- 1Department of Science and Research, Anning First People’s Hospital Affiliated to Kunming University of Science and Technology, Kunming, Yunnan, China
- 2Department of Medical Imaging, Yanan Hospital Affiliated to Kunming Medical University, Kunming, Yunnan, China
- 3Department of Immunology, Institute of Basic Medical Sciences Chinese Academy of Medical Sciences, School of Basic Medicine Peking Union Medical College, Beijing, China
- 4Department of Pulmonary and Critical Care Medicine, Anning First People’s Hospital Affiliated to Kunming University of Science and Technology, Kunming, Yunnan, China
- 5School of Basic Medical Sciences, Kunming Medical University, Kunming, Yunnan, China
- 6The School of Medicine, Kunming University, Kunming, China
- 7Department of Gerontology 2, The Second People’s Hospital of Kunming, Kunming, China
- 8Department of Medical Imaging, Anning First People’s Hospital Affiliated to Kunming University of Science and Technology, Kunming, Yunnan, China
Objectives: We aimed to evaluate the indeterminate rate of interferon gamma release assays (IGRAs) in the detection of latent tuberculosis infection (LTBI).
Methods: On 15 November 2022, we searched the PubMed® (National Library of Medicine, Bethesda, MD, USA), Embase® (Elsevier, Amsterdam, the Netherlands), and Cochrane Library databases in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Two investigators independently extracted the study data and assessed their quality using a modified quality assessment of diagnostic accuracy studies (i.e., QUADAS-2) tool. A random-effects model was used to calculate pooled results.
Results: We included 403 studies involving 486,886 individuals and found that the pooled indeterminate rate was 3.9% (95% CI 3.5%–4.2%). The pooled indeterminate rate for QuantiFERON®-TB (QFT) was similar to that for T-SPOT®.TB (T-SPOT) [odds ratio (OR) = 0.88, 95% CI 0.59–1.32]; however, the indeterminate rate for a new generation of QFT (QFT-plus) was lower than that of T-SPOT (OR = 0.24, 95% CI 0.16–0.35). The indeterminate rate in the immunocompromised population was significantly higher than that in healthy controls (OR = 3.51, 95% CI 2.11–5.82), and it increased with the reduction of CD4+ cell count in HIV-positive patients. Children’s pooled indeterminate rates (OR = 2.56, 95% CI 1.79–3.57) were significantly higher than those of adults, and the rates increased as the children’s age decreased.
Conclusion: On average, 1 in 26 tests yields indeterminate IGRA results in LTBI screening. The use of advanced versions of the QuantiFERON-TB assay (QFT-plus), may potentially reduce the occurrence of an indeterminate result. Our study emphasizes the high risk of immunosuppression and young age in relation to indeterminate IGRA, which should receive more attention in the management of LTBI.
Systematic review registration: PROSPERO https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020211363, CRD42020211363.
1 Introduction
Latent tuberculosis infection (LTBI) is defined as a persistent immune response to Mycobacterium tuberculosis antigen stimulation, with no evidence of clinically manifested active tuberculosis (1, 2). Globally, approximately one-quarter of the population is affected by LTBI (3). The diagnosis and preventive treatment of LTBI are critical for TB elimination (4). Two immune-based tests, that is, the interferon gamma (interferon-γ) release assay (IGRA) and the tuberculin skin test (TST), are currently used to diagnose LTBI (5, 6).
Bacillus Calmette–Guérin (BCG) and non-tuberculosis mycobacteria (NTM) have no effect on IGRAs, which assess the level of interferon-γ responses to the TB-specific antigens early secreted antigenic target 6 kDa (ESAT-6) and culture filtrate protein-10 kDa (CFP-10) (7). Two types of IGRA are now commercially available, the T-SPOT®.TB (T-SPOT, Oxford Immunotec Ltd, Oxford, UK) and QuantiFERON®-TB (QFT, Cellestis Ltd., Carnegie, Australia), which use enzyme-linked immunosorbent spot (ELISPOT) and enzyme-linked immunosorbent assay (ELISA) formats respectively (8). A mitogen tube is used as positive control to assess the performance of the test and the functionality of the individual's T-cells in both types of commercially available IGRAs, and a nil tube is used as a negative control to adjust for background interferon-γ. Either high interferon-γ levels in the negative control or a low response in the positive control can result in an indeterminate result (9).
Previous meta-analyses have evaluated the indeterminate rate of IGRAs. Two studies assessed the indeterminate rate in HIV-positive patients (10, 11), two studies assessed the indeterminate rate in inflammatory bowel disease patients (12, 13), and one study assessed the indeterminate rate in patients that underwent an organ transplant (14). Two large meta-analyses assessed the indeterminate rate in the entire population (15, 16); however, active TB, suspected TB, and LTBI were included. As the immune response may differ depending on the type of TB infection, the indeterminate rate of IGRAs may vary among these groups. For example, Santin et al.’s meta-analysis, focusing on HIV-positive patients, found that the difference in indeterminate rates for QuantiFERON-TB Gold In-Tube (QFT-GIT) in active TB, suspected TB, and LTBI, were 15.3% (95% CI 10.8%−21.2%), 12.3% (95% CI 6.9%−39.4%), and 3.9% (95% CI 2.4%−6.4%), respectively (11). However, to our knowledge, no meta-analysis has comprehensively assessed the indeterminate rate of IGRAs specifically in the detection of LTBI. Therefore, we included 403 studies and conducted a systematic review and meta-analysis to evaluate the indeterminate rate of IGRAs in the screening of LTBI.
2 Methods
We strictly adhered to the standards of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) in reporting the findings of this review (17). The study is registered in PROSPERO as CRD42020211363; however, the protocol was greatly modified before it was implemented. The adjusted protocol is shown in the Appendix, page 67.
2.1 Search strategy and selection criteria
In this systematic review and meta-analysis, we searched the PubMed, Cochrane Library, and Embase® (Elsevier, Amsterdam, the Netherlands) databases on 15 November 2022, with no language or time constraints. Table S1 in the appendix shows the keywords. The reference lists of relevant articles were manually checked for other potentially relevant papers.
Studies were included if they met all of the following criteria (1): they screened for LTBI in healthy or high-risk adults and/or children (2); IGRAs were used to detect LTBI, with initial indeterminate results reported; and (3) they were cross-sectional or longitudinal studies. Referring to Campbell et al.’s study (18) and our previous study (19), Table S2 in the appendix provides the classification of the high-risk population, which accounted for recent contacts, populations with the possibility of contact, immunocompromised populations, and populations with the possibility of immunosuppression.
Studies were excluded based on the following criteria (1): individuals who were active TB and suspected active TB were not excluded at baseline (2); only IGRA- or TST-positive/-negative individuals were included at baseline (3); non-commercial or modified IGRAs were used (4); they included invalid results caused by technical errors, including insufficient cells or blood, machine failure, blood contamination, or prolonged incubation time that exceeded the manufacturer’s recommendation; and (5) they were abstracts, letters, case reports, or reviews.
Two investigators (GZZ and QYL) independently screened the article titles and abstracts retrieved from the literature search. The full texts of the potentially eligible studies were further reviewed before being included in the analysis. A third investigator (CS) cross-checked the extracted data. Disagreements were resolved through consensus.
2.2 Data extraction and quality assessment
Using a preconceived and standardized data extraction form, we collected information including the first author’s name, year of publication, title, study area, study design, the timing of data collection (prospective or retrospective), investigated population, participant demographics, IGRA type and manufacturer, number of individuals screened, and number of individuals with initial indeterminate results. Two investigators (GZZ and QYL) independently extracted data from individual studies. The third investigator (CS) cross-checked extracted data. Disagreements were resolved through consensus.
There is currently no reference diagnostic test for LTBI. Furthermore, the Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2) tool indicated that some items would not be appropriate for inclusion in this study. Therefore, with reference to previous studies (18–20), the QUADAS-2 tool was modified with six quality items to improve the assessment of study diagnostic accuracy. High-quality studies were defined as those that met at least five of the criteria, those of moderate quality met three or four criteria, and those of low quality met two or fewer criteria (Appendix Table S3). Two investigators (GZZ and QYL) independently assessed the methodological quality of one-quarter of the studies. A third investigator (CS) independently reviewed those assessments. Disagreements were resolved by consensus.
2.3 Statistical analysis
A random-effects model was used to calculate the pooled prevalence rates and risks, and their 95% CIs. A random-effects metaregression was used to examine the factors that influenced the pooled prevalence rates. The I²-statistic was used to assess the heterogeneity of the included studies (21), with I² > 50% indicating significant heterogeneity. Publication bias was not statistically calculated because factors other than results, including investigator motivation and funding, may influence the publication of a study (18, 22), and publication bias is only one possible explanation of funnel plot asymmetry (23, 24). The “meta” package in R statistical software, version 3.4.3 (Schwarzer, 2007; Team, 2017), was used to conduct the meta-analysis.
3 Results
3.1 Characteristics of the included studies
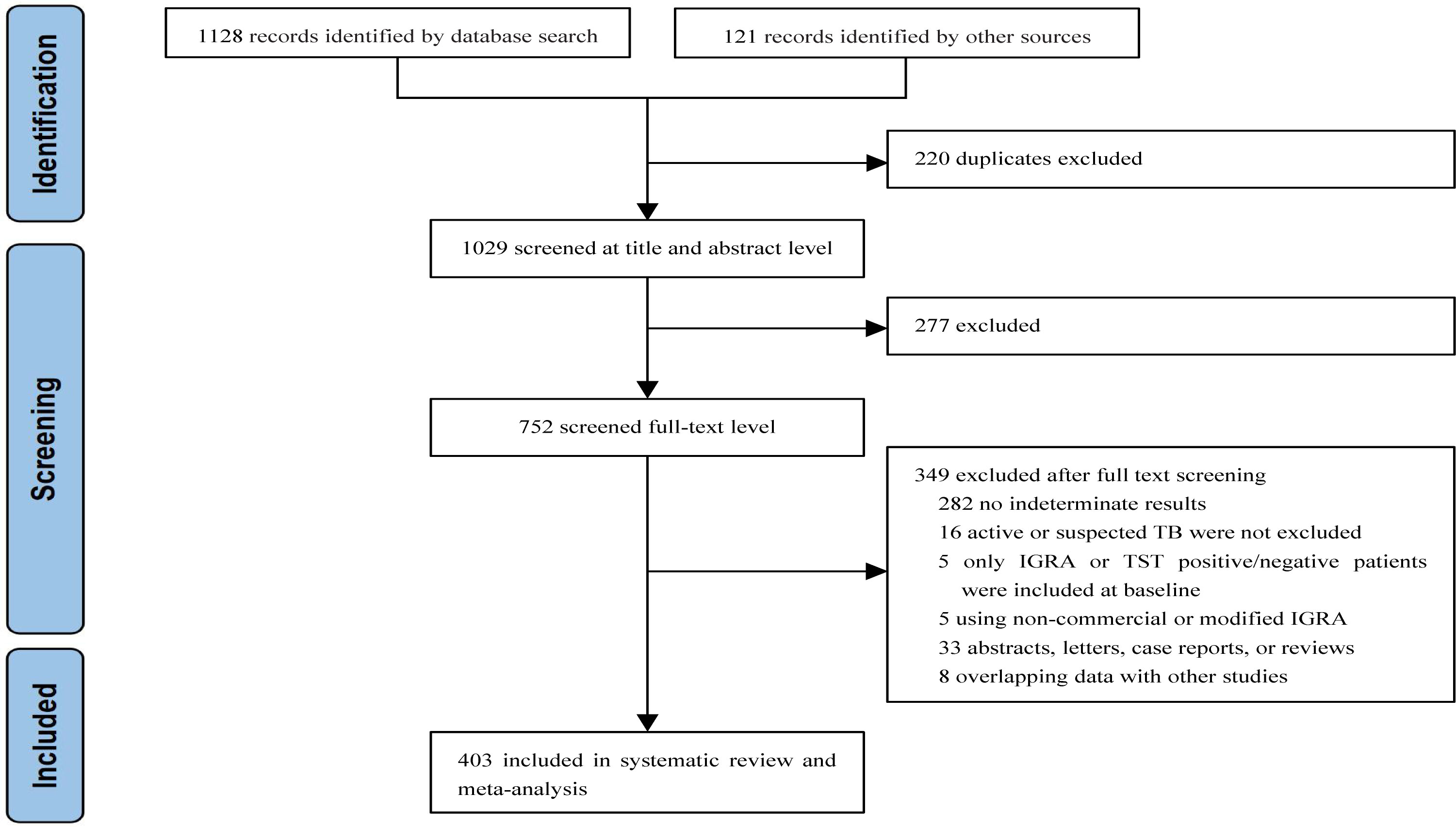
We identified 1,249 articles after removing duplicates. Of these, 277 articles were excluded after reviewing the titles and abstracts. The full texts of 752 articles were assessed. Finally, 403 studies involving 486,886 individuals were included in the analyses (Figure 1).
Of the 403 studies, 358 were from areas with a TB burden of less than 100 cases per 100,000 people, whereas 45 studies were from areas with a TB burden of more than 100 cases per 100,000 people. In addition, 183 studies were cohort studies, 125 were cross-sectional studies, 4 studies were randomized controlled trials, and 91 studies did not state the study type. Detailed characteristics of study participants can be found in Appendix Table S4.
Of the studies analyzed, 315 and 53 were considered to be of high and moderate quality, respectively. The main limitation found was that the definition of indeterminate for IGRA was not reported in 65 (16.1%) studies, which poses a potential risk of inconsistent definitions of indeterminate IGRA results. In addition, the reason for participants’ withdrawal from the study was not reported in 55 (13.6%) studies. See Appendix Table S5 for details.
3.2 Comparison of IGRA indeterminate rates among different populations
Our calculation of the pooled indeterminate rate in all populations showed an overall rate of 3.9% (95% CI 3.5%–4.2%; I2 = 97%). Subgroup analysis showed that immunity status significantly affected indeterminate results. The pooled indeterminate rate in the immunocompromised population (5.7%, 95% CI 4.8%–6.6%; I2 = 94%) was significantly higher than in the immunocompetent population (1.9%, 95% CI 1.5%–2.3%; I2 = 97%). In the population with a possibility of immunosuppression, the pooled indeterminate rate was 4.8% (95% CI 4.1%–5.6%; I2 = 97%) (see Table 1).
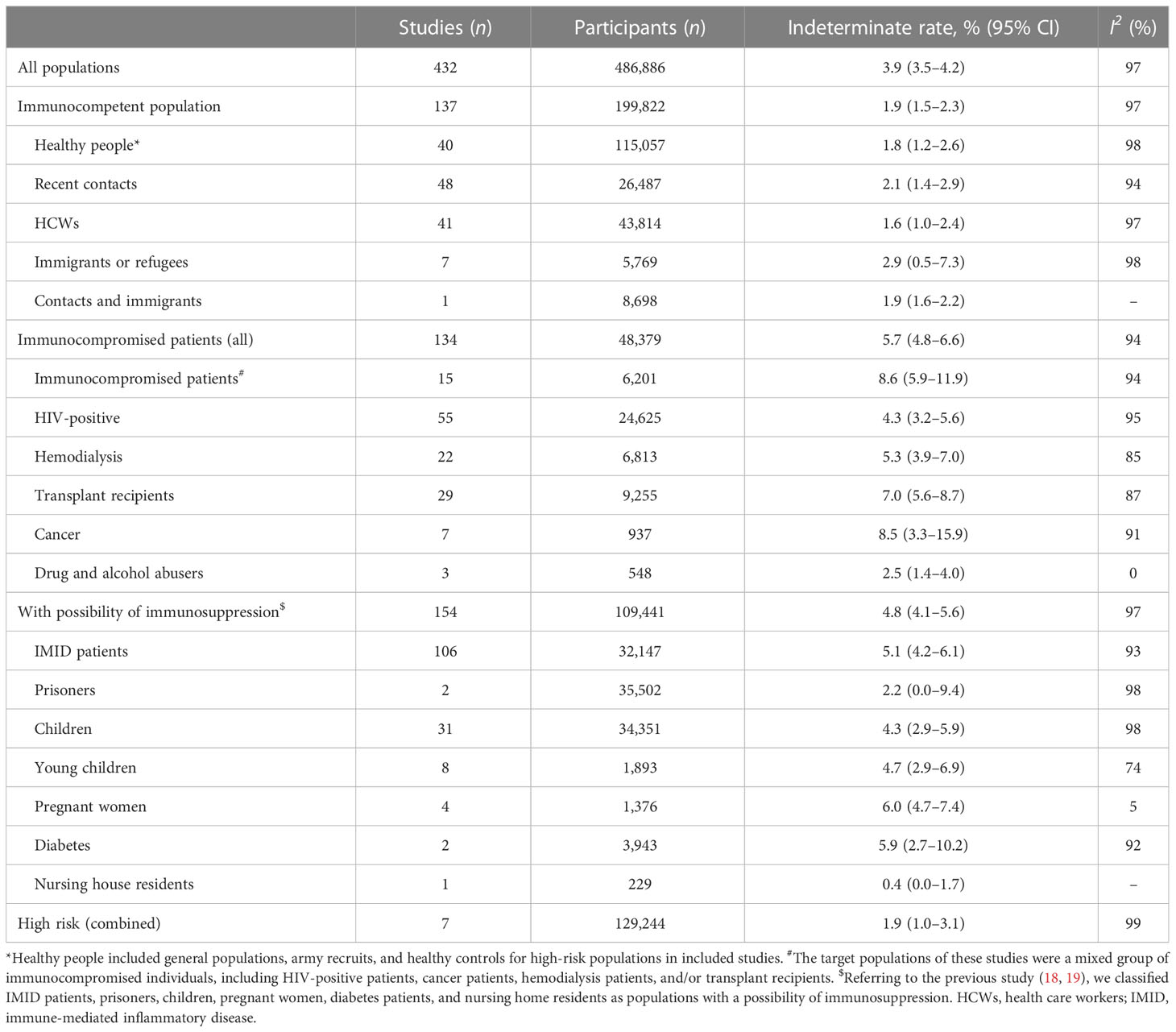
Table 1 Comparison of interferon gamma release assay (IGRA) indeterminate rates among different populations.
Appendix Table S6 shows that in multivariable models of metaregression, the population (i.e., immunity status), the timing of data collection, age, proportion of men, the type of IGRA, area, and the number of participants were all significantly associated with the pooled indeterminate rate (p < 0.05). We performed subgroup analyses stratified by the year of publication, type of study, timing of data collection, TB burden of the areas, age group, proportion of men, number of participants, type of IGRA, and quality of included studies (Appendix Table S7).
3.3 Comparison of IGRA indeterminate rates between QFT and T-SPOT in head-to-head studies
We compared the indeterminate rates in 55 head-to-head studies and found that the indeterminate rate for QFT was similar to that for T-SPOT (pooled OR = 0.88, 95% CI 0.59%–1.32%; I2 = 91%). However, the subgroup analysis stratified by the generation of QFT showed differences in indeterminate rate. Although not reaching statistical significance, we observed that the indeterminate rate for the second generation of QFT (QFT-G) was higher than that for T-SPOT (pooled OR = 1.68, 95% CI 0.76–3.70; I2 = 39%). The indeterminate rate for the third generation of QFT (QFT-GIT) was similar to that for T-SPOT (pooled OR = 0.86, 95% CI 0.54–1.37; I2 = 93%). The fourth generation of QFT (QFT-plus) had a significantly lower indeterminate rate than that for T-SPOT (pooled OR = 0.24, 95% CI 0.16–0.35; I2 = 0%) (see Figure 2A, and Appendix Table S8 and Figure S1).
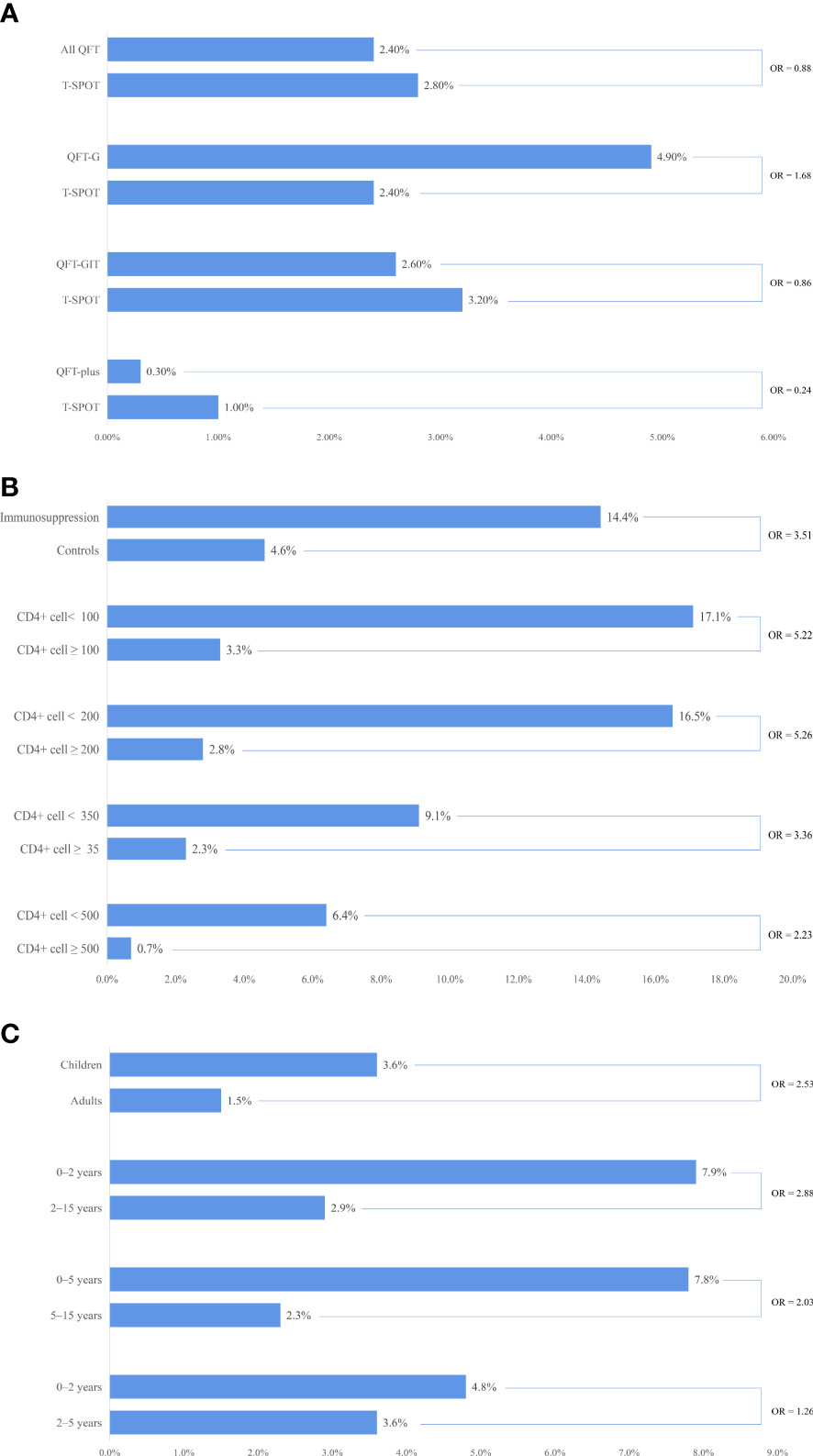
Figure 2 Comparisons of indeterminate interferon gamma release assay (IGRA) rates: immune status, age groups, and test types. (A) comparison of indeterminate IGRA rates between QuantiFERON®-TB (QFT) and T-SPOT®.TB (T-SPOT) in head-to-head studies; (B) comparison of indeterminate IGRA rates among different immune statuses; (C) comparison of indeterminate IGRA rates among different age groups.
We compared the indeterminate rate for QFT in head-to-head studies among different generations. Although not reaching statistical significance, we observed that the indeterminate rate for QFT-GIT was lower than that for QFT-G (pooled OR = 0.64, 95% CI 0.23–1.75; I2 = 62%), but that the indeterminate rate for QFT-GIT was higher than that for QFT-plus (pooled OR = 1.49, 0.98–2.17; I2 = 0%) (see Appendix Table S9).
3.4 Comparison of IGRA indeterminate rates among different immune statuses
We compared the indeterminate rate between the immunocompromised population and their healthy controls in 16 studies and found that the indeterminate rate for the immunocompromised population was significantly higher than that for the healthy control (pooled OR = 3.51, 95% CI 2.11–5.82; I2 = 61%). We further compared the indeterminate rate in HIV-positive patients among different groups stratified by CD4+ cell count. The indeterminate rate for the group with a CD4+ cell count lower than 100 cells/mm3 was significantly higher than that for the group with a CD4+ cell count greater than 100 cells/mm3 (pooled OR = 5.22, 95% CI 2.66–10.25; I2 = 52%). A similar result was found in other subgroup analyses stratified by CD4+ cell count (see Table 3). We also found that the indeterminate rate decreased with the increase of CD4+ cell count (see Figure 2B, Appendix Tables S10–11 and Figures S2–S4).
3.5 Comparison of indeterminate rates of IGRA among different age groups
We compared the indeterminate rate between children and adults in seven studies and found that the indeterminate rate for children was significantly higher than that for adults (pooled OR = 2.56, 95% CI 1.79–3.57; I2 = 41%) (see Appendix Figure S5). We further compared the indeterminate rate in children among different age groups. The indeterminate rate for groups aged less than 2 years was significantly higher than that for groups aged 2–15 years (pooled OR = 2.88, 95% CI 1.70–4.87; I2 = 44%). A similar result was found in other subgroup analyses stratified by age (see Table 4). We also found that the indeterminate rate decreased with an increase in age in children (see Figure 2C, and Appendix Table S12 and Figure S6).
3.6 Comparison of indeterminate rates of IGRA caused by failed positive and failed negative controls among different populations
Finally, we compared the indeterminate rate of IGRA caused by failed positive and negative controls among the different populations. In all populations, the proportion of failed positive controls in indeterminate cases was 94.6% (95% CI 89.6%–98.0%; I2 = 95%) and the proportion of failed negative controls was 4.0% (95% CI 1.4%–12.4%; I2 = 93%). In the immunocompetent population, the proportion of failed positive controls in indeterminate cases was 99.4% (95% CI 97.3%–100%; I2 = 51%) and the proportion of failed negative controls was 1.0% (95% CI 0.0%–3.5%; I2 = 52%).
Furthermore, in immunocompromised patients, the proportion of failed positive controls in indeterminate cases was 95.7% (95% CI 85.6%–99.9%; I2 = 95%) and the proportion of failed negative controls was 2.6% (95% CI 0.2%–7.2%; I2 = 72%). In the population with a possibility of immunosuppression, the proportion of failed positive controls in indeterminate cases was 91.7% (95% CI 83.2%–97.5%; I2 = 93%) and the proportion of failed negative controls was 5.6% (95% CI 1.4%–12.4%; I2 = 90%) (see Table 2). For more details, see Appendix Table S13.
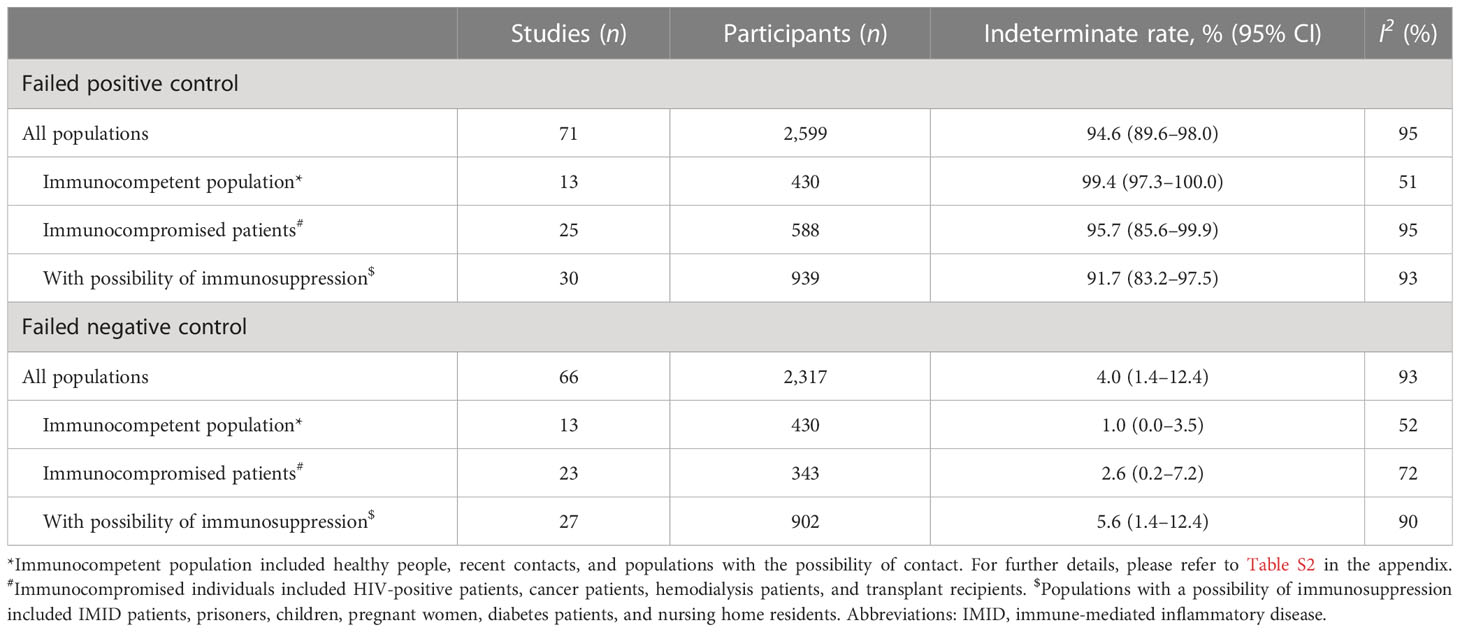
Table 2 Comparison of indeterminate rates of interferon gamma release assay (IGRA) caused by failed positive controls and failed negative controls among different populations.
4 Discussion
To our knowledge, our meta-analysis of 403 studies involving 486,886 participants is the largest to systematically assess the indeterminate rate of IGRAs in the screening for LTBI. Our study revealed five main findings. First, the pooled indeterminate rate of IGRAs was 3.9% in all populations. Second, the indeterminate rate for QFT was similar to that for T-SPOT, but the indeterminate rate for a new generation of QFT (QFT-plus) was significantly lower than that for T-SPOT. Third, the indeterminate rate for the immunocompromised population was significantly higher than that for healthy controls, and the indeterminate rate increased as CD4+ cell count decreased in HIV-positive patients. Fourth, the indeterminate rate for children was significantly higher than that of adults, and the indeterminate rate in children increased as age decreased. Fifth, 94.6% of indeterminate cases were caused by a failed positive control.
The End TB Strategy of the World Health Organization (WHO) recommends that all countries should aim to provide preventive treatment for LTBI for > 90% of people living with HIV and living with children who are contacts of TB cases by 2025 (25), which means that a large number of people worldwide require screening for LTBI. Although our study reveals a low indeterminate rate of 3.9%, it is estimated that hundreds of thousands of IGRAs might not produce a conclusive result in the screening of LTBI. Indeterminate results pose diagnostic challenges for healthcare providers, in addition to causing frustration for individuals undergoing testing, particularly when determining whether or not to initiate preventive treatment, as this therapy has toxic side effects (26). Similarly, indeterminate IGRA results have significant consequences for individual patients (27), because an equivocal result can lead to repeat testing and even a workup for immunologic disease, with concomitant uncertainty and inconvenience (9). Therefore, further research is needed to reduce the indeterminate rate of IGRA.
The results from previous studies that compared the indeterminate rates of QFT and T-SPOT are controversial. In Diel et al.’s study (15), the pooled indeterminate rate for QFT-GIT (2.1%, 95% CI 2.0%–2.3%) was lower than that for T-SPOT (3.8%, 95% CI 3.5%−4.2%). Huo et al. found a significantly lower pooled indeterminate rate for QFT-GIT than for T-SPOT among HIV-positive individuals (10). The authors suggested that the more demanding laboratory work for the T-SPOT likely explained the higher indeterminate rates (15). However, although not reaching statistical significance, Meier et al. reported a higher indeterminate rate for QFT (5.0%, 95% CI 4.0%–6.0%) than for T-SPOT (3.0%, 95% CI 2.0%–5.0%) (16). Santin et al. also reported a higher pooled indeterminate rate for QFT-GIT than for T-SPOT (11). Our meta-analysis included more head-to-head studies and provided more comprehensive results. The comparison of 55 head-to-head studies revealed that the pooled indeterminate rate for QFT was similar to the pooled indeterminate rate for T-SPOT. Nevertheless, subgroup analysis revealed that the indeterminate rate for QFT-G was significantly higher than that for T-SPOT, the indeterminate rate for QFT-GIT was similar to that for T-SPOT, and the indeterminate rate of QFT-plus was significantly lower than that for T-SPOT. Although the finding did not reach statistical significance, further analysis found that the indeterminate rate for QFT-plus was the lowest across all generations of QFT. Therefore, our study revealed a potential advantage to using the newest generation of QFT (QFT-plus), owing to its low indeterminate rate.
Immunosuppression is an important risk factor for indeterminate IGRA results. Park et al. included six studies and reported that the indeterminate rate of IGRA was higher in patients on immunosuppression treatment than in those not on immunosuppression treatment (pooled OR = 2.91, 95% CI 1.36–6.24) (12). Santin et al. included 11 studies and found that the indeterminate rates of IGRA were higher in HIV-infected than in HIV-uninfected individuals; however, the difference did not reach statistical significance (11). Santin et al. also reported that the pooled indeterminate rate of IGRA for those with a CD4+ cell count of ≥200 was significantly lower than for those with a CD4+ cell count of <200 (11). Meier et al. reported that immunocompromised patients contributed to the indeterminate results in their meta-analysis (16). Our meta-analysis included 16 studies and found that the indeterminate rate for the immunocompromised population was significantly higher than that for the healthy control population (pooled OR = 3.51, 95% CI 2.11–5.82). In HIV-positive patients, we found that the indeterminate rate increased as the CD4+ cell count decreased. Therefore, our study adds to the evidence supporting the correlation between immunosuppression and highly indeterminate results.
There is also concern about the routine use of IGRA in young children (< 5 years), owing to a higher indeterminate rate of IGRA than older children (28, 29). In our study, the indeterminate rate for children was significantly higher than that for adults, and further analysis revealed that the indeterminate rate increased as the age of the children decreased. Our results differ from those reported in Meier et al.’s meta-analysis (16), which assessed the indeterminate rate of IGRA in children and found that the pooled indeterminate rate in the group with median or mean ages of 0–7 years (4.0%, 95% CI 3.0%–6.0%) was similar to those in the group with median or mean ages ≥8 years (4.0%, 95% CI 3.0%–5.0%). Therefore, to our knowledge, this is the first meta-analysis that reported that young children were related to a high indeterminate rate.
We identified that most of the indeterminate results (94.6%, 95% CI 89.6%–98.0%) were caused by failed positive controls and a small portion of indeterminate results (4.0%, 95% CI 1.4% to 12.4%) were caused by failed negative controls (see Table 5). Failed positive controls may be due to an impaired cellular immune response associated with a decrease in the number or function of T lymphocytes, as seen in HIV infection and cancer (30). Although the proportion of indeterminate results caused by failed positive controls was highest in the immunocompetent population (99.4%, 95% CI 97.3%–100%), most of the indeterminate results in the immunocompetent population can be attributed to technical errors, as few indeterminate cases were confirmed as being caused by immunosuppression (31, 32). Failed negative controls may be due to the presence of heterophilic antibodies (e.g., human anti-mouse) or spontaneous IFN-γ secretion during an infection or following vaccination (33). Although the 95% CIs overlapped, the proportion of indeterminate results caused by failed negative controls was higher in the immunocompromised population than in the immunocompetent population. This observation may be due to the higher possibility of infection in the immunocompromised population than in the immunocompetent population. In addition, immunometric assays are inherently vulnerable to interference from heterophilic antibodies, which is particularly relevant in dialysis patients and people with autoimmune diseases or an infection (34–36).
This meta-analysis had several limitations. First, 65 studies (16.1%) did not provide a definition of indeterminate IGRA results, which may have led to inconsistent interpretations of the test results. Second, although we carefully reviewed the methods sections of all included studies and attempted to exclude indeterminate cases caused by technical errors, few included studies reported the relevant information. Therefore, it was difficult to determine the extent to which technical errors may have influenced our overall findings. Third, as 358 studies (88.8%) included in the analysis were conducted in areas with low TB burden, the generalizability of the meta-analysis findings to areas with high TB burden may be limited. Finally, while obvious heterogeneity was present in several groups, subgroup analyses to identify the source of heterogeneity were not possible.
5 Conclusion
On average, 1 in 26 tests yields indeterminate IGRA results in LTBI screening. The use of advanced versions of the QuantiFERON-TB assay (QFT-plus) may reduce the occurrence of indeterminate results. Our study emphasizes the high risk of indeterminate IGRA in relation to immunosuppression and young age, which should receive more attention in the management of LTBI.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
GZ, YZZ, and CS initiated the project and were responsible for protocol design. GZ and QL performed the literature review, collected the data, assessed the quality of studies, and analyzed the data. SL, HC, SC, XG, JH, YX, HL, YCZ, and JH interpreted the data. GZ wrote the initial draft of the manuscript. All authors were responsible for critical revision of the manuscript and provided important intellectual content. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Talent Project of Kunming Health Science and Technology under grant number 2022-SW (Leading Talents)-001 and the Special Project “Spring City Plan” Famous Doctor under grant number C202012016.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1170579/full#supplementary-material
References
1. World Health Organization. Global tuberculosis report 2020. Available at: www.who.int/teams/global-tuberculosis-programme/tb-reports (Accessed January 10, 2023).
2. World Health Organization. Latent TB infection: updated and consolidated guidelines for programmatic management (2018). Geneva: World Health Organization. Available at: www.who.int/tb/publications/2018/latent-tuberculosis (Accessed January 10, 2023).
3. Cohen A, Mathiasen VD, Schön T, Wejse C. The global prevalence of latent tuberculosis: a systematic review and meta-analysis. Eur Respir J (2019) 54(3):1900655. doi: 10.1183/13993003.00655-2019
4. Dye C, Glaziou P, Floyd K, Raviglione M. Prospects for tuberculosis elimination. Annu Rev Public Health (2013) 34:271–86. doi: 10.1146/annurev-publhealth-031912-114431
5. National Tuberculosis Advisory Committee. Position statement on interferon-γ release assays in the detection of latent tuberculosis infection. Commun Dis Intell Q Rep (2012) 36(1):125–31.
6. US Centers for Disease Control and Prevention. Latent tuberculosis infection: a guide for primary health care providers. Available at: www.cdc.gov/tb/publications/ltbi/default.htm (Accessed January 10, 2023).
7. Pai M, Zwerling A, Menzies D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med (2008) 149(3):177–84. doi: 10.7326/0003-4819-149-3-200808050-00241
8. Bastian I, Coulter C. Position statement on interferon-γ release assays for the detection of latent tuberculosis infection. Commun Dis Intell Q Rep (2017) 41(4):E322–36.
9. Brown J, Kumar K, Reading J, Harvey J, Murthy S, Capocci S, et al. Frequency and significance of indeterminate and borderline quantiferon gold TB IGRA results. Eur Respir J (2017) 50(4):1701267. doi: 10.1183/13993003.01267-2017
10. Huo ZY, Peng L. Accuracy of the interferon-γ release assay for the diagnosis of active tuberculosis among HIV-seropositive individuals: a systematic review and meta-analysis. BMC Infect Dis (2016) 16:350. doi: 10.1186/s12879-016-1687-8
11. Santin M, Muñoz L, Rigau D. Interferon-γ release assays for the diagnosis of tuberculosis and tuberculosis infection in HIV-infected adults: a systematic review and meta-analysis. PloS One (2012) 7(3):e32482. doi: 10.1371/journal.pone.0032482
12. Park CH, Park JH, Jung YS. Impact of immunosuppressive therapy on the performance of latent tuberculosis screening tests in patients with inflammatory bowel disease: a systematic review and meta-analysis. J Pers Med (2022) 12(3):507. doi: 10.3390/jpm12030507
13. Shahidi N, Fu YT, Qian H, Bressler B. Performance of interferon-gamma release assays in patients with inflammatory bowel disease: a systematic review and meta-analysis. Inflamm Bowel Dis (2012) 18(11):2034–42. doi: 10.1002/ibd.22901
14. Rahimifard N, Mahmoudi S, Mamishi S, Pourakbari B. Prevalence of latent tuberculosis infection in transplant candidates: a systematic review and meta-analysis. Microb Pathog (2018) 125:401–10. doi: 10.1016/j.micpath.2018.09.040
15. Diel R, Loddenkemper R, Nienhaus A. Evidence-based comparison of commercial interferon-gamma release assays for detecting active TB: a metaanalysis. Chest (2010) 137(4):952–68. doi: 10.1378/chest.09-2350
16. Meier NR, Volken T, Geiger M, Heininger U, Tebruegge M, Ritz N. Risk factors for indeterminate interferon-gamma release assay for the diagnosis of tuberculosis in children-a systematic review and meta-analysis. Front Pediatr (2019) 7:208. doi: 10.3389/fped.2019.00208
17. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-p) 2015 statement. Syst Rev (2015) 4(1):1. doi: 10.1186/2046-4053-4-1
18. Campbell JR, Winters N, Menzies D. Absolute risk of tuberculosis among untreated populations with a positive tuberculin skin test or interferon-gamma release assay result: systematic review and meta-analysis. BMJ (2020) 368:m549. doi: 10.1136/bmj.m549
19. Zhou G, Luo Q, Luo S, He J, Chen N, Zhang Y, et al. Positive rates of interferon-γ release assay and tuberculin skin test in detection of latent tuberculosis infection: a systematic review and meta-analysis of 200,000 head-to-head comparative tests. Clin Immunol (2022) 245:109132. doi: 10.1016/j.clim.2022.109132
20. Hoffmann M, Tsinalis D, Vernazza P, Fierz W, Binet I. Assessment of an interferon-gamma release assay for the diagnosis of latent tuberculosis infection in haemodialysis patient. Swiss Med Wkly (2010) 140(19-20):286–92. doi: 10.4414/smw.2010.12960
21. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (2003) 327(7414):557–60. doi: 10.1136/bmj.327.7414.557
22. Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, Brozek J, et al. GRADE guidelines: 5. rating the quality of evidence–publication bias. J Clin Epidemiol (2011) 64(12):1277–82. doi: 10.1016/j.jclinepi.2011.01.011
23. Mueller M, D'Addario M, Egger M, Cevallos M, Dekkers O, Mugglin C, et al. Methods to systematically review and meta-analyse observational studies: a systematic scoping review of recommendations. BMC Med Res Methodol (2018) 18(1):44. doi: 10.1186/s12874-018-0495-9
24. Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ (2011) 343:d4002. doi: 10.1136/bmj.d4002
25. World Health Organization. The end TB strategy 2014. Available at: https://www.who.int/teams/global-tuberculosis-programme/the-end-tb-strategy (Accessed January 10, 2023).
26. Kobashi Y, Mouri K, Obase Y, Fukuda M, Miyashita N, Oka M. Clinical evaluation of QuantiFERON TB-2G test for immunocompromised patients. Eur Respir J (2007) 30(5):945–50. doi: 10.1183/09031936.00040007
27. Yun JW, Chung HS, Koh WJ, Chung DR, Kim YJ, Kang ES. Significant reduction in rate of indeterminate results of the QuantiFERON-TB gold in-tube test by shortening incubation delay. J Clin Microbiol (2014) 52(1):90–4. doi: 10.1128/jcm.01547-13
28. Alsdurf H, Hill PC, Matteelli A, Getahun H, Menzies D. The cascade of care in diagnosis and treatment of latent tuberculosis infection: a systematic review and meta-analysis. Lancet Infect Dis (2016) 16(11):1269–78. doi: 10.1016/S1473-3099(16)30216-X
29. Starke JR. Interferon-γ release assays for diagnosis of tuberculosis infection and disease in children. Pediatrics (2014) 134(6):e1763–73. doi: 10.1542/peds.2014-2983
30. Wigg AJ, Narayana SK, Anwar S, Ramachandran J, Muller K, Chen JW, et al. High rates of indeterminate interferon-gamma release assays for the diagnosis of latent tuberculosis infection in liver transplantation candidates. Transpl Infect Dis (2019) 21(3):e13087. doi: 10.1111/tid.13087
31. Cummings KJ, Smith TS, Shogren ES, Khakoo R, Nanda S, Bunner L, et al. Prospective comparison of tuberculin skin test and QuantiFERON-TB gold in-tube assay for the detection of latent tuberculosis infection among healthcare workers in a low-incidence setting. Infect Control Hosp Epidemiol (2009) 30(11):1123–6. doi: 10.1086/644754
32. Rose W, Read SE, Bitnun A, Rea E, Stephens D, Pongsamart W, et al. Relating tuberculosis (TB) contact characteristics to QuantiFERON-TB-Gold and tuberculin skin test results in the Toronto pediatric TB clinic. J Pediatr Infect Dis Soc (2015) 4(2):96–103. doi: 10.1093/jpids/piu024
33. Zrinski Topić R, Zoričić-Letoja I, Pavić I, Dodig S. Indeterminate results of QuantiFERON-TB gold in-tube assay in nonimmunosuppressed children. Arch Med Res (2011) 42(2):138–43. doi: 10.1016/j.arcmed.2011.02.001
34. Bolstad N, Warren DJ, Nustad K. Heterophilic antibody interference in immunometric assays. Best Pract Res Clin Endocrinol Metab (2013) 27(5):647–61. doi: 10.1016/j.beem.2013.05.011
35. Hu YF, Ho SY. Heterophilic antibodies influence immunometric assay: a case report and reviews. Immunol Res (2020) 68(2):104–6. doi: 10.1007/s12026-020-09128-6
Keywords: interferon gamma release assay (IGRA), latent tuberculosis infection (LTBI), indeterminate, diagnosis, meta-analysis
Citation: Zhou G, Luo Q, Luo S, Chen H, Cai S, Guo X, He J, Xia Y, Li H, Zhou Y, Zhang Y and Song C (2023) Indeterminate results of interferon gamma release assays in the screening of latent tuberculosis infection: a systematic review and meta-analysis. Front. Immunol. 14:1170579. doi: 10.3389/fimmu.2023.1170579
Received: 21 February 2023; Accepted: 19 April 2023;
Published: 15 May 2023.
Edited by:
Subash Babu, International Centers for Excellence in Research (ICER), IndiaReviewed by:
Luke Elizabeth Hanna, National Institute of Research in Tuberculosis (ICMR), IndiaReinout Van Crevel, Radboud University Medical Centre, Netherlands
Copyright © 2023 Zhou, Luo, Luo, Chen, Cai, Guo, He, Xia, Li, Zhou, Zhang and Song. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yazhou Zhang, 15887117301@163.com; Chao Song, chaoge6870@163.com
†ORCID: Guozhong Zhou, orcid.org/0009-0001-9112-6631
 Guozhong Zhou1†
Guozhong Zhou1† Chao Song
Chao Song