- 1TB Elimination & Implementation Science, Burnet Institute, Melbourne, VIC, Australia
- 2Department of Paediatrics, University of Melbourne, Melbourne, VIC, Australia
- 3RID-TB Project Burnet Institute, Daru, Papua New Guinea
- 4PNG Country Program, Burnet Institute, Port Moresby, Papua New Guinea
- 5Public Health Services, Western Provincial Health Authority, Daru, Papua New Guinea
- 6National TB Program, National Department of Health, Port Moresby, Papua New Guinea
Daru, South Fly District, Papua New Guinea is a high transmission setting for multidrug-resistant tuberculosis (MDR-TB). An emergency response by the Government in 2014 established a high-quality model for treatment and care. Household contact screening and management commenced in 2016 with TB preventive treatment (TPT) for well young child (<5 years) contacts of people with drug-susceptible TB and later expanded to young child contacts of MDR-TB. The model of care is community-based and led by non-specialist health workers, under supervision. An electronic medical record system supports care, reporting and operational research. Community engagement and education has been central, with a concerted focus on peer-led counselling and patient-centred services to improve TPT uptake and completion. Challenges include the application of households as the unit of intervention for detection of active TB and TPT provision. Our implementation experience in Daru has highlighted significant population mixing dynamics with most transmission likely occurring outside the household. We propose a community-wide screening approach with the provision of TPT based on testing to include older children, adolescents, and young adults. As there is the possibility of MDR-TB infection irrespective of the drug susceptibility of the household index case, a novel option is a combination TPT regimen of 6 months of daily isoniazid and levofloxacin (6HLfx). A sensitive aged-related algorithm to detect and exclude active TB is being developed. Ongoing community engagement, quality data systems with operational research to evaluate approaches are critical in high transmission MDR-TB settings.
Introduction
Tuberculosis (TB) is the leading infectious cause of morbidity and mortality in Papua New Guinea (PNG) (1, 2). The emergence and increasing transmission of multidrug-resistant TB (MDR-TB) is a major challenge (3). On Daru Island in the South Fly District of Western Province, PNG, there is an unprecedented MDR-TB outbreak with a TB notification rate of 2,021 per 100,000 for all TB and 594 per 100,000 for MDR/RR-TB (rifampicin-resistant) in 2015 (4, 5). In 2014, the PNG National Department of Health and Western Provincial Health Authority (WPHA) initiated an emergency response to MDR-TB, which has resulted in the stabilisation of TB notifications, improved treatment outcomes and a reduction in TB-related mortality (5, 6). A description of the geographical, social and demographic context in Daru, which is located in the transboundary region between PNG and the Australian Torres Strait, has been detailed elsewhere (7, 8).
A comprehensive community-based model of care for TB was established through the introduction of evidence-based practice, with significantly improved outcomes and introduction of novel tools including bedaquiline-containing treatment regimens (5, 9, 10). Community engagement and patient-centred support services have been central to delivery of care (11). An electronic patient-level medical record system (EMRS - Bahmni, Thoughtworks) was implemented in 2016 to support quality patient care, program monitoring and operational research (5, 9). The screening and management of household contacts of people with TB commenced in 2016. TB preventive treatment (TPT) for well young child (<5 years) contacts of drug-susceptible (DS) TB index cases was introduced in 2017 and extended to those of MDR-TB index cases in 2019 (12).
In Daru, as in many other high-transmission and resource-limited settings, best practice strategies and tools for TB and MDR-TB are often under-utilised or not prioritised (13). Active case finding through contact screening and TPT for eligible at-risk contacts is a programmatic priority. However, application to high transmission MDR-TB settings presents additional challenges that require specific considerations and novel approaches. Table 1 summarises the current approach, challenges and solutions for contact screening and management in Daru, PNG.
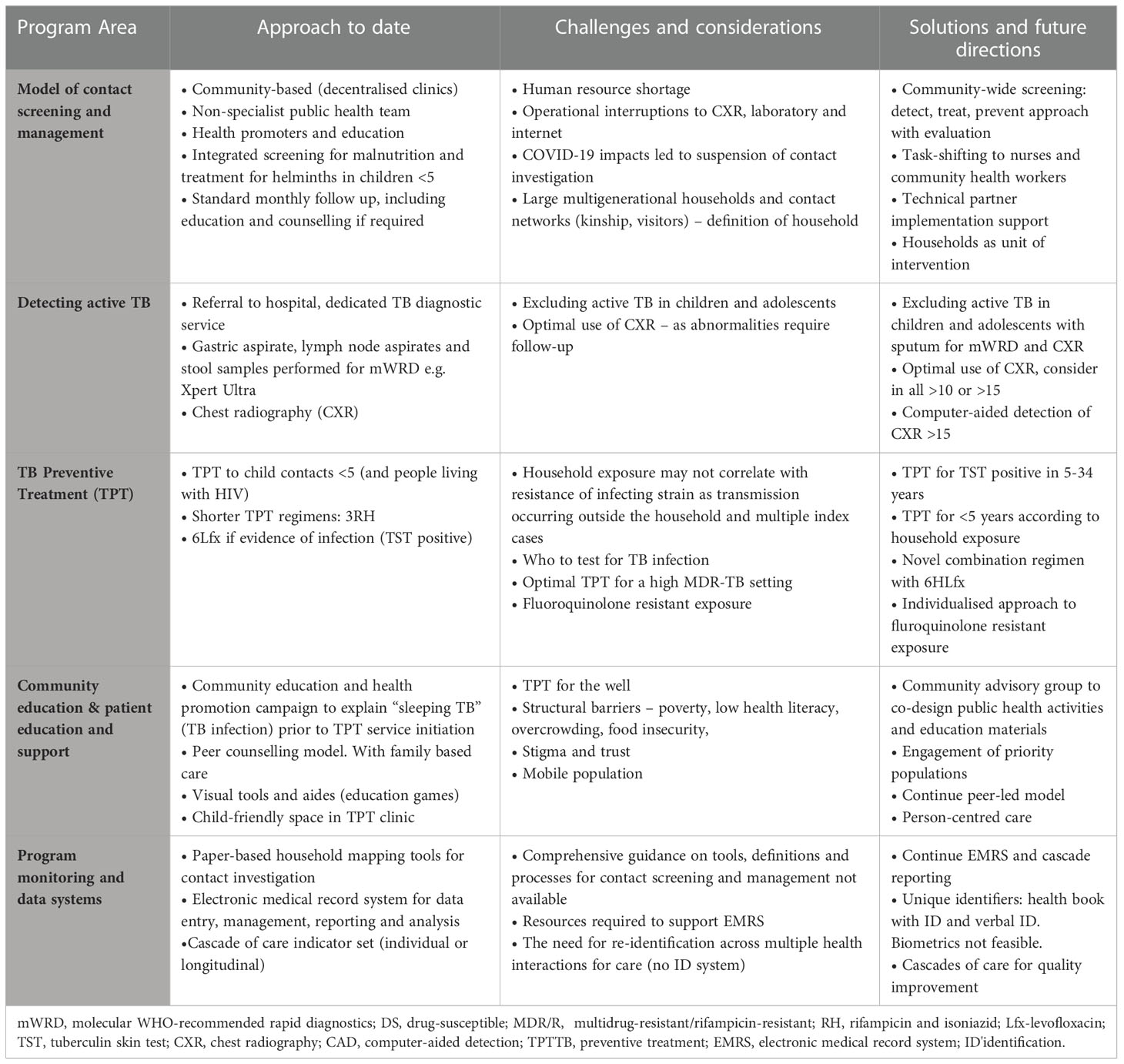
Table 1 Contact screening and management – current approach, challenges and solutions in Daru, PNG 2017 - 2022.
Current approach to household contact screening and management
In Daru, a dedicated public health TB Team consisting of a health extension officer (an auxiliary medical officer), nursing officers, community health workers and health promoters conduct contact screening for all newly detected bacteriologically confirmed pulmonary or extrapulmonary TB under the supervision of a medical officer. Following verbal consent by index cases or their guardian and the household head, contact screening occurs at the household level. Education is provided by trained health promoters to household contacts, who are defined as any person who lives in the same house as the index case within three months prior to diagnosis, including spending time during the day. Contacts with a positive symptom screen are referred to the TB diagnostic clinic for investigation and asymptomatic young child contacts to the TPT clinic. Young children are also screened for malnutrition which is managed according to local guidance and includes empiric treatment for soil transmitted helminths. Screening and management are provided by non-specialist nursing community health worker teams, in a community-based model of care (decentralised clinics) following standard operating procedures.
Detecting active TB in contacts
TB detection with treatment of disease is a critical role of contact screening and management along with determining eligibility for TPT. In Daru, there is a TB diagnostic clinic in the hospital where contacts with TB-related symptoms are referred for further investigation including chest radiography (CXR) and appropriate specimens such as sputum or lymph node aspirate for Xpert Ultra as per PNG and World Health Organisation guidelines (14). If RR-TB is detected, a specimen is sent to the national laboratory for culture and drug-susceptibility testing and molecular assays for early diagnosis of fluoroquinolone-resistance. Gastric aspirate and/or stool samples for Xpert Ultra are taken from symptomatic child contacts unable to expectorate sputum. Asymptomatic contacts are not routinely referred for investigation, including asymptomatic young child contacts who are eligible for TPT.
TB preventive treatment
The provision of TPT to eligible household contacts is widely recommended but implementation in resource-constrained settings remains limited (1, 15). TPT should be routinely offered to well young child (<5 years) household contacts of people with confirmed or presumptive pulmonary DS-TB (16). The protective efficacy of TPT is well established (17) and the recently recommended shorter regimens are associated with high rates of completion and very few adverse events (16). The program in Daru was the first in PNG to systematically introduce a community-based model of TPT in 2016 using 6 months of daily isoniazid (6H) for young child contacts. A TPT regimen of 3 months of daily rifampicin and isoniazid (3RH) – recommended by WHO in 2018 – has been associated with high uptake, completion and safety in young child contacts under programmatic conditions in resource-limited settings (18). The availability in PNG of dispersible, child-friendly combined formulations for active TB since 2016 (19), led to the successful implementation of 3RH for young child contacts of DS-TB in Daru from 2019.
In Daru, up to 20% of incident TB is MDR/RR-TB (5). TPT as 3RH or 6H may not be protective for child contacts of MDR-TB and is not recommended in that context (16). Furthermore, known exposure to DS-TB does not necessarily determine that infection is limited to a drug-susceptible strain only (20, 21). The optimal TPT regimen following infection with MDR-TB is unknown. A regimen that includes daily levofloxacin for at least six months (6Lfx) is widely used. Observational evidence from a number of settings suggests that a fluoroquinolone-based TPT regimen in MDR-TB contacts with evidence of TB infection (TBI) can substantially reduce the risk of developing MDR-TB, and with no significant safety concerns (22–26). The findings from two randomised-controlled trials of 6Lfx for MDR-TB contacts are expected in 2023 (27, 28).
The use of TPT in MDR-TB contacts requires an assessment of potential benefit versus risk, and benefit is greatest in contacts with infection (17). In 2019, tuberculin skin test (TST) was introduced in Daru to determine eligibility for 6Lfx as TPT for young child contacts of index cases with bacteriologically confirmed MDR-TB (without fluoroquinolone resistance) as part of a pilot project. This age group was selected as being high risk for disease due to care-giving relationships and high likelihood that exposure occurred in the household of the index case. A similar model of implementation was used as for 3RH for young child contacts of DS-TB, with the addition of TST. An operational research protocol with ethical approval was obtained and following informed consent, young child contacts with a positive TST and no evidence of disease were offered 6Lfx. To date, an effective model of care has been established with high uptake and completion.
Community engagement and patient education
Community engagement and education is critical to support contact screening and management, and especially to support TPT uptake and adherence given that this requires giving medication daily for months to a child or adolescent who is well. In Daru, several approaches are utilised to deliver education to people on treatment, their families and the wider community. An international community-based organisation has been providing TB health promotion and social mobilisation activities since 2011, focussing on enhancing detection and treatment support for active TB (6). A peer counselling model was introduced in 2017 involving People who have been Affected by, Living with or having Survived TB, known as PALS, who are trained and mentored to provide education and counselling support (11). The counselling program initially focused on support for people on treatment for active TB and expanded in 2018 to include families of children on TPT.
Locally tailored education materials including visual tools were developed, tested with community, and guides created for standard counselling sessions scheduled at key points of treatment. A child-friendly space with activities was initiated at the TPT follow-up clinic. Counselling and education are provided in the family’s preferred language where possible. Due to the limited health literacy and linguistic diversity (>3 languages) of the people South Fly District, visual tools and aides have been important. The term “sleeping TB” was used to explain the concepts of TBI. Community education and health promotion campaigns to increase understanding of “sleeping TB” and TPT were conducted including promotion from the town mayor and local leaders, public education sessions at churches, schools, sports events, community locations and local radio. The above measures have contributed to high rates of adherence and TPT completion.
Program monitoring and data systems
Monitoring and evaluation of contact screening and management programs are essential. However, comprehensive guidance does not exist regarding tools, data definitions or processes for programs to implement contextually adapted solutions. The WHO has defined minimum data and indicator definitions for TPT and the Zero TB Initiative has outlined a comprehensive set of indicators for the search-treat-prevent approach (29, 30). The EMRS in Daru was adapted to include modules for contact screening and TPT in 2018. A household mapping tool that documents investigation of all close contacts of an index case is used as previously described (12). The public health team records patient data using paper-based records. A data entry team subsequently enters this data into the EMRS. Contact screening and management is monitored using a cascade-of-care framework for TB detection and prevention. The EMRS enables reporting of an individual-level cascade, rather than aggregate. Resources required to support the EMRS, including information technology expertise, programming skills and data entry capacity, are provided through partner support. Despite challenges, implementation has been successful, with the data system monitoring TPT uptake and outcome.
Implementation challenges and considerations
Operational challenges include a chronic shortage of health care workers, interruption of CXR services, laboratory reagents and power and internet outages. The availability of international partner support has been critical to the TB program in Daru since 2011. Adopting a community-based model with mobile teams and task-shifting from medical and para-medical staff to nurses and community-health workers with training and technical support from partners has helped to address human resource issues. The COVID-19 pandemic has had a major impact on the delivery of TB services. From March 2020 until April 2022, contact screening was suspended initially due to public health restrictions and infection risks and subsequently health workforce shortages. Programmatic focus over this period was active TB treatment and care with case-finding limited to facility-based only (passive). Household contact screening and management restarted in April 2022.
Daru, and South Fly District have significant particular challenges in the social determinants of health which contribute to increased risk of TB transmission, with one of the highest measures of household poverty in the region, low levels of health literacy overcrowding food insecurity and low access to health services due to service availability, remoteness and lack of trust (7) (8). The TB program has had a concerted focus on community engagement, health promotion and patient-centred services. A major challenge has been identifying and reaching households which has been successfully addressed through the engagement of community leaders, household heads and community education. This patient and family-centred approach has built trust. Timely referrals for eligible TPT candidates for pre-initiation counselling also reduced delays in time to initiation of TPT. In the early implementation of TPT for young children, adherence issues were noted prompting an exploration and development of solutions for contributing issues through counsellors.
A Community Advisory Group, self-named TB Nanito Kopia Kodu (the Voice to Kill TB Forever) was formed from community consultations in late 2019 with members representing key stakeholder groups (e.g. churches, schools, businesses, key populations). The group’s primary focus is providing community input and co-design for TB public health activities, including the development of community education materials and models to reach key populations such as adolescents and students, working adults (not present at home), prisoners, people with disabilities and the elderly. Accordingly, a comprehensive community education campaign has been developed with a participatory approach involving training of representatives from key populations.
Re-identification of individuals during multiple episodes across the screening and prevention cascades of care has been a particular challenge due to poor civil registration coverage (31). In PNG, people may give different names, age and residence from visit to visit with addresses not specific enough. Paper-based recording at multiple points of care also contributes to the problem by making it difficult to check a patient’s details against existing information. The linkage of contacts to multiple index cases needs to be reconciled for reporting. This is currently addressed through local health worker and community knowledge of households and engagement via peer counsellors. Biometric systems have been proposed as a solution for patient identification, but cost, complexity and acceptability are barriers to implementing them. An immediate solution is patient-held records, such as health books or a card that can be issued and used to identify patients. However, these may get lost or not brought to patient visits. We have designed a potential solution for re-identification at low cost that supplements patient-held records with a memorisable unique identifier consisting of three common objects, represented with images. Even partial recall of this identifier can aid re-identification.
The primary challenge in Daru is the application of the household as the unit of intervention for contact screening and to deliver post-exposure management including detection of active TB and TPT. This is both from an implementation perspective and the fact that households may not be the optimal unit of intervention for timely detection of active TB and identifying those at risk of recent exposure for TPT (32, 33). The socio-cultural context in Daru, as elsewhere in PNG, involves large multi-generational family structures based on kinship. However, there are some unique features with significant overcrowding in settlements and frequent travel between mainland villages and Daru Island, often for extended periods of time. This results in large “households” with complex family structures and population movement. Therefore, the operational household contact definition used is broad and includes those who have spent more than one night in the household.
Our experience in Daru is that most transmission is likely occurring outside the household, as noted even in children in other high-incidence settings (32). The likelihood of exposure and infection occurring outside of the household increases with age. Adolescence is a peak age for close and casual social contact and so in a highly endemic setting such as Daru, the majority of the population may be infected by adulthood (34). TST testing in MDR-TB household contacts in Daru has demonstrated a high prevalence of infection similar to reported elsewhere (35). The majority of TB and MDR-TBnotifications are in older adolescents and young adults, with around 70% of TB notified in those 15-34 years (5). Furthermore, with large household sizes and a very mobile population, an individual can be exposed to multiple index cases concurrently, including DS and MDR-TB. Therefore, consideration of alternative approaches in Daru to household contact management are warranted.
Future directions – moving beyond household contact screening and management
Until recently in high TB transmission settings, recommendations for TPT have been limited to specific high-risk groups for disease such as young child contacts and people living with HIV (16). Whilst important in reducing tuberculosis-related morbidity and mortality in these groups, the impact of TPT on community transmission by preventing new cases is small, likely negligible for young child contacts (32, 36). Therefore, a community-wide strategy has been considered in the design of an enhanced public health intervention and model of care that aims to increase TB detection and treatment while providing TPT to a broader population to reduce transmission in addition to TB morbidity and mortality (37, 38). Figure 1 outlines our considerations for a comprehensive detect-treat-prevent strategy in a high TB transmission setting by age.
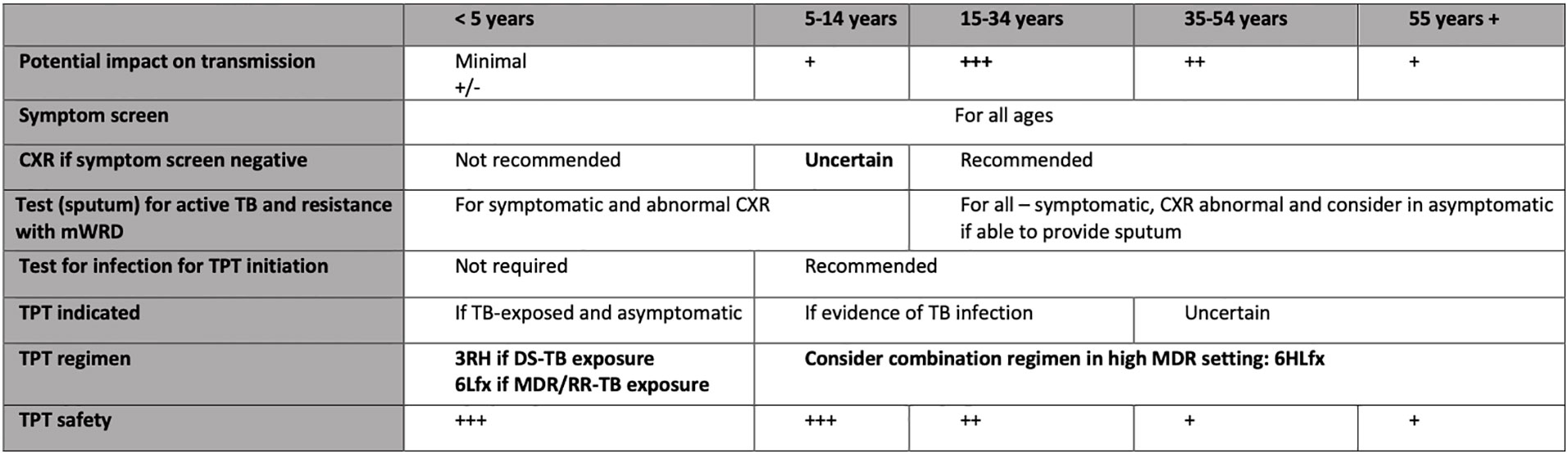
Figure 1 Considerations for a comprehensive detect-treat-prevent strategy in a high MDR-TB transmission setting by age. mWRD, molecular WHO-recommended rapid diagnostics; DS-TB, drug-susceptible tuberculosis; MDR/RR, multidrug-resistant/rifampicin-resistant tuberculosis; RH, rifampicin and isoniazid; Lfx, levofloxacin; CXR, chest. NB. Different considerations apply in high HIV transmission settings and special populations.
First, programmatic scaling-up of contact screening and management that includes older children, adolescents and young adults in the community is planned. The 2020 WHO recommendations include a wider age range of TPT options for HIV-uninfected contacts (16). We therefore propose community-wide TST testing for Daru residents aged 5-34 years irrespective of household contact, with TPT provided to those with infection and no evidence of active TB, as part of a comprehensive approach to complement active case-finding. The model of community engagement and implementation described above will be applied and the household will be the unit of intervention. The application of a community-wide systematic screening for case detection aligns with recent WHO recommendations for settings with a TB prevalence of 0.5% such as Daru, and is based on new evidence of public health benefit (39). The decision to provide TPT for 5-34 years with infection is based on the following considerations: high-risk age group for active TB; high risk age group for transmission in the community; and lower risk of isoniazid-related toxicity due to TPT compared 35 years and older.
Second, in high transmission MDR-TB settings, consideration of a TPT regimen for older children, adolescents and adults that considers the possibility of infection with an MDR-TB strain irrespective of the index case is needed. A novel option is a combination TPT regimen of 6 months of daily isoniazid and levofloxacin (6HLfx) for 5–34 year-olds with evidence of infection and no active TB. This TPT regimen is chosen to effectively treat infection with either DS or MDR-TB as a positive TST cannot determine drug susceptibility. Whilst exposure to both is common, the majority (>80%) of TB in Daru is DS-TB. Isoniazid or rifampicin may not effectively treat infection with an MDR-TB strain. While it is plausible that levofloxacin alone may cover treatment of DS in addition to MDR infection, there is no definitive evidence of efficacy at present. There is circumstantial evidence that isoniazid may also be effective in the treatment of MDR infection (20). Rifapentine is currently not available in PNG and a three-drug regimen may have additional adverse effects over two. Therefore, isoniazid is included in the proposed regimen. Young child (<5 years) contacts can be managed as per the strain of the index case. The optimal approach for management of fluoroquinolone-resistant TB infection is uncertain and should be individualised.
Third, it is critical to exclude active disease especially in adolescents and adults. An optimal age-related contact management algorithm is required to mitigate the risk – individual and public health – of generating resistance including to fluoroquinolones. This risk needs to be negligible, similar to isoniazid and rifampicin (40, 41). In addition to symptom screening, the inclusion of CXR and sputum Xpert Ultra in the algorithm will provide high sensitivity for detection. The optimal age-related use of chest-radiography in asymptomatic, HIV-uninfected people aged 5–34 years to rule out active TB before TPT is unclear. WHO guidelines recommend that asymptomatic young child contacts or PLHIV do not require CXR before TPT initiation. It is likely that the same approach could be applied to all well children (<10 years) with infection without risk of not detecting sub-clinical disease. This is the approach that is being considered in Daru. In addition to a negative symptom screen, CXR will be routinely included in screening adolescents and adults (≥10 years), as it is important to exclude active TB with a sensitive algorithm prior to TPT initiation. Computer-aided detection of CXR is recommended for TB screening in people ≥15 and this will also be used for screening young adolescents (10-14 years). This is because adolescents with TB commonly present with adult-type disease such as with cavitation, most critical to rule-out before initiating TPT.
The significant health individual and health system burden of ongoing MDR-TB transmission is a compelling argument for the potential benefit of the community-wide approach to detect, treat and prevent DS and MDR-TB and likely outweighs potential individual treatment risks with 6HLfx (13, 42). Operational mixed-methods research will evaluate the impact, acceptability, feasibility and cost-effectiveness. The intervention will build on the local leadership, programmatic experience and platform of innovation that has been established in Daru. Ongoing engagement and placing the affected community at the centre of the response remains critical to success and towards ending tuberculosis in high-transmission settings.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
SM, SG and GKH contributed to conception and design of the study. GD, SB, TK, GC, SF, AM, JG, CM, TM, SU, SG and SM developed sections of the manuscript. SM wrote the first draft of the manuscript. All authors contributed to manuscript revision and approved the submitted version.
Funding
The Reducing the Impact of Drug-resistant TB (RID-TB) project is supported by the Australian Government and implemented by the Burnet Institute as part of the Western Province TB Program in partnership with the Western Provincial Health Authority, PNG Australia Transition to Health (PATH) and World Vision International. The Stronger Systems for Drug-resistant Tuberculosis and Malaria (STRATUM) GNT 1152867 project was supported by the Australian Government and implemented by the Menzies School of Health Research and the Burnet Institute. The Comprehensive community-based solutions to reduce TB in high-burden MDR-TB settings (CURB-TB) project received grant funding from the Australian Government (GNT1201008) SM is a recipient of an NHMRC Postgraduate Scholarship (GNT 1191614) and the RACP NHMRC Woolcock Scholarship.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. World Health Organisation. Global tuberculosis report 2022. Geneva: World Health Organisation (2022). Available at: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2022.
2. Aia P, Wangchuk L, Morishita F, Kisomb J, Yasi R, Kal M, et al. Epidemiology of tuberculosis in Papua new Guinea: analysis of case notification and treatment-outcome data, 2008–2016. West Pac Surveill Response J (2018) 9:9–19. doi: 10.5365/wpsar.2018.9.1.006
3. Aia P, Kal M, Lavu E, John LN, Johnson K, Coulter C, et al. The burden of drug-resistant tuberculosis in Papua new Guinea: Results of a Large population-based survey. PloS One (2016) 11:e0149806. doi: 10.1371/journal.pone.0149806
4. Kase P, Dakulala P, Bieb S. Outbreak of multidrug-resistant tuberculosis on daru island: an update. Lancet Respir (2016) 4(8):e40. doi: 10.1016/s2213-2600(16)30094-7
5. Morris L, Hiasihri S, Chan G, Honjepari A, Tugo O, Taune M, et al. The emergency response to multidrug-resistant tuberculosis in daru, Western province, Papua new Guinea, 2014–2017. Public Health Action (2019) 9:S4–S11. doi: 10.5588/pha.18.0074
6. Specialist Health Service. TB prevention and control in PNG. report of the review of contribution of DFAT investments 2011-2018 (2019). Available at: https://www.dfat.gov.au/publications/aid/review-dfat-support-tb-response-papua-new-guinea-2011-2018-management-response.
7. Busilacchi S, Butler J, Putten IV, Maru Y, Posu J. Asymmetrical development across transboundary regions: The case of the Torres strait treaty region (Australia and Papua new Guinea). Sustainability (2018) 10:4200–18. doi: 10.3390/su10114200
8. Jops P, Kupul M, Trumb RN, Cowan J, Graham SM, Bell S, et al. Exploring tuberculosis riskscapes in a Papua new guinean ‘Hotspot.’. Qual Health Res (2022) 32(11):1747-62. doi: 10.1177/10497323221111912
9. Taune M, Ustero P, Hiashiri S, Huang K, Aia P, Morris L, et al. Successful implementation of bedaquiline for multidrug-resistant TB treatment in remote Papua new Guinea. Public Health Action (2019) 9:S73–9. doi: 10.5588/pha.18.0071
10. Huang GKL, Pawape G, Taune M, Hiasihri S, Ustero P, O’Brien DP, et al. Telemedicine in resource-limited settings to optimize care for multidrug-resistant tuberculosis. Front Public Health (2019) 7:222. doi: 10.3389/fpubh.2019.00222
11. Adepoyibi T, Keam T, Kuma A, Haihuie T, Hapolo M, Islam S, et al. A pilot model of patient education and counselling for drug-resistant tuberculosis in daru, Papua new Guinea. Public Health Action (2019) 9:S80–2. doi: 10.5588/pha.18.0096
12. Honjepari A, Madiowi S, Madjus S, Burkot C, Islam S, Chan G, et al. Implementation of screening and management of household contacts of tuberculosis cases in daru, Papua new Guinea. Public Health Action (2019) 9:S25–31. doi: 10.5588/pha.18.0072
13. Kendall EA, Sahu S, Pai M, Fox GJ, Varaine F, Cox H, et al. What will it take to eliminate drug-resistant tuberculosis? Int J Tuberc Lung Dis (2019) 23:535–46. doi: 10.5588/ijtld.18.0217
14. World Health Organisation. Consolidated guidelines on tuberculosis. module 3: diagnosis - rapid diagnostics for tuberculosis detection, 2021 update. Geneva: World Health Organsation (2021). Available at: https://www.who.int/publications/i/item/9789240029415.
15. Hill PC, Rutherford ME, Audas R, van CR, Graham SM. Closing the policy-practice gap in the management of child contacts of tuberculosis cases in developing countries. PloS Med (2011) 8:e1001105. doi: 10.1371/journal.pmed.1001105.t003
16. World Health Organisation, World Health Organisation. WHO consolidated guidelines on tuberculosis: module 1 preventative treatment. Geneva: World Health Organisation (2020). Available at: https://www.who.int/publications/i/item/9789240001503.
17. Martinez L, Cords O, Horsburgh CR, Andrews JR, Consortium PTCS, Acuna-Villaorduna C, et al. The risk of tuberculosis in children after close exposure: a systematic review and individual-participant meta-analysis. Lancet (2020) 395:973–84. doi: 10.1016/s0140-6736(20)30166-5
18. Schwoebel V, Koura KG, Adjobimey M, Gnanou S, Wandji AG, Gody J-C, et al. Tuberculosis contact investigation and short-course preventive therapy among young children in Africa. Int J Tuberc Lung Dis (2020) 24:452–60. doi: 10.5588/ijtld.19.0712
19. Apis V, Landi M, Graham SM, Islam T, Amini J, Sabumi G, et al. Outcomes in children treated for tuberculosis with the new dispersible fixed-dose combinations in port Moresby. Public Heal Action (2019) 9:S32–7. doi: 10.5588/pha.18.0062
20. Huang C-C, Becerra MC, Calderon R, Contreras C, Galea J, Grandjean L, et al. Isoniazid preventive therapy in contacts of multidrug-resistant tuberculosis. Am J Resp Crit Care (2020) 202:1159–68. doi: 10.1164/rccm.201908-1576oc
21. Demers A-M, Kim S, McCallum S, Eisenach K, Hughes M, Naini L, et al. Drug susceptibility patterns of mycobacterium tuberculosis from adults with multidrug-resistant tuberculosis and implications for a household contact preventive therapy trial. BMC Infect Dis (2021) 21:205. doi: 10.1186/s12879-021-05884-4
22. Byrne AL, Fox GJ, Marais BJ. Better than a pound of cure: preventing the development of multidrug-resistant tuberculosis. Future Microbiol (2018) 13:577–88. doi: 10.2217/fmb-2017-0236
23. Malik AA, Gandhi NR, Lash TL, Cranmer LM, Omer SB, Ahmed JF, et al. Effectiveness of preventive therapy for persons exposed at home to drug-resistant tuberculosis, Karachi, Pakistan. Emerg Infect Dis (2021) 27:805–12. doi: 10.3201/eid2703.203916
24. Seddon J, Fred D, Amanullah F, Schaaf H, Starke J, Keshavjee S, et al. Post-exposure management of multidrug-resistant tuberculosis contacts: evidence-based recommendations. policy brief no. 1. Dubai, United Arab Emirates: Harvard Medical School Center for Global Health Delivery, Dubai (2015). Available at: https://www.zerotbinitiative.org/resources-for-coalitions.
25. Seddon JA, Hesseling AC, Finlayson H, Fielding K, Cox H, Hughes J, et al. Preventive therapy for child contacts of multidrug-resistant tuberculosis: A prospective cohort study. Clin Infect Dis (2013) 57:1676–84. doi: 10.1093/cid/cit655
26. Bamrah S, Brostrom R, Dorina F, Setik L, Song R, Kawamura LM, et al. Treatment for LTBI in contacts of MDR-TB patients, federated states of Micronesia, 2009-2012. Int J Tuberc Lung Dis (2014) 18:912–8. doi: 10.5588/ijtld.13.0028
27. Seddon JA, Garcia-Prats AJ, Purchase SE, Osman M, Demers A-M, Hoddinott G, et al. Levofloxacin versus placebo for the prevention of tuberculosis disease in child contacts of multidrug-resistant tuberculosis: study protocol for a phase III cluster randomised controlled trial (TB-CHAMP). Trials (2018) 19:693. doi: 10.1186/s13063-018-3070-0
28. Fox GJ, Nguyen CB, Nguyen T-A, Tran PT, Marais BJ, Graham SM, et al. Levofloxacin versus placebo for the treatment of latent tuberculosis among contacts of patients with multidrug-resistant tuberculosis (the VQUIN MDR trial): a protocol for a randomised controlled trial. BMJ Open (2020) 10:e033945-8. doi: 10.1136/bmjopen-2019-033945
29. World Health Organisation. WHO operational handbook on tuberculosis module 2: Screening – systematic screening for tuberculosis disease (2021). Available at: https://www.who.int/publications/i/item/9789240022614.
30. Zero TB Initiative. A best-practice framework of program indicators for monitoring a comprehensive approach to the tuberculosis epidemic (2017). Available at: https://www.zerotbinitiative.org/resources-for-coalitions.
31. Pacific Community (SPC). Papua New Guinea civil registration and vital statistics action plan (2020-2021). Noumea, New Caledonia: The Pacific Community (SPC) (2021). Available at: https://sdd.spc.int/digital_library/papua-new-guinea-civil-registration-and-vital-statistics-action-plan-2020-2021.
32. Martinez L, Lo NC, Cords O, Hill PC, Khan P, Hatherill M, et al. Paediatric tuberculosis transmission outside the household: challenging historical paradigms to inform future public health strategies. Lancet Respir Med (2019) 7:544–52. doi: 10.1016/s2213-2600(19)30137-7
33. McCreesh N, White RG. An explanation for the low proportion of tuberculosis that results from transmission between household and known social contacts. Sci Rep-uk (2018) 8:5382. doi: 10.1038/s41598-018-23797-2
34. McCreesh N, Morrow C, Middelkoop K, Wood R, White RG. Estimating age-mixing patterns relevant for the transmission of airborne infections. Epidemics-neth (2019) 28:100339. doi: 10.1016/j.epidem.2019.03.005
35. Shah NS, Yuen CM, Heo M, Tolman AW, Becerra MC. Yield of contact investigations in households of patients with drug-resistant tuberculosis: systematic review and meta-analysis. Clin Infect Dis (2014) 58:381–91. doi: 10.1093/cid/cit643
36. Coleman M, Martinez L, Theron G, Wood R, Marais B. Mycobacterium tuberculosis transmission in high-incidence settings–new paradigms and insights. Pathogens (2022) 11:1228. doi: 10.3390/pathogens11111228
37. Rangaka M, Cavalcante S, Marais BJ, Thim S, Martinson N, Swaminathan S, et al. Controlling the seedbeds of tuberculosis: diagnosis and treatment of tuberculosis infection. Lancet (2015) 386(10010):2344-53. doi: 10.1016/s0140-6736(15)00323-2
38. Comstock G, Ferebee S, Hammes L. A controlled trial of community-wide isoniazid prophylaxis in Alaska. Am Rev Respir Dis (1967) 95(6):935-43. doi: doi: 10.1164/arrd.1967.95.6.935.
39. World Health Organisation. WHO consolidated guidelines on tuberculosis module 2: Screening – systematic screening for tuberculosis disease. Geneva: World Health Organisation (2021). Available at: https://www.who.int/publications/i/item/9789240022676. doi: 10.30978/tb2021-2-86
40. Balcells ME, Thomas SL, Godfrey-Faussett P, Grant AD. Isoniazid preventive therapy and risk for resistant tuberculosis. Emerg Infect Dis (2006) 12:744–51. doi: 10.3201/eid1205.050681
41. den Boon S, Matteelli A, Getahun H. Rifampicin resistance after treatment for latent tuberculous infection: a systematic review and meta-analysis. Int J Tuberc Lung Dis (2016) 20:1065–71. doi: 10.5588/ijtld.15.0908
Keywords: tuberculosis, contact management, multidrug-resistant TB (MDR-TB), Papua New Guinea, contact screening, implementation research
Citation: Majumdar SS, Islam S, Huang GKL, Morris L, Bauri M, Chan G, Kama G, Keam T, Peacock-Smith A, Finch S, Marukutira T, Bhatt S, Drewett G, Wratten M, Murray A, Pank N, Masah C, Bala R, Umali S, Kalon S, Greig J, Chani K, Kal M and Graham SM (2023) Contact screening and management in a high-transmission MDR-TB setting in Papua New Guinea: Progress, challenges and future directions. Front. Trop. Dis 3:1085401. doi: 10.3389/fitd.2022.1085401
Received: 31 October 2022; Accepted: 19 December 2022;
Published: 10 January 2023.
Edited by:
Amyn Abdul Malik, Yale University, United StatesReviewed by:
Jennifer Furin, Harvard University, United StatesCopyright © 2023 Majumdar, Islam, Huang, Morris, Bauri, Chan, Kama, Keam, Peacock-Smith, Finch, Marukutira, Bhatt, Drewett, Wratten, Murray, Pank, Masah, Bala, Umali, Kalon, Greig, Chani, Kal and Graham. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Suman S. Majumdar, suman.majumdar@burnet.edu.au
 Suman S. Majumdar
Suman S. Majumdar Shahidul Islam4
Shahidul Islam4 G. Khai Lin Huang
G. Khai Lin Huang Tafireyi Marukutira
Tafireyi Marukutira George Drewett
George Drewett Scott Umali
Scott Umali Stobdan Kalon
Stobdan Kalon Kudakwashe Chani
Kudakwashe Chani