- 1Department of Biology, College of Science, United Arab Emirates University, Al-Ain, United Arab Emirates
- 2Department of Veterinary Medicine, College of Agriculture and Veterinary Medicine, United Arab Emirates University, Al-Ain, United Arab Emirates
- 3Department of Parasitology, University of Agriculture, Faisalabad, Pakistan
- 4Department of Chemistry, College of Science, United Arab Emirates University, Al-Ain, United Arab Emirates
- 5Department of Infectious Diseases and Public Health, Jockey Club College of Veterinary Medicine and Life Sciences, City University of Hong Kong, Kowloon, Hong Kong SAR, China
Haematophagous arthropods can harbor various pathogens including viruses, bacteria, protozoa, and nematodes. Insects possess an innate immune system comprising of both cellular and humoral components to fight against various infections. Haemocytes, the cellular components of haemolymph, are central to the insect immune system as their primary functions include phagocytosis, encapsulation, coagulation, detoxification, and storage and distribution of nutritive materials. Plasmatocytes and granulocytes are also involved in cellular defense responses. Blood-feeding arthropods, such as mosquitoes and ticks, can harbour a variety of viral pathogens that can cause infectious diseases in both human and animal hosts. Therefore, it is imperative to study the virus-vector-host relationships since arthropod vectors are important constituents of the ecosystem. Regardless of the complex immune response of these arthropod vectors, the viruses usually manage to survive and are transmitted to the eventual host. A multidisciplinary approach utilizing novel and strategic interventions is required to control ectoparasite infestations and block vector-borne transmission of viral pathogens to humans and animals. In this review, we discuss the arthropod immune response to viral infections with a primary focus on the innate immune responses of ticks and mosquitoes. We aim to summarize critically the vector immune system and their infection transmission strategies to mammalian hosts to foster debate that could help in developing new therapeutic strategies to protect human and animal hosts against arthropod-borne viral infections.
Introduction
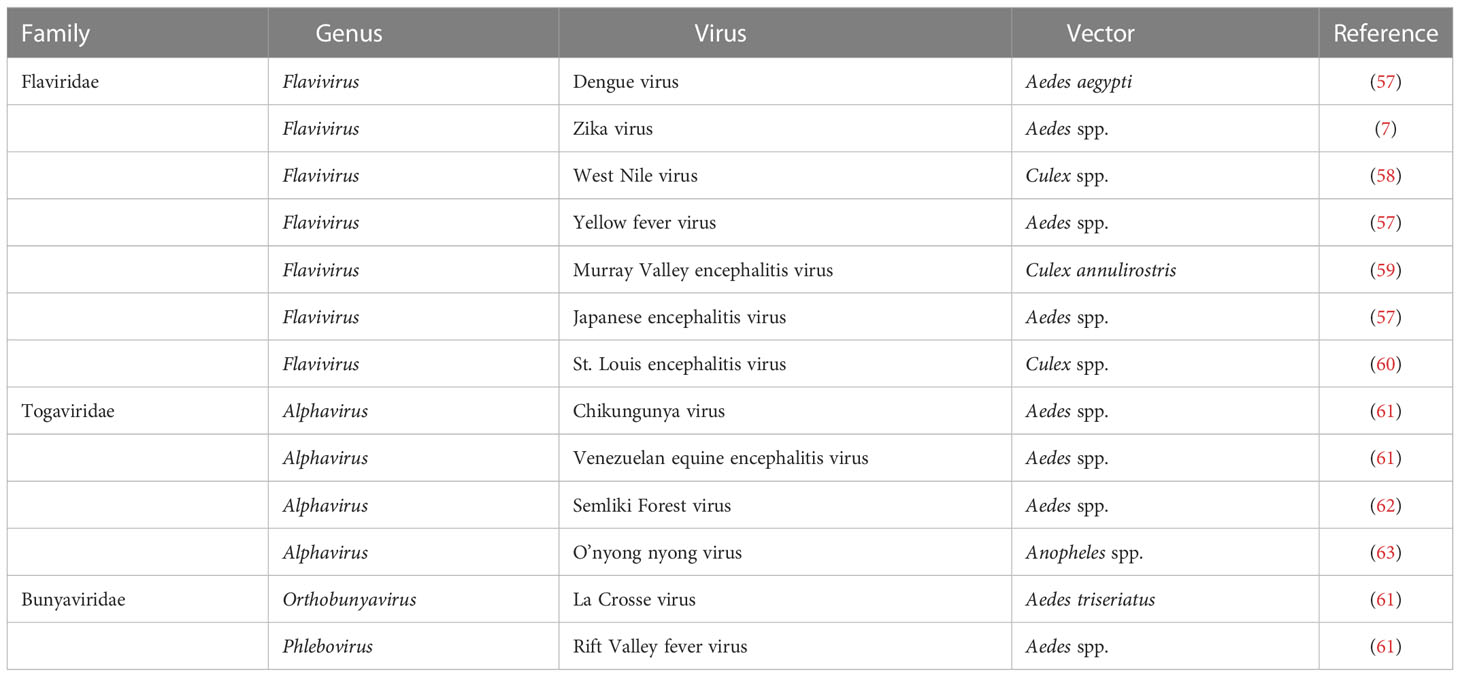
Insecta is the most abundant group of terrestrial animals, both in terms of numbers as well as species (approx. 5.5 million) (1). However, most insect species are not described (1, 2); about 80% of species yet to be identified (2). Insects provide many essential services in the natural ecosystem as pollinators and biological control agents, and help in nutrient recycling and food resources (3). However, many insect species are serious pests of cash crops or staple food crops. In addition, some insects (including mosquitoes, lice, fleas, and bed bugs), and ticks (Acari) serve as vectors transmitting a variety of pathogens (bacteria, viruses, nematodes and protozoa) to humans and animals (4). Among these, mosquito-borne and tick-borne viruses cause some of the most severe diseases with high fatality rates in humans and animals (5–7). In particular vector-borne viruses, including Zika, West Nile fever, Rift Valley fever, dengue, yellow fever, chikungunya, Japanese encephalitis, Crimean-Congo hemorrhagic fever, tick-borne encephalitis and Alkhurma hemorrhagic fever virus, are a continued threat to human and livestock health globally. Due to globalization and climate change, ticks and mosquitoes are occupying new geographic areas expanding the remit of vector-borne diseases.
Insects have an open circulatory system, the blood (haemolymph) mixes with the interstitial fluid and circulates in a body cavity called the haemocoel (8). Haemocytes or blood cells, the cellular components of haemolymph (9), are classified into various cell types such as prohemocytes, plasmatocytes, granulocytes, coagulocytes, oenocytoids, spherulocytes, thrombocytoids, and crystal cells (not all haemocyte types are present in most insects) (10, 11), with their main functions being immune response to pathogens including detoxification, coagulation, encapsulation and phagocytosis; phagocytosis; other cells are involved in carrying and transferring nutritive materials to various organs (12, 13). Viral transmission in insects is dependent on innate immune response (8). In this review, we discuss the innate immunity of arthropod vectors with specific reference to anti-viral immune responses in ticks and mosquitoes.
Insect innate immune system and pathogen clearance mechanisms
Insect antiviral innate immunity has mostly been studied in Drosophila melanogaster (14). The innate immune system of insects acts through cellular and humoral components (15, 16), which together coordinate against bacterial and viral infections. Cellular immune responses include phagocytosis, nodulation, and encapsulation of pathogens by haemocytes (11, 17). However, during humoral response, pattern-recognition receptors (PRRs), which are germline-encoded, recognize pathogen-associated molecular patterns (PAMPs) and damage-associated molecules patterns (DAMPs). PAMPs include bacterial lipopolysaccharide (LPS), peptidoglycan, and fungal β-1,3-glucans (15). PRRs bind to the PAMPs and initiate opsonization of pathogens. The activation of downstream signaling induced by PRRs leads to the synthesis and secretion of effector molecules, for example reactive oxygen species (ROS), antimicrobial peptides (AMPs), and components of the phenoloxidase cascade; these effector molecules restrict infections and clear the intruding pathogens (8, 18–20). Blood sucking insects such as mosquitoes acquire various pathogens during blood feeding and their midgut epithelial cells act as the first line of defense and produce ROS and several AMPs. The expression of genes coding for AMPs depends on various signaling pathways such as Toll, Immune Deficiency (IMD), and Janus Kinase and Signal Transducer and Activator of Transcription (JAK/STAT) pathways; activation of these pathways inhibits viral replication (8, 21, 22). PRRs detect microbial invaders and initiate signaling cascades that hinder their proliferation in the host. This leads to engagement of adaptor molecules establishing multi-protein complexes comprised of kinases, transcription factors, and other regulatory molecules (23). Figure 1 shows the complex interplay of insect innate immune responses.
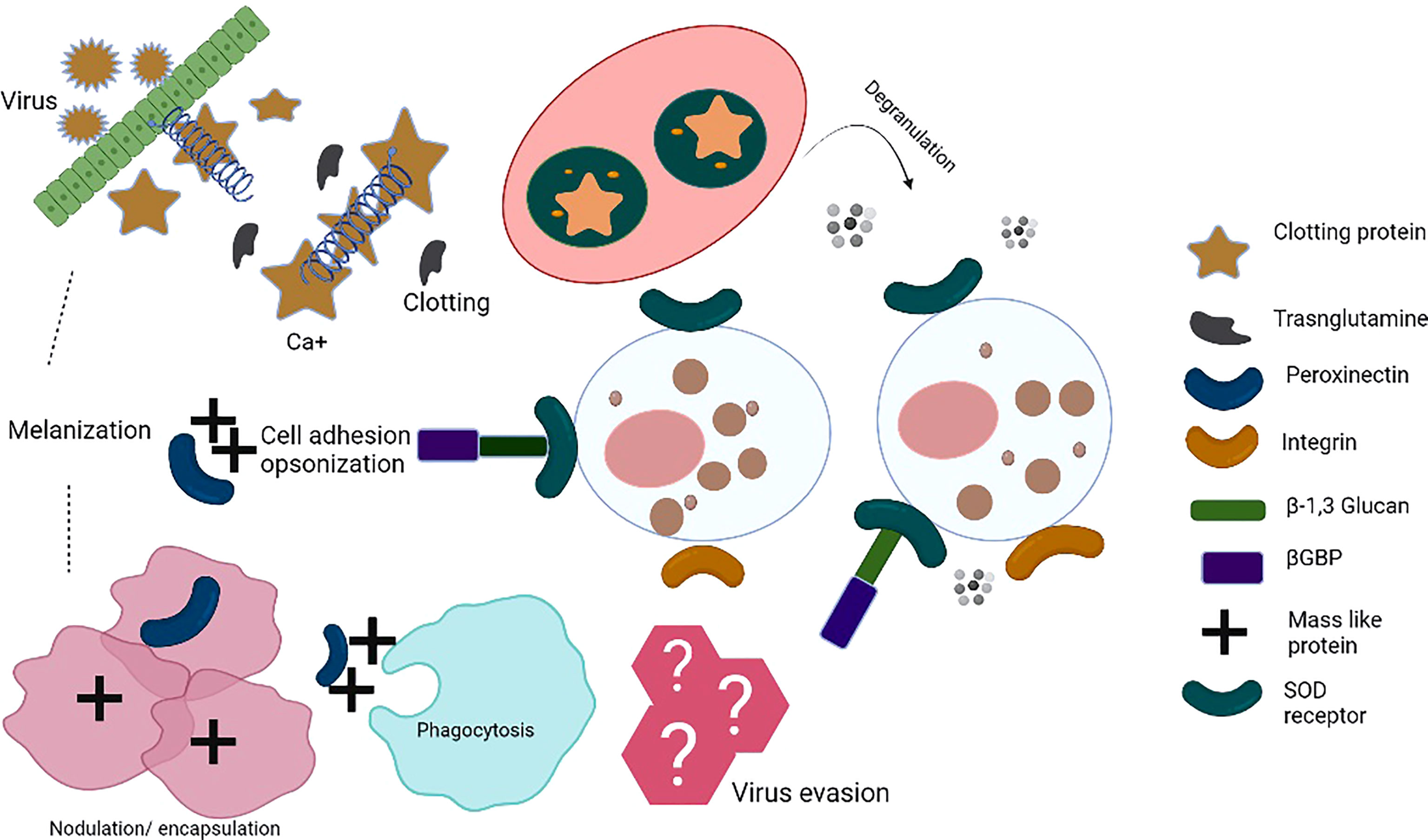
Figure 1 Insect innate immune mechanisms: When the insect’s immune system recognizes pathogen-associated molecular patterns (PAMPs) by pattern-recognition receptors (PRRs), pathogens encounter a complex system of humoral and cellular responses. Humoral responses include the production of antimicrobial peptides (AMPs), reactive nitrogen intermediates (RNI) or reactive oxygen intermediates (ROI), and a complex enzymatic cascade to regulate clotting or melanization. Cellular immune response involves various haemocytes that participate in phagocytosis/nodulation/encapsulation for pathogen clearance from haemolymph. In addition, different immune cells release toxic factors to kill pathogens, i.e. cell-mediated killing (complement components, etc.). These events take place in the haemocoel of the ticks. The series of immune reactions depict innate immunity. Clotting protein is a unique lipoprotein. Hemolymph clotting is induced upon transglutaminase (TGase) release from hemocytes/tissues. Calcium ions play an important role in the cascade leading to coagulation. TGase is involved in the coagulation, disturbing the chemical nature of the target pathogens. Peroxinectin is an opsonin that does attachment, spreads, and induces degranulation. SOD produces H2O2 from which hypohalic acid is released, which is a toxic substance, Prophenoloxidase activating system (proPO system) is an efficient part of the innate immune response. bGBP is a pattern recognition protein known to bind β-1,3, glucan. Masquerade (Mas-) like protein is also a multifunctional innate immune protein, an opsonin-like peroxinectin, capable of doing pathogen binding, inducing degranulation, and pathogen clearance. TGase, transglutaminase; Ca2+, Calcium, SOD, superoxide dismutase; βGBP, glucan with β-1,3 glucan binding protein; Mas-like protein,-- Masquerade- like protein.
Viral replication may be limited by activation of above-mentioned pathways. However, RNA interference (RNAi) is considered the most potent antiviral defense mechanism; it hinders viral replication yielding small RNAs (using viral double-stranded RNA as a template) targeting viral RNA degradation (24). Thus, innate immune system works through various effector mechanisms including phagocytosis, encapsulation, melanization, nodulation, lysis, RNAi, autophagy, and apoptosis.
Phagocytosis of pathogens by haemocytes
Phagocytosis by haemocytes starts when PRRs such as thioester-containing proteins, Nimrod proteins, β-integrins, and peptidoglycan recognition proteins (PGRPs) bind PAMPs (25, 26). For instance, PGRP-LC initiates the phagocytosis of E. coli in D. melanogaster (26). The pathogen is then taken into a membrane-delimited phagosome. The phagosome fuses with a lysosome (phagolysosome), where the pathogen is digested by hydrolytic enzymes such as lysozyme, proteases, lipases, nucleases, and glycosylases (25). There are haemocytes that circulate in the haemolymph (circulating haemocytes) and tissue-resident haemocytes (sessile haemocytes) (13, 27). Insects have morphologically and functionally distinct haemocyte subpopulations. The classification of haemocytes has not been standardized across Insecta (25); however, the majority of haemocytes are phagocytic (25). In Lepidopterans and Hemipterans, the phagocytic haemocytes are granulocytes, whereas in fruit flies, plasmatocytes show phagocytic activities (13, 27). In mosquitoes, circulating and sessile haemocytes initiate phagocytosis within seconds of making contact with the pathogens (25, 27) and can ingest hundreds of bacteria in a short time.
Encapsulation
Encapsulation is the most common type of defense reaction against parasites. Haemocyte-mediated encapsulation occurs in insects when large pathogens enter the haemolymph, for example, the eggs or larvae of parasites, protozoa and nematodes (25). Encapsulation in insects is of two types: cellular and humoral encapsulation. Haemocytes may or may not be involved in the humoral encapsulation that is associated with phenoloxidase; however, the cellular process may occur without melanization (11). In Galleria mellonella, during encapsulation, granulocytes release the adhesion protein, peroxinectin. It is a multifunctional molecule which is not only used for attachment and spreading but it also enhances encapsulation, degranulation, opsonization and peroxidase activity (28). Thus, this adhesion helps plasmatocytes attach to the layer of granulocytes which is then enclosed by several layers of plasmatocytes, followed by additional granulocytes (11, 25). Multiple layers of plasmatocytes surrounding the pathogen produce a capsule of multi-layered overlapping cells to sequester pathogen. In Drosophila, lamellocytes may commonly be observed in capsules; however, in Lepidoptera, both granulocytes and plasmatocytes are commonly observed in capsules (11, 16). In some cases, accompanied by encapsulation, plasmatocytes deposit pigment around the parasite (melanization). These defense responses, either alone or together, successfully kill parasites (29). In mosquitoes, the complement C3-like protein (AgTEP1) induces an immune response against Plasmodium berghei. Parasite death occurs when protein binds to the surface of the parasite and initiatesencapsulation by haemocytes (30).
Melanization
Melanization is an immune effector mechanism that kills pathogens including bacteria, protozoa, and nematodes; it is also involved in wound healing (25). In melanization reaction, tyrosine converts to melanin precursors and then proteins cross-link to form a melanin layer that impounds an attacking pathogen and establishes a dark proteinaceous capsule. This can cause starvation or oxidative damage resulting in the pathogen’s death (31). Furthermore, this process also facilitates clearing of dead microbes. Melanization occurs with the coordination of PRRs, serine proteases, serine protease inhibitors, and enzymes and starts when PRRs (for example β-1,3 glucan, C-type lectins and Gram-negative binding proteins) recognize PAMPs and initiate a serine protease cascade. Phenoloxidase begins the production of melanin by hydroxylation of tyrosine. Many enzymes and PRRs involved in the melanization process are produced by haemocytes, for instance, oenocytoids (which are large and oval cells, their cytoplasm contains agglomerates of microtubules) (32) are the main producers of prophenoloxidase. Prophenoloxidase (ProPO) participates in melanization (25, 33). Therefore, haemocytes play an important role in clearing microbes from the insects’ haemolymph by forming melanotic capsules (29). Recently, the activity of the phenoloxidase/melanization responses was investigated in Drosophila; it was found that injection of Zika virus into wild-type adult flies caused melanin formation at the injection site by increasing phenoloxidase activity in the hemolymph (34).
Nodulation
The nodulation process involves coordinated adherence of haemocytes in order to enclose large clusters of pathogens followed by melanization (25). In the case of bacterial infections, several haemocytes attach to the aggregates of microbes. This helps in the removal of a considerable number of bacteria from insects’ haemolymph through phagocytosis (11). Thus, haemocytes bind together to form a capsule around the pathogen (25) and nodulation is accomplished by the stimulation of prophenoloxidase and mature nodules’ melanization (11).
Lysis
Lysis is immune-based disruption of the cellular membrane to kill the pathogens. This mechanism is difficult to observe because an immune-based reduction in infection intensity leads to pathogen death that is not an easily visible immune phenotype as compared to phagocytosis, encapsulation, nodulation, and melanization where pathogens can be seen inside haemocytes. Factors such as AMPs induce pathogen death through lysis (25). The AMPs include defensin and defensin-like peptides, cecropin and cecropin-like peptides, attacins and gloverins, and lebocins mostly detected in Diptera, Coleoptera and Lepidoptera and act against bacteria and fungi. Immune signaling pathways govern the production of AMPs. For example, in D. melanogaster, activation of the Toll pathway induces the transcription of drosomycin while activation of the Imd pathway induces the transcription of diptericin (35). Lysozymes are also involved in lytic activity. ROS and RNS (reactive nitrogen intermediates) affect lytic activity in the extracellular environment. Furthermore, reactive species are also involved in the antimicrobial response in the haemocoel (35, 36).
RNA interference
RNAi is a process in which RNA molecules inhibit gene expression or translation by neutralizing targeted mRNA molecules; it is considered an ancient gene silencing pathway connected to antiviral defense (37). Small RNA-guided antiviral immunity was first revealed in plants, and subsequently, in fruit flies (D. melanogaster) and round worms (Caenorhabditis elegans) (37). By studying the function of insect genes, RNAi could be used for insect pest management (38). Among various defenses, activation of the RNAi pathway is the key antiviral mechanism in mosquitoes that leads to viral RNA degradation and replication inhibition (8, 39). The production of small RNAs from long viral double-stranded RNA (dsRNA) has a major role in the RNAi pathway and small RNAs include small interfering RNAs (siRNAs), microRNAs (miRNAs), and PIWI-interacting RNAs (piRNAs) (40). In the small interfering RNA (siRNA) pathway, a ribonuclease, Dicer-2 (Dcr2) cleaves the viral double-stranded RNA (dsRNA) to generate viral siRNAs (41). siRNA leads the Argonaute-2 (Ago-2) protein to target viral RNAs to initiate degradation (41). Ago-2 is a slicer protein and slicer activity is crucial for an effective RNAi response. Furthermore, virus replication is negatively controlled by the transcription of Vago, a cysteine-rich polypeptide that is activated by the binding of viral RNA to Dcr2 (42). In mosquitoes, the PIWI-interacting RNA pathway (piRNA) is also used in antiviral response in addition to the siRNA pathway (39, 43). piRNAs are small non-coding RNAs that interact with proteins, for instance, PIWI, Ago-3, and Aubergine (Aub) to form the piRNA-induced silencing complex (piRISC) and regulate RNA silencing (44). However, piRNA biogenesis seems to vary considerably between germline and somatic cells. Virus-derived piRNAs have been shown to be produced in whole Aedes mosquitoes upon infection with chikungunya and dengue virus (43).
Apoptosis
Apoptosis, the programmed cell death, is characterized by distinct morphological features and energy-dependent biochemical mechanisms which is essential for proper development and functioning of the immune system and normal cell turnover (45). A range of cellular anti-viral mechanisms exist in insects, including incompatibility of viruses with hosts, apoptosis, and shutdown of protein synthesis (1). For example, certain species of Lepidoptera respond to viral infections by inducing apoptosis in infected cells (46). There are two major apoptotic pathways: the intrinsic pathway that is mediated by mitochondria, and the extrinsic pathway mediated by death receptors [CD95, TRAIL-R1 (TNF-related apoptosis-inducing ligand-R1) or TRAIL-R2] activated by their natural ligands, the TNF family. The caspases (cysteine aspartyl-specific proteases) activate in both pathways which cleave cellular substrates leading to the biochemical/morphological changes that cause cell death and inflammation. These two pathways may be linked and influenced by the molecules of one another (47). In Drosophila, apoptosis occurs following intrinsic pathway activated by intracellular signals such as shutdown of protein or mRNA production, or DNA damage, and involves the formation of large complexes at the mitochondrial membrane in the case of baculovirus (46).
Autophagy
Autophagy is the process employed for degradation of intracellular materials and elimination of intracellular pathogens such as bacteria and viruses (48–50). Bacteria can be eliminated by autophagy in Drosophila. During this process, peptidoglycan recognition protein LE (PGRP-LE) on haemocytes recognizes the bacterial peptidoglycan and induces LC3/Atg8 proteins targeting autophagy to clear the infection of Listeria monocytogenes (51). Furthermore, autophagy is employed in the immune reaction against Rift Valley fever virus (52).
Coagulation
In case of injury, an insect’s cuticle serves as the first line of defense, providing a physical and chemical barrier. The open circulatory system of arthropods has efficient mechanisms that can prevent haemolymph loss in case of injury and also trap microbes before their entry and spreading in the body cavity/hemocoel. Haemolymph clotting is of significance in the innate immune system, which has been studied in arthropods, crayfish and horseshoe crab. In the lepidopteran species, there are four steps in clotting system (28) First, haemocyte degranulation establishes extracellular aggregates that seal the wound and makes a soft clot. Then, initiation of the prophenoloxidase cascade/tranglutaminase (TGase) facilitates crosslinking to form the hard clot. Subsequently, plasmatocytes induce scab formation by spreading and sealing the clot from the haemocoel. Finally, epidermis regenerates, grows and replaces the scab (15).
Arthropod vectors, viruses, and innate immune responses
Mosquitoes
Mosquitoes (Diptera: Culicidae) are the vectors of pathogens that cause dengue, malaria, Japanese encephalitis, and filariasis (53). Out of 3000 documented mosquito species worldwide, more than 100 species are known to transmit infections to humans (54) (Figure 2). Mosquito-borne diseases are prevalent worldwide and infect over 0.7 billion population annually (54). Immune responses in vectors and vaccine strategies have been studied to circumvent the disease outbreaks (55, 56).
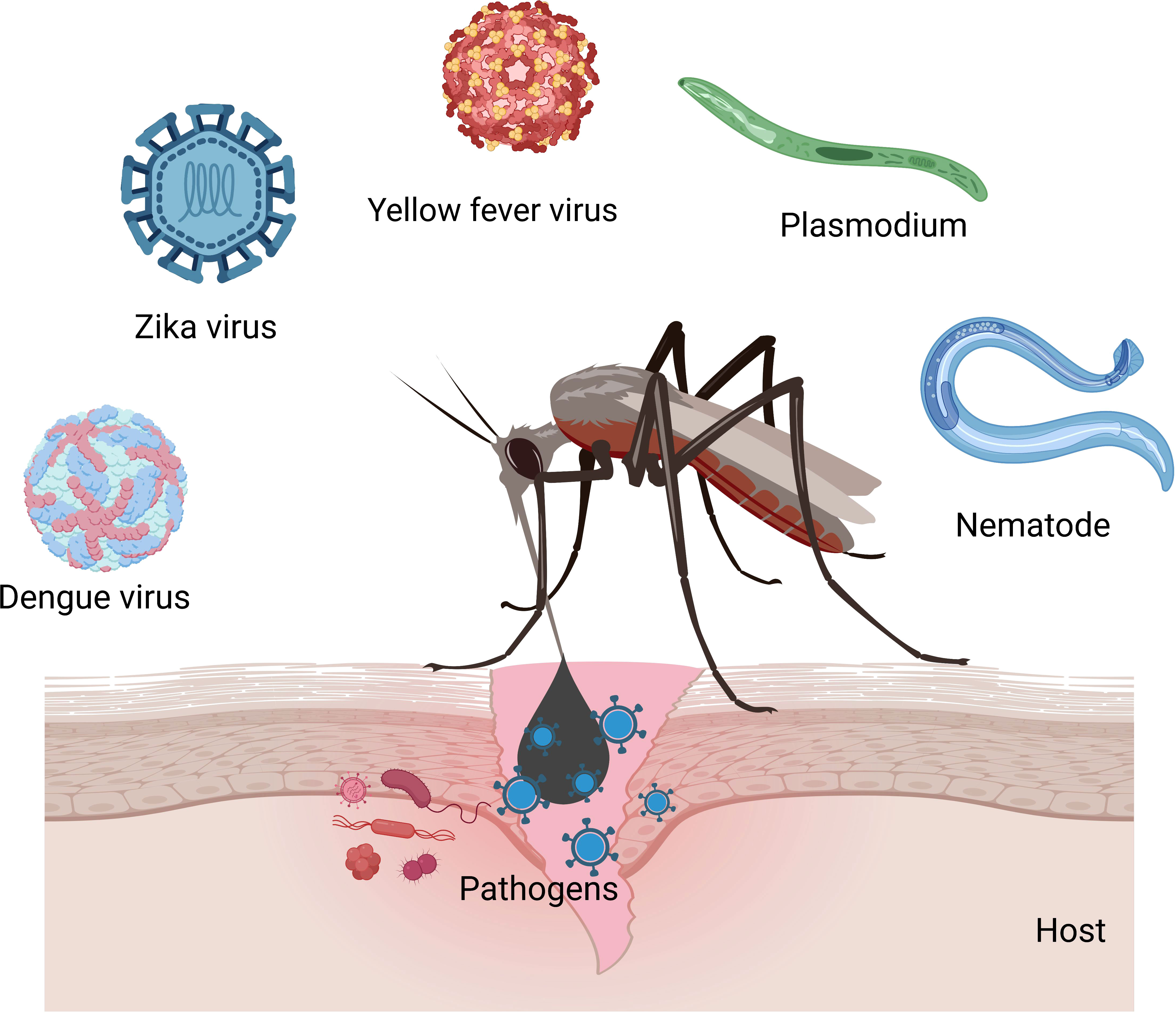
Figure 2 Mosquitoes transmit viruses (Zika, yellow fever, chikungunya, dengue, and West Nile), nematodes (filariasis), and Plasmodium parasites (malaria) in humans.
Most mosquito-borne viruses are RNA viruses, which belong to families Flaviviridae, Togaviridae, and Bunyaviridae (Table 1). Dengue virus is a member of the family Flaviviridae that causes 390 million infections every year (8). Furthermore, other members of the Flaviviridae such as Zika virus, West Nile virus, yellow fever virus, and Japanese encephalitis virus are also a cause of global concern due to increasing incidence and geographic expansion (64). In addition, their circulation poses serious health threats, for example, Zika virus has been linked to Guillain-Barré syndrome in adults and birth defects (microcephaly in prenatally infected infants) (65).
In mosquitoes, virus enters the midgut when it ingests the infected blood meal. The antiviral defense mechanism of mosquitoes is triggered as the virus is recognized by PRRs; however, the dissemination of the virus to the salivary glands is poorly understood (8). It is assumed that when the virus enters midgut epithelium, it replicates in the tissue and subsequently it gets disseminated to the haemocoel (66). The virus may spread through the haemolymph circulation to other tissues/organs including salivary glands, trachea, and neural tissues (21, 67) (Figure 3). The translocation of the virus to the salivary glands is crucial for their transmission to vertebrate hosts (68, 69).
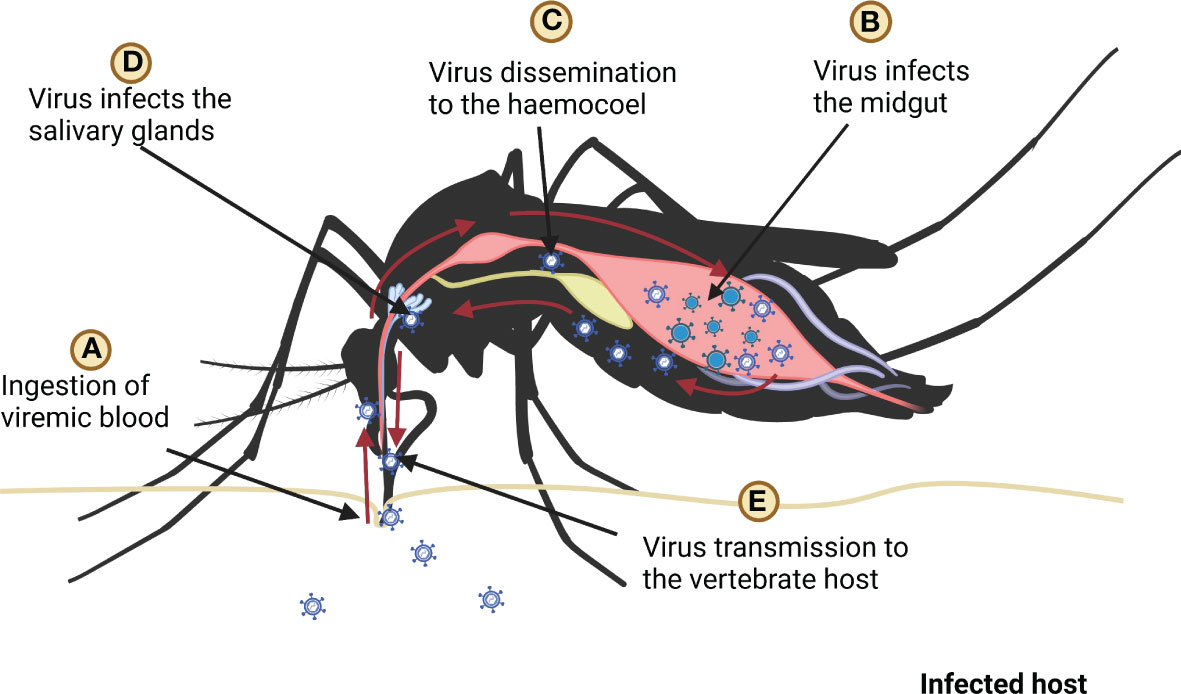
Figure 3 Ingestion, replication, and dissemination of virus in the mosquito, (A) mosquito ingests the blood of infected host, (B) ingested virus infects the midgut (C) after replication in the midgut virus spreads in the haemocoel, (D) after dissemination virus infects salivary glands (E) finally mosquito transmits virus to host by biting it.
In mosquitoes, during viral infection, PRRs recognize virus-conserved PAMPs to instigate immune responses. Degradation and thereby inhibition of viral replication occurs through activation of the RNAi pathway which is the main antiviral mechanism (70). Despite antiviral defense mechanisms that hinder/limit viral replication, the immune system of the mosquito may not be able to effectively clear the virus (8, 71). Without significant lethal effects of viral infection to hosts (mosquitoes), mosquitoes tolerate/resist long-lasting infections that make them efficient vectors for the emergence or re-emergence of viral diseases. Therefore, to conceptualize anti-vectorial strategies, understanding the mechanisms of mosquito tolerance/resistance to viral infection is vital. Furthermore, we can develop novel anti-vector strategies such as use of genetically modified mosquitoes (72–76) and Wolbachia-infected mosquitoes (77–80) for resistance to viral pathogens to prevent human infections through understanding of the innate immune responses of mosquitoes (8). In 2021, in United States, first genetically engineered mosquitoes were released in a field trial after many years of fight for regulatory approval and its public acceptance for controlling populations of wild mosquitoes (Aedes aegypti), which carry viruses such as chikungunya, dengue, yellow fever, and Zika (81). Aedes aegypti was transinfected with the endosymbiont Wolbachia pipientis as a vector control strategy for dengue virus transmission. The Wolbachia infected mosquitoes showed a decreased biting success due to bendy proboscis (78), therefore limit virus transmission in populations. Furthermore, Wolbachia-harboring mosquitoes could reduce Zika virus transmission and can be effective vector control strategy (79).
Ticks
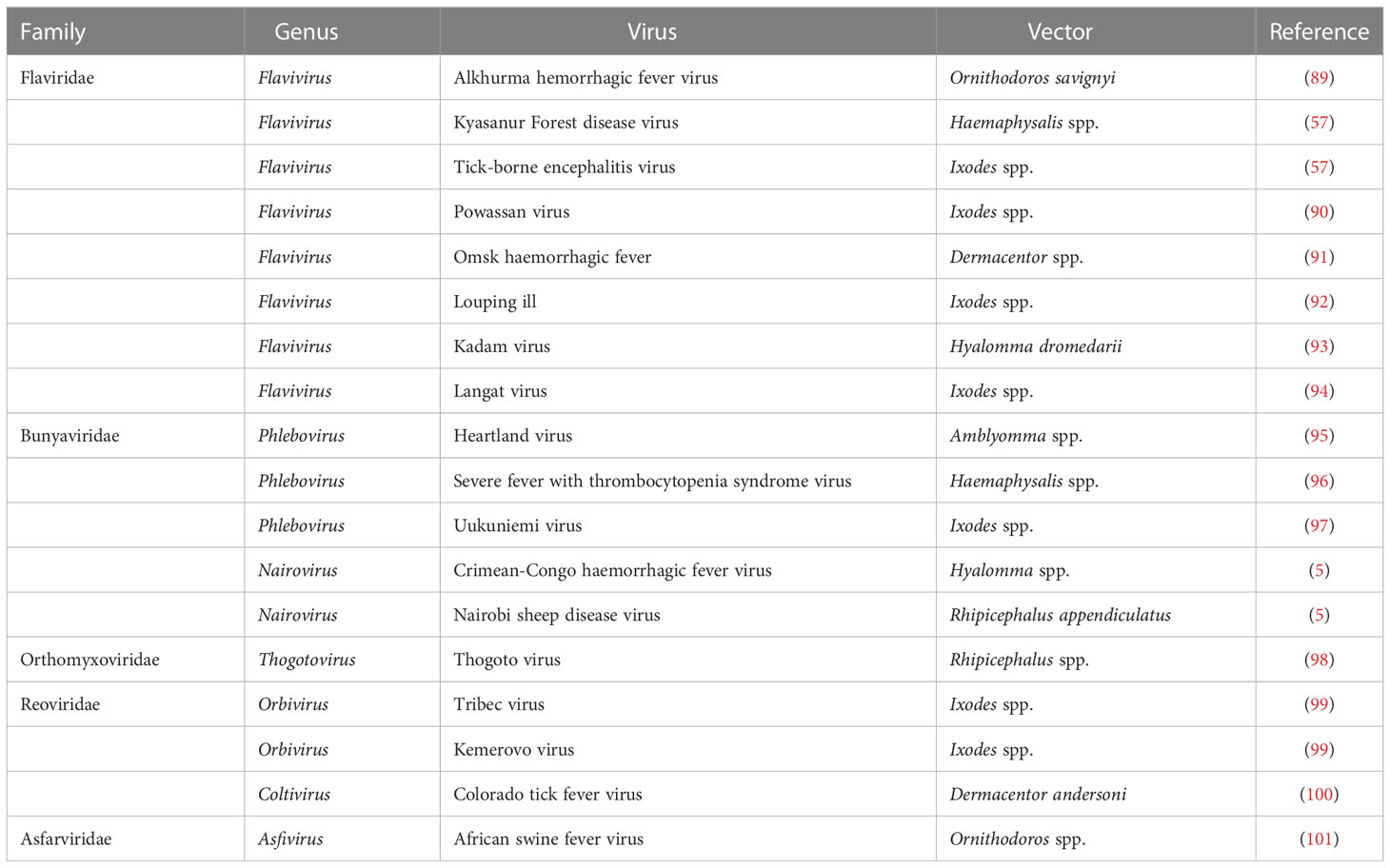
Ticks are responsible for the transmission of a variety of pathogens that cause diseases in humans and animals (82–84), including various arboviruses (85–87). However, the majority of human viral diseases transmitted by tick vectors are caused by flaviviruses, while other tick-borne viruses belong to other families such as Asfarviridae, Bunyaviridae, Flaviridae, Orthomyxoviridae, and Reoviridae (88) (Table 2). Tick-borne viruses are mostly RNA viruses that replicate in ticks as well as in vertebrate cells (86); however, antiviral innate immune mechanism is yet to be explored.
After mosquitoes, ticks are the second most important arthropod vectors (102), feeding on the blood of various vertebrate hosts including birds, mammals, amphibians and reptiles (103). Tick feeding can cause anemia in vertebrate hosts as a single adult female hard tick is able to consume more than 1 mL of blood (104), thereby adversely impacting on livestock health and productivity.
Tick feeding can also result in virus transmission. In the natural ecosystem, ticks become infected during viremic and non-viremic transmissions. In the case of viremic transmission, ticks become infected by feeding on the viremic vertebrate, whereas non-viremic transmission occurs when virus from an infected tick is transferred to an uninfected tick during co-feeding in a contained area on the skin of vertebrate host (88). During feeding, ticks secrete salivary molecules (105) that contain a mixture of proteins, peptides and non-peptide molecules that modulate host hemostasis and immune responses (106). Tick saliva can facilitate the transmission of viruses and other pathogens to the host during blood feeding by modulating host immune response (107). However, how pathogens evade different protective pathways and persist in the vector need to be explored. Tick-borne pathogens such as Anaplasma, Borrelia burgdorferi sensu lato, Ehrlichia, Francisella, and relapsing fever spirochetes have evolved various immune evasion strategies such as altering surface components, complement inhibition, antimicrobial molecule blocking, and inhibiting cytokines (108). Several tick-borne microbes modulate their outer-surface constituents via differential expression of various surface proteins through transcriptional regulation and intragenic recombination. Therefore, alteration of surface antigens allows microorganisms, for example, B. burgdorferi and relapsing fever spirochetes, to evade neutralizing host antibody response and encourage persistent infections in animals (109, 110). Surface components of bacteria may serve as PAMPs through recognition by TLRs (Toll-like receptors). Certain pathogens, for example Francisella tularensis mask their surfaces (to evade immune sensing) by synthesizing a carbohydrate-based capsule that inhibits the antibody and complement deposition on the cell wall, and provide protection against microbicidal host responses (for instance, opsonization) (111). Some bacteria, for example, B. burgdorferi and Anaplasma use host lipids (as a building block for the biogenesis of their membranes) that helps in bypassing host immune responses by avoiding the immune cells (108, 112). The cytokines play crucial roles in the integration of the innate and adaptive immune responses that are important for host defense against pathogens; various tick-borne microbes inhibit or enhance cytokine expression (108). For example, Anaplasma phagocytophilum infection leads to disruption of the IFN-γ (interferon-gamma) signaling pathways and downstream phagocytosis events by neutrophils (113). Ehrlichia chaffeensis may change early immune responses by inhibiting transcription of interleukin (IL)-12, IL-15, and IL-18 genes that allow Ehrlichia survival in macrophages (114). Borrelia burgdorferi or A. phagocytophilum infections trigger ticks (Ixodes) JAK/STAT or IMD pathways that stimulate a robust microbicidal response (115, 116). Anaplasma phagocytophilum activates the expression of tick antifreeze glycoprotein (IAFGP), which enhances the cold tolerance and survival of Ixode scapularis that eventually assist in persistence of pathogen in nature (117). Furthermore, A. phagocytophilum may modulate expression of salivary gland proteins (such as Salp16 and P11) from I. scapularis that aid its survival (118, 119). A pathogen ingested within the blood meal interacts with the tick gut (Figure 4) (104), colonizes the gut epithelial cells and/or crosses the gut epithelium to enter the haemocoel and may spread through haemolymph circulation to all tissues and organs (104). In haemolymph, complement-like molecules such as C3, C4, and C5 proteins opsonise pathogens that can be phagocytosed by haemocytes (30). Pathogens can also be destroyed by various types of effector molecules including AMPs, complement-like molecules, and factors of redox metabolism. Thereafter, the pathogen reaches the salivary glands to successfully transmit through saliva to the vertebrate host during the next blood feeding. Some pathogens, e.g. bacteria, also have the ability to invade tick ovaries and may transmit to progeny trans-ovarially (Figure 4). In the tick’s salivary glands, ovaries and midgut, pathogens have to deal with resident microorganisms as well as tick immune responses that impact the vector competence. Understanding the immune factors involved in interactions between ticks and tick-borne pathogens is essential to delineate the biology of tick-transmitted diseases and could help to detect targets for developing new strategies to block pathogen transmission (120). The acquisition, development, and transmission of diseases by ticks are explained in Figure 4.

Figure 4 Acquisition, development, and transmission of pathogens by ticks. 1. When ticks bite, pathogens are ingested with the meal. 2. Pathogens either stay until the next meal or move through the gastrointestinal epithelium 3. The extraneous pathogen can potentially harm the tick’s body, depending on the type of pathogen. 4. Pathogens enter the salivary gland and attack the acini through the epithelium. 5,6. Pathogens, along with saliva, are introduced into a new host during feeding, disrupting host homeostasis and setting off inflammatory responses.
To understand host-virus interaction, studies have been performed by injecting virus into D. melanogaster flies (121). This method has been shown to be relevant for identifying pathogen virulence factors and host defense mechanisms; however, injecting the virus amounts to bypassing the hosts’ natural protection barriers. Various studies have revealed that the transmission route used by pathogens has a substantial impact on the intensity of an infection and differential immune responses (122, 123). Drosophila and mosquitoes have a holometabolous life-cycle; they undergo metamorphosis between four life stages: egg, larva, pupa, and imago (adult), whereas ticks exhibit hemimetabolous development with life-cycles consisting of four stages: egg, larva, nymph and adult (103). The pathogen tropism and infection outcome may thus depend on the route of infection of the pathogen and the developmental stages of the arthropod (123, 124).
Conclusion
Presently, treatments are available for many vector-borne viral diseases. However, vaccines are not available for the majority of mosquito- or tick-transmitted viral diseases. There is a focus on diagnosis, treatment and on vector control strategies to help prevent viral disease transmission and spread. In the case of mosquitoes, field tests are being conducted on the utility of genetically modified mosquitoes for control, however, there are concerns regarding the introduction of transgenic organisms into the wild. This approach is new and there is limited knowledge about how mosquito antiviral defense mechanisms may evolve with this strategy. The tick immune system and antiviral defense responses remain poorly understood. It is crucial to know how the mosquito and tick innate immune systems respond to viral infection and replication, and how the viruses are actually transmitted to a healthy mammalian host, which may help in the development of novel strategies to block or control vector-borne virus transmission. A continued integration of the expertise of ecologists, animal virologists, immunologists, entomologists, molecular biologists, geneticists, and public health personnel are required to achieve optimal health outcome for human, animals and the environment, and to eliminate the risk of these vector-borne diseases in the future.
Author contributions
Conceptualization: NP, AW, and UK. Writing—original draft preparation: NP. Writing—review and editing: NP, UK, AW, KM, SM, OS, BG, and NM. Visualization: NP, TZ, UK, and KM. Supervision: AW. Project administration: AW. All authors contributed to the article, read and approved the final manuscript. All authors contributed to the article and approved the submitted version.
Funding
The funding of this study was provided by the UAE University through UPAR Grant # G00003709 to AW.
Acknowledgments
We also appreciate the library facility provided by the United Arab Emirates University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Terenius O. Anti-parasitic and anti-viral immune responses in insects. Ph D Thesis Dep Genet Microbiol Toxicol Stock Univ Stock Sweden (2004). p. 68.
2. Stork NE. How many species of insects and other terrestrial arthropods are there on earth? Annu Rev Entomol (2018) 63:31–45. doi: 10.1146/annurev-ento-020117-043348
3. Martelli F, Zhongyuan Z, Wang J, Wong CO, Karagas NE, Roessner U, et al. Low doses of the neonicotinoid insecticide imidacloprid induce ROS triggering neurological and metabolic impairments in drosophila. Proc Natl Acad Sci U.S.A. (2020) 117:25840–50. doi: 10.1073/pnas.2011828117
4. Baxter RHG, Contet A, Krueger K. Arthropod innate immune systems and vector-borne diseases. Biochemistry (2017) 56:907–18. doi: 10.1021/acs.biochem.6b00870
5. Labuda M, Nuttall PA. Tick-borne viruses. Parasitology (2004) 129:S221–45. doi: 10.1017/S0031182004005220
6. Shi J, Hu Z, Deng F, Shen S. Tick-borne viruses. Virol Sin (2018) 33:21–43. doi: 10.1007/s12250-018-0019-0
7. Paixão ES, Teixeira MG, Rodrigues LC. Zika, chikungunya and dengue: The causes and threats of new and reemerging arboviral diseases. BMJ Glob Heal (2018) 3:1–6. doi: 10.1136/bmjgh-2017-000530
8. Lee WS, Webster JA, Madzokere ET, Stephenson EB, Herrero LJ. Mosquito antiviral defense mechanisms: A delicate balance between innate immunity and persistent viral infection. Parasites Vectors (2019) 12:1–12. doi: 10.1186/s13071-019-3433-8
9. Ribeiro C, Brehélin M. Insect haemocytes: What type of cell is that? J Insect Physiol (2006) 52:417–29. doi: 10.1016/j.jinsphys.2006.01.005
10. Pandey JP, Tiwari RK. An overview of insect hemocyte science and its future application in applied and biomedical fields. Amer J Biochem Mol Biol (2012) 2:82–105. doi: 10.3923/ajbmb.2012.82.105
11. Browne N, Heelan M, Kavanagh K. An analysis of the structural and functional similarities of insect hemocytes and mammalian phagocytes. Virulence (2013) 4:597–603. doi: 10.4161/viru.25906
12. Sapcaliu A, Radoi I, Pavel C, Tudor N, Cauia E, Siceanu A, et al. Research regarding haemocyte profile from apis mellifera carpatica bee haemolymph originated in the south of Romania. Lucr stiinłifice Med Vet (2009) 42(2):393–7.
13. Strand MR. Insect hemocytes and their role in immunity. Insect Immunol (2008), 25–47. doi: 10.1016/B978-0-12-373976-6.50004-5
14. Hanson MA, Hamilton PT, Perlman SJ. Immune genes and divergent antimicrobial peptides in flies of the subgenus drosophila. BMC Evol Biol (2016) 16:1–14. doi: 10.1186/s12862-016-0805-y
15. Lavine MD, Strand MR. Insect hemocytes and their role in immunity. Insect Biochem Mol Biol (2002) 32:1295–309. doi: 10.1016/B978-012373976-6.50004-5
16. Schmidt O, Theopold U, Strand M. Innate immunity and its evasion and suppression by hymenopteran endoparasitoids. BioEssays (2001) 23:344–51. doi: 10.1002/bies.1049
17. Satyavathi VV, Minz A, Nagaraju J. Nodulation: An unexplored cellular defense mechanism in insects. Cell Signal (2014) 26:1753–63. doi: 10.1016/j.cellsig.2014.02.024
18. Nainu F, Tanaka Y, Shiratsuchi A, Nakanishi Y. Protection of insects against viral infection by apoptosis-dependent phagocytosis. J Immunol (2015) 195:5696–706. doi: 10.4049/jimmunol.1500613
19. Tsuzuki S, Matsumoto H, Furihata S, Ryuda M, Tanaka H, Sung EJ, et al. Switching between humoral and cellular immune responses in drosophila is guided by the cytokine GBP. Nat Commun (2014) 5:1–11. doi: 10.1038/ncomms5628
20. Santiago PB, De Araújo CN, Motta FN, Praça YR, Charneau S, Bastos IMD, et al. Proteases of haematophagous arthropod vectors are involved in blood-feeding, yolk formation and immunity - a review. Parasites Vectors (2017) 10:1–20. doi: 10.1186/s13071-017-2005-z
21. Xi Z, Ramirez JL, Dimopoulos G. The aedes aegypti toll pathway controls dengue virus infection. PloS Pathog (2008) 4:1–12. doi: 10.1371/journal.ppat.1000098
22. Weng SC, Li HH, Li JC, Liu WL, Chen CH, Shiao SH. A thioester-containing protein controls dengue virus infection in aedes aegypti through modulating immune response. Front Immunol (2021) 12:670122. doi: 10.3389/fimmu.2021.670122
23. Stokes BA, Yadav S, Shokal U, Smith LC, Eleftherianos I. Bacterial and fungal pattern recognition receptors in homologous innate signaling pathways of insects and mammals. Front Microbiol (2015) 6:19. doi: 10.3389/fmicb.2015.00019
24. Goic B, Stapleford KA, Frangeul L, Doucet AJ, Gausson V, Blanc H, et al. Virus-derived DNA drives mosquito vector tolerance to arboviral infection. Nat Commun (2016) 7:1–10. doi: 10.1038/ncomms12410
25. Hillyer FJ. Insect immunology and hematopoiesis. Dev Comp Immunol 2016 (2016) 58:102–18. doi: 10.1016/j.dci.2015.12.006.Insect
26. Rämet M, Manfruelli P, Pearson A, Mathey-Prevot B, Ezekowitz RAB. Functional genomic analysis of phagocytosis and identification of a drosophila receptor for e. Coli. Nat (2002) 416:644–8. doi: 10.1038/nature735
27. Hillyer JF, Strand MR. Mosquito hemocyte-mediated immune responses. Curr Opin Insect Sci (2014) 3:14–21. doi: 10.1016/j.cois.2014.07.002
28. Jiravanichpaisal P, Lee BL, Söderhäll K. Cell-mediated immunity in arthropods: Hematopoiesis, coagulation, melanization and opsonization. Immunobiology (2006) 211:213–36. doi: 10.1016/j.imbio.2005.10.015
29. Poinar GO, Leutenegger R, Götz P. Ultrastructure of the formation of a melanotic capsule in diabrotica (Coleoptera) in response to a parasitic nematode (Mermithidae). J Ultrasructure Res (1968) 25:293–306. doi: 10.1016/S0022-5320(68)80075-9
30. Shokal U, Eleftherianos I. Evolution and function of thioester-containing proteins and the complement system in the innate immune response. Front Immunol (2017) 8:759. doi: 10.3389/fimmu.2017.00759
31. Christensen BM, Li J, Chen CC, Nappi AJ. Melanization immune responses in mosquito vectors. Trends Parasitol (2005) 21:192–9. doi: 10.1016/j.pt.2005.02.007
32. Eleftherianos I, Heryanto C, Bassal T, Zhang W, Tettamanti G, Mohamed A. Haemocyte-mediated immunity in insects: Cells, processes and associated components in the fight against pathogens and parasites. Immunology (2021) 164:401–32. doi: 10.1111/imm.13390
33. Hillyer JF, Schmidt SL, Christensen BM. Hemocyte-mediated phagocytosis and melanization in the mosquito armigeres subalbatus following immune challenge by bacteria. Cell Tissue Res (2003) 313:117–27. doi: 10.1007/s00441-003-0744-y
34. Harsh S, Tafesh-Edwards G, Eleftherianos I. Zika virus infection triggers the melanization response in drosophila. Biochim Biophys Acta (BBA)-Molecular Basis Dis (2022) 1868:166424. doi: 10.1016/j.bbadis.2022.166424
35. Lemaitre B, Hoffmann J. The host defense of drosophila melanogaster. Annu Rev Immunol (2007) 25:697–743. doi: 10.1146/annurev.immunol.25.022106.141615
36. Ha EM, Oh CT, Bae YS, Lee WJ. A direct role for dual oxidase in drosophila gut immunity. Sci (80- ) (2005) 310:847–50. doi: 10.1126/science.1117311
37. Swevers L, Liu J, Smagghe G. Defense mechanisms against viral infection in drosophila: RNAi and non-RNAi. Viruses (2018) 10:1–35. doi: 10.3390/v10050230
38. Yu N, Christiaens O, Liu J, Niu J, Cappelle K, Caccia S, et al. Delivery of dsRNA for RNAi in insects: An overview and future directions. Insect Sci (2013) 20:4–14. doi: 10.1111/j.1744-7917.2012.01534.x
39. Blair CD, Olson KE. Mosquito immune responses to arbovirus infections. Curr Opin Insect Sci (2014) 3:22–9. doi: 10.1016/j.cois.2014.07.005
40. Ding SW, Voinnet O. Antiviral immunity directed by small RNAs. Cell (2007) 130:413–26. doi: 10.1016/j.cell.2007.07.039
41. Bronkhorst AW, van Rij RP. The long and short of antiviral defense: Small RNA-based immunity in insects. Curr Opin Virol (2014) 7:19–28. doi: 10.1016/j.coviro.2014.03.010
42. Deddouche S, Matt N, Budd A, Mueller S, Kemp C, Galiana-Arnoux D, et al. The DExD/H-box helicase dicer-2 mediates the induction of antiviral activity in drosophila. Nat Immunol (2008) 9:1425–32. doi: 10.1038/ni.1664
43. Rückert C, Bell-Sakyi L, Fazakerley JK, Fragkoudis R. Antiviral responses of arthropod vectors: an update on recent advances. VirusDisease (2014) 25:249–60. doi: 10.1007/s13337-014-0217-9
44. Siomi MC, Sato K, Pezic D, Aravin AA. PIWI−interacting small RNAs: the vanguard of genome defence. Nat Rev Mol Cell Biol (2011) 12:246–58. doi: 10.1038/nrm3089
45. Elmore S. Apoptosis: A review of programmed cell death. Toxicol Pathol (2007) 35:495–516. doi: 10.1080/01926230701320337
46. Clarke TE, Clem RJ. Insect defenses against virus infection: The role of apoptosis. Int Rev Immunol (2003) 22:401–24. doi: 10.1080/08830180305215
47. Igney FH, Krammer PH. Death and anti-death: Tumour resistance to apoptosis. Nat Rev Cancer (2002) 2:277–88. doi: 10.1038/nrc776
48. Yano T, Kurata S. Intracellular recognition of pathogens and autophagy as an innate immune host defence. J Biochem (2011) 150:143–9. doi: 10.1093/jb/mvr083
49. Moy RH, Cherry S. Antimicrobial autophagy: A conserved innate immune response in drosophila. J Innate Immun (2013) 5:444–55. doi: 10.1159/000350326
50. Shibutani ST, Saitoh T, Nowag H, Münz C, Yoshimori T. Autophagy and autophagy-related proteins in the immune system. Nat Immunol (2015) 16:1014–24. doi: 10.1038/ni.3273
51. Yano T, Mita S, Ohmori H, Oshima Y, Fujimoto Y, Ueda R, et al. Autophagic control of listeria through intracellular innate immune recognition in drosophila. Nat Immunol (2008) 9:908–16. doi: 10.1038/ni.1634
52. Moy RH, Gold B, Molleston JM, Schad V, Yanger K, Salzano MV, et al. Antiviral autophagy restricts rift valley fever virus infection and is conserved from flies to mammals. Immunity (2014) 40:51–65. doi: 10.1016/j.immuni.2013.10.020
53. Prudêncio M. In fairness to mosquitoes. Trends Parasitol (2020) 36:876–7. doi: 10.1016/j.pt.2020.08.003
54. Tehri K, Singh N. The role of botanicals as green pesticides in integrated mosquito management – a review. Int J Mosq Res (2015) 2:18–23.
55. Ferluga J, Singh I, Rout S, Al-Qahtani A, Yasmin H, Kishore U. Immune responses in malaria and vaccine strategies. Adv Exp Med Biol (2021) 1313:273–91. doi: 10.1007/978-3-030-67452-6_12
56. Yasmin H, Adhikary A, Al-Ahdal MN, Roy S, Kishore U. Host–pathogen interaction in leishmaniasis: Immune response and vaccination strategies. Immuno (2022) 2:218–54. doi: 10.3390/immuno2010015
57. Gould EA, Solomon T. Pathogenic flaviviruses. Lancet (2008) 371:500–9. doi: 10.1016/S0140-6736(08)60238-X
58. Kramer LD, Li J, Shi PY. West Nile Virus. Lancet Neurol (2007) 6:171–81. doi: 10.1016/S1474-4422(07)70030-3
59. Broom AK, Lindsay MD, Plant AJ, Wright AE, Condon RJ, Mackenzie JS. Epizootic activity ofMurray valley encephalitis virus in an aboriginal community in the southeast Kimberley region of Western Australia: results of cross-sectional and longitudinal serologic studies. Am J Trop Med Hyg (2002) 67:319–23.
60. Day JF. Predicting st. Louis encephalitis virus epidemics: lessons from recent, and not so recent, outbreaks. Annu Rev Entomol (2001) 46:111–38.
61. Hollidge BS, González-Scarano F, Soldan SS. Arboviral encephalitides: Transmission, emergence, and pathogenesis. J Neuroimmune Pharmacol (2010) 5:428–42. doi: 10.1007/s11481-010-9234-7
62. Mathiot CC, Grimaud G, Garry P, Bouquety JC, Mada A, Daguisy AM, et al. An outbreak of human semliki forest virus infections in central African republic. Am J Trop Med Hyg (1990) 42:386–93. doi: 10.4269/ajtmh.1990.42.386
63. Williams MC, Woodall JP, Corbet PS, Gillett JD. O’nyong-nyong fever: An epidemic virus disease in East Africa VIII. virus isolations from anopheles mosquitoes. Trans R Soc Trop Med Hyg (1965) 59:300–6. doi: 10.1016/0035-9203(65)90012-X
64. Trammell CE, Goodman AG. Host factors that control mosquito-borne viral infections in humans and their vector. Viruses (2021) 13:1–16. doi: 10.3390/v13050748
65. Weaver SC, Costa F, Garcia-Blanco MA, Ko AI, Ribeiro GS, Saade G, et al. Zika virus: History, emergence, biology, and prospects for control. Antiviral Res (2016) 130:69–80. doi: 10.1016/j.antiviral.2016.03.010
66. Franz AWE, Kantor AM, Passarelli AL, Clem RJ. Tissue barriers to arbovirus infection in mosquitoes. Viruses (2015) 7:3741–67. doi: 10.3390/v7072795
67. Salazar MI, Richardson JH, Sánchez-Vargas I, Olson KE, Beaty BJ. Dengue virus type 2: Replication and tropisms in orally infected aedes aegypti mosquitoes. BMC Microbiol (2007) 7:1–13. doi: 10.1186/1471-2180-7-9
68. Bennett KE, Flick D, Fleming KH, Jochim R, Beaty BJ, Black WC IV. Quantitative trait loci that control dengue-2 virus dissemination in the mosquito aedes aegypti. Genetics (2005) 170:185–94. doi: 10.1534/genetics.104.035634
69. Chauhan C, Behura SK, deBruyn B, Lovin DD, Harker BW, Gomez-Machorro C, et al. Comparative expression profiles of midgut genes in dengue virus refractory and susceptible aedes aegypti across critical period for virus infection. PloS One (2012) 7:1–13. doi: 10.1371/journal.pone.0047350
70. Wei Y, He YL, Zheng XL. Research progress in RNA interference against the infection of mosquito-borne viruses. Yi Chuan= Hered (2020) 42:153–60. doi: 10.16288/j.yczz.19-262
71. Cheng G, Liu Y, Wang P, Xiao X. Mosquito defense strategies against viral infection. Trends Parasitol (2016) 32:177–86. doi: 10.1016/j.pt.2015.09.009
72. Wilke ABB, de Castro Gomes A, Natal D, Marrelli MT. Control of vector populations using genetically modified mosquitoes. Rev Saude Publica (2009) 43:869–74. doi: 10.1590/S0034-89102009005000050
73. Okorie PN, Marshall JM, Akpa OM, Ademowo OG. Perceptions and recommendations by scientists for a potential release of genetically modified mosquitoes in Nigeria. Malar J (2014) 13:1–8. doi: 10.1186/1475-2875-13-154
74. Touré YT, Oduola AM, Sommerfeld J, Morel CM. Biosafety and risk assessment in the use of genetically modified mosquitoes for disease control. Frontis (2004) 2:217–22.
75. Ramsey JM, Bond JG, Macotela ME, Facchinelli L, Valerio L, Brown DM, et al. A regulatory structure for working with genetically modified mosquitoes: Lessons from Mexico. PloS Negl Trop Dis (2014) 8:1–9. doi: 10.1371/journal.pntd.0002623
76. Marshall JM. The cartagena protocol and genetically modified mosquitoes. Nat Biotechnol (2010) 28:896–7. doi: 10.1038/nbt0910-896
77. Anders KL, Indriani C, Ahmad RA, Tantowijoyo W, Arguni E, Andari B, et al. Update to the AWED (Applying wolbachia to eliminate dengue) trial study protocol: A cluster randomised controlled trial in yogyakarta, Indonesia. Trials (2020) 21:1–16. doi: 10.1186/s13063-020-04367-2
78. Turley AP, Moreira LA, O’Neill SL, McGraw EA. Wolbachia infection reduces blood-feeding success in the dengue fever mosquito, aedes aegypti. PloS Negl Trop Dis (2009) 3:1–6. doi: 10.1371/journal.pntd.0000516
79. Dutra HLC, Rocha MN, Dias FBS, Mansur SB, Caragata EP, Moreira LA. Wolbachia blocks currently circulating zika virus isolates in Brazilian aedes aegypti mosquitoes. Cell Host Microbe (2016) 19:771–4. doi: 10.1016/j.chom.2016.04.021
80. O’Reilly KM, Hendrickx E, Kharisma DD, Wilastonegoro NN, Carrington LB, Elyazar IRF, et al. Estimating the burden of dengue and the impact of release of wMel wolbachia-infected mosquitoes in Indonesia: A modelling study. BMC Med (2019) 17:1–14. doi: 10.1186/s12916-019-1396-4
81. Waltz E. First genetically modified mosquitoes released in the united states. Nature (2021) 593:175–6. doi: 10.1038/d41586-021-01186-6
82. Jongejan F, Uilenberg G. Ticks and control methods. Rev Sci tech Off Int Epiz (1994) 13:1201–26. doi: 10.20506/rst.13.4.818
83. Jongejan F, Uilenberg G. The global importance of ticks. Parasitology (2004) 129:S3–S14. doi: 10.1017/S0031182004005967
84. Perveen N, Muzaffar SB, Al-Deeb MA. Ticks and tick-borne diseases of livestock in the middle East and north Africa: A review. Insects (2021) 12:1–34. doi: 10.3390/insects12010083
85. de la Fuente J, Antunes S, Bonnet S, Cabezas-cruz A. Tick-pathogen interactions and vector Competence : Identification of molecular drivers for tick-borne diseases. Front Cell Infect Microbiol (2017) 7:114. doi: 10.3389/fcimb.2017.00114
86. Kazimírová M, Thangamani S, Bartíková P, Hermance M, Holíková V, Štibrániová I, et al. Tick-borne viruses and biological processes at the tick-Host-Virus interface. Front Cell Infect Microbiol (2017) 7:339. doi: 10.3389/fcimb.2017.00339
87. Talactac MR, Hernandez EP, Hatta T, Yoshii K, Kusakisako K, Tsuji N, et al. The antiviral immunity of ticks against transmitted viral pathogens. Dev Comp Immunol (2021) 119:104012. doi: 10.1016/j.dci.2021.104012
88. Grabowski JM, Offerdahl DK, Bloom ME. The use of ex vivo organ cultures in tick-borne virus research. ACS Infect Dis (2018) 4:247–56. doi: 10.1021/acsinfecdis.7b00274
89. Charrel RN, Fagbo S, Moureau G. Alkhurma hemorrhagic fever virus in ornithodoros savignyi ticks. Emerg Infect Dis (2007) 13:153–5. doi: 10.3201/eid1301.061094
90. Ebel GD. Update on powassan virus: Emergence of a north American tick-borne flavivirus. Annu Rev Entomol (2010) 55:95–110. doi: 10.1146/annurev-ento-112408-085446
91. Růžek D, Yakimenko VV, Karan LS, Tkachev SE. Omsk haemorrhagic fever. Lancet (2010) 376:2104–13. doi: 10.1016/S0140-6736(10)61120-8
92. Jeffries CL, Mansfield KL, Phipps LP, Wakeley PR, Mearns R, Schock A, et al. Louping ill virus: An endemic tick-borne disease of great Britain. J Gen Virol (2014) 95:1005–14. doi: 10.1099/vir.0.062356-0
93. Wood OL, Moussa MI, Hoogstraal H, Büttiker W. Kadam virus (Togaviridae, flavivirus) infecting camel-parasitizing hyalomma dromedarii ticks (Acari: Ixodidae) in Saudi Arabia. J Med Entomol (1982) 19:207–8. doi: 10.1093/jmedent/19.2.207
94. Rumyantsev AA, Murphy BR, Pletnev AG. A tick-borne langat virus mutant that is temperature sensitive and host range restricted in neuroblastoma cells and lacks neuroinvasiveness for immunodeficient mice. J Virol (2006) 80:1427–39. doi: 10.1128/jvi.80.3.1427-1439.2006
95. Savage HM, Godsey MS, Amy L, Panella NA, Burkhalter KL, Harmon JR, et al. First detection of heartland virus (bunyaviridae: Phlebovirus) from field collected arthropods. Am J Trop Med Hyg (2013) 89:445–52. doi: 10.4269/ajtmh.13-0209
96. Yu X-J, Liang M-F, Zhang S-Y, Liu Y, Li J-D, Sun Y-L, et al. Fever with thrombocytopenia associated with a novel bunyavirus in China. N Engl J Med (2011) 364:1523–32. doi: 10.1056/nejmoa1010095
97. Hubálek Z, Rudolf I. Tick-borne viruses in Europe. Parasitol Res (2012) 111:9–36. doi: 10.1007/s00436-012-2910-1
98. Haig DA, Woodall JP, Danskin D. Thogoto virus: a hitherto underscribed agent isolated from ticks in. J Gen Microbiol (1965) 38:389–94. doi: 10.1099/00221287-38-3-389
99. Dilcher M, Hasib L, Lechner M, Wieseke N, Middendorf M, Marz M, et al. Genetic characterization of tribeč virus and kemerovo virus, two tick-transmitted human-pathogenic orbiviruses. Virology (2012) 423:68–76. doi: 10.1016/j.virol.2011.11.020
100. Attoui H, Jaafar FM, De Micco P, De Lamballerie X. Coltiviruses and seadornaviruses in north America, Europe, and Asia. Emerg Infect Dis (2005) 11:1673–9. doi: 10.3201/eid1111.050868
101. Dixon LK, Chapman DAG, Netherton CL, Upton C. African Swine fever virus replication and genomics. Virus Res (2013) 173:3–14. doi: 10.1016/j.virusres.2012.10.020
102. Parola P, Raoult D. Ticks and tickborne bacterial diseases in humans: An emerging infectious threat. Clin Infect Dis (2001) 32:897–928. doi: 10.1086/319347
103. Sonenshine DE, Roe RM. Ticks, people, and animals. In: Biology of ticks volume 1. (2013) New York: Oxford University Press.
104. Fogaça AC, Sousa G, Pavanelo DB, Esteves E, Martins LA, Urbanová V, et al. Tick immune system: What is known, the interconnections, the gaps, and the challenges. Front Immunol (2021) 12:628054. doi: 10.3389/fimmu.2021.628054
105. Bowman AS, Sauer JR. Tick salivary glands: Function, physiology and future. Parasitology (2004) 129:S67–81. doi: 10.1017/S0031182004006468
106. Kotál J, Langhansová H, Lieskovská J, Andersen JF, Francischetti IMB, Chavakis T, et al. Modulation of host immunity by tick saliva. J Proteomics (2015) 128:58–68. doi: 10.1016/j.jprot.2015.07.005
107. Kazimírová M, Štibrániová I. Tick salivary compounds: Their role in modulation of host defences and pathogen transmission. Front Cell Infect Microbiol (2013) 4:43. doi: 10.3389/fcimb.2013.00043
108. Rana VS, Kitsou C, Dumler JS, Pal U. Immune evasion strategies of major tick-transmitted bacterial pathogens. Trends Microbiol (2023) 31(1):62–75. doi: 10.1016/j.tim.2022.08.002
109. Ting Liang F, Jacobs MB, Bowers LC, Philipp MT. An immune evasion mechanism for spirochetal persistence in Lyme borreliosis. J. Exp. Med. (2002) 195(4):415–42. doi: 10.1084/jem.20011870
110. Porcella SF, Raffel SJ, Anderson DE, Gilk SD, Bono JL, Schrumpf ME, et al. Variable tick protein in two genomic groups of the relapsing fever spirochete borrelia hermsii in Western north America. Infect Immun (2005) 73:6647–58. doi: 10.1128/IAI.73.10.6647-6658.2005
111. Freudenberger Catanzaro KC, Inzana TJ. The francisella tularensis polysaccharides: What is the real capsule? Microbiol Mol Biol Rev (2020) 84:1–18. doi: 10.1128/mmbr.00065-19
112. Toledo A, Benach JL. Hijacking and use of host lipids by intracellular pathogens. Virulence Mech Bact Pathog (2016) 3:635–66. doi: 10.1128/9781555819286.ch22
113. Johns JL, MacNamara KC, Walker NJ, Winslow GM, Borjesson DL. Infection with anaplasma phagocytophilum induces multilineage alterations in hematopoietic progenitor cells and peripheral blood cells. Infect Immun (2009) 77:4070–80. doi: 10.1128/IAI.00570-09
114. Moumène A, Meyer DF. Ehrlichia’s molecular tricks to manipulate their host cells. Microbes Infect (2016) 18:172–9. doi: 10.1016/j.micinf.2015.11.001
115. Shaw DK, Wang X, Brown LJ, Chávez ASO, Reif KE, Smith AA, et al. Infection-derived lipids elicit an immune deficiency circuit in arthropods. Nat Commun (2017) 8:1–13. doi: 10.1038/ncomms14401
116. Smith AA, Navasa N, Yang X, Wilder CN, Buyuktanir O, Marques A, et al. Cross-species interferon signaling boosts microbicidal activity within the tick vector. Cell Host Microbe (2016) 20:91–8. doi: 10.1016/j.chom.2016.06.001
117. Abraham NM, Liu L, Jutras BL, Yadav AK, Narasimhan S, Gopalakrishnan V, et al. Pathogen-mediated manipulation of arthropod microbiota to promote infection. Proc Natl Acad Sci U.S.A. (2017) 114:E781–90. doi: 10.1073/pnas.1613422114
118. Sukumaran B, Narasimhan S, Anderson JF, DePonte K, Marcantonio N, Krishnan MN, et al. An ixodes scapularis protein required for survival of anaplasma phagocytophilum in tick salivary glands. J Exp Med (2006) 203:1507–17. doi: 10.1084/jem.20060208
119. Liu L, Narasimhan S, Dai J, Zhang L, Cheng G, Fikrig E. Ixodes scapularis salivary gland protein P11 facilitates migration of anaplasma phagocytophilum from the tick gut to salivary glands. EMBO Rep (2011) 12:1196–203. doi: 10.1038/embor.2011.177
120. Schorderet-Weber S, Noack S, Selzer PM, Kaminsky R. Blocking transmission of vector-borne diseases. Int J Parasitol Drugs Drug Resist (2017) 7:90–109. doi: 10.1016/j.ijpddr.2017.01.004
121. Jennings BH. Drosophila-a versatile model in biology & medicine. Mater Today (2011) 14:190–5. doi: 10.1016/S1369-7021(11)70113-4
122. Gupta V, Vasanthakrishnan RB, Siva-Jothy J, Monteith KM, Brown SP, Vale PF. The route of infection determines wolbachia antibacterial protection in drosophila. Proc R Soc B Biol Sci (2017) 284:1–9. doi: 10.1098/rspb.2017.0809
123. Martins NE, Faria VG, Teixeira L, Magalhães S, Sucena É. Host adaptation is contingent upon the infection route taken by pathogens. PloS Pathog (2013) 9:1–8. doi: 10.1371/journal.ppat.1003601
Keywords: immune system, innate immunity, virus circulation, haemocoel, haemocytes, antiviral defense
Citation: Perveen N, Muhammad K, Muzaffar SB, Zaheer T, Munawar N, Gajic B, Sparagano OA, Kishore U and Willingham AL (2023) Host-pathogen interaction in arthropod vectors: Lessons from viral infections. Front. Immunol. 14:1061899. doi: 10.3389/fimmu.2023.1061899
Received: 05 October 2022; Accepted: 17 January 2023;
Published: 31 January 2023.
Edited by:
Balachandran Ravindran, Institute of Life Sciences (ILS), IndiaReviewed by:
Taruna Madan, National Institute for Research in Reproductive Health (ICMR), IndiaAbhishek Shastri, Central and North West London NHS Foundation Trust, United Kingdom
Copyright © 2023 Perveen, Muhammad, Muzaffar, Zaheer, Munawar, Gajic, Sparagano, Kishore and Willingham. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Arve Lee Willingham, awillingham@uaeu.ac.ae
 Nighat Perveen
Nighat Perveen Khalid Muhammad
Khalid Muhammad Sabir Bin Muzaffar
Sabir Bin Muzaffar Tean Zaheer
Tean Zaheer Nayla Munawar
Nayla Munawar Bojan Gajic2
Bojan Gajic2 Olivier Andre Sparagano
Olivier Andre Sparagano Uday Kishore
Uday Kishore Arve Lee Willingham
Arve Lee Willingham