- 1Department of Rheumatology, Fukushima Medical University School of Medicine, Fukushima, Fukushima, Japan
- 2Department of Gastroenterology, Fukushima Medical University School of Medicine, Fukushima, Fukushima, Japan
- 3Department of Immunology, Fukushima Medical University School of Medicine, Fukushima, Fukushima, Japan
Altered expression of adhesion molecules in immune cells has been demonstrated in rheumatoid arthritis (RA). Carcinoembryonic–antigen–related cell–adhesion molecule 1 (CEACAM1) is an adhesion molecule that acts as a coinhibitory receptor in the immune system. We investigated the role of CEACAM1 in immune cell subsets of patients with RA. Peripheral blood was obtained from 37 patients with RA and 20 healthy controls (HC). The expression of CEACAM1 and T–cell immunoglobulin mucin domain molecule (TIM) –3 on peripheral blood mononuclear cells and neutrophils was analyzed by flow cytometry. Intracellular TIM–3 expression was analyzed using cellular lysates by Western blot analysis. Serum levels of soluble CEACAM1 (sCEACAM1) were estimated by an enzyme-linked immunosorbent assay. CEACAM1 expression was not detected in peripheral blood mononuclear cells, including in CD14(+) monocytes and CD3(+) lymphocytes isolated from patients with RA or HC. However, substantial cell–surface expression of CEACAM1 was detected in peripheral blood neutrophils, and it was significantly elevated in samples from patients with RA without remission compared to those in remission. There was no significant difference in serum levels of sCEACAM1 between patients with RA and HC. Cell-surface expression of TIM-3 was not detected in peripheral blood neutrophils from patients with RA or HC but was seen in CD14(+) monocytes. However, there was no significant difference in TIM–3 expression on monocytes between patients with RA and HC. Our data indicate that cell-surface expression of CEACAM1 on peripheral blood neutrophils are higher in patients with RA and that it is associated with rheumatoid inflammation. Further studies are needed to explore the potential role of CEACAM1 in rheumatoid inflammatory pathways.
1 Introduction
Rheumatoid arthritis (RA) is a chronic systemic autoimmune disorder that is characterized by inflammatory cell infiltrations into the synovial tissues, which results in the joint destruction (1). The rheumatoid synovium is also characterized by the increased expression of adhesion molecules of the infiltrating immune cells (2), and ligand binding on these adhesion molecules induces immune cell activation and inflammatory cytokine production (3). Carcinoembryonic–antigen–related cell–adhesion molecule 1 (CEACAM1), also known as cluster of differentiation (CD) 66a, is an adhesion molecule expressed by immune cells that interact with integrin or extracellular matrix proteins to modulate the immune response (4). Thus, CEACAM1 possesses diverse biological functions in cell adhesion, migration, and intercellular signaling during inflammation (5). Also, CEACAM1 is thought to be a coinhibitory molecule in the immune system (6) as it acts synergistically with TIM–3, which is a negative regulatory molecule that plays a critical role in immune tolerance (7). We have previously demonstrated that serum levels of soluble TIM–3 (sTIM–3) are elevated in patients with RA and that it is associated with proinflammatory markers and RA disease activity (8). Recently, it was demonstrated that CEACAM1 serves as a heterophilic ligand for TIM–3 in activated T cells and that this interaction is crucial for regulating autoimmunity (9). These findings suggest that CEACAM1 also plays a functional role in T cells during inflammatory or autoimmune disorders (10). CEACAM1 expression was shown to be up-regulated on the innate immune cells when cells are activated by pathogens (11). Furthermore, CEACAM1 expressing myeloid cells control angiogenesis suggesting a causal relation between CEACAM1 expression and its inhibitory effect during the inflammatory processes (12). However, very few studies have investigated the role of CEACAM1 in regulating autoimmunity in human autoimmune diseases. Hence, we evaluated CEACAM1 and TIM–3 expression in peripheral blood immune cells isolated from patients with RA by flow cytometry and also investigated the relationship between their expression and rheumatoid inflammatory parameter in patients with RA.
2 Methods
2.1 Patients
This single–center study observational study included 37 patients with RA who were diagnosed based on the 2010ACR/EULAR classification criteria (13). RA patients were enrolled between August 2021 and March 2022, and we reviewed the records of these patients with RA. All patients were treated at the Department of Rheumatology, Fukushima Medical University.
The following clinical and demographic data were retrieved from the Medical Records Unit of the Fukushima University Hospital: age, gender, simplified disease activity score (SDAI), and disease activity score–28 for RA with C-reactive protein (DAS28–CRP). For comparison with healthy controls (HC), 20 individuals, i.e., 6 males and 14 females with a median age of 44.5 years at blood test [interquartile range (IQR); 30–51.25 years], were included. This study conformed to the principles of the Declaration of Helsinki. Ethical approval for this study (No.2021–158) was provided by the Ethics Committee of Fukushima Medical University.
2.2 Measurement of clinical disease activity
All patients underwent clinical assessment at baseline, including 28–swollen joint and tender joint counts (28–SJC and 28–TJC, respectively), physician and global patient assessment with visual analogue scales (0–100 mm), and CRP (mg/dL). Composite disease activity indices were subsequently calculated based on SDAI and DAS28–CRP. This score was reported as a quantitative value that could be categorized as remission and non-remission (low, intermediate, and high disease activity).
2.3 Blood sampling and cell isolation
Peripheral blood (2 mL) from patients with RA and HC was collected into precoated EDTA tubes, diluted with 6 ml of cold Ammonium–Chloride Potassium (ACK) lysis buffer (0.15 M NH4Cl, 13 mM KCl, 0.1 mM Na2EDTA, pH 7.4) (14), and incubated for 5 min on ice. A cell pellet was obtained by centrifugation (10 min, 3,000 ×g, 4°C) and washed once with 6 ml cold ACK buffer. The supernatant was discarded after the last centrifugation step and the cell pellet was resuspended in cold PBS.
2.4 Neutrophil isolation and cytokine stimulation
HC`s neutrophils were isolated on the basis of the density gradient centrifugation method. In brief, peripheral blood was layered on a Polymorphprep™ (Axis-Shield, Oslo, Norway) cushion, and cells were isolated according to the manufacturer’s protocol. Isolated neutrophils were stimulated with IL-6 (20 ng/mL and 100 ng/mL) and TNF-α (20 ng/mL and 100 ng/mL) for 24 hr in vitro. Cell surface expressions of CEACAM1 on neutrophils were analyzed by flow cytometry.
2.5 Flow cytometry
Freshly isolated white blood cells were used for flow cytometric analysis of the surface expression of CEACAM1, TIM–3, CD3, CD14, and CD16 using antihuman CEACAM1 (PE; mouse. No. 283340, diluent 2:100; R&D Systems, Minneapolis, MN, USA), anti–TIM3 (APC; rat. No. 344823, diluent 2:100; R&D Systems), antihuman CD3 (PerCP–CY5.5; mouse. No. 344806, diluent 2:100; Biolegend, San Diego, CA, USA), antihuman CD14 (FITC; mouse. No.301804, diluent 2:100; Biolegend), and antihuman CD16 antibodies (PerCP; mouse. No. 302029, diluent 2:100; Biolegend), respectively. Dead cells were excluded using LIVE/DEAD™ Fixable Dead Cell Stain Kits. Anti–CEACAM1–specific mouse monoclonal antibody (clone number 283340) was shown to be non-cross-reactive with other CEACAM families as described previously (15). Flow cytometry was performed on a FACS–Canto II (BD Biosciences, Franklin Lakes, NJ, USA) and data were analyzed using FlowJo software (BD Biosciences). Flow cytometry gating strategies were shown in Supplemental Figures 1, 2.
2.6 ELISA methods
Serum concentration of CEACAM1 was measured using enzyme–linked immunosorbent assay (ELISA) kits (Thermo Fisher Scientific, Waltham, MA, USA; Catalog No. EHCEACAM1) according to the manufacturer’s instructions. Similarly, serum TIM–3 was measured using an ELISA Kit (R&D Systems; Catalog No. DTIM30). The CEACAM1 ELISA kit uses the solid-phase sandwich enzyme immunoassay technique, and the TIM–3 ELISA kit uses the quantitative sandwich enzyme immunoassay technique. The CEACAM1 ELISA kit involves specific detection of captured target proteins using biotinylated detection antibody and the interaction was then analyzed using streptavidin conjugated with horseradish peroxidase according to the manufacturer’s protocol. Monoclonal antibodies specific for CEACAM1 and TIM–3 have been pre-coated onto a microplate. Standards and samples are pipetted into the wells and any CEACAM1 and TIM–3 present are bound by the immobilized antibody. After washing away any unbound substances, enzyme–linked polyclonal antibodies specific for CEACAM1 and TIM–3 are added to the wells. Following a wash to remove any unbound antibody–enzyme reagent, a substrate solution is added to the wells and color develops in proportion to the amounts of CEACAM1 and TIM–3 bound in the initial step. The color development is stopped and the intensity of the color is measured. Determine the optical density of each well within 30 minutes using a microplate reader set to 450 nm.
2.7 Western blotting for intracellular TIM–3 expression
Freshly isolated neutrophils were washed in ice–cold PBS and lysed on ice for 20 min with RIPA buffer (Sigma–Aldrich, La Jolla, CA, USA) supplemented with protease inhibitor cocktail. Cell lysates were centrifuged at 10,000×g for 10 min at 4°C and the supernatant was collected. Equal amounts of total protein (30 μg) were subjected to 12% SDS–PAGE under reducing condition and electrotransferred onto polyvinylidene fluoride membranes that were then blocked with 5% bovine serum albumin for 1 h at room temperature. The membrane was incubated with primary antibodies against human TIM–3, followed by incubation with secondary antibodies at room temperature and visualization using the ECL reagent (Amersham, Little Chalfont, UK). Immunoblots were imaged using the LAS–3000 Imaging System (Fuji Film, Tokyo, Japan). β–actin was also detected to normalize the loading amount of total cell lysate between samples.
2.8 Statistical analyses
Nonnormally distributed data are presented throughout the manuscript as median with 25–75th centiles and were compared by Mann–Whitney U test. Pearson’s Chi-square tests were performed for dichotomic variables. Correlation between continuous variables was analyzed using the Spearman rank correlation test. All data entry and statistical analyses were performed using SPSS Statistics version 22.0 (IBM, Armonk, NY, USA). All statistical analyses were 2–tailed, and statistical significance was defined as p < 0.05. Mean fluorescence intensity (MFI) of any given marker within the defined immune cells subpopulation were recorded.
3 Results
3.1 Characteristics of patients with RA
The demographic, clinical, and laboratory characteristics of the 37 patients with RA are summarized in Table 1. The median age (IQR) at blood test for this group was 69 years (58–77 years). Among 37 patients with RA, 34 (91.9%) were female. The median values (IQR) for SDAI and DAS28–CRP were 4.12 (2.03–13.13) and 1.93 (1.28–2.97) respectively. Patients with RA were classified into stages I to IV according to Steinbrocker radiographic staging (16), and there were 7 (19%) in stage I, 9 (24%) in stage II, 7 (19%) in stage III, and 12 (32%) in stage IV. The staging was not possible in some patients (n=2) due to a lack of radiological information.
3.2 Expression of CEACAM1 on blood cells
Anticoagulated whole blood from patients with RA and HC were used to evaluate the expression of CEACAM1 on peripheral immune cells. As shown in representative images, minimal CEACAM1 expression was seen on the cell surface of CD3(+) lymphocytes and CD14(+) monocytes (Figure 1). In contrast, CEACAM1 was expressed on the surface of CD16(+) CD14 (–) neutrophil populations (Figure 1). These results indicate that cell–surface expression of CEACAM1 is seen in the neutrophils in human peripheral blood.
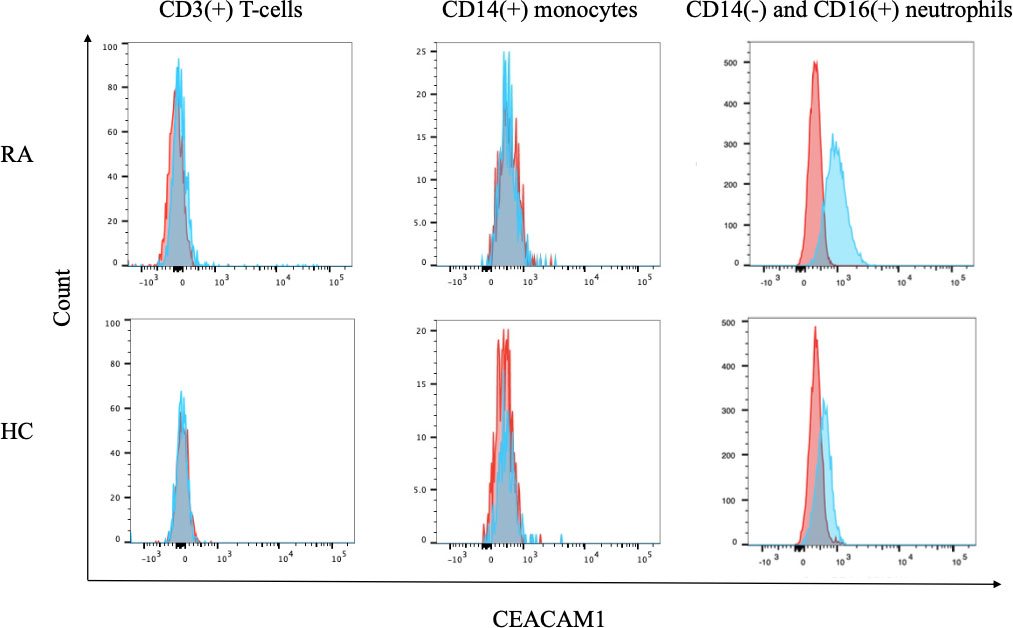
Figure 1 The expression of CEACAM1 on peripheral blood immune cells. Flow cytometry overlay histograms for CEACAM1 demonstrating typical cell profiles from subdivided peripheral blood immune cells from RA patients and HC. CEACAM1 expression was detected using anti–CEACAM1 antibody (blue shaded histogram) or isotype control antibody (red shaded histograms). In contrast to lymphocytes or monocytes, peripheral blood neutrophils expressed CEACAM1 on their surface. RA, rheumatoid arthritis; HC, healthy controls.
3.3 Evaluation of CEACAM1 expression on neutrophils
To further evaluate the expression of CEACAM1 on neutrophils, we compared the expression levels of CEACAM1 in peripheral blood neutrophils from 20 HC and 37 patients with RA. As shown in Figure 2, the MFI of CEACAM1 (median 1,534, IQR 1,055–1,748) was significantly higher in patients with RA compared to HC (median 696, IQR 438.5–936) (p < 0.001). Additionally, the MFI of CEACAM1 were significantly higher in the CRP–positive RA group (median 1603, IQR 1,434–1,920) than in the CRP–negative RA group (median 1,534, IQR 1,055–1748) (p = 0.001) (Figure 3A). Furthermore, we investigated the differences in CEACAM1 expression in neutrophils between RA patients with and without remission state. The MFI of CEACAM1 was significantly higher in the non-remission group than in the remission group for SDAI (Figure 3B). The clinical background between the remission group and non-remission group were shown in Supplemental Table 1. However, there was no significant difference between the remission group and the non-remission group for DAS28–CRP (Supplemental Figure 3). The MFI of CEACAM1 was not correlated with SDAI or DAS28–CRP (Supplemental Figure 4), even though the association with disease activity was not clear. Furthermore, the MFI of CEACAM1 was significantly lower in the RA patients who used biologics or Janus kinase (JAK) inhibitors compared to the non-used patients (Figures 3C, D). Elevated levels of inflammatory cytokines such as TNF-α and IL-6 in RA patients are thought to play a crucial role in the development and progression of RA. To analyze whether these proinflammatory cytokines modulate the CEACAM1 expressions of neutrophils, peripheral blood neutrophils were simulated with TNF-α or IL-6 for 24 hr in vitro. Cell surface expressions of CEACAM1 on neutrophils were analyzed by FCM. The expression of CEACAM1 levels was marginally elevated in IL-6-stimulated neutrophils. However, levels of CEACAM1 expression were significantly up-regulated in TNF-α-stimulated neutrophils (Figure 4). As shown in Supplemental Figure 5, after removing the dead cells, the expression of CEACAM1 levels was elevated in live cells.
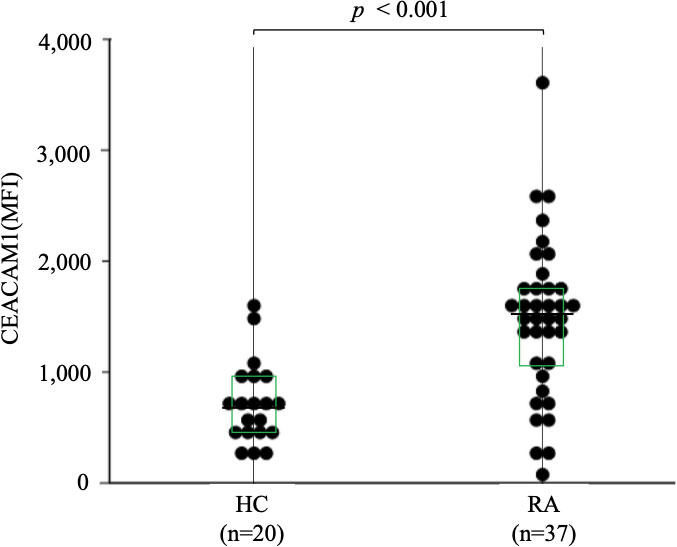
Figure 2 The expression of CEACAM1 on peripheral blood neutrophils isolated from HC (n=20) and RA patients (n=37). The MFI of CEACAM1 in RA patients was significantly higher compared to those in HC. Statistical significance was determined by the Mann–Whitney U test. Horizontal bars and squares indicate median and IQR, respectively. HC, healthy controls; RA, rheumatoid arthritis; MFI, mean fluorescence intensity; IQR, interquartile range.
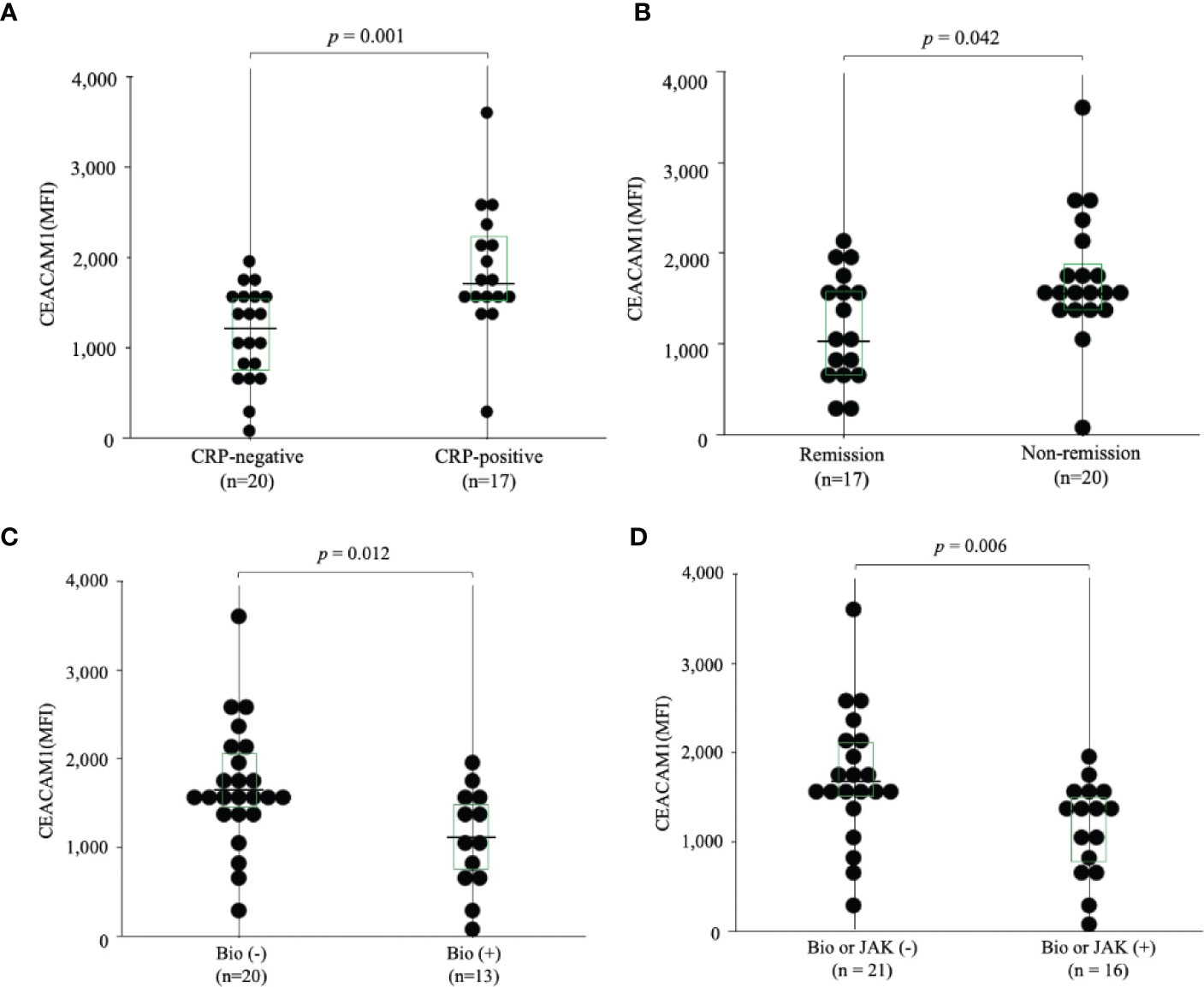
Figure 3 The expression of CEACAM1 on peripheral blood neutrophils in RA patients. (A) The expression of CEACAM1 on peripheral blood neutrophils isolated from RA patients with (n=17) or without (n=20) elevated serum inflammatory marker (CRP >0.30 mg/dL). The MFI of CEACAM1 positive neutrophils in RA patients with elevated serum inflammatory marker was significantly higher compared to RA patients without them. (B) The expression of CEACAM1 on peripheral blood neutrophils in RA patients with or without remission according to SDAI. The MFI of CEACAM1 in the non–remission group was significantly higher compared to the remission group. (C, D) The MFI of CEACAM1 was significantly higher in the RA patients who used Bio or JAK inhibitors than in the non-used patients. Statistical significance was determined by the Mann–Whitney U test. Horizontal bars and squares indicate the median and IQR, respectively. RA, rheumatoid arthritis; CRP, C–reactive protein; MFI, mean fluorescence intensity; Bio, Biologics; JAK, Janus kinase; IQR, interquartile range.
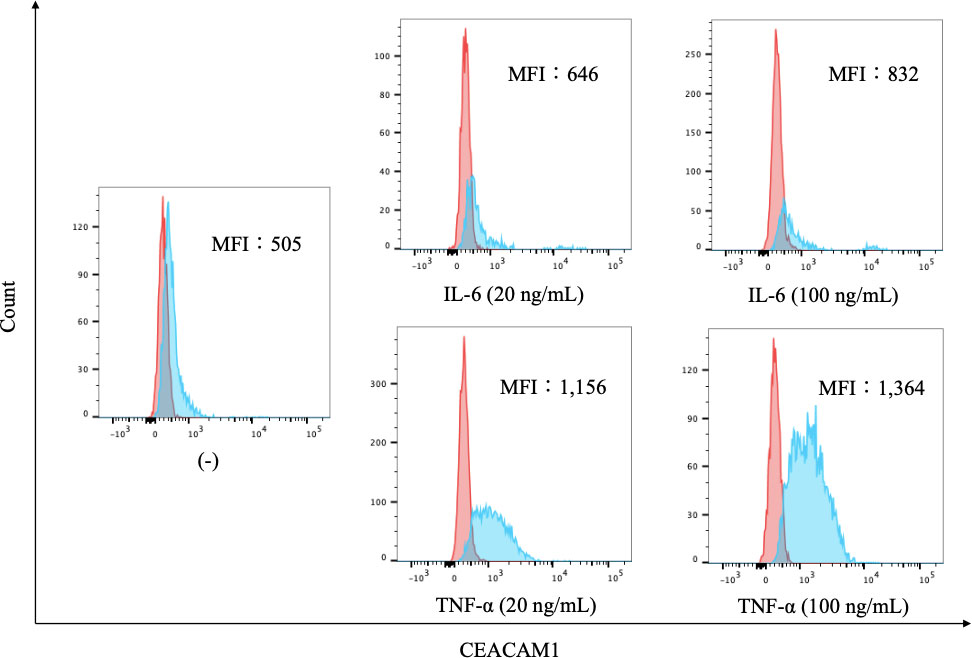
Figure 4 The expression of CEACAM1 on cytokine-stimulated neutrophils. Flow cytometry overlay histograms for CEACAM1 demonstrating typical cell profiles from subdivided peripheral blood immune cells from HC. CEACAM1 expression was detected using anti–CEACAM1 antibody (blue shaded histogram) or isotype control antibody (red shaded histograms). Neutrophils were stimulated with cytokines associated with inflammatory pathogenesis of RA [IL-6 (20 ng/mL and 100 ng/mL) and TNF-α (20 ng/mL and 100 ng/mL)]. The MFI of CEACAM1 differed depending on the cytokine and its concentrations. HC, healthy controls; IL-6, interleukin-6; TNF-α: tumor necrosis factor; MFI, mean fluorescence intensity.
3.4 Evaluation of sCEACAM1 levels
Levels of sCEACAM1 were measured using serum samples from patients with RA and HC, and as shown in Figure 5, there were no significant differences between the two groups [12,060 (8,622–14,556) pg/mL versus 15486 (13,014–19,865) pg/mL, p = 0.184]. CEACAM1 is known to interact with TIM–3 during immune regulation; hence, its correlation with serum TIM–3 levels were also investigated, but there was no significant correlation (the data was not shown).
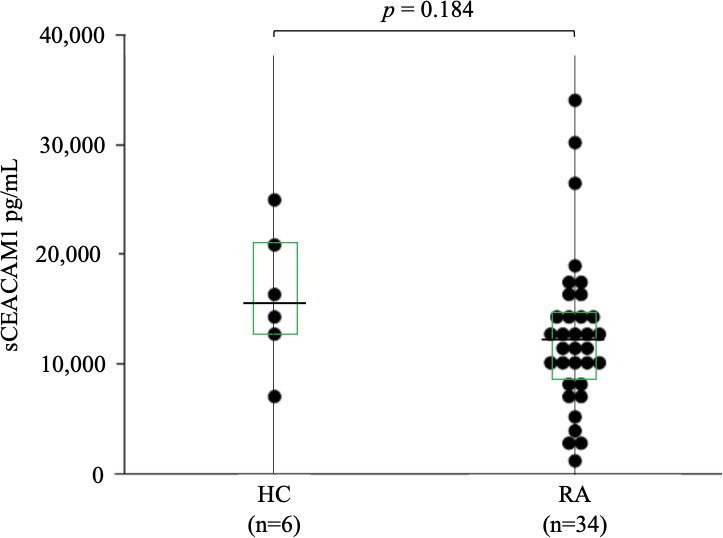
Figure 5 Serum levels of sCEACAM1 in RA. The comparison of serum levels of sCEACAM1 between HC (n=6) and RA patients (n=34). There were no significant differences between HC and RA patients. Statistical significance was determined by the Mann–Whitney U test. Horizontal bars and squares indicate median and IQR, respectively. sCEACAM1, soluble CEACAM1; HC, healthy controls; RA, rheumatoid arthritis; IQR, interquartile range.
3.5 Expression of TIM–3 on peripheral blood immune cells
To assess TIM–3 expression in RA, we subjected fresh peripheral blood samples from HC and patients with RA to flow cytometry and found that TIM–3 is barely expressed on peripheral blood CD3(+) T cells or CD14(−) and CD16(+) neutrophils, both in HC and patients with RA (Figure 6). To analyze the expression of TIM–3 on monocytes, CD14(+) monocytes in HC and patients with RA were gated, and the surface expression of TIM-3 was analyzed (Supplemental Figure 1). While we observed TIM–3 expression on peripheral blood CD14(+) monocytes, there was no significant difference in TIM–3 expression levels between HC and patients with RA (Figure 6).
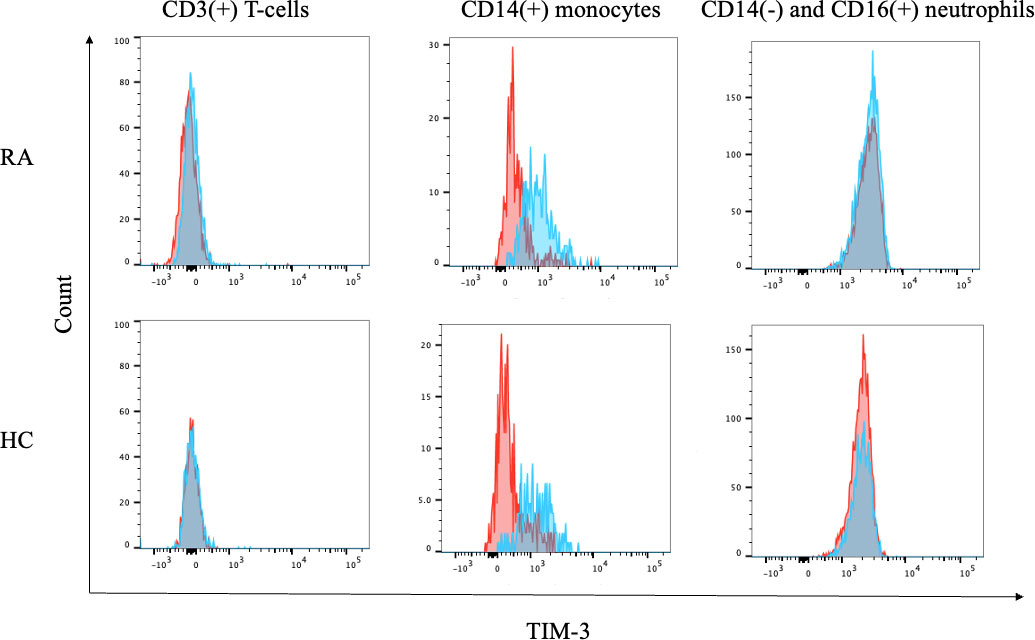
Figure 6 Expression of TIM–3 on blood cells. Flow cytometry overlay histograms for TIM–3 demonstrating typical cell profiles from subdivided peripheral blood cells from RA patients and HC. TIM–3 expression was detected using anti-TIM–3 antibody (blue shaded histogram) or isotype control antibody (red shaded histograms). In contrast to lymphocytes or neutrophils, peripheral blood monocytes expressed CEACAM1 on their surface. RA, rheumatoid arthritis.
3.6 Intracellular expression of TIM–3 in neutrophils
As flow cytometry did not detect cell-surface expression of TIM–3 on peripheral blood neutrophils, we analyzed its intracellular expression using neutrophil cellular lysates by Western blot. Whole neutrophil lysates displayed bands characteristic of TIM–3 (Figure 7), and in agreement with the previous reports on the molecular mass of TIM–3, the fully glycosylated form of the protein (64 kDa) was found in neutrophils (17). The results indicate that the intracellular expression of TIM–3 was similar between active and inactive patients with RA.
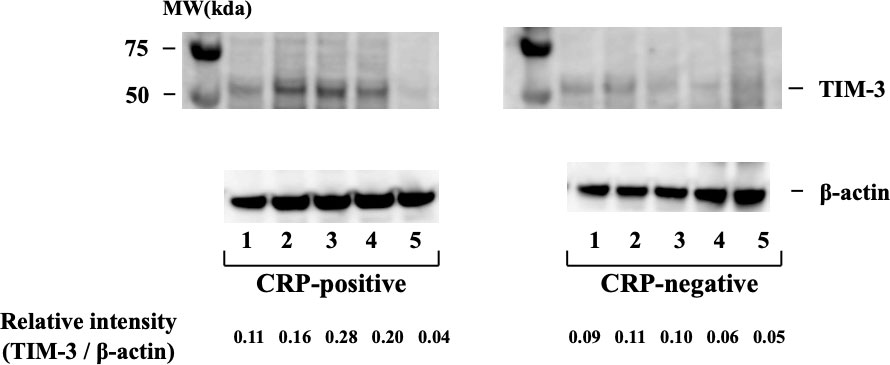
Figure 7 Intracellular expression of TIM–3 in neutrophils isolated from RA patients with or without inflammatory sign (CRP >0.30 mg/dL). Intracellular expression of TIM–3 was determined by Western blotting using neutrophils cellular lysate in RA patients with or without elevated serum CRP levels. TIM–3 expression was normalized β–actin bands and the ratio of TIM–3 to β–actin was calculated. They indicate that the intracellular expression of TIM–3 was similar between active and inactive patients with RA. RA, rheumatoid arthritis; CRP, C-reactive protein.
4 Discussion
The rheumatoid synovium displays the increased expression of adhesion molecules on the infiltrating immune cells (18). Importantly, binding of these adhesion molecules to the cells in the synovial microenvironment can induce immune cell activation (19). CEACAM1, also known as CD66a, is a member of the carcinoembryonic antigen family and is widely expressed in different cell types (4). It is involved in intercellular adhesion and in the regulation of cellular growth and differentiation, and when expressed on cell membranes, it interacts with integrin or extracellular matrix proteins to modulate immune response (5). However, as little is known about the role of CEACAM1 in rheumatoid inflammatory cells, we investigated its expression in peripheral blood immune cells isolated from RA patients with systemic inflammation. We demonstrate that CEACAM1 is unlikely expressed on monocytes or lymphocytes, while it is exclusively expressed on peripheral blood neutrophils in RA patients. Furthermore, cell-surface expression of CEACAM1 was significantly higher in neutrophils isolated from patients with RA and systemic inflammation compared to those without systemic inflammation as well as HC.
Many studies have evaluated the expression of various activation–dependent surface markers in neutrophils isolated from the synovial fluid of patients with inflammatory arthritis (20) and they report greater expression of members from the CD66 family in neutrophils from the synovial fluid compared to peripheral blood neutrophils (21). The enhanced expression of CEACAM1 on neutrophils in patients with RA suggests that CEACAM1 expression can be modulated by the rheumatoid inflammatory process. Nevertheless, this should be further validated by investigating the relationship between CEACAM1 expression and interacting molecules or immune cells seen during the rheumatoid inflammatory process.
Neutrophils are regarded as the first line of defense pathogens (22), and while much is known about their migration to inflamed tissue, knowledge of their regulation during the inflammatory process is relatively scant (23). In rheumatoid synovium, activated neutrophils secrete inflammatory cytokines, which are implicated in RA pathogenesis (24). Also, circulating neutrophils isolated from RA patients were shown to exhibit several features indicative of partial activation (25). Taken together, our data suggest that inflammatory cytokines can be implicated in the increased CEACAM1 expressions on circulating neutrophils in RA. CEACAM1 is one of the adhesion molecules on neutrophils that plays an important role in the cell’s biological functions, including adhesion or phagocytosis (26). Initially, there is little CEACAM1 expression on resting human neutrophils but their activation causes rapid translocation of CEACAM1 to the cell surface (27). Although CEACAM1 is an activation marker for neutrophils, its role in neutrophil-dependent inflammation is not completely elucidated. In our data, RA patients with inflammation exhibit an increased expression of CEACAM1 on their peripheral blood neutrophils. In contrast, CEACAM1 expression is barely seen in peripheral blood neutrophils of HC, and these observations support the hypothesis that inflammatory stimuli upregulate CEACAM1 expression on neutrophils. The levels of CEACAM1 expression were significantly up-regulated in TNF-α-stimulated neutrophils (Figure 4). Indeed, the MFI of CEACAM1 was significantly lower in the RA patients treated with cytokine-targeting treatments compared to those without these treatments. In rheumatoid synovium, activated neutrophils secrete many cytokines and are implicated in RA pathogenesis (24). Also, circulating neutrophils isolated from RA patients were shown to exhibit several features indicative of partial activation (25). Taken together, our data suggest that inflammatory cytokines can be implicated in the increased CEACAM1 expressions on circulating neutrophils in RA. To the best of our knowledge, this is the first report of describing the role of CEACAM1 in rheumatoid inflammatory processes.
Although CEACAM1 is designated as an activation marker in immune cells, it also acts as a co-inhibitory receptor in the immune system (11). CEACAM1 is an ITIM type 1 membrane protein and its main functional role in lymphocytes is to generate inhibitory signals (28); however, the inhibitory effects of CEACAM1 have not been completely elucidated. CEACAM1 can bind to TIM–3 intracellularly, which appears to be important for the function of TIM–3 (29). In vitro, CEACAM1 is co–expressed with TIM–3 in activated T cells and is thought to contribute to the establishment of T–cell tolerance (30).
Although TIM–3 has been considered to be expressed only by T cells, it is now clear that TIM–3 is expressed by multiple cell types, including myeloid cells (31). In our results, the surface expression of TIM–3 on neutrophils was not detected using the flow cytometry technique, however intracellular TIM–3 expression was detectable in neutrophils isolated from patients with RA. Even if the precise role of TIM–3 in the neutrophils isolated from RA patients was not defined in the present study, it is possible that CEACAM1 and TIM–3 can be co–ordinately regulated in patients with RA. Many pathogens utilize CEACAM1 as a receptor on neutrophils resulting in a potential inhibition of the inflammatory responses. In fact, a feedback loop provided by CEACAM1 was demonstrated to downregulate inflammasome activation in neutrophils (32). However, CEACAM1–TIM–3 interaction in neutrophils could be highly complex. Further and careful investigations are required to delineate the underlying molecular mechanisms concerning the changes in CEACAM1 expression and its intracellular signaling in RA.
There are several limitations in this study. The sample size was relatively small and the numbers of RA patients with disease activity were limited. A second limitation is the restricted number of cell types were analyzed by flow cytometry with a major focus on the immune compartment, so that additional studies would be needed to analyze the more detailed lymphoid and myeloid lineage cells. The protein interactions between CEACAM1 and TIM–3 were not investigated in this study.
In summary, our data indicate that CEACAM1 is expressed in peripheral blood neutrophils in patients with RA and that its expression was upregulated in response to rheumatoid inflammation. Inflammation-related cell-surface expression of CEACAM1 on neutrophils, in the context of a rheumatoid environment, might be implicated with the rheumatoid inflammatory process.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by ethics committee of Fukushima Medical University. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
HM, YF, KS, YS, SY, NM, JT, MY-F, TA, SS, ES, and HW were involved in the acquisition of the clinical data. HM, YF, and KM drafted the manuscript and carried out the molecular biochemical studies. MO and TM contributed as an advisor to study protocols of flow cytometry. HM, YF, MO, and KM participated in the design of the study. HM and YF performed the statistical analysis. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Japan Grant-in-Aid for Scientific Research (JP20K08777) to KM.
Acknowledgments
We are grateful to Ms. Sachiyo Kanno for her technical assistance in this study and would like to thank Enago (www.enago.jp) for English language review.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.978435/full#supplementary-material
Abbreviations
ACK, Ammonium–Chloride Potassium; CCP, cyclin citrullinated peptide; CD, cluster of differentiation; CEACAM1, carcinoembryonic–antigen–related cell–adhesion molecule 1; DAS28–CRP, disease activity score–28 for RA with C-reactive protein; ELISA, enzyme–linked immunosorbent assay; FSC, forward scatter; HC, healthy controls; IL-6, interleukin-6; IQR, interquartile range; JAK, Janus kinase; MFI, mean fluorescence intensity; RA, rheumatoid arthritis; RF, rheumatoid factor; sCEACAM1, soluble carcinoembryonic–antigen–related cell–adhesion molecule 1; SDAI, simplified disease activity score; SSC, side scatter; sTIM; soluble T–cell immunoglobulin mucin domain molecule–3; TIM–3, T–cell immunoglobulin mucin domain molecule–3; TNF-α, tumor necrosis factor.
References
1. Rivellese F, Pitzalis C. Cellular and molecular diversity in rheumatoid arthritis. Semin Immunol (2022) 53:101519. doi: 10.1016/j.smim.2021.101519
2. Szekanecz Z, Haines GK, Harlow LA, Shah MR, Fong TW, Fu R, et al. Increased synovial expression of the adhesion molecules Cd66a, Cd66b, and Cd31 in rheumatoid and osteoarthritis. Clin Immunol Immunopathol (1995) 76(2):180–6. doi: 10.1006/clin.1995.1113
3. Shimizu Y, van Seventer GA, Horgan KJ, Shaw S. Roles of adhesion molecules in T-cell recognition: Fundamental similarities between four integrins on resting human T cells (Lfa-1, vla-4, vla-5, vla-6) in expression, binding, and costimulation. Immunol Rev (1990) 114:109–43. doi: 10.1111/j.1600-065x.1990.tb00563.x
4. Kim WM, Huang YH, Gandhi A, Blumberg RS. Ceacam1 structure and function in immunity and its therapeutic implications. Semin Immunol (2019) 42:101296. doi: 10.1016/j.smim.2019.101296
5. Gray-Owen SD, Blumberg RS. Ceacam1: Contact-dependent control of immunity. Nat Rev Immunol (2006) 6(6):433–46. doi: 10.1038/nri1864
6. Zhang Q, Vignali DA. Co-Stimulatory and Co-inhibitory pathways in autoimmunity. Immunity (2016) 44(5):1034–51. doi: 10.1016/j.immuni.2016.04.017
7. Anderson AC, Anderson DE. Tim-3 in autoimmunity. Curr Opin Immunol (2006) 18(6):665–9. doi: 10.1016/j.coi.2006.09.009
8. Matsumoto H, Fujita Y, Asano T, Matsuoka N, Temmoku J, Sato S, et al. Association between inflammatory cytokines and immune-checkpoint molecule in rheumatoid arthritis. PloS One (2021) 16(11):e0260254. doi: 10.1371/journal.pone.0260254
9. Huang YH, Zhu C, Kondo Y, Anderson AC, Gandhi A, Russell A, et al. Ceacam1 regulates Tim-3-Mediated tolerance and exhaustion. Nature (2015) 517(7534):386–90. doi: 10.1038/nature13848
10. Nagaishi T, Iijima H, Nakajima A, Chen D, Blumberg RS. Role of Ceacam1 as a regulator of T cells. Ann N Y Acad Sci (2006) 1072:155–75. doi: 10.1196/annals.1326.004
11. Kammerer R, Stober D, Singer BB, Obrink B, Reimann J. Carcinoembryonic antigen-related cell adhesion molecule 1 on murine dendritic cells is a potent regulator of T cell stimulation. J Immunol (2001) 166(11):6537–44. doi: 10.4049/jimmunol.166.11.6537
12. Horst AK, Bickert T, Brewig N, Ludewig P, van Rooijen N, Schumacher U, et al. Ceacam1+ myeloid cells control angiogenesis in inflammation. Blood (2009) 113(26):6726–36. doi: 10.1182/blood-2008-10-184556
13. Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, et al. 2010 rheumatoid arthritis classification criteria: An American college of Rheumatology/European league against rheumatism collaborative initiative. Arthritis Rheum (2010) 62(9):2569–81. doi: 10.1002/art.27584
14. Farschtschi S, Mattes M, Hildebrandt A, Chiang D, Kirchner B, Kliem H, et al. Development of an advanced flow cytometry based high-resolution immunophenotyping method to benchmark early immune response in dairy cows. Sci Rep (2021) 11(1):22896. doi: 10.1038/s41598-021-02405-w
15. Saiz-Gonzalo G, Hanrahan N, Rossini V, Singh R, Ahern M, Kelleher M, et al. Regulation of ceacam family members by ibd-associated triggers in intestinal epithelial cells, their correlation to inflammation and relevance to ibd pathogenesis. Front Immunol (2021) 12:655960. doi: 10.3389/fimmu.2021.655960
16. Steinbrocker O, Traeger CH, Batterman RC. Therapeutic criteria in rheumatoid arthritis. J Am Med Assoc (1949) 140(8):659–62. doi: 10.1001/jama.1949.02900430001001
17. van de Weyer PS, Muehlfeit M, Klose C, Bonventre JV, Walz G, Kuehn EW. A highly conserved tyrosine of Tim-3 is phosphorylated upon stimulation by its ligand galectin-9. Biochem Biophys Res Commun (2006) 351(2):571–6. doi: 10.1016/j.bbrc.2006.10.079
18. Postigo AA, Garcia-Vicuna R, Laffon A, Sanchez-Madrid F. The role of adhesion molecules in the pathogenesis of rheumatoid arthritis. Autoimmunity (1993) 16(1):69–76. doi: 10.3109/08916939309010649
19. Lowin T, Straub RH. Integrins and their ligands in rheumatoid arthritis. Arthritis Res Ther (2011) 13(5):244. doi: 10.1186/ar3464
20. Jarvis JN, Petty HR, Tang Y, Frank MB, Tessier PA, Dozmorov I, et al. Evidence for chronic, peripheral activation of neutrophils in polyarticular juvenile rheumatoid arthritis. Arthritis Res Ther (2006) 8(5):R154. doi: 10.1186/ar2048
21. Honig M, Peter HH, Jantscheff P, Grunert F. Synovial pmn show a coordinated up-regulation of Cd66 molecules. J Leukoc Biol (1999) 66(3):429–36. doi: 10.1002/jlb.66.3.429
22. Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol (2018) 18(2):134–47. doi: 10.1038/nri.2017.105
23. van Rees DJ, Szilagyi K, Kuijpers TW, Matlung HL, van den Berg TK. Immunoreceptors on neutrophils. Semin Immunol (2016) 28(2):94–108. doi: 10.1016/j.smim.2016.02.004
24. Pillinger MH, Abramson SB. The neutrophil in rheumatoid arthritis. Rheum Dis Clin North Am (1995) 21(3):691–714. doi: 10.1016/S0889-857X(21)00463-4
25. Felzmann T, Gadd S, Majdic O, Maurer D, Petera P, Smolen J, et al. Analysis of function-associated receptor molecules on peripheral blood and synovial fluid granulocytes from patients with rheumatoid and reactive arthritis. J Clin Immunol (1991) 11(4):205–12. doi: 10.1007/BF00917426
26. Gray-Owen SD, Dehio C, Haude A, Grunert F, Meyer TF. Cd66 carcinoembryonic antigens mediate interactions between opa-expressing neisseria gonorrhoeae and human polymorphonuclear phagocytes. EMBO J (1997) 16(12):3435–45. doi: 10.1093/emboj/16.12.3435
27. Stocks SC, Ruchaud-Sparagano MH, Kerr MA, Grunert F, Haslett C, Dransfield I. Cd66: Role in the regulation of neutrophil effector function. Eur J Immunol (1996) 26(12):2924–32. doi: 10.1002/eji.1830261218
28. Nagaishi T, Pao L, Lin SH, Iijima H, Kaser A, Qiao SW, et al. Shp1 phosphatase-dependent T cell inhibition by Ceacam1 adhesion molecule isoforms. Immunity (2006) 25(5):769–81. doi: 10.1016/j.immuni.2006.08.026
29. Lake CM, Voss K, Bauman BM, Pohida K, Jiang T, Dveksler G, et al. Tim-3 drives temporal differences in restimulation-induced cell death sensitivity in effector Cd8(+) T cells in conjunction with Ceacam1. Cell Death Dis (2021) 12(4):400. doi: 10.1038/s41419-021-03689-6
30. Zhu C, Anderson AC, Kuchroo VK. Tim-3 and its regulatory role in immune responses. Curr Top Microbiol Immunol (2011) 350:1–15. doi: 10.1007/82_2010_84
31. Han G, Chen G, Shen B, Li Y. Tim-3: An activation marker and activation limiter of innate immune cells. Front Immunol (2013) 4:449. doi: 10.3389/fimmu.2013.00449
Keywords: rheumatoid arthritis, CEACAM1, TIM–3, neutrophils, monocytes
Citation: Matsumoto H, Fujita Y, Onizawa M, Saito K, Sumichika Y, Yoshida S, Temmoku J, Matsuoka N, Yashiro-Furuya M, Asano T, Sato S, Suzuki E, Machida T, Watanabe H and Migita K (2022) Increased CEACAM1 expression on peripheral blood neutrophils in patients with rheumatoid arthritis. Front. Immunol. 13:978435. doi: 10.3389/fimmu.2022.978435
Received: 26 June 2022; Accepted: 28 November 2022;
Published: 14 December 2022.
Edited by:
Ram P. Singh, United States Department of Veterans Affairs, United StatesReviewed by:
Amit Gandhi, Brigham and Women’s Hospital and Harvard Medical School, United StatesYasser M. El-Sherbiny, Nottingham Trent University, United Kingdom
Copyright © 2022 Matsumoto, Fujita, Onizawa, Saito, Sumichika, Yoshida, Temmoku, Matsuoka, Yashiro-Furuya, Asano, Sato, Suzuki, Machida, Watanabe and Migita. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kiyoshi Migita, migita@fmu.ac.jp
 Haruki Matsumoto
Haruki Matsumoto Yuya Fujita
Yuya Fujita Michio Onizawa
Michio Onizawa Kenji Saito1
Kenji Saito1 Shuhei Yoshida
Shuhei Yoshida Jumpei Temmoku
Jumpei Temmoku Shuzo Sato
Shuzo Sato Eiji Suzuki
Eiji Suzuki Takeshi Machida
Takeshi Machida Kiyoshi Migita
Kiyoshi Migita