- 1Department of General Surgery, The Affiliated Cancer Hospital of Zhengzhou University & Henan Cancer Hospital, Zhengzhou, Henan, China
- 2Department of Pathophysiology, School of Basic Medical Sciences, Zhengzhou University, Zhengzhou, Henan, China
- 3Department of Molecular and Cellular Biology, China-United States (US) Hormel (Henan) Cancer Institute, Zhengzhou, Henan, China
- 4Research Center of Basic Medicine, Academy of Medical Sciences, Zhengzhou University, Zhengzhou, Henan, China
Digestive tract cancers, including esophageal, gastric, and colorectal cancers, are the major cause of death among cancer patients worldwide due to the heterogeneity of cancer cells, which limits the effectiveness of traditional treatment methods. Immunotherapy represents a promising treatment strategy for improving the prognosis of patients with digestive tract cancers. However, the clinical application of this approach is limited by the absence of optimal targets. Cancer/testis antigens are characterized by low or absent expression in normal tissues, but high expression in tumor tissues, making them an attractive target for antitumor immunotherapy. Recent preclinical trials have shown promising results for cancer/testis antigen-targeted immunotherapy in digestive cancer. However, practical problems and difficulties in clinical application remain. This review presents a comprehensive analysis of cancer/testis antigens in digestive tract cancers, covering their expression, function, and potential as an immunotherapy target. Additionally, the current state of cancer/testis antigens in digestive tract cancer immunotherapy is discussed, and we predict that these antigens hold great promise as an avenue for breakthroughs in the treatment of digestive tract cancers.
1 Introduction
Cancers affecting the digestive tract, such as esophageal cancer, gastric cancer, and colorectal cancer (CRC), continue to be the primary cause of death among cancer patients worldwide (1). Due to application of endoscopic screening, the detection rate of early-stage digestive tract cancers has increased. However, the mortality is still very high because of the heterogeneity of cancers and little improvement in the standard gold therapy suitable for tumors of the digestive tract. It is therefore essential to search for specific prognostic and predictive molecular signatures to guide targeted, individualized therapy. Immunotherapy, which aims to enhance the body’s natural defenses to eliminate malignant cells, represents a monumental breakthrough in cancer treatment and has revolutionized the field of oncology (2). To develop effective immunotherapy treatments, it is crucial to first identify tumor antigens. Cancer/testis antigens (CTAs) are expressed in the testes and various types of cancer but have limited expression in normal adult somatic cells and tissues. These antigens can be recognized by cytolytic T lymphocytes (CTLs) (3, 4). Moreover, CTAs have been reported to be expressed in digestive tract tumors and exhibit specific biological functions. The upregulation of CTAs has been linked to several unfavorable outcomes commonly associated with cancer (5), including promotion of tumor cell stemness (6, 7), elevation of cancer cell tumorigenicity (8), enhancement of mobility (9) and metastasis (10), and conferment of drug resistance (11). These characteristics render CTAs ideal candidates as novel immunotherapeutic targets in digestive tract cancers. The aim of this review is to highlight the latest advances and hypotheses regarding the involvement of CTAs in the pathogenesis of digestive tract cancers and to investigate their potential as targets for cancer immunotherapy.
2 CTAs in the digestive tract cancers
2.1 Discovery and types of CTAs
Melanoma antigen-1 (MAGE-1, MAGE-A1, MA2-E), a member of the MAGE family, was the first CTA discovered by Alexander Knuth and Thierry Boon in 1991 (12). With a new method called serological identification of antigens by recombinant expression cloning (SEREX) (13), many more CTAs were uncovered, such as synovial sarcoma, X breakpoint 2 (HOM-MEL-40/SSX2) (13), New York’s esophageal squamous cell carcinoma 1 (CTAG1B, NY-ESO-1) (14, 15), synaptonemal complex protein 1 (SCP1) (16), and CT7 (17). Although the first CTAs was discovered in 1991, the name was defined in 1998 (17). To facilitate the organization of the expanding collection of CTAs, the Cancer-Testis database (CTdatabase, http://www.cta.Incc.br/) was established as a user-friendly interface. Over 730 CTAs belonging to over 100 gene families have been identified in many cancer tissues, where their expression is significantly elevated compared with normal tissues and predominantly restricted to germ cells and trophoblasts. Although not all of them have been demonstrated to induce immune responses, they are collectively referred to as CTAs (18).
Cancer/testis (CT) genes are typically expressed in germ line cells, trophoblasts, and certain cancer cells. CT genes are classified into three groups based on their expression profiles: testis-restricted, testis/brain-restricted, and testis-selective. The majority of CTAs are encoded by CT genes (19). However, due to the lack of a clear and universally applicable definition for CT genes, Oliver Hofmann used multiple in silico gene expression analysis technologies to investigate the expression patterns of a set of 153 CTAs in normal and cancer tissues. The CTA genes are further classified into two categories: CT-X and non-X CT genes. CT-X family members are subject to more stringent transcriptional regulation in somatic tissues, making them more suitable for immunotherapy applications (20). The CTdatabase has identified a total of 276 CTA genes, of which 127 (46%) are located on the X chromosome, whereas the remaining are distributed across the autosomes and Y chromosome.
2.2 Expression of CTAs in the digestive tract cancers
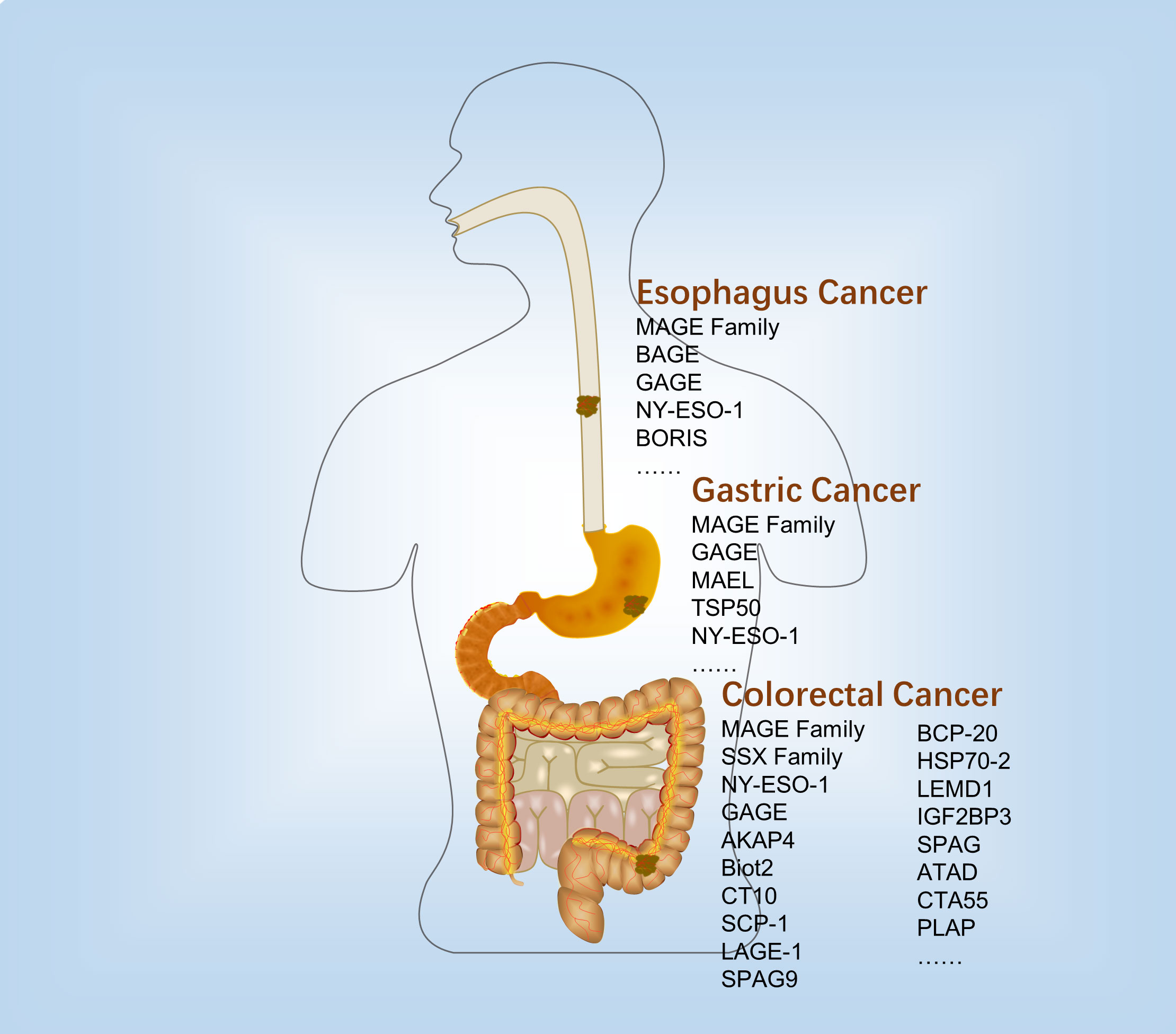
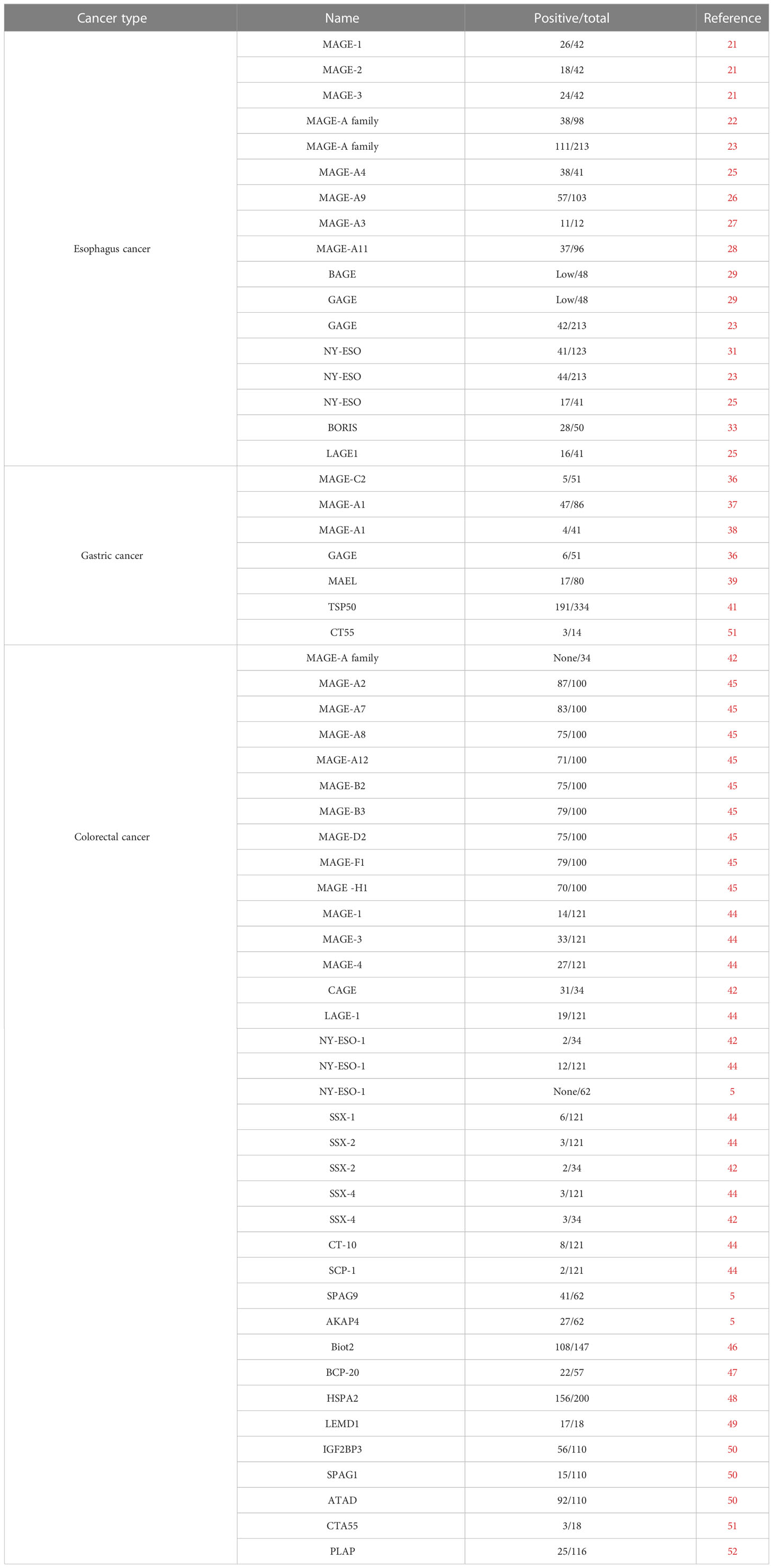
Many CTAs were expressed in the human digestive tract cancers. However, the expression profile was diverse in the different digestive tract cancer tissues and cell lines (Figure 1 and Table 1).
2.2.1 Esophagus cancer
In 1995, Masaki Mori found that MAGE-1, -2, -3 were expressed in 26, 18, and 24 of 42 surgical esophageal cancer tissues and 5, 4, and 4 of 12 human esophageal cancer cell lines, respectively. At least one of the them were expressed in 33 of 42 esophageal tumor tissues, and all of them expressed in 12 of 42 esophageal tumor tissues. However, none of them were expressed in the 42 normal esophageal tissues (21). Apart from that, MAGE-A was detected in 38 of 98 (22) and 111 of 213 (23) esophageal cancer patients.
The MAGE gene family consists of several subfamilies, one of which is the MAGE-A subfamily that includes MAGE-A1 to -A12 (24). In esophageal cancer, several members of the MAGE-A subfamily, including MAGE-A4 (25), MAGE-A9 (26), MAGE-A3 (27), and MAGE-A11 (28) have been detected. In addition to the MAGE-gene family, B melanoma antigen (BAGE) and G antigen (GAGE) families were also expressed in various tumors of different histological origins, including the esophageal squamous and esophageal adenocarcinoma (29). Similarly, they were not expressed in normal tissues other than testis (30).
Additionally, NY-ESO-1, which is also known as cancer/testis antigen 1B (CTAG1), is a prototypical member of the cancer-testis gene family and was originally identified from esophageal cancer (31). NY-ESO-1 is a major CTA in several studies. A study reported that 33% (41 out of 123) of esophageal squamous specimens showed positive mRNA expression for NY-ESO-1 (31). In two other studies, the proportions are 20.7% (23) and 41.4% (25), respectively. Usually, the expression of CTAs was not independent. A reported strong correlation was observed between the expression of cancer/testis antigen 2 (LAGE-1) and the expression of NY-ESO-1 and MAGE genes in esophageal squamous cancer (25, 32). Yutaka Kawakami discovered a new CTA called brother of the regulator of imprinted sites (BORIS), which is expressed in esophageal cancer and may serve as a novel prognostic indicator for patients with this type of cancer (33). BORIS could bind to the promoter of NY-ESO-1 (34) and MAGE-A1 (35) genes to regulate their expression.
2.2.2 Gastric cancer
There is growing evidence showed that several CTAs were expressed in the gastric cancer. The expression of MAGE-C2, also known as CT10, and GAGE was detected in 5 out of 51 and 6 out of 51 gastrointestinal stromal tumor tissues, respectively (36). MAGE-A1 as an important member of MAGE family was detected positive expression in 47/86 (37) and 4/41 (38) gastric cancer tissues. Another CTA, maelstrom spermatogenic transposon silencer (MAEL), was detected in gastric cancer using RT-PCR to measure its mRNA levels. The results indicated that MAEL over- and underexpressions were 17 and 28 out of 80 gastric cancer patients, respectively (39). A 50-kDa serine protease-like protein called testis-specific protease-like protein 50 (TSP50), which is encoded by a CTA gene, was discovered in human breast cancer cells through the isolation of a hypomethylated DNA fragment (40). According to Rongcheng Luo and his colleagues, a study found that the expression of TSP50 was upregulated in a significant proportion of human gastric cancer cases, with 57.2% of samples (191 out of 334) showing overexpression (41).
2.2.3 Colorectal cancer
The expression of the MAGE family in CRC tissues is contradictory. The analysis of 34 CRC samples revealed no expression of the MAGE antigen, specifically MAGE-A1, -A2, -1, -A3, -A12, and -C1 (42). Achim A. Jungbluth and his colleagues detected that MAGE antigens were not expressed in CRC (43). However, it was found that MAGE-1 (11.6%), -3 (27.3%), and -4 (22.3%) were detected to have a positive expression in the CRC tumor samples (44). A different study reported significant overexpression of MAGE-A2 (87%), MAGE-A7 (83%), MAGE-A8 (75%), MAGE-A12 (71%), MAGE-B2 (75%), MAGE-B3 (79%), MAGE-D2 (75%), MAGE-F1 (79%), and MAGE-H1 (70%) in CRC tissues (45). Therefore, more research would be required to better understand the expression pattern of the MAGE family in the CRC. In addition to the MAGE family, other cancer/testis antigens (CTAs) have also been identified in CRC, with NY-ESO-1 being one of the most extensively studied. In a cohort of 121 CRC patients, NY-ESO-1 gene expression was detected. The same study reported that several other CTAs, SSX family gene (10%), CT10 (6.6%), SCP-1 (1.7%), and LAGE-1 (15.7%), were overexpressed in CRC tissues compared with matched adjacent non-cancerous tissues (44). Similarly, the researchers analyzed the CTA levels in 34 CRC tissues and found that two of them were NY-ESO-1 positive. The expressions of SSX-2, SSX-4, and CAGE were respectively 2, 3, and 31 (42). However, a study including 62 Iranian CRC samples was not detected the expression of NY-ESO-1. Approximately 66% and 44% of tumors were observed to express the genes encoding for sperm associated antigen 9 (SPAG9) and a-kinase anchoring protein 4 (AKAP4), respectively (5). Other CTAs expressed in CRC include coiled-coil domain containing 7 (Biot2) (46), F-box protein 39 (BCP-20, FBXO39) (47), heat shock protein family A member 1B (HSP70-2) (48), LEM domain containing 1 (LEMD1) (49), insulin-like growth factor 2 mRNA binding protein 3 (IGF2BP3), sperm-associated antigen 1 (SPAG), acute type A aortic dissection (ATAD) (50), CTA55 (51), and recombinant phospholipase A2 activating protein (PLAP) (52). However, due to the small sample size used in these studies, it is necessary to confirm the results in a larger cohort to validate their findings.
2.3 The role of CTAs in the digestive tract cancers
Expressions of CTAs in tumors are perceived as the result of widespread DNA hypomethylation in the carcinogenesis (53). The special expression patterns made them as promising biomarkers and therapeutic targets. There have been numerous clinical research studies and trials conducted to investigate the potential clinical applications of CTAs, but their precise role in cancers is still not well understood.
2.3.1 Prognostic and biomarkers
An increasing body of evidence suggests that CTA expression may have a prognostic role in esophageal, gastric, and colorectal cancers. However, there also a number of CTAs which had no relationship to clinical features of tumors.
2.3.1.1 In esophageal cancer
Studies have found that the presence of MAGE is irrelevant to age, sex, histologic type, depth of wall invasion, lymph-node metastasis, or disease stage (21–23). Additionally, no significant difference was observed between MAGE-A expression and TNM stage, grading, or survival period in patients with the disease (22). A separate study indicated a correlation between tumor progression and the expression levels of MAGE-A4. Specifically, the expression levels of MAGE-A4 were found to be correlated with tumor metastasis to the lymph nodes, and the number of involved lymph nodes was also associated with the level of MAGE-A4 expression (25). Another study found that the expressions of MAGE-A11 (28) and MAGE-A9 (26) in esophageal cancer tissues were significantly correlated with larger tumor size and more advanced tumor stage. Moreover, the expression levels of MAGE-A9 and lymph node metastasis were found to be independent prognostic factors for the overall survival rate of patients with esophageal cancer (26). However, the role of NY-ESO-1 in esophageal cancer is controversial due to conflicting reports on its prognostic value as well as its potential as a target for immunotherapy. One study found that no significant difference was observed in survival rates between NY-ESO-1 protein-positive and -negative cases (31). Nonetheless, co-expression of NY-ESO-1 and MAGE-A4 was significantly correlated with differentiation of esophageal cancer (25). Expressions of MAGE genes have been found to be significantly related to a good prognosis in the absence of BAGE and GAGE expressions. Conversely, the expressions of BAGE or GAGE has been linked to a poor prognosis in cancer patients (29), although there was no significant difference in disease progression, TNM factors, or survival curves with the expression of GAGE (23). BORIS is another biomarker for prognostic diagnosis of esophageal cancer patients. Patients with tumors that tested positive for BORIS had poor overall survival according to one study. Additionally, BORIS expression was identified as an independent poor prognostic factor and was significantly associated with lymph node metastasis (33).
2.3.1.2 In gastric cancer
MAGE-A expression has been linked with lymph node metastasis, poor differentiation, high clinical TNM stage, and inferior patient survival (54). However, MAGE-A expression alone is not deemed an independent prognostic factor in patients with the disease. Conversely, MAGE-A1 expression has been proposed as a predictive marker for resistance to taxane-based chemotherapy in patients with gastric cancer, although it does not directly contribute to drug resistance (38). In high-grade gastrointestinal stromal tumors, MAGE-C2 co-expression with GAGE was significantly correlated with mitotic rate, tumor size, and neoplasm recurrence (36). Additionally, markers for poor relapse-free survival in gastric cancer include MAGE-A1, MAGE-A3, MAGE-A4, MAGE-C1, and NY-ESO-1 (55). High levels of TSP50 were significantly associated with shorter survival time, later TNM stage, and presence of lymph node metastases in patients with the disease. Furthermore, TSP50 overexpression was identified as a significant independent prognostic factor in gastric cancer patients (41). Moreover, in patients with H. pylori-negative gastric cancer, there was a significant correlation between MAEL expression and tumor stage, tumor grade and depth of invasion (39).
2.3.1.3 In CRC
Who exhibited a high protein expression of MAGE-D4 (56) or MAGE-A9 (57), had significantly shorter overall survival compared with those with a low protein expression. Nevertheless, there was no correlation found between MAGE-D4 expression and clinicopathological parameters (56). In patients with colorectal cancer, a high expression of MAGE-A9 was significantly associated with venous invasion, lymph node metastasis, and poor prognostic (57). Similarly, a study conducted in Taiwanese patients with colorectal cancer revealed that MAGE-B3, MAGE-D2, and MAGE-H1 expressions were correlated with tumor size and stage, whereas MAGE-B3 was also correlated with lymph node metastasis (45). In addition, NY-ESO-1 (44) and AKAP4 (5) were found to be significantly correlated with tumor stages and local lymph node metastasis in CRC patients. Biot2 expression was also found to be associated with poor prognosis in early-stage patients with CRC (58).
However, a high expression of CTAs in digestive tract cancers may have prognostic significance or simply exist as a tumor marker without indicating patient prognosis. Differences in detection methods can lead to different rates of CTA detection in various studies, which may result in biased analysis of patient prognosis. Additionally, some patients’ clinical characteristics may be associated with CTA expression, but further research is needed to identify such patients.
2.3.2 Tumorigenesis, development, and metastasis
In addition to being biomarkers for digestive tract cancers, some CTAs also play a key role in the tumorigenesis, development, and metastasis. The absence of BORIS resulted in a decrease of cell proliferation and invasion in the esophageal cancer cell lines (33).Overexpression of MAGE-A1 in the gastric cancer cell lines increased the sensitivity to paclitaxel and docetaxel (38). In a study, SCRN1 was found to be expressed in five of seven gastric cancer patients, and it promoted growth of NIH3T3 cells (59). The knockdown of Biot2 in CRC cell lines has been shown to cause cell cycle arrest in the G1 phase and induce apoptosis by regulating p16 and p21, both in vitro and in vivo (60). Furthermore, according to research, ablation of HSP70-2 significantly reduced cellular growth, the colony-forming, migratory, and invasive abilities of CRC cells, and tumor growth of human CRC cell line xenograft (48). Additionally, when researchers screened the transcriptome of cancer stem cells (CSC) of human CRC, they found that LEMD1 was preferentially expressed and its presence was essential for the maintenance of CSC (61). Moreover, according to research, CT55 functions as a stimulator of nuclear factor-κB (NF-κB) signaling induced by tumor necrosis factor (TNF)-α by binding to the IκB kinase complex. Deficiency of CT55 suppresses the development of colitis-associated CRC (62).
CTAs can be used as targets for immunotherapy in digestive tract tumors, allowing immune cells or related immune agents to selectively eliminate tumor cells that express CTAs but minimizing side effects on normal cells. Therefore, the application of CTAs to immunotherapy has become an area of active research and a subject of intense interest. These findings suggest that CTAs represent a promising focus for the treatment of digestive tract cancers.
3 CTAs in the immunotherapy of digestive tract tumor therapy
During the course of cancer development, tumor antigens can be identified as aggressor by the immune system, which triggers cellular immune responses. While T-cell-related immunotherapy has received significant attention, research has also demonstrated that other immune cells of both the innate and adaptive immune systems, such as DCs, macrophages, NK cells, and B cells, play a crucial role in facilitating immunotherapy responses. In cancer treatment, the major types of immunotherapies applied are oncolytic virus therapies, cancer vaccines, adoptive cell transfer (ACT), and immune checkpoint inhibitors (ICIs). Due to the limited expression of CTAs in tumors, their high immunogenicity, and their biased expression, CTA-based immunotherapy has emerged as a promising approach in cancer treatment, showing encouraging results in preclinical and early clinical trials (Figure 2).
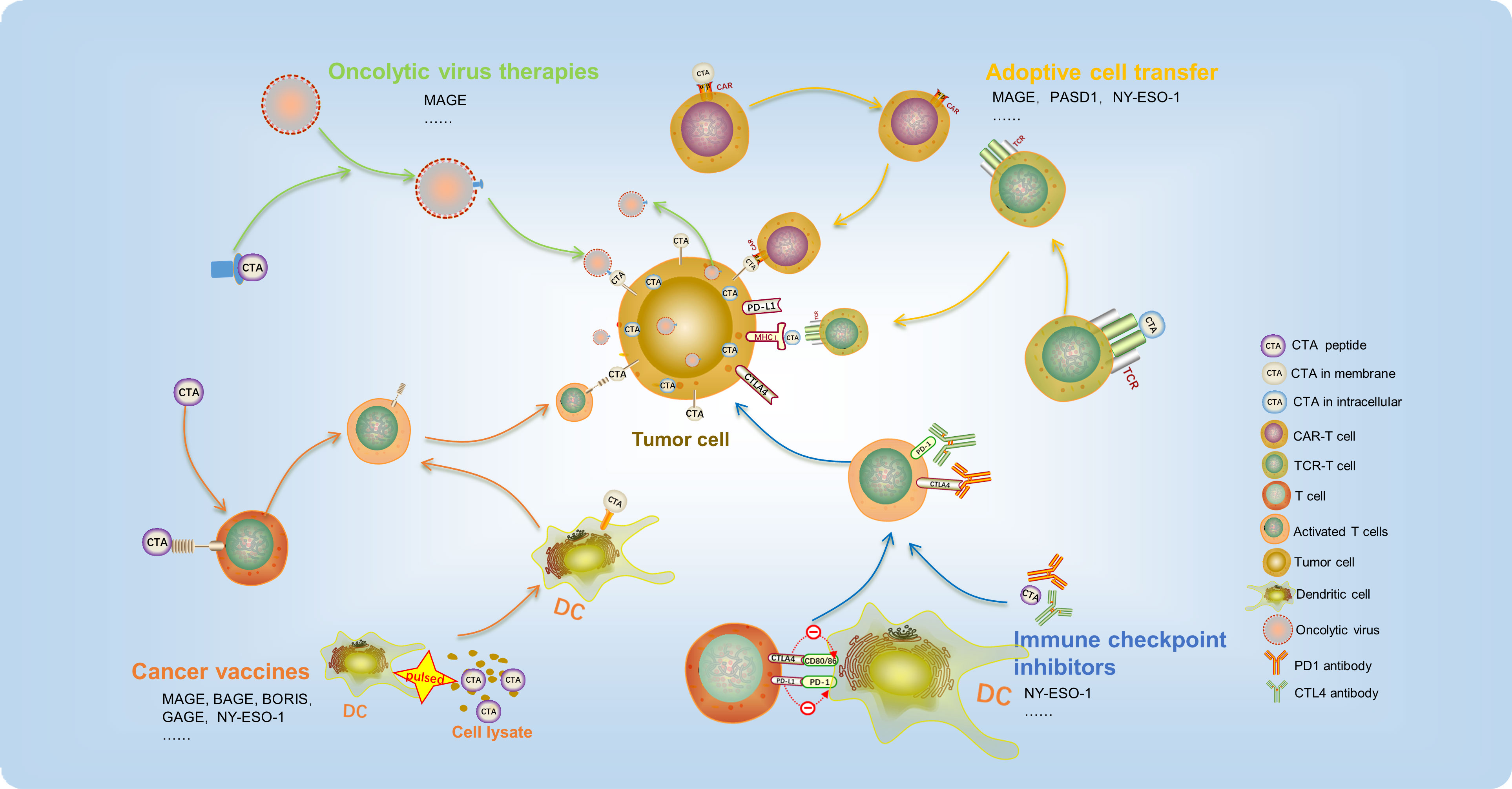
Figure 2 CTA-based immunotherapy has recently been used in cancer treatment and achieved promising outcomes. It mainly includes oncolytic virus therapies, cancer vaccines, adoptive cell transfer, and immune checkpoint inhibitors.
During cancer development, tumor antigens can be recognized as aggressors by the immune system, triggering cellular immune responses. While T-cell-related immunotherapy has received significant attention, research has demonstrated that other immune cells of both the innate and adaptive immune systems, such as DCs, macrophages, NK cells, and B cells, play a crucial role in facilitating immunotherapy responses. Types of CTA-based immunotherapy applied in digestive tract tumors treatment include oncolytic virus therapies, cancer vaccines, adoptive cell transfer (ACT), and immune checkpoint inhibitors (ICIs). Due to their limited tumor expression, high immunogenicity, and biased expression, CTAs have emerged as a promising strategy in cancer treatment (Table 2), showing encouraging results in preclinical and early clinical trials.
3.1 Oncolytic virus therapies
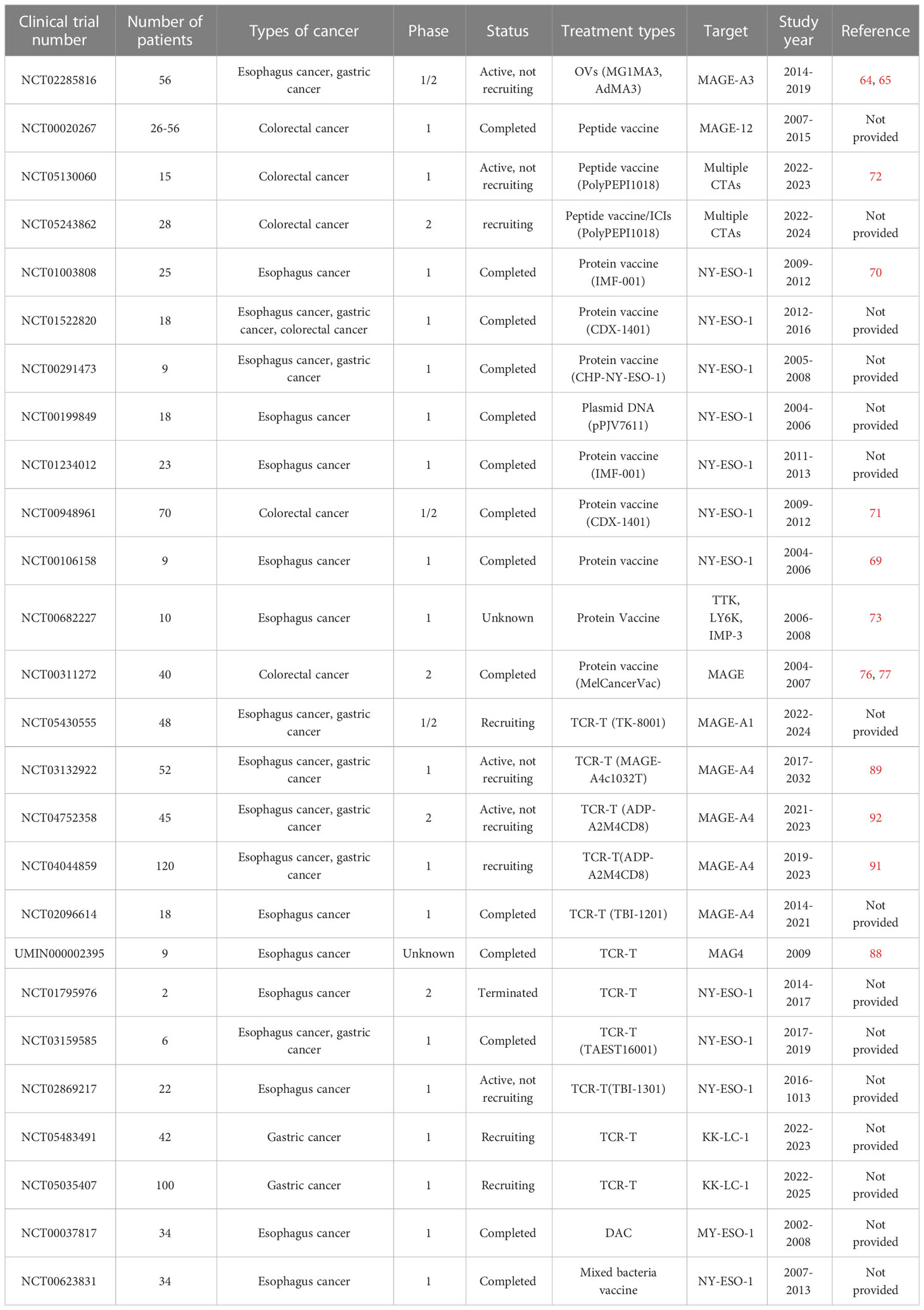
Oncolytic viruses (OVs) are a unique category of viruses that selectively infect and destroy tumor cells while leaving normal cells unharmed, thanks to their exceptional oncolytic activity and targeting ability. Once inside the tumor cells, OVs can multiply and release new viral particles, which then infect other nearby tumor cells. Additionally, OVs stimulate an antitumor immune response at the local or systemic level, modify the tumor microenvironment, and amplify their antitumor effects. Currently, five types of oncolytic viruses have been approved for clinical use, and many other preclinical studies are underway (63). The application of CTA in oncolytic virus therapy is also currently under investigation. Ad-MAGEA3 (AdMA3) is a replication-deficient adenovirus (E1/E3-deleted) of human serotype 5 that carries a transgene encoding human MAGE-A3 gene. MG1-MAGEA3 (MG1MA3) is an oncolytic rhabdovirus Maraba with replication competency, created by introducing the human MAGE-A3 transgene between the G and L genes of the attenuated MG1 strain. Jonathan G. Pol confirmed the safety of the Ad : MG1 oncolytic vaccination approach in non-human primates (64). Moreover, they initiated clinical trials for solid tumor treatment, including esophageal cancer and gastric cancer (NCT02285816). The Ad : MG1 oncolytic virus has the ability to replicate within the bloodstream and activate an adaptive, antitumor cellular response in cancer patients. In three out of six evaluated patients, antitumor immunity was observed, with over 1% of total circulating CD8+ T cells reacting against MAGE-A3 in one participant (65). This strategy that modified oncolytic viruses with CTAs as target could eliminate the tumor cells specifically and provides an immunotherapy tool for future digestive tract tumor therapy clinical application.
3.2 Cancer vaccines
Cancer vaccines are utilized to deliver tumor antigens into antigen-presenting cells and stimulate T-cell-mediated antitumor immune responses. Vaccines made from a peptide expressed specifically in the tumor may induce the tumor immune response. In patients with digestive tract cancer, specific T-cell responses can be induced by immunogenic epitopes derived from CTAs such as MAGE, BAGE, GAGE, and NY-ESO-1 (66, 67). Up to now, there has been some clinical trial focused on targeting MAGE and NY-ESO-1 that have employed peptide vaccines as a treatment option for digestive tract tumor. (Table 2). Three peptide vaccines, CHP-NY-ESO-1, IMF-001, and CDX-1401, have been constructed targeting NY-ESO-1. CHP-NY-ESO-1 is a recombinant protein that consists of NY-ESO-1 and a polysaccharide-based delivery system. The safety of this peptide vaccine has been demonstrated through in vitro and animal experiments, indicating their potential for use in clinical trials (68). A clinical trial (NCT00106158) was conducted using CHP-NY-ESO-1 vaccine for 13 patients with advanced esophageal cancer. The study observed the induction of CHP-NY-ESO-1 immunity and some favorable clinical outcomes in patients, without any major toxicities or adverse events (69). Results from other clinical trials (NCT01003808) have demonstrated that CHP-NY-ESO-1 can trigger an immune response in patients with esophageal cancer, leading to a reduction in tumor size. The degree of reduction was observed to increase with increasing dosage (70). CDX-1401 is a vaccine that consists of a human monoclonal antibody specific for DEC-205 fused to the full-length tumor antigen NY-ESO-1. CDX-1401 has the capacity to deliver NY-ESO-1 to DCs through DEC-205 and augment the body’s immune response. Clinical trial (NCT00948961) results have demonstrated that two out of four patients with colorectal cancer experienced stabilized conditions after treatment (71).
Additionally, PolyPEPI1018 is a readily available, multipeptide vaccine consisting of 12 immunogenic epitopes derived from seven cancer testis antigens (CTAs) that are frequently expressed in patients with colorectal cancer. In clinical trials of metastatic colorectal cancer, PolyPEPI1018 was found to elicit an immune response and T-cell infiltration in MSS-type patients. In comparison with TAS-102 alone, the combination of PolyPEPI1018 plus TAS-102 has demonstrated good tolerability, and it can elicit immune responses in peripheral blood and tumor tissue of patients with a lower likelihood of causing grade 3 adverse events (NCT05130060) (72). A phase I clinical trial (NCT00682227) was conducted to examine the safety, immunogenicity, and antitumor effect of a cancer vaccine targeting TTK protein kinase, lymphocyte antigen 6 family member K (LY6K), and insulin-like growth factor 2 mRNA binding protein 3 (IMP-3) against esophageal squamous cell carcinoma. 50% of the 10 enrolled patients showed favorable clinical responses after receiving the vaccination (73). SCRN1 is another CTA identified in gastric cancer tumor tissue. The CTL clones stimulated by SCRN1 were able to recognize tumor cells that expressed the natural SCRN1 protein endogenously (59).
DCs are considered the most efficient antigen-presenting cells and play critical roles in eliciting antitumor immunity (74). In addition to serving as tumor antigens, CTAs have also been utilized in the development of vaccines delivered by dendritic cells (DCs), which have demonstrated significant clinical outcomes. After 4T1 mammary tumor implantation, mice that were vaccinated with a BORIS-based DC vaccine showed a robust anticancer immune response. The tumor growth was inhibited, and the number of spontaneous clonogenic metastases was also lowered significantly (75). In addition, the effect of DC vaccine on patients with advanced CRC were evaluated. The process of generating MAGE-DCs involves pulsing autologous peripheral blood mononuclear cells with allogeneic tumor cell lysate that contains high levels of MAGE (NCT00311272). The MAGE-DCs can present MAGE antigen to T cells and stimulate an antitumor immune response (76). Moreover, the MAGE-DCs were safe and non-toxic. After treatment with the MAGE-DC vaccine, 24% (4/17) of the patients showed stable disease (77). Taken together, these findings provide compelling evidence for the potential utility of CTAs as vaccines in immunotherapy for digestive tract tumors.
3.3 ICIs
Immune checkpoints are molecules involved in co-inhibitory signaling pathways that help maintain immune tolerance. However, cancer cells often hijack these pathways to evade immunosurveillance (78). To counteract this, ICIs such as programmed cell death 1 (PD-1), programmed cell death 1 ligand 1 (PD-L1), and cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) antibodies have been developed. These drugs aim to reactivate antitumor immune responses by blocking coinhibitory signaling pathways and promoting immune-mediated elimination of cancer cells. However, remarkable efficacy has been observed with ICI only in a subset of patients. The most widely used methods for ICIs was combination with other chemicals to treat cancers. Similarly, combination treatment with ICIs and CTAs can enhance the body’s immune response. McAuliffe et al. developed a vaccine consisting of a chimpanzee adenovirus (ChAdOx1) and a modified vaccinia Ankara (MVA) that encodes MAGE-type antigens. In murine tumor models expressing P1A, the combination of ChAdOx1/MVA with anti-PD-1 antibody produced superior tumor clearance and survival when compared with treatment with anti-PD-1 alone (79). Thus far, favorable outcomes have been observed in other types of tumors through the utilization of a combination of CTAs and ICIs (80). Clinical trials are currently underway to investigate the combined treatment of PolyPEPI1018 and atezolizumab for colorectal cancer, and results are pending (NCT05243862). Thus, CTA antibodies are also potentially biomarkers predicting and monitoring response to ICI therapy.
3.4 ACT
ACT therapies refer to the use of autologous immune cells, mainly T cells, that are extracted, modified, and reinfused into patients to target and eliminate cancer cells. These therapies have demonstrated long-lasting clinical efficacy. There are two types of ACT therapies, namely, chimeric antigen receptor-modified T-cell (CAR-T) immunotherapy and T-cell receptor T cell (TCR-T) immunotherapy (81, 82).
While CAR T-cell therapy has demonstrated impressive outcomes in certain types of B-cell cancers, its applicability to other malignancies, including solid tumors is impeded by the absence of appropriate surface antigens (83). An example of the successful application of CAR T-cell therapy in solid tumors is the MAGE-A1-specific CAR, which demonstrated cytotoxic activity in vitro and in vivo. It was able to infiltrate tumors that express MAGEA1 and specifically inhibit the growth of lung adenocarcinoma xenografts in nude mice (84). Furthermore, PAS domain-containing repressor 1 (PASD1) is another CTA that has been found to be immunogenic in CRC samples. CD8+ T cells, induced by the PASD1 peptide, were shown to be capable of killing HLA-A*24:02+ PASD1+ cells (85). The researchers, led by Vita Golubovskaya, utilized a single-chain Fv fragment from a mouse monoclonal antibody clone specific to alkaline phosphatase, placental (PLAP), to engineer PLAP-CAR-T cells. These humanized PLAP-CAR-T cells were then shown to significantly inhibit tumor growth in a colon cancer xenograft model (52). However, the expression of CTAs is mainly intracellular, which limits their potential as targets for CAR therapy.
Major histocompatibility complexes (MHCs) present intracellular antigens associated with tumors, which can be targeted by T-cell receptors (TCRs). One type of antigenic target for TCR T cells are cancer-testis antigens (86). The growth of MAGE-A4-expressing esophageal cancer was hindered in NOG mice through the use of genetically engineered T cells that expressed a MAGE-A4-specific TCR designed to target the MAGE-A4 143-151 peptide-NYKRCFPVI, which is restricted to HLA-A24 (87). Furthermore, the use of MAGE-A4-specific TCR in adoptive immunotherapy for patients with recurrent esophageal cancer has been reported as safe (UMIN000002395) (88). In a phase I clinical trial (NCT03132922), Hong et al. evaluated the safety, clinical activity, and translational effects of MAGE-A4-specific TCR (89)in the treatment of solid tumors such as gastric cancer and esophageal cancer. All 38 patients across nine different tumor types experienced grade ≥3 hematologic toxicities; cytokine release syndrome was reported in 55% of patients, with 90% of these being grade ≤2. The objective response rate (ORR) (all partial response) was 24% (9/38). Phase II clinical trials are currently enrolling participants (NCT04044768) (90). In the phase I SURPASS trial (NCT04044859), the safety and efficacy of next-generation ADP-A2M4CD8 SPEAR T-cells that co-express the CD8a coreceptor with an engineered TCR targeting MAGE-A4 were evaluated. In this study which included 18 patients (two with esophageal cancer and four with gastric cancer), the results indicated that the TCR-T cells were safe for use within the human body. The best overall responses observed in the study were one partial response (gastric cancer), four cases of stable disease (two of which were gastric cancer and two were esophageal), and one case of progressive disease (gastric cancer) (91). The phase II clinical trial (NCT04752358) of this TCR-T-cell therapy in esophageal and gastric cancer is currently ongoing, and preliminary results suggest that the clinical outcomes are promising (92).
Furthermore, additional clinical trials have assessed the safety, tolerability, and efficacy of NY-ESO-1 and KK-LC-1-specific TCR gene-transduced T lymphocytes in treating tumors of the digestive tract (Table 2). However, as with the prior studies, only one trial has been completed thus far, and its results are pending publication. These findings form the foundation for future clinical investigations aimed at targeting CTAs with ACT therapies in digestive tract tumors.
4 Opportunity and challenge
Currently, surgery, radiation, and chemotherapy remain the major treatment means of patients with digestive tract tumors. The immune therapeutics have not been used as the first line of digestive tract tumor therapy in the clinical setting. Although recent progresses in cancer immunotherapy therapies have been very rapid, their efficacy is still limited to a very small subset of cancer patients. While CTA-based immunotherapies show great potential, the full therapeutic benefits of CTA-targeted digestive tract tumors have yet to be fully realized. There are also many detours and challenges along the way. To overcome the barriers and increase the efficacy of CTA-targeted digestive tract tumor immunotherapy, new strategies and cutting-edge technology should be applied (Figure 3).
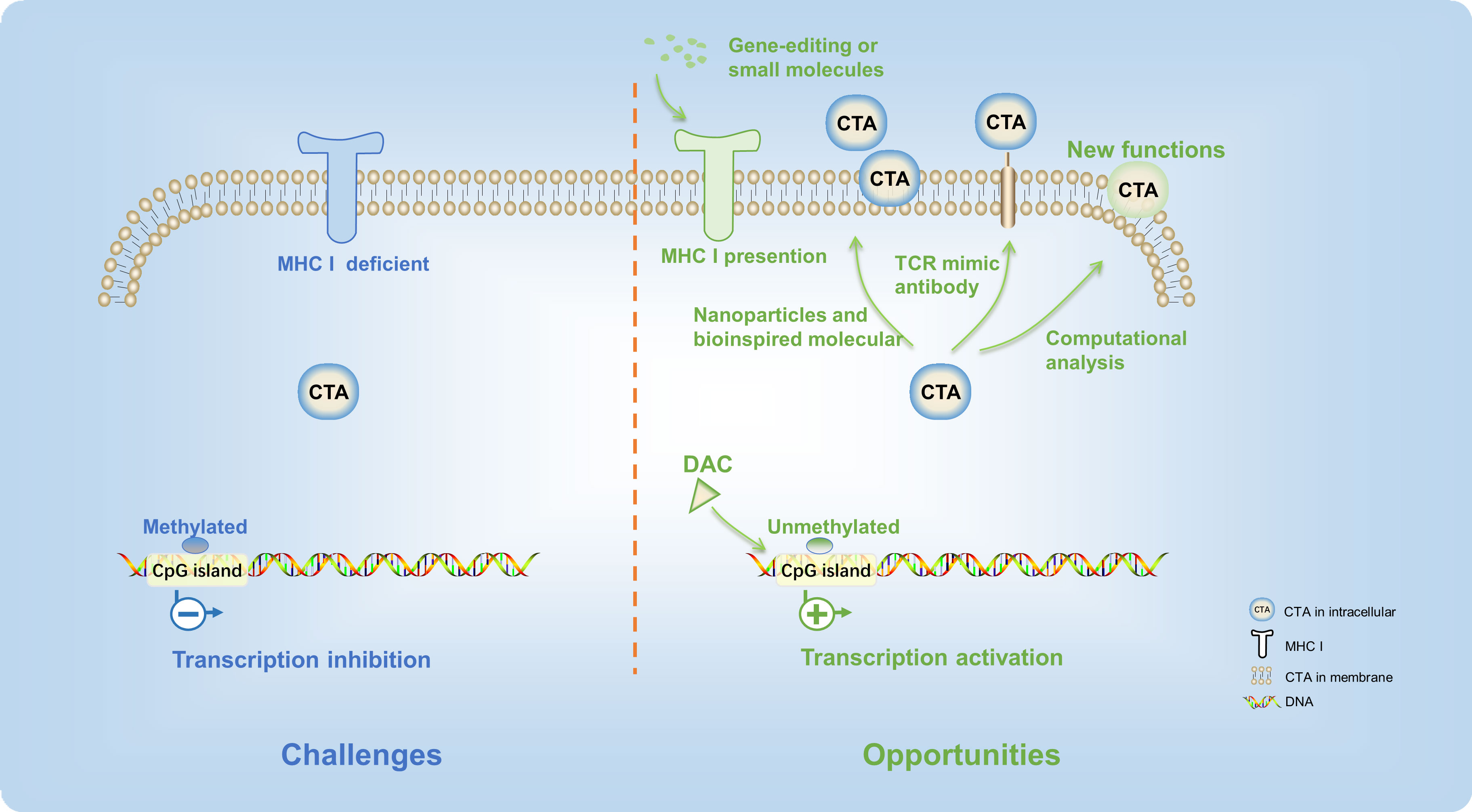
Figure 3 There are many detours and challenges in the CTA-based digestive tract tumor immunotherapy. New strategies and cutting-edge technology provided opportunities to overcome these difficulties.
First of all, since the majority of CTA protein targets are intracellular, tumor cells are often not recognized by specific antibodies or active immune cells, thus presenting a major challenge in CTA-targeted therapies (93, 94). Consequently, most CTAs are unable to elicit a robust immune response in cancer patients. With the defect of cytoplasmic localization, TCR mimic antibodies present new opportunities for additional CAR strategies targeting CTAs (82). TCR mimic antibodies have specificities that resemble those of T-cell receptors, targeting peptides presented in complex with MHC or HLA-I (95). This method enabled HLA-A2/NY-ESO-1 peptide-specific CARs to recognize tumors, offering a promising avenue to expand the range of CAR T-cell targets (96). Likewise, there has been considerable interest in bispecific antibody-based therapeutics that aim to target intracellular oncoproteins (97). This approach expands the range of CTAs that can be targeted and enhances the effectiveness of conventional antibody-based therapeutics. Meanwhile, screening more CTAs located in the membrane of digestive tract cancer cells is an alternative approach. PRAME, a CTA, was previously recognized as an intracellular protein. In recent years, a computational analysis of transmembrane proteins has predicted that a particular protein has an extracellular region that could be targeted specifically by PRAME-specific antibodies in vitro and in vivo (98). Therefore, the advances of science and technology could help to find new functions of existing CTAs.
After that, in cancer cells, the MHC-I protein is usually deficient (99), leading to low amounts of CTA epitopes on the cell membrane surface. Therefore, the T cells could not capture tumor antigen. Promoting the transcription of the MHC gene through gene-editing technology or stimulation of small molecules is an effective strategy to improve the efficacy of CTA presentation. Advanced biomaterials, such as nanoparticles and bioinspired molecular (100), could also effectively harness immunotherapies of CTA and improve their potency.
Finally, the inconsistent expression level of CTAs in the digestive tract cancer patients limited their clinical application. Although many CTAs were expressed in digestive tract cancers, only few of CTA-targeted immune therapeutics exhibited high anticancer efficacy. One of the main reasons is that the expression level of CTAs was inhibited by the high DNA methylation level at the promoter regions (101). On account of this, the demethylation agent, such as decitabine (5-aza-2′-deoxycytidine, DAC), was applied to improve antigen-specific T-cell immune responses (102). Expressions of MAGE-A (27, 103), MAGE-3, NY-ESO-1 (104), beta-2-microglobulin, calreticulin, CD58, proteasome 20S subunit beta 8 (PSMB8), and PSMB9 (105) were increased significantly in esophageal cancer and CRC after the treatment of decitabine. Moreover, clinical studies are currently underway to investigate the regulation of CTA expression by DAC (NCT00037817). Furthermore, research has also demonstrated that a mixed bacterial vaccine can activate the body’s immune response and serve as an immune modulator, thereby promoting the combination of NY-ESO-1-positive tumor cells with antigen-specific cancer vaccines (NCT00623831) (106). Reports suggest that therapeutic interventions such as radiotherapy may enhance the release of the NY-ESO-1 antigen from the tumor, which could play a critical role in directing tumor immunotherapy (107, 108). Moreover, the overexpression of CTA in the tumor cells not only improved the antitumor efficacy of T cells but also increased sensitivity of tumor cells for immunotherapy in the digestive tract tumors (109). These approaches have the potential to modulate the extent and phenotype of the antitumor immune response, thus increasing the efficacy of CTA-targeted immunotherapy for digestive tract tumors.
In summary, CTA-based immunotherapies provided a new platform and opportunity for the development of therapeutics for digestive tract tumors. It is anticipated that these novel strategies and approaches will bring about significant breakthroughs in the field of digestive tract tumors immunotherapy in the near future.
Author contributions
HA: Conceptualization, Investigation, Writing - review & editing, Writing - original draft. HY: Conceptualization, Investigation, Data curation, Writing - original draft. LL: Conceptualization, Investigation, Data curation, Methodology. JM: Conceptualization, Investigation, Methodology. KL: Conceptualization, Investigation, Funding acquisition. ZL: Conceptualization, Investigation, Methodology, Writing - review & editing. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (grant number 81872335), The Central Plains Science and Technology Innovation Leading Talents (No. 224200510015), the Health Commission of Henan Province (grant number 232102311077), and the Health and Family Planning Commission of Henan Province (grant number LHGJ20220198).
Acknowledgments
We would like to thank Guanglong Chen and Weijie Zhao for helpful guidance in data collection, and Ye Kong and Jialin Zhang for technical support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel RL, KD M, Jemal A. Cancer statistics, 2020. CA Cancer J Clin (2020) 70:7–30. doi: 10.3322/caac.21590
2. van Weverwijk A, de Visser KE. Mechanisms driving the immunoregulatory function of cancer cells. Nat Rev Cancer (2023) 23:193–215. doi: 10.1038/s41568-022-00544-4
3. Hogan B, Fellous M, Avner P, Jacob F. Isolation of a human teratoma cell line which expresses F9 antigen. NATURE (1977) 270:515–8. doi: 10.1038/270515a0
4. Holden S, Bernard O, Artzt K, Whitmore WJ, Bennett D. Human and mouse embryonal carcinoma cells in culture share an embryonic antigen (F9). NATURE (1977) 270:518–20. doi: 10.1038/270518a0
5. Tavakoli KA, Mahjoubi B, Mirzaei R, Shabani S, Mahjoubi F. AKAP4, SPAG9 and NY-ESO-1 in Iranian colorectal cancer patients as probable diagnostic and prognostic biomarkers. Asian Pac J Cancer Prev (2018) 19:463–9. doi: 10.22034/APJCP.2018.19.2.463
6. Gordeeva O. Cancer-testis antigens: unique cancer stem cell biomarkers and targets for cancer therapy. Semin Cancer Biol (2018) 53:75–89. doi: 10.1016/j.semcancer.2018.08.006
7. Costa FF, Le Blanc K, Brodin B. Concise review: cancer/testis antigens, stem cells, and cancer. Stem Cells (2007) 25:707–11. doi: 10.1634/stemcells.2006-0469
8. Pan J, Yu H, Guo Z, Liu Q, Ding M, Xu K, et al. Emerging role of sperm-associated antigen 9 in tumorigenesis. BioMed Pharmacother (2018) 103:1212–6. doi: 10.1016/j.biopha.2018.04.168
9. Szender JB, Papanicolau-Sengos A, Eng KH, Miliotto AJ, Lugade AA, Gnjatic S, et al. NY-ESO-1 expression predicts an aggressive phenotype of ovarian cancer. GYNECOL Oncol (2017) 145:420–5. doi: 10.1016/j.ygyno.2017.03.509
10. Cao QH, Liu F, CZ Li, Liu N, Shu M, Lin Y, et al. Testes-specific protease 50 (TSP50) promotes invasion and metastasis by inducing EMT in gastric cancer. BMC Cancer (2018) 18:94. doi: 10.1186/s12885-018-4000-y
11. Kwon Y, Kim Y, HS J, Jeoung D. Role of HDAC3-miRNA-CAGE network in anti-cancer drug-resistance. Int J Mol Sci (2018) 20:51. doi: 10.3390/ijms20010051
12. van der Bruggen P, Traversari C, Chomez P, Lurquin C, De Plaen E, Van den Eynde B, et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science (1991) 254:1643–7. doi: 10.1126/science.1840703
13. Sahin U, Türeci O, Schmitt H, Cochlovius B, Johannes T, Schmits R, et al. Human neoplasms elicit multiple specific immune responses in the autologous host. Proc Natl Acad Sci U.S.A. (1995) 92:11810–3. doi: 10.1073/pnas.92.25.11810
14. Chen YT, MJ S, Sahin U, Türeci O, Gure AO, Tsang S, et al. A testicular antigen aberrantly expressed in human cancers detected by autologous antibody screening. Proc Natl Acad Sci U.S.A. (1997) 94:1914–8. doi: 10.1073/pnas.94.5.1914
15. Chen YT, Boyer AD, Viars CS, Tsang S, Old LJ, Arden KC. Genomic cloning and localization of CTAG, a gene encoding an autoimmunogenic cancer-testis antigen NY-ESO-1, to human chromosome Xq28. Cytogenet Cell Genet (1997) 79:237–40. doi: 10.1159/000134734
16. Türeci O, Sahin U, Zwick C, Koslowski M, Seitz G, Pfreundschuh M. Identification of a meiosis-specific protein as a member of the class of cancer/testis antigens. Proc Natl Acad Sci U.S.A. (1998) 95:5211–6. doi: 10.1073/pnas.95.9.5211
17. Old LJ, Chen YT. New paths in human cancer serology. J Exp Med (1998) 187:1163–7. doi: 10.1084/jem.187.8.1163
18. Wang C, Gu Y, Zhang K, Xie K, Zhu M, Dai N, et al. Systematic identification of genes with a cancer-testis expression pattern in 19 cancer types. Nat Commun (2016) 7:10499. doi: 10.1038/ncomms10499
19. McFarlane RJ, Feichtinger J, Larcombe L. Cancer germline gene activation: friend or foe? Cell Cycle (2014) 13:2151–2. doi: 10.4161/cc.29661
20. Hofmann O, Caballero OL, Stevenson BJ, Chen YT, Cohen T, Chua R, et al. Genome-wide analysis of cancer/testis gene expression. Proc Natl Acad Sci U.S.A. (2008) 105:20422–7. doi: 10.1073/pnas.0810777105
21. Inoue H, Mori M, Li J, Mimori K, Honda M, Nakashima H, et al. Human esophageal carcinomas frequently express the tumor-rejection antigens of MAGE genes. Int J Cancer (1995) 63:523–6. doi: 10.1002/ijc.2910630411
22. Haier J, Owzcareck M, Guller U, Spagnoli GC, Bürger H, Senninger N, et al. Expression of MAGE-a cancer/testis antigens in esophageal squamous cell carcinomas. Anticancer Res (2006) 26:2281–7.
23. Akcakanat A, Kanda T, Tanabe T, Komukai S, Yajima K, Nakagawa S, et al. Heterogeneous expression of GAGE, NY-ESO-1, MAGE-a and SSX proteins in esophageal cancer: implications for immunotherapy. Int J Cancer (2006) 118:123–8. doi: 10.1002/ijc.21219
24. Schooten E, Maggio AD, van Bergen En Henegouwen PMP, Kijanka MM. MAGE-a antigens as targets for cancer immunotherapy. Cancer Treat Rev (2018) 67:54–62. doi: 10.1016/j.ctrv.2018.04.009
25. Forghanifard MM, Gholamin M, Farshchian M, Moaven O, Memar B, Forghani MN, et al. Cancer-testis gene expression profiling in esophageal squamous cell carcinoma: identification of specific tumor marker and potential targets for immunotherapy. Cancer Biol Ther (2011) 12:191–7. doi: 10.4161/cbt.12.3.15949
26. Qi Y, KX C, FC X, CY Z, Huang Q, Wu K, et al. High expression of MAGE-A9 is associated with unfavorable survival in esophageal squamous cell carcinoma. Oncol Lett (2017) 14:3415–20. doi: 10.3892/ol.2017.6614
27. Shi X, Chen X, Fang B, Ping Y, Qin G, Yue D, et al. Decitabine enhances tumor recognition by T cells through upregulating the MAGE-A3 expression in esophageal carcinoma. BioMed Pharmacother (2019) 112:108632. doi: 10.1016/j.biopha.2019.108632
28. Wu Y, Sang M, Liu F, Zhang J, Li W, Li Z, et al. Epigenetic modulation combined with PD-1/PD-L1 blockade enhances immunotherapy based on MAGE-A11 antigen-specific CD8+T cells against esophageal carcinoma. Carcinogenesis (2020) 41:894–903. doi: 10.1093/carcin/bgaa057
29. D AZP DSMP, D APM D BETM, D PZP. MAGE, BAGE, and GAGE gene expression in patients with esophageal squamous cell carcinoma and adenocarcinoma of the gastric cardia. CANCER-AM Cancer Soc (2015) 91:1882–8. doi: 10.1002/1097-0142(20010515)91:10<1882::AID-CNCR1210>3.0.CO;2-H
30. Eynde B, Boon T. Tumor antigens recognized by T lymphocytes. Int J Clin Lab Res (1997) 27:81–6. doi: 10.1007/BF02912440
31. Fujita S. NY-ESO-1 expression and immunogenicity in esophageal cancer. Clin Cancer Res Off J Am Assoc Cancer Res (2004) 10:6551. doi: 10.1158/1078-0432.CCR-04-0819
32. Zhang Y, Zhang Y, Zhang L. Expression of cancer-testis antigens in esophageal cancer and their progress in immunotherapy. J Cancer Res Clin Oncol (2019) 145:281–91. doi: 10.1007/s00432-019-02840-3
33. Okabayashi K, Fujita T, Miyazaki J, Okada T, Iwata T, Hirao N, et al. Cancer-testis antigen BORIS is a novel prognostic marker for patients with esophageal cancer. Cancer Sci (2012) 103:1617–24. doi: 10.1111/j.1349-7006.2012.02355.x
34. Kang Y, Hong JA, Chen GA, Nguyen DM, Schrump DS. Dynamic transcriptional regulatory complexes including BORIS, CTCF and Sp1 modulate NY-ESO-1 expression in lung cancer cells. Oncogene (2007) 26:4394–403. doi: 10.1038/sj.onc.1210218
35. Vatolin S, Abdullaev Z, Pack SD, Flanagan PT, Custer M, Loukinov DI, et al. Conditional expression of the CTCF-paralogous transcriptional factor BORIS in normal cells results in demethylation and derepression of MAGE-A1 and reactivation of other cancer-testis genes. Cancer Res (2005) 65:7751–62. doi: 10.1158/0008-5472.CAN-05-0858
36. Ghadban T, Perez DR, Vashist YK, Bockhorn M, Koenig AM, El GA, et al. Expression of cancer testis antigens CT10 (MAGE-C2) and GAGE in gastrointestinal stromal tumors. Eur J Surg Oncol (2014) 40:1307–12. doi: 10.1016/j.ejso.2014.03.011
37. Lian Y, Sang M, Gu L, Liu F, Yin D, Liu S, et al. MAGE-a family is involved in gastric cancer progression and indicates poor prognosis of gastric cancer patients. Pathol Res Pract (2017) 213:943–8. doi: 10.1016/j.prp.2017.05.007
38. Suzuki T, Yoshida K, Wada Y, Hamai Y, Sentani K, Oue N, et al. Melanoma-associated antigen-A1 expression predicts resistance to docetaxel and paclitaxel in advanced and recurrent gastric cancer. Oncol Rep (2007) 18:329–36. doi: 10.3892/or.18.2.329
39. Abbaszadegan MR, Taghehchian N, Aarabi A, Moghbeli M. MAEL cancer-testis antigen as a diagnostic marker in primary stages of gastric cancer with helicobacter pylori infection. J Gastrointest Cancer (2020) 51:17–22. doi: 10.1007/s12029-018-0183-3
40. Yuan L, Shan J, De Risi D, Broome J, Lovecchio J, Gal D, et al. Isolation of a novel gene, TSP50, by a hypomethylated DNA fragment in human breast cancer. Cancer Res (1999) 59:3215–21.
41. Liu F, Cao Q, Liu N, Li C, You C, Liu C, et al. Overexpression of testes-specific protease 50 (TSP50) predicts poor prognosis in patients with gastric cancer. Gastroenterol Res Pract (2014) 2014:498246. doi: 10.1155/2014/498246
42. Dakshinamurthy AG, Ramesar R, Goldberg P, Blackburn JM. Infrequent and low expression of cancer-testis antigens located on the X chromosome in colorectal cancer: implications for immunotherapy in south African populations. Biotechnol J (2008) 3:1417–23. doi: 10.1002/biot.200800144
43. Jungbluth AA, Busam KJ, Kolb D, Iversen K, Coplan K, Chen YT, et al. Expression of MAGE-antigens in normal tissues and cancer. Int J Cancer (2000) 85:460–5. doi: 10.1002/(SICI)1097-0215(20000215)85:4<460::AID-IJC3>3.0.CO;2-N
44. Li M, Yuan YH, Han Y, YX L, Yan L, Wang Y, et al. Expression profile of cancer-testis genes in 121 human colorectal cancer tissue and adjacent normal tissue. Clin Cancer Res (2005) 11:1809–14. doi: 10.1158/1078-0432.CCR-04-1365
45. Chung FY, Cheng TL, Chang HJ, Chiu HH, Huang MY, Chang MS, et al. Differential gene expression profile of MAGE family in taiwanese patients with colorectal cancer. J Surg Oncol (2010) 102:148–53. doi: 10.1002/jso.21580
46. Shen YM, Arbman G, Sandstrom P, Gullstrand P, YQ W, Zhang H, et al. Novel gene hBiot2 is an independent prognostic factor in colorectal cancer patients. Oncol Rep (2012) 27:376–82. doi: 10.3892/or.2011.1521
47. Song MH, Ha JC, Lee SM, Park YM, Lee SY. Identification of BCP-20 (FBXO39) as a cancer/testis antigen from colon cancer patients by SEREX. Biochem Biophys Res Commun (2011) 408:195–201. doi: 10.1016/j.bbrc.2011.02.077
48. Jagadish N, Parashar D, Gupta N, Agarwal S, Suri V, Kumar R, et al. Heat shock protein 70-2 (HSP70-2) is a novel therapeutic target for colorectal cancer and is associated with tumor growth. BMC Cancer (2016) 16:561. doi: 10.1186/s12885-016-2592-7
49. Yuki D, YM L, Fujii Y, Nakamura Y, Furukawa Y. Isolation of LEM domain-containing 1, a novel testis-specific gene expressed in colorectal cancers. Oncol Rep (2004) 12:275–80. doi: 10.3892/or.12.2.275
50. Vasaikar S, Huang C, Wang X, Petyuk VA, Savage SR, Wen B, et al. Proteogenomic analysis of human colon cancer reveals new therapeutic opportunities. CELL (2019) 177:1035–49. doi: 10.1016/j.cell.2019.03.030
51. Dong XY, YY Li, XA Y, Chen WF. BJ-HCC-20, a potential novel cancer-testis antigen. Biochem Cell Biol (2004) 82:577–82. doi: 10.1139/o04-056
52. Li X, Berahovich R, Zhou H, Liu X, Li F, Xu S, et al. PLAP -CAR T cells mediate high specific cytotoxicity against colon cancer cells. Front Biosci (Landmark Ed) (2020) 25:1765–86. doi: 10.2741/4877
53. Kim R, Kulkarni P, Hannenhalli S. Derepression of Cancer/Testis antigens in cancer is associated with distinct patterns of DNA hypomethylation. BMC Cancer (2013) 13:144. doi: 10.1186/1471-2407-13-144
54. Lian Y, Sang M, Gu L, Liu F, Yin D, Liu S, et al. MAGE-a family is involved in gastric cancer progression and indicates poor prognosis of gastric cancer patients. Pathol - Res Pract (2017) 213:943–948. doi: 10.1016/j.prp.2017.05.007
55. Perez D, Hauswirth F, Jäger D, Metzger U, Samartzis EP, Went P, et al. Protein expression of cancer testis antigens predicts tumor recurrence and treatment response to imatinib in gastrointestinal stromal tumors. Int J Cancer (2011) 128:2947–52. doi: 10.1002/ijc.25836
56. Zhang QM, SJ He, Shen N, Luo B, Fan R, Fu J, et al. Overexpression of MAGE-D4 in colorectal cancer is a potentially prognostic biomarker and immunotherapy target. Int J Clin Exp Pathol (2014) 7:3918–27.
57. Zhan W, Zhang Z, Zhang Y, Ma J, Wu T, Gu Y, et al. Prognostic value of MAGE-A9 expression in patients with colorectal cancer. Clin Res Hepatol Gastroenterol (2016) 40:239–45. doi: 10.1016/j.clinre.2015.08.005
58. Sun X-F. Novel gene hBiot2 is an independent prognostic factor in colorectal cancer patients. Oncol Rep (2012) 27:376–382. doi: 10.3892/or.2011.1521
59. Suda T, Tsunoda T, Uchida N, Watanabe T, Hasegawa S, Satoh S, et al. Identification of secernin 1 as a novel immunotherapy target for gastric cancer using the expression profiles of cDNA microarray. Cancer Sci (2006) 97:411–9. doi: 10.1111/j.1349-7006.2006.00194.x
60. Zhou C, Zhang P, GC Xu, DM Wu, RY L, Zeng Q, et al. RNA Interference of Biot2 induces G1 phase arrest and apoptosis in mouse colorectal cancer cell line. Oncol Res (2015) 22:93–103. doi: 10.3727/096504014X14146137738583
61. Takeda R, Hirohashi Y, Shen M, Wang L, Ogawa T, Murai A, et al. Identification and functional analysis of variants of a cancer/testis antigen LEMD1 in colorectal cancer stem-like cells. Biochem Biophys Res Commun (2017) 485:651–7. doi: 10.1016/j.bbrc.2017.02.081
62. Zhao H, Pan WM, Zhang HH, Song Y, Chen J, Xiang Y, et al. Cancer testis antigen 55 deficiency attenuates colitis-associated colorectal cancer by inhibiting NF-κB signaling. Cell Death Dis (2019) 10:304. doi: 10.1038/s41419-019-1537-x
63. Shalhout SZ, Miller DM, Emerick KS, Kaufman HL. Therapy with oncolytic viruses: progress and challenges. Nat Rev Clin Oncol (2023) 20:160–77. doi: 10.1038/s41571-022-00719-w
64. Pol JG, Acuna SA, Yadollahi B, Tang N, Stephenson KB, Atherton MJ, et al. Preclinical evaluation of a MAGE-A3 vaccination utilizing the oncolytic maraba virus currently in first-in-human trials. ONCOIMMUNOLOGY (2019) 8:e1512329. doi: 10.1080/2162402X.2018.1512329
65. Pol JG, Atherton MJ, Bridle BW, Stephenson KB, Le Boeuf F, Hummel JL, et al. Development and applications of oncolytic maraba virus vaccines. Oncolytic Virother (2018) 7:117–28. doi: 10.2147/OV.S154494
66. Yang P, Meng M, Zhou Q. Oncogenic cancer/testis antigens are a hallmarker of cancer and a sensible target for cancer immunotherapy. Biochim Biophys Acta Rev Cancer (2021) 1876:188558. doi: 10.1016/j.bbcan.2021.188558
67. Bender A, Karbach J, Neumann A, Jger D, Al-Batran SE, Atmaca A, et al. LUD 00-009: phase 1 study of intensive course immunization with NY-ESO-1 peptides in HLA-A2 positive patients with NY-ESO-1-expressing cancer. Cancer Immun A J Acad Cancer Immunol (2007) 7:16.
68. Harada N, Hoshiai K, Takahashi Y, Sakaguchi Y, Kuno T, Hishida T, et al. Preclinical safety pharmacology study of a novel protein-based cancer vaccine CHP-NY-ESO-1. Kobe J Med Sci (2008) 54:E23–34.
69. Wada H, Sato E, Uenaka A, Isobe M, Kawabata R, Nakamura Y, et al. Analysis of peripheral and local anti-tumor immune response in esophageal cancer patients after NY-ESO-1 protein vaccination. Int J Cancer (2008) 123:2362–9. doi: 10.1002/ijc.23810
70. Kageyama S, Wada H, Muro K, Niwa Y, Ueda S, Miyata H, et al. Dose-dependent effects of NY-ESO-1 protein vaccine complexed with cholesteryl pullulan (CHP-NY-ESO-1) on immune responses and survival benefits of esophageal cancer patients. J Transl Med (2013) 11:246. doi: 10.1186/1479-5876-11-246
71. Dhodapkar MV, Sznol M, Zhao B, Wang D, Carvajal RD, Keohan ML, et al. Induction of antigen-specific immunity with a vaccine targeting NY-ESO-1 to the dendritic cell receptor DEC-205. Sci Transl Med (2014) 6:232r–51r. doi: 10.1126/scitranslmed.3008068
72. Hubbard JM, Zemla TJ, Graham RP, Jin Z, Zhu M, Mitchell JL, et al. Phase ib open-label study to evaluate safety, tolerability, immunogenicity, and efficacy of multiple subcutaneous injections of PolyPEPI1018 vaccine as an add-on immunotherapy to TAS-102 in participants with late-stage microsatellite-stable metastatic colorectal cancer (MSS mCRC; OBERTO-201). J Clin Oncol (2023) 41:147. doi: 10.1200/JCO.2023.41.4_suppl.147
73. Kono K, Mizukami Y, Daigo Y, Takano A, Masuda K, Yoshida K, et al. Vaccination with multiple peptides derived from novel cancer-testis antigens can induce specific T-cell responses and clinical responses in advanced esophageal cancer. Cancer Sci (2009) 100:1502–9. doi: 10.1111/j.1349-7006.2009.01200.x
74. Verneau J, Sautes-Fridman C, Sun CM. Dendritic cells in the tumor microenvironment: prognostic and theranostic impact. Semin Immunol (2020) 48:101410. doi: 10.1016/j.smim.2020.101410
75. Mkrtichyan M, Ghochikyan A, Davtyan H, Movsesyan N, Loukinov D, Lobanenkov V, et al. Cancer-testis antigen, BORIS based vaccine delivered by dendritic cells is extremely effective against a very aggressive and highly metastatic mouse mammary carcinoma. Cell Immunol (2011) 270:188–97. doi: 10.1016/j.cellimm.2011.05.007
76. Burgdorf SK, Claesson MH, Nielsen HJ, Rosenberg J. Changes in cytokine and biomarker blood levels in patients with colorectal cancer during dendritic cell-based vaccination. Acta Oncol (2009) 48:1157–64. doi: 10.3109/02841860903099964
77. Burgdorf SK, Fischer A, Myschetzky PS, Munksgaard SB, Zocca MB, Claesson MH, et al. Clinical responses in patients with advanced colorectal cancer to a dendritic cell based vaccine. Oncol Rep (2008) 20:1305–11. doi: 10.3892/or_00000145
78. Zhang Y, Zhang Z. The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol Immunol (2020) 17:807–21. doi: 10.1038/s41423-020-0488-6
79. McAuliffe J, HF C, Noblecourt L, Ramirez-Valdez RA, Pereira-Almeida V, Zhou Y, et al. Heterologous prime-boost vaccination targeting MAGE-type antigens promotes tumor T-cell infiltration and improves checkpoint blockade therapy. J Immunother Cancer (2021) 9:e003218. doi: 10.1136/jitc-2021-003218
80. Haag GM, Zoernig I, JC H, Halama N, Dick J, Lang N, et al. Phase II trial of ipilimumab in melanoma patients with preexisting humoural immune response to NY-ESO-1. Eur J Cancer (2018) 90:122–9. doi: 10.1016/j.ejca.2017.12.001
81. Fesnak AD, June CH, Levine BL. Engineered T cells: the promise and challenges of cancer immunotherapy. Nat Rev Cancer (2016) 16:566–81. doi: 10.1038/nrc.2016.97
82. Jakobsen MK, Gjerstorff MF. CAR T-cell cancer therapy targeting surface Cancer/Testis antigens. Front Immunol (2020) 11:1568. doi: 10.3389/fimmu.2020.01568
83. Sadelain M, Riviere I, Riddell S. Therapeutic T cell engineering. Nature (2017) 545:423–31. doi: 10.1038/nature22395
84. Mao Y, Fan W, Hu H, Zhang L, Michel J, Wu Y, et al. MAGE-A1 in lung adenocarcinoma as a promising target of chimeric antigen receptor T cells. J Hematol Oncol (2019) 12:106. doi: 10.1186/s13045-019-0793-7
85. Soh JE, Abu N, Sagap I, Mazlan L, Yahaya A, Mustangin M, et al. Validation of immunogenic PASD1 peptides against HLA-A*24:02 colorectal cancer. Immunotherapy-UK (2019) 11:1205–19. doi: 10.2217/imt-2019-0073
86. Linnemann C, Heemskerk B, Kvistborg P, Kluin RJ, Bolotin DA, Chen X, et al. High-throughput identification of antigen-specific TCRs by TCR gene capture. Nat Med (2013) 19:1534–41. doi: 10.1038/nm.3359
87. Ottaviani S, Colau D, van der Bruggen P, van der Bruggen P. A new MAGE-4 antigenic peptide recognized by cytolytic T lymphocytes on HLA-A24 carcinoma cells. Cancer Immunol Immunother (2006) 55:867–72. doi: 10.1007/s00262-005-0053-2
88. Kageyama S, Ikeda H, Miyahara Y, Imai N, Ishihara M, Saito K, et al. Adoptive transfer of MAGE-A4 T-cell receptor gene-transduced lymphocytes in patients with recurrent esophageal cancer. Clin Cancer Res (2015) 21:2268–77. doi: 10.1158/1078-0432.CCR-14-1559
89. Sanderson JP, Crowley DJ, Wiedermann GE, Quinn LL, Crossland KL, Tunbridge HM, et al. Preclinical evaluation of an affinity-enhanced MAGE-A4-specific T-cell receptor for adoptive T-cell therapy. ONCOIMMUNOLOGY (2020) 9:1682381. doi: 10.1080/2162402X.2019.1682381
90. Hong DS, Van Tine BA, Biswas S, McAlpine C, Johnson ML, Olszanski AJ, et al. Autologous T cell therapy for MAGE-A4(+) solid cancers in HLA-A*02(+) patients: a phase 1 trial. Nat Med (2023) 29:104–14. doi: 10.1038/s41591-022-02128-z
91. Hong DS, Clarke JM, Asch A, Charlson J, Johanns TM, Calvo E, et al. 540P safety and efficacy from the SURPASS trial with ADP-A2M4CD8, a SPEAR T-cell therapy incorporating a CD8α co-receptor and an affinity optimized TCR targeting MAGE-A4. Ann Oncol (2021) 32:S604–5. doi: 10.1016/j.annonc.2021.08.1062
92. Hong DS, Jalal SI, Elimova E, Ajani JA, Blum Murphy MA, Cervantes A, et al. SURPASS-2 trial design: a phase 2, open-label study of ADP-A2M4CD8 SPEAR T cells in advanced esophageal or esophagogastric junction cancers. J Clin Oncol (2022) 40:S363. doi: 10.1200/JCO.2022.40.4_suppl.TPS363
93. Zajac P, Schultz-Thater E, Tornillo L, Sadowski C, Trella E, Mengus C, et al. MAGE-a antigens and cancer immunotherapy. Front Med (Lausanne) (2017) 4:18. doi: 10.3389/fmed.2017.00018
94. Schultz-Thater E, Noppen C, Gudat F, Durmuller U, Zajac P, Kocher T, et al. NY-ESO-1 tumour associated antigen is a cytoplasmic protein detectable by specific monoclonal antibodies in cell lines and clinical specimens. Br J Cancer (2000) 83:204–8. doi: 10.1054/bjoc.2000.1251
95. Chang AY, Dao T, Gejman RS, Jarvis CA, Scott A, Dubrovsky L, et al. A therapeutic T cell receptor mimic antibody targets tumor-associated PRAME peptide/HLA-I antigens. J Clin Invest (2017) 127:3557. doi: 10.1172/JCI96860
96. Maruta M, Ochi T, Tanimoto K, Asai H, Saitou T, Fujiwara H, et al. Direct comparison of target-reactivity and cross-reactivity induced by CAR- and BiTE-redirected T cells for the development of antibody-based T-cell therapy. Sci Rep (2019) 9:13293. doi: 10.1038/s41598-019-49834-2
97. Xu G, Luo Y, Wang H, Wang Y, Liu B, Wei J. Therapeutic bispecific antibodies against intracellular tumor antigens. Cancer Lett (2022) 538:215699. doi: 10.1016/j.canlet.2022.215699
98. Pankov D, Sjostrom L, Kalidindi T, SG L, Sjostrom K, Gardner R, et al. In vivo immuno-targeting of an extracellular epitope of membrane bound preferentially expressed antigen in melanoma (PRAME). Oncotarget (2017) 8:65917–31. doi: 10.18632/oncotarget.19579
99. Garrido F, Romero I, Aptsiauri N, Garcia-Lora AM. Generation of MHC class I diversity in primary tumors and selection of the malignant phenotype. Int J Cancer (2016) 138:271–80. doi: 10.1002/ijc.29375
100. Riley RS, June CH, Langer R, Mitchell MJ. Delivery technologies for cancer immunotherapy. Nat Rev Drug Discovery (2019) 18:175–96. doi: 10.1038/s41573-018-0006-z
101. Ziller MJ, Gu H, Muller F, Donaghey J, Tsai LT, Kohlbacher O, et al. Charting a dynamic DNA methylation landscape of the human genome. Nature (2013) 500:477–81. doi: 10.1038/nature12433
102. Chou J, Voong LN, Mortales CL, Towlerton AM, Pollack SM, Chen X, et al. Epigenetic modulation to enable antigen-specific T-cell therapy of colorectal cancer. J Immunother (2012) 35:131–41. doi: 10.1097/CJI.0b013e31824300c7
103. Weinert BT, Krishnadath KK, Milano F, Pedersen AW, Claesson MH, Zocca MB. Real-time PCR analysis of genes encoding tumor antigens in esophageal tumors and a cancer vaccine. Cancer Immun (2009) 9:9.
104. Schrump DS, Fischette MR, Nguyen DM, Zhao M, Li X, Kunst TF, et al. Phase I study of decitabine-mediated gene expression in patients with cancers involving the lungs, esophagus, or pleura. Clin Cancer Res (2006) 12:5777–85. doi: 10.1158/1078-0432.CCR-06-0669
105. Siebenkas C, Chiappinelli KB, Guzzetta AA, Sharma A, Jeschke J, Vatapalli R, et al. Inhibiting DNA methylation activates cancer testis antigens and expression of the antigen processing and presentation machinery in colon and ovarian cancer cells. PloS One (2017) 12:e179501. doi: 10.1371/journal.pone.0179501
106. Karbach J, Neumann A, Brand K, Wahle C, Siegel E, Maeurer M, et al. Phase I clinical trial of mixed bacterial vaccine (Coley's toxins) in patients with NY-ESO-1 expressing cancers: immunological effects and clinical activity. Clin Cancer Res (2012) 18:5449–59. doi: 10.1158/1078-0432.CCR-12-1116
107. Klein O, Davis ID, McArthur GA, Chen L, Haydon A, Parente P, et al. Low-dose cyclophosphamide enhances antigen-specific CD4(+) T cell responses to NY-ESO-1/ISCOMATRIX vaccine in patients with advanced melanoma. Cancer Immunol Immunother (2015) 64:507–18. doi: 10.1007/s00262-015-1656-x
108. Klar AS, Gopinadh J, Kleber S, Wadle A, Renner C. Treatment with 5-Aza-2'-Deoxycytidine induces expression of NY-ESO-1 and facilitates cytotoxic T lymphocyte-mediated tumor cell killing. PloS One (2015) 10:e139221. doi: 10.1371/journal.pone.0139221
Keywords: cancer/testis antigens, digestive tract cancers, immunotherapy, target, esophagus cancer (adenocarcinoma), gastrointestinal carcinoma
Citation: Ai H, Yang H, Li L, Ma J, Liu K and Li Z (2023) Cancer/testis antigens: promising immunotherapy targets for digestive tract cancers. Front. Immunol. 14:1190883. doi: 10.3389/fimmu.2023.1190883
Received: 21 March 2023; Accepted: 26 May 2023;
Published: 16 June 2023.
Edited by:
Ganesan Ramamoorthi, Moffitt Cancer Center, United StatesReviewed by:
Olga Gordeeva, Russian Academy of Sciences, RussiaErwin Goldberg, Northwestern University, United States
Copyright © 2023 Ai, Yang, Li, Ma, Liu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kangdong Liu, kdliu@zzu.edu.cn; Zhi Li, zlyylizhi0316@zzu.edu.cn
†These authors share last authorship
 Huihan Ai
Huihan Ai Hang Yang
Hang Yang Liang Li1
Liang Li1 Jie Ma
Jie Ma Kangdong Liu
Kangdong Liu Zhi Li
Zhi Li