- Guangdong Provincial Key Laboratory of Autophagy and Major Chronic Non-communicable Diseases, Affiliated Hospital of Guangdong Medical University, Zhanjiang, China
Systemic lupus erythematosus (SLE) is a highly heterogeneous autoimmune disease that primarily affects women. Currently, in the search for the mechanisms of SLE pathogenesis, the association of lifestyle factors such as diet, cigarette smoking, ultraviolet radiation exposure, alcohol and caffeine-rich beverage consumption with SLE susceptibility has been systematically investigated. The cellular and molecular mechanisms mediating lifestyle effects on SLE occurrence, including interactions between genetic risk loci and environment, epigenetic changes, immune dysfunction, hyper-inflammatory response, and cytotoxicity, have been proposed. In the present review of the reports published in reputable peer-reviewed journals and government websites, we consider the current knowledge about the relationships between lifestyle factors and SLE incidence and outline directions of future research in this area. Formulation of practical measures with regard to the lifestyle in the future will benefit SLE patients and may provide potential therapy strategies.
Introduction
Systemic lupus erythematosus (SLE) is a highly heterogeneous autoimmune disease that primarily affects women, especially in the reproductive age. The prevalence rate of SLE worldwide is about 20–70 per 100,000 general population (1, 2). The exact etiology of SLE remains unclear, but genetic risk loci, such as N-acetyltransferase 2 (NAT2) slow acetylator genotype, and environmental factors are crucial in the development of susceptibility to SLE (3, 4). Although many SLE susceptibility genes have been identified recently, gene therapy approaches remain a distant prospect from the point of view of the clinical treatment (5). Furthermore, the significant side effects of high-dose immunosuppressive therapy for SLE, such as osteoporosis, hypertension and infection, have caused much concern (4, 6). Thus, the knowledge of environmental and lifestyle risk factors, especially those that can be controlled, may offer new promising therapeutic strategies for SLE.
Here we review evidence from reports published in reputable peer-reviewed journals and government websites and consider recent advances in our understanding of the links between lifestyle factors with SLE susceptibility and development. In particular, we analyze the effects of the 1) diet including N-3 polyunsaturated fatty acids (N-3 PUFA), N-6 PUFA, calorie restriction, vitamins, as well as 2) other lifestyle factors, including cigarette smoking, ultraviolet radiation exposure, consumption of alcohol and caffeine-rich beverages, etc. Implementation of practical measures with regard to these lifestyle factors will benefit SLE patients and may provide potential therapy strategies.
Diet effects on SLE
N-3 PUFA and N-6 PUFA
In the last thirty years, numerous studies in murine SLE models such as NZBWF1, BXSB/MpJ, and MRL-1pr/1pr mice reported that fish and olive oils containing N-3 PUFA effectively attenuated plasma auto-antibodies, proteinuria, and kidney glomerulonephritis as well as increased lifespan of animals, compared with the phenotypes of mice fed with beef tallow that contained saturated fatty acids, N-6 PUFA, or N-9 monounsaturated fatty acids (N-9 MUFA) (Figure 1) (7–12). Furthermore, an increasing number of human clinical trials demonstrated that consumption of N-3 PUFA had positive effects on autoimmune glomerulonephritis conditions, such as lupus nephritis and others (13–17). Since the earliest clinical trial in 1989, there have been seven major published clinical studies focusing on the relationship between N-3 PUFA and SLE. All but one of the clinical studies reported beneficial effects, including the improvement in endothelial function, disease activity, or inflammatory markers following the implementation of N-3 PUFA in SLE patients (18). A clinical nutritional study of SLE patients found that dietary patterns low in N-3 PUFA and high in carbohydrates positively correlated with the severity of disease activity, adverse serum lipids, and the presence of plaque (19). A double-blind, double placebo-controlled factorial trial in 52 patients with SLE (15) reported a significant decline in SLAM-R score (revised Systemic Lupus Activity Measure) from 6.12 to 4.69 in the subjects receiving eicosapentaenoic acid (EPA)/docosahexaenoic acid (DHA) compared to those on placebo. In the study carried out by Das and colleagues (20), daily oral supplementation of even moderate EPA and DHA (EPA 162 mg, DHA 144 mg) induced prolonged remission of SLE in ten patients. Furthermore, EPA and DHA also suppressed both T-cell proliferation and the production of inflammatory cytokines.
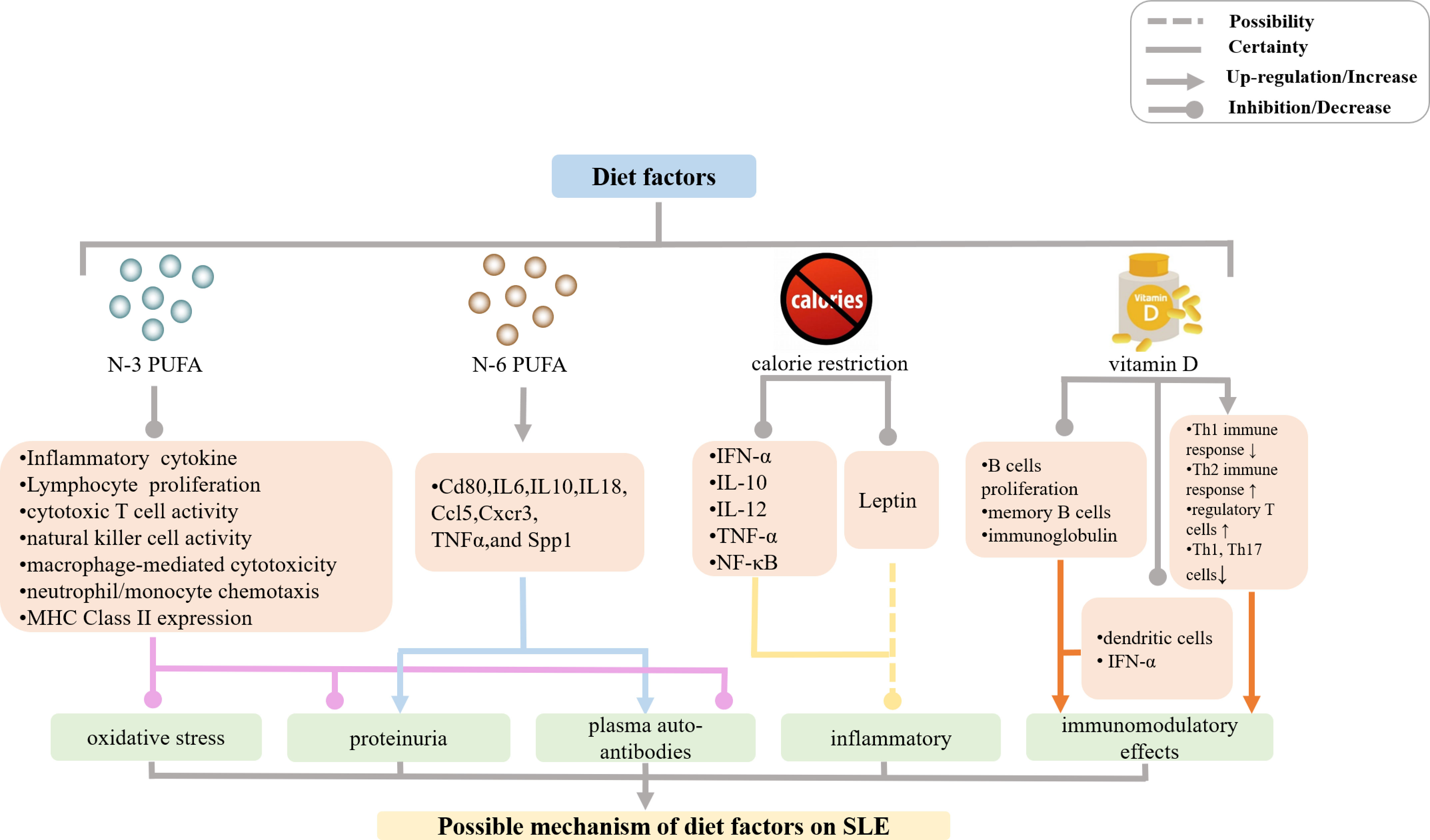
Figure 1 Mechanisms of diet effects on SLE incidence and manifestations. (PUFA, polyunsaturated fatty acids; MHC, major histocompatibility complex; IL, interleukin; IFN, interferon; TNF, tumor necrosis factor; NF, nuclear factor).
Mechanistically, N-3 PUFA prevented inflammatory and autoimmune responses mainly via anti-inflammatory and immune-modulating effects as it suppressed pro-inflammatory cytokine production, lymphocyte proliferation, cytotoxic T cell activity, natural killer cell activity, macrophage-mediated cytotoxicity, neutrophil/monocyte chemotaxis, MHC Class II expression, and antigen presentation (21–38). A large body of experimental evidence has shown that N-3 PUFA decreased plasma levels of interleukin (IL)-1β, IL-6, IL-10, IL-12, IL-18, tumor necrosis factor alpha (TNF-α), transforming growth factor beta 1 (TGF-β1), intercellular adhesion molecule 1 (ICAM-1), and fibronectin. N-3 PUFA increased the production of antioxidant enzymes and down-regulated mRNA expression of CD4+ T cell-associated genes, such as Cd80, Il6, Il10, Il18, Ccl5, Cxcr3, Tnfa, and Spp1, thereby reducing inflammatory response, oxidative stress, and autoimmune reactions in murine SLE models (11, 39–46). In contrast, N-6 PUFA-containing corn oil, safflower oil, and sunflower oil, which all induced the production of plasma auto-antibodies, proteinuria, and glomerulonephritis by increasing mRNA expression levels of the above-mentioned CD4+ T cell-associated genes in the kidney and/or spleen, contributed to the development of autoimmune reactions in NZBWF1 mice (11). The N-6 PUFA precursor was also shown to participate in the inflammatory process in SLE patients in a clinical study (13). However, the precise molecular mechanisms of N-3 PUFA and N-6 PUFA effects in SLE models remain unclear, and further studies are needed to confirm and correctly interpret the results of the published accounts.
Calorie restriction
There have been many studies that examined the association between calorie restriction and autoimmune diseases such as SLE (Figure 1). Calorie restriction has been shown to alleviate SLE manifestations such as proteinuria, glomerulonephritis, and deposition of immune complexes as well as to prolong the lifespan of lupus mouse models by down-regulating mRNA expression of genes encoding the proinflammatory mediators IFN-α, IL-10, IL-12, TNF-α, NF-κB, and polymeric immune globulin receptor (47–52). This, in turn, reduced lymphoproliferation and antibody production, increased antioxidant defense, and decreased the extent of T lymphocyte shift (53–56). It is known that circulating levels of adipokine leptin markedly decrease with calorie restriction (57). Leptin has pro-inflammatory effects and may inhibit regulatory T cells as well as promote autoimmune responses (58–65). Hypoleptinemia and deficient leptin signaling led to the expansion of the population of regulatory T cells in NZB × NZW F1 mice (57), and a reduction in the number of Th17 cells in MRL/Mp-Faslpr mice (66), which contributed to the amelioration of SLE lesions. In addition, caloric restriction was also shown to significantly improve fatigue in subjects with SLE in a clinical study (67).
Vitamin D
A large body of evidence in the last decade has suggested that vitamin D deficiency plays a key role in the development of autoimmune diseases such as SLE. Moreover, the degree of vitamin D deficiency in SLE patients correlates with the severity of SLE manifestations (Figure 1) (68–86). However, a study of a large prospective cohort of women born between 1980 and 2002 indicated that vitamin D consumption did not significantly affect the risk of SLE or rheumatoid arthritis (87). Furthermore, other prospective cohort studies suggested that dietary vitamin D intake during adolescence did not modify SLE risk in adulthood (88). Hiraki et al. suggested the association between dietary vitamin D intake and SLE risk may be misleading, because only 20% of vitamin D comes from food, whereas 80% of vitamin D is generated in the skin following exposure to UVB. Therefore, vitamin D consumption may not accurately reflect the extent of vitamin D deficiency or insufficiency (89). A clinical study conducted in 2017 showed that individuals with vitamin D deficiency are more prone to develop SLE compared with those relatives with SLE (90). In summary, there is a relationship between the degree of vitamin D deficiency or insufficiency and SLE incidence or exacerbation.
Immunomodulatory effects of vitamin D were examined in patients with SLE and it was then shown that 1,25-(OH)2-D3 suppressed the proliferation of activated B cells, decreased the number of memory B cells, and reduced the production of immunoglobulin, which also inhibited the maturation and activation of dendritic cells and reduced the production of IFN-α. In addition, 1,25-(OH)2-D3 also prevented Th1 immune response and simultaneously enhanced Th2 immune response, increased the number of regulatory T cells as well as decreased the numbers of Th1 and Th17 cells. These multiple effects lead to the recovery and maintenance of immune homeostasis, and an overall protective effect in SLE patients (86, 91–104). Although these observations justify the recommendation of vitamin D supplementation in SLE patients, the role of Vitamin D is not fully elucidated (105–107).
Effects of cigarette smoking and consumption of alcohol and caffeine-rich beverages on susceptibility to SLE
Cigarette smoking
Numerous epidemiologic studies revealed that exposure to cigarette smoke is associated with increased risk of SLE (Figure 2) (108–115). Furthermore, strong and consistent evidence suggests that current smoking is more risky than previous smoking (116–122). A study conducted by the Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index that involved 105 patients with SLE with 8.98-year follow-up indicated that smoking exposure may have deleterious effects on lupus morbidity (123). According to a meta-analysis conducted in 2004 that included seven case-control and two cohort studies, there was a modest association between current smoking and risk of SLE, whereas the effect of former smoking was not statistically significant (119). Subsequently, an updated meta-analysis in 2015, which contained 12 published articles encompassing 13 separate studies, found that the odds ratio (OR) values for SLE of current smokers and ex-smokers were 1.56 and 1.23, respectively, compared with the probability of SLE in nonsmokers (121). Recent research focused on cigarette smoking affecting clinical manifestations of patients with SLE has indicated that cigarette smoking was associated with photosensitivity, cutaneous damage, active SLE rash (124–127), higher SLE Disease Activity Index (SLEDAI) score (128), pleuritis, peritonitis, metabolic syndrome (129), neuropsychiatric symptoms (130, 131), vascular necrosis (132), thrombotic events (133–136), cardiovascular disease (137), peripheral vascular disease (138, 139), and production of anti-phospholipid antibodies (136). Moreover, smoking lowers the efficacy of medicines used to treat SLE (3, 140, 141). Likewise, a prospective cross-sectional study of Chinese SLE patients performed in 2015 reported that cigarette smoking causes the development and worsening of symptoms in SLE patients, including photosensitivity, nephropathy, proteinuria, compared with those in nonsmokers (after adjustment for age and gender), whereas SLEDAI scores were not significantly different in smokers and non-smokers (142). Taken together, these studies indicated that smoking is associated with increased risk for the development of SLE.
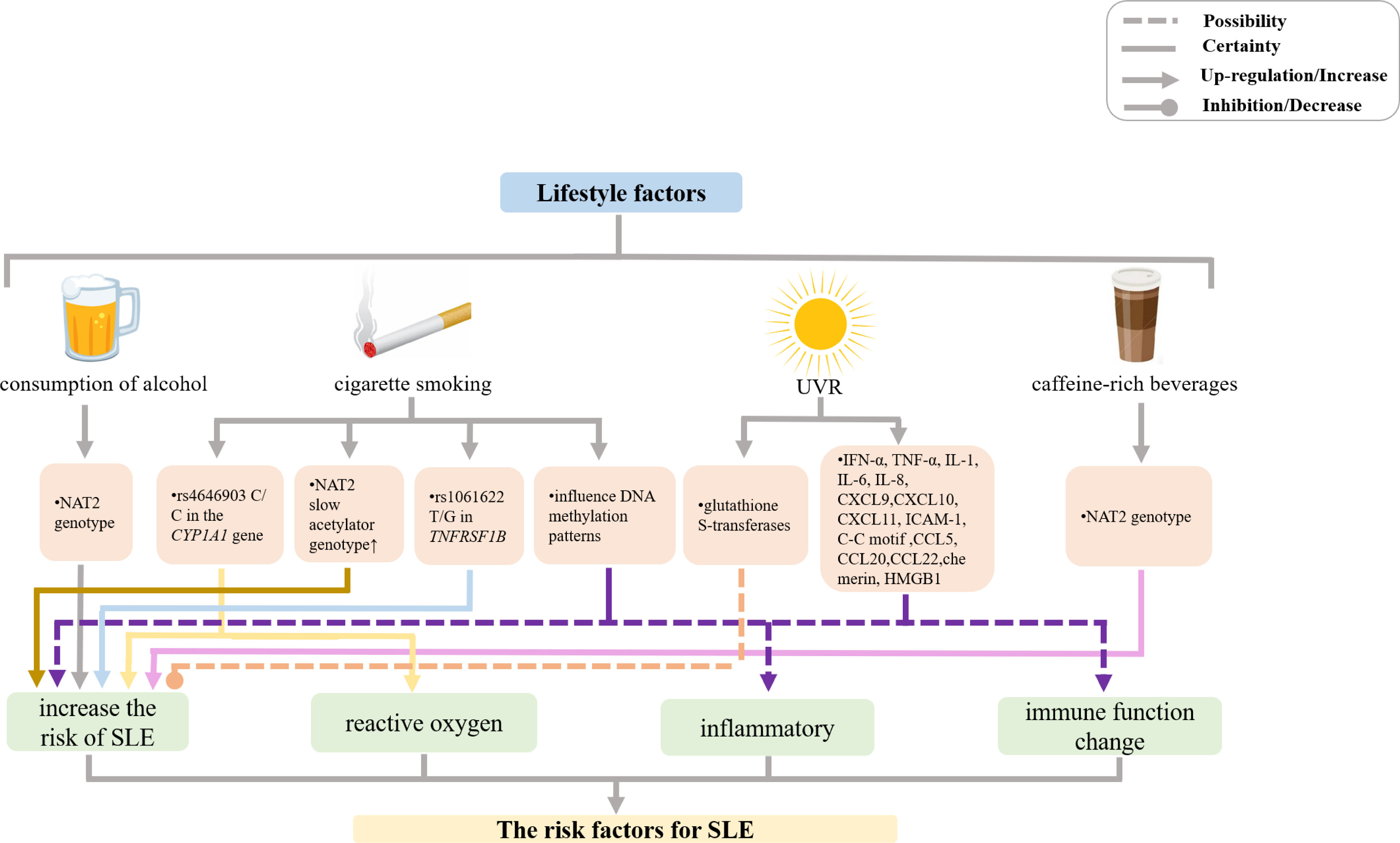
Figure 2 Mechanisms of other lifestyle factors effects on SLE incidence and manifestations. (UVR, ultraviolet radiation; NAT, N-acetyltransferase; IFN, interferon; TNF, tumor necrosis factor; IL, interleukin; CXCL, chemokine (C-X-C motif) ligand; CCL, chemokine (C-C motif) ligand; ICAM, intercellular adhesion molecule; HMG, high-mobility group protein).
The mechanism whereby smoking affects SLE pathogenesis remains unclear. In recent years, several new lines of evidence have suggested that the effect of smoking in SLE may be modulated by gene polymorphisms and epigenetic changes. The studies of Japanese population by Kiyohara et al. showed that smokers with the N-acetyltransferase 2 (NAT2) slow acetylator genotype were at a significantly higher risk of SLE (OR 2.34, 95% CI 1.21–4.52) compared with nonsmokers carrying the rapid acetylator genotype (143). Moreover, Kiyohara et al. also demonstrated that smokers with rs1061622 T/G in TNFRSF1B that confers an increased risk for SLE (OR 1.56, 95% CI 0.99–2.47) had 49% of the excess risk for SLE resulting from the gene-environment interactions. In addition, although a significant association between the TT genotype of STAT4 rs7574865 and increased risk of SLE (OR 2.21, 95% CI 1.10–4.68) was found in that study, there was no significant interaction between STAT4 polymorphisms and smoking (144). Further, smokers carrying rs4646903 C/C in the CYP1A1 gene that encodes a monooxygenase that generates various reactive oxygen species were also at a significantly increased risk of SLE (OR 9.72, 95% CI 2.73–34.6), as the presence of rs4646903 contributed over 60% excess risk of SLE (145). Therefore, several gene polymorphism-smoking interactions increase the risk of SLE. In addition, cigarette smoking, as a lifestyle factor, may influence DNA methylation patterns and thereby change the expression levels of disease-relevant genes (146–151). In a genome-wide DNA methylation analysis of peripheral blood mononuclear cells by Dogan et al., it was found that methylation levels of genes implicated in inflammatory and immune function pathways were altered by cigarette smoking, which could consequently cause complex illnesses with inflammatory components (152). Notably, there are indications that DNA methylation state may repair after the cessation of cigarette smoking (153, 154). However, much more remains to be done with respect to the elucidation of the interactions between gene polymorphisms and epigenetic changes on the one hand and smoking on the other hand.
Ultraviolet radiation
Ultraviolet radiation (UVR) is an important environmental factor inducing SLE, as demonstrated in various studies of human populations and experimental studies (155) (Figure 2). It plays a crucial role in the pathogenesis of lupus by inducing a proinflammatory environment and leading to abnormal long-lasting photoreactivity via inflammatory mediators, such as proinflammatory cytokines, chemokines, and adhesion molecules. UVR exposure upregulates proinflammatory cytokines expression, such as IFN-α, IL-1, IL-6, and TNF-α (156). IFNs increase the expression of proinflammatory chemokines, including chemokine (C-XC motif) ligand (CXCL) 9, CXCL10, and CXCL11, which recruit chemokine (C-X-C motif) receptor 3 effector cells and induce keratinocyte apoptosis (157).
UVR also upregulates intracellular adhesion molecules, such as intercellular adhesion molecule 1 (ICAM-1) and lymphocyte function-associated antigen 1, and increases the secretion of chemokines, including IL-8, chemokine (C-C motif) ligand (CCL) 5, CCL20, CCL22, and chemerin, which are important for recruiting immune cells to areas of inflammation (158, 159).
In addition, one study revealed that UVR exposure induced high-mobility group protein B1 (HMGB1) release, which is related to the number of apoptotic cells in patients with SLE. HMGB1 released from apoptotic keratinocytes exerts inflammatory effects through binding to its receptors, resulting in the development of inflammatory lesions in the skin of patients with SLE upon UVR exposure (160).
If UVR is a trigger for SLE onset, glutathione S-transferases (GSTs, detoxification enzymes that protect cells from attack by reactive electrophiles that are produced by certain stressors, such as infection) may play a key role (3). The isoenzyme Mu of GST (GSTM1) is dominantly inherited. A population-based case-control study reported a threefold increased risk of SLE associated with 24 or more months of occupational sun exposure among Caucasian participants with the null GST Mu 1 (GSTM1) genotype (which leads to decreased activity of the GST enzyme). No effect of occupational sun exposure (on SLE risk) was seen in participants with the positive genotype (i.e., with the full activity of the GST enzyme) (161). However, more mechanisms of UVR affecting SLE disease progression need to be discovered and explored.
Consumption of alcohol and caffeine-rich beverages
Previously, epidemiological studies showed that there was no significant association between alcohol consumption and SLE (110, 162–166). However, in the last several decades, several studies have consistently suggested that moderate alcohol consumption was negatively associated with the risk of SLE, irrespective of the type of alcoholic beverage (3, 112, 115, 167, 168). A meta-analysis of six case-control studies and one cohort study published in 2008 revealed that moderate alcohol consumption likely has a protective effect against the development of SLE (169). Furthermore, a case-control study from Japan suggested that consumption of black tea (OR = 1.88, 95% CI 1.03–3.41) and coffee (OR = 1.57, 95% CI 0.95–2.61) increased the risk of SLE (Figure 2) (170). Gene-environment interactions may be implicated in the mechanisms responsible for protective effects of alcohol consumption and SLE-aggravating action of caffeine-rich beverages. Kiyohara et al. showed that NAT2 genotype significantly affected the association between SLE risk on the one hand and alcohol and black tea consumption on the other hand (170). Another study that enrolled 505 patients with SLE from the Korean Lupus Network (KORNET) SLE registry between January 2014 and January 2016 showed that current alcohol consumption likely influenced the development of cutaneous damage in patients with SLE (166).
In conclusion, the available evidence reflects that cigarette smoking, caffeine-rich beverages, and UVR may promote the progression of SLE, while alcohol consumption is controversial and needs more research.
Future directions
Modifying lifestyle risk factors could be the basis of potential preventative measures or therapy for SLE in the future. Insights into cellular and molecular mechanisms of negative and positive effects of lifestyle preferences on SLE incidence and manifestations are still being researched. These mechanisms involve gene-environment interactions, epigenetic changes, immune dysfunction, hyper-inflammatory response, cytotoxicity, and others. Practical measures with regard to these lifestyle choices in the future will benefit SLE patients and may provide potential therapy strategies.
Author contributions
JC and SL wrote the manuscript and designed the figures. WP, FG, LY, H-FL, and QP revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China (no. 82070757, 81471530), the Department of established positions for the Zhujiang Scholar from Guangdong Medical University, and Guangdong Basic and Applied Basic Research Foundation (no. 2019A1515012203).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Barber MRW, Drenkard C, Falasinnu T, Hoi A, Mak A, Kow NY, et al. Global epidemiology of systemic lupus erythematosus. Nat Rev Rheumatol (2021) 17:515–32. doi: 10.1038/s41584-021-00668-1
2. Danchenko N, Satia JA, Anthony MS. Epidemiology of systemic lupus erythematosus: a comparison of worldwide disease burden. Lupus (2006) 15:308–18. doi: 10.1191/0961203306lu2305xx
3. Woo JMP, Parks CG, Jacobsen S, Costenbader KH, Bernatsky S. The role of environmental exposures and gene-environment interactions in the etiology of systemic lupus erythematous. J Internal Med (2022) 291:755–78. doi: 10.1111/joim.13448
4. Felten R, Scher F, Sibilia J, Chasset F, Arnaud L. Advances in the treatment of systemic lupus erythematosus: From back to the future, to the future and beyond. Joint Bone Spine (2019) 86:429–36. doi: 10.1016/j.jbspin.2018.09.004
5. Chen J, Liao S, Zhou H, Yang L, Guo F, Chen S, et al. Humanized mouse models of systemic lupus erythematosus: Opportunities and challenges. Front Immunol (2021) 12:816956. doi: 10.3389/fimmu.2021.816956
6. Albrecht K, Redeker I, Aringer M, Marschall U, Strangfeld A, Callhoff J. Comorbidity and healthcare utilisation in persons with incident systemic lupus erythematosus followed for 3 years after diagnosis: analysis of a claims data cohort. Lupus Sci Med (2021) 8(1):e000526. doi: 10.1136/lupus-2021-000526
7. Prickett JD, Robinson DR, Steinberg AD. Effects of dietary enrichment with eicosapentaenoic acid upon autoimmune nephritis in female NZBxNZW/F1 mice. Arthritis Rheumatol (1983) 26:133–9. doi: 10.1002/art.1780260203
8. Robinson DR, Prickett JD, Polisson R, Steinberg AD, Levine L. The protective effect of dietary fish oil on murine lupus. Prostaglandins (1985) 30:51–75. doi: 10.1016/S0090-6980(85)80010-1
9. Robinson DR, Prickett JD, Makoul GT, Steinberg AD, Colvin RB. Dietary fish oil reduces progression of established renal disease in (NZB× NZW) F1 mice and delays renal disease in BXSB and MRL/1 strains. Arthritis Rheumatol (1986) 29:539–46. doi: 10.1002/art.1780290412
10. Pestka JJ. N-3 polyunsaturated fatty acids and autoimmune-mediated glomerulonephritis. prostaglandins. Leukotrienes Essential Fatty Acids (PLEFA) (2010) 82:251–8. doi: 10.1016/j.plefa.2010.02.013
11. Pestka JJ, Vines LL, Bates MA, He K, Langohr I. Comparative effects of n-3, n-6 and n-9 unsaturated fatty acid-rich diet consumption on lupus nephritis, autoantibody production and CD4+ T cell-related gene responses in the autoimmune NZBWF1 mouse. PLoS One (2014) 9(6):e100255. doi: 10.1371/journal.pone.0100255
12. Aparicio-Soto M, Sanchez-Hidalgo M, Cardeno A, Rosillo MA, Sanchez-Fidalgo S, Utrilla J, et al. Dietary extra virgin olive oil attenuates kidney injury in pristane-induced SLE model via activation of HO-1/Nrf-2 antioxidant pathway and suppression of JAK/STAT, NF-kappaB and MAPK activation. J Nutr Biochem (2016) 27:278–88. doi: 10.1016/j.jnutbio.2015.09.017
13. Gorczyca D, Szponar B, Paściak M, Czajkowska A, Szmyrka M. Serum levels of n-3 and n-6 polyunsaturated fatty acids in patients with systemic lupus erythematosus and their association with disease activity: a pilot study. Scandinavian J Rheumatol (2022) 51:230–6. doi: 10.1080/03009742.2021.1923183
14. Walton AJ, Snaith ML, Locniskar M, Cumberland AG, Morrow WJ, Isenberg DA. Dietary fish oil and the severity of symptoms in patients with systemic lupus erythematosus. Ann rheumatic Dis (1991) 50:463–6. doi: 10.1136/ard.50.7.463
15. Duffy EM, Meenagh GK, McMillan SA, Strain JJ, Hannigan BM, Bell AL. The clinical effect of dietary supplementation with omega-3 fish oils and/or copper in systemic lupus erythematosus. J Rheumatol (2004) 31:1551–6.
16. Wright SA, O’Prey FM, McHenry MT, Leahey WJ, Devine AB, Duffy EM, et al. A randomised interventional trial of omega-3-polyunsaturated fatty acids on endothelial function and disease activity in systemic lupus erythematosus. Ann rheumatic Dis (2008) 67:841–8. doi: 10.1136/ard.2007.077156
17. Arriens C, Hynan LS, Lerman RH, Karp DR, Mohan C. Placebo-controlled randomized clinical trial of fish oil’s impact on fatigue, quality of life, and disease activity in systemic lupus erythematosus. Nutr J (2015) 14:82. doi: 10.1186/s12937-015-0068-2
18. Li X, Bi X, Wang S, Zhang Z, Li F, Zhao AZ. Therapeutic potential of ω-3 polyunsaturated fatty acids in human autoimmune diseases. Front Immunol (2019) 10:2241. doi: 10.3389/fimmu.2019.02241
19. Elkan AC, Anania C, Gustafsson T, Jogestrand T, Hafström I, Frostegård J. Diet and fatty acid pattern among patients with SLE: associations with disease activity, blood lipids and atherosclerosis. Lupus (2012) 21:1405–11. doi: 10.1177/0961203312458471
20. Das UN. Beneficial effect of eicosapentaenoic and docosahexaenoic acids in the management of systemic lupus erythematosus and its relationship to the cytokine network. Prostaglandins leukotrienes Essential Fatty Acids (1994) 51:207–13. doi: 10.1016/0952-3278(94)90136-8
21. Gil A. Polyunsaturated fatty acids and inflammatory diseases. Biomedicine pharmacotherapy = Biomedecine pharmacotherapie (2002) 56:388–96. doi: 10.1016/s0753-3322(02)00256-1
22. Calder PC. N-3 polyunsaturated fatty acids and inflammation: from molecular biology to the clinic. Lipids (2003) 38:343–52. doi: 10.1007/s11745-003-1068-y
23. Yaqoob P. Fatty acids and the immune system: from basic science to clinical applications. Proc Nutr Soc (2004) 63:89–104. doi: 10.1079/pns2003328
24. Calder PC. N-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr (2006) 83:1505s–19s. doi: 10.1093/ajcn/83.6.1505S
25. Calder PC. The relationship between the fatty acid composition of immune cells and their function. Prostaglandins leukotrienes essential Fatty Acids (2008) 79:101–8. doi: 10.1016/j.plefa.2008.09.016
26. Wall R, Ross RP, Fitzgerald GF, Stanton C. Fatty acids from fish: the anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr Rev (2010) 68:280–9. doi: 10.1111/j.1753-4887.2010.00287.x
27. Calder PC. The 2008 ESPEN sir David cuthbertson lecture: Fatty acids and inflammation–from the membrane to the nucleus and from the laboratory bench to the clinic. Clin Nutr (Edinburgh Scotland) (2010) 29:5–12. doi: 10.1016/j.clnu.2009.11.003
28. Hou TY, McMurray DN, Chapkin RS. Omega-3 fatty acids, lipid rafts, and T cell signaling. Eur J Pharmacol (2016) 785:2–9. doi: 10.1016/j.ejphar.2015.03.091
29. Lorente-Cebrián S, Costa AG, Navas-Carretero S, Zabala M, Laiglesia LM, Martínez JA, et al. An update on the role of omega-3 fatty acids on inflammatory and degenerative diseases. J Physiol Biochem (2015) 71:341–9. doi: 10.1007/s13105-015-0395-y
30. Calder PC. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim Biophys Acta (2015) 1851:469–84. doi: 10.1016/j.bbalip.2014.08.010
31. Crowe W, Allsopp PJ, Nyland JF, Magee PJ, Strain JJ, Doherty LC, et al. Inflammatory response following in vitro exposure to methylmercury with and without n-3 long chain polyunsaturated fatty acids in peripheral blood mononuclear cells from systemic lupus erythematosus patients compared to healthy controls. Toxicol vitro: an Int J published Assoc BIBRA (2018) 52:272–8. doi: 10.1016/j.tiv.2018.05.008
32. Akerele OA, Cheema SK. A diet enriched in longer chain omega-3 fatty acids reduced placental inflammatory cytokines and improved fetal sustainability of C57BL/6 mice. Prostaglandins leukotrienes essential Fatty Acids (2018) 137:43–51. doi: 10.1016/j.plefa.2018.08.002
33. Harvey LD, Yin Y, Attarwala IY, Begum G, Deng J, Yan HQ, et al. Administration of DHA reduces endoplasmic reticulum stress-associated inflammation and alters microglial or macrophage activation in traumatic brain injury. ASN Neuro (2015) 7(6):1759091415618969. doi: 10.1177/1759091415618969
34. Allaire J, Couture P, Leclerc M, Charest A, Marin J, Lépine MC, et al. A randomized, crossover, head-to-head comparison of eicosapentaenoic acid and docosahexaenoic acid supplementation to reduce inflammation markers in men and women: the comparing EPA to DHA (ComparED) study. Am J Clin Nutr (2016) 104:280–7. doi: 10.3945/ajcn.116.131896
35. He J, Neumann D, Kakazu A, Pham TL, Musarrat F, Cortina MS, et al. PEDF plus DHA modulate inflammation and stimulate nerve regeneration after HSV-1 infection. Exp Eye Res (2017) 161:153–62. doi: 10.1016/j.exer.2017.06.015
36. El-Mowafy AM, Katary MM, Pye C, Ibrahim AS, Elmarakby AA. Novel molecular triggers underlie valproate-induced liver injury and its alleviation by the omega-3 fatty acid DHA: role of inflammation and apoptosis. Heliyon (2016) 2:e00130. doi: 10.1016/j.heliyon.2016.e00130
37. Talamonti E, Pauter AM, Asadi A, Fischer AW, Chiurchiù V, Jacobsson A. Impairment of systemic DHA synthesis affects macrophage plasticity and polarization: implications for DHA supplementation during inflammation. Cell Mol Life Sciences: CMLS (2017) 74:2815–26. doi: 10.1007/s00018-017-2498-9
38. Wang P, Xiang K, Xu YY, He YS, Hu YQ, Ni J, et al. Genetically predicted circulating omega-3 fatty acids levels are causally associated with increased risk for systemic lupus erythematosus. Front Nutr (2022) 9:783338. doi: 10.3389/fnut.2022.783338
39. Kelley VE, Ferretti A, Izui S, Strom T. A fish oil diet rich in eicosapentaenoic acid reduces cyclooxygenase metabolites, and suppresses lupus in MRL-lpr mice. J Immunol (1985) 134:1914–9.
40. Chandrasekar B, Fernandes G. Decreased proinflammatory cytokines and increased antioxidant enzyme gene expression by ω-3 lipids in murine lupus nephritis. Biochem Biophys Res Commun (1994) 200:893–8. doi: 10.1006/bbrc.1994.1534
41. Chandrasekar B, Troyer DA, Venkatraman JT, Fernandes G. Dietary omega-3 lipids delay the onset and progression of autoimmune lupus nephritis by inhibiting transforming growth factor β mRNA and protein expression. J Autoimmun (1995) 8:381–93. doi: 10.1006/jaut.1995.0030
42. Venkatraman JT, W-c C. Effects of dietary ω-3 and ω-6 lipids and vitamin e on serum cytokines, lipid mediators and anti-DNA antibodies in a mouse model for rheumatoid arthritis. J Am Coll Nutr (1999) 18:602–13. doi: 10.1080/07315724.1999.10718895
43. Bhattacharya A, Lawrence RA, Krishnan A, Zaman K, Sun D, Fernandes G. Effect of dietary n-3 and n-6 oils with and without food restriction on activity of antioxidant enzymes and lipid peroxidation in livers of cyclophosphamide treated autoimmune-prone NZB/W female mice. J Am Coll Nutr (2003) 22:388–99. doi: 10.1080/07315724.2003.10719322
44. Kim YJ, Kim HJ, No JK, Chung HY, Fernandes G. Anti-inflammatory action of dietary fish oil and calorie restriction. Life Sci (2006) 78:2523–32. doi: 10.1016/j.lfs.2005.10.034
45. Halade GV, Rahman MM, Bhattacharya A, Barnes JL, Chandrasekar B, Fernandes G. Docosahexaenoic acid-enriched fish oil attenuates kidney disease and prolongs median and maximal life span of autoimmune lupus-prone mice. J Immunol (2010) 184:5280–6. doi: 10.4049/jimmunol.0903282
46. Halade GV, Williams PJ, Veigas JM, Barnes JL, Fernandes G. Concentrated fish oil (Lovaza®) extends lifespan and attenuates kidney disease in lupus-prone short-lived (NZBxNZW) F1 mice. Exp Biol Med (2013) 238:610–22. doi: 10.1177/1535370213489485
47. Imoto AM, Gottems LB, Salomon AL, Silva H, Júnior IL, Peccin MS, et al. The impact of a low-calorie, low-glycemic diet on systemic lupus erythematosus: a systematic review. Adv Rheumatol (London England) (2021) 61:66. doi: 10.1186/s42358-021-00224-1
48. Fernandes G, Yunis EJ, Good RA. Influence of diet on survival of mice. Proc Natl Acad Sci USA (1976) 73:1279–83. doi: 10.1073/pnas.73.4.1279
49. Muthukumar AR, Jolly CA, Zaman K, Fernandes G. Calorie restriction decreases proinflammatory cytokines and polymeric ig receptor expression in the submandibular glands of autoimmune prone (NZB x NZW)F1 mice. J Clin Immunol (2000) 20:354–61. doi: 10.1023/a:1006620130114
50. Leiba A, Amital H, Gershwin ME, Shoenfeld Y. Diet and lupus. Lupus (2001) 10:246–8. doi: 10.1191/096120301674681790
51. Jolly CA, Muthukumar A, Avula CP, Troyer D, Fernandes G. Life span is prolonged in food-restricted autoimmune-prone (NZB x NZW)F(1) mice fed a diet enriched with (n-3) fatty acids. J Nutr (2001) 131:2753–60. doi: 10.1093/jn/131.10.2753
52. Kim YJ, Yokozawa T, Chung HY. Effects of energy restriction and fish oil supplementation on renal guanidino levels and antioxidant defences in aged lupus-prone B/W mice. Br J Nutr (2005) 93:835–44. doi: 10.1079/bjn20051440
53. Muthukumar AR, Jolly CA, Zaman K, Fernandes G. Calorie restriction decreases proinflammatory cytokines and polymeric ig receptor expression in the submandibular glands of autoimmune prone (NZB× NZW) F1 mice. J Clin Immunol (2000) 20:354–61. doi: 10.1023/A:1006620130114
54. Jolly CA, Muthukumar A, Avula CR, Troyer D, Fernandes G. Life span is prolonged in food-restricted autoimmune-prone (NZB× NZW) f (1) mice fed a diet enriched with (n-3) fatty acids. J Nutr (2001) 131:2753–60. doi: 10.1093/jn/131.10.2753
55. Jung Kim Y, Yokozawa T, Chung HY. Effects of energy restriction and fish oil supplementation on renal guanidino levels and antioxidant defences in aged lupus-prone B/W mice. Br J Nutr (2005) 93:835–44. doi: 10.1079/BJN20051440
56. Jolly CA. Dietary restriction and immune function. J Nutr (2004) 134(8):1853–6. doi: 10.1093/jn/134.8.1853
57. Liu Y, Yu Y, Matarese G, La Cava A. Cutting edge: fasting-induced hypoleptinemia expands functional regulatory T cells in systemic lupus erythematosus. J Immunol (2012) 188:2070–3. doi: 10.4049/jimmunol.1102835
58. Chen H, Qi J, Liu T, Zou M, Hu Y, Wei J, et al. Leptin accelerates b cell dysfunctions via activating JAK/STAT3/5 and ERK1/2 pathways in patients with systemic lupus erythematosus. Clin Exp Rheumatol (2022). doi: 10.55563/clinexprheumatol/84syjo
59. Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature (1998) 394:897–901. doi: 10.1038/29795
60. Loffreda S, Yang SQ, Lin HZ, Karp CL, Brengman ML, Wang DJ, et al. Leptin regulates proinflammatory immune responses. FASEB journal: Off Publ Fed Am Societies Exp Biol (1998) 12:57–65. doi: 10.1096/fsb2fasebj.12.1.57
61. La Cava A, Matarese G. The weight of leptin in immunity. Nat Rev Immunol (2004) 4:371–9. doi: 10.1038/nri1350
63. Procaccini C, Jirillo E, Matarese G. Leptin as an immunomodulator. Mol aspects Med (2012) 33:35–45. doi: 10.1016/j.mam.2011.10.012
64. Cojocaru M, Cojocaru IM, Siloşi I, Rogoz S. Role of leptin in autoimmune diseases. Maedica (2013) 8:68–74.
65. Procaccini C, Pucino V, Mantzoros CS, Matarese G. Leptin in autoimmune diseases. Metabolism: Clin Exp (2015) 64:92–104. doi: 10.1016/j.metabol.2014.10.014
66. Fujita Y, Fujii T, Mimori T, Sato T, Nakamura T, Iwao H, et al. Deficient leptin signaling ameliorates systemic lupus erythematosus lesions in MRL/Mp-faslpr mice. J Immunol (2014) 192:979–84. doi: 10.4049/jimmunol.1301685
67. Davies RJ, Lomer MC, Yeo SI, Avloniti K, Sangle SR, D’Cruz DP. Weight loss and improvements in fatigue in systemic lupus erythematosus: a controlled trial of a low glycaemic index diet versus a calorie restricted diet in patients treated with corticosteroids. Lupus (2012) 21:649–55. doi: 10.1177/0961203312436854
68. Jiang Z, Pu R, Li N, Chen C, Li J, Dai W, et al. High prevalence of vitamin d deficiency in Asia: A systematic review and meta-analysis. Crit Rev Food Sci Nutr (2021) 16:1–10. doi: 10.1080/10408398.2021.1990850
69. Vanderlinden LA, Bemis EA, Seifert J, Guthridge JM, Young KA, Demoruelle MK, et al. Relationship between a vitamin d genetic risk score and autoantibodies among first-degree relatives of probands with rheumatoid arthritis and systemic lupus erythematosus. Front Immunol (2022) 13:881332. doi: 10.3389/fimmu.2022.881332
70. Kamen DL, Cooper GS, Bouali H, Shaftman SR, Hollis BW, Gilkeson GS. Vitamin d deficiency in systemic lupus erythematosus. Autoimmun Rev (2006) 5:114–7. doi: 10.1016/j.autrev.2005.05.009
71. Borba VZ, Vieira JG, Kasamatsu T, Radominski SC, Sato EI, Lazaretti-Castro M. Vitamin d deficiency in patients with active systemic lupus erythematosus. Osteoporosis international: J established as result cooperation between Eur Foundation Osteoporosis Natl Osteoporosis Foundation USA (2009) 20:427–33. doi: 10.1007/s00198-008-0676-1
72. Toloza SM, Cole DE, Gladman DD, Ibañez D, Urowitz MB. Vitamin d insufficiency in a large female SLE cohort. Lupus (2010) 19:13–9. doi: 10.1177/0961203309345775
73. Amital H, Szekanecz Z, Szücs G, Dankó K, Nagy E, Csépány T, et al. Serum concentrations of 25-OH vitamin d in patients with systemic lupus erythematosus (SLE) are inversely related to disease activity: is it time to routinely supplement patients with SLE with vitamin d? Ann Rheumatic Dis (2010) 69:1155–7. doi: 10.1136/ard.2009.120329
74. Souto M, Coelho A, Guo C, Mendonça L, Argolo S, Papi J, et al. Vitamin d insufficiency in Brazilian patients with SLE: prevalence, associated factors, and relationship with activity. Lupus (2011) 20:1019–26. doi: 10.1177/0961203311401457
75. Hamza RT, Awwad KS, Ali MK, Hamed AI. Reduced serum concentrations of 25-hydroxy vitamin d in Egyptian patients with systemic lupus erythematosus: relation to disease activity. Med Sci monitor: Int Med J Exp Clin Res (2011) 16:1–10. doi: 10.12659/msm.882131
76. Mok CC, Birmingham DJ, Leung HW, Hebert LA, Song H, Rovin BH. Vitamin d levels in Chinese patients with systemic lupus erythematosus: relationship with disease activity, vascular risk factors and atherosclerosis. Rheumatol (Oxford England) (2012) 51:644–52. doi: 10.1093/rheumatology/ker212
77. Petri M, Bello KJ, Fang H, Magder LS. Vitamin d in systemic lupus erythematosus: modest association with disease activity and the urine protein-to-creatinine ratio. Arthritis Rheumatism (2013) 65:1865–71. doi: 10.1002/art.37953
78. de Souza VA, Bastos MG, Fernandes NM, Mansur HN, Raposo NR, de Souza DM, et al. Association of hypovitaminosis d with systemic lupus erythematosus and inflammation. Jornal brasileiro nefrologia: ‘orgao oficial Sociedades Bras e Latino-Americana Nefrologia (2014) 36:430–6. doi: 10.5935/0101-2800.20140062
79. McGhie TK, DeCeulaer K, Walters CA, Soyibo A, Lee MG. Vitamin d levels in Jamaican patients with systemic lupus erythematosus. Lupus (2014) 23:1092–6. doi: 10.1177/0961203314528556
80. Robinson AB, Tangpricha V, Yow E, Gurion R, McComsey GA, Schanberg LE. Vitamin d deficiency is common and associated with increased c-reactive protein in children and young adults with lupus: an atherosclerosis prevention in pediatric lupus erythematosus substudy. Lupus Sci Med (2014) 1:e000011. doi: 10.1136/lupus-2014-000011
81. AlSaleem A, AlE’ed A, AlSaghier A, Al-Mayouf SM. Vitamin d status in children with systemic lupus erythematosus and its association with clinical and laboratory parameters. Clin Rheumatol (2015) 34:81–4. doi: 10.1007/s10067-014-2811-z
82. Mandal M, Tripathy R, Panda AK, Pattanaik SS, Dakua S, Pradhan AK, et al. Vitamin d levels in Indian systemic lupus erythematosus patients: association with disease activity index and interferon alpha. Arthritis Res Ther (2014) 16:R49. doi: 10.1186/ar4479
83. Yap KS, Northcott M, Hoi AB, Morand EF, Nikpour M. Association of low vitamin d with high disease activity in an Australian systemic lupus erythematosus cohort. Lupus Sci Med (2015) 2:e000064. doi: 10.1136/lupus-2014-000064
84. Stagi S, Cavalli L, Bertini F, de Martino M, Cerinic MM, Brandi ML, et al. Vitamin d levels in children, adolescents, and young adults with juvenile-onset systemic lupus erythematosus: a cross-sectional study. Lupus (2014) 23:1059–65. doi: 10.1177/0961203314532564
85. Yap KS, Morand EF, Vitamin D. And systemic lupus erythematosus: continued evolution. Int J rheumatic Dis (2015) 18:242–9. doi: 10.1111/1756-185x.12489
86. Handono K, Marisa D, Kalim H. Association between the low levels of vitamin d and treg function in systemic lupus erythematosus patients. Acta Med Indonesiana (2013) 45:26–31.
87. Costenbader KH, Feskanich D, Holmes M, Karlson E, Benito-Garcia E. Vitamin d intake and risks of systemic lupus erythematosus and rheumatoid arthritis in women. Ann Rheumatol Dis (2008) 67:530–5. doi: 10.1136/ard.2007.072736
88. Hiraki LT, Munger KL, Costenbader KH, Karlson EW. Dietary intake of vitamin d during adolescence and risk of adult-onset systemic lupus erythematosus and rheumatoid arthritis. Arthritis Care Res (2012) 64:1829–36. doi: 10.1002/acr.21776
89. Liao M, Liu G, Feng R, Zhang L. Vitamin d intake cannot represent the extent of vitamin d deficiency or insufficiency: Comment on the article by hiraki et al. Arthritis Care Res (2013) 65:491–1. doi: 10.1002/acr.21840
90. Young KA, Munroe ME, Guthridge JM, Kamen DL, Niewold TB, Gilkeson GS, et al. Combined role of vitamin d status and CYP24A1 in the transition to systemic lupus erythematosus. Ann rheumatic Dis (2017) 76:153–8. doi: 10.1136/annrheumdis-2016-209157
91. Boonstra A, Barrat FJ, Crain C, Heath VL, Savelkoul HF, O’Garra A. 1α, 25-dihydroxyvitamin D3 has a direct effect on naive CD4+ T cells to enhance the development of Th2 cells. J Immunol (2001) 167:4974–80. doi: 10.4049/jimmunol.167.9.4974
92. Ben-Zvi I, Aranow C, Mackay M, Stanevsky A, Kamen DL, Marinescu LM, et al. The impact of vitamin d on dendritic cell function in patients with systemic lupus erythematosus. PloS One (2010) 5:e9193. doi: 10.1371/journal.pone.0009193
93. Baeke F, Takiishi T, Korf H, Gysemans C, Mathieu C, Vitamin D. Modulator of the immune system. Curr Opin Pharmacol (2010) 10:482–96. doi: 10.1016/j.coph.2010.04.001
94. Terrier B, Derian N, Schoindre Y, Chaara W, Geri G, Zahr N, et al. Restoration of regulatory and effector T cell balance and b cell homeostasis in systemic lupus erythematosus patients through vitamin d supplementation. Arthritis Res Ther (2012) 14:R221. doi: 10.1186/ar4060
95. Zhang H, Shih D, Zhang X. Mechanisms underlying effects of 1, 25-dihydroxyvitamin D3 on the Th17 cells. Eur J Microbiol Immunol (2013) 3:237–40. doi: 10.1556/EuJMI.3.2013.4.1
96. Schneider L, dos Santos ASP, Santos M, da Silva Chakr RM, Monticielo OA. Vitamin d and systemic lupus erythematosus: state of the art. clin. Rheumatol. (2014) 33:1033–8.
97. Wahono CS, Rusmini H, Soelistyoningsih D, Hakim R, Handono K, Endharti AT, et al. Effects of 1, 25 (OH) 2D3 in immune response regulation of systemic lupus erithematosus (SLE) patient with hypovitamin d. Int J Clin Exp Med (2014) 7:22.
98. Piantoni S, Andreoli L, Scarsi M, Zanola A, Dall’Ara F, Pizzorni C, et al. Phenotype modifications of T-cells and their shift toward a Th2 response in patients with systemic lupus erythematosus supplemented with different monthly regimens of vitamin d. Lupus (2015) 24:490–8. doi: 10.1177/0961203314559090
99. Yap KS, Morand EF. Vitamin d and systemic lupus erythematosus: continued evolution. Int J Rheumatol Dis (2015) 18:242–9. doi: 10.1111/1756-185X.12489
100. He XJ, Ding Y, Xiang W, Dang XQ. Roles of 1,25(OH)2D3 and vitamin d receptor in the pathogenesis of rheumatoid arthritis and systemic lupus erythematosus by regulating the activation of CD4+ T cells and the PKCdelta/ERK signaling pathway. Cell Physiol biochemistry: Int J Exp Cell physiology biochemistry Pharmacol (2016) 40:743–56. doi: 10.1159/000453135
101. Mak A. The impact of vitamin d on the immunopathophysiology, disease activity, and extra-musculoskeletal manifestations of systemic lupus erythematosus. Int J Mol Sci (2018) 19(8):2355. doi: 10.3390/ijms19082355
102. Marinho A, Carvalho C, Boleixa D, Bettencourt A, Leal B, Guimaraes J, et al. Vitamin d supplementation effects on FoxP3 expression in T cells and FoxP3(+)/IL-17A ratio and clinical course in systemic lupus erythematosus patients: a study in a Portuguese cohort. Immunologic Res (2017) 65:197–206. doi: 10.1007/s12026-016-8829-3
103. Ding Y, Liao W, He XJ, Xiang W. Effects of 1,25(OH)2 D3 and vitamin d receptor on peripheral CD4(+)/CD8(+) double-positive T lymphocytes in a mouse model of systemic lupus erythematosus. J Cell Mol Med (2017) 21:975–85. doi: 10.1111/jcmm.13037
104. Reynolds JA, Rosenberg AZ, Smith CK, Sergeant JC, Rice GI, Briggs TA, et al. Brief report: Vitamin d deficiency is associated with endothelial dysfunction and increases type I interferon gene expression in a murine model of systemic lupus erythematosus. Arthritis Rheumatol (Hoboken N.J.) (2016) 68:2929–35. doi: 10.1002/art.39803
105. Pocovi-Gerardino G, Correa-Rodríguez M, Callejas-Rubio JL, Ríos-Fernández R, Ortego-Centeno N, Rueda-Medina B. Dietary intake and nutritional status in patients with systemic lupus erythematosus. Endocrinologia Diabetes y nutricion (2018) 65:533–9. doi: 10.1016/j.endinu.2018.05.009
106. Aparicio-Soto M, Sánchez-Hidalgo M, Alarcón-de-la-Lastra C. An update on diet and nutritional factors in systemic lupus erythematosus management. Nutr Res Rev (2017) 30:118–37. doi: 10.1017/s0954422417000026
107. Charoenngam N, Vitamin D. And rheumatic diseases: A review of clinical evidence. Int J Mol Sci (2021) 22(19):10659. doi: 10.3390/ijms221910659
108. Kudsi M, Nahas LD, Alsawah R, Hamsho A, Omar A. The prevalence of oral mucosal lesions and related factors in systemic lupus erythematosus patients. Arthritis Res Ther (2021) 23:229. doi: 10.1186/s13075-021-02614-8
109. Raymond WD, Hamdorf M, Furfaro M, Eilertsen GO, Nossent JC. Smoking associates with increased BAFF and decreased interferon-γ levels in patients with systemic lupus erythematosus. Lupus Sci Med (2021) 8(1):e000537. doi: 10.1136/lupus-2021-000537
110. Washio M, Horiuchi T, Kiyohara C, Kodama H, Tada Y, Asami T, et al. Smoking, drinking, sleeping habits, and other lifestyle factors and the risk of systemic lupus erythematosus in Japanese females: findings from the KYSS study. Modern Rheumatol (2006) 16:143–50. doi: 10.1007/s10165-006-0474-6
111. Ekblom-Kullberg S, Kautiainen H, Alha P, Leirisalo-Repo M, Julkunen H. Smoking and the risk of systemic lupus erythematosus. Clin Rheumatol (2013) 32:1219–22. doi: 10.1007/s10067-013-2224-4
112. Takvorian SU, Merola JF, Costenbader KH. Cigarette smoking, alcohol consumption and risk of systemic lupus erythematosus. Lupus (2014) 23:537–44. doi: 10.1177/0961203313501400
113. Versini M, Tiosano S, Comaneshter D, Shoenfeld Y, Cohen AD, Amital H. Smoking and obesity in systemic lupus erythematosus: a cross-sectional study. Eur J Clin Invest (2017) 47:422–7. doi: 10.1111/eci.12757
114. Barbhaiya M, Tedeschi SK, Lu B, Malspeis S, Kreps D, Sparks JA, et al. Cigarette smoking and the risk of systemic lupus erythematosus, overall and by anti-double stranded DNA antibody subtype, in the nurses’ health study cohorts. Ann rheumatic Dis (2018) 77:196–202. doi: 10.1136/annrheumdis-2017-211675
115. Cozier YC, Barbhaiya M, Castro-Webb N, Conte C, Tedeschi SK, Leatherwood C, et al. Relationship of cigarette smoking and alcohol consumption to incidence of systemic lupus erythematosus in a prospective cohort study of black women. Arthritis Care Res (2019) 71:671–7. doi: 10.1002/acr.23703
116. Nagata C, Fujita S, Iwata H, Kurosawa Y, Kobayashi K, Kobayashi M, et al. Systemic lupus erythematosus: a case-control epidemiologic study in Japan. Int J Dermatol (1995) 34:333–7. doi: 10.1111/j.1365-4362.1995.tb03614.x
117. Hardy CJ, Palmer BP, Muir KR, Sutton AJ, Powell RJ. Smoking history, alcohol consumption, and systemic lupus erythematosus: a case-control study. Ann Rheumatol Dis (1998) 57:451–5. doi: 10.1136/ard.57.8.451
118. Ghaussy NO, Sibbitt WL, Qualls CR. Cigarette smoking, alcohol consumption, and the risk of systemic lupus erythematosus: a case-control study. J Rheumatol (2001) 28:2449–53.
119. Costenbader KH, Kim DJ, Peerzada J, Lockman S, Nobles-Knight D, Petri M, et al. Cigarette smoking and the risk of systemic lupus erythematosus: a meta-analysis. Arthritis Rheumatol (2004) 50:849–57. doi: 10.1002/art.20049
120. Kiyohara C, Washio M, Horiuchi T, Asami T, Ide S, Atsumi T, et al. Cigarette smoking, alcohol consumption, and risk of systemic lupus erythematosus: a case-control study in a Japanese population. J Rheumatol (2012) 39:1363–70. doi: 10.3899/jrheum.111609
121. Jiang F, Li S, Jia C. Smoking and the risk of systemic lupus erythematosus: an updated systematic review and cumulative meta-analysis. Clin Rheumatol (2015) 34(11):1885–92. doi: 10.1007/s10067-015-3008-9
122. Zhou A, Liu X, Xia T, Li F, Wang J, Li J. Estrogen receptor alpha gene (ESR1) polymorphism and its interaction with smoking and drinking contribute to susceptibility of systemic lupus erythematosus. Immunologic Res (2017) 65:951–6. doi: 10.1007/s12026-017-8935-x
123. Montes RA, Mocarzel LO, Lanzieri PG, Lopes LM, Carvalho A, Almeida JR. Smoking and its association with morbidity in systemic lupus erythematosus evaluated by the systemic lupus international collaborating Clinics/American college of rheumatology damage index: Preliminary data and systematic review. Arthritis Rheumatol (Hoboken N.J.) (2016) 68:441–8. doi: 10.1002/art.39427
124. Cui B, He K, Zhang X, Zhou W, Sun Z, Zhang M, et al. Association of cigarette smoking with retinal thickness and vascular structure in an elderly Chinese population. Photodiagnosis Photodyn Ther (2021) 36:102481. doi: 10.1016/j.pdpdt.2021.102481
125. Turchin I, Bernatsky S, Clarke AE, St-Pierre Y, Pineau CA. Cigarette smoking and cutaneous damage in systemic lupus erythematosus. J Rheumatol (2009) 36:2691–3. doi: 10.3899/jrheum.090403
126. Bourré-Tessier J, Peschken CA, Bernatsky S, Joseph L, Clarke AE, Fortin PR, et al. Association of smoking with cutaneous manifestations in systemic lupus erythematosus. Arthritis Care Res (2013) 65:1275–80. doi: 10.1002/acr.21966
127. Böckle BC, Sepp NT. Smoking is highly associated with discoid lupus erythematosus and lupus erythematosus tumidus: analysis of 405 patients. Lupus (2015) 24:669–74. doi: 10.1177/0961203314559630
128. Ghaussy NO, Sibbitt W, Bankhurst AD, Qualls CR. Cigarette smoking and disease activity in systemic lupus erythematosus. J Rheumatol (2003) 30:1215–21.
129. Medeiros MM, Xavier de Oliveira IM, Ribeiro AT. Prevalence of metabolic syndrome in a cohort of systemic lupus erythematosus patients from northeastern Brazil: association with disease activity, nephritis, smoking, and age. Rheumatol Int (2016) 36:117–24. doi: 10.1007/s00296-015-3316-z
130. Rubin RL, Hermanson TM, Bedrick EJ, McDonald JD, Burchiel SW, Reed MD, et al. Effect of cigarette smoke on autoimmunity in murine and human systemic lupus erythematosus. Toxicol Sci (2005) 87:86–96. doi: 10.1093/toxsci/kfi217
131. Moraes-Fontes MF, Lúcio I, Santos C, Campos MM, Riso N, Vaz Riscado M. Neuropsychiatric features of a cohort of patients with systemic lupus erythematosus. ISRN Rheumatol (2012) 2012:989218. doi: 10.5402/2012/989218
132. Mont M, Glueck C, Pacheco I, Wang P, Hungerford D, Petri M. Risk factors for osteonecrosis in systemic lupus erythematosus. J Rheumatol (1997) 24:654–62.
133. Ho K, Ahn C, Alarcon G, Baethge B, Tan F, Roseman J, et al. Systemic lupus erythematosus in a multiethnic cohort (LUMINA): XXVIII. factors predictive of thrombotic events. Rheumatol (Oxford) (2005) 44:1303–7. doi: 10.1093/rheumatology/kei014
134. Calvo-Alén J, Toloza S, Fernández M, Bastian HM, Fessler BJ, Roseman JM, et al. Systemic lupus erythematosus in a multiethnic US cohort (LUMINA): XXV. smoking, older age, disease activity, lupus anticoagulant, and glucocorticoid dose as risk factors for the occurrence of venous thrombosis in lupus patients. Arthritis Rheumatol (2005) 52:2060–8. doi: 10.1002/art.21149
135. Kaiser R, Cleveland CM, Criswell LA. Risk and protective factors for thrombosis in systemic lupus erythematosus: results from a large, multi-ethnic cohort. Ann Rheumatol Dis (2009) 68:238–41. doi: 10.1136/ard.2008.093013
136. Gustafsson JT, Gunnarsson I, Källberg H, Pettersson S, Zickert A, Vikerfors A, et al. Cigarette smoking, antiphospholipid antibodies and vascular events in systemic lupus erythematosus. Ann Rheumatol Dis (2014) 74(8):1537–43
137. Amaya-Amaya J, Sarmiento-Monroy JC, Caro-Moreno J, Molano-González N, Mantilla RD, Rojas-Villarraga A, et al. Cardiovascular disease in latin american patients with systemic lupus erythematosus: a cross-sectional study and a systematic review. Autoimmune Dis (2013) 2013:794383. doi: 10.1155/2013/794383
138. June RR, Scalzi LV. Peripheral vascular disease in systemic lupus patients. JCR: J Clin Rheumatol (2013) 19:367–72. doi: 10.1097/RHU.0000000000000017
139. Erdozain JG, Villar I, Nieto J, Ruiz-Irastorza G. Peripheral arterial disease in systemic lupus erythematosus: prevalence and risk factors. J Rheumatol (2014) 41:310–7. doi: 10.3899/jrheum.130817
140. Parodis I, Gomez A, Frodlund M, Jönsen A, Zickert A, Sjöwall C, et al. Smoking reduces the efficacy of belimumab in mucocutaneous lupus. Expert Opin Biol Ther (2018) 18:911–20. doi: 10.1080/14712598.2018.1494719
141. Parodis I, Sjöwall C, Jönsen A, Ramsköld D, Zickert A, Frodlund M, et al. Smoking and pre-existing organ damage reduce the efficacy of belimumab in systemic lupus erythematosus. Autoimmun Rev (2017) 16:343–51. doi: 10.1016/j.autrev.2017.02.005
142. Xu D, You X, Wang Z, Zeng Q, Xu J, Jiang L, et al. Chinese Systemic lupus erythematosus treatment and research group registry VI: Effect of cigarette smoking on the clinical phenotype of Chinese patients with systemic lupus erythematosus. PloS One (2015) 10:e0134451. doi: 10.1371/journal.pone.0134451
143. Kiyohara C, Washio M, Horiuchi T, Tada Y, Asami T, Ide S, et al. Cigarette smoking, n-acetyltransferase 2 polymorphisms and systemic lupus erythematosus in a Japanese population. Lupus (2009) 18:630–8. doi: 10.1177/0961203309102809
144. Kiyohara C, Washio M, Horiuchi T, Tada Y, Asami T, Ide S, et al. Cigarette smoking, STAT4 and TNFRSF1B polymorphisms, and systemic lupus erythematosus in a Japanese population. J Rheumatol (2009) 36:2195–203. doi: 10.3899/jrheum.090181
145. Kiyohara C, Washio M, Horiuchi T, Asami T, Ide S, Atsumi T, et al. Risk modification by CYP1A1 and GSTM1 polymorphisms in the association of cigarette smoking and systemic lupus erythematosus in a Japanese population. Scand J Rheumatol (2012) 41:103–9. doi: 10.3109/03009742.2011.608194
146. Andersen A, Reimer R, Dawes K, Becker A, Hutchens N, Miller S, et al. DNA Methylation differentiates smoking from vaping and non-combustible tobacco use. Epigenetics (2022) 17:178–90. doi: 10.1080/15592294.2021.1890875
147. Richmond RC, Sillero-Rejon C, Khouja JN, Prince C, Board A, Sharp G, et al. Investigating the DNA methylation profile of e-cigarette use. Clin Epigenet (2021) 13:183. doi: 10.1186/s13148-021-01174-7
148. Mathers JC, Strathdee G, Relton CL. Induction of epigenetic alterations by dietary and other environmental factors. Adv Genet (2010) 71:3–39. doi: 10.1016/b978-0-12-380864-6.00001-8
149. Zeilinger S, Kühnel B, Klopp N, Baurecht H, Kleinschmidt A, Gieger C, et al. Tobacco smoking leads to extensive genome-wide changes in DNA methylation. PloS One (2013) 8:e63812. doi: 10.1371/journal.pone.0063812
150. Tsaprouni LG, Yang TP, Bell J, Dick KJ, Kanoni S, Nisbet J, et al. Cigarette smoking reduces DNA methylation levels at multiple genomic loci but the effect is partially reversible upon cessation. Epigenetics (2014) 9:1382–96. doi: 10.4161/15592294.2014.969637
151. Dogan MV, Shields B, Cutrona C, Gao L, Gibbons FX, Simons R, et al. The effect of smoking on DNA methylation of peripheral blood mononuclear cells from African American women. BMC Genomics (2014) 15:151. doi: 10.1186/1471-2164-15-151
152. Dogan MV, Shields B, Cutrona C, Gao L, Gibbons FX, Simons R, et al. The effect of smoking on DNA methylation of peripheral blood mononuclear cells from African American women. BMC Genomics (2014) 15:151. doi: 10.1186/1471-2164-15-151
153. Zeilinger S, Kühnel B, Klopp N, Baurecht H, Kleinschmidt A, Gieger C, et al. Tobacco smoking leads to extensive genome-wide changes in DNA methylation. PLoS One (2013) 8(5):e63812. doi: 10.1371/journal.pone.0063812
154. Tsaprouni LG, Yang T-P, Bell J, Dick KJ, Kanoni S, Nisbet J, et al. Cigarette smoking reduces DNA methylation levels at multiple genomic loci but the effect is partially reversible upon cessation. Epigenetics (2014) 9:1382–96. doi: 10.4161/15592294.2014.969637
155. Oke V, Wahren-Herlenius M. Cutaneous lupus erythematosus: clinical aspects and molecular pathogenesis. J Internal Med (2013) 273:544–54. doi: 10.1111/joim.12057
156. Foltyn VN, Golan TD. In vitro ultraviolet irradiation induces pro-inflammatory responses in cells from premorbid SLE mice. Lupus (2001) 10:272–83. doi: 10.1191/096120301680416968
157. Zahn S, Rehkämper C, Kümmerer BM, Ferring-Schmidt S, Bieber T, Tüting T, et al. Evidence for a pathophysiological role of keratinocyte-derived type III interferon (IFNλ) in cutaneous lupus erythematosus. J Invest Dermatol (2011) 131:133–40. doi: 10.1038/jid.2010.244
158. Nyberg F, Hasan T, Skoglund C, Stephansson E. Early events in ultraviolet light-induced skin lesions in lupus erythematosus: expression patterns of adhesion molecules ICAM-1, VCAM-1 and e-selectin. Acta dermato-venereologica (1999) 79:431–6. doi: 10.1080/000155599750009852
159. Yin Q, Xu X, Lin Y, Lv J, Zhao L, He R. Ultraviolet b irradiation induces skin accumulation of plasmacytoid dendritic cells: a possible role for chemerin. Autoimmunity (2014) 47:185–92. doi: 10.3109/08916934.2013.866105
160. Abdulahad DA, Westra J, Reefman E, Zuidersma E, Bijzet J, Limburg PC, et al. High mobility group box1 (HMGB1) in relation to cutaneous inflammation in systemic lupus erythematosus (SLE) lupus. (2013) 22:597–606. doi: 10.1177/0961203313483377
161. Fraser PA, Ding WZ, Mohseni M, Treadwell EL, Dooley MA, St Clair EW, et al. Glutathione s-transferase m null homozygosity and risk of systemic lupus erythematosus associated with sun exposure: a possible gene-environment interaction for autoimmunity. J Rheumatol (2003) 30:276–82.
162. Wang P, Dan YL, Wu Q, Tao SS, Yang XK, Wang DG, et al. Non-causal effects of smoking and alcohol use on the risk of systemic lupus erythematosus. Autoimmun Rev (2021) 20:102890. doi: 10.1016/j.autrev.2021.102890
163. Nagata C, Fujita S, Iwata H, Kurosawa Y, Kobayashi K, Kobayashi M, et al. Systemic lupus erythematosus: a case-control epidemiologic study in Japan. Int J Dermatol (1995) 34:333–7. doi: 10.1111/j.1365-4362.1995.tb03614.x
164. Ghaussy NO, Sibbitt WL Jr., Qualls CR. Cigarette smoking, alcohol consumption, and the risk of systemic lupus erythematosus: a case-control study. J Rheumatol (2001) 28:2449–53.
165. Formica MK, Palmer JR, Rosenberg L, McAlindon TE. Smoking, alcohol consumption, and risk of systemic lupus erythematosus in the black women’s health study. J Rheumatol (2003) 30:1222–6.
166. Kim SK, Lee SS, Choe JY, Park SH, Lee H. Effect of alcohol consumption and smoking on disease damage in systemic lupus erythematosus: data from the Korean lupus network (KORNET) registry. Lupus (2017) 26:1540–9. doi: 10.1177/0961203317709346
167. Kiyohara C, Washio M, Horiuchi T, Asami T, Ide S, Atsumi T, et al. Cigarette smoking, alcohol consumption, and risk of systemic lupus erythematosus: a case-control study in a Japanese population. J Rheumatol (2012) 39:1363–70. doi: 10.3899/jrheum.111609
168. Barbhaiya M, Lu B, Sparks JA, Malspeis S, Chang SC, Karlson EW, et al. Influence of alcohol consumption on the risk of systemic lupus erythematosus among women in the nurses’ health study cohorts. Arthritis Care Res (2017) 69:384–92. doi: 10.1002/acr.22945
169. Wang J, Pan H-F, Ye D-Q, Su H, Li X-P. Moderate alcohol drinking might be protective for systemic lupus erythematosus: a systematic review and meta-analysis. Clin Rheumatol (2008) 27:1557–63. doi: 10.1007/s10067-008-1004-z
Keywords: systemic lupus erythematosus, lifestyle, diet, smoking, alcohol, ultraviolet radiation
Citation: Chen J, Liao S, Pang W, Guo F, Yang L, Liu H-f and Pan Q (2022) Life factors acting on systemic lupus erythematosus. Front. Immunol. 13:986239. doi: 10.3389/fimmu.2022.986239
Received: 05 July 2022; Accepted: 31 August 2022;
Published: 15 September 2022.
Edited by:
Francesca Romana Spinelli, Sapienza University of Rome, ItalyReviewed by:
Sarfaraz Ahmed Hasni, National Institutes of Health (NIH), United StatesFaten Mohammad, Minia University, Egypt
Copyright © 2022 Chen, Liao, Pang, Guo, Yang, Liu and Pan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hua-feng Liu, liuhf@gdmu.edu.cn; Qingjun Pan, pqj@gdmu.edu.cn
†These authors have contributed equally to this work and share first authorship
 Jiaxuan Chen
Jiaxuan Chen Shuzhen Liao
Shuzhen Liao Wanxian Pang
Wanxian Pang Fengbiao Guo
Fengbiao Guo Lawei Yang
Lawei Yang Hua-feng Liu
Hua-feng Liu Qingjun Pan
Qingjun Pan