- Department of Basic Research on Social Recognition and Memory, Research Center for Child Mental Development, Kanazawa University, Kanazawa, Japan
Nicotinamide adenine dinucleotide (NAD) is a substrate of adenosine diphosphate (ADP)-ribosyl cyclase and is catalyzed to cyclic ADP-ribose (cADPR) by CD38 and/or CD157. cADPR, a Ca2+ mobilizing second messenger, is critical in releasing oxytocin from the hypothalamus into the brain. Although NAD precursors effectively play a role in neurodegenerative disorders, muscular dystrophy, and senescence, the beneficial effects of elevating NAD by NAD precursor supplementation on brain function, especially social interaction, and whether CD38 is required in this response, has not been intensely studied. Here, we report that oral gavage administration of nicotinamide riboside, a perspective NAD precursor with high bioavailability, for 12 days did not show any suppressive or increasing effects on sociability (mouse’s interest in social targets compared to non-social targets) in both CD157KO and CD38KO male mice models in a three-chamber test. CD157KO and CD38KO mice displayed no social preference (that is, more interest towards a novel mouse than a familiar one) behavior. This defect was rescued after oral gavage administration of nicotinamide riboside for 12 days in CD157KO mice, but not in CD38KO mice. Social memory was not observed in CD157KO and CD38KO mice; subsequently, nicotinamide riboside administration had no effect on social memory. Together with the results that nicotinamide riboside had essentially no or little effect on body weight during treatment in CD157KO mice, nicotinamide riboside is less harmful and has beneficial effect on defects in recovery from social behavioral, for which CD38 is required in mice.
1 Introduction
Prosocial behavior is important for humans and in the human diverse society (1–3), and humans naturally possess social cognition and memory ability at different levels (4, 5). Social cognition is a behavior that covers aspects of information about one’s self and others within their social groups (4, 6). Alternatively, social recognition is an ability to discriminate familiar and unfamiliar social objects and to interact between them, which is processed as social memory. Therefore, such abilities are required for living in a society to learn members’ identities, to maintain groups between friendly individuals or at the working places, and for interpersonal communication. In our society, we need to acquire social skills to make social decisions (2, 3).
In experimental neurosciences, social recognition is usually defined as an interest towards novel social objects (social motivation), and social memory is defined as a decrease in investigative behaviors toward re-exposed (and thus have become) familiar conspecifics (7, 8). Among the various neurotransmitters involved in social recognition, oxytocin is reported to be involved in social interaction, social recognition, and memory in the social brain (9). Disruption of the oxytocin system leads to impaired social recognition and mutual interactions in humans with psychiatric disorders, such as autism spectrum disorders (ASDs) or schizophrenia (10–12). Defects in the oxytocin system is involved in anxiety-, depression-, avoidance-, and hyperactivity-like behaviors, which are useful psychiatric disorder models (13, 14). For the past 20 years, studies have shown that CD38 and CD157 are critical molecules in prosocial behavior (9, 14–28), as described below.
Oxytocin is synthesized in neurons in the paraventricular nucleus and supraoptic nucleus of the hypothalamus, and secreted somato-dendritically from oxytocin-producing neurons into the brain. It plays the role of a neuromodulator (24, 29, 30). CD38 is a cell-surface antigen with adenosine diphosphate (ADP)-ribosyl cyclase activity, which catalyzes cyclic ADP-ribose (cADPR) from nicotinamide adenine dinucleotide (NAD) (31, 32). cADPR functions as a second messenger to trigger Ca2+ mobilization from endoplasmic Ca2+ pools (31, 33). In the hypothalamus, cADPR elevates intracellular free Ca2+ concentrations and subsequently releases oxytocin from oxytocinergic neurons (24). The linkage between this signaling cascade and social behavior was, for the first time, shown in Cd38 knockout (CD38KO) mice, in which social memory and recognition or parental nurturing behavior were disrupted mainly owing to reduced oxytocin secretion (27). The importance of CD38 and oxytocin in social memory was further confirmed by local re-expression of human CD38 in the hypothalamus by CD38-containing lentivirus infection or simple subcutaneous supply of oxytocin in CD38 KO mice, in which social behavioral impairment was rescued (28).
CD157 (originally found as BST-1) (34) is a sister molecule of CD38 and is expressed in neuroprogenitor or neurolineage cells in the subventricular zone of the fetal brain (21). The functional role of CD157 on oxytocin’s reaction is nearly identical to that of CD38. CD157 produces cADPR at one third of the CD38’s catalyzing level but with almost no nicotinic acid adenine dinucleotide phosphate producing ability, differing from CD38. Thus, various functions of CD157 seem to be mediated by not only cADPR with respect to Ca2+ homeostasis, but also by migrating powers with homophilic binding on the cell surface or adhesive properties associated with integrin (35). Though the phenotypes of Cd157 knockout (CD157KO) and CD38KO mice in social behavior are largely similar, CD157 invokes multiple circuits to control anxiety- and depression-like behaviors (14, 20, 21).
Nicotinamide riboside, a NAD precursor, is converted to nicotinamide mononucleotide through nicotinamide riboside kinase 1. Subsequently, nicotinamide mononucleotide is converted to NAD by the action of nicotinamide mononucleotide adenylyl transferase. Nicotinamide riboside kinase 1 and nicotinamide riboside kinase 2 genes are utilized in a de novo fashion (36–39). Evidence shows that nicotinamide riboside supplementation in humans increases intracellular NAD concentrations and subsequently improves NAD-dependent activities in the cell by increasing silent mating-type information through nicotinamide riboside kinase 2-dependent gene silencing, and longevity via nicotinamide riboside kinase 1-dependent NAD synthesis (39, 40). Thus, it is possible that the exogenous application of nicotinamide riboside can promote the biosynthesis of NAD via nicotinamide mononucleotide. Subsequently, NAD is degraded to cADPR and nicotinamide by poly(ADP-ribose) polymerases (PARPS) and CD38, respectively. cADPR helps produce the beneficial effects of NAD (41). The most fundamental use of NAD precursor molecules, nicotinic acid and nicotinamide mononucleotide, is the prevention of pellagra. Similar to nicotinic acid and nicotinamide mononucleotide, nicotinamide riboside is a natural product found in milk (38), which is incorporated into the intracellular NAD pool (42–44), and thus can be used as a general supplement, potentially for people who have adverse reactions to nicotinic acid or nicotinamide mononucleotide. However, more significantly, the specific utilization of nicotinamide riboside by neurons may provide qualitative advantages over niacin in promoting function in the central and peripheral nervous systems. Nicotinamide riboside has been already used as a supplement or therapeutic agent to elevate or maintain cellular NAD contents because of increase in CD38 in aged subjects (45, 46).
Nicotinamide riboside is beneficial for treating social impairments in young and aged people, some of which are based on impairments of oxytocin and/or oxytocin release (17); however, this finding is less reported. To assess this question, we previously investigated the role of orally applied nicotinamide riboside (gavage route) for 12 days on impaired social behaviors in CD157KO mice. We re-examined the effects of gavage administration of nicotinamide riboside in CD157KO mice by using a single protocol (17). Furthermore, we studied the effect of nicotinamide riboside in CD38KO mice. These comparative results clarified the functional roles of CD157 and CD38 as neuromodulators, rather than immune factors in diseases including cancer (36, 47).
2 Methods
2.1 Animals
CD157KO (Cd157-/-/Cd38+/+ of the C57BL/6 genetic background) and CD38KO (Cd157+/+/Cd38-/- of the ICR genetic background) mice were created as described previously (28, 34, 48, 49), and were kindly provided by Ishihara and Okamoto, respectively. The mice were maintained by crossbreeding homozygous mutant mice. Slc : ICR (CD-1) outbred male mice (8 weeks old, 25–30 g body weight) and C57BL6/N (8 weeks old, 23–27 g body weight) mice were obtained from Japan SLC Inc. (Hamamatsu, Japan) through a local distributor (Sankyo Laboratory Service Corporation, Toyama, Japan) and used as controls for CD38KO and CD157KO mice, respectively. Half of the offspring of wild-type mice and all KO mice were bred in our laboratory colony, weaned at 21–32 days of age, and housed in same-sex groups of five sibling pairs in one cage in the animal center under standard conditions (24°C; 12/12-h light/dark cycle, with lights on at 8:45 a.m.) with food and water ad libitum. After pretest and during the test each mouse was housed in individual cage.
All animal experiments were carried out in accordance with the Fundamental Guidelines for Proper Conduct of Animal Experiment and Related Activities in Academic Research Institutions under the jurisdiction of the Ministry of Education, Culture, Sports, Science and Technology of Japan and approved by the Committee on Animal Experimentation of Kanazawa University Graduate School of Medical Sciences.
2.2 Animal treatment
Nicotinamide riboside was supplied by Brenner from ChromaDex, Irvine, CA, USA. Male mice were gavage administered with nicotinamide riboside in a dose of 13 mg in a 50 μl solution, or the equivalent volume of physiological saline (PBS) as placebo control (17). Mice were treated daily at around 3-5 p.m. for 12 days.
2.3 Social behavior test in three-chamber boxes
The sociability, social preference, and social memory were tested using a three-chamber box to assess whether subject mice tended to spend time with mice in different chambers, as described previously (50) (Supplementary Figure 1).
2.3.1 Habituation
Test mice were first habituated for 5 min in an empty three-chamber box.
2.3.2 Sociability
Sociability was examined in a 5-min interval by the duration for which an experimental naive male mouse stayed in either a left chamber with a non-social target (usually a 50-ml conical plastic tube) or in the right chamber with a male mouse in a small metal mesh cage. Usually, the mouse stays longer with the target mouse due to its prosocial nature.
2.3.3 Social preference
At the end of the 5-min sociability test, these subject and target mice were immediately tested in a third 5-min session to quantitate the preference to spend time with a new unfamiliar mouse (Stranger 2; an experiment naïve male) placed in the wire cage (in the right chamber), replacing the plastic tube in the previous 5-min session. The test mouse had a choice to stay with either the first (already investigated, now familiar mouse; Stranger 1) or the novel unfamiliar mouse (Stranger 2). The mice usually interact more with the unfamiliar mouse.
2.3.4 Social memory
At the end of the 5-min social preference test, the mouse (Stranger 1) in the previous stage was utilized in another (fourth) 5-min session to quantitate social memory after 30 min. It was examined if the test subject spent more time with a second new stranger (Stranger 3; an experiment naïve male) than Stranger 1 to test for short-term social memory after posing the 30-min separation. Stranger 3 was placed in the wire cage (in the right chamber) that had been occupied by Stranger 2 during the previous 5-min session, and after 30 min separation. The test mouse had a choice between the first, already-investigated, now-most familiar mouse (Stranger 1) and the novel unfamiliar mouse (Stranger 3).
The trial was recorded for 5 min using the ANY-maze video system, as described previously (14, 17). Latency to enter (defined by all four paws entering), time spent, entries, and distance travelled in the light chamber were recorded. Experiments were repeated thrice on one day after treatment for 12 days.
2.4 Statistical analysis
The data are expressed as the means ± standard error of mean. The comparisons were evaluated using Student’s t-test and One-way or Two-way ANOVA, followed by post-hoc Bonferroni test. In all analyses, P < 0.05 indicated statistical significance.
3 Results
3.1 On CD157 knockout mice
Adult male C57BL6/N wild-type and CD157KO mice treated with saline or 13 mg nicotinamide riboside (approximately 350-400 mg/kg of body weight) for 12 days did not demonstrate any apparent changes in coordinated movement dysfunction (data not shown). The body weight gain during gavage had no or little difference between treatments with saline and nicotinamide riboside (Figures 1A, B).

Figure 1 Nicotinamide riboside (NR) treatment has no effect on mouse body weight. (A) Body weight of C57BL6 male mice, before (0 day) and after NR (13 mg/mouse) or placebo (PL) treatment for 12 days (PL: n = 6, unpaired t-test, t(10) = 0.3571, P = 0.7285; NR: n = 6, t(10) = 1.598, P = 0.1412). (B) Body weight of CD157 KO mice before (0 day) and after NR or placebo (PL) treatment for 12 days (PL: n = 6, unpaired t-test, t(10) = 0.2399, P = 0.8152; NR: n = 6, unpaired t-test, t(10) = 0.4341, P = 0.6734).
3.2 Social interaction in the three-chamber box test in CD157KO mice
We performed three types of social interaction tests with novel social targets each time (that is, sociability, social preference, and social memory). A target adult male mouse was placed in the left chamber (Stranger 1) and a non-social target in the right chamber, under which condition we could test sociability (mouse’s general interest towards social targets). Wild-type and CD157KO mice interacted with the social target mouse (Stranger 1) significantly longer than with non-social targets (two-tailed Student’s t-test, P < 0.0001 for both; Figures 2A, B). The result indicated that the typical phenotype of the social mouse, called sociability, was not disrupted even in CD157KO mice. This sociability phenotype in both genotypes was not much affected by gavage treatment of saline (placebo) or nicotinamide riboside (13 mg/mouse) daily for 12 days (Figures 2C, D; two-tailed Student’s t-test, P < 0.001), similar to previously reported results (17).
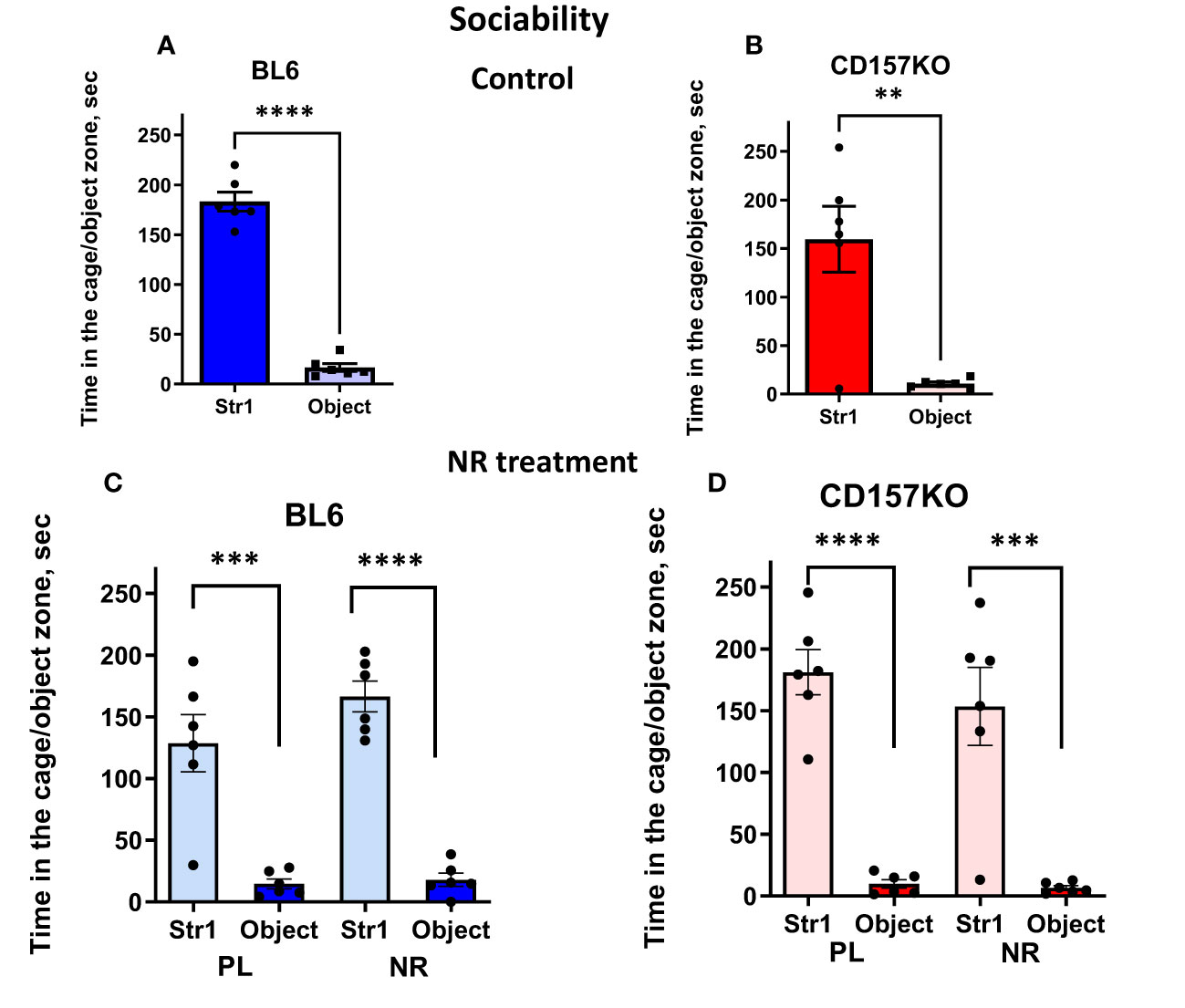
Figure 2 Nicotinamide riboside (NR) treatment has no apparent effect on sociability in C57BL6 (BL6) and CD157KO male mice in the three-chamber test. Duration spent in the chamber with Stranger 1 mouse in a cage (Str1) or a non-social target (object) by wild-type (BL6; A) or CD157KO (B) mice. (C, D) Duration spent in the chamber with Stranger 1 mouse in a cage (Str1) or a non-social target (object) by wild-type (BL6; C) or CD157KO (D) mice, which were treated with gavage administration of saline (PL) or nicotinamide riboside (NR) for 12 days and then examined. Two-tailed Student’s t-test, **P < 0.01, ***P < 0.001, ****P < 0.0001.
The non-social target was replaced with the second new target male mouse in the right chamber (Stranger 2) to examine social novelty (social preference between familiar (Stranger 1) and new unfamiliar (Stranger 2) mice; Supplementary Figure 1; Figure 3). Figure 3A shows the time spent in the zone with Stranger 2 is significantly longer for wild-type mice (two-tailed Student’s t-test, P < 0.0001). Contrastingly, in CD157KO mice, the time spent in the two zones are similar (Figure 3B). The result indicated that, unlike in the wild-type mice, social preference to new social targets was completely lost in CD157KO mice.
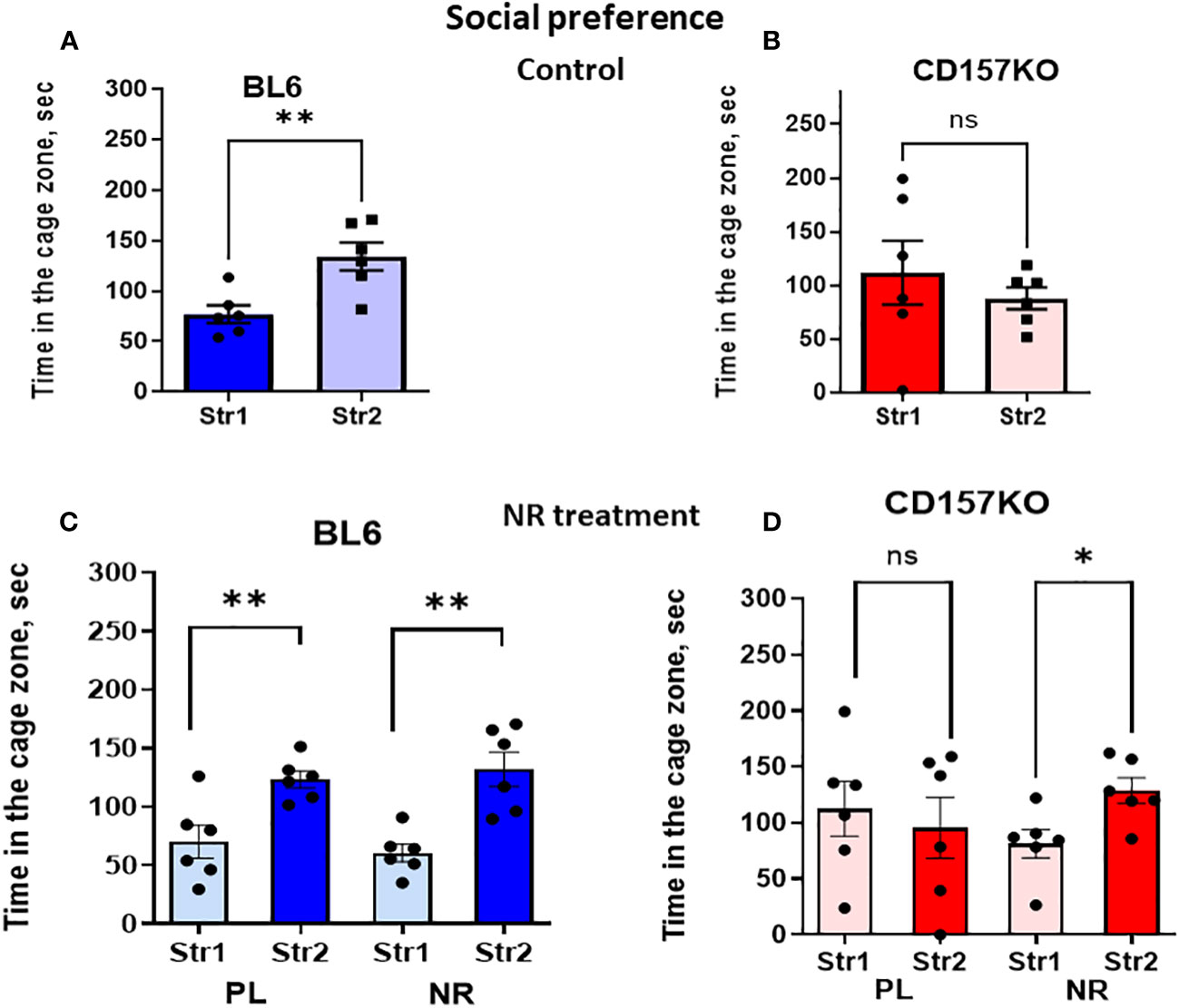
Figure 3 Nicotinamide riboside (NR) treatment corrects social preference deficit in CD157KO mice. Duration spent in the chamber with the familiar mouse in a cage (Str1) or a novel mouse (Str2) by wild-type (BL6; A, C) or CD157KO (B, D) mice. Mice were treated with gavage administration of saline (PL) or nicotinamide riboside (NR) for 12 days (C, D) or were without any treatment (A, B). Two-tailed Student’s t-test, *P < 0.05, **P < 0.01. ns, not significant.
No social preference phenotype in CD157KO mice was changed after gavage treatment with saline for 12 days (Figure 3D). In sharp contrast, CD157KO mice treated with gavage nicotinamide riboside (13 mg daily for 12 days) interacted much more frequently with Stranger 2 compared with stranger 1 (Figure 3D); the time spent in the area with Stranger 2 was significantly longer than that in the zone with Stranger 1 (two-tailed Student’s t-test, P < 0.01). Wild type mice treated with nicotinamide riboside or saline displayed significant social preference (two-tailed Student’s t-test, P < 0.01; Figure 3C).
In the social memory stage, the Stranger 2 mouse was replaced with a novel social stimulus (Stranger 3) (Supplementary Figure 1). Next behavior tests were performed after 30 min, which allowed to measure short-term memory in mice. Both genotypes of wild-type and CD157KO mice lacked significant social memory, without any preference to stranger 3 compared to Stranger 1 (Figures 4A, B); nicotinamide riboside did not affect this preference (Figures 4C, D).
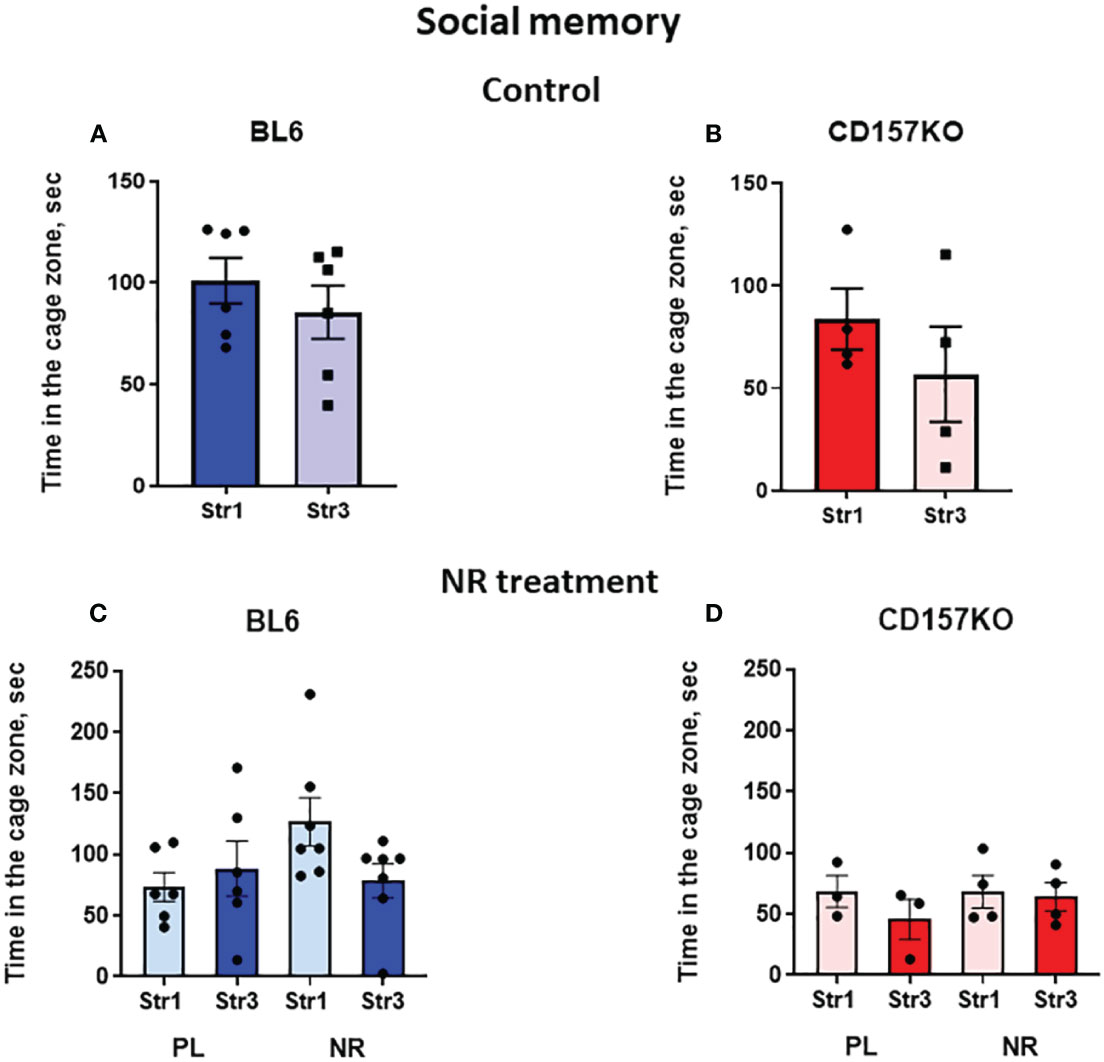
Figure 4 Wild-type C57BL6 (BL6) and C157KO mice show absence of social memory and no or little effect of nicotinamide riboside (NR) treatment. Duration spent in the chamber with the familiar mouse in a cage (Str1) or a novel mouse (Str3) by wild-type (BL6; A, C) or CD157KO (B, D) mice. Mice were treated with gavage administration of saline (PL) or NR for 12 days (C, D) or were without any treatment (A, B).
3.3 On CD38 knockout mice
Finally, we examined the effects of nicotinamide riboside in CD38KO mice to assess whether CD38 was necessary for the recovery of social behavior defects. As done with the CD157KO mice, social behavior was examined in the three-chamber box test. CD38KO mice displayed the sociability phenotype, similar to the wild-type ICR mice (Figures 5A, B). This sociability in CD38KO mice was unaffected by gavage administration of saline (Student’s t-test P < 0.0001) or treatment with nicotinamide riboside (Student’s t-test P < 0.0001; Figures 5C, D).
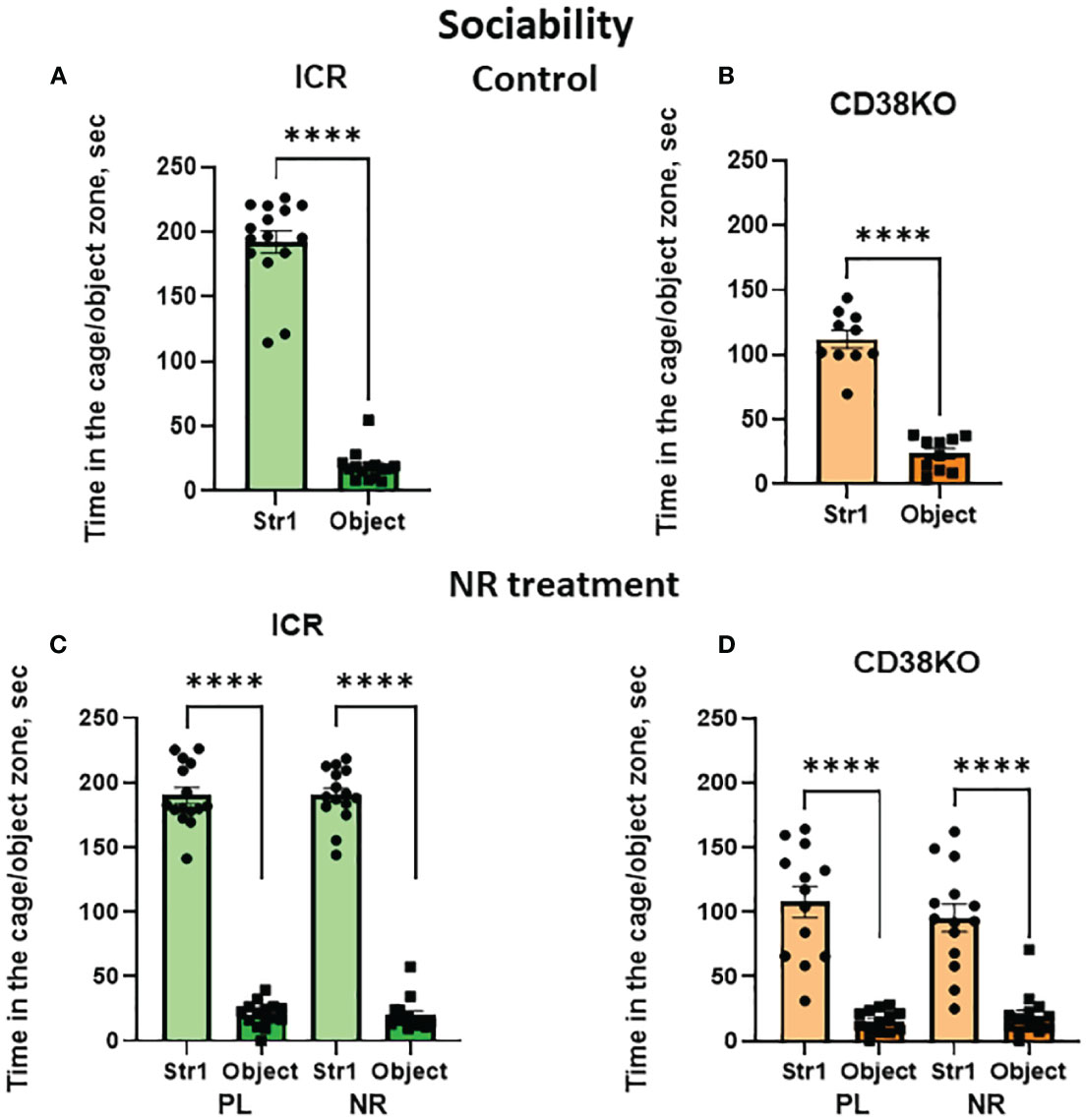
Figure 5 Nicotinamide riboside (NR) treatment has no apparent effect on sociability in wild-type (ICR) and CD38KO male mice in the three-chamber test. Duration spent in the chamber with a novel mouse in a cage (Str1) or a non-social target (object) by ICR (A, C) or CD38KO (B, D) mice. Mice were treated with gavage administration of saline (PL) or NR for 12 days (C, D) or were without any treatment (A, B), and then were examined for sociability behavior. Two-tailed Student’s t-test, ****P < 0.0001.
Unlike the wild-type mice (Figure 6A; Student’s t-test P < 0.0001), CD38KO displayed no or little social preference (Figure 6B; Student’s t-test P = 0.055). Such social preference was unaffectedly observed in wild-type mice under treatment with saline as well as with nicotinamide riboside (Student’s t-test P < 0.0001; Figure 6C). CD38KO mice treated with gavage administration of nicotinamide riboside (13 mg/day) for 12 days showed a complete lack of social preference (two-tailed Student’s t-test, P > 0.05; Figure 6D). Unexpectedly, significance in social preference was observed in CD38KO mice treated with saline gavage (Figure 6D; two-tailed Student’s t-test, P < 0.01). The reasons for why such unexpected result was obtained are not clear, but one reason may reside on the handling effect of mice during gavage, because handling animals sometimes causes undesirable results is well reported (51). Note that, although some activities in the central zone were lower in CD38KO mice, probably because of moving around other places due to hyperactive features (24, 28), CD38KO mice displayed the sociability.
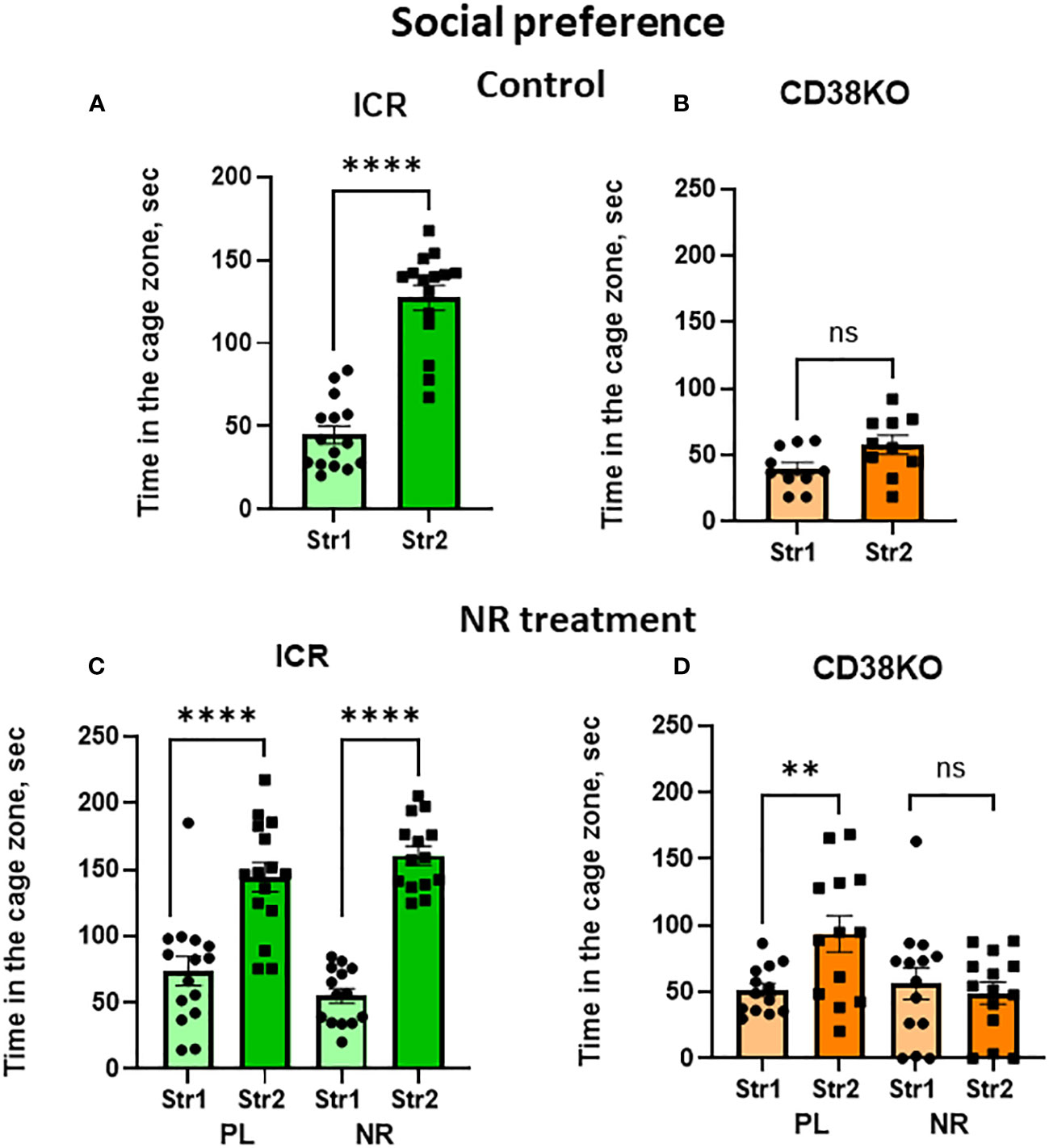
Figure 6 Effects of nicotinamide riboside (NR) or saline (PL) treatment on social preference behavior in wild-type (ICR) or CD38KO mice. Duration spent in the chamber with the familiar mouse in a cage (Str1) or a novel mouse (Str2) by wild-type (A, C) or CD38KO (B, D) male mice. Mice were treated with gavage administration of saline (PL) or NR for 12 days (C, D) or were without any treatment (A, B). Two-tailed Student’s t-test, **P < 0.01, ****P < 0.0001. ns, not significant.
Social short-term memory which was performed with the 30-min separation between trials was observed in wild-type ICR mice (Figure 7A; two-tailed Student’s t-test, P < 0.001). CD38KO mice were not able to distinguish between Stranger 1 and Stranger 3 in this test (Figure 7B). Interestingly, nicotinamide riboside-treated ICR and CD38KO mice showed a lack of social memory (Two-tailed Student’s t-test, P > 0.05; Figures 7C, D), unlike those treated with saline.
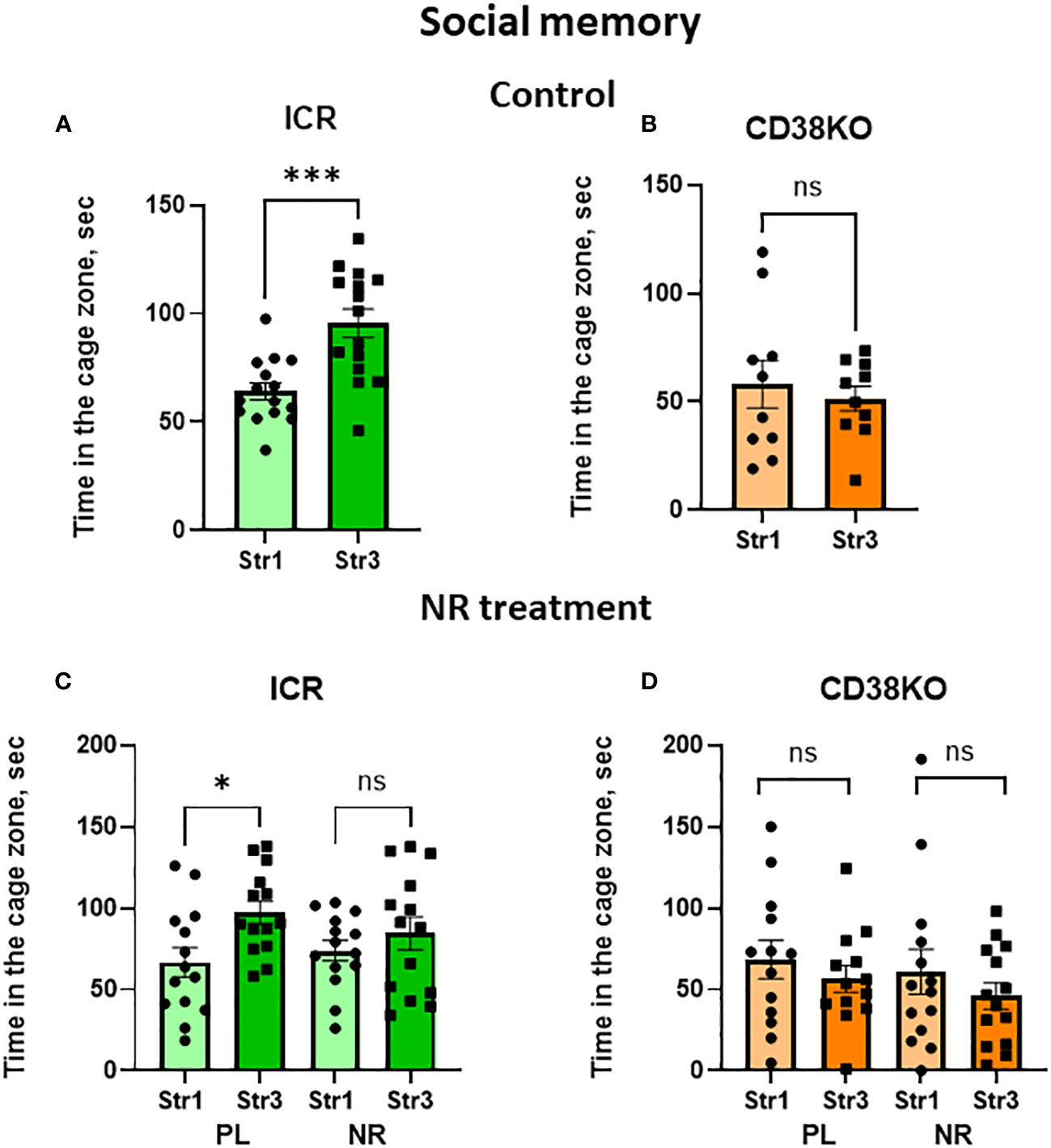
Figure 7 Social memory behavior in wild-type (ICR) and CD38KO mice and no or little effect of nicotinamide riboside (NR) treatment. Duration spent in the chamber with the familiar mouse in a cage (Str1) or a novel mouse (Str3) by ICR (A, C) or CD38KO (B, D) mice. Mice were treated with gavage administration of saline (PL) or NR for 12 days (C, D) or were without any treatment (A, B). Note that social memory behavior was observed in ICR mice (A, C) but was not affected by NR treatment (C). Two-tailed Student’s t-test, *P < 0.05.
4 Discussion
The results showed that CD157KO and CD38KO mice displayed sociability (mouse’s motivation to engage with social (mouse) targets compared to non-social targets), which is an essential characteristic of mice, and gavage administration of nicotinamide riboside had no significant effects on their sociability. We also demonstrated that social preference (social novelty to new social targets) was disrupted in both CD157KO and CD38KO mice, and gavage administration of nicotinamide riboside daily for 12 days ameliorated social preference defects only in CD157KO mice, but not in CD38KO mice. Social memory (preference to novel mice rather than already familiar mice) was displayed in wild-type (ICR strain) mice. This behavior, unfortunately, disappeared after nicotinamide riboside gavage administration. Social memory was not observed in CD38KO (genetic background of the ICR strain) and no social memory was affected by either treatment with saline or nicotinamide riboside. In the current study, the effect of nicotinamide riboside on social memory in CD157KO mice was not investigated, because C57BL6 wild-type mice did not display social memory. Of particular interests, it will be expected to get more sharp results on effects of decreases in NAD concentrations and supplementation effects of nicotinamide riboside at the tissues and behavior levels in double knockout mice which delete both CD157 and CD38 genes in future.
Here, apart from confirming the beneficial effects of nicotinamide riboside on social behavior defects in CD157 KO mice, but we also tested its effect on body weight. No apparent effect on body weight was observed during the 12 days of gavage administration. Application of nicotinamide riboside had similar effect as saline in some cases. Such results are important, as they suggest no or little risk by nicotinamide on animal health and fewer side effects.
Nicotinamide riboside is a prospective NAD precursor with high bioavailability (52). Nicotinamide riboside does not demonstrate sirtuin (SIRT) inhibition as a backside effect, unlike nicotinamide (53, 54). Moreover, unlike nicotinamide mononucleotide, nicotinamide riboside can be freely transported across the cell membrane. NAD is synthesized from tryptophan in the de novo pathway, as well as from the vitamin precursors, nicotinic acid, nicotinamide mononucleotide, and nicotinamide riboside, in a salvage pathway (38, 39, 55). Thus, it is likely that the exogenous application of nicotinamide riboside nay increase the biosynthesis of NAD through the production of nicotinamide mononucleotide in the first step (Figure 8) (40, 41, 56). In terms of elevation of mouse liver NAD, it has been reported that nicotinamide riboside is more efficiently orally bioavailable compared with nicotinamide mononucleotide and nicotinic acid, validating nicotinamide riboside as the favored NAD precursor (52). CD38 catalyzes the pathway of biosynthesis from nicotinamide riboside. Therefore, in agreement to this, the current results are reasonable; CD38 is critical in the recovery of social behavior defects.
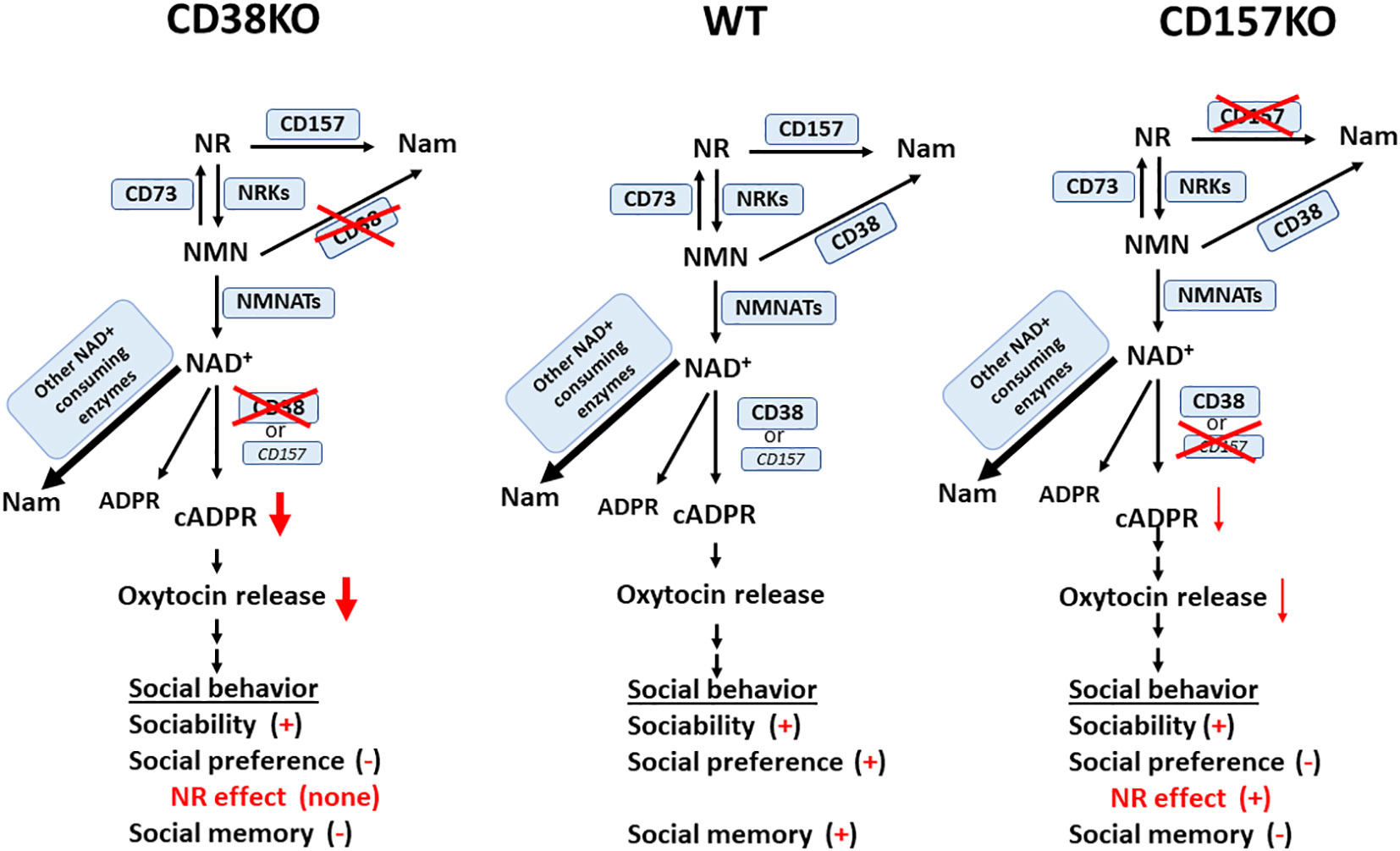
Figure 8 A simplified scheme for possible NAD catabolism from nicotinamide riboside (NR) and metabolism from NAD to cyclic ADP-ribose (cADPR) in wild-type (WT), CD38KO, and CD157KO mice. This is a reciprocal pathway from NR supplementation to NAD and nicotinamide (Nam) via nicotinamide mononucleotide (NMN) catalyzed by CD157, CD73 nicotinamide riboside kinases (NRKs), CD38, and nicotinamide mononucleotide adenylyl transferase (NMNATs); NAD is metabolized to Nam, ADP-ribose (ADPR), and cADPR. cADPR production is greatly reduced in CD38KO mice, but less affected in CD157KO mice, which induces different levels of oxytocin release. Social behaviors consist of sociability, social preference, and social memory. Different levels of oxytocin release elicit different levels of impairment of three subclasses of social behaviors. Note that impaired social preference is recovered in CD157KO, but not in CD38KO mice. It is noted that NAD concentration increase in the brain after nicotinamide riboside was confirmed in CD157KO mice (17), but not examined in CD38KO supplemented with nicotinamide riboside.
NAD concentration increases in the cortex or hypothalamus and oxytocin release to the cerebrospinal fluid by nicotinamide riboside were confirmed in CD157KO mice (17). Although not examined in CD38KO supplemented with nicotinamide riboside, it is highly likely that NAD increase may occur in CD38KO mice as well, because the salvage synthesizing pathway is not disrupted in either KO mice (Figure 8). However, in CD38KO mice, the CD38-dependent cADPR system existed in ICR wild-type mice is greatly disrupted, which is why beneficial effects were observed in CD157KO mice but not in CD38KO mice. Existence of CD38 is critical for social behavior, as previously demonstrated (9, 15, 21, 28, 57).
A decrease in volume of the amygdala, an important constituent of the “social brain,” might be caused by a loss of CD157 in the neural stem cells during the developmental stages (14, 21). The behavioral impairments in CD157KO mice were rescued by oxytocin, likely because oxytocin directly targets the intracellular signaling networks in the social brain downstream of CD157, which might be independent of CD38; its downstream signaling networks are indicated in Figure 8. Of course, to completely conclude needs to wait until actual measuring of oxytocin release in CD38KO mice treated with nicotinamide riboside. The above observation, however, suggests that oxytocin can be used for the treatment of social avoidance in psychological disorders. Furthermore, whether the results obtained here with mice are applicable to human behavioral recovery is of interest, especially since there have been reports of the impact of nicotinamide riboside on the effectiveness of oxytocin for treating impaired social interaction in cases of ASD. Together, current results suggest nicotinamide riboside is possibly an alternative substitute of oxytocin as a clinical usage (15, 18).
Nicotinamide riboside could be used as a general supplement, potentially for people who have adverse reactions to nicotinic acid or nicotinamide mononucleotide (52). In the brain, the tissue activity of NAD synthesis by nicotinic acid is very low, because nicotinic acid is not suitably recruited with supplement administration (58). Nicotinamide riboside has already been demonstrated as a favorable supplement or therapeutic agent to elevate or maintain cellular NAD contents (39, 42–44). Recently, a study demonstrated an increased consumption of NAD due to increased CD38 in aged subjects and, thus, proposed that inhibition of CD38 or increase in NAD might lead to the longer life span (45, 46). Furthermore, with respect to CD38 inhibition, it would be nice to analyze the area of the big sample of myeloma patients (ranging over thousands) treated with anti-CD38 antibodies: At the moment there are only two approved antibodies, one with total blocking NADase activity (59–61) and a second with partial inhibition of ADP-ribosyl cyclase activity of human CD38 (62, 63).
NAD is an abundant biomolecule and participates in multiple vital processes such as ATP synthesis, redox homeostasis, and signaling pathways (39). These effects can be explained by the enhancement of energy metabolism and activation of SIRT, for which NAD is a co-factor, and by the influence on mTOR pathway regulation (64). NAD as a substrate in ADP-ribosyl cyclase reactions could be a crucial component for oxytocin release induced by nicotinamide riboside. This pathway of elevating of NAD contents may enhance nicotinamide riboside-induced oxytocin release and, thus, influence social behavior. Recent findings suggest that hyper-activation of mTOR may play a crucial role in ASD (65, 66), predicting mTOR as a potential target in ASD therapy. On the other hand, SIRT utilizes NAD as a co-factor and can lead to mTOR pathway inhibition (64). For this, there is evidence that cADP-ribose acts as an endogenous inhibitor of mTOR (67).
In summary, we demonstrated that CD157 and/or CD38 are essential for social behavior. This implies that CD157 and CD38 possess a critical function in the nervous system; they play a role in neuromodulation via NAD metabolism, rather than entero-immune regulation (68). Together with benefits in mouse brain tissues induced by supplementation of nicotinamide riboside (17), the current results indicate potential therapeutic applications of nicotinamide riboside in ASD patients, as proposed by Cercillieux et al. (39).
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was reviewed and approved by the Committee on Animal Experimentation of Kanazawa University Graduate School of Medical Sciences.
Author contributions
HH and MG designed and performed experiments. HH and MG wrote article and drew figures. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1166609/full#supplementary-material
References
1. Curioni A. What makes us act together? on the cognitive models supporting humans’ decisions for joint action. Front Integr Neurosci (2022) 16:900527. doi: 10.3389/fnint.2022.900527
2. Rand DG, Nowak MA. Human cooperation. Trends Cognit Sci (2013) 17:413–25. doi: 10.1016/j.tics.2013.06.003
3. Tomasello M, Vaish A. Origins of human cooperation and morality. Annu Rev Psychol (2013) 64:231–55. doi: 10.1146/annurev-psych-113011-143812
4. Teixeira GC, Araújo AMM, de Andrade MJO. Social neuroscience and mental processes: how does our brain process social information? Innov Clin Neurosci (2022) 19:33–5.
5. Adolphs R. The social brain: neural basis of social knowledge. Annu Rev Psychol (2009) 60:693–716. doi: 10.1146/annurev.psych.60.110707.163514
6. Bicks LK, Koike H, Akbarian S, Morishita H. Prefrontal cortex and social cognition in mouse and man. Front Psychol (2015) 6:1805. doi: 10.3389/fpsyg.2015.01805
7. Tanimizu T, Kenney JW, Okano E, Kadoma K, Frankland PW, Kida S. Functional connectivity of multiple brain regions required for the consolidation of social recognition memory. J Neurosci (2017) 37:4103–16. doi: 10.1523/JNEUROSCI.3451-16.2017
8. Kogan JH, Frankland PW, Silva AJ. Long-term memory underlying hippocampus-dependent social recognition in mice. Hippocampus (2000) 10:47–56. doi: 10.1002/(SICI)1098-1063(2000)10:1<47::AID-HIPO5>3.0.CO;2-6
9. Higashida H, Yokoyama S, Huang J-J, Liu L, Ma W-J, Akther S, et al. Social memory, amnesia, and autism: brain oxytocin secretion is regulated by NAD+ metabolites and single nucleotide polymorphisms of CD38. Neurochem Int (2012) 61:828–38. doi: 10.1016/j.neuint.2012.01.030
10. Goerlich KS, Votinov M. Hormonal abnormalities in alexithymia. Front Psychiatry (2022) 13:1070066. doi: 10.3389/fpsyt.2022.1070066
11. Ghazy AA, Soliman OA, Elbahnasi AI, Alawy AY, Mansour AM, Gowayed MA. Role of oxytocin in different neuropsychiatric, neurodegenerative, and neurodevelopmental disorders. Rev Physiol Biochem Pharmacol (2023) 186:95–134. doi: 10.1007/112_2022_72
12. Yamasue H, Domes G, Hurlemann R, Grinevich V. “Oxytocin and autism spectrum disorders.,”. In: Editors. behavioral pharmacology of neuropeptides: oxytocin. current topics in behavioral neurosciences. Cham: Springer International Publishing (2017). p. 449–65. doi: 10.1007/7854_2017_24
13. Peñagarikano O. Oxytocin in animal models of autism spectrum disorder: oxytocin in animal models of autism. Devel Neurobio (2017) 77:202–13. doi: 10.1002/dneu.22449
14. Lopatina O, Yoshihara T, Nishimura T, Zhong J, Akther S, Fakhrul AAKM, et al. Anxiety- and depression-like behavior in mice lacking the CD157/BST1 gene, a risk factor for parkinson’s disease. Front Behav Neurosci (2014) 8:133. doi: 10.3389/fnbeh.2014.00133
15. Higashida H, Furuhara K, Lopatina O, Gerasimenko M, Hori O, Hattori T, et al. Oxytocin dynamics in the body and brain regulated by the receptor for advanced glycation end-products, CD38, CD157, and nicotinamide riboside. Front Neurosci (2022) 16:858070. doi: 10.3389/fnins.2022.858070
16. Gerasimenko M, Lopatina O, Shabalova AA, Cherepanov SM, Salmina AB, Yokoyama S, et al. Distinct physical condition and social behavior phenotypes of CD157 and CD38 knockout mice during aging. PloS One (2020) 15:e0244022. doi: 10.1371/journal.pone.0244022
17. Gerasimenko M, Cherepanov SM, Furuhara K, Lopatina O, Salmina AB, Shabalova AA, et al. Nicotinamide riboside supplementation corrects deficits in oxytocin, sociability and anxiety of CD157 mutants in a mouse model of autism spectrum disorder. Sci Rep (2020) 10:10035. doi: 10.1038/s41598-019-57236-7
18. Higashida H, Hashii M, Tanaka Y, Matsukawa S, Higuchi Y, Gabata R, et al. CD38, CD157, and RAGE as molecular determinants for social behavior. Cells (2019) 9:62. doi: 10.3390/cells9010062
19. Shabalova AA, Liang M, Zhong J, Huang Z, Tsuji C, Shnayder NA, et al. Oxytocin and CD38 in the paraventricular nucleus play a critical role in paternal aggression in mice. Horm Behav (2020) 120:104695. doi: 10.1016/j.yhbeh.2020.104695
20. Kasai S, Yoshihara T, Lopatina O, Ishihara K, Higashida H. Selegiline ameliorates depression-like behavior in mice lacking the CD157/BST1 gene, a risk factor for parkinson’s disease. Front Behav Neurosci (2017) 11:75. doi: 10.3389/fnbeh.2017.00075
21. Higashida H, Liang M, Yoshihara T, Akther S, Fakhrul A, Stanislav C, et al. An immunohistochemical, enzymatic, and behavioral study of CD157/BST-1 as a neuroregulator. BMC Neurosci (2017) 18:35. doi: 10.1186/s12868-017-0350-7
22. Zhong J, Amina S, Liang M, Akther S, Yuhi T, Nishimura T, et al. Cyclic ADP-ribose and heat regulate oxytocin release via CD38 and TRPM2 in the hypothalamus during social or psychological stress in mice. Front Neurosci (2016) 10:304. doi: 10.3389/fnins.2016.00304
23. Yokoyama S, Al Mahmuda N, Munesue T, Hayashi K, Yagi K, Yamagishi M, et al. Association study between the CD157/BST1 gene and autism spectrum disorders in a Japanese population. Brain Sci (2015) 5:188–200. doi: 10.3390/brainsci5020188
24. Salmina AB, Lopatina O, Kuvacheva NV, Higashida H. Integrative neurochemistry and neurobiology of social recognition and behavior analyzed with respect to CD38-dependent brain oxytocin secretion. Curr Top Med Chem (2013) 13:2965–77. doi: 10.2174/15680266113136660211
25. Akther S, Korshnova N, Zhong J, Liang M, Cherepanov SM, Lopatina O, et al. CD38 in the nucleus accumbens and oxytocin are related to paternal behavior in mice. Mol Brain (2013) 6:41. doi: 10.1186/1756-6606-6-41
26. Lopatina O, Inzhutova A, Salmina AB, Higashida H. The roles of oxytocin and CD38 in social or parental behaviors. Front Neurosci (2012) 6:182. doi: 10.3389/fnins.2012.00182
27. Higashida H, Yokoyama S, Munesue T, Kikuchi M, Minabe Y, Lopatina O. CD38 gene knockout juvenile mice: a model of oxytocin signal defects in autism. Biol Pharm Bull (2011) 34:1369–72. doi: 10.1248/bpb.34.1369
28. Jin D, Liu H-X, Hirai H, Torashima T, Nagai T, Lopatina O, et al. CD38 is critical for social behaviour by regulating oxytocin secretion. Nature (2007) 446:41–5. doi: 10.1038/nature05526
29. Higashida H. Somato-axodendritic release of oxytocin into the brain due to calcium amplification is essential for social memory. J Physiol Sci (2016) 66:275–82. doi: 10.1007/s12576-015-0425-0
30. Insel TR, Winslow JT, Wang Z, Young LJ. Oxytocin, vasopressin, and the neuroendocrine basis of pair bond formation. Adv Exp Med Biol (1998) 449:215–24. doi: 10.1007/978-1-4615-4871-3_28
31. Lee HC. Cyclic ADP-ribose and nicotinic acid adenine dinucleotide phosphate (NAADP) as messengers for calcium mobilization. J Biol Chem (2012) 287:31633–40. doi: 10.1074/jbc.R112.349464
32. Kim U-H. Multiple enzymatic activities of CD38 for Ca 2+ signaling messengers. messenger (2014) 3:6–14. doi: 10.1166/msr.2014.1030
33. Wei W, Graeff R, Yue J. Roles and mechanisms of the CD38/cyclic adenosine diphosphate ribose/Ca(2+) signaling pathway. World J Biol Chem (2014) 5:58–67. doi: 10.4331/wjbc.v5.i1.58
34. Ishihara K, Hirano T. BST-1/CD157 regulates the humoral immune responses. vivo. Chem Immunol (2000) 75:235–55. doi: 10.1159/000058772
35. Lo Buono N, Parrotta R, Morone S, Bovino P, Nacci G, Ortolan E, et al. The CD157-integrin partnership controls transendothelial migration and adhesion of human monocytes. J Biol Chem (2011) 286:18681–91. doi: 10.1074/jbc.M111.227876
36. Yaku K, Okabe K, Nakagawa T. NAD metabolism: implications in aging and longevity. Ageing Res Rev (2018) 47:1–17. doi: 10.1016/j.arr.2018.05.006
37. Yaku K, Palikhe S, Izumi H, Yoshida T, Hikosaka K, Hayat F, et al. BST1 regulates nicotinamide riboside metabolism via its glycohydrolase and base-exchange activities. Nat Commun (2021) 12:6767. doi: 10.1038/s41467-021-27080-3
38. Bieganowski P, Brenner C. Discoveries of nicotinamide riboside as a nutrient and conserved NRK genes establish a preiss-handler independent route to NAD+ in fungi and humans. Cell (2004) 117:495–502. doi: 10.1016/s0092-8674(04)00416-7
39. Cercillieux A, Ciarlo E, Canto C. Balancing NAD+ deficits with nicotinamide riboside: therapeutic possibilities and limitations. Cell Mol Life Sci (2022) 79:463. doi: 10.1007/s00018-022-04499-5
40. Belenky P, Bogan KL, Brenner C. NAD+ metabolism in health and disease. Trends Biochem Sci (2007) 32:12–9. doi: 10.1016/j.tibs.2006.11.006
41. Braidy N, Guillemin G, Grant R. Promotion of cellular NAD(+) anabolism: therapeutic potential for oxidative stress in ageing and alzheimer’s disease. Neurotox Res (2008) 13:173–84. doi: 10.1007/BF03033501
42. Cantó C, Houtkooper RH, Pirinen E, Youn DY, Oosterveer MH, Cen Y, et al. The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab (2012) 15:838–47. doi: 10.1016/j.cmet.2012.04.022
43. Ryu D, Zhang H, Ropelle ER, Sorrentino V, Mázala DAG, Mouchiroud L, et al. NAD+ repletion improves muscle function in muscular dystrophy and counters global PARylation. Sci Transl Med (2016) 8:361ra139. doi: 10.1126/scitranslmed.aaf5504
44. Khan NA, Auranen M, Paetau I, Pirinen E, Euro L, Forsström S, et al. Effective treatment of mitochondrial myopathy by nicotinamide riboside, a vitamin B3. EMBO Mol Med (2014) 6:721–31. doi: 10.1002/emmm.201403943
45. Tarragó MG, Chini CCS, Kanamori KS, Warner GM, Caride A, de Oliveira GC, et al. A potent and specific CD38 inhibitor ameliorates age-related metabolic dysfunction by reversing tissue NAD+ decline. Cell Metab (2018) 27:1081–1095.e10. doi: 10.1016/j.cmet.2018.03.016
46. Camacho-Pereira J, Tarragó MG, Chini CCS, Nin V, Escande C, Warner GM, et al. CD38 dictates age-related NAD decline and mitochondrial dysfunction through an SIRT3-dependent mechanism. Cell Metab (2016) 23:1127–39. doi: 10.1016/j.cmet.2016.05.006
47. Quarona V, Zaccarello G, Chillemi A, Brunetti E, Singh VK, Ferrero E, et al. CD38 and CD157: a long journey from activation markers to multifunctional molecules. Cytometry B Clin Cytom (2013) 84:207–17. doi: 10.1002/cyto.b.21092
48. Lopatina O, Inzhutova A, Pichugina YA, Okamoto H, Salmina AB, Higashida H. Reproductive experience affects parental retrieval behaviour associated with increased plasma oxytocin levels in wild-type and CD38-knockout mice. J Neuroendocrinol (2011) 23:1125–33. doi: 10.1111/j.1365-2826.2011.02136.x
49. Kato I, Yamamoto Y, Fujimura M, Noguchi N, Takasawa S, Okamoto H. CD38 disruption impairs glucose-induced increases in cyclic ADP-ribose, [Ca2+]i, and insulin secretion. J Biol Chem (1999) 274:1869–72. doi: 10.1074/jbc.274.4.1869
50. Zhang J-B, Chen L, Lv Z-M, Niu X-Y, Shao C-C, Zhang C, et al. Oxytocin is implicated in social memory deficits induced by early sensory deprivation in mice. Mol Brain (2016) 9:98. doi: 10.1186/s13041-016-0278-3
51. Saré RM, Lemons A, Smith CB. Behavior testing in rodents: highlighting potential confounds affecting variability and reproducibility. Brain Sci (2021) 11:522. doi: 10.3390/brainsci11040522
52. Trammell SA, Yu L, Redpath P, Migaud ME, Brenner C. Nicotinamide riboside is a major NAD+ precursor vitamin in cow milk. J Nutr (2016) 146:957–63. doi: 10.3945/jn.116.230078
53. Avalos JL, Bever KM, Wolberger C. Mechanism of sirtuin inhibition by nicotinamide: altering the NAD(+) cosubstrate specificity of a Sir2 enzyme. Mol Cell (2005) 17:855–68. doi: 10.1016/j.molcel.2005.02.022
54. Dai H, Sinclair DA, Ellis JL, Steegborn C. Sirtuin activators and inhibitors: promises, achievements, and challenges. Pharmacol Ther (2018) 188:140–54. doi: 10.1016/j.pharmthera.2018.03.004
55. Nikiforov A, Kulikova V, Ziegler M. The human NAD metabolome: functions, metabolism and compartmentalization. Crit Rev Biochem Mol Biol (2015) 50:284–97. doi: 10.3109/10409238.2015.1028612
56. Braidy N, Berg J, Clement J, Khorshidi F, Poljak A, Jayasena T, et al. Role of nicotinamide adenine dinucleotide and related precursors as therapeutic targets for age-related degenerative diseases: rationale, biochemistry, pharmacokinetics, and outcomes. Antioxid Redox Signal (2019) 30:251–94. doi: 10.1089/ars.2017.7269
57. Higashida H, Munesue T, Kosaka H, Yamasue H, Yokoyama S, Kikuchi M. Social interaction improved by oxytocin in the subclass of autism with comorbid intellectual disabilities. Diseases (2019) 7:24. doi: 10.3390/diseases7010024
58. Mori V, Amici A, Mazzola F, Di Stefano M, Conforti L, Magni G, et al. Metabolic profiling of alternative NAD biosynthetic routes in mouse tissues. PloS One (2014) 9:e113939. doi: 10.1371/journal.pone.0113939
59. Zhu C, Song Z, Wang A, Srinivasan S, Yang G, Greco R, et al. Isatuximab acts through fc-dependent, independent, and direct pathways to kill multiple myeloma cells. Front Immunol (2020) 11:1771. doi: 10.3389/fimmu.2020.01771
60. Martino EA, Bruzzese A, Iaccino E, Mendicino F, Mimmi S, Lucia E, et al. Isatuximab in multiple myeloma. Expert Opin Biol Ther (2023) 23:7–10. doi: 10.1080/14712598.2023.2193289
61. Martin TG, Corzo K, Chiron M, van de Velde H, Abbadessa G, Campana F, et al. Therapeutic opportunities with pharmacological inhibition of CD38 with isatuximab. Cells (2019) 8:1522. doi: 10.3390/cells8121522
62. Donk NWCJ, Janmaat ML, Mutis T, Lammerts van Bueren JJ, Ahmadi T, Sasser AK, et al. Monoclonal antibodies targeting CD 38 in hematological malignancies and beyond. Immunol Rev (2016) 270:95–112. doi: 10.1111/imr.12389
63. van de Donk NWCJ, Richardson PG, Malavasi F. CD38 antibodies in multiple myeloma: back to the future. Blood (2018) 131:13–29. doi: 10.1182/blood-2017-06-740944
64. Ghosh HS, McBurney M, Robbins PD. SIRT1 negatively regulates the mammalian target of rapamycin. PloS One (2010) 5:e9199. doi: 10.1371/journal.pone.0009199
65. Sato A. mTOR, a potential target to treat autism spectrum disorder. CNSNDDT (2016) 15:533–43. doi: 10.2174/1871527315666160413120638
66. Pagani M, Barsotti N, Bertero A, Trakoshis S, Ulysse L, Locarno A, et al. mTOR-related synaptic pathology causes autism spectrum disorder-associated functional hyperconnectivity. Nat Commun (2021) 12:6084. doi: 10.1038/s41467-021-26131-z
67. Higashida H, Kamimura S, Inoue T, Hori O, Islam MS, Lopatina O, et al. Cyclic ADP-ribose as an endogenous inhibitor of the mTOR pathway downstream of dopamine receptors in the mouse striatum. J Neural Transm (2018) 125:17–24. doi: 10.1007/s00702-016-1666-7
Keywords: NAD, nicotinamide riboside, CD157, CD38, social behavior, neuromodulator
Citation: Gerasimenko M and Higashida H (2023) Remission of social behavior impairment by oral administration of a precursor of NAD in CD157, but not in CD38, knockout mice. Front. Immunol. 14:1166609. doi: 10.3389/fimmu.2023.1166609
Received: 15 February 2023; Accepted: 18 April 2023;
Published: 04 May 2023.
Edited by:
Katsuhiko Ishihara, Kawasaki University of Medical Welfare, JapanCopyright © 2023 Gerasimenko and Higashida. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haruhiro Higashida, haruhiro@med,kanazawa-u.ac.jp
 Maria Gerasimenko
Maria Gerasimenko Haruhiro Higashida
Haruhiro Higashida