Light spectrum impacts on development respiratory metabolism and antioxidant capacity of larval swimming crab Portunus trituberculatus
- 1Key Laboratory of Aquacultural Biotechnology, Ningbo University, Chinese Ministry of Education, Ningbo, China
- 2Marine Economic Research Center, Dong Hai Strategic Research Institute, Ningbo University, Ningbo University, Ningbo, China
- 3Collaborative Innovation Center for Zhejiang Marine High-efficiency and Healthy Aquaculture, Ningbo University, Ningbo, China
- 4Key Laboratory of Green Mariculture (Co-construction by Ministry and Province), Ministry of Agriculture and Rural, Ningbo, China
- 5Zhejiang Provincial Engineering Research Center for the Safety of Pressure Vessel and Pipeline, Ningbo University, Ningbo, China
- 6Institute of Semiconductors, Chinese Academy of Sciences, Beijing, China
The effects of the spectrum on the development, respiratory metabolism, and antioxidant capacity of the larval swimming crab Portunus trituberculatus were studied. Seven light spectra, i.e., purple (400 nm), blue (425 nm), cyan (510 nm), green (525 nm), yellow (598 nm), red (638 nm), and white (full spectrum), were estimated. The larvae had the optimum survival rate and development under cyan light. On the contrary, larvae in red and yellow lights had poor growth performance. The oxygen consumption rate (OCR) dropped while the ammonia excretion rate (AER) rose as the larvae developed. Early larvae’s oxygen-nitrogen ratio (O: N) fell when exposed to red light, suggesting more protein was utilized in the respiratory process. Regarding the antioxidant system, crab had the lowest malondialdehyde (MDA) under green, cyan and yellow light, and the highest total antioxidant capacity (T-AOC) in cyan light. Taken together, the current results suggest that cyan was the optimum spectrum for the development of P. trituberculatus larvae.
Introduction
The swimming crab, Portunus trituberculatus, is an important commercial marine crab species in China (Dou et al., 2021), with the yield increased to 100,895 tons in 2020. (Fisheries Bureau of Agriculture Ministry of China, 2021). Currently, P. trituberculatus larvae are mainly bred in ponds with natural live food, resulting in inconsistent production because of unpredictable circumstances (Shi et al., 2019). Consequently, the indoor nursery of swimming crabs, which could supply the larvae stability, has expanded quickly in recent years. In the indoor nursery, light is an indispensable environmental factor.
Three components of light, including intensity, spectrum, and photoperiod, are among the most crucial environmental parameters in indoor farming (Boeuf and Le Bail, 1999; Hoang et al., 2002; Chen et al., 2021; Chen et al., 2022). Spectrum directly impacts early larvae’s physiology, development, growth, and behavior (Pierce et al., 2008; Wu et al., 2020).The effects of the light spectrum on aquatic creatures are species-specific (Cheng and Flamarique, 2007; Migaud et al., 2010). For example, the giant freshwater prawn (Macrobrachium rosenbergii) had better development under green and white light (Wei et al., 2021). Blue or cyan light can enhance the growth performance of mud crabs (Scylla paramamosain) (Chen et al., 2022). For larval and juvenile P. trituberculatus, the optimal light intensity (Dou et al., 2021; Xu et al., 2022a) and photoperiod (Xu et al., 2022b) have been determined. However, the ideal light spectrum for P. trituberculatus larvae was poorly understood.
The respiratory system metabolizes most energy in the anmials (Leiva et al., 2015). Light modulates the respiratory metabolism of crustaceans by affecting endogenous motor rhythms and hormones (Hoang et al., 2003). As crucial markers of respiratory metabolism in aquatic organisms, oxygen consumption rate (OCR), ammonia excretion rate (AER), and oxygen-nitrogen ratio (O:N) can be used to evaluate crustacean energy consumption patterns (Zhang et al., 2014).
Reactive oxygen species (ROS) are oxygen radicals continuously produced in the immune system (Aguirre et al., 2005; Wu et al., 2016). Hydroxyl radicals (OH), superoxide anions (O2-), and hydrogen peroxide (H2O2) make up the majority of ROS (Jacob, 1995). A suboptimal environment could produce excess ROS, causing oxidative damage to cells and tissues (Lushchak, 2011). Antioxidase, including Superoxide dismutase (SOD), and catalase (CAT) could efficiently remove the excessive ROS and maintain homeostasis. (Craig et al., 2007; Oliva et al., 2012; Malandrakis et al., 2014; Makrinos and Bowden, 2016).
In this study, light-emitting diodes (LEDs) were used as light sources to investigate the effects of different light spectra on the survival, development, respiratory metabolism, and antioxidant capacity of P. trituberculatus larvae to explore the optimum light spectrum for P. trituberculatus larvae development.
Material and methods
Experimental design, management, and sampling
The experiment was carried out at the Donghai nursery in Xiangshan County, Ningbo City, Zhejiang Province, from May to June 2021. Experimental Zoea I was collected and evenly mixed from four female parent P. trituberculatus. In this experiment, the larvae were reared in plastic culture tanks (19.0 cm*12.2 cm*10.9 cm) with 2000mL seawater (Wu et al., 2010). One hunderd Zoea I larvae were placed in each tank in sextuplicate. Larvae were fed newly hatched Artemia nauplii at 10 individuals mL−1 at 6:00, 12:00, 18:00, and 24:00 (Shi et al., 2019). Seven treatments were set up with seven distinct spectral LED strips (Yamingjie Intelligent Technology Co., Ltd.), i.e., purple (400 nm), blue (425 nm), cyan (510 nm), green (525 nm), yellow (598 nm), red (638 nm), and white (634 nm, full spectrum). The light intensity was kept at 1W m-2 (Fei et al., 2020; Chen et al., 2022) by adjusting dimmers and the distance between lamps and the water surface, and a light cycle of 12L:12D was used throughout the experiment (6:00-18:00 light). During the experiment, the water temperature was maintained at 25 ± 1 °C (Dou et al., 2021; Xu et al., 2022b).
Morality and molting were observed daily until all the larvae died or developed into C1 juvenile crabs. At the end of the experiment, the alive C1 were quickly snap-frozen in liquid nitrogen. The level of SOD, CAT, total antioxidant capacity (T-AOC), hydrogen peroxide (H2O2), and malondialdehyde (MDA) were measured with commercial kits (Nanjing Jiancheng Institute of Biological Engineering) (Chen et al., 2022).
The same batch of larvae was used to test respiratory metabolism under different spectrums. The larvae were kept in a cement tank to develop to the targeted stage. After two hours in darkness, 100 larvae of the same developmental stage were put in polypropylene containers (19.0 cm 12.2 cm 10.9 cm) and treated with a specific light spectrum in quadruplicate. The OCR, AER and O: N ratio were measured and calculated as described by (Liu et al., 2022).
Data processing and statistical analysis
Statistical calculations were made using the SPSS 22.0 program. All the data is expressed as Mean ± SD. The homogeneity and normality of the survival, development, respiratory metabolism and antioxidant capability were examined with Levene’s and Kolmogorov-Smirnov tests. One-way ANOVA and Duncan’s test were performed to determine whether there were significant differences among treatments.
Results
Effect of light spectrum on the survival and development
The survival rate of larvae under red light was significantly lower than that of all other groups (P<0.05). On the contrary, the larvae of all the developmental stages had a higher survival rate in cyan light (Figure 1). The larval development under cyan light was shortened from Zoea I to Zoea III (Figures 2A-C). The molt interval of Zoea IV to megalopa was much shorter under purple, blue, cyan, and white light than it was under green, yellow, and red light (Figure 2D). The shortest molt interval was observed under green light from megalopa to C1 (Figure 2E). Overall, the cumulative developmental duration of the larvae under cyan light was the shortest, whereas a retarded development was found in yellow and red light (Figure 2F).
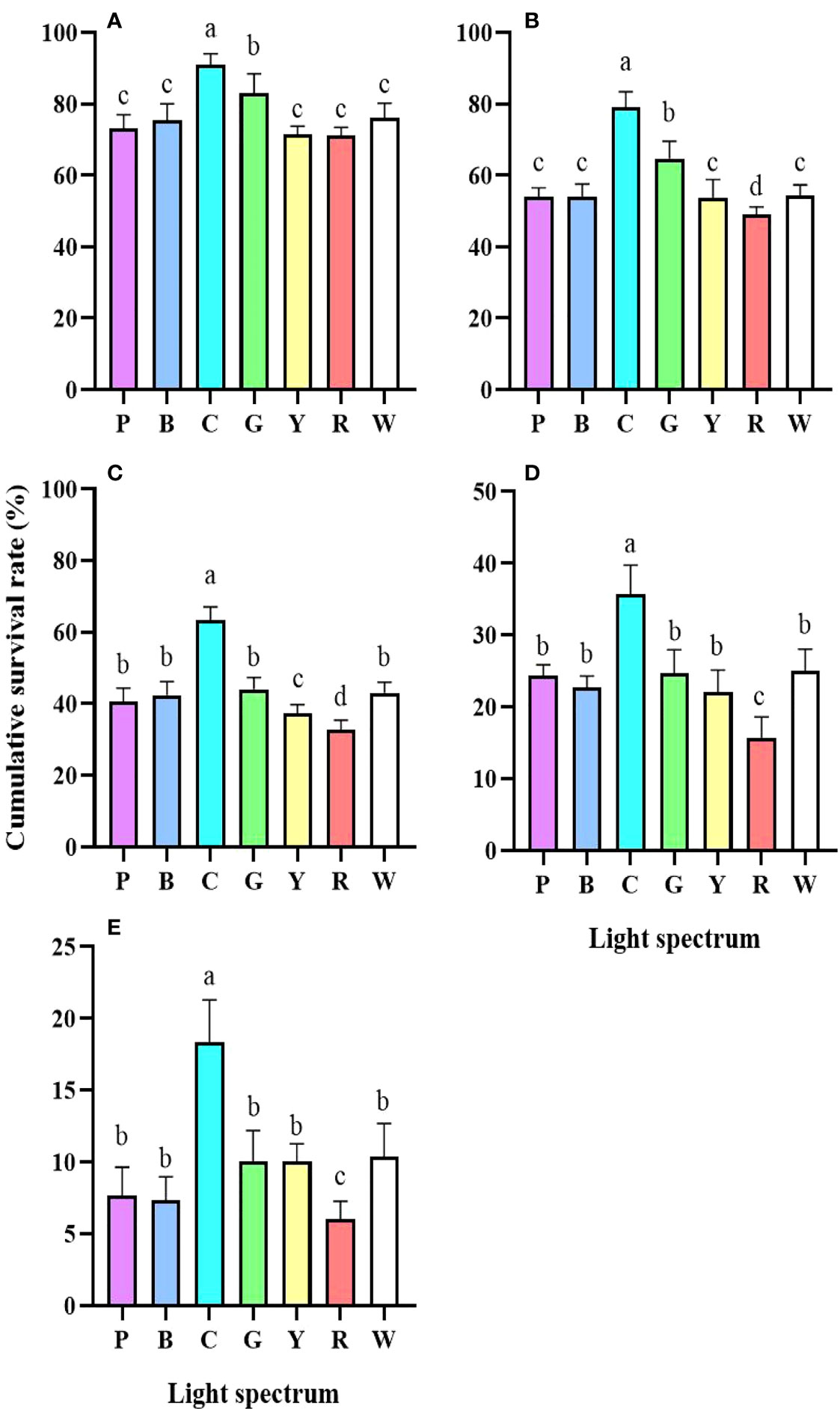
Figure 1 Cumulative survival rate of P. trituberculatus larvae under different light spectrum (mean ± SD) (n=6). (A) Zoea I, (B) Zoea II, (C) Zoea III, (D) Zoea IV, (E) Megalopa. P, Purple; B, Blue; C, Cyan; G, Green; Y, Yellow; R, Red; W, White. Different letters indicate significant differences between treatment groups (P<0.05).
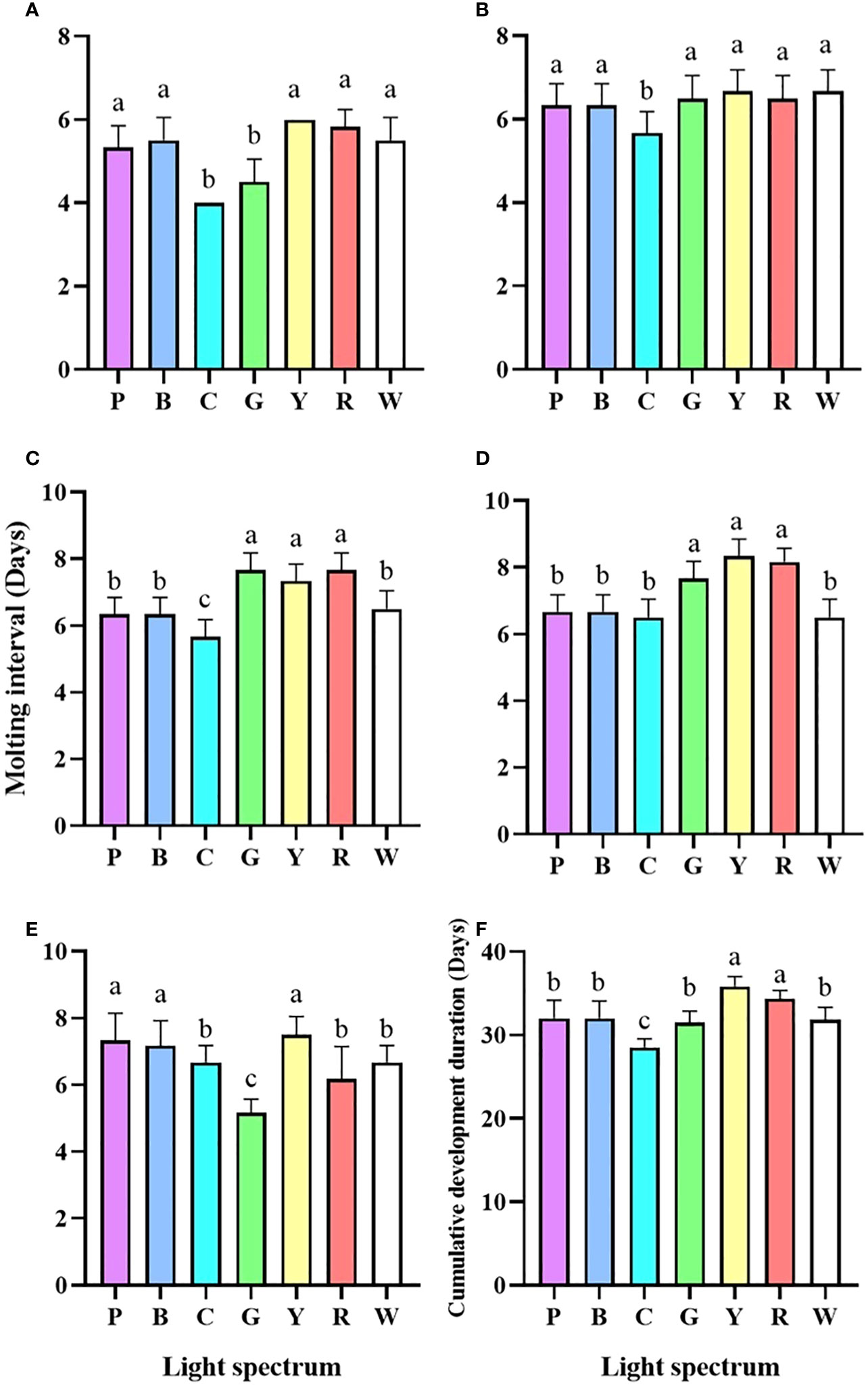
Figure 2 Molting interval (Zoea I - Zoea II) (A), Molting interval (Zoea II - Zoea III) (B), Molting interval (Zoea III - Zoea IV) (C), Molting interval (Zoea IV - Megalopa) (D), Molting interval (Megalopa - C1) (E) and Cumulative development duration (F) of P. trituberculatus larvae under different light spectrum (mean ± SD) (n=6). P, Purple; B, Blue; C, Cyan; G, Green; Y, Yellow; R, Red; W, White. Different letters indicate significant differences between treatment groups (P<0.05).
Effect of light spectrum on respiratory metabolism and antioxidant stress
As larvae developed, OCR significantly decreased (Figure S1). Zoea I to Zoea III had a considerably higher AER under red light compared to the other treatment (P<0.05) (Figures S2A-C). As a result, the early larvae had the lowest O:N ratio when exposed to red light (Figures 3A-C). Zoea IV has the highest O:N ratio under yellow light (Figure 3D). However, at the end of the experiment, the lowest O:N ratio of megalopa was found in the green light, which was significantly lower than those in the purple light (Figure 3E).
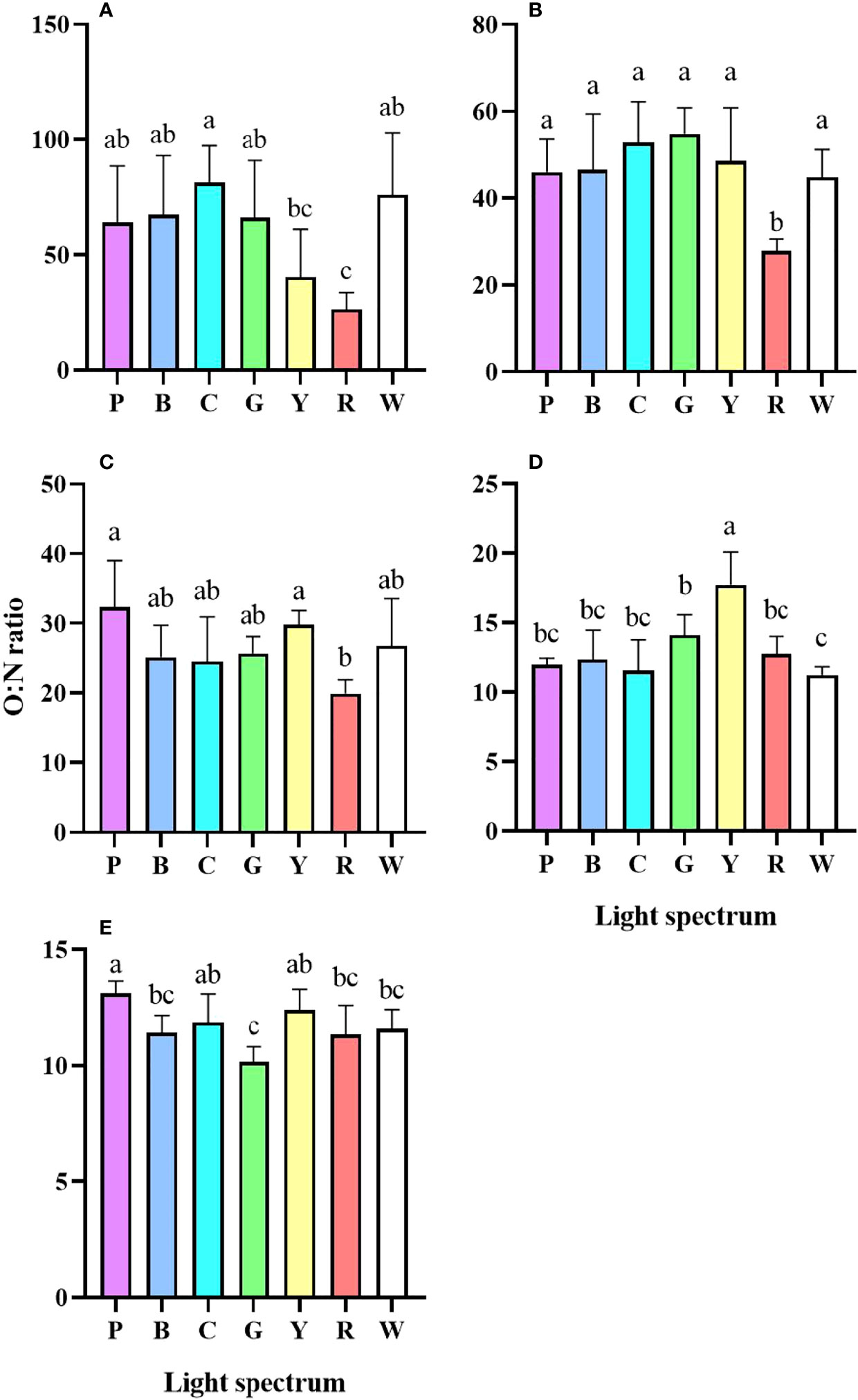
Figure 3 Oxygen-nitrogen ratio (O:N ratio) of P. trituberculatus larvae under different light spectrum (mean ± SD) (n=4). (A) Zoea I, (B) Zoea II, (C) Zoea III, (D) Zoea IV, (E) Megalopa. P, Purple; B, Blue; C, Cyan; G, Green; Y, Yellow; R, Red; W, White. Different letters indicate significant differences between treatment groups (P<0.05).
For the antioxidant capacity, under cyan light, the SOD and CAT activity of the crab was considerably reduced (P<0.05) (Figures 4A, B), while the T-AOC was elevated (Figure 4C). Crab under purple light had the lowest T-AOC activity (Figure 4C) and the group also had high H2O2 accumulation (P<0.05) (Figure 4D). In comparison to the cyan, green, and yellow light groups, the larvae exposed to purple and red light had higher levels of MDA (P<0.05) (Figure 4E).
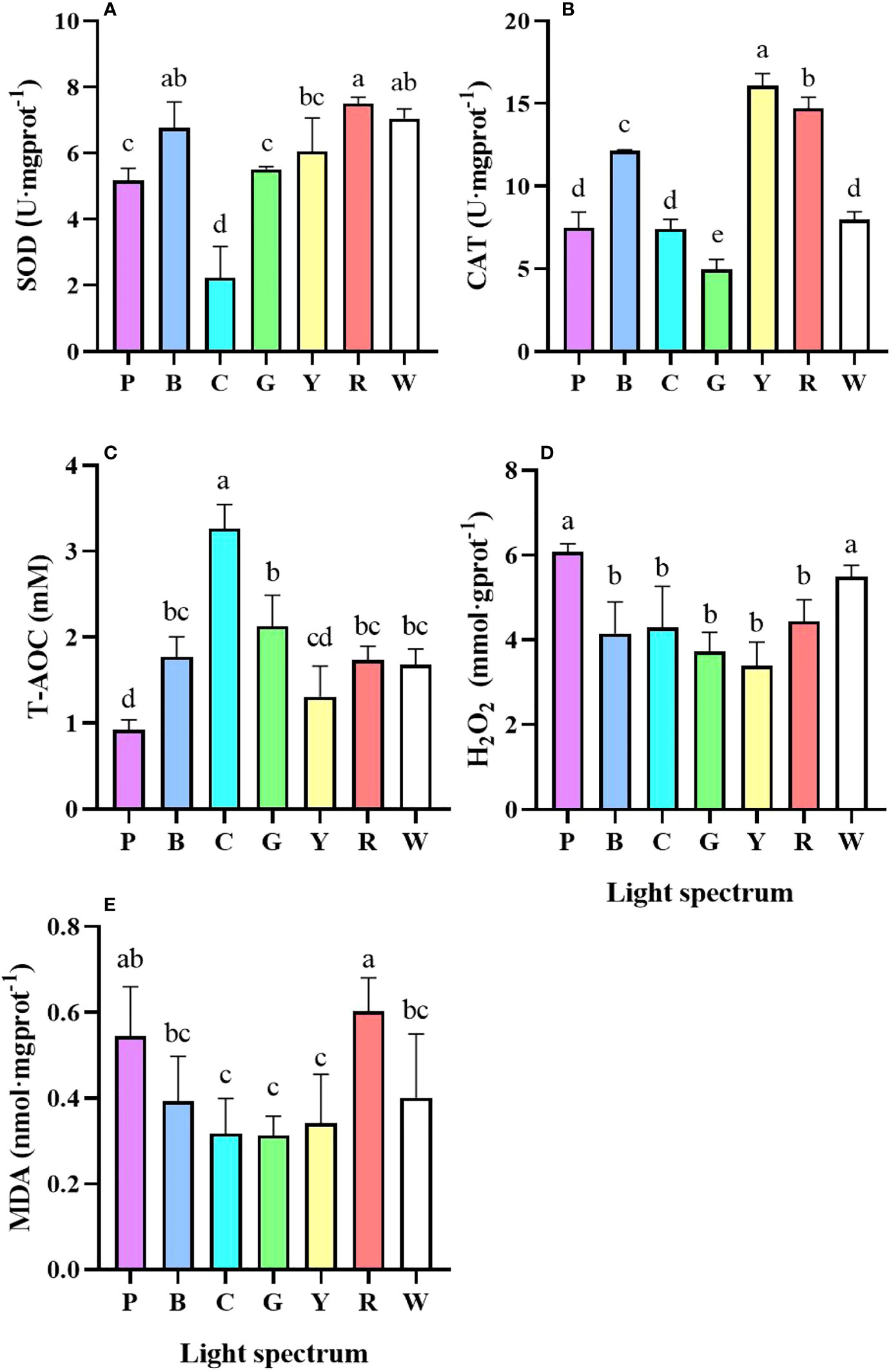
Figure 4 The effect of light spectrum on the antioxidant capacity of P. trituberculatus larvae (n = 6). (A) SOD, (B) CAT, (C) T-AOC, (D) H2O2, (E) MDA. P, Purple; B, Blue; C, Cyan; G, Green; Y, Yellow; R, Red; W, White. Different letters indicate significant differences (P<0.05).
Discussion
Light spectrum substantially influences the growth and molting of crustaceans (Wang et al., 2003; Guo et al., 2012; Fei et al., 2020). In the present study, the survival rate of larvae treated with cyan light in the current study was much greater than that of the other groups. The wavelength of cyan light was between blue and green light. Similarly, shortwave light as cyan and blue light also positively affected S. paramamosain growth performance (Chen et al., 2022). In contrast, the survival rate of larvae treated with red light was significantly lower. However, a previous study suggested that though the P. trituberculatus fed more frequently under blue light, those under yellow and red light had a better growth performance (Wang et al., 2014). This could be attributed to two reasons, i) in Wang’s study, the crab used were subadult P. trituberculatus, and ii) the light source used Wang’s study was fluorescent lamps, which has a wider light spectrum compared with LED (Kim et al., 2015).
The O: N ratio has been frequently used to identify the oxidative metabolic substrates (Dall and Smith, 1986). The catabolism of pure protein yields 3–16 O: N. High O:N denotes enhanced catabolism of lipids and carbonhydrate, 50–60 O: N denotes similar amounts of protein and lipids were used as metabolic substrates (Mayzaud and Conover, 1988). As the larvae developed, the OCR and the O: N continued to fall, indicates that the larvae’s metabolic substrate shifted to protein. In the present study, a significant low O: N ratio of early larvae was observed under red light. The result suggests that the larvae mobilized more proteins during respiratory metabolism in under red light, leading to suboptimal survival rate and development.
Unsuitable lighting conditions have been reported to cause xidative stress in crustaceans (Chen et al., 2021; Dou et al., 2021; Xu et al., 2022a; Chen et al., 2022). In this study, crab under white, purple and red light showed not only high levels of SOD and CAT, but also higher H2O2 and MDA, indicating substantial oxidative stress over activated antioxidase. On the contrary, for cyan and green light, T-AOC activity of the crab was significantly increased, while MDA and H2O2 decreased. These results suggest the crab had a better adaptability to these spectrum.
Conclusion
In this research, we looked into how the light spectrum affected the growth, respiratory metabolism, and antioxidant capability of P. trituberculatus larvae. Larvae showed the best growth performance and lower oxidative stress damage under cyan light. On the contrary, yellow and red light resulted in a low survival rate by altering respiratory metabolism and antioxidant pathways. Overall, these findings demonstrated that cyan light was the optimal spectrum for larval P. trituberculatus.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
FZ, SZ and ZR: Investigation, Data curation, Visualization, Writing - original draft. CM, ChS, CW and YY: Review & editing, Funding acquisition. CeS: Conceptualization, Writing - review & editing, Project administration, Funding acquisition. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 31972783, 41776164, 41676140), the National Key Research and Development Program of China (Project No. 2019YFD0901000), the Province Key Research and Development Program of Zhejiang (2021C02047), Key Scientifific and Technological Grant of Zhejiang for Breeding New Agricultural Varieties (2021C02069-6), 2025 Technological Innovation for Ningbo (2019B10010), China Agriculture Research System of MOF and MARA, SanNongLiuFang Zhejiang Agricultural Science and Technology Cooperation Project (2021SNLF029), K. C. Wong Magna Fund in Ningbo University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.1071469/full#supplementary-material
References
Aguirre J., Ríos-Momberg M., Hewitt D., Hansberg W. (2005). Reactive oxygen species and development in microbial eukaryotes. Trends Microbiol. 13 (3), 111–118. doi: 10.1016/j.tim.2005.01.007
Boeuf G., Le Bail P. Y. (1999). Does light have an influence on fish growth? Aquaculture 177 (1-4), 129–152. doi: 10.1016/S0044-8486(99)00074-5
Cheng C. L., Flamarique I. N. (2007). Chromatic organization of cone photoreceptors in the retina of rainbow trout: single cones irreversibly switch from UV (SWS1) to blue (SWS2) light sensitive opsin during natural development. J. Exp. Biol. 210 (23), 4123–4135. doi: 10.1242/jeb.009217
Chen S., Migaud H., Shi C., Song C., Wang C., Ye Y., et al. (2021). Light intensity impacts on growth, molting and oxidative stress of juvenile mud crab Scylla paramamosain. Aquaculture 545, 737159. doi: 10.1016/j.aquaculture.2021.737159
Chen S., Shi C., Migaud H., Song C., Mu C., Ye Y., et al. (2022). Light spectrum impacts on growth, molting, and oxidative stress response of the mud crab Scylla paramamosain. Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.840353
Craig P. M., Wood C. M., McClelland G. B. (2007). Oxidative stress response and gene expression with acute copper exposure in zebrafish (Danio rerio). Am. J. Physiology-Regul. Integr. Comp. Physiol. 293 (5), R1882–R1892. doi: 10.1152/ajpregu.00383.2007
Dall W., Smith D. M. (1986). Oxygen consumption and ammonia-n excretion in fed and starved tiger prawns, penaeus esculentus haswell. Aquaculture 55 (1), 23–33. doi: 10.1016/0044-8486(86)90052-9
Dou J., Zhang G., Shi C., Song C., Mu C., Ye Y., et al. (2021). High-intensity light of full-spectrum LED promotes survival rate but not development of the larval swimming crab Portunus trituberculatus. Aquacult. Eng. 93, 102158. doi: 10.1016/j.aquaeng.2021.102158
Fei F., Gao X., Wang X., Liu Y., Bin H., Liu B. (2020). Effect of spectral composition on growth, oxidative stress responses, and apoptosis-related gene expression of the shrimp, Penaeus vannamei. Aquacult. Rep. 16, 100267. doi: 10.1016/j.aqrep.2019.100267
Fisheries Bureau of Agriculture Ministry of China (2021). China Fisheries statistical yearbook (Beijing: China Agriculture Press).
Guo B., Mu Y., Wang F., Dong S. (2012). Effect of periodic light color change on the molting frequency and growth of Litopenaeus vannamei. Aquaculture 362, 67–71. doi: 10.1016/j.aquaculture.2012.07.034
Hoang T., Barchiesis M., Lee S. Y., Keenan C. P., Marsden G. E. (2003). Influences of light intensity and photoperiod on moulting and growth of Penaeus merguiensis cultured under laboratory conditions. Aquaculture 216 (1-4), 343–354. doi: 10.1016/S0044-8486(02)00460-X
Hoang T., Lee S. Y., Keenan C. P., Marsden G. E. (2002). Spawning behaviour of Penaeus (Fenneropenaeus) merguiensis de man and the effect of light intensity on spawning. Aquacult. Res. 33 (5), 351–357. doi: 10.1046/j.1365-2109.2002.00685.x
Jacob R. A. (1995). The integrated antioxidant system. Nutr. Res. 15 (5), 755–766. doi: 10.1016/0271-5317(95)00041-G
Kim J. K., Mao Y., Kraemer G., Yarish C. (2015). Growth and pigment content of Gracilaria tikvahiae McLachlan under fluorescent and LED lighting. Aquaculture 436, 52–57. doi: 10.1016/j.aquaculture.2014.10.037
Leiva F. P., Urbina M. A., Cumillaf J. P., Gebauer P., Paschke K. (2015). Physiological responses of the ghost shrimp Neotrypaea uncinata (Milne edwards 1837)(Decapoda: Thalassinidea) to oxygen availability and recovery after severe environmental hypoxia. Comp. Biochem. Physiol. Part A.: Mol. Integr. Physiol. 189, 30–37. doi: 10.1016/j.cbpa.2015.07.008
Liu J., Shi C., Ye Y., Ma Z., Mu C., Ren Z., et al. (2022). Effects of temperature on growth, molting, feed intake, and energy metabolism of individually cultured juvenile mud crab Scylla paramamosain in the recirculating aquaculture system. Water 14 (19), 2988. doi: 10.3390/w14192988
Lushchak V. I. (2011). Environmentally induced oxidative stress in aquatic animals. Aquat. Toxicol. 101 (1), 13–30. doi: 10.1016/j.aquatox.2010.10.006
Makrinos D. L., Bowden T. J. (2016). Natural environmental impacts on teleost immune function. Fish. Shellfish. Immunol. 53, 50–57. doi: 10.1016/j.fsi.2016.03.008
Malandrakis E. E., Exadactylos A., Dadali O., Golomazou E., Klaoudatos S., Panagiotaki P. (2014). Molecular cloning of four glutathione peroxidase (GPx) homologs and expression analysis during stress exposure of the marine teleost Sparus aurata. Comp. Biochem. Physiol. Part B.: Biochem. Mol. Biol. 168, 53–61. doi: 10.1016/j.cbpb.2013.11.005
Mayzaud P., Conover R. J. (1988). O: N atomic ratio as a tool to describe zooplankton metabolism. Mar. Ecol. Prog. Series. Oldendorf. 45 (3), 289–302. doi: 10.3354/meps045289
Migaud H., Davie A., Taylor J. F. (2010). Current knowledge on the photoneuroendocrine regulation of reproduction in temperate fish species. J. Fish. Biol. 76 (1), 27–68. doi: 10.1111/j.1095-8649.2009.02500.x
Oliva M., Vicente J. J., Gravato C., Guilhermino L., Galindo-Riaño M. D. (2012). Oxidative stress biomarkers in Senegal sole, Solea senegalensis, to assess the impact of heavy metal pollution in a huelva estuary (SW spain): seasonal and spatial variation. Ecotoxicol. Environ. Saf. 75, 151–162. doi: 10.1016/j.ecoenv.2011.08.017
Pierce L. X., Noche R. R., Ponomareva O., Chang C., Liang J. O. (2008). Novel functions for period 3 and exo-rhodopsin in rhythmic transcription and melatonin biosynthesis within the zebrafish pineal organ. Brain Res. 1223, 11–24. doi: 10.1016/j.brainres.2008.05.020
Shi C., Wang J., Peng K., Mu C., Ye Y., Wang C. (2019). The effect of tank colour on background preference, survival and development of larval swimming crab Portunus trituberculatus. Aquaculture 504, 454–461. doi: 10.1016/j.aquaculture.2019.01.032
Wang F., Dong S., Huang G., Wu L., Tian X., Ma S. (2003). The effect of light color on the growth of Chinese shrimp Fenneropenaeus chinensis. Aquaculture 228 (1-4), 351–360. doi: 10.1016/S0044-8486(03)00312-0
Wang F., Wang X., Liu D. T., Dong S. L. (2014). Effects of light color on growth and energy budget of swimming crab Portunus trituberculatus. Periodical. Ocean. Univ. China 44 (11), 25–29.
Wei J., Tian L., Wang Y., Yu L., Zhu X. (2021). Effects of salinity, photoperiod, and light spectrum on larval survival, growth, and related enzyme activities in the giant freshwater prawn, Macrobrachium rosenbergii. Aquaculture 530, 735794. doi: 10.1016/j.aquaculture.2020.735794
Wu C., Cao F., Lu Z., Chen L., Ye J. (2016). Molecular cloning, characterization and mRNA expression of six peroxiredoxins from black carp Mylopharyngodon piceus in response to lipopolysaccharide challenge or dietary carbohydrate. Fish. Shellfish. Immunol. 50, 210–222. doi: 10.1016/j.fsi.2016.01.033
Wu X., Cheng Y., Zeng C., Wang C., Cui Z. (2010). Reproductive performance and offspring quality of the first and the second brood of female swimming crab, portunus trituberculatus. Aquaculture 303 (1-4), 94–100. doi: 10.1016/j.aquaculture.2010.03.006
Wu L., Wang Y., Han M., Song Z., Song C., Xu S., et al. (2020). Growth, stress and non-specific immune responses of turbot (Scophthalmus maximus) larvae exposed to different light spectra. Aquaculture 520, 734950. doi: 10.1016/j.aquaculture.2020.734950
Xu H., Dou J., Wu Q., Ye Y., Song C., Mu C., et al. (2022a). Investigation of the light intensity effect on growth, molting, hemolymph lipid, and antioxidant capacity of juvenile swimming crab Portunus trituberculatus. Front. Mar. Sci. 851. doi: 10.3389/fmars.2022.922021
Xu H., Dou J., Wu Q., Ye Y., Song C., Mu C., et al. (2022b). Photoperiod affects the survival rate but not the development of larval swimming crab Portunus trituberculatus. Aquacult. Int. 30, 1769–1778. doi: 10.1007/s10499-022-00875-x
Keywords: swimming crab, light spectrum, respiratory metabolism, antioxidant capacity, development
Citation: Zhang F, Zhang S, Ren Z, Song C, Ye Y, Mu C, Wang C and Shi C (2022) Light spectrum impacts on development respiratory metabolism and antioxidant capacity of larval swimming crab Portunus trituberculatus. Front. Mar. Sci. 9:1071469. doi: 10.3389/fmars.2022.1071469
Received: 16 October 2022; Accepted: 26 October 2022;
Published: 10 November 2022.
Edited by:
Xiaolong Gao, Xiamen University, ChinaReviewed by:
Baoquan Gao, Yellow Sea Fisheries Research Institute (CAFS), ChinaBin Wen, Shanghai Ocean University, China
Copyright © 2022 Zhang, Zhang, Ren, Song, Ye, Mu, Wang and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhiming Ren, renzhiming@nbu.edu.cn; Ce Shi, shice3210@126.com
 Feifei Zhang
Feifei Zhang Shuai Zhang1,3,4
Shuai Zhang1,3,4  Zhiming Ren
Zhiming Ren Yangfang Ye
Yangfang Ye Changkao Mu
Changkao Mu Chunlin Wang
Chunlin Wang Ce Shi
Ce Shi