- 1Lab of Gastroenterology and Hepatology, State Key Laboratory of Biotherapy, West China Hospital, Sichuan University, Chengdu, China
- 2Department of Gastroenterology, West China Hospital, Sichuan University, Chengdu, China
The biliary system is comprised of cholangiocytes and plays an important role in maintaining liver function. Under normal conditions, cholangiocytes remain in the stationary phase and maintain a very low turnover rate. However, the robust biliary repair is initiated in disease conditions, and different repair mechanisms can be activated depending on the pathological changes. During biliary disease, immune cells including monocytes, lymphocytes, neutrophils, and mast cells are recruited to the liver. The cellular interactions between cholangiocytes and these recruited immune cells as well as hepatic resident immune cells, including Kupffer cells, determine disease outcomes. However, the role of immune cells in the initiation, regulation, and suspension of biliary repair remains elusive. The cellular processes of cholangiocyte proliferation, progenitor cell differentiation, and hepatocyte-cholangiocyte transdifferentiation during biliary diseases are reviewed to manifest the underlying mechanism of biliary repair. Furthermore, the potential role of immune cells in crucial biliary repair mechanisms is highlighted. The mechanisms of biliary repair in immune-mediated cholangiopathies, inherited cholangiopathies, obstructive cholangiopathies, and cholangiocarcinoma are also summarized. Additionally, novel techniques that could clarify the underlying mechanisms of biliary repair are displayed. Collectively, this review aims to deepen the understanding of the mechanisms of biliary repair and contributes potential novel therapeutic methods for treating biliary diseases.
1 Introduction
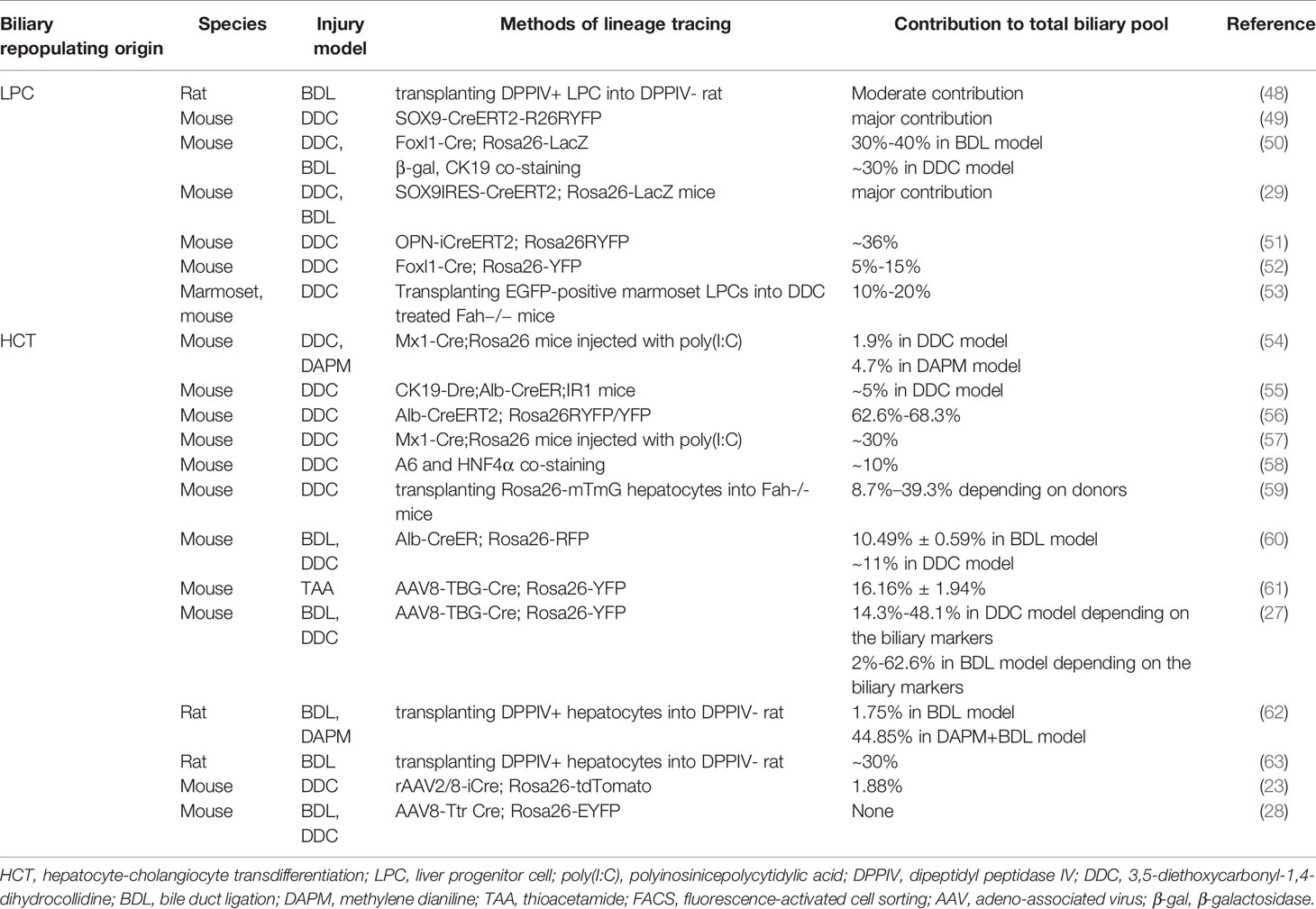
Cholangiocytes represent less than 5% of the cell population in the liver; however, they are indispensable for maintaining healthy liver function (1). Cholangiocytes line the biliary system from the canal of hering to the duodenum and are responsible for modifying bile formation and drainage (Figure 1A) (2). In addition, cholangiocytes secrete mucins, defensins, and immunoglobins during a quiescent state, protecting the biliary system from injury (3).
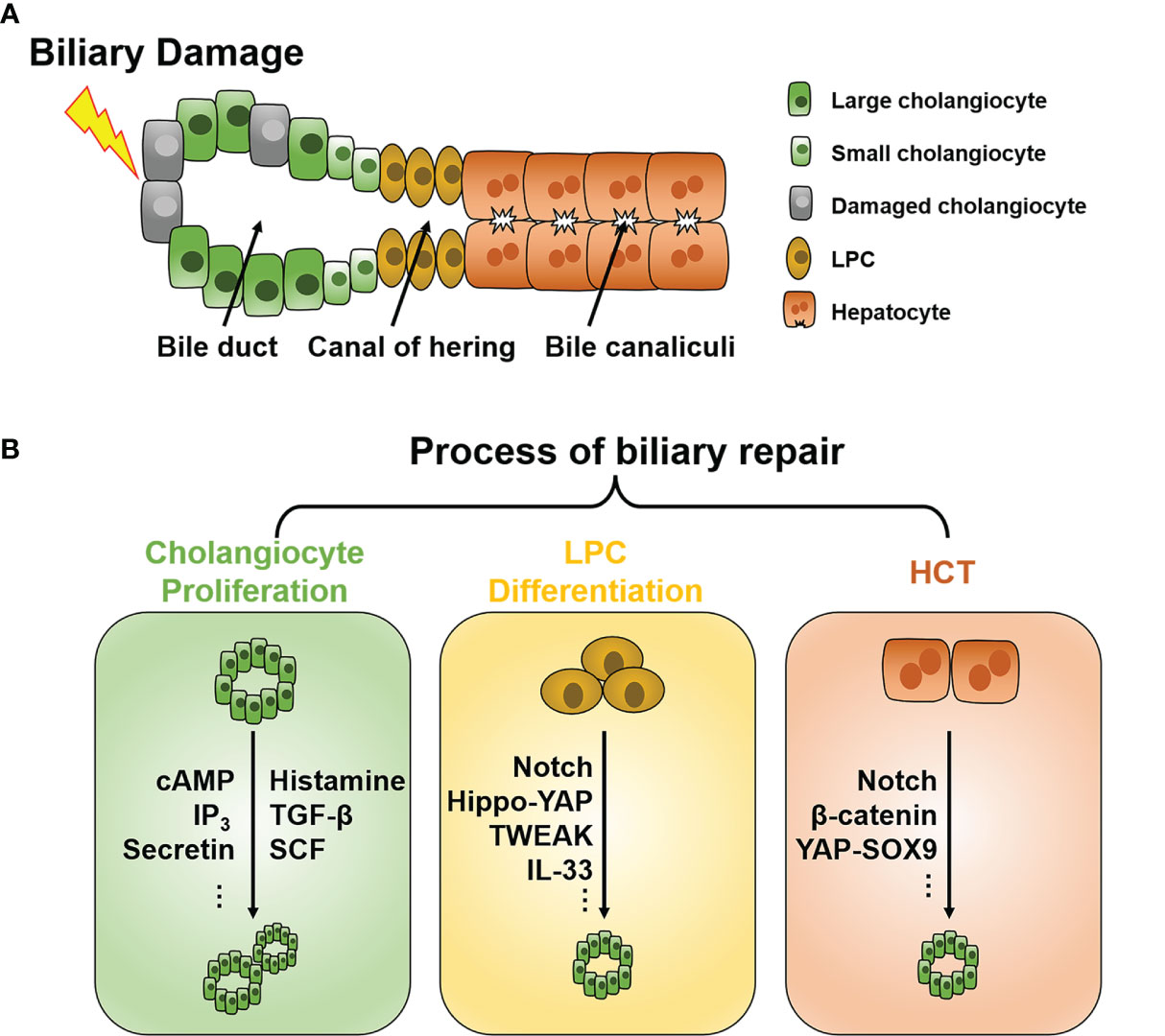
Figure 1 Schematic of the intrahepatic biliary tree structure and major mechanisms of biliary repair during biliary damage. (A) The intrahepatic biliary tree consists of bile canaliculi surrounded by apical membranes of hepatocytes, the canal of hering where LPC resides, and the bile duct lined by small and large cholangiocytes. (B) When subjected to insults, different repair mechanisms such as cholangiocyte proliferation, LPC differentiation, and HCT can be triggered depending on the pathological conditions. LPC, liver progenitor cell; HCT, hepatocyte-cholangiocyte transdifferentiation; cAMP, cyclic adenosine monophosphate; IP3, inositol 1,4,5-trisphosphate; TGF-β, tissue growth factor-β; TWEAK, TNF-like weak inducer of apoptosis; SCF, stem cell factor; YAP, Yes-associated protein; SOX9, Sry HMG box protein 9; IL, interleukin.
Genetic disorders and the majority of liver injuries could impair biliary function, namely cholangiopathies. Cholangiopathies are characterized by dysregulation of bile formation and bile flow (including bile acid circulation), biliary inflammation and fibrosis, and ultimately, cholangiocarcinoma (CCA) (4). When subjected to pathogenic factors, cholangiocytes can be activated and secrete various cytokines and chemokines, resulting in multiple pathophysiological changes and triggering cellular crosstalk, including activation of myofibroblasts and hepatic resident immune cells such as Kupffer cells, and infiltration of peripheral immune cells, such as monocytes, lymphocytes, mast cells, and neutrophils. These pathogenic changes in cholangiocytes eventually lead to biliary dysfunction and cholangiopathies (5). Cholangiopathies consist of a broad disease spectrum and can be classified etiologically as inherited, immune-mediated, infectious, vascular, malignant, and others. Although these diseases are relatively uncommon, they still cause considerable morbidity and mortality yearly. Even worse, limited therapies and treatments are available for cholangiopathies (4). Thus, research is warranted to elucidate the pathogenesis and discover promising therapeutic targets for cholangiopathies.
The liver possesses vigorous regenerative capability and is the only solid organ in the human body that can fully recover to a normal state under compensated injury (6). Liver regeneration is a complicated process regulated by multiple mechanisms, including classical signaling pathways as well as epigenetic and posttranscriptional modulation (6–8). As a vital part of the liver, the biliary system also harbors great reparative capacity. After biliary damage and consequent destruction of the biliary epithelium, a robust repopulation of cholangiocytes can be exerted to restore the equilibrium of the biliary epithelium. This specific tissue repair is defined as biliary repair.
Biliary repair involves diverse repopulating origins of cholangiocytes and complicated regulating systems and is essential for tissue homeostasis maintenance and disease regression. Nevertheless, aberrant and excessive biliary repair might play a role in carcinogenesis and the development of several cholangiopathies (9, 10). This review focused on the mechanisms and regulation of biliary repair in different cholangiopathies and highlighted the role of immune cell-mediated modulation of biliary repair. The role of aberrant biliary repair in the pathogenesis of some cholangiopathies is also summarized.
2 Mechanism of Biliary Repair
Under normal conditions, cholangiocytes are retained in the stationary phase and maintain a very low turnover rate to counterbalance occasional cell loss, such as apoptosis and senescence (11). When subjected to injuries, different repair mechanisms can be triggered depending on the pathological conditions. To date, three chief processes of biliary repair have been demonstrated (Figure 1B): 1) cholangiocyte proliferation, which is the replication of pre-existing cholangiocytes; 2) liver progenitor cell (LPC) differentiation toward cholangiocytes; and 3) hepatocytes with high plasticity transform to cholangiocytes, namely hepatocyte-cholangiocyte transdifferentiation (HCT). These processes generally orchestrate the reparation of biliary injury, although they contribute unequally in different cholangiopathies.
2.1 Cholangiocyte Proliferation
In a healthy liver, most cholangiocytes remain dormant mitotically, and proliferation occasionally occurs to achieve cellular equilibrium (11). In this review, we mainly focus on cholangiocyte proliferation in pathological conditions. The cell size of cholangiocytes gradually increases with morphological and functional changes along with the canal of hering to the large bile duct (12). This cell heterogeneity along with the biliary tree endows them with different responsiveness to insults (13). Major signaling pathways driving proliferation in both small and large cholangiocytes are Ca2+ and cyclic adenosine monophosphate (cAMP)-dependent (14). Secretin can bind to secretin receptors, and only large cholangiocytes express and elevate intracellular cAMP, initiating cell proliferation through the protein kinase A (PKA)/mitogen-activated protein kinase (MEK)/extracellular signal-regulated protein kinase1/2 (ERK1/2) signaling pathway (15–17). For small cholangiocytes, agents such as forskolin and follicle-stimulating hormone can also trigger cAMP increase and lead to cholangiocyte proliferation (17, 18). In addition, activation of the inositol 1,4,5-trisphosphate (IP3)/Ca2+ signaling pathway can also stimulate the proliferation of small cholangiocytes (14, 19).
Usually, large bile ducts are more susceptible to damage, resulting in compromised proliferative capability (20). Under this condition, small cholangiocytes can acquire the phenotype of large cholangiocytes and help to restore biliary epithelium as a compensatory method (21, 22). However, the mechanisms of such differentiation remain elusive. Heterogeneity of regenerating capacity was also found independent of cell size in cholangiocytes. It was indicated that cholangiocyte proliferation predominantly contributes to biliary repair. Proliferation is chiefly generated by a subgroup of cholangiocytes with persistent regenerating capability, which is not predestined but rather “stochastically” regulated during injury (23). In general, the biliary system does not uniformly proliferate as a whole due to significant cellular heterogeneity among the biliary tract, and this highly dynamic and adaptive replenishment process plays a fundamental role in biliary repair.
2.2 Liver Progenitor Cell Differentiation
Although cholangiocytes possess immense regenerative capacity, severe acute injury and prolonged chronic injury could exhaust their proliferative potential (24). The liver could also activate alternative reparation methods, such as the LPC expansion, to avoid abnormal repair, which eventually leads to ductular fibrosis and cirrhosis. LPCs, also known as oval cells, are considered bipotent progenitor cells that reside adjacent to the canal of hering and can differentiate into cholangiocytes or hepatocytes (25, 26). However, whether LPCs can give rise to cholangiocytes or hepatocytes under physiological conditions has obtained controversial results (27–30), so as in the biliary repair of cholangiopathies. While LPCs expand in response to insults, the cell fate of LPCs is chiefly determined by the type of liver injury (11, 31). When biliary damage causes regenerative capacity exhaustion of cholangiocytes, LPCs can differentiate toward cholangiocytes and impede hepatocyte differentiation simultaneously. Such lineage specification is modulated by an intricate signaling network, including the Notch (31), Wnt-β-catenin (31–34), and Hippo-Yes-associated protein (YAP) signaling pathways (35, 36).
LPC differentiation is also, to a large extent, modulated by their surrounding cellular milieu, called the “LPC niche”. The LPC niche comprises hepatic stellate cells (HSCs), macrophages and other immune cells, endothelial cells, and extracellular matrix (ECM) (37–39). Based on their adjacent distributions, HSCs (31, 40, 41) and macrophages (31, 42–44) can regulate parenchymal infiltration and differentiation orientation of LPC by intimate cellular crosstalk. In addition, ECM composition and remodeling during liver injury were also found to be crucial to LPC expansion and activation (45, 46). Though the contribution of LPCs for liver repair has been confirmed in several disease models, the cellular source of LPCs during liver repair is still elusive. LPCs originating from pre-existing hepatic stem cells is one of the hypotheses; however, compelling evidence, i.e., exclusive molecular markers is lacking (47). Moreover, the contribution of LPCs to the regenerating biliary pool differs according to the liver injury model and lineage tracing methods utilized (Table 1).
2.3 Hepatocyte-Cholangiocyte Transdifferentiation
Another theory with nonnegligible evidence is that hepatocytes and cholangiocytes actually serve as facultative progenitor cells with reprogramming capacity and can transdifferentiate into each other. The intermediate phase of cells undergoing this process expresses both hepatocyte and cholangiocyte phenotypes and might be interpreted as LPCs, which seem inappropriate as they are not independent cell clusters and harbor considerable heterogeneity within the cluster (27, 47, 64). The more moderate theory is that hepatocytes or cholangiocytes first de-differentiate to LPCs and then differentiate to each other (65, 66). Each postulation obtains proponents and opponents, and in this review, we focus on those relevant to biliary repair.
Several studies failed to observe cell plasticity of lineage-tagged hepatocytes in the condition of biliary injury caused by bile duct ligation (28) or 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC) diet model (23). Conversely, other researchers have confirmed HCT in vivo in rodent biliary injury models by utilizing the lineage-tracing technique. This HCT process seems to occur without LPC involvement. However, the proportion of HCT-derived cholangiocytes varied extensively in different models (Table 1). Transplanting labeled hepatocytes into the liver of recipients can also repopulate the biliary pool through HCT after biliary damage, whereas its contributions to the biliary pool remain varied (Table 1). During acute biliary injury, almost all hepatocytes are capable of HCT (67), while a subpopulation of Sry HMG box protein 9 (SOX9)-positive hepatocytes was identified as most potent in biliary reprogramming because they comprise 3% of all hepatocytes but contribute to nearly one-third of HCT (60, 68). These results correspond with clinical findings that cells coexpressing hepatocyte and cholangiocyte markers exist in liver samples from patients with cholangiopathies (69).
Although HCT derived cholangiocytes can acquire a genetic phenotype similar to that of bona fide cholangiocytes, it remains inconclusive whether these cells have sufficient biliary function. It was reported that cholangiocytes of HCT origin could obtain featured cellular polarity (positive staining of apical and basolateral markers of cholangiocytes, including primary cilia) (27) and even form mature bile ducts capable of bile drainage when the biliary system develops abnormally (70). In contrast, some studies found that HCT-derived cholangiocytes are functionally immature, and unable to coalesce with the pre-existing bile duct. These HCT-derived cholangiocytes might be a transient form that will revert to hepatocytes after injury subsides (23, 56, 59, 71). The possibility that these maladaptive cells contribute to CCA formation was also raised (9, 72).
In general, the role of HCT in biliary repair is not yet fully understood. It seems to be a double-edged sword that can respond to biliary damage and rescue the biliary system by replenishing the biliary pool. However, it might not be sufficient to form a mature bile duct to counterbalance compromised biliary proliferative capacity and function, even giving rise to carcinogenesis.
2.4 Other Sources of Biliary Repair
Except for the processes mentioned above, there are some less common sources of biliary repair. Some progenitor cells reside in the peribiliary gland in the extrahepatic biliary tree (73). They resemble LPCs in many ways, such as similar genetic phenotypes and are likewise capable of bipotent differentiation to hepatocytes and cholangiocytes. In comparison, they are primarily activated when large intrahepatic or extrahepatic bile ducts are affected (74–76). Moreover, it was reported that bone marrow stem cells can be recruited to the liver during several liver diseases and can differentiate into LPCs and cholangiocytes (77–79). Compared to the abovementioned process, these approaches may play a less important role in biliary repair. However, they remain worthy of further study as they, especially bone marrow stem cells, serve as a potential cellular therapy for liver disease (80).
3 Crosstalk Between the Immune System and Biliary Repair
Cholangiocytes are susceptible to various exogenous and endogenous insults and were once deemed innocent victims of liver injury. However, they are now unmasked as dynamic participants in the pathogenesis and development of several liver diseases (5). Cholangiocytes subjected to insults can switch from the quiescent form to the active form, which is also called “reactive ductular cells (RDCs)” and is characterized by an anomalous morphology and secretory phenotype (81). RDCs play a multifaceted role in orchestrating biliary repair by autocrine and paracrine signaling of several chemokines and cytokines (82). Similarly, immune cells have long been recognized as an essential player in tissue hemostasis and repair (83). Recent studies further expand our knowledge about the communication between cholangiocytes and the immune system in biliary repair.
In the quiescent stage, cholangiocytes contribute to immune function by secreting immunoglobin A and antimicrobial peptides into the bile duct (84). Under disease conditions, damage-associated molecular patterns originate from adjacent damaged hepatocytes or nonparenchymal cells, and pathogen-associated molecular patterns derived from the gut-liver axis and bloodstream can activate Toll-like receptors constitutively expressed on cholangiocytes and provoke far more vigorous biliary immune responses (85, 86). RDCs can release a repertoire of chemokines and cytokines, such as the CC-motif and CXC-motif chemokine family, interleukin (IL) -6, 8, 17, etc., and can recruit and activate various immune cells such as T cells, macrophages, and neutrophils to the periductular niche (87–94). These immune cells in turn modify biliary repair by adjusting the proliferating capability of adjacent cholangiocytes (95), expansion and differentiation of LPC (31, 42–44, 96), or plasticity of hepatocytes (61), etc. Thus, the crosstalk between cholangiocytes and immune cells and the specific role of different immune cell subsets in biliary repair are summarized.
3.1 Macrophages
Macrophages are the most abundant immune cells in the liver and comprise liver-resident Kupffer cells located in the space of Disse and macrophages derived from monocytes recruited from peripheral circulation (97). Macrophages are functionally heterogenous and are traditionally categorized into the proinflammatory “M1” subtype and the anti-inflammatory “M2” subtype (98). However, accumulating evidence indicates that macrophages have a more complicated phenotype spectrum, as they perform manifold or even opposing functions and express both M1 and M2 markers concurrently during liver injury (99, 100). Macrophages generally play a dual role in the pathogenesis of cholangiopathies; for example, during cholestasis, macrophages can either be activated to release proinflammatory factors and promote liver fibrosis (101), or perform an enhanced anti-inflammatory response mediated by the bile acid signaling pathway (102, 103). The balance between the proinflammatory and anti-inflammatory effects of macrophages was shown to be associated with the severity of cholestasis diseases and played a crucial role in biliary repair (104).
In cholangiopathies, CC-chemokine receptor 2 (CCR2) positive macrophages can be recruited to the periductular niche by CC-chemokine ligand 2 (CCL2) and other chemokines released from injured cholangiocytes (90). These peribiliary macrophages can provoke cholangiocyte proliferation by inducing the expression of Integrin αvβ6 (ITGB6) on cholangiocytes (105). Additionally, macrophages can eliminate senescent cholangiocytes and cell debris by phagocytosis favoring cholangiocyte proliferation (106). In addition, surrounding macrophages can incite expansion of LPC by secreting TNF-like weak inducer of apoptosis (TWEAK) even in the absence of liver injury. Depletion of CD11b+ macrophages can ameliorate this effect (107). After LPC expansion, macrophages can further regulate the lineage specification of LPC by paracrine signaling (31).
Using single-cell RNA sequencing (scRNA-seq), functionally distinct subpopulations of macrophages were discovered, and the composition of the macrophage population changes dynamically during liver diseases (108). For example, TREM2+CD9+ profibrogenic macrophages expand in liver fibrosis and induce HSC proliferation and activation (109). MACRO+ and MACRO- macrophages were also found responding differently to inflammatory stimulations (110). Immune-related genes expression of macrophages are significantly altered in the DDC mouse model, and macrophage interactions with other liver cells are largely inhibited (111). However, contributions of heterogenous macrophages to biliary repair during cholangiopathies are largely unknown at the single-cell level. Studies further dissecting the specific role of different subsets of macrophages in biliary repair are warranted.
3.2 Neutrophils
Neutrophils respond swiftly to liver injury and are the first immune cells recruited to the injury sites. Neutrophils are considered proinflammatory cells and are responsible for exacerbating tissue damage (112). The infiltration of neutrophils is ubiquitous in liver injury and is involved in many cholangiopathies (97). For example, neutrophils can directly interact with cholangiocytes via integrin β1 and elicit cholestasis (113). Neutrophils were also indicated as central immune cells in the pathogenesis of immune-mediated cholangiopathies such as primary biliary cholangitis (PBC) and primary sclerosing cholangitis (PSC) (114). Recently their contribution to tissue repair was also discovered. Neutrophils can clear the injury site by phagocytosis, degranulation, and neutrophil extracellular traps to create an appropriate milieu for tissue reconstruction (115). Moreover, monocytes can be recruited by neutrophil-released IL-37 and help tissue repair (116). Nevertheless, their specific role in biliary repair has scarcely been studied, and further research is needed.
3.3 Mast Cells
Mast cells, a kind of inflammatory cell capable of releasing histamine and other particles, are emerging as novel participants in cholangiopathies (117). The infiltration of mast cells was detected in close proximity to cholangiocytes during cholestasis, PSC, PBC, and CCA (118–120). In vivo studies using antagonists and gene knockout mouse models further revealed a mast cell-mediated histamine-tissue growth factor-β (TGF-β)/stem cell factor (SCF) axis in the pathogenesis of PSC and CCA (117, 121). Concerning biliary repair, mast cells mainly regulate cholangiocyte proliferation. Coculture of cholangiocytes with mast cells isolated from bile duct ligated (BDL) rats significantly increased cholangiocyte proliferation via mast cell-derived histamine (118). Moreover, in vivo H1/H2 histamine receptor blockage dampened the proliferation of small cholangiocytes and large cholangiocytes, respectively (119). Whether other mechanisms of mast cells also contribute to biliary repair remains unclear and needs more studies.
3.4 Lymphocytes
Lymphocytes encompass many cell types, including T lymphocytes, B lymphocytes, natural killer cells, etc. ScRNA-seq of the human liver revealed distinct transcriptomic profiles of intrahepatic lymphocytes compared to peripheral lymphocytes (109). In the condition of liver cirrhosis, phenotype shift was observed in T cells and natural killer cells, such as expansion of a SELL+CD4+ memory T cell population and loss of CD56+CD16− natural killer cells population (109, 122). Moreover, significant T cell heterogeneity was observed in liver of CCA and PSC patients and associated with disease pathogenesis and prognosis (123, 124). In contrast, there was no significant phenotypic changes of intrahepatic B cells in cirrhotic livers (109).
Nevertheless, the effect of these lymphocytes on biliary repair remains largely unknown. Innate lymphoid cells (ILCs), a newly defined, less enriched lymphocyte population located on the mucosal surface, are involved in regulating cholangiocyte proliferation. IL-33 can trigger cholangiocyte proliferation in a mouse model of biliary atresia, and type 2 ILCs (ILC2s) and ILC2-secreted IL-13 were found downstream of IL-33 in this mitogenic effect, which was abolished in the absence of ILC2s (95). IL-13 was also reported to be potent to drive LPC expansion and differentiation toward biliary fate (125). ScRNA-sec further revealed that two CD45+ ILC2 subsets, canonical ILC2, and newly defined “biliary immature myeloid cells”, interaction played an important role in IL-33 induced cholangiocyte proliferation (126). However, 10 weeks of IL-33 injection resulted in biliary tumorigenesis in mice genetically predisposed to CCA (95). It seems that the effect of lymphocytes on biliary repair is less well-defined.
3.5 Hepatic Stellate Cells
HSCs, the primary producer of hepatic ECM, are also closely related to biliary repair during cholangiopathies (127). Therefore, we included HSCs and introduced their role in biliary repair. HSCs and infiltrated immune cells together constitute the LPC niche (39). Likewise, HSCs can be activated and transformed to collagen-depositing myofibroblasts through immune cells-mediated signaling pathways, such as those involving macrophage-derived TGF-β and platelet-derived growth factor (PDGF) (127). Myofibroblast activation and ECM deposition are essential for LPC expansion. Myofibroblast-secreted Jagged1 could promote LPC differentiation toward the biliary lineage in the condition of biliary injury (31, 41, 128). Moreover, myofibroblasts can also regulate HCT by interacting with laminin and ITGB6 (61). Except for the abovementioned functions, HSCs were even reported to gain progenitor features and function as bipotent LPCs to repopulate the liver parenchyma after partial hepatectomy (PHx) (129, 130). By utilizing scRNA-seq, functional zonation patterns and heterogeneous subpopulations of HSCs were discovered (131). However, its critical role in biliary repair remains to be elucidated.
The role of different immune cells in biliary repair is summarized in Figure 2. Notably, immune cells are the key participants in the inflammatory response and have a double-sided effect on tissue repair. RDCs are highly proliferative to refurbish damaged biliary trees. Recruited immune cells could perpetuate the proliferation of RDCs, whereas protracted inflammation and excessive immune cell infiltration disturb biliary function and induce cell death (132, 133), ultimately leading to ductopenia caused by overbalanced apoptosis (134, 135). Similarly, cholangiocyte senescence, a mechanism to avoid neoplasia by irreversible cell cycle arrest, also has a secretory phenotype comparable to RDCs (5). Although senescence means decreased proliferation, mild senescence can provoke tissue repair by other mechanisms, including secretion of growth factors and proteins facilitating the expansion of LPCs and recruitment of immune cells regulating tissue repair (136, 137). Nonetheless, preserving senescence leads to tissue damage and impaired tissue repair (137). The immune cells show a double-edged sword role in biliary repair, and further studies that focus on the crosstalk of immune cells and cholangiocytes are urgently needed. As the heterogeneity of immune cells exists during biliary repair, it is also crucial to clarify the specific properties and factors secreted by these immune cells.
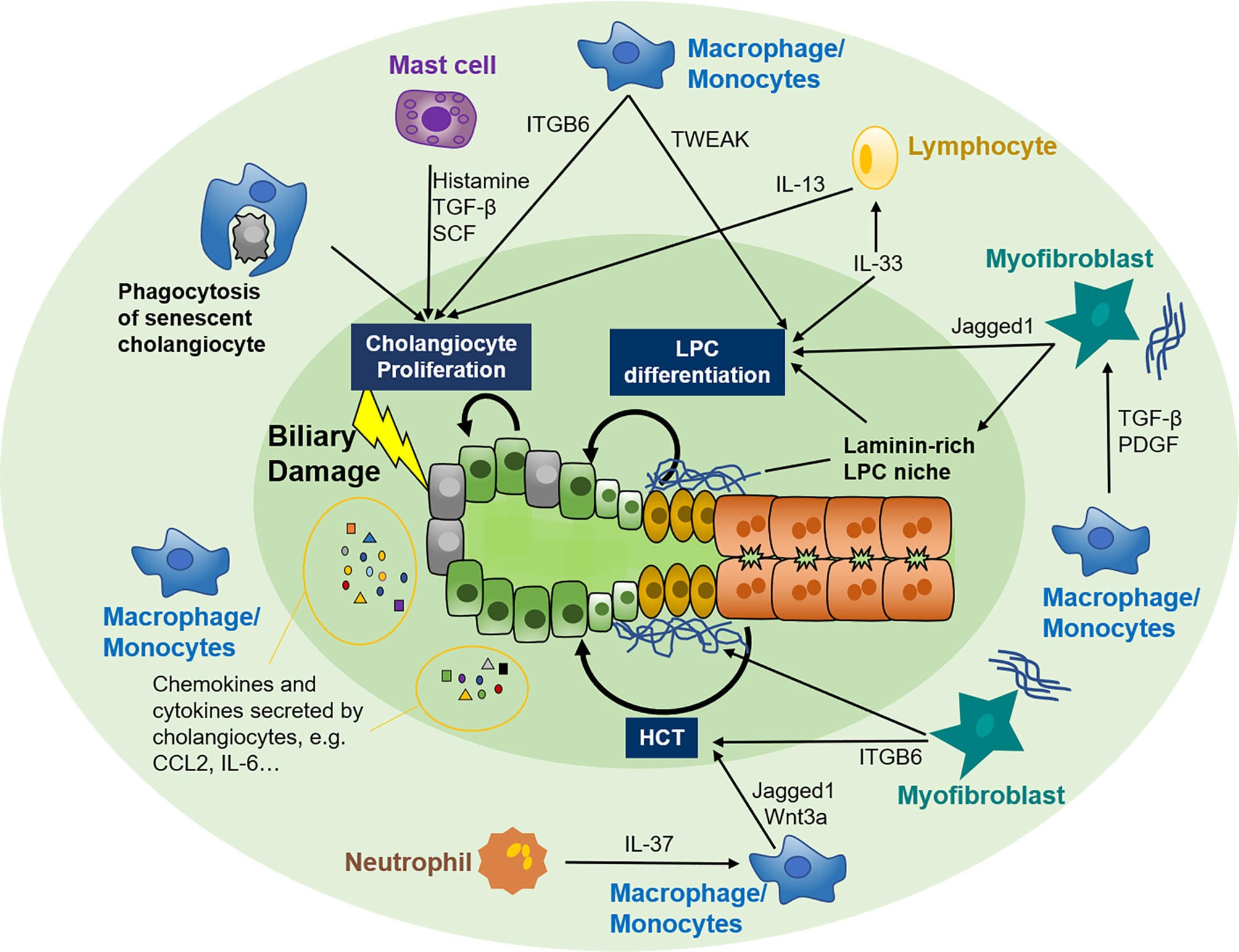
Figure 2 Crosstalk between the immune system and biliary repair. Cholangiocytes are susceptible to various exogenous and endogenous insults that facilitate the switch from quiescent to active. Active cholangiocytes can recruit various immune cells, including macrophages, mast cells, neutrophils, and lymphocytes, to biliary injury sites by autocrine and paracrine of several chemokines and cytokines. In turn, these immune cells modify biliary repair by adjusting the proliferating capability of adjacent cholangiocytes, expansion and differentiation of LPC, or plasticity of hepatocytes. Myofibroblasts are also involved in the crosstalk between immune cells and biliary repair. LPC, liver progenitor cell; HCT, hepatocyte-cholangiocyte transdifferentiation; TGF-β, tissue growth factor β; SCF, stem cell factor; IL, interleukin; TWEAK, TNF-like weak inducer of apoptosis; ITGB6, integrin αvβ6; CCL2, CC-chemokine ligand 2; CXCL2, CXC-chemokine ligand 2; PDGF, platelet-derived growth factor.
4 Role of Immune Cells in Biliary Repair in Cholangiopathies
Genetic disorders and liver injuries could impair biliary function, namely cholangiopathies. The role of immune cells in the development of cholangiopathies remains elusive. We next summarize the effect of biliary repair mediated by immune cells in cholangiopathies, such as immune-mediated cholangiopathies. As few studies are available on immune cells and biliary repair in inherited cholangiopathies, obstructive cholangiopathies, and CCA, other mechanisms of biliary repair are reviewed for these diseases.
4.1 Immune-Mediated Cholangiopathies
PBC and PSC are two major chronic cholestatic diseases caused by disordered immune conditions (82). Putatively, the pathogenesis of PSC is associated with the detrimental effect caused by the crosstalk of active cholangiocytes with innate and adaptive immune systems, which are activated by proinflammatory mediators originating from gut microbiota (138). Toxic bile acid was also proposed to contribute to PSC development (138). For PBC, a breach of biliary immune tolerance of the E2 subunit of the pyruvate dehydrogenase complex and an aberrantly activated immune response were postulated to play a central role in PBC pathogenesis (139). Despite these hypotheses, the pathogenesis of the two diseases remains incompletely understood, and effective treatment is lacking. Studies concerning biliary repair in these diseases are warranted to facilitate understanding of disease development and provide potential therapeutic targets.
4.1.1 Cholangiocyte Activation
An increase in cholangiocyte activation and senescence along with the subsequent secretion of proinflammatory factors was observed in both PBC and PSC, indicating impaired biliary repair (140, 141). Overactivation of the Notch signaling pathway was found in both diseases, further indicating aberrant biliary repair conditions (142, 143). As immune-mediated diseases, the immune cells recruited by cholangiocyte secretion significantly altered the composition of intrahepatic immune cells and are involved in disease pathogenesis and regulation of biliary repair (144). ScRNA-seq of liver of PBC patients indicated that ORMDL3+ cholangiocytes displayed higher interaction with immune cells such as macrophages and monocytes, which might play a role in the pathogenesis of PBC (145).
4.1.2 Macrophages
Macrophages induce ITGB6 expression on cholangiocytes in animal models of biliary epithelium injury and congenital hepatic fibrosis (105, 146). Furthermore, ITGB6 was critical to LPC function in animal models of sclerosing cholangitis mimicking PSC (147). The above results indicate a potential role of macrophages in the regulation of LPC-mediated biliary repair. Moreover, distinct LPC differentiation patterns were observed between PSC and PBC. Generally, LPCs exhibit a predominant biliary phenotype in PBC, while LPCs are more likely to differentiate into hepatocytes in PSC (148).
Moreover, expansion of progenitor cells in the peribiliary gland was also reported in PSC but not in PBC (149). This finding can be partly explained by the different distributions of biliary lesions. Interlobular bile ducts are mainly affected in PBC, thus LPCs residing in the canal of hering around the portal tract are activated to refurbish injured ductules. In contrast, large intrahepatic and extrahepatic bile ducts are the major damaged targets in PSC, favoring activation of peribiliary gland stem cells rather than LPCs (149). In addition, biliary stenosis and resultant cholestasis-induced hepatocytic injury in the PSC also drive LPCs toward hepatocyte differentiation (148). Another theory is associated with immune cell crosstalk with LPC. Macrophages can determine the lineage orientation of LPCs through secretion of the Wnt-β-catenin signaling pathway, which induces hepatocyte differentiation and antagonizes biliary differentiation by inhibiting the Notch signaling pathway (31). Correspondingly, the relative numbers of macrophages in PSC were found to be three-fold higher than those in PBC (150). Enriched Wnt3a positive staining was observed in PSC patients (148), indicating the intriguing role of macrophages against biliary repair in PSC.
Furthermore, macrophages might also contribute to disease regression by eliminating senescent cells (106). LPCs also contribute to disease regression by releasing extracellular vesicles containing microRNA (miRNA) lethal-7, which can inhibit cholangiocyte activation and biliary fibrosis in multidrug-resistant protein 2 (Mdr2) -/- mouse model resembling features of human PSC (151). For PBC, biliary differentiation of LPCs might be promoted by Notch signaling pathway activation through cellular crosstalk with myofibroblasts (31). A laminin-rich stem cell niche also contributes to LPC commitment to the biliary lineage in PBC (39). These observations indicate that macrophages play a crucial role in the development and biliary repair of PBC and PSC.
4.1.3 Other Immune Cells and Epigenetic Factors
Other types of immune cells, such as mast cells recruited by RDCs, can prompt cholangiocyte senescence and proliferation, partly through histamine and TGF-β signaling pathways. However, mast cells might also lead to cholestatic liver injury in Mdr2 -/- mouse model (119, 121, 152). Another study found proinflammatory cytokines induced the upregulation of nitric oxide synthase 2 (NOS2) in cholangiocytes of PSC patients. NOS2 inhibits cAMP production and subsequent fluid secretion into the bile duct, thus exacerbating cholestasis. This might also impair cAMP dependent cholangiocyte proliferation (133). Whether other immune cells and inflammatory cytokines are also involved in the pathogenesis of PBC and PSC needs more solid evidence.
A recent study also indicated an important role of long non-coding RNAs (lncRNAs) in the pathogenesis of PSC. LncRNA ACTA-AS1 was found upregulated in cholangiocytes of PSC patients. ACTA-AS1 facilitated cholangiocyte proliferation by binding p300 and promoting transcription of proliferative genes through acetylates lysine 27 on histone 3 (153). Knockdown of ACTA-AS1 significantly impaired cholangiocyte proliferation, indicating a potential role of epigenetic treatment in cholangiopathies, although more validations are needed.
4.2 Biliary Repair in Inherited Cholangiopathies
Inherited cholangiopathies are a subset of cholangiopathies caused by hereditary or genetic factors, including Alagille syndrome (ALGS), polycystic liver diseases (PLD), biliary atresia (BA), etc. Inherited cholangiopathies are rare but can lead to chronic and progressive damage to cholangiocytes (10). The pathogenesis of inherited cholangiopathies remains elusive and effective treatment is limited. Thus, understanding the mechanism and regulation of biliary repair during inherited cholangiopathies is of great significance in expanding our knowledge of these diseases and searching for potential therapeutic targets.
4.2.1 Alagille Syndrome
ALGS is an autosomal dominant dysmorphogenetic disorder caused by mutations in the Notch ligand Jagged1 or rarely in Notch receptor 2 (154). ALGS is a multisystem disorder involving the liver, vasculature, heart, eyes, and skeleton and is characterized by dysplasia of the intrahepatic bile ducts and cholestasis (155).
SOX9 is a biliary marker and regulator of biliary development (156). In ALGS patients, the SOX9 level was inversely correlated with disease severity (157). In a Jagged1+/– mouse model, the expression of SOX9 was decreased in the pericortical region of the embryonic liver (158). Removing one copy of SOX9 in Jagged1+/– livers could enhance the ductular reaction, inflammatory cell infiltration, and fibrosis and decrease the number of cholangiocytes per portal vein (157). Additionally, SOX9 overexpression activates Notch2 expression, promotes bipotential mouse embryonic liver cell differentiation into cholangiocytes, and eventually improves the morphogenesis of biliary structures, while HCT was not influenced (157). Conversely, in the Alb-Cre+/-; Rbpjf/f; Hnf6f/f mouse model, HCT contributed to the mature bile duct formation independent of the Notch signaling pathway. Additionally, TGF-β signaling pathway is another potent driver of the biliary system formation from hepatocytes (70). In the zebrafish model of ALGS, multipotent progenitors reside in the extrahepatic duct, contributing to intrahepatic duct regeneration by balancing the Jagged1/Notch and fibroblast growth factor signaling pathways (159).
Collectively, LPC differentiation plays a major role in ALGS and is mainly regulated by the Notch signaling pathway, while HCT also contributes to the development of ALGS. Many kinds of animal mutation models have been developed to exploit the role of the biliary system in ALGS. Organoid culture systems also improve the cell models from 2D to 3D structures to better explore the mechanism of liver diseases and repair (160). However, few studies have focused on the crosstalk of immune cells and surrounding cells in the development of ALGS.
4.2.2 Polycystic Liver Diseases
PLD is an autosomal dominant polycystic disease caused by mutations in the PRKCSH or SEC63 genes, both of which are expressed in cholangiocytes (4). Additionally, cAMP is an essential regulator of dysregulated signaling pathways in PLD, which increases in cholangiocytes lining liver cysts. Takeda G protein-coupled receptor 5 (TGR5) is a bile acid receptor, which is expressed in sinusoidal cells, Kupffer cells, gallbladder epithelia, and cholangiocytes. TGR5 increases cAMP expression and promotes cell proliferation and cyst growth in PLD. Inhibition of TGR5 alleviates this phenotype (161). Additionally, depleting SOX9 leads to cholangiocyte hyperproliferation and induces the formation of hepatic cysts via the Wnt/β-catenin signaling pathway (162). Moreover, diminished intracellular Ca2+ level also leads to cholangiocyte hyperproliferation in PLD development. In contrast, this hyperproliferation could be inhibited by ursodeoxycholic acid, a kind of bile acid regulating the intracellular Ca2+ level through the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT)/MEK/ERK1/2 signaling pathway (163).
Inflammatory cytokines, such as IL-6 and IL-8, are also involved in the pathogenesis of PLD (164). In addition to traditional pathogenesis factors, epigenetic elements, such as miRNAs, a kind of small non-coding RNA oligonucleotides that can regulate cell survival and proliferation, also contributed to the development of PLD. About 80% of miRNAs were found reduced in PLD cholangiocytes, which resulted in the accumulation of miRNA-targeted proteins. MiR-345 targeted cell-proliferation proteins were found most significantly increased in PLD cholangiocytes, leading to cholangiocyte hyperproliferation and cyst growth (165). MiR-15a was also decreased in PLD cholangiocytes and was associated with cholangiocyte hyperproliferation and cyst growth by increasing Cdc25A expression (166).
In general, cholangiocyte hyperproliferation is the core mechanism in PLD. SOX9, cAMP, and Ca2+ play important roles in cystic growth via various signaling pathways. However, further studies that deepen the understanding of the physiological and pathological function of immune cells in PLD are still urgently needed.
4.2.3 Biliary Atresia
BA is a leading cause of neonatal cholestasis and leads to advanced cirrhosis. BA is characterized by fibrosing obstruction of extrahepatic bile ducts, and its pathogenesis includes genetic factors, autoimmunity, abnormal fetal or prenatal circulation, inflammatory response, etc. (167). Moreover, epigenetic regulations, such as lncRNAs and miRNAs, are also involved in the development of BA.
H19 is a lncRNA and contributes to cell proliferation and differentiation. In BA patients, the expression of H19 in the liver and hepatic macrophages is significantly upregulated (168, 169). In addition, the expression level of hepatic H19 and serum exosomal H19 is positively correlated with the severity of liver fibrosis. In the BDL mouse model, knockout of H19 can inhibit cholangiocyte hyperproliferation and alleviate liver fibrosis via the sphingosine 1-phosphate receptor 2 (S1PR2)/sphingosine kinase 2 (SphK2) and lethal-7/high-mobility group AT-hook 2 (HMGA2) axes (168). H19 also regulates cholangiocyte proliferation via the Rho-GTPase signaling pathway by promoting the activation and polarization of macrophages (169).
The expression of miR-200s decreases in BA, while the expression of miR-124 increases in BA. MiR-200s promote the expression of IL-6 in cholangiocytes by inhibiting Forkhead Box A2 expression, while miR-124 reduces cholangiocyte proliferation via the inhibition of the IL-6/signal transducer and activator of transcription 3 (STAT3) signaling pathway (170). Thus, maintaining the balance of miR-200s and miR-124 may serve as potential targets for the treatment of BA. Inflammatory cytokines other than IL-6 are also involved in the development of BA. IL-33 is a member of the IL-1 cytokine family. In BA, increased IL-33 is beneficial to cholangiocyte proliferation via the IL-33/ILC2/IL-13 signaling pathway (95). In addition to the aforementioned epigenetic elements and inflammatory cytokines, transcription factors GATA6 can also control the proliferation and differentiation of cholangiocytes. Overexpression of GATA6 leads to HCT in vitro (171), and GATA6 deficiency suppresses cholangiocyte proliferation in BDL mice (172).
BA is a common cause of cirrhosis in children, and abnormal cholangiocyte proliferation is one of the most important mechanisms. Mechanically, lncRNAs, miRNAs, inflammatory cytokines, and transcription factors contribute to abnormal cholangiocyte proliferation. Apart from this, mitophagy, beta-amyloid deposition, and immune cells crosstalk are also involved in the development of BA (173–175).
4.3 Biliary Repair in Obstructive Cholangiopathies
Cholestasis induced by obstructive cholangiopathies is one of the important causes of chronic liver disease and cirrhosis. A significant role of LPC differentiation and HCT in biliary repair has been observed in animal cholestatic models.
4.3.1 Hepatocyte-Cholangiocyte Transdifferentiation
In cholestasis models, activation of Notch-Hes1 signaling can promote HCT to form primitive ductules and induce the expression of SOX9 in mature hepatocytes (56). Furthermore, SOX9+ hepatocytes can differentiate into cholangiocytes with apical-basal polarity (positive staining of apical and basolateral markers of cholangiocytes) and form neo-lumens leading to biliary repair (27, 60). These HCT-derived cholangiocytes can transform back to hepatocytes after injuring (59). SOX9+ epithelial adhesion molecule (EpCAM)− cells were found converted from mature hepatocytes with DDC injury. These cells were biphenotypic cells that can differentiate into functional hepatocytes and form bile duct-like luminal structures by converting into cholangiocyte-like cells and contributing to tissue repair (57).
The Wnt/β-catenin signaling pathway also regulates HCT-mediated biliary repair. DDC injury induces EpCAM+ cells to secrete Wnts, and activation of β-catenin with Wnt7a can promote HCT and reduce liver damage and mortality. Moreover, Kupffer cells and endothelial cells are necessary to activate β-catenin (58). However, some studies indicate that DDC only induces hepatocyte transdifferentiation to a biliary-like phenotype without EpCAM, cytokeratin 19 (CK19), and prominin-1 expression (23). One study even failed to identify hepatocyte-derived biliary epithelial cells in the left hepatic duct ligation mouse model (28). The reason may be that the damage induced by the left hepatic duct ligation mouse model instead of BDL is not severe enough to trigger HCT. Moreover, the study only detected cells without both CK19 and enhanced yellow fluorescent protein expression but did not attach importance to cholangiocyte-like cells.
PHx can stimulate hepatocyte proliferation and is a suitable model to study liver regeneration. In BDL and methylene diamiline (DAPM) after the PHx rat model, HCT was observed after injection of dipeptidyl-peptidase IV (DPPIV) positive hepatocytes into portal circulation (62). Another study showed that DPPIV positive hepatocytes could also be converted to cholangiocyte-like cells in the BDL rat model without PHx (63).
4.3.2 Liver Progenitor Cell Differentiation
LPC differentiation into cholangiocytes also contributes to biliary repair in the BDL and DDC animal models. The winged-helix transcription factor Forkhead Box l1 (Foxl1) is a putative marker of LPC in mice. The expression of Foxl1 increases in the portal tracts with liver injury by BDL or DDC, and Foxl1 positive cells differentiate into hepatocytes and cholangiocytes (50). Deleting Foxl1 in LPC can decrease cholangiocyte proliferation, leading to more severe oxidative stress and liver function injury (52). In biliary repair, SOX9+ hepatocytes play an important role in HCT. Moreover, SOX9+ LPCs show biphenotypic potential to differentiate into cholangiocytes or hepatocytes (29). The ductular structures formed by LPC were observed degenerating after recovery from DDC injury (51). Biliary obstruction induced by BDL in rats led to exogenous DPPIV positive LPC differentiation into bile duct epithelial cells (48). Moreover, immortalized marmoset hepatic progenitor cells injected into DDC mice through the spleen were also capable of differentiating into cholangiocytes (53).
Cholestatic liver diseases are critical pathogenic factors resulting in cirrhosis and liver cancer, Whereas medicine management still faces huge challenges. Although accumulating mechanisms of HCT and LPC differentiation in cholestatic liver diseases have been discovered, new technologies are still needed to explore tissue microarchitecture in bile duct development and repair. Tissue engineering of the biliary tract can recapitulate site-specific characteristics of cholestatic liver disease and allow the study of the interrelation of bile ducts with other systems and cell-to-matrix interactions (176).
4.4 Biliary Repair in Cholangiocarcinoma
CCA is a biliary epithelial tumor categorized according to anatomical location as intrahepatic cholangiocarcinoma (iCCA) and extrahepatic cholangiocarcinoma (ECC). CCA is the second most frequent type of hepatic malignancy after hepatocellular carcinoma (HCC), accounting for 10% to 20% of newly diagnosed primary liver cancer cases (177). Abnormal biliary repair and chronic inflammation are known to be involved in the development of CCA.
4.4.1 Intrahepatic Cholangiocarcinoma
iCCA is located proximally to the second-order bile ducts within the liver parenchyma, originating from mature cholangiocytes and hepatocytes (178). Abnormal cholangiocyte proliferation is the primary source of tumor cells in iCCA. IL-6 is a pleiotropic inflammatory cytokine and plays an important role in tumorigenesis and cancer progression. IL-6 is highly expressed in human iCCA (179) and contributes to CCA cell proliferation and invasion through the IL-6/STAT3 signaling pathway (180, 181). Furthermore, loss of c-Jun N-terminal kinases (JNKs) in hepatocytes affects cholesterol metabolism and bile acid synthesis, conjugation, and transportation (182), leading to overexpression of IL-6. Deletion of JNK1 and JNK2 contributes to diminished HCC and promotes iCCA development by stimulating cholangiocyte hyperproliferation (182, 183). It seems that the JNK-STAT-IL-6 signaling pathway is crucial for cholangiocyte proliferation in the development of iCCA.
Moreover, LPC proliferation is also involved in the development of iCCA. Brahma-related gene 1 (BRG1) encodes the enzymatic subunit of the Switch/Sucrose Non-Fermentable complex and contributes to stem cell maintenance and tumor development. BRG1 expression is upregulated in iCCA and enhances the Wnt/β-catenin signaling pathway to promote LPC proliferation, while inhibiting BRG1 prevents iCCA development (184). Furthermore, LPC regulated by macrophages is also involved in biliary repair in immune-mediated cholangiopathies and may be associated with CCA. Expression of TWEAK increases with the maturation of macrophages and promotes LPC proliferation and ductular reactions (107). In CCA, the TWEAK/Fibroblast growth factor-inducible molecule 14 signaling pathway provokes the recruitment and polarization of macrophages by increasing monocyte chemoattractant protein 1.
HCT also contributes to the development of iCCA. Notch2 is the major determinant of transdifferentiation of hepatocytes into malignant cholangiocytes in mice (185). In the thioacetamide (TAA) mouse model, Kupffer cells can express Jagged1 transiently to activate Notch signaling and induce HCT leading to the development of iCCA (186). Tumor necrosis factor receptor-related factor 3 (TRAF3) is a highly versatile regulator of organ development and tissue homeostasis via the tumor necrosis factor receptor family, the IL-1 receptor family, and the retinoic acid-inducible gene I -like receptors family in immune responses (187). In iCCA patients, low TRAF3 expression is associated with a poor prognosis. In liver-specific Traf3- and Pten-deficient mice, inactivation of TRAF3 induces HCT to promote iCCA development via NF-κB inducing kinase (NIK) upregulation, and NIK inhibition suppresses cholangiocyte overgrowth (188).
iCCA accounts for approximately 20% of all CCAs (189). Cholangiocyte proliferation, LPC differentiation, and HCT are all involved in the development of iCCA. However, mechanism studies are warranted to discover effective therapeutic targets in favor of the survival of iCCA patients.
4.4.2 Extrahepatic Cholangiocarcinoma
ECC is divided into perihilar cholangiocarcinoma (pCCA) and distal cholangiocarcinoma (dCCA). pCCA is localized between the second-order bile ducts and the insertion of the cystic duct into the common bile duct, while dCCA is confined to the common bile duct below the cystic duct insertion (190). Several studies have focused on inflammatory cytokines in the carcinogenesis of ECC. IL-33 is a member of the IL-1 cytokine family and plays a crucial role in innate and adaptive immune responses, inflammatory responses, dysmetabolism, cardiovascular disorders, and cancer (191). In the liver, IL-33 promotes epithelial repair by the proliferation of hilar bile ducts via activation of the IL-33/ILC2/IL-13 axis (95). Consistently, IL-33 induces cholangiocyte proliferation from extrahepatic bile ducts but not intrahepatic bile ducts in human cholangiocyte cell lines (95). Additionally, IL-33 promotes ECC formation in mice with mutations of Kras and TGF-β Receptor 2 via activation of AKT and YAP, which predispose them to CCA (95, 192). IL-33 also upregulates the expression of IL-6, further accelerating the formation of CCA with activation of AKT and YAP (193). It seems that IL-33 could promote ECC formation by coordinating with other proinflammatory signaling pathways. ECC accounts for approximately 80% of all CCAs with poor prognoses (189). Studies in ECC are currently focused on clinical management and therapeutic targets. Further mechanisms studies on biliary repair and immune cell crosstalk are also needed.
5 Potential Novel Techniques in Biliary Repair
Although accumulating studies have proven dynamic biliary repair during cholangiopathies, the contributions of different regenerating pools of cholangiocytes remain an open question. Related studies are summarized in Table 1. More novel techniques remain needed to clarify the underlying mechanisms of biliary repair.
5.1 Lineage Tracing Techniques
In many studies, conclusions have been drawn utilizing the lineage tracing technique by labeling specific cell subsets, which enables researchers to distinguish the hepatocyte or biliary origin of regenerating cholangiocytes. The lineage tracing technique is a powerful tool to trace the progeny of certain cell types and reveal cell plasticity. However, one common drawback exists: the lack of exclusive cell biomarkers. For example, the most frequently adopted biomarkers of LPC, such as SOX9, EpCAM, and osteopontin, are shared by cholangiocytes or other liver cell types. Therefore, they are insufficient to detect LPC identity and might bias the conclusions, particularly in research regarding biliary repair (38). The identification of more specific and sensitive makers will benefit the understanding of liver regeneration. A feasible resolution is the application of a dual-recombinase system. Combining two separate recombinase systems, Dre-rox and Cre-lox, could effectively avoid non-specific labeling in lineage tracing (194). One study intercrossed CK19-Dre mice with Alb-CreER mice and yielded CK19-Dre/Alb-CreER/IR1 mice which showed significantly increased labeling accuracy of hepatocytes and cholangiocytes and further confirmed the appearance of HCT in DDC and BDL models (55). Another study concerning biliary repair designed a different labeling strategy using a dual-recombinase system. They labeled SOX9+ hepatocytes by SOX9-CreER; HNF4a-DreER mice and found that hepatocytes highly expressing SOX9 were the most vigorous origin of HCT during BDL and DDC induced biliary injury (60).
5.2 Single-Cell Related Techniques
Due to varied biliary injuries, pathological changes, and profound cell heterogeneity, cholangiocytes respond diversely to mitogenic mediators, and different repair processes are present in cholangiopathies. Additionally, LPC, hepatocytes, and liver resident or infiltrated immune cells also present varied subtypes and contribute differentially to biliary repair. However, traditional experimental tools such as immunostaining or RNA-sequencing are insufficient to reveal cell heterogeneity. In this case, scRNA-seq is a powerful technique to unmask transcriptional differences at the single-cell level. Utilizing scRNA-seq, researchers further confirmed heterogeneity within cholangiocytes and hepatocytes during cholangiocyte proliferation and HCT induced by biliary injury. It is identified that YAP enriched in a subset of liver epithelial cells dynamically drives liver regeneration (195). It is also suggested that some broadly-used biliary markers such as CK19 were inconsistently even low expressed in cholangiocytes. This might account for the contradictory results of studies applying these markers as biliary labels in lineage tracing (Table 1). Hepatic immune cells, as crucial regulators of biliary repair, also present significant heterogeneity (196, 197). scRNA-seq and more advanced techniques such as single-cell lineage tracing combined with single-cell transcriptomics (198), single-cell chromosome immunoprecipitation-sequencing, and single-cell assays for transposase-accessible chromatin sequencing are waiting for further application to dissect the role of different subsets of cells involved in biliary repair and their specific regulatory mechanisms.
5.3 Liver Organoids
The liver organoid model might also serve as a novel therapeutic method and experimental tool for liver injury and repair. Organoids are typically defined as in vitro three-dimensional organ-like architectures derived from isolated stem/progenitor cells and in many aspects superior to traditional two-dimensional culture systems (199). Liver organoids were reported to contain hepatocytes, cholangiocytes and bile ducts of normal function, which enable better recapitulation of liver disease and liver regeneration (200). Ductal organoids and hepatocyte organoids could be generated using a culture medium supplemented with different growth factors and could help to enhance the understanding of cholangiocyte proliferation and HCT (201). Moreover, liver organoids are currently deemed potential candidates for transplantation therapy for liver disease. Transplantation of ductal cell-derived and primary hepatocyte-derived liver organoids yielded promising results as they perform considerable repopulation capacity of liver parenchyma, including biliary tree, and physiological functions, such as albumin secretion (202–205). Notwithstanding, the possibility that transplantation therapy exacerbates liver disease by activating the LPC niche and raising cancer risk has not been fully ruled out (206). Thus, there is still a long way to go before the clinical application of cell transplantation therapy for liver diseases.
6 Summary and Prospects
The role of immune cells in the regulation of biliary repair is highlighted in this review. As mentioned above, immune cells, such as Kupffer cells, monocytes, lymphocytes, neutrophils, and mast cells, modulate cholangiocyte proliferation, LPC expansion, and differentiation as well as HCT. What is thought-provoking is that unbalanced biliary repair might escalate liver injury and harbor the risk of carcinogenesis (38, 207). Ductal-mesenchymal interplay was reported as a potential rheostat to control the extent of biliary repair and equilibrate its contradictory effects (208). However, the underlying signaling pathways and other candidate mechanisms that decide the timing of initiation and suspension of biliary repair are largely unknown and worth further study.
Following the discovery of the considerable regenerating capability possessed by LPC, hepatocytes, and cholangiocytes, transplanting these cells into the injured liver in aid of compromised regeneration seems a feasible and tempting treatment strategy. However, the results of hepatocyte transplantation were unsatisfactory as the regenerating effect was limited with fluctuating duration (80). In contrast, transplantation of LPCs that could differentiate into functional liver parenchymal cells yielded more promising results in the acute liver injury model (204). More studies are still needed to evaluate the potential clinical use of cell transplantation in the treatment of cholangiopathies.
Currently, several animal models of biliary injury have been established and extensively help investigation of the mechanisms of biliary repair and potential treatments. Unfortunately, they share some drawbacks, such as nonconformity in pathological and immunity changes with human patients (209). Thus, animal models better recapitulating the pathophysiological conditions of clinical patients would greatly benefit further unveiling the detailed machinery of biliary repair.
Although an increasing number of studies have shed light on our comprehensive understanding of the biliary repair, several vital questions remain to be solved: 1) Are LPC and HCT-derived cholangiocytes functionally competitive? 2) What mechanism determines the proportion of newly regenerated cholangiocytes from different origins? 3) During HCT, does the hepatocyte directly transform into a cholangiocyte or first undergo an intermediate progenitor-cell-like phase? 4) To what extent do immune cells regulate biliary repair? What is the underlying mechanism? 5) What mechanism suspends biliary repair and avoids excessive repair-induced carcinogenesis? Thus, what we already know about biliary repair is still far from the truth. Further studies are still urgently needed to fully understand the underlying mechanism and translate these observations into the clinical treatment of cholangiopathies.
In conclusion, this review summarizes the main regeneration methods of biliary repair and their varied regulations in different cholangiopathies. Great efforts are still needed to expand our knowledge regarding the detailed mechanisms of biliary repair in a broad spectrum of cholangiopathies and exploit promising therapeutic targets.
Author Contributions
JG and CT conceived and supervised the study; TL, SQ and JG analyzed articles and finalized the figures.TL, SQ, CT and JG wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Fund of China (82170623, 82170625, U1702281, 81873584, 82000613, and 82000574), the National Key R&D Program of China (2017YFA0205404), Sichuan Science and Technology Program (2020YJ0084 and 2021YFS0147), and the 135 projects for disciplines of excellence of West China Hospital, Sichuan University (ZYGD18004).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Boyer JL. Bile Formation and Secretion. Compr Physiol (2013) 3(3):1035–78. doi: 10.1002/cphy.c120027
2. Tabibian JH, Masyuk AI, Masyuk TV, O’Hara SP, LaRusso NF. Physiology of Cholangiocytes. Compr Physiol (2013) 3(1):541–65. doi: 10.1002/cphy.c120019
3. Farina A, Dumonceau JM, Delhaye M, Frossard JL, Hadengue A, Hochstrasser DF, et al. A Step Further in the Analysis of Human Bile Proteome. J Proteome Res (2011) 10(4):2047–63. doi: 10.1021/pr200011b
4. Lazaridis KN, LaRusso NF. The Cholangiopathies. Mayo Clin Proc (2015) 90(6):791–800. doi: 10.1016/j.mayocp.2015.03.017
5. Banales JM, Huebert RC, Karlsen T, Strazzabosco M, LaRusso NF, Gores GJ. Cholangiocyte Pathobiology. Nat Rev Gastroenterol Hepatol (2019) 16(5):269–81. doi: 10.1038/s41575-019-0125-y
6. Michalopoulos GK, Bhushan B. Liver Regeneration: Biological and Pathological Mechanisms and Implications. Nat Rev Gastroenterol Hepatol (2021) 18(1):40–55. doi: 10.1038/s41575-020-0342-4
7. Zeng X, Yuan X, Cai Q, Tang C, Gao J. Circular Rna as an Epigenetic Regulator in Chronic Liver Diseases. Cells (2021) 10(8):1945. doi: 10.3390/cells10081945
8. Cai Q, Gan C, Tang C, Wu H, Gao J. Mechanism and Therapeutic Opportunities of Histone Modifications in Chronic Liver Disease. Front Pharmacol (2021) 12:784591. doi: 10.3389/fphar.2021.784591
9. Sekiya S, Suzuki A. Intrahepatic Cholangiocarcinoma Can Arise From Notch-Mediated Conversion of Hepatocytes. J Clin Invest (2012) 122(11):3914–8. doi: 10.1172/JCI63065
10. Fabris L, Fiorotto R, Spirli C, Cadamuro M, Mariotti V, Perugorria MJ, et al. Pathobiology of Inherited Biliary Diseases: A Roadmap to Understand Acquired Liver Diseases. Nat Rev Gastroenterol Hepatol (2019) 16(8):497–511. doi: 10.1038/s41575-019-0156-4
11. Stanger BZ. Cellular Homeostasis and Repair in the Mammalian Liver. Annu Rev Physiol (2015) 77:179–200. doi: 10.1146/annurev-physiol-021113-170255
12. Kanno N, LeSage G, Glaser S, Alvaro D, Alpini G. Functional Heterogeneity of the Intrahepatic Biliary Epithelium. Hepatology (2000) 31(3):555–61. doi: 10.1002/hep.510310302
13. Georgiev P, Jochum W, Heinrich S, Jang JH, Nocito A, Dahm F, et al. Characterization of Time-Related Changes After Experimental Bile Duct Ligation. Br J Surg (2008) 95(5):646–56. doi: 10.1002/bjs.6050
14. Francis HL, Demorrow S, Franchitto A, Venter JK, Mancinelli RA, White MA, et al. Histamine Stimulates the Proliferation of Small and Large Cholangiocytes by Activation of Both Ip3/Ca2+ and Camp-Dependent Signaling Mechanisms. Lab Invest (2012) 92(2):282–94. doi: 10.1038/labinvest.2011.158
15. Ueno Y, Alpini G, Yahagi K, Kanno N, Moritoki Y, Fukushima K, et al. Evaluation of Differential Gene Expression by Microarray Analysis in Small and Large Cholangiocytes Isolated From Normal Mice. Liver Int (2003) 23(6):449–59. doi: 10.1111/j.1478-3231.2003.00876.x
16. Glaser S, Lam IP, Franchitto A, Gaudio E, Onori P, Chow BK, et al. Knockout of Secretin Receptor Reduces Large Cholangiocyte Hyperplasia in Mice With Extrahepatic Cholestasis Induced by Bile Duct Ligation. Hepatology (2010) 52(1):204–14. doi: 10.1002/hep.23657
17. Francis H, Glaser S, Ueno Y, LeSage G, Marucci L, Benedetti A, et al. Camp Stimulates the Secretory and Proliferative Capacity of the Rat Intrahepatic Biliary Epithelium Through Changes in the Pka/Src/Mek/Erk1/2 Pathway. J Hepatol (2004) 41(4):528–37. doi: 10.1016/j.jhep.2004.06.009
18. Mancinelli R, Onori P, Gaudio E, DeMorrow S, Franchitto A, Francis H, et al. Follicle-Stimulating Hormone Increases Cholangiocyte Proliferation by an Autocrine Mechanism Via Camp-Dependent Phosphorylation of Erk1/2 and Elk-1. Am J Physiology-Gastrointest Liver Physiol (2009) 297(1):G11–26. doi: 10.1152/ajpgi.00025.2009
19. Alpini G, Franchitto A, Demorrow S, Onori P, Gaudio E, Wise C, et al. Activation of Alpha(1) -Adrenergic Receptors Stimulate the Growth of Small Mouse Cholangiocytes Via Calcium-Dependent Activation of Nuclear Factor of Activated T Cells 2 and Specificity Protein 1. Hepatology (2011) 53(2):628–39. doi: 10.1002/hep.24041
20. LeSage GD, Benedetti A, Glaser S, Marucci L, Tretjak Z, Caligiuri A, et al. Acute Carbon Tetrachloride Feeding Selectively Damages Large, But Not Small, Cholangiocytes From Normal Rat Liver. Hepatology (1999) 29(2):307–19. doi: 10.1002/hep.510290242
21. Mancinelli R, Franchitto A, Gaudio E, Onori P, Glaser S, Francis H, et al. After Damage of Large Bile Ducts by Gamma-Aminobutyric Acid, Small Ducts Replenish the Biliary Tree by Amplification of Calcium-Dependent Signaling and De Novo Acquisition of Large Cholangiocyte Phenotypes. Am J Pathol (2010) 176(4):1790–800. doi: 10.2353/ajpath.2010.090677
22. Mancinelli R, Franchitto A, Glaser S, Meng F, Onori P, DeMorrow S, et al. Gaba Induces the Differentiation of Small Into Large Cholangiocytes by Activation of Ca2+/Camk I-Dependent Adenylyl Cyclase 8. Hepatology (2013) 58(1):251–63. doi: 10.1002/hep.26308
23. Kamimoto K, Kaneko K, Kok CY, Okada H, Miyajima A, Itoh T. Heterogeneity and Stochastic Growth Regulation of Biliary Epithelial Cells Dictate Dynamic Epithelial Tissue Remodeling. Elife (2016) 5:e15034. doi: 10.7554/eLife.15034
24. Ko S, Russell JO, Molina LM, Monga SP. Liver Progenitors and Adult Cell Plasticity in Hepatic Injury and Repair: Knowns and Unknowns. Annu Rev Pathol (2020) 15:23–50. doi: 10.1146/annurev-pathmechdis-012419-032824
25. Paku S, Schnur J, Nagy P, Thorgeirsson SS. Origin and Structural Evolution of the Early Proliferating Oval Cells in Rat Liver. Am J Pathol (2001) 158(4):1313–23. doi: 10.1016/s0002-9440(10)64082-5
26. Duncan AW, Dorrell C, Grompe M. Stem Cells and Liver Regeneration. Gastroenterology (2009) 137(2):466–81. doi: 10.1053/j.gastro.2009.05.044
27. Yanger K, Zong Y, Maggs LR, Shapira SN, Maddipati R, Aiello NM, et al. Robust Cellular Reprogramming Occurs Spontaneously During Liver Regeneration. Genes Dev (2013) 27(7):719–24. doi: 10.1101/gad.207803.112
28. Malato Y, Naqvi S, Schurmann N, Ng R, Wang B, Zape J, et al. Fate Tracing of Mature Hepatocytes in Mouse Liver Homeostasis and Regeneration. J Clin Invest (2011) 121(12):4850–60. doi: 10.1172/JCI59261
29. Furuyama K, Kawaguchi Y, Akiyama H, Horiguchi M, Kodama S, Kuhara T, et al. Continuous Cell Supply From a Sox9-Expressing Progenitor Zone in Adult Liver, Exocrine Pancreas and Intestine. Nat Genet (2011) 43(1):34–41. doi: 10.1038/ng.722
30. Carpentier R, Suñer RE, van Hul N, Kopp JL, Beaudry JB, Cordi S, et al. Embryonic Ductal Plate Cells Give Rise to Cholangiocytes, Periportal Hepatocytes, and Adult Liver Progenitor Cells. Gastroenterology (2011) 141(4):1432–8, 8.e1-4. doi: 10.1053/j.gastro.2011.06.049
31. Boulter L, Govaere O, Bird TG, Radulescu S, Ramachandran P, Pellicoro A, et al. Macrophage-Derived Wnt Opposes Notch Signaling to Specify Hepatic Progenitor Cell Fate in Chronic Liver Disease. Nat Med (2012) 18(4):572–9. doi: 10.1038/nm.2667
32. Russell JO, Monga SP. Wnt/Beta-Catenin Signaling in Liver Development, Homeostasis, and Pathobiology. Annu Rev Pathol (2018) 13:351–78. doi: 10.1146/annurev-pathol-020117-044010
33. Apte U, Thompson MD, Cui S, Liu B, Cieply B, Monga SP. Wnt/Beta-Catenin Signaling Mediates Oval Cell Response in Rodents. Hepatology (2008) 47(1):288–95. doi: 10.1002/hep.21973
34. Williams JM, Oh SH, Jorgensen M, Steiger N, Darwiche H, Shupe T, et al. The Role of the Wnt Family of Secreted Proteins in Rat Oval “Stem” Cell-Based Liver Regeneration: Wnt1 Drives Differentiation. Am J Pathol (2010) 176(6):2732–42. doi: 10.2353/ajpath.2010.080486
35. Yimlamai D, Christodoulou C, Galli GG, Yanger K, Pepe-Mooney B, Gurung B, et al. Hippo Pathway Activity Influences Liver Cell Fate. Cell (2014) 157(6):1324–38. doi: 10.1016/j.cell.2014.03.060
36. Aloia L, McKie MA, Vernaz G, Cordero-Espinoza L, Aleksieva N, van den Ameele J, et al. Epigenetic Remodelling Licences Adult Cholangiocytes for Organoid Formation and Liver Regeneration. Nat Cell Biol (2019) 21(11):1321–33. doi: 10.1038/s41556-019-0402-6
37. Carpino G, Renzi A, Franchitto A, Cardinale V, Onori P, Reid L, et al. Stem/Progenitor Cell Niches Involved in Hepatic and Biliary Regeneration. Stem Cells Int (2016) 2016:3658013. doi: 10.1155/2016/3658013
38. Lukacs-Kornek V, Lammert F. The Progenitor Cell Dilemma: Cellular and Functional Heterogeneity in Assistance or Escalation of Liver Injury. J Hepatol (2017) 66(3):619–30. doi: 10.1016/j.jhep.2016.10.033
39. Lorenzini S, Bird TG, Boulter L, Bellamy C, Samuel K, Aucott R, et al. Characterisation of a Stereotypical Cellular and Extracellular Adult Liver Progenitor Cell Niche in Rodents and Diseased Human Liver. Gut (2010) 59(5):645–54. doi: 10.1136/gut.2009.182345
40. Ge JY, Zheng YW, Tsuchida T, Furuya K, Isoda H, Taniguchi H, et al. Hepatic Stellate Cells Contribute to Liver Regeneration Through Galectins in Hepatic Stem Cell Niche. Stem Cell Res Ther (2020) 11(1):425. doi: 10.1186/s13287-020-01942-x
41. Pintilie DG, Shupe TD, Oh SH, Salganik SV, Darwiche H, Petersen BE. Hepatic Stellate Cells’ Involvement in Progenitor-Mediated Liver Regeneration. Lab Invest (2010) 90(8):1199–208. doi: 10.1038/labinvest.2010.88
42. Elsegood CL, Chan CW, Degli-Esposti MA, Wikstrom ME, Domenichini A, Lazarus K, et al. Kupffer Cell-Monocyte Communication Is Essential for Initiating Murine Liver Progenitor Cell-Mediated Liver Regeneration. Hepatology (2015) 62(4):1272–84. doi: 10.1002/hep.27977
43. Van Hul N, Lanthier N, Espanol Suner R, Abarca Quinones J, van Rooijen N, Leclercq I. Kupffer Cells Influence Parenchymal Invasion and Phenotypic Orientation, But Not the Proliferation, of Liver Progenitor Cells in a Murine Model of Liver Injury. Am J Pathol (2011) 179(4):1839–50. doi: 10.1016/j.ajpath.2011.06.042
44. Thomas JA, Pope C, Wojtacha D, Robson AJ, Gordon-Walker TT, Hartland S, et al. Macrophage Therapy for Murine Liver Fibrosis Recruits Host Effector Cells Improving Fibrosis, Regeneration, and Function. Hepatology (2011) 53(6):2003–15. doi: 10.1002/hep.24315
45. Kallis YN, Robson AJ, Fallowfield JA, Thomas HC, Alison MR, Wright NA, et al. Remodelling of Extracellular Matrix Is a Requirement for the Hepatic Progenitor Cell Response. Gut (2011) 60(4):525–33. doi: 10.1136/gut.2010.224436
46. Wang Y, Cui CB, Yamauchi M, Miguez P, Roach M, Malavarca R, et al. Lineage Restriction of Human Hepatic Stem Cells to Mature Fates Is Made Efficient by Tissue-Specific Biomatrix Scaffolds. Hepatology (2011) 53(1):293–305. doi: 10.1002/hep.24012
47. Michalopoulos GK, Khan Z. Liver Stem Cells: Experimental Findings and Implications for Human Liver Disease. Gastroenterology (2015) 149(4):876–82. doi: 10.1053/j.gastro.2015.08.004
48. Yovchev MI, Lee EJ, Rodriguez-Silva W, Locker J, Oertel M. Biliary Obstruction Promotes Multilineage Differentiation of Hepatic Stem Cells. Hepatol Commun (2019) 3(8):1137–50. doi: 10.1002/hep4.1367
49. Dorrell C, Erker L, Schug J, Kopp JL, Canaday PS, Fox AJ, et al. Prospective Isolation of a Bipotential Clonogenic Liver Progenitor Cell in Adult Mice. Genes Dev (2011) 25(11):1193–203. doi: 10.1101/gad.2029411
50. Sackett SD, Li Z, Hurtt R, Gao Y, Wells RG, Brondell K, et al. Foxl1 Is a Marker of Bipotential Hepatic Progenitor Cells in Mice. Hepatology (2009) 49(3):920–9. doi: 10.1002/hep.22705
51. Español-Suñer R, Carpentier R, Van Hul N, Legry V, Achouri Y, Cordi S, et al. Liver Progenitor Cells Yield Functional Hepatocytes in Response to Chronic Liver Injury in Mice. Gastroenterology (2012) 143(6):1564–75.e7. doi: 10.1053/j.gastro.2012.08.024
52. Shin S, Upadhyay N, Greenbaum LE, Kaestner KH. Ablation of Foxl1-Cre-Labeled Hepatic Progenitor Cells and Their Descendants Impairs Recovery of Mice From Liver Injury. Gastroenterology (2015) 148(1):192–202.e3. doi: 10.1053/j.gastro.2014.09.039
53. Guo Z, Jing R, Rao Q, Zhang L, Gao Y, Liu F, et al. Immortalized Common Marmoset (Callithrix Jacchus) Hepatic Progenitor Cells Possess Bipotentiality in Vitro and in Vivo. Cell Discov (2018) 4:23. doi: 10.1038/s41421-018-0020-7
54. Nagahama Y, Sone M, Chen X, Okada Y, Yamamoto M, Xin B, et al. Contributions of Hepatocytes and Bile Ductular Cells in Ductular Reactions and Remodeling of the Biliary System After Chronic Liver Injury. Am J Pathol (2014) 184(11):3001–12. doi: 10.1016/j.ajpath.2014.07.005
55. He L, Li Y, Li Y, Pu W, Huang X, Tian X, et al. Enhancing the Precision of Genetic Lineage Tracing Using Dual Recombinases. Nat Med (2017) 23(12):1488–98. doi: 10.1038/nm.4437
56. Sekiya S, Suzuki A. Hepatocytes, Rather Than Cholangiocytes, Can Be the Major Source of Primitive Ductules in the Chronically Injured Mouse Liver. Am J Pathol (2014) 184(5):1468–78. doi: 10.1016/j.ajpath.2014.01.005
57. Tanimizu N, Nishikawa Y, Ichinohe N, Akiyama H, Mitaka T. Sry Hmg Box Protein 9-Positive (Sox9+) Epithelial Cell Adhesion Molecule-Negative (Epcam-) Biphenotypic Cells Derived From Hepatocytes Are Involved in Mouse Liver Regeneration. J Biol Chem (2014) 289(11):7589–98. doi: 10.1074/jbc.M113.517243
58. Okabe H, Yang J, Sylakowski K, Yovchev M, Miyagawa Y, Nagarajan S, et al. Wnt Signaling Regulates Hepatobiliary Repair Following Cholestatic Liver Injury in Mice. Hepatology (2016) 64(5):1652–66. doi: 10.1002/hep.28774
59. Tarlow BD, Pelz C, Naugler WE, Wakefield L, Wilson EM, Finegold MJ, et al. Bipotential Adult Liver Progenitors Are Derived From Chronically Injured Mature Hepatocytes. Cell Stem Cell (2014) 15(5):605–18. doi: 10.1016/j.stem.2014.09.008
60. Han X, Wang Y, Pu W, Huang X, Qiu L, Li Y, et al. Lineage Tracing Reveals the Bipotency of Sox9(+) Hepatocytes During Liver Regeneration. Stem Cell Rep (2019) 12(3):624–38. doi: 10.1016/j.stemcr.2019.01.010
61. Xu T, Lu Z, Xiao Z, Liu F, Chen Y, Wang Z, et al. Myofibroblast Induces Hepatocyte-To-Ductal Metaplasia Via Laminin-Avbeta6 Integrin in Liver Fibrosis. Cell Death Dis (2020) 11(3):199. doi: 10.1038/s41419-020-2372-9
62. Michalopoulos GK, Barua L, Bowen WC. Transdifferentiation of Rat Hepatocytes Into Biliary Cells After Bile Duct Ligation and Toxic Biliary Injury. Hepatology (2005) 41(3):535–44. doi: 10.1002/hep.20600
63. Yovchev MI, Locker J, Oertel M. Biliary Fibrosis Drives Liver Repopulation and Phenotype Transition of Transplanted Hepatocytes. J Hepatol (2016) 64(6):1348–57. doi: 10.1016/j.jhep.2016.01.036
64. Zheng YW, Taniguchi H. Diversity of Hepatic Stem Cells in the Fetal and Adult Liver. Semin Liver Dis (2003) 23(4):337–48. doi: 10.1055/s-2004-815557
65. He J, Lu H, Zou Q, Luo L. Regeneration of Liver After Extreme Hepatocyte Loss Occurs Mainly Via Biliary Transdifferentiation in Zebrafish. Gastroenterology (2014) 146(3):789–800.e8. doi: 10.1053/j.gastro.2013.11.045
66. Verfaillie CM. Biliary Cells to the Rescue of Prometheus. Gastroenterology (2014) 146(3):611–4. doi: 10.1053/j.gastro.2014.01.039
67. Gadd VL, Aleksieva N, Forbes SJ. Epithelial Plasticity During Liver Injury and Regeneration. Cell Stem Cell (2020) 27(4):557–73. doi: 10.1016/j.stem.2020.08.016
68. Font-Burgada J, Shalapour S, Ramaswamy S, Hsueh B, Rossell D, Umemura A, et al. Hybrid Periportal Hepatocytes Regenerate the Injured Liver Without Giving Rise to Cancer. Cell (2015) 162(4):766–79. doi: 10.1016/j.cell.2015.07.026
69. Limaye PB, Alarcon G, Walls AL, Nalesnik MA, Michalopoulos GK, Demetris AJ, et al. Expression of Specific Hepatocyte and Cholangiocyte Transcription Factors in Human Liver Disease and Embryonic Development. Lab Invest (2008) 88(8):865–72. doi: 10.1038/labinvest.2008.56
70. Schaub JR, Huppert KA, Kurial SNT, Hsu BY, Cast AE, Donnelly B, et al. De Novo Formation of the Biliary System by Tgfbeta-Mediated Hepatocyte Transdifferentiation. Nature (2018) 557(7704):247–51. doi: 10.1038/s41586-018-0075-5
71. Tanimizu N, Ichinohe N, Yamamoto M, Akiyama H, Nishikawa Y, Mitaka T. Progressive Induction of Hepatocyte Progenitor Cells in Chronically Injured Liver. Sci Rep (2017) 7:39990. doi: 10.1038/srep39990
72. Fan B, Malato Y, Calvisi DF, Naqvi S, Razumilava N, Ribback S, et al. Cholangiocarcinomas Can Originate From Hepatocytes in Mice. J Clin Invest (2012) 122(8):2911–5. doi: 10.1172/JCI63212
73. Cardinale V, Wang Y, Carpino G, Mendel G, Alpini G, Gaudio E, et al. The Biliary Tree–a Reservoir of Multipotent Stem Cells. Nat Rev Gastroenterol Hepatol (2012) 9(4):231–40. doi: 10.1038/nrgastro.2012.23
74. DiPaola F, Shivakumar P, Pfister J, Walters S, Sabla G, Bezerra JA. Identification of Intramural Epithelial Networks Linked to Peribiliary Glands That Express Progenitor Cell Markers and Proliferate After Injury in Mice. Hepatology (2013) 58(4):1486–96. doi: 10.1002/hep.26485
75. Carpino G, Nevi L, Overi D, Cardinale V, Lu WY, Di Matteo S, et al. Peribiliary Gland Niche Participates in Biliary Tree Regeneration in Mouse and in Human Primary Sclerosing Cholangitis. Hepatology (2020) 71(3):972–89. doi: 10.1002/hep.30871
76. Lanzoni G, Cardinale V, Carpino G. The Hepatic, Biliary, and Pancreatic Network of Stem/Progenitor Cell Niches in Humans: A New Reference Frame for Disease and Regeneration. Hepatology (2016) 64(1):277–86. doi: 10.1002/hep.28326
77. Oh SH, Witek RP, Bae SH, Zheng D, Jung Y, Piscaglia AC, et al. Bone Marrow-Derived Hepatic Oval Cells Differentiate Into Hepatocytes in 2-Acetylaminofluorene/Partial Hepatectomy-Induced Liver Regeneration. Gastroenterology (2007) 132(3):1077–87. doi: 10.1053/j.gastro.2007.01.001
78. Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, et al. Multi-Organ, Multi-Lineage Engraftment by a Single Bone Marrow-Derived Stem Cell. Cell (2001) 105(3):369–77. doi: 10.1016/s0092-8674(01)00328-2
79. Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, et al. Bone Marrow as a Potential Source of Hepatic Oval Cells. Science (New York NY) (1999) 284(5417):1168–70. doi: 10.1126/science.284.5417.1168
80. Huebert RC, Rakela J. Cellular Therapy for Liver Disease. Mayo Clin Proc (2014) 89(3):414–24. doi: 10.1016/j.mayocp.2013.10.023
81. Roskams TA, Theise ND, Balabaud C, Bhagat G, Bhathal PS, Bioulac-Sage P, et al. Nomenclature of the Finer Branches of the Biliary Tree: Canals, Ductules, and Ductular Reactions in Human Livers. Hepatology (2004) 39(6):1739–45. doi: 10.1002/hep.20130
82. O’Hara SP, Tabibian JH, Splinter PL, LaRusso NF. The Dynamic Biliary Epithelia: Molecules, Pathways, and Disease. J Hepatol (2013) 58(3):575–82. doi: 10.1016/j.jhep.2012.10.011
83. Forbes SJ, Rosenthal N. Preparing the Ground for Tissue Regeneration: From Mechanism to Therapy. Nat Med (2014) 20(8):857–69. doi: 10.1038/nm.3653
84. Keulemans YC, Mok KS, de Wit LT, Gouma DJ, Groen AK. Hepatic Bile Versus Gallbladder Bile: A Comparison of Protein and Lipid Concentration and Composition in Cholesterol Gallstone Patients. Hepatology (1998) 28(1):11–6. doi: 10.1002/hep.510280103
85. Yang L, Seki E. Toll-Like Receptors in Liver Fibrosis: Cellular Crosstalk and Mechanisms. Front Physiol (2012) 3:138. doi: 10.3389/fphys.2012.00138
86. Chen XM, O’Hara SP, Nelson JB, Splinter PL, Small AJ, Tietz PS, et al. Multiple Tlrs Are Expressed in Human Cholangiocytes and Mediate Host Epithelial Defense Responses to Cryptosporidium Parvum Via Activation of Nf-Kappa B. J Immunol (2005) 175(11):7447–56. doi: 10.4049/jimmunol.175.11.7447
87. Oo YH, Banz V, Kavanagh D, Liaskou E, Withers DR, Humphreys E, et al. Cxcr3-Dependent Recruitment and Ccr6-Mediated Positioning of Th-17 Cells in the Inflamed Liver. J Hepatol (2012) 57(5):1044–51. doi: 10.1016/j.jhep.2012.07.008
88. Heydtmann M, Lalor PF, Eksteen JA, Hübscher SG, Briskin M, Adams DH. Cxc Chemokine Ligand 16 Promotes Integrin-Mediated Adhesion of Liver-Infiltrating Lymphocytes to Cholangiocytes and Hepatocytes Within the Inflamed Human Liver. J Immunol (2005) 174(2):1055–62. doi: 10.4049/jimmunol.174.2.1055
89. Kamihira T, Shimoda S, Nakamura M, Yokoyama T, Takii Y, Kawano A, et al. Biliary Epithelial Cells Regulate Autoreactive T Cells: Implications for Biliary-Specific Diseases. Hepatology (2005) 41(1):151–9. doi: 10.1002/hep.20494
90. Guicciardi ME, Trussoni CE, Krishnan A, Bronk SF, Lorenzo Pisarello MJ, O’Hara SP, et al. Macrophages Contribute to the Pathogenesis of Sclerosing Cholangitis in Mice. J Hepatol (2018) 69(3):676–86. doi: 10.1016/j.jhep.2018.05.018
91. Kaffe E, Fiorotto R, Pellegrino F, Mariotti V, Amenduni M, Cadamuro M, et al. B-Catenin and Interleukin-1β-Dependent Chemokine (C-X-C Motif) Ligand 10 Production Drives Progression of Disease in a Mouse Model of Congenital Hepatic Fibrosis. Hepatology (2018) 67(5):1903–19. doi: 10.1002/hep.29652
92. Kruglov EA, Nathanson RA, Nguyen T, Dranoff JA. Secretion of Mcp-1/Ccl2 by Bile Duct Epithelia Induces Myofibroblastic Transdifferentiation of Portal Fibroblasts. Am J Physiol Gastrointest Liver Physiol (2006) 290(4):G765–71. doi: 10.1152/ajpgi.00308.2005
93. O’Hara SP, Splinter PL, Trussoni CE, Gajdos GB, Lineswala PN, LaRusso NF. Cholangiocyte N-Ras Protein Mediates Lipopolysaccharide-Induced Interleukin 6 Secretion and Proliferation. J Biol Chem (2011) 286(35):30352–60. doi: 10.1074/jbc.M111.269464
94. Harada K, Shimoda S, Sato Y, Isse K, Ikeda H, Nakanuma Y. Periductal Interleukin-17 Production in Association With Biliary Innate Immunity Contributes to the Pathogenesis of Cholangiopathy in Primary Biliary Cirrhosis. Clin Exp Immunol (2009) 157(2):261–70. doi: 10.1111/j.1365-2249.2009.03947.x
95. Li J, Razumilava N, Gores GJ, Walters S, Mizuochi T, Mourya R, et al. Biliary Repair and Carcinogenesis Are Mediated by Il-33-Dependent Cholangiocyte Proliferation. J Clin Invest (2014) 124(7):3241–51. doi: 10.1172/jci73742
96. Irvine KM, Clouston AD, Gadd VL, Miller GC, Wong WY, Melino M, et al. Deletion of Wntless in Myeloid Cells Exacerbates Liver Fibrosis and the Ductular Reaction in Chronic Liver Injury. Fibrogene Tissue Repair (2015) 8:19. doi: 10.1186/s13069-015-0036-7
97. Heymann F, Tacke F. Immunology in the Liver–from Homeostasis to Disease. Nat Rev Gastroenterol Hepatol (2016) 13(2):88–110. doi: 10.1038/nrgastro.2015.200
98. Murray PJ. Macrophage Polarization. Annu Rev Physiol (2017) 79:541–66. doi: 10.1146/annurev-physiol-022516-034339
99. Tacke F, Zimmermann HW. Macrophage Heterogeneity in Liver Injury and Fibrosis. J Hepatol (2014) 60(5):1090–6. doi: 10.1016/j.jhep.2013.12.025
100. Ramachandran P, Pellicoro A, Vernon MA, Boulter L, Aucott RL, Ali A, et al. Differential Ly-6c Expression Identifies the Recruited Macrophage Phenotype, Which Orchestrates the Regression of Murine Liver Fibrosis. Proc Natl Acad Sci USA (2012) 109(46):E3186–95. doi: 10.1073/pnas.1119964109
101. Duwaerts CC, Gehring S, Cheng CW, van Rooijen N, Gregory SH. Contrasting Responses of Kupffer Cells and Inflammatory Mononuclear Phagocytes to Biliary Obstruction in a Mouse Model of Cholestatic Liver Injury. Liver Int (2013) 33(2):255–65. doi: 10.1111/liv.12048
102. Guo C, Xie S, Chi Z, Zhang J, Liu Y, Zhang L, et al. Bile Acids Control Inflammation and Metabolic Disorder Through Inhibition of Nlrp3 Inflammasome. Immunity (2016) 45(4):944. doi: 10.1016/j.immuni.2016.10.009
103. Péan N, Doignon I, Garcin I, Besnard A, Julien B, Liu B, et al. The Receptor Tgr5 Protects the Liver From Bile Acid Overload During Liver Regeneration in Mice. Hepatology (2013) 58(4):1451–60. doi: 10.1002/hep.26463
104. Fabris L, Spirli C, Cadamuro M, Fiorotto R, Strazzabosco M. Emerging Concepts in Biliary Repair and Fibrosis. Am J Physiol Gastrointest Liver Physiol (2017) 313(2):G102–16. doi: 10.1152/ajpgi.00452.2016
105. Guillot A, Guerri L, Feng D, Kim SJ, Ahmed YA, Paloczi J, et al. Bile Acid-Activated Macrophages Promote Biliary Epithelial Cell Proliferation Through Integrin Alphavbeta6 Upregulation Following Liver Injury. J Clin Invest (2021) 131(9):e132305. doi: 10.1172/JCI132305
106. Hoenicke L, Zender L. Immune Surveillance of Senescent Cells–Biological Significance in Cancer- and Non-Cancer Pathologies. Carcinogenesis (2012) 33(6):1123–6. doi: 10.1093/carcin/bgs124
107. Bird TG, Lu WY, Boulter L, Gordon-Keylock S, Ridgway RA, Williams MJ, et al. Bone Marrow Injection Stimulates Hepatic Ductular Reactions in the Absence of Injury Via Macrophage-Mediated Tweak Signaling. Proc Natl Acad Sci USA (2013) 110(16):6542–7. doi: 10.1073/pnas.1302168110
108. Ramachandran P, Matchett KP, Dobie R, Wilson-Kanamori JR, Henderson NC. Single-Cell Technologies in Hepatology: New Insights Into Liver Biology and Disease Pathogenesis. Nat Rev Gastroenterol Hepatol (2020) 17(8):457–72. doi: 10.1038/s41575-020-0304-x
109. Ramachandran P, Dobie R, Wilson-Kanamori JR, Dora EF, Henderson BEP, Luu NT, et al. Resolving the Fibrotic Niche of Human Liver Cirrhosis at Single-Cell Level. Nature (2019) 575(7783):512–8. doi: 10.1038/s41586-019-1631-3
110. MacParland SA, Liu JC, Ma XZ, Innes BT, Bartczak AM, Gage BK, et al. Single Cell Rna Sequencing of Human Liver Reveals Distinct Intrahepatic Macrophage Populations. Nat Commun (2018) 9(1):4383. doi: 10.1038/s41467-018-06318-7
111. Wang J, Hu W, Shen Z, Liu T, Dai W, Shen B, et al. Dissecting the Single-Cell Transcriptomeunderlying Chronic Liver Injury. Mol Ther Nucleic Acids (2021) 26:1364–73. doi: 10.1016/j.omtn.2021.11.008
112. Kolaczkowska E, Kubes P. Neutrophil Recruitment and Function in Health and Inflammation. Nat Rev Immunol (2013) 13(3):159–75. doi: 10.1038/nri3399
113. Takeuchi M, Vidigal PT, Guerra MT, Hundt MA, Robert ME, Olave-Martinez M, et al. Neutrophils Interact With Cholangiocytes to Cause Cholestatic Changes in Alcoholic Hepatitis. Gut (2021) 70(2):342–56. doi: 10.1136/gutjnl-2020-322540
114. Zimmer CL, von Seth E, Buggert M, Strauss O, Hertwig L, Nguyen S, et al. A Biliary Immune Landscape Map of Primary Sclerosing Cholangitis Reveals a Dominant Network of Neutrophils and Tissue-Resident T Cells. Sci Trans Med (2021) 13(599):eabb3107. doi: 10.1126/scitranslmed.abb3107
115. Wang J, Hossain M, Thanabalasuriar A, Gunzer M, Meininger C, Kubes P. Visualizing the Function and Fate of Neutrophils in Sterile Injury and Repair. Science (New York NY) (2017) 358(6359):111–6. doi: 10.1126/science.aam9690
116. Soehnlein O, Lindbom L. Phagocyte Partnership During the Onset and Resolution of Inflammation. Nat Rev Immunol (2010) 10(6):427–39. doi: 10.1038/nri2779
117. Pham L, Kennedy L, Baiocchi L, Meadows V, Ekser B, Kundu D, et al. Mast Cells in Liver Disease Progression: An Update on Current Studies and Implications. Hepatology (2022) 75(1):213–8. doi: 10.1002/hep.32121
118. Hargrove L, Graf-Eaton A, Kennedy L, Demieville J, Owens J, Hodges K, et al. Isolation and Characterization of Hepatic Mast Cells From Cholestatic Rats. Lab Invest (2016) 96(11):1198–210. doi: 10.1038/labinvest.2016.89
119. Kennedy L, Hargrove L, Demieville J, Karstens W, Jones H, DeMorrow S, et al. Blocking H1/H2 Histamine Receptors Inhibits Damage/Fibrosis in Mdr2(-/-) Mice and Human Cholangiocarcinoma Tumorigenesis. Hepatology (2018) 68(3):1042–56. doi: 10.1002/hep.29898
120. Jarido V, Kennedy L, Hargrove L, Demieville J, Thomson J, Stephenson K, et al. The Emerging Role of Mast Cells in Liver Disease. Am J Physiol Gastrointest Liver Physiol (2017) 313(2):G89–G101. doi: 10.1152/ajpgi.00333.2016
121. Meadows V, Kennedy L, Hargrove L, Demieville J, Meng F, Virani S, et al. Downregulation of Hepatic Stem Cell Factor by Vivo-Morpholino Treatment Inhibits Mast Cell Migration and Decreases Biliary Damage/Senescence and Liver Fibrosis in Mdr2(-/-) Mice. Biochim Biophys Acta Mol Basis Dis (2019) 1865(12):165557. doi: 10.1016/j.bbadis.2019.165557
122. Fasbender F, Widera A, Hengstler JG, Watzl C. Natural Killer Cells and Liver Fibrosis. Front Immunol (2016) 7:19. doi: 10.3389/fimmu.2016.00019
123. Ma L, Hernandez MO, Zhao Y, Mehta M, Tran B, Kelly M, et al. Tumor Cell Biodiversity Drives Microenvironmental Reprogramming in Liver Cancer. Cancer Cell (2019) 36(4):418–30.e6. doi: 10.1016/j.ccell.2019.08.007
124. Poch T, Krause J, Casar C, Liwinski T, Glau L, Kaufmann M, et al. Single-Cell Atlas of Hepatic T Cells Reveals Expansion of Liver-Resident Naive-Like Cd4(+) T Cells in Primary Sclerosing Cholangitis. J Hepatol (2021) 75(2):414–23. doi: 10.1016/j.jhep.2021.03.016
125. Gieseck RL 3rd, Ramalingam TR, Hart KM, Vannella KM, Cantu DA, Lu WY, et al. Interleukin-13 Activates Distinct Cellular Pathways Leading to Ductular Reaction, Steatosis, and Fibrosis. Immunity (2016) 45(1):145–58. doi: 10.1016/j.immuni.2016.06.009
126. Peters AL, Luo Z, Li J, Mourya R, Wang Y, Dexheimer P, et al. Single Cell Rna Sequencing Reveals Regional Heterogeneity of Hepatobiliary Innate Lymphoid Cells in a Tissue-Enriched Fashion. PLoS One (2019) 14(4):e0215481. doi: 10.1371/journal.pone.0215481
127. Tsuchida T, Friedman SL. Mechanisms of Hepatic Stellate Cell Activation. Nat Rev Gastroenterol Hepatol (2017) 14(7):397–411. doi: 10.1038/nrgastro.2017.38
128. Van Hul NK, Abarca-Quinones J, Sempoux C, Horsmans Y, Leclercq IA. Relation Between Liver Progenitor Cell Expansion and Extracellular Matrix Deposition in a Cde-Induced Murine Model of Chronic Liver Injury. Hepatology (2009) 49(5):1625–35. doi: 10.1002/hep.22820
129. Chen Z, Wan L, Jin X, Wang W, Li D. Transforming Growth Factor-B Signaling Confers Hepatic Stellate Cells Progenitor Features After Partial Hepatectomy. J Cell Physiol (2020) 235(3):2655–67. doi: 10.1002/jcp.29169
130. Swiderska-Syn M, Syn WK, Xie G, Krüger L, Machado MV, Karaca G, et al. Myofibroblastic Cells Function as Progenitors to Regenerate Murine Livers After Partial Hepatectomy. Gut (2014) 63(8):1333–44. doi: 10.1136/gutjnl-2013-305962
131. Dobie R, Wilson-Kanamori JR, Henderson BEP, Smith JR, Matchett KP, Portman JR, et al. Single-Cell Transcriptomics Uncovers Zonation of Function in the Mesenchyme During Liver Fibrosis. Cell Rep (2019) 29(7):1832–47.e8. doi: 10.1016/j.celrep.2019.10.024
132. Yasoshima M, Kono N, Sugawara H, Katayanagi K, Harada K, Nakanuma Y. Increased Expression of Interleukin-6 and Tumor Necrosis Factor-Alpha in Pathologic Biliary Epithelial Cells: In Situ and Culture Study. Lab Invest (1998) 78(1):89–100.
133. Spirli C, Fabris L, Duner E, Fiorotto R, Ballardini G, Roskams T, et al. Cytokine-Stimulated Nitric Oxide Production Inhibits Adenylyl Cyclase and Camp-Dependent Secretion in Cholangiocytes. Gastroenterology (2003) 124(3):737–53. doi: 10.1053/gast.2003.50100
134. Pinto C, Giordano DM, Maroni L, Marzioni M. Role of Inflammation and Proinflammatory Cytokines in Cholangiocyte Pathophysiology. Biochim Biophys Acta Mol Basis Dis (2018) 1864(4 Pt B):1270–8. doi: 10.1016/j.bbadis.2017.07.024
135. Lazaridis KN, Strazzabosco M, LaRusso NF. The Cholangiopathies: Disorders of Biliary Epithelia. Gastroenterology (2004) 127(5):1565–77. doi: 10.1053/j.gastro.2004.08.006
136. Rodier F, Campisi J. Four Faces of Cellular Senescence. J Cell Biol (2011) 192(4):547–56. doi: 10.1083/jcb.201009094
137. He S, Sharpless NE. Senescence in Health and Disease. Cell (2017) 169(6):1000–11. doi: 10.1016/j.cell.2017.05.015
138. Karlsen TH, Folseraas T, Thorburn D, Vesterhus M. Primary Sclerosing Cholangitis - a Comprehensive Review. J Hepatol (2017) 67(6):1298–323. doi: 10.1016/j.jhep.2017.07.022
139. Zhang H, Leung PSC, Gershwin ME, Ma X. How the Biliary Tree Maintains Immune Tolerance? Biochim Biophys Acta Mol Basis Dis (2018) 1864(4 Pt B):1367–73. doi: 10.1016/j.bbadis.2017.08.019
140. Tabibian JH, O’Hara SP, Splinter PL, Trussoni CE, LaRusso NF. Cholangiocyte Senescence by Way of N-Ras Activation Is a Characteristic of Primary Sclerosing Cholangitis. Hepatology (2014) 59(6):2263–75. doi: 10.1002/hep.26993
141. Elssner C, Goeppert B, Longerich T, Scherr AL, Stindt J, Nanduri LK, et al. Nuclear Translocation of Relb Is Increased in Diseased Human Liver and Promotes Ductular Reaction and Biliary Fibrosis in Mice. Gastroenterology (2019) 156(4):1190–205.e14. doi: 10.1053/j.gastro.2018.11.018
142. Nijjar SS, Wallace L, Crosby HA, Hubscher SG, Strain AJ. Altered Notch Ligand Expression in Human Liver Disease: Further Evidence for a Role of the Notch Signaling Pathway in Hepatic Neovascularization and Biliary Ductular Defects. Am J Pathol (2002) 160(5):1695–703. doi: 10.1016/s0002-9440(10)61116-9
143. Nijjar SS, Crosby HA, Wallace L, Hubscher SG, Strain AJ. Notch Receptor Expression in Adult Human Liver: A Possible Role in Bile Duct Formation and Hepatic Neovascularization. Hepatology (2001) 34(6):1184–92. doi: 10.1053/jhep.2001.29399
144. Trussoni CE, O’Hara SP, LaRusso NF. Cellular Senescence in the Cholangiopathies: A Driver of Immunopathology and a Novel Therapeutic Target. Semin Immunopathol (2022). doi: 10.1007/s00281-022-00909-9
145. Xiang B, Deng C, Qiu F, Li J, Li S, Zhang H, et al. Single Cell Sequencing Analysis Identifies Genetics-Modulated Ormdl3(+) Cholangiocytes Having Higher Metabolic Effects on Primary Biliary Cholangitis. J Nanobiotechnol (2021) 19(1):406. doi: 10.1186/s12951-021-01154-2
146. Locatelli L, Cadamuro M, Spirli C, Fiorotto R, Lecchi S, Morell CM, et al. Macrophage Recruitment by Fibrocystin-Defective Biliary Epithelial Cells Promotes Portal Fibrosis in Congenital Hepatic Fibrosis. Hepatology (2016) 63(3):965–82. doi: 10.1002/hep.28382
147. Peng ZW, Ikenaga N, Liu SB, Sverdlov DY, Vaid KA, Dixit R, et al. Integrin Alphavbeta6 Critically Regulates Hepatic Progenitor Cell Function and Promotes Ductular Reaction, Fibrosis, and Tumorigenesis. Hepatology (2016) 63(1):217–32. doi: 10.1002/hep.28274
148. Carpino G, Cardinale V, Folseraas T, Overi D, Floreani A, Franchitto A, et al. Hepatic Stem/Progenitor Cell Activation Differs Between Primary Sclerosing and Primary Biliary Cholangitis. Am J Pathol (2018) 188(3):627–39. doi: 10.1016/j.ajpath.2017.11.010
149. Carpino G, Cardinale V, Renzi A, Hov JR, Berloco PB, Rossi M, et al. Activation of Biliary Tree Stem Cells Within Peribiliary Glands in Primary Sclerosing Cholangitis. J Hepatol (2015) 63(5):1220–8. doi: 10.1016/j.jhep.2015.06.018
150. Cameron RG, Blendis LM, Neuman MG. Accumulation of Macrophages in Primary Sclerosing Cholangitis. Clin Biochem (2001) 34(3):195–201. doi: 10.1016/s0009-9120(01)00215-6
151. McDaniel K, Wu N, Zhou T, Huang L, Sato K, Venter J, et al. Amelioration of Ductular Reaction by Stem Cell Derived Extracellular Vesicles in Mdr2 Knockout Mice Via Lethal-7 Microrna. Hepatology (2019) 69(6):2562–78. doi: 10.1002/hep.30542
152. Kyritsi K, Kennedy L, Meadows V, Hargrove L, Demieville J, Pham L, et al. Mast Cells Induce Ductular Reaction Mimicking Liver Injury in Mice Through Mast Cell-Derived Transforming Growth Factor Beta 1 Signaling. Hepatology (2021) 73(6):2397–410. doi: 10.1002/hep.31497
153. Navarro-Corcuera A, Sehrawat TS, Jalan-Sakrikar N, Gibbons HR, Pirius NE, Khanal S, et al. Long Non-Coding Rna Acta2-As1 Promotes Ductular Reaction by Interacting With the P300/Elk1 Complex. J Hepatol (2021) 76(4):921–33. doi: 10.1016/j.jhep.2021.12.014
154. McDaniell R, Warthen DM, Sanchez-Lara PA, Pai A, Krantz ID, Piccoli DA, et al. Notch2 Mutations Cause Alagille Syndrome, a Heterogeneous Disorder of the Notch Signaling Pathway. Am J Hum Genet (2006) 79(1):169–73. doi: 10.1086/505332
155. Turnpenny PD, Ellard S. Alagille Syndrome: Pathogenesis, Diagnosis and Management. Eur J Hum Genet: EJHG (2012) 20(3):251–7. doi: 10.1038/ejhg.2011.181
156. Antoniou A, Raynaud P, Cordi S, Zong Y, Tronche F, Stanger BZ, et al. Intrahepatic Bile Ducts Develop According to a New Mode of Tubulogenesis Regulated by the Transcription Factor Sox9. Gastroenterology (2009) 136(7):2325–33. doi: 10.1053/j.gastro.2009.02.051
157. Adams JM, Huppert KA, Castro EC, Lopez MF, Niknejad N, Subramanian S, et al. Sox9 Is a Modifier of the Liver Disease Severity in a Mouse Model of Alagille Syndrome. Hepatology (2020) 71(4):1331–49. doi: 10.1002/hep.30912
158. Thakurdas SM, Lopez MF, Kakuda S, Fernandez-Valdivia R, Zarrin-Khameh N, Haltiwanger RS, et al. Jagged1 Heterozygosity in Mice Results in a Congenital Cholangiopathy Which Is Reversed by Concomitant Deletion of One Copy of Poglut1 (Rumi). Hepatology (2016) 63(2):550–65. doi: 10.1002/hep.28024
159. Zhao C, Lancman JJ, Yang Y, Gates KP, Cao D, Barske L, et al. Intrahepatic Cholangiocyte Regeneration From an Fgf-Dependent Extrahepatic Progenitor Niche in a Zebrafish Model of Alagille Syndrome. Hepatology (2022) 75(3):567–83. doi: 10.1002/hep.32173
160. Shiota J, Samuelson LC, Razumilava N. Hepatobiliary Organoids and Their Applications for Studies of Liver Health and Disease: Are We There Yet? Hepatology (2021) 74(4):2251–63. doi: 10.1002/hep.31772
161. Masyuk TV, Masyuk AI, Lorenzo Pisarello M, Howard BN, Huang BQ, Lee PY, et al. Tgr5 Contributes to Hepatic Cystogenesis in Rodents With Polycystic Liver Diseases Through Cyclic Adenosine Monophosphate/Gαs Signaling. Hepatology (2017) 66(4):1197–218. doi: 10.1002/hep.29284
162. Wills ES, Roepman R, Drenth JP. Polycystic Liver Disease: Ductal Plate Malformation and the Primary Cilium. Trends Mol Med (2014) 20(5):261–70. doi: 10.1016/j.molmed.2014.01.003
163. Munoz-Garrido P, Marin JJ, Perugorria MJ, Urribarri AD, Erice O, Sáez E, et al. Ursodeoxycholic Acid Inhibits Hepatic Cystogenesis in Experimental Models of Polycystic Liver Disease. J Hepatol (2015) 63(4):952–61. doi: 10.1016/j.jhep.2015.05.023
164. Urribarri AD, Munoz-Garrido P, Perugorria MJ, Erice O, Merino-Azpitarte M, Arbelaiz A, et al. Inhibition of Metalloprotease Hyperactivity in Cystic Cholangiocytes Halts the Development of Polycystic Liver Diseases. Gut (2014) 63(10):1658–67. doi: 10.1136/gutjnl-2013-305281
165. Masyuk T, Masyuk A, Trussoni C, Howard B, Ding J, Huang B, et al. Autophagy-Mediated Reduction of Mir-345 Contributes to Hepatic Cystogenesis in Polycystic Liver Disease. JHEP Rep: Innovation Hepatol (2021) 3(5):100345. doi: 10.1016/j.jhepr.2021.100345
166. Lee SO, Masyuk T, Splinter P, Banales JM, Masyuk A, Stroope A, et al. Microrna15a Modulates Expression of the Cell-Cycle Regulator Cdc25a and Affects Hepatic Cystogenesis in a Rat Model of Polycystic Kidney Disease. J Clin Invest (2008) 118(11):3714–24. doi: 10.1172/jci34922
167. Verkade HJ, Bezerra JA, Davenport M, Schreiber RA, Mieli-Vergani G, Hulscher JB, et al. Biliary Atresia and Other Cholestatic Childhood Diseases: Advances and Future Challenges. J Hepatol (2016) 65(3):631–42. doi: 10.1016/j.jhep.2016.04.032
168. Xiao Y, Liu R, Li X, Gurley EC, Hylemon PB, Lu Y, et al. Long Noncoding Rna H19 Contributes to Cholangiocyte Proliferation and Cholestatic Liver Fibrosis in Biliary Atresia. Hepatology (2019) 70(5):1658–73. doi: 10.1002/hep.30698
169. Tian X, Wang Y, Lu Y, Wang W, Du J, Chen S, et al. Conditional Depletion of Macrophages Ameliorates Cholestatic Liver Injury and Fibrosis Via Lncrna-H19. Cell Death Dis (2021) 12(7):646. doi: 10.1038/s41419-021-03931-1
170. Xiao Y, Wang J, Yan W, Zhou Y, Chen Y, Zhou K, et al. Dysregulated Mir-124 and Mir-200 Expression Contribute to Cholangiocyte Proliferation in the Cholestatic Liver by Targeting Il-6/Stat3 Signalling. J Hepatol (2015) 62(4):889–96. doi: 10.1016/j.jhep.2014.10.033
171. Soini T, Pihlajoki M, Andersson N, Lohi J, Huppert KA, Rudnick DA, et al. Transcription Factor Gata6: A Novel Marker and Putative Inducer of Ductal Metaplasia in Biliary Atresia. Am J Physiol Gastrointest Liver Physiol (2018) 314(5):G547–g58. doi: 10.1152/ajpgi.00362.2017
172. Pihlajoki M, Soini T, Cochran RB, Liljeström E, Huang J, Rudnick DA, et al. Gata6 Modulates the Ductular Reaction to Bile Duct Ligation. Hepatol Int (2021) 15(1):166–78. doi: 10.1007/s12072-020-10119-w
173. Babu RO, Lui VCH, Chen Y, Yiu RSW, Ye Y, Niu B, et al. Beta-Amyloid Deposition Around Hepatic Bile Ducts Is a Novel Pathobiological and Diagnostic Feature of Biliary Atresia. J Hepatol (2020) 73(6):1391–403. doi: 10.1016/j.jhep.2020.06.012
174. Xiao Y, Zhou Y, Lu Y, Zhou K, Cai W. Phb2 Interacts With Lc3 and Sqstm1 Is Required for Bile Acids-Induced Mitophagy in Cholestatic Liver. Cell Death Dis (2018) 9(2):160. doi: 10.1038/s41419-017-0228-8
175. Russi AE, Bezerra JA. A Single-Cell View of Biliary Atresia. Nat Rev Gastroenterol Hepatol (2021) 18(4):219–20. doi: 10.1038/s41575-021-00417-5
176. Brevini T, Tysoe OC, Sampaziotis F. Tissue Engineering of the Biliary Tract and Modelling of Cholestatic Disorders. J Hepatol (2020) 73(4):918–32. doi: 10.1016/j.jhep.2020.05.049
177. Brindley PJ, Bachini M, Ilyas SI, Khan SA, Loukas A, Sirica AE, et al. Cholangiocarcinoma. Nat Rev Dis Primers (2021) 7(1):65. doi: 10.1038/s41572-021-00300-2
178. Hill MA, Alexander WB, Guo B, Kato Y, Patra K, O’Dell MR, et al. Kras and Tp53 Mutations Cause Cholangiocyte- and Hepatocyte-Derived Cholangiocarcinoma. Cancer Res (2018) 78(16):4445–51. doi: 10.1158/0008-5472.Can-17-1123
179. Zhang M, Yang H, Wan L, Wang Z, Wang H, Ge C, et al. Single-Cell Transcriptomic Architecture and Intercellular Crosstalk of Human Intrahepatic Cholangiocarcinoma. J Hepatol (2020) 73(5):1118–30. doi: 10.1016/j.jhep.2020.05.039
180. Li H, Lan T, Liu H, Liu C, Dai J, Xu L, et al. Il-6-Induced Cggnbp2 Encodes a Protein to Promote Cell Growth and Metastasis in Intrahepatic Cholangiocarcinoma. Hepatology (2021) 1-18. doi: 10.1002/hep.32232
181. Zheng T, Hong X, Wang J, Pei T, Liang Y, Yin D, et al. Gankyrin Promotes Tumor Growth and Metastasis Through Activation of Il-6/Stat3 Signaling in Human Cholangiocarcinoma. Hepatology (2014) 59(3):935–46. doi: 10.1002/hep.26705
182. Manieri E, Folgueira C, Rodríguez ME, Leiva-Vega L, Esteban-Lafuente L, Chen C, et al. Jnk-Mediated Disruption of Bile Acid Homeostasis Promotes Intrahepatic Cholangiocarcinoma. Proc Natl Acad Sci USA (2020) 117(28):16492–9. doi: 10.1073/pnas.2002672117
183. Cubero FJ, Mohamed MR, Woitok MM, Zhao G, Hatting M, Nevzorova YA, et al. Loss of C-Jun N-Terminal Kinase 1 and 2 Function in Liver Epithelial Cells Triggers Biliary Hyperproliferation Resembling Cholangiocarcinoma. Hepatol Commun (2020) 4(6):834–51. doi: 10.1002/hep4.1495
184. Zhou Y, Chen Y, Zhang X, Xu Q, Wu Z, Cao X, et al. Brahma-Related Gene 1 Inhibition Prevents Liver Fibrosis and Cholangiocarcinoma by Attenuating Progenitor Expansion. Hepatology (2021) 74(2):797–815. doi: 10.1002/hep.31780
185. Wang J, Dong M, Xu Z, Song X, Zhang S, Qiao Y, et al. Notch2 Controls Hepatocyte-Derived Cholangiocarcinoma Formation in Mice. Oncogene (2018) 37(24):3229–42. doi: 10.1038/s41388-018-0188-1
186. Terada M, Horisawa K, Miura S, Takashima Y, Ohkawa Y, Sekiya S, et al. Kupffer Cells Induce Notch-Mediated Hepatocyte Conversion in a Common Mouse Model of Intrahepatic Cholangiocarcinoma. Sci Rep (2016) 6:34691. doi: 10.1038/srep34691
187. Häcker H, Tseng PH, Karin M. Expanding Traf Function: Traf3 as a Tri-Faced Immune Regulator. Nat Rev Immunol (2011) 11(7):457–68. doi: 10.1038/nri2998
188. Shiode Y, Kodama T, Shigeno S, Murai K, Tanaka S, Newberg JY, et al. TNF Receptor-Related Factor 3 Inactivation Promotes the Development of Intrahepatic Cholangiocarcinoma Through NF-κB-Inducing Kinase-Mediated Hepatocyte Transdifferentiation. Hepatology (2022) 1–17. doi: 10.1002/hep.32317
189. Rizvi S, Khan SA, Hallemeier CL, Kelley RK, Gores GJ. Cholangiocarcinoma - Evolving Concepts and Therapeutic Strategies. Nat Rev Clin Oncol (2018) 15(2):95–111. doi: 10.1038/nrclinonc.2017.157
190. Montal R, Sia D, Montironi C, Leow WQ, Esteban-Fabró R, Pinyol R, et al. Molecular Classification and Therapeutic Targets in Extrahepatic Cholangiocarcinoma. J Hepatol (2020) 73(2):315–27. doi: 10.1016/j.jhep.2020.03.008
191. Mantovani A, Dinarello CA, Molgora M, Garlanda C. Interleukin-1 and Related Cytokines in the Regulation of Inflammation and Immunity. Immunity (2019) 50(4):778–95. doi: 10.1016/j.immuni.2019.03.012
192. Nakagawa H, Suzuki N, Hirata Y, Hikiba Y, Hayakawa Y, Kinoshita H, et al. Biliary Epithelial Injury-Induced Regenerative Response by Il-33 Promotes Cholangiocarcinogenesis From Peribiliary Glands. Proc Natl Acad Sci USA (2017) 114(19):E3806–e15. doi: 10.1073/pnas.1619416114
193. Yamada D, Rizvi S, Razumilava N, Bronk SF, Davila JI, Champion MD, et al. Il-33 Facilitates Oncogene-Induced Cholangiocarcinoma in Mice by an Interleukin-6-Sensitive Mechanism. Hepatology (2015) 61(5):1627–42. doi: 10.1002/hep.27687
194. Wells JM, Watt FM. Diverse Mechanisms for Endogenous Regeneration and Repair in Mammalian Organs. Nature (2018) 557(7705):322–8. doi: 10.1038/s41586-018-0073-7
195. Pepe-Mooney BJ, Dill MT, Alemany A, Ordovas-Montanes J, Matsushita Y, Rao A, et al. Single-Cell Analysis of the Liver Epithelium Reveals Dynamic Heterogeneity and an Essential Role for Yap in Homeostasis and Regeneration. Cell Stem Cell (2019) 25(1):23–38.e8. doi: 10.1016/j.stem.2019.04.004
196. Guilliams M, Bonnardel J, Haest B, Vanderborght B, Wagner C, Remmerie A, et al. Spatial Proteogenomics Reveals Distinct and Evolutionarily Conserved Hepatic Macrophage Niches. Cell (2022) 185(2):379–96.e38. doi: 10.1016/j.cell.2021.12.018
197. Seidman JS, Troutman TD, Sakai M, Gola A, Spann NJ, Bennett H, et al. Niche-Specific Reprogramming of Epigenetic Landscapes Drives Myeloid Cell Diversity in Nonalcoholic Steatohepatitis. Immunity (2020) 52(6):1057–74.e7. doi: 10.1016/j.immuni.2020.04.001
198. Kester L, van Oudenaarden A. Single-Cell Transcriptomics Meets Lineage Tracing. Cell Stem Cell (2018) 23(2):166–79. doi: 10.1016/j.stem.2018.04.014
199. Lancaster MA, Knoblich JA. Organogenesis in a Dish: Modeling Development and Disease Using Organoid Technologies. Science (New York NY) (2014) 345(6194):1247125. doi: 10.1126/science.1247125
200. Weiss P, Taylor AC. Reconstitution of Complete Organs From Single-Cell Suspensions of Chick Embryos in Advanced Stages of Differentiation. Proc Natl Acad Sci USA (1960) 46(9):1177–85. doi: 10.1073/pnas.46.9.1177
201. Prior N, Inacio P, Huch M. Liver Organoids: From Basic Research to Therapeutic Applications. Gut (2019) 68(12):2228–37. doi: 10.1136/gutjnl-2019-319256
202. Hu H, Gehart H, Artegiani B, LÖpez-Iglesias C, Dekkers F, Basak O, et al. Long-Term Expansion of Functional Mouse and Human Hepatocytes as 3d Organoids. Cell (2018) 175(6):1591–606.e19. doi: 10.1016/j.cell.2018.11.013
203. Peng WC, Logan CY, Fish M, Anbarchian T, Aguisanda F, Álvarez-Varela A, et al. Inflammatory Cytokine Tnfα Promotes the Long-Term Expansion of Primary Hepatocytes in 3d Culture. Cell (2018) 175(6):1607–19.e15. doi: 10.1016/j.cell.2018.11.012
204. Huch M, Gehart H, van Boxtel R, Hamer K, Blokzijl F, Verstegen MM, et al. Long-Term Culture of Genome-Stable Bipotent Stem Cells From Adult Human Liver. Cell (2015) 160(1-2):299–312. doi: 10.1016/j.cell.2014.11.050
205. Sampaziotis F, Muraro D, Tysoe OC, Sawiak S, Beach TE, Godfrey EM, et al. Cholangiocyte Organoids Can Repair Bile Ducts After Transplantation in the Human Liver. Science (New York NY) (2021) 371(6531):839–46. doi: 10.1126/science.aaz6964
206. Bayart E, Cohen-Haguenauer O. Technological Overview of Ips Induction From Human Adult Somatic Cells. Curr Gene Ther (2013) 13(2):73–92. doi: 10.2174/1566523211313020002
207. Sia D, Villanueva A, Friedman SL, Llovet JM. Liver Cancer Cell of Origin, Molecular Class, and Effects On Patient Prognosis. Gastroenterology (2017) 152(4):745–61. doi: 10.1053/j.gastro.2016.11.048
208. Cordero-Espinoza L, Dowbaj AM, Kohler TN, Strauss B, Sarlidou O, Belenguer G, et al. Dynamic Cell Contacts Between Periportal Mesenchyme and Ductal Epithelium Act as a Rheostat for Liver Cell Proliferation. Cell Stem Cell (2021) 28(11):1907–21.e8. doi: 10.1016/j.stem.2021.07.002
Keywords: cholangiopathy, biliary repair, liver progenitor cell, macrophage, hepatocyte-cholangiocyte transdifferentiation, immune cells
Citation: Lan T, Qian S, Tang C and Gao J (2022) Role of Immune Cells in Biliary Repair. Front. Immunol. 13:866040. doi: 10.3389/fimmu.2022.866040
Received: 30 January 2022; Accepted: 08 March 2022;
Published: 30 March 2022.
Edited by:
Niloufar Safinia, King’s College London, United KingdomReviewed by:
James Ahodantin, University of Maryland, Baltimore, United StatesMatthias Bartneck, University Hospital RWTH Aachen, Germany
Copyright © 2022 Lan, Qian, Tang and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinhang Gao, Gao.jinhang@scu.edu.cn; Gao.jinhang@qq.com; Chengwei Tang, shcqcdmed@163.com
†These authors have contributed equally to this work
 Tian Lan
Tian Lan Shuaijie Qian
Shuaijie Qian Chengwei Tang
Chengwei Tang Jinhang Gao
Jinhang Gao