- 1Division of Translational Cell Genetics, Medical University of Innsbruck, Innsbruck, Austria
- 2Department of Neurology, Klinik Floridsdorf, Wien, Austria
- 3Department of Neurology, Medical University of Innsbruck, Innsbruck, Austria
This review focuses on current clinical and immunological aspects of cerebral malaria induced by Plasmodium falciparum infection. Albeit many issues concerning the inflammatory responses remain unresolved and need further investigations, current knowledge of the underlying molecular mechanisms is highlighted. Furthermore, and in the light of significant limitations in preventative diagnosis and treatment of cerebral malaria, this review mainly discusses our understanding of immune mechanisms in the light of the most recent research findings. Remarkably, the newly proposed CD8+ T cell-driven pathophysiological aspects within the central nervous system are summarized, giving first rational insights into encouraging studies with immune-modulating adjunctive therapies that protect from symptomatic cerebral participation of Plasmodium falciparum infection.
Introduction
Malaria is a mosquito-borne infectious disease that is self-limiting even without therapy. However, in 1–2% of cases, mostly among children under the age of five, malaria becomes severe and life-threatening (1). Why young children are especially prone to develop severe and cerebral malaria (CM) is not fully understood. The vector responsible for most complicated malaria cases is the parasite Plasmodium falciparum (Pf). However, P. vivax infections also can cause CM (2), and P. knowlesi occasionally provokes severe malaria signs and symptoms (3).
Although it has become a declared global target of the World Health Organisation (WHO) to eliminate malaria worldwide and malaria cases and death have been reduced within the last ten years, in 2021, this progress was impeded as the COVID-19 pandemic disrupted malaria services and diagnosis. The immense number of malaria cases in 2021 (241 million cases) and the distressing number of deaths (627 000) revealed how fragile medical services are. Especially in the African region, where 96% of all malaria deaths occurred, 80% were children under five, significantly more effort is needed to reverse the last year’s trend for 2022 (4).
The definition of severe malaria by the WHO is Pf parasitemia together with one or more of the following medical conditions: impaired consciousness, prostration, multiple convulsions, acidosis, hypoglycemia, anaemia, renal impairment, jaundice, pulmonary oedema, significant bleeding and shock (5). Apart from infants, pregnant women are also more likely to develop severe malaria, particularly in the second and third trimesters. Placental malaria can lead to fetal and maternal death when untreated, and premature labour and children with low birth weight are common complications even with intermittent preventive treatment in pregnancy (4). Other susceptible population groups are non-semi-immune persons, e.g. travellers or migrant workers, moving to holo-endemic areas.
Cerebral malaria (CM) is characterized by unarousable coma not attributable to other neuro-pathologies combined with Pf parasitemia. Of all severe malaria complications, CM is the most prevalent and deadly, with a mortality rate of 100% if untreated and a patient fatality of 15-25% if treated with current first-line anti-malaria therapy (6). Children with CM present acutely in a coma often precipitated by seizure and a one to three-day history of fever, vomiting, and anorexia. Within two days, most children will have recovered from or succumbed to the disease (7). 14-25% of children recovering from CM suffer from long-term sequelae like cognitive and hearing impairments (8, 9). As there is no specific therapy beyond symptomatic neurocritical management, all strategies aim to eliminate the parasite from the system while targeting symptoms arising from severe complications like respiratory distress or convulsions. Furthermore, it is impossible to predict which children are likely to develop cerebral complications because the exact molecular mechanisms in human pathology remain unsolved. In animal studies, which are a reasonable way to investigate molecular pathophysiology, it has been shown that CM is, at least in part, an immune-mediated disease. Most likely, cerebral complications are caused by a misguided host immune response provoked by the parasite infection. Therefore adjunctive therapies focusing on the regulation of immune cells are interesting new treatment strategies (10).
Treatment of Severe and Cerebral Malaria
Artemisinins, which have the fastest parasite clearing time of all anti-malarial drugs, have become the drugs of the moment, and artesunate is the first-line therapy for treating severe and cerebral malaria in both children and adults (5).
The main difference between the treatment of uncomplicated and severe malaria is the route of administration of the artemisinin-based therapy. Artesunate should be administered intravenously for 24 hours in severe and cerebral malaria cases. If an intravenous application is not possible, the intramuscular route is the second-best option, and artemether is recommended in case of missing artesunate. After that, a three-day oral artemisinin-based combination therapy (ACT) should be followed (5).
Beyond intravenous artesunate, no “brain-specific” drug is available. Symptomatic therapy strategies are the only possibility to manage organ manifestations and intracranial complications of Pf malaria. Although not widely available, respiratory support and artificial ventilation are crucial (11). Increasing intracranial pressure decreases cerebral perfusion and leads to secondary transtentorial herniation, the primary cause of death in children with CM (12). Seizure management is another critical treatment, as up to 70% of children with severe and cerebral malaria have seizures, which, when treated with benzodiazepines, are less likely to cause secondary neurological damage, and fever should be controlled to reduce high fever convulsions and long-term neurologic damage (12).
Although it is a breakthrough, the first-ever malaria vaccination only provides partial protection. A four-dose regimen (i.m.) of the MosquirixTM (RTS,S/AS01) vaccine showed protection from severe and cerebral malaria in 32.2% of children aged 5-17 months (13). Therefore, seasonal chemoprevention should continue in addition to vaccination (14).
Genetic Coevolution
There have been millenniums of coevolution between humanity and Plasmodium species. Considering this, it is no surprise that both parasite and host have undergone genetic alterations for successful coexistence. On the host side, protective variations include sickle-cell anaemia (15), thalassemia (16), G6PD deficiency (17) and other haemoglobinopathies with high incidence in malaria-endemic countries. These severe inherited haemoglobin disorders are nicely reviewed elsewhere (18).
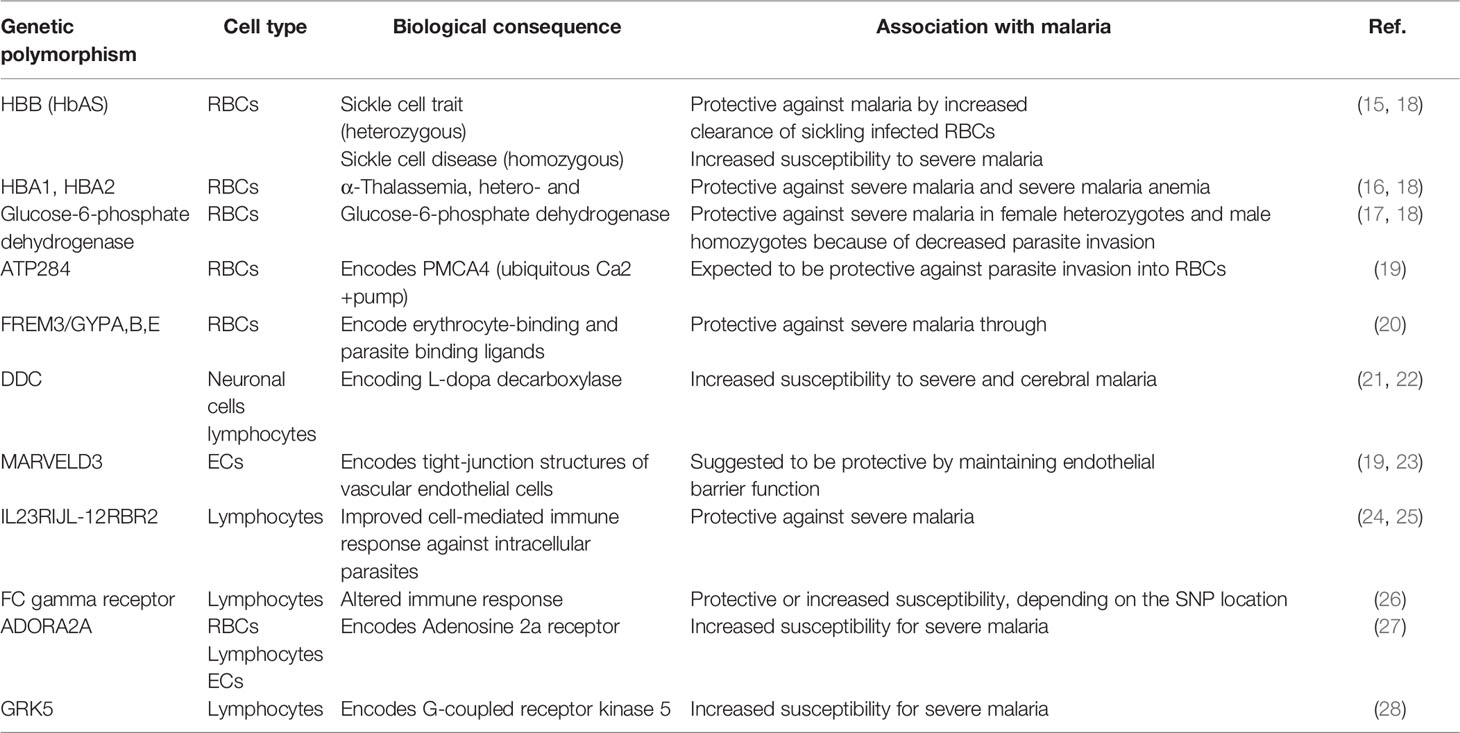
Several novel genetic variants have been identified with new technologies for genome-wide association studies (GWAS). Many genetic polymorphisms related to malaria are relevant for red blood cell (RBC) biology, like variants of the ATP2B4 gene, a plasma membrane calcium transporter contributing to resistance against severe malaria (19), or variants of the FREM3 gene and a cluster of three glycophorin genes (GYPE, GYPB and GYPA) associated with 33% protection against severe malaria (20). However, newly identified single nucleotide polymorphisms (SNPs) indicate the importance of other cells for malaria disease progression, as variations in genes encoding proteins necessary for neurons, endothelial cells and immune cell-signalling have been identified with GWAS. For example, a SNP close to the DDC (L-DOPA decarboxylase) gene is associated with cerebral malaria susceptibility (21). DDC is an enzyme that catalyzes the decarboxylation of L-DOPA to dopamine, which is involved in neuronal signalling and regulating the activation of specific lymphocyte subtypes (22). SNPs near the MARVELD3 gene have been suggested to protect against the severe progression of malaria (19, 23). Structural variants of the gene product of MARVELD3 or alterations in its expression could influence the barrier function and endothelial adherence of parasitized erythrocytes. A more recent GWAS identified the IL-23R and IL-12RBR2 genes associated with alterations in malaria severity (24). Both genes are essential for immune cell signalling, especially for T-cells. Variants of IL-12B are protective against cerebral malaria in children (25). Polymorphisms in Fc gamma receptors on the surface of immune cells such as B-cells, NK cells and macrophages have been linked to resistance and susceptibility to malaria in different population studies (26).
Accumulating evidence indicates that the G protein-coupled signal transduction pathways are involved in the regulation of malaria, specifically in the severe, life-threatening manifestations of the disease. In a case-controlled study of adults, SNPs of ADORA2A and GRK5 genes were associated with virulence and infectivity of the malaria parasite (27). The ADORA2A gene was associated with severe Pf malaria in children in a meta-analysis that evaluated several G protein-coupled signalling pathways (28). Identified genes and proteins listed in Table 1 might be as new drug targets and require further exploration in malaria studies to validate and functionally characterize causalities to enhance our understanding of cerebral malaria pathology.
Pathophysiology of Cerebral Malaria
Magnetic resonance imaging has achieved vital progress in elucidating cerebral malaria pathology in children suffering from cerebral manifestations. It has become evident that brain swelling and brain stem herniation lead to respiratory arrest and death (6). Further investigations could link the swelling of the brain to a dysfunctional blood-brain barrier, indicating increased vascular permeability (29). Causative for the penetration of vascular fluid into brain tissue might be an excessive inflammatory response to sequestration of parasitized erythrocytes in the cerebral vasculature (30). Overall, CM seems to be caused by a combination of host factors and parasite factors, leading to inflammation and coagulation in the brain vasculature (31). Endothelial cell dysfunction due to inflammation is the main driver for brain pathology concerning host factors. On the parasite side, PfEMP-1 (Pf erythrocyte membrane protein-1) molecules on the surface of iRBCs are responsible for most parasite-host cell interactions.
Parasite Virulence Factors
In order to escape the clearance by the spleen, iRBCs adhere to vessel walls in several organs (32). The surface molecule PfEMP-1 mediates this sequestration by binding to the host vasculature via interaction with specific adhesion molecules. PfEMP1 is encoded by the var gene family with approximately 60 members (33). The extracellular (interacting) part of PfEMP1 consists of Duffy-binding-like (DBL) domains and cysteine-rich interdomain regions (CIDR), each of which can be subdivided into seven and three main sequence classes, respectively (34). Among the host endothelial adhesion molecules, intercellular adhesion molecule-1 (ICAM-1) has long been suggested as the main anchor point for PfEMP-1 (35). Still, more recently, CD36 and endothelial protein C receptor (EPCR) were identified as more probable binding sites (36, 37). CIDRα2-6 domains of PfEMP1 bind to CD36 (38), and CIDRα1 binds to EPCR on endothelial cells (39).
Host Virulence Factors
On the host side of malaria pathology, activated endothelial cells play a significant role in mediating cerebral manifestations. Endothelial cells are activated permanently upon Pf infection and sequestration of iRBCs to the vasculature. This chronic activation leads to dysregulation in the endothelial barrier function and brain oedema (40).
Activation of the Brain Endothelium
Brain endothelial cells are activated during the first phase of Pf infection, even before the sequestration of iRBCs. The exact mechanism of early endothelial cell activation is unknown, but it is hypothesized that soluble factors from iRBCs such as Pf histidine-rich-protein 2 (PfHRP2) might activate brain endothelial cells (41). PfHRP2 has been suggested as a prognostic marker for CM as plasma levels increase with malaria severity (42). However, studies outside the African continent could not confirm the correlation between plasma levels and disease complications (43).
Changes in the bioavailability of nitric oxide (NO), being the principal protector of endothelium homeostasis, have been reported in children with Pf malaria (44). However, inhaled NO as adjunctive therapy for severe malaria proved insufficient for change of outcome (45). Another vasoactive substance elevated in the plasma of malaria patients is endothelin-1 (ET-1) (46). This peptide is among the most effective vasoconstrictive peptides in the human body, but whether ET-1 is involved in vasculopathy in cerebral manifestations of malaria remains unanswered.
Under hypoxic conditions, after vasoconstriction due to sequestration of iRBCs, vascular endothelial growth factor-A (VEGF-A) is released by endothelial cells (47). VEGF-A binds to its receptor (VEGFR2) on endothelial cells, inducing vascular permeability. On the other hand, VEGF-A exerts protective, anti-apoptotic effects in endothelial and neuronal cells. As reviewed elsewhere, the role of VEGF-A remains controversial (48). However, a study investigating serum samples from malaria patients showed significantly lower levels of VEGF-A in cerebral malaria non-survivors, pointing instead to a protective effect of VEGF-A in cerebral complications of malaria (49). Similar protective effects on the vascular brain endothelium are mediated by angiopoietin-1 (Ang-1) and its receptor Tie-2. Ang-1 is essential for endothelial quiescence but can be blocked by Ang-2, which renders a natural competing antagonist for Ang-1 regarding the binding to their shared Tie-2 receptor. Ang-2 destabilizes existing vessels as part of the initiation of angiogenesis, the formation of new blood vessels (50), and is released from activated endothelial cells. Elevated levels of Ang-2 are found in severe and cerebral malaria (47, 51). Although anti-malarial therapy decreases Ang-2 levels (52), direct targeting of the Ang-2/Tie-2 pathway remains challenging and has been reviewed elsewhere (53).
Many of these peptides from endothelial activation have been suggested as possible biomarkers to predict the fatal outcome of CM. However, currently, there is no test available to confirm the diagnosis of cerebral manifestations prior to the appearance of clinical symptoms.
Blood-Brain Barrier Disruption
The blood-brain barrier (BBB) comprises brain endothelial cells connected by tight junctions formed by transmembrane proteins occludin, claudin and zonula occludens protein-1 (ZO-1). Endothelial cells are in contact with surrounding pericytes and astrocytes situated in the perivascular space. Together, these cells provide a highly functional barrier between the blood and the brain interstitial fluid. In cerebral malaria, an impaired barrier function allows leakage of plasma proteins and fluids into the perivascular space causing vasogenic oedema and brain swelling (6). One reason for the disintegration of the BBB is the decrease in endothelial tight junction proteins occludin and ZO-1 (54). Another reason for the destruction of the BBB lies in the apoptosis of endothelial cells caused by a hyperinflammatory environment upon lymphocyte sequestration.
Cytokine Mediated Inflammation of the Brain
Numerous studies showed that serum levels of the pro-inflammatory cytokine TNF-α were higher in CM than in severe forms of malaria in children and adults (55). Nevertheless, a more recent study showed that brain swelling of children suffering from CM is independent of peripheral plasma cytokine levels (56), and therapy with a monoclonal antibody against TNF-α did not improve survival in CM patients (57).
Likewise, IFN-γ is released from immune cells like CD4+ and CD8+ T cells, natural killer cells and γδ-T cells during malaria infection. IFN-γ is a potent activator of macrophages, increasing their phagocytosis activity, vital in the early control of parasite growth. On the other side, IFN-γ induces brain endothelium activation and an increase of adhesion molecules (58). However, targeting the IFN-γ pathway as adjunctive therapy for CM is questionable as this important cytokine is involved in many distinctive processes important for gaining immunity against malaria (59).
Chemokine Induced Migration to the Brain
Besides promoting the expression of adhesion molecules in the brain vasculature, IFN-γ is active in up-regulating CXCL10 released from endothelial cells (60). Confirming, CXCL10 has been described as a biomarker of CM and a predictor of mortality (61). The receptor to which CXCL10 binds is CXCR3, which is predominately expressed on immune cells such as CD4+ and CD8+ T-cells (62). Recently, the adhesion of CD8+ T cells to the brain vascular endothelium was shown to be involved in manifestations of human CM for the first time (63).
CD8+ T Cells Sequestration
From murine cerebral malaria research, we know that T cells play a crucial role in the experimental cerebral malaria (ECM) model, and functional studies using neutralizing antibodies or T cell-deficient mice have demonstrated a significant role of CD8+ T cells in inducing brain damage (64). However, evidence for the involvement of CD8+ T cells in human CM has long been missing. Recent studies have investigated the presence of CD8+ T cells in human CM post-mortem sections. A first study successfully showed the presence of CD8+ T cells in the brains of children who died from malaria. However, a clear correlation to cerebral manifestations could not be drawn (65). Finally, Riggle et al. provided evidence for the significant involvement of CD8+ T cells in the human setting by investigating 31 brains of children who had died from CM, using multiplexed histology (63). Interestingly, the presence of CD8+ T cells was correlated with the number of iRBCs within the lumen of brain veins. Additionally, sequestered CD8+ T cells showed positive staining for the cytolytic protease granzyme B (GrB) (63). As enzymes such as GrB (66) and perforin (67) are responsible for apoptosis of endothelial cells in ECM, the findings presented by Riggle et al. indicate a similar mechanism in human CM and underline the relevance of the murine malaria model.
Immunological Aspects of CM Pathology
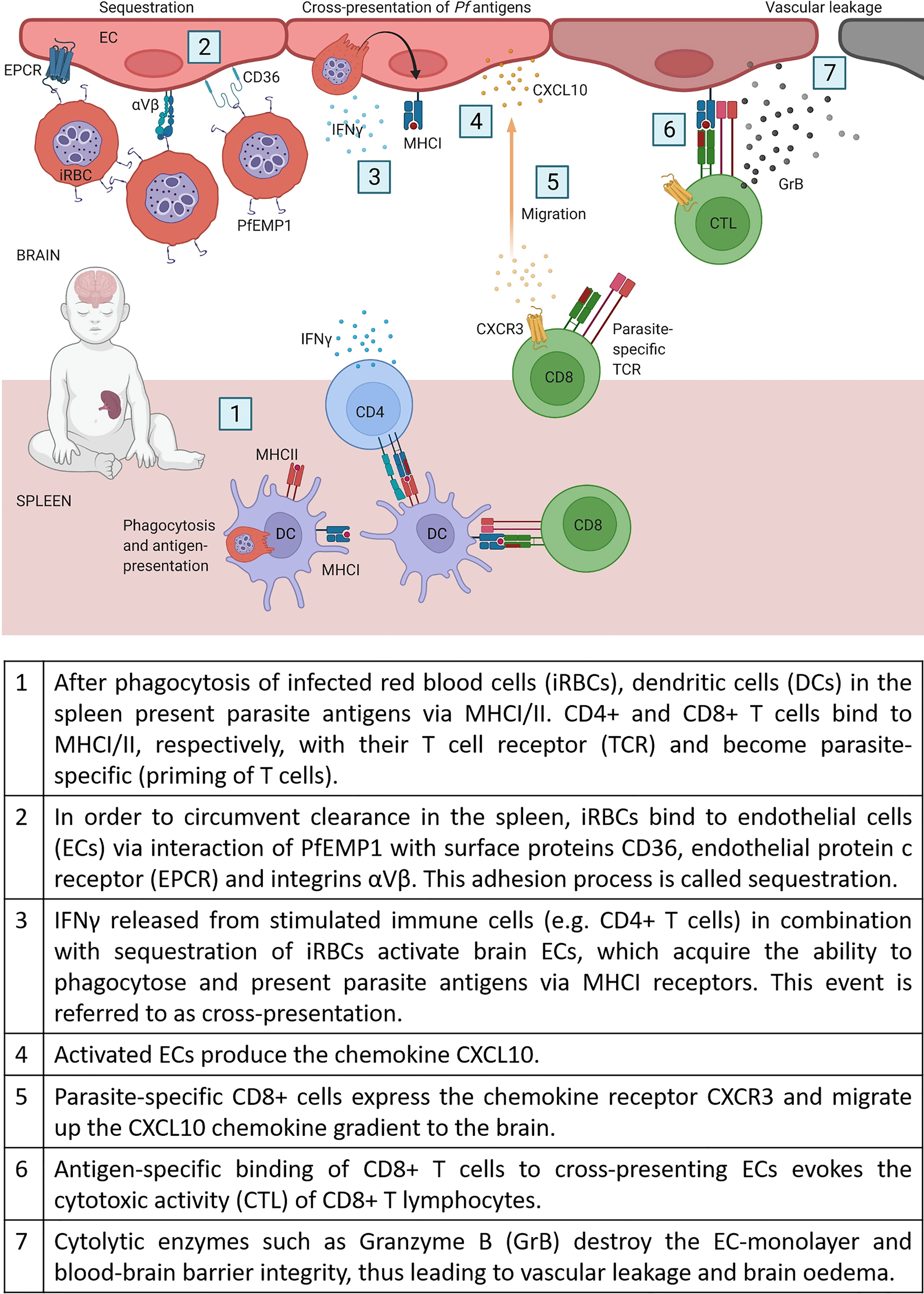
CD8+ T cells recognize pathogens through major histocompatibility complex I molecules (MHCI) on the surface of antigen-presenting cells or infected cells and contribute, therefore, to clearance and immunity against intracellular pathogens. However, as erythrocytes lack MHCI receptors, CD8+ T cells do not recognize Pf infected RBCs and are therefore unable to add to clearance of blood-stage infections. Instead, CD8+ T cells are suspected to be the main drivers for cerebral pathology in humans and experimental mouse models (68, 69). One important step for enabling cytotoxic CD8+ T cells (CTLs) to interfere with brain ECs, is a specific process called cross-presentation. As a result of chronic activation of ECs by sequestration of iRBCs in combination with high IFNγ levels, ECs start to phagocytose parasites (e.g. free merozoites) and present these antigens via their major histocompatibility complex class I (MHCI) (70, 71). In Figure 1, we compile the current knowledge of all the stepwise mechanisms which, as we propose, may lead to CM in non-immune humans.
Experimental Cerebral Malaria
The mouse model to examine CM named experimental cerebral malaria (ECM) is studied with C57BL/6 mice infected with Plasmodium berghei ANKA (PbA). In this mouse model, RBCs bearing asexual forms of the parasite are injected intraperitoneal (106 infected RBCs) or intravenously (104 infected RBCs). As asexual forms do not enter hepatocytes, the liver stage of the disease is circumvented, and the blood stage is visible in blood smears after approximately three days. Mice show signs of neurological symptoms such as ataxia, convulsions or paralysis starting from day four and die between days 6-8. Mice that do not develop cerebral manifestations die from hyper-parasitemia and anaemia at later time points (day 14 to 21) (72). Similarly to human CM, brain pathology includes dysfunction of the BBB, brain haemorrhaging and brain swelling (68). In addition, mice with neurological symptoms treated with anti-malarial drugs survive with long-lasting cognitive deficits (73). The most significant difference between the murine model and human CM is the extent of iRBCs sequestration in the brain’s microvasculature (74) due to the lack of molecules on RBCs infected with PbA. Therefore, the malaria mouse model is mainly used to study host virulence factors of malaria. However, the presence of parasites in the brain vasculature is also critical for ECM (75, 76). Several studies searched for functional equivalents of PfEMP1 in mouse and non-falciparum human Plasmodium species (77, 78). Two proteins necessary for the transport of PfEMP1 to the erythrocyte surface, SBP1 and MAHRP1, are evolutionarily conserved between Plasmodium species, and their orthologues have been identified in Plasmodium berghei, although the transported surface protein is not known (79–81).
Mouse Models of CM and Their Translational Potential
In experimental malaria models, multiple pathways have been classified as essential for developing CM. After the deletion of specific genes, several knockout mice infected with PbA showed resistance against cerebral manifestations of malaria. The identified genes can serve as potential therapeutic targets. Many of the involved genes discussed hereafter encode proteins that function in leukocyte migration to the brain and modulate T-cell effector functions. However, the limitations of the experimental murine model for translational research will also be discussed in the following passage.
Interferon-Gamma
Cytokines such as interferon-gamma (IFNγ) and its receptors (IFNγR) seem crucial for CM development. In mouse models, IFNγR2 knockout proved protective against CM, whereas specific knockout on T cells (CD4-Cre+ IFN-γR2flox/flox) did not prevent cerebral symptoms and early death (82). A probable explanation for the survival benefits in an IFNγ deficient system is that this cytokine is involved in activating the brain endothelium, enabling the cross-presentation of parasite proteins by endothelial cells. Therefore, endothelial cells act as antigen-presenting cells (APCs) and are thus recognized by cytotoxic T cells and destroyed. As a result, the endothelial layer gets leaky and infiltrating fluid causes brain swelling (proposed in Figure 1).
Outside the model of ECM, however, IFNγ is essential for a functional immune system, and its stimulation of innate and adaptive immune responses is crucial to attack hepatic stages of Pf during the early stage of infection (83). Therefore, blocking IFNγ during a Pf infection might do more harm in the long run than prevent cerebral complications.
Tumour Necrosis Factor-Alpha
Tumour necrosis factor receptor 2 (TNFR2) deficient mice were resistant to CM pathology and maintained BBB integrity during infection with PbA (84). Accordingly, in the human setting, TNFα has been suggested to mediate the sequestration of iRBCs by upregulation of adhesion molecules on endothelial cells and induce the fatal inflammatory cascade together with IFNγ and nitric oxide (85).
Instead, Lymphotoxin alpha (LTα), which binds the same receptors as TNFα (TNFR1 and TNFR2), might be a more specific target and control late-stage inflammation to prevent neurological complications. LTα knockout animals and LIGHT-lymphotoxin beta receptor (LTβR) deficient mice were protected from CM (86), and LTβR-/- mice showed reduced lymphocyte recruitment to the brain resulting in a survival benefit (87).
However, the translational potential of TNFα and LTα for human CM is questionable, as, similar to IFNγ, TNFα and its receptors are associated with gaining immunity against malaria (88).
Interleukins
Interleukins (IL) are other cytokine family members playing a role in cerebral malaria complications. For instance, prophylactic treatment with IL-4 increased survival time in PbA infected mice by reducing parasitemia by stimulation of Th2 CD4+ T cells and the phagocytic system. However, IL-4 treatment was only effective as a preventive strategy, whereas starting therapy on day five after infection did not abrogate CM fatality (89). However, knockout of the interleukin-4 receptor alpha (IL-4Rα) specifically on dendritic cells (DCs) resulted in decreased numbers of cytotoxic CD8+ T cells in the brain (90). In human studies, increased serum levels of IL-4 are associated with severe and cerebral forms of malaria (91), probably by increasing the fatal inflammatory response.
Interleukin-12 receptor beta2 (IL-12Rβ2) deficient mice are protected against cerebral complications, whereas IL-12-p40 deficient mice show similar susceptibility as wild-type animals (92), suggesting that ECM induction through IL-12Rβ2 can occur independently of IL-12 ligands. Interestingly, IL-12Rβ2 primarily occurs on activated T cells and NK cells, emphasizing the role of these cells in ECM pathology. In human CM, decreased plasma levels of IL-12 occur in severe childhood malaria (93). Unfortunately, no study differentiated between severe and cerebral pathologies of malaria to give data on IL-12 involvement in human brain pathology.
The Interleukin-33 receptor (ST2) is expressed on brain endothelial cells, and ST2 knockout mice are protected from CM due to less cytotoxic CD8+ T in the brain microvasculature (94).
Adhesion Molecules
The role of the adhesion molecule ICAM1 in ECM development has long been an undoubted truth as ICAM-/- mice are protected from brain pathologies (95). With newer technologies enabling ICAM1 knock-down selectively on endothelial cells (ECs), this theory has become questionable, as these EC-specific knockout mice still developed CM with cellular sequestration independent from ICAM-1 expression on cerebral microvasculature (96). Recently, PfEMP1 A-Type ICAM-1-binding domains have been shown to be not associated with CM in children (97). Instead, the role of PfEMP1 binding to endothelial protein C receptor (EPCR) was significantly linked with brain swelling (98).
Integrin αDβ2 on the surface of lymphocytes is involved in adhesion to brain vessels (99), a critical step in ECM development. In a preclinical study investigating PfEMP1 interacting partners, integrins αVβ3 and αVβ6 have been shown to bind to the DBLδ_D4 domain of a specific parasite line expressing the single var gene PFL2665c (100). Therefore, the authors have suggested that endothelial cells expressing αVβ3 and αVβ6 integrins potentially add to the sequestration of iRBCs via the DBLδ_D4 domain of PfEMP1.
CD36 (cluster of differentiation 36) is a membrane protein found on the surface of ECs and has been described as essential for cytoadherence of iRBCs in ECM, although survival of CD36 knockout mice was not improved (101). In human CM, polymorphisms of CD36 are associated with protection from neurological complications (102), and CD36 is a common target of the PfEMP1 protein on iRBCs for adherence to endothelial receptors (103).
Chemokines
Chemokine receptor CXCR3-/- mice are protected from fatal ECM by decreased infiltration of perforin-positive CD8+ T cells to the brain. The results suggest that CXCR3 is necessary for the migration of CD8+ cells to the brain (104). The ligands of the CXCR3 receptor, IP-10 (CXCL10) and Mig (CXCL9), however, did only partially protect mice from fatal CM when genetically knocked out (105). In patients, CXCL10 serum levels have been significantly associated with a high risk for cerebral complications (61) and suggested as prognostic biomarkers for severe and cerebral complications of malaria (106).
Transcription Factors
When the transcription factor Batf3 is knocked out in mice, animals lack a specific DC subset necessary for T cell priming. As a result, Batf3-/- mice show decreased cytolytic active CD8+ T cells and are protected from developing CM (107). Batf3 is a potential target for immunotherapy in humans, although its impact on CM has not been investigated so far (108).
Lipoproteins
Apolipoprotein E (ApoE) is the dominant apolipoprotein in the brain, and ApoE−/− mice are protected against the development of ECM through decreased sequestration of parasites and T cells within the brain. Additionally, treating mice with the ApoE antagonist heparin octasaccharide significantly decreased ECM incidence (109). Whether heparin-based therapies might be promising for reestablishing blood perfusion in congested brain microvasculature, however, is unclear, as bleedings are a possible complication in the neurological complex of CM.
Protein-Kinases
Protein kinase C-theta (PKC-theta) deficient mice do not show neurologic symptoms typical for CM, such as abrogated cerebral microcirculation or brain ischemia. Interestingly, recruitment and activation of CD8+ T cells were reduced in the brain of resistant mice (110). Further investigation with specific pharmacological inhibitors of the PKC-theta pathway may present a new treatment strategy that needs to be investigated.
Conclusion
Summing up the latest research data from mouse and human CM studies, the importance of lymphocyte sequestration in the brain vasculature has achieved objective evidence. Although substantial progress has been made in elucidating the cause of death in CM, specific treatment is still missing, and solutions are not within sight. For this reason, research regarding targets, which are drugable in the human setting, is urgently needed, and focus should be concentrated on the development of adjunctive therapies for treating and preventing the potentially fatal evolution into CM.
Author Contributions
KA-S wrote the manuscript, performed literature research and prepared the table and figure. PL reviewed the manuscript and added an essential part due to his expertise in malaria research and neurology. ES reviewed the manuscript and added essential parts due to his expertise in malaria research and neurology. GB revised the manuscript and added an essential part due to his expertise in T cell biology. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Austrian Science Fund TAI80-B to KA-S.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Figure 1 was created with BioRender.com.
References
2. Tanwar GS, Khatri PC, Sengar GS, Kochar A, Kochar SK, Middha S, et al. Clinical Profiles of 13 Children With Plasmodium Vivax Cerebral Malaria. Ann Trop Paediatr (2011) 31(4):351–6. doi: 10.1179/1465328111Y.0000000040
3. Barber BE, Grigg MJ, Piera KA, William T, Cooper DJ, Plewes K, et al. Intravascular Haemolysis in Severe Plasmodium Knowlesi Malaria: Association With Endothelial Activation, Microvascular Dysfunction, and Acute Kidney Injury. Emerg Microbes Infect (2018) 7(1):106. doi: 10.1038/s41426-018-0105-2
4. WHO. World Malaria Report 2021 Vol. 322. World Health Organization (2021). Geneva, Licence: CC BY-NC-SA 3.0 IGO.
5. WHO. Guidelines for the Treatment of Malaria - Third Edition. (2015). Geneva, World Health Organization, Licence: CC BY-NC-SA 3.0 IGO
6. Seydel KB, Kampondeni SD, Valim C, Potchen MJ, Milner DA, Muwalo FW, et al. Brain Swelling and Death in Children With Cerebral Malaria. N Engl J Med (2015) 372(12):1126–37. doi: 10.1056/NEJMoa1400116
7. Idro R, Jenkins NE, Newton CR. Pathogenesis, Clinical Features, and Neurological Outcome of Cerebral Malaria. Lancet Neurol (2005) 4(12):827–40. doi: 10.1016/S1474-4422(05)70247-7
8. Kariuki SM, Abubakar A, Newton CR, Kihara M. Impairment of Executive Function in Kenyan Children Exposed to Severe Falciparum Malaria With Neurological Involvement. Malar J (2014) 13:365. doi: 10.1186/1475-2875-13-365
9. Schmutzhard J, Lackner P, Helbok R, Hurth HV, Aregger FC, Muigg V, et al. Erratum to: Severe Malaria in Children Leads to a Significant Impairment of Transitory Otoacoustic Emissions - A Prospective Multicenter Cohort Study. BMC Med (2016) 14:70. doi: 10.1186/s12916-016-0616-4
10. Riggle BA, Miller LH, Pierce SK. Desperately Seeking Therapies for Cerebral Malaria. J Immunol (2020) 204(2):327–34. doi: 10.4049/jimmunol.1900829
11. Karnad DR, Nor MBM, Richards GA, Baker T, Amin P, Societies CWF. Intensive Care in Severe Malaria: Report From the Task Force on Tropical Diseases by the World Federation of Societies of Intensive and Critical Care Medicine. J Crit Care (2018) 43:356–60. doi: 10.1016/j.jcrc.2017.11.007
12. Taylor TE, Molyneux ME. The Pathogenesis of Pediatric Cerebral Malaria: Eye Exams, Autopsies, and Neuroimaging. Ann N Y Acad Sci (2015) 1342(1):44–52. doi: 10.1111/nyas.12690
13. Tinto H, D’Alessandro U, Sorgho H, Valea I, Tahita MC, Kabore W, et al. Efficacy and Safety of RTS,s/AS01 Malaria Vaccine With or Without a Booster Dose in Infants and Children in Africa: Final Results of a Phase 3, Individually Randomised, Controlled Trial. Lancet (2015) 386(9988):31–45. doi: 10.1016/S0140-6736(15)60721-8
14. Chandramohan D, Zongo I, Sagara I, Cairns M, Yerbanga RS, Diarra M, et al. Seasonal Malaria Vaccination With or Without Seasonal Malaria Chemoprevention. New Engl J Med (2021) 385(11):1005–17. doi: 10.1056/NEJMoa2026330
15. Luzzatto L. Sickle Cell Anaemia and Malaria. Mediterr J Hematol Infect Dis (2012) 4(1):e2012065. doi: 10.4084/mjhid.2012.065
16. Taylor SM, Parobek CM, Fairhurst RM. Haemoglobinopathies and the Clinical Epidemiology of Malaria: A Systematic Review and Meta-Analysis. Lancet Infect Dis (2012) 12(6):457–68. doi: 10.1016/S1473-3099(12)70055-5
17. Uyoga S, Ndila CM, Macharia AW, Nyutu G, Shah S, Peshu N, et al. Glucose-6-Phosphate Dehydrogenase Deficiency and the Risk of Malaria and Other Diseases in Children in Kenya: A Case-Control and a Cohort Study. Lancet Haematol (2015) 2(10):E437–E44. doi: 10.1016/S2352-3026(15)00152-0
18. Hedrick PW. Population Genetics of Malaria Resistance in Humans. Heredity (Edinb) (2011) 107(4):283–304. doi: 10.1038/hdy.2011.16
19. Timmann C, Thye T, Vens M, Evans J, May J, Ehmen C, et al. Genome-Wide Association Study Indicates Two Novel Resistance Loci for Severe Malaria. Nature (2012) 489(7416):443–6. doi: 10.1038/nature11334
20. Malaria Genomic Epidemiology N, Band G, Rockett KA, Spencer CC, Kwiatkowski DP. A Novel Locus of Resistance to Severe Malaria in a Region of Ancient Balancing Selection. Nature (2015) 526(7572):253–7. doi: 10.1038/nature15390
21. Jallow M, Teo YY, Small KS, Rockett KA, Deloukas P, Clark TG, et al. Genome-Wide and Fine-Resolution Association Analysis of Malaria in West Africa. Nat Genet (2009) 41(6):657–65. doi: 10.1038/ng.388
22. Capellino S, Claus M, Watzl C. Regulation of Natural Killer Cell Activity by Glucocorticoids, Serotonin, Dopamine, and Epinephrine. Cell Mol Immunol (2020) 17(7):705–11. doi: 10.1038/s41423-020-0477-9
23. Steed E, Rodrigues NT, Balda MS, Matter K. Identification of Marveld3 as a Tight Junction-Associated Transmembrane Protein of the Occludin Family. BMC Cell Biol (2009) 10:95. doi: 10.1186/1471-2121-10-95
24. Ravenhall M, Campino S, Sepulveda N, Manjurano A, Nadjm B, Mtove G, et al. Novel Genetic Polymorphisms Associated With Severe Malaria and Under Selective Pressure in North-Eastern Tanzania. PloS Genet (2018) 14(1):e1007172. doi: 10.1371/journal.pgen.1007172
25. Zhang L, Prather D, Vanden Eng J, Crawford S, Kariuki S, ter Kuile F, et al. Polymorphisms in Genes of Interleukin 12 and Its Receptors and Their Association With Protection Against Severe Malarial Anaemia in Children in Western Kenya. Malar J (2010) 9:87. doi: 10.1186/1475-2875-9-87
26. Amiah MA, Ouattara A, Okou DT, N’Guetta SA, Yavo W. Polymorphisms in Fc Gamma Receptors and Susceptibility to Malaria in an Endemic Population. Front Immunol (2020) 11:561142. doi: 10.3389/fimmu.2020.561142
27. Gupta H, Jain A, Saadi AV, Vasudevan TG, Hande MH, D’Souza SC, et al. Categorical Complexities of Plasmodium Falciparum Malaria in Individuals is Associated With Genetic Variations in ADORA2A and GRK5 Genes. Infect Genet Evol (2015) 34:188–99. doi: 10.1016/j.meegid.2015.06.010
28. Auburn S, Fry AE, Clark TG, Campino S, Diakite M, Green A, et al. Further Evidence Supporting a Role for Gs Signal Transduction in Severe Malaria Pathogenesis. PloS One (2010) 5(4):e10017. doi: 10.1371/journal.pone.0010017
29. Brown H, Rogerson S, Taylor T, Tembo M, Mwenechanya J, Molyneux M, et al. Blood-Brain Barrier Function in Cerebral Malaria in Malawian Children. Am J Trop Med Hyg (2001) 64(3-4):207–13. doi: 10.4269/ajtmh.2001.64.207
30. Storm J, Craig AG. Pathogenesis of Cerebral Malaria–Inflammation and Cytoadherence. Front Cell Infect Microbiol (2014) 4:100–. doi: 10.3389/fcimb.2014.00100
31. Wassmer SC, Grau GER. Severe Malaria: What’s New on the Pathogenesis Front? Int J Parasitol (2017) 47(2-3):145–52. doi: 10.1016/j.ijpara.2016.08.002
32. Hviid L, Jensen AT. Pfemp1 - A Parasite Protein Family of Key Importance in Plasmodium Falciparum Malaria Immunity and Pathogenesis. Adv Parasitol (2015) 88:51–84. doi: 10.1016/bs.apar.2015.02.004
33. Gardner MJ, Hall N, Fung E, White O, Berriman M, Hyman RW, et al. Genome Sequence of the Human Malaria Parasite Plasmodium Falciparum. Nature (2002) 419(6906):498–511. doi: 10.1038/nature01097
34. Jensen AR, Adams Y, Hviid L. Cerebral Plasmodium Falciparum Malaria: The Role of Pfemp1 in Its Pathogenesis and Immunity, and Pfemp1-Based Vaccines to Prevent it. Immunol Rev (2020) 293(1):230–52. doi: 10.1111/imr.12807
35. Berendt AR, Simmons DL, Tansey J, Newbold CI, Marsh K. Intercellular-Adhesion Molecule-1 Is an Endothelial-Cell Adhesion Receptor for Plasmodium-Falciparum. Nature (1989) 341(6237):57–9. doi: 10.1038/341057a0
36. Turner L, Lavstsen T, Berger SS, Wang CW, Petersen JE, Avril M, et al. Severe Malaria Is Associated With Parasite Binding to Endothelial Protein C Receptor. Nature (2013) 498(7455):502–5. doi: 10.1038/nature12216
37. Bernabeu M, Danziger SA, Avril M, Vaz M, Babar PH, Brazier AJ, et al. Severe Adult Malaria is Associated With Specific Pfemp1 Adhesion Types and High Parasite Biomass. Proc Natl Acad Sci (2016) 113(23):E3270–E9. doi: 10.1073/pnas.1524294113
38. Hsieh FL, Turner L, Bolla JR, Robinson CV, Lavstsen T, Higgins MK. The Structural Basis for CD36 Binding by the Malaria Parasite. Nat Commun (2016) 7:12837. doi: 10.1038/ncomms12837
39. Lau CKY, Turner L, Jespersen JS, Lowe ED, Petersen B, Wang CW, et al. Structural Conservation Despite Huge Sequence Diversity Allows EPCR Binding by the Pfemp1 Family Implicated in Severe Childhood Malaria. Cell Host Microbe (2015) 17(1):118–29. doi: 10.1016/j.chom.2014.11.007
40. Pais TF, Penha-Goncalves C. Brain Endothelium: The “Innate Immunity Response Hypothesis” in Cerebral Malaria Pathogenesis. Front Immunol (2018) 9:3100. doi: 10.3389/fimmu.2018.03100
41. Pal P, Daniels BP, Oskman A, Diamond MS, Klein RS, Goldberg DE. Plasmodium Falciparum Histidine-Rich Protein II Compromises Brain Endothelial Barriers and may Promote Cerebral Malaria Pathogenesis. mBio (2016) 7(3):e00617–16. doi: 10.1128/mBio.00617-16
42. Rubach MP, Mukemba J, Florence S, John B, Crookston B, Lopansri BK, et al. Plasma Plasmodium Falciparum Histidine-Rich Protein-2 Concentrations are Associated With Malaria Severity and Mortality in Tanzanian Children. PloS One (2012) 7(5):e35985. doi: 10.1371/journal.pone.0035985
43. Manning L, Laman M, Stanisic D, Rosanas-Urgell A, Bona C, Teine D, et al. Plasma Plasmodium Falciparum Histidine-Rich Protein-2 Concentrations do Not Reflect Severity of Malaria in Papua New Guinean Children. Clin Infect Dis (2011) 52(4):440–6. doi: 10.1093/cid/ciq105
44. Yeo TW, Lampah DA, Kenangalem E, Tjitra E, Weinberg JB, Granger DL, et al. Decreased Endothelial Nitric Oxide Bioavailability, Impaired Microvascular Function, and Increased Tissue Oxygen Consumption in Children With Falciparum Malaria. J Infect Dis (2014) 210(10):1627–32. doi: 10.1093/infdis/jiu308
45. Hawkes MT, Conroy AL, Opoka RO, Hermann L, Thorpe KE, McDonald C, et al. Inhaled Nitric Oxide as Adjunctive Therapy for Severe Malaria: A Randomized Controlled Trial. Malar J (2015) 14:421. doi: 10.1186/s12936-015-0946-2
46. Dietmann A, Lackner P, Helbok R, Spora K, Issifou S, Lell B, et al. Opposed Circulating Plasma Levels of Endothelin-1 and C-Type Natriuretic Peptide in Children With Plasmodium Falciparum Malaria. Malaria J (2008) 7(1):253. doi: 10.1186/1475-2875-7-253
47. Yeo TW, Lampah DA, Gitawati R, Tjitra E, Kenangalem E, Piera K, et al. Angiopoietin-2 Is Associated With Decreased Endothelial Nitric Oxide and Poor Clinical Outcome in Severe Falciparum Malaria. Proc Natl Acad Sci U.S.A. (2008) 105(44):17097–102. doi: 10.1073/pnas.0805782105
48. Canavese M, Spaccapelo R. Protective or Pathogenic Effects of Vascular Endothelial Growth Factor (VEGF) as Potential Biomarker in Cerebral Malaria. Pathog Glob Health (2014) 108(2):67–75. doi: 10.1179/2047773214Y.0000000130
49. Jain V, Armah HB, Tongren JE, Ned RM, Wilson NO, Crawford S, et al. Plasma IP-10, Apoptotic and Angiogenic Factors Associated With Fatal Cerebral Malaria in India. Malar J (2008) 7:83. doi: 10.1186/1475-2875-7-83
50. Fiedler U, Augustin HG. Angiopoietins: A Link Between Angiogenesis and Inflammation. Trends Immunol (2006) 27(12):552–8. doi: 10.1016/j.it.2006.10.004
51. Lovegrove FE, Tangpukdee N, Opoka RO, Lafferty EI, Rajwans N, Hawkes M, et al. Serum Angiopoietin-1 and -2 Levels Discriminate Cerebral Malaria From Uncomplicated Malaria and Predict Clinical Outcome in African Children. PloS One (2009) 4(3):e4912. doi: 10.1371/journal.pone.0004912
52. Conroy AL, Phiri H, Hawkes M, Glover S, Mallewa M, Seydel KB, et al. Endothelium-Based Biomarkers Are Associated With Cerebral Malaria in Malawian Children: A Retrospective Case-Control Study. PloS One (2010) 5(12):e15291. doi: 10.1371/journal.pone.0015291
53. de Jong GM, Slager JJ, Verbon A, van Hellemond JJ, van Genderen PJ. Systematic Review of the Role of Angiopoietin-1 and Angiopoietin-2 in Plasmodium Species Infections: Biomarkers or Therapeutic Targets? Malar J (2016) 15(1):581. doi: 10.1186/s12936-016-1624-8
54. Medana IM, Turner GDH. Human Cerebral Malaria and the Blood-Brain Barrier. Int J Parasitol (2006) 36(5):555–68. doi: 10.1016/j.ijpara.2006.02.004
55. Akanmori BD, Kurtzhals JA, Goka BQ, Adabayeri V, Ofori MF, Nkrumah FK, et al. Distinct Patterns of Cytokine Regulation in Discrete Clinical Forms of Plasmodium Falciparum Malaria. Eur Cytokine Netw (2000) 11(1):113–8.
56. Harawa V, Njie M, Kessler A, Choko A, Kumwenda B, Kampondeni S, et al. Brain Swelling Is Independent of Peripheral Plasma Cytokine Levels in Malawian Children With Cerebral Malaria. Malaria J (2018) 17(1):435. doi: 10.1186/s12936-018-2590-0
57. van Hensbroek MB, Palmer A, Onyiorah E, Schneider G, Jaffar S, Dolan G, et al. The Effect of a Monoclonal Antibody to Tumor Necrosis Factor on Survival From Childhood Cerebral Malaria. J Infect Dis (1996) 174(5):1091–7. doi: 10.1093/infdis/174.5.1091
58. Dunst J, Kamena F, Matuschewski K. Cytokines and Chemokines in Cerebral Malaria Pathogenesis. Front Cell Infect Microbiol (2017) 7:324. doi: 10.3389/fcimb.2017.00324
59. King T, Lamb T. Interferon-Gamma: The Jekyll and Hyde of Malaria. PloS Pathog (2015) 11(10):e1005118. doi: 10.1371/journal.ppat.1005118
60. Loos T, Dekeyzer L, Struyf S, Schutyser E, Gijsbers K, Gouwy M, et al. TLR Ligands and Cytokines Induce CXCR3 Ligands in Endothelial Cells: Enhanced CXCL9 in Autoimmune Arthritis. Lab Invest (2006) 86(9):902–16. doi: 10.1038/labinvest.3700453
61. Wilson NO, Jain V, Roberts CE, Lucchi N, Joel PK, Singh MP, et al. CXCL4 and CXCL10 Predict Risk of Fatal Cerebral Malaria. Dis Markers (2011) 30:39–49. doi: 10.1155/2011/828256
62. Griffith JW, Sokol CL, Luster AD. Chemokines and Chemokine Receptors: Positioning Cells for Host Defense and Immunity. Annu Rev Immunol (2014) 32(1):659–702. doi: 10.1146/annurev-immunol-032713-120145
63. Riggle BA, Manglani M, Maric D, Johnson KR, Lee MH, Neto OLA, et al. CD8+ T Cells Target Cerebrovasculature in Children With Cerebral Malaria. J Clin Invest (2020) 130(3):1128–38. doi: 10.1172/JCI133474
64. Belnoue E, Kayibanda M, Vigario AM, Deschemin JC, van Rooijen N, Viguier M, et al. On the Pathogenic Role of Brain-Sequestered Alphabeta CD8+ T Cells in Experimental Cerebral Malaria. J Immunol (2002) 169(11):6369–75. doi: 10.4049/jimmunol.169.11.6369
65. Barrera V, Haley MJ, Strangward P, Attree E, Kamiza S, Seydel KB, et al. Comparison of CD8(+) T Cell Accumulation in the Brain During Human and Murine Cerebral Malaria. Front Immunol (2019) 10:1747. doi: 10.3389/fimmu.2019.01747
66. Haque A, Best SE, Unosson K, Amante FH, de Labastida F, Anstey NM, et al. Granzyme B Expression by CD8+ T Cells is Required for the Development of Experimental Cerebral Malaria. J Immunol (2011) 186(11):6148–56. doi: 10.4049/jimmunol.1003955
67. Nitcheu J, Bonduelle O, Combadiere C, Tefit M, Seilhean D, Mazier D, et al. Perforin-Dependent Brain-Infiltrating Cytotoxic CD8+ T Lymphocytes Mediate Experimental Cerebral Malaria Pathogenesis. J Immunol (2003) 170(4):2221–8. doi: 10.4049/jimmunol.170.4.2221
68. Swanson PA 2nd, Hart GT, Russo MV, Nayak D, Yazew T, Pena M, et al. CD8+ T Cells Induce Fatal Brainstem Pathology During Cerebral Malaria via Luminal Antigen-Specific Engagement of Brain Vasculature. PloS Pathog (2016) 12(12):e1006022. doi: 10.1371/journal.ppat.1006022
69. Renia L, Grau GE, Wassmer SC. CD8+ T Cells and Human Cerebral Malaria: A Shifting Episteme. J Clin Invest (2020) 130(3):1109–11. doi: 10.1172/JCI135510
70. Howland SW, Poh CM, Renia L. Activated Brain Endothelial Cells Cross-Present Malaria Antigen. PloS Pathogens (2015) 11(6):e1004963. doi: 10.1371/journal.ppat.1004963
71. Razakandrainibe R, Pelleau S, Grau GE, Jambou R. Antigen Presentation by Endothelial Cells: What Role in the Pathophysiology of Malaria? Trends Parasitol (2012) 28(4):151–60. doi: 10.1016/j.pt.2012.01.004
72. Engwerda C, Belnoue E, Gruner AC, Renia L. Experimental Models of Cerebral Malaria. Curr Top Microbiol Immunol (2005) 297:103–43.
73. Reis PA, Comim CM, Hermani F, Silva B, Barichello T, Portella AC, et al. Cognitive Dysfunction Is Sustained After Rescue Therapy in Experimental Cerebral Malaria, and Is Reduced by Additive Antioxidant Therapy. PloS Pathog (2010) 6(6):e1000963. doi: 10.1371/journal.ppat.1000963
74. Ghazanfari N, Mueller SN, Heath WR. Cerebral Malaria in Mouse and Man. Front Immunol (2018) 9:2016–. doi: 10.3389/fimmu.2018.02016
75. Strangward P, Haley MJ, Shaw TN, Schwartz JM, Greig R, Mironov A, et al. A Quantitative Brain Map of Experimental Cerebral Malaria Pathology. PloS Pathog (2017) 13(3):e1006267. doi: 10.1371/journal.ppat.1006267
76. Baptista FG, Pamplona A, Pena AC, Mota MM, Pied S, Vigario AM. Accumulation of Plasmodium Berghei-Infected Red Blood Cells in the Brain is Crucial for the Development of Cerebral Malaria in Mice. Infect Immun (2010) 78(9):4033–9. doi: 10.1128/IAI.00079-10
77. Batinovic S, McHugh E, Chisholm SA, Matthews K, Liu B, Dumont L, et al. An Exported Protein-Interacting Complex Involved in the Trafficking of Virulence Determinants in Plasmodium-Infected Erythrocytes. Nat Commun (2017) 8(1):16044. doi: 10.1038/ncomms16044
78. Fonager J, Pasini EM, Braks JA, Klop O, Ramesar J, Remarque EJ, et al. Reduced CD36-Dependent Tissue Sequestration of Plasmodium-Infected Erythrocytes Is Detrimental to Malaria Parasite Growth In Vivo. J Exp Med (2012) 209(1):93–107. doi: 10.1084/jem.20110762
79. De Niz M, Ullrich A-K, Heiber A, Blancke Soares A, Pick C, Lyck R, et al. The Machinery Underlying Malaria Parasite Virulence Is Conserved Between Rodent and Human Malaria Parasites. Nat Commun (2016) 7(1):11659. doi: 10.1038/ncomms11659
80. Blisnick T, Morales Betoulle ME, Barale JC, Uzureau P, Berry L, Desroses S, et al. Pfsbp1, a Maurer’s Cleft Plasmodium Falciparum Protein, Is Associated With the Erythrocyte Skeleton. Mol Biochem Parasitol (2000) 111(1):107–21. doi: 10.1016/S0166-6851(00)00301-7
81. Spycher C, Klonis N, Spielmann T, Kump E, Steiger S, Tilley L, et al. MAHRP-1, a Novel Plasmodium Falciparum Histidine-Rich Protein, Binds Ferriprotoporphyrin IX and Localizes to the Maurer’s Clefts. J Biol Chem (2003) 278(37):35373–83. doi: 10.1074/jbc.M305851200
82. Villegas-Mendez A, Strangward P, Shaw TN, Rajkovic I, Tosevski V, Forman R, et al. Gamma Interferon Mediates Experimental Cerebral Malaria by Signaling Within Both the Hematopoietic and Nonhematopoietic Compartments. Infect Immun (2017) 85(11):e01035–16. doi: 10.1128/IAI.01035-16
83. D’Ombrain MC, Robinson LJ, Stanisic DI, Taraika J, Bernard N, Michon P, et al. Association of Early Interferon-Gamma Production With Immunity to Clinical Malaria: A Longitudinal Study Among Papua New Guinean Children. Clin Infect Dis (2008) 47(11):1380–7. doi: 10.1086/592971
84. Piguet PF, Da Kan C, Vesin C. Role of the Tumor Necrosis Factor Receptor 2 (TNFR2) in Cerebral Malaria in Mice. Lab Invest (2002) 82(9):1155–66. doi: 10.1097/01.LAB.0000028822.94883.8A
85. Tripathi AK, Sullivan DJ, Stins MF. Plasmodium Falciparum-Infected Erythrocytes Increase Intercellular Adhesion Molecule 1 Expression on Brain Endothelium Through NF-Kappa B. Infect Immun (2006) 74(6):3262–70. doi: 10.1128/IAI.01625-05
86. Engwerda CR, Mynott TL, Sawhney S, De Souza JB, Bickle QD, Kaye PM. Locally Up-Regulated Lymphotoxin Alpha, Not Systemic Tumor Necrosis Factor Alpha, is the Principle Mediator of Murine Cerebral Malaria. J Exp Med (2002) 195(10):1371–7. doi: 10.1084/jem.20020128
87. Randall LM, Amante FH, Zhou Y, Stanley AC, Haque A, Rivera F, et al. Cutting Edge: Selective Blockade of LIGHT-Lymphotoxin Beta Receptor Signaling Protects Mice From Experimental Cerebral Malaria Caused by Plasmodium Berhei ANKA. J Immunol (2008) 181(11):7458–62. doi: 10.4049/jimmunol.181.11.7458
88. Walk J, Stok JE, Sauerwein RW. Can Patrolling Liver-Resident T Cells Control Human Malaria Parasite Development? Trends Immunol (2019) 40(3):186–96. doi: 10.1016/j.it.2019.01.002
89. Wu XZ, Thylur RP, Dayanand KK, Punnath K, Norbury CC, Gowda DC. IL-4 Treatment Mitigates Experimental Cerebral Malaria by Reducing Parasitemia, Dampening Inflammation, and Lessening the Cytotoxicity of T Cells. J Immunol (2021) 206(1):118–31. doi: 10.4049/jimmunol.2000779
90. Wu XZ, Brombacher F, Chroneos ZC, Norbury CC, Gowda DC. IL-4R Alpha Signaling by CD8 Alpha(+) Dendritic Cells Contributes to Cerebral Malaria by Enhancing Inflammatory, Th1, and Cytotoxic CD8(+) T Cell Responses. J Biol Chem (2021) 296:100615. doi: 10.1016/j.jbc.2021.100615
91. Tangteerawatana P, Pichyangkul S, Hayano M, Kalambaheti T, Looareesuwan S, Troye-Blomberg M, et al. Relative Levels of IL4 and IFN-Gamma in Complicated Malaria: Association With IL4 Polymorphism and Peripheral Parasiternia. Acta Trop (2007) 101(3):258–65. doi: 10.1016/j.actatropica.2007.02.008
92. Fauconnier M, Palomo J, Bourigault ML, Meme S, Szeremeta F, Beloeil JC, et al. IL-12R Beta 2 Is Essential for the Development of Experimental Cerebral Malaria. J Immunol (2012) 188(4):1905–14. doi: 10.4049/jimmunol.1101978
93. Perkins DJ, Weinberg JB, Kremsner PG. Reduced Interleukin-12 and Transforming Growth Factor-Ss 1 in Severe Childhood Malaria: Relationship of Cytokine Balance With Disease Severity. J Infect Dis (2000) 182(3):988–92. doi: 10.1086/315762
94. Palomo J, Reverchon F, Piotet J, Besnard AG, Couturier-Maillard A, Maillet I, et al. Critical Role of IL-33 Receptor ST2 in Experimental Cerebral Malaria Development. Eur J Immunol (2015) 45(5):1354–65. doi: 10.1002/eji.201445206
95. Favre N, Da Laperousaz C, Ryffel B, Weiss NA, Imhof BA, Rudin W, et al. Role of ICAM-1 (CD54) in the Development of Murine Cerebral Malaria. Microbes Infect (1999) 1(12):961–8. doi: 10.1016/S1286-4579(99)80513-9
96. Ramos TN, Bullard DC, Darley MM, McDonald K, Crawford DF, Barnum SR. Experimental Cerebral Malaria Develops Independently of Endothelial Expression of Intercellular Adhesion Molecule-1 (ICAM-1). J Biol Chem (2013) 288(16):10962–6. doi: 10.1074/jbc.C113.457028
97. Joste V, Guillochon E, Fraering J, Vianou B, Watier L, Jafari-Guemouri S, et al. Pfemp1 a-Type ICAM-1-Binding Domains Are Not Associated With Cerebral Malaria in Beninese Children. Mbio (2020) 11(6):e02103–20. doi: 10.1128/mBio.02103-20
98. Kessler A, Dankwa S, Bernabeu M, Harawa V, Danziger SA, Duffy F, et al. Linking EPCR-Binding Pfemp1 to Brain Swelling in Pediatric Cerebral Malaria. Cell Host Microbe (2017) 22(5):601–14.e5. doi: 10.1016/j.chom.2017.09.009
99. de Azevedo-Quintanilha IG, Vieira-de-Abreu A, Ferreira AC, Reis PA, Silva TI, Nascimento DD, et al. Integrin Alpha(D)Beta(2) Influences Cerebral Edema, Leukocyte Accumulation and Neurologic Outcomes in Experimental Severe Malaria. PloS One (2019) 14(12):e0224610. doi: 10.1371/journal.pone.0224610
100. Chesnokov O, Merritt J, Tcherniuk SO, Milman N, Oleinikov AV. Plasmodium Falciparum Infected Erythrocytes can Bind to Host Receptors Integrins Alpha V Beta 3 and Alpha V Beta 6 Through DBL Delta 1_D4 Domain of PFL2665c Pfemp1 Protein. Sci Rep (2018) 8:17871. doi: 10.1038/s41598-018-36071-2
101. Franke-Fayard B, Janse CJ, Cunha-Rodrigues M, Ramesar J, Buscher P, Que I, et al. Murine Malaria Parasite Sequestration: CD36 is the Major Receptor, But Cerebral Pathology Is Unlinked to Sequestration. P Natl Acad Sci USA (2005) 102(32):11468–73. doi: 10.1073/pnas.0503386102
102. Omi K, Ohashi J, Patarapotikul J, Hananantachai H, Naka I, Looareesuwan S, et al. CD36 Polymorphism Is Associated With Protection From Cerebral Malaria. Am J Hum Genet (2003) 72(2):364–74. doi: 10.1086/346091
103. Oquendo P, Hundt E, Lawler J, Seed B. Cd36 Directly Mediates Cytoadherence of Plasmodium-Falciparum Parasitized Erythrocytes. Cell (1989) 58(1):95–101. doi: 10.1016/0092-8674(89)90406-6
104. Miu J, Mitchell AJ, Muller M, Carter SL, Manders PM, McQuillan JA, et al. Chemokine Gene Expression During Fatal Murine Cerebral Malaria and Protection Due to CXCR3 Deficiency. J Immunol (2008) 180(2):1217–30. doi: 10.4049/jimmunol.180.2.1217
105. Campanella GSV, Tager AM, El Khoury JK, Thomas SY, Abrazinski TA, Manice LA, et al. Chemokine Receptor CXCR3 and Its Ligands CXCL9 and CXCL10 Are Required for the Development of Murine Cerebral Malaria. P Natl Acad Sci USA (2008) 105(12):4814–9. doi: 10.1073/pnas.0801544105
106. Sahu PK, Satpathi S, Behera PK, Mishra SK, Mohanty S, Wassmer SC. Pathogenesis of Cerebral Malaria: New Diagnostic Tools, Biomarkers, and Therapeutic Approaches. Front Cell Infect Microbiol (2015) 5. doi: 10.3389/fcimb.2015.00075
107. Kuehlwein JM, Borsche M, Korir PJ, Risch F, Mueller AK, Hubner MP, et al. Protection of Batf3-Deficient Mice From Experimental Cerebral Malaria Correlates With Impaired Cytotoxic T-Cell Responses and Immune Regulation. Immunology (2020) 159(2):193–204. doi: 10.1111/imm.13137
108. Ataide MA, Komander K, Knopper K, Peters AE, Wu H, Eickhoff S, et al. BATF3 Programs CD8(+)T Cell Memory. Nat Immunol (2020) 21(11):1397–407. doi: 10.1038/s41590-020-0786-2
109. Kassa FA, Van Den Ham K, Rainone A, Fournier S, Boilard E, Olivier M. Absence of Apolipoprotein E Protects Mice From Cerebral Malaria. Sci Rep (2016) 6:33615. doi: 10.1038/srep33615
Keywords: cerebral malaria (CM), CD8+, T cell sequestration, blood brain barrier (BBB), pathophysiology of CM, activation of the brain endothelium, parasite virulence factors, host virulence factors
Citation: Albrecht-Schgoer K, Lackner P, Schmutzhard E and Baier G (2022) Cerebral Malaria: Current Clinical and Immunological Aspects. Front. Immunol. 13:863568. doi: 10.3389/fimmu.2022.863568
Received: 28 January 2022; Accepted: 21 March 2022;
Published: 20 April 2022.
Edited by:
Irene S. Soares, University of São Paulo, BrazilCopyright © 2022 Albrecht-Schgoer, Lackner, Schmutzhard and Baier. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Karin Albrecht-Schgoer, karin.albrecht@i-med.ac.at
 Karin Albrecht-Schgoer
Karin Albrecht-Schgoer Peter Lackner2
Peter Lackner2 Erich Schmutzhard
Erich Schmutzhard Gottfried Baier
Gottfried Baier