- 1Department of Medical Genetics, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 2Men’s Health and Reproductive Health Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 3Skull Base Research Center, Loghman Hakin Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran
Neuromyelitis optica spectrum disorders (NMOSD) comprise a variety of disorders being described by optic neuritis and myelitis. This disorder is mostly observed in sporadic form, yet 3% of cases are familial NMO. Different series of familial NMO cases have been reported up to now, with some of them being associated with certain HLA haplotypes. Assessment of HLA allele and haplotypes has also revealed association between some alleles within HLA-DRB1 or other loci and sporadic NMO. More recently, genome-wide SNP arrays have shown some susceptibility loci for NMO. In the current manuscript, we review available information about the role of genetic factors in NMO.
Introduction
Neuromyelitis optica spectrum disorders (NMOSD) comprise a variety of disorders being described by acute inflammatory responses in the optic nerve and spinal cord, i.e., optic neuritis and myelitis, respectively (1). NMO is mostly triggered by IgG autoantibodies against aquaporin 4 (AQP4) (2). AQP4 monomers comprise six transmembrane helical domains and two small helical parts around a thin aqueous pore (3). These monomers lump together to make corresponding tetramers with the ability of being aggregated in cell plasma membranes. The constructed supramolecular collections are named as orthogonal arrays of particles (OAPs) (3). AQP4 is the supreme ample water-channel protein in the central nervous system (CNS) (1). A number of NMO patients do not have AQP4-IgG, yet they have IgG antibodies against myelin oligodendrocyte glycoprotein, a glycoprotein in the outer myelin sheath of CNS neurons (4).
Following the discovery of AQP4-specific proliferative T cells in NMO patients, it has been recognized that AQP4-specific T cells exhibit Th17 features and display molecular mimicry with a peptide sequence encoded by the commensal bacterium Clostridium perfringens. Further studies have revealed distinct features of gut microbiota in NMO cases versus both multiple sclerosis (MS) cases and healthy subjects (5).
Although this disorder has some similarities with MS, it is important to distinguish between these two conditions, particularly at early stages of the disorder, since therapeutic modalities for these disorders are different (6). Most importantly, a number of prescribed agents for MS might be harmful for patients with NMO (7, 8). NMO and MS can be differentiated through assessment of NMO antibody. Although the existence of cerebral lesions has been formerly regarded as a criterion for differentiation between these two conditions, it is currently acknowledged that these lesions do not exclude NMO. In fact, with the advent of NMO antibody assessment techniques, some cases diagnosed as MS for a long time have been found to have NMO (9).
Typically, NMO manifests around the ages of 35 to 45 years, yet less than 20% of cases occur in children, and elderlies account for 18% of cases. NMO is recognized as a condition with female predominance. Although 70% to 90% of total NMO patients are female, such sex bias is not seen in children (6, 10). In NMO-AQP4 cases, gender influences both age at disease onset and site of attack (11).
NMO is most probably a complex multifactorial disorder. Most cases of this disorder are sporadic, yet 3% of cases are familial (12). A previous meta-analysis of whole-genome association studies in NMO has shown association of AQP4-IgG positive NMO with two independent signals in the MHC region. Notably, one of these signals has been suggested to be related with structural variations in the complement component 4 region. Moreover, a significant causal effect has been found between AQP4-IgG positive NMO and recognized risk variant for systemic lupus erythematosus (SLE). Most notably, such causal link has not been observed with MS risk variants (13). A number of other studies have reported an association between genetic variants and gene expressions alterations and NMO. In the current manuscript, we review available information about the role of genetic factors in NMO.
Family Studies
Familial and sporadic NMO are similar in terms of clinical manifestations, age onset of disease, gender-based effects, and proportion of AQP4-IgG positive cases (12). A pioneer study in this field has reported occurrence of NMO in identical twin sisters at the ages of 24 and 26, respectively (14). A subsequent study reported NMO manifestations such as sudden loss of vision and transverse myelopathy in two sisters at the age of 3. Notably, HLA haplotyping revealed a shared haplotype between these two sisters, yet an unaffected sib also had this haplotype (15). More recently, a group of researchers described a series of familial NMO cases including siblings, parent–child, and aunt–niece pairs, more than 80% of them being female. A number of reported cases had either maternal or paternal transmission. More than 75% of cases had AQP4-IgG. About half of cases had clinical manifestations or serologic markers of another immune-related condition. The observed familial transmission of NMO suggested a complex genetic etiology for this disorder (12). A number of other studies also reported familial clustering of NMO cases, with some of them reported the presence of a shared haplotype among affected cases. Table 1 summarizes the results of family studies in NMO.
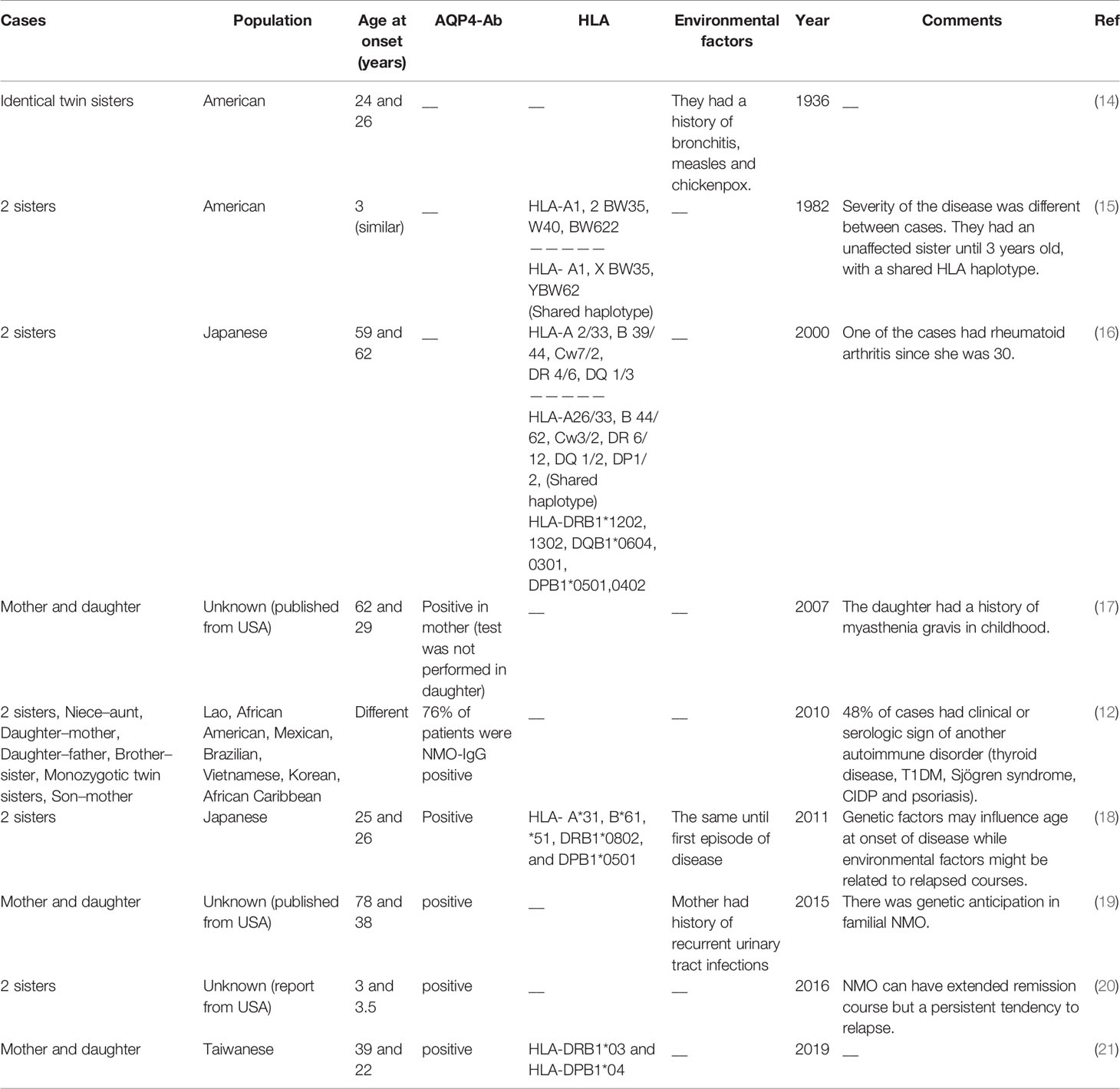
Table 1 Summary of the results of family studies in neuromyelitis optica [HLA, human leukocyte antigen, AQP4-Ab, aquaporin-4 antibody (NMO-IgG)].
HLA Studies
An HLA genotyping study in seropositive Brazilian NMO patients has revealed some susceptibility loci for NMO, most importantly HLA-DRB1*04:05 and *16:02. A number of alleles within HLA class I showed association with NMO, yet this association did not remain significant after corrections for multiple comparisons (22). Another study in Afro-Caribbean NMO cases has shown higher frequency of HLA-DRB1*03 in NMO patients. On the other hand, HLA-DRB1*15, but not DRB1*03 allele has been recognized as a susceptibility locus for MS. In brief, distribution of HLA-DRB1 and DQB1 has been different among NMO and MS cases in this population (23). Another study in seropositive Brazilian NMO patients has shown overrepresentation of the HLA-DRB1*03 allele group in NMO cases compared with unaffected individuals. On the other hand, MS patients have shown higher frequency of the HLA-DRB1*15 allele group. DRB3 and DRB5 have had higher frequencies in NMO and MS cases, respectively (24). Another study has confirmed overrepresentation of HLA-DRB1*03 and HLA-DRB1*10 alleles in another group of Brazilian NMO patients compared with controls, in spite of no significant overrepresentation of MS-associated alleles (25). In addition, the DR3 and DR15 haplotypes have been found to be more common in NMO and MS, respectively. The association between HLA-DRB1*03:01 allele and NMO has not been dependent on seropositivity (26). In a study in Japanese patients, HLA-DRB1*08:02 and HLA-DRB1*16:02 have been found as risk loci, while HLA-DRB1*09:01 has been a protective allele (27). Table 2 shows the results of HLA studies in NMO cases in different populations.
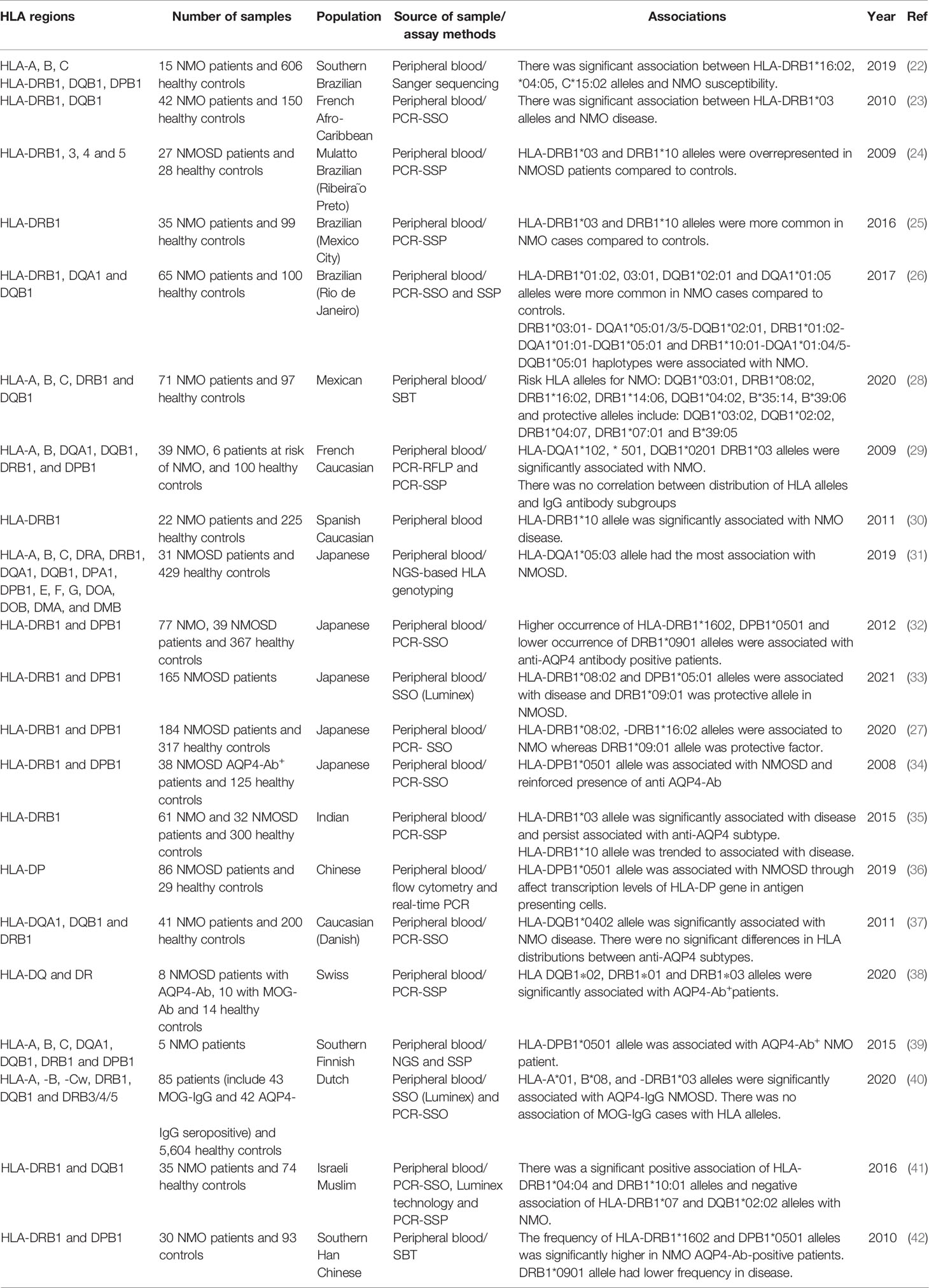
Table 2 HLA studies in neuromyelitis optica (SSP-PCR, sequence-specific primers–polymerase chain reaction; PCR-SSO, polymerase chain reaction–sequence specific oligoprobes; SBT, sequencing-based typing; MOG-Ab, myelin oligodendrocyte glycoprotein antibody).
Genomic Studies
Whole-exome sequencing (WES) has facilitated identification of risk loci for NMO. Application of this method in addition to HLA sequencing in seropositive NMO cases of Chinese origin has shown significant association between HLA-DQB1*05:02 and NMO. Additionally, the frequency of “HLA-DQB1*05:02-DRB1*15:01” haplotype has been higher in the NMO group compared with controls. Besides, this study has shown higher frequency of loss-of-function mutations in NOP16 in these patients compared with healthy subjects. The G390R of IgG1, which decreases the threshold for BCR activation, has been another NMO-associated variant. Notably, most of the NMO-associated genetic factors have been enriched pathways related with nervous system and immune responses (43).
Another genome-wide study using an SNP array has identified the rs1964995 in the MHC region as a risk locus for NMO. Notably, three MS-associated variants have also been found to be associated with NMO. A variant within KCNMA1 gene has been associated with disability score as well as presence of transverse myelitis (27).
The importance of copy number variations (CNVs) in conferring risk of NMO has been previously assessed using a genome-wide method. The majority of identified CNVs have been located at TCRγ and TCRα regions. These CNVs have been mostly deletions with sizes of 5 to 50 kb. Since they have been only in the peripheral blood T cells, it has been deduced that they are most probably somatically acquired CNVs. Moreover, it has been an association between the presence of CNVs in NMO cases and seronegativity for AQP4-IgG or low antibody titer (44).
Several SNPs within AQP4 gene have been genotyped in NMO cases to find possible risk loci for this condition in different ethnic groups. For instance, Matiello et al. have compared genotype frequencies of 8 SNPs within AQP4 gene in sporadic and familial NMO cases as well as healthy controls. One of these SNPs has been found to be associated with risk of NMO. Moreover, two missense mutations at Arg19 have been found in three NMO patients. The authors have reported that apart from one infrequent SNP, no other examined SNP or haplotype has been linked to NMO, possibly excluding the importance of AQP4 variants in conferring risk of NMO (45). Qiu et al. have also genotyped eight SNPs in AQP4 in a group of AQP4-IgG-positive NMO cases. They have shown associations between a number of SNPs and clinical manifestations of NMO such as extensive transverse myelitis, optic neuritis, or simultaneous systemic autoimmune disorders (46). Table 3 shows the results of genomic studies in NMO cases.
Expression Studies
Expressions of several immune-related genes have been assessed in NMO cases at transcript or protein levels. Moreover, a number of high-throughput sequencing strategies have been employed to assess expression of different subtypes of transcripts. For instance, lncRNA and mRNA profile has been assessed in these patients using microarray technique. Such type of analysis has led to the identification of more than 1,300 lncRNAs with differential expression between NMO cases and normal controls. Moreover, more than 700 mRNAs have been found to be differentially expressed between NMO cases and normal subjects. These genes have been functionally correlated with IL-23-related cascades, IFN-γ signaling, natural killer-κB pathway, and a number of other immune-related mechanisms (74). Another RNA expression profiling experiment has shown possible contribution of T-cell-related genes and the TNF/NF-kB cascade in the pathogenesis of NMO. Notably, IL7Ra (CD127) has been found to be downregulated in the circulation of NMO patients compared with control subjects. Moreover, transcription factors located in the upstream of CD127 and survival pathways in its downstream have been considerably downregulated. These expression changes have been accompanied by decrease in the quantities of naïve T cells, reduction of BID-mediated T-cell survival signaling and activation of cell apoptosis. Taken together, these observations indicate the importance of IL7Ra signaling in the pathoetiology of NMO (75). A high-throughput expression profiling in brain tissue samples obtained from an NMO patient as well as patients with Parkinson’s disease and amyotrophic lateral sclerosis has shown upregulation of more than 200 genes in brain lesions of NMO patients with the mostly upregulated ones being associated with immune response. Upregulation of IFI30, CD163, and SPP1 has also been confirmed by further RNA and protein-based techniques. Genes with high expression in NMO brain lesions has been functionally related with NF-κB and Blimp-1, indicating the importance macrophage-mediated inflammatory responses in the pathoetiology of NMO brain lesions (76).
With the aim of finding effective markers for the assessment of response of NMO patients to therapeutic options, Vaknin-Dembinsky et al. have assessed miRNAs profile in the blood of NMO patients before and following treatment with rituximab. They have reported upregulation of 14 miRNAs and downregulation of 32 miRNAs in NMO patients after treatment with rituximab. Moreover, they have shown higher levels of 17 miRNAs and lower levels of 25 miRNAs in untreated cases compared with healthy controls. Notably, rituximab could normalize expression of a number of these miRNAs, among them have been brain-specific or brain-enriched miRNAs. Cumulatively, circulatory miRNA profile can be used as a biomarker for therapeutic response (77).
The pleiotropic cytokine IL-6 is also implicated in the pathogenesis of NMO through enhancement of survival of plasmablasts, induction of release of antibodies against AQP4, disruption of integrity of blood–brain barrier and its functionality, as well as increasing differentiation and activity of proinflammatory T cells (78). Expression of this cytokine has been reported to be elevated in CSF and blood samples of NMO patients (79). Table 4 shows the results of expression studies in NMO.
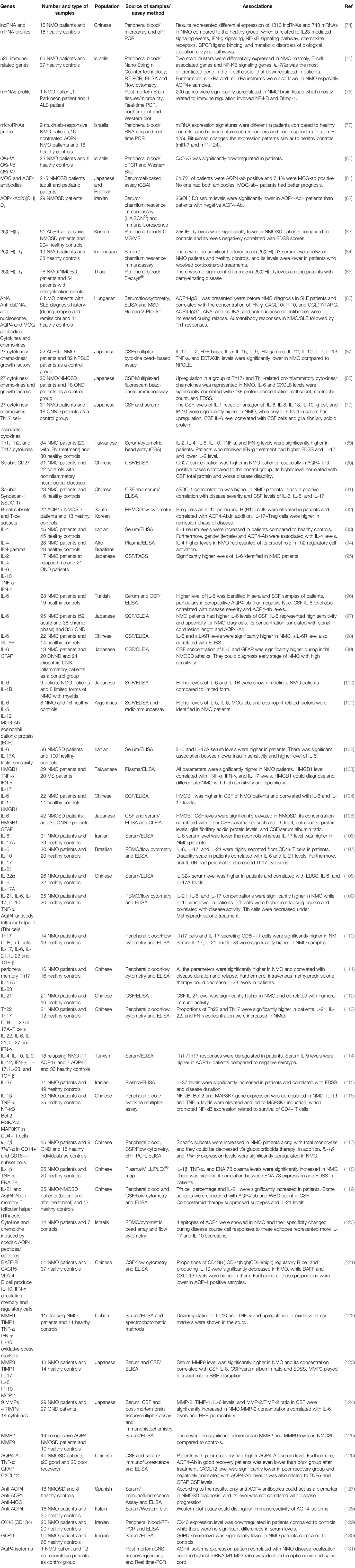
Table 4 Expression studies in neuromyelitis optica (NPSLE, neuropsychiatric systemic lupus erythematosus; ONND, other non-inflammatory neurological disorders; OND, other neurological disorders).
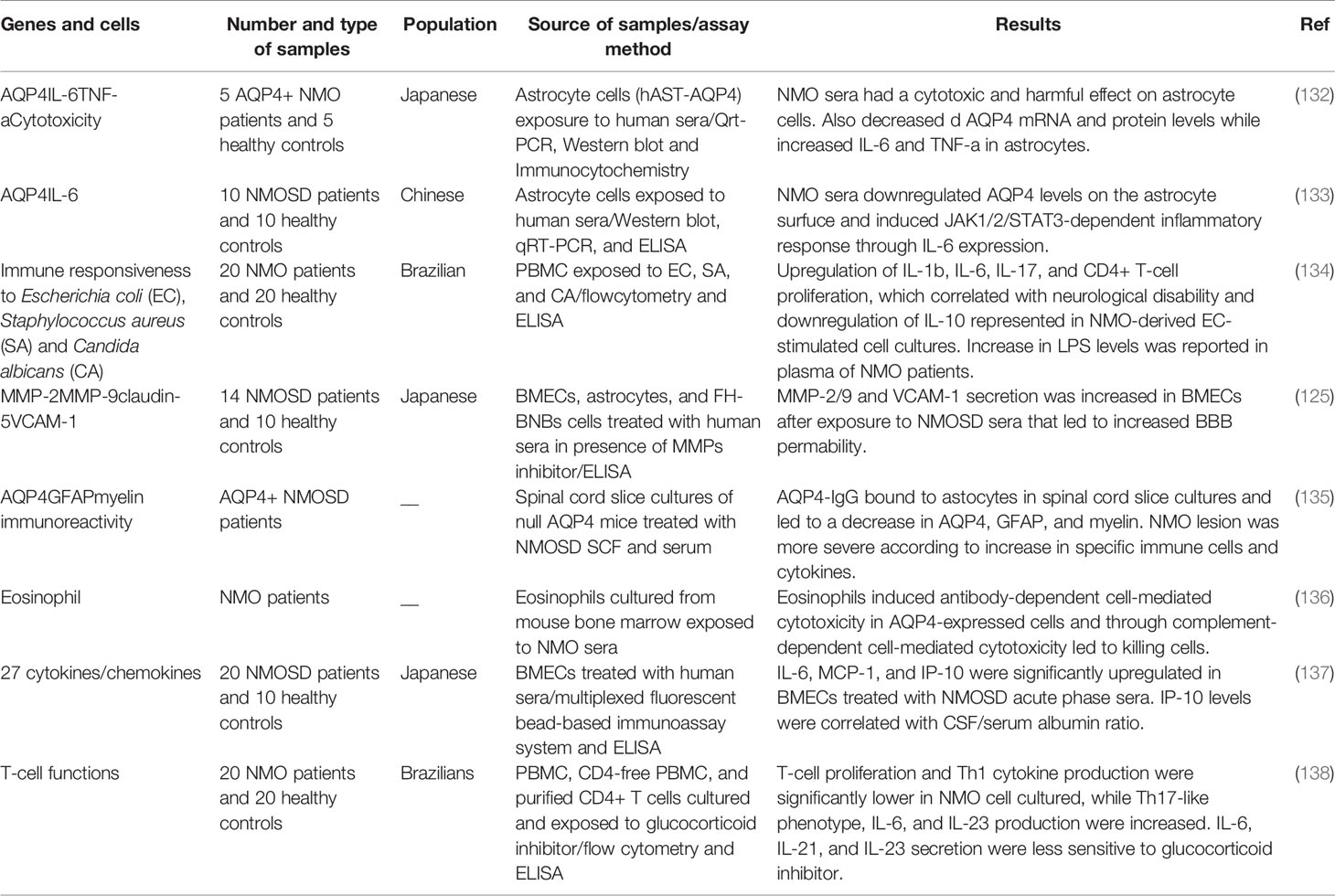
In Vitro Studies
A number of in vitro studies have appraised the functional mechanisms of development of NMO. In an effort to find the impact humoral factors on astrocyte injury in NMO, Haruki et al. have conducted a series of experiments on immortalized human primary astrocytes. Moreover, they assessed the effect of TY09 human brain microvascular endothelial on the quantity and localization of AQP4 protein in astrocytes. Serum samples of NMO patients have been shown to induce cytotoxic effects on AQP4-expressing astrocytes. Moreover, these serum samples could decrease AQP4 expression at both mRNA and protein levels, while increasing release of TNF-α and IL-6 from astrocytes. Experiments in an in vitro BBB model has shown localization of AQP4 protein at the astrocytic membrane following co-culture with TY09, in contact with these cells (132).
Sera samples of these patients or even NMO-IgG have also been shown to rapidly downregulate AQP4 levels on the surface of astrocytes. Astrocytes treated with NMO-IgG, IL-6/R, and NMO-IgG + IL-6/R have shown over-production of IL-6 transcripts. Moreover, NMO-IgG could elicit alterations in gene transcription via the JAK/STAT3 pathway. Cumulatively, NMO-IgG has been reported to induce the JAK1/2/STAT3 pathway in astrocytes, representing a crucial event in the pathoetiology of NMO. Besides, suppression of JAK1/2 signaling might be a therapeutic modality for NMOSD (133).
Another in vitro study has shown similar magnitude of lymphoproliferation and cytokine profiles in peripheral blood mononuclear cells of NMO cases and healthy controls in reponse to Staphylococcus aureus and Candida albicans. However, NMO-originated Escherichia coli-induced cell cultures have exhibited higher proliferation of CD4+ T cells in association with higher production of IL-1β, IL-6, and IL-17. IL-10 release has been lower in NMO-derived cells compared with controls. Notably, the in vitro E. coli-stimulated expressions of IL-6 and IL-17 have been correlated with neurological debilities. Overproduction of Th17-associated cytokines has been associated with the production of IL-23 and IL-6 by LPS-stimulated monocytes. Consistently, LPS levels have been higher in the plasma samples of NMO cases. Therefore, increase in Th17 type response to E. coli might contribute in the pathogenesis of NMO (134). Table 5 shows the results of in vitro mechanistical studies in NMO.
Discussion
NMO comprises a group of immune-meditaed conditions with complex etiology. While family studies have shown clustering of NMO cases in some familites, the exact genetic background of this disorder has not been clarified yet. Since the first report of familial NMO cases in 1936 (14), several studies have attempted to find susceptibility loci for NMO. The first attempts have been focused on the HLA region, based on the importance of this region in the regulation of immune responses and their association with MS, a disorder that clinically resembles NMO. However, various studies have shown that HLA-related susceptibility loci for NMO is distinct from MS. The HLA-DRB1*03 allele has been the mostly appreciated risk locus for NMO. Several other HLA-DRB1, DQB1, and DPB1 alleles have been found to be associated with NMO. Yet, the results of these studies have not been validated in independent cohorts from different ethnic backgrounds.
Exome sequencing and genome-wide SNP arrays have also validated the significance of the HLA region in conferring risk of NMO. In addition, they have shown other risk loci within AQP4, CYP27B1, CYP7A1, CD226, CD58, CD6, FCRL3, GPC5, MIF, ATG5, PD-1.3, IL2RA, IL7RA, and IL17A. With the exception of AQP4 and CD58, almost other genes have been assessed in single studies, needing confirmation in independent cohorts. Moreover, a number of variants, particularly within SLC28A3 and SLC29A1, have been associated with clinical course or some immune markers in patients with NMO.
Deletion-type CNVs can also been regarded as predisposing factors for NMO. Notably, these CNVs have been found to occur as somatic changes.
In addition to several cytokines that are altered in the course of NMO development, expressions of numerous mRNAs, lncRNAs, and miRNAs have been found to be deregulated in the peripheral blood or brain lesions of NMO patients. Not surprisingly, these genes are mostly enriched in pathways related to functions of the immune system.
Finally, in vitro studies have shown the effects of NMO sera on deregulation of function of astrocytes, suggesting the impact of humoral responses on pathoetiology of this condition. Moreover, these circulatory markers could negatively affect permeability of the blood–brain barrier.
Taken together, NMO has a complex genetic background with prominent roles of immune-related genes, particularly cytokine coding genes and those coding cytokine receptors. Future genome-wide studies in NMO patients from different ethnic background would facilitate identification of risk loci for this condition. Finally, systematic review and meta-analysis studies are recommended to produce quantitative results without any bias along with an overview of genetic aspects of disease. Also, further studies should assess treatment responses in association with distinct genetic backgrounds. Finally, a limitation of studies conducted in this filed is that the expression profiles of genes and cytokines have not been assessed in association with different treatment options.
Author Contributions
MT and SG-F wrote the draft and revised it. TA collected the tables and data. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Jarius S, Paul F, Weinshenker BG, Levy M, Kim HJ, Wildemann B. Neuromyelitis Optica. Nat Rev Dis Primers (2020) 6(1):85. doi: 10.1038/s41572-020-0214-9
2. Papadopoulos MC, Verkman AS. Aquaporin 4 and Neuromyelitis Optica. Lancet Neurol (2012) 11(6):535–44. doi: 10.1016/S1474-4422(12)70133-3
3. Ho JD, Yeh R, Sandstrom A, Chorny I, Harries WE, Robbins RA, et al. Crystal Structure of Human Aquaporin 4 at 1.8 A and Its Mechanism of Conductance. Proc Natl Acad Sci USA (2009) 106(18):7437–42. doi: 10.1073/pnas.0902725106
4. Ambrosius W, Michalak S, Kozubski W, Kalinowska A. Myelin Oligodendrocyte Glycoprotein Antibody-Associated Disease: Current Insights Into the Disease Pathophysiology, Diagnosis and Management. Int J Mol Sci (2020) 22(1):100. doi: 10.3390/ijms22010100
5. Zamvil SS, et al. The Gut Microbiome in Neuromyelitis Optica. Neurotherapeutics (2018) 15(1):92–101. doi: 10.1007/s13311-017-0594-z
6. Lana-Peixoto MA, Talim N. Neuromyelitis Optica Spectrum Disorder and Anti-MOG Syndromes. Biomedicines (2019) 7(2):42. doi: 10.3390/biomedicines7020042
7. Kleiter I, et al. Failure of Natalizumab to Prevent Relapses in Neuromyelitis Optica. Arch Neurol (2012) 69(2):239–45. doi: 10.1001/archneurol.2011.216
8. Min J-H, Kim BJ, Lee KH. Development of Extensive Brain Lesions Following Fingolimod (FTY720) Treatment in a Patient With Neuromyelitis Optica Spectrum Disorder. Multiple Sclerosis J (2012) 18(1):113–5. doi: 10.1177/1352458511431973
9. Lalan S, et al. Differentiation of Neuromyelitis Optica From Multiple Sclerosis on Spinal Magnetic Resonance Imaging. Int J MS Care (2012) 14(4):209–14. doi: 10.7224/1537-2073-14.4.209
10. McKeon A, et al. CNS Aquaporin-4 Autoimmunity in Children. Neurology (2008) 71(2):93–100. doi: 10.1212/01.wnl.0000314832.24682.c6
11. Kim SM, et al. Gender Effect on Neuromyelitis Optica Spectrum Disorder With Aquaporin4-Immunoglobulin G. Mult Scler (2017) 23(8):1104–11. doi: 10.1177/1352458516674366
12. Matiello M, Kim HJ, Kim W, Brum DG, Barreira AA, Kingsbury DJ, et al. Familial Neuromyelitis Optica. Neurology (2010) 75(4):310–5. doi: 10.1212/WNL.0b013e3181ea9f15
13. Estrada K, Whelan CW, Zhao F, Bronson P, Handsaker RE, Sun C, et al. A Whole-Genome Sequence Study Identifies Genetic Risk Factors for Neuromyelitis Optica. Nat Commun (2018) 9(1):1–10. doi: 10.1038/s41467-018-04332-3
14. McAlpine D. Familial Neuromyelitis Optica: Its Occurrence in Identical Twins. Brain (1938) 61(4):430–48. doi: 10.1093/brain/61.4.430
15. Ch’ien LT, Medeiros MO, Belluomini JJ, Lemmi H, Whitaker JN. Neuromyelitis Optica (Devic’s Syndrome) in Two Sisters. Clin Electroencephalogr (1982) 13(1):36–9. doi: 10.1177/155005948201300104
16. Yamakawa K, Kuroda H, Fujihara K, Sato S, Nakashima I, Takeda A, et al. Familial Neuromyelitis Optica (Devic’s Syndrome) With Late Onset in Japan. Neurology (2000) 55(2):318–20. doi: 10.1212/WNL.55.2.318
17. Braley T, Mikol DD. Neuromyelitis Optica in a Mother and Daughter. Arch Neurol (2007) 64(8):1189–92. doi: 10.1001/archneur.64.8.1189
18. Tanaka Y, et al. Neuromyelitis Optica in Japanese Sisters. Internal Med (2011) 50(22):2829–32. doi: 10.2169/internalmedicine.50.5613
19. Kavoussi SC, Lesser RL. Genetic Anticipation in Familial Neuromyelitis Optica: Case and Literature Review. Connecticut Med (2015) 79(4):239–47. doi: 10.1590/0004-282X20190031.
20. Chuquilin M, Mullaguri N, Weinshenker B. Pediatric Familial Neuromyelitis Optica in Two Sisters With Long Term Follow-Up. J Clin Neurosci (2016) 29:183–4. doi: 10.1016/j.jocn.2016.01.009
21. Lee J-J, et al. Intra-Family Phenotype Variations in Familial Neuromyelitis Optica Spectrum Disorders. Mult Scler Relat Disord (2019) 30:57–62. doi: 10.1016/j.msard.2019.02.002
22. Kay CSK, Scola RH, Arndt RC, Lorenzoni PJ, Werneck LC, et al. HLA-Alleles Class I and II Associated With Genetic Susceptibility to Neuromyelitis Optica in Brazilian Patients. Arq Neuropsiquiatr (2019) 77(4):239–47. doi: 10.1590/0004-282x20190031
23. Deschamps R, et al. Different HLA Class II (DRB1 and DQB1) Alleles Determine Either Susceptibility or Resistance to NMO and Multiple Sclerosis Among the French Afro-Caribbean Population. Mult Scler (2011) 17(1):24–31. doi: 10.1177/1352458510382810
24. Brum DG, Barreira AA, dos Santos AC, Kaimen-Maciel DR, Matiello M, Costa RM, et al. HLA-DRB Association in Neuromyelitis Optica Is Different From That Observed in Multiple Sclerosis. Mult Scler (2010) 16(1):21–9. doi: 10.1177/1352458509350741
25. Alonso VR, de Jesus Flores Rivera J, Garci YR, Granados J, Sanchez T, Mena-Hernandez L, et al. Neuromyelitis Optica (NMO IgG+) and Genetic Susceptibility, Potential Ethnic Influences. Cent Nerv Syst Agents Med Chem (2018) 18(1):4–7. doi: 10.2174/1871524916666160229115047
26. Alvarenga MP, Fernandez O, Leyva L, Campanella L, Vasconcelos CF, Alvarenga M, et al. The HLA DRB1*03:01 Allele Is Associated With NMO Regardless of the NMO-IgG Status in Brazilian Patients From Rio De Janeiro. J Neuroimmunol (2017) 310:1–7. doi: 10.1016/j.jneuroim.2017.05.018
27. Matsushita T, Masaki K, Isobe N, Sato S, Yamamoto K, Nakamura Y, et al. Genetic Factors for Susceptibility to and Manifestations of Neuromyelitis Optica. Ann Clin Transl Neurol (2020) 7(11):2082–93. doi: 10.1002/acn3.51147
28. Romero-Hidalgo S, Flores-Rivera J, Rivas-Alonso V, Barquera R, Villarreal-Molina MT, Antuna-Puente B, et al. Native American Ancestry Significantly Contributes to Neuromyelitis Optica Susceptibility in the Admixed Mexican Population. Sci Rep (2020) 10(1):1–11.
29. Zephir H, Fajardy I, Outteryck O, Blanc F, Roger N, Fleury M, et al. Is Neuromyelitis Optica Associated With Human Leukocyte Antigen? Multiple Sclerosis J (2009) 15(5):571–9. doi: 10.1177/1352458508102085
30. Blanco Y, Ercilla-Gonzalez G, Llufriu S, Casanova-Estruch B, Magraner M, Ramio-Torrenta L, et al. HLA-DRB1 Typing in Caucasians Patients With Neuromyelitis Optica. Rev neurologia (2011) 53(3):146–52.
31. Ogawa K, Okuno T, Hosomichi K, Hosokawa A, Hirata J, Suzuki K, et al. Next-Generation Sequencing Identifies Contribution of Both Class I and II HLA Genes on Susceptibility of Multiple Sclerosis in Japanese. J Neuroinflamm (2019) 16(1):1–9. doi: 10.1186/s12974-019-1551-z
32. Yoshimura S, Isobe N, Matsushita T, Yonekawa T, Masaki K, Sato S, et al. Distinct Genetic and Infectious Profiles in Japanese Neuromyelitis Optica Patients According to Anti-Aquaporin 4 Antibody Status. J Neurology Neurosurgery Psychiatry (2013) 84(1):29–34. doi: 10.1136/jnnp-2012-302925
33. Watanabe M, Nakamura Y, Sato S, Niino M, Fukaura H, Tanaka M, et al. HLA Genotype-Clinical Phenotype Correlations in Multiple Sclerosis and Neuromyelitis Optica Spectrum Disorders Based on Japan MS/NMOSD Biobank Data. Sci Rep (2021) 11. doi: 10.1038/s41598-020-79833-7
34. Matsushita T, Matsuoka T, Isobe N, Kawano Y, Minohara M, Shi N, et al. Association of the HLA-DPB1* 0501 Allele With Anti-Aquaporin-4 Antibody Positivity in Japanese Patients With Idiopathic Central Nervous System Demyelinating Disorders. Tissue Antigens (2009) 73(2):171–6. doi: 10.1111/j.1399-0039.2008.01172.x
35. Pandit L, Malli C, D’Cunha A, Mustafa S. Human Leukocyte Antigen Association With Neuromyelitis Optica in a South Indian Population. Mult Scler (2015) 21(9):1217–8. doi: 10.1177/1352458515574149
36. Chang Y, Wang Y, Fan P, Wang J, Shu Y, Li R, et al. Expression of HLA-DP in Patients With Neuromyelitis Optica Spectrum Disorders. Zhonghua yi xue za zhi (2019) 99(45):3574–80.. doi: 10.3760/cma.j.issn.0376-2491.2019.45.009
37. Asgari N, Nielsen C, Stenager E, Kyvik KO, Lillevang ST. HLA. HLA, PTPN22 and PD-1 Associations as Markers of Autoimmunity in Neuromyelitis Optica. Multiple Sclerosis J (2012) 18(1):23–30. doi: 10.1177/1352458511417480
38. Hofer LS, Ramberger M, Gredler V, Pescoller AS, Rostásy K, Sospedra M, et al. Comparative Analysis of T-Cell Responses to Aquaporin-4 and Myelin Oligodendrocyte Glycoprotein in Inflammatory Demyelinating Central Nervous System Diseases. Front Immunol (2020) 11.
39. Siuko M, Valori M, Kivelä T, Setälä K, Morin A, Kwan T, et al. Exome and Regulatory Element Sequencing of Neuromyelitis Optica Patients. J Neuroimmunol (2015) 289:139–42. doi: 10.1016/j.jneuroim.2015.11.002
40. Bruijstens AL, Wong YYM, van Pelt DE, van der Linden PJ, Haasnoot GW, Hintzen RQ, et al. HLA Association in MOG-IgG–and AQP4-IgG–related Disorders of the CNS in the Dutch Population. Neurol-Neuroimmunol Neuroinflamm (2020) 7(3). doi: 10.1212/NXI.0000000000000702
41. Brill L, Mandel M, Karussis D, Petrou P, Miller K, Ben-Hur T, et al. Increased Occurrence of Anti-AQP4 Seropositivity and Unique HLA Class II Associations With Neuromyelitis Optica (NMO), Among Muslim Arabs in Israel. J Neuroimmunol (2016) 293:65–70. doi: 10.1016/j.jneuroim.2016.02.006
42. Wang H, Dai Y, Qiu W, Zhong X, Wu A, Wang Y, et al. HLA-DPB1* 0501 Is Associated With Susceptibility to Anti-Aquaporin-4 Antibodies Positive Neuromyelitis Optica in Southern Han Chinese. J Neuroimmunol (2011) 233(1-2):181–4. doi: 10.1016/j.jneuroim.2010.11.004
43. Zhong X, Chen C, Sun X, Wang J, Li R, Chang Y, et al. Whole-Exome Sequencing Reveals the Major Genetic Factors Contributing to Neuromyelitis Optica Spectrum Disorder in Chinese Patients With Aquaporin 4-IgG Seropositivity. Eur J Neurol (2021) 28(7):2294–304. doi: 10.1111/ene.14771
44. Sato S, Yamamoto K, Matsushita T, Isobe N, Kawano Y, Iinuma K, et al. Copy Number Variations in Multiple Sclerosis and Neuromyelitis Optica. Ann Neurol (2015) 78(5):762–74. doi: 10.1002/ana.24511
45. Matiello M, Schaefer-Klein JL, Hebrink DD, Kingsbury DJ, Atkinson EJ, Weinshenker BG. Genetic Analysis of Aquaporin-4 in Neuromyelitis Optica. Neurology (2011) 77(12):1149–55. doi: 10.1212/WNL.0b013e31822f045b
46. Qiu W, Chang Y, Li R, Long Y, Huang J, Mai W, et al. Correlation of AQP4 Gene Polymorphism With NMO Clinical Phenotypes and Its Underlying Mechanism. Zhonghua Yi Xue Za Zhi (2015) 95(7):501–6.
47. Yang T-T, He Y, Xiang Y-J, Ao D-H, Wang Y-Y, Zhang Q, et al. No Association of AQP4 Polymorphisms With Neuromyelitis Optica and Multiple Sclerosis. Trans Neurosci (2016) 7(1):76–83. doi: 10.1515/tnsci-2016-0012
48. Wang Q-S, Xiao H-Q, Chen H-X, Liu Y-P, Ding X-D. The Single Nucleotide Polymorphism Site of Aquaporin-4 Gene in Patients With Neuromyelitis Optica. Exp Ther Med (2017) 14(6):6017–21. doi: 10.3892/etm.2017.5267
49. Wei Q, Yanyu C, Rui L, Caixia L, Youming L, Jianhua H, et al. Human Aquaporin 4 Gene Polymorphisms in Chinese Patients With Neuromyelitis Optica. J Neuroimmunol (2014) 274(1-2):192–6. doi: 10.1016/j.jneuroim.2014.07.003
50. Chu L, Dai Q, Xu Z, He D, Wang H, Wang Q, et al. Association Between the Single Nucleotide Polymorphism and the Level of Aquaporin-4 Protein Expression in Han and Minority Chinese With Inflammatory Demyelinating Diseases of the Central Nervous System. Mol Neurobiol (2016) 53(5):2878–85. doi: 10.1007/s12035-015-9171-9
51. Mai W, Hu X, Lu Z, Qiu W, Peng F, Wang Y. Preliminary Study on the Association of AQP4 Promoter Polymorphism With Anti-Aquaporin-4 Antibody Positivity in Southern Han Chinese Patients With Idiopathic Demyelinating Disorders of Central Nervous System. J Neuroimmunol (2013) 255(1-2):75–80. doi: 10.1016/j.jneuroim.2012.10.004
52. Ogasawara M, Meguro A, Sakai T, Mizuki N, Takahashi T, Fujihara K, et al. Genetic Analysis of the Aquaporin-4 Gene for Anti-AQP4 Antibody-Positive Neuromyelitis Optica in a Japanese Population. Japanese J Ophthalmol (2016) 60(3):198–205. doi: 10.1007/s10384-016-0441-5
53. Liu Q-B, Li Z-X, Zhao G-X, Yu H, Wu Z-Y. No Association Between Identified Multiple Sclerosis Non-MHC Risk Loci and Neuromyelitis Optica. Neurosci Bull (2014) 30(6):1036–44. doi: 10.1007/s12264-013-1457-1
54. Mei S, Li X, Gong X, Li X, Yang L, Zhou H, et al. LC-MS/MS Analysis of Erythrocyte Thiopurine Nucleotides and Their Association With Genetic Variants in Patients With Neuromyelitis Optica Spectrum Disorders Taking Azathioprine. Ther Drug Monitoring (2017) 39(1):5–12. doi: 10.1097/FTD.0000000000000362
55. Zhuang J-C, Huang Z-Y, Zhao G-X, Yu H, Li Z-X, Wu Z-Y. Variants of CYP27B1 Are Associated With Both Multiple Sclerosis and Neuromyelitis Optica Patients in Han Chinese Population. Gene (2015) 557(2):236–9. doi: 10.1016/j.gene.2014.12.045
56. Kim HJ, Park H-Y, Kim E, Lee K-S, Kim K-K, Choi B-O, et al. Common CYP7A1 Promoter Polymorphism Associated With Risk of Neuromyelitis Optica. Neurobiol Dis (2010) 37(2):349–55. doi: 10.1016/j.nbd.2009.10.013
57. Zhao G-X, Liu Y, Li Z-X, Lv C-Z, Traboulsee A, Sadovnick AD, et al. Variants in the Promoter Region of CYP7A1 Are Associated With Neuromyelitis Optica But Not With Multiple Sclerosis in the Han Chinese Population. Neurosci Bull (2013) 29(5):525–30. doi: 10.1007/s12264-013-1347-6
58. Liu C, Wang G, Liu H, Li Y, Li J, Dai Y, et al. CD226 Gly307Ser Association With Neuromyelitis Optica in Southern Han Chinese. Can J Neurological Sci (2012) 39(4):488–90. doi: 10.1017/S0317167100014001
59. Kim JY, Bae JS, Kim HJ, Shin HD. CD58 Polymorphisms Associated With the Risk of Neuromyelitis Optica in a Korean Population. BMC Neurol (2014) 14(1):1–6. doi: 10.1186/1471-2377-14-57
60. Liu J, Shi Z, Lian Z, Chen H, Zhang Q, Feng H, et al. Association of CD58 Gene Polymorphisms With NMO Spectrum Disorders in a Han Chinese Population. J Neuroimmunol (2017) 309:23–30. doi: 10.1016/j.jneuroim.2017.05.003
61. Park TJ, Kim H, Kim JH, Bae J, Cheong H, Park BL, et al. Associations of CD6, TNFRSF1A and IRF8 Polymorphisms With Risk of Inflammatory Demyelinating Diseases. Neuropathology Appl Neurobiol (2013) 39(5):519–30. doi: 10.1111/j.1365-2990.2012.01304.x
62. Wang X, Yu T, Yan Q, Wang W, Meng N, Li X, et al. Significant Association Between Fc Receptor-Like 3 Polymorphisms (-1901A> G and-658C> T) and Neuromyelitis Optica (NMO) Susceptibility in the Chinese Population. Mol Neurobiol (2016) 53(1):686–94. doi: 10.1007/s12035-014-9036-7
63. Lan W, Fang S, Zhang H, Wang DTJ, Wu J. The Fc Receptor-Like 3 Polymorphisms (Rs7528684, Rs945635, Rs3761959 and Rs2282284) and the Risk of Neuromyelitis Optica in a Chinese Population. Medicine (2015) 94(38). doi: 10.1097/MD.0000000000001320
64. Shin J-G, Kim HJ, Park BL, Bae JS, Kim LH, Cheong HS, et al. Putative Association of GPC5 Polymorphism With the Risk of Inflammatory Demyelinating Diseases. J neurological Sci (2013) 335(1-2):82–8. doi: 10.1016/j.jns.2013.08.031
65. Brill L, Vaknin-Dembinsky A, Zveik O, Haham N, Miller K, Benedek G. MIF-173g/C Polymorphism Is Associated With NMO Disease Severity. J Neuroimmunol (2020) 339:577120. doi: 10.1016/j.jneuroim.2019.577120
66. Cai P-P, Wang H-X, Zhuang J-C, Liu Q-B, Zhao G-X, Li Z-X, et al. Variants of Autophagy-Related Gene 5 Are Associated With Neuromyelitis Optica in the Southern Han Chinese Population. Autoimmunity (2014) 47(8):563–6. doi: 10.3109/08916934.2014.929668
67. Ainiding G, Kawano Y, Sato S, Isobe N, Matsushita T, Yoshimura S, et al. Interleukin 2 Receptor α Chain Gene Polymorphisms and Risks of Multiple Sclerosis and Neuromyelitis Optica in Southern Japanese. J neurological Sci (2014) 337(1-2):147–50. doi: 10.1016/j.jns.2013.11.037
68. Dai Y, Li J, Zhong X, Wang Y, Qiu W, Lu Z, et al. IL2RA Allele Increases Risk of Neuromyelitis Optica in Southern Han Chinese. Can J Neurological Sci (2013) 40(6):832–5. doi: 10.1017/S0317167100015973
69. Zhuang J-C, Wu L, Qian M-Z, Cai P-P, Liu Q-B, Zhao G-X, et al. Variants of Interleukin-7/Interleukin-7 Receptor Alpha Are Associated With Both Neuromyelitis Optica and Multiple Sclerosis Among Chinese Han Population in Southeastern China. Chin Med J (2015) 128(22):3062. doi: 10.4103/0366-6999.169093
70. Kim JY, Cheong HS, Kim HJ, Kim LH, Namgoong S, Shin HD. Association Analysis of IL7R Polymorphisms With Inflammatory Demyelinating Diseases. Mol Med Rep (2014) 9(2):737–43. doi: 10.3892/mmr.2013.1863
71. Wang H, Zhong X, Wang K, Qiu W, Li J, Dai Y, et al. Interleukin 17 Gene Polymorphism Is Associated With Anti-Aquaporin 4 Antibody-Positive Neuromyelitis Optica in the Southern Han Chinese—a Case Control Study. J neurological Sci (2012) 314(1-2):26–8. doi: 10.1016/j.jns.2011.11.005
72. Liu Q-B, Wu L, Zhao G-X, Cai P-P, Li Z-X, Wu Z-Y. Variants of Interferon Regulatory Factor 5 Are Associated With Neither Neuromyelitis Optica Nor Multiple Sclerosis in the Southeastern Han Chinese Population. Chin Med J (2015) 128(13):1743. doi: 10.4103/0366-6999.159347
73. Forwell AL, Bernales CQ, Ross JP, Yee IM, Encarnacion M, Lee JD, et al. Analysis of CH25H in Multiple Sclerosis and Neuromyelitis Optica. J Neuroimmunol (2016) 291:70–2. doi: 10.1016/j.jneuroim.2015.12.014
74. Xu J, Zhang F, Gao C, Ma X, Peng X, Kong D, et al. Microarray Analysis of lncRNA and mRNA Expression Profiles in Patients With Neuromyelitis Optica. Mol Neurobiol (2017) 54(3):2201–8. doi: 10.1007/s12035-016-9754-0
75. Brill L, Lavon I, Vaknin-Dembinsky A. Reduced Expression of the IL7Ra Signaling Pathway in Neuromyelitis Optica. J Neuroimmunol (2018) 324:81–9. doi: 10.1016/j.jneuroim.2018.08.011
76. Satoh J, Obayashi S, Misawa T, Tabunoki H, Yamamura T, Arima K, et al. Neuromyelitis Optica/Devic’s Disease: Gene Expression Profiling of Brain Lesions. Neuropathology (2008) 28(6):561–76.
77. Vaknin-Dembinsky A, Charbit H, Brill L, Abramsky O, Gur-Wahnon D, Ben-Dov IZ, et al. Circulating microRNAs as Biomarkers for Rituximab Therapy, in Neuromyelitis Optica (NMO). J Neuroinflamm (2016) 13(1):1–8. doi: 10.1186/s12974-016-0648-x
78. Fujihara K, et al. Interleukin-6 in Neuromyelitis Optica Spectrum Disorder Pathophysiology. Neurol-Neuroimmunol Neuroinflamm (2020) 7(5). doi: 10.1212/NXI.0000000000000841
79. Uzawa A, Mori M, Arai K, Sato Y, Hayakawa S, Masuda S, et al. Cytokine and Chemokine Profiles in Neuromyelitis Optica: Significance of Interleukin-6. Multiple Sclerosis J (2010) 16(12):1443–52. doi: 10.1177/1352458510379247
80. Lavon I, et al. QKI-V5 Is Downregulated in CNS Inflammatory Demyelinating Diseases. Mult Scler Relat Disord (2020) 39:101881. doi: 10.1016/j.msard.2019.101881
81. Sato DK, Callegaro D, Lana-Peixoto MA, Waters PJ, de Haidar Jorge FM, Takahashi T, et al. Distinction Between MOG Antibody-Positive and AQP4 Antibody-Positive NMO Spectrum Disorders. Neurology (2014) 82(6):474–81. doi: 10.1212/WNL.0000000000000101
82. Shaygannejad V, Maljaei MB, Bank SS, Mirmosayyeb O, Maracy MR, Askari G. Association Between Sun Exposure, Vitamin D Intake, Serum Vitamin D Level, and Immunoglobulin G Level in Patients With Neuromyelitis Optica Spectrum Disorder. Int J Prev Med (2018) 9. doi: 10.4103/ijpvm.IJPVM_45_16
83. Min J-H, Waters P, Vincent A, Cho H-J, Joo B-E, Woo S-Y, et al. Low Levels of Vitamin D in Neuromyelitis Optica Spectrum Disorder: Association With Disease Disability. PloS One (2014) 9(9):e107274. doi: 10.1371/journal.pone.0107274
84. Kusumadewi W, Imran D, Witjaksono F, Pakasi TA, Rusmana AI, Pangeran D, et al. Low Vitamin D-25 (OH) Level in Indonesian Multiple Sclerosis and Neuromyelitis Optic Patients. Mult Scler Relat Disord (2018) 25:329–33. doi: 10.1016/j.msard.2018.08.030
85. Jitprapaikulsan J, Siritho S, Prayoonwiwat N. Vitamin D Level Status in Thai Neuromyelitis Optica Patients. J Neuroimmunol (2016) 295:75–8. doi: 10.1016/j.jneuroim.2016.03.016
86. Kovacs KT, Kalluri SR, Boza-Serrano A, Deierborg T, Csepany T, Simo M, et al. Change in Autoantibody and Cytokine Responses During the Evolution of Neuromyelitis Optica in Patients With Systemic Lupus Erythematosus: A Preliminary Study. Multiple Sclerosis J (2016) 22(9):1192–201. doi: 10.1177/1352458515613165
87. Ichinose K, Arima K, Ushigusa T, Nishino A, Nakashima Y, Suzuki T, et al. Distinguishing the Cerebrospinal Fluid Cytokine Profile in Neuropsychiatric Systemic Lupus Erythematosus From Other Autoimmune Neurological Diseases. Clin Immunol (2015) 157(2):114–20. doi: 10.1016/j.clim.2015.01.010
88. Matsushita T, Tateishi T, Isobe N, Yonekawa T, Yamasaki R, Matsuse D, et al. Characteristic Cerebrospinal Fluid Cytokine/Chemokine Profiles in Neuromyelitis Optica, Relapsing Remitting or Primary Progressive Multiple Sclerosis. PloS One (2013) 8(4):e61835. doi: 10.1371/journal.pone.0061835
89. Wang KC, et al. Distinct Serum Cytokine Profiles in Neuromyelitis Optica and Multiple Sclerosis. J Interferon Cytokine Res (2013) 33(2):58–64. doi: 10.1089/jir.2012.0040
90. Liu B, Zhong X, Lu Z, Qiu W, Hu X, Wang H. Cerebrospinal Fluid Level of Soluble CD27 Is Associated With Disease Severity in Neuromyelitis Optica Spectrum Disorder. Neuroimmunomodulation (2018) 25(4):185–92. doi: 10.1159/000489561
91. Pei S, Zheng D, Wang Z, Hu X, Pan S, Wang H. Elevated Soluble Syndecan-1 Levels in Neuromyelitis Optica Are Associated With Disease Severity. Cytokine (2018) 111:140–5. doi: 10.1016/j.cyto.2018.08.017
92. Cho EB, Cho H-J, Seok JM, Min J-H, Kang E-S, Kim BJ. The IL-10-Producing Regulatory B Cells (B10 Cells) and Regulatory T Cell Subsets in Neuromyelitis Optica Spectrum Disorder. Neurological Sci (2018) 39(3):543–9. doi: 10.1007/s10072-018-3248-y
93. Tahani S, Dehghani L, Jahanbani-Ardakani H, Shaygannejad V, Fazli A, Hamidavi A, et al. Elevated Serum Level of IL-4 in Neuromyelitis Optica and Multiple Sclerosis Patients. J Immunoassay Immunochem (2019) 40(5):555–63. doi: 10.1080/15321819.2019.1655649
94. Alves-Leon SV, Pimentel MLV, Sant'Anna G, Malfetano FR, Estrada CD, Quirico-Santos T. Immune System Markers of Neuroinflammation in Patients With Clinical Diagnose of Neuromyelitis Optica. Arquivos neuro-psiquiatria (2008) 66(3B):678–84. doi: 10.1590/S0004-282X2008000500013
95. Uzawa A, Mori M, Ito M, Uchida T, Hayakawa S, Masuda S, et al. Markedly Increased CSF Interleukin-6 Levels in Neuromyelitis Optica, But Not in Multiple Sclerosis. J Neurol (2009) 256(12):2082–4. doi: 10.1007/s00415-009-5274-4
96. İİçöz S, Tüzün E, Kürtüncü M, Durmuşş H, Mutlu M, Eraksoy M, et al. Enhanced IL-6 Production in Aquaporin-4 Antibody Positive Neuromyelitis Optica Patients. Int J Neurosci (2010) 120(1):71–5.
97. Uzawa A, Mori M, Masuda H, Ohtani R, Uchida T, Sawai S, et al. Interleukin-6 Analysis of 572 Consecutive CSF Samples From Neurological Disorders: A Special Focus on Neuromyelitis Optica. Clinica Chimica Acta (2017) 469:144–9. doi: 10.1016/j.cca.2017.03.006
98. Wang H, Wang K, Zhong X, Dai Y, Qiu W, Wu A, et al. Notable Increased Cerebrospinal Fluid Levels of Soluble Interleukin-6 Receptors in Neuromyelitis Optica. Neuroimmunomodulation (2012) 19(5):304–8. doi: 10.1159/000339302
99. Uzawa A, Mori M, Sawai S, Masuda S, Muto M, Uchida T, et al. Cerebrospinal Fluid Interleukin-6 and Glial Fibrillary Acidic Protein Levels Are Increased During Initial Neuromyelitis Optica Attacks. Clinica Chimica Acta (2013) 421:181–3. doi: 10.1016/j.cca.2013.03.020
100. Yanagawa K, Kawachi I, Toyoshima Y, Yokoseki A, Arakawa M, Hasegawa A, et al. Pathologic and Immunologic Profiles of a Limited Form of Neuromyelitis Optica With Myelitis. Neurology (2009) 73(20):1628–37. doi: 10.1212/WNL.0b013e3181c1deb9
101. Correale J, Fiol M. Activation of Humoral Immunity and Eosinophils in Neuromyelitis Optica. Neurology (2004) 63(12):2363–70. doi: 10.1212/01.WNL.0000148481.80152.BF
102. Maghbooli Z, Moghadasi AN, Rezaeimanesh N, Omidifar A, Varzandi T, Sahraian MA. The Possible Role of Interleukin-6 as a Regulator of Insulin Sensitivity in Patients With Neuromyelitis Optica Spectrum Disorder. BMC Neurol (2021) 21(1):1–9.
103. Wang K-C, Tsai C-P, Lee C-L, Chen S-Y, Chin L-T, Chen S-J. Elevated Plasma High-Mobility Group Box 1 Protein Is a Potential Marker for Neuromyelitis Optica. Neuroscience (2012) 226:510–6. doi: 10.1016/j.neuroscience.2012.08.041
104. Wang H, Wang K, Wang C, Xu F, Zhong X, Qiu W, et al. Cerebrospinal Fluid High-Mobility Group Box Protein 1 in Neuromyelitis Optica and Multiple Sclerosis. Neuroimmunomodulation (2013) 20(2):113–8. doi: 10.1159/000345994
105. Uzawa A, et al. CSF High-Mobility Group Box 1 Is Associated With Intrathecal Inflammation and Astrocytic Damage in Neuromyelitis Optica. J Neurology Neurosurgery Psychiatry (2013) 84(5):517–22. doi: 10.1136/jnnp-2012-304039
106. Ashtari F, Madanian R, Shaygannejad V, Zarkesh SH, Ghadimi K. Serum Levels of IL-6 and IL-17 in Multiple Sclerosis, Neuromyelitis Optica Patients and Healthy Subjects. Int J physiol pathophysiol Pharmacol (2019) 11(6):267.
107. Barros P, Cassano T, Hygino J, Ferreira T, Centurião N, Kasahara T, et al. Prediction of Disease Severity in Neuromyelitis Optica by the Levels of Interleukin (IL)-6 Produced During Remission Phase. Clin Exp Immunol (2016) 183(3):480–9. doi: 10.1111/cei.12733
108. Wang H, Wang K, Wang C, Xu F, Qiu W, Hu X. Increased Plasma Interleukin-32 Expression in Patients With Neuromyelitis Optica. J Clin Immunol (2013) 33(3):666–70. doi: 10.1007/s10875-012-9837-2
109. Li Y-J, Zhang F, Qi Y, Chang G-Q, Fu Y, Su L, et al. Association of Circulating Follicular Helper T Cells With Disease Course of NMO Spectrum Disorders. J Neuroimmunol (2015) 278:239–46. doi: 10.1016/j.jneuroim.2014.11.011
110. Wang H, Dai Y, Qiu W, Lu Z, Peng F, Wang Y, et al. Interleukin-17-Secreting T Cells in Neuromyelitis Optica and Multiple Sclerosis During Relapse. J Clin Neurosci (2011) 18(10):1313–7. doi: 10.1016/j.jocn.2011.01.031
111. Li Y, Wang H, Long Y, Lu Z, Hu X. Increased Memory Th17 Cells in Patients With Neuromyelitis Optica and Multiple Sclerosis. J Neuroimmunol (2011) 234(1-2):155–60. doi: 10.1016/j.jneuroim.2011.03.009
112. Wu A, Zhong X, Wang H, Xu W, Cheng C, Dai Y, et al. Cerebrospinal Fluid IL-21 Levels in Neuromyelitis Optica and Multiple Sclerosis. Can J neurological Sci (2012) 39(6):813–20. doi: 10.1017/S0317167100015663
113. Xu W, Dai Y, Wu A, Wang H, Cheng C, Qiu W, et al. IL-22 Secreting CD4+ T Cells in the Patients With Neuromyelitis Optica and Multiple Sclerosis. J Neuroimmunol (2013) 261(1-2):87–91. doi: 10.1016/j.jneuroim.2013.04.021
114. Ulusoy C, Tüzün E, Kürtüncü M, Türkoğlu R, Akman-Demir G, Eraksoy M. Comparison of the Cytokine Profiles of Patients With Neuronal-Antibody-Associated Central Nervous System Disorders. Int J Neurosci (2012) 122(6):284–9. doi: 10.3109/00207454.2011.648762
115. Farrokhi M, Rezaei A, Amani-Beni A, Etemadifar M, Kouchaki E, Zahedi A. Increased Serum Level of IL-37 in Patients With Multiple Sclerosis and Neuromyelitis Optica. Acta Neurologica Belgica (2015) 115(4):609–14. doi: 10.1007/s13760-015-0491-3
116. Yang T, Wang S, Yang X, Zheng Q, Wang L, Li Q, et al. Upregulation of Bcl-2 and Its Promoter Signals in CD4+ T Cells During Neuromyelitis Optica Remission. Front Neurosci (2017) 11:11. doi: 10.3389/fnins.2017.00011
117. Zeng Q, Dong X, Ruan C, Hu B, Luo Y, Luo Z, et al. CD14+ CD16++ Monocytes Are Increased in Patients With NMO and Are Selectively Suppressed by Glucocorticoids Therapy. J Neuroimmunol (2016) 300:1–8. doi: 10.1016/j.jneuroim.2016.09.011
118. Yang T, Wang S, Zheng Q, Wang L, Li Q, Wei M, et al. Increased Plasma Levels of Epithelial Neutrophil-Activating Peptide 78/CXCL5 During the Remission of Neuromyelitis Optica. BMC Neurol (2016) 16(1):1–6. doi: 10.1186/s12883-016-0622-3
119. Fan X, Jiang Y, Han J, Liu J, Wei Y, Jiang X, et al. Circulating Memory T Follicular Helper Cells in Patients With Neuromyelitis Optica/Neuromyelitis Optica Spectrum Disorders. Mediators Inflamm (2016) 2016. doi: 10.1155/2016/3678152
120. Vaknin-Dembinsky A, Brill L, Kassis I, Petrou P, Ovadia H, Ben-Hur T, et al. T-Cell Responses to Distinct AQP4 Peptides in Patients With Neuromyelitis Optica (NMO). Mult Scler Relat Disord (2016) 6:28–36. doi: 10.1016/j.msard.2015.12.004
121. Quan C, Yu H, Qiao J, Xiao B, Zhao G, Wu Z, et al. Impaired Regulatory Function and Enhanced Intrathecal Activation of B Cells in Neuromyelitis Optica: Distinct From Multiple Sclerosis. Multiple Sclerosis J (2013) 19(3):289–98. doi: 10.1177/1352458512454771
122. Pentón-Rol G, Cervantes-Llanos M, Martínez-Sánchez G, Cabrera-Gómez JA, Valenzuela-Silva CM, Ramírez-Nuñez O, et al. TNF-α and IL-10 Downregulation and Marked Oxidative Stress in Neuromyelitis Optica. J Inflammation (2009) 6(1):1–9.
123. Hosokawa T, Nakajima H, Doi Y, Sugino M, Kimura F, Hanafusa T, et al. Increased Serum Matrix Metalloproteinase-9 in Neuromyelitis Optica: Implication of Disruption of Blood–Brain Barrier. J Neuroimmunol (2011) 236(1-2):81–6. doi: 10.1016/j.jneuroim.2011.04.009
124. Uchida T, Mori M, Uzawa A, Masuda H, Muto M, Ohtani R, et al. Increased Cerebrospinal Fluid Metalloproteinase-2 and Interleukin-6 Are Associated With Albumin Quotient in Neuromyelitis Optica: Their Possible Role on Blood–Brain Barrier Disruption. Multiple Sclerosis J (2017) 23(8):1072–84. doi: 10.1177/1352458516672015
125. Tasaki A, Shimizu F, Sano Y, Fujisawa M, Takahashi T, Haruki H, et al. Autocrine MMP-2/9 Secretion Increases the BBB Permeability in Neuromyelitis Optica. J Neurology Neurosurgery Psychiatry (2014) 85(4):419–30. doi: 10.1136/jnnp-2013-305907
126. Kang H, Cao S, Chen T, Jiang Z, Liu Z, Li Z, et al. The Poor Recovery of Neuromyelitis Optica Spectrum Disorder Is Associated With a Lower Level of CXCL12 in the Human Brain. J Neuroimmunol (2015) 289:56–61. doi: 10.1016/j.jneuroim.2015.10.005
127. García-Miranda P, Morón-Civanto FJ, Martínez-Olivo MdM, Suárez-Luna N, Ramírez-Lorca R, Lebrato-Hernández L, et al. Predictive Value of Serum Antibodies and Point Mutations of AQP4, AQP1 and MOG in A Cohort of Spanish Patients With Neuromyelitis Optica Spectrum Disorders. Int J Mol Sci (2019) 20(22):5810. doi: 10.3390/ijms20225810
128. Marnetto F, Hellias B, Granieri L, Frau J, Patanella AK, Nytrova P, et al. Western Blot Analysis for the Detection of Serum Antibodies Recognizing Linear Aquaporin-4 Epitopes in Patients With Neuromyelitis Optica. J neuroimmunol (2009) 217(1-2):74–9. doi: 10.1016/j.jneuroim.2009.10.002
129. Alidadiani P, Eskandari N, Shaygannejad V, Dabiri A, Manian M, Jahanbani-Ardakani H, et al. Expression of OX40 Gene and Its Serum Levels in Neuromyelitis Optica Patients. Biomolecular concepts (2018) 10(1):62–7.
130. Chitsaz N, Dehghani L, Safi A, Esmalian-Afyouni N, Shaygannejad V, Rezvani M, et al. Evaluation of Glucose-6-Phosphate Dehydrogenase Serum Level in Patients With Multiple Sclerosis and Neuromyelitis Optica. Iranian J Neurol (2019) 18(4):150.
131. Saini H, Fernandez G, Kerr D, Levy M. Differential Expression of Aquaporin-4 Isoforms Localizes With Neuromyelitis Optica Disease Activity. J Neuroimmunol (2010) 221(1-2):68–72. doi: 10.1016/j.jneuroim.2010.02.007
132. Haruki H, Sano Y, Shimizu F, Omoto M, Tasaki A, Oishi M, et al. NMO Sera Down-Regulate AQP4 in Human Astrocyte and Induce Cytotoxicity Independent of Complement. J Neurol Sci (2013) 331(1-2):136–44. doi: 10.1016/j.jns.2013.05.035
133. Du L, Chang H, Xu W, Wei Y, Wang Y, Yin L, et al. Effect of NMO-IgG on the Interleukin-6 Cascade in Astrocytes via Activation of the JAK/STAT3 Signaling Pathway. Life Sci (2020) 258:118217. doi: 10.1016/j.lfs.2020.118217
134. Barros PO, Linhares UC, Teixeira B, Kasahara TM, Ferreira TB, Alvarenga R, et al. High In Vitro Immune Reactivity to Escherichia Coli in Neuromyelitis Optica Patients Is Correlated With Both Neurological Disabilities and Elevated Plasma Lipopolysaccharide Levels. Hum Immunol (2013) 74(9):1080–7. doi: 10.1016/j.humimm.2013.06.016
135. Zhang H, Bennett JL, Verkman A. Ex Vivo Spinal Cord Slice Model of Neuromyelitis Optica Reveals Novel Immunopathogenic Mechanisms. Ann Neurol (2011) 70(6):943–54. doi: 10.1002/ana.22551
136. Zhang H, Verkman A. Eosinophil Pathogenicity Mechanisms and Therapeutics in Neuromyelitis Optica. J Clin Invest (2013) 123(5):2306–16. doi: 10.1172/JCI67554
137. Shimizu F, Nishihara H, Sano Y, Takeshita Y, Takahashi S, Maeda T, et al. Markedly Increased IP-10 Production by Blood-Brain Barrier in Neuromyelitis Optica. PloS One (2015) 10(3):e0122000. doi: 10.1371/journal.pone.0122000
Keywords: genetics, HLA, association, neuromyelitis optica spectrum disorder, expression
Citation: Ghafouri-Fard S, Azimi T and Taheri M (2021) A Comprehensive Review on the Role of Genetic Factors in Neuromyelitis Optica Spectrum Disorder. Front. Immunol. 12:737673. doi: 10.3389/fimmu.2021.737673
Received: 07 July 2021; Accepted: 10 September 2021;
Published: 05 October 2021.
Edited by:
Clio Mavragani, National and Kapodistrian University of Athens, GreeceReviewed by:
Amin Safa, Complutense University of Madrid, SpainMasayuki Tahara, Utano Hospital (NHO), Japan
Rezvan Noroozi, Jagiellonian University, Poland
Copyright © 2021 Ghafouri-Fard, Azimi and Taheri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohammad Taheri, Mohammad_823@yahoo.com
 Soudeh Ghafouri-Fard
Soudeh Ghafouri-Fard Tahereh Azimi
Tahereh Azimi Mohammad Taheri
Mohammad Taheri