- Center for Infectious Diseases, Beijing Youan Hospital, Capital Medical University, Beijing, China
Background: There is conflicting evidence about whether a double dose of the hepatitis B virus (HBV) vaccine induces better immunity than the standard-dose vaccine for people living with HIV (PLWH). This study provides a meta-analysis that summarizes the efficacy of HBV vaccine regimens among HIV-infected patients, clarifying the role of particular factors such as dose and frequency of vaccination in vaccine responsiveness and highlighting the need for evidence-based practice to assess HBV vaccination among PLWH.
Methods: Randomized clinical trials (RCTs) and prospective studies reporting vaccination response rates among PLWH were found through a search of PubMed, Cochrane, and the Web of Science. The key outcome was vaccine response. A random-effects model was used to estimate the pooled response rate. Subgroup analysis was conducted to evaluate key factors and explore sources of heterogeneity. Possible biases were assessed using quality and publication bias assessment.
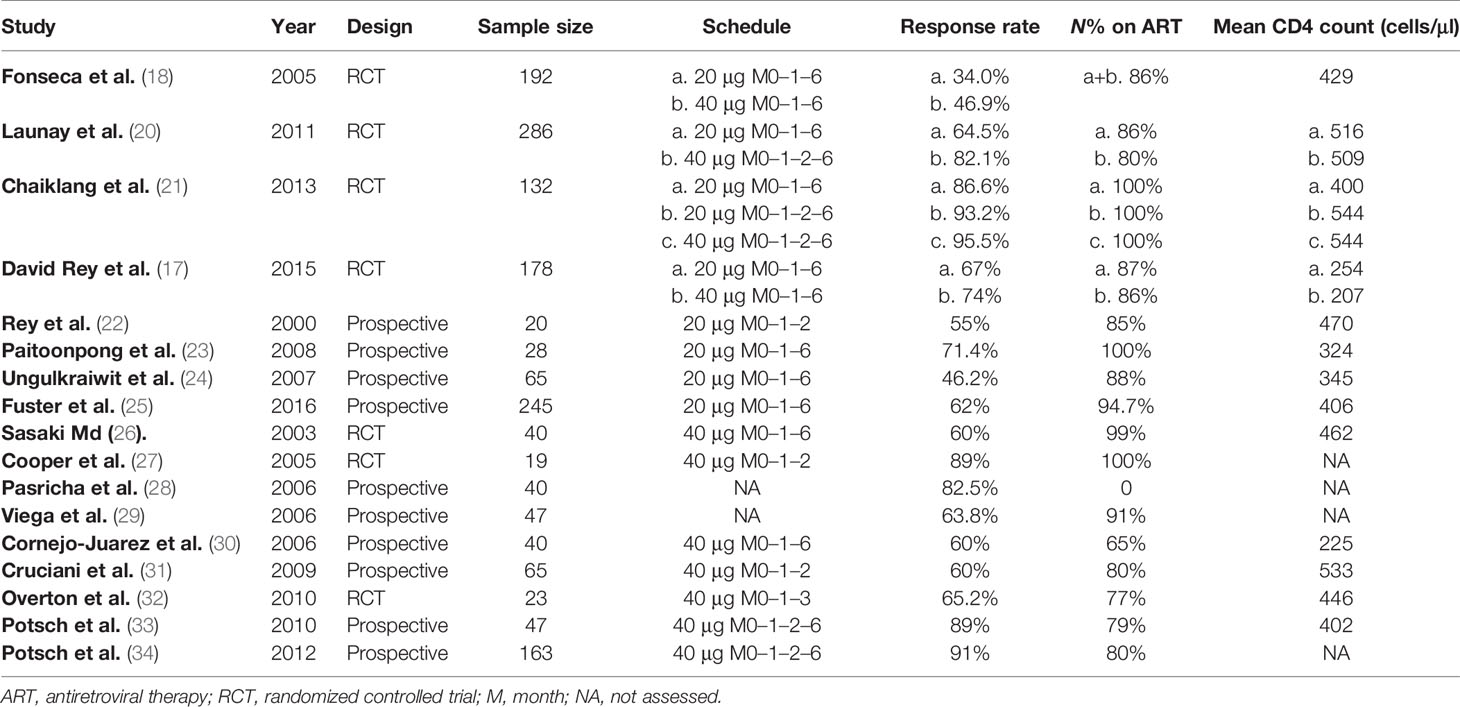
Results: Eligible studies included controlled trials that examined the effects of 17 interventional studies with 1,821 participants. Among PLWH who received the HBV vaccine, the pooled response rate of HBV vaccination was 71.5% (95% CI 64.0%–77.9%, p < 0.001). Compared with the standard dose (65.5%, 95% CI 53.1%–76.1%), the double dose (75.2%, 95% CI 66.2%–82.5%) was associated with a better response rate [Q(1) = 19.617, p < 0.001]. When stratified by schedule, the four-dose schedule (89.7%, 95% CI 83.1%–93.9%) had a higher response rate than the three-dose schedule (63.3%, 95% CI 56.6%–69.4%) and the difference was significant [Q(1) = 88.305, p < 0.001]. PLWH with higher CD4+ T-cell counts (>500 cells/mm3) at the time of vaccination had better response rates [Q(1) = 88.305, p < 0.001].
Conclusions: In this meta-analysis, the double dose of the HBV vaccine and multiple injections were associated with better immune responses than the standard HBV vaccine regimen in PLWH. Higher seroconversion rates were observed in PLWH with high CD4+ T-cell levels, indicating that individuals infected with HIV should receive the HBV vaccine as soon as possible after diagnosis.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/.
Introduction
Given that human immunodeficiency virus (HIV) and hepatitis B virus (HBV) share similar routes of transmission, coinfection with the two viruses is common (1). Globally, almost 10% of people living with HIV (PLWH) are infected with HBV (2, 3). HBV-related liver disease affects the life expectancy of PLWH. In individuals infected with HIV, HBV more often becomes a chronic infection, with patients more likely to experience progression to cirrhosis, hepatocellular carcinoma (4, 5), and liver failure (6, 7). While antiretroviral therapy (ART) has led to a decline in AIDS-related mortality, liver disease remains a major cause of morbidity and mortality in PLWH (8–10).
International guidelines highly recommend HBV immunization (11, 12). HBV infection has declined with the popularization of HBV vaccines, especially as current A2 recombinant HBV vaccines show cross-reactivity and cross-protection against the non-A2 HBV genotype (13). U.S. and British guidelines specify that PLWH should also be vaccinated for HBV. HBV vaccine responsiveness is measured by assessing seroconversion after vaccination, with HBV surface antibody [anti-HBs] ≥10 IU/L measured at 4–8 weeks after the last dose defined as a positive response. While the HBV vaccine has been certified by the CDC as an effective way to prevent HBV infection (14), PLWH often have a lower seroresponse after HBV vaccination than HIV-negative individuals. Indeed, immune responses to most vaccines are impaired in PLWH (15, 16). Several studies have worked on improving the response rate by changing the inoculation time and dose of the vaccine. While there is no consensus on an appropriate HBV vaccination schedule for PLWH, high-dose vaccine schedules may induce stronger response rates against HBV (17–20).
Several questions remain about the appropriate HBV vaccine schedule and dose for PLWH. This is in part because the number of PLWH who are susceptible to vaccine-preventable infectious diseases remains unknown and historic data from the pre-ART era may skew findings. Effective ART can increase CD4+ T-cell counts in PLWH, which strengthens both humoral and cellular immunity. The meta-analysis presented here summarizes the efficacy of HBV vaccines among PLWH. A subgroup analysis is conducted based on PLWH vaccination and treatment background, clarifying response rates and associated factors of the HBV vaccine among PLWH and providing evidence-based prevention recommendations.
Methods
This review was registered in the International Prospective Register of Systematic Reviews (PROSPERO, CRD42018081009) and is reported fully in line with the Preferred Reporting Items for Systematic Reviews and Meta-analysis.
Data Sources and Search Strategy
A comprehensive search of articles published in PubMed, Cochrane, and the Web of Science during January 2000 to April 2021 was conducted. Additional searches were conducted in Google Scholar and ClinicalTrials.gov. Keywords represented the intersection of HIV-related terms (HIV OR AIDS) and HBV-related terms (HBV vaccine OR vaccination). The reference lists of the included studies were also screened to ensure that no studies were omitted. Only peer-reviewed articles written in English were included.
Study Selection and Data Extraction
Search results were initially imported into Endnote X8 to exclude duplicates. All titles and abstracts were screened by two authors to narrow the scope. Two investigators selected the remaining articles independently by full-text assessment if they 1) included adult HIV-positive patients, 2) evaluated the efficacy of the HBV vaccine, 3) provided vaccination at doses of 20 or 40 μg, and 4) provided sufficient data to calculate the effect size. Studies were excluded if 1) patients were positive for one of the HBV serological markers, 2) they were retrospective studies or case reports, and 3) they were not written in the English language.
Two authors independently extracted information using an Excel spreadsheet. The key outcome was vaccine response. Other information, such as study design, year of publication, author, sample size, sex distribution, CD4+ T-cell count schedule, and dose of HBV vaccine, was also extracted from the articles. Disagreements during the process were solved through negotiation.
Frequency of vaccination and dose of vaccination were defined to avoid ambiguous discussion. The “three-dose schedule” was defined as three intramuscular injections of HBV vaccine at months 0, 1, and 2 (or 6). The “four-dose schedule” was defined as four intramuscular injections at months 0, 1, 2, and 3 (or 6). Besides, standard dose (20 μg) and double dose (40 μg) were involved.
Statistical Analysis
Comprehensive Meta-Analysis (CMA) Version 2.0 was used to conduct single rate and subgroup analysis. Combined event rate (ER) was calculated to measure the vaccine response rate among PLWH. A random-effects model was adopted using the ER to estimate the vaccine response. The I2 statistic was used to assess heterogeneity across studies. Potential publication bias across included studies was measured using the Egger’s regression test and adjusted with trim-and-fill. For categorical moderators, subgroup analysis was also performed according to the HBV vaccine schedule (three-dose or four-dose), dose (standard dose or double dose), and CD4+ T-cell count (<500 or ≥500 cells/mm3). p <0.05 was considered statistically significant.
Quality Assessment of Individual Studies
As there are prospective cohort and RCT studies included, we referred to the Newcastle-Ottawa quality assessment scale (NOS) and Cochrane Collaboration’s tool. The NOS evaluated the quality of prospective cohort studies with eight items from three areas: sample selection, comparability of cohorts, and outcome assessment. RCT studies were assessed for selection bias, performance bias, detection bias, attrition bias, reporting bias, and other biases.
Results
Characteristics of the Included Studies
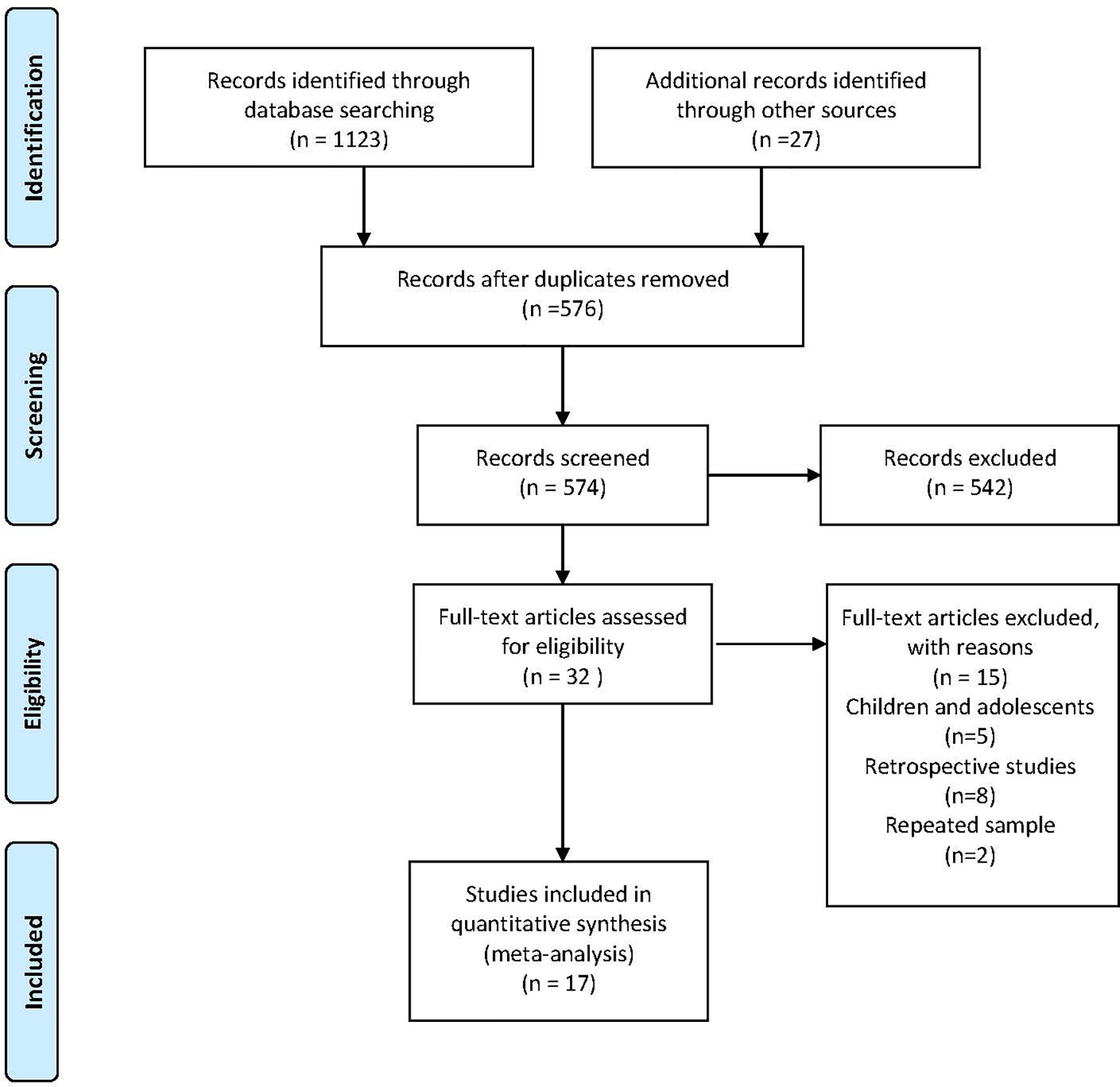
Our search yielded 1,123 studies and 27 were identified by checking references and conferences. After discarding duplicates and screening the titles and abstracts, 32 articles remained for full-text assessment. Seventeen eligible studies consisting of 1,821 participants, with sample sizes ranging from 20 to 286, were included in the meta-analysis. The flow diagram of study selection is shown in Figure 1. The age of the patients ranged from 18 to 77 years old, and approximately 36.2% were female. Table 1 summarizes the characteristics of the included studies which varied in study design, schedule, dose, comparison, CD4+ T-cell count, and HBV vaccine regimen. Seven studies were RCTs (17, 18, 20, 21, 26, 27, 32), while the remaining were prospective observational studies (22–25, 28–31, 33, 34). More than 80% (and sometimes even 100%) of the participants were on ART in the included studies. Of the 4 four-dose studies, all participants received the HBV vaccine at months 0, 1, 2, and 6 (20, 21, 33, 34). Unlike the four-dose regimen studies, the three-dose regimen studies differed in the time interval of the three injections. Eleven included studies with participants receiving the M0–1–6 regimen, three included studies with patients receiving the M0–1–2 regimen (22, 27, 31), and one included patients receiving the M0–1–3 regimen (32).
Three RCTs showed high risk in the process of random sequence generation and two showed attrition bias and reporting bias, respectively. Two cohort studies included different populations in the two groups and one study did not assess potential confounding factors. In addition, four prospective cohort studies were single-arm studies and lacked information about non-exposed patients and comparability. Details of quality assessment of individual studies are shown in Supplementary Tables S1, S2.
Vaccine Response
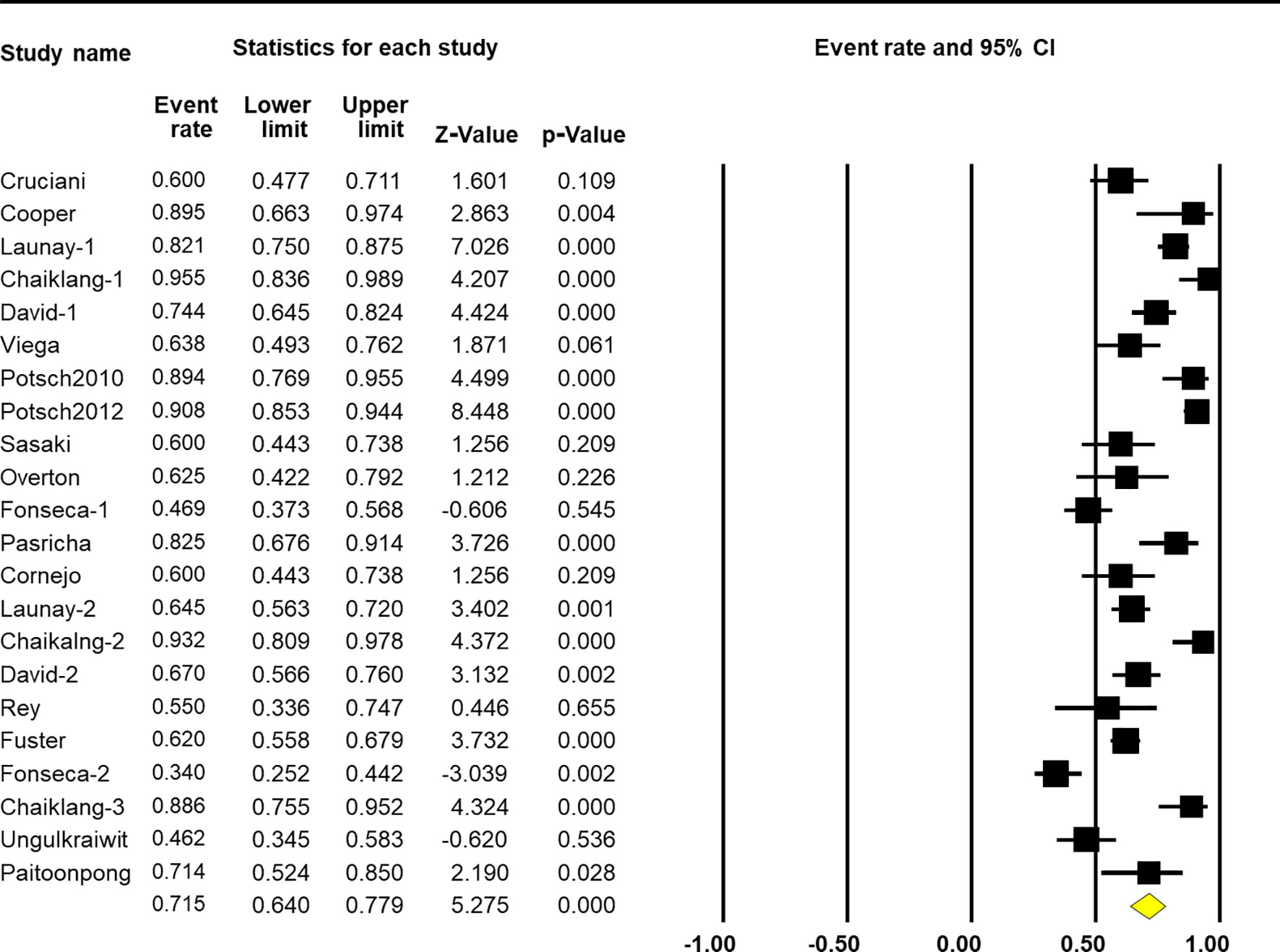
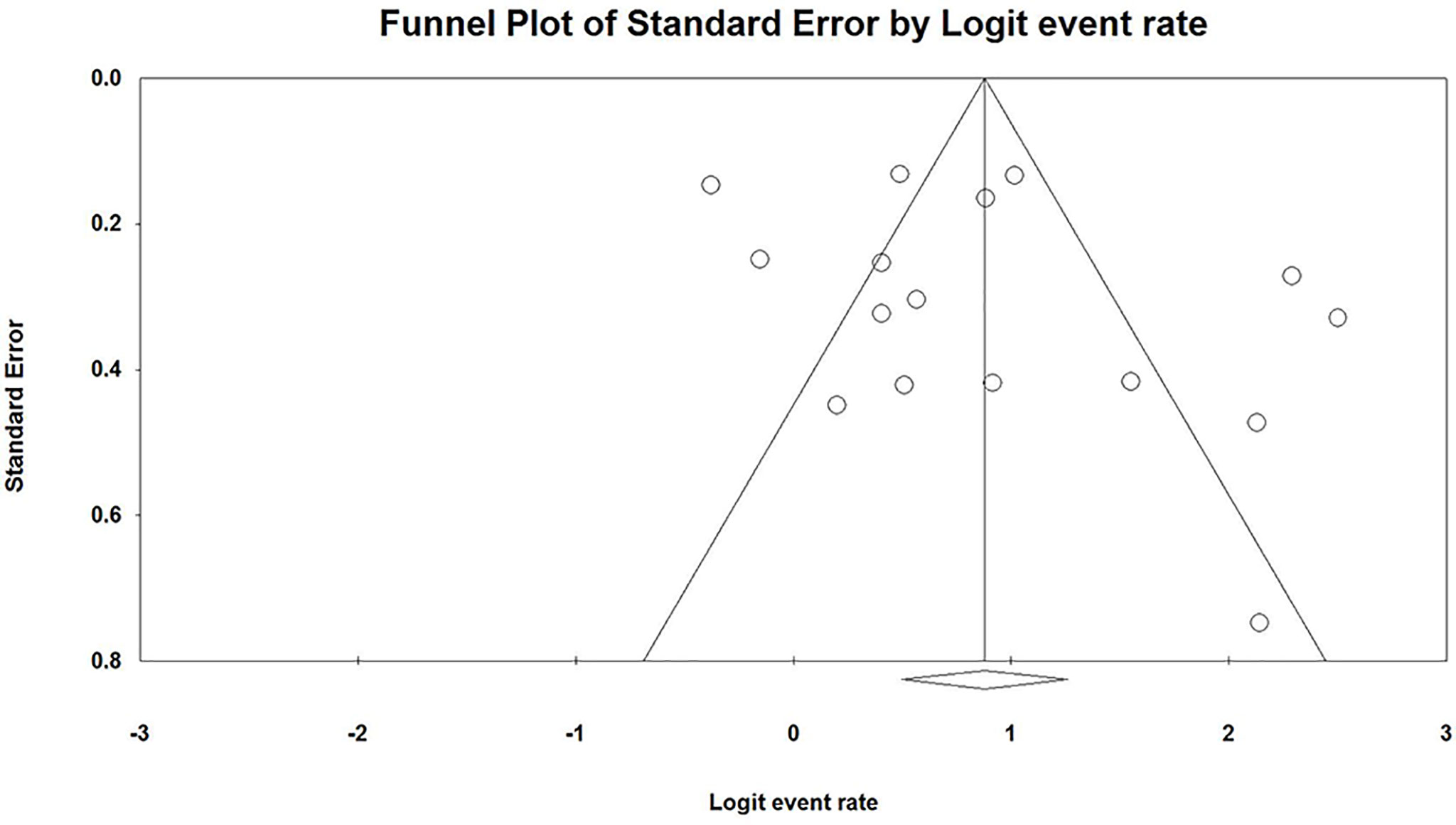
The pooled response rate of the HBV vaccine was 71.5% (95% CI 64.0%–77.9%, p < 0.001, Figure 2). However, there was significant heterogeneity across individual studies [Q(22) = 165.005, p < 0.001, I2 = 86.7%]. Egger’s regression showed no publication bias across studies with p-values of 0.096 and 0.191 in one-tailed and two-tailed analyses, respectively. The funnel plots are shown in Figure 3.
Factors Associated With Response Rate
When stratified by vaccine dose, results from the subgroup analysis showed a significant difference across groups [Q(1) = 19.617, p < 0.001, Table 2]. Nine arms reported the effect of HBV vaccination at a standard dose (20 μg), so the combined ER of the vaccine response rate was 65.5% (95% CI 53.1%–76.1%, p = 0.015, Figure 4). Thirteen arms reported the effect of HBV vaccination at a double dose (40 μg), so the combined ER of the response rate was 75.2% (95% CI 66.2%–82.5%, p < 0.001, Figure 4).
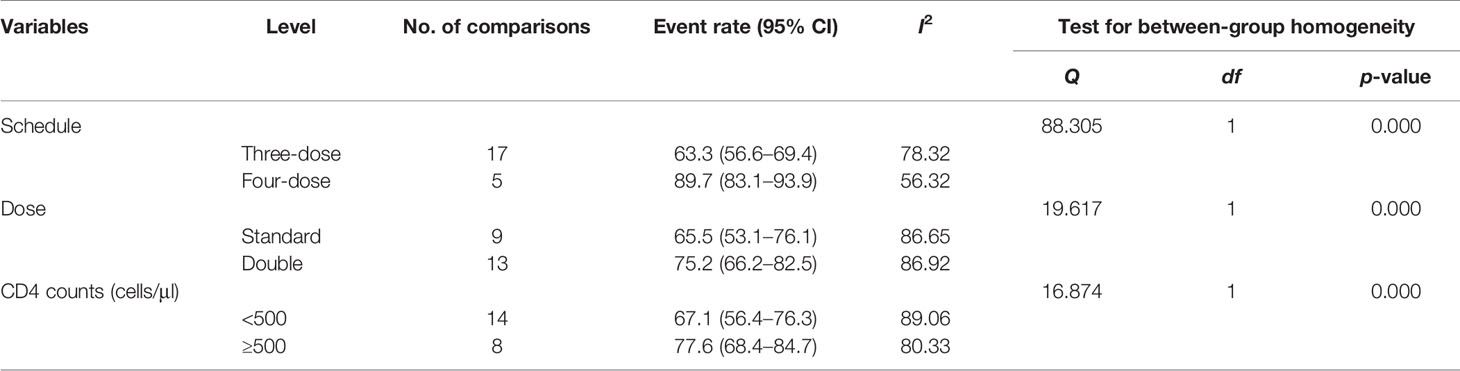
Table 2 Meta-analysis results assessing the efficacy of HBV vaccination in response rates across subgroups.
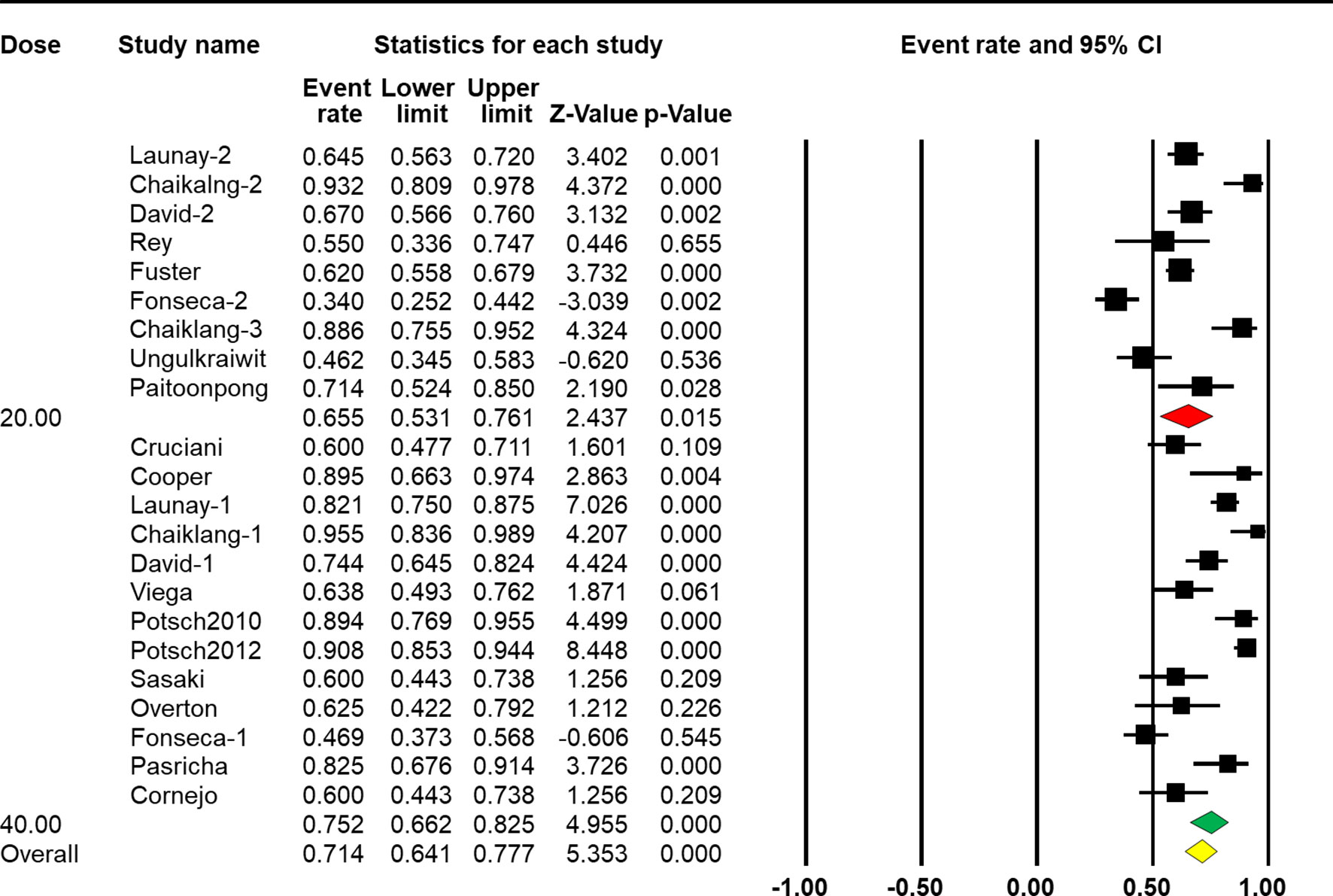
Figure 4 Efficacy of HBV vaccination at different doses (standard dose vs. double dose) on the response rate.
The four-dose schedule showed a higher response rate than the three-dose schedule [Q(1) = 88.305, p < 0.001, Table 2]. Seventeen arms reported the effect of HBV vaccination with three intramuscular injections, and the combined vaccine rate was 63.3% (95% CI 56.6%–69.4%, p < 0.001, Figure 5). From five arms, the combined response rate of HBV vaccination with four intramuscular injections was 89.7% (95% CI 83.1%–93.9%, p < 0.001, Figure 5).
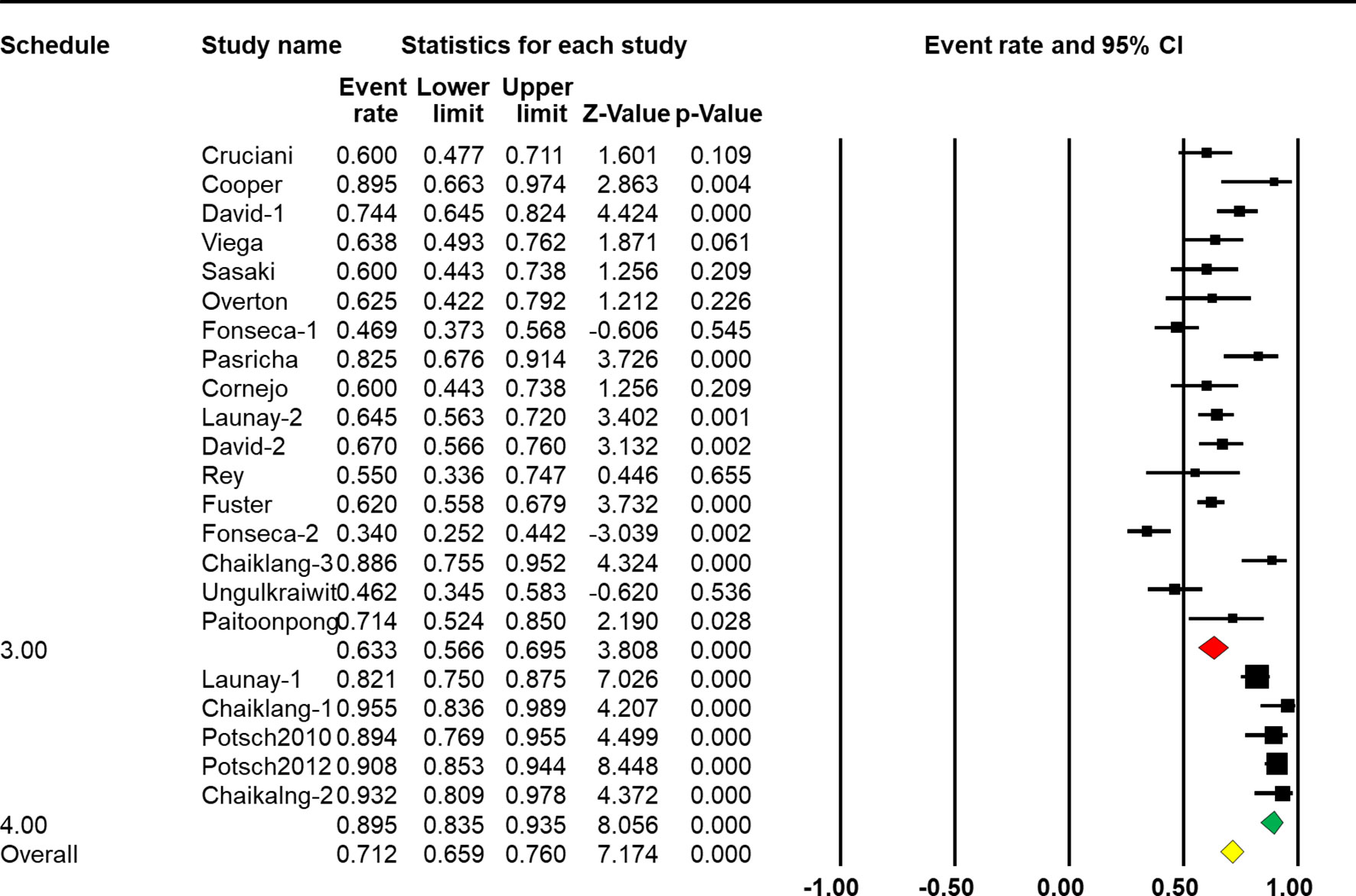
Figure 5 Efficacy of HBV vaccination with different schedules (three-dose vs. four-dose) on the response rate.
Subgroup analysis based on CD4+ T-cell count showed that a higher CD4+ T-cell count was associated with a better response rate [Q(1) = 88.305, p < 0.001, Table 2]. Eight arms reported the effect of HBV vaccination with more than 500 cells/mm3 and the combined ER of the response rate was 77.6% (95% CI 68.4%–84.7%, p < 0.001, Figure 6). Fourteen arms reported the effect of HBV vaccination with less than 500 cells/mm3 and the combined ER of the response rate was 67.1% (95% CI 56.4%–76.3%, p = 0.002, Figure 6).
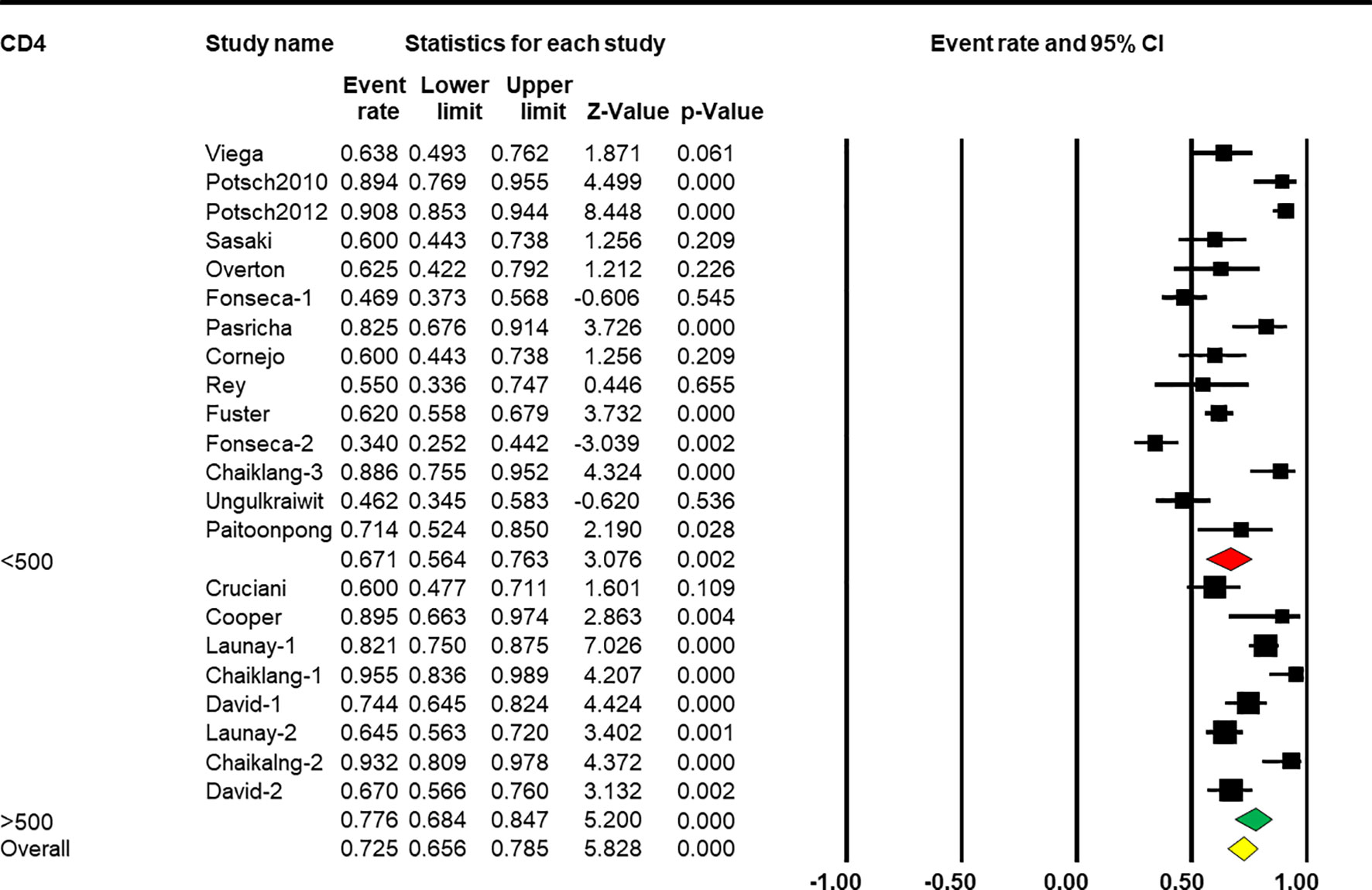
Figure 6 Efficacy of HBV vaccination with different CD4 stratification (<500 vs. ≥500 cells/mm3) on the response rate.
Discussion
The aim of this meta-analysis was to pool available data on the response rates of HBV vaccines in PLWH. To provide credible evidence, only RCT and prospective studies were included. The findings indicated that both a double dose of the HBV vaccine and the four-dose schedule were associated with a better immune response than the standard HBV vaccine regimen in PLWH. In addition, higher seroconversion rates were observed in PLWH with CD4+ T-cell levels >500 cells/mm3.
By analyzing 17 studies that met the inclusion criteria during 2000 to 2016, the pooled response rate of HBV vaccination for PLWH was 71.5% (95% CI 64.0%–77.9%, p < 0.001, Figure 2). Using the “classic schedule” (20 μg of HBsAg at months 0–1–6), PLWH response rates to the vaccine were lower than those of non-HIV-infected people (20%–70% vs. 90%–95%) (22, 35–38). According to a previous study, high plasma viral load, prevaccination total serum IgG, and elevated prevaccination IgG1 among PLWH were associated with poor vaccine response (38).
The double-dose HBV vaccine regimen had a significantly higher response rate than the standard-dose regimen (75.2% and 65.5%, respectively; p < 0.001). Several studies (20, 33, 34, 39) have shown that PLWH who have never received the HBV vaccine had higher response rates after the double-dose vaccination. Moreover, two meta-analyses found that a higher dose vaccination could improve the anti-HB immune reaction in previously unvaccinated patients and could ultimately result in a high response rate (OR 1.96, 95% CI 1.47–2.61) (19, 40). Beginning in 2013, the British HIV Association recommended 40-μg doses of HBV vaccine for primary vaccination and revaccination of PLWH (41). In some studies, double-dose groups had higher and more long-lived anti-HB titers, which warrants additional study. However, other studies indicated that there is no relationship between vaccine dose and level of anti-HB response (17, 18, 21). In an open-label RCT, a total of 178 PLWH were randomized to receive either standard-dose (20 mg, three-dose) or double-dose (40 mg, three-dose) HBV vaccines at months 0, 1, and 6. A similar response was seen between the standard-dose and double-dose groups (67% vs. 74%, p > 0.05) (17). Another RCT by Fonseca et al. reported that seroconversion in response to 20 and 40 mg of the three-dose regimen among PLWH were 34% and 47%, respectively, at week 28. However, this difference was not significant (p > 0.05) (18).
In addition to double dose, several studies have shown that increased HBV vaccine schedules are more effective in PLWH. Launay et al. (20) proved that PLWH vaccinated with a four-double-dose regimen had higher anti-HBV titers and stronger immune responses than those vaccinated with the standard three-dose regimen (82% vs. 65%, p < 0.05). Chaiklang et al. compared the immunogenicity and safety of three-standard dose and four-double dose vs. four-standard dose in an RCT (21). Higher response rates were not observed in the double-dose or four-double-dose groups. This may have been impacted by low CD4+ T cell counts, high viral load of the participants, and small sample size. The meta-analysis described here showed a similar result. However, the subgroup analysis did not include the time interval of the three-dose regimen studies because the number of studies including three subgroups varied. Further studies are needed to explore the relationship between immune response and time interval for three- or four-dose HBV vaccination regimens in PLWH.
Several studies have found that the response to HBV vaccination is significantly associated with undetectable virus load (29, 42) and high or even close to normal CD4+ T-cell counts (18, 22, 29) in PLWH. Veiga et al. (29) found that vaccine responders had higher CD4+ T-cell numbers than non-responders (452 vs. 359 cells/mm3). The results shown in this meta-analysis were similar. PLWH with higher CD4+ T-cell counts had a stronger response to HBV vaccination than those with low CD4+ T-cell counts, especially those with a baseline CD4+ T-cell count >500 cells/mm3 (p < 0.001). It is suggested that vaccination as early as possible after diagnosis with high CD4+ T-cell counts is necessary for PLWH. However, according to the CDC, vaccination should not be deferred until CD4+ T-cell counts return to a certain level because patients with low CD4+ T-cell counts can still mount a response to the HBV vaccine (43). The lack of response to the HBV vaccine is attributed to a variety of immunological mechanisms, including antigen presentation of the peptide-based vaccine to T cells as well as B-cell activity. The functions of T and B cells in HBV vaccine non-responders are complicated. S gene escape mutants created after HBV vaccination may change host susceptibility or lead to vaccine escape from immune attack, as shown previously (44).
Immune responses are lower following HBV vaccination of PLWH than individuals without HIV infection. For PLWH, achieving a durable and protective level of immunity remains a challenge, especially for those with detectable HIV RNA or low CD4+ T-cell counts at the time of vaccination. For multiple reasons, completing and maintaining adequate HBV immunity in HIV-infected individuals is complex, and new strategies have emerged to overcome these barriers (45). Some specialists suggest waiting for HIV viral suppression and immune reconstitution before vaccination (30, 33). One study recommended a second vaccine series for PLWH with high CD4+ T-cell counts and undetectable HIV RNA who do not respond to HBV vaccination (17). The benefits of double dosage and increased dose frequency remain controversial. In this meta-analysis, results indicated that a higher vaccine dose, higher CD4+ T-cell levels, and multiple injections are associated with a better response to HBV vaccination in PLWH. In patients with lower CD4+ T-cell counts, higher HBV vaccine doses or increased dose frequency may be warranted to accentuate the immune response, and in patients with higher CD4+ T-cell counts, standard HBV vaccination should be performed as soon as possible after diagnosis. Importantly, the role of ART in vaccination is not evaluated in our study and there is only one study conducted in treatment-naive PLWH (28). Data showed that even after a double dose of vaccine, HBsAb titers were much lower in HIV-positive patients than HIV-negative adults.
This study has some limitations. The HIV patients included were from many different regions, including the Americas (USA 2, Brazil 5, Mexico 1, and Chile 1), Europe (France 3 and Italy 1), and East Asia and Southeast Asia (India 1 and Thailand 3). However, studies from the country with the highest HBsAg prevalence, Africa (46), are absent. The heterogeneity of this analysis was significant (I2 > 50%) and the observed differences may be due to differences in sample size, demographics, and CD4+ T-cell counts or inadequate statistical power. The results of this study are credible but need to be interpreted with caution.
Besides, the funnel plot showed that some studies are outside the scope of the confidence interval (triangle jurisdiction). The scattered funnel plot also revealed significant heterogeneity in our study. However, the random-effect model and the performed subgroup analysis were adopted to reduce the effect of heterogeneity. The asymmetric funnel plot also suggested that there was possible publication bias; however, we conducted a comprehensive search to make sure no study was omitted.
Conclusion
PLWH have significantly lower initial response rates after HBV vaccination than immunocompetent individuals. Additional vaccine doses and other methods for strengthening immunity should be considered for PLWH. This meta-analysis indicated that there were higher seroprotection rates to HBV vaccine in response to double-dose vaccination, increased dose frequency, and higher baseline CD4+ T-cell counts. To obtain the best response to hepatitis B vaccine in PLWH, additional large-scale studies that explore the role of other potential factors should be conducted in the future.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
XH, CG, and HW conceptualized the study and developed the research protocol. YT, WH, and YW identified articles for full-text review and extracted data that matched the inclusion criteria. YT performed the statistical analyses. All authors contributed to the writing of the manuscript. XH, TZ, and WW polished and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Science and Technology Major Project of China During the 13th Five-year Plan Period (2017ZX10201101, 2018ZX10715-005-002-002), the Beijing Excellent Talent Plan (2018000021223ZK04), the Beijing Talent Project in the New Millennium (2020A35), and Beijing Hospitals Authority “Peak Climbing” Planning (DFL20191701).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We gratefully acknowledge the help provided by Jianhua Hou for his valuable suggestions and discussions.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2021.745541/full#supplementary-material
References
1. Parikh N, Nonnemacher MR, Pirrone V, Block T, Mehta A, Wigdahl B. Substance Abuse, HIV-1 and Hepatitis. Curr HIV Res (2012) 10(7):557–71. doi: 10.2174/157016212803306023
2. Thio CL. Hepatitis B and Human Immunodeficiency Virus Coinfection. Hepatology (2009) 49(5 Suppl):S138–45. doi: 10.1002/hep.22883
3. Kourtis AP, Bulterys M, Hu DJ, Jamieson DJ. HIV-HBV Coinfection--A Global Challenge. N Engl J Med (2012) 366(19):1749–52. doi: 10.1056/NEJMp1201796
4. Sterling RK, Wahed AS, King WC, Kleiner DE, Khalili M, Sulkowski M, et al. Spectrum of Liver Disease in Hepatitis B Virus (HBV) Patients Co-Infected With Human Immunodeficiency Virus (HIV): Results of the HBV-HIV Cohort Study. Am J Gastroenterol (2019) 114(5):746–57. doi: 10.1038/s41395-018-0409-9
5. Colin JF, Cazals-Hatem D, Loriot MA, Martinot-Peignoux M, Pham BN, Auperin A, et al. Influence of Human Immunodeficiency Virus Infection on Chronic Hepatitis B in Homosexual Men. Hepatol (Baltimore Md) (1999) 29(4):1306–10. doi: 10.1002/hep.510290447
6. Laurence JC. Hepatitis A and B Immunizations of Individuals Infected With Human Immunodeficiency Virus. Am J Med (2005) 118 Suppl 10A:75S–83S. doi: 10.1016/j.amjmed.2005.07.024
7. Thio CL, Seaberg EC, Skolasky R Jr., Phair J, Visscher B, Muñoz A, et al. HIV-1, Hepatitis B Virus, and Risk of Liver-Related Mortality in the Multicenter Cohort Study (MACS). Lancet (Lond Engl) (2002) 360(9349):1921–6. doi: 10.1016/s0140-6736(02)11913-1
8. Lewden C, Salmon D, Morlat P, Bevilacqua S, Jougla E, Bonnet F, et al. Causes of Death Among Human Immunodeficiency Virus (HIV)-Infected Adults in the Era of Potent Antiretroviral Therapy: Emerging Role of Hepatitis and Cancers, Persistent Role of AIDS. Int J Epidemiol (2005) 34(1):121–30. doi: 10.1093/ije/dyh307
9. Lewden C, May T, Rosenthal E, Burty C, Bonnet F, Costagliola D, et al. Changes in Causes of Death Among Adults Infected by HIV Between 2000 and 2005: The “Mortalite 2000 and 2005” Surveys (ANRS EN19 and Mortavic). J Acquir Immune Defic Syndr (2008) 48(5):590–8. doi: 10.1097/QAI.0b013e31817efb54
10. Palella FJ Jr., Baker RK, Moorman AC, Chmiel JS, Wood KC, Brooks JT, et al. Mortality in the Highly Active Antiretroviral Therapy Era: Changing Causes of Death and Disease in the HIV Outpatient Study. J Acquir Immune Defic Syndr (2006) 43(1):27–34. doi: 10.1097/01.qai.0000233310.90484.16
11. Kaplan JE, Benson C, Holmes KK, Brooks JT, Pau A, Masur H. Guidelines for Prevention and Treatment of Opportunistic Infections in HIV-Infected Adults and Adolescents: Recommendations From CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recommend Rep: Morbid Mortal Weekly Rep Recommend Rep (2009) 58(Rr-4):1–207; quiz CE1-4.
12. Wilkins E, Nelson M, Agarwal K, Awoyemi D, Barnes E, Bhagani S, et al. British HIV Association Guidelines for the Management of Hepatitis Viruses in Adults Infected With HIV 2013. HIV Med (2013) 14 Suppl 4:1–71. doi: 10.1111/hiv.12106
13. Cassidy A, Mossman S, Olivieri A, De Ridder M, Leroux-Roels G. Hepatitis B Vaccine Effectiveness in the Face of Global HBV Genotype Diversity. Expert Rev Vaccines (2011) 10(12):1709–15. doi: 10.1586/erv.11.151
14. Schillie S, Vellozzi C, Reingold A, Harris A, Haber P, Ward JW, et al. Prevention of Hepatitis B Virus Infection in the United States: Recommendations of the Advisory Committee on Immunization Practices. MMWR Recommend Rep: Morbid Mortal Weekly Rep Recommend Rep (2018) 67(1):1–31. doi: 10.15585/mmwr.rr6701a1
15. Bruguera M, Cremades M, Salinas R, Costa J, Grau M, Sans J. Impaired Response to Recombinant Hepatitis B Vaccine in HIV-Infected Persons. J Clin Gastroenterol (1992) 14(1):27–30. doi: 10.1097/00004836-199201000-00007
16. Kottilil S, Jackson JO, Polis MA. Hepatitis B & Hepatitis C in HIV-Infection. Indian J Med Res (2005) 121(4):424–50.
17. Rey D, Piroth L, Wendling MJ, Miailhes P, Michel ML, Dufour C, et al. Safety and Immunogenicity of Double-Dose Versus Standard-Dose Hepatitis B Revaccination in Non-Responding Adults With HIV-1 (ANRS HB04 B-BOOST): A Multicentre, Open-Label, Randomised Controlled Trial. Lancet Infect Dis (2015) 15(11):1283–91. doi: 10.1016/s1473-3099(15)00220-0
18. Fonseca MO, Pang LW, de Paula Cavalheiro N, Barone AA, Heloisa Lopes M. Randomized Trial of Recombinant Hepatitis B Vaccine in HIV-Infected Adult Patients Comparing a Standard Dose to a Double Dose. Vaccine (2005) 23(22):2902–8. doi: 10.1016/j.vaccine.2004.11.057
19. Lee JH, Hong S, Im JH, Lee JS, Baek JH, Kwon HY. Systematic Review and Meta-Analysis of Immune Response of Double Dose of Hepatitis B Vaccination in HIV-Infected Patients. Vaccine (2020) 38(24):3995–4000. doi: 10.1016/j.vaccine.2020.04.022
20. Launay O, van der Vliet D, Rosenberg AR, Michel ML, Piroth L, Rey D, et al. Safety and Immunogenicity of 4 Intramuscular Double Doses and 4 Intradermal Low Doses vs Standard Hepatitis B Vaccine Regimen in Adults With HIV-1: A Randomized Controlled Trial. JAMA (2011) 305(14):1432–40. doi: 10.1001/jama.2011.351
21. Chaiklang K, Wipasa J, Chaiwarith R, Praparattanapan J, Supparatpinyo K. Comparison of Immunogenicity and Safety of Four Doses and Four Double Doses vs. Standard Doses of Hepatitis B Vaccination in HIV-Infected Adults: A Randomized, Controlled Trial. PloS One (2013) 8(11):e80409. doi: 10.1371/journal.pone.0080409
22. Rey D, Krantz V, Partisani M, Schmitt MP, Meyer P, Libbrecht E, et al. Increasing the Number of Hepatitis B Vaccine Injections Augments Anti-HBs Response Rate in HIV-Infected Patients. Effects on HIV-1 Viral Load. Vaccine (2000) 18(13):1161–5. doi: 10.1016/s0264-410x(99)00389-8
23. Paitoonpong L, Suankratay C. Immunological Response to Hepatitis B Vaccination in Patients With AIDS and Virological Response to Highly Active Antiretroviral Therapy. Scand J Infect Dis (2008) 40(1):54–8. doi: 10.1080/00365540701522975
24. Ungulkraiwit P, Jongjirawisan Y, Atamasirikul K, Sungkanuparph S. Factors for Predicting Successful Immune Response to Hepatitis B Vaccination in HIV-1 Infected Patients. Southeast Asian J Trop Med Public Health (2007) 38(4):680–5. doi: 10.1049/cp:19970140
25. Fuster F, Vargas JI, Jensen D, Sarmiento V, Acuña P, Peirano F, et al. CD4/CD8 Ratio as a Predictor of the Response to HBV Vaccination in HIV-Positive Patients: A Prospective Cohort Study. Vaccine (2016) 34(16):1889–95. doi: 10.1016/j.vaccine.2016.02.055
26. Sasaki M, Foccacia R, de Messias-Reason IJ. Efficacy of Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF) as a Vaccine Adjuvant for Hepatitis B Virus in Patients With HIV Infection. Vaccine (2003) 21(31):4545–9. doi: 10.1016/s0264-410x(03)00500-0
27. Cooper CL, Davis HL, Angel JB, Morris ML, Elfer SM, Seguin I, et al. CPG 7909 Adjuvant Improves Hepatitis B Virus Vaccine Seroprotection in Antiretroviral-Treated HIV-Infected Adults. AIDS (Lond Engl) (2005) 19(14):1473–9. doi: 10.1097/01.aids.0000183514.37513.d2
28. Pasricha N, Datta U, Chawla Y, Singh S, Arora SK, Sud A, et al. Immune Responses in Patients With HIV Infection After Vaccination With Recombinant Hepatitis B Virus Vaccine. BMC Infect Dis (2006) 6:65. doi: 10.1186/1471-2334-6-65
29. Veiga AP, Casseb J, Duarte AJ. Humoral Response to Hepatitis B Vaccination and its Relationship With T CD45RA+ (Naïve) and CD45RO+ (Memory) Subsets in HIV-1-Infected Subjects. Vaccine (2006) 24(49-50):7124–8. doi: 10.1016/j.vaccine.2006.06.079
30. Cornejo-Juárez P, Volkow-Fernández P, Escobedo-López K, Vilar-Compte D, Ruiz-Palacios G, Soto-Ramírez LE. Randomized Controlled Trial of Hepatitis B Virus Vaccine in HIV-1-Infected Patients Comparing Two Different Doses. AIDS Res Ther (2006) 3:9. doi: 10.1186/1742-6405-3-9
31. Cruciani M, Mengoli C, Serpelloni G, Lanza A, Gomma M, Nardi S, et al. Serologic Response to Hepatitis B Vaccine With High Dose and Increasing Number of Injections in HIV Infected Adult Patients. Vaccine (2009) 27(1):17–22. doi: 10.1016/j.vaccine.2008.10.040
32. Overton ET, Kang M, Peters MG, Umbleja T, Alston-Smith BL, Bastow B, et al. Immune Response to Hepatitis B Vaccine in HIV-Infected Subjects Using Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF) as a Vaccine Adjuvant: ACTG Study 5220. Vaccine (2010) 28(34):5597–604. doi: 10.1016/j.vaccine.2010.06.030
33. Potsch DV, Oliveira ML, Ginuíno C, Miguel JC, Oliveira SA, Silva EF, et al. High Rates of Serological Response to a Modified Hepatitis B Vaccination Schedule in HIV-Infected Adults Subjects. Vaccine (2010) 28(6):1447–50. doi: 10.1016/j.vaccine.2009.11.066
34. Potsch DV, Camacho LA, Tuboi S, Villar LM, Miguel JC, Ginuíno C, et al. Vaccination Against Hepatitis B With 4-Double Doses Increases Response Rates and Antibodies Titers in HIV-Infected Adults. Vaccine (2012) 30(41):5973–7. doi: 10.1016/j.vaccine.2012.07.028
35. Wilson CM, Ellenberg JH, Sawyer MK, Belzer M, Crowley-Nowick PA, Puga A, et al. Serologic Response to Hepatitis B Vaccine in HIV Infected and High-Risk HIV Uninfected Adolescents in the REACH Cohort. Reaching for Excellence in Adolescent Care and Health. J Adolesc Health (2001) 29(3 Suppl):123–9. doi: 10.1016/S1054-139X(01)00278-6
36. Arundel C, Lewis JH. Drug-Induced Liver Disease in 2006. Curr Opin Gastroenterol (2007) 23(3):244–54. doi: 10.1097/MOG.0b013e3280b17dfb
37. Sulkowski MS, Thomas DL, Chaisson RE, Moore RD. Hepatotoxicity Associated With Antiretroviral Therapy in Adults Infected With Human Immunodeficiency Virus and the Role of Hepatitis C or B Virus Infection. JAMA (2000) 283(1):74–80. doi: 10.1001/jama.283.1.74
38. Chonwattana W, Raengsakulrach B, Holtz TH, Wasinrapee P, Tongtoyai J, Chaikummao S, et al. Hepatitis B Vaccination Uptake and Correlates of Serologic Response Among HIV-Infected and Uninfected Men Who Have Sex With Men (MSM) in Bangkok, Thailand. Vaccine (2016) 34(17):2044–50. doi: 10.1016/j.vaccine.2015.11.071
39. Flynn PM, Cunningham CK, Rudy B, Wilson CM, Kapogiannis B, Worrell C, et al. Hepatitis B Vaccination in HIV-Infected Youth: A Randomized Trial of Three Regimens. J Acquir Immune Defic Syndr (2011) 56(4):325–32. doi: 10.1097/QAI.0b013e318203e9f2
40. Ni JD, Xiong YZ, Wang XJ, Xiu LC. Does Increased Hepatitis B Vaccination Dose Lead to a Better Immune Response in HIV-Infected Patients Than Standard Dose Vaccination: A Meta-Analysis? Int J STD AIDS (2013) 24(2):117–22. doi: 10.1177/0956462412472309
41. Geretti AM, Brook G, Cameron C, Chadwick D, French N, Heyderman R, et al. British HIV Association Guidelines on the Use of Vaccines in HIV-Positive Adults 2015. HIV Med (2016) 17(Suppl 3):s2–s81. doi: 10.1111/hiv.12424
42. Overton ET, Sungkanuparph S, Powderly WG, Seyfried W, Groger RK, Aberg JA. Undetectable Plasma HIV RNA Load Predicts Success After Hepatitis B Vaccination in HIV-Infected Persons. Clin Infect Dis (2005) 41(7):1045–8. doi: 10.1086/433180
43. Panel on Opportunistic Infections in Adults and Adolescents With HIV. In: Guidelines for the Prevention and Treatment of Opportunistic Infections in Adults and Adolescents With HIV:recommendations From the Centers for Disease Control and Prevention. the National Institutes of Health atHMAotIDSoA, editor.: the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. Available at http://aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf.
44. Patterson SB, Landrum ML, Okulicz JF. Delayed-Type Hypersensitivity and Hepatitis B Vaccine Responses, In Vivo Markers of Cellular and Humoral Immune Function, and the Risk of AIDS or Death. Vaccine (2014) 32(27):3341–4. doi: 10.1016/j.vaccine.2014.04.049
45. Farooq PD, Sherman KE. Hepatitis B Vaccination and Waning Hepatitis B Immunity in Persons Living With HIV. Curr HIV/AIDS Rep (2019) 16(5):395–403. doi: 10.1007/s11904-019-00461-6
Keywords: human immunodeficiency virus, hepatitis B virus, vaccine, immune response, response rate
Citation: Tian Y, Hua W, Wu Y, Zhang T, Wang W, Wu H, Guo C and Huang X (2021) Immune Response to Hepatitis B Virus Vaccine Among People Living With HIV: A Meta-Analysis. Front. Immunol. 12:745541. doi: 10.3389/fimmu.2021.745541
Received: 22 July 2021; Accepted: 06 December 2021;
Published: 22 December 2021.
Edited by:
Carla Osiowy, Public Health Agency of Canada (PHAC), CanadaReviewed by:
Jose-Abrego Alexis, University of Guadalajara, MexicoEmmanuel Ochola, St. Mary’s Hospital Lacor, Uganda
Copyright © 2021 Tian, Hua, Wu, Zhang, Wang, Wu, Guo and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaojie Huang, huangxiaojie78@ccmu.edu.cn; Caiping Guo, gcpdt001@126.com
†These authors have contributed equally to this work
 Yakun Tian
Yakun Tian Wei Hua
Wei Hua Yaxin Wu
Yaxin Wu Tong Zhang
Tong Zhang Wen Wang
Wen Wang Hao Wu
Hao Wu Caiping Guo
Caiping Guo Xiaojie Huang
Xiaojie Huang