- 1Women and Children's Hospital Affiliated to Qingdao University, Heart center, Qingdao, China
- 2University of California, Los Angeles, Department of Cardiology, Los Angeles, CA, United States
- 3School of Laboratory Medicine and Bioengineering, Key Laboratory of Biomarkers and In Vitro Diagnosis Translation of Zhejiang province, Key Laboratory of Bio-tech Vaccine of Zhejiang Province, Engineering Research Center of Novel Vaccine of Zhejiang Province, Hangzhou Medical College, Hangzhou, China
COVID-19 is an inflammatory disease with multiple organs involved, mainly respiratory symptoms. Although the majority of patients with COVID-19 present with a mild to moderate self-limited course of illness, about 5-10% of patients with inflammatory disorders in severe COVID-19 have life-threatening progression. With the exception of a few drugs that have shown outstanding anti-COVID-19 effects, the efficacy of most drugs remains controversial. An increasing number of animal and clinical studies have shown that neuromodulation has a significant effect on reducing inflammatory markers of COVID-19, thus exerting an effective neuroimmunotherapeutic value. Currently, the main neuroimmunomodulatory measures effective against COVID-19 include vagus nerve stimulation, electroacupuncture, and cholinergic drugs. In this review, we will summarize the research progress of potential value of this neuroimmunotherapy measures for COVID-19 and elaborate its efficacies and mechanisms, in order to provide reliable evidence for clinical intervention.
1 Introduction
The COVID-19 pandemic continues to spread in an unprecedented way, causing inconvenience and challenges around the world. In particular, asymptomatic infections can lead to rapid spread of the virus (1–3). Although the majority of cases are mild or moderate, about 5%-10% of cases with inflammatory disorders are severe and life-threatening. This means that in most cases COVID-19 patients do not need antiviral treatment. However, waiting until a patient is seriously ill to start treatment may miss the window for early treatment, during which the course of the disease is more likely to change. Therefore, it is urgent to explore effective interventional measures (4).
Since the outbreak of COVID-19 more than three years ago, the search for intervention strategies to prevent or combat novel coronavirus has become the focus of clinical research (5). It is generally considered that antiviral and non-antiviral therapy, inflammatory regulation, and critical supportive care constitute the entire COVID-19 treatment system. In particular, anti-inflammatory therapy is an important part of the whole treatment. As treatment protocols are constantly updated and optimized during the pandemic, clinicians are required to keep an eye on treatment plan for COVID-19. Moreover, vaccines are a routine medical strategy against the virus, and many countries have been preparing them since the beginning of the pandemic (6, 7). As a result, restarting antiviral vaccine research will take more time and cost. Although there are no specific drugs or treatments officially approved to treat COVID-19, some therapies and drugs have shown promising results in preclinical or clinical trials (8, 9). However, many factors such as technical requirements and virus variation have always restricted the clinical efficacy. At the same time, immediate validation of COVID-19 with known antiviral drugs is a highly effective screening route. The process of discovering a new drug typically takes a decade or more, which is clearly not appropriate for the current emergency outbreak. One effective method currently in use is to test whether existing drugs or methods have antiviral/anti-inflammatory activity against SARS-COV-2, and computer-aided and structure-based means design approaches play an important role in this process (10). Therefore, antiviral measures repurposing is currently the optimal choice for screening anti-COVID-19 methods due to its time-saving and labor-saving characteristics.
As a variety of existing neuromodulation methods, vagus nerve stimulation, electroacupuncture, and cholinergic drugs have been shown to have significant anti-inflammatory effects on a variety of inflammatory diseases (11–13). Especially since COVID-19 pandemic, it has been gradually applied to the anti-inflammatory regulation function after SARS-COV-2 infection, and has obtained obvious antiviral value. This further proves the important value of neuroimmunomodulatory in the fight against cytokine reaction due to SARS-COV-2. Therefore, we will comprehensively elaborate and sort out the treatment approaches in these three major neuroimmunomodulatory aspects, so as to provide clinicians with standards and guidance for the selection of measures against COVID-19.
2 Virology
Common symptoms reported for COVID-19 were dry cough, fever, fatigue and shortness of breath (14). In addition, multiple organ manifestations, mainly digestive symptoms, were gradually reported (15). Most COVID-19 cases have mild symptoms, but in some cases, cytokine storms can be triggered, followed by acute respiratory distress syndrome (ARDS), septic shock, and even multiple organ failure, leading to death (16). Therefore, it is particularly important to further clarify the characteristics of COVID-19 and explore effective interventions.
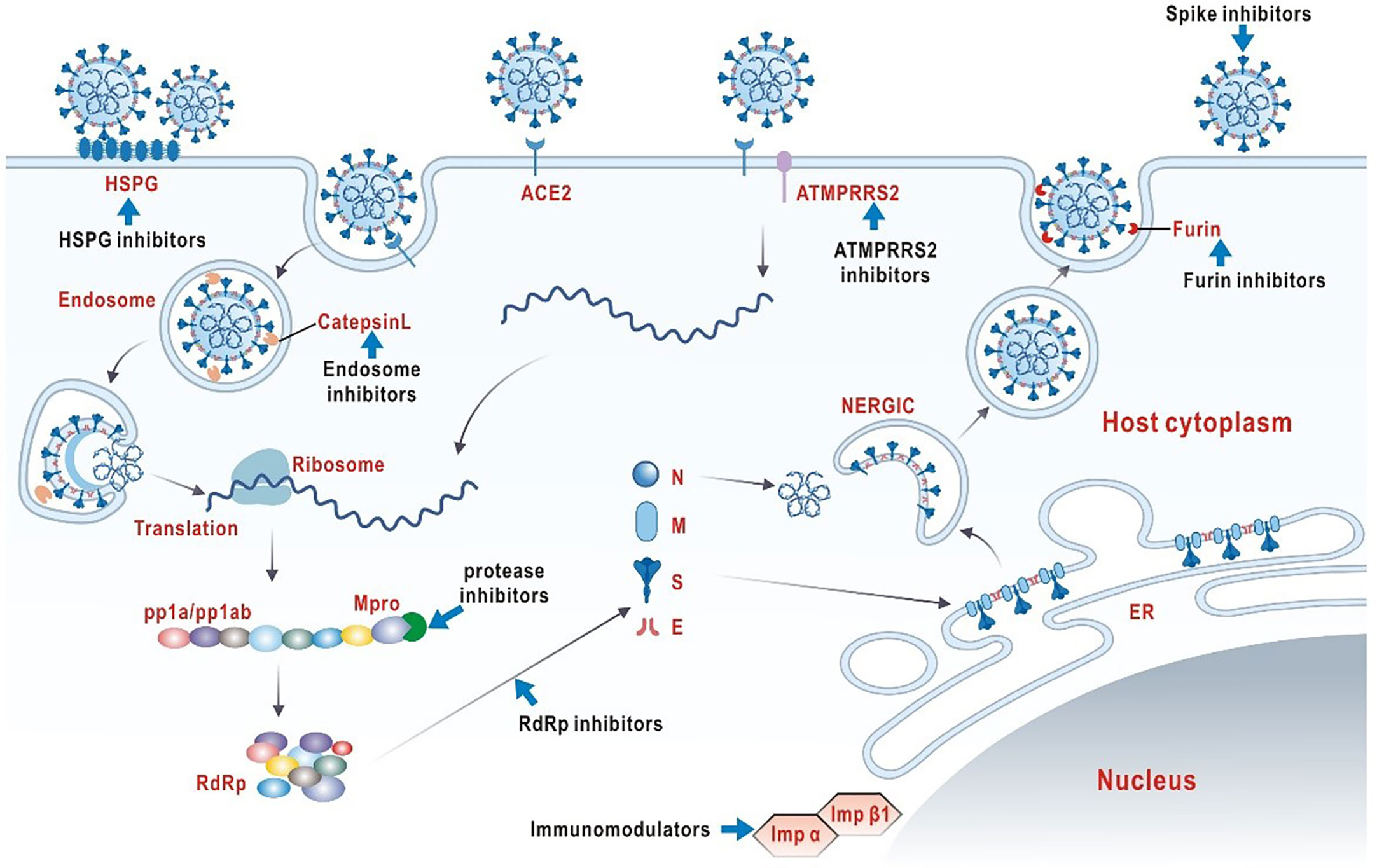
It is vital to clarify the structural and biological characteristics of SARS-COV-2 before exploring effective antiviral drugs (Figure 1). SARS-COV-2 is a member of the single-stranded RNA coronavirus family, as are previously known severe acute respiratory syndrome (SARS) and middle east respiratory syndrome (MERS) (17). The high diversity of SARS-COV-2 is attributed to its susceptibility to mutation and recombination. Humans, mammals and birds are the hosts of infection. In particular, novel coronavirus infection in humans mainly shows symptoms of multi-system infection mainly in respiratory tract (18). Coronavirus enters host cells via a trimer spike glycoprotein that gives the virus its coronavirus appearance, which is now recognized as the main mode of infection. It should be emphasized that SARS-COV-2 spikes have a stronger binding affinity for host receptors than other coronavirus, which may account for the relatively high rate of transmission and incidence of SARS-COV-2 (19, 20). The spike consists of two subunits, S1 and S2. The top of the S1 subunit, binds to the angiotensin converting enzyme 2 (ACE2) receptor on the surface of the host cell.S2 subunit fuses with the host cell membrane. When the S1 subunit binds to the receptor, the host trans-membrane serine protease 2 (TMPRSS2) activates the spikes and cleaves ACE2 by acting on the S2 subunit. This cleavage promotes the fusion of the virus with the cell membrane (21). Another infection mechanism is a PH-dependent endocytosis pathway (22). In summary, viral replication requires the help of different biological processes in the infected host cell, including endocytosis on the plasma membrane, uncoating and release of viral genomes into the cytoplasm, transcription and translation of viral RNA, and release of mature virions through golgi vesicles (23). In theory, blocking any infection process could have an antiviral effect.
At present, the selection and development of laboratory or clinical anti-SARS-COV-2 drugs are mainly based on the above understanding of the structure, the infection mechanisms of SARS-COV-2 and the clinical symptoms COVID-19 infection. Till now, antiviral therapy options are still very limited (24, 25). On the other hand, COVID-19 is a rapidly evolving inflammatory disease. In severe cases, uncontrolled inflammatory responses and cytokine storms are common (26). Therefore, regulating the inflammatory state is essential to improve the prognosis of severely ill COVID-19 patients. In this review, our goal is to systematically summarize the current effective means of neuro-immune regulation worldwide for COVID-19 to better facilitate clinical interventions and future antiviral strategies.
3 Clinically or laboratory effective neuroimmunotherapy for COVID-19
In epidemics of viral etiology, past experience suggests that early and indiscriminate use of neuroimmunotherapy is logical and beneficial for certain aggressive and rapidly mutating types. Therefore, this is also the empirical basis for the tentative application of existing means of neuro-immune regulation at the beginning of the COVID-19 outbreak. In this section we will list those anti-COVID-19 neuroimmunomodulatory tools that have shown some efficacy in clinical trials or that look promising in this regard.
3.1 Vagus nerve stimulation against COVID-19
COVID-19 is an infectious disease caused by SARS-CoV-2 invading cells through angiotensin 2 converting enzyme (ACE2) receptors that are heavily expressed in alveolar epithelial cells, which is how SARS-CoV-2 infects other organs (27). COVID-19 causes a storm of inflammatory factors (increased levels of interleukin-1β, IL-2, IL-6, IL-10, TNF, interferon, etc.), promoting the sympathetic-vagus balance disorder (1, 28). In turn, the body’s neural immunity regulates inflammatory states in response to infection (29). It is clear that nervous system immunity plays an important role in the inflammatory process after infection.
To the best of our knowledge, the immune system is mediated and regulated by afferent and efferent fibers of the vagus nerve, the main nerve of the parasympathetic nervous system (30). As a major component of the neuroendocrine-immune axis, the nerve is playing a crucial role in the response to infection (29). This axis is the three information systems of nerve, endocrine and immune system, which regulate the functions of various organs and systems through the connection of information (cytokines, hormones, chemical transmitters, etc.) and cooperate with each other, so as to achieve the coordination and unity of the overall functions. From an immunological perspective, the parasympathetic vagus nerve plays an anti-inflammatory role, while the sympathetic nervous system may have both pro-inflammatory and anti-inflammatory effects (29). In particular, the anti-inflammatory potential of the vagus nerve has been gradually validated in laboratory and clinical studies in recent years. The pathway of vagus nerve involved in immune reflex mainly includes hypothalamic-pituitary-adrenal axis anti-inflammatory pathway and cholinergic anti-inflammatory pathway (29). Therefore, vagus nerve stimulation to regulate parasympathetic nervous system, activate related anti-inflammatory pathways, and rebuild abnormal sympathetically vagus balance play a potential clinical value in the treatment of COVID-19.
The vagus nerve originates from the 10th cranial nerve and its branches are widely distributed and circulated in the neck, chest and abdomen (31). The extensive projection of the vagus nerve means that it is involved in many functions of the body’s autonomic nervous system (32). The vagus nerve connects specific sensors and effectors around it to the central nervous system. Vagus-mediated connections involve projection to the hypothalamus and cerebral cortex, thus allowing vagus regulation into the subcortical and cortical regions of the brain. Thus, signals generated by the vagus nerve have the potential to affect a wide range of functions, and thus overall body protection (32). Vagus nerve stimulation can be performed through both invasive and non-invasive methods (33). Invasive stimulation usually involves implantation of electrodes around the left cervical branch of the vagus nerve, but implantation risks and costs are high. Non-invasive stimulation in the outer ear using skin electrodes is easy to use. The afferent branch of the cervical vagus nerve can also be applied to the neck for non-invasive stimulation via the surface skin electrode of the handheld device (34).
Sensory fibers in the vagus nerve sense peripheral homeostasis (e.g. immune state) and respond accordingly (e.g. immune regulation). The auricular branch of the vagus nerve has its natural advantages as the preferred stimulation site for laboratory research or clinical application (35). The outer ear is the only peripheral branch of the vagus nerve from which it emanates. This area is susceptible to peripheral nerve stimulation.
With the COVID-19 pandemic, there is increasing laboratory or clinical evidence that auricular vagus nerve stimulation has a positive effect on COVID-19. It has been reported that stimulating auricular vagus nerve in severe COVID-19 patients can significantly reduce the levels of several pro-inflammatory mediators and increase the levels of related anti-inflammatory mediators (36). A similar study observed the same phenomenon, however, the overall clinical outcomes of COVID-19 patients treated with auricular vagus nerve stimulation did not improve (37). In addition, there have been cases where administering auricular vagus nerve stimulation to patients with COVID-19 significantly reduced blood levels of interleukin-6 (38). Animal experiments showed that auricular vagus nerve stimulation significantly restored endotoxin-induced tissue damage, and reduced the level of inflammatory cytokines and immune cells infiltrated by tissue, suggesting that the selection of auricular vagus nerve stimulation is of great significance as a feasible intervention for the treatment of acute inflammation (39). However, it has also been reported that although auricular vagus nerve stimulation can reduce some inflammatory indicators, it does not improve clinical symptoms (40). These studies have shown that vagus nerve stimulation has been used in treatment of cytokine reaction due to a virus and it was specific. Therefore, adequate basic experimental verification and randomized controlled studies are necessary to explore the role of auricular vagus nerve stimulation in COVID-19 regulation.
3.2 Electroacupuncture against COVID-19
To the best of our knowledge, COVID-19 is a respiratory infectious disease mainly with respiratory symptoms. However, other systemic symptoms, including gastrointestinal symptoms, accompany the disease process (18). We should accurately assess the potential threat of COVID-19 to the gastrointestinal (GI) tract and actively respond to it. Clinicians and scientists continue to try a variety of drug interventions, but their clinical effects and side effects have always affected the clinical treatment (5). However, to date there is still no clinically effective intervention for COVID-19. Therefore, the exploration and trial of effective and less invasive interventions will greatly improve the clinical course of patients with COVID-19. Acupuncture as a supplementary treatment is one of the fastest growing replacement therapy.
Acupuncture is a traditional Chinese medicine therapy with a long history in China, involves inserting fine needles into specific acupoints (41). The world health organization recommends acupuncture was applied to a wide range of diseases, including muscle bone disease, nervous system diseases, etc (42). According to traditional medical theory, acupuncture stimulation can promote the flow of qi, a life force that is said to circulate in the meridians known as meridians (43, 44). Acupoints are thought to be pathophysiological mechanisms associated with and possibly reflecting visceral and systemic conditions, and thus stimulation of specific acupoints may elicit responses that control the unbalanced internal environment and improve physical symptoms. Electroacupuncture (EA) is a modern modified acupuncture technique, which uses electric current to stimulate points rather than manual operation, and has the advantage of strong repeatability (45, 46). Over the past decade, several studies have explored the efficacy of EA in the treatment of functional gastrointestinal disorders, evaluating the effects of electricity on gastrointestinal secretory function, sensory, motor, and electromyographic activity (45, 47). To the best of our knowledge, EA is widely used in the treatment of various diseases due to its economical, reusable and low side effects. Some scholars used EA to stimulate the lung Shu point (BL13) to regulate inflammatory mediators, thus reducing the severity of viral pneumonia (48). Studies have also shown that electrical stimulation of Zusanli can significantly reduce abdominal pain and abdominal distension and other gastrointestinal symptoms caused by severe acute pancreatitis (49, 50). Therefore, these studies provide sufficient basis and reference value for EA to alleviate the gastrointestinal symptoms of COVID-19.
Currently, it is known that COVID-19 is mainly mediated by angiotensin converting enzyme II (ACE2) to infect cells, and the respiratory tract is the main target organ, while ACE2 is expressed to varying degrees in intestinal and gallbladder organs. This means a potential risk of infection of digestive tract organs (51). The experience of TCM in regulating gastrointestinal dysfunction suggests that electroacupuncture has potential clinical value in regulating gastrointestinal function of COVID-19.
Compared to respiratory symptoms, GI symptoms, although less common, have recently become more important (52). Studies shows that the GI tract symptoms can be as high as 50% (39.6 50%), including nausea (17.3%), diarrhea (12.9%), anorexia (12.2%), abdominal pain (5.8%), gas (5%) and vomiting (5%) (53, 54). The virus can also cause GI bleeding, acute pancreatitis, hepatitis, colitis and other GI diseases. Given the high rate of GI symptoms (diarrhea, nausea, etc.) in patients with COVID-19, screening these patients is critical. It is speculated that the virus can be re-transmitted through feces through aerosol containing virus droplets, which may constitute part of the cause of GI symptoms of COVID-19. However, this has not been confirmed (55). Based on this, sewage was evaluated to detect the presence of SARS-CoV-2 virus, thus identifying the occurrence of fecal-oral transmission and determining response strategies (56).
In addition to its high expression in the respiratory tract, ACE2 is highly expressed in GI cells, such as esophageal epithelial cells and absorptive enterocytes cells in the ileum and colon (57–59). SARS-CoV-2 virus can also enter the portal vein through the GI tract and affect vagus nerve function through vascular or lymphatic pathways. In addition, SARS-CoV-2 can further stimulate the central and peripheral nervous systems by binding cytokines, culminating in GI symptoms such as nausea (with or without vomiting).Therefore, the expression of ACE2 determines whether different organs are involved in the occurrence of symptoms and the degree of inflammation.
EA plays a role in regulating GI function by stimulating acupoints (Figure 2). Some scholars used EA to stimulate the lung Shu point (BL13) to regulate inflammatory mediators, thus reducing the severity of viral pneumonia (48). Studies have also shown that electrical stimulation of Zusanli can significantly reduce abdominal pain and abdominal distension and other GI symptoms caused by severe acute pancreatitis (49, 50, 60). Although the mechanism by which EA alleviates GI symptoms has not been fully elucidated, its clinical effect of significantly regulating inflammation and alleviating disease has potential value. Therefore, exploring the specific mechanism of EA regulating GI function will help to understand the principle of its function.
The esophagus is the passage that carries food to the stomach. At the gastroesophageal junction (GEJ), there is a thickened layer of muscle called the lower esophageal sphincter (LES). Esophageal motility abnormalities are classified into different types according to the function and contraction mode of LES (61). Recent studies have reported the effect of EA on esophageal dyskinesia.EA stimulation increases LES pressure (LESP) and peak peristalsis amplitude (62).
Gastric motility is one of the most important physiological functions of the human intestine. Without coordinated movement, digestion and absorption of dietary nutrients cannot occur. To perform its function efficiently, the intestine needs to produce not only simple contractions, but also coordinated contractions to produce transport (peristalsis) of luminal contents. Studies have shown that EA at ST36 restored the impaired gastric regulation induced by vagal nerve transection in dogs, but had no effect on gastric regulation in normal dogs (63). In addition, electrical stimulation significantly increased the number and amplitude of peak gastric EMG activity, suggesting increased gastric contractions after stimulation (64).
The small intestine moves in two different patterns: fasting and eating. The typical manifestation of fasting is the migration motor complex (MMC). Intestinal dyskinesia include loss of MMC, MMC damage. In experiments with rats, the investigators observed that EA at hind limb points (ST36 and SP6) significantly enhanced small intestinal transport (65).
There are staged contractions and large transitional contractions in the colon. Disrupted colonic motility is associated with a variety of functional disorders, such as irritable bowel syndrome (IBS), constipation, and diarrhea. EA stimulation increased colonic transport processes through the sacral parasympathetic efferent pathway (66). Similarly, EA stimulation of ST36 significantly increased contractility in the distal colon.
As for the pathophysiological mechanism of EA in regulating GI symptoms of COVID-19, we may find a speculative answer through a recent relevant study. In 2021, Ma et al. conducted an in-depth study on the neuroanatomy of EA stimulation therapy, and they confirmed that the physiological mechanism of electroacupuncture stimulation at ST36 site in mice is realized by driving the vagal adrenal axis (67). Therefore, EA has become an important method for neuroimmune regulation of COVID-19 to play an anti-inflammatory role. With the threat of infection from the ongoing spread of COVID-19, the effectiveness of EA in regulating the GI symptoms of COVID-19 is noteworthy.
3.3 Cholinergic drugs against COVID-19
With the COVID-19 pandemic, the cholinergic anti-inflammatory pathway (CAP), first articulated in 2003, has attracted widespread attention. Recent research into the pathogenesis of COVID-19 suggests that cholinergic anti-inflammatory systems may play a role. It is based on cholinergic neurotransmission and neuronal nicotinic acetylcholine receptor (nAChR). Acetylcholine (ACh) works by reducing pro-inflammatory cytokines and acts directly on the brain, released by cholinergic neurons to act on immune cells. It is important to emphasize that voluntary cholinergic neuron stimulation by nAChR activates skeletal muscle contraction.
The cholinergic system is primarily composed of nerve cells that communicate with other neurons and cells using or in response to the neurotransmitter Ach (68). Neuronal nAChR is a pentamer receptor-operated cationic channel composed of the is oplastid or heteromeric assembly of nine different isomers (69). Certain forms of nAChR can be activated by ACh. The homologous α7 receptor has been the focus of research in recent years. It is a high Ca2+ permeability nAChRs that responds to both choline and Ach (70). Studies in α7 nAChR ko mice have shown that responses to different inflammatory agents enhance tissue inflammation, suggesting that these receptors can negatively regulate inflammatory responses (71). The cholinergic regulation of macrophage function by nAChRα7 is a very extensive cholinergic anti-inflammatory pathway of vagus nerve in the regulation of tissue inflammation (72). Ach on macrophages exerts anti-inflammatory effects throughα7nAChR along the splenic nerve 9-11 via ganglia in the peritoneal - superior mesenteric plexus (73). α7nAChRs activation in macrophages down-regulates the production of pro-inflammatory cytokines through JAK2-STAT3 signaling pathway and blocking the activation of NF-κB pathway (74). Since the COVID-19 pandemic, studies have found that nicotine agonists play a key role in fighting the COVID-19 induced cytokine storm (75). Therefore, the cholinergic anti-inflammatory pathway, in which ACh is the key anti-inflammatory mediator.
The cholinergic anti-inflammatory pathway can be activated by external vagus nerve stimulation and drug intervention. One of the most hotly debated issues at the moment is the use of tobacco products (nicotine) and the risk of COVID-19 infection (76). The potential role of nicotine in COVID-19 pathology is that it itself may be used as an anti-inflammatory for COVID-19 through its interaction with the nicotine cholinergic system. Given its anti-inflammatory and potential role in interfering with the entry and/or replication of SARS-CoV-2, nicotine, nicotine agonists, or positive allosteric regulators of nicotine cholinergic receptors may play a therapeutic role in COVID-19. However, the negative effects of smoking on COVID-19 patients cannot be ignored, so a large number of studies are needed to prove it.
There is some preliminary evidence suggesting that the spike protein of SARS-CoV-2 interacts with human nicotinic acetylcholine receptors may have therapeutic potential for COVID-19 (77–79). As spike protein is similar to neurotoxins, it can bind to nAChR, including in the vagus nerve and in the brain (80). nAChRs are a type of receptor found on the surface of many different types of cells, including immune cells. They are involved in a wide range of physiological processes, including the regulation of the immune system, and their activation can lead to inflammation and other harmful effects (81). Therefore, if the spike protein does indeed bind to these receptors, it could potentially have a number of effects on the human body, including exacerbating the inflammatory response associated with COVID-19. Therefore, nicotinic receptors may affect the pathogenesis of SARS-CoV-2 infection. The dysregulation of nAChR by SARS-CoV-2 could promote a cholinergic dysfunction thus a central sympathetic drive leading to the cytokine storm.
Despite the potential harmful effects of this interaction, there is also some evidence to suggest that drugs that target nAChRs may have therapeutic potential for COVID-19. A drug called varenicline, which is used to treat nicotine addiction and also targets nAChRs, may have potential as a treatment for COVID-19 (82). The researchers found that varenicline was able to inhibit the replication of SARS-CoV-2 in vitro, and they suggested that it may have potential as a treatment for COVID-19.
Overall, while there is some evidence to suggest that the interaction between the spike protein of SARS-CoV-2 and human nAChRs may have therapeutic potential for COVID-19, more research is needed to fully understand the implications of this interaction and to develop effective and safe treatments.
4 Comparison of treatment plans
Each of the neuroimmunotherapy for COVID-19 has its own set of advantages and disadvantages. It is important now is that choosing appropriate treatment strategies depends on a better understanding of the pathophysiological background of COVID-19. We summarized some potential pros and cons of each treatment approach for better treatment decision making.
Vagus nerve stimulation has shown potential to reduce the cytokine storm and inflammation in severe cases of COVID-19. Non-invasive treatment that is relatively safe and well-tolerated, and can be administered through a wearable device that patients can use at home. Therefore, it could be assessed as a first resort early treatment for COVID-19 vulnerable population. On the contrary, cholinergic drugs, requiring a cardio-vascular monitoring, could be assessed as a therapeutic option for hospitalized patients. For instance, it could be interesting to evaluate whether the early treatment is sufficient to spare hospitalization or to improve the patient’s outcome thanks to an additive beneficial effect with cholinergic drugs used later in the course of the illness. However, the use of cholinergic drugs can have side effects, including nausea, vomiting, and diarrhea. Some cholinergic drugs can interact with other medications, making it important to monitor patients carefully. Electroacupuncture is also relatively safe and well-tolerated. It can be administered by licensed acupuncturists and can be incorporated into standard care. However, the use of needles may not be suitable for all patients, especially those who are immunocompromised or have bleeding disorders. The effectiveness of electroacupuncture may vary depending on the skill of the practitioner and the patient’s individual response.
In summary, while each treatment has its own advantages and disadvantages, more research is needed to fully understand their effectiveness and safety for treating COVID-19. It is important to consult with a healthcare professional to determine which treatment, if any, is appropriate for a particular patient.
5 Conclusion
The impact of neuro-immune regulation on COVID-19 is an area that should be studied in greater depth, as there may be an intersection of multiple pathophysiological processes. At present, neural immunity plays a key role in the process of COVID-19 and is also an important route for intervention. This evidence will guide further research, as there are many promising therapies that can prevent the SARS-CoV-2 virus. Vagus nerve stimulation effectively reduces inflammatory cytokine storm, providing a new strategy for the clinical treatment of COVID-19. Electroacupuncture, as an emerging neuroregulatory anti-inflammatory tool whose mechanism of action has just been elucidated, has a wide range of clinical applications including COVID-19 treatment. Cholinergic drugs, represented by nicotine agonists, can bind to nAChR and regulate inflammation by activating this receptor, bringing more thinking and challenges to COVID-19 prevention. What is important now is that choosing appropriate treatment strategies depends on a better understanding of the pathophysiological background of COVID-19, and neuro-immune regulation is a recurring theme in the growing body of scientific evidence.
Author contributions
XY completed all of the content of the article, including analysis, writing and so on. QK is responsible for the overall design and review of the article.
Funding
Zhejiang Provincial Program for the Cultivation of High-level Innovative Health Talents (WJW2021002) (QK) Science Foundation of National Health Commission of the PRC (WJW-ZJ-2203) (QK).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in wuhan, China. Lancet (2020) 395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5
2. Rothe C, Schunk M, Sothmann P, Bretzel G, Froeschl G, Wallrauch C, et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Eng J Med (2020) 382(10):970–1. doi: 10.1056/NEJMc2001468
3. Li R, Pei S, Chen B, Song Y, Zhang T, Yang W, et al. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV-2. Science (2020) 368(6490):489–93. doi: 10.1126/science.abb3221
4. Hoffmann C, Camp R, Kamps BS. Covid reference, Edition 2020-2. Available at: www.covidreference.com.
5. Şimşek Yavuz S, Ünal S. Antiviral treatment of COVID-19. Turkish J Med Sci (2020) 50(SI-1):611–9. doi: 10.3906/sag-2004-145
6. Simsek-Yavuz S, Komsuoglu CF. An update of anti-viral treatment of COVID-19. Turk J Med Sci (2021) 51(SI-1):3372–90. doi: 10.3906/sag-2106-250
7. Ashraf MU, Kim Y, Kumar S, Seo D, Ashraf M, Bae YS. COVID-19 vaccines (Revisited) and oral-mucosal vector system as a potential vaccine platform. Vaccines (Basel) (2021) 9(2). doi: 10.3390/vaccines9020171
8. Liu C, Zhou Q, Li Y, Garner LV, Watkins SP, Carter LJ, et al. Research and development on therapeutic agents and vaccines for COVID-19 and related human coronavirus diseases. ACS Cent Sci (2020) 6(3):315–31. doi: 10.1021/acscentsci.0c00272
9. Li G, De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV). Nat Rev Drug Discov (2020) 19(3):149–50. doi: 10.1038/d41573-020-00016-0
10. Zumla A, Chan JF, Azhar EI, Hui DS, Yuen KY. Coronaviruses–drug discovery and therapeutic options. Nat Rev Drug Discov (2016) 15(5):327–47. doi: 10.1038/nrd.2015.37
11. Tornero C, Pastor E, Garzando M, Orduna J, Forner MJ, Bocigas I, et al. Non-invasive vagus nerve stimulation for COVID-19: results from a randomized controlled trial (SAVIOR I). Front Neurol (2022) 13:820864. doi: 10.3389/fneur.2022.820864
12. Yu X. Potential value of electroacupuncture in alleviating COVID-19 by stimulating acupuncture point. NEUROMODULATION (2021) 24(8):1497. doi: 10.1111/ner.13542
13. Gromova OA, Torshin IY, Semenov VA, Putilina MV, Chuchalin AG. Direct and indirect neurological signs of COVID-19. NeurosciBehav Physiol (2021) 51(7):856–66. doi: 10.1007/s11055-021-01144-9
15. Ungaro RC, Agrawal M, Brenner EJ, Zhang X, Colombel JF, Kappelman MD, et al. New gastrointestinal symptoms are common in inflammatory bowel disease patients with COVID-19: data from an international registry. Inflamm Bowel Dis (2022) 28:314–7. doi: 10.1093/ibd/izab184
16. Ye Q, Wang B, Mao J. The pathogenesis and treatment of the ‘Cytokine storm’ in COVID-19. J Infect (2020) 80:607–13. doi: 10.1016/j.jinf.2020.03.037
18. Wu Y, Chen C, Chan Y. The outbreak of COVID-19: an overview. J Chin Med Assoc (2020) 83(3):217–20. doi: 10.1097/JCMA.0000000000000270
19. Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell (2020) 181(2):281–92. doi: 10.1016/j.cell.2020.02.058
20. Yan R, Zhang Y, Li Y, Xia L, Guo Y, Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science (2020) 367(6485):1444–8. doi: 10.1126/science.abb2762
22. Glebov OO. Understanding SARS-CoV-2 endocytosis for COVID-19 drug repurposing. FEBS J (2020) 287(17):3664–71. doi: 10.1111/febs.15369
23. Dolgin E. The race for antiviral drugs to beat COVID - and the next pandemic. Nature (2021) 592(7854):340–3. doi: 10.1038/d41586-021-00958-4
24. Laws M, Surani YM, Hasan MM, Chen Y, Jin P, Al-Adhami T, et al. Current trends and future approaches in small-molecule therapeutics for COVID-19. Curr Med Chem (2021) 28(19):3803–24. doi: 10.2174/0929867327666200721161840
25. National Institutes of Health. Coronavirus disease 2019 (COVID-19) treatment guidelines (2021). Available at: www.covid19treatmentguidelines.nih.gov/ (Accessed 5 Nov 2021).
26. Liu BM, Martins TB, Peterson LK, Hill HR. Clinical significance of measuring serum cytokine levels as inflammatory biomarkers in adult and pediatric COVID-19 cases: a review. CYTOKINE (2021) 142:155478. doi: 10.1016/j.cyto.2021.155478
27. Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell (2020) 181(2):271–280.e8. doi: 10.1016/j.cell.2020.02.052
28. Zhu Z, Cai T, Fan L, Lou K, Hua X, Huang Z, et al. Clinical value of immune-inflammatory parameters to assess the severity of coronavirus disease 2019. Int J Infect Dis (2020) 95:332–9. Ningbo, China: official publication of the International Society for Infectious Diseases. doi: 10.1016/j.ijid.2020.04.041
29. Bonaz B, Sinniger V, Pellissier S. Anti-inflammatory properties of the vagus nerve: potential therapeutic implications of vagus nerve stimulation. J Physiol (2016) 594(20):5781–90. doi: 10.1113/JP271539
30. Pavlov VA, Tracey KJ. The vagus nerve and the inflammatory reflex-linking immunity and metabolism. Nat Rev Endocrinol (2012) 8(12812):743–54. doi: 10.1038/nrendo.2012.189
32. Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Auton Neurosci (2000) 85:1–17. doi: 10.1016/S1566-0702(00)00215-0
33. Kaniusas E, Kampusch S, Tittgemeyer M, Panetsos F, Gines RF, Papa M, et al. Current directions in the auricular vagus nerve stimulation I - a physiological perspective. Front Neurosci (2019) 13:854. doi: 10.3389/fnins.2019.00854
34. Barbanti P, Grazzi L, Egeo G, Padovan AM, Liebler E, Bussone G. Non-invasive vagus nerve stimulation for acute treatment of high-frequency and chronic migraine: an open-label study. J Headache Pain (2015) 16(1):61–5. doi: 10.1186/s10194-015-0542-4
35. Mercante B, Ginatempo F, Manca A, Melis F, Enrico P, Deriu F. Anatomo-physiologic basis for auricular stimulation. Med Acupunct (2018) 30:141–50. doi: 10.1089/acu.2017.1254
36. Seitz T, Szeles JC, Kitzberger R, Holbik J, Grieb A, Wolf H, et al. Percutaneous auricular vagus nerve stimulation reduces inflammation in critical covid-19 patients. Front Physiol (2022) 13:897257. doi: 10.3389/fphys.2022.897257
37. Uehara L, Correa J, Ritti R, Leite P, de Faria DRG, Pacheco-Barrios K, et al. Transcutaneous auricular vagus nerve stimulation effects on inflammatory markers and clinical evolution of patients with COVID-19: a pilot randomized clinical trial. Expert Rev Med Devices (2022) 19(11):915–20. doi: 10.1080/17434440.2022.2154147
38. Correa FI, Souza P, Uehara L, Ritti-Dias RM, da Silva GO, Segheto W, et al. Transcutaneous auricular vagus nerve stimulation improves inflammation but does not interfere with cardiac modulation and clinical symptoms of individuals with COVID-19: a randomized clinical trial. Life (Basel) (2022) 12(10). doi: 10.3390/life12101644
39. Go YY, Ju WM, Lee CM, Chae SW, Song JJ. Different transcutaneous auricular vagus nerve stimulation parameters modulate the anti-inflammatory effects on lipopolysaccharide-induced acute inflammation in mice. Biomedicines (2022) 10(2). doi: 10.3390/biomedicines10020247
40. Rangon CM, Barruet R, Mazouni A, Le Cossec C, Thevenin S, Guillaume J, et al. Auricular neuromodulation for mass vagus nerve stimulation: insights from SOS COVID-19 a multicentric, randomized, controlled, double-blind French pilot study. Front Physiol (2021) 12:704599. doi: 10.3389/fphys.2021.704599
41. Yin J, Chen JD. Gastrointestinal motility disorders and acupuncture. AutonNeurosci (2010) 157(1-2):31–7. doi: 10.1016/j.autneu.2010.03.007
42. World Health Organization. Medicines publications and documentation. Available at: http://apps.who.int/medicinedocs/en/ (Accessed 20 Jun 2011).
43. World Health Organization. WHO international standard terminologies on traditional medicine in the Western pacific region. Geneva: WHO (2007).
45. Li Y, Tougas G, Chiverton SG, Hunt RH. The effect of acupuncture on gastrointestinal function and disorders. Am J Gastroenterol (1992) 87:1372–81.
46. Lux G, Hagel J, Backer P, Backer G, Vogl R, Ruppin H, et al. Acupuncture inhibits vagal gastric acid secretion stimulated by sham feeding in healthy subjects. Gut. (1994) 35:1026–9. doi: 10.1136/gut.35.8.1026
47. Diehl DL. Acupuncture for gastrointestinal and hepatobiliary disorders. J Altern Complement Med (1999) 5:27–45. doi: 10.1089/acm.1999.5.27
48. Luo W, Wang JY, Huang C. "[Effect of EA stimulation of "Feishu" (BL 13) on lung index, serum and lung IL-10 and TNF-alpha levels in mice with viral pneumonia]. Zhen Ci Yan Jiu (2014) 39(4):293–7.
49. Li J, Zhao Y, Wen Q, Xue Q, Lv J, Li N. EA for severe acute pancreatitis accompanied with paralytic ileus: a randomized controlled trial. Zhongguo Zhen Jiu. (2016) 36(11):1126–30. doi: 10.13703/j.0255-2930.2016.11.002
50. Wang X. EA for treatment of acute pancreatitis and its effect on the intestinal permeability of the patient. Zhongguo Zhen Jiu. (2007) 27(6):421–3.
51. Cheng H, Wang Y, Wang GQ. Organ-protective effect of angiotensin-converting enzyme 2 and its effect on the prognosis of COVID-19. J Med Virol (2020) 92(7):726–30. doi: 10.1002/jmv.25785
52. Kopel J, Perisetti A, Gajendran M, Boregowda U, Goyal H. Clinical insights into the gastrointestinal manifestations of COVID-19. Dig Dis Sci (2020) 65:1932–9. doi: 10.1007/s10620-020-06362-8
53. Bezerra JA, Pochapin MB, El-Serag HB, Vargo JJ. COVID-19 clinical insights for our community of gastroenterologists and gastroenterology care providers. Bethesda: American College of Gastroenterology (2020).
54. Zhang J, Dong X, Cao Y, Yuan YD, Yang YB, Yan YQ, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in wuhan, China. Allergy (2020) 75:1730–41. doi: 10.1111/all.14238
55. Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, et al. First case of 2019 novel coronavirus in the united states. N Engl J Med (2020) 382:929–36. doi: 10.1056/NEJMoa2001191
56. Randazzo W, Truchado P, Cuevas-Ferrando E, Simón P, Allende A, Sánchez G. SARS-CoV-2 RNA in wastewater anticipated COVID-19 occurrence in a low prevalence area. Water Res (2020) 181:115942. doi: 10.1016/j.watres.2020.115942
57. Burguen˜o JF, Reich A, Hazime H, Quintero MA, Fernandez I, Fritsch J, et al. Expression of SARSCoV-2 entry molecules ACE2 and TMPRSS2 in the gut of patients with IBD. Inflamm Bowel Dis (2020) 26(6):797e808. doi: 10.1093/ibd/izaa085
58. Harmer D, Gilbert M, Borman R, Clark KL. Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Lett (2002) 532(1e2):107e110. doi: 10.1016/S0014-5793(02)03640-2
59. Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology (2020) 158(6):1831e1833. doi: 10.1053/j.gastro.2020.02.055
60. Zhao L, Li X, Shi Z. Clinical observation on severe acute pancreatitis treated with EA at dachangshu (BL 25) and shangjuxu (ST 37) combined with ulinastatin. Zhongguo Zhen Jiu. (2018) 38(2):132–6. doi: 10.13703/j.0255-2930.2018.02.005
61. Nebel OT, Fornes MF, Castell DO. Symptomatic gastroesophageal reflux: incidence and precipitating factors. Am J Dig Dis (1976) 21:953–6. doi: 10.1007/BF01071906
62. Shuai X, Xie P, Liu J, Xiang Y, Li J, Lan Y. Different effects of EA on esophageal motility and serum hormones in cats with esophagitis. Dis Esophagus (2008) 21:170–5. doi: 10.1111/j.1442-2050.2007.00757.x
63. Ouyang H, Xing J, Chen J. EA restores impaired gastric accommodation in vagotomized dogs. Dig Dis Sci (2004) 49:1418–24. doi: 10.1023/B:DDAS.0000042240.05247.01
64. Tabosa A, Yamamura Y, Forno ER, Mello LE. A comparative study of the effects of EA and moxibustion in the gastrointestinal motility of the rat. Dig Dis Sci (2004) 49:602–10. doi: 10.1023/B:DDAS.0000026305.20852.41
65. Iwa M, Matsushima M, Nakade Y, Pappas TN, Fujimiya M, Takahashi T. EA at ST-36 accelerates colonic motility and transit in freely moving conscious rats. Am J PhysiolGastrointest Liver Physiol (2006) 290:G285–92. doi: 10.1152/ajpgi.00068.2005
66. Luo D, Liu S, Xie X, Hou X. EA at acupoint ST-36 promotes contractility of distal colon via a cholinergic pathway in conscious rats. Dig Dis Sci (2008) 53:689–93. doi: 10.1007/s10620-007-9929-7
67. Liu S, Wang Z, Su Y, Qi L, Yang W, Fu M, et al. A neuroanatomical basis for electroacupuncture to drive the vagal-adrenal axis. NATURE (2021) 598(7882):641–5. doi: 10.1038/s41586-021-04001-4
68. Halder N, Lal G. Cholinergic system and its therapeutic importance in inflammation and autoimmunity. Front Immunol (2021) 12:660342. doi: 10.3389/fimmu.2021.660342
69. Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med (2005) 11:875–9. doi: 10.1038/nm1267
70. Wang Y, Pereira EF, Maus AD, Ostlie NS, Navaneetham D, Lei S, et al. Human bronchial epithelial and endothelial cells express alpha7 nicotinic acetylcholine receptors. Mol Pharmacol (2001) 60:1201–9. doi: 10.1124/mol.60.6.1201
71. Su X, Matthay MA, Malik AB. Requisite role of the cholinergic alpha7 nicotinic acetylcholine receptor pathway in suppressing gram-negative sepsis-induced acute lung inflammatory injury. J Immunol (2010) 184:401–10. doi: 10.4049/jimmunol.0901808
72. MacKenzie A. Endothelium-derived vasoactive agents, AT1 receptors and inflammation. Pharmacol Ther (2011) 131:187–203. doi: 10.1016/j.pharmthera.2010.11.001
73. van Maanen MA, Stoof SP, Larosa GJ, Vervoordeldonk MJ, Tak PP. Role of the cholinergic nervous system in rheumatoid arthritis: aggravation of arthritis in nicotinic acetylcholine receptor α7 subunit gene knockout mice. Ann Rheum Dis (2010) 69:1717–23. doi: 10.1136/ard.2009.118554
74. Báez-Pagán CA, Delgado-Vélez M, Lasalde-Dominicci JA. Activation of the macrophage α7 nicotinic acetylcholine receptor and control of inflammation. J Neuroimmune Pharmacol (2015) 10:468–76. doi: 10.1007/s11481-015-9601-5
75. Khani MA, SalehiRad M, Darbeheshti S, Motaghinejad M. Survival of COVID-19 patients requires precise immune regulation: the hypothetical immunoprotective role of nicotinic agonists. Med Hypotheses (2020) 143:109871. doi: 10.1016/j.mehy.2020.109871
76. Benowitz NL, Goniewicz ML, Halpern-Felsher B, Krishnan-Sarin S, Ling PM, O'Connor RJ, et al. Tobacco product use and the risks of SARS-CoV-2 infection and COVID-19: current understanding and recommendations for future research. Lancet Respir Med (2022) 10:900–15. doi: 10.1016/S2213-2600(22)00182-5
77. Changeux JP, Amoura Z, Rey FA, Miyara M. A nicotinic hypothesis for covid-19 with preventive and therapeutic implications. C R Biol (2020) 343:33–9. doi: 10.5802/crbiol.8
78. Farsalinos K, Eliopoulos E, Leonidas DD, Papadopoulos GE, Tzartos S, Poulas K, et al. Nicotinic cholinergic system and COVID-19: in silico identification of an interaction between SARS-CoV-2 and nicotinic receptors with potential therapeutic targeting implications. Int J Mol Sci (2020) 21. doi: 10.3390/ijms21165807
79. Tanmay S, Labrou D, Farsalinos K, Poulas K. Is SARS-CoV-2 spike glycoprotein impairing macrophage function via alpha7-nicotinic acetylcholine receptors? food chem. Toxicol (2021) 152:112184. doi: 10.1016/j.fct.2021.112184
80. Oliveira A, Ibarra AA, Bermudez I, Casalino L, Gaieb Z, Shoemark D K, et al. A potential interaction between the SARS-CoV-2 spike protein and nicotinic acetylcholine receptors. Biophys J (2021) 120:983–93. doi: 10.1016/j.bpj.2021.01.037
81. Carlson EC, Macsai M, Bertrand S, Bertrand D, Nau J. The SARS-CoV-2 virus and the cholinergic system: spike protein interaction with human nicotinic acetylcholine receptors and the 88. nicotinic agonist varenicline. Int J Mol Sci (2023) 24. doi: 10.3390/ijms24065597
Keywords: COVID-19, SARS-CoV-2, neuroimmunotherapy, vagus nerve, electroacupuncture, cholinergic drugs
Citation: Yu X and Kong Q (2023) Potential value of neuroimmunotherapy for COVID-19: efficacies and mechanisms of vagus nerve stimulation, electroacupuncture, and cholinergic drugs. Front. Immunol. 14:1197467. doi: 10.3389/fimmu.2023.1197467
Received: 31 March 2023; Accepted: 23 May 2023;
Published: 05 July 2023.
Edited by:
Bruno Bonaz, Centre Hospitalier Universitaire de Grenoble, FranceReviewed by:
Claire Marie Rangon, Fondation Ophtalmologique Adolphe de Rothschild, FrancePeter S. Staats, National Spine and Pain Centers, United States
Marie-Francoise Joelle Doursout, University of Texas Health Science Center at Houston, United States
Copyright © 2023 Yu and Kong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qingming Kong, qmkong_1025@163.com
 Xianqiang Yu
Xianqiang Yu Qingming Kong3*
Qingming Kong3*