- College of Life Sciences, Nankai University, Tianjin, China
Fibrosis is the extensive deposition of fibrous connective tissue, and it is characterized by the accumulation of collagen and other extracellular matrix (ECM) components. Fibrosis is essential for wound healing and tissue repair in response to a variety of triggers, which include infection, inflammation, autoimmune disorder, degenerative disease, tumor, and injury. Fibrotic remodeling in various diseases, such as liver cirrhosis, pulmonary fibrosis, renal interstitial fibrosis, myocardial infarction, systemic sclerosis (SSc), and graft-versus-host disease (GVHD), can impair organ function, causing high morbidity and mortality. Both innate and adaptive immunity are involved in fibrogenesis. Although the roles of macrophages in fibrogenesis have been studied for many years, the underlying mechanisms concerning the manner in which T cells regulate fibrosis are not completely understood. The T cell receptor (TCR) engages the antigen and shapes the repertoire of antigen-specific T cells. Based on the divergent expression of surface molecules and cell functions, T cells are subdivided into natural killer T (NKT) cells, γδ T cells, CD8+ cytotoxic T lymphocytes (CTL), regulatory T (Treg) cells, T follicular regulatory (Tfr) cells, and T helper cells, including Th1, Th2, Th9, Th17, Th22, and T follicular helper (Tfh) cells. In this review, we summarize the pro-fibrotic or anti-fibrotic roles and distinct mechanisms of different T cell subsets. On reviewing the literature, we conclude that the T cell regulations are commonly disease-specific and tissue-specific. Finally, we provide perspectives on microbiota, viral infection, and metabolism, and discuss the current advancements of technologies for identifying novel targets and developing immunotherapies for intervention in fibrosis and fibrotic diseases.
Introduction
As a leading cause of mortality, fibrotic diseases can occur in virtually every organ and tissue. Extracellular matrix (ECM) component deposition is observed in many fibroproliferative diseases, including liver cirrhosis (LC), pulmonary fibrosis, renal interstitial fibrosis, myocardial infarction, systemic sclerosis (SSc), and graft-versus-host disease (GVHD) (1, 2). Numerous studies have demonstrated that the immune response plays an essential role in fibrosis and fibrotic diseases. Systematic investigation of the immune cells and signaling pathways remains fundamental for developing novel therapies (3). Therefore, it is essential to understand the key factors influencing fibrosis.
After hepatic injuries, immune cells participate in wound healing and tissue repair by initiating inflammation. The infiltrated T cells, macrophages, neutrophils, dendritic cells (DCs), and the liver-resident macrophages, Kupffer cells, cooperatively contribute to the liver fibrotic cascade and lead to the activation of hepatic stellate cells (HSCs) and the generation of myofibroblasts (4, 5). Non-alcoholic fatty liver disease (NAFLD) is characterized by hepatic steatosis with the presence of T cells, including natural killer T (NKT) cells, γδ T cells, CD8+ cytotoxic T lymphocytes (CTL), regulatory T (Treg) cells, and T helper cells. These T cells exert their function by attenuating or aggravating the liver injury and fibrosis progression (6).
Further, pulmonary fibrosis is a highly lethal pathological process, in which T cell responses contribute to the pathogenesis of idiopathic pulmonary fibrosis (IPF), cystic fibrosis (CF), and various other lung diseases (7, 8). However, the functions of each T cell subset appear to be perplexing and are influenced by interactions with epithelial cells or fibroblasts, the pulmonary localization of fibrosis in the bronchial or alveolar region, and the disease progression stage (9, 10). T cells can regulate the pulmonary fibrosis outcome through cAMP-regulated chloride channels (11), Fas-Fas ligand (FasL) interactions (12), or T cell exhaustion (13). Given the strong relationship between fibrosis and inflammation, the determination of precise functions of T cells may also be beneficial for inflammatory and autoimmune diseases.
Cardiovascular disease (CVD) is a class of heart or blood vessel-related disease. T cells modulate cardiac fibroblasts and MMP activity during cardiac fibrosis and hypertension (14–16). Using T cell-deficient mice disease model, previous studies have demonstrated the pivotal role of T cells in heart failure (HF) (17, 18), myocardial fibrosis (19), ischemia (20), and myocardial infarction (21).
Furthermore, intestinal fibrosis occurs in the gastrointestinal tract during chronic diseases such as Crohn's disease (CD), ulcerative colitis (UC), ulcerative jejunoileitis, and radiation enteritis. T cells participate in persistent dysregulated inflammation and lead to excessive myofibroblast proliferation, ECM deposition, and scar tissue formation (15, 22).
Systemic sclerosis (SSc) affects the skin and multiple internal organs, causing excessive ECM deposition and vasculopathy. Many studies have suggested that some T cell subsets, such as Th17, Treg, Th2, Th9, and Th22, can serve as a hallmark of SSc. Therefore, discussing the mechanisms of each T cell subset may help identify leash the pro-fibrotic T cells and cytokines in SSc (2, 23).
Renal fibrosis is considered to be a consequence of immune response involving myofibroblast accumulation and matrix deposition. Researchers have observed that T cell activation and infiltration can cause interstitial fibrosis and glomerular injury in the kidney (24).
Furthermore, GVHD patients may suffer vascular injury caused by the immune response between recipient endothelial cells and circulating alloreactive donor T cells. T helper cells, including Th17 and Tfh cells, secrete IL-17 and IL-21 cytokines and augment this immune response and fibrosis outcome (25).
T cell-dependent fibrosis plays a crucial role in muscle regeneration, and sclerotic scar tissue in muscles is attributed to Duchenne muscular dystrophy (DMD). Although T cells can undermine the muscle regeneration (26), they are also important for biomaterial scaffold muscle tissue repair (27).
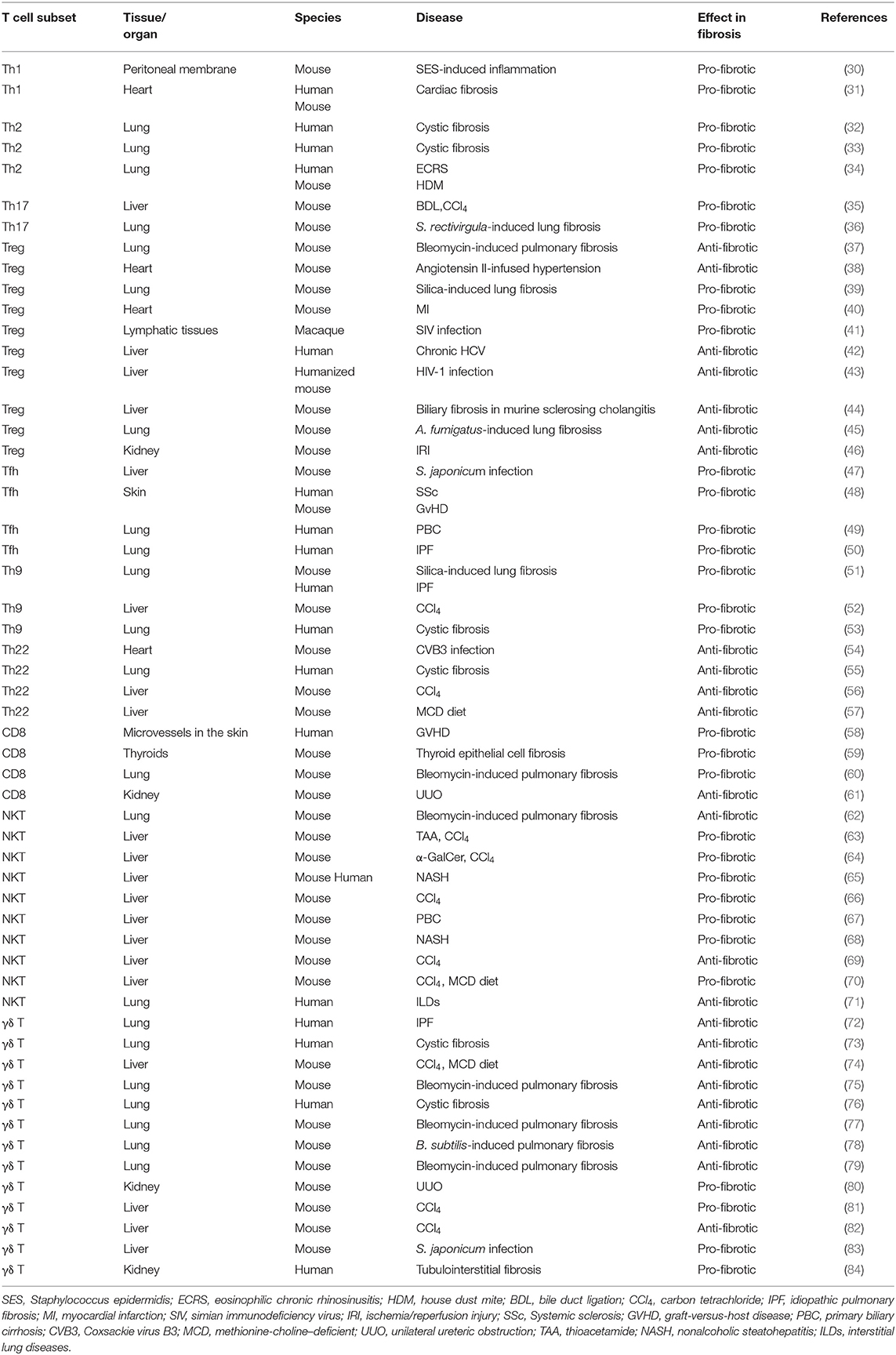
In summary, T cells are essential for fibrosis and fibrotic diseases. The orchestration of fibrotic tissue remodeling is programed by multiple cytokines, chemokines, and growth factors (28, 29). In this review, we briefly discuss the role of all T cell subsets and their underlying mechanisms in fibrosis and fibrotic diseases (Table 1, Figure 1).
The Role and Mechanisms of T Cells in Fibrosis and Fibrotic Diseases
Th1 Cells and Fibrosis
Inflammation is considered to be one of the major steps leading to fibrosis (29). However, the production of pro-inflammatory cytokines is not always pro-fibrotic. IL-12 induces the differentiation of naïve CD4 cells to Th1 cells to produce the pro-inflammatory cytokine IFNγ. IFNγ suppresses fibroblast-induced collagen synthesis and attenuates fibrosis. Therefore, Th1 cells are largely considered to play an anti-fibrotic role (85). Wynn et al. previously used IL-12 for treating Schistosoma mansoni infection in mice. This not only inhibited Th2-dominated immune response by elevating Th1 cytokine expression but also drastically ameliorated fibrosis (86). IFNγ production up-regulates the expression of matrix metalloproteinases (MMPs), including MMP-2, MMP-7, MMP-9, and MMP-13, to degrade ECM components. This proteolytic activity helps alter ECM remodeling and ameliorates fibrosis (87). Th1 cells and cytokine IFNγ are not always anti-fibrotic. On the contrary, they can also play a harmful role in bone regeneration (88), liver injury (89), and fibrotic diseases (30, 31). In cardiac fibrosis, Th1 cell infiltration leads to the activation of cardiac fibroblasts (CFBs) which then transform into myofibroblasts via integrin alpha4. Further, Th1 cells induce TGFβ expression in myofibroblasts, which forms a fibrillary ECM in the myocardium (31).
Th2 Cells and Fibrosis
Th2 cells are characterized by the production of signature cytokines IL-4, IL-5, and IL-13. Th2 cells, along with eosinophils, basophils, macrophages, and type 2 innate lymphoid cells (ILC2), contribute to the type 2 immunity-induced pathological process of fibrosis (90). As a commonly recognized opponent of Th1 cells, Th2 cells can alter Th1-associated IFNγ expression levels. In Pseudomonas aeruginosa-infected cystic fibrosis patients, an elevated ratio of pulmonary CCR4+ Th2 cells to CXCR3+ Th1 lymphocytes was found in the bronchoalveolar lavage fluid, with significantly higher levels of Th2 cytokines IL-4 and IL-13 (32). In addition to infection, allergic inflammation also triggers a Th2 response. Asthma is a chronic allergic inflammatory disease with fibrotic airway remodeling. However, the mechanism underlying airway fibrosis remains poorly understood. Morimoto et al. found that IL-33 induces the production of ST2hi memory-type pathogenic Th2 cells which enhance amphiregulin (Areg) levels. Furthermore, amphiregulin-epidermal growth factor receptor (EGFR) signal results in osteopontin-producing eosinophils and fibrotic responses. Their study highlighted how Th2 memory cells are critical for allergy-induced airway fibrosis (34). It has been reported that aberrant and spontaneous development of Th2 cells in the lamina propria of TRAF6-knockout mice with eosinophilic enteritis causes fibrosis in the small intestine (91). Further, IL-13 is considered to be an essential Th2 cytokine for fibrosis (90). By specifically disrupting IL-13 signaling in liver-resident tissue fibroblasts, also known as hepatic stellate cells (HSCs), Gieseck et al. demonstrated that PDGFRB+ fibroblasts are necessary for mediating IL-13-induced pathological fibrosis in mice (92). Furthermore, IL-13-regulated lipogenesis, bile acid synthesis, and biliary-dependent steatosis seem to be distinct cellular pathways from fibrosis, suggesting the possible intervention of IL-13 for the promotion of hepatobiliary expansion without aggravating fibrosis (92). Th2-targeted treatment has been tested in fibrosis-related diseases. For example, vitamin D3 was found to attenuate Th2 response in cystic fibrosis patients with allergic bronchopulmonary aspergillosis by substantially reducing DC-expressed costimulatory molecule OX40 ligand (OX40L) and increasing TGFβ expression (33).
Th9 Cells and Fibrosis
Th9 cells were originally described in parasitic infections and allergic diseases. As a newly defined subset of T helper cells, it responds to environmental cues and cytokine milieu to produce IL-9 (93). Pleiotropic cytokine IL-9 activates various target cells including dendritic cells, mast cells, and CD8+ T cells, and is involved in the pathological processes of multiple diseases including inflammatory diseases, infectious diseases, autoimmune diseases, and cancer (93–95). Elevated serum IL-9 levels have been reported in patients with periportal fibrosis caused by Schistosoma mansoni infection (96). In both the silica-induced lung fibrosis mouse model and human patients with idiopathic pulmonary fibrosis (IPF) and cystic fibrosis, IL-9 levels were found to be correspondingly elevated (51, 53). While the administration of IL-9 neutralizing antibody protects mice from IPF and cystic fibrosis (CF) (51, 53). During an infection event, IL-33 activates ILC2 to produce IL-9, and the Th9 cells and IL-9 cytokine production is further amplified by IL-9-activated mast cell-driven ILC2 expansion (53). In liver cirrhosis patients, IL-9 is also significantly increased and has been proven to play an important role in hepatic fibrosis progression. IL-9 has further been reported to activate Raf/MEK/ERK signaling pathway in a commonly used carbon tetrachloride (CCl4)-induced liver fibrosis mouse model. Consistent with studies in lung fibrotic diseases, IL-9 antibody inactivates hepatic stellate cells (HSCs) and ameliorates liver fibrosis (52). These results offer a possible treatment strategy for reducing fibrosis by blocking IL-9 signaling.
Th17 Cells and Fibrosis
Th17 cells have been discovered and characterized as the third subset of T helper cells. Characterized by their IL-17-producing ability, Th17 cells are critically involved in inflammatory responses (97). The relation between Th17 cells and fibrosis has been investigated in recent years. Th17 cells serve as a key component in mucosal immunity including the respiratory tract (98). In a hypersensitivity pneumonitis mouse model established by repeated exposure to Saccharopolyspora rectivirgula, increased Th17 were responsible for the inflammatory and fibrotic responses (36). Similarly, Th17 cells infiltrated into the bronchial submucosa of human patients with cystic fibrosis (CF) (99, 100). In the liver, IL-17 targets multiple types of cells, including Kupffer cells and hepatic stellate cells (HSCs). Gao et al. observed increased levels of IL-17A and IL-17 receptor in a liver fibrosis model induced by intragastric gavage with CCl4 or bile duct ligation (35). Mechanistically, IL-17 activates the STAT3 signaling pathway in HSCs to produce type 1 collagen. Interestingly, the deletion of IL-23, or administration of IL-17E (also known as IL-25), attenuates liver fibrosis (35). Given the fact that IL-17 is not only produced by Th17 but also by γδ T cells (101), NKT cells (102), and type 3 innate lymphoid cells (ILC3) (103), and the differentiation and function of Th17 cells highly rely on the cytokine milieu, such as IL-1β, IL-6, IL-23, and TGFβ (104), it is important to identify novel factors that may regulate Th17 cell differentiation in fibrosis-related diseases. Angiogenesis contributes to fibroproliferative diseases. Recently, Jameson et al. reported that placental growth factor (PlGF), an angiogenic factor of the VEGF family, is specifically secreted from Th17 cells. PIGF is also required for the progression of collagen-induced arthritis in mice (105). Th17 cell differentiation program is highly heterogeneous (106) and is controlled by numerous factors, including febrile temperature (107). The fever shapes Th17 cell differentiation by SUMOylation of the transcription factor SMAD4 (107), which is tightly associated with T cell function during inflammation (108, 109). SMAD4 has been reported to control Th17 function with an oncoprotein SKI (110, 111), which has been linked to wound healing and fibrosis (112). This evidence suggests that TGFβ signaling in T cells also plays an important role in regulating inflammatory responses (104). TGFβ controls fibrosis in different cell types. Elevated mechanical tension activates the TGFβ signaling loop in mouse alveolar stem cells (AT2) and causes progressive pulmonary fibrosis (113). The complexity of the cytokine regimen responsible for Th17 differentiation may be harnessed to treat fibrotic diseases.
Th22 Cells and Fibrosis
Th22 cells are characterized by the production of cytokine IL-22. Although there exists a Th17-expressed IL-22 population, the Th22 cells exhibit transcriptomes distinct from those of Th17 cells (114). Importantly, Th22 cells express several members of the fibroblast growth factor (FGF) family, such as FGF1, FGF-5, FGF-12, and FGF-13, which are master regulators for wound healing, tissue repair, tissue regeneration, and fibrosis (114). Th22 represents a key T cell subset for epidermal immunity, and its levels are significantly increased in the epidermis of patients with morphea and SSc (115). Th22 cells are also increased in patients with cystic fibrosis in response to P. aeruginosa infection; this suggests the involvement of Th22 cells in pulmonary immunity (55). Th22 cells are also highly associated with liver fibrosis and are considered to play a hepatoprotective role (116). Th22 cell levels are elevated in mice with CCl4-induced liver fibrosis (56), mice with methionine choline-deficient (MCD) diet-induced non-alcoholic steatohepatitis (NASH)(57), and human patients with liver cirrhosis (LC) (117). Liver-infiltrated Th22 cells, or recombinant IL-22 treatment, ameliorates fibrogenesis by attenuating hepatic stellate cell activation (56). Similarly, increased levels of Th22 and IL-22 are observed in mice with Coxsackie virus B3 (CVB3)-induced chronic myocarditis and dilated cardiomyopathy. Consistently, the expression of matrix metalloproteinase-9 (MMP9) is increased and that of metalloproteinase-1 (TIMP-1) inhibitor is decreased (54). Furthermore, the administration of an IL-22-neutralizing antibody exacerbated myocardial fibrosis as well as mortality. It is important to note that although IL-22 is mainly produced by immune cells including Th22 cells, Th17 cells, γδ T cells, NKT cells, and type 3 innate lymphoid cells (ILC3), it primarily targets non-immune cells (118). These facts suggest that targeting crosstalk between immune cells and tissues via the IL-22 signaling pathway is possible in fibrogenesis.
Regulatory T Cells and Fibrosis
Regulatory T cells (Tregs) play a pivotal role in modulating self-tolerance and immune homeostasis. It has been reported that cystic fibrosis patients with P. aeruginosa infection have impaired Tregs (119). Further, depletion of Treg cells by anti-CD25 antibody in silica-induced lung fibrosis attenuates fibrosis, and this process is probably dependent on the indirect function of Treg-secreted IL-10 and TGFβ (39). However, intranasal administration of TGFβ1-expressing plasmid results in increased TGFβ1- and IL-10-producing Treg cells and ameliorates bleomycin-induced lung fibrosis in mice (37). Recently, Ichikawa et al. found that depletion of CD69hiCD103hiFoxp3+ Treg cells resulted in substantially higher levels of lung fibrosis in mice exposed to Aspergillus fumigatus because of the pathology promoted by tissue-resident CD103loCD44hiCD69hiCD4+ T cells, which express high level of fibrosis-related genes. Their work thus defined a new tissue-resident Treg subpopulation in the lungs (45). The role of Tregs in fibrosis can be controversial and may be associated with a specific type of disease model. In angiotensin II-infused hypertensive mice, the adoptive transfer of CD4+CD25+ Treg cells improves cardiac hypertrophy and ameliorates cardiac fibrosis (38). However, in mice with ischemic cardiomyopathy, Treg ablation alleviates hypertrophy and cardiac fibrosis (40). Under the context of viral infections, TGFβ1+ Treg cells induce deleterious collagen deposition and lymphatic tissue fibrosis in simian immunodeficiency virus (SIV)-infected rhesus macaques, showing a pro-fibrotic effect (41). However, in hepatitis C virus (HCV)-infected human patients, or in human immunodeficiency virus type 1 (HIV-1)-infected humanized mice, Treg cells prevent liver immunopathogenesis and limit liver fibrosis (42, 43). Treg cells exhibit different transcriptional changes in response to regenerative or fibrogenic environmental cues. During kidney fibrosis, remarkably increased levels of tissue-resident Treg cells express elevated fibrosis-related transcription factors, such as Id2, Nfκb, Rgs2, and Junb (46). Depletion or restoration of Treg cells may become a viable approach in controlling fibrosis in this case. In contrast, in Mdr2 (Abcb4) deficient mice with sclerosing cholangitis, a low dose of IL-2 treatment diminishes biliary injury and fibrosis by the expansion of intrahepatic Tregs (44).
Tfh Cells and Fibrosis
T-follicular helper (Tfh) cells, characterized by the expression of the lineage-specific transcription factor Bcl6 and production of IL-21, are essential for B cell function. They express high level of surface markers, such as CXCR5, CD40L, ICOS, and PD-1 (120). Upon Schistosoma japonicum infection, macrophages drive the differentiation of Tfh cells through CD40-CD40L and ICOS-ICOSL interactions. Following this, the infiltrated Tfh cells increase the hepatic granuloma formation and lead to severe liver fibrosis (47). The levels of these cell are also increased in patients with primary biliary cirrhosis (PBC) (49). In patients with idiopathic pulmonary fibrosis, the levels of CXCR5+ICOS+PD-1+ Tfh cells are increased in the peripheral blood (50). Further, the levels of CXCR5+ICOS+PD-1+ Tfh cells are strongly associated with dermal fibrosis in patients with systemic sclerosis (SSc). A mouse sclerodermatous GVHD (GVHD-SSc) model suggested that the level of these profibrotic Tfh cells are IL-21- and MMP-12-dependent. Furthermore, it has been shown that both IL-21 and ICOS antibody administration can effectively reduce skin fibrosis (48). As a new subset of T cells, Tfh cells may bring novel insights into fibrotic disease therapies.
Tfr Cells and Fibrosis
T follicular regulatory (Tfr) cells, sharing Tfh makers including CXCR5, Bcl6, ICOS, and PD-1, are characterized by Foxp3 expression and have a regulatory function (121). In patients with primary biliary cholangitis (PBC), the levels of CD4+CXCR5+CD127loCD25hi Tfr cells, as well as CCR7hiPD-1lo central memory Tfr cells, are dramatically decreased, whereas those of CCR7loPD-1hi effector memory Tfr cells are increased. This evidence suggests the involvement of Tfr cells in primary biliary cholangitis regulation (122). In patients with chronic hepatitis B (CHB) infection, levels of circulating Tfr cells are significantly increased and are positively associated with FIB-4, which is the fibrosis index based on four factors (123). Another study including patients with CHB and chronic hepatitis C (CHC) also indicated that Tfr cells possibly modulated liver fibrosis by secreting the regulatory cytokine IL-10 and TGFβ (124). Thus, evidence suggests an emerging role of Tfr cells in virus-induced liver fibrosis (123).
Cytotoxic T Cells (CTLs, CD8+ T Cells) and Fibrosis
Cytotoxic T cells (CTLs, CD8+ T cells), expressing CD8 glycoprotein as an identity marker, are vital for killing infected cells and tumor cells. They also play important roles in many fibrosis-related diseases. Perivascular infiltrated CD8+ T cells are found in patients with GVHD of the skin (58). In a mouse model of acute cerebral ischemia, CD8+ T cells infiltrated into the perivascular space and expressed IL-16 to recruit monocytes and CD4+ T cells, resulting in reduced hindlimb muscle fibrosis (125). Furthermore, in a renal fibrosis model, CD8+ T cells and IFNγ reduced the CD4+ T cell-induced monocyte-to-fibroblast transition (61). Activated CD8+ T cells can produce TNFα and induce thyroid fibrosis (59). They can also secrete IL-13 to mediate bleomycin-induced pulmonary fibrogenesis in an IL-21-dependent manner (60). In contrast, fibrosis also has an impact on the immunosurveillance functions of CD8+ T cells. For example, mouse liver fibrosis during HBV infection can jeopardize hepatocellular antigen recognition by intravascular CD8+ T cells when crawling along liver sinusoids (126). Further, liver fibrosis and non-alcoholic steatohepatitis (NASH) lead to the accumulation of liver resident IgA+PD-L1+IL-10+ cells that directly impair CTL/CD8+ T cell functions against tumor-associated antigens, resulting in the development of hepatocellular carcinoma (HCC) (127). The fibrosis of secondary lymph nodes may result in CD8+ T cell depletion after vaccine responses (128). Therefore, while developing vaccine strategies and triggering pro-fibrotic inflammatory responses for infectious diseases, the impact on CD8+ T cells must be considered.
NKT Cells and Fibrosis
Natural killer T (NKT) cells express the αβ T cell receptor and recognize the glycolipid antigens presented by MHC I-liked protein CD1d. Being largely presented in the liver, NKT cells are central components of the immune response during liver injury, repair, inflammation, and fibrosis (129–131). A xenobiotics-induced liver fibrosis model is commonly used for determining the effect of NKT cells on liver fibrogenesis. NKT cells are critically involved in liver fibrosis induced by thioacetamide (TAA) (63), CCl4 (63, 64, 66, 69), α-galactosylceramide (α-GalCer) (64), or 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC) (69). It seems that the function of NKT cells in fibrosis is subject to different contexts. In TAA-induced liver fibrosis, the CD1d deficient mice show ameliorated liver fibrogenesis with blunted TIMP-1 expression (63), whereas in CCl4-induced liver fibrosis, NKT-deficient mice are more susceptible (64). Furthermore, infiltrated NKT cells increase NKG2D ligand expression to activate HSCs and ameliorate liver fibrosis (66, 69). The participation of NKT cells at different fibrosis stages may also cause divergence (64). In a methionine choline-deficient (MCD) diet-induced non-alcoholic steatohepatitis (NASH) model, NKT cells accumulated, Hedgehog (Hh) and osteopontin (OPN) levels increased, and HSCs activated and differentiate to myofibroblasts (65, 68). Additionally, in a xenobiosis-induced model of primary biliary cirrhosis (PBC), the invariant natural killer T cell activator α-GalCer induced exacerbated fibrosis (67). Moreover, it has been reported that IL-30 treatment (69) or targeting CXCR6/CLCL16 (70) may serve as a potential approach for the amelioration of liver fibrosis. Further, NKT cells play a role in lung diseases (62, 71). In a bleomycin-induced pulmonary mouse fibrosis model, the genetic deletion of CD1d was reported to result in severe fibrosis, although adoptive transfer of NKT cells protected the mice from fibrosis. NKT cells attenuate pulmonary fibrosis by producing IFNγ and reducing TGFβ levels (62). Given the unique characteristics of NKT cells, the precise mechanism for NKT cells in fibrosis warrants further investigation.
γδ T Cells and Fibrosis
γδ T cells are unconventional T cells characterized by a T cell receptor γ chain and δ chain, which are not restricted by MHC. These cells may either instigate fibrosis or resolve fibrosis in a cytokine-dependent and disease-dependent manner (132). Although the overall number of γδ T cells is small, these cells represent a major T cell population in the skin, gut, and lung. The dendritic γδ T cells in the dermis are also named DETC, dendritic epidermal T cells. Further, it has been reported that skin fibroblasts exhibit higher proliferative activity and stronger collagen synthesis ability when cultured with γδ T cell supernatant (133). Studies on patients with systemic sclerosis have demonstrated the involvement of γδ T cells (134–136). The level of pathogenic CD27+ γδ T cells were reported to increase, with upregulation of granzyme B or perforin expression (134). Further, Vγ9Vδ2, a subset of human γδ T cells, shows anti-fibrotic potential with the production of IFNγ (135, 136).
An abundance of approximately 8–20% γδ T cells in resident pulmonary lymphocytes shows the possible engagement of γδ T cells in lung infection, chronic inflammation, and subsequent pulmonary fibrosis (137). Sarcoidosis and idiopathic pulmonary fibrosis have shown to cause an increase in γδ T cell levels in the bronchoalveolar lavage fluid of patients (72). γδ T cells are also expanded in both cystic fibrosis patients with P. aeruginosa infection and mice with Bacillus subtilis infection (76, 138). In a bleomycin-induced fibrosis model, ablation of γδ T cells results in severe pulmonary fibrosis. Moreover, γδ T cells may prevent fibrosis by expressing CXCL10 (79). An important response of γδ T cells to infection and injury is the production of cytokine IL-17. Thus, γδ T cells were found to be the predominant source of IL-17 in mice with fibrosis induced by bleomycin (77), B. subtilis (139), and silica (140). IL-22 also contributes to the inhibition of pulmonary fibrosis. Deficiency in aryl hydrocarbon receptor (AhR) signaling or IL-22 enhances collagen deposition and accelerates fibrosis. Vγ6Vδ1 γδ T cells are the predominant source of IL-22 in protecting the lung from pulmonary fibrosis (78). Another major cytokine produced by γδ T cells is IFNγ. In a bleomycin-induced pulmonary fibrosis model, IFNγ produced by γδ T cells was found to attenuate fibrosis by indirectly inhibiting IL-17-secreting Th17 cells (75).
γδ T cells are also enriched in the liver and play an important role in liver fibrosis and cirrhosis (141). IL-17-producing γδ T cells are crucial in a variety of liver diseases (101). The chemokine receptor CCR6 is required for generating IL-17-producing γδ T cells. Hepatic γδ T cells contact and promote the FasL-induced HSC apoptosis to protect the liver from excessive fibrosis in an IL-17-dependent manner (74). IFNγ-producing γδ T cells are also protective in liver fibrosis by showing direct cytotoxicity against activated HSCs (82). Furthermore, hepatocyte-derived exosomes may mediate the activation of TLR3, which in turn enhances the production of IL-17 in γδ T cells (81). IL-17-producing γδ T cells are also enriched in patients with biliary atresia (BA), which is characterized by the destruction of the biliary system and liver fibrosis (142).
In addition, γδ T cells also display critical roles during fibrosis of other tissues. As γδ T cell is a key source of IL-17, the number of γδ T cells is elevated in patients with tubulointerstitial fibrosis, as determined by their biopsies (84). Further, in mice with kidney obstructive injury, γδ T cells are a major source of IL-17 and contribute to the pathogenesis of renal fibrosis with myofibroblast activation and ECM deposition (80). Moreover, in another study on a myocardial infarction mouse model, the IL-17-producing γδ T cells were found to promote fibroblast proliferation and aggravate fibrosis (143).
The aforementioned studies suggest that γδ T cells may be protective or deleterious in fibrosis in a cytokine-specific and tissue-specific manner. Thus, understanding the roles and underlying mechanisms of γδ T cells in the pathogenesis of fibrotic diseases would be useful for developing γδ T cell-based immunotherapies.
Concluding Remarks and Perspectives
As a key process of wound healing, a variety of immune cells engage in the manifestation of inflammation and fibrosis. To date, the involvement of T cells has been well-established in orchestrating the fibrous tissue microenvironment. Although extensive studies have been conducted on T cells and fibrosis, many questions remain elusive.
What is the initial trigger of fibrosis? How do we identify risk factors leading to a severe fibrotic disease? How do we utilize T cells in other fibrosis-related narratives? The pandemic of 2019 coronavirus disease COVID-19, caused by the SARS-CoV-2 virus infection, has caused worldwide mortality (144). COVID-19 is accompanied by fibrosis (145, 146), and is particularly dangerous for patients with pulmonary fibrotic diseases including cystic fibrosis (147). Pulmonary fibrosis is also commonly developed in patients with other virus-induced respiratory diseases, including severe acute respiratory syndrome coronavirus (SARS-CoV) (148–150) and Middle East respiratory syndrome (MERS) (151). A better understanding of cellular and molecular mechanisms will help treat fibrosis in patients with severe virus-induced diseases. Some T cell subsets participate in tissue repair and wound healing (152, 153) and are useful for tissue engineering. For instance, the recruitment of antigen-specific T cells followed by Th2 adjuvant vaccination can help biomaterials for tissue repair (154). Studies on the influence of T cells on fibrosis are especially useful for the treatment of hypertrophic scarring, which is a severe form of fibrosis frequently developed in case of severe burn injuries. In early studies, T cells were found to be heavily infiltrated into the dermis and epidermis of human patients (155). In burn patients with hypertrophic scars, Th1 and Th2 cell subsets and cytokines were identified to be strongly associated with the development of fibrosis (156, 157). In a previous study, TGFβ-producing T cells were found in burn patients (158); Th22 cells were also found to promote fibroblast-mediated wound repair in an acute skin wounding mouse model (159). These T cells interact with and remodel ECM and shape the local immune and fibrogenic responses in both the epidermis and dermis of hypertrophic tissues (160). The initial trigger event for T cell activation and the crosstalk between T cells and keratinocytes, fibroblast, and epithelial cells thus become critical questions for resolving the niche and local environment. Although these types of studies are largely limited in human patients, several animal models have been developed to serve as powerful tools to study human hypertrophic scarring. Momtazi et al. grafted human skin onto several immune-deficient mice, including TCRαβ−/−γδ−/−, RAG-1−/−, and RAG-2−/−γc−/− mice. The proliferative scars showed histological and immunohistochemical similarities to human hypertrophic scars. The study therefore not only proves the importance of T cells in scar formation but also provides a useful tool for human study (161). Similar studies were also performed with nude mice and SCID pig as animal models for studying the roles and functions of T cells in hypertrophic scar tissue (162, 163). Moreover, given the fact that T cells and T cell associated cytokines and chemokines differ over the period of scar formation (164), the findings of these animal model studies are particularly important for studying the temporal and spatial characteristic of fibrosis in terms of T cell-shaped local environment.
Are there novel pathways and mechanisms that are essential for T cell-mediated fibrotic diseases? How do we render T cells capable of halting the irreversible fibrotic response? There have been all kinds of approaches and clinical trials for fibrosis-related diseases (25, 165). T cell function can be modulated by lowering the level of cytokines, such as IFNγ (166), or by inhibiting kinases, such as hyperactivated focal adhesion kinase (FAK) (167). In some cases, altering the intestinal microbiota can reduce fibrosis (168). The checkpoint inhibitor for cancer therapy has drawn extensive attention in recent years and is also a promising way for fibrosis remodeling. The blockade of costimulatory signals such as OX40L or CTLA4 prevents fibrosis and induces the regression of established fibrosis (169, 170). Targeting a metabolic regulator is a prominent strategy for fibrosis reduction (171). Furthermore, mitochondrial biogenesis and endoplasmic reticulum (ER) stress have been associate with fibrosis (172, 173). Obesity-associated oxidative stress also contributes to fibrosis and fibrotic diseases (174). Accordingly, discoveries on fibrosis mechanisms warrant new opportunities to develop metabolic reprogramming drugs.
Technological advances shed light on the research as well as approaches for controlling fibrotic diseases. Single-cell sequencing provides the necessary tools to delineate the transcriptomic profiles of all types of individual cells. Thus, it has become possible to identify subpopulations that are critical for fibrogenesis and reveal new fibrogenic pathways (175). Chimeric antigen receptor (CAR) T cell therapy has been used in cancer treatment. Further, T cells engineered with fibroblast activation protein CAR have shown great potential to ablate cardiac fibroblasts, and significantly reduce cardiac fibrosis (176). In summary, this review discusses recent notable studies, and provides a framework for T cell-mediated fibrosis paving the way for rational targets and effective immunotherapies.
Author Contributions
MZ prepared and wrote the manuscript. SZ edited the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (81921004, 31972896).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor declared a past co-authorship with one of the authors SZ.
References
1. Wynn TA. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Investig. (2007) 117:524–9. doi: 10.1172/JCI31487
2. Chizzolini C. T cells, B cells, and polarized immune response in the pathogenesis of fibrosis and systemic sclerosis. Curr Opin Rheumatol. (2008) 20:707–12. doi: 10.1097/BOR.0b013e32830c45ae
3. Sugimoto MA, Vago JP, Perretti M, Teixeira MM. Mediators of the Resolution of the Inflammatory Response. Trends Immunol. (2019) 40:212–7. doi: 10.1016/j.it.2019.01.007
4. Pellicoro A, Ramachandran P, Iredale JP, Fallowfield JA. Liver fibrosis and repair: immune regulation of wound healing in a solid organ. Nat Rev Immunol. (2014) 14:181–94. doi: 10.1038/nri3623
5. Koyama Y, Brenner DA. Liver inflammation and fibrosis. J Clin Investig. (2017) 127:55–64. doi: 10.1172/JCI88881
6. Van Herck MA, Weyler J, Kwanten WJ, Dirinck EL, De Winter BY, Francque SM, et al. The differential roles of t cells in non-alcoholic fatty liver disease and obesity. Front Immunol. (2019) 10:82. doi: 10.3389/fimmu.2019.00082
7. Desai O, Winkler J, Minasyan M, Herzog EL. The role of immune and inflammatory cells in idiopathic pulmonary fibrosis. Front Med. (2018) 5:43. doi: 10.3389/fmed.2018.00043
8. Wynn TA. Integrating mechanisms of pulmonary fibrosis. J Exp Med. (2011) 208:1339–50. doi: 10.1084/jem.20110551
9. Lo Re S, Lison D, Huaux F. CD4+ T lymphocytes in lung fibrosis: diverse subsets, diverse functions. J Leukocyte Biol. (2013) 93:499–510. doi: 10.1189/jlb.0512261
10. Luzina IG, Todd NW, Iacono AT, Atamas SP. Roles of T lymphocytes in pulmonary fibrosis. J Leukocyte Biol. (2008) 83:237–44. doi: 10.1189/jlb.0707504
11. Chen JH, Schulman H, Gardner P. A cAMP-regulated chloride channel in lymphocytes that is affected in cystic fibrosis. Science. (1989) 243:657–60. doi: 10.1126/science.2464852
12. Hao Z, Hampel B, Yagita H, Rajewsky K. T cell-specific ablation of Fas leads to Fas ligand-mediated lymphocyte depletion and inflammatory pulmonary fibrosis. J Exp Med. (2004) 199:1355–65. doi: 10.1084/jem.20032196
13. McKinney EF, Lee JC, Jayne DR, Lyons PA, Smith KG. T-cell exhaustion, co-stimulation and clinical outcome in autoimmunity and infection. Nature. (2015) 523:612–6. doi: 10.1038/nature14468
14. Wei L. Immunological aspect of cardiac remodeling: T lymphocyte subsets in inflammation-mediated cardiac fibrosis. Exp Mol Pathol. (2011) 90:74–8. doi: 10.1016/j.yexmp.2010.10.004
15. Norlander AE, Madhur MS, Harrison DG. The immunology of hypertension. J Exp Med. (2018) 215:21–33. doi: 10.1084/jem.20171773
16. Abdullah CS, Jin ZQ. Targeted deletion of T-cell S1P receptor 1 ameliorates cardiac fibrosis in streptozotocin-induced diabetic mice. FASEB J. (2018) 32:5426–35. doi: 10.1096/fj.201800231R
17. Laroumanie F, Douin-Echinard V, Pozzo J, Lairez O, Tortosa F, Vinel C, et al. CD4+ T cells promote the transition from hypertrophy to heart failure during chronic pressure overload. Circulation. (2014) 129:2111–24. doi: 10.1161/CIRCULATIONAHA.113.007101
18. Kaya Z, Goser S, Buss SJ, Leuschner F, Ottl R, Li J, et al. Identification of cardiac troponin I sequence motifs leading to heart failure by induction of myocardial inflammation and fibrosis. Circulation. (2008) 118:2063–72. doi: 10.1161/CIRCULATIONAHA.108.788711
19. Ramos G, Hofmann U, Frantz S. Myocardial fibrosis seen through the lenses of T-cell biology. J Mol Cell Cardiol. (2016) 92:41–5. doi: 10.1016/j.yjmcc.2016.01.018
20. Stabile E, Burnett MS, Watkins C, Kinnaird T, Bachis A, la Sala A, et al. Impaired arteriogenic response to acute hindlimb ischemia in CD4-knockout mice. Circulation. (2003) 108:205–10. doi: 10.1161/01.CIR.0000079225.50817.71
21. Borg N, Alter C, Gorldt N, Jacoby C, Ding Z, Steckel B, et al. CD73 on T cells orchestrates cardiac wound healing after myocardial infarction by purinergic metabolic reprogramming. Circulation. (2017) 136:297–313. doi: 10.1161/CIRCULATIONAHA.116.023365
22. Lenti MV, Di Sabatino A. Intestinal fibrosis. Mol Aspects Med. (2019) 65:100–9. doi: 10.1016/j.mam.2018.10.003
23. Liu M, Wu W, Sun X, Yang J, Xu J, Fu W, et al. New insights into CD4(+) T cell abnormalities in systemic sclerosis. Cytokine Growth Factor Rev. (2016) 28:31–6. doi: 10.1016/j.cytogfr.2015.12.002
24. Nikolic-Paterson DJ. CD4+ T cells: a potential player in renal fibrosis. Kidney Int. (2010) 78:333–5. doi: 10.1038/ki.2010.182
25. MacDonald KP, Blazar BR, Hill GR. Cytokine mediators of chronic graft-versus-host disease. J Clin Investig. (2017) 127:2452–63. doi: 10.1172/JCI90593
26. Morrison J, Lu QL, Pastoret C, Partridge T, Bou-Gharios G. T-cell-dependent fibrosis in the mdx dystrophic mouse. Lab Investig. (2000) 80:881–91. doi: 10.1038/labinvest.3780092
27. Sadtler K, Estrellas K, Allen BW, Wolf MT, Fan H, Tam AJ, et al. Developing a pro-regenerative biomaterial scaffold microenvironment requires T helper 2 cells. Science. (2016) 352:366–70. doi: 10.1126/science.aad9272
28. Eming SA, Wynn TA, Martin P. Inflammation and metabolism in tissue repair and regeneration. Science. (2017) 356:1026–30. doi: 10.1126/science.aam7928
29. Distler JHW, Gyorfi AH, Ramanujam M, Whitfield ML, Konigshoff M, Lafyatis R. Shared and distinct mechanisms of fibrosis. Nat Rev Rheumatol. (2019) 15:705–30. doi: 10.1038/s41584-019-0322-7
30. Fielding CA, Jones GW, McLoughlin RM, McLeod L, Hammond VJ, Uceda J, et al. Interleukin-6 signaling drives fibrosis in unresolved inflammation. Immunity. (2014) 40:40–50. doi: 10.1016/j.immuni.2013.10.022
31. Nevers T, Salvador AM, Velazquez F, Ngwenyama N, Carrillo-Salinas FJ, Aronovitz M, et al. Th1 effector T cells selectively orchestrate cardiac fibrosis in nonischemic heart failure. J Exp Med. (2017) 214:3311–29. doi: 10.1084/jem.20161791
32. Hartl D, Griese M, Kappler M, Zissel G, Reinhardt D, Rebhan C, et al. Pulmonary T(H)2 response in Pseudomonas aeruginosa-infected patients with cystic fibrosis. J Allergy Clin Immunol. (2006) 117:204–11. doi: 10.1016/j.jaci.2005.09.023
33. Kreindler JL, Steele C, Nguyen N, Chan YR, Pilewski JM, Alcorn JF, et al. Vitamin D3 attenuates Th2 responses to Aspergillus fumigatus mounted by CD4+ T cells from cystic fibrosis patients with allergic bronchopulmonary aspergillosis. J Clin Investig. (2010) 120:3242–54. doi: 10.1172/JCI42388
34. Morimoto Y, Hirahara K, Kiuchi M, Wada T, Ichikawa T, Kanno T, et al. Amphiregulin-producing pathogenic memory t helper 2 cells instruct eosinophils to secrete osteopontin and facilitate airway fibrosis. Immunity. (2018) 49:134–50 e6. doi: 10.1016/j.immuni.2018.04.023
35. Meng F, Wang K, Aoyama T, Grivennikov SI, Paik Y, Scholten D, et al. Interleukin-17 signaling in inflammatory, Kupffer cells, and hepatic stellate cells exacerbates liver fibrosis in mice. Gastroenterology. (2012) 143:765–76 e3. doi: 10.1053/j.gastro.2012.05.049
36. Simonian PL, Roark CL, Wehrmann F, Lanham AK, Diaz del Valle F, Born WK, et al. Th17-polarized immune response in a murine model of hypersensitivity pneumonitis and lung fibrosis. J Immunol. (2009) 182:657–65. doi: 10.4049/jimmunol.182.1.657
37. Kitani A, Fuss I, Nakamura K, Kumaki F, Usui T, Strober W. Transforming growth factor (TGF)-beta1-producing regulatory T cells induce Smad-mediated interleukin 10 secretion that facilitates coordinated immunoregulatory activity and amelioration of TGF-beta1-mediated fibrosis. J Exp Med. (2003) 198:1179–88. doi: 10.1084/jem.20030917
38. Kvakan H, Kleinewietfeld M, Qadri F, Park JK, Fischer R, Schwarz I, et al. Regulatory T cells ameliorate angiotensin II-induced cardiac damage. Circulation. (2009) 119:2904–12. doi: 10.1161/CIRCULATIONAHA.108.832782
39. Liu F, Liu J, Weng D, Chen Y, Song L, He Q, et al. CD4+CD25+Foxp3+ regulatory T cells depletion may attenuate the development of silica-induced lung fibrosis in mice. PLoS ONE. (2010) 5:e15404. doi: 10.1371/journal.pone.0015404
40. Bansal SS, Ismahil MA, Goel M, Zhou G, Rokosh G, Hamid T, et al. Dysfunctional and proinflammatory regulatory T-lymphocytes are essential for adverse cardiac remodeling in ischemic cardiomyopathy. Circulation. (2019) 139:206–21. doi: 10.1161/CIRCULATIONAHA.118.036065
41. Estes JD, Wietgrefe S, Schacker T, Southern P, Beilman G, Reilly C, et al. Simian immunodeficiency virus-induced lymphatic tissue fibrosis is mediated by transforming growth factor beta 1-positive regulatory T cells and begins in early infection. J Infect Dis. (2007) 195:551–61. doi: 10.1086/510852
42. Claassen MA, de Knegt RJ, Tilanus HW, Janssen HL, Boonstra A. Abundant numbers of regulatory T cells localize to the liver of chronic hepatitis C infected patients and limit the extent of fibrosis. J Hepatol. (2010) 52:315–21. doi: 10.1016/j.jhep.2009.12.013
43. Nunoya J, Washburn ML, Kovalev GI, Su L. Regulatory T cells prevent liver fibrosis during HIV type 1 infection in a humanized mouse model. J Infect Dis. (2014) 209:1039–44. doi: 10.1093/infdis/jit548
44. Taylor AE, Carey AN, Kudira R, Lages CS, Shi T, Lam S, et al. Interleukin 2 promotes hepatic regulatory T cell responses and protects from biliary fibrosis in murine sclerosing cholangitis. Hepatology. (2018) 68:1905–21. doi: 10.1002/hep.30061
45. Ichikawa T, Hirahara K, Kokubo K, Kiuchi M, Aoki A, Morimoto Y, et al. CD103(hi) Treg cells constrain lung fibrosis induced by CD103(lo) tissue-resident pathogenic CD4 T cells. Nat Immunol. (2019) 20:1469–80. doi: 10.1038/s41590-019-0494-y
46. do Valle Duraes F, Lafont A, Beibel M, Martin K, Darribat K, Cuttat R, et al. Immune cell landscaping reveals a protective role for regulatory T cells during kidney injury and fibrosis. JCI insight. (2020) 5:130651. doi: 10.1172/jci.insight.130651
47. Chen X, Yang X, Li Y, Zhu J, Zhou S, Xu Z, et al. Follicular helper T cells promote liver pathology in mice during Schistosoma japonicum infection. PLoS Pathog. (2014) 10:e1004097. doi: 10.1371/journal.ppat.1004097
48. Taylor DK, Mittereder N, Kuta E, Delaney T, Burwell T, Dacosta K, et al. T follicular helper-like cells contribute to skin fibrosis. Sci Transl Med. (2018) 10:aaf5307. doi: 10.1126/scitranslmed.aaf5307
49. Wang L, Sun Y, Zhang Z, Jia Y, Zou Z, Ding J, et al. CXCR5+ CD4+ T follicular helper cells participate in the pathogenesis of primary biliary cirrhosis. Hepatology. (2015) 61:627–38. doi: 10.1002/hep.27306
50. Asai Y, Chiba H, Nishikiori H, Kamekura R, Yabe H, Kondo S, et al. Aberrant populations of circulating T follicular helper cells and regulatory B cells underlying idiopathic pulmonary fibrosis. Respir Res. (2019) 20:244. doi: 10.1186/s12931-019-1216-6
51. Sugimoto N, Suzukawa M, Nagase H, Koizumi Y, Ro S, Kobayashi K, et al. IL-9 Blockade suppresses silica-induced lung inflammation and fibrosis in mice. Am J Respir Cell Mol Biol. (2019) 60:232–43. doi: 10.1165/rcmb.2017-0287OC
52. Guo X, Cen Y, Wang J, Jiang H. CXCL10-induced IL-9 promotes liver fibrosis via Raf/MEK/ERK signaling pathway. Biomed Pharmacother. (2018) 105:282–9. doi: 10.1016/j.biopha.2018.05.128
53. Moretti S, Renga G, Oikonomou V, Galosi C, Pariano M, Iannitti RG, et al. A mast cell-ILC2-Th9 pathway promotes lung inflammation in cystic fibrosis. Nat Commun. (2017) 8:14017. doi: 10.1038/ncomms14017
54. Guo Y, Wu W, Cen Z, Li X, Kong Q, Zhou Q. IL-22-producing Th22 cells play a protective role in CVB3-induced chronic myocarditis and dilated cardiomyopathy by inhibiting myocardial fibrosis. Virol J. (2014) 11:230. doi: 10.1186/s12985-014-0230-z
55. Bayes HK, Bicknell S, MacGregor G, Evans TJ. T helper cell subsets specific for Pseudomonas aeruginosa in healthy individuals and patients with cystic fibrosis. PLoS ONE. (2014) 9:e90263. doi: 10.1371/journal.pone.0090263
56. Lu DH, Guo XY, Qin SY, Luo W, Huang XL, Chen M, et al. Interleukin-22 ameliorates liver fibrogenesis by attenuating hepatic stellate cell activation and downregulating the levels of inflammatory cytokines. World J Gastroenterol. (2015) 21:1531–45. doi: 10.3748/wjg.v21.i5.1531
57. Rolla S, Alchera E, Imarisio C, Bardina V, Valente G, Cappello P, et al. The balance between IL-17 and IL-22 produced by liver-infiltrating T-helper cells critically controls NASH development in mice. Clin Sci. (2016) 130:193–203. doi: 10.1042/CS20150405
58. Biedermann BC, Sahner S, Gregor M, Tsakiris DA, Jeanneret C, Pober JS, et al. Endothelial injury mediated by cytotoxic T lymphocytes and loss of microvessels in chronic graft versus host disease. Lancet. (2002) 359:2078–83. doi: 10.1016/S0140-6736(02)08907-9
59. Yu S, Fang Y, Sharav T, Sharp GC, Braley-Mullen H. CD8+ T cells induce thyroid epithelial cell hyperplasia and fibrosis. J Immunol. (2011) 186:2655–62. doi: 10.4049/jimmunol.1002884
60. Brodeur TY, Robidoux TE, Weinstein JS, Craft J, Swain SL, Marshak-Rothstein A. IL-21 Promotes Pulmonary Fibrosis through the Induction of Profibrotic CD8+ T Cells. J Immunol. (2015) 195:5251–60. doi: 10.4049/jimmunol.1500777
61. Dong Y, Yang M, Zhang J, Peng X, Cheng J, Cui T, et al. Depletion of CD8+ T Cells Exacerbates CD4+ T Cell-Induced Monocyte-to-Fibroblast Transition in Renal Fibrosis. J Immunol. (2016) 196:1874–81. doi: 10.4049/jimmunol.1501232
62. Kim JH, Kim HY, Kim S, Chung JH, Park WS, Chung DH. Natural killer T (NKT) cells attenuate bleomycin-induced pulmonary fibrosis by producing interferon-gamma. Am J Pathol. (2005) 167:1231–41. doi: 10.1016/S0002-9440(10)61211-4
63. Ishikawa S, Ikejima K, Yamagata H, Aoyama T, Kon K, Arai K, et al. CD1d-restricted natural killer T cells contribute to hepatic inflammation and fibrogenesis in mice. J Hepatol. (2011) 54:1195–204. doi: 10.1016/j.jhep.2010.08.022
64. Park O, Jeong WI, Wang L, Wang H, Lian ZX, Gershwin ME, et al. Diverse roles of invariant natural killer T cells in liver injury and fibrosis induced by carbon tetrachloride. Hepatology. (2009) 49:1683–94. doi: 10.1002/hep.22813
65. Syn WK, Oo YH, Pereira TA, Karaca GF, Jung Y, Omenetti A, et al. Accumulation of natural killer T cells in progressive nonalcoholic fatty liver disease. Hepatology. (2010) 51:1998–2007. doi: 10.1002/hep.23599
66. Jin Z, Sun R, Wei H, Gao X, Chen Y, Tian Z. Accelerated liver fibrosis in hepatitis B virus transgenic mice: involvement of natural killer T cells. Hepatology. (2011) 53:219–29. doi: 10.1002/hep.23983
67. Wu SJ, Yang YH, Tsuneyama K, Leung PS, Illarionov P, Gershwin ME, et al. Innate immunity and primary biliary cirrhosis: activated invariant natural killer T cells exacerbate murine autoimmune cholangitis and fibrosis. Hepatology. (2011) 53:915–25. doi: 10.1002/hep.24113
68. Syn WK, Agboola KM, Swiderska M, Michelotti GA, Liaskou E, Pang H, et al. NKT-associated hedgehog and osteopontin drive fibrogenesis in non-alcoholic fatty liver disease. Gut. (2012) 61:1323–9. doi: 10.1136/gutjnl-2011-301857
69. Mitra A, Satelli A, Yan J, Xueqing X, Gagea M, Hunter CA, et al. IL-30 (IL27p28) attenuates liver fibrosis through inducing NKG2D-rae1 interaction between NKT and activated hepatic stellate cells in mice. Hepatology. (2014) 60:2027–39. doi: 10.1002/hep.27392
70. Wehr A, Baeck C, Heymann F, Niemietz PM, Hammerich L, Martin C, et al. Chemokine receptor CXCR6-dependent hepatic NK T Cell accumulation promotes inflammation and liver fibrosis. J Immunol. (2013) 190:5226–36. doi: 10.4049/jimmunol.1202909
71. Bergantini L, Cameli P, d'Alessandro M, Vagaggini C, Refini RM, Landi C, et al. NK and NKT-like cells in granulomatous and fibrotic lung diseases. Clin Exp Med. (2019) 19:487–94. doi: 10.1007/s10238-019-00578-3
72. Utsumi K, Kawanishi K, Kuriyama Y, Nakano M, Ichinose Y, Toyama K. [Gamma delta T cells in peripheral blood and in bronchoalveolar lavage fluid from patients with sarcoidosis and idiopathic pulmonary fibrosis]. Nihon Kyobu Shikkan Gakkai zasshi. (1995) 33:1186–90.
73. Perez-Payarols J, Julia Benique MR, Matamoros Flori N, Roman Pinana JM. [An increase in gamma-delta T-lymphocytes in the peripheral blood of cystic fibrosis patients]. Anales espanoles de pediatria. (1996) 44:239–41.
74. Hammerich L, Bangen JM, Govaere O, Zimmermann HW, Gassler N, Huss S, et al. Chemokine receptor CCR6-dependent accumulation of gammadelta T cells in injured liver restricts hepatic inflammation and fibrosis. Hepatology. (2014) 59:630–42. doi: 10.1002/hep.26697
75. Segawa S, Goto D, Iizuka A, Kaneko S, Yokosawa M, Kondo Y, et al. The regulatory role of interferon-gamma producing gamma delta T cells via the suppression of T helper 17 cell activity in bleomycin-induced pulmonary fibrosis. Clin Exp Immunol. (2016) 185:348–60. doi: 10.1111/cei.12802
76. Raga S, Julia MR, Crespi C, Figuerola J, Martinez N, Mila J, et al. Gammadelta T lymphocytes from cystic fibrosis patients and healthy donors are high TNF-alpha and IFN-gamma-producers in response to Pseudomonas aeruginosa. Respir Res. (2003) 4:9. doi: 10.1186/1465-9921-4-9
77. Braun RK, Ferrick C, Neubauer P, Sjoding M, Sterner-Kock A, Kock M, et al. IL-17 producing gammadelta T cells are required for a controlled inflammatory response after bleomycin-induced lung injury. Inflammation. (2008) 31:167–79. doi: 10.1007/s10753-008-9062-6
78. Simonian PL, Wehrmann F, Roark CL, Born WK, O'Brien RL, Fontenot AP. gammadelta T cells protect against lung fibrosis via IL-22. J Exp Med. (2010) 207:2239–53. doi: 10.1084/jem.20100061
79. Pociask DA, Chen K, Choi SM, Oury TD, Steele C, Kolls JK. gammadelta T cells attenuate bleomycin-induced fibrosis through the production of CXCL10. Am J Pathol. (2011) 178:1167–76. doi: 10.1016/j.ajpath.2010.11.055
80. Peng X, Xiao Z, Zhang J, Li Y, Dong Y, Du J. IL-17A produced by both gammadelta T and Th17 cells promotes renal fibrosis via RANTES-mediated leukocyte infiltration after renal obstruction. J Pathol. (2015) 235:79–89. doi: 10.1002/path.4430
81. Seo W, Eun HS, Kim SY, Yi HS, Lee YS, Park SH, et al. Exosome-mediated activation of toll-like receptor 3 in stellate cells stimulates interleukin-17 production by gammadelta T cells in liver fibrosis. Hepatology. (2016) 64:616–31. doi: 10.1002/hep.28644
82. Liu M, Hu Y, Yuan Y, Tian Z, Zhang C. gammadeltaT cells suppress liver fibrosis via strong cytolysis and enhanced NK cell-mediated cytotoxicity against hepatic stellate cells. Front Immunol. (2019) 10:477. doi: 10.3389/fimmu.2019.00477
83. Zheng L, Hu Y, Wang Y, Huang X, Xu Y, Shen Y, et al. Recruitment of Neutrophils Mediated by Vgamma2 gammadelta T Cells Deteriorates Liver Fibrosis Induced by Schistosoma japonicum Infection in C57BL/6 Mice. Infect Immunity. (2017) 85:16. doi: 10.1128/IAI.01020-16
84. Law BM, Wilkinson R, Wang X, Kildey K, Lindner M, Beagley K, et al. Effector gammadelta T cells in human renal fibrosis and chronic kidney disease. Nephrol Dialysis, Transpl. (2019) 34:40–8. doi: 10.1093/ndt/gfy098
85. Wynn TA. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat Rev Immunol. (2004) 4:583–94. doi: 10.1038/nri1412
86. Wynn TA, Cheever AW, Jankovic D, Poindexter RW, Caspar P, Lewis FA, et al. An IL-12-based vaccination method for preventing fibrosis induced by schistosome infection. Nature. (1995) 376:594–6. doi: 10.1038/376594a0
87. Roderfeld M, Rath T, Pasupuleti S, Zimmermann M, Neumann C, Churin Y, et al. Bone marrow transplantation improves hepatic fibrosis in Abcb4-/- mice via Th1 response and matrix metalloproteinase activity. Gut. (2012) 61:907–16. doi: 10.1136/gutjnl-2011-300608
88. Liu Y, Wang L, Kikuiri T, Akiyama K, Chen C, Xu X, et al. Mesenchymal stem cell-based tissue regeneration is governed by recipient T lymphocytes via IFN-gamma and TNF-alpha. Nat Med. (2011) 17:1594–601. doi: 10.1038/nm.2542
89. Zhang S, Liang R, Luo W, Liu C, Wu X, Gao Y, et al. High susceptibility to liver injury in IL-27 p28 conditional knockout mice involves intrinsic interferon-gamma dysregulation of CD4+ T cells. Hepatology. (2013) 57:1620–31. doi: 10.1002/hep.26166
90. Gieseck RL III, Wilson MS, Wynn TA. Type 2 immunity in tissue repair and fibrosis. Nat Rev Immunol. (2018) 18:62–76. doi: 10.1038/nri.2017.90
91. Han D, Walsh MC, Cejas PJ, Dang NN, Kim YF, Kim J, et al. Dendritic cell expression of the signaling molecule TRAF6 is critical for gut microbiota-dependent immune tolerance. Immunity. (2013) 38:1211–22. doi: 10.1016/j.immuni.2013.05.012
92. Gieseck RL 3rd, Ramalingam TR, Hart KM, Vannella KM, Cantu DA, Lu WY, et al. Interleukin-13 activates distinct cellular pathways leading to ductular reaction, steatosis, and fibrosis. Immunity. (2016) 45:145–58. doi: 10.1016/j.immuni.2016.06.009
93. Kaplan MH. Th9 cells: differentiation and disease. Immunol Rev. (2013) 252:104–15. doi: 10.1111/imr.12028
94. Noelle RJ, Nowak EC. Cellular sources and immune functions of interleukin-9. Nat Rev Immunol. (2010) 10:683–7. doi: 10.1038/nri2848
95. Vegran F, Apetoh L, Ghiringhelli F. Th9 cells: a novel CD4 T-cell subset in the immune war against cancer. Cancer Res. (2015) 75:475–9. doi: 10.1158/0008-5472.CAN-14-2748
96. Barreto AV, Lacerda GA, Figueiredo AL, Diniz GT, Gomes EC, Domingues AL, et al. Evaluation of serum levels of IL-9 and IL-17 in human Schistosoma mansoni infection and their relationship with periportal fibrosis. Immunobiology. (2016) 221:1351–4. doi: 10.1016/j.imbio.2016.07.014
97. Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Ann Rev Immunol. (2009) 27:485–517. doi: 10.1146/annurev.immunol.021908.132710
98. Iwanaga N, Kolls JK. Updates on T helper type 17 immunity in respiratory disease. Immunology. (2019) 156:3–8. doi: 10.1111/imm.13006
99. Tan HL, Regamey N, Brown S, Bush A, Lloyd CM, Davies JC. The Th17 pathway in cystic fibrosis lung disease. Am J Respir Critic Care Med. (2011) 184:252–8. doi: 10.1164/rccm.201102-0236OC
100. Bacher P, Hohnstein T, Beerbaum E, Rocker M, Blango MG, Kaufmann S, et al. Human anti-fungal Th17 immunity and pathology rely on cross-reactivity against Candida albicans. Cell. (2019) 176:1340–55 e15. doi: 10.1016/j.cell.2019.01.041
101. Xi C, Jia Z, Xiaoli W, Na Z, He W, Hao J. New aspect of Liver IL-17(+)gammadelta T Cells. Mol Immunol. (2019) 107:41–3. doi: 10.1016/j.molimm.2018.12.030
102. Tsagaratou A. Unveiling the regulation of NKT17 cell differentiation and function. Mol Immunol. (2019) 105:55–61. doi: 10.1016/j.molimm.2018.11.013
103. Domingues RG, Hepworth MR. Immunoregulatory Sensory Circuits in Group 3 Innate Lymphoid Cell (ILC3) Function and Tissue Homeostasis. Front Immunol. (2020) 11:116. doi: 10.3389/fimmu.2020.00116
104. Zhang S. The role of transforming growth factor beta in T helper 17 differentiation. Immunology. (2018) 155:24–35. Epub 2018/04/24. doi: 10.1111/imm.12938
105. Jameson J, Ugarte K, Chen N, Yachi P, Fuchs E, Boismenu R, et al. A role for skin gammadelta T cells in wound repair. Science. (2002) 296:747–9. Epub 2002/04/27. doi: 10.1126/science.1069639
106. Zhang S. Hero or villain? The heterogeneity of Th17 cells. Mol Immunol. (2019) 112:358–9. doi: 10.1016/j.molimm.2019.06.014
107. Wang X, Ni L, Wan S, Zhao X, Ding X, Dejean A, et al. Febrile temperature critically controls the differentiation and pathogenicity of T Helper 17 Cells. Immunity. (2020) 52, 1–14. doi: 10.1016/j.immuni.2020.01.006
108. Gu AD, Wang Y, Lin L, Zhang SS, Wan YY. Requirements of transcription factor Smad-dependent and -independent TGF-beta signaling to control discrete T-cell functions. Proc Natl Acad Sci USA. (2012) 109:905–10. doi: 10.1073/pnas.1108352109
109. Gu AD, Zhang S, Wang Y, Xiong H, Curtis TA, Wan YY. A critical role for transcription factor Smad4 in T cell function that is independent of transforming growth factor beta receptor signaling. Immunity. (2015) 42:68–79. doi: 10.1016/j.immuni.2014.12.019
110. Zhang S, Takaku M, Zou L, Gu AD, Chou WC, Zhang G, et al. Reversing SKI-SMAD4-mediated suppression is essential for TH17 cell differentiation. Nature. (2017) 551:105–9. doi: 10.1038/nature24283
111. Zhang S, Zhang G, Wan YY. SKI and SMAD4 are essential for IL-21-induced Th17 differentiation. Mol Immunol. (2019) 114:260–8. doi: 10.1016/j.molimm.2019.07.029
112. Tecalco-Cruz AC, Rios-Lopez DG, Vazquez-Victorio G, Rosales-Alvarez RE, Macias-Silva M. Transcriptional cofactors Ski and SnoN are major regulators of the TGF-beta/Smad signaling pathway in health and disease. Signal Transduc Targeted Therap. (2018) 3:15. doi: 10.1038/s41392-018-0015-8
113. Wu H, Yu Y, Huang H, Hu Y, Fu S, Wang Z, et al. Progressive pulmonary fibrosis is caused by elevated mechanical tension on alveolar stem cells. Cell. (2020) 180:107–21 e17. doi: 10.1016/j.cell.2019.11.027
114. Eyerich S, Eyerich K, Pennino D, Carbone T, Nasorri F, Pallotta S, et al. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Investig. (2009) 119:3573–85. doi: 10.1172/JCI40202
115. Brembilla NC, Dufour AM, Alvarez M, Hugues S, Montanari E, Truchetet ME, et al. IL-22 capacitates dermal fibroblast responses to TNF in scleroderma. Ann Rheum Dis. (2016) 75:1697–705. doi: 10.1136/annrheumdis-2015-207477
116. Khawar MB, Azam F, Sheikh N, Abdul Mujeeb K. How does interleukin-22 mediate liver regeneration and prevent injury and fibrosis? J Immunol Res. (2016) 2016:2148129. doi: 10.1155/2016/2148129
117. Qin S, Chen M, Guo X, Luo W, Wang J, Jiang H. The clinical significance of intrahepatic Th22 cells in liver cirrhosis. Adv Clin Exp Med. (2019) 28:765–70. doi: 10.17219/acem/94062
118. Dudakov JA, Hanash AM, van den Brink MR. Interleukin-22: immunobiology and pathology. Ann Rev Immunol. (2015) 33:747–85. doi: 10.1146/annurev-immunol-032414-112123
119. Hector A, Schafer H, Poschel S, Fischer A, Fritzsching B, Ralhan A, et al. Regulatory T-cell impairment in cystic fibrosis patients with chronic pseudomonas infection. Am J Respir Crit Care Med. (2015) 191:914–23. doi: 10.1164/rccm.201407-1381OC
120. Liu X, Nurieva RI, Dong C. Transcriptional regulation of follicular T-helper (Tfh) cells. Immunol Rev. (2013) 252:139–45. doi: 10.1111/imr.12040
121. Fonseca VR, Ribeiro F, Graca L. T follicular regulatory (Tfr) cells: Dissecting the complexity of Tfr-cell compartments. Immunol Rev. (2019) 288:112–27. doi: 10.1111/imr.12739
122. Zheng J, Wang T, Zhang L, Cui L. Dysregulation of circulating Tfr/Tfh ratio in primary biliary cholangitis. Scand J Immunol. (2017) 86:452–61. doi: 10.1111/sji.12616
123. Wu X, Su Z, Cai B, Yan L, Li Y, Feng W, et al. Increased circulating follicular regulatory t-like cells may play a critical role in chronic hepatitis B virus infection and disease progression. Viral immunol. (2018) 31:379–88. doi: 10.1089/vim.2017.0171
124. Wang L, Qiu J, Yu L, Hu X, Zhao P, Jiang Y. Increased numbers of CD5+CD19+CD1dhighIL-10+ Bregs, CD4+Foxp3+ Tregs, CD4+CXCR5+Foxp3+ follicular regulatory T (TFR) cells in CHB or CHC patients. J Transl Med. (2014) 12:251. doi: 10.1186/s12967-014-0251-9
125. Stabile E, Kinnaird T, la Sala A, Hanson SK, Watkins C, Campia U, et al. CD8+ T lymphocytes regulate the arteriogenic response to ischemia by infiltrating the site of collateral vessel development and recruiting CD4+ mononuclear cells through the expression of interleukin-16. Circulation. (2006) 113:118–24. doi: 10.1161/CIRCULATIONAHA.105.576702
126. Guidotti LG, Inverso D, Sironi L, Di Lucia P, Fioravanti J, Ganzer L, et al. Immunosurveillance of the liver by intravascular effector CD8(+) T cells. Cell. (2015) 161:486–500. doi: 10.1016/j.cell.2015.03.005
127. Shalapour S, Lin XJ, Bastian IN, Brain J, Burt AD, Aksenov AA, et al. Inflammation-induced IgA+ cells dismantle anti-liver cancer immunity. Nature. (2017) 551:340–5. doi: 10.1038/nature24302
128. Kityo C, Makamdop KN, Rothenberger M, Chipman JG, Hoskuldsson T, Beilman GJ, et al. Lymphoid tissue fibrosis is associated with impaired vaccine responses. J Clin Investig. (2018) 128:2763–73. doi: 10.1172/JCI97377
129. Notas G, Kisseleva T, Brenner D. NK and NKT cells in liver injury and fibrosis. Clin Immunol. (2009) 130:16–26. doi: 10.1016/j.clim.2008.08.008
130. Mattner J. Natural killer T (NKT) cells in autoimmune hepatitis. Curr Opin Immunol. (2013) 25:697–703. doi: 10.1016/j.coi.2013.09.008
131. Gao B, Radaeva S, Park O. Liver natural killer and natural killer T cells: immunobiology and emerging roles in liver diseases. J Leukocyte Biol. (2009) 86:513–28. doi: 10.1189/jlb.0309135
132. Bank I. The Role of gammadelta T Cells in Fibrotic Diseases. Rambam Maimonides Med J. (2016) 7:10256. doi: 10.5041/RMMJ.10256
133. Ohtsuka T. Effect of gammadelta T cell supernatant on human skin fibroblast proliferation and collagen production–possible role of transforming growth factor-beta and basic fibroblast growth factor. Int J Dermatol. (2008) 47:1135–40. doi: 10.1111/j.1365-4632.2008.03805.x
134. Henriques A, Silva C, Santiago M, Henriques MJ, Martinho A, Trindade H, et al. Subset-specific alterations in frequencies and functional signatures of gammadelta T cells in systemic sclerosis patients. Inflamm Res. (2016) 65:985–94. doi: 10.1007/s00011-016-0982-6
135. Markovits N, Bendersky A, Loebstein R, Brusel M, Kessler E, Bank I. Anti-fibrotic characteristics of Vgamma9+ gammadelta T cells in systemic sclerosis. Clin Exp Rheumatol. (2016) 34 Suppl 100:23–9.
136. Migalovich Sheikhet H, Villacorta Hidalgo J, Fisch P, Balbir-Gurman A, Braun-Moscovici Y, Bank I. Dysregulated CD25 and cytokine expression by gammadelta T cells of systemic sclerosis patients stimulated with cardiolipin and zoledronate. Front Immunol. (2018) 9:753. doi: 10.3389/fimmu.2018.00753
137. Cheng M, Hu S. Lung-resident gammadelta T cells and their roles in lung diseases. Immunology. (2017) 151:375–84. doi: 10.1111/imm.12764
138. Simonian PL, Roark CL, Diaz del Valle F, Palmer BE, Douglas IS, Ikuta K, et al. Regulatory role of gammadelta T cells in the recruitment of CD4+ and CD8+ T cells to lung and subsequent pulmonary fibrosis. J Immunol. (2006) 177:4436–43. doi: 10.4049/jimmunol.177.7.4436
139. Simonian PL, Roark CL, Born WK, O'Brien RL, Fontenot AP. Gammadelta T cells and Th17 cytokines in hypersensitivity pneumonitis and lung fibrosis. Transl Res. (2009) 154:222–7. doi: 10.1016/j.trsl.2009.08.006
140. Lo Re S, Dumoutier L, Couillin I, Van Vyve C, Yakoub Y, Uwambayinema F, et al. IL-17A-producing gammadelta T and Th17 lymphocytes mediate lung inflammation but not fibrosis in experimental silicosis. J Immunol. (2010) 184:6367–77. doi: 10.4049/jimmunol.0900459
141. Wang X, Tian Z. gammadelta T cells in liver diseases. Front Med. (2018) 12:262–8. doi: 10.1007/s11684-017-0584-x
142. Klemann C, Schroder A, Dreier A, Mohn N, Dippel S, Winterberg T, et al. Interleukin 17, produced by gammadelta T cells, contributes to hepatic inflammation in a mouse model of biliary atresia and is increased in livers of patients. Gastroenterology. (2016) 150:229–41 e5. doi: 10.1053/j.gastro.2015.09.008
143. Yan X, Shichita T, Katsumata Y, Matsuhashi T, Ito H, Ito K, et al. Deleterious effect of the IL-23/IL-17A axis and gammadeltaT cells on left ventricular remodeling after myocardial infarction. J Am Heart Assoc. (2012) 1:e004408. doi: 10.1161/JAHA.112.004408
144. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. (2020) 382:1708–20. doi: 10.1101/2020.02.06.20020974
145. Wu J, Feng CL, Xian XY, Qiang J, Zhang J, Mao QX, et al. [Novel coronavirus pneumonia (COVID-19) CT distribution and sign features]. Zhonghua jie he he hu xi za zhi. (2020) 43:E030.
146. Xu YH, Dong JH, An WM, Lv XY, Yin XP, Zhang JZ, et al. Clinical and computed tomographic imaging features of novel coronavirus pneumonia caused by SARS-CoV-2. J Infect. (2020). doi: 10.1016/j.jinf.2020.02.017
147. Deschamp AR, Hatch JE, Slaven JE, Gebregziabher N, Storch G, Hall GL, et al. Early respiratory viral infections in infants with cystic fibrosis. J Cystic Fibrosis. (2019) 18:844–50. doi: 10.1016/j.jcf.2019.02.004
148. Xie L, Liu Y, Fan B, Xiao Y, Tian Q, Chen L, et al. Dynamic changes of serum SARS-coronavirus IgG, pulmonary function and radiography in patients recovering from SARS after hospital discharge. Respir Res. (2005) 6:5. doi: 10.1186/1465-9921-6-5
149. Venkataraman T, Coleman CM, Frieman MB. Overactive epidermal growth factor receptor signaling leads to increased fibrosis after severe acute respiratory syndrome coronavirus infection. J Virol. (2017) 91(12). doi: 10.1128/JVI.00182-17
150. Venkataraman T, Frieman MB. The role of epidermal growth factor receptor (EGFR) signaling in SARS coronavirus-induced pulmonary fibrosis. Antiviral Res. (2017) 143:142–50. doi: 10.1016/j.antiviral.2017.03.022
151. Kim J, Yang YL, Jeong Y, Jang YS. Middle East respiratory syndrome-coronavirus infection into established hDDP4-transgenic mice accelerates lung damage via activation of the pro-inflammatory response and pulmonary fibrosis. J Microbiol Biotechnol. (2019) 30, 427–38. doi: 10.4014/jmb.1910.10055
152. Burzyn D, Kuswanto W, Kolodin D, Shadrach JL, Cerletti M, Jang Y, et al. A special population of regulatory T cells potentiates muscle repair. Cell. (2013) 155:1282–95. doi: 10.1016/j.cell.2013.10.054
153. Ali N, Zirak B, Rodriguez RS, Pauli ML, Truong HA, Lai K, et al. Regulatory T cells in skin facilitate epithelial stem cell differentiation. Cell. (2017) 169:1119–29 e11. doi: 10.1016/j.cell.2017.05.002
154. Kwee BJ, Seo BR, Najibi AJ, Li AW, Shih TY, White D, et al. Treating ischemia via recruitment of antigen-specific T cells. Sci Adv. (2019) 5:eaav6313. doi: 10.1126/sciadv.aav6313
155. Castagnoli C, Trombotto C, Ondei S, Stella M, Calcagni M, Magliacani G, et al. Characterization of T-cell subsets infiltrating post-burn hypertrophic scar tissues. Burns. (1997) 23:565–72. doi: 10.1016/S0305-4179(97)00070-3
156. Bernabei P, Rigamonti L, Ariotti S, Stella M, Castagnoli C, Novelli F. Functional analysis of T lymphocytes infiltrating the dermis and epidermis of post-burn hypertrophic scar tissues. Burns. (1999) 25:43–8. doi: 10.1016/S0305-4179(98)00128-4
157. Tredget EE, Yang L, Delehanty M, Shankowsky H, Scott PG. Polarized Th2 cytokine production in patients with hypertrophic scar following thermal injury. J Interferon Cytokine Res. (2006) 26:179–89. doi: 10.1089/jir.2006.26.179
158. Wang J, Jiao H, Stewart TL, Shankowsky HA, Scott PG, Tredget EE. Increased TGF-beta-producing CD4+ T lymphocytes in postburn patients and their potential interaction with dermal fibroblasts in hypertrophic scarring. Wound Repair Regen. (2007) 15:530–9. doi: 10.1111/j.1524-475X.2007.00261.x
159. McGee HM, Schmidt BA, Booth CJ, Yancopoulos GD, Valenzuela DM, Murphy AJ, et al. IL-22 promotes fibroblast-mediated wound repair in the skin. J Invest Dermatol. (2013) 133:1321–9. doi: 10.1038/jid.2012.463
160. Kwan PO, Tredget EE. Biological principles of scar and contracture. Hand Clin. (2017) 33:277–92. doi: 10.1016/j.hcl.2016.12.004
161. Momtazi M, Ding J, Kwan P, Anderson CC, Honardoust D, Goekjian S, et al. Morphologic and histologic comparison of hypertrophic scar in nude mice, T-cell receptor, and recombination activating gene knockout mice. Plast Reconstr Surg. (2015) 136:1192–204. doi: 10.1097/PRS.0000000000001782
162. Ding J, Tredget EE. Transplanting human skin grafts onto nude mice to model skin scars. Methods Mol Biol. (2017) 1627:65–80. doi: 10.1007/978-1-4939-7113-8_5
163. Singer AJ, Tuggle C, Ahrens A, Sauer M, McClain SA, Tredget E, et al. Survival of human cadaver skin on severe combined immune deficiency pigs: Proof of concept. Wound Repair Regen. (2019) 27:426–30. doi: 10.1111/wrr.12715
164. Chen B, Li H, Xia W. The role of Th1/Th2 cell chemokine expression in hypertrophic scar. Int Wound J. (2020) 17:197–205. doi: 10.1111/iwj.13257
165. Schuppan D, Kim YO. Evolving therapies for liver fibrosis. J Clin Investig. (2013) 123:1887–901. doi: 10.1172/JCI66028
166. Kosaka H, Yoshimoto T, Yoshimoto T, Fujimoto J, Nakanishi K. Interferon-gamma is a therapeutic target molecule for prevention of postoperative adhesion formation. Nat Med. (2008) 14:437–41. doi: 10.1038/nm1733
167. Jiang H, Hegde S, Knolhoff BL, Zhu Y, Herndon JM, Meyer MA, et al. Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nat Med. (2016) 22:851–60. doi: 10.1038/nm.4123
168. Tedesco D, Thapa M, Chin CY, Ge Y, Gong M, Li J, et al. Alterations in intestinal microbiota lead to production of interleukin 17 by intrahepatic gammadelta T-cell receptor-positive cells and pathogenesis of cholestatic liver disease. Gastroenterology. (2018) 154:2178–93. doi: 10.1053/j.gastro.2018.02.019
169. Elhai M, Avouac J, Hoffmann-Vold AM, Ruzehaji N, Amiar O, Ruiz B, et al. OX40L blockade protects against inflammation-driven fibrosis. Proc Natl Acad Sci USA. (2016) 113:E3901–10. doi: 10.1073/pnas.1523512113
170. Boleto G, Guignabert C, Pezet S, Cauvet A, Sadoine J, Tu L, et al. T-cell costimulation blockade is effective in experimental digestive and lung tissue fibrosis. Arthritis Res Therap. (2018) 20:197. doi: 10.1186/s13075-018-1694-9
171. Zhao X, Kwan JYY, Yip K, Liu PP, Liu FF. Targeting metabolic dysregulation for fibrosis therapy. Nat Rev Drug Discov. (2020) 19:57–75. doi: 10.1038/s41573-019-0040-5
172. Dai DF, Santana LF, Vermulst M, Tomazela DM, Emond MJ, MacCoss MJ, et al. Overexpression of catalase targeted to mitochondria attenuates murine cardiac aging. Circulation. (2009) 119:2789–97. doi: 10.1161/CIRCULATIONAHA.108.822403
173. Kropski JA, Blackwell TS. Endoplasmic reticulum stress in the pathogenesis of fibrotic disease. J Clin Investig. (2018) 128:64–73. doi: 10.1172/JCI93560
174. Grohmann M, Wiede F, Dodd GT, Gurzov EN, Ooi GJ, Butt T, et al. Obesity Drives STAT-1-Dependent NASH and STAT-3-Dependent HCC. Cell. (2018) 175:1289–306 e20. doi: 10.1016/j.cell.2018.09.053
175. Ramachandran P, Dobie R, Wilson-Kanamori JR, Dora EF, Henderson BEP, Luu NT, et al. Resolving the fibrotic niche of human liver cirrhosis at single-cell level. Nature. (2019) 575:512–8. doi: 10.1038/s41586-019-1631-3
Keywords: T cells, fibrosis, fibrotic diseases, T helper, CD8, Treg, NKT, γδ T
Citation: Zhang M and Zhang S (2020) T Cells in Fibrosis and Fibrotic Diseases. Front. Immunol. 11:1142. doi: 10.3389/fimmu.2020.01142
Received: 14 March 2020; Accepted: 11 May 2020;
Published: 26 June 2020.
Edited by:
Jianlei Hao, Jinan University, ChinaReviewed by:
Lei He, University of Chicago, United StatesEdward Tredget, University of Alberta Hospital, Canada
Copyright © 2020 Zhang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Song Zhang, z@immlab.org
 Mengjuan Zhang
Mengjuan Zhang Song Zhang
Song Zhang