Cucumaria in Russian Waters of the Barents Sea: Biological Aspects and Aquaculture Potential
- Murmansk Marine Biological Institute, Murmansk, Russia
Sea cucumbers are a popular luxury and delicacy food items in Asian markets. These echinoderms possess a wide range of bioactive substances that can be used to produce pharmaceutical products. Recent depletion of natural populations of sea cucumbers requires involving new objects both in commercial harvesting and aquaculture. The northern sea cucumber Cucumaria frondosa is the most abundant sea cucumber in the Barents Sea. In this paper, we summarized literature data on the biology of this polar species to evaluate its fishery and aquaculture potential in the area. This eurythermic sea cucumber is typically occurs at 20–100 m depth. Cucumaria mainly colonize rocky or pebbly bottoms. Their main food items are detritus, pellets, phytoplankton, and small planktonic crustaceans. Spawning is registered in February–May. The age of commercial specimens (body length 25–30 cm, wet weight 300–350 g) is 10 years. The most abundant stocks of C. frondosa are registered in the central and south-eastern parts of the sea. Due to the low growth rate of Cucumaria the most appropriate cultivation method for these holothurians is a combination of larval culture and sea ranching. Coastal sites of the Barents Sea merit all the criteria for sea ranching of Cucumaria, but the development of their extensive aquaculture requires significant investments with long pay-back periods.
Introduction
The Barents Sea is a large marine ecosystem with a wide range of environmental conditions resulting from interactions of the cold Arctic and warm Atlantic waters (Dvoretsky and Dvoretsky, 2013, 2018a, 2019). This region is considered to be the most productive shelf region of the Arctic (Wassmann et al., 2006). In Russian waters of the Barents Sea, traditional marine fisheries are focused on two main fish species including Northeast Arctic cod Gadus morhua and Northeast Arctic haddock Melanogrammus aeglefinus (Dvoretsky and Dvoretsky, 2015) with annual catch rates of 305,500 and 88,000 t, respectively (Anonymous, 2021). Two non-indigenous crabs (the red king crab Paralithodes camtschaticus and the snow crab Chionoecetes opilio) provide commercial stocks for Russian trap fisheries with relatively high total annual catches (10,820 t for king crabs and 13,202 t for snow crabs) (Dvoretsky and Dvoretsky, 2015, 2018b, 2021; Bakanev and Pavlov, 2021).
Non-traditional marine resources of the Barents Sea include mollusks and echinoderms. Russian small-scale fisheries focused on the Iceland scallop Chlamys islandica (annual landings 100 t per year) and the green sea urchin Strongylocentrotus droebachiensis (<500 t) have a negligible economic income for the region (Dvoretsky and Dvoretsky, 2020b). Other potential candidates for fishery are blue mussels and sea cucumbers (Dvoretsky and Dvoretsky, 2016; Matishov, 2016).
Sea cucumbers are highly marketable echinoderms (Han et al., 2016). Being a good food product the flesh of these marine animals also contains a wide range of bioactive substances that can be used in the pharmaceutical industry (Hamel and Mercier, 2008; Han et al., 2016). Among holothurian species living in the Barents Sea, only the dendrochirotid sea cucumber Cucumaria frondosa has commercial importance due to its relatively large size, wide distribution, and high abundance. Fisheries of sea cucumbers are done by hand using scuba diving or trawling (Conand, 2004; Hamel and Mercier, 2008). The last method, however, can lead to degradation of benthic communities including changes in the seafloor structure, reductions in habitat complexity, and decreases in species richness and abundance (Anderson et al., 2011). In recent decades, sea cucumber fisheries have declined substantially mainly due to overfishing (Purcell et al., 2013).
Aquaculture has been proving to be an excellent alternative to a fishery of sea cucumbers (Mills et al., 2012; Purcell et al., 2012) and other invertebrates (Shumway, 2011; Dvoretsky and Dvoretsky, 2020b). Growing interest in echinoderm products from Asian markets requires involving new species from the north hemisphere to aquaculture (Mercier and Hamel, 2013). Taking into account the importance of the Arctic as a key development priority, Russian President has approved the strategy for the development of the Arctic zone of the Russian Federation and the provision of national security in the period through 2035. Opportunities include further development of all important economic sectors (Anonymous, 2020). In particular, Paragraph i in Section IV of the Strategy declares “the growth of fishery industries including technological re-equipment of local enterprises, construction of new vessels, introduction of new production facilities for full processing of aquatic biological resources and development of aquaculture.” Cucumaria from the Barents Sea are considered potential sources both for fishery and aquaculture in the area (Gudimova, 1998a). For this reason, the aim of our study is to summarize important data on the biology of these commercially important sea cucumbers in the Barents Sea and provide a baseline for the development of their aquaculture in coastal waters of the Kola Peninsula.
Biological Aspects
The sea pumpkin C. frondosa (Gunner, 1767) is a member of the phylum Echinodermata, class Holothurioidea, order Dendrochirotida, and family Cucumariidae. This sea cucumber has a worm-like soft body with relatively large skin thickness (0.5 cm). Size of adult animals can reach 20–40 cm (Gudimova, 1998a). The color of C. frondosa varies from light purple to dark brown depending on depth, habitat, sex, and age (Hamel and Mercier, 2008).
Distribution
Cucumaria frondosa occurs in the North Atlantic from the Arctic to Cape Cod and from the Arctic to the northern latitudes of the United Kingdom, in Iceland, in the North Sea (to the south of the Dogger Bank) and along the coast of Greenland, in the Barents and Norwegian Seas (Gudimova, 1998a; Hamel and Mercier, 2008). Occasionally, this species was registered in the southwestern Kara Sea and in coastal waters of the White Sea (Gudimova, 1998a, 2000). In the Barents Sea, the northern border of C. frondosa is registered near Franz Josef Land, while the eastern limit is the Kara Gate (south of Novaya Zemlya). The sea cucumbers also occur on Spitsbergen Bank, at the area from Kharlov Island to Cape Svyatoy Nos, at Murmansk Shallowness, North Kanin Bank and on Goose Bank (Gudimova, 2000).
In the Barents Sea, the distribution of Cucumaria is determined by a variety of environmental conditions. This species is typically distributed between 20 and 100 m (Gudimova, 1999). Small animals (body length <15 cm) prefer tide pools of the lower intertidal zone (3–15 m) (Propp, 1971). Abundant concentrations of larger sea cucumbers usually occur at 100 m depth (Gudimova, 1998a, 1999). Occasional findings of C. frondosa were registered at deep-water sites (250 m). The northern sea cucumber is a eurythermic species living at a temperature diapason from –2°C to +10°C (Gudimova, 1998a). The most abundant aggregations are registered at temperatures –1°C to +4°C. However, water temperature is not the only factor determining the distribution of C. frondosa. The species has low salinity tolerance (30–35 psu) and can survive only short-term salinity fluctuations typical to the tidal zone of the Barents Sea in spring (Gudimova, 1998a, 1999). In these stress conditions, however, the animals do not feed (Gudimova, 1998a). Cucumaria mainly colonize rocky or pebbly bottoms at coastal sites of the Kola Peninsula, although they have been observed on sandy bottoms on slopes of banks and troughs in the open sea areas (Gudimova, 1998a, 1999). Recently, this species has been reported as an epibiont of red king crab (Dvoretsky and Dvoretsky, 2021).
Bottom topography and velocity of currents also play an important role in distribution of Cucumaria because these factors affect the attachment behavior and food habitats of these animals.
Feeding
Dendrochirotid sea cucumbers are mainly sessile (Hamel and Mercier, 2008). They use ten ramified tentacles distributed around the mouth to capture particles suspended in the water column. Feeding is considered to be optimal at flow velocities from 55 to 130 cm s–1 (Holtz and MacDonald, 2009). Cucumaria exhibit a seasonal feeding rhythm that starts in March and ends in October. The feeding activity is directly correlated to high tide, increasing water temperature, and day length (Singh et al., 1999). In the Barents Sea, their main food items are detritus, pellets, mineral particles, phytoplankton (the microalgae Fragilaria oceanica and Coscinodiscus concinnus), fragments of macroalgae and tiny animalcules of the zooplankton (predominantly crustaceans) (Gudimova, 1998a; Gudimova et al., 2004). The most preferable feeding habits of C. frondosa are scallop beds. In the coastal Barents Sea, this species also prefers the communities dominated by calcareous red algae Lithothamnion spp. and barnacles Balanus spp. (Propp, 1971).
Reproduction
Sex ratio of C. frondosa is close to 1:1. Sexual dimorphism in Cucumaria is not expressed and the gonad analysis is required to sex determination. An unpaired gonad of C. frondosa consists of two bundles of unbranched tubules (500 tubules in medium- and 700 tubules in large-sized animals) (Gudimova, 1998a). The typical colors of female gonads are dark red and brown, while male gonads are bright pink and purple. The reproductive cycle of C. frondosa is characterized by an annual spawning and a generally highly synchronized gamete release (Gudimova et al., 2004). Gametogenesis in the Barents Sea Cucumaria is clearly initiated by an increase in day length while spawning appears to be triggered by a mix of factors, including tide cycles, phytoplankton concentrations, and diet components (Gudimova, 1998a, 1999). Specialists from Murmansk Marine Biological Institute (MMBI, Murmansk, Russia) found that the oocytes are 0.65 mm in diameter and that the fecundity varies between 60,000 and 150,000 oocytes (Gudimova, 1998a, 1999). Annual levels of the gonad index are higher than 20%, and the maximum (50%) is registered in June-July (Supplementary Figure 1). Spawning period of C. frondosa in the Barents Sea is registered from February to May with a peak from April to May (Gudimova, 1998a; Antsiferova, 2007, 2008). Settlement occurs predominantly on rocky bottoms or pebbles, usually in crevices located in the shaded area under hard substrata (Gudimova, 1998a).
Growth
The growth rate of C. frondosa is extremely slow (Supplementary Figure 2). Data gathered in the coastal Barents Sea show that a 25–30-cm size of commercial sea cucumbers is reached after approximately 10 years of growth (Gudimova, 1998a). The weight of such specimens is 300–350 g. The maximum age of C. frondosa is 22 years (Gudimova et al., 2004). The main factors affecting the Cucumaria growth rates are water temperature and concentrations of phytoplankton (Hamel and Mercier, 1996).
Stocks and Products
There are two abundant stocks of C. frondosa in the Barents Sea: (i) Spitsbergen Bank (central part of the sea), and (ii) south-eastern part of the sea (Figure 1).
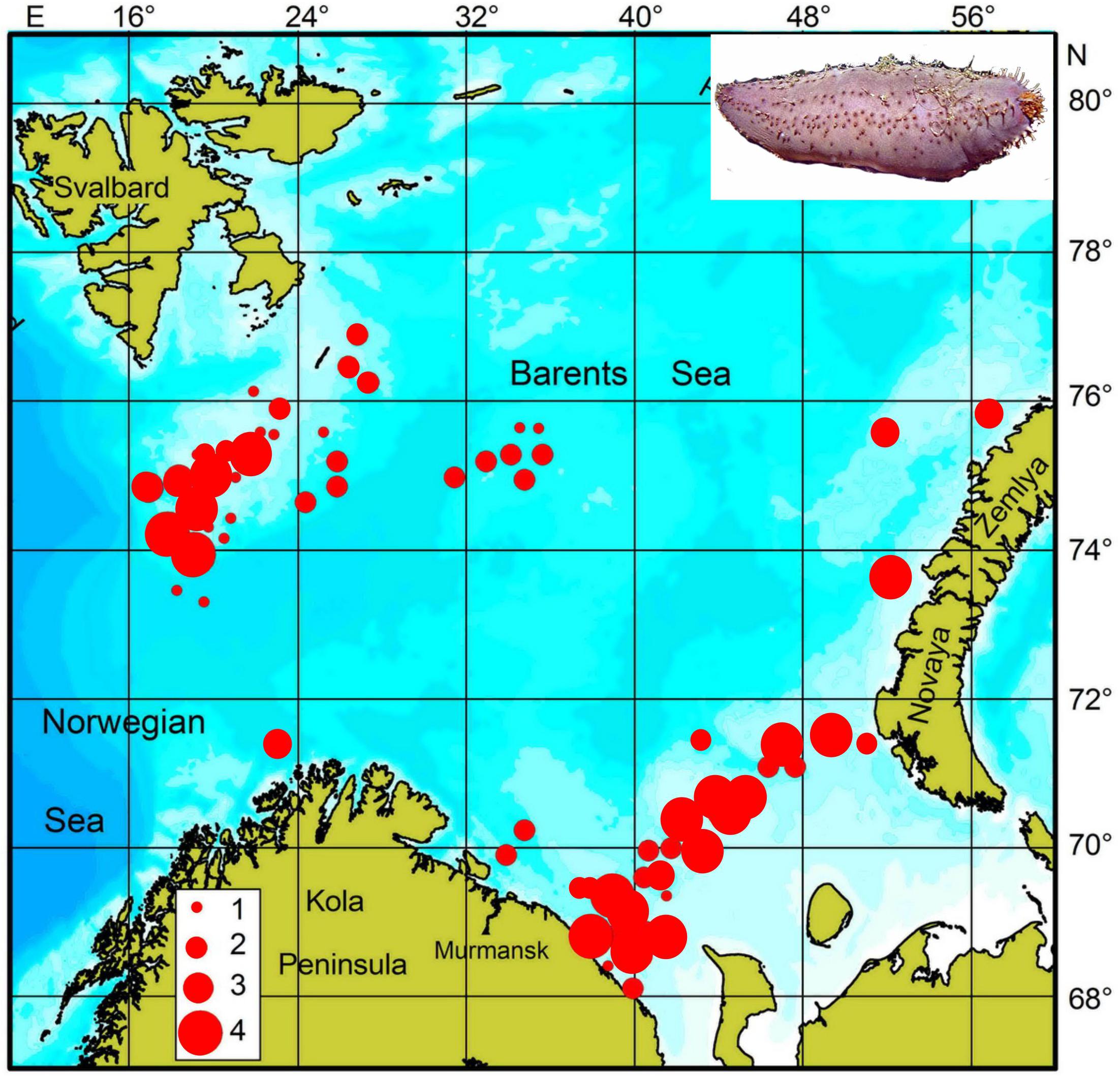
Figure 1. Distribution of Cucumaria frondosa in the central part (Spitsbergen Bank) and in the south-eastern part of the Barents Sea (integrated data from scientific trawl surveys conducted in the 1980–1990s and 2005–2015). 1 – <10 g⋅m–2, 2 – 10–30 g⋅m–2, 3 – 30–70 g⋅m–2, 4 – >70 g⋅m–2 (adopted from Gudimova, 1998a; Zakharov et al., 2018).
Within the boundaries of these clusters the square of sites colonized by Cucumaria varies from 600 to 3,000 km2 (Figure 1; Gudimova,1998a,b). The maximum biomass of the sea cucumbers is 400–500 g⋅m–2, mean value is 10–20 g⋅m–2. The total stocks of Cucumaria were estimated to be 150,000 ± 30,000 t on Spitsbergen Bank and 215,000 ± 50,000 t in the south-eastern Barents Sea (Gudimova,1998a,b).
The main products derived from C. frondosa are the muscle bands and the boiled and dried body wall (Gudimova, 1998a; Hamel and Mercier, 2008). The organs and muscles are typically boiled, dried and salted before export, while lesser proportions are marketed as frozen, pickled or live products (Hamel and Mercier, 2008; Mercier and Hamel, 2013). Now the price per kilogram of processed C. frondosa can vary from $20–$360 US depending on the thickness and texture of the body wall, skin color as well as processing integrity (Hossain et al., 2020). Although the muscle of Cucumaria contains less protein than the meat of mollusks and crustaceans (Kizevetter, 1962; Nasedkina et al., 1973) it is much richer in minerals (P, Ca, Mg, I, Fe, Mn, Cu) and vitamins (thiamine, riboflavin) (Supplementary Table 1). The most common amino acids are alanine, glutamine, and leucine (Supplementary Table 2). The levels of amino acids in C. frondosa from the Barents Sea differ significantly in comparison to the Gulf of St. Lawrence (Supplementary Table 2) reflecting different habitat conditions in these areas.
Since a lot of valuable biochemical components such as monosulfated triterpenoid glycoside Frondoside A, disulfated glycoside Frondoside B, trisulfated glycoside Frondoside C, eicosapentaenoic acid, fucoidan, 12-methyltetradecanoic acid, cerebrosides, and fucosylated chondroitin sulfate were reported in C. frondosa (Avilov et al., 1998; Janakiram et al., 2015; Hossain et al., 2020), this species has a promising potential for the pharmaceutical industry as well. Body wall, tentacles, muscles, eggs, and coelomocytes of C. frondosa were found to have antibacterial, anticoagulant, antiaging, and anti-hyperglycemic properties (Haug et al., 2002) whereas the anti-oxidant and anti-fatigue activity was registered for the gonads, respiratory structures, and digestive tract (Mamelona et al., 2007; Zhong et al., 2007). Metabolites of C. frondosa were reported as anti-cancer and anti-inflammatory agents (Bruckner, 2005; Janakiram et al., 2015).
Aquaculture
Experimental Studies
An experimental study was conducted by specialists from MMBI to analyze the feeding behavior of C. frondosa. A total of 12 sea cucumbers (weight range 20–250 g) were collected in the wild. Juveniles were harvested from Laminaria kelps in a subtidal zone while adult animals were captured by trawls at 80–90 m depth in the Sem Ostrovov area (68°47′11″ N, 37°25′37″ E). The sea cucumbers were held in aquariums. Each aquarium was supplied with seawater at a rate of 1,200–1,400 ml⋅min–1. The acclimation period lasted for 2 weeks. Feeding intensity was estimated as the total number of animals that fed simultaneously. This index was found to be closely associated with variations of water temperature during the tidal cycle. An increase in water temperature led to a decrease in feeding intensity. Since there were no salinity fluctuations in this experiment, the pattern observed was more likely associated with a reaction of Cucumaria on a decreased water density (Gudimova, 1998a). Experiments by Singh et al. (1999) showed that the rate of tentacle insertions increases with increasing seston chloropigment concentration but decreases with increasing current speed. Canadian authors found that Cucumaria can capture relatively large food items such as fish eggs (diameter 1,500 m) that make this food source a promising candidate for aquaculture of northern sea cucumbers (Gianasi et al., 2017).
A number of observations were undertaken to reveal growth patterns in tagged juvenile sea cucumbers (weight 3.03–90.43 g) reared in the same conditions as described above. A one-year study showed that some animals demonstrated zero or even negative growth of their weight, while other animals had as high growth rates as 80–140% (Gudimova, 1998a). These results indicated high individual variability in growth rates of Cucumaria at different seasons. In addition, environmental conditions such as water temperature, current velocity, light regime, and food availability could affect growth in C. frondosa (Gudimova, 1998a, 1999). For comparison, in the St. Lawrence Estuary, the average growth rate of reared Cucumaria was 2 mm per month, with the fastest monthly growth of 5–6 mm occurring in the spring–summer period (from May to June) (Hamel and Mercier, 1996). Recent Canadian studies have shown that adult Cucumaria are not photosensitive and they do not exhibit any preference for either illuminated or shaded areas (Sun et al., 2020).
Perspectives
Taking into account short metamorphosis and relatively high survival rate of larvae as well as the slow growth rate and low mobility of C. frondosa, the most appropriate cultivation method for this species in the Barents Sea is accepted to be a combination of larval culture and sea ranching of juvenile and adult sea cucumbers (Gudimova, 1998a).
The process of artificial reproduction of juvenile seed-stock includes broodstock collection and maintenance, spawning, larval development, and nursery of juveniles (Figure 2). The method for cultivation of the Japanese sea cucumber Apostichopus japonicus developed by specialists from Pacific Research Fisheries Center (TINRO Center, Vladivostok, Russia) (Gavrilova, 2005; Gavrilova et al., 2005, 2006; Gavrilova and Kucherjavenko, 2010) could be adopted for C. frondosa. This commercial rearing system includes pumps, tanks for sea cucumbers, filters, and UF-equipment (Figure 2).
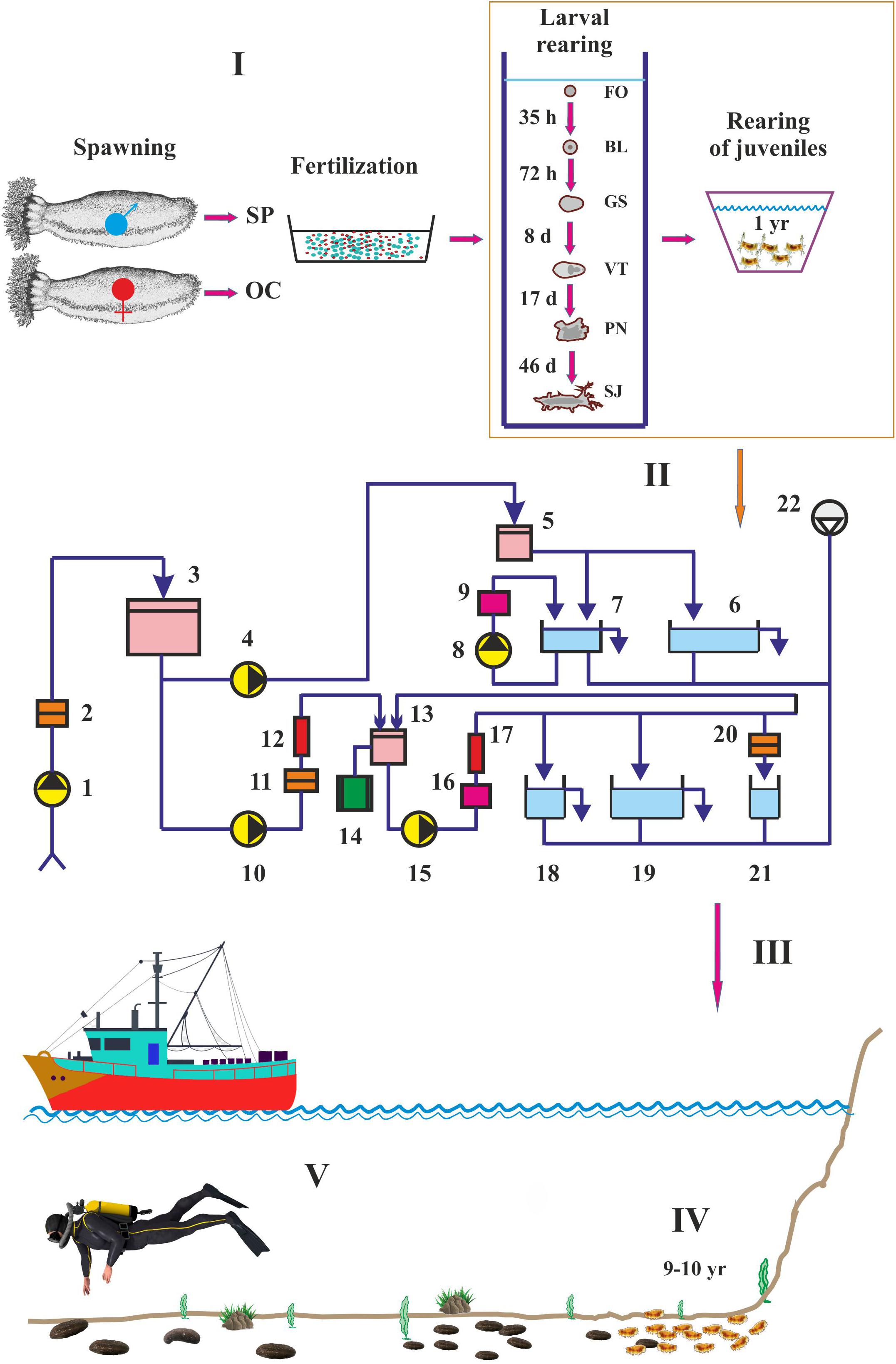
Figure 2. Cultivation of Cucumaria frondosa. I – fertilization under laboratory conditions, II – schematic diagram of the TINRO commercial rearing system, III – transfer of juveniles on the seafloor, IV – sea ranching, V – harvesting (adopted from Hamel and Mercier, 1996; Gavrilova et al., 2006). SP, sperm, OC, oocytes. FO, fertilized oocytes, BL, blastula, GS, gastrula, VT, vitellaria, PN, pentactula, SJ, settled juvenile. 1, 4, 8, 10, 15 – pump for seawater; 2 – filter (pore diameter 150–200 μm); 3 – septic tank; 5, 13 – water can; 6 – holding tank for juveniles; 7 – broodstock tank; 9, 16 – chiller, 11 – micro-filter (pore diameter 20–40 μm); 12, 17 – UV-sterilizer with ozonation; 14 – heater with thermoregulation; 18 – tank for spawning and fertilization; 19 – rearing tank for larvae; 20 – ultra-filter (pore diameter 0.1–0.2 μm); 21 – tank for food items; 22 – aerator.
The system works as follows (Gavrilova et al., 2005). Before entering the tanks for cultivation, seawater should first be filtered and allowed to drainage in septic tanks for 12–48 h. The water should be heated or cooled, aerated, ozonated and sterilized with ultraviolet. Broodstock sea cucumbers are collected when individuals reach sexual maturity (weight 300–350 g). The females spawn after a period of acclimation to adapt to new conditions. Live phytoplankton have been found to be the most suitable spawning trigger for C. frondosa whereas desiccation, thermal shock, potassium chloride, and serotonin did not induce spawning in this species (Gianasi et al., 2019). After spawning and fertilization, the larvae proceed through the gastrula, blastula, vitellaria, and pentactula stages in the rearing tank (Hamel and Mercier, 1996). The larvae are fed both artificial and natural diets. The settled juveniles are held in a holding tank with artificial attachment substrates such as corrugated polyvinyl chloride plates. The juveniles are fed with microalgae for 12 months. The second stage of the Cucumaria farming is sea ranching in bottom closures where juveniles with a body length of 3 mm are released and adults are harvested after reaching a commercial size (Gudimova, 1998a). Alternatively, the juveniles could be used in the stock enhancement of depleted natural populations. Yields of Cucumaria from intensive ocean-based systems could be increased considerably by using additional food items. Although natural seston, microalgae diets, and fish eggs could be consumed successfully by C. frondosa (Nelson et al., 2012; Gianasi et al., 2017), these items have low relevance for sea ranching due to seasonal fluctuations in their abundance in the case of the natural seston and high prices in the case of fish eggs and commercial shellfish diets. More promising results have been recently reported by Yu et al. (2020) for the brown seaweeds Ascophyllum nodosum and Saccharina latissima. Both species are very common in the coastal Barents Sea (Malavenda et al., 2017) and can be explored as diets for intensive aquaculture of Cucumaria.
Since environmental conditions at coastal sites of the Kola Peninsula are quite suitable for C. frondosa (Dvoretsky and Dvoretsky, 2018b, 2020a), the area has a good potential for aquaculture of this sea cucumber and other echinoderms.
The development of extensive sea cucumber aquaculture is a great challenge for Polar regions of Russia because of environmental limitations (low temperatures, pronounced winter, ice cover) and slow growth rates of Cucumaria. For these reasons, a significant investment must be made in this industry and the pay-back period for this investment is as long as 15–20 years.
Conclusion
Although C. frondosa is a widely distributed and intensively studied sea cucumber of the world, the commercial aquaculture of this species has not yet been developed. In the Barents Sea, Cucumaria form two large stocks registered in the central and south-eastern parts of the sea. Spawning period of C. frondosa in is registered from February to May. Size-at-age data obtained from coastal sites of the Kola Peninsula showed very slow growth rate in C. frondosa. Commercial sea cucumbers at a body length of 25–30 cm and wet weight of 300–350 g are 10-year-old. The life span of C. frondosa lasts 22 years. The total abundances of Cucumaria are 150,000 t on Spitsbergen Bank and 215,000 t in the south-eastern part of the sea. A combination of larval culture in rearing tanks and sea ranching at coastal sites is the most appropriate cultivation method for this species in the Barents Sea. Russian scientists and businessmen are interested in developing the technologies for holoturian farming in the Barents Sea but substantial funding is required for this industry. New investors such as large Russian gas and oil companies need to be involved in the establishment of echinoderm aquaculture in the Barents Sea.
Author Contributions
AD conceived the manuscript and wrote the manuscript. VD drew the figures and reviewed the manuscript. Both authors contributed to the manuscript and approved the submitted version.
Funding
This work was supported by the Ministry of Science and Higher Education of the Russian Federation.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We are grateful to the two reviewers for the highly constructive comments that improved the manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2021.613453/full#supplementary-material
References
Anderson, S. C., Flemming, J. M., Watson, R., and Lotze, H. K. (2011). Serial exploitation of global sea cucumber fisheries. Fish. Fish. 12, 317–339. doi: 10.1111/j.1467-2979.2010.00397.x
Anonymous (2020). On the Development the Strategy of the Arctic Zone of the Russian Federation and National Security for the Period Up to 2035. 645. Available online at: http://static.kremlin.ru/media/events/files/ru/J8FhckYOPAQQfxN6Xlt6ti6XzpTVAvQy.pdf (accessed October 26, 2020)
Anonymous (2021). Results of the Activities of the Federal Agency for Fisheries in 2020 and Tasks for 2021. Available online at: http://fish.gov.ru/files/documents/ob_agentstve/kollegiya/itogi_2021.pdf (accessed May 27, 2021).
Antsiferova, A. V. (2007). Reproduction Biology of the Commercial Holoturian Cucumaria Frondosa (Gunner, 1767) From the Barents Sea. Ph. D, Thesis. Moscow: Moscow State Universtity.
Antsiferova, A. V. (2008). Gonad status in the Barents Sea holothurian Cucumaria frondosa in the autumn-winter period. Vestnik MGTU 9, 762–765.
Avilov, S. A., Drozdova, O. A., Kalinin, V. I., Kalinovsky, A. I., Stonik, V. A., Gudimova, E. N., et al. (1998). Frondoside C, a new nonholostane triterpene glycoside from the sea cucumber Cucumaria frondosa: structure and cytotoxicity of its desulfated derivative. Can. J. Chem. 76, 137–141. doi: 10.1139/cjc-76-2-137
Bakanev, S. V., and Pavlov, V. A. (2021). “Snow crab (Chionoecetes opilio),” in Materials of Total Allowable Catches of Water Biological Resources in Fishing Areas in Inland Seas of the Russian Federation, on the Continental Shelf of the Russian Federation, in the Exclusive Economical Zone of the Russian Federation, in the Azov and Caspian Seas in 2022, ed. D. O. Sologub (Murmansk), 17–28.
Bruckner, A. W. (2005). The recent status of sea cucumber fisheries in the continental United States of America. SPC Beche De Mer Inf. Bul. 22, 39–46.
Conand, C. (2004). Present status of world sea cucumber resources and utilisation: an international overview. FAO Fish. Tech. Pap. 463, 13–23. doi: 10.1007/978-3-662-44730-7_3
Dvoretsky, V. G., and Dvoretsky, A. G. (2013). Epiplankton in the Barents Sea: summer variations of mesozooplankton biomass, community structure and diversity. Cont. Shelf Res. 52, 1–11. doi: 10.1016/j.csr.2012.10.017
Dvoretsky, A. G., and Dvoretsky, V. G. (2015). Commercial fish and shellfish in the Barents Sea: have introduced crab species affected the population trajectories of commercial fish? Rev. Fish Biol Fish. 25, 297–322. doi: 10.1007/s11160-015-9382-1
Dvoretsky, A. G., and Dvoretsky, V. G. (2016). Problems and perspectives of the bivalve mollusks aquaculture in the Barents Sea. Herald Kola Sci. Centre RAS 3, 59–74.
Dvoretsky, V. G., and Dvoretsky, A. G. (2018a). Mesozooplankton in the Kola Transect (Barents Sea): autumn and winter structure. J. Sea Res. 142, 18–22.
Dvoretsky, A. G., and Dvoretsky, V. G. (2018b). Red king crab (Paralithodes camtschaticus) fisheries in Russian waters: historical review and present status. Rev. Fish Biol. Fish. 28, 331–353. doi: 10.1007/s11160-017-9510-1
Dvoretsky, A. G., and Dvoretsky, V. G. (2019). Summer macrozooplankton assemblages of Arctic shelf: a latitudinal study. Cont. Shelf Res. 188:103967. doi: 10.1016/j.csr.2019.103967
Dvoretsky, A. G., and Dvoretsky, V. G. (2020a). Aquaculture of green sea urchin in the Barents Sea: a brief review of Russian studies. Rev. Aquaculture 12, 2080–2090. doi: 10.1111/raq.12423
Dvoretsky, A. G., and Dvoretsky, V. G. (2020b). Effects of environmental factors on the abundance, biomass, and individual weight of juvenile red king crabs in the Barents Sea. Front. Mar. Sci. 7:726.
Dvoretsky, A. G., and Dvoretsky, V. G. (2021). New echinoderm-crab epibiotic associations from the coastal Barents Sea. Animals 11:917. doi: 10.3390/ani11030917
Gavrilova, G. S. (2005). Mariculture of the invertebrate in the Far East: stages, results, problems. Izv. TINRO 141, 103–120.
Gavrilova, G. S., and Kucherjavenko, A. V. (2010). Farming of sea cucumber apostichopus japonicus in peter the great bay: methodical specifics and business results of the aquaculture farm in the Sukhodol Bight. Izv. TINRO 162, 342–354.
Gavrilova, G. S., Gostyukhina, O. B., and Zakharova, E. A. (2005). The first experimental plant rearing of far eastern trepang in primorye. Rybn. Khoz 3, 47–49.
Gavrilova, G. S., Kurganskij, G. N., Bocharov, L. N., and Akulin, V. N. (2006). Method for Commercial Cultivation of Young Specimens of Trepang and Apparatus for Performing the Same. Patent of the Russian Federation No. 2284105.
Gianasi, B. L., Hamel, J.-F., and Mercier, A. (2019). Triggers of spawning and oocyte maturation in the commercial sea cucumber Cucumaria frondosa. Aquaculture 498, 50–60. doi: 10.1016/j.aquaculture.2018.08.030
Gianasi, B. L., Parrish, C. C., Hamel, J.-F., and Mercier, A. (2017). Influence of diet on growth, reproduction and lipid and fatty acid composition in the sea cucumber Cucumaria frondosa. Aquac. Res. 48, 3413–3432. doi: 10.1111/are.13168
Gudimova, E. N. (1998a). “Sea cucumber Cucumaria frondosa (Gunner, 1767),” in Harvesting and Perspective for Uses Algae and Invertebrates of the Barents and White Seas, ed. G. G. Matishov (Apatity: KSC RAS Press), 453–528.
Gudimova, E. N. (1998b). Prospects of sea cucumber fishing in the Barents Sea. Rybn. Khoz. 3, 45–46.
Gudimova, E. N. (1999). Holoturian Cucumaria frondosa (Gunner, 1767) in the Barents Sea: Taxonomy, Biology, Usage. Ph. D, Thesis. St. Petersburg: Zoological Institute RAS.
Gudimova, E. N. (2000). “Distribution of Cucumaria frondosa (Holothuroidea) in the Barents Sea,” in Benthos of the Barents and Kara Seas, ed. G. G. Matishov (Apatity: KSC RAS Press), 347–360.
Gudimova, E. N., Gudimov, A., and Collin, P. (2004). “A study of the biology for fishery in two populations of Cucumaria frondosa: in the Barents Sea (Russia) and in the Gulf of Maine (USA),” in Proceeding of the Echinoderms Munchen 11th Internation Echinoderm Conference, eds T. Heinzeller and J. H. Nebelsick 269–275. doi: 10.1201/9780203970881.ch46
Gunner, J. E. (1767). Beskrifning pä trenne Norrska Sjo-Kräk, Sjo-Pungar kallade. Stockholm Vetensk. Acad. Handls Ar. 28, 114–124.
Hamel, J.-F., and Mercier, A. (1996). Early development, settlement, growth, and spatial distribution of the sea cucumber Cucumaria frondosa (Echinodermata: Holothuroidea). Can. J. Fish. Aquat. Sci. 53, 253–271. doi: 10.1139/f95-186
Hamel, J.-F., and Mercier, A. (2008). “Population status, fisheries and trade of sea cucumbers in temperate areas of the Northern Hemisphere,” in Sea Cucumbers. A Global Review of Fisheries and Trade. FAO Fisheries and Aquaculture Technical Paper. No. 516, eds V. Toral-Granda, A. Lovatelli, and M. Vasconcellos (Rome: FAO), 257–291.
Han, Q., Keesing, J. K., and Liu, D. (2016). A review of sea cucumber aquaculture, ranching, and stock enhancement in China. Rev. Fish. Sci. Aquaculture 24, 326–341. doi: 10.1080/23308249.2016.1193472
Haug, T., Kjuul, A. K., Styrvold, O. B., Sandsdalen, E., Olsen, O. M., and Stensvag, K. (2002). Antibacterial activity in Strongylocentrotus droebachiensis (Echinoidea), Cucumaria frondosa (Holothuroidea), and Asterias rubens (Asteroidea). J. Invert. Pathol. 81, 94–102. doi: 10.1016/s0022-2011(02)00153-2
Holtz, E., and MacDonald, B. (2009). Feeding behaviour of the sea cucumber Cucumaria frondosa (Echinodermata: Holothuroidea) in the laboratory and the field: Relationships between tentacle insertion rate, flow speed, and ingestion. Mar. Biol. 156, 1389–1398. doi: 10.1007/s00227-009-1179-0
Hossain, A., Dave, D., and Shahidi, F. (2020). Northern sea cucumber (Cucumaria frondosa): a potential candidate for functional food, nutraceutical, and pharmaceutical sector. Mar. Drugs 18:274. doi: 10.3390/md18050274
Janakiram, N. B., Mohammed, A., and Rao, C. V. (2015). Sea cucumbers metabolites as potent anti-cancer agents. Mar. Drugs 13, 2909–2923. doi: 10.3390/md13052909
Kizevetter, I. V. (1962). Catch and Processing of Commercial Invertebrates Form the Far Eastern Seas. Vladivostok: Primorskoe kniznoe izdatelstvo.
Malavenda, S. V., Shoshina, E. V., and Kapkov, V. I. (2017). Species diversity of seaweeds in different areas of the Barents Sea. Vestn. MGTU 20, 336–351. doi: 10.21443/1560-9278-2017-20-2-336-351
Mamelona, J., Pelletier, E., Girard-LaLancette, K., Legault, J., Karboune, S., and Kermasha, S. (2007). Quantification of phenolic contents and antioxidant capacity of Atlantic sea cucumber. Cucumaria frondosa. Food Chem. 104, 1040–1047. doi: 10.1016/j.foodchem.2007.01.016
Matishov, G. G. (2016). Arctic Hydrobiology as the Basis of modern Technologies for Industry, and Agriculture: Collection of Selected Papers. Rostov-on-Don: SSC RAS.
Mercier, A., and Hamel, J.-F. (2013). “Sea cucumber aquaculture: hatchery production, juvenile growth and industry challenges,” in Advances in Aquaculture Hatchery Technology, Woodhead Publishing Series in Food Science, Technology and Nutrition No. 242, eds G. Allan and G. Burnell (Oxford: Elsevier), 431–454. doi: 10.1533/9780857097460.2.431
Mills, D. J., Duy, N. D. Q., Juinio-Meñez, M. A., Raison, C. M., and Zarate, J. M. (2012). “Overview of sea cucumber aquaculture and sea-ranching research in the South-East Asian region,” in Asia-Pacific Tropical Sea Cucumber Aquaculture. Proceedings of an International Symposium Held in Noumea, New Caledonia, 15–17 February 2011, ACIAR Proceedings No. 136, eds C. A. Hair, T. D. Pickering, and D. J. Mills (Canberra: Australian Centre for International Agricultural Research), 22–31.
Nasedkina, E. A., Kasianenko, Y. I., and Slutskaya, T. N. (1973). Chemical composition features of echinoderm muscles. Rybn. Khoz. 7, 81–82.
Nelson, E. J., MacDonald, B. A., and Robinson, S. M. C. (2012). The absorption efficiency of the suspension-feeding sea cucumber, Cucumaria frondosa, and its potential as an extractive integrated multi-trophic aquaculture (IMTA) species. Aquaculture 370–371, 19–25. doi: 10.1016/j.aquaculture.2012.09.029
Propp, M. V. (1971). Ecology of Coastal Benthic Communities of Murmansk Shallow Waters of the Barents Sea on Materials of Underwater Hydrobiological Studies. Leningrad: Nauka.
Purcell, S. W., Hair, C. A., and Mills, D. J. (2012). Sea cucumber culture, farming and sea ranching in the tropics: progress, problems and opportunities. Aquaculture 368–369, 68–81. doi: 10.1016/j.aquaculture.2012.08.053
Purcell, S. W., Mercier, A., Conand, C., Hamel, J.-F., Toral, M. V., Lovatelli, G. A., et al. (2013). Sea cucumber fisheries: global analysis of stocks, management measures and drivers of overfishing. Fish Fish. 14, 34–59. doi: 10.1111/j.1467-2979.2011.00443.x
Singh, R., MacDonald, B. A., Thomas, M. L. H., and Lawton, P. (1999). Patterns of seasonal and tidal feeding activity in the dendrochirote sea cucumber Cucumaria frondosa (Echinodermata: Holothuroidea) in the Bay of Fundy, Canada. Mar. Ecol. Prog. Ser. 187, 133–145. doi: 10.3354/meps187133
Sun, J., Hamel, J.-F., Stuckless, B., Small, T. J., and Mercier, A. (2020). Effect of light, phytoplankton, substrate types and colour on locomotion, feeding behaviour and microhabitat selection in the sea cucumber Cucumaria frondosa. Aquaculture 526:735369. doi: 10.1016/j.aquaculture.2020.735369
Wassmann, P., Reigstad, M., Haug, T., Rudels, B., Carroll, M. L., Hop, H., et al. (2006). Food webs and carbon flux in the Barents Sea. Prog. Oceanogr. 71, 232–287. doi: 10.1016/j.pocean.2006.10.003
Yu, Z., Robinson, S., Macdonald, B., Lander, T., and Smith, C. (2020). Effect of diets on the feeding behavior and physiological properties of suspension-feeding sea cucumber Cucumaria frondosa. J. Oceanol. Limnol. 38, 883–893. doi: 10.1007/s00343-019-9190-x
Zakharov, D. V., Strelkova, N. A., Manushin, I. E., Zimina, O. L., Jørgensen, L. L., Luybin, P. A., et al. (2018). Atlas of the Megabenthic Organisms of the Barents Sea and Adjacent Waters. Murmansk: PINRO.
Keywords: Cucumaria frondosa, Barents Sea, growth, distribution, polar aquaculture
Citation: Dvoretsky AG and Dvoretsky VG (2021) Cucumaria in Russian Waters of the Barents Sea: Biological Aspects and Aquaculture Potential. Front. Mar. Sci. 8:613453. doi: 10.3389/fmars.2021.613453
Received: 02 October 2020; Accepted: 19 July 2021;
Published: 06 August 2021.
Edited by:
Seyed Hossein Hoseinifar, Gorgan University of Agricultural Sciences and Natural Resources, IranReviewed by:
Giovanni Daneri, Patagonian Ecosystems Investigation Research Center (CIEP), ChileYunwei Dong, Ocean University of China, China
Copyright © 2021 Dvoretsky and Dvoretsky. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alexander G. Dvoretsky, ag-dvoretsky@yandex.ru
 Alexander G. Dvoretsky
Alexander G. Dvoretsky Vladimir G. Dvoretsky
Vladimir G. Dvoretsky