- 1West China School of Medicine, Sichuan University, Chengdu, Sichuan, China
- 2Precision Medicine Research Center, West China Hospital, Sichuan University, Chengdu, Sichuan, China
- 3Department of Laboratory Medicine, West China Hospital, Sichuan University, TCM Regulating Metabolic Diseases Key Laboratory of Sichuan Province, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, Sichuan, China
Background: Neoadjuvant immunochemotherapy may benefit patients with non-small cell lung cancer (NSCLC), but its impact requires further investigation.
Methods: A meta-analysis was conducted. PubMed, Embase, Web of Science, and the Cochrane Library were searched. The study was registered in PROSPERO (registration no. CRD42022360893).
Results: 60 studies of 3,632 patients were included. Comparing with neoadjuvant chemotherapy, neoadjuvant immunochemotherapy showed higher pCR (RR: 4.71, 95% CI: 3.69, 6.02), MPR (RR, 3.20, 95% CI: 2.75, 3.74), and ORR (RR, 1.46, 95% CI: 1.21, 1.77), fewer surgical complications (RR: 0.67, 95%CI: 0.48, 0.94), higher R0 resection rate (RR: 1.06, 95%CI: 1.03, 1.10, I2 = 52%), and longer 1-year and 2-year OS, without affecting TRAEs. For neoadjuvant immunochemotherapy in NSCLC, the pooled pCR rate was 0.35 (95% CI: 0.31, 0.39), MPR was 0.59 (95% CI: 0.54, 0.63), and ORR was 0.71 (95% CI: 0.66, 0.76). The pooled incidence of all grade TRAEs was 0.70 (95% CI: 0.60, 0.81), and that of >= grade 3 TRAEs was 0.24 (95% CI: 0.16, 0.32). The surgical complications rate was 0.13 (95% CI: 0.07, 0.18) and R0 resection rate was 0.98 (95% CI: 0.96, 0.99). The pooled 1-year OS was 0.97 (95%CI: 0.96, 0.99), and 2-year OS was 0.89 (95%CI: 0.83, 0.94). Patients with squamous cell carcinoma, stage III or higher PD-L1 performed better. Notably, no significant differences were observed in pCR, MPR, and ORR between 2 or more treatment cycles. Pembrolizumab-, or toripalimab-based neoadjuvant immunochemotherapy demonstrated superior efficacy and tolerable toxicity.
Conclusion: According to our analysis, reliable efficacy, safety, and survival of neoadjuvant immunochemotherapy for operable NSCLC were demonstrated.
Systematic review registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022360893, identifier CRD42022360893.
1 Introduction
Non-small cell lung cancer (NSCLC) remains the main reason of tumor-related deaths (1). Of patients with NSCLC, 20-25% are surgically resectable, but 30-55% of patients treated with surgery still experience cancer relapse, metastasis, or death (2, 3). Due to the large tumor burden in advanced NSCLC, direct surgical treatment is challenging, while neoadjuvant therapy can shrink the tumor and make unresectable tumors resectable (4, 5). As a result, the NCCN guidelines recommend preoperative neoadjuvant therapy and postoperative adjuvant therapy as the standard therapy for NSCLC (6). But neoadjuvant chemotherapy may only provide a 5-6% benefit of 5-year overall survival (OS) and few patients achieve pathologic complete response (pCR) (7).
Immune checkpoint inhibitor (ICI) plays an important role in the first-line and second-line therapy of patients with NSCLC, showing a better survival benefit than chemotherapy (8–12). A growing view is that earlier immunotherapy may provide greater benefits. Several clinical studies have shown that neoadjuvant immunotherapy can was crucial in the comprehensive treatment of NSCLC, with controllable adverse events and less surgical delay (13, 14). CheckMate 159 showed that the pCR and MPR rates of nivolumab were 10% and 45%, respectively (15). The LCCMC 3 study revealed that the MPR rate of patients receiving 2 cycles of atezolizumab neoadjuvant therapy was 20.4%, and the pCR rate was 6.8% (16). These results were superior to those of previous neoadjuvant chemotherapy.
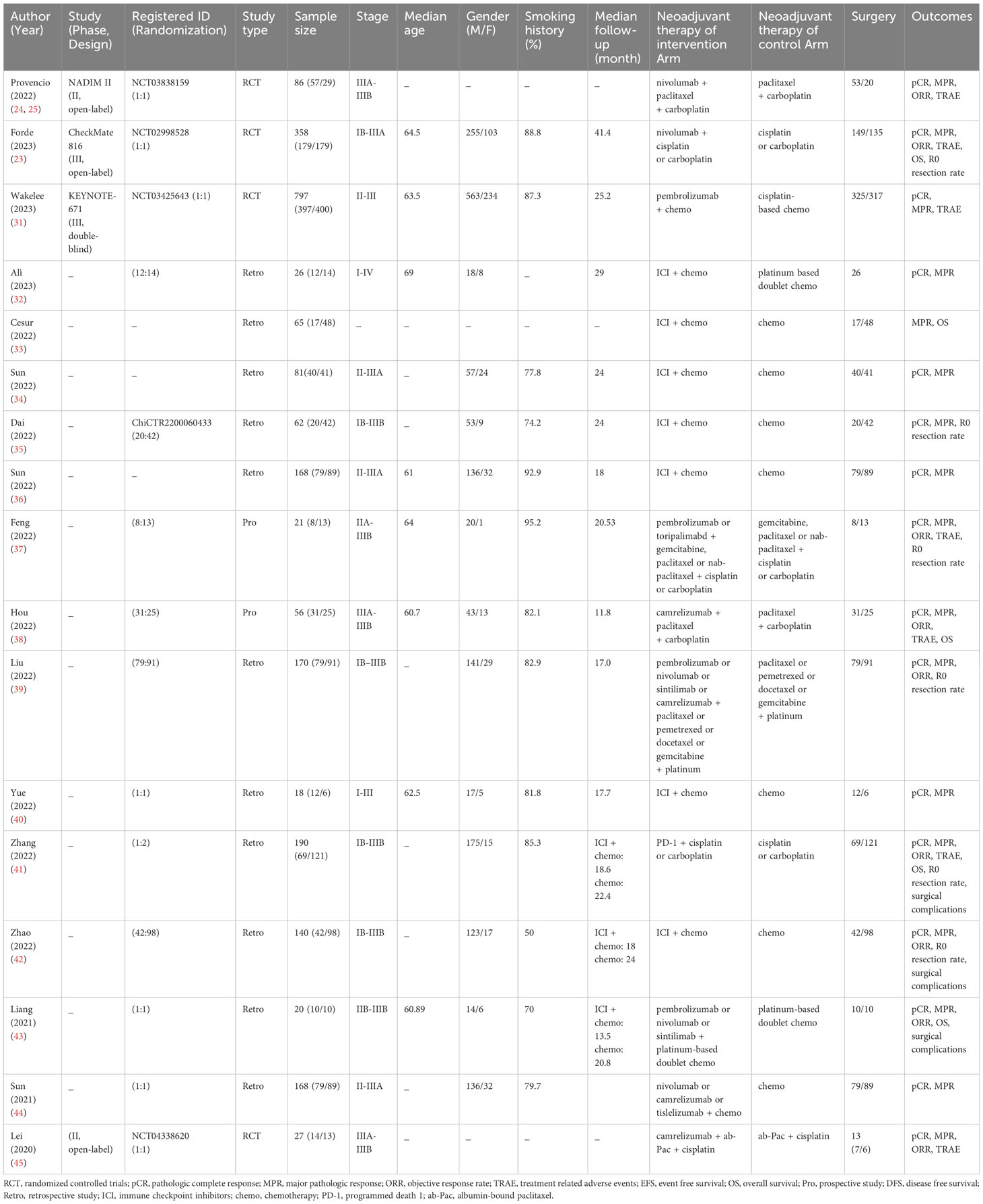
In the NADIM study, neoadjuvant immunochemotherapy for operable NSCLC had a pCR rate of 69.2% and an MPR rate of 84.6% (17, 18). In the NeoTPD01, and NCT02716038, and SAKK 16/14 studies, the MPR was around 60% (19–21). CheckMate 816, the first successful phase III trial of neoadjuvant immunochemotherapy versus chemotherapy in operable stage IB-III NSCLC, showed that MPR (36.9% vs. 8.9%) and pCR (24% vs. 2.2%) were significantly improved (22, 23). Updated data from the NADIM II study also revealed that neoadjuvant immunochemotherapy can effectively shrink tumors, increase pCR (36.8% vs. 6.9%), MPR (52.6% vs. 13.8%), and ORR (75.4% vs. 48.2%), and help patients obtain better survival in locally advanced IIIA-IIIB resectable NSCLC (24, 25). These studies provide encouraging results of neoadjuvant immunochemotherapy in patients with NSCLC.
Despite the promising results, concerns about the efficacy, safety, and survival of neoadjuvant immunochemotherapy remain. To address these concerns, we conducted a meta-analysis to combine all related trials and evaluate the efficacy, safety, and survival rates of neoadjuvant immunochemotherapy in operable NSCLC. Additionally, we compared the results among different subgroups, such as age, gender, smoking history, histology, stage, treatment cycles, pretreatment programmed death-ligand 1 (PD-L1), and ICI type. Our goal was to provide guidance for the clinical treatment of NSCLC.
2 Methods
To ensure the accuracy and transparency of our study, we followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) and AMSTAR (Assessing the methodological quality of systematic reviews) guidelines (26, 27). This study was registered in PROSPERO (registration no.CRD42022360893, available at: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022360893).
2.1 Data search
We searched PubMed, Embase, Web of Science, and Cochrane Library, using keywords such as “neoadjuvant”, “immunotherapy”, “chemotherapy”, and “non-small cell lung cancer”. The search was conducted independently by two authors and included papers updated until July 2023. Language restrictions were not applied.
2.2 Study criteria
To be eligible for our study, trials were required to have administered neoadjuvant immunochemotherapy to patients diagnosed with operable NSCLC and acquired radiological or pathological response data. Enrolled patients should not have received any prior systemic anti-neoplastic treatment for NSCLC, must have had no history of lung radiotherapy, and should have undergone surgical resection for NSCLC. Excluded were trials involving patients with concurrent progressive or actively treated additional malignancies, those who had received previous systemic antineoplastic therapy for NSCLC, or those with a history of lung radiotherapy. Studies falling into categories such as reviews, comments, case reports, trial protocols, or animal experiments were also eliminated. In the context of randomized controlled trials (RCTs), preference was given to those that compared non-combination therapy with combination therapy, establishing a basis for a comparison group. In cases where multiple publications reported results from the same study population across different journals, the most comprehensive or most current study was selected for inclusion.
2.3 Data extraction and quality assessment
We screened these literatures based on pre-determined inclusion and exclusion criteria. Two authors independently screened the records, read the full-text papers, and extracted details from the included studies. The primary endpoints were pCR (no residual vital cancer cells), major pathologic response (MPR, <= 10% residual vital cancer cells), the incidence of grade 3 or higher treatment-related adverse events (TRAEs), and 1-year and 2-year OS (the duration from randomization to death from any reason). The secondary endpoints were objective response rate (ORR, proportion of patients with a partial or complete response), total grade TRAEs, R0 resection rate, and the incidence of surgical complications. We assessed the quality of RCTs using the Cochrane Collaboration’s tool (28). For dual-arm non-RCTs, we used the Newcastle-Ottawa scale, and for single-arm non-RCTs, we used the Methodological Index for Non-Randomized Studies criteria (MINORS) to assess quality (29, 30). We consulted a third referee to resolve discrepancies.
2.4 Data synthesis and statistical analysis
For single-arm studies, we combined the proportion of each endpoint with a 95% confidence interval (95% CI) to draw forest plots. For dual-arm studies, we calculated the risk ratio (RR) and 95% CI. We used the Cochrane Q test and I2 test to determine statistical heterogeneity. If I2 > 50% or p < 0.05, we used the random effects model. If I2 < 50% or p > 0.05, we used the fixed effects model. We carried out subgroup analysis according to age, gender, smoking history, histology, stage, treatment cycles, pretreatment PD-L1, and ICI type. The sensitivity analysis evaluated the stability of the results by ruling out each trial separately. We evaluated publication bias using funnel plots. We used R 4.3.1 software and the meta_v6.2-1 package for the analysis.
3 Results
3.1 Study selection
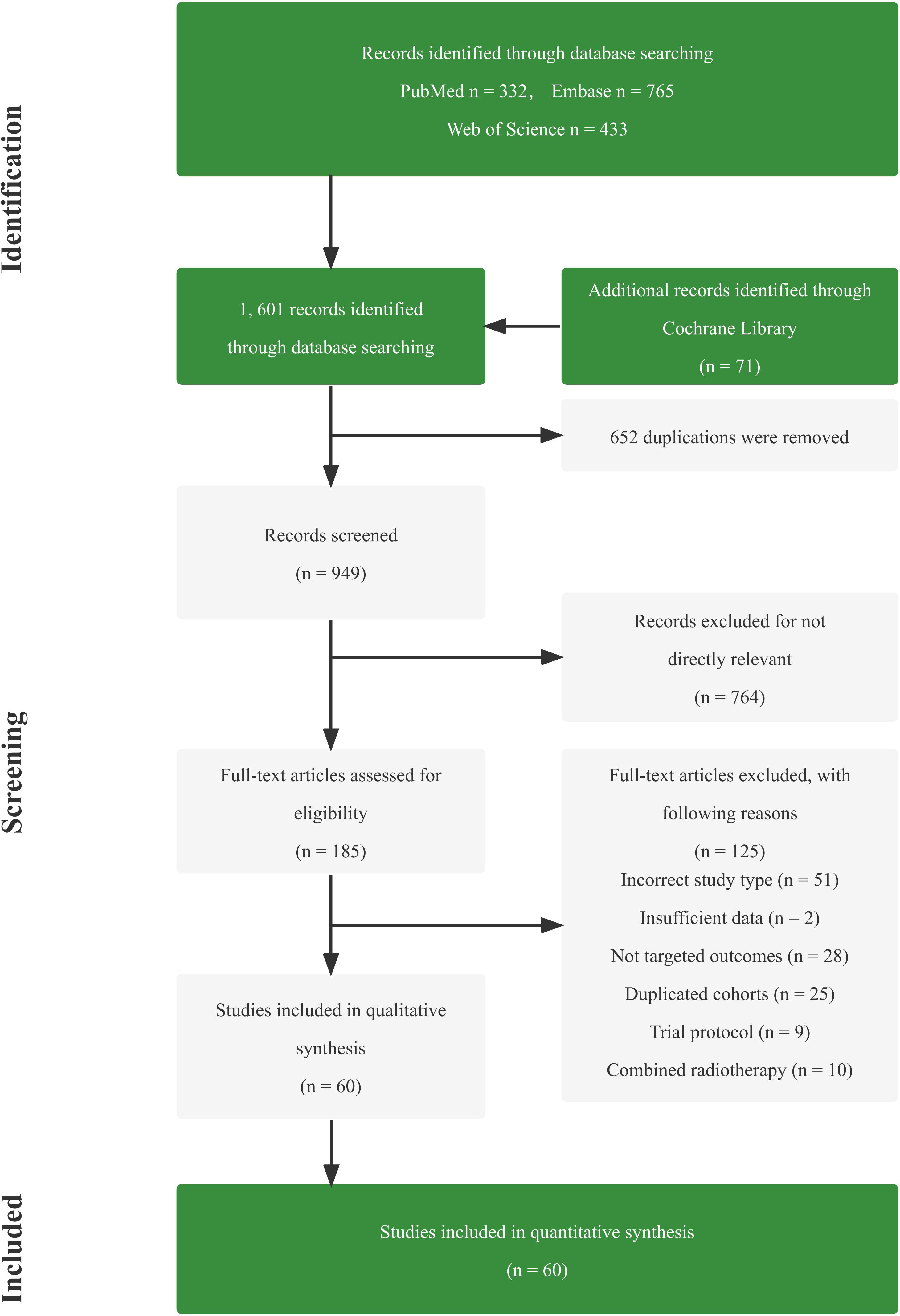
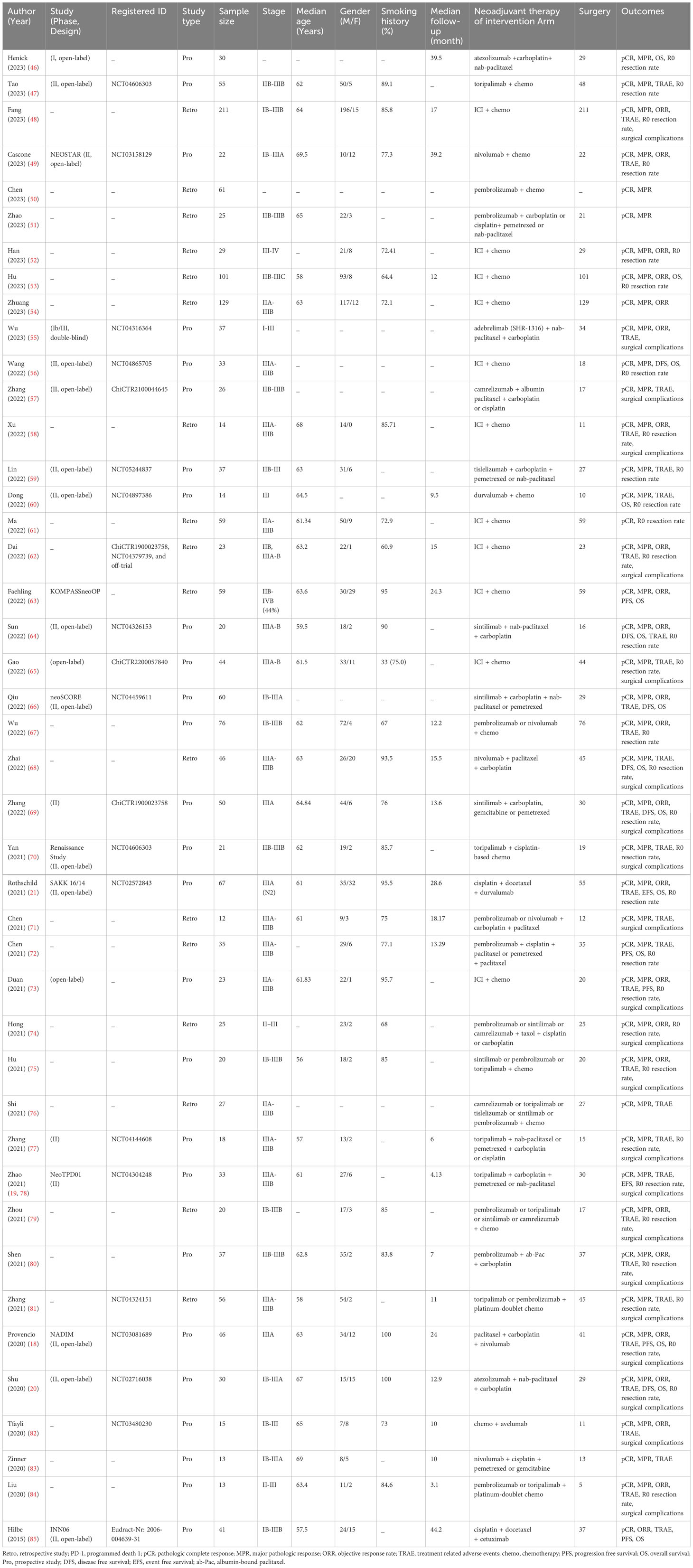
Totally, 1,601 studies were screened. After eliminating duplicates and irrelevant studies based on their titles and abstracts, 1,416 were excluded, and the remaining 185 studies were reviewed in detail. Out of these, 125 studies were further excluded due to reasons such as incorrect study type, insufficient data, non-targeted outcomes, duplicated cohorts, trial protocol, and treatment combined with radiotherapy. Ultimately, 60 studies comprising 4 RCTs, 13 dual-arm cohorts, and 43 single-arm studies were selected for analysis, with a total of 3,632 eligible patients included. Figure 1; Supplementary Table 1 provides details of this literature search. Tables 1, 2 suggest the characteristics of the eligible studies. All the included studies were considered moderately or highly credible, and Supplementary Figure 3 provides funnel plots. The quality scores of each eligible study are presented in Supplementary Tables 2-4.
3.2 Efficacy of neoadjuvant immunochemotherapy
The efficacy of neoadjuvant immunochemotherapy was assessed based on pCR, MPR, and ORR rates, with neoadjuvant immunochemotherapy showing significantly better efficacy than neoadjuvant chemotherapy. The estimated RR was 4.71 (95% CI: 3.69, 6.02, I2 = 0%) for pCR, 3.20 (95% CI: 2.75, 3.74, I2 = 26%) for MPR, and 1.46 (95% CI: 1.21, 1.77, I2 = 62%) for ORR (Figures 2A, B; Supplementary Figure 1A). For neoadjuvant immunochemotherapy in NSCLC, the pooled pCR rate was 0.35 (95% CI: 0.31, 0.39, I2 = 78%), the MPR rate was 0.59 (95% CI: 0.54, 0.63, I2 = 85%), and the ORR rate was 0.71 (95% CI: 0.66, 0.76, I2 = 82%) (Figure 3).
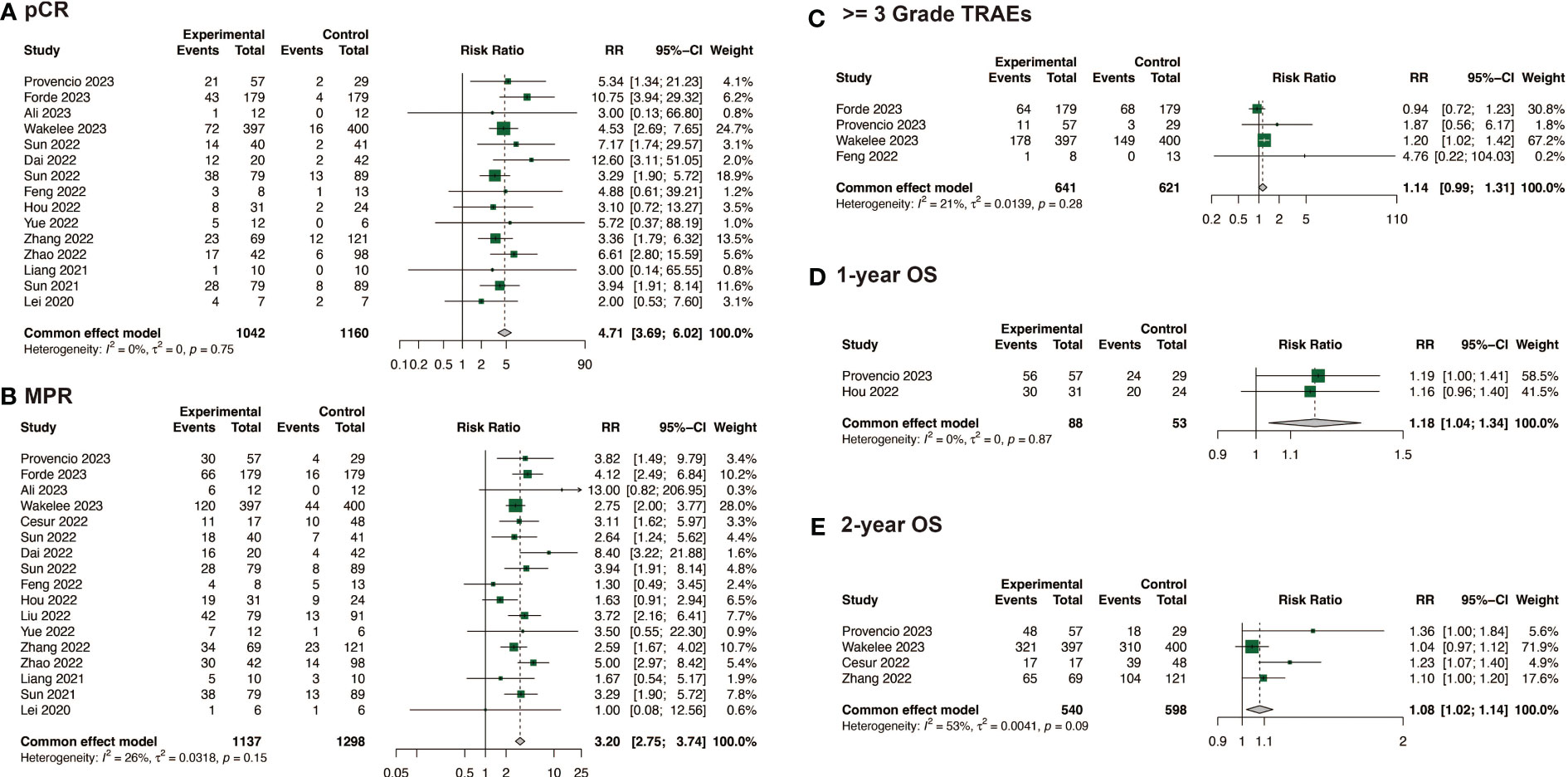
Figure 2 Comparison of efficacy, safety and survival between neoadjuvant immunochemotherapy with neoadjuvant chemotherapy alone. (A) Comparison of pCR; (B) Comparison of MPR; (C) Comparison of >= 3 Grade TRAEs; (D) Comparison of 1-year OS; (E) Comparison of 2-year OS.
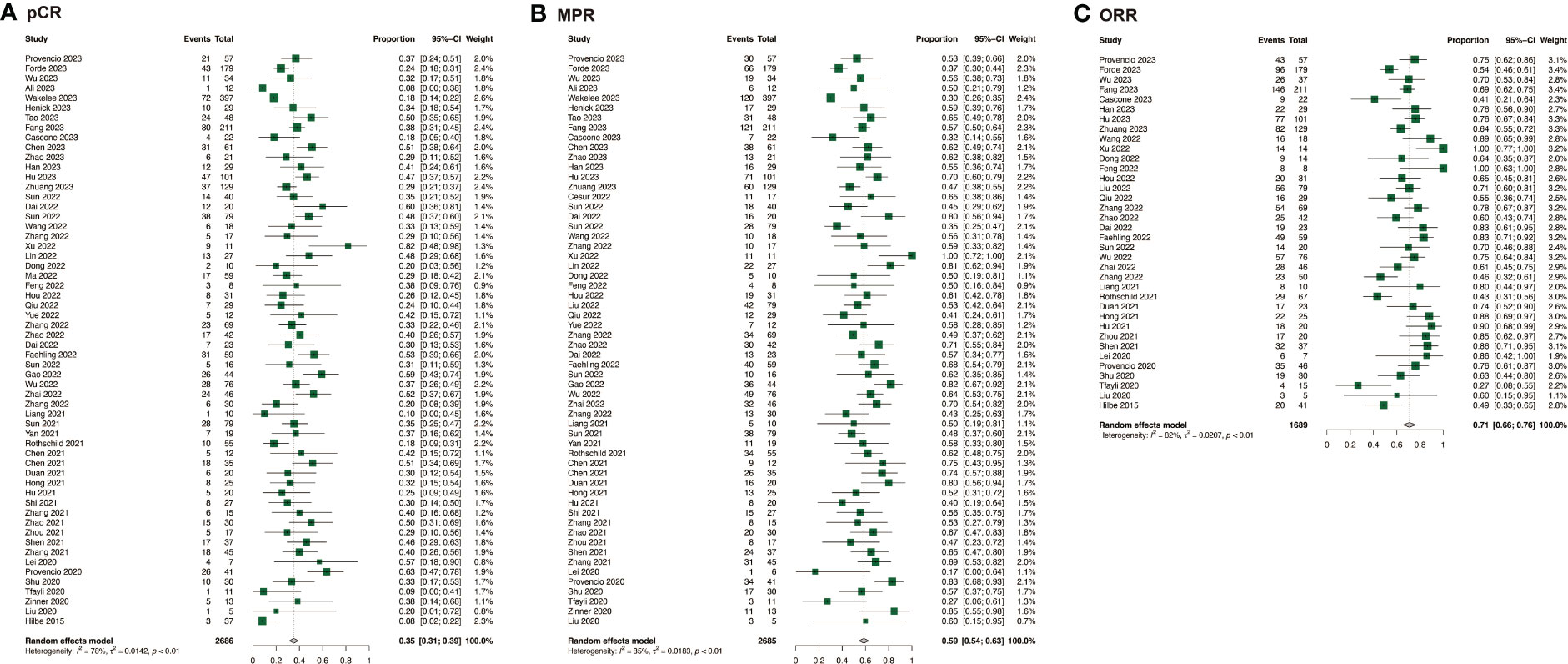
Figure 3 Efficacy of neoadjuvant immunochemotherapy in resectable non-small cell lung cancer. (A) pCR of neoadjuvant immunochemotherapy in resectable non-small cell lung cancer; (B) MPR of neoadjuvant immunochemotherapy in resectable non-small cell lung cancer; (C) ORR of neoadjuvant immunochemotherapy in resectable non-small cell lung cancer.
3.3 Safety of neoadjuvant immunochemotherapy
In the comparison of safety and surgical outcomes between neoadjuvant immunochemotherapy and neoadjuvant chemotherapy, the estimated RR for >= grade 3 TRAEs was 1.14 (95%CI: 0.99, 1.31, I2 = 21%) (Figure 2C) and for all grade TRAEs, the RR was 1.00 (95%CI: 0.96, 1.03, I2 = 19%), suggesting no significant difference (Supplementary Figure 1B). However, neoadjuvant immunochemotherapy may result in fewer surgical complications (RR: 0.67, 95%CI: 0.48, 0.94, I2 = 0%) and higher R0 resection rate (RR: 1.06, 95%CI: 1.03, 1.10, I2 = 52%) (Supplementary Figures 1C, D). The pooled incidence of >= grade 3 TRAEs was 0.24 (95%CI: 0.16, 0.32, I2 = 96%)(Figure 4A). The pooled incidence of all grade TRAEs was 0.70 (95%CI: 0.60, 0.81, I2 = 97%) and that of surgical complications was 0.13 (95%CI: 0.07, 0.18, I2 = 82%), and the R0 resection rate was 0.98 (95%CI: 0.96, 0.99, I2 = 61%) (Supplementary Figure 2).
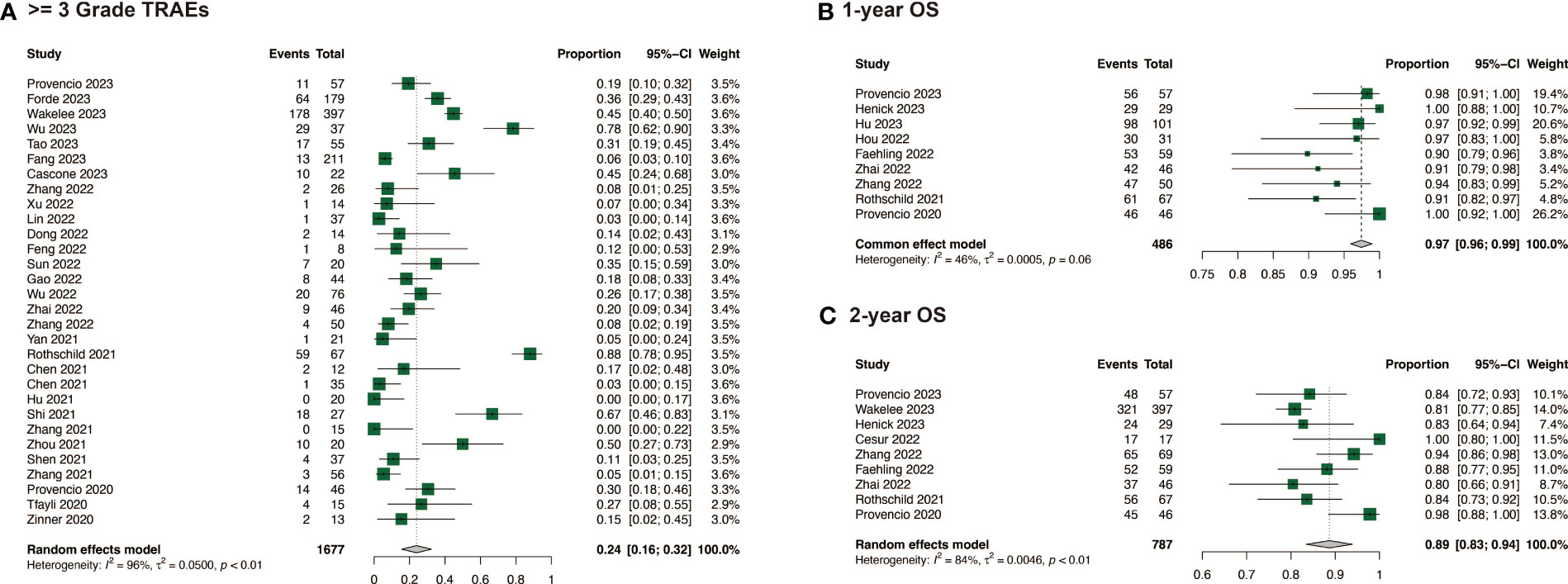
Figure 4 Safety and survival of neoadjuvant immunochemotherapy in resectable non-small cell lung cancer. (A) >= 3 Grade TRAEs of neoadjuvant immunochemotherapy in resectable non-small cell lung cancer; (B) 1-year OS of neoadjuvant immunochemotherapy in resectable non-small cell lung cancer; (C) 2-year OS of neoadjuvant immunochemotherapy in resectable non-small cell lung cancer.
3.4 Survival of neoadjuvant immunochemotherapy
When compared with neoadjuvant chemotherapy, neoadjuvant immunotherapy may significantly enable long survival for patients, with a RR of 1.18 (95%CI: 1.04, 1.34, I2 = 0%) for 1-year OS, and 1.08 (95%CI: 1.02, 1.14, I2 = 53%) for 2-year OS (Figures 2D, E). Among the studies that reported specific survival data for patients with NSCLC receiving neoadjuvant immunochemotherapy, the pooled results were 0.97 (95%CI: 0.96, 0.99, I2 = 46%) for 1-year OS, and 0.89 (95%CI: 0.83, 0.94, I2 = 84%) for 2-year OS (Figures 4B, C).
3.5 Sensitivity analysis and subgroup analysis
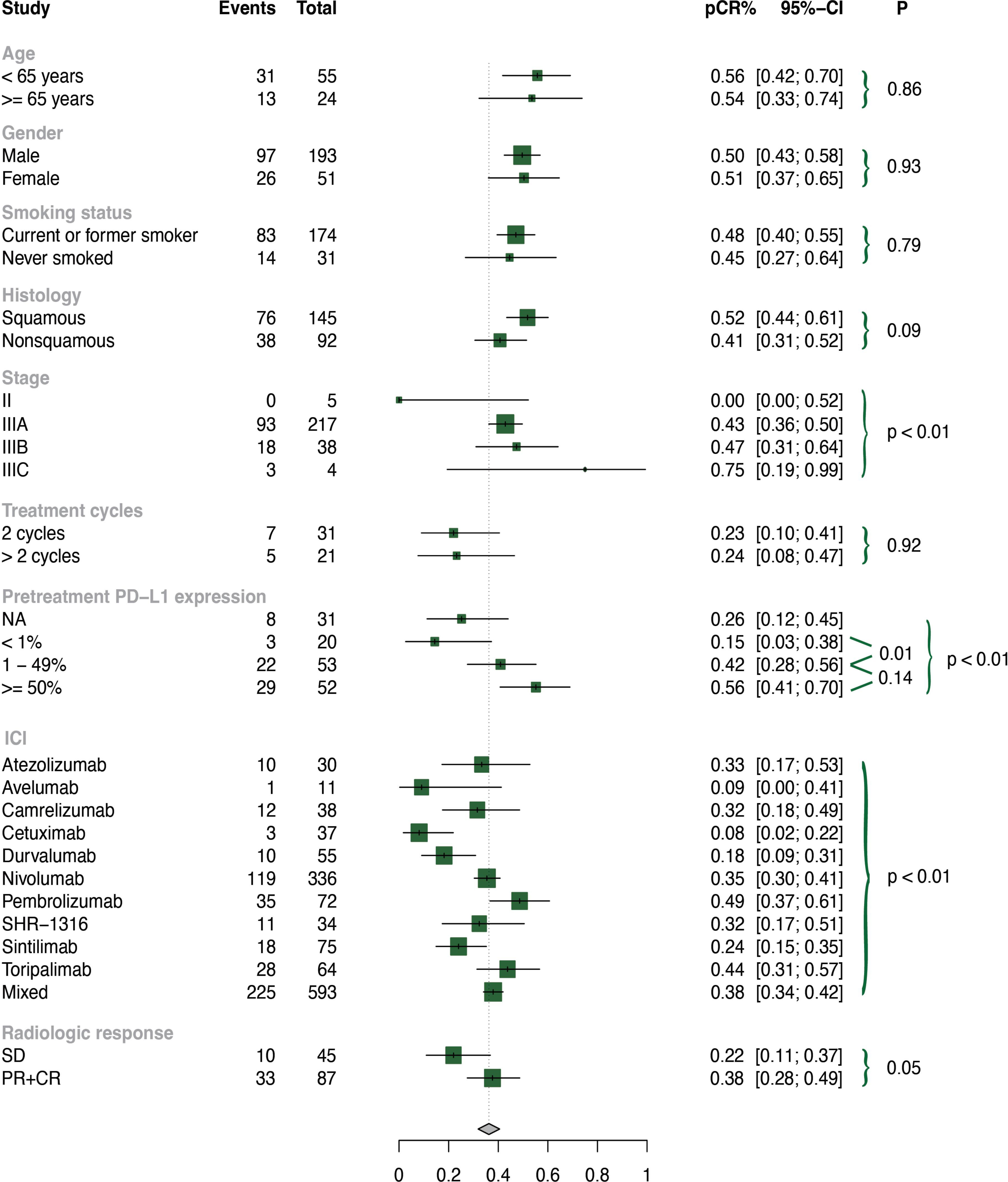
To test the stability, we performed sensitivity analyses by removing each individual trial, and found that our selected studies were reliable (Supplementary Figure 4). We also performed subgroup analyses, and the results are presented in Figure 5; Supplementary Figures 5-7.
As basic clinical characteristics may contribute to heterogeneity, we conducted subgroup analyses based on age, gender, and smoking history in the neoadjuvant immunochemotherapy group. However, no significant differences were found in these subgroups (all p values > 0.05).
Among the included patients, the histology subtypes were divided into squamous and non-squamous. Neoadjuvant immunochemotherapy treatment in patients with squamous lung cancer performed significantly higher rates of MPR (p = 0.03) and ORR (p < 0.01), and a tendency towards better pCR without reaching statistical significance (p = 0.09). Stage is also a key factor of heterogeneity, so we further explored subgroups based on stage (II, IIIA, IIIB, IIIC). We found that patients with stage II NSCLC experienced less benefit in terms of pCR (p < 0.01) and ORR (p = 0.05) than those with advanced stage.
The optimal treatment cycle for neoadjuvant immunochemotherapy remains uncertain, with no clear evidence indicating whether 2 or more cycles are superior. To investigate this, we conducted a subgroup analysis of treatment cycles (2 cycles vs. >2 cycles) and found no significant discrepancies in pCR (p = 0.92), MPR (p = 0.80), or ORR (p = 0.61) between these subgroups. We also examined the effect of pretreatment PD-L1 expression and found that patients with higher PD-L1 (TPS ≥ 50% or TPS = 1-49%) had significantly improved pCR, MPR, and ORR compared to those with lower PD-L1 (TPS < 1%). Patients who achieved partial response (PR) or complete response (CR) had higher MPR rates than those with stable disease (SD) (p < 0.01).
We observed significant differences among subgroups in pCR, MPR, ORR, and 3 or higher grade TRAEs for different ICI types (p < 0.01). Pembrolizumab-based neoadjuvant immunochemotherapy demonstrated higher pCR (0.49, 95% CI: 0.37-0.61), MPR (0.69, 95% CI: 0.57-0.80), and ORR (0.86, 95% CI: 0.71-0.95) rates. Toripalimab-based neoadjuvant immunochemotherapy showed higher pCR (0.44, 95% CI: 0.31-0.57) and MPR (0.61, 95% CI: 0.48-0.73) rates. Nivolumab-based neoadjuvant immunochemotherapy had higher pCR (0.35, 95% CI: 0.30-0.41) and ORR (0.62, 95% CI: 0.56-0.67) rates. In contrast, avelumab-based neoadjuvant immunochemotherapy demonstrated relatively lower pCR (0.09, 95% CI: 0.00-0.41), MPR (0.27, 95% CI: 0.06-0.61), and ORR (0.27, 95% CI: 0.08-0.55) rates. Pembrolizumab- (0.06, 95% CI: 0.00-0.13) and toripalimab-based (0.02, 95% CI: 0.00-0.08) neoadjuvant immunochemotherapy had significantly lower incidence of 3 or higher grade TRAEs than other ICIs (p < 0.01).
4 Discussion
ICIs plus chemotherapy have emerged in the neoadjuvant therapy of NSCLC. This approach has demonstrated good therapeutic effects and safety, offering new hope for the prolonged survival of patients with NSCLC (86). It represents the current direction of NSCLC neoadjuvant therapy. However, there is still a need to further evaluate the efficacy, safety, and survival of this treatment for operable NSCLC. To address this, we conducted this meta-analysis. Our analysis, which included 60 studies and 3,632 patients, found that neoadjuvant immunochemotherapy was superior to neoadjuvant chemotherapy in terms of achieving higher rates of pCR, MPR, and ORR. Additionally, neoadjuvant immunochemotherapy was related to a lower incidence of surgical complications and longer 1-year and 2-year OS, without affecting TRAEs and R0 resection rates. These findings provide valuable reference for the clinical treatment of NSCLC.
Our study investigated the efficacy of neoadjuvant immunochemotherapy for NSCLC, and the results showed that the pooled pCR was 0.35 (95% CI: 0.31, 0.39), MPR was 0.59 (95% CI: 0.54, 0.64), and ORR was 0.71 (95% CI: 0.66, 0.76). These rates were significantly higher than those for neoadjuvant chemotherapy (pooled pCR of 0.04) and neoadjuvant immunotherapy (pCR of no more than 0.10) reported in previous studies (7, 15, 16). Combination therapy can achieve optimal treatment effects by stimulating tumor cell mutations, releasing new tumor antigens, and restructuring the immune microenvironment (87). Our study found that neoadjuvant immunochemotherapy performed better for patients with squamous cell carcinoma, or stage III (p < 0.01). Previous studies have also shown that neoadjuvant systemic therapy brings greater clinical benefits to patients with stage III, but caution is needed when assessing pathologic response due to bias introduced by non-operative patients (22). It is possible that patients with squamous cell carcinoma, or stage III are associated with a high level of tumor mutational burden (TMB), inflammation, and PD-L1 expression, which may make them more responsive to immunotherapy (88). However, it is crucial to remember that these factors are not absolute for individual patients.
In neoadjuvant immunochemotherapy for NSCLC, the pooled 1-year OS was 0.97 (95%CI: 0.96, 0.99), and 2-year OS was 0.89 (95%CI: 0.83, 0.94). The benefit of neoadjuvant chemotherapy of OS, compared to operation, is only 5%-6%. The CheckMate 816 trial showed that preoperative nivolumab in combination with chemotherapy resulted in a 37% lower risk of disease recurrence, progression, or death than chemotherapy (22). The SAKK 16/14 trial revealed an encouraging 1-year event-free survival (EFS) of 73% and 2-year EFS of 68% in the neoadjuvant durvalumab plus chemotherapy group (21). EFS measures the time from treatment initiation to the occurrence of any disease-related event and can provide an early assessment of treatment efficacy. However, we did not evaluate EFS in our study because the endpoint of survival is non-uniform, including EFS, OS, progression-free survival (PFS), and disease-free survival, making the survival outcomes difficult to analyze.
Our study suggested that neoadjuvant immunochemotherapy did not increase TRAEs compared with neoadjuvant chemotherapy and may lead to fewer surgical complications, fully confirming its safety. The pooled rate of all grade TRAEs was 0.60 (95% CI: 0.60, 0.81), and that of grade 3 or higher TRAEs was 0.24 (95% CI: 0.16, 0.32). In NSCLC, immune-related adverse events, including pneumonitis, thyroid dysfunction, and skin rash, are the most common types of TRAEs associated with ICIs used in neoadjuvant immunochemotherapy. The pooled rate of surgical complications of neoadjuvant immunochemotherapy was 0.13 (95% CI: 0.07, 0.18), and the pooled R0 resection rate of neoadjuvant immunochemotherapy was 0.98 (95% CI: 0.96, 0.99). Although these adverse events could be serious and potentially life-threatening, they are relatively rare and can usually be managed effectively if detected and treated early. Close monitoring and prompt reporting of any symptoms to the healthcare provider are essential for ensuring the safety of neoadjuvant immunochemotherapy in patients with NSCLC.
Accurately identifying the population for neoadjuvant immunotherapy is critical. Our data show that higher PD-L1 expression (TPS >= 50% or TPS = 1-49%) performed better in neoadjuvant immunochemotherapy (p < 0.01). In the published NADIM trial, pCR patients had a higher proportion of PD-L1 positive tumors, but PD-L1 expression was not related to patient survival (18). The results of the CheckMate 816 study revealed that patients with pretherapy PD-L1 above 1% had longer EFS than those with PD-L1 below 1%, supporting PD-L1 as a predictor of neoadjuvant immunotherapy (22). However, in the phase II study of atezolizumab plus chemotherapy, no significant difference was found in MPR and pretreatment PD-L1 (20). TMB is a measure of the number of mutations in a tumor’s DNA and has been suggested as a potential predictive biomarker for response to neoadjuvant immunochemotherapy (22). Additionally, the preoperative ctDNA clearance rate may be related to a high predictive effect on postoperative recurrence (22, 40). However, the mechanism and predictive value of ctDNA clearance still need further exploration in basic research. Although our data suggest that these biomarkers can be used as predictors, more marker guidance is needed for patient selection and precise treatment due to the heterogeneity of the data.
Moreover, no significant differences were observed in pCR (p = 0.92), MPR (p = 0.80), and ORR (p = 0.61) between 2 or more treatment cycles, suggesting that increasing cycles of therapy may not increase efficacy. Patients who were PR or CR were related to a higher MPR rate than those in SD (p < 0.01). We also found that pembrolizumab- or toripalimab-based neoadjuvant immunochemotherapy performed better in efficacy without affecting >= 3 grade TRAEs. In most included studies, neoadjuvant immunotherapy combined with chemotherapy was used for 2-4 cycles, and operation was performed 4–6 weeks after neoadjuvant immunotherapy (14).
Our study has some limitations. Firstly, the follow-up time of some trials was not long enough to adequately report on long-term survival. Additionally, existing studies are still limited regarding the selection of effective predictors such as ctDNA and the timing of neoadjuvant immunotherapy or adjuvant therapy, making it difficult to obtain more novel results. Thirdly, the study outcomes were non-uniform, making it difficult to pool the survival results of EFS. Therefore, more innovative long-term RCTs are needed to overcome the above obstacles, and the internal mechanism of neoadjuvant immunochemotherapy needs to be further explored. Despite these limitations, this meta-analysis provides objective information on the efficacy, safety, and survival of neoadjuvant immunochemotherapy in operable NSCLC.
5 Conclusion
Our study demonstrates the reliable efficacy, safety, and survival of neoadjuvant immunochemotherapy for operable NSCLC, making it a promising direction for neoadjuvant treatment in the future.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
YZ: Conceptualization, Data curation, Formal analysis, Methodology, Software, Writing – original draft, Writing – review & editing. BF: Formal analysis, Methodology, Writing – original draft. JC: Formal analysis, Project administration, Writing – review & editing, Funding acquisition. LY: Conceptualization, Funding acquisition, Supervision, Investigation, Resources, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by Department of Science and Technology of Sichuan Province (2022YFS0205), the Project of Sichuan Province Key Laboratory Open Funding (grant No.SZKF202210), Natural Science Foundation of Sichuan Province of China (2023NSFSC1883) and the 2024 College Students’ Innovative Entrepreneurial Training Plan Program (Project No. C2024130171).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1273220/full#supplementary-material
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA: A Cancer J Clin (2022) 72:7–33. doi: 10.3322/caac.21708
2. Uramoto H, Tanaka F. Recurrence after surgery in patients with NSCLC. Transl Lung Cancer Res (2014) 3:242–9. doi: 10.3978/j.issn.2218-6751.2013.12.05
3. Taylor MD, Nagji AS, Bhamidipati CM, Theodosakis N, Kozower BD, Lau CL, et al. Tumor recurrence after complete resection for non-small cell lung cancer. Ann Thorac Surg (2012) 93:1813–20. doi: 10.1016/j.athoracsur.2012.03.031
4. Saw SPL, Ong BH, Chua KLM, Takano A, Tan DSW. Revisiting neoadjuvant therapy in non-small-cell lung cancer. Lancet Oncol (2021) 22:e501–e16. doi: 10.1016/s1470-2045(21)00383-1
5. Broglio KR, Quintana M, Foster M, Olinger M, McGlothlin A, Berry SM, et al. Association of pathologic complete response to neoadjuvant therapy in HER2-positive breast cancer with long-term outcomes: A meta-analysis. JAMA Oncol (2016) 2:751–60. doi: 10.1001/jamaoncol.2015.6113
6. Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman JR, Bharat A, et al. Non-small cell lung cancer, version 3.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw (2022) 20:497–530. doi: 10.6004/jnccn.2022.0025
7. Group NM-aC. Preoperative chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual participant data. Lancet (2014) 383:1561–71. doi: 10.1016/s0140-6736(13)62159-5
8. Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med (2018) 378:2078–92. doi: 10.1056/NEJMoa1801005
9. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med (2015) 373:1627–39. doi: 10.1056/NEJMoa1507643
10. Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med (2015) 373:123–35. doi: 10.1056/NEJMoa1504627
11. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Updated analysis of KEYNOTE-024: pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol (2019) 37:537–46. doi: 10.1200/jco.18.00149
12. Desai AP, Adashek JJ, Reuss JE, West HJ, Mansfield AS. Perioperative immune checkpoint inhibition in early-stage non-small cell lung cancer: A review. JAMA Oncol (2023) 9:135–42. doi: 10.1001/jamaoncol.2022.5389
13. Kang J, Zhang C, Zhong WZ. Neoadjuvant immunotherapy for non-small cell lung cancer: State of the art. Cancer Commun (Lond). (2021) 41:287–302. doi: 10.1002/cac2.12153
14. Liang W, Cai K, Chen C, Chen H, Chen Q, Fu J, et al. Expert consensus on neoadjuvant immunotherapy for non-small cell lung cancer. Transl Lung Cancer Res (2020) 9:2696–715. doi: 10.21037/tlcr-2020-63
15. Forde PM, Chaft JE, Pardoll DM. Neoadjuvant PD-1 blockade in resectable lung cancer. New Engl J Med (2018) 379:e14. doi: 10.1056/NEJMc1808251
16. Carbone D, Lee J, Kris M, Wistuba I, Kwiatkowski D, Owen D, et al. OA06.06 clinical/biomarker data for neoadjuvant atezolizumab in resectable stage IB-IIIB NSCLC: primary analysis in the LCMC3 study. J Thorac Oncol (2021) 16:S115–S6. doi: 10.1016/j.jtho.2021.01.294
17. Provencio M, Nadal E, Insa A, García Campelo R, Huidobro G, Domine M, et al. Phase II study of neo-adjuvant chemo/immunotherapy for resectable stages IIIA non-small cell lung cancer- nadim study-SLCG. J Thorac Oncol (2018) 13:S320. doi: 10.1016/j.jtho.2018.08.236
18. Provencio M, Nadal E, Insa A, García-Campelo MR, Casal-Rubio J, Dómine M, et al. Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol (2020) 21:1413–22. doi: 10.1016/s1470-2045(20)30453-8
19. Zhao Z, Chen S, Qi H, Yang CP, Lin YB, Jin JT, et al. Phase II trial of toripalimab plus chemotherapy as neoadjuvant treatment in resectable stage III non-small cell lung cancer (NeoTPD01 Study). J Clin Oncol (2021) 39:8541. doi: 10.1200/JCO.2021.39.15-suppl.8541
20. Shu CA, Gainor JF, Awad MM, Chiuzan C, Grigg CM, Pabani A, et al. Neoadjuvant atezolizumab and chemotherapy in patients with resectable non-small-cell lung cancer: an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol (2020) 21:786–95. doi: 10.1016/s1470-2045(20)30140-6
21. Rothschild SI, Zippelius A, Eboulet EI, Prince SS, Betticher D, Bettini A, et al. SAKK 16/14: durvalumab in addition to neoadjuvant chemotherapy in patients with stage IIIA(N2) non-small-cell lung cancer-A multicenter single-arm phase II trial. J Clin Oncol (2021) 39:2872–80. doi: 10.1200/jco.21.00276
22. Forde PM, Spicer J, Lu S, Provencio M, Mitsudomi T, Awad MM, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med (2022) 386:1973–85. doi: 10.1056/NEJMoa2202170
23. Forde PM, Spicer J, Girard N, Provencio M, Lu S, Wang C, et al. 84O Neoadjuvant nivolumab (N) + platinum-doublet chemotherapy (C) for resectable NSCLC: 3-y update from CheckMate 816. J Thorac Oncol (2023) 18:S89–90. doi: 10.1016/S1556-0864(23)00338-6
24. Provencio-Pulla M, Nadal E, Larriba JLG, Martinez-Marti A, Bernabé R, Bosch-Barrera J, et al. Nivolumab + chemotherapy versus chemotherapy as neoadjuvant treatment for resectable stage IIIA NSCLC: Primary endpoint results of pathological complete response (pCR) from phase II NADIM II trial. J Clin Oncol (2022) 40:8501. doi: 10.1200/JCO.2022.40.16_suppl.8501
25. Provencio M, Nadal E, González-Larriba JL, Martínez-Martí A, Bernabé R, Bosch-Barrera J, et al. Perioperative nivolumab and chemotherapy in stage III non-small-cell lung cancer. N Engl J Med (2023) 389:504–13. doi: 10.1056/NEJMoa2215530
26. Higgins JPT, Green SE. Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Collaboration website United Kingdom (2022). Available at: https://training.cochrane.org/handbook/current.
27. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (2021) 372:n71. doi: 10.1136/bmj.n71
28. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. Bmj (2011) 343:d5928. doi: 10.1136/bmj.d5928
29. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
30. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg (2003) 73:712–6. doi: 10.1046/j.1445-2197.2003.02748.x
31. Wakelee H, Liberman M, Kato T, Tsuboi M, Lee SH, Gao S, et al. Perioperative pembrolizumab for early-stage non-small-cell lung cancer. N Engl J Med (2023) 389: 491–503. doi: 10.1056/NEJMoa2302983
32. Alì G, Poma AM, Di Stefano I, Zirafa CC, Lenzini A, Martinelli G, et al. Different pathological response and histological features following neoadjuvant chemotherapy or chemo-immunotherapy in resected non-small cell lung cancer. Front Oncol (2023) 13:1115156. doi: 10.3389/fonc.2023.1115156
33. Cesur E, Ozer KB, Erus S, Bulutay P, Selcukbiricik F, Tanju S, et al. EP05.02-005 is immunotherapy safer than radiotherapy in combination with chemotherapy in neoadjuvant therapy? J Thorac Oncol (2022) 17:S284. doi: 10.1016/j.jtho.2022.07.487
34. Sun X, Liu W, Sun L, Mo H, Feng Y, Wu X, et al. Maturation and abundance of tertiary lymphoid structures are associated with the efficacy of neoadjuvant chemoimmunotherapy in resectable non-small cell lung cancer. J ImmunoTher Cancer. (2022) 10:e005531. doi: 10.1136/jitc-2022-005531
35. Dai F, Wu X, Wang X, Li K, Wang Y, Shen C, et al. Neoadjuvant immunotherapy combined with chemotherapy significantly improved patients' overall survival when compared with neoadjuvant chemotherapy in non-small cell lung cancer: A cohort study. Front Oncol (2022) 12:1022123. doi: 10.3389/fonc.2022.1022123
36. Sun X, Feng Y, Zhang B, Huang W, Zhao X, Zhang H, et al. The role of neutrophil-to-lymphocyte ratio in predicting pathological response for resectable non-small cell lung cancer treated with neoadjuvant chemotherapy combined with PD-1 checkpoint inhibitors. Cancer Res Treat (2022) 54:1017–29. doi: 10.4143/crt.2021.1007
37. Feng Y, Sun W, Zhang J, Wang Y, Chen J, Liu X, et al. Neoadjuvant PD-1 inhibitor combines with chemotherapy versus neoadjuvant chemotherapy in resectable squamous cell carcinoma of the lung. Thorac Cancer. (2022) 13:442–52. doi: 10.1111/1759-7714.14280
38. Hou X, Shi X, Luo J. Efficacy and safety of camrelizumab (a PD-1 inhibitor) combined with chemotherapy as a neoadjuvant regimen in patients with locally advanced non-small cell lung cancer. Oncol Lett (2022) 24:215. doi: 10.3892/ol.2022.13336
39. Liu Z, Gao Z, Zhang M, Wang X, Gong J, Jiang S, et al. Real-world effectiveness and prognostic factors analysis of stages I-III non-small cell lung cancer following neoadjuvant chemo-immunotherapy or neoadjuvant chemotherapy. Ann Thorac Cardiovasc Surg (2022) 28:111–20. doi: 10.5761/atcs.oa.21-00143
40. Yue D, Liu W, Chen C, Zhang T, Ma Y, Cui L, et al. Circulating tumor DNA predicts neoadjuvant immunotherapy efficacy and recurrence-free survival in surgical non-small cell lung cancer patients. Transl Lung Cancer Res (2022) 11:263–76. doi: 10.21037/tlcr-22-106
41. Zhang B, Xiao H, Pu X, Zhou C, Yang D, Li X, et al. A real-world comparison between neoadjuvant chemoimmunotherapy and chemotherapy alone for resectable non-small cell lung cancer. Cancer Med (2023) 12:274–86. doi: 10.1002/cam4.4889
42. Zhao D, Xu L, Wu J, She Y, Su H, Hou L, et al. Comparison of perioperative outcomes among non-small cell lung cancer patients with neoadjuvant immune checkpoint inhibitor plus chemotherapy, EGFR-TKI, and chemotherapy alone: a real-world evidence study. Transl Lung Cancer Res (2022) 11:1468–78. doi: 10.21037/tlcr-22-476
43. Liang H, Yang C, Gonzalez-Rivas D, Zhong Y, He P, Deng H, et al. Sleeve lobectomy after neoadjuvant chemoimmunotherapy/ chemotherapy for local advanced non-small cell lung cancer. Trans Lung Cancer Res (2021) 10:143–55. doi: 10.21037/tlcr-20-778
44. Sun X, Feng Y, Zhang B, Huang W, Zhao X, Zhang H, et al. The role of neutrophil-to-lymphocyte ratio in predicting pathological response for resectable NSCLC treated with neoadjuvant chemotherapy combined with PD-1 checkpoint inhibitors. Cancer Res Treat (2022) 54:1017–29. doi: 10.4143/crt.2021.1007
45. Lei J, Yan X, Zhao J, Tian F, Lu Q, Jiang T. 62MO A randomised, controlled, multicenter phase II trial of camrelizumab combined with albumin-bound paclitaxel and cisplatin as neoadjuvant treatment in locally advanced NSCLC. Ann Oncol (2020) 31:S1441–S2. doi: 10.1016/j.annonc.2020.10.550
46. Henick BS, Gainor JF, Awad MM, Chiuzan C, Izard S, Georgis Y, et al. 3-year update of neoadjuvant atezolizumab + chemotherapy in patients with resectable non-small cell lung cancer. Cancer Res (2023) 83:CT217. doi: 10.1158/1538-7445.AM2023-CT217
47. Tao Y, Li X, Liu B, Wang J, Lv C, Li S, et al. Association of early immune-related adverse events with treatment efficacy of neoadjuvant Toripalimab in resectable advanced non-small cell lung cancer. Front Oncol (2023) 13:1135140. doi: 10.3389/fonc.2023.1135140
48. Fang M, Hang Q, Jiang H, Cai L, Hu J, Ying H, et al. Efficacy and safety evaluation of neoadjuvant immunotherapy plus chemotherapy for resectable non–small cell lung cancer in real world. Front Oncol (2023) 12:1055610. doi: 10.3389/fonc.2022.1055610
49. Cascone T, Leung CH, Weissferdt A, Pataer A, Carter BW, Godoy MCB, et al. Neoadjuvant chemotherapy plus nivolumab with or without ipilimumab in operable non-small cell lung cancer: the phase 2 platform NEOSTAR trial. Nat Med (2023) 29:593–604. doi: 10.1038/s41591-022-02189-0
50. Chen Y, Yan B, You J. Neoadjuvant immunochemotherapy of pembrolizumab plus chemotherapy in resectable non-small cell lung cancer. J Thorac Oncol (2023) 18:S108–S9. doi: 10.1016/S1556-0864(23)00375-1
51. Zhao G, Zhang H, Xu F, Lu C, Zhu Q, Grossi F, et al. Neoadjuvant pembrolizumab and chemotherapy in resectable clinical stage III non-small-cell lung cancer: a retrospective cohort study. Transl Lung Cancer Res (2023) 12:141–9. doi: 10.21037/tlcr-22-871
52. Han R, Zhang Y, Wang T, Xiao H, Luo Z, Shen C, et al. Tumor immune microenvironment predicts the pathologic response of neoadjuvant chemoimmunotherapy in non–small-cell lung cancer. Cancer Science. (2023) 114:2569–83. doi: 10.1111/cas.15778
53. Hu X, Hu C, Liu X, Ma F, Xie J, Zhong P, et al. Tumor regression rate, PD-L1 expression, pembrolizumab/nab-paclitaxel–based regimens, squamous cell carcinoma, and comorbidities were independently associated with efficacy of neoadjuvant chemoimmunotherapy in non-small cell lung cancer. Front Oncol (2023) 12:1057646. doi: 10.3389/fonc.2022.1057646
54. Zhuang F, Haoran E, Huang J, Wu J, Xu L, Zhang L, et al. Utility of 18F-FDG PET/CT uptake values in predicting response to neoadjuvant chemoimmunotherapy in resectable non-small cell lung cancer. Lung Cancer. (2023) 178:20–7. doi: 10.1016/j.lungcan.2023.02.001
55. Yan W, Zhong WZ, Liu YH, Chen Q, Xing W, Zhang Q, et al. Adebrelimab (SHR-1316) in combination with chemotherapy as perioperative treatment in patients with resectable stage II to III NSCLCs: an open-label, multicenter, phase 1b trial. J Thorac Oncol (2023) 18:194–203. doi: 10.1016/j.jtho.2022.09.222
56. Wang T, Li L, Huang L, Liu J, Zhu DD, Qin C, et al. 82P Preliminary analysis of tislelizumab (TIS) and chemotherapy as neoadjuvant therapy for potentially resectable stage IIIA/IIIB non-small cell lung cancer (NSCLC). Immuno-Oncol Technol (2022) 16: 100186. doi: 10.1016/j.iotech.2022.100186
57. Zhang Y, Liu S, Yang L, Liu Y, Wang C, Han Y, et al. Camrelizumab combined with albumin paclitaxel and platinum in perioperative treatment of resectable squamous cell lung cancer: A single-arm, open-label, phase II clinical trial. Ann Oncol (2022) 33:S978. doi: 10.1016/j.annonc.2022.07.1067
58. Xu H, Wang W, Yin J, Song C, Li L, Sun Z. Efficacy and safety of the PD-1 inhibitor combined with albumin-bound paclitaxel and nedaplatin in preoperative neoadjuvant therapy of unresectable stage III lung squamous cell carcinoma. Drug Des Devel Ther (2022) 16:4269–77. doi: 10.2147/dddt.S388777
59. Lin YB, Long H, Chen YH, Zhai WY, Wang YZ, Rao BY. EP05.02-011 phase II trial of neoadjuvant tislelizumab with chemotherapy for resectable stage IIB-III non-small cell lung cancer. J Thorac Oncol (2022) 17:S287. doi: 10.1016/j.jtho.2022.07.493
60. Dong X, Tong F, Zhang R, Liang B, Zhai W, Wang S, et al. Neoadjuvant durvalumab plus chemotherapy in stage III non-small cell lung cancer: A phase II single-center exploratory study. Immuno-Oncol Technol (2022) 16:100240. doi: 10.1016/j.iotech.2022.100240
61. Ma T, Wen T, Cheng X, Wang Y, Wei P, Yang B, et al. Pathological complete response to neoadjuvant chemoimmunotherapy correlates with peripheral blood immune cell subsets and metastatic status of mediastinal lymph nodes (N2 lymph nodes) in non-small cell lung cancer. Lung Cancer. (2022) 172:43–52. doi: 10.1016/j.lungcan.2022.08.002
62. Dai J, Zhu X, Li D, Huang Y, Liu X, He W, et al. Sleeve resection after neoadjuvant chemoimmunotherapy in the treatment of locally advanced non-small cell lung cancer. Trans Lung Cancer Res (2022) 11:188–200. doi: 10.21037/tlcr-22-56
63. Faehling M, Witte H, Sebastian M, Ulmer M, Saetzler R, Steinestel K, et al. Real-world multicentre analysis of neoadjuvant immunotherapy and chemotherapy in localized or oligometastatic non-small cell lung cancer (KOMPASSneoOP). Ther Adv Med Oncol (2022) 14:17588359221085333. doi: 10.1177/17588359221085333
64. Sun C, Liu Y, Zhang P, Wang X, Xu Y, Lin X, et al. Interim analysis of the efficiency and safety of neoadjuvant PD-1 inhibitor (sintilimab) combined with chemotherapy (nab-paclitaxel and carboplatin) in potentially resectable stage IIIA/IIIB non-small cell lung cancer: a single-arm, phase 2 trial. J Cancer Res Clin Oncol (2023) 149:819–31. doi: 10.1007/s00432-021-03896-w
65. Gao Y, Jiang J, Xiao D, Zhou Y, Chen Y, Yang H, et al. Robotic-assisted thoracic surgery following neoadjuvant chemoimmunotherapy in patients with stage III non-small cell lung cancer: A real-world prospective cohort study. Front Oncol (2022) 12:969545. doi: 10.3389/fonc.2022.969545
66. Qiu F, Fan J, Shao M, Yao J, Zhao L, Zhu L, et al. Two cycles versus three cycles of neoadjuvant sintilimab plus platinum-doublet chemotherapy in patients with resectable non-small-cell lung cancer (neoSCORE): A randomized, single center, two-arm phase II trial. J Clin Oncol (2022) 40:8500. doi: 10.1200/JCO.2022.40.16_suppl.8500
67. Wu J, Hou L EH, Zhao Y, Yu X, Xu L, Ning Y, et al. Real-world clinical outcomes of neoadjuvant immunotherapy combined with chemotherapy in resectable non-small cell lung cancer. Lung Cancer. (2022) 165:115–23. doi: 10.1016/j.lungcan.2022.01.019
68. Zhai H, Li W, Jiang K, Zhi Y, Yang Z. Neoadjuvant nivolumab and chemotherapy in patients with locally advanced non-small cell lung cancer: A retrospective study. Cancer Manag Res (2022) 14:515–24. doi: 10.2147/cmar.S344343
69. Zhang P, Dai J, Sun F, Xia H, He W, Duan L, et al. Neoadjuvant sintilimab and chemotherapy for resectable stage IIIA non-small cell lung cancer. Ann Thorac Surg (2022) 114:949–58. doi: 10.1016/j.athoracsur.2022.01.039
70. Yan S, Chen J, Wang J, Lv C, Bi J, Yang X, et al. 64P Neoadjuvant toripalimab plus chemotherapy in patients with potentially resectable non-small cell lung cancer: A prospective, single-arm, phase II trial (Renaissance Study). Ann Oncol (2021) 32:S1400. doi: 10.1016/j.annonc.2021.10.082
71. Chen T, Ning J, Campisi A, Dell'Amore A, Ciarrocchi AP, Li Z, et al. Neoadjuvant PD-1 inhibitors and chemotherapy for locally advanced NSCLC: A retrospective study. Ann Thorac Surg (2021) 113:993–9. doi: 10.1016/j.athoracsur.2021.03.041
72. Chen Y, Yan B, Xu F, Hui Z, Zhao G, Liu J, et al. Neoadjuvant chemoimmunotherapy in resectable stage IIIA/IIIB non-small cell lung cancer. Transl Lung Cancer Res (2021) 10:2193–204. doi: 10.21037/tlcr-21-329
73. Duan H, Wang T, Luo Z, Tong L, Dong X, Zhang Y, et al. Neoadjuvant programmed cell death protein 1 inhibitors combined with chemotherapy in resectable non-small cell lung cancer: an open-label, multicenter, single-arm study. Transl Lung Cancer Res (2021) 10:1020–8. doi: 10.21037/tlcr-21-130
74. Hong T, Sun T, Zhang M, Liu X, Yuan Y, Dolo PR, et al. Surgical perspective in neoadjuvant chemoimmunotherapy for stage II–III non-small cell lung cancer. Thorac Cancer. (2021) 12:2796–802. doi: 10.1111/1759-7714.14127
75. Hu Y, Ren S-Y, Wang R-Y, Zeng C, Li J-N, Xiao P, et al. Surgical outcomes after neoadjuvant chemoimmunotherapy for resectable non-small cell lung cancer. Front Oncol (2021) 11:684070. doi: 10.3389/fonc.2021.684070
76. Shi L, Liu Z, Meng Q, Tong L, Li H. Pathologic response to neoadjuvant PD-1 inhibitors and chemotherapy in squamous non-small-cell lung cancer. J Thorac Oncol (2021) 16:S979. doi: 10.1016/j.jtho.2021.08.269
77. Zhang Y, Zeng L, Zhang X, Zhou Y, Zhang B, Jiang W, et al. P15.02 toripalimab and platinum-doublet chemotherapy as neoadjuvant therapy for potentially resectable non-small cell lung cancer. J Thorac Oncol (2021) 16:S1014–S5. doi: 10.1016/j.jtho.2021.08.339
78. Zhao ZR, Long H. Updated event-free survival of neoadjuvant toripalimab with chemotherapy for resectable stage III NSCLC (NeoTAP01 study). Ann Oncol (2022) 33:S984. doi: 10.1016/j.annonc.2022.07.1081
79. Zhou S, Hao X, Yu D, Liu S, Cao X, Su C, et al. Preliminary efficacy evaluation of neoadjuvant immunotherapy combined with chemotherapy in resectable non-small cell lung cancer. Chin J Lung Cancer. (2021) 24:420–5. doi: 10.3779/j.issn.1009-3419.2021.102.13
80. Shen D, Wang J, Wu J, Chen S, Li J, Liu J, et al. Neoadjuvant pembrolizumab with chemotherapy for the treatment of stage IIB-IIIB resectable lung squamous cell carcinoma. J Thorac Dis (2021) 13:1760–8. doi: 10.21037/jtd-21-103
81. Zhang Y, Zeng L, Zhang X, Zhou Y, Zhang B, Guo L, et al. 1160P Efficacy and biomarker identification of neoadjuvant chemo-immunotherapy in potentially resectable non-small cell lung cancer. Ann Oncol (2021) 32:S934. doi: 10.1016/j.annonc.2021.08.1763
82. Tfayli A, Al Assaad M, Fakhri G, Akel R, Atwi H, Ghanem H, et al. Neoadjuvant chemotherapy and Avelumab in early stage resectable nonsmall cell lung cancer. Cancer Med (2020) 9:8406–11. doi: 10.1002/cam4.3456
83. Zinner R, Axelrod R, Solomides CC, Cowan S, Leiby B, Bhatia AK, et al. Neoadjuvant nivolumab (N) plus cisplatin (C)/pemetrexed (P) or cisplatin /gemcitabine (G) in resectable NSCLC. J Clin Oncol (2020) 38:9051. doi: 10.1200/JCO.2020.38.15-suppl.9051
84. Liu YT, Gao YS, Mao YS, Jiang J, Yang L, Yang JL, et al. [The outcome and safety of neoadjuvant PD-1 blockade plus chemotherapy in stage II~III non-small cell lung cancer]. Zhonghua Zhong Liu Za Zhi. (2020) 42:480–5. doi: 10.3760/cma.j.cn112152-20200213-00087
85. Hilbe W, Pall G, Kocher F, Pircher A, Zabernigg A, Schmid T, et al. Multicenter phase II study evaluating two cycles of docetaxel, cisplatin and cetuximab as induction regimen prior to surgery in Chemotherapy-Naive patients with NSCLC stage IB-IIIA (INN06-Study). PloS One (2015) 10:e0125364. doi: 10.1371/journal.pone.0125364
86. Zhang B, Zhong H, Han B. Neoadjuvant immunotherapy for patients with non-small cell lung cancer-is a new era coming? JAMA Oncol (2023) 9:301–2. doi: 10.1001/jamaoncol.2022.6898
87. Pfirschke C, Engblom C, Rickelt S, Cortez-Retamozo V, Garris C, Pucci F, et al. Immunogenic chemotherapy sensitizes tumors to checkpoint blockade therapy. Immunity (2016) 44:343–54. doi: 10.1016/j.immuni.2015.11.024
88. Haddad RI, Seiwert TY, Chow LQM, Gupta S, Weiss J, Gluck I, et al. Influence of tumor mutational burden, inflammatory gene expression profile, and PD-L1 expression on response to pembrolizumab in head and neck squamous cell carcinoma. J Immunother Cancer. (2022) 10:e003026. doi: 10.1136/jitc-2021-003026
Keywords: non-small cell lung cancer, neoadjuvant immunochemotherapy, efficacy, safety, survival
Citation: Zheng Y, Feng B, Chen J and You L (2023) Efficacy, safety, and survival of neoadjuvant immunochemotherapy in operable non-small cell lung cancer: a systematic review and meta-analysis. Front. Immunol. 14:1273220. doi: 10.3389/fimmu.2023.1273220
Received: 05 August 2023; Accepted: 21 November 2023;
Published: 01 December 2023.
Edited by:
Jiaxi He, First Affiliated Hospital of Guangzhou Medical University, ChinaCopyright © 2023 Zheng, Feng, Chen and You. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liting You, youlitin_med@163.com
†These authors have contributed equally to this work
 Yue Zheng
Yue Zheng Baijie Feng1†
Baijie Feng1†