- 1College of Animal Science and Technology, Yangzhou University, Yangzhou, China
- 2Joint International Research Laboratory of Agriculture & Agri-Product Safety, Ministry of Education, Yangzhou University, Yangzhou, China
- 3College of Medical, Yangzhou University, Yangzhou, China
Throughout history, pollution has become a part of our daily life with the improvement of life quality and the advancement of industry and heavy industry. In recent years, the adverse effects of heavy metals, such as cadmium (Cd), on human health have been widely discussed, particularly on the immune system. Here, this review summarizes the available evidence on how Cd exposure may affect health. By analyzing the general manifestations of inflammation caused by Cd exposure, we find that the role of omega-3 (n-3) polyunsaturated fatty acids (PUFAs) in vivo can counteract Cd-induced harm. Additionally, we elucidate the effects of n-3 PUFAs on the immune system, and analyze their prophylactic and therapeutic effects on Cd exposure. Overall, this review highlights the role of n-3 PUFAs in the pathological changes induced by Cd exposure. Although n-3 PUFAs remain to be verified whether they can be used as therapeutic agents, as rehabilitation therapy, supplementation with n-3 PUFAs is reliable and effective.
Introduction
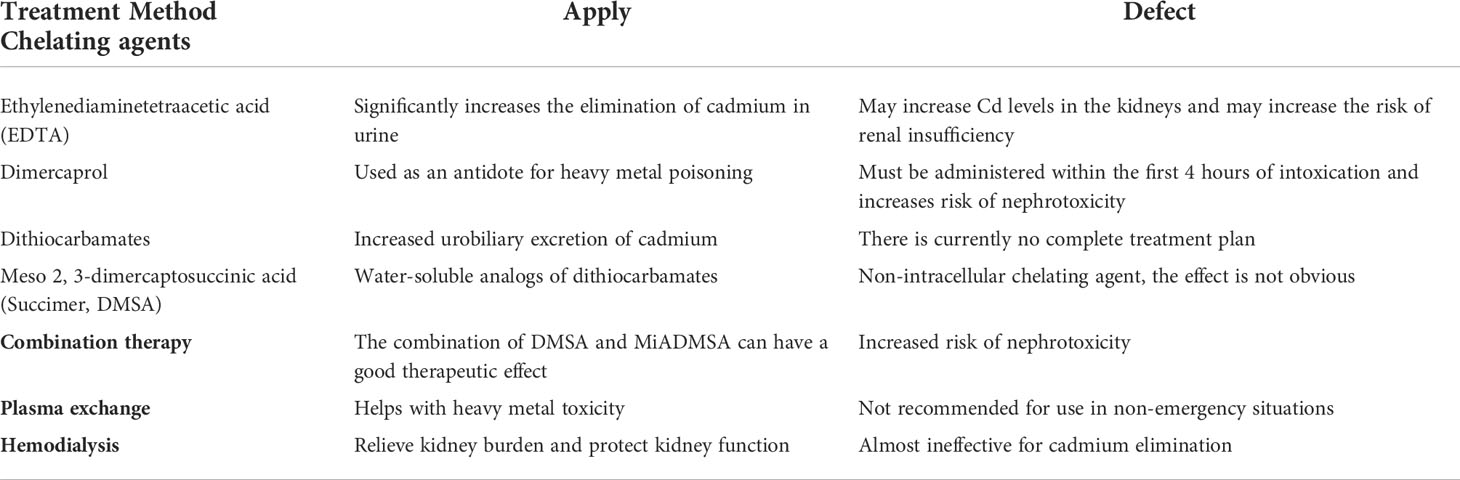
Heavy metals exist everywhere. It is dangerous to consume excessive amounts of any metal, including those normally found in the environment, such as zinc, copper, and iron, as well as discolored metals like lead, arsenic, mercury, and cadmium (1). Among them, cadmium (Cd), upon entering the body, could cause huge damage to a series of important organs, as well as the nervous system, reproductive system, immune system, and other systems. Additionally, it has strong toxic effects on cells. Short-term exposure to Cd can cause apoptosis and necrosis in cells, and the long-term exposure can induce cancerous cells and lead to tumors. So far, only chelation therapy has proven to be effective for Cd poisoning (2) (Table 1).
In view of the fact that oxidative stress is one of the necessary mechanisms of Cd-induced damage, the administration of some antioxidants is expected to be a crucial therapeutic approach. In rehabilitation therapy, omega-3 (n-3) polyunsaturated fatty acids (PUFAs), which have been shown to respond to oxidative stress in the body, are widely used as antioxidants and anti-inflammatory agents. Besides, some existing studies have pointed out n-3 PUFAs could be a possible treatment for Cd exposure in the body (3–5), thus it is necessary to explain and analyze their mechanisms for further advancement of relevant research.
The protective function of n-3 PUFAs
More than 15,000 studies have shown that n-3 PUFAs are anti-inflammatory, regulating the immune system and showing a wide range of beneficial benefits in mammals. Generally, fatty acids (FAs) can be divided into short-chain, medium-chain, long-chain, and very-long-chain FAs. According to the degree of unsaturation on the carbon chain, they are also divided into saturated FAs, monounsaturated FAs (containing one double bond), and polyunsaturated FAs (containing multiple double bonds) (6, 7). As nutrition has developed, studies have found that FAs with double bonds in different positions have different nutritional value. Thus, they are classified into n-3 PUFAs and omega-6 (n-6) PUFAs according to the position of the last double bond relative to the methyl terminal of the molecule. Among them, n-3 PUFAs mainly include α-linolenic acid (ALA; 18:3 n-3), eicosapentaenoic acid (EPA; 20:5 n-3), and docosahexaenoic acid (DHA; 22:6 n-3). In addition, stearidonic acid (SDA, 18:4 n-3) and docosapentaenoic acid (DPA, 22:5 n-3) are intermediates of n-3 PUFAs that have attracted extensive attention. Among them, DPA is an intermediate product between EPA and DHA, and SDA, derived from ALA, exists in the biosynthetic pathway of DPA and DHA (Figure 1).
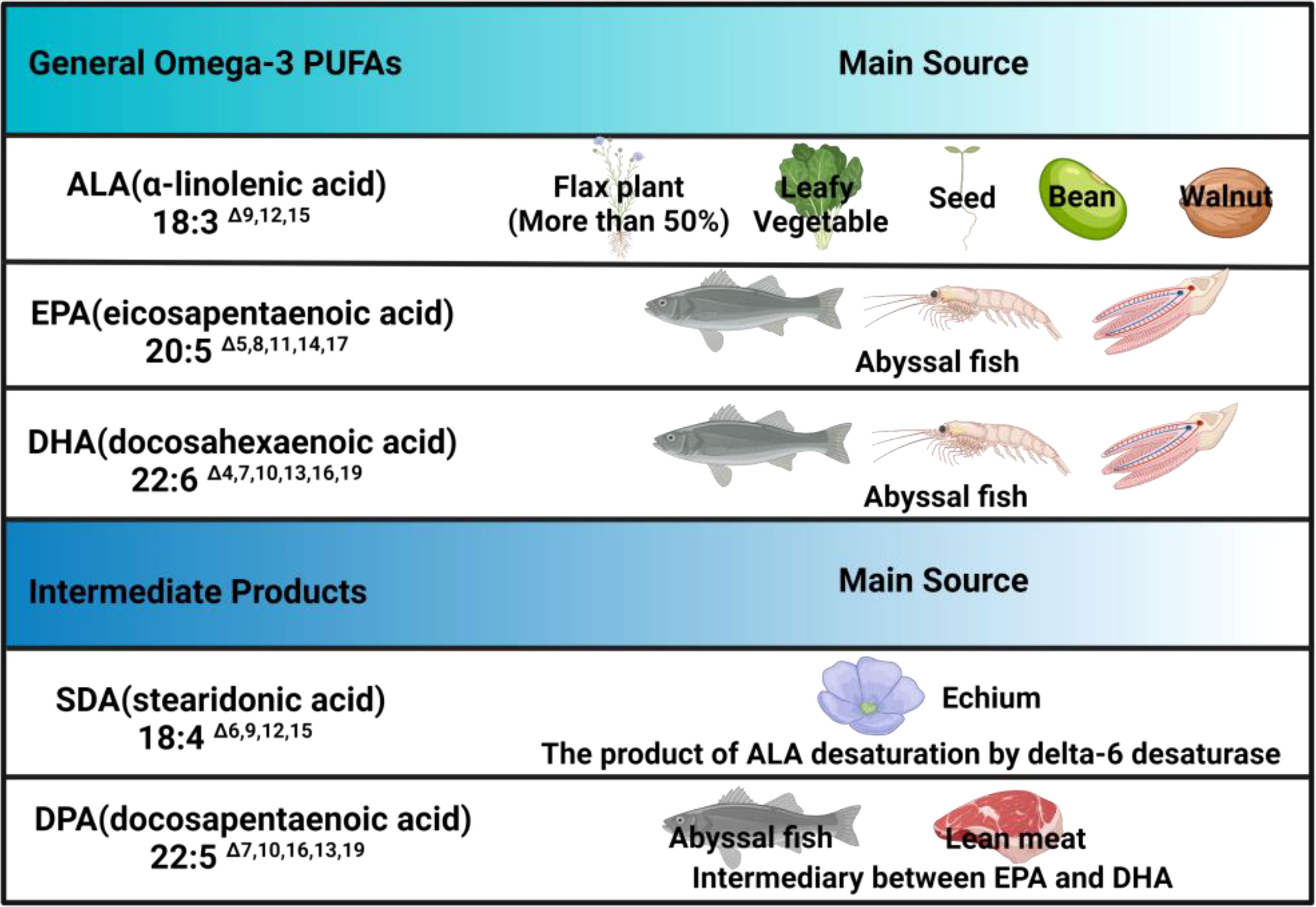
Figure 1 Omega-3 PUFAs and their main sources. N-3 PUFAs mainly consist ALA, EPA, DHA and intermediate derivatives, among which only SDA and DPA are listed in the table, but other derivatives, such as eicosatetraenoic acid (ETA), are not included, which means there is a lack of clarity regarding these derivatives currently.
In vivo
EPA and DHA are two forms of n-3 PUFAs that are widely distributed in mammals as essential FAs (8). In order to prolong and transform, it is necessary for n-3 PUFAs to enter the gastrointestinal tract and combine with chylomicrons before being transferred into the liver as glycerol (9). The liver processes ALA and EPA into docosapentaenoic acids (DPA, an n-3 fatty acid) and DHA by desaturases and fatty acid chain elongases, but they cannot be processed unlimitedly. Studies have shown that platelet membranes are involved in regulating the concentrations of DHA (10, 11). When DHA is saturated, they even promote the reverse conversion of excess DHA to EPA and DPA which are then bound to triglyceride in the form of very low-density lipoprotein cholesterol and release it into the blood. Subsequent studies have shown that the DHA content in the platelet membrane may be responsible for mechanism of n-3 PUFAs (12). In mice, highly purified DHA could significantly increase DHA, DPA, and EPA contents in the platelet membrane, while highly purified EPA could only increase EPA and DPA contents (13). This again proves that DHA supplementation is effective in rehabilitation therapy or treatment. In addition, n-6 PUFAs also affect the metabolism of n-3 PUFAs (14–16), since liver is the main site of desaturations and elongations of n-3 PUFAs and n-6 PUFAs. Their metabolisms are based on the same enzymes, which makes them competitive. There is evidence that higher concentration of n-6 PUFAs diet could result in lower conversion rate of ALA, and conversely, high concentrations of ALA could also reduce conversion of n-6 PUFAs (17–19). In light of this, numerous studies have revealed that the ratio of n-3/n-6 is closely related to body health, which again highlights the importance of the ratio of healthy dietary intake.
In vitro
In the immune system, n-3 PUFAs are found in almost all known immune cells, among which the macrophages are the most commonly mentioned. To date, the effects of n-3 PUFAs on the production and secretion of cytokines and chemokines by macrophages have extensively been investigated, as well as the regulatory mechanisms behind them. As part of the innate immune system, macrophages have the function of locating pathogens. Studies have found that n-3 PUFAs can participate in regulating the production and secretion of cytokines and chemokines in macrophages, thus having the phagocytic ability of macrophages (20). In addition, some studies have also pointed out that n-3 PUFAs could change the activation state of macrophages (21–23). In the course of research, n-3 PUFAs are found to play an important role in neutrophils, which is related to the n-3/n-6 competition. When n-3 is heavily involved in the phospholipid composition of neutrophil membrane, n-6 PUFAs will be metabolized to other fatty acid derivatives with anti-inflammatory properties, such as prostaglandins and protectins (24–26). In addition to this, neutrophils rich in n-3 PUFAs are able to migrate better, which has also been validated in vivo (27–29). Interestingly, as metabolites derived from n-3 PUFAs appear to inhibit the migration ability of neutrophils, we speculate that this is the result of their metabolism. It is worth mentioning that when oxidative stress occurs in the body, there will be inflammatory infiltration of neutrophils, increase of proteases secretion, and the production of a large number of oxidative intermediates.
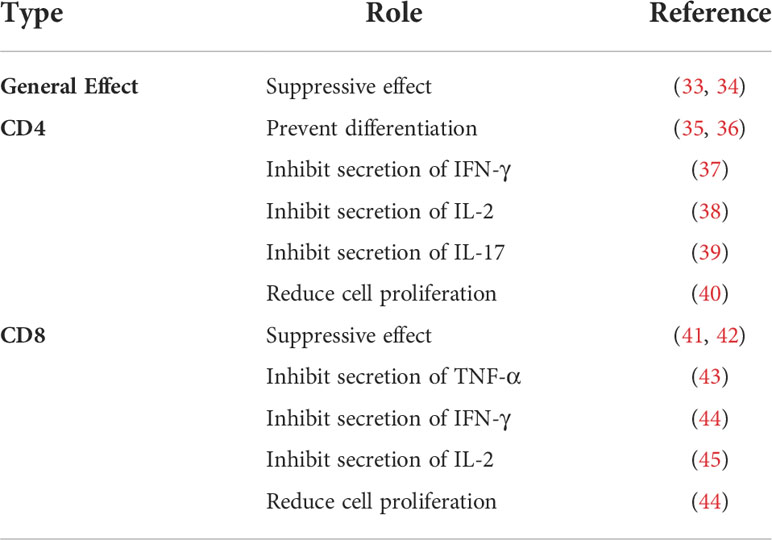
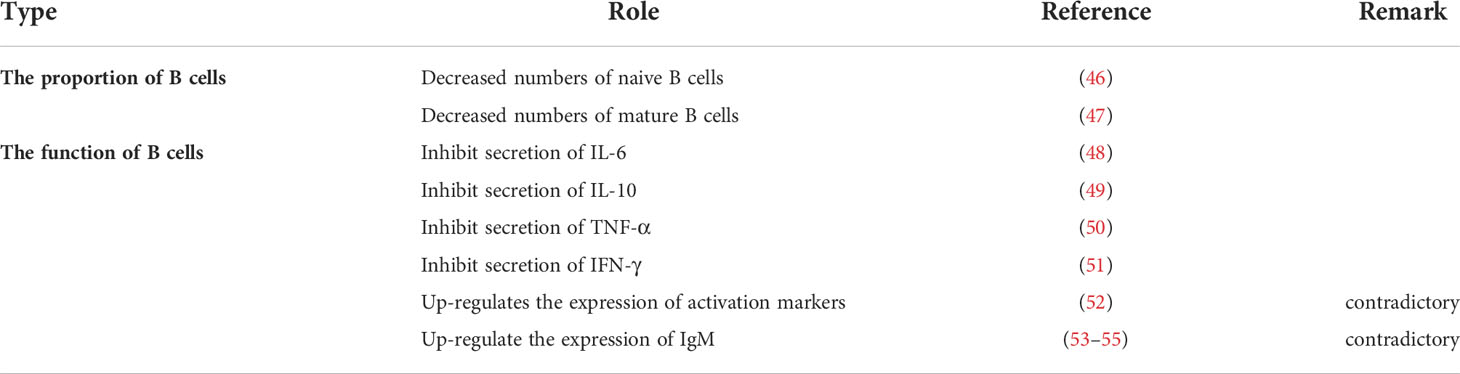
Moreover, n-3 PUFAs also seem to be present in oxidative stress. Studies have shown that supplementation of n-3 PUFAs could increase production of reactive oxygen species (ROS) in neutrophils (30–32). Although this performance appears to be age-related, we are more interested to know whether this phenomenon simply supplements the missing ROS or unrestricted production, which will have a substantial impact on our subsequent utilization of n-3 PUFAs. For the main lymphocytes in the immune system, many studies have demonstrated the role of n-3 PUFAs in T cells (Table 2) and B cells (Table 3), but many conclusions are not unified because they have different subgroups/populations. Thus, in this article, we only list the references without further analysis. Also, some other cells related to the immune system are regulated by n-3 PUFAs, such as eosinophils and basophils (56–59). However, they will not be investigated here because current research cannot form a complete system, instead we will discuss the experimental methods on n-3 PUFAs. In the future, more research will look at supplementation of EPA or DHA to observe changes in the body, but at this point it is unknown whether single or multiple PUFAs play a role. It may be necessary, therefore, to improve the current commonly used experimental methods to be supplemented alone or inhibited alone.
Clinical aspects
In what ways did n-3 PUFAs affect the harmful? There is evidence that the n-3 PUFAs can inhibit the inflammation by activating various genes and pathways including IL1, IL-2, IL-6, IL-12, TNF- α, peroxisome proliferator-activated receptors (PPARs), and so forth (60–64), through absorbing supplemented n-3 PUFAs. Also, some studies have delved into the pathway and found that n-3 PUFAs regulate these factors through a number of master transcription factors, including sterol regulatory element-binding protein (SREBP), PPAR, nuclear factor-kappa B (NF-κB), and carbohydrate response element-binding protein (ChREBP) (65–68) (Figure 2). In addition, intermediates formed by oxidative synthesis of n-3 PUFAs are also important, and dietary n-3 PUFAs are stored in the form of triacylglycerols and phospholipids. During the resolution of acute inflammation, the organism promotes the conversion of n-3 DHA into d-series resolvins and protectins/neuroprotectins (69). In macrophages, DHA is also converted to maresins (macrophage mediators in resolving inflammation) via 14-lipoxygenation (70). Moreover, these mediators are capable of limiting neutrophil recruitment and potently stimulating macrophage phagocytosis of apoptotic cells in a stereospecific manner. Aside from that, neuronal transient receptor potential vanilloid 1 (TRPV1) currents are also inhibited by them, which regulates inflammation and chemotherapy-induced pain.
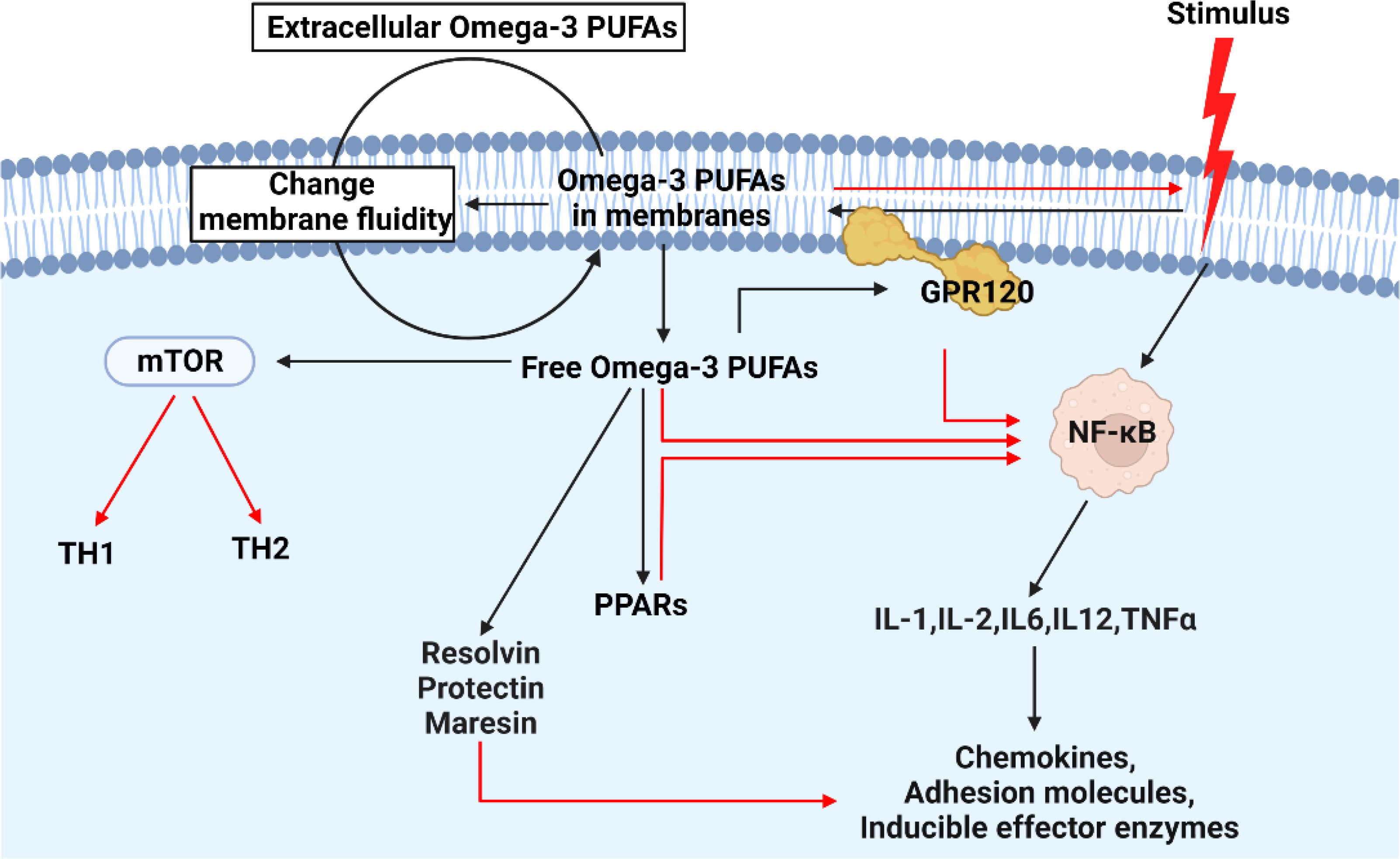
Figure 2 The mechanism of action of n-3 in cells when subjected to external inflammatory stimuli. When cells encounter external inflammatory stimuli, n-3 PUFAs will be activated. On the one hand, more extracellular n-3 PUFAs enter the cell by changing the fluidity of the cell membrane. On the other hand, free intracellular n-3 PUFAs inhibit the expression of intracellular inflammatory factors by combing with other factors. Among them, GPR120: free fatty acid receptor 4 (FFA4). In the figure, the red line indicates the inhibitory effect.
In conclusion, normally n-3 PUFAs have a regulatory effect on immune cells of the innate and adaptive branches. Here, the ability of n-3 PUFAs in controlling the inflammatory response and switching inflammation into a regressive state is emphasized, which is the basis to combat inflammation caused by Cd exposure. Additionally, many mechanisms have not been fully elucidated, such as whether n-3 PUFAs play a role in relation to cellular localization and metabolic inactivation pathways. With the advent of new technologies such as molecular imaging and lipidomics, it may not be too late to reveal how these mechanisms work.
The possible harm of Cd exposure to the body
Cd, a toxic metal, poses a health risk to both humans and animals (Figure 3). In fact, Cd exposure occurs naturally in the environment since Cd is a pollutant from agricultural and industrial sources. In addition to inhalation by smoking and pulmonary ingestion (71), studies have indicated that Cd can also be ingested by food (72). Although only 5% of Cd in food is absorbed into the human body through the gastrointestinal tract, it is much lower than the absorption capacity of the lungs. Ingestion through food, however, is the most significant route of exposure for general non-occupational populations. In addition, Cd can be ingested through skin contact (73). Although this kind of penetration is not ideal, there is still some risk.
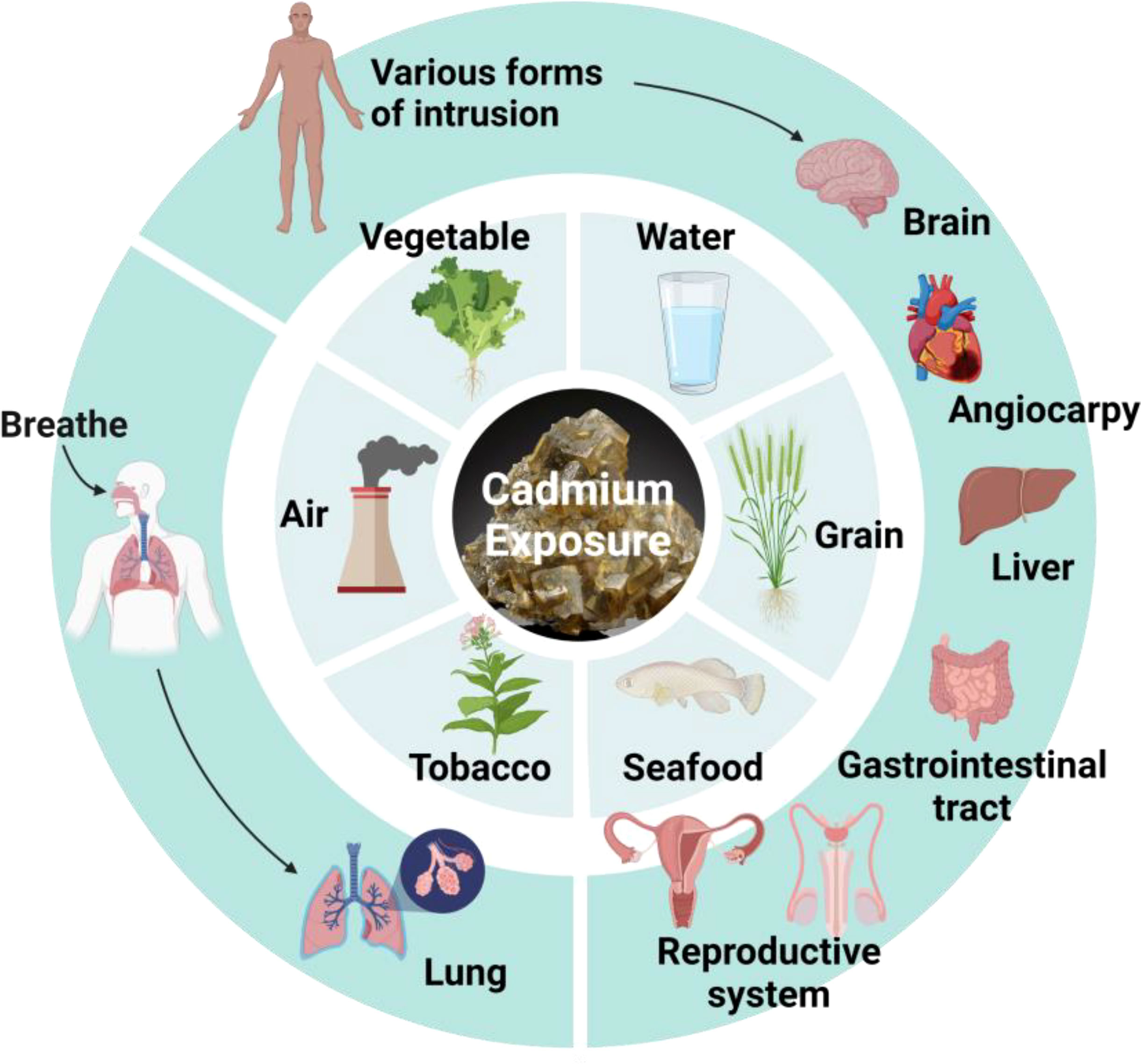
Figure 3 Hazards from Cd exposure. Cd can enter the body through air, tobacco, seafood, grain, water, vegetable, and damage different organs.
Liver
After Cd enters the human body, it can cause damage to a series of important organs such as liver, kidney, bone, brain and lung, as well as nervous system, reproductive system, immune system, and other systems, among which liver and kidney are the most important target organs. It is reported that the toxicity of Cd is obviously time-dependent and concentration-dependent (74–77). The Cd-induced liver injury is mainly due to the competitive replacement of Cd with the metal prosthetic groups in the liver antioxidant enzymes, which inactivates the antioxidant enzymes and reduces the scavenging ability of free radicals, thereby causing cellular lipid peroxidation and oxidative stress (78, 79). In addition, the entry of Cd can also cause cells to secrete pro-inflammatory factors and chemokines, resulting in apoptosis and pathological changes, and eventually the formation of tumors. A study found that acute Cd poisoning could bring about an increase in the level of a series of proteases related to liver in the blood, which in turn results in the incidence of non-alcoholic hepatitis and fatty liver (80).
Kidney
Low concentrations or chronic Cd poisoning mainly cause kidney damage. In clinical manifestations, renal tubular reabsorption dysfunction occurs in the early stage of Cd poisoning, and low molecular weight proteins appear in the urine (81–83). With the aggravation of Cd damage to the kidney, macromolecular proteinuria would occur in the body. At the same time, a large quantity of proximal convoluted tubule cells undergo apoptosis, and the activities of Na/K-ATPase and other proteins decrease significantly. In addition, the glomerulus and distal convoluted tubules are found to be affected in different degrees. It is worth noting that the damage caused by Cd to the kidney tissue is extremely difficult to repair on account of urinary physiology (84, 85). The Cd would travel to the kidneys with the blood and filter through the glomeruli, and then almost all of Cd would be reabsorbed by the proximal convoluted tubule with only trace amounts of them could be excreted. And Cd in excess of the treatment range will severely damage the function of the glomerulus, bringing about a series of health problems. Studies have pointed out that the kidneys will undergo fibrosis and even necrosis if glomeruli fail to function (86, 87).
Bone
In addition, patients in incident of Cd-contaminated water source in Japan in the 1960s suffered from some pathological injuries, including bone pain, susceptibility to fractures, developmental deformities of long bones, osteoporosis, osteomalacia, and so on (88). It is reported that these damages are related to kidney disease with a large number of calcium ions being excreted from urine, thus affecting the growth and development of bones (89, 90). Aside from that, Cd can directly damage bones by inhibiting the differentiation of osteoblasts and promoting the formation and function of osteoclasts, leading to disorder in the normal bone formation and pathological damage of bones (91).
Other organs
In addition to liver, kidney and bones, exposure to Cd can also cause serious damage to other organs. As a result of Cd’s serious neurotoxicity, it can cause a series of neuropathy throughout the body. A study found that the lesions are mainly caused by embryonic transmission and respiratory pathways (92–95). Similarly, in addition to contributing to toxic effects on the peripheral nervous system, the absorbed Cd also damages olfactory neurons, central nervous cells, and oligodendrocytes through competitive replacement, as well as generates a large number of free radicals after entering the brain, which can induce many diseases including headache, dizziness, olfactory dysfunction, Parkinson-like symptoms, vasomotor control impairment, and learning impairment. Notably, this mode of transmission could bring more damage to babies whose immune systems are not yet fully developed. Numerous studies have shown that Cd damages the central nervous system in infants at lower doses than in adults, which is also related to a weaker blood-brain barrier in infants (96, 97).
In addition, people involved in the Cd industry frequently suffer from respiratory and lung diseases, such as chronic rhinitis, pharyngitis, pneumonia, pulmonary fibrosis, and emphysema (98–100). As another target of Cd, the gastrointestinal tract is also affected, and Cd exposure could cause gastrointestinal cell apoptosis, intestinal tissue villi damage, and changes in the structure of the intestinal microbiome (101–103). In the cardiovascular aspect, contrary to previous studies that did not attribute to Cd on this aspect, recent studies have shown that Cd poisoning can cause vascular inflammation, promote vascular arteriosclerosis, damage the structural integrity of blood vessels, and diminish myocardial contractility and cardiac conduction excitation (104–107). A series of conditions can also occur, such as the decline in libido and coronary blood flow. Although mechanism of Cd action has not been fully elucidated, there is no doubt that it causes damage to cardiovascular system.
Also, certain pathological changes, including cancer and other chronic diseases, can be associated with Cd exposure (108). Several studies examining Cd exposure suggest that Cd is responsible for some malignancies, such as pancreatic cancer (109), and both human and mouse models have indicated a link between Cd and pancreatic cancer (110, 111). Another cancer thought to be induced by Cd is kidney cancer (112), which is associated with kidney damage as previously described, as Cd can cause a wide range of pathologies in renal tissue from renal insufficiency to renal cancer. In addition, despite several studies suggesting that heavy metals are involved in the development of breast cancer (113), more research is needed to prove whether Cd is involved. According to studies, breast cancer tissues accumulate large amount of Cd after Cd exposure, and DNA methylation levels correlate positively with cadmium exposure (114), whereas in a study on humans, Cd levels in diet and urine do not appear to be correlated with breast cancer incidence and mortality (115). Aside from that, research has determined that Cd contributes to diabetes occurrence (116, 117). By acting on islet β cells, up-regulation of inflammatory factors (such as TNF-α,IL-1,IL-6) significantly increases the incidence of diabetes by inducing intracellular lipid accumulation and affecting its insulin secretion function.
As an immunotoxic inhibitor, Cd interacts with almost all immune cells, impairing the immune system in a time- and dose-dependent manner (Figure 4). In summary, its toxic effects are achieved by competitive replacement of proteases (essentially replacing essential metals in proteases), thereby inducing apoptosis or oxidative stress. So far, most studies on Cd exposure have focused on exploring the effects of Cd ions on the body, but few have examined its toxic mechanism and corresponding potential detoxification mechanism. An urgent need is to unravel the toxic effects and mechanisms of Cd in immune cells and develop effective immunotherapies to mitigate its toxic effects.
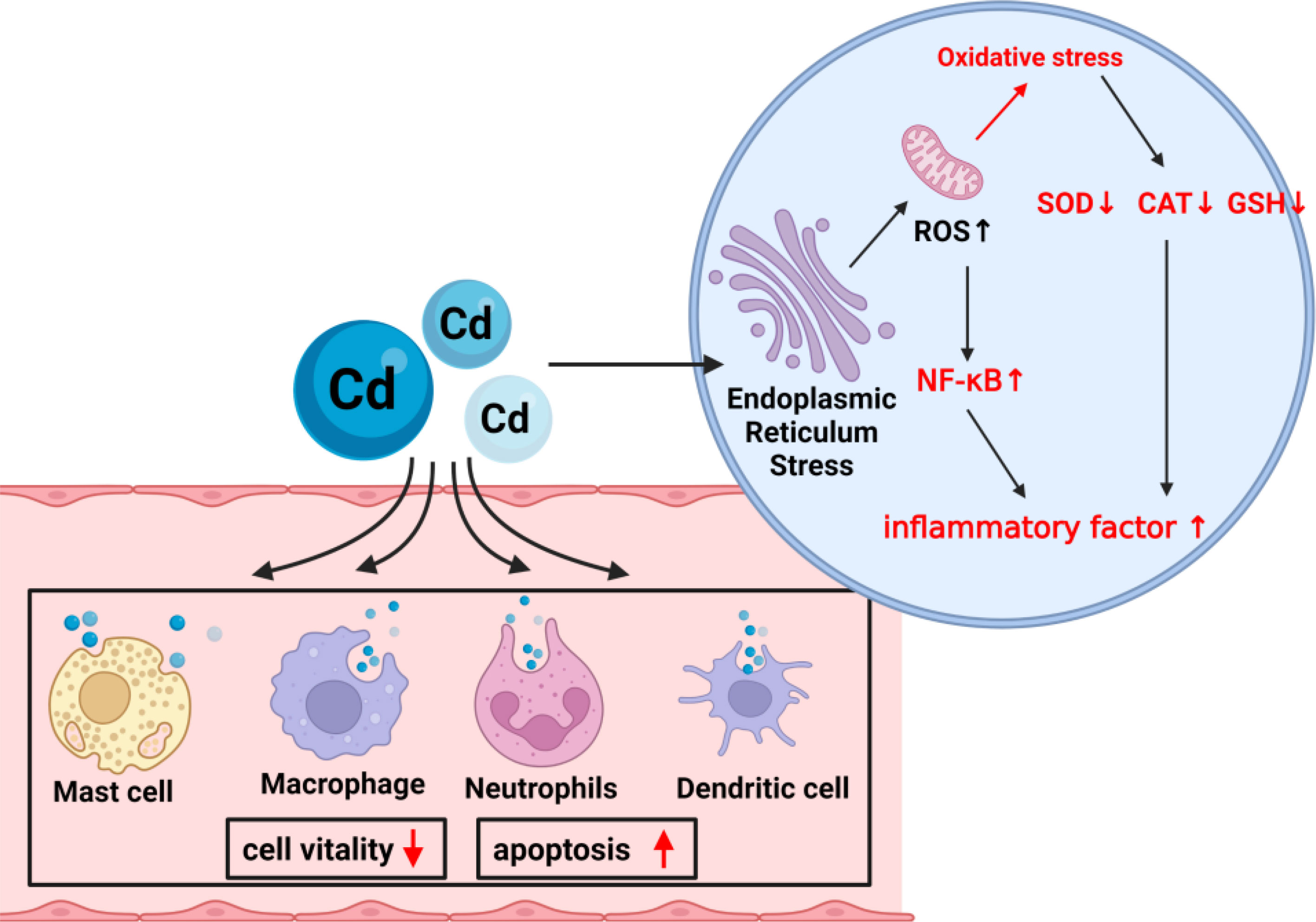
Figure 4 The mechanism of Cd exposure on cells. Cd exposure damages most of the immune cells in the immune system, including decreased vitality and increased apoptosis rate. Oxidative stress in cells occurs mainly through damage to the endoplasmic reticulum and mitochondria.
The role of n-3 PUFAs in Cd exposure
As mentioned previously, it is well known that Cd poisoning is extremely harmful to the body. Research on its treatment was initiated as early as the 20th century, and a wide variety of measures were adopted. As a result of in-depth research in recent years, the exploration of a wide range of antidotes to Cd poisoning has become a hot spot, including metal ion supplements, microorganisms, antioxidants, and so on (118–121). As new experimental advances continue to emerge, although more and more studies have proven that these methods can counteract Cd poisoning, problems such as inability to use them in practice or causing side effects of treatment inevitably arise. Therefore, n-3 PUFAs, a possible natural product as a potential treatment for Cd poisoning, is proposed here.
The earliest research on the interaction between n-3 PUFAs and Cd was conducted in 1997, when Howlett et al. explored the relationship between Cd and n-3 PUFAs in Saccharomyces cerevisiae (122). Their results showed that the cellular fatty acid unsaturation and plasma membrane would increase significantly when Cd enters Saccharomyces cerevisiae, especially DHA and EPA. In addition, compared with cells enriched in n-3 PUFAs, cells with low levels of n-3 PUFAs shows a more pronounced decrease in their cell viability. Interestingly, the study also found that potassium efflux is much higher in cells enriched in n-3 PUFAs than in cells with lower levels. Although the author failed to make a reasonable analysis of this phenomenon, in light of recent research, we believe that protective effects of Cd ions may be responsible, and they also have a protective effect on the cardiovascular system. Unfortunately, limited by species and technology, the study did not address the doubt, but for sure, the study demonstrated some kind of interaction between n-3 PUFAs and Cd. There were few studies exploring this relationship after that. It was not until 2007 when another related study mentioned this relationship again that researchers began to look at its implications (123). The study sheds light on the permeability of the gut through DHA supplementation at different concentrations and finds a significant increase in the accumulation of Cd in DHA-supplemented cells compared with the normal human colon carcinoma cell line. Further research found that Cd is absorbed and processed through the paracellular pathway, which reconfirms the previous conclusion that the role of n-3 PUFAs may be closely related to the Na-K-Ca ATP channel.
In vivo
Among all body organs, the brain is most susceptible to oxidative stress. As previously mentioned, Cd induces oxidative stress by enhancing ROS production and damages mitochondria as a result of various factors. A more serious concern is that elevated ROS levels can cause antioxidant systems to malfunction and lead to neurodegenerative diseases, including Alzheimer’s and Parkinson’s (124–127). In the face of this damage, can n-3 PUFAs play a role? According to many studies, Cd may entry the brain and exerts its effects through the blood-brain barrier as an enzyme protein that protects this barrier (128, 129). A study showed that supplementation with n-3 PUFAs could increase monoamine oxidase and acetylcholinesterase levels in the body, and these indicators even return to near-normal levels in Cd-exposed mice, demonstrating that n-3 PUFAs have the potential to counteract Cd exposure (130). In addition, a study showed that, by supplementing ALA in Cd poisoned mice, the originally elevated ROS levels in the brain are alleviated, and n-3 PUFAs are found to further reduce neuroinflammation and neurodegeneration through regulated Nrf2/HO-1/JNK signaling pathway (131). It should be added that another study reported similar findings, albeit not for the damage caused by Cd, specifically demonstrating that ALA has an anti-apoptotic effect (132). Therefore, further studies are necessary to gain a deeper understanding of the mechanistic effects of ALA on Cd-induced oxidative stress and neurodegeneration.
As for the hippocampus, a part of the limbic system and located in the medial temporal lobe of the brain, has been shown to play an important role in learning and memory (133, 134). A study in rats showed that after Cd exposure, the number of neurons near the hippocampus would significantly reduce with the appearance of edema around the nerve, and training memory would significantly diminish, and after DHA supplementation, the memory function and hippocampal structure of the rats are improved (135). Interestingly, the study also found that the content of DHA decreases significantly as Cd enters the brain, but does not make quantitative tests to fully explain the whole process of this phenomenon. We suppose that this phenomenon may be explained by indirect involvement of n-3 PUFAs in pro-oxidative, pro-inflammatory, and memory-disrupting effects. In addition to this, several studies have been conducted on a range of antioxidant enzymes with positive results, showing significant decreases in the levels of SOD, CAT, and GST after exposure to Cd (136, 137). However, after supplementation with n-3 PUFAs, they all recover to varying degrees. Overall, based on the available studies, it is conceivable to consider supplementation of n-3 PUFAs as a possible medical adjunct in the suppression of inflammatory brain injury caused by Cd exposure.
In the liver, different studies have reported the effects of Cd exposure on oxidative stress and fatty acid composition, and the results consistently show changes across different animals, with the most important change in FAs composition being the reduction in the percentage of DHA in the body. Although the antioxidant system apparently responds to ROS generation under Cd exposure, it appears that the body’s own antioxidant capacity is insufficient to counteract cellular damage. Supplementation of n-3 PUFAs has a positive effect on the body’s antioxidant defense system, lipid peroxidation, and oxidative damage. It is pivotal to emphasize here that a study evaluating therapeutic concentrations showed that, n-3 supplementation could reduce liver tissue and cell damage from Cd exposure only for Cd exposure below 1 μM, whereas the antagonistic effect of supplementation with n-3 PUFAs is not obvious after Cd exposure above 1 μM (138). Therefore, although some studies have suggested that n-3 PUFAs can be used as a primary treatment for fatty liver disease, n-3 PUFAs may not be a primary treatment for liver disease caused by acute Cd exposure. However, we suggest exploiting preventive and rehabilitative therapeutic roles of n-3 PUFAs in the liver. Existing studies have shown that n-3 PUFAs supplementation can effectively resist oxidative damage caused by long-term chronic Cd exposure and have anti-inflammatory and anti-apoptotic effects (139–141). Studies have shown that pretreatment with n-3 PUFAs in stressed mice can significantly reduce subsequent stress-induced liver injury (142). Therefore, supplementing n-3 PUFAs in daily life is a good preventive measure. In addition, conventional drugs in the treatment of liver disease may have adverse effects, and their efficacy and safety are also questionable, while as natural products, n-3 PUFAs can be safely used. At the moment, we are predominantly concerned about the ratio and dosage, which do not appear to have a unified answer. In addition, it would be worthwhile to explore the possibility to gain a deeper understanding of the specific mechanisms of n-3 PUFAs in response to Cd exposure from genomic, proteomic, and metabolomic analysis.
In terms of the reproductive system, identification of factors that affect fertility has important clinical and public health implications. Many studies have pointed out that Cd exposure affects fertility (143–145), and FAs, as important substrates for early reproductive events, have received much attention. Human and animal studies have shown that ingestion of n-3 PUFAs through diet or dietary supplements can be effective in reducing the risk of early preterm birth, and n-3 PUFAs supplementation during pregnancy could also reduce the risk of allergic disease in childhood (146, 147). At the same time, supplementation of n-3 PUFAs in men also has a protective effect on their reproductive systems (148–150). In response to damage from Cd exposure, n-3 PUFAs are found to have positive effects on hormones that control reproduction (151–153). Serum testosterone and LH concentrations significantly go up after adding n-3 PUFAs to the diet of male model animals, thus we reckon the mechanisms behind these effects are related to the antioxidant properties of n-3 PUFAs. Therefore, it is reasonable to believe that n-3 PUFAs have ameliorating effects on Cd exposure-induced reproductive impairment. Indeed, significant reductions in sperm count, sperm motility, and the percentage of sperm with normal morphology were studied in Cd exposure-induced mice, and supplementation with n-3 PUFAs could largely alleviate the effects of Cd on semen quality (154). Researchers in another study thought that this improvement might be mediated by the antioxidant properties of n-3 PUFAs (155). As observed in all results, Cd exposure in both sexes decreased n-3 PUFAs levels in the gonads. Unfortunately, few studies have specifically investigated the effects of n-3 PUFAs on Cd exposure in the female reproductive system (156), which we think may be related to different metabolic demands during the reproductive period. However, a study pointed out that the concentration of n-3 PUFAs in the maternal placenta is significantly lower than the normal tissue level, which may explain why Cd can be transmitted through the placenta (157). In summary, although the reproductive system is not the primary target of Cd toxicity, n-3 PUFAs do counteract it to a certain extent, but the use of it as primary therapy requires further research, including specific action mechanisms in the male and female reproductive system.
The process of fat metabolism is greatly affected by Cd exposure which greatly increases the incidence of diabetes. Do n-3 PUFAs play a role here? This is a long-standing controversial issue. Some studies have indicated that supplementation with n-3 PUFAs increases fasting blood glucose, but changing the intake of n-3 PUFAs does not alter diabetes prevalence or have a therapeutic effect (158, 159). In addition, n-3 PUFAs do not appear to provide any protection to the renal function of diabetic patients. The use of n-3 PUFAs supplements is therefore unsupported by many studies. However, there is convincing evidence that n-3 PUFAs can lower triglyceride levels in vivo (160). A long-term study suggests that n-3 PUFAs supplementation can reduce triglyceride concentrations in people at risk for diabetes (158), confirming their preventive role in Cd exposure-mediated diabetes.
In cancer, n-3 PUFAs have been confirmed to exert anticancer effects by regulating the expression levels of transcription factors such as NF-κB, p53, and cyclooxygenase-2 (COX2) (161). Among the Cd exposure, the role of n-3 PUFAs in colorectal cancer is of great concern, as Cd toxicity is strongly associated with colorectal cancer. Studies have shown that Cd exposure leads to abnormal COX-2 expression in HT-29 cells (162), which is consistent with the signaling pathway of n-3 PUFAs against carcinogenesis. In both human and animal models, n-3 PUFAs have also been shown to effectively reduce the development of colorectal cancer (163).
In vitro
More research has focused on in vitro. The first is the composition of the plasma membrane. Different studies have found changes in plasma membrane permeability and the increasing sensitivity to Cd when cells are supplemented with n-3 PUFAs. A study noted that with the addition of Cd, cell membrane order would significantly dwindle, especially the contents of n-3 PUFAs as well as levels of antioxidant enzymes (164). We consider that this can be counteracted by n-3 PUFAs, since glutathione peroxidase activity can block the production of antioxidant enzymes during normal cellular metabolism, and glutathione has been found to be the main cellular target or sequestration site of Cd. Therefore, the decreased levels of antioxidant enzymes in cells after Cd exposure partly reflect the depletion of glutathione in cells. Combined with existing research, it is evident that n-3 PUFAs can participate in the regulation of glutathione levels in cells, thereby repairing oxidative damage. Cellular sensitivity is similar to plasma membrane permeability, with one study suggesting that fish daily supplemented with n-3 PUFAs are more sensitive to Cd exposure (165). After Cd exposure, the ROS in macrophages, granulocytes, and lymphocytes would change significantly, which apparently comes from the effects of n-3 PUFAs on the immune system. Studies show that a DHA-rich diet enhances the expression of the immunoglobulin M (IgM) gene, while an EPA-rich diet induces transcriptional down-regulation of genes involved in the Toll/NF-κB pathway, which in turn suppresses pro-inflammatory cytokines and induces detrimental damage to cells (166–168). By supplementing n-3 PUFAs to respond more efficiently to pathogen infections, the body benefits in the context of Cd contamination. In conclusion, we re-emphasize that the best response to long-term chronic Cd exposure might be daily supplementation with n-3 PUFAs.
Discussion and outlook
It is always a priority for scientists to find ways to reduce the toxic effects of Cd, many of which are determined by its physical and chemical properties. Typically, Cd expresses pro-inflammatory activity, causing primary and secondary tissue damage by infiltrating innate immune cells (neutrophils, monocytes, and macrophages). The best defenses against Cd toxicity may be to inhibit ROS production, reduce oxidative stress levels, maintain redox balance, and inhibit abnormal immune signaling activation. Here we found that n-3 PUFAs are perfect for these tasks (Figure 5). It is worth noted that the relationship between n-3 PUFAs and Cd exposure is still complex from the current research. If bodyself has low levels of n-3 PUFAs, it would likely to fall into a vicious cycle of Cd exposure, producing less n-3 PUFAs but consuming more n-3 PUFAs. On the other hand, at present, most studies agree it is unreasonable to supplement n-3 PUFAs in related inflammatory diseases, and mainly because of the inability to adjust the internal ratio of n-3 PUFAs, the external ratio of n-3/n-6, and the possible lipid peroxidation. Recently, more research has begun to focus on this problem and provide a solution, along with the administration of other nutrients and drugs that may have oxidative or antioxidant effects. It is unfortunate that this solution is not yet perfect, and reducing the impact of these variables would possibly be one of the future research directions. However, n-3 PUFAs have been shown to play a significant role in rehabilitation therapy. Among the different research findings, we recommend the selection of supplemental doses up to 4.4 g/d.
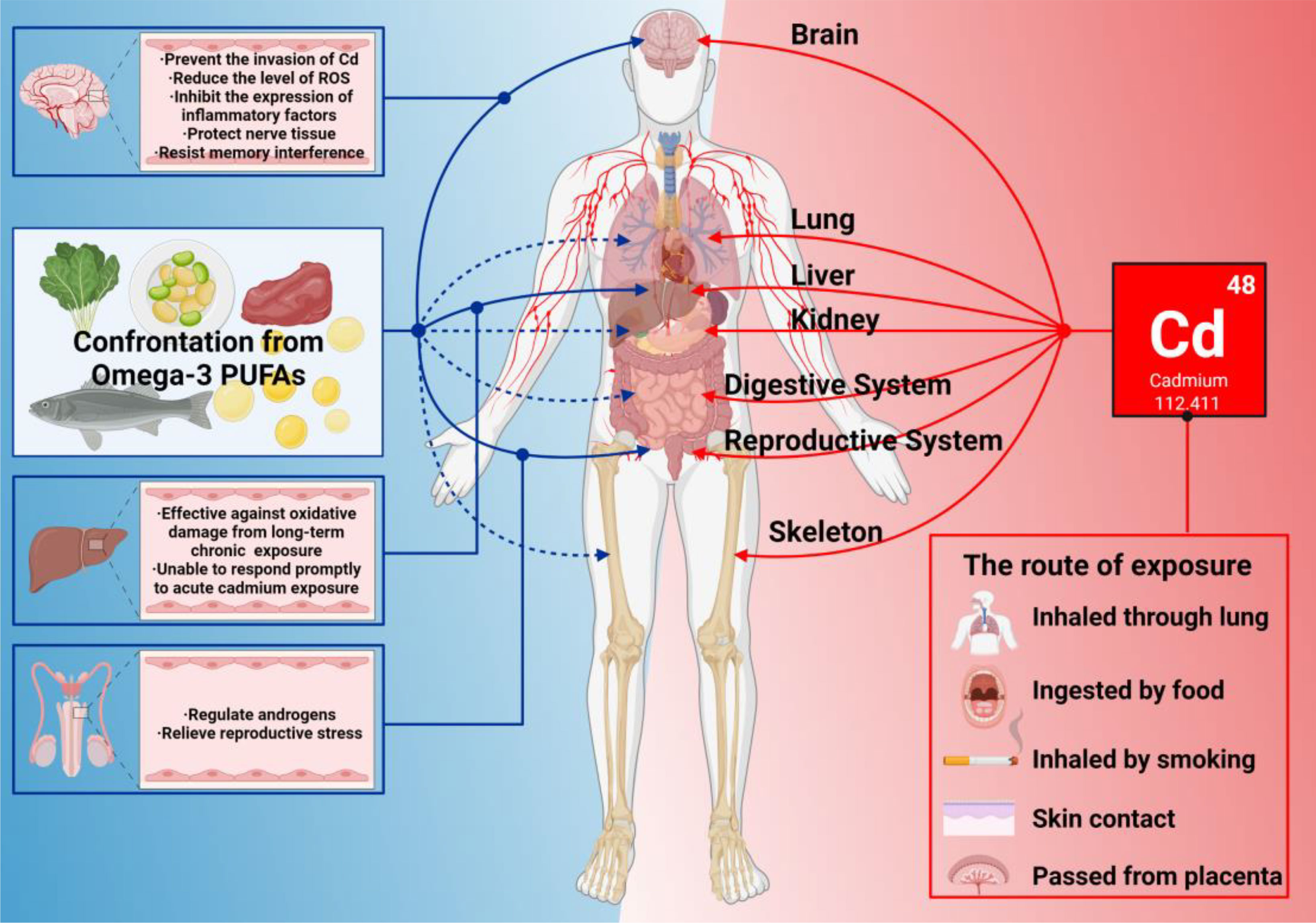
Figure 5 What n-3 PUFAs can do when Cd exposure harms bodies. In this figure, the red line represents the damage to various organs of the body caused by Cd exposure. The solid blue line represents the organ that has been studied so far with n-3 PUFAs that can resist damage from Cd exposure. The blue dashed line indicates that n-3 PUFAs are known to have a positive effect on the organ’s immune system.
Current research shows, n-3 PUFAs control the expression of a great variety of genes through different transcriptional factors, such as SREBP (169), PPARs (170), ChREBP (171), and NF-κB (172), which mainly regulate target gene transcription that encodes proteins involved in lipid and carbohydrate metabolism, thermogenesis, and inflammatory processes. However, it is important to point out that further studies are needed to elucidate these roles and to better understand the beneficial role of n-3 PUFAs in the mechanism of Cd exposure-induced disease, and to probe into their function as protective nutrients, aiming to prevent or treat the development of Cd exposure-related diseases.
Author contributions
ZC and QL contributed equally to this work. ZC, QL, JW, XC, KW, YNW, and YHW: Writing and Editing. ZC and ZY: Conceptualization, Editing and funding acquisition. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by the Jiangsu Agricultural Science and Technology Independent Innovation Fund (CX(21)3119).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Fu Z, Xi S. The effects of heavy metals on human metabolism. Toxicol Mech Methods (2020) 30(3):167–76. doi: 10.1080/15376516.2019.1701594
2. Kim JJ, Kim YS, Kumar V. Heavy metal toxicity: An update of chelating therapeutic strategies. J Trace elements Med biology: Organ Soc Minerals Trace Elements (GMS) (2019) 54:226–31. doi: 10.1016/j.jtemb.2019.05.003
3. Kyriakidou Y, Wood C, Ferrier C, Dolci A, Elliott B. The effect of omega-3 polyunsaturated fatty acid supplementation on exercise-induced muscle damage. J Int Soc Sports Nutr (2021) 18(1):9. doi: 10.1186/s12970-020-00405-1
4. Okereke OI, Vyas CM, Mischoulon D, Chang G, Cook NR, Weinberg A, et al. Effect of long-term supplementation with marine omega-3 fatty acids vs placebo on risk of depression or clinically relevant depressive symptoms and on change in mood scores: A randomized clinical trial. Jama (2021) 326(23):2385–94. doi: 10.1001/jama.2021.21187
5. Hahn J, Cook NR, Alexander EK, Friedman S, Walter J, Bubes V, et al. Vitamin d and marine omega 3 fatty acid supplementation and incident autoimmune disease: Vital randomized controlled trial. BMJ (Clinical Res ed) (2022) 376:e066452. doi: 10.1136/bmj-2021-066452
6. Chen Z, Cao X, Lu Q, Zhou J, Wang Y, Wu Y, et al. Circ01592 regulates unsaturated fatty acid metabolism through adsorbing mir-218 in bovine mammary epithelial cells. Food Funct (2021) 12(23):12047–58. doi: 10.1039/d1fo02797b
7. Chen Z, Lu Q, Liang Y, Cui X, Wang X, Mao Y, et al. Circ11103 interacts with mir-128/Ppargc1a to regulate milk fat metabolism in dairy cows. J Agric Food Chem (2021) 69(15):4490–500. doi: 10.1021/acs.jafc.0c07018
8. Djuricic I, Calder PC. Beneficial outcomes of omega-6 and omega-3 polyunsaturated fatty acids on human health: An update for 2021. Nutrients (2021) 13(7):2421. doi: 10.3390/nu13072421
9. Haidari F, Abiri B, Iravani M, Ahmadi-Angali K, Vafa M. Randomized study of the effect of vitamin d and omega-3 fatty acids cosupplementation as adjuvant chemotherapy on inflammation and nutritional status in colorectal cancer patients. J dietary Suppl (2020) 17(4):384–400. doi: 10.1080/19390211.2019.1600096
10. Mackay I, Ford I, Thies F, Fielding S, Bachoo P, Brittenden J. Effect of omega-3 fatty acid supplementation on markers of platelet and endothelial function in patients with peripheral arterial disease. Atherosclerosis (2012) 221(2):514–20. doi: 10.1016/j.atherosclerosis.2011.12.041
11. Block RC, Shearer GC, Holub A, Tu XM, Mousa S, Brenna JT, et al. Aspirin and omega-3 fatty acid status interact in the prevention of cardiovascular diseases in framingham heart study. Prostaglandins leukotrienes essential Fatty Acids (2021) 169:102283. doi: 10.1016/j.plefa.2021.102283
12. Véricel E, Colas R, Calzada C, Lê QH, Feugier N, Cugnet C, et al. Moderate oral supplementation with docosahexaenoic acid improves platelet function and oxidative stress in type 2 diabetic patients. Thromb haemostasis (2015) 114(2):289–96. doi: 10.1160/th14-12-1003
13. Pisaniello AD, Psaltis PJ, King PM, Liu G, Gibson RA, Tan JT, et al. Omega-3 fatty acids ameliorate vascular inflammation: A rationale for their atheroprotective effects. Atherosclerosis (2021) 324:27–37. doi: 10.1016/j.atherosclerosis.2021.03.003
14. Liput KP, Lepczyński A, Ogłuszka M, Nawrocka A, Poławska E, Grzesiak A, et al. Effects of dietary n-3 and n-6 polyunsaturated fatty acids in inflammation and cancerogenesis. Int J Mol Sci (2021) 22(13):6965. doi: 10.3390/ijms22136965
15. Van Name MA, Savoye M, Chick JM, Galuppo BT, Feldstein AE, Pierpont B, et al. A low Ω-6 to Ω-3 pufa ratio (N-6:N-3 pufa) diet to treat fatty liver disease in obese youth. J Nutr (2020) 150(9):2314–21. doi: 10.1093/jn/nxaa183
16. Li N, Jia M, Deng Q, Wang Z, Huang F, Hou H, et al. Effect of low-ratio n-6/N-3 pufa on blood lipid level: A meta-analysis. Hormones (Athens Greece) (2021) 20(4):697–706. doi: 10.1007/s42000-020-00248-0
17. Yue H, Liu W, Zhang W, Jia M, Huang F, Du F, et al. Dietary low ratio of n-6/N-3 polyunsaturated fatty acids improve type 2 diabetes mellitus Via activating brown adipose tissue in Male mice. J Food Sci (2021) 86(3):1058–65. doi: 10.1111/1750-3841.15645
18. Ding D, Zhong QW, Zuo LS, Ling CW, Xiong F, Ke YB, et al. Association between erythrocyte membrane n-3 and n-6 polyunsaturated fatty acids and carotid atherosclerosis: A prospective study. Atherosclerosis (2020) 298:7–13. doi: 10.1016/j.atherosclerosis.2020.02.013
19. Ghasemi E, Golabadi D, Piadeh A. Effect of supplementing palmitic acid and altering the dietary ratio of n-6:N-3 fatty acids in low-fibre diets on production responses of dairy cows. Br J Nutr (2021) 126(3):355–65. doi: 10.1017/s0007114520004183
20. Calder PC. Fatty acids and inflammation: The cutting edge between food and pharma. Eur J Pharmacol (2011) 668 Suppl 1:S50–8. doi: 10.1016/j.ejphar.2011.05.085
21. Fernandes PF, Galassi TO, Horewicz VV, Salgado ASI, Mack JM, Baldança HDS, et al. Immunoregulatory effect of preventive supplementation of omega-3 fatty acid in a complex regional pain syndrome type I model in mice. Front Integr Neurosci (2022) 16:818692. doi: 10.3389/fnint.2022.818692
22. Liang P, Henning SM, Grogan T, Elashoff D, Ye H, Cohen P, et al. Effects of dietary omega-3 fatty acids on orthotopic prostate cancer progression, tumor associated macrophages, angiogenesis and T-cell activation-dependence on Gpr120. Prostate Cancer prostatic Dis (2022) 25(3):539–46. doi: 10.1038/s41391-021-00440-2
23. Koppelmann T, Pollak Y, Ben-Shahar Y, Gorelik G, Sukhotnik I. The mechanisms of the anti-inflammatory and anti-apoptotic effects of omega-3 polyunsaturated fatty acids during methotrexate-induced intestinal damage in cell line and in a rat model. Nutrients (2021) 13(3):888. doi: 10.3390/nu13030888
24. Rodrigues FG, Campos JB, Silva GD, Wexner SD. Endoscopic ultrasound in the diagnosis of foreign bodies of the colon and rectum. Rev da Associacao Med Bras (1992) (2016) 62(9):818–21. doi: 10.1590/1806-9282.62.09.818
25. Serhan CN, Chiang N, Dalli J, Levy BD. Lipid mediators in the resolution of inflammation. Cold Spring Harbor Perspect Biol (2014) 7(2):a016311. doi: 10.1101/cshperspect.a016311
26. Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature (2014) 510(7503):92–101. doi: 10.1038/nature13479
27. Salas-Hernández A, Espinoza-Pérez C, Vivar R, Espitia-Corredor J, Lillo J, Parra-Flores P, et al. Resolvin D1 and E1 promote resolution of inflammation in rat cardiac fibroblast in vitro. Mol Biol Rep (2021) 48(1):57–66. doi: 10.1007/s11033-020-06133-8
28. Quiros M, Feier D, Birkl D, Agarwal R, Zhou DW, García AJ, et al. Resolvin E1 is a pro-repair molecule that promotes intestinal epithelial wound healing. Proc Natl Acad Sci U S A (2020) 117(17):9477–82. doi: 10.1073/pnas.1921335117
29. Tułowiecka N, Kotlęga D, Prowans P, Szczuko M. The role of resolvins: Epa and dha derivatives can be useful in the prevention and treatment of ischemic stroke. Int J Mol Sci (2020) 21(20):7628. doi: 10.3390/ijms21207628
30. Zhang Y, Liu L, Sun D, He Y, Jiang Y, Cheng KW, et al. Dha protects against monosodium urate-induced inflammation through modulation of oxidative stress. Food Funct (2019) 10(7):4010–21. doi: 10.1039/c9fo00573k
31. Olmo I, Teuber S, Larrazabal C, Alarcon P, Raipane F, Burgos RA, et al. Docosahexaenoic acid and tug-891 activate free fatty acid-4 receptor in bovine neutrophils. Veterinary Immunol immunopathol (2019) 209:53–60. doi: 10.1016/j.vetimm.2019.02.008
32. Basyreva LY, Vakhrusheva TV, Letkeman ZV, Maximov DI, Fedorova EA, Panasenko ОM, et al. Effect of vitamin D3 in combination with omega-3 polyunsaturated fatty acids on netosis in type 2 diabetes mellitus patients. Oxid Med Cell Longevity (2021) 2021:8089696. doi: 10.1155/2021/8089696
33. Farjadian S, Moghtaderi M, Kalani M, Gholami T, Hosseini Teshnizi S. Effects of omega-3 fatty acids on serum levels of T-helper cytokines in children with asthma. Cytokine (2016) 85:61–6. doi: 10.1016/j.cyto.2016.06.002
34. Li Y, Tang Y, Wang S, Zhou J, Zhou J, Lu X, et al. Endogenous n-3 polyunsaturated fatty acids attenuate T cell-mediated hepatitis Via autophagy activation. Front Immunol (2016) 7:350. doi: 10.3389/fimmu.2016.00350
35. Cucchi D, Camacho-Muñoz D, Certo M, Niven J, Smith J, Nicolaou A, et al. Omega-3 polyunsaturated fatty acids impinge on Cd4+ T cell motility and adipose tissue distribution via direct and lipid mediator-dependent effects. Cardiovasc Res (2020) 116(5):1006–20. doi: 10.1093/cvr/cvz208
36. Liddle DM, Hutchinson AL, Monk JM, Power KA, Robinson LE. Dietary Ω-3 polyunsaturated fatty acids modulate Cd4(+) T-cell subset markers, adipocyte antigen-presentation potential, and Nlrp3 inflammasome activity in a coculture model of obese adipose tissue. Nutr (Burbank Los Angeles County Calif) (2021) 91-92:111388. doi: 10.1016/j.nut.2021.111388
37. Yang J, Zhang X, Li K, Zhou Y, Hu Y, Chen X, et al. Effects of en combined with pn enriched with n-3 polyunsaturated fatty acids on immune related indicators and early rehabilitation of patients with gastric cancer: A randomized controlled trial. Clin Nutr (Edinburgh Scotland) (2022) 41(6):1163–70. doi: 10.1016/j.clnu.2022.03.018
38. Li LY, Wang X, Zhang TC, Liu ZJ, Gao JQ. Cardioprotective effects of omega 3 fatty acids from fish oil and it enhances autoimmunity in porcine cardiac myosin-induced myocarditis in the rat model. Z fur Naturforschung C J Biosci (2021) 76(9-10):407–15. doi: 10.1515/znc-2021-0057
39. Nienaber A, Ozturk M, Dolman R, Blaauw R, Zandberg LL, van Rensburg S, et al. N-3 long-chain pufa promote antibacterial and inflammation-resolving effects in mycobacterium tuberculosis-infected C3heb/Fej mice, dependent on fatty acid status. Br J Nutr (2022) 127(3):384–97. doi: 10.1017/s0007114521001124
40. Lee J, Choi YR, Kim M, Park JM, Kang M, Oh J, et al. Common and differential effects of docosahexaenoic acid and eicosapentaenoic acid on helper T-cell responses and associated pathways. BMB Rep (2021) 54(5):278–83. doi: 10.5483/BMBRep.2021.54.5.267
41. Chiurchiù V, Leuti A, Dalli J, Jacobsson A, Battistini L, Maccarrone M, et al. Proresolving lipid mediators resolvin D1, resolvin D2, and maresin 1 are critical in modulating T cell responses. Sci Trans Med (2016) 8(353):353ra111. doi: 10.1126/scitranslmed.aaf7483
42. Liddle DM, Monk JM, Hutchinson AL, Ma DWL, Robinson LE. Cd8(+) T Cell/Adipocyte inflammatory cross talk and ensuing M1 macrophage polarization are reduced by fish-Oil-Derived n-3 polyunsaturated fatty acids, in part by a tnf-A-Dependent mechanism. J Nutr Biochem (2020) 76:108243. doi: 10.1016/j.jnutbio.2019.108243
43. Lu S, Yang Z, Tang H, Sun X, Wang B, Qu J, et al. Associations between omega-3 polyunsaturated fatty acids supplementation and surgical prognosis in patients with gastrointestinal cancer: A systematic review and meta-analysis. Food Chem Mol Sci (2022) 4:100099. doi: 10.1016/j.fochms.2022.100099
44. Wu S, Wang S, Wang L, Peng H, Zhang S, Yang Q, et al. Docosahexaenoic acid supplementation represses the early immune response against murine cytomegalovirus but enhances nk cell effector function. BMC Immunol (2022) 23(1):17. doi: 10.1186/s12865-022-00492-6
45. Chiurchiù V, Leuti A, Saracini S, Fontana D, Finamore P, Giua R, et al. Resolution of inflammation is altered in chronic heart failure and entails a dysfunctional responsiveness of T lymphocytes. FASEB J (2019) 33(1):909–16. doi: 10.1096/fj.201801017R
46. Onodera T, Fukuhara A, Shin J, Hayakawa T, Otsuki M, Shimomura I. Eicosapentaenoic acid and 5-hepe enhance macrophage-mediated treg induction in mice. Sci Rep (2017) 7(1):4560. doi: 10.1038/s41598-017-04474-2
47. Barman M, Rabe H, Hesselmar B, Johansen S, Sandberg AS, Wold AE. Cord blood levels of epa, a marker of fish intake, correlate with infants’ T- and b-lymphocyte phenotypes and risk for allergic disease. Nutrients (2020) 12(10):3000. doi: 10.3390/nu12103000
48. Patel D, Goruk S, Richard C, Field CJ. Combined supplementation of arachidonic and docosahexaenoic acids in T helper type-2 skewed brown Norway rat offspring is beneficial in the induction of oral tolerance towards ovalbumin and immune system development. J Nutr (2022) 152(9):2165–78. doi: 10.1093/jn/nxac118
49. Guesdon W, Kosaraju R, Brophy P, Clark A, Dillingham S, Aziz S, et al. Effects of fish oils on ex vivo b-cell responses of obese subjects upon Bcr/Tlr stimulation: A pilot study. J Nutr Biochem (2018) 53:72–80. doi: 10.1016/j.jnutbio.2017.10.009
50. Elsayed HRH, Anbar HS, Rabei MR, Adel M, El-Gamal R. Eicosapentaenoic and docosahexaenoic acids attenuate methotrexate-induced apoptosis and suppression of splenic T, b-lymphocytes and macrophages with modulation of expression of Cd3, Cd20 and Cd68. Tissue Cell (2021) 72:101533. doi: 10.1016/j.tice.2021.101533
51. Attaman JA, Stanic AK, Kim M, Lynch MP, Rueda BR, Styer AK. The anti-inflammatory impact of omega-3 polyunsaturated fatty acids during the establishment of endometriosis-like lesions. Am J Reprod Immunol (New York NY: 1989) (2014) 72(4):392–402. doi: 10.1111/aji.12276
52. Illesca P, Valenzuela R, Espinosa A, Echeverría F, Soto-Alarcon S, Campos C, et al. Protective effects of eicosapentaenoic acid plus hydroxytyrosol supplementation against white adipose tissue abnormalities in mice fed a high-fat diet. Molecules (Basel Switzerland) (2020) 25(19):4433. doi: 10.3390/molecules25194433
53. Teague H, Harris M, Fenton J, Lallemand P, Shewchuk BM, Shaikh SR. Eicosapentaenoic and docosahexaenoic acid ethyl esters differentially enhance b-cell activity in murine obesity. J Lipid Res (2014) 55(7):1420–33. doi: 10.1194/jlr.M049809
54. Teague H, Fhaner CJ, Harris M, Duriancik DM, Reid GE, Shaikh SR. N-3 pufas enhance the frequency of murine b-cell subsets and restore the impairment of antibody production to a T-independent antigen in obesity. J Lipid Res (2013) 54(11):3130–8. doi: 10.1194/jlr.M042457
55. Ramon S, Gao F, Serhan CN, Phipps RP. Specialized proresolving mediators enhance human b cell differentiation to antibody-secreting cells. J Immunol (Baltimore Md: 1950) (2012) 189(2):1036–42. doi: 10.4049/jimmunol.1103483
56. Moustaka K, Maleskou E, Lambrianidou A, Papadopoulos S, Lekka ME, Trangas T, et al. Docosahexaenoic acid inhibits proliferation of eol-1 leukemia cells and induces cell cycle arrest and cell differentiation. Nutrients (2019) 11(3):574. doi: 10.3390/nu11030574
57. Hirakata T, Lee HC, Ohba M, Saeki K, Okuno T, Murakami A, et al. Dietary Ω-3 fatty acids alter the lipid mediator profile and alleviate allergic conjunctivitis without modulating T(H)2 immune responses. FASEB journal: Off Publ Fed Am Societies Exp Biol (2019) 33(3):3392–403. doi: 10.1096/fj.201801805R
58. Mochimaru T, Fukunaga K, Miyata J, Matsusaka M, Masaki K, Kabata H, et al. 12-Oh-17,18-Epoxyeicosatetraenoic acid alleviates eosinophilic airway inflammation in murine lungs. Allergy (2018) 73(2):369–78. doi: 10.1111/all.13297
59. Cho E, Park Y. Association between serum fatty acid composition and innate immune markers in healthy adults. Nutr Res Pract (2016) 10(2):182–7. doi: 10.4162/nrp.2016.10.2.182
60. Zgorzynska E, Dziedzic B, Markiewicz M, Walczewska A. Omega-3 pufas suppress il-1β-Induced hyperactivity of immunoproteasomes in astrocytes. Int J Mol Sci (2021) 22(11):5410. doi: 10.3390/ijms22115410
61. Oner F, Alvarez C, Yaghmoor W, Stephens D, Hasturk H, Firatli E, et al. Resolvin E1 regulates Th17 function and T cell activation. Front Immunol (2021) 12:637983. doi: 10.3389/fimmu.2021.637983
62. McSorley EM, van Wijngaarden E, Yeates AJ, Spence T, Mulhern MS, Harrington D, et al. Methylmercury and long chain polyunsaturated fatty acids are associated with immune dysregulation in young adults from the Seychelles child development study. Environ Res (2020) 183:109072. doi: 10.1016/j.envres.2019.109072
63. Madison AA, Belury MA, Andridge R, Renna ME, Rosie Shrout M, Malarkey WB, et al. Omega-3 supplementation and stress reactivity of cellular aging biomarkers: An ancillary substudy of a randomized, controlled trial in midlife adults. Mol Psychiatry (2021) 26(7):3034–42. doi: 10.1038/s41380-021-01077-2
64. Tang W, Wang Y, Xu F, Fan W, Zhang Y, Fan K, et al. Omega-3 fatty acids ameliorate cognitive dysfunction in schizophrenia patients with metabolic syndrome. Brain behavior Immun (2020) 88:529–34. doi: 10.1016/j.bbi.2020.04.034
65. Zhang A, Guan Z, Ockerman K, Dong P, Guo J, Wang Z, et al. Regulation of glial size by eicosapentaenoic acid through a novel golgi apparatus mechanism. PLoS Biol (2020) 18(12):e3001051. doi: 10.1371/journal.pbio.3001051
66. Taha A, Sharifpanah F, Wartenberg M, Sauer H. Omega-3 and omega-6 polyunsaturated fatty acids stimulate vascular differentiation of mouse embryonic stem cells. J Cell Physiol (2020) 235(10):7094–106. doi: 10.1002/jcp.29606
67. Chen X, Chen C, Fan S, Wu S, Yang F, Fang Z, et al. Omega-3 polyunsaturated fatty acid attenuates the inflammatory response by modulating microglia polarization through Sirt1-mediated deacetylation of the Hmgb1/Nf-Kb pathway following experimental traumatic brain injury. J Neuroinflamm (2018) 15(1):116. doi: 10.1186/s12974-018-1151-3
68. Dentin R, Benhamed F, Pégorier JP, Foufelle F, Viollet B, Vaulont S, et al. Polyunsaturated fatty acids suppress glycolytic and lipogenic genes through the inhibition of chrebp nuclear protein translocation. J Clin Invest (2005) 115(10):2843–54. doi: 10.1172/jci25256
69. Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. Lipid mediator class switching during acute inflammation: Signals in resolution. Nat Immunol (2001) 2(7):612–9. doi: 10.1038/89759
70. Serhan CN, Dalli J, Colas RA, Winkler JW, Chiang N. Protectins and maresins: New pro-resolving families of mediators in acute inflammation and resolution bioactive metabolome. Biochim Biophys Acta (2015) 1851(4):397–413. doi: 10.1016/j.bbalip.2014.08.006
71. Wang DH, Xu H, Zheng YH, Gu DS, Zhu YJ, Ren Y, et al. Environmental exposure to lead and cadmium and hearing loss in Chinese adults: A case-control study. PLoS One (2020) 15(5):e0233165. doi: 10.1371/journal.pone.0233165
72. Lv Q, He Q, Wu Y, Chen X, Ning Y, Chen Y. Investigating the bioaccessibility and bioavailability of cadmium in a cooked rice food matrix by using an 11-day rapid caco-2/Ht-29 Co-culture cell model combined with an in vitro digestion model. Biol Trace element Res (2019) 190(2):336–48. doi: 10.1007/s12011-018-1554-0
73. Chavatte L, Juan M, Mounicou S, Leblanc Noblesse E, Pays K, Nizard C, et al. Elemental and molecular imaging of human full thickness skin after exposure to heavy metals. Metallomics: Integrated biometal Sci (2020) 12(10):1555–62. doi: 10.1039/d0mt00121j
74. Genchi G, Sinicropi MS, Lauria G, Carocci A, Catalano A. The effects of cadmium toxicity. Int J Environ Res Public Health (2020) 17(11):3782. doi: 10.3390/ijerph17113782
75. Yan LJ, Allen DC. Cadmium-induced kidney injury: Oxidative damage as a unifying mechanism. Biomolecules (2021) 11(11):1575. doi: 10.3390/biom11111575
76. Hong D, Min JY, Min KB. Association between cadmium exposure and liver function in adults in the united states: A cross-sectional study. J Prev Med Public Health = Yebang Uihakhoe chi (2021) 54(6):471–80. doi: 10.3961/jpmph.21.435
77. Zhang A, Matsushita M, Zhang L, Wang H, Shi X, Gu H, et al. Cadmium exposure modulates the gut-liver axis in an alzheimer’s disease mouse model. Commun Biol (2021) 4(1):1398. doi: 10.1038/s42003-021-02898-1
78. Sarmiento-Ortega VE, Moroni-González D, Díaz A, Eduardo B, Samuel T. Oral subacute exposure to cadmium loael dose induces insulin resistance and impairment of the hormonal and metabolic liver-adipose axis in wistar rats. Biol Trace element Res (2021) 200(10):4370–84. doi: 10.1007/s12011-021-03027-z
79. Alshehri AS, El-Kott AF, El-Kenawy AE, Khalifa HS, AlRamlawy AM. Cadmium chloride induces non-alcoholic fatty liver disease in rats by stimulating mir-34a/Sirt1/Fxr/P53 axis. Sci total Environ (2021) 784:147182. doi: 10.1016/j.scitotenv.2021.147182
80. Andjelkovic M, Buha Djordjevic A, Antonijevic E, Antonijevic B, Stanic M, Kotur-Stevuljevic J, et al. Toxic effect of acute cadmium and lead exposure in rat blood, liver, and kidney. Int J Environ Res Public Health (2019) 16(2):274. doi: 10.3390/ijerph16020274
81. Tsai KF, Hsu PC, Kung CT, Lee CT, You HL, Huang WT, et al. The risk factors of blood cadmium elevation in chronic kidney disease. Int J Environ Res Public Health (2021) 18(23):12337. doi: 10.3390/ijerph182312337
82. Sun X, Wang Y, Jiang T, Yuan X, Ren Z, Tuffour A, et al. Nephrotoxicity profile of cadmium revealed by proteomics in mouse kidney. Biol Trace element Res (2021) 199(5):1929–40. doi: 10.1007/s12011-020-02312-7
83. Sotomayor CG, Groothof D, Vodegel JJ, Eisenga MF, Knobbe TJ, IJmker J, et al. Plasma cadmium is associated with increased risk of long-term kidney graft failure. Kidney Int (2021) 99(5):1213–24. doi: 10.1016/j.kint.2020.08.027
84. Kawada T. Urinary cadmium and some urinary indicators of kidney tubular damage in a population exposed to chronic environmental cadmium. Int Arch Occup Environ Health (2020) 93(8):1039–40. doi: 10.1007/s00420-020-01541-6
85. Madrim MF, Ja’afar MH, Hod R. Prevalence of abnormal urinary cadmium and risk of albuminuria as a primary bioindicator for kidney problems among a healthy population. PeerJ (2021) 9:e12014. doi: 10.7717/peerj.12014
86. Liang L, Huang K, Yuan W, Liu L, Zou F, Wang G. Dysregulations of mir-503-5p and Wnt/B-catenin pathway coordinate in mediating cadmium-induced kidney fibrosis. Ecotoxicol Environ Saf (2021) 224:112667. doi: 10.1016/j.ecoenv.2021.112667
87. Tsai KF, Hsu PC, Lee CT, Kung CT, Chang YC, Fu LM, et al. Association between enzyme-linked immunosorbent assay-measured kidney injury markers and urinary cadmium levels in chronic kidney disease. J Clin Med (2021) 11(1):156. doi: 10.3390/jcm11010156
88. Kazantzis G. Cadmium, osteoporosis and calcium metabolism. Biometals: An Int J role metal ions biology biochem Med (2004) 17(5):493–8. doi: 10.1023/b:biom.0000045727.76054.f3
89. Sakurai M, Suwazono Y, Nishijo M, Nogawa K, Watanabe Y, Yoneda K, et al. The relationship between the urinary cadmium concentration and cause-specific mortality in subjects without severe renal damage: A 35-year follow-up study in a cadmium-polluted area of Japan. Int J Environ Res Public Health (2021) 18(15):7747. doi: 10.3390/ijerph18157747
90. Sun XL, Kido T, Nakagawa H, Nishijo M, Sakurai M, Ishizaki M, et al. The relationship between cadmium exposure and renal volume in inhabitants of a cadmium-polluted area of Japan. Environ Sci pollut Res Int (2021) 28(18):22372–9. doi: 10.1007/s11356-020-12278-7
91. Luo H, Gu R, Ouyang H, Wang L, Shi S, Ji Y, et al. Cadmium exposure induces osteoporosis through cellular senescence, associated with activation of nf-Kb pathway and mitochondrial dysfunction. Environ pollut (Barking Essex: 1987) (2021) 290:118043. doi: 10.1016/j.envpol.2021.118043
92. Pamphlett R, Cherepanoff S, Too LK, Kum Jew S, Doble PA, Bishop DP. The distribution of toxic metals in the human retina and optic nerve head: Implications for age-related macular degeneration. PloS One (2020) 15(10):e0241054. doi: 10.1371/journal.pone.0241054
93. Tsentsevitsky AN, Zakyrjanova GF, Petrov AM. Cadmium desynchronizes neurotransmitter release in the neuromuscular junction: Key role of ros. Free Radical Biol Med (2020) 155:19–28. doi: 10.1016/j.freeradbiomed.2020.05.017
94. Huang Y, Dai Y, Li M, Guo L, Cao C, Huang Y, et al. Exposure to cadmium induces neuroinflammation and impairs ciliogenesis in hesc-derived 3d cerebral organoids. Sci total Environ (2021) 797:149043. doi: 10.1016/j.scitotenv.2021.149043
95. Graniel-Amador MA, Torres-Rodríguez HF, Jiménez-Andrade JM, Hernández-Rodríguez J, Arteaga-Silva M, Montes S. Cadmium exposure negatively affects the microarchitecture of trabecular bone and decreases the density of a subset of sympathetic nerve fibers innervating the developing rat femur. Biometals: An Int J role metal ions biology biochem Med (2021) 34(1):87–96. doi: 10.1007/s10534-020-00265-x
96. Canale S, Blute N, Xia T, Thomas M, Gee M, Chang CH. Arsenic, cadmium, lead, and mercury in lactation foods and prenatal vitamins: Potentially avoidable exposure for breastfeeding mothers and infants. Breastfeeding med: Off J Acad Breastfeeding Med (2021) 16(7):558–63. doi: 10.1089/bfm.2020.0359
97. Yang W, Vuong AM, Xie C, Dietrich KN, Karagas MR, Lanphear BP, et al. Maternal cadmium exposure and neurobehavior in children: The home study. Environ Res (2020) 186:109583. doi: 10.1016/j.envres.2020.109583
98. Hu X, Kim KH, Lee Y, Fernandes J, Smith MR, Jung YJ, et al. Environmental cadmium enhances lung injury by respiratory syncytial virus infection. Am J Pathol (2019) 189(8):1513–25. doi: 10.1016/j.ajpath.2019.04.013
99. Yang G, Sun T, Han YY, Rosser F, Forno E, Chen W, et al. Serum cadmium and lead, current wheeze, and lung function in a nationwide study of adults in the united states. J Allergy Clin Immunol In Pract (2019) 7(8):2653–60.e3. doi: 10.1016/j.jaip.2019.05.029
100. Larson-Casey JL, Gu L, Fiehn O, Carter AB. Cadmium-mediated lung injury is exacerbated by the persistence of classically activated macrophages. J Biol Chem (2020) 295(46):15754–66. doi: 10.1074/jbc.RA120.013632
101. Liu Y, Wu J, Xiao Y, Liu Q, Yu L, Tian F, et al. Relief of cadmium-induced intestinal motility disorder in mice by lactobacillus plantarum Ccfm8610. Front Immunol (2020) 11:619574. doi: 10.3389/fimmu.2020.619574
102. Zheng R, Wang P, Cao B, Wu M, Li X, Wang H, et al. Intestinal response characteristic and potential microbial dysbiosis in digestive tract of bufo gargarizans after exposure to cadmium and lead, alone or combined. Chemosphere (2021) 271:129511. doi: 10.1016/j.chemosphere.2020.129511
103. Jiang Z, Mu W, Yang Y, Sun M, Liu Y, Gao Z, et al. Cadmium exacerbates dextran sulfate sodium-induced chronic colitis and impairs intestinal barrier. Sci total Environ (2020) 744:140844. doi: 10.1016/j.scitotenv.2020.140844
104. Tubsakul A, Sangartit W, Pakdeechote P, Kukongviriyapan V, Apaijit K, Kukongviriyapan U. Curcumin mitigates hypertension, endothelial dysfunction and oxidative stress in rats with chronic exposure to lead and cadmium. Tohoku J Exp Med (2021) 253(1):69–76. doi: 10.1620/tjem.253.69
105. Arbi S, Bester MJ, Pretorius L, Oberholzer HM. Adverse cardiovascular effects of exposure to cadmium and mercury alone and in combination on the cardiac tissue and aorta of sprague-dawley rats. J Environ Sci Health Part A Toxic/hazardous substances Environ Eng (2021) 56(6):609–24. doi: 10.1080/10934529.2021.1899534
106. Fujie T, Ozaki Y, Takenaka F, Nishio M, Hara T, Fujiwara Y, et al. Induction of metallothionein isoforms in cultured bovine aortic endothelial cells exposed to cadmium. J toxicological Sci (2020) 45(12):801–6. doi: 10.2131/jts.45.801
107. Che L, Wu ZL, Huang LY, Wu JS, Du ZB, Lin JX, et al. Microrna-101 inhibits cadmium-induced angiogenesis by targeting cyclooxygenase-2 in primary human umbilical vein endothelial cells. Biochem Pharmacol (2021) 189:114192. doi: 10.1016/j.bcp.2020.114192
108. Luparello C. Cadmium-associated molecular signatures in cancer cell models. Cancers (2021) 13(11):2823. doi: 10.3390/cancers13112823
109. Buha A, Wallace D, Matovic V, Schweitzer A, Oluic B, Micic D, et al. Cadmium exposure as a putative risk factor for the development of pancreatic cancer: Three different lines of evidence. BioMed Res Int (2017) 2017:1981837. doi: 10.1155/2017/1981837
110. Djordjevic VR, Wallace DR, Schweitzer A, Boricic N, Knezevic D, Matic S, et al. Environmental cadmium exposure and pancreatic cancer: Evidence from case control, animal and in vitro studies. Environ Int (2019) 128:353–61. doi: 10.1016/j.envint.2019.04.048
111. Chen C, Xun P, Nishijo M, Sekikawa A, He K. Cadmium exposure and risk of pancreatic cancer: A meta-analysis of prospective cohort studies and case-control studies among individuals without occupational exposure history. Environ Sci pollut Res Int (2015) 22(22):17465–74. doi: 10.1007/s11356-015-5464-9
112. Satarug S, CG G, AV D, Phelps KR. Cadmium and lead exposure, nephrotoxicity, and mortality. Toxics (2020) 8(4):86. doi: 10.3390/toxics8040086
113. Wallace DR. Nanotoxicology and metalloestrogens: Possible involvement in breast cancer. Toxics (2015) 3(4):390–413. doi: 10.3390/toxics3040390
114. Larsson SC, Orsini N, Wolk A. Urinary cadmium concentration and risk of breast cancer: A systematic review and dose-response meta-analysis. Am J Epidemiol (2015) 182(5):375–80. doi: 10.1093/aje/kwv085
115. Filippini T, Torres D, Lopes C, Carvalho C, Moreira P, Naska A, et al. Cadmium exposure and risk of breast cancer: A dose-response meta-analysis of cohort studies. Environ Int (2020) 142:105879. doi: 10.1016/j.envint.2020.105879
116. Hong H, Xu Y, Xu J, Zhang J, Xi Y, Pi H, et al. Cadmium exposure impairs pancreatic B-cell function and exaggerates diabetes by disrupting lipid metabolism. Environ Int (2021) 149:106406. doi: 10.1016/j.envint.2021.106406
117. Xiao L, Li W, Zhu C, Yang S, Zhou M, Wang B, et al. Cadmium exposure, fasting blood glucose changes, and type 2 diabetes mellitus: A longitudinal prospective study in China. Environ Res (2021) 192:110259. doi: 10.1016/j.envres.2020.110259
118. García-Risco M, Calatayud S, Pedrini-Martha V, Albalat R, Dallinger R, Palacios Ò, et al. Metal-specificity divergence between metallothioneins of nerita peloronta (Neritimorpha, Gastropoda) sets the starting point for a novel chemical Mt classification proposal. Int J Mol Sci (2021) 22(23):13114. doi: 10.3390/ijms222313114
119. Tian X, Ding Y, Kong Y, Wang G, Wang S, Cheng D. Purslane (Portulacae oleracea l.) attenuates cadmium-induced hepatorenal and colonic damage in mice: Role of chelation, antioxidant and intestinal microecological regulation. Phytomed: Int J phytother phytopharmacol (2021) 92:153716. doi: 10.1016/j.phymed.2021.153716
120. Thakur B, Yadav R, Mukherjee A, Melayah D, Marmeisse R, Fraissinet-Tachet L, et al. Protection from metal toxicity by Hsp40-like protein isolated from contaminated soil using functional metagenomic approach. Environ Sci pollut Res Int (2021) 28(14):17132–45. doi: 10.1007/s11356-020-12152-6
121. Yadav M, Soni R, Chauhan MK, Sandal N. Cellular and physiological approaches to evaluate the chelating effect of chlorella on metal ion stressed lymphocytes. Biometals (2021) 34(2):351–63. doi: 10.1007/s10534-021-00285-1
122. Howlett NG, Avery SV. Induction of lipid peroxidation during heavy metal stress in saccharomyces cerevisiae and influence of plasma membrane fatty acid unsaturation. Appl Environ Microbiol (1997) 63(8):2971–6. doi: 10.1128/aem.63.8.2971-2976.1997
123. Aspenström-Fagerlund B, Ring L, Aspenström P, Tallkvist J, Ilbäck NG, Glynn AW. Oleic acid and docosahexaenoic acid cause an increase in the paracellular absorption of hydrophilic compounds in an experimental model of human absorptive enterocytes. Toxicology (2007) 237(1-3):12–23. doi: 10.1016/j.tox.2007.04.014
124. Zhou J, Li XY, Liu YJ, Feng J, Wu Y, Shen HM, et al. Full-coverage regulations of autophagy by ros: From induction to maturation. Autophagy (2022) 18(6):1240–55. doi: 10.1080/15548627.2021.1984656
125. Arab HH, Safar MM, Shahin NN. Targeting ros-dependent Akt/Gsk-3β/Nf-Kb and dj-1/Nrf2 pathways by dapagliflozin attenuates neuronal injury and motor dysfunction in rotenone-induced parkinson’s disease rat model. ACS Chem Neurosci (2021) 12(4):689–703. doi: 10.1021/acschemneuro.0c00722
126. Moulton MJ, Barish S, Ralhan I, Chang J, Goodman LD, Harland JG, et al. Neuronal ros-induced glial lipid droplet formation is altered by loss of alzheimer’s disease-associated genes. Proc Natl Acad Sci United States America (2021) 118(52):e2112095118. doi: 10.1073/pnas.2112095118
127. Davies DA, Adlimoghaddam A, Albensi BC. Role of Nrf2 in synaptic plasticity and memory in alzheimer’s disease. Cells (2021) 10(8):1884. doi: 10.3390/cells10081884
128. Kania KD, Wagner W, Pułaski Ł. Cdse/Zns core-Shell-Type quantum dot nanoparticles disrupt the cellular homeostasis in cellular blood-brain barrier models. Int J Mol Sci (2021) 22(3):1068. doi: 10.3390/ijms22031068
129. Zhang T, Xu Z, Wen L, Lei D, Li S, Wang J, et al. Cadmium-induced dysfunction of the blood-brain barrier depends on ros-mediated inhibition of ptpase activity in zebrafish. J hazardous materials (2021) 412:125198. doi: 10.1016/j.jhazmat.2021.125198
130. Alnahdi HS, Sharaf IA. Possible prophylactic effect of omega-3 fatty acids on cadmium-induced neurotoxicity in rats’ brains. Environ Sci pollut Res Int (2019) 26(30):31254–62. doi: 10.1007/s11356-019-06259-8
131. Alam SI, Kim MW, Shah FA, Saeed K, Ullah R, Kim MO. Alpha-linolenic acid impedes cadmium-induced oxidative stress, neuroinflammation, and neurodegeneration in mouse brain. Cells (2021) 10(9):2274. doi: 10.3390/cells10092274
132. Sharmin MM, Islam MA, Yamamoto I, Taniguchi S, Yonekura S. 5-ala attenuates the palmitic acid-induced er stress and apoptosis in bovine mammary epithelial cells. Molecules (Basel Switzerland) (2021) 26(4):1183. doi: 10.3390/molecules26041183
133. Wilckens KA, Stillman CM, Waiwood AM, Kang C, Leckie RL, Peven JC, et al. Exercise interventions preserve hippocampal volume: A meta-analysis. Hippocampus (2021) 31(3):335–47. doi: 10.1002/hipo.23292
134. Gomes-Leal W. Adult hippocampal neurogenesis and affective disorders: New neurons for psychic well-being. Front Neurosci (2021) 15:594448. doi: 10.3389/fnins.2021.594448
135. Shati AA, El-Kott AF. Resolvin D1 protects against cadmium chloride-induced memory loss and hippocampal damage in rats: A comparison with docosahexaenoic acid. Hum Exp Toxicol (2021) 40(12_suppl):S215–s32. doi: 10.1177/09603271211038739
136. Katrenčíková B, Vaváková M, Paduchová Z, Nagyová Z, Garaiova I, Muchová J, et al. Oxidative stress markers and antioxidant enzymes in children and adolescents with depressive disorder and impact of omega-3 fatty acids in randomised clinical trial. Antioxidants (Basel Switzerland) (2021) 10(8):1256. doi: 10.3390/antiox10081256
137. Ibrahim Fouad G. Combination of omega 3 and coenzyme Q10 exerts neuroprotective potential against hypercholesterolemia-induced alzheimer’s-like disease in rats. Neurochemical Res (2020) 45(5):1142–55. doi: 10.1007/s11064-020-02996-2
138. Linhartova P, Sampels S. Combined incubation of cadmium, docosahexaenoic and eicosapentaenoic acid results in increased uptake of cadmium and elevated docosapentaenoic acid content in hepatocytes in vitro. Lipids Health Dis (2015) 14:156. doi: 10.1186/s12944-015-0159-2
139. Ferain A, Delbecque E, Neefs I, Dailly H, De Saeyer N, Van Larebeke M, et al. Interplay between dietary lipids and cadmium exposure in rainbow trout liver: Influence on fatty acid metabolism, metal accumulation and stress response. Aquat Toxicol (Amsterdam Netherlands) (2021) 231:105676. doi: 10.1016/j.aquatox.2020.105676
140. Owumi SE, Olayiwola YO, Alao GE, Gbadegesin MA, Odunola OA. Cadmium and nickel Co-exposure exacerbates genotoxicity and not oxido-inflammatory stress in liver and kidney of rats: Protective role of omega-3 fatty acid. Environ Toxicol (2020) 35(2):231–41. doi: 10.1002/tox.22860
141. Linhartova P, Gazo I, Sampels S. Combined incubation of cadmium, docosahexaenoic and eicosapentaenoic acid affecting the oxidative stress and antioxidant response in human hepatocytes in vitro. Physiol Res (2016) 65(4):609–16. doi: 10.33549/physiolres.933247
142. Sugimoto K, Tanizaki T, Shimizu E, Hosomi R, Fukunaga K, Yoshida M, et al. Single and repeated dose 28-day and 13-week toxicity studies of oil prepared from the internal organs of the Japanese giant scallop (Patinopecten yessoensis) in mice. Foods (Basel Switzerland) (2020) 9(6):691. doi: 10.3390/foods9060691
143. Skogheim TS, Weyde KVF, Engel SM, Aase H, Surén P, Øie MG, et al. Metal and essential element concentrations during pregnancy and associations with autism spectrum disorder and attention-Deficit/Hyperactivity disorder in children. Environ Int (2021) 152:106468. doi: 10.1016/j.envint.2021.106468
144. Arteaga-Silva M, Arenas-Rios E, Bonilla-Jaime H, Damian-Matzumura P, Limon-Morales O, Hernandez-Rodriguez J, et al. Neuroendocrine effects of cadmium exposure on Male reproductive functions. Front bioscience (Landmark edition) (2021) 26(2):286–326. doi: 10.2741/4895
145. Saintilnord WN, Tenlep SYN, Preston JD, Duregon E, DeRouchey JE, Unrine JM, et al. Chronic exposure to cadmium induces differential methylation in mice spermatozoa. Toxicological sciences: an Off J Soc Toxicol (2021) 180(2):262–76. doi: 10.1093/toxsci/kfab002
146. Serra R, Peñailillo R, Monteiro LJ, Monckeberg M, Peña M, Moyano L, et al. Supplementation of omega 3 during pregnancy and the risk of preterm birth: A systematic review and meta-analysis. Nutrients (2021) 13(5):1704. doi: 10.3390/nu13051704
147. Ciesielski TH, Williams SM. Low omega-3 intake is associated with high rates of depression and preterm birth on the country level. Sci Rep (2020) 10(1):19749. doi: 10.1038/s41598-020-76552-x
148. Mohammadi H, Golbabaei F, Dehghan SF, Imani H, Ramezani Tehrani F, Khodakarim Ardakani S. The influence of vitamin e and omega-3 fatty acids on reproductive health indices among Male workers exposed to electromagnetic fields. Am J men’s Health (2022) 16(1):15579883221074821. doi: 10.1177/15579883221074821
149. Wang S, Chen Q, Zhang Y, Zheng F, Xue T, Ge X, et al. Omega-3 polyunsaturated fatty acids alleviate hydrogen sulfide-induced blood-testis barrier disruption in the testes of adult mice. Reprod Toxicol (Elmsford NY) (2020) 98:233–41. doi: 10.1016/j.reprotox.2020.10.007
150. Moustafa A. Effect of omega-3 or omega-6 dietary supplementation on testicular steroidogenesis, adipokine network, cytokines, and oxidative stress in adult Male rats. Oxid Med Cell Longevity (2021) 2021:5570331. doi: 10.1155/2021/5570331
151. Komal F, Khan MK, Imran M, Ahmad MH, Anwar H, Ashfaq UA, et al. Impact of different omega-3 fatty acid sources on lipid, hormonal, blood glucose, weight gain and histopathological damages profile in pcos rat model. J Trans Med (2020) 18(1):349. doi: 10.1186/s12967-020-02519-1
152. Childs CE. Sex hormones and n-3 fatty acid metabolism. Proc Nutr Soc (2020) 79(2):219–24. doi: 10.1017/s0029665119001071
153. Stanhiser J, Jukic AMZ, Steiner AZ. Serum omega-3 and omega-6 fatty acid concentrations and natural fertility. Hum Reprod (Oxford England) (2020) 35(4):950–7. doi: 10.1093/humrep/dez305
154. Ekhoye EI, Olerimi SE, Ehebha SE. Comparison of the deleterious effects of yaji and cadmium chloride on testicular physiomorphological and oxidative stress status: The gonadoprotective effects of an omega-3 fatty acid. Clin Exp Reprod Med (2020) 47(3):168–79. doi: 10.5653/cerm.2019.03517
155. Akhigbe RE, Hamed MA, Odetayo AF, Akhigbe TM, Ajayi AF, Ajibogun FAH. Omega-3 fatty acid rescues Ischaemia/Perfusion-induced testicular and sperm damage Via modulation of lactate transport and xanthine Oxidase/Uric acid signaling. Biomed pharmacother = Biomedecine pharmacotherapie (2021) 142:111975. doi: 10.1016/j.biopha.2021.111975
156. Parajuli RP, Goodrich JM, Chan HM, Lemire M, Ayotte P, Hegele RA, et al. Variation in biomarker levels of metals, persistent organic pollutants, and omega-3 fatty acids in association with genetic polymorphisms among Inuit in nunavik, Canada. Environ Res (2021) 200:111393. doi: 10.1016/j.envres.2021.111393
157. Sakamoto M, Chan HM, Domingo JL, Koriyama C, Murata K. Placental transfer and levels of mercury, selenium, vitamin e, and docosahexaenoic acid in maternal and umbilical cord blood. Environ Int (2018) 111:309–15. doi: 10.1016/j.envint.2017.11.001
158. Brown TJ, Brainard J, Song F, Wang X, Abdelhamid A, Hooper L. Omega-3, omega-6, and total dietary polyunsaturated fat for prevention and treatment of type 2 diabetes mellitus: Systematic review and meta-analysis of randomised controlled trials. BMJ (Clinical Res ed) (2019) 366:l4697. doi: 10.1136/bmj.l4697
159. de Boer IH, Zelnick LR, Ruzinski J, Friedenberg G, Duszlak J, Bubes VY, et al. Effect of vitamin d and omega-3 fatty acid supplementation on kidney function in patients with type 2 diabetes: A randomized clinical trial. Jama (2019) 322(19):1899–909. doi: 10.1001/jama.2019.17380
160. Liu QK. Triglyceride-lowering and anti-inflammatory mechanisms of omega-3 polyunsaturated fatty acids for atherosclerotic cardiovascular risk reduction. J Clin lipidology (2021) 15(4):556–68. doi: 10.1016/j.jacl.2021.05.007
161. D’Angelo S, Motti ML, Meccariello R. Ω-3 and Ω-6 polyunsaturated fatty acids, obesity and cancer. Nutrients (2020) 12(9):2751. doi: 10.3390/nu12092751
162. Naji S, Issa K, Eid A, Iratni R, Eid AH. Cadmium induces migration of colon cancer cells: Roles of reactive oxygen species, P38 and cyclooxygenase-2. Cell Physiol Biochem (2019) 52(6):1517–34. doi: 10.33594/000000106
163. Tu M, Wang W, Zhang G, Hammock BD. Ω-3 polyunsaturated fatty acids on colonic inflammation and colon cancer: Roles of lipid-metabolizing enzymes involved. Nutrients (2020) 12(11):3301. doi: 10.3390/nu12113301
164. Howlett NG, Avery SV. Relationship between cadmium sensitivity and degree of plasma membrane fatty acid unsaturation in saccharomyces cerevisiae. Appl Microbiol Biotechnol (1997) 48(4):539–45. doi: 10.1007/s002530051093
165. Lundebye AK, Lock EJ, Rasinger JD, Nøstbakken OJ, Hannisdal R, Karlsbakk E, et al. Lower levels of persistent organic pollutants, metals and the marine omega 3-fatty acid dha in farmed compared to wild Atlantic salmon (Salmo salar). Environ Res (2017) 155:49–59. doi: 10.1016/j.envres.2017.01.026
166. Ouyang L, Dan Y, Hua W, Shao Z, Duan D. Therapeutic effect of omega-3 fatty acids on T cell-mediated autoimmune diseases. Microbiol Immunol (2020) 64(8):563–9. doi: 10.1111/1348-0421.12800
167. Rajasinghe LD, Li QZ, Zhu C, Yan M, Chauhan PS, Wierenga KA, et al. Omega-3 fatty acid intake suppresses induction of diverse autoantibody repertoire by crystalline silica in lupus-prone mice. Autoimmunity (2020) 53(7):415–33. doi: 10.1080/08916934.2020.1801651
168. Shati AA, El-Kott AF, Alkhateeb MA. Resolvin D1 prevents cadmium chloride-induced memory loss and hippocampal damage in rats by Activation/Upregulation of pten-induced suppression of Pi3k/Akt/Mtor signaling pathway. Clin Exp Pharmacol Physiol (2022) 49(2):275–90. doi: 10.1111/1440-1681.13596
169. Zhang L, Jia NN, Yang RH, Wang F. Eicosapentaenoic acid reduces inflammation and apoptosis by Srebp1/Tlr4/Myd88. Bratislavske lekarske listy (2020) 121(11):822–9. doi: 10.4149/bll_2020_135
170. Damsgaard CT, Vuholm S, Teisen MN, Stark KD, Lauritzen L. Does polymorphisms in ppar and apoe genes modify associations between fatty acid desaturase (Fads), n-3 long-chain pufa and cardiometabolic markers in 8-11-Year-Old Danish children? Br J Nutr (2021) 125(4):369–76. doi: 10.1017/s0007114520002822
171. Kandeil MA, Hashem RM, Mahmoud MO, Hetta MH, Tohamy MA. Zingiber officinale extract and omega-3 fatty acids ameliorate endoplasmic reticulum stress in a nonalcoholic fatty liver rat model. J Food Biochem (2019) 43(12):e13076. doi: 10.1111/jfbc.13076
Keywords: cadmium, omega-3 PUFAs, immune system, oxidative stress, health
Citation: Chen Z, Lu Q, Wang J, Cao X, Wang K, Wang Y, Wu Y and Yang Z (2022) The function of omega-3 polyunsaturated fatty acids in response to cadmium exposure. Front. Immunol. 13:1023999. doi: 10.3389/fimmu.2022.1023999
Received: 20 August 2022; Accepted: 09 September 2022;
Published: 29 September 2022.
Edited by:
Weicheng Hu, Huaiyin Normal University, ChinaReviewed by:
Jianmei Zhang, Sungkyunkwan University, South KoreaJianGuo Wang, Northwest A&F University, China
Shoupeng Fu, Jilin University, China
Copyright © 2022 Chen, Lu, Wang, Cao, Wang, Wang, Wu and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhangping Yang, yzp@yzu.edu.cn
 Zhi Chen
Zhi Chen Qinyue Lu
Qinyue Lu Jiacheng Wang3
Jiacheng Wang3 Xiang Cao
Xiang Cao Zhangping Yang
Zhangping Yang