- 1Division of Thoracic Oncology, Department of Thoracic Medicine, Linkou Chang Gung Memorial Hospital, College of Medicine, Chang Gung University, Taoyuan, Taiwan
- 2Department of Internal Medicine, Linkou Chang Gung Memorial Hospital, College of Medicine, Chang Gung University, Taoyuan, Taiwan
- 3Department of Pathology, Linkou Chang Gung Memorial Hospital, College of Medicine, Chang Gung University, Taoyuan, Taiwan
- 4Institute of European and American Studies, Academia Sinica, Taipei, Taiwan
- 5Division of Chest Medicine, Department of Internal Medicine, Taichung Veterans General Hospital, Taichung, Taiwan
- 6Department of Family Medicine, Shuang Ho Hospital, Taipei Medical University, New Taipei, Taiwan
- 7Department of Thoracic Medicine, Taipei Chang Gung Memorial Hospital, Taipei, Taiwan
- 8Department of Pulmonary and Critical Care Medicine, Chiayi Chang Gung Memorial Hospital, College of Medicine, Chang Gung University, Puzi, Taiwan
- 9Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Keelung Chang Gung Memorial Hospital, College of Medicine, Chang Gung University, Keelung, Taiwan
- 10Department of Thoracic Medicine, Taoyuan Chang Gung Memorial Hospital, Taoyuan, Taiwan
- 11Department of Respiratory Therapy, College of Medicine, Chang Gung University, Taoyuan, Taiwan
- 12Divisions of Pulmonary & Critical Care Medicine, Department of Internal Medicine, Kaohsiung Chang Gung Memorial Hospital, College of Medicine, Chang Gung University, Kaohsiung, Taiwan
Introduction: Uncommon epidermal growth factor receptor (EGFR) mutations include single and complex mutations. However, the association of the smoking status of patients with uncommon and complex EGFR mutations remains unclear.
Methods: This retrospective study evaluates the spectrum of uncommon EGFR mutations and investigates the influence of smoking status on the frequency of various uncommon EGFR mutations using a multi-institutional medical database.
Results: Between 2010 and 2019, 5,608 non-small cell lung cancer (NSCLC) patients were analyzed. EGFR mutations were detected in 3,155 (56.3%) patients. Among the 399 (12.6%) patients with uncommon mutations, 198 had single uncommon and 201 complex mutations, including 87 exon 20 insertions, 79 de novo T790M, 70 complex common, and 52 complex uncommon mutations. For comparison, we also included 402 patients with common EGFR mutations. The percentage of ever-smokers was significantly higher in patients with uncommon EGFR mutations than in patients with common EGFR mutations (25.8% vs. 17.4%, p = 0.005). Furthermore, the percentage of ever-smokers was higher in those with a complex mutation than in those with a single uncommon mutation (30.3% vs. 21.2%, p = 0.040). Among patients carrying uncommon EGFR mutations, ever-smokers had significantly more complex uncommon EGFR mutations than never-smokers (22.3% vs. 9.8%, p = 0.002). Among patients carrying G719X, L861Q, and S768I, ever-smokers tended to have complex EGFR mutations more frequently than never-smokers (64.7% vs. 28.7%, 50.0% vs. 18.7%, 88.9% vs. 81.2%, respectively).
Conclusions: Our study demonstrates not only a comprehensive spectrum of uncommon EGFR mutations, but also a positive relationship between smoking status and uncommon EGFR mutation frequency, especially complex uncommon EGFR mutations. The results suggest that smoking contributes to the development of complex EGFR mutations.
Introduction
Lung cancer has been the leading cause of cancer-related deaths for several years worldwide, and non-small cell lung cancer (NSCLC) accounts for 85–90% of all cases (1). Smoking is the most important risk factor that causes lung cancer (2). Studies have shown that the pathogenesis and clinical manifestations of ever- and never-smokers are different, and it has also been verified that smoking is associated with poor therapeutic outcomes and decreased survival (2).
Epidermal growth factor receptor (EGFR) mutations are the most commonly detected and targetable driver mutations in NSCLC (3). Approximately 50% of Asian and 8–16% of non-Asian patients with NSCLC harbor EGFR mutation (4). Exon 19 deletions and L858R substitutions in exon 21 account for approximately 85–90% of all EGFR mutations, which are known to be common mutations and are sensitive to EGFR-tyrosine kinase inhibitors (TKIs). Patients with advanced NSCLC and common EGFR mutations have a higher response rate and longer progression-free survival (PFS) when treated with first-line EGFR-TKIs than with platinum-based chemotherapy (5). Further randomized clinical trials have validated the overall survival (OS) advantage of second- and third-generation over first-EGFR-TKIs (6, 7). Hence, routine testing for EGFR mutation status has become a standard-of-care recommendation for advanced NSCLC patients, especially those with lung adenocarcinoma histology (8, 9).
EGFR mutations, other than exon 19 deletions and L858R mutations, are uncommon. These uncommon mutations are highly heterogeneous and demonstrate variable responses to EGFR-TKIs, which remain poorly characterized. The most frequently found uncommon mutations include G719X, L861Q, S768I, exon 20 insertions (Ex20ins), and de novo T790M (10). NSCLC carrying Ex20ins and de novo T790M are resistant to both first- and second-generation EGFR-TKI therapies, whereas other uncommon mutations are usually sensitive (11). Information regarding the activity of EGFR-TKIs against uncommon EGFR mutations are limited (11, 12). Chiu et al. (13) reported that first-generation EGFR-TKIs were less effective in patients with the G719X, L861Q, and S768I mutations than in those with common mutations. Recently, Yang et al. conducted a post-hoc analysis of 1023 cases and showed that a second-generation EGFR-TKI, afatinib, was highly effective in patients with certain types of uncommon mutations (14).
Uncommon EGFR mutations can occur alone or coexist with either common or other uncommon EGFR mutations, including complex or compound mutations. The incidence of complex EGFR mutations varies among different study populations and detection methods, ranging from 4% to 26% of all EGFR mutations (15). Data regarding the effect of EGFR-TKIs on complex EGFR mutations are even fewer, and the results based on a relatively small number of cases were highly heterogeneous (15). Generally, the result in patients carrying complex mutations is similar to that in patients with single uncommon mutations and is less favorable than that in patients with common mutations (15, 16). Nevertheless, the characteristics of NSCLC patients with complex EGFR mutations are not fully understood.
Previous studies have identified several clinical features related to the prevalence of EGFR mutations in NSCLC such as female sex, Asian ethnicity, lung adenocarcinoma, and never-smoking status (17–19). EGFR mutations occur more frequently in non-smokers than in smokers. In these earlier studies, the majority of the detected EGFR mutations were common (20, 21). Despite the strong association between the prevalence of EGFR mutations and never-smoking status, EGFR mutations can still be detected in smokers. Smoking is known to increase tumor mutation burden (22). Moreover, advanced detection techniques have broadened EGFR mutation spectrum, and uncommon or complex EGFR mutations have been identified (10, 23). It remains unclear whether smoking status correlates with the pattern of uncommon EGFR mutations and affects the development of complex EGFR mutations.
In the present study, we use a multi-institutional database from 2010 to 2019 to examine the uncommon EGFR mutation spectrum. We compare the frequencies of uncommon EGFR mutation subtypes between ever- and never-smokers, while investigating the smoking status of patients harboring uncommon EGFR genotypes.
Materials and methods
Patient population
This retrospective cohort study accesses patient data from the Chang Gung Research Database, a multi-institutional electronic medical record collection system in Taiwan (24). The screening criteria were as follows (1): NSCLC patients who were treated at any one of the institutions of Chang Gung Memorial Hospitals, including Linkou, Kaohsiung Medical Centers, Taipei, Taoyuan, Keelung, and Chiayi branches; and (2) patients with histologically or cytologically confirmed NSCLC who underwent EGFR mutation analysis at Chang Gung Memorial Hospital, Linkou Medical Center between 2010 and 2019. A total of 5,608 patients were screened. Of the 3,155 patients carrying EGFR mutations, 399 had uncommon EGFR mutations (Figure 1). Clinical data of patients with uncommon EGFR mutations were recorded, including age at diagnosis, sex, smoking status, Eastern Cooperative Oncology Group (ECOG) performance status, histology type, tumor stage (according to the 8th edition of the American Joint Committee on Cancer staging system), and EGFR mutation type. Two thousand, seven hundred and fifty-six patients had common EGFR mutations. We filtered patients’ information using the following criteria: (1) NSCLC patients who were treated between 2016 and 2017; (2) positive common EGFR mutation; and (3) who were at advanced or metastatic stage and received Taiwan’s National Health Insurance reimbursed first-line treatment with EGFR-TKIs. Thus, 402 patients were enrolled for comparison. This study was approved by the Institutional Review Board of Chang Gung Memorial Hospital (No. 202200840B0).
EGFR mutation testing
EGFR mutation status was detected using polymerase chain reaction (PCR)-direct sequencing or mutant type-specific sensitive methods based on the tumor purity of tissue samples (25). Mutant-type-specific sensitive methods include the Scorpions amplification-refractory mutation system (ARMS) (Therascreen EGFR RGQ PCR Kit, Qiagen) and competitive allele-specific TaqMan PCR (Life Technologies) (25, 26). For PCR-direct sequencing, DNA was extracted from tumor specimens for EGFR mutation analysis, as described previously (25, 27). For mutant type-specific sensitive methods, DNA extraction and analysis were performed using commercial kits in accordance with the manufacturer’s instructions.
Exon 19 deletions (Ex19del) and L858R are common EGFR mutations. All EGFR mutations other than the common ones were uncommon, including G719X, L861Q, S768I, exon 20 insertion (Ex20ins), and de novo T790M. Two or more EGFR mutations within the same tumor tissue were defined as complex EGFR mutations, encompassing de novo T790M, complex common, and complex uncommon EGFR mutations. De novo T790M mutations are primary T790M mutations that are accompanied by common or uncommon EGFR mutations. Complex common EGFR mutations are common mutations that coexist with one or more uncommon mutations. Complex uncommon EGFR mutations are two or more distinct uncommon mutations within the same tumor tissue. The distribution of uncommon EGFR mutations is shown in Figure 1.

Figure 1 NSCLC patients receiving EGFR mutation testing and distribution of uncommon EGFR mutation. EGFR, epidermal growth factor receptor; Ex19del, exon 19 deletion; NSCLC, non-small cell lung cancer.
Statistical analysis
Fisher's exact test and Pearson’s chi-squared test were used to compare categorical variables, including clinical factors, the type and frequency of EGFR mutations, and smoking status. A logistic regression was employed to calculate the odds ratios (ORs) and 95% confidence intervals (CIs) for univariate and multivariate analyses to assess the independent association between different variables and presence of common versus uncommon EGFR mutations. For evaluation of the association of smoking status with the type and frequency of uncommon EGFR mutations, patients with NSCLC carrying uncommon EGFR mutations were categorized as never-smokers or ever-smokers based on their self-reported smoking status. Never-smokers were defined as those who smoked fewer than 100 cigarettes during their lifetime. Other patients were defined as ever-smokers, including both current and former smokers. We evaluated the correlation between smoking status and the frequency of uncommon EGFR mutations, and compared the mutation types between ever- and never-smokers with NSCLC histology carrying uncommon EGFR mutations. A two-sided p value less than 0.05 was considered statistically significant. Statistical analyses were performed using GraphPad Prism (version 5.02, GraphPad Software, La Jolla, CA, USA) and R software (version 4.1.2).
Results
Patient characteristics
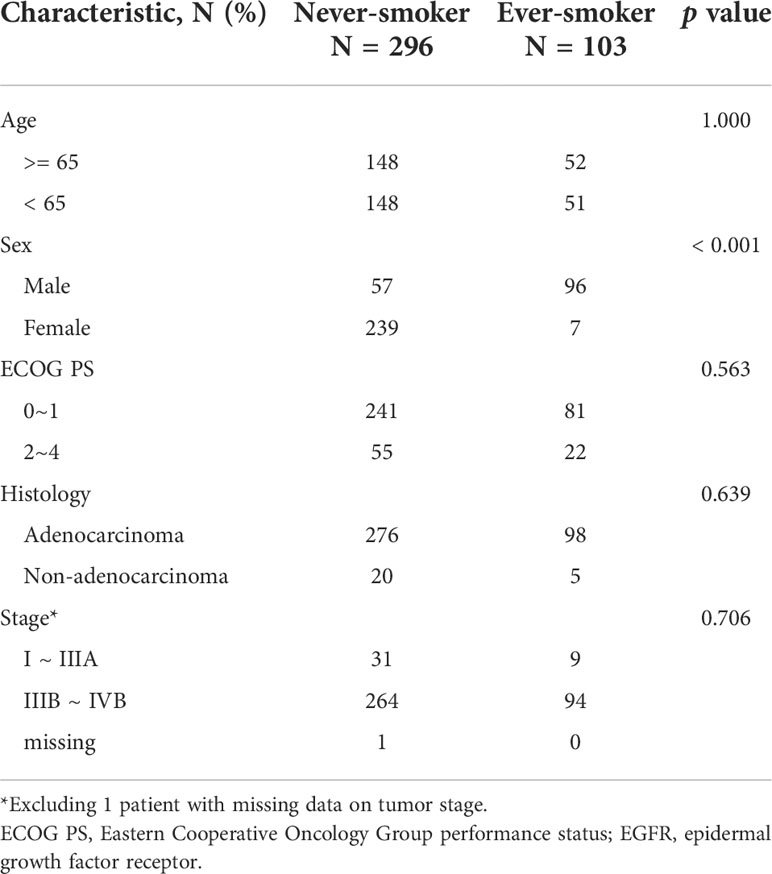
A total of 399 patients with NSCLC harboring uncommon EGFR mutations were enrolled in the study. The baseline characteristics of the patients are shown in Table 1. The median age during diagnosis was 65 years (range:32–94 years). Most patients were female (61.7%), never-smokers (74.2%), had an ECOG performance status of 0–1 (80.7%), were diagnosed with adenocarcinoma histology (93.7%), and had advanced- or metastatic- staged diseases at baseline (89.7%).
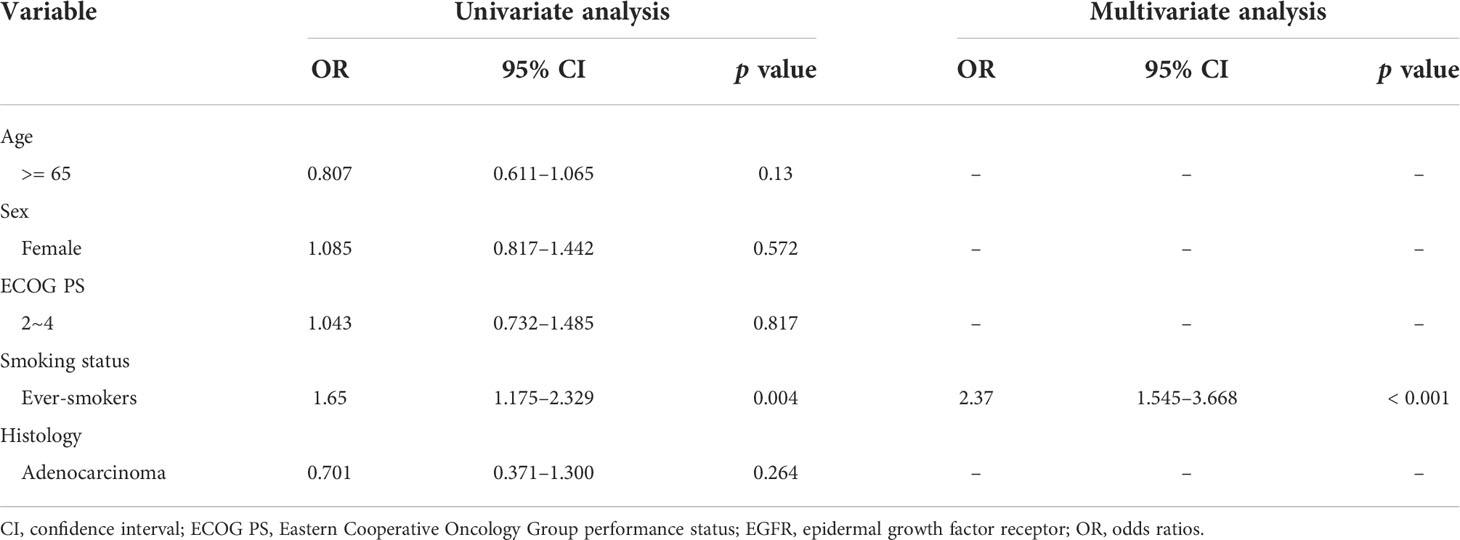
Another set of 402 NSCLC patients with common EGFR mutations who were diagnosed between 2016 and 2017 were included for comparison. All patients had stage IIIB/IIIC or stage IV cancer. The demographic data of the 402 patients were similar to those of the 399 patients, except for smoking status (Table 1). The percentage of ever-smokers among patients with uncommon EGFR mutations was significantly higher than that among patients with common EGFR mutations (25.8% vs. 17.4%, p = 0.005). Furthermore, we performed logistic regression to assess the independent association between different variables and presence of common versus uncommon EGFR mutations. We found that ever smoking history was significantly correlated with uncommon EGFR mutation status (Table 2).
Of all the patients with uncommon EGFR mutations, 198 had one EGFR mutation and 201 had complex EGFR mutations. The clinical features and results of univariate analysis of these two populations are summarized in Table 1. The percentage of ever-smokers among patients with complex EGFR mutations was significantly greater than that among patients with single uncommon EGFR mutations (30.3% vs. 21.2%, p = 0.040).
Smoking status and sex in patients with uncommon EGFR mutation
Since we had observed that the percentage of ever-smokers was higher in patients with uncommon EGFR mutations than in those with common EGFR mutations, as a next step, we examined the pattern of uncommon EGFR mutations according to smoking status and sex. The baseline characteristics between never- and ever-smokers were similar except sex (Table 3). Of the 198 patients with one uncommon EGFR mutation, 86 had EGFR exon 20 insertions and 112 had other single uncommon EGFR mutations. Of the 201 patients with complex EGFR mutations, 79 had de novo T790M mutations, 70 had complex common mutations, and 52 had complex uncommon EGFR mutations. The associations of smoking status and sex with uncommon EGFR mutations are shown in Table 4. Differences in the frequency of various types of uncommon EGFR mutations were only seen in the distinct smoking status but not in sex (p = 0.017 and 0.803, respectively). To elucidate the interaction between sex and smoking behaviors, we evaluated smoking status and observed that the proportion of ever-smokers was higher in males than in females, both in patients with common and uncommon EGFR mutations (41.4% vs. 1.3%, 62.7% vs. 2.8%; both p < 0.001).
Among the 399 patients with uncommon EGFR mutations, ever-smokers had a significantly higher frequency of complex uncommon EGFR mutations than never-smokers (22.3% vs. 9.8%, p = 0.001). As for the remainders having exon 20 insertion, other single uncommon EGFR mutations, de novo T790M, and complex common EGFR mutations, the frequency was similar between ever- and never-smokers (Table 4). The smoking rates of patients with each uncommon EGFR mutation type are shown in Figure 2. Compared with the common EGFR mutation group, the percentage of ever-smokers was significantly higher in the complex common and uncommon mutation groups (p = 0.033 and < 0.001, respectively). In contrast, there were no significant differences in the various uncommon EGFR mutation types between the sexes (Table 4).
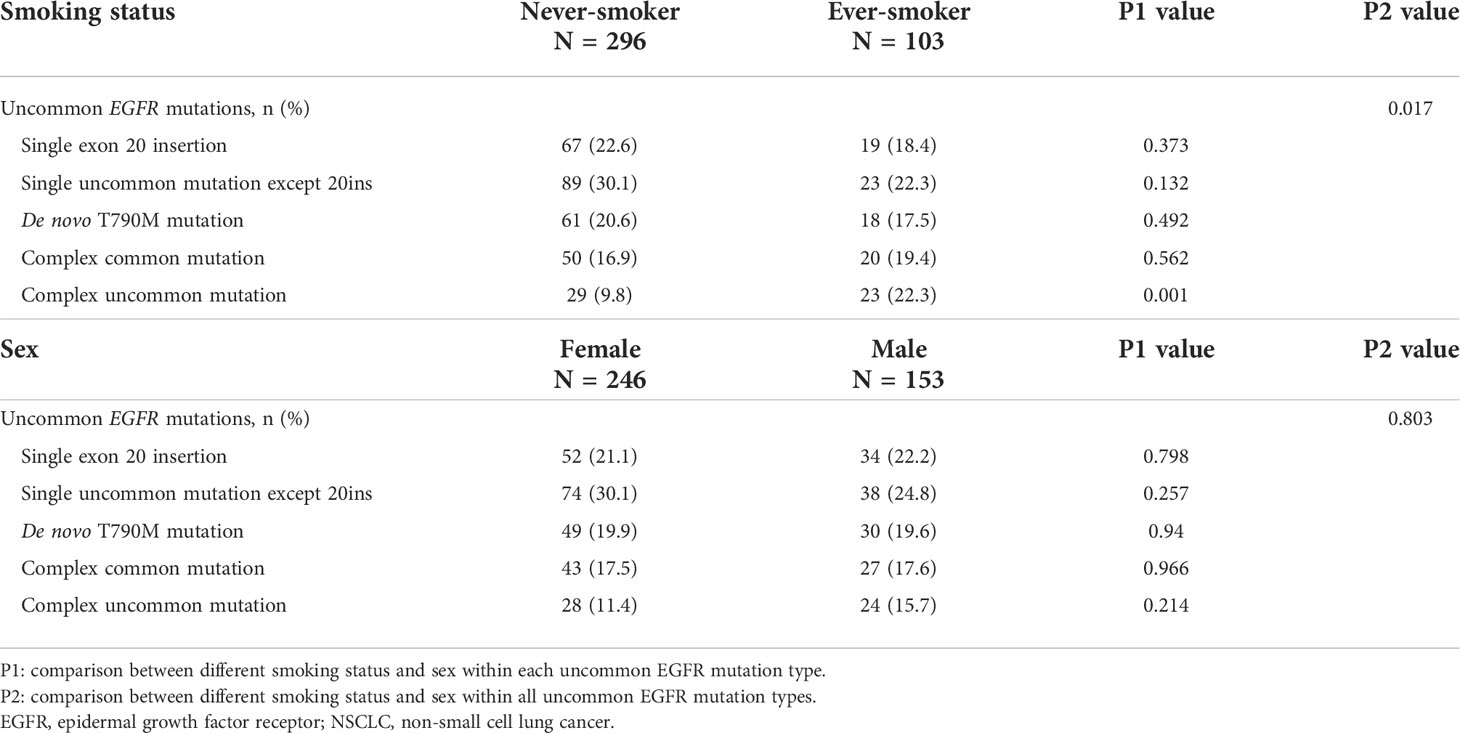
Table 4 Frequency of uncommon EGFR mutation types in NSCLC patients with different smoking status and sex.
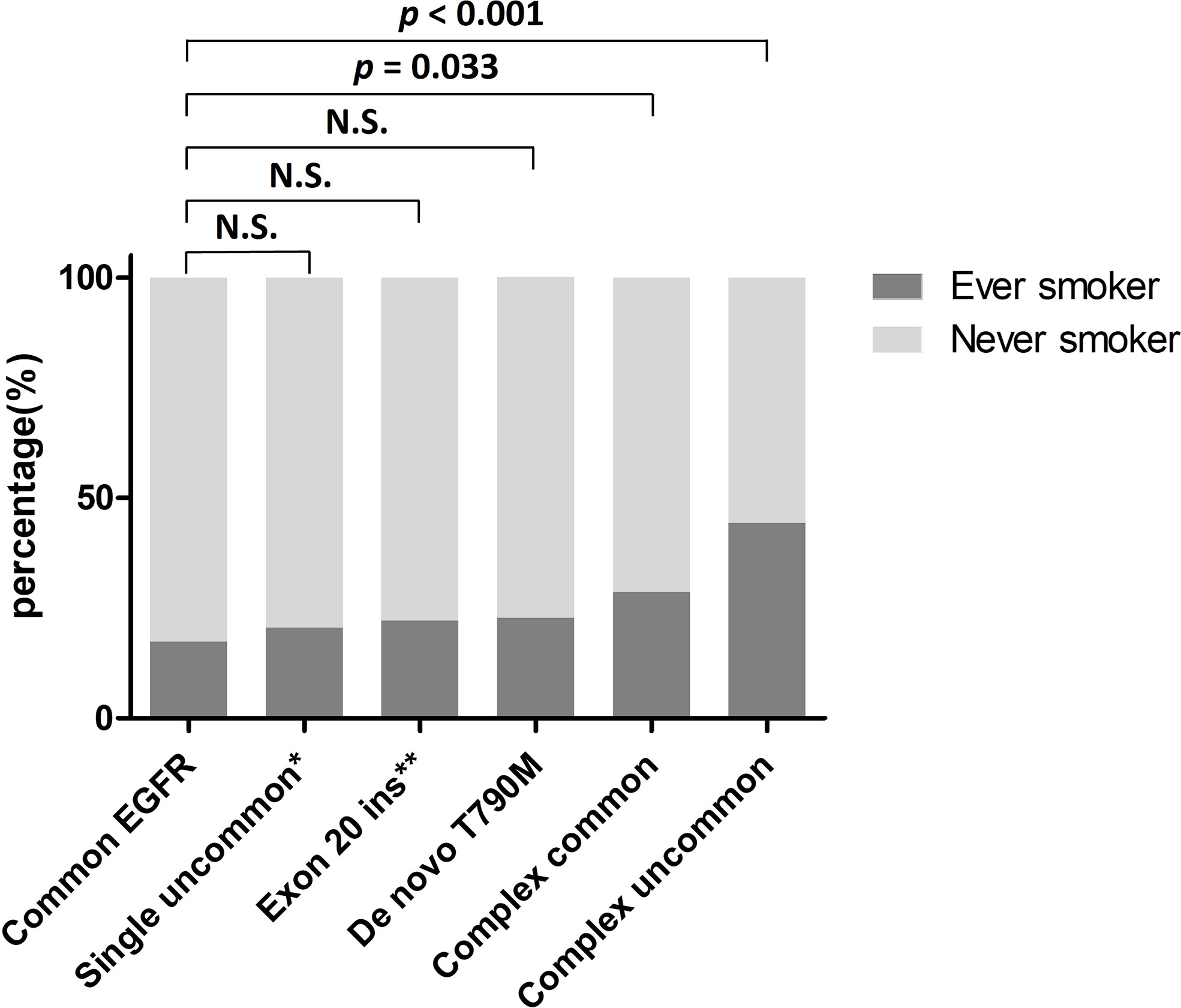
Figure 2 Percentage of ever-smokers in NSCLC patients harboring various EGFR mutations. *Excluding exon 20 insertion. **Including single exon 20 insertion only. EGFR, epidermal growth factor receptor; Exon 20 ins, exon 20 insertion; N.S., not significant; NSCLC, non-small cell lung cancer.
Association of smoking status with specific uncommon EGFR genotypes
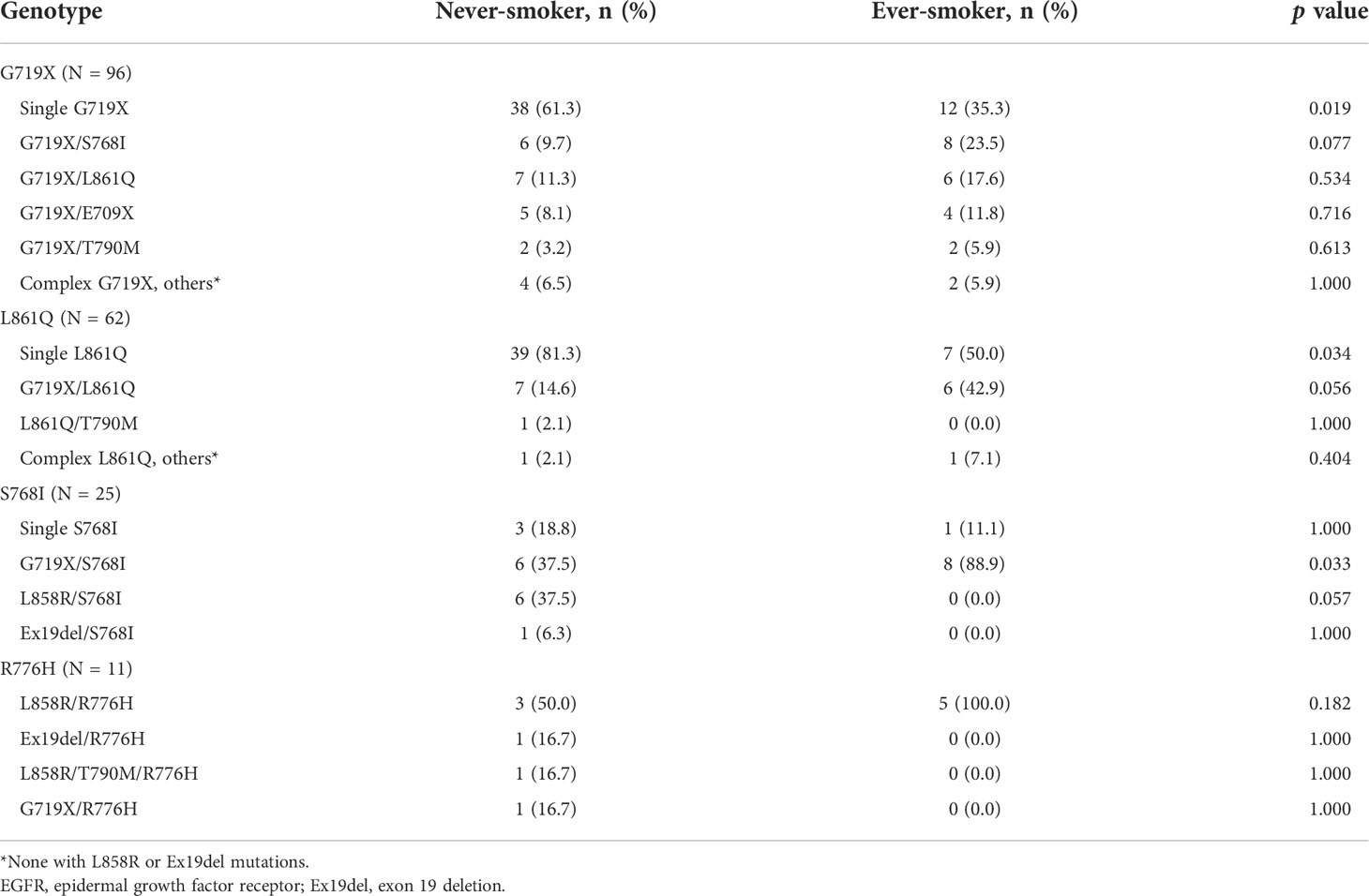
To study the association of smoking behavior with specific genotypes of uncommon EGFR mutations, we analyzed the smoking status of patients with G719X, L861Q, and S768I. We also included patients with R776H mutations in the analysis because we noticed a high smoking rate in this population. Among all the patients with uncommon EGFR mutations, 96 had G719X (including 13 G719X/L861Q, 14 G719X/S768I, and 1 G719X/R776H co-mutation), 62 had L861Q (including 13 G719X/L861Q co-mutations), 25 had S768I (including 14 G719X/S768I co-mutations), and 11 had R776H (all were complex mutations). None of the patients had complex common EGFR mutations, with either G719X or L861Q. The smoking status of patients with G719X, L861Q, S768I, and R776H mutations are shown in Table 3. In patients with G719X and L861Q mutations, ever-smokers had single G719X or L861Q less frequently than never-smokers (35.3% vs. 61.3%, p = 0.019; 50% vs. 81.3%, p = 0.034, respectively). In patients with S768I mutations, ever-smokers had more G719X/S768I co-mutations (88.9% vs. 37.5%, p = 0.033) than never-smokers. The frequency of R776H was similar between never- and ever-smokers.
Discussion
Several studies have identified smoking as a negative predictive marker for EGFR mutation status in patients with lung adenocarcinoma or NSCLC (20, 21, 28, 29). Most of these studies focused on common EGFR mutations. Few investigations have explored the influence of smoking on the prevalence of uncommon EGFR mutations, and the number of cases in these studies was limited. To the best of our knowledge, this is the largest study to explore the association between smoking status and uncommon EGFR mutation prevalence. In the present study, we demonstrate a comprehensive EGFR mutation spectrum of 3,155 patients by assessing a multi-institutional medical record database over a 10-year period. The percentage of ever-smokers was higher in the 399 patients carrying uncommon mutations than in the 402 patients carrying common mutations. A logistic regression analysis demonstrated the independent association of smoking history with uncommon EGFR mutation status. The frequency of complex uncommon mutations in ever-smokers was higher than that in never-smokers. Our observations suggest that smoking status is a positive predictor of specific EGFR mutations.
Tobacco smoke is a mixture of chemicals that contain many carcinogens (30). These carcinogens may induce DNA damage, leading to an increased burden of somatic mutations, thereby enhancing the chance of driver mutation development during tumorigenesis (22). A previous study has shown that increased somatic mutation burdens associated with smoking, including base substitutions (point mutations), small insertions and deletions (indels), and copy number changes, contribute to different extents in different cancers, but almost all contribute to lung cancer (22). Lung cancer is characterized by molecular heterogeneity. Another study demonstrated that smokers with NSCLC histology carry a 10-fold higher mutation frequency and possess a more distinct mutation spectrum than nonsmokers (31). Smoking can also trigger apolipoprotein B mRNA-editing enzyme catalytic polypeptide-like (APOBEC) activity, a major putative enzymatic source of mutation in cancers (32). One study reported that smokers with lung adenocarcinoma carried more APOBEC-associated genomic alterations compared to non-smokers (22). Furthermore, a recently proteogenomic study investigating wild-type and mutant-EGFR lung cancer in Taiwan revealed that APOBEC mutagenesis potentially contributed to the early onset of non-smoking lung adenocarcinoma in females (33). The study also found that high APOBEC signature was associated with younger females harboring wild-type EGFR and smokers with EGFR mutations other than L858R and Ex19del tended to have low APOBEC signature (33). In our study, we found that the frequency of complex uncommon EGFR mutations was significantly higher in ever-smokers than in never-smokers (Table 4). Compared with the common EGFR mutation group, the percentage of ever-smokers was significantly higher in the complex common and uncommon mutation groups (Figure 2). Tumor mutation burden increased by smoking may partially explain these observations. However, whether APOBEC mutagenesis contributes to the development of complex uncommon EGFR mutations needs to be clarified.
Although several lines of evidence have verified the negative association between smoking status and the prevalence of common EGFR mutations, only limited investigations have confirmed the relationship between smoking behavior and uncommon EGFR mutation frequency. A European study by Lohinai et al. (34) reported that rare EGFR mutations were significantly correlated with smoking status. In their report, 23 of 33 patients with rare mutations and 14 of 16 patients with complex uncommon mutations were smokers. Two other European studies reported similar results from cohorts of 14 and 23 patients carrying rare mutations (35, 36). However, three Asian studies showed a higher percentage of smokers in patients with uncommon EGFR mutations than in those with common mutations without statistical significance, even though the number of cases was higher than that in the aforementioned European studies (13, 37, 38). The Asian ethnicity is also associated with a higher prevalence of EGFR mutations (18). The discrepancy between eastern and western data implies that smoking may play a crucial role in uncommon EGFR mutagenesis. In our analysis, we enrolled a relatively large population and observed a significant difference in smoking rates between patients with common and uncommon EGFR mutations (Table 1). We further found that, compared to never-smokers, ever-smokers had a significantly divergent uncommon EGFR mutation spectrum (Table 4) and identified that patients carrying complex uncommon EGFR mutations had the highest smoking rate among all subgroups (Figure 2). Our results validate the positive relationship between smoking status and uncommon EGFR mutations, and provide strong evidence that smoking contributes to the development of complex EGFR mutations.
In addition to exon 20 insertion and de novo T790M mutation, G719X, L861Q, and S768I are the most frequently detected uncommon EGFR mutations. In the subgroup of patients harboring G719X or L861Q, we observed that never-smokers had a significantly higher frequency of single uncommon mutations than ever-smokers. Since no complex common mutation with G719X or L861Q was detected, a positive correlation between smoking status and complex uncommon mutations in the subgroup with G719X or L861Q can be implied. In patients harboring S768I, the only complex uncommon mutation was G719X/S768I, and consistently, ever-smokers had a significantly higher frequency of G719X/S768I co-mutation than never-smokers. In other words, G719X/L861Q and G719X/S768I co-mutations were the most frequent complex uncommon mutations in patients carrying these three mutations, and ever-smokers had a significantly higher frequency of these two co-mutations than never-smokers (p = 0.004). G719X is a mutation in exon 18. Previous surveys of exon 18 have shown that these mutations are associated with current or former smokers (39, 40). E709X and exon 18 deletions are also exon 18 mutations. Our investigation identified that 7 out of 21 patients with E709X and 1 out of 3 patients with exon 18 were ever-smokers. These findings are consistent with previous studies. Furthermore, in patients with L861Q and S768I, we first disclosed the detailed mutation spectra and their relationship with smoking status. In patients with exon 20 insertion and de novo T790M mutations, a low percentage of ever-smokers was observed, similar to previous reports (39, 41). In particular, we identified a subgroup of patients carrying exon 20 R776H/C mutations. All of these were co-mutations, and L858R/R776H was the most common. The smoking rate was high since six of the 12 patients were ever-smokers. A report (42) showed that R776H/C is associated with a never-smoking history, which is different from our observations. Further studies are required to confirm this relationship. Taken together, our results imply that smoking may be important in the tumorigenesis of NSCLC harboring specific uncommon EGFR genotypes such as G719X, L861Q, and S768I co-mutations.
Female sex has also been reported to be an important factor associated with EGFR mutations (19). An international prospective trial demonstrated that female sex had a statistically significant association with a higher EGFR mutation rate in univariate analysis, but had no association when data were stratified by smoking status (18). Gender differences in tobacco smoking were also investigated. Generally, adult males smoked more cigarettes than adult females, especially in Asian countries (43, 44). Indeed, the smoking rate was significantly higher in males than females in both the common and uncommon EGFR mutation cohorts in our analysis (p < 0.001; data not illustrated). However, the divergences in the frequency of various types of uncommon EGFR mutations were only seen in distinct smoking statuses but not in sex (Table 5). Another Japanese study revealed that EGFR mutations were correlated with light smoking status and adenocarcinoma histology but not sex (45). The authors proposed that a higher percentage of adenocarcinoma in females may be the reason for their predominance in the EGFR mutation rate. It needs to be further clarified whether sex is a determinant for EGFR mutation prevalence.
The current study has several limitations. The first is its retrospective nature. Although this analysis was assessed through a multi-institutional database, potential biases, such as selection bias, could not be avoided. The second limitation was the underestimation of uncommon EGFR mutations. In our study, not all EGFR mutation detection methods used were PCR-direct sequencing. Mutant type-specific methods covered the majority of EGFR mutation genotypes; however, some uncommon EGFR mutations could not be detected. Third, no history of secondhand smoking was recorded in the medical database. Similar to first-hand smoking, second-hand smoking may have a great impact on EGFR mutagenesis and influence the frequency of EGFR mutations in never-smokers. Fourth, this study did not show the actual prevalence of uncommon EGFR mutations in ever- and never-smokers. We only focused on the 399 patients carrying uncommon EGFR mutations but did not collect data on smoking status or other epidemiological data of the entire population of 5,608 people. The comparison between ever- and never-smokers was restricted to uncommon EGFR mutations.
Conclusion
EGFR mutations are heterogeneous genetic alterations. NSCLC harboring common EGFR mutations may be distinct from the ones harboring uncommon mutations. Unlike the negative association between smoking and common EGFR mutations, our results demonstrate a positive correlation between smoking and uncommon EGFR mutations. Ever-smokers had complex uncommon EGFR mutations more frequently than never-smokers, such as G719X, L861Q, and S768I co-mutations. Our study suggests that smoking contributes to the development of complex EGFR mutations.
Data availability statement
The original contributions presented in the study are included in the article/supplementary materials. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Institutional Review Board of Chang Gung Memorial Hospital (No. 202200840B0). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
H-WK, T-YY, and S-CC initiated the study concept. H-WK, T-YY, and C-CW designed the study. H-WK, S-SS, C-LW, C-YL, C-HK, Y-CL, L-FL, C-TY, and C-CW contributed to the data acquisition. C-WW validated the pathology data. H-WK and C-TC performed the data analysis. H-WK and C-CW interpreted the data. H-WK contributed to the funding acquisition and drafted the manuscript. C-TY and C-CW supervised the study. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the grant MOST 108-2314-B-182A-131 from the Ministry of Science and Technology, Taiwan, and grants CMRPG3J1951 and CMRPG5L0171 from Linkou Chang Gung Memorial Hospital.
Acknowledgments
We thank the research assistant, Ms. Shin-Yu Huang, for this study. We also thank Editage, a division of Cactus Communications, for editorial assistance.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin (2022) 72:7–33. doi: 10.3322/caac.21708
2. Warren GW, Cummings KM. Tobacco and lung cancer: Risks, trends, and outcomes in patients with cancer. Am Soc Clin Oncol Educ Book (2013) 33:359–64. doi: 10.14694/EdBook_AM.2013.33.359
3. Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature (2018) 553:446–54. doi: 10.1038/nature25183
4. Graham RP, Treece AL, Lindeman NI, Vasalos P, Shan M, Jennings LJ, et al. Worldwide frequency of commonly detected EGFR mutations. Arch Pathol Lab Med (2018) 142:163–7. doi: 10.5858/arpa.2016-0579-CP
5. Recondo G, Facchinetti F, Olaussen KA, Besse B, Friboulet L. Making the first move in EGFR-driven or ALK-driven NSCLC: First-generation or next-generation TKI? Nat Rev Clin Oncol (2018) 15:694–708. doi: 10.1038/s41571-018-0081-4
6. Wu YL, Cheng Y, Zhou X, Lee KH, Nakagawa K, Niho S, et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): A randomised, open-label, phase 3 trial. Lancet Oncol (2017) 18:1454–66. doi: 10.1016/S1470-2045(17)30608-3
7. Ramalingam SS, Vansteenkiste J, Planchard D, Cho BC, Gray JE, Ohe Y, et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med (2020) 382:41–50. doi: 10.1056/NEJMoa1913662
8. Wu YL, Planchard D, Lu S, Sun H, Yamamoto N, Kim DW, et al. Pan-Asian adapted clinical practice guidelines for the management of patients with metastatic non-small-cell lung cancer: A CSCO-ESMO initiative endorsed by JSMO, KSMO, MOS, SSO and TOS. Ann Oncol (2019) 30:171–210. doi: 10.1093/annonc/mdy554
9. Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman JR, Bharat A, et al. Non-small cell lung cancer, version 3.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw (2022) 20:497–530. doi: 10.6004/jnccn.2022.0025
10. O'Kane GM, Bradbury PA, Feld R, Leighl NB, Liu G, Pisters KM, et al. Uncommon EGFR mutations in advanced non-small cell lung cancer. Lung Cancer (2017) 109:137–44. doi: 10.1016/j.lungcan.2017.04.016
11. John T, Taylor A, Wang H, Eichinger C, Freeman C, Ahn MJ. Uncommon EGFR mutations in non-small-cell lung cancer: A systematic literature review of prevalence and clinical outcomes. Cancer Epidemiol (2022) 76:102080. doi: 10.1016/j.canep.2021.102080
12. Zhang T, Wan B, Zhao Y, Li C, Liu H, Lv T, et al. Treatment of uncommon EGFR mutations in non-small cell lung cancer: New evidence and treatment. Transl Lung Cancer Res (2019) 8:302–16. doi: 10.21037/tlcr.2019.04.12
13. Chiu CH, Yang CT, Shih JY, Huang MS, Su WC, Lai RS, et al. Epidermal growth factor receptor tyrosine kinase inhibitor treatment response in advanced lung adenocarcinomas with G719X/L861Q/S768I mutations. J Thorac Oncol (2015) 10:793–9. doi: 10.1097/JTO.0000000000000504
14. Yang JC, Schuler M, Popat S, Miura S, Park K, Passaro A, et al. Afatinib for the treatment of non-small cell lung cancer harboring uncommon EGFR mutations: An updated database of 1023 cases brief report. Front Oncol (2022) 12:834704. doi: 10.3389/fonc.2022.834704
15. Attili I, Passaro A, Pisapia P, Malapelle U, de Marinis F. Uncommon EGFR compound mutations in non-small cell lung cancer (NSCLC): A systematic review of available evidence. Curr Oncol (2022) 29:255–66. doi: 10.3390/curroncol29010024
16. Zhang B, Wang S, Qian J, Yang W, Qian F, Lu J, et al. Complex epidermal growth factor receptor mutations and their responses to tyrosine kinase inhibitors in previously untreated advanced lung adenocarcinomas. Cancer (2018) 124:2399–406. doi: 10.1002/cncr.31329
17. Shigematsu H, Lin L, Takahashi T, Nomura M, Suzuki M, Wistuba II, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst (2005) 97:339–46. doi: 10.1093/jnci/dji055
18. Shi Y, Au JS, Thongprasert S, Srinivasan S, Tsai CM, Khoa MT, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol (2014) 9:154–62. doi: 10.1097/JTO.0000000000000033
19. Zhang YL, Yuan JQ, Wang KF, Fu XH, Han XR, Threapleton D, et al. The prevalence of EGFR mutation in patients with non-small cell lung cancer: a systematic review and meta-analysis. Oncotarget (2016) 7:78985–93. doi: 10.18632/oncotarget.12587
20. D'Angelo SP, Pietanza MC, Johnson ML, Riely GJ, Miller VA, Sima CS, et al. Incidence of EGFR exon 19 deletions and L858R in tumor specimens from men and cigarette smokers with lung adenocarcinomas. J Clin Oncol (2011) 29:2066–70. doi: 10.1200/JCO.2010.32.6181
21. Tseng JS, Wang CL, Yang TY, Chen CY, Yang CT, Chen KC, et al. Divergent epidermal growth factor receptor mutation patterns between smokers and non-smokers with lung adenocarcinoma. Lung Cancer (2015) 90:472–6. doi: 10.1016/j.lungcan.2015.09.024
22. Alexandrov LB, Ju YS, Haase K, Van Loo P, Martincorena I, Nik-Zainal S, et al. Mutational signatures associated with tobacco smoking in human cancer. Science (2016) 354:618–22. doi: 10.1126/science.aag0299
23. Mao L, Zhao W, Li X, Zhang S, Zhou C, Zhou D, et al. Mutation spectrum of EGFR from 21,324 Chinese patients with non-small cell lung cancer (NSCLC) successfully tested by multiple methods in a CAP-accredited laboratory. Pathol Oncol Res (2021) 27:602726. doi: 10.3389/pore.2021.602726
24. Shao SC, Chan YY, Kao Yang YH, Lin SJ, Hung MJ, Chien RN, et al. The Chang gung research database-a multi-institutional electronic medical records database for real-world epidemiological studies in Taiwan. Pharmacoepidemiol Drug Saf (2019) 28:593–600. doi: 10.1002/pds.4713
25. Tseng JS, Wang CL, Huang MS, Chen CY, Chang CY, Yang TY, et al. Impact of EGFR mutation detection methods on the efficacy of erlotinib in patients with advanced EGFR-wild type lung adenocarcinoma. PloS One (2014) 9:e107160. doi: 10.1371/journal.pone.0107160
26. Didelot A, Le Corre D, Luscan A, Cazes A, Pallier K, Emile JF, et al. Competitive allele specific TaqMan PCR for KRAS, BRAF and EGFR mutation detection in clinical formalin fixed paraffin embedded samples. Exp Mol Pathol (2012) 92:275–80. doi: 10.1016/j.yexmp.2012.03.001
27. Chang LC, Lim CK, Chang LY, Chen KY, Shih JY, Yu CJ. Non-small cell lung cancer harbouring non-resistant uncommon EGFR mutations: Mutation patterns, effectiveness of epidermal growth factor receptor-tyrosine kinase inhibitors and prognostic factors. Eur J Cancer (2019) 119:77–86. doi: 10.1016/j.ejca.2019.06.025
28. Pao W, Miller V, Zakowski M, Doherty J, Politi K, Sarkaria I, et al. EGF receptor gene mutations are common in lung cancers from "never smokers" and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci USA (2004) 101:13306–11. doi: 10.1073/pnas.0405220101
29. Pham D, Kris MG, Riely GJ, Sarkaria IS, McDonough T, Chuai S, et al. Use of cigarette-smoking history to estimate the likelihood of mutations in epidermal growth factor receptor gene exons 19 and 21 in lung adenocarcinomas. J Clin Oncol (2006) 24:1700–4. doi: 10.1200/JCO.2005.04.3224
30. Hecht SS. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat Rev Cancer (2003) 3:733–44. doi: 10.1038/nrc1190
31. Govindan R, Ding L, Griffith M, Subramanian J, Dees ND, Kanchi KL, et al. Genomic landscape of non-small cell lung cancer in smokers and never-smokers. Cell (2012) 150:1121–34. doi: 10.1016/j.cell.2012.08.024
32. Petljak M, Maciejowski J. Molecular origins of APOBEC-associated mutations in cancer. DNA Repair (Amst) (2020) 94:102905. doi: 10.1016/j.dnarep.2020.102905
33. Chen YJ, Roumeliotis TI, Chang YH, Chen CT, Han CL, Lin MH, et al. Proteogenomics of non-smoking lung cancer in East Asia delineates molecular signatures of pathogenesis and progression. Cell (2020) 182(1):226–44.e17. doi: 10.1016/j.cell.2020.06.012
34. Lohinai Z, Hoda MA, Fabian K, Ostoros G, Raso E, Barbai T, et al. Distinct epidemiology and clinical consequence of classic versus rare EGFR mutations in lung adenocarcinoma. J Thorac Oncol (2015) 10:738–46. doi: 10.1097/JTO.0000000000000492
35. Pallis AG, Voutsina A, Kalikaki A, Souglakos J, Briasoulis E, Murray S, et al. 'Classical' but not 'other' mutations of EGFR kinase domain are associated with clinical outcome in gefitinib-treated patients with non-small cell lung cancer. Br J Cancer (2007) 97:1560–6. doi: 10.1038/sj.bjc.6604068
36. Kauffmann-Guerrero D, Huber RM, Reu S, Tufman A, Mertsch P, Syunyaeva Z, et al. NSCLC patients harbouring rare or complex EGFR mutations are more often smokers and might not benefit from first-line tyrosine kinase inhibitor therapy. Respiration (2018) 95:169–76. doi: 10.1159/000484175
37. Wu JY, Yu CJ, Chang YC, Yang CH, Shih JY, Yang PC. Effectiveness of tyrosine kinase inhibitors on "uncommon" epidermal growth factor receptor mutations of unknown clinical significance in non-small cell lung cancer. Clin Cancer Res (2011) 17:3812–21. doi: 10.1158/1078-0432.CCR-10-3408
38. Keam B, Kim DW, Park JH, Lee JO, Kim TM, Lee SH, et al. Rare and complex mutations of epidermal growth factor receptor, and efficacy of tyrosine kinase inhibitor in patients with non-small cell lung cancer. Int J Clin Oncol (2014) 19:594–600. doi: 10.1007/s10147-013-0602-1
39. Beau-Faller M, Prim N, Ruppert AM, Nanni-Metellus I, Lacave R, Lacroix L, et al. Rare EGFR exon 18 and exon 20 mutations in non-small-cell lung cancer on 10 117 patients: A multicentre observational study by the French ERMETIC-IFCT network. Ann Oncol (2014) 25:126–31. doi: 10.1093/annonc/mdt418
40. Klughammer B, Brugger W, Cappuzzo F, Ciuleanu T, Mok T, Reck M, et al. Examining treatment outcomes with erlotinib in patients with advanced non-small cell lung cancer whose tumors harbor uncommon EGFR mutations. J Thorac Oncol (2016) 11:545–55. doi: 10.1016/j.jtho.2015.12.107
41. Li H, Hu H, Wang R, Pan Y, Wang L, Li Y, et al. Primary concomitant EGFR T790M mutation predicted worse prognosis in non-small cell lung cancer patients. Onco Targets Ther (2014) 7:513–24. doi: 10.2147/OTT.S60122
42. Ruan Z, Kannan N. Mechanistic insights into R776H mediated activation of epidermal growth factor receptor kinase. Biochemistry (2015) 54:4216–25. doi: 10.1021/acs.biochem.5b00444
43. Jamal A, King BA, Neff LJ, Whitmill J, Babb SD, Graffunder CM. Current cigarette smoking among adults - united states, 2005-2015. MMWR Morb Mortal Wkly Rep (2016) 65:1205–11. doi: 10.15585/mmwr.mm6544a2
44. Yang JJ, Yu D, Wen W, Shu XO, Saito E, Rahman S, et al. Tobacco smoking and mortality in Asia: A pooled meta-analysis. JAMA Netw Open (2019) 2:e191474. doi: 10.1001/jamanetworkopen.2019.1474
Keywords: smoking, epidermal growth factor receptor (EGFR), non-small cell lung cancer (NSCLC), uncommon EGFR mutation, complex EGFR mutation
Citation: Ko H-W, Shie S-S, Wang C-W, Chiu C-T, Wang C-L, Yang T-Y, Chou S-C, Liu C-Y, Kuo C-HS, Lin Y-C, Li L-F, Yang C-T and Wang C-C (2022) Association of smoking status with non-small cell lung cancer patients harboring uncommon epidermal growth factor receptor mutation. Front. Immunol. 13:1011092. doi: 10.3389/fimmu.2022.1011092
Received: 03 August 2022; Accepted: 05 October 2022;
Published: 20 October 2022.
Edited by:
Joanna Julia Domagala-Kulawik, Medical University of Warsaw, PolandReviewed by:
Maria Anna Smolle, Medical University of Graz, AustriaKatharina Leithner, Medical University of Graz, Austria
Copyright © 2022 Ko, Shie, Wang, Chiu, Wang, Yang, Chou, Liu, Kuo, Lin, Li, Yang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chin-Chou Wang, ccwang5202@yahoo.com.tw
 How-Wen Ko
How-Wen Ko Shian-Sen Shie2
Shian-Sen Shie2 Chih-Hsi Scott Kuo
Chih-Hsi Scott Kuo Yu-Ching Lin
Yu-Ching Lin Chin-Chou Wang
Chin-Chou Wang