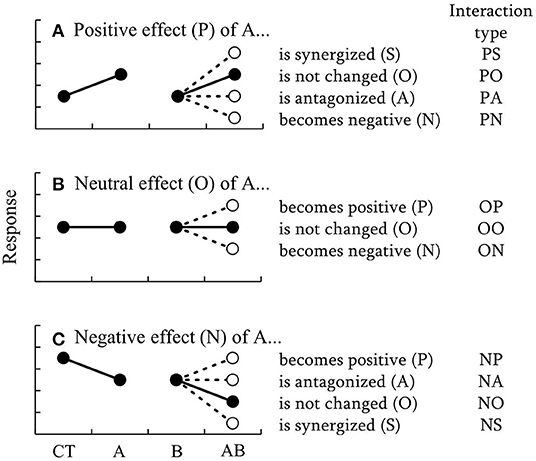
Figure 1. Schematic diagrams of 11 types of interactions proposed as a new classification to describe how the effect of a certain factor, A, varies with some other factor, B. The changes of the (A) positive, (B) neutral, (C) negative effects of A by B are shown separately. CT, A, and B indicate control, and treatments with elevated level of factors A and B, respectively. Black and white circles indicate the results of additive and non-additive effects, respectively.
The directions and changes of two factors can be described by pairing the interaction types, such as PS-PS or PA-PA, because the changes of effect of factor B are basically accompanied by changes of factor A (Figure 2). For example, when a positive effect of A is synergized by B (PS), the positive effect of B must be synergized by A (PS), resulting in PS-PS. When a positive effect of A is antagonized by B (PA), a positive effect of B can be antagonized (PA) or reversed by A (PN), resulting PA-PA or PA-PN, respectively.
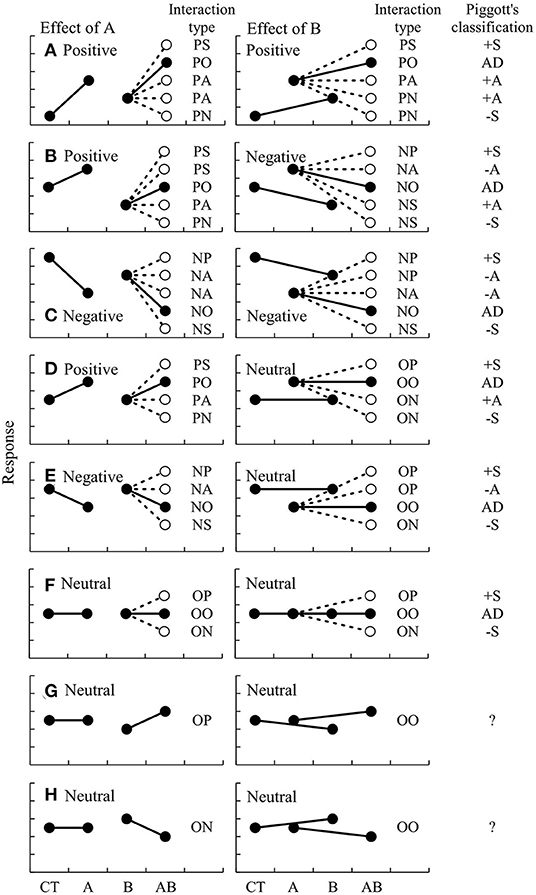
Figure 2. Schematic diagrams of potential types of interactions between two factors, A and B, and comparison with the classification by Piggott et al. (2015). The changes of A and B are separately shown at left and right, respectively, when the effects of A and B are (A) double positive, (B) opposing, (C) double negative, (D) positive and neutral, (E) negative and neutral, (F–H) double neutral, respectively.
This classification of interaction types is compared with that proposed by Piggott et al. (2015) in Figure 2. There are several similarities between the two schemes. For example, when the factors have opposing effects (e.g., one is positive, but the other is negative), both schemes classify the possible interactions into five types, including PS-NP (positive synergism, +S), PS-NA (negative antagonism, –A), PO-NO (additive effect, AD), PA-NS (positive antagonism, +A), and PN-NS (negative synergism, –S). Similarly, the interactions are classified into PS-OP (+S), PO-OO (AD), PA-ON (+A), and PN-ON (–S) when the two factors have positive and neutral effects, respectively; and into NP-OP (+S), NA-OP (–A), NO-OO (AD), and NS-ON (–S) when the two factors have negative and neutral effects, respectively. When both factors have neutral effects, they can be classified as OP-OP (+S), OO-OO (AD), or ON-ON (–S).
However, there are critical differences in some cases. When both factors have positive effects, the interactions can be classified only into 4 types by Piggott's classification (+S, AD, +A, and –S); but into 5 types according to the new classification: PS-PS (+S), PO-PO (AD), PA-PA (+A), PA-PN (+A), and PN-PN (–S). Note that type +A is here subdivided into PA-PA and PA-PN. Similarly, when both factors have negative effects, the interactions are classified as NP-NP (+S), NA-NP (–A), NA-NA (–A), NO-NO (AD), and NS-NS (–S). Moreover, there are two new possible types of interaction, OP-OO and ON-OO, which are not recognized by the scheme of Piggott et al. (2015).
Overall, there are few differences between the two schemes but the new classification, including the information about the directions and changes of both factors, provide more comprehensive coverage of the possible combination of interactions than that of Piggott et al. (2015). For example, +S of the Piggott's scheme can be subdivided according to the new scheme into PS-PS, PS-NP, NP-NP, PS-OP, NP-OP, and OP-OP.
Review of The Combined Effects of Temperature and Nutrients on Macroalgae
To perform a retrospective study to evaluate the new scheme, academic publications reporting the combined effects of temperature and nutrients on the growth of marine macroalgae were searched with Google Scholar by combining four categories of keywords: category 1, combined and interactive effects; category 2, temperature and warming; category 3, nutrient, nitrate, and dissolved inorganic nitrogen (DIN); and category 4, macroalga, alga, and seaweed. This search identified 31 articles where the combined effects on growth (i.e., relative growth rate and size) or recruitment density (the result of growth after settlement) of several factors including, at least, temperature and nutrients, were reported using ANOVA. The direction of changes in temperature and nutrients were unified to “elevated temperature” (i.e., warming) and “nutrient enrichment,” respectively, because a negative effect of decreased temperature results in changes in the same direction as a positive effect of elevated temperature (keeping them separate complicates understanding of the trends of the interactions).
The effect of each factor on growth was classified into 11 types of interaction based on the results of ANOVA and/or multiple comparison test, even when a significant probability of interaction was not described in the article. When the study cultured macroalgae at 3–5 levels of temperature (e.g., 5, 10, and 15°C) or nutrients, the classification was conducted for each consecutive level separately (e.g., 5–10 and 10–15°C). When multiple comparison tests were not conducted in a study, the potential type of interaction was speculated from figures (indicated by a question mark in Supplementary Table 1; such as PS?) but this was excluded from subsequent analysis.
Modification of Nutrient Effects by Elevated Temperature
A total of 78 cases (including all species, variables, temperature range, and nutrient range) involving temperature-nutrient interactions were identified and analyzed using the new scheme (Supplementary Table 1). The results are presented below in two parts: modification of nutrient effects by elevated temperature; and modification of temperature effects by nutrient enrichment.
The effects of nutrient enrichment on macroalgal growth were positive in 35 cases, neutral in 38 cases, and negative in 5 cases (Table 1). These nutrient effects varied due to elevated temperature in 27 cases but not in 51 cases.

Table 1. Distribution among different groups of macroalgae of interaction types involving a nutrient effect (from published experiments that included species, variables, temperature range, and nutrient range).
The positive effect of nutrients was synergized (PS) by elevated temperature in 7 cases (Lotze and Worm, 2002; Steen, 2003, 2004; Gao G. et al., 2017; Gao X. et al., 2017). For example, the effect of nutrient enrichment on recruit density of the green alga Ulva intestinalis was weak at the relatively low temperature of 5°C but was strong at 12 and 17°C; both the latter seemingly optimal temperatures for recruitment (Lotze and Worm, 2002). Also in the other cases, the ranges of temperature elevation were between relatively low and optimal levels for their growth: 7–17°C for Ulva compressa (Steen, 2004); 14–18°C for Ulva rigida (Gao G. et al., 2017); and 5–15°C for juveniles and 5–10°C for larger size classes of the kelp Saccharina japonica (Gao X. et al., 2017).
Additionally, neutral nutrient effect became a positive effect (OP) as a result of elevated temperature from relatively low to around optimal levels in other 3 cases: 7–17°C for the fucoid brown algae Fucus vesiculosus and Sargassum muticum (Steen and Rueness, 2004); and 10–15°C for the red alga Porphyra amplissima (Kim et al., 2007). These results can be explained by a lowered nutrient uptake rate (Pedersen et al., 2004 and references therein) and down-regulation of nitrogen transporter genes (Takahashi et al., 2020) under relatively low temperatures.
In contrast, a positive nutrient effect was antagonized (PA) by elevated temperature in 10 cases (Kim et al., 2007; Gao et al., 2013; de Faveri et al., 2015; Kay et al., 2016; Gao X. et al., 2017; Gouvêa et al., 2017; Piñeiro-Corbeira et al., 2019). For example, the nutrient effect on relative growth rate of the brown alga Saccharina japonica was synergized by temperature elevated from 5°C to the optimal level of 10°C; but was antagonized by further temperature elevation from 10 to 15°C (Gao X. et al., 2017). Also in other cases, the antagonized effects of nutrients were found between around optimal growth and relatively high temperatures: 10–15°C for Porphyra linearis and 15–20°C for P. amplissima (Kim et al., 2007); 15–24°C for the kelp Undaria pinnatifida (Gao et al., 2013); 15–25°C for the red alga Hypnea musciformis (de Faveri et al., 2015); 16–20 and 20–24°C for the fucoid brown alga Ascophyllum nodosum (Kay et al., 2016); 24–28°C for the red alga Laurencia catarinensis (Gouvêa et al., 2017); and 14–26°C for F. vesiculosus (Piñeiro-Corbeira et al., 2019). Additionally, the negative effect of a high level of nutrient enrichment (from 20 to 100 μM DIN) was also antagonized (NA) by elevated temperature from optimal growth to higher levels (16–20°C for A. nodosum; Kay et al., 2016). Although the physiological mechanism of these types of interactions is unclear, it may be related to changes in nutrient demands with elevated temperature, because increased phosphorus uptake rates with temperature elevation have been demonstrated in a brown macroalgae species (Ohtake et al., 2020) and corals (Ezzat et al., 2016), whereas nitrogen uptake rate was not affected (Ohtake et al., 2020) or was decreased by warming (Ezzat et al., 2016).
Thus, the effect of nutrient enrichment on macroalgal growth is often synergized by elevated temperature (PS) from relatively low to optimal levels but was antagonized by further temperature elevation (PA). However, there were few cases where neutral nutrient effect became positive (OP) in response to temperature elevated to relatively high levels: 10–15°C for P. amplissima and Porphyra umbilicalis, and 15–20°C for P. linearis (Kim et al., 2007); and 25–35°C for H. musciformis (de Faveri et al., 2015). Because elevated temperature can decrease the nitrogen content of plants and macroalgae (Hay et al., 2010; Wu et al., 2019), nutrient enrichment may have a positive effect when the macroalgal thallus is nitrogen-starved under warm conditions.
Further, there was just one case where a positive effect of relatively high levels of nutrient enrichment (DIN 50 to 120 μM) on relative growth rate (of L. catarinensis) became negative (PN) when temperature was elevated from 24 to 28°C, whereas the effect of relatively low levels of nutrient enrichment (DIN 1.3–50 μM) was still positive at the higher temperature (Gouvêa et al., 2017). This indicates that the magnitude of nutrient enrichment can affect whether or not a positive nutrient effect can become negative.
Modification of Temperature Effects by Nutrient Enrichment
The effects of elevated temperature on macroalgal growth were positive in 28 cases, neutral in 26 cases, and negative in 24 cases (Table 2). These temperature effects varied due to nutrient enrichment in 28 cases but not in 50 cases.
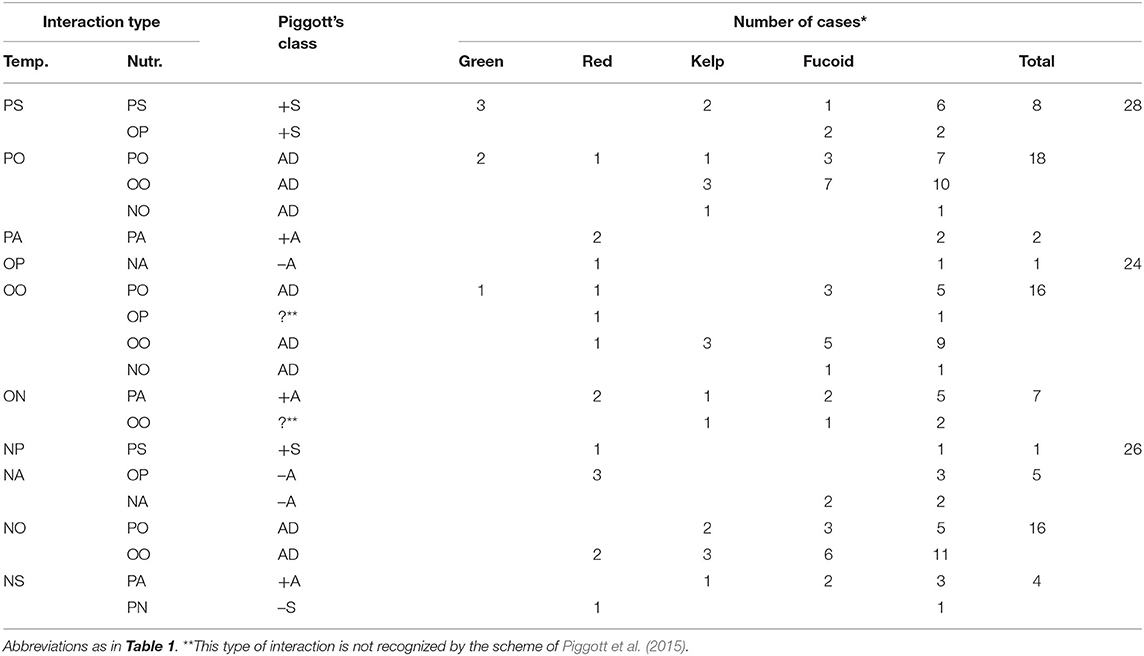
Table 2. Distribution among different groups of macroalgae of interaction types involving a temperature effect (from published experiments that included species, variables, temperature range and nutrient range).
The positive effect of elevated temperature was synergized (PS) by nutrient enrichment in 8 cases. This type of interaction simultaneously occurred with the synergism of nutrient effect by elevated temperature (PS-PS) in 6 cases (Lotze and Worm, 2002; Steen, 2003, 2004; Gao G. et al., 2017; Gao X. et al., 2017) and turning a neutral nutrient effect into positive (PS-OP) in 2 cases (Steen and Rueness, 2004). These results indicate that this interaction occurred when the elevated temperature weakened the negative effect of low temperature on nutrient uptake rates, as discussed above.
The negative effect of elevated temperature was synergized (NS) by nutrient enrichment in 4 cases (Kay et al., 2016; Gao X. et al., 2017; Gouvêa et al., 2017; Piñeiro-Corbeira et al., 2019). A neutral effect of elevated temperature became negative under the influence of nutrient enrichment (ON) in 7 cases. Most of these cases occurred with the antagonism of the nutrient effect by elevated temperature (NS-PA and ON-PA). Therefore, this interaction can occur when the strong effect of nutrient enrichment around the optimal growth temperature is suppressed by further temperature elevation. Note that the nutrient effect is still positive even when it synergizes the negative effect of elevated temperature in this type of interaction.
In contrast, the negative effect of elevated temperature was antagonized (NA) by nutrient enrichment in 5 cases. Many of these cases occurred when a neutral nutrient effect became positive because of elevated temperature (NA-OP) in 3 cases: 15–20°C for P. linearis and 10–15°C for P. umbilicalis (Kim et al., 2007); and 25–35°C for H. musciformis (de Faveri et al., 2015). In other cases, NA occurred when the negative effect of a high level of nutrient enrichment (DIN 20–100 μM) was antagonized by elevated temperature (NA-NA) in 2 cases: 16–20°C for changes in weight and length of A. nodosum (Kay et al., 2016). Hence, this interaction can occur when temperature is elevated between optimal and higher levels. Even in both cases, macroalgal growth was enhanced by nutrient enrichment.
There was only one report showing that a negative effect of elevated temperature can become positive and can be synergized by nutrient enrichment: Gouvêa et al. (2017) showed that the negative effect of temperature elevated from 20 to 24°C became positive because of nutrient enrichment (between 1.3 and 50–120 μM DIN) on relative growth rate of L. catarinensis. In addition, they showed that neutral effect of further temperature elevation from 24 to 28°C (at 1.3 μM DIN) became negative under low levels of nutrient enrichment (between 1.3 and 50 μM DIN), whereas the negative effect of temperature elevated from 24 to 28°C (at 50 μM DIN) was synergized by further nutrient enrichment (between 50 and 120 μM DIN). Thus, the effect of elevated temperature strongly depends on the range of temperature elevation and nutrient concentrations.
Ecological Implications
Marine macroalgal forests dominated by kelp and fucoid brown algae are highly productive and play an important role in coastal ecosystems through the provision of habitat and spawning grounds for a wide range of marine organisms (Steneck et al., 2002). Recent ocean warming has resulted in the increased abundance and/or expansion of range of marine forests in arctic reefs (Smale, 2020). The elevated temperature from relatively low to higher levels in these areas may enhance a bottom-up effects on macroalgae, as shown in this review. In contrast, ocean warming has caused range contraction, decreased abundance, and local extinction of marine forests in temperate reefs worldwide (Smale, 2020). Previous studies have shown that above-average temperatures combined with low nutrient availability during summer causes mass mortality and failed recruitment in kelp species (Dayton and Tegner, 1984; Dean and Jacobsen, 1984, 1986; Gerard, 1997; Tegner et al., 1997), but nutrient enrichment can enhance their recruitment, growth, and survival (North and Zimmerman, 1984; Dean and Jacobsen, 1986; Hernández-Carmona et al., 2001).
From the viewpoint of the interaction between temperature and nutrients, the present review has demonstrated that negative warming effects were antagonized by nutrient enrichment according to some studies but was synergized in others. However, even when the negative warming effect was synergized by nutrient enrichment, the positive effect of nutrient enrichment was still positive in many cases. Therefore, negative warming effects on macroalgal forests may be mitigated by in situ nutrient enrichment in coastal waters. Specifically, nutrient enrichment is predicted to be effective on macroalgae growing in regions, where nutrient concentrations in the surface water has been declining because vertical mixing of nutrient-poor surface water and nutrient-rich deep water has been suppressed by ocean warming or long-term natural climate change (Watanabe et al., 2005; D'Alelio et al., 2020).
The aquaculture production of commercially important macroalgae (genera Undaria, Saccharina, Sargassum, Neopyropia, Gracilaria, Eucheuma, and Kappaphycus), is increasing worldwide due to the rising demand for their utilization as human food, bait, fertilizer and raw industrial materials (Hurd et al., 2014; Chung et al., 2017). Specifically, the kelp Undaria pinnatifida has been extensively cultivated in China, Korea, and Japan, and has recently been introduced to Europe for commercial culture (Peteiro et al., 2016). Although the negative warming effect on this species is reported to be offset by nutrient enrichment (Gao et al., 2013), both the elevated temperature and nutrient enrichment are predicted to increase the risk of herbivory by isopods during autumn, when the outdoor cultivation of this species is started (Endo et al., 2021). Hence, warming may force the beginning of cultivation to be delayed by 1 month or more until the temperature drops into optimal levels. In contrast, elevated temperatures during winter may enhance macroalgal growth by the synergistic positive effect of increased nutrient concentration during this season, as shown in the present review, although there is also a possibility that warming might decrease nitrogen concentration in the surface water via suppression of vertical water mixing, as mentioned above (Watanabe et al., 2005; D'Alelio et al., 2020).
Green algae belonging to the genus Ulva are known to cause severe algal bloom phenomena (so-called green tides) in eutrophied coastal waters and have negative ecological impacts such as decreased oxygen levels in coastal environments due to their decomposition (Gao G. et al., 2017; Lee and Kang, 2020). The present review has shown that the positive effects of nutrient enrichment on Ulva species was often synergized by temperatures elevated from 5–7 to 11–17°C (Lotze and Worm, 2002; Steen, 2004) but there was no change with further temperature elevation from 15 to 30°C (Lee and Kang, 2020), whereas the effect was often antagonized by similar temperature elevations in red and brown algae. Hence, blooms of Ulva under eutrophication are predicted to be enhanced by warming in cold seasons and areas, but might not strongly be affected under hot conditions (i.e., around 30°C).
The combined effects of temperature and nutrient availability have also been examined in a number of other groups of primary producers, including terrestrial plants (Ordóñez et al., 2015 and references therein), seagrasses (Egea et al., 2018; Ontoria et al., 2019; and references therein), and phytoplankton (Thomas et al., 2017 and references therein), although the number of reports showing the results of ANOVA and multiple comparison tests is quite limited. For example, Ordóñez et al. (2015) reported that the negative effect of elevated temperature on the yield of maize (Zea mays) was synergized by nutrient enrichment. Although a similar result was obtained from macroalgal studies, the present review showed that whether the negative warming effect was synergized or antagonized depended on the magnitude of nutrient enrichment. Based on this information, there is still a possibility that reducing the amount of fertilizer might mitigate the negative warming effect on the crop yield, although this hypothesis needs to be tested.
Thomas et al. (2017) revealed that the optimal growth temperature of the diatom Thalassiosira pseudonana increased as a result of nutrient enrichment. This result is consistent with a macroalgal study conducted by Gouvêa et al. (2017), who showed that the optimal growth temperature of the red macroalga L. catarinensis was around 20°C in low nutrient treatments but increased to 24°C in nutrient enriched treatments. Further evaluation of optimal growth temperature under both nutrient-deficient and nutrient-rich conditions, using thermal performance curve (e.g., Fernández et al., 2020) or response surface methodology (e.g., Mendes et al., 2012; Sato et al., 2020), will contribute to understand the relationships between nutrient availability and optimal growth temperature of primary producers.
Conclusions
Using a more detailed scheme to classify the interactions of different factors on the growth of plants, this review was able to show that the effects of elevated temperature and nutrient enrichment on the growth of marine macroalgae are often synergized within the temperature range between relatively low to optimal growth levels. Thus, global warming is predicted to enhance a bottom-up effect on macroalgal productivity in cold-temperate and subarctic zones. In contrast, a negative effect of further temperature elevation on macroalgal growth can be both antagonized and synergized by nutrient enrichment, depending on the range of temperature elevation. Although the positive effect of nutrient enrichment was often antagonized by this temperature elevation, the effect was still positive in most cases even when it was antagonized. Therefore, the negative effect of warming on primary production in warming hotspots may be mitigated by nutrient enrichment. However, caution is advised in determining the amount of fertilizer, because there is evidence that a positive effect of relatively high levels of nutrient enrichment can become negative as a result of increased temperature. Additionally, nutrient enrichment under warm conditions was predicted to strengthen the top-down (herbivory) effects on primary producers (e.g., Endo et al., 2021). Further studies on the effect of elevated temperature on the nutrient requirement and interactive effects of temperature, nutrients, and herbivory on the productivity of marine macroalgae and terrestrial plants are needed in order to understand how to use fertilizer to mitigate negative warming effect on these primary producers.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author/s.
Author Contributions
HE: idea and conceptualization. HE and XG: literature review, writing, and editing. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We sincerely thank Prof. Yukio Agatsuma of Tohoku University for supporting this work.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.774801/full#supplementary-material
References
Bita, C., and Gerats, T. (2013). Plant tolerance to high temperature in a changing environment: scientific fundamentals and production of heat stress-tolerant crops. Front. Plant Sci. 4, 273. doi: 10.3389/fpls.2013.00273
Brown, C. J., Saunders, M. I., Possingham, H. P., and Richardson, A. J. (2014). Interactions between global and local stressors of ecosystems determine management effectiveness in cumulative impact mapping. Divers. Distrib. 20, 538–546. doi: 10.1111/ddi.12159
Chung, I. K., Sondak, C. F., and Beardall, J. (2017). The future of seaweed aquaculture in a rapidly changing world. Eur. J. Phycol. 52, 495–505. doi: 10.1080/09670262.2017.1359678
Côté, I. M., Darling, E. S., and Brown, C. J. (2016). Interactions among ecosystem stressors and their importance in conservation. Proc. R. Soc. B Biol. Sci. 283, 20152592. doi: 10.1098/rspb.2015.2592
Crain, C. M., Kroeker, K., and Halpern, B. S. (2008). Interactive and cumulative effects of multiple human stressors in marine systems. Ecol. Lett. 11, 1304–1315. doi: 10.1111/j.1461-0248.2008.01253.x
D'Alelio, D., Rampone, S., Cusano, L. M., Morfino, V., Russo, L., Sanseverino, N., et al. (2020). Machine learning identifies a strong association between warming and reduced primary productivity in an oligotrophic ocean gyre. Sci. Rep. 10, 1–12. doi: 10.1038/s41598-020-59989-y
Dayton, P. K., and Tegner, M. J. (1984). Catastrophic storms, El Niño, and patch stability in a southern California kelp community. Science. 224, 283–285. doi: 10.1126/science.224.4646.283
de Faveri, C., Schmidt, E. C., Simioni, C., Martins, C. D., Bonomi-Barufi, J., Horta, P. A., et al. (2015). Effects of eutrophic seawater and temperature on the physiology and morphology of Hypnea musciformis JV Lamouroux (Gigartinales, Rhodophyta). Ecotoxicology 24, 1040–1052. doi: 10.1007/s10646-015-1444-6
Dean, T. A., and Jacobsen, F. R. (1984). Growth of juvenile Macrocystis pyrifera (Laminariales) in relation to environmental factors. Mar. Biol. 83, 301–311. doi: 10.1007/BF00397463
Dean, T. A., and Jacobsen, F. R. (1986). Nutrient-limited growth of juvenile kelp, Macrocystis pyrifera, during the 1982–1984 ‘El Niño' in southern California. Mar. Biol. 90, 597–601. doi: 10.1007/BF00409280
Egea, L. G., Jiménez-Ramos, R., Vergara, J. J., Hernández, I., and Brun, F. G. (2018). Interactive effect of temperature, acidification and ammonium enrichment on the seagrass Cymodocea nodosa. Mar. Pollut. Bull. 134, 14–26. doi: 10.1016/j.marpolbul.2018.02.029
Endo, H., Sato, Y., Kaneko, K., Takahashi, D., Nagasawa, K., Okumura, Y., et al. (2021). Ocean warming combined with nutrient enrichment increases the risk of herbivory during cultivation of the marine macroalga Undaria pinnatifida. ICES J. Mar. Sci. 78, 402–409. doi: 10.1093/icesjms/fsaa069
Ezzat, L., Maguer, J. F., Grover, R., and Ferrier-Pagès, C. (2016). Limited phosphorus availability is the Achilles heel of tropical reef corals in a warming ocean. Sci. Rep. 6, 1–11. doi: 10.1038/srep31768
Fernández, P. A., Gaitán-Espitia, J. D., Leal, P. P., Schmid, M., Revill, A. T., and Hurd, C. L. (2020). Nitrogen sufficiency enhances thermal tolerance in habitat-forming kelp: implications for acclimation under thermal stress. Sci. Rep. 10, 1–12. doi: 10.1038/s41598-020-60104-4
Folt, C. L., Chen, C. Y., Moore, M. V., and Burnaford, J. (1999). Synergism and antagonism among multiple stressors. Limnol. Oceanogr. 44, 864–877. doi: 10.4319/lo.1999.44.3_part_2.0864
Gao, G., Clare, A. S., Rose, C., and Caldwell, G. S. (2017). Eutrophication and warming-driven green tides (Ulva rigida) are predicted to increase under future climate change scenarios. Mar. Pollut. Bull. 114, 439–447. doi: 10.1016/j.marpolbul.2016.10.003
Gao, X., Endo, H., Nagaki, M., and Agatsuma, Y. (2017). Interactive effects of nutrient availability and temperature on growth and survival of different size classes of Saccharina japonica (Laminariales, Phaeophyceae). Phycologia 56, 253–260. doi: 10.2216/16-91.1
Gao, X., Endo, H., Taniguchi, K., and Agatsuma, Y. (2013). Combined effects of seawater temperature and nutrient condition on growth and survival of juvenile sporophytes of the kelp Undaria pinnatifida (Laminariales; Phaeophyta) cultivated in northern Honshu, Japan. J. Appl. Phycol. 25, 269–275. doi: 10.1007/s10811-012-9861-x
Gerard, V. A.. (1997). The role of nitrogen nutrition in high-temperature tolerance of the kelp, Laminaria saccharina (Chromophyta). J. Phycol. 33, 800–810. doi: 10.1111/j.0022-3646.1997.00800.x
Gouvêa, L. P., Schubert, N., Martins, C. D. L., Sissini, M., Ramlov, F., Rodrigues, E. R. D. O., et al. (2017). Interactive effects of marine heatwaves and eutrophication on the ecophysiology of a widespread and ecologically important macroalga. Limnol. Oceanogr. 62, 2056–2075. doi: 10.1002/lno.10551
Gruner, D. S., Smith, J. E., Seabloom, E. W., Sandin, S. A., Ngai, J. T., Hillebrand, H., et al. (2008). A cross-system synthesis of consumer and nutrient resource control on producer biomass. Ecol. Lett. 11, 740–755. doi: 10.1111/j.1461-0248.2008.01192.x
Gunderson, A. R., Armstrong, E. J., and Stillman, J. H. (2016). Multiple stressors in a changing world: the need for an improved perspective on physiological responses to the dynamic marine environment. Ann. Rev. Mar. Sci. 8, 357–378. doi: 10.1146/annurev-marine-122414-033953
Hay, K. B., Millers, K. A., Poore, A. G., and Lovelock, C. E. (2010). The use of near infrared reflectance spectrometry for characterization of brown algal tissue. J. Phycol. 46, 937–946. doi: 10.1111/j.1529-8817.2010.00890.x
Hernández-Carmona, G., Robledo, D., and Serviere-Zaragoza, E. (2001). Effect of nutrient availability on Macrocystis pyrifera recruitment and survival near its southern limit off Baja California. Bot. Mar. 44, 221–229. doi: 10.1515/BOT.2001.029
Hurd, C. L., Harrison, P. J., Bischof, K., and Lobban, C. S. (2014). Seaweed Ecology and Physiology, 2nd Edn. Cambridge: Cambridge University Press.
Kay, L. M., Schmidt, A. L., Wilson, K. L., and Lotze, H. K. (2016). Interactive effects of increasing temperature and nutrient loading on the habitat-forming rockweed Ascophyllum nodosum. Aquat. Bot. 133, 70–78. doi: 10.1016/j.aquabot.2016.06.002
Kim, J. K., Kraemer, G. P., Neefus, C. D., Chung, I. K., and Yarish, C. (2007). Effects of temperature and ammonium on growth, pigment production and nitrogen uptake by four species of Porphyra (Bangiales, Rhodophyta) native to the New England coast. J. Appl. Phycol. 19, 431–440. doi: 10.1007/s10811-006-9150-7
Lee, J. E., and Kang, J. W. (2020). The interactive effects of elevated temperature and nutrient concentrations on the physiological responses of Ulva linza Linnaeus (Ulvales, Chlorophyta). J. Appl. Phycol. 32, 2459–2476. doi: 10.1007/s10811-019-02031-0
Lobell, D. B., Sibley, A., and Ortiz-Monasterio, J. I. (2012). Extreme heat effects on wheat senescence in India. Nat. Clim. Change. 2, 186–189. doi: 10.1038/nclimate1356
Lotze, H. K., and Worm, B. (2002). Complex interactions of climatic and ecological controls on macroalgal recruitment. Limnol. Oceanogr. 47, 1734–1741. doi: 10.2307/3096546
Mendes, L. F., Vale, L. A., Martins, A. P., Yokoya, N. S., Marinho-Soriano, E., and Colepicolo, P. (2012). Influence of temperature, light and nutrients on the growth rates of the macroalga Gracilaria domingensis in synthetic seawater using experimental design. J. Appl. Phycol. 24, 1419–1426. doi: 10.1007/s10811-012-9797-1
North, W. J., and Zimmerman, R. C. (1984). Influences of macronutrients and water temperatures on summertime survival of Macrocystis canopies. Hydrobiologia 116/117, 419–424. doi: 10.1007/BF00027713
Nunes, L. J., Meireles, C. I., Pinto Gomes, C. J., and Almeida Ribeiro, N. (2020). Forest contribution to climate change mitigation: management oriented to carbon capture and storage. Climate 8, 21. doi: 10.3390/cli8020021
Ohtake, M., Natori, N., Sugai, Y., Tsuchiya, K., Aketo, T., Nishihara, G. N., et al. (2020). Growth and nutrient uptake characteristics of Sargassum macrocarpum cultivated with phosphorus-replete wastewater. Aquat. Bot. 163, 103208. doi: 10.1016/j.aquabot.2020.103208
Ontoria, Y., Gonzalez-Guedes, E., Sanmartí, N., Bernardeau-Esteller, J., Ruiz, J. M., Romero, J., et al. (2019). Interactive effects of global warming and eutrophication on a fast-growing Mediterranean seagrass. Mar. Environ. Res. 145, 27–38. doi: 10.1016/j.marenvres.2019.02.002
Ordóñez, R. A., Savin, R., Cossani, C. M., and Slafer, G. A. (2015). Yield response to heat stress as affected by nitrogen availability in maize. Field Crops Res. 183, 184–203. doi: 10.1016/j.fcr.2015.07.010
Ostrowski, A., Connolly, R. M., and Sievers, M. (2021). Evaluating multiple stressor research in coastal wetlands: a systematic review. Mar. Environ. Res. 105239. doi: 10.1016/j.marenvres.2020.105239
Pedersen, A., Kraemer, G., and Yarish, C. (2004). The effects of temperature and nutrient concentrations on nitrate and phosphate uptake in different species of Porphyra from Long Island Sound (USA). J. Exp. Mar. Biol. Ecol. 312, 235–252. doi: 10.1016/j.jembe.2004.05.021
Peteiro, C., Sánchez, N., and Martínez, B. (2016). Mariculture of the Asian kelp Undaria pinnatifida and the native kelp Saccharina latissima along the Atlantic coast of Southern Europe: an overview. Algal Res. 15, 9–23. doi: 10.1016/j.algal.2016.01.012
Piggott, J. J., Townsend, C. R., and Matthaei, C. D. (2015). Reconceptualizing synergism and antagonism among multiple stressors. Ecol. Evol. 5, 1538–1547. doi: 10.1002/ece3.1465
Piñeiro-Corbeira, C., Barreiro, R., Franco, J. N., Cremades, J., Cunha, J., and Arenas, F. (2019). Unexpected nutrient influence on the thermal ecophysiology of seaweeds that recently followed opposite abundance shifts. Mar. Environ. Res. 151, 104747. doi: 10.1016/j.marenvres.2019.06.009
Sato, Y., Endo, H., Oikawa, H., Kanematsu, K., Naka, H., Mogamiya, M., et al. (2020). Sexual difference in the optimum environmental conditions for growth and maturation of the brown alga Undaria pinnatifida in the gametophyte stage. Genes. 11, 944. doi: 10.3390/genes11080944
Sellers, A. J., Leung, B., Altieri, A. H., Glanz, J., Turner, B. L., and Torchin, M. E. (2021). Seasonal upwelling reduces herbivore control of tropical rocky intertidal algal communities. Ecology 102, e03335. doi: 10.1002/ecy.3335
Smale, D. A.. (2020). Impacts of ocean warming on kelp forest ecosystems. New Phytol. 225, 1447–1454. doi: 10.1111/nph.16107
Steen, H.. (2003). Intraspecific competition in Sargassum muticum (Phaeophyceae) germlings under various density, nutrient and temperature regimes. Bot. Mar. 46, 36–43. doi: 10.1515/BOT.2003.006
Steen, H.. (2004). Interspecific competition between Enteromorpha (Ulvales: Chlorophyceae) and Fucus (Fucales: Phaeophyceae) germlings: effects of nutrient concentration, temperature, and settlement density. Mar. Ecol. Prog. Ser. 278, 89–101. doi: 10.3354/meps278089
Steen, H., and Rueness, J. (2004). Comparison of survival and growth in germlings of six fucoid species (Fucales, Phaeophyceae) at two different temperature and nutrient levels. Sarsia 89, 175–183. doi: 10.1080/00364820410005818
Steneck, R. S., Graham, M. H., Bourque, B. J., Corbett, D., Erlandson, J. M., Estes, J. A., et al. (2002). Kelp forest ecosystems: biodiversity, stability, resilience and future. Environ. Conserv. 29, 436–459. doi: 10.1017/S0376892902000322
Strain, E. M. A., Thomson, R. J., Micheli, F., Mancuso, F. P., and Airoldi, L. (2014). Identifying the interacting roles of stressors in driving the global loss of canopy-forming to mat-forming algae in marine ecosystems. Global Change Biol. 20, 3300–3312. doi: 10.1111/gcb.12619
Takahashi, M., Kumari, P., Li, C., and Mikami, K. (2020). Low temperature causes discoloration by repressing growth and nitrogen transporter gene expression in the edible red alga Pyropia yezoensis. Mar. Environ. Res. 159, 105004. doi: 10.1016/j.marenvres.2020.105004
Tegner, M. J., Dayton, P. K., Edwards, P. B., and Riser, K. L. (1997). Large-scale, low-frequency oceanographic effects on kelp forest succession: a tale of two cohorts. Mar. Ecol. Prog. Ser. 146, 117–134. doi: 10.3354/meps146117
Thomas, M. K., Aranguren-Gassis, M., Kremer, C. T., Gould, M. R., Anderson, K., Klausmeier, C. A., et al. (2017). Temperature-nutrient interactions exacerbate sensitivity to warming in phytoplankton. Global Change Biol. 23, 3269–3280. doi: 10.1111/gcb.13641
Watanabe, K., Yoshida, G., Hori, M., Umezawa, Y., Moki, H., and Kuwae, T. (2020). Macroalgal metabolism and lateral carbon flows can create significant carbon sinks. Biogeosciences 17, 2425–2440. doi: 10.5194/bg-17-2425-2020
Watanabe, Y. W., Ishida, H., Nakano, T., and Nagai, N. (2005). Spatiotemporal decreases of nutrients and chlorophyll-a in the surface mixed layer of the western North Pacific from 1971 to 2000. J. Oceanogr. 61, 1011–1016. doi: 10.1007/s10872-006-0017-y
Wu, T., Qu, C., Li, Y., Li, X., Zhou, G., Liu, S., et al. (2019). Warming effects on leaf nutrients and plant growth in tropical forests. Plant Ecol. 220, 663–674. doi: 10.1007/s11258-019-00943-y
Keywords: climate change, cumulative effect, bottom-up control, ecosystem primary producer, interactions between global and local stressors, multiple stressors, non-additive effect
Citation: Endo H and Gao X (2022) A New Classification Tool and a Systematic Review of Macroalgal Studies Disentangle the Complex Interactive Effects of Warming and Nutrient Enrichment on Primary Production. Front. Mar. Sci. 9:774801. doi: 10.3389/fmars.2022.774801
Received: 13 September 2021; Accepted: 10 January 2022;
Published: 02 February 2022.
Edited by:
JInghui Fang, Chinese Academy of Fishery Sciences (CAFS), ChinaReviewed by:
Alejandra Moenne, University of Santiago, ChileInés G. Viana, Spanish Institute of Oceanography, Spain
Copyright © 2022 Endo and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xu Gao, gaoxu@ouc.edu.cn
 Hikaru Endo
Hikaru Endo Xu Gao
Xu Gao