- 1Department of Surgery, Duke University, Durham, NC, United States
- 2School of Medicine, Duke University, Durham, NC, United States
- 3Department of Medicine, Division of Nephrology, Duke University, Durham, NC, United States
Donor specific transfusions have been the basis of tolerance inducing protocols since Peter Medawar showed that it was experimentally feasible in the 1950s. Though trials of cellular therapies have become increasingly common in solid organ transplantation, they have not become standard practice. Additionally, whereas some protocols have focused on cellular therapies as a method for donor antigen delivery—thought to promote tolerance in and of itself in the correct immunologic context—other approaches have alternatively focused on the intrinsic immunosuppressive properties of the certain cell types with less emphasis on their origin, including mesenchymal stem cells, regulatory T cells, and regulatory dendritic cells. Regardless of intent, all cellular therapies must contend with the potential that introducing donor antigen in a new context will lead to sensitization. In this review, we focus on the variety of cellular therapies that have been applied in human trials and non-human primate models, describe their efficacy, highlight data regarding their potential for sensitization, and discuss opportunities for cellular therapies within our current understanding of the immune landscape.
Introduction
Presently, solid organ allotransplantation is hampered by poor outcomes due to the nonspecific effects of immunosuppressive medications, especially calcineurin inhibtors (1, 2). Indeed, improvements in long term graft outcomes in both liver and kidney transplant have been minimal and disappointing in the past 20 years (3–5). In spite of this, there has been a consolidation of immunosuppressive management, with the vast majority of patients now managed on a calcineurin-based regimen (6).
In a distinct vein, there has long been an interest in the ability to specifically modify the immune response to donor antigens. This research has focused on utilizing donor derived biological products across a number of cell types and preparations to condition the immune system to accept the allograft and is based, conceptually, on the work of Sir Peter Medawar (7). In spite of Medawar’s early experiments, however, the ability to not simply blunt the total immune response but rather specifically inhibit the response to the donor has been elusive. Indeed, it has been haunted by the fact that specific inhibition requires that an immune system be exposed to the antigens which it may respond to, giving rise to a risk of sensitization. The use of regulatory cell therapies may be a way to avoid this, but they remain incompletely explored. An overview of cellular therapies currently under investigation may be found in Figure 1.
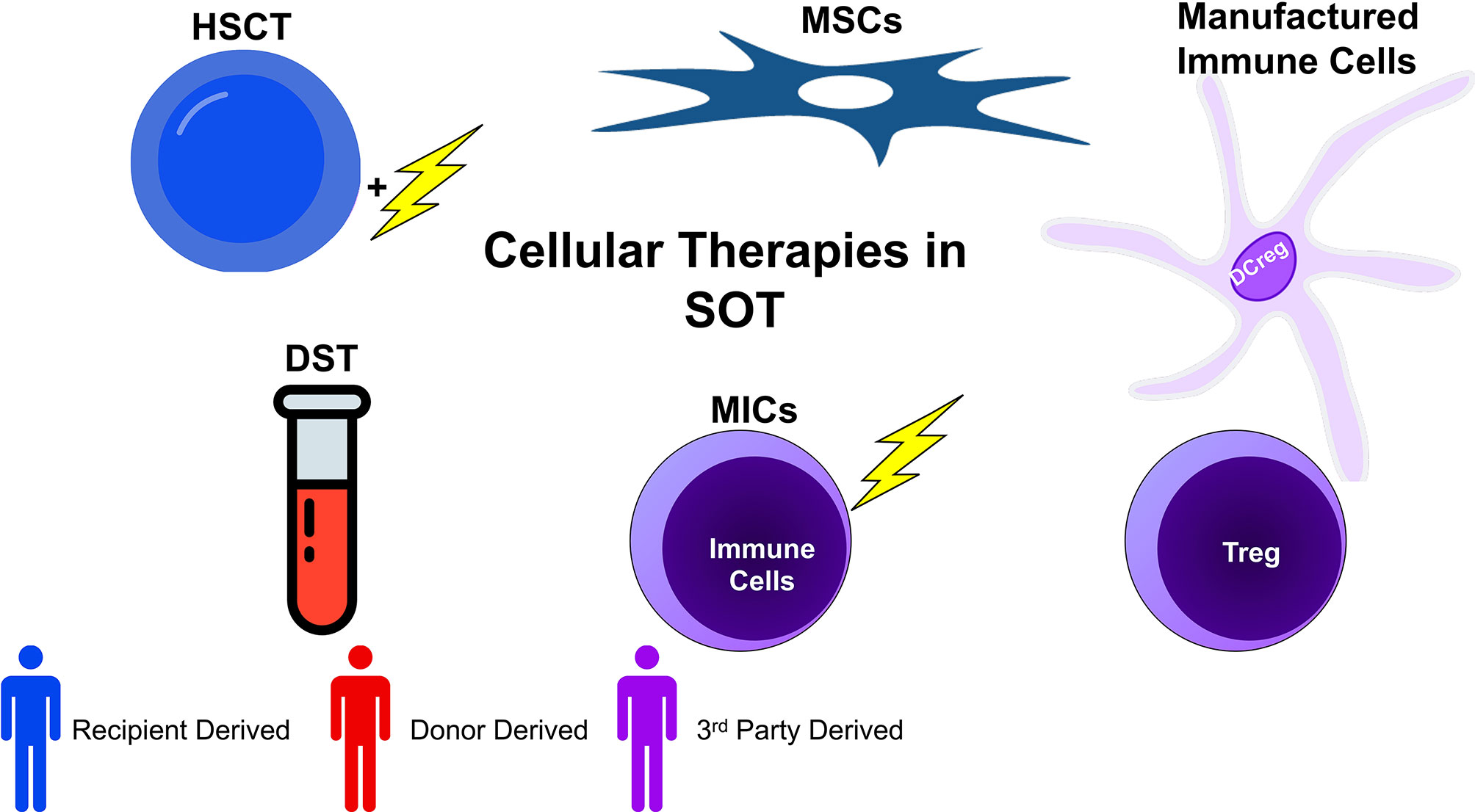
Figure 1 Cellular therapies in transplantation can be broadly categorized into five groups: donor specific transfusions (DSTs), hematopoietic stem cell transplant (HSCT), mesenchymal stem cell based therapy (MSC), manufactured immune cell therapy (using various regulatory cells), and modified immune cell (MIC) therapy. These broad categories can be further subdivided by the source of the cells. Whereas recipient derived cells carry no risk of sensitization (or very little), donor derived cells inherently may sensitize the recipient. Additionally, 3rd party cells, depending on their genetic similarity with donor and recipient, may or may not be sensitizing. When evaluating cellular therapies, both the function and origin of cells are of import.
In the present manuscript, we will review the progress made in the application of donor specific cellular therapies starting with an examination of early evidence. We will further describe the multiple cellular strategies currently being investigated in clinical trials including the combination of donor specific transfusion (DST) with novel immunosuppressive regimens, modified cellular therapies, and specific cell type based therapies. Finally, we will examine the future landscape of donor directed therapies and the unmet research need.
The Origins of Cellular Therapy in Transplantation
Few experiments in transplantation are as well-known as those of Sir Peter Medawar. In his seminal set of studies, Dr. Medawar built on the work of Dr. Ray Owen (8) and demonstrated that intra-embryonic injection of cells from future skin donors led to tolerance among a subset of mice (7). Ultimately, Medawar was awarded the Nobel Prize for this work and it remains the theoretical basis for many tolerance inducing protocols today. A few specific aspects of the experiment are worth noting. First, the injections occurred early in the development of the mice; therefore, the thymus was robust and actively educating T cells. Thymic development continues to adolescence when involution begins (9). Therefore, these injections in the fetal period had the advantage of potentially utilizing the positive and negative selection programs of the thymus to abrogate responses to the alloantigens.
Additionally, they utilized a mix of donor cells from the “testis, kidney, and splenic tissue” (7). Here, the splenic tissue used suggests a high proportion of B cells as well as professional antigen presenting cells (APCs) which are known to express higher levels of major histocompatibility complex (MHC) class II as well as class I, which is constitutively expressed. That is, there were a variety of antigens expressed by these tissues and therefore available to the recipients. Taken together, these details suggest that both the character of donor derived infusions and the overall immunologic context (in this case most modified by the pre-natal environment) may determine the fate of the immune response.
Though his experiments were conceptually very important, the clinical reality of early transplantation was more complex and the application of cellular products of as treatment remains elusive (10). As adults were the first recipients of transplanted organs, the selective programs of the thymus were less available. Early experience with DST showed the now predictable result of allosensitization leading to the inability to transplant and a divisive discussion within the transplant community regarding the benefit of transfusion pre-operatively (11). Still, results were heterogenous: some early animal models hinted at the benefit of DST (12) and a number of early studies indeed showed that non-leukoreduced transfusions in general (not donor specific) appeared to lengthen graft survival, though the mechanism was unknown (13, 14).
Salvatierra and colleagues were some of the first to specifically evaluate the role of DST on graft survival in a systematic way (15). In this seminal work, they showed that while 1/3 of patients became sensitized (as measured by persistently high levels of alloantibody), 2/3 of patients were able to be transplanted. These patients had improved graft survival compared to similarly mismatched controls (94% vs. 56%). Despite a high degree of donor responsiveness as measured by mixed-lymphocyte reaction (MLR), patients who received DST had lower rates of acute rejection in the first 3 months using similar immunosuppression (44% vs. 82%). From these experiments, it was difficult to determine the exact differentiating factor which caused sensitization in some and tolerogenic effects in others. However, the authors did show that all patients who became sensitized with DST had also received multiple other blood transfusions. In this instance, the treatment was standardized (non-frozen, non-leukoreduced DST) but the context, potentially measured by the amount of 3rd party (i.e., neither donor nor recipient) blood transfusions, may have modified the ultimate immune response to donor antigen. Concomitantly, experimentation in mouse models showed similar results with improved skin graft survival with DST but not with 3rd party transfusion (16).
Over the next decade, several studies continued to illustrate the principles by which DST may be beneficial. First, evidence showed that the salutary effect of DST was most apparent in human leukocyte antigen (HLA) mismatched recipients, whereas it had little effect in fully haploidentical recipients (17). This was predictable as HLA matched recipients may have fewer antigens to tolerize to and therefore the effect would be of a lower magnitude. Additionally, it is worth noting that even in the Salvatierra study, the graft survival of the high reactivity MLR group was worse than that of the low reactivity MLR group (which did not receive DST), even with the addition of DST (15). Other studies suggested that at least some degree of HLA similarity was needed for improved graft survival with DST (18). Further mechanistic studies were also undertaken around this time which showed that it was possible that APC-depleted DST were more effective at inducing tolerance (19), suggesting that the type of cell was of import. However, due to the continued risk of sensitization, DST fell out of favor throughout the 1990s and early 2000s, culminating in a meta-analysis in 2010 which concluded that the risks of DST outweighed the benefits in the modern immunosuppressive era (20).
Combining DST and Costimulation Blockade
One area where DST continues to be investigated is in conjunction with costimulation blockade. Early murine experiments showed indefinite islet graft survival when DST with small lymphocytes (with or without T-cell depletion) was combined with anti-CD154 (21) or when DST with splenocytes was combined with CTLA4Ig (22) or anti-CD154 (23). Elegant mechanistic experiments showed that these effects may be mediated by deletion of alloreactive cells (24), the production of allospecific suppressor cells (25), or the induction of anergy (26) and appeared to depend at least in part on indirect presentation of donor antigens by recipient MHCs, whereas direct presentation was dispensible (27). Indeed, improved outcomes were achieved using DST in conjunction with rapamycin and anti-CD154 in a rhesus macaque model of skin transplantation, compared to rapamycin and anti-CD154 alone (28). This prompted further trials which showed operational tolerance in kidney transplant in both rhesus macaque (29) and cynomologous (30) models. Trials using belatacept based costimulation blockade, rapamycin, and DST in the form of donor bone marrow have been completed with many patients able to be maintained on belatacept monotherapy with excellent renal function outcomes (31, 32). In these studies, alemtuzumab—an anti-CD52 monoclonal antibody which is a potent depletional induction agent which causes near immediate (33) and long lasting lymphopenia, up to 6 months (34)—was used. This potentially allowed for remodeling of the immune system during its reconstitution (35). Of note, a total of n = 5 (12%) of patients on this protocol developed de novo DSA after transplant, though it is unclear what clinical significance these antibodies have.
DST Using Modified Cellular Products
Another distinct line of investigations has attempted to deliver donor antigen itself in a more tolerogenic manner. Beginning with multiple early studies in mice, Luo and colleagues have shown that pre-treating cellular infusions (in this case splenocytes) with ethylene carbodiimide (ECDI) leads to long term tolerance in a high proportion of mice undergoing islet transplantation into the kidney capsule (36). Of note, they showed that this effect was donor specific, with no prolongation of 3rd party grafts. Multiple findings from this first study are mechanistically interesting. First, they found that the ablation of Treg via an anti-CD25 antibody prior to ECDI-treated cell infusion abrogated tolerance induction but late (120 days after transplantation) depletion of Treg did not. Additionally, they showed that the PD-1 axis was necessary for tolerance induction.
Following on from these studies, it was demonstrated that ECDI treated splenocytes could condition tolerance in murine cardiac allografts (37). Additionally this tolerance could be achieved not only with splenocytes but also culture-expanded B cells or cryopreserved cellular products and could be combined with clinically relevant immunosuppressive regimens (38). Mechanistically, these infusions cause an expansion of myeloid derived suppressor cells (MDSCs) both at the site of the allograft (37) and systemically (39) which appear to be important in abrogating deleterious T-cell responses. ECDI treatment leads to apoptosis of the infused cells and therefore presentation of donor antigen in an apoptotic context. This should attenuate any inflammatory immune response and is generally termed efferocytosis—the clearing of apoptotic cells in a non-inflammatory manner (40). Specifically, it has been demonstrated that this anti-inflammatory program is mediated by the receptor tyrosine kinase MerTK which participates in the phagocytosis of apoptotic cells in an allotransplantation context via suppression of IFN-α signaling and subsequent expansion of MDSCs (41).
Recently, this strategy has also expanded into a non-human primate (NHP) model of islet cell transplantation, again, using splenocytes as the donor cell source supplemented by culture-expanded donor B cells from the peripheral blood. This lead to long term tolerance in a high proportion of recipients (42). Multiple experiments confirmed that there was a decrease in donor-specific T-cell response and an increase in the proportion of MDSCs. Of note, this protocol was not successful when primates had previous sensitization to donor antigens. However, in mice, ECDI treated splenocyte infusion when combined with rapamycin and anti-CD154 (MR1) led to prolonged survival in sensitized recipients relative to rapamycin and anti-CD 154 alone (43). Overall, ECDI treated cells represent one cellular therapy that is donor specific, that does not appear to cause sensitization, and may allow for the reduction or withdrawal of immunosuppression in select patients. Further work is needed to translate this therapy into vascularized grafts in NHP models in anticipation of human trials.
Another line of investigation that parallels the treatment of splenocytes with ECDI is the use of modified immune cells (MICs). Terness and colleagues initially observed that DCs treated with mitomycin C become tolerogenic in an in vitro context, potentially via downregulation of costimulatory molecules CD80/CD86 and ICAM-1. Intriguingly, T cells exposed to these DCs became stably tolerized to the antigens presented as these cells could not be restimulated after co-culture with these MICs (44). Further experiments showed that MICs prolonged graft survival in rat heart transplantation and again showed a phenotype of downregulated costimulatory molecules. Indeed, they achieved the same clinical effect using antibodies that coated DC costimulatory molecules and blocked the productive interaction of other cells with these DCs (45). They further extended these results from sorted DCs to whole PBMC in both rat and porcine contexts with improved graft survival with mitomycin C treated PBMC infusion (46). They also showed improved survival in a vascular composite allograft context in rats (47). Recently, they completed a trial in humans where they showed both safety of MICs derived from whole PBMC as well as specific inhibition of donor responses in patients treated with MICs (48). However, as this was a phase 1 study, patients were maintained on CNI for immunosuppression and no comparator group was examined. Of note, they also showed an increase in regulatory B cells and did not observe any sensitization in the study. In sum, modified cellular therapies are a promising but incompletely studied form of cellular therapy that may specifically inhibit the recipient response to donor.
Combined Hematopoietic Stem Cell Transplant and Solid Organ Transplantation
Another area where a form of DST continues to be investigated is in chimerism protocols. Early murine models showed tolerance could be achieved by the depletion of the recipient immune system followed by concomitant hematopoietic stem cell transplant (HSCT) and solid organ transplant from the same donor (49). Within a few years, this result had also been replicated in NHP (50). Mechanistically, this tolerance is thought to be due to chimerism (51). Though the outcomes were exciting, there was some hesitation as macrochimerism can lead to graft vs. host disease (GVHD), especially with the use of HLA-mismatched donors (52). Studies using combined HSCT in conjunction with solid organ transplant in humans were first pursued among patients with multiple myeloma who also qualified for kidney transplant. In these patients, they were conditioned using cyclophosphamide, anti-thymocyte globulin (ATG), and thymic irradiation. Though initial results with one (53), and then six subsequent patients (54) were encouraging, long term follow-up demonstrated GVHD (either acute or chronic) in 4/7 patients and the need for at least some immunosuppression in 3/7 patients (55).
In a more targeted way, the same group as above also performed combined HSCT and kidney transplant in five patients using a non-myeloablative regimen consisting of cyclophosphamide, thymic irradiation, and anti-CD2 monoclonal antibody, and a short course of cyclosporine. Four out of five patients were able to be weaned off all immunosuppression with stable graft function. Interestingly, the second patient on this protocol developed acute humoral rejection with early graft loss (day 10) in spite of a negative crossmatch, though none of the patients developed GVHD (56). Other groups have also attempted to achieve tolerance using combined HSCT and kidney transplant with variable success. One group has utilized total lymphoid irradiation and ATG for conditioning with a T-cell depleted HSCT infusion which has led to at least transient chimerism and the ability to withdraw immunosuppression in 16/22 (72% of patients) who were HLA-matched (57–59). However, none of the patients on this protocol who were HLA-mismatched have yet been able to undergo immunosuppression withdrawal. A final group has attempted HSCT with fludrabine, cyclophosphamide, and whole-body irradiation condition in conjunction with kidney transplant and shown the ability to withdraw immunosuppression in most patients that have stable chimerism, with some now up to 9.5 years off all immunosuppression (60–62). However, only 6/20 total patients and 6/15 who underwent weaning are currently still off immunosuppression. Though these protocols have been very successful in achieving tolerance in a small number of patients, the high morbidity and continued concern regarding GVHD has limited their widespread adoption.
A Shift to Cell Type Based Therapies—The Rise of Mesenchymal Stem Cells
As the field of transplantation shifted away from DST based strategies and early enthusiasm for non-CNI based therapies waned due to concerns about increased rates of acute rejeciton (63–66), there was a concomitant increase in interest in cellular therapies of specific cell types, especially ones that may be derived from the recipient themselves. One early stream of investigation targeted the use of mesenchymal stem cells (MSC). MSCs are heterogenous progenitor cells which can differentiate into mesodermal tissues, are adherent to plastic, and express certain cell surface markers. Of note, MSCs may be derived from many tissues but in practice are mostly isolated from bone marrow via culture methods that take advantage of the specific propensity of MSCs to adhere to plastic (67). Regardless, expansion of cells is required as the dose used is approximately 1.5 x 106 cells/kg body weight (68). It should be mentioned that the preparations of MSCs do vary widely, and some groups have shown that both cryopreservation itself and the way in which MSCs are prepared after cryopreservation, for example, influences their immunosuppressive effect (69).
The first description of MSCs as immunosuppressive was in 2002 when investigators utilized MSCs in a skin transplantation model among baboons (70). They noted that MSCs inhibited MLRs to alloantigens and that MSC administration to baboons prolonged skin graft survival. Importantly, they also noted that the effect of MSCs was independent of their origin. That is, MSCs prolonged skin grafts regardless of whether the grafts were from the same donor as the MSCs or 3rd party, suggesting a general immunosuppressive effect, not an allospecific one.
Mechanisms of MSC’s immunosuppressive effect are various and have been reviewed extensively in the literature (71). For the purposes of this review, the most salient mechanisms are the generation of tolerogenic APC. Early experiments showed that MSCs decreased ability of co-cultured dendritic cells (DCs) to stimulate T cells, including in an allostimulatory context (72). Further experimentation showed that this effect was in part due to the inhibition of DCs from entering the cell cycle (73) and potentially mediated by the soluble factors IL-6, IL-10, and hepatocyte growth factor (74). Additionally, MSC conditioned DCs have been shown to preference the generation of Treg via a CCL18 dependent mechanism (75). Indeed, taken together, these data together suggest that MSCs may ultimately change the context in which antigen is presented and therefore promote a tolerogenic phenotype. However, it is sobering to think that while MSCs may modify APCs to prevent a productive response to certain antigens, other cellular therapies may instead potentiate responses.
Consistent with the above mechanisms, further animal studies after Bartholemew’s initial description showed prolongation of graft survival regardless of origin of MSC. An early study in rat liver transplantation showed graft survival prolongation with MSC derived from donor, third-party, or syngeneic animals (76). Further studies in rat corneal transplant also showed prolongation of graft survival with third-party derived MSCs (77). Experiments in mouse models of heart (78) or kidney (79) transplantation showed prolongation of graft survival with the infusion of syngeneic MSCs when administered pre-transplant.
The first human trials of MSCs in transplantation were spurred on by safety in other fields (80, 81) and culminated in early clinical studies which showed the safety of MSC infusion among kidney transplant recipients (82, 83). Due to the nonspecific nature of the immunosuppressive effects of MSCs, early trials generally used autologously derived MSCs—thought to be the safest product—combined with kidney transplantation (84–86). In a randomized controlled trial setting, autologous MSC infusion with kidney transplantation and tacrolimus showed a lower incidence of acute rejection and opportunistic infection (83). However, the incidence of rejection in the standard therapy group was high (approximately 20%) and longer follow-up remains to be reported. Later trials in kidney (87, 88), liver (89, 90), and lung (91) transplantation utilized 3rd party derived MSCs and demonstrated a similarly good safety profile.
One study did use donor derived MSCs which have the theoretical benefit of both nonspecific immunosuppression combined with antigen specificity. Again, the authors demonstrated a good safety profile and were able to use a lower dose of tacrolimus in the MSC group compared to the standard immunosuppression group (92). Of note, there was no assessment of the development of alloantibody in this trial. In the opposite manner, a study used MSCs which were mismatched to the recipient HLA and the donor HLA (i.e., they were no “repeated mismatches”) and showed good safety (93). This study did assess for sensitization to the specific 3rd party alloantigen and did not observe it. Though one recipient had pre-existing allo-antibody to an MSC donor antigen, the antibody titer did not change with MSC infusion.
An interesting result from an early autologous MSC safety trial was the detection of an MSC infiltrate in a kidney graft when MSCs were administered 7 days after transplant (82). Though this did not lead to any production of antibody (the MSCs, again, were autologous) or lasting graft damage, there was a transient increase in creatinine. This may have been due in part to the proinflammatory environment of the recently implanted kidney. Indeed, a body of research exists that shows that innate signaling via molecules such as damage associated molecular patterns (DAMPs) (94) may modify the alloresponse. Similarly, these factors may be important when considering the infusion of dynamic cellular therapies.
One other important consideration of cellular therapies generally is their safety, which has been extensively reviewed previously for MSCs (95, 96). Important to transplant, prior studies have found that MSCs may promote a pro-coagulable state via the instant blood mediated inflammatory reaction (97), though administration directly into the bone marrow may mitigate this (98). Regardless, the clinical context of MSC therapy must be taken into consideration as this hypercoagulability may be deleterious to certain clinical conditions, for examples, in COVID-19 infeciton (99). In vascularized organ transplant, the potential for thrombosis of newly anastomosed vessels should lead to the tracking of these types of events when cellular therapies are utilized.
Overall, the data show that, while safe and slightly immunosuppressive in humans in vivo, MSCs do not themselves appear to condition for tolerance or cause drastic shifts in the recipient immune system. Indeed, a recent meta-analysis came to much the same conclusion (100). Of special note, present data do not suggest that MSCs are sensitizing, potentially due to their concomitant immunosuppressive effects, which raises the possibility of utilizing them as a delivery vector for alloantigen.
Other Cell Type Based Therapies—Treg and Beyond
Besides MSCs, many other cell types have been investigated. The largest study of regulatory cell based immunosuppressive products, the ONE study (101), was recently published. In this study, the safety of six different autologously derived cellular products was assessed using seven single arm studies that were harmonized for comparison. A single control arm was standard of care therapy for living donor renal transplant recipients using basiliximab induction, tapered steroid, mycophenolate mofetil, and tacrolimus. The six phase 1/2a interventional single arm studies consisted of two polyclonal Treg studies, two donor-antigen specific Treg studies, a tolerogenic DC study, and a regulatory macrophage study. As these were safety studies, no minimal graft outcomes were reported but all infusions were well tolerated and rejection events were similar between the control and interventional studies. Additionally, there were similar rates alloantibody production between the control and interventional studies. As all products were derived from recipients, the risk of sensitization was low, though the donor specific Treg were incubated with donor cells in order to achieve their specificity. A recent subset analysis of the natural Treg infusion data from this study has shown that these patients were more likely to be weaned to a monotherapy immunosuppression regimen (102). Still, the preparation of these cells is laborious and the appropriate timing of infusions remains unknown.
Conclusions
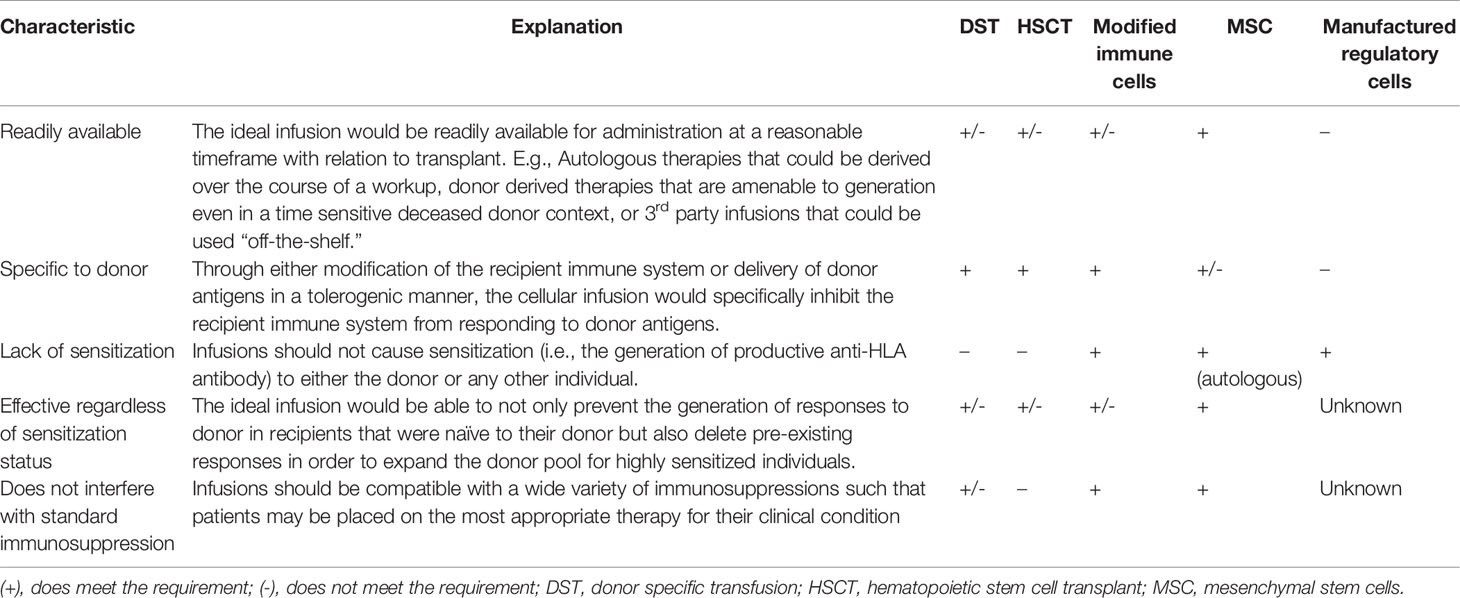
Cellular therapies, especially those derived from donors, carry great potential but also great risk. Early studies showed that DST may have a salutary effect in certain patients, but an inability to determine who may benefit from these transfusions and the high degree of sensitization (up to 1/3 of patients) led clinicians to abandon these therapies. A shift towards specific cell type based therapies has been productive in improving the field’s ability to manufacture and deliver consistent therapies but has yet to revolutionize the way in which we approach immunosuppression. Modified cellular therapies are on the horizon and represent an exciting development that is grounded in long standing understanding of mechanisms of the immune response. Combinations of DST with costimulation blockade have emerged as a promising approach. However, questions remain regarding sensitization, long-term outcomes, and the pool of patients appropriate for costimulation blockade-based therapy. Hematopoietic stem cell transplant in conjunction with solid organ transplant is less clinically applicable due to the morbidity involved with conditioning, even in the absence of high rates of GVHD. Regardless of the cellular therapies pursued, there are key characteristics that these therapies must possess to be most useful (summarized in Table 1).
Further phase 1 studies should continue to follow the example of the ONE study and others who attempt to gain as much information as possible with the most parsimonious control group. Additionally, further investigations into modifications of donor cells may be one way to overcome the significant hurdle that is sensitization. Transplantation is the original precision medicine, with HLA matching and crossmatch testing ensuring that organs implanted function appropriately and for the longest possible time. Cellular therapies ought to consider this precision in their development given the cost and potential for adverse events. In the future, the extent to which a therapy can inhibit a donor specific response relative to its overall immunosuppressive effect may become the most important metric, rather than a simplistic view which preferences only the inhibition of rejection episodes.
Author Contributions
BS drafted the manuscript. JO performed critical review and revision of the manuscript. CN performed critical review and revision of the manuscript. XL performed critical review and revision of the manuscript and oversaw the drafting process. All authors contributed to the article and approved the submitted version.
Funding
BS was supported by NIH grant R38 AI140297.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Naesens M, Kuypers DR, Sarwal M. Calcineurin Inhibitor Nephrotoxicity. Clin J Am Soc Nephrol (2009) 4(2):481–508. doi: 10.2215/cjn.04800908
2. Webster AC, Woodroffe RC, Taylor RS, Chapman JR, Craig JC. Tacrolimus versus Ciclosporin as Primary Immunosuppression for Kidney Transplant Recipients: Meta-Analysis and Meta-Regression of Randomised Trial Data. Bmj (2005) 331(7520):810. doi: 10.1136/bmj.38569.471007.AE
3. Lamb KE, Lodhi S, Meier-Kriesche HU. Long-Term Renal Allograft Survival in the United States: A Critical Reappraisal. Am J Transplant (2011) 11(3):450–62. doi: 10.1111/j.1600-6143.2010.03283.x
4. Coemans M, Süsal C, Döhler B, Anglicheau D, Giral M, Bestard O, et al. Analyses of the Short- And Long-Term Graft Survival after Kidney Transplantation in Europe between 1986 and 2015. Kidney Int (2018) 94(5):964–73. doi: 10.1016/j.kint.2018.05.018
5. Rana A, Ackah RL, Webb GJ, Halazun KJ, Vierling JM, Liu H, et al. No Gains in Long-term Survival After Liver Transplantation Over the Past Three Decades. Ann Surg (2019) 269(1):20–7. doi: 10.1097/sla.0000000000002650
6. Hart A, Lentine KL, Smith JM, et al. OPTN/SRTR 2019 Annual Data Report: Kidney. Am J Transplantation (2021) 21(S2):21–137. doi: 10.1111/ajt.16502
7. Billingham RE, Brent L, Medawar PB. ‘Actively Acquired Tolerance’ of Foreign Cells. Nature (1953) 172(4379):603–6. doi: 10.1038/172603a0
8. Owen RD. Immunogenetic Consequences Of Vascular Anastomoses Between Bovine Twins. Science (1945) 102(2651):400–1. doi: 10.1126/science.102.2651.400
9. Cowan JE, Takahama Y, Bhandoola A, Ohigashi I. Postnatal Involution and Counter-Involution of the Thymus. Front Immunol (2020) 11:897. doi: 10.3389/fimmu.2020.00897
10. Hickson LJ, Herrmann SM, McNicholas BA, Griffin MD. Progress toward the Clinical Application of Mesenchymal Stromal Cells and Other Disease-Modulating Regenerative Therapies: Examples from the Field of Nephrology. Kidney360 (2021) 2(3):542–57. doi: 10.34067/kid.0005692020
11. Van Rood JJ, Balner H. Blood Transfusion And Transplantation. Transplantation (1978) 26(5):275–7. doi: 10.1097/00007890-197811000-00001
12. Halasz NA, Orloff MJ, Hirose F. Increased Survival Of Renal Homografts In Dogs After Injection Of Graft Donor Blood. Transplantation (1964) 2(4):453–8. doi: 10.1097/00007890-196407000-00001
13. Hourmant M, Soulillou JP, Bui-Quang D. Beneficial Effect Of Blood Transfusion: Role Of The Time Interval Between The Last Transfusion And Transplantation. Transplantation (1979) 28(1):40–3. doi: 10.1097/00007890-197907000-00009
14. Stiller CR, Sinclair NR, Sheppard RR, Lockwood BL, Ulan RA, Sharpe JA, et al. Beneficial Effect Of Operation-Day Blood-Transfusions On Human Renal-Allograft SURVIVAL. Lancet (1978) 311(8057):169–70. doi: 10.1016/S0140-6736(78)90608-6
15. Salvatierra O Jr, Vincenti F, Amend W, Potter D, Iwaki Y, Opelz G, et al. Deliberate Donor-Specific Blood Transfusions Prior To Living Related Renal Transplantation. A New Approach. Ann Surg (1980) 192(4):543–52. doi: 10.1097/00000658-198010000-00012
16. Okazaki H, Maki T, Wood M, Monaco AP. Effect of a Single Transfusion of Donor-Specific and Nonspecific Blood On Skin Allograft Survival in Mice. Transplantation (1980) 30(6):421–4. doi: 10.1097/00007890-198012000-00007
17. Sanfilippo F, Thacker L, Vaughn WK. Living-Donor Renal Transplantation in SEOPF. The Impact of Histocompatibility, Transfusions, and Cyclosporine on Outcome. Transplantation (1990) 49(1):25–9. doi: 10.1097/00007890-199001000-00006
18. Twuyver EV, Mooijaart RJD, Berge IJMT, Horst ARVD, Wilmink JM, Kast WM, et al. Pretransplantation Blood Transfusion Revisited. N Engl J Med (1991) 325(17):1210–3. doi: 10.1056/nejm199110243251704
19. Hori S, Sato S, Kitagawa S, Azuma T, Kokudo S, Hamaoka T, et al. Tolerance Induction of Allo-Class II H-2 Antigen-Reactive L3T4+ Helper T Cells and Prolonged Survival of the Corresponding Class II H-2-Disparate Skin Graft. J Immunol (1989) 143(5):1447–52.
20. Mackie F. CARI Donor-Specific Transfusions. Nephrology (2010) 15(s1):S101–5. doi: 10.1111/j.1440-1797.2009.01217.x
21. Parker DC, Greiner DL, Phillips NE, Appel MC, Steele AW, Durie FH, et al. Survival of Mouse Pancreatic Islet Allografts in Recipients Treated With Allogeneic Small Lymphocytes and Antibody to CD40 Ligand. Proc Natl Acad Sci (1995) 92(21):9560–4. doi: 10.1073/pnas.92.21.9560
22. Sayegh MH, Zheng X-G, Magee C, Hancock WW, Turka LA. Donor Antigen Is Necessary for the Prevention of Chronic Rejection In CTLA4Ig-Treated Murine Cardiac Allograft Recipients. Transplantation (1997) 64(12):1646–50. doi: 10.1097/00007890-199712270-00003
23. Hancock WW, Sayegh MH, Zheng X-G, Peach R, Linsley PS, Turka LA. Costimulatory Function and Expression of CD40 Ligand, CD80, and CD86 in Vascularized Murine Cardiac Allograft Rejection. Proc Natl Acad Sci (1996) 93(24):13967–72. doi: 10.1073/pnas.93.24.13967
24. Iwakoshi NN, Mordes JP, Markees TG, Phillips NE, Rossini AA, Greiner DL. Treatment of Allograft Recipients With Donor-Specific Transfusion and anti-CD154 Antibody Leads to Deletion of Alloreactive CD8+ T Cells and Prolonged Graft Survival in a CTLA4-Dependent Manner. J Immunol (2000) 164(1):512–21. doi: 10.4049/jimmunol.164.1.512
25. Vignes C, Chiffoleau E, Douillard P, Josien R, Pêche H, Heslan J-M, et al. Anti-TCR-Specific DNA Vaccination Demonstrates a Role for a CD8+ T Cell Clone in the Induction of Allograft Tolerance by Donor-Specific Blood Transfusion. J Immunol (2000) 165(1):96–101. doi: 10.4049/jimmunol.165.1.96
26. Quezada SA, Fuller B, Jarvinen LZ, Gonzalez M, Blazar BR, Rudensky AY, et al. Mechanisms of Donor-Specific Transfusion Tolerance: Preemptive Induction of Clonal T-Cell Exhaustion via Indirect Presentation. Blood (2003) 102(5):1920–6. doi: 10.1182/blood-2003-02-0586
27. Kishimoto K, Yuan X, Auchincloss H, Sharpe AH, Mandelbrot DA, Sayegh MH. Mechanism of Action of Donor-Specific Transfusion in Inducing Tolerance: Role of Donor MHC Molecules, Donor Co-Stimulatory Molecules, and Indirect Antigen Presentation. J Am Soc Nephrol (2004) 15(9):2423–8. doi: 10.1097/01.Asn.0000137883.20961.2d
28. Xu H, Montgomery SP, Preston EH, Tadaki DK, Hale DA, Harlan DM, et al. Studies Investigating Pretransplant Donor-Specific Blood Transfusion, Rapamycin, and the CD154-Specific Antibody IDEC-131 in a Nonhuman Primate Model of Skin Allotransplantation. J Immunol (2003) 170(5):2776–82. doi: 10.4049/jimmunol.170.5.2776
29. Preston EH, Xu H, Dhanireddy KK, Pearl JP, Leopardi FV, Starost MF, et al. IDEC-131 (Anti-CD154), Sirolimus and Donor-Specific Transfusion Facilitate Operational Tolerance in Non-Human Primates. Am J Transplant (2005) 5(5):1032–41. doi: 10.1111/j.1600-6143.2005.00796.x
30. Pearl JP, Xu H, Leopardi F, Preston E, Kirk AD. CD154 Blockade, Sirolimus, and Donor-Specific Transfusion Prevents Renal Allograft Rejection in Cynomolgus Monkeys Despite Homeostatic T-Cell Activation. Transplantation (2007) 83(9):1219–25. doi: 10.1097/01.tp.0000259929.04596.d5
31. Kirk AD, Guasch A, Xu H, Cheeseman J, Mead SI, Ghali A, et al. Renal Transplantation Using Belatacept Without Maintenance Steroids or Calcineurin Inhibitors. Am J Transplant (2014) 14(5):1142–51. doi: 10.1111/ajt.12712
32. Schmitz R, Fitch ZW, Xu H, Ghali A, Mehta AK, Guasch A, et al. Kidney Transplantation Using Alemtuzumab, Belatacept, and Sirolimus: Five-year Follow-Up. Am J Transplantation (2020) 20(12):3609–19. doi: 10.1111/ajt.16121
33. Pearl JP, Parris J, Hale DA, Hoffmann SC, Bernstein WB, McCoy KL, et al. Immunocompetent T-Cells With a Memory-Like Phenotype are the Dominant Cell Type Following Antibody-Mediated T-Cell Depletion. Am J Transplant (2005) 5(3):465–74. doi: 10.1111/j.1600-6143.2005.00759.x
34. Calne R, Friend P, Moffatt S, Bradley A, Hale G, Firth J, et al. Prope Tolerance, Perioperative Campath 1H, and Low-Dose Cyclosporin Monotherapy in Renal Allograft Recipients. Lancet (1998) 351(9117):1701–2. doi: 10.1016/s0140-6736(05)77739-4
35. Stock P, Kirk AD. The risk and Opportunity of Homeostatic Repopulation. Am J Transplant (2011) 11(7):1349–50. doi: 10.1111/j.1600-6143.2011.03543.x
36. Luo X, Pothoven KL, McCarthy D, DeGutes M, Martin A, Getts DR, et al. ECDI-Fixed Allogeneic Splenocytes Induce Donor-Specific Tolerance for Long-Term Survival of Islet Transplants via Two Distinct Mechanisms. Proc Natl Acad Sci (2008) 105(38):14527–32. doi: 10.1073/pnas.0805204105
37. Chen G, Kheradmand T, Bryant J, Wang S, Tasch J, Wang JJ, et al. Intragraft CD11b(+) IDO(+) Cells Mediate Cardiac Allograft Tolerance by ECDI-Fixed Donor Splenocyte Infusions. Am J Transplant (2012) 12(11):2920–9. doi: 10.1111/j.1600-6143.2012.04203.x
38. Wang S, Zhang X, Zhang L, Bryant J, Kheradmand T, Hering BJ, et al. Preemptive Tolerogenic Delivery of Donor Antigens for Permanent Allogeneic Islet Graft Protection. Cell Transplant (2015) 24(6):1155–65. doi: 10.3727/096368914x681027
39. Bryant J, Lerret NM, Wang J-J, Kang H-K, Tasch J, Zhang Z, et al. Preemptive Donor Apoptotic Cell Infusions Induce IFN-γ–Producing Myeloid-Derived Suppressor Cells for Cardiac Allograft Protection. J Immunol (2014) 192(12):6092–101. doi: 10.4049/jimmunol.1302771
40. Arandjelovic S, Ravichandran KS. Phagocytosis of Apoptotic Cells in Homeostasis. Nat Immunol (2015) 16(9):907–17. doi: 10.1038/ni.3253
41. Zhang L, DeBerge M, Wang J, Dangi A, Zhang X, Schroth S, et al. Receptor Tyrosine Kinase MerTK Suppresses an Allogenic Type I IFN Response to Promote Transplant Tolerance. Am J Transplantation (2019) 19(3):674–85. doi: 10.1111/ajt.15087
42. Singh A, Ramachandran S, Graham ML, Daneshmandi S, Heller D, Suarez-Pinzon WL, et al. Long-Term Tolerance of Islet Allografts in Nonhuman Primates Induced by Apoptotic Donor Leukocytes. Nat Commun (2019) 10(1):3495. doi: 10.1038/s41467-019-11338-y
43. Dangi A, Yu S, Lee FT, Burnette M, Knechtle S, Kwun J, et al. Donor Apoptotic Cell-Based Therapy for Effective Inhibition of Donor-Specific Memory T and B Cells to Promote Long-Term Allograft Survival in Allosensitized Recipients. Am J Transplant (2020) 20(10):2728–39. doi: 10.1111/ajt.15878
44. Jiga LP, Bauer TM, Chuang JJ, Opelz G, Terness P. Generation of Tolerogenic Dendritic Cells by Treatment with Mitomycin C: Inhibition of Allogeneic T-Cell Response Is Mediated by Downregulation of ICAM-1, CD80, and CD86. Transplantation (2004) 77(11):1761–4. doi: 10.1097/01.tp.0000131165.37177.6e
45. Jiga LP, Ehser S, Kleist C, Opelz G, Terness P. Inhibition of Heart Allograft Rejection with Mitomycin C-Treated Donor Dendritic Cells. Transplantation (2007) 83(3):347–50. doi: 10.1097/01.tp.0000248854.30016.11
46. Kleist C, Sandra-Petrescu F, Jiga L, Dittmar L, Mohr E, Greil J, et al. Generation of Suppressive Blood Cells for Control of Allograft Rejection. Clin Science (2015) 128(9):593–607. doi: 10.1042/cs20140258
47. Radu CA, Fischer S, Diehm Y, Hetzel O, Neubrech F, Dittmar L, et al. The Combination of Mitomycin-Induced Blood Cells with a Temporary Treatment of Ciclosporin A Prolongs Allograft Survival in Vascularized Composite Allotransplantation. Langenbecks Arch Surg (2018) 403(1):83–92. doi: 10.1007/s00423-017-1616-3
48. Morath C, Schmitt A, Kleist C, Daniel V, Opelz G, Süsal C, et al. Phase I Trial of Donor-Derived Modified Immune Cell Infusion in Kidney Transplantation. J Clin Invest (2020) 130(5):2364–76. doi: 10.1172/jci133595
49. Sharabi Y, Sachs DH. Mixed Chimerism and Permanent Specific Transplantation Tolerance Induced By a Nonlethal Preparative Regimen. J Exp Med (1989) 169(2):493–502. doi: 10.1084/jem.169.2.493
50. Kawai T, Cosimi AB, Colvin RB, Powelson J, Eason J, Kozlowski T, et al. Mixed Allogeneic Chimerism and Renal Allograft Tolerance in Cynomolgus Monkeys. Transplantation (1995) 59(2):256–62. doi: 10.1097/00007890-199501000-00018
51. Kawai T, Poncelet A, Sachs DH, Mauiyyedi S, Boskovic S, Wee SL, et al. Long-Term Outcome And Alloantibody Production In A Non-Myeloablative Regimen For Induction Of Renal Allograft Tolerance. Transplantation (1999) 68(11):1767–75. doi: 10.1097/00007890-199912150-00022
52. Lee SJ, Klein J, Haagenson M, Baxter-Lowe LA, Confer DL, Eapen M, et al. High-Resolution Donor-Recipient HLA Matching Contributes to the Success of Unrelated Donor Marrow Transplantation. Blood (2007) 110(13):4576–83. doi: 10.1182/blood-2007-06-097386
53. Spitzer TR, Delmonico F, Tolkoff-Rubin N, McAfee S, Sackstein R, Saidman S, et al. Combined Histocompatibility Leukocyte Antigenmatched Donor Bone Marrow and Renal Transplantation for Multiple Myeloma with End Stage Renal Disease: The Induction of Allograft Tolerance through Mixed Lymphohematopoietic Chimerism. Transplantation (1999) 68(4):480–4. doi: 10.1097/00007890-199908270-00006
54. Fudaba Y, Spitzer T, Shaffer J, Kawai T, Fehr T, Delmonico F, et al. Myeloma Responses and Tolerance Following Combined Kidney and Nonmyeloablative Marrow Transplantation: In Vivo and In Vitro Analyses. Am J Transplantation (2006) 6(9):2121–33. doi: 10.1111/j.1600-6143.2006.01434.x
55. Spitzer TR, Sykes M, Tolkoff-Rubin N, Kawai T, McAfee SL, Dey BR, et al. Long-Term Follow-Up of Recipients of Combined Human Leukocyte Antigen-Matched Bone Marrow and Kidney Transplantation for Multiple Myeloma with End-Stage Renal Disease. Transplantation (2011) 91(6):672–6. doi: 10.1097/TP.0b013e31820a3068
56. Kawai T, Cosimi AB, Spitzer TR, Tolkoff-Rubin N, Suthanthiran M, Saidman SL, et al. HLA-Mismatched Renal Transplantation without Maintenance Immunosuppression. N Engl J Med (2008) 358(4):353–61. doi: 10.1056/NEJMoa071074
57. Scandling JD, Busque S, Dejbakhsh-Jones S, Benike C, Millan MT, Shizuru JA, et al. Tolerance and Chimerism after Renal and Hematopoietic-Cell Transplantation. N Engl J Med (2008) 358(4):362–8. doi: 10.1056/NEJMoa074191
58. Scandling JD, Busque S, Dejbakhsh-Jones S, Benike C, Sarwal M, Millan MT, et al. Tolerance and Withdrawal of Immunosuppressive Drugs in Patients Given Kidney and Hematopoietic Cell Transplants. Am J Transplantation (2012) 12(5):1133–45. doi: 10.1111/j.1600-6143.2012.03992.x
59. Scandling JD, Busque S, Shizuru JA, Lowsky R, Hoppe R, Dejbakhsh-Jones S, et al. Chimerism, Graft Survival, and Withdrawal of Immunosuppressive Drugs in HLA Matched and Mismatched Patients After Living Donor Kidney and Hematopoietic Cell Transplantation. Am J Transplantation (2015) 15(3):695–704. doi: 10.1111/ajt.13091
60. Leventhal J, Abecassis M, Miller J, Gallon L, Ravindra K, Tollerud DJ, et al. Chimerism and Tolerance without GVHD or Engraftment Syndrome in HLA-Mismatched Combined Kidney and Hematopoietic Stem Cell Transplantation. Sci Trans Med (2012) 4(124):124ra28–124ra28. doi: 10.1126/scitranslmed.3003509
61. Leventhal J, Abecassis M, Miller J, Gallon L, Tollerud D, Elliott MJ, et al. Tolerance Induction in HLA Disparate Living Donor Kidney Transplantation by Donor Stem Cell Infusion: Durable Chimerism Predicts Outcome. Transplantation (2013) 95(1):169. doi: 10.1097/TP.0b013e3182782fc1
62. Leventhal JR, Miller J, Mathew JM, Kurian S, Tambur AR, Friedewald J, et al. Updated Follow-Up of a Tolerance Protocol in HLA-Identical Renal Transplant Pairs Given Donor Hematopoietic Stem Cells. Hum Immunol (2018) 79(5):277–82. doi: 10.1016/j.humimm.2018.01.010
63. Vincenti F, Charpentier B, Vanrenterghem Y, Rostaing L, Bresnahan B, Darji P, et al. A Phase III Study of Belatacept-Based Immunosuppression Regimens versus Cyclosporine in Renal Transplant Recipients (BENEFIT Study). Am J Transplant (2010) 10(3):535–46. doi: 10.1111/j.1600-6143.2009.03005.x
64. Vincenti F, Larsen C, Durrbach A, Wekerle T, Nashan B, Blancho G, et al. Costimulation Blockade with Belatacept in Renal Transplantation. N Engl J Med (2005) 353(8):770–81. doi: 10.1056/NEJMoa050085
65. Vincenti F, Rostaing L, Grinyo J, Rice K, Steinberg S, Gaite L, et al. Belatacept and Long-Term Outcomes in Kidney Transplantation. N Engl J Med (2016) 374(4):333–43. doi: 10.1056/NEJMoa1506027
66. Cornell LD, Smith RN, Colvin RB. Kidney Transplantation: Mechanisms of Rejection and Acceptance. Annu Rev Pathol (2008) 3:189–220. doi: 10.1146/annurev.pathmechdis.3.121806.151508
67. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy (2006) 8(4):315–7. doi: 10.1080/14653240600855905
68. Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, et al. Mesenchymal Stem Cells for Treatment of Steroid-Resistant, Severe, Acute Graft-Versus-Host Disease: A Phase II Study. Lancet (2008) 371(9624):1579–86. doi: 10.1016/s0140-6736(08)60690-x
69. Chinnadurai R, Copland IB, Garcia MA, Petersen CT, Lewis CN, Waller EK, et al. Cryopreserved Mesenchymal Stromal Cells Are Susceptible to T-Cell Mediated Apoptosis Which Is Partly Rescued by IFNγ Licensing. Stem Cells (2016) 34(9):2429–42. doi: 10.1002/stem.2415
70. Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, et al. Mesenchymal Stem Cells Suppress Lymphocyte Proliferation in Vitro and Prolong Skin Graft Survival in Vivo. Exp Hematol (2002) 30(1):42–8. doi: 10.1016/S0301-472X(01)00769-X
71. Weiss ARR, Dahlke MH. Immunomodulation by Mesenchymal Stem Cells (MSCs): Mechanisms of Action of Living, Apoptotic, and Dead MSCs. Review. Front Immunol (2019) 10:1191. doi: 10.3389/fimmu.2019.01191
72. Jiang X-X, Zhang Y, Liu B, Zhang S-X, Wu Y, Yu X-D, et al. Human Mesenchymal Stem Cells Inhibit Differentiation and Function of Monocyte-Derived Dendritic Cells. Blood (2005) 105(10):4120–6. doi: 10.1182/blood-2004-02-0586
73. Ramasamy R, Fazekasova H, Lam EW-F, Soeiro I, Lombardi G, Dazzi F. Mesenchymal Stem Cells Inhibit Dendritic Cell Differentiation and Function by Preventing Entry into the Cell Cycle. Transplantation (2007) 83(1):71–6. doi: 10.1097/01.tp.0000244572.24780.54
74. Deng Y, Zhang Y, Ye L, Zhang T, Cheng J, Chen G, et al. Umbilical Cord-Derived Mesenchymal Stem Cells Instruct Monocytes Towards an Il10-Producing Phenotype by Secreting IL6 and HGF. Sci Rep (2016) 6(1):1–9. doi: 10.1038/srep37566
75. Melief SM, Schrama E, Brugman MH, Tiemessen MM, Hoogduijn MJ, Fibbe WE, et al. Multipotent Stromal Cells Induce Human Regulatory T Cells through a Novel Pathway Involving Skewing Of Monocytes toward Anti-Inflammatory Macrophages. Stem Cells (2013) 31(9):1980–91. doi: 10.1002/stem.1432
76. Wang Y, Zhang A, Ye Z, Xie H, Zheng S. Bone Marrow–Derived Mesenchymal Stem Cells Inhibit Acute Rejection of Rat Liver Allografts in Association with Regulatory T-Cell Expansion. Transplant Proc (2009) 41(10):4352–6. doi: 10.1016/j.transproceed.2009.08.072
77. Lohan P, Murphy N, Treacy O, Lynch K, Morcos M, Chen B, et al. Third-Party Allogeneic Mesenchymal Stromal Cells Prevent Rejection in a Pre-Sensitized High-Risk Model of Corneal Transplantation. Transplant Proc (2009) 41(10):4352–6. doi: 10.3389/fimmu.2018.02666
78. Casiraghi F, Azzollini N, Cassis P, Imberti B, Morigi M, Cugini D, et al. Pretransplant Infusion of Mesenchymal Stem Cells Prolongs the Survival of a Semiallogeneic Heart Transplant through the Generation of Regulatory T Cells. J Immunol (2008) 181(6):3933–46. doi: 10.4049/jimmunol.181.6.3933
79. Casiraghi F, Azzollini N, Todeschini M, Cavinato R, Cassis P, Solini S, et al. Localization of Mesenchymal Stromal Cells Dictates their Immune or Proinflammatory Effects in Kidney Transplantation. Am J Transplantation (2012) 12(9):2373–83. doi: 10.1111/j.1600-6143.2012.04115.x
80. Pal R, Venkataramana NK, Bansal A, Balaraju S, Jan M, Chandra R, et al. Ex Vivo-Expanded Autologous Bone Marrow-Derived Mesenchymal Stromal Cells in Human Spinal Cord Injury/Paraplegia: A Pilot Clinical Study. Cytotherapy (2009) 11(7):897–911. doi: 10.3109/14653240903253857
81. Lazarus HM, Koc ON, Devine SM, Curtin P, Maziarz RT, Holland HK, et al. Cotransplantation of HLA-Identical Sibling Culture-Expanded Mesenchymal Stem Cells and Hematopoietic Stem Cells in Hematologic Malignancy Patients. Biol Blood Marrow Transplant (2005) 11(5):389–98. doi: 10.1016/j.bbmt.2005.02.001
82. Perico N, Casiraghi F, Introna M, Gotti E, Todeschini M, Cavinato RA, et al. Autologous Mesenchymal Stromal Cells and Kidney Transplantation: A Pilot Study of Safety and Clinical Feasibility. Clin J Am Soc Nephrol (2011) 6(2):412–22. doi: 10.2215/cjn.04950610
83. Tan J, Wu W, Xu X, Liao L, Zheng F, Messinger S, et al. Induction Therapy with Autologous Mesenchymal Stem Cells in Living-Related Kidney Transplants: A Randomized Controlled Trial. JAMA (2012) 307(11):1169–77. doi: 10.1001/jama.2012.316
84. Reinders ME, de Fijter JW, Roelofs H, Bajema IM, de Vries DK, Schaapherder AF, et al. Autologous Bone Marrow-Derived Mesenchymal Stromal Cells for the Treatment of Allograft Rejection After Renal Transplantation: Results of a Phase I Study. Stem Cells Trans Med (2013) 2(2):107–11. doi: 10.5966/sctm.2012-0114
85. Mudrabettu C, Kumar V, Rakha A, Yadav AK, Ramachandran R, Kanwar DB, et al. Safety and Efficacy of Autologous Mesenchymal Stromal Cells Transplantation in Patients Undergoing Living Donor Kidney Transplantation: A Pilot Study. Nephrology (2015) 20(1):25–33. doi: 10.1111/nep.12338
86. Pan G-h, Chen Z, Xu L, Zhu J-h, Xiang P, Ma J-j, et al. Low-Dose Tacrolimus Combined with Donor-Derived Mesenchymal Stem Cells after Renal Transplantation: A Prospective, Non-Randomized Study. Oncotarget (2016) 7(11):12089. doi: 10.18632/oncotarget.7725
87. Sun Q, Huang Z, Han F, Zhao M, Cao R, Zhao D, et al. Allogeneic Mesenchymal Stem Cells as Induction Therapy Are Safe and Feasible In Renal Allografts: Pilot Results of a Multicenter Randomized Controlled Trial. J Trans Med (2018) 16(1):1–10. doi: 10.1186/s12967-018-1422-x
88. Erpicum P, Weekers L, Detry O, Bonvoisin C, Delbouille M-H, Grégoire C, et al. Infusion of Third-Party Mesenchymal Stromal Cells after Kidney Transplantation: A Phase I-II, Open-Label, Clinical Study. Kidney Int (2019) 95(3):693–707. doi: 10.1016/j.kint.2018.08.046
89. Soeder Y, Loss M, Johnson CL, Hutchinson JA, Haarer J, Ahrens N, et al. First-In-Human Case Study: Multipotent Adult Progenitor Cells for Immunomodulation after Liver Transplantation. Stem Cells Trans Med (2015) 4(8):899–904. doi: 10.5966/sctm.2015-0002
90. Detry O, Vandermeulen M, Delbouille M-H, Somja J, Bletard N, Briquet A, et al. Infusion of Mesenchymal Stromal Cells after Deceased Liver Transplantation: A Phase I–Ii, Open-Label, Clinical Study. J Hepatol (2017) 67(1):47–55. doi: 10.1016/j.jhep.2017.03.001
91. Keller CA, Gonwa TA, Hodge DO, Hei DJ, Centanni JM, Zubair AC. Feasibility, Safety, and Tolerance of Mesenchymal Stem Cell Therapy for Obstructive Chronic Lung Allograft Dysfunction. Stem Cells Trans Med (2018) 7(2):161–7. doi: 10.1002/sctm.17-0198
92. Peng Y, Ke M, Xu L, Liu L, Chen X, Xia W, et al. Donor-Derived Mesenchymal Stem Cells Combined With Low-Dose Tacrolimus Prevent Acute Rejection After Renal Transplantation: A Clinical Pilot Study. Transplantation (2013) 95(1):161–8. doi: 10.1097/TP.0b013e3182754c53
93. Dreyer GJ, Groeneweg KE, Heidt S, Roelen DL, van Pel M, Roelofs H, et al. Human Leukocyte Antigen Selected Allogeneic Mesenchymal Stromal Cell Therapy in Renal Transplantation: The Neptune Study, A Phase I Single-Center Study. Am J Transplantation (2020) 20(10):2905–15. doi: 10.1111/ajt.15910
94. Todd JL, Palmer SM. Danger Signals in Regulating the Immune Response to Solid Organ Transplantation. J Clin Invest (2017) 127(7):2464–72. doi: 10.1172/JCI90594
95. Moll G, Hoogduijn MJ, Ankrum JA. Editorial: Safety, Efficacy and Mechanisms of Action of Mesenchymal Stem Cell Therapies. Front Immunol (2020) 11:243. doi: 10.3389/fimmu.2020.00243
96. Caplan H, Olson SD, Kumar A, George M, Prabhakara KS, Wenzel P, et al. Mesenchymal Stromal Cell Therapeutic Delivery: Translational Challenges to Clinical Application. Front Immunol (2019) 10:1645. doi: 10.3389/fimmu.2019.01645
97. Moll G, Rasmusson-Duprez I, von Bahr L, Connolly-Andersen AM, Elgue G, Funke L, et al. Are Therapeutic Human Mesenchymal Stromal Cells Compatible with Human Blood? Stem Cells (2012) 30(7):1565–74. doi: 10.1002/stem.1111
98. Moll G, Ankrum JA, Kamhieh-Milz J, Bieback K, Ringden O, Volk HD, et al. Intravascular Mesenchymal Stromal/Stem Cell Therapy Product Diversification: Time for New Clinical Guidelines. Trends Mol Med (2019) 25(2):149–63. doi: 10.1016/j.molmed.2018.12.006
99. Moll G, Drzeniek N, Kamhieh-Milz J, Geissler S, Volk HD, Reinke P. MSC Therapies for COVID-19: Importance of Patient Coagulopathy, Thromboprophylaxis, Cell Product Quality and Mode of Delivery for Treatment Safety and Efficacy. Front Immunol (2020) 11:1091. doi: 10.3389/fimmu.2020.01091
100. Zhao L, Hu C, Han F, Chen D, Cheng J, Wu J, et al. Induction Therapy With Mesenchymal Stem Cells in Kidney Transplantation: A Meta-Analysis. Stem Cell Res Ther (2021) 12(1):158. doi: 10.1186/s13287-021-02219-7
101. Sawitzki B, Harden PN, Reinke P, Moreau A, Hutchinson JA, Game DS, et al. Regulatory Cell Therapy in Kidney Transplantation (The ONE Study): A Harmonised Design and Analysis of Seven Non-Randomised, Single-Arm, Phase 1/2A Trials. Lancet (2020) 395(10237):1627–39. doi: 10.1016/s0140-6736(20)30167-7
Keywords: allotransplantation, donor specific transfusion (DST), donor specific antibodies, mesenchymal stem cell, sensitization, allosensitization, tolerance
Citation: Shaw BI, Ord JR, Nobuhara C and Luo X (2021) Cellular Therapies in Solid Organ Allotransplantation: Promise and Pitfalls. Front. Immunol. 12:714723. doi: 10.3389/fimmu.2021.714723
Received: 25 May 2021; Accepted: 04 August 2021;
Published: 30 August 2021.
Edited by:
Jayme E. Locke, University of Alabama at Birmingham, United StatesReviewed by:
Rusan Ali Catar, Charité – Universitätsmedizin Berlin, GermanyMeng Lv, Peking University People’s Hospital, China
Copyright © 2021 Shaw, Ord, Nobuhara and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xunrong Luo, Xunrong.Luo@duke.edu
†ORCID: Brian I. Shaw, orcid.org/0000-0002-2317-6549
 Brian I. Shaw
Brian I. Shaw Jeffrey R. Ord2
Jeffrey R. Ord2 Xunrong Luo
Xunrong Luo