- 1Unité Mixte de Recherche CNRS 7276, INSERM 1262, Université de Limoges, Limoges, France
- 2Segal Cancer Center, Lady Davis Institute for Medical Research and Departments of Oncology and Medicine, McGill University, Montréal, QC, Canada
Class switch recombination (CSR) changes antibody isotype by replacing Cμ constant exons with different constant exons located downstream on the immunoglobulin heavy (IgH) locus. During CSR, transcription through specific switch (S) regions and processing of non-coding germline transcripts (GLTs) are essential for the targeting of activation-induced cytidine deaminase (AID). While CSR to IgG1 is abolished in mice lacking an Iγ1 exon donor splice site (dss), many questions remain regarding the importance of I exon dss recognition in CSR. To further clarify the role of I exon dss in CSR, we first evaluated RNA polymerase II (RNA pol II) loading and chromatin accessibility in S regions after activation of mouse B cells lacking Iγ1 dss. We found that deletion of Iγ1 dss markedly reduced RNA pol II pausing and active chromatin marks in the Sγ1 region. We then challenged the post-transcriptional function of I exon dss in CSR by using antisense oligonucleotides (ASOs) masking I exon dss on GLTs. Treatment of stimulated B cells with an ASO targeting Iγ1 dss, in the acceptor Sγ1 region, or Iμ dss, in the donor Sμ region, did not decrease germline transcription but strongly inhibited constitutive splicing and CSR to IgG1. Supporting a global effect on CSR, we also observed that the targeting of Iμ dss reduced CSR to IgG3 and, to a lesser extent, IgG2b isotypes. Altogether, this study reveals that the recognition of I exon dss first supports RNA pol II pausing and the opening of chromatin in targeted S regions and that GLT splicing events using constitutive I exon dss appear mandatory for the later steps of CSR, most likely by guiding AID to S regions.
Introduction
During immune responses, B cells can diversify the immunoglobulin (Ig) repertoire through class switch recombination (CSR) and somatic hypermutation (SHM). SHM introduces mutations in the variable (V) regions of Ig genes modifying antibody affinity for a cognate antigen. The mouse Ig heavy chain (IgH) locus comprises eight constant genes (CH). CSR involves long-range interactions at the IgH locus and occurs between GC-rich repetitive switch (S) DNA regions preceding each CH gene due to the enzymatic activity of activation-induced deaminase (AID) (1, 2). Thus, CSR replaces the Cμ exons by a downstream constant gene Cγ, Cε, or Cα allowing expression of antibodies with different isotypes (from IgM to IgG, IgE, or IgA) and effector functions.
Transcription through S regions is required for CSR (3). Germline (GL) transcription is initiated from intervening (I) promoters a few kilobases upstream of both the donor and the acceptor S regions. The μ I promoter drives constitutive transcription through Sμ while γ, ε, or α I promoters are inducible. Primary GL transcripts (GLTs) exhibit a conserved structure composed of a non-coding I exon, an intronic S region, and CH exons (4, 5). In mature GLTs, the I exon is spliced to the first exon (CH1) of the adjacent constant gene. Because multiple stop codons are present in the three reading frames of I exons, these GLTs do not encode peptides of significant lengths.
During CSR, AID initiates double-strand DNA breaks (DSBs) by deaminating cytidines inside the transcribed S regions. GL transcription through S regions of CH gene favors AID accessibility to S regions (3). GL transcription promotes generation of RNA:DNA hybrid structures (R-loops) (6, 7) revealing single-stranded DNA (ssDNA) that serves as a substrate for AID (8). The impairment of transcription elongation upon R-loop formation (9) may favor RNA polymerase II (RNA pol II) pausing. RNA pol II pausing then promotes AID recruitment to S regions (10, 11). Paused RNA pol II and histone modifications associated with “open” chromatin, such as histone H3 lysine 4 trimethylation (H3K4me3) and histone H3 lysine 9 acetylation (H3K9ac), are enriched in transcribed I–S regions and have been involved in AID targeting to S regions primed for CSR (12–16). Moreover, the suppressor of Ty 5 homolog (Spt5) transcription elongation factor and the RNA exosome, a cellular RNA-processing degradation complex, associate with AID together with paused RNA pol II in transcribed S regions and are required for CSR (17, 18).
Beyond the prerequisite transcription of S regions, splicing of GLTs has been proposed to be important for the CSR process. Notably, CSR to IgG1 is severely impaired in a mouse model lacking the Iγ1 exon donor splice site (dss) (19, 20). Further supporting a role for splicing of GLTs in CSR, several RNA processing and splicing factors are critical regulators of CSR (21, 22). Interestingly, it has recently been proposed that intronic switch RNAs produced by the splicing of primary GLTs act as guide RNAs and target AID to DNA in a sequence-specific manner (23). After lariat debranching by the RNA debranching enzyme (DBR1), these switch RNAs are folded into G-quadruplexes. G-quadruplexes and AID are targeted to the S region DNA through the post-transcriptional action of the DEAD-box RNA helicase 1 (DDX1) (24).
Even though these data suggest that processing of GLTs by the splicing machinery is necessary for CSR, the precise role of I exon dss recognition in antibody class switching remains largely unknown. To address this issue, we first analyzed whether the presence of Iγ1 exon dss could influence RNA pol II pausing and chromatin accessibility of the Sγ1 region, as early events leading to CSR to IgG1. For that, chromatin immunoprecipitation (ChIP) experiments were performed in stimulated B cells from the previously described human metallothionein IIA (hMT) and s-hMT (splice hMT) mouse models, lacking or harboring Iγ1 exon dss, respectively (19, 20). We next specifically evaluated the impact of GLT splicing on CSR to IgG1 by using antisense oligonucleotides (ASOs) targeting specific I exon dss on primary GLTs, from both donor and acceptor S regions. Contrary to the models used previously to study the impact of I exons on CSR, treatment of mouse B cells by ASOs masks only a short RNA sequence (23–25 nucleotides) surrounding the I exon dss on primary GLTs. This antisense strategy bypassing the impact of I exon dss recognition on transcription is very useful for studying the involvement of I exon dss recognition in CSR at the post-transcriptional level. Collectively, our data indicate that the recognition of I exon dss exerts both transcriptional and post-transcriptional roles during CSR.
Materials and Methods
Mice
Two- to 8-month-old C57BL/6, s-hMT, and hMT mice were used. hMT and s-hMT mice were kindly obtained from Dr. A. Radbruch (Leibniz Institute, Berlin, Germany). Mice were housed, and procedures were conducted in agreement with European Directive 2010/63/EU on animals used for scientific purposes applied in France as the “Décret n°2012-118 du 1er février 2013 relatif à la protection des animaux utilisés à des fins scientifiques.” Accordingly, the present project APAFIS#15279-2018052915087229 v3 was authorized by the “Ministère de l'Education Nationale, de l'Enseignement Supérieur et de la Recherche.”
Splenic B Cell in vitro Stimulation and ASO Treatments
Splenic B cells isolated from C57BL/6, hMT, and s-hMT mice were purified with the EasySep Mouse B Cell Isolation Kit (Stemcell Technologies). B cells were cultured for 2–4 days in RPMI 1640 with UltraGlutamine (Lonza) containing 10% fetal calf serum (FCS) (Dominique Dutscher), 1 mM sodium pyruvate (Eurobio), 1% AANE (Eurobio), 50 U/ml penicillin/50 μg/ml streptomycin (Gibco), and 129 μM 2-mercaptoethanol (Sigma-Aldrich). Splenic B cells were stimulated with either 1 or 5 μg/ml lipopolysaccharide (LPS) (LPS-EB Ultrapure, InvivoGen), 1 μg/ml LPS + 20 ng/ml interleukin 4 (IL4) (recombinant murine IL-4, PeproTech), or 5 μg/ml anti-CD40 [mouse CD40/TNFRSF5 MAb (Clone 1C10), Bio-Techne, USA] + 40 ng/ml IL4. For ASO treatments, vivo-morpholino ASOs (Iγ1 dss ASO: 5′-CCCACTCCCCTGGTCACTTACCG-3′; Iμ dss ASO: 5′-GGCTGCCTCTGGCTTACCATTTG-3′) and an irrelevant ASO (control: 5′-CCTCTTACCTCAGTTACAATTTATA-3′) were designed and purchased at Gene Tools, LLC (Philomath, USA). Stimulated splenic B cells were cultured in the presence of 2, 3, or 4 μM ASO, as indicated.
Culture samples were harvested at day 2, 3, or 4 for subsequent flow cytometry analysis, ChIP assays, or RNA/protein extraction, as described below and in the Supplementary Methods. Supernatants of stimulated B cells were collected at day 3 or 4 and stored at −20°C until used for enzyme-linked immunosorbent assays (ELISAs).
Flow Cytometry
Cell suspensions of in vitro stimulated splenic B cells were washed in phosphate-buffered saline (PBS). To reduce Fc receptor-mediated binding by antibodies of interest, B cells were preincubated for 15 min with 0.5 μg/ml anti-mouse CD16/CD32 (Clone 2.4G2, BD Pharmingen, ref 553142) in FACS buffer [PBS supplemented with 2% FCS and 2 mM (ethylenedinitrilo)tetraacetic acid (EDTA) (Sigma-Aldrich)]. Cells were then labeled with 0.5 μg/ml anti-mouse B220-BV421 (clone RA3-6B2, BioLegend, ref 103240) and 0.5 μg/ml anti-mouse IgG1-FITC (clone A85-1, BD Pharmingen, ref 553443) antibodies or with 0.25 μg/ml anti-mouse B220-APC-Cy7 (clone RA3-6B2, BD Pharmingen, ref 552094) and 0.5 μg/ml anti-mouse IgG1-FITC (clone A85-1, BD Pharmingen, ref 553443) or 0.5 μg/ml anti-mouse IgG3-FITC (clone R40-82, BD Pharmingen, ref 553403) and anti-mouse IgG2a/2b-BV421 (clone R2-40, BD OptiBuild, ref 744292) antibodies.
After 45 min, cells were washed in PBS and suspended in FACS buffer. For nonviable-cell exclusion, 7-AAD (BD Pharmingen, ref 559925) was added on cells 10 min before flow cytometry analysis. Data were acquired on a BD Pharmingen Fortessa LSR2 (BD Biosciences, San Jose, CA, USA) and analyzed using Flowlogic™ software (Miltenyi Biotec).
ELISAs
Culture supernatants and sera were analyzed for the presence of IgM, IgG1, or IgG2b by ELISA. Blood samples were collected from 12-week-old hMT and s-hMT mice. Serum samples were recovered by centrifugation and stored at −20°C until used. ELISAs were performed in polycarbonate 96-multiwell plates, coated overnight at 4°C (100 μl per well) with 2 μg/ml IgM, IgG1, or IgG2b antibodies (Southern Biotechnologies: goat anti-mouse IgM human ads UNLB, ref 1020-01; goat anti-mouse IgG1 human ads-UNLB, ref 1070-01; and goat anti-mouse IgG2b human ads-UNLB, ref 1090-01) in PBS. After three successive washing steps in PBS with 0.05% Tween® 20 (Sigma-Aldrich), a blocking step with 100 μl of 3% bovine serum albumin (BSA) (Euromedex) in PBS was performed for 30 min at 37°C. After three washing steps, 50 μl of sera/supernatant or standards IgM, IgG1, or IgG2b (Southern Biotechnologies, 400 ng/ml) were diluted into successive wells in 1% BSA/PBS buffer and incubated for 2 h at 37°C. After three washing steps, 100 μl per well of 1 μg/l alkaline phosphatase (AP)-conjugated goat anti-mouse antibodies (Southern Biotechnologies: goat anti-mouse IgG1 human ads-AP, ref 1070-04 and goat anti-mouse IgM-AP, ref 1021-04; Beckman Coulter: goat anti-mouse IgG2b human ads-AP, ref 1090-04) was incubated in PBS with 0.05% Tween® 20 for 2 h at 37°C. After three washing steps, AP activity was assayed: 100 μl of substrate for AP (SIGMAFAST™ p-nitrophenyl phosphate tablets, Sigma-Aldrich) was added, and after 15 min, the reaction was blocked with the addition of 50 μl of 3 M NaOH (Sigma-Aldrich). Optic density was then measured at 405 nm on a Multiskan FC microplate photometer (Thermo Scientific).
RT-PCR and Quantitative RT-PCR
In vitro stimulated splenic B cells were harvested, and RNA was extracted using the TRIzol™ reagent (Invitrogen) procedure. Reverse transcription was carried out on 1 μg of DNase I (Invitrogen)-treated RNA using a High-Capacity cDNA Reverse Transcription kit (Applied Biosystems). Priming for reverse transcription was done with random hexamers.
To analyze GL transcription, PCRs were performed on cDNA using the Taq Core Kit (MP Biomedicals) and appropriate primer pairs (primer pairs are provided in Supplementary Table 1). PCR amplification of the β-actin transcript was used as an internal loading control.
To determine nucleotide sequence of normal and alternative transcripts, PCRs were performed on cDNA using the Phusion® High-Fidelity DNA Polymerase (New England BioLabs) and appropriate primer pairs. After purification of the RT-PCR products using the NucleoSpin Gel and PCR clean-up kit (Macherey-Nagel) according to the manufacturer's instructions, sequencing was performed using the BigDye™ Terminator v3.1 Cycle Sequencing Kit on a 3130 × l Genetic Analyzer ABI PRISM (Applied Biosystems). Quantification of the purified RT-PCR products was also performed using ImageJ software.
Quantitative PCRs were performed on cDNA using Premix Ex Taq™ (probe qPCR), ROX Plus (Takara), or TB Green Premix Ex Taq™ II (Tli RNase H Plus), ROX Plus (Takara) on a StepOnePlus Real-Time PCR system (Applied Biosystems). Transcripts were quantified according to the standard 2−ΔΔCt method after normalization to Gapdh. Primers and probes used for determination of transcripts are listed in Supplementary Table 1.
ChIP Assays
ChIP assays were performed using anti-H3ac (Millipore, 06-599), anti-H3K4me3 (Millipore, 07-473), anti-H3K9ac (Millipore, 06-942), anti-RNA pol II (CTD4H8, Santa Cruz Biotechnology, sc-47701), anti-RNA pol II ser2P (Abcam, ab5095), and anti-RNA pol II ser5P (Abcam, ab5131) as previously described (25). In brief, 1 ×107 LPS-stimulated B cells from hMT and s-hMT mice were harvested at day 2, washed twice in PBS, and cross-linked at 37°C for 15 min in 15 ml of PBS with 1% formaldehyde (Sigma-Aldrich). The reaction was quenched with 0.125 M glycine (Sigma-Aldrich). After lysis, chromatin was sonicated to 0.5–1 kb using a Vibracell 75043 (Thermo Fisher Scientific). After dilution in ChIP buffer [0.01% SDS (Sigma-Aldrich), 1.1% Triton X-100 (Sigma-Aldrich), 1.2 mM EDTA (Eurobio), 16.7 mM Tris-HCl (Sigma-Aldrich), pH 8.1, and 167 mM NaCl (Sigma-Aldrich)], chromatin was precleared by rotating for 2 h at 4°C with 100 μl of 50% protein A/G slurry (0.2 mg/ml sheared salmon sperm DNA, 0.5 mg/ml BSA, and 50% protein A/G; Sigma-Aldrich), 0.3–0.5 ×106 cell equivalents were saved as input, and 3–5 ×106 cell equivalents were incubated overnight with specific or control antibodies. Immune complexes were precipitated by the addition of protein A/G. Cross-linking was reversed by overnight incubation (70°C) in Tris-EDTA (Sigma-Aldrich) buffer with 0.02% SDS, and genomic DNA was obtained after phenol/chloroform extraction. Analysis of immunoprecipitated DNA sequences was done by quantitative PCR using the primer pairs described in Supplementary Table 1.
Statistical Analysis
Results are expressed as means ± SEM, and overall differences between variables were evaluated by an unpaired two-tailed Student's t test using Prism GraphPad software (San Diego, CA).
Results
RNA pol II Pausing and Histone Modifications Upstream of the Sγ1 Region Are Altered in Mice Lacking Iγ1 dss
To clarify the role of I exon dss in CSR, we first performed comparative experiments in homozygous s-hMT and hMT mice (19, 20). In these models, the promoter and Iγ1 exon have been replaced by the LPS-inducible hMT promoter and an artificial IhMT exon containing (s-hMT) or lacking (hMT) Iγ1 dss (Figure 1A). As previously described by Lorenz and collaborators (20), IgG1 class switching was almost abolished in hMT mice, whereas CSR to other Ig isotypes remained unaffected (Supplementary Figures 1A–C). This was not associated with a difference in AID mRNA expression between splenic B cells isolated from hMT and s-hMT mice (Supplementary Figure 1D). Previous nuclear run-on assays have shown that the transcription rate of the Sγ1 region was higher in LPS-stimulated B cells from s-hMT mice than that from hMT mice (20), suggesting a role for Iγ1 exon dss in GL transcription of the Sγ1 region. In agreement with these results, we found low levels of unspliced γ1 GLTs in LPS-stimulated B cells from hMT mice, compared to s-hMT (Figure 1B).
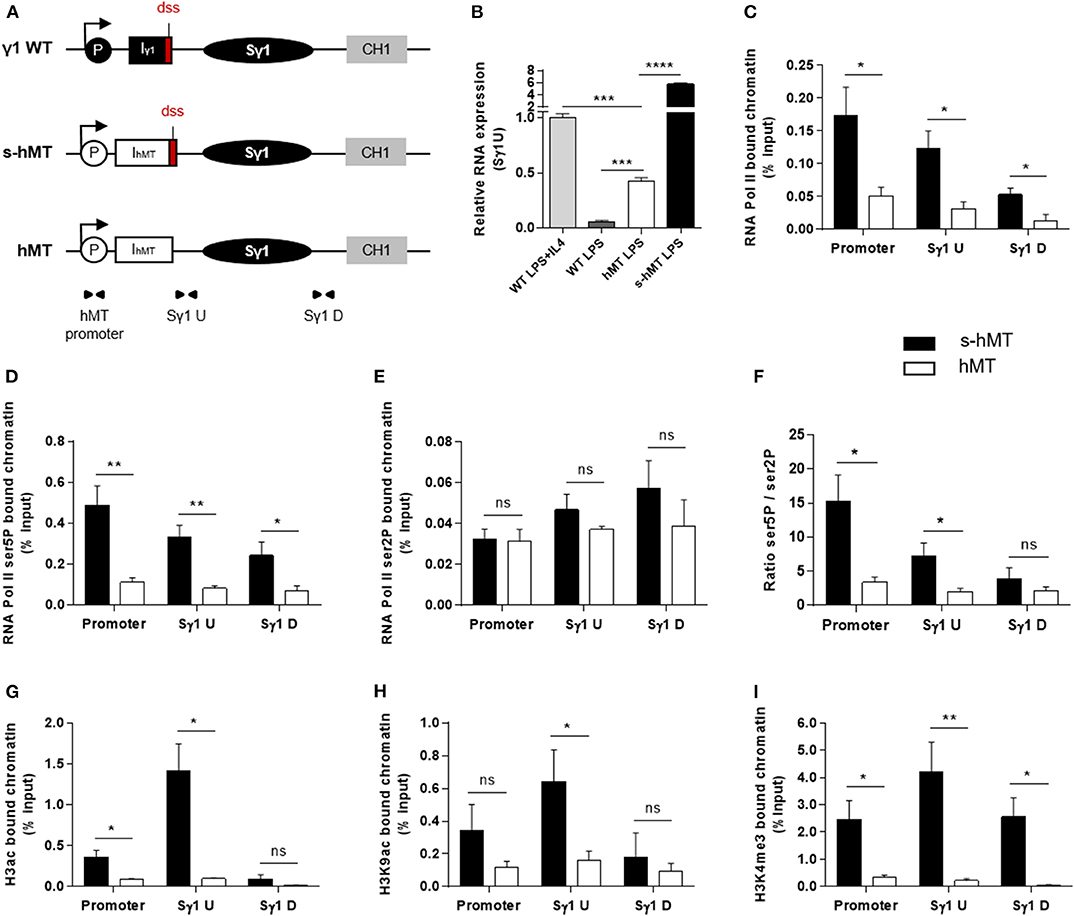
Figure 1. Deletion of Iγ1 exon dss decreased RNA pol II pausing and chromatin accessibility in the Sγ1 region. (A) Wild-type (WT) γ1 locus and s-hMT and hMT murine models. Black and gray elements represent gene segments of the γ1 WT locus. The white elements are gene segments specific to s-hMT and hMT models and are not present in WT animals. The red fragment corresponds to the sequence containing the I exon donor splice site (dss), retained in the s-hMT model and absent in the hMT model. P, promoter; Iγ1, γ1 I exon; IhMT, hMT I exon; Sγ1, γ1 switch region; CH1, γ1 constant exon 1. (B) Splenic B cells were isolated from WT, homozygous s-hMT and homozygous hMT mice and stimulated with LPS + IL4 or LPS. After 2 days of stimulation, γ1 GLT expression relative to GAPDH mRNA expression was monitored by quantitative RT-PCR using Sγ1U primers described in schema A. Expression of γ1 GLTs in B cells isolated from WT mice and stimulated by LPS + IL4 was normalized to 1. (C–I) Splenic B cells were isolated from homozygous s-hMT and hMT mice and stimulated with LPS. After 2 days of stimulation, the cells were analyzed for RNA pol II (C), Ser5P RNA pol II (D), and Ser2P RNA pol II (E) levels in the γ1 locus by ChIP coupled to quantitative PCR. Ser5P RNA pol II/Ser2P RNA pol II ratios are indicated (F). Cells were also analyzed for H3ac (G), H3K9ac (H), and H3K4me3 (I) levels in the γ1 locus by ChIP coupled to quantitative PCR. Background signals from mock samples with irrelevant antibody were subtracted. Values were normalized to total input DNA. Position and sequence of primers used for quantitative PCR are described in schema A (triangles) and Supplementary Table 1. (B–I) Data are means ± SEM of at least two independent experiments, n = 3–5 for each genotype. Unpaired two-tailed Student's t test was used to determine significance. ns: non-significant; *P <0.05, **P <0.01, ***P <0.001, ****P <0.0001.
Paused RNA pol II and histone modifications associated with “open” chromatin are involved in AID recruitment to S regions during CSR (10, 12, 13, 15, 16, 18). To study the role of I exon dss in RNA pol II pausing and chromatin remodeling in the Sγ1 region, we performed ChIP experiments in splenic B cells isolated from hMT and s-hMT mice after 2 days of LPS stimulation. First, antibodies directed against total RNA pol II, elongating RNA pol II (Ser2P), or pausing RNA pol II (Ser5P) were used. In agreement with the low level of γ1 GLTs described in Figure 1B, stimulated B cells from hMT mice displayed significantly decreased total RNA pol II loading throughout the Sγ1 region, compared to s-hMT mice (Figure 1C). Interestingly, a significant decrease in Ser5P RNA pol II loading (Figure 1D), but not Ser2P RNA pol II loading (Figure 1E), was observed in the promoter–Sγ1 region in stimulated B cells from hMT compared to s-hMT mice. Accordingly, stimulated B cells from s-hMT mice exhibited significantly higher Ser5P/Ser2P ratios upstream from the Sγ1 region than B cells from hMT animals (Figure 1F), suggesting that the recognition of I exon dss regulated RNA pol II pausing upstream from the Sγ1 region. As a control, we found similar Ser5P and Ser2P RNA pol II loading in Sμ and Sγ2b regions in stimulated B cells from both hMT and s-hMT mice (Supplementary Figure 2).
We next performed similar ChIP experiments using antibodies directed against variants of histone H3. We observed very low levels of acetylated histone H3 (H3ac), a general marker of chromatin accessibility, throughout the whole promoter–Sγ1 region in LPS-stimulated B cells from hMT compared to s-hMT mice (Figure 1G). In B cells from s-hMT mice, the highest levels of H3ac, H3K9ac, and H3K4me3 histone forms were observed in the region upstream from Sγ1 (Figures 1G–I). In contrast, this increase was not anymore observed in B cells from hMT mice.
Collectively, our data indicate that Iγ1 exon dss recognition is of key importance for transcriptional activity of the Sγ1 region by supporting RNA pol II pausing and chromatin remodeling, two occurrences required for AID recruitment in S regions and efficient CSR.
ASO Strategy Targeting I Exon dss Allows Uncoupling GL Transcription and Splicing in Wild-Type B Cells
We next wanted to study the function of I exon splicing on CSR. For this purpose, we treated stimulated splenic B cells isolated from wild-type (WT) mice with vivo-morpholino ASO targeting the dss sequence of Iγ1 exon (Iγ1 dss ASO) (Figure 2A). Indeed, we previously demonstrated that passive administration of these phosphorodiamidate morpholino oligonucleotides complexed with an octaguanidine dendrimer could very efficiently modulate the splicing of Ig transcripts (26). First, we wanted to verify that masking the dss sequence of Iγ1 exon on GLTs by ASO did not inhibit γ1 GL transcription. After 2 days of culture with LPS + IL4 and 2 μM ASO, expression of unspliced γ1 GLTs in activated B cells was significantly increased after treatment with Iγ1 dss ASO (Figure 2B), compared to cells treated with an irrelevant control ASO. This suggested an accumulation of unspliced γ1 GLTs due to Iγ1 dss ASO-induced splicing inhibition. In order to study the consequences of Iγ1 dss ASO on spliced γ1 GLTs, we next performed PCR using the Iγ1–Cγ1 primer pair (Iγ1-for and Cγ1-rev, described in Figure 2C and Supplementary Table 1) on cDNA from ASO-treated WT splenic B cells stimulated by LPS + IL4. After 2 days, the γ1 GLT profile was strikingly different in control and Iγ1 dss ASO conditions (Figure 2C, top). A band corresponding to the constitutively spliced γ1 GLT (involving the constitutive Iγ1 exon dss) was strongly detected in control but slightly detected upon Iγ1 dss ASO treatment (Figure 2C, top, and Supplementary Figure 3). Remarkably, in both control and Iγ1 dss ASO conditions, additional bands were also detected that could account for new splicing isoforms. Sanger sequencing indeed revealed alternative transcripts so far not described in the literature (alternative transcripts 1, 2, and 3; Figure 2C, middle, and Supplementary Figure 3) involving donor and acceptor splice sites internal to the Iγ1 exon. The fact that these alternative splice sites were predicted with high consensus value by the HSF 3.1 tool [HSF 3.1, 07/24/2019, http://www.umd.be/HSF/HSF.shtml, (27)] (Figure 2C, bottom) indicated that the different PCR products were bona fide spliced transcripts. As a proof of ASO efficiency, treatment with Iγ1 dss ASO almost abolished the major constitutive GLT and prevented alternative transcript 1 detection (Figure 2C, top). Thus, after treatment with Iγ1 dss ASO, transcript isoforms using the constitutive Iγ1 dss (constitutive transcript and alternative transcript 1) represented only 16% of the detected transcripts (vs. 85% in control). Another consequence of Iγ1 dss ASO treatment is the detection of alternative transcript 3 and a better detection of alternative transcript 2.
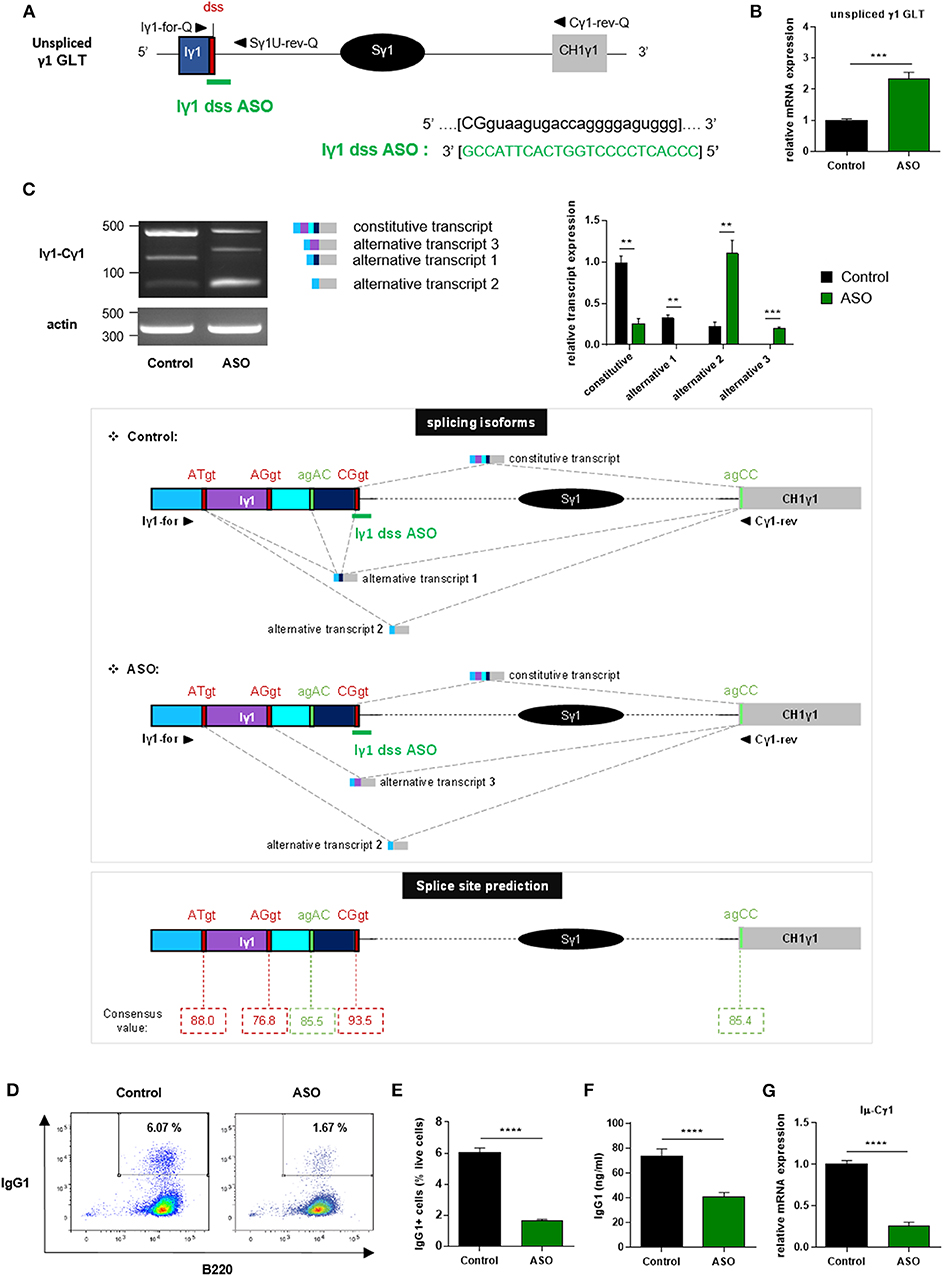
Figure 2. Treatment with Iγ1 exon dss ASO inhibited γ1 GLT constitutive splicing and IgG1 class switching in B cells. (A) Antisense oligonucleotide targeting the donor splice site of Iγ1 exon (Iγ1 dss ASO) was designed and synthetized as “vivo-morpholino ASO” permitting passive administration of ASO in cells (Gene Tools, LLC). Targeted γ1 GLT (uppercase: exon sequence; lowercase: intron sequence) and Iγ1 dss ASO sequences are indicated. Iγ1, γ1 I exon; Sγ1, γ1 switch region; CH1γ1, γ1 constant exon 1; dss, donor splice site. (B–G) Splenic B cells were isolated from C57BL/6 mice, stimulated with LPS + IL4, and treated with 2 μM Iγ1 dss ASO (ASO) or an irrelevant ASO (control) for 2 days (B,C) or 3 days (D–G). (B) Unspliced γ1 GLT expression relative to GAPDH mRNA expression was monitored by quantitative RT-PCR using Iγ1-for-Q and Sγ1U-rev-Q primers described in schema A. Expression of γ1 GLTs in control B cells was normalized to 1. (C, top) RT-PCR was performed using Iγ1-for and Cγ1-rev primers (position described in schema C, middle) to identify constitutively and alternatively spliced transcripts. PCR products were analyzed on agarose gels. Expression of actin mRNA is also shown. Molecular markers in base pairs are indicated. Schematic representation of the different γ1 spliced transcripts is indicated on the right, and transcript sequences are given in Supplementary Figure 3. One experiment out of three is shown. Relative quantification of amplification products was done using ImageJ software and expressed after normalization to actin band intensity. (C, middle) Schematic representation of γ1 spliced transcripts detected in B cells from C57BL/6 mice after treatment with an irrelevant ASO (control) or Iγ1 dss ASO (ASO). Gray hatched lines represent splicing events involving constitutive and alternative splice sites. Donor and acceptor splice sites are indicated in red and green, respectively. (C, bottom) Consensus value (ranging from 0 to 100) of each predicted splice site determined using the HSF 3.1 tool. (D) Flow cytometry analysis of purified B-cell populations using the indicated cell surface markers. Plots are gated on live cells. The percentage of B220+IgG1+ cells is indicated. One experiment out of five is shown. (E) Percentage of IgG1-positive cells determined by flow cytometry. (F) Quantification of IgG1 in culture supernatants by ELISA. (G) Post-switch Iμ-Cγ1 mRNA expression relative to GAPDH expression was monitored by quantitative RT-PCR. Expression of post-switch mRNAs in control B cells was normalized to 1. Sequences of primers used for RT-PCR and quantitative RT-PCR are indicated in Supplementary Table 1. (B,C,E–G) Data are means ± SEM of two independent experiments, n = 3–8 for each group. Unpaired two-tailed Student's t test was used to determine significance. **P <0.01, ***P <0.001, ****P <0.0001.
Since our data showed that Iγ1 dss ASO strongly decreased γ1 GLT constitutive splicing, the ASO strategy is a good tool to study the function of Iγ1 exon splicing on CSR.
Treatment With Iγ1 Exon dss ASO Specifically Inhibits IgG1 Class Switching
We next investigated the effect of ASO-mediated γ1 GLT splicing inhibition on CSR to IgG1. After 3 days of culture with LPS + IL4 and 2 μM ASO, CSR to IgG1 was greatly diminished in B cells treated with Iγ1 dss ASO compared to B cells treated with an irrelevant control ASO. The percentage of IgG1-positive B cells determined by flow cytometry was decreased 4-fold in Iγ1 dss ASO-treated B cells (Figures 2D,E), and IgG1 levels determined in culture supernatants by ELISA were almost 2-fold lower in Iγ1 dss ASO-treated cells (Figure 2F) than in control B cells. In agreement with the CSR defect, Iμ-Cγ1 switched transcripts were decreased by 4-fold in Iγ1 dss ASO-treated B cells (Figure 2G).
In order to control Iγ1 dss ASO specificity, we realized similar experiments in B cells stimulated by anti-CD40 + IL4. Similar to what was observed in LPS + IL4-stimulated B cells, after 2 days of culture with anti-CD40 + IL4 and 2 μM ASO, expression of unspliced γ1 GLTs was significantly increased (Figure 3B), and CSR to IgG1 was significantly diminished (Figures 3C,D) in B cells treated with Iγ1 dss ASO compared to B cells treated with an irrelevant control ASO. However, expression of unspliced Iε GLTs (Figures 3A,E) and CSR to IgE (Figures 3F,G) were similar in B cells treated with the irrelevant control ASO or Iγ1 dss ASO. This showed that Iγ1 dss ASO specifically decreased CSR to IgG1 by inhibiting splicing precisely on γ1 GLTs.
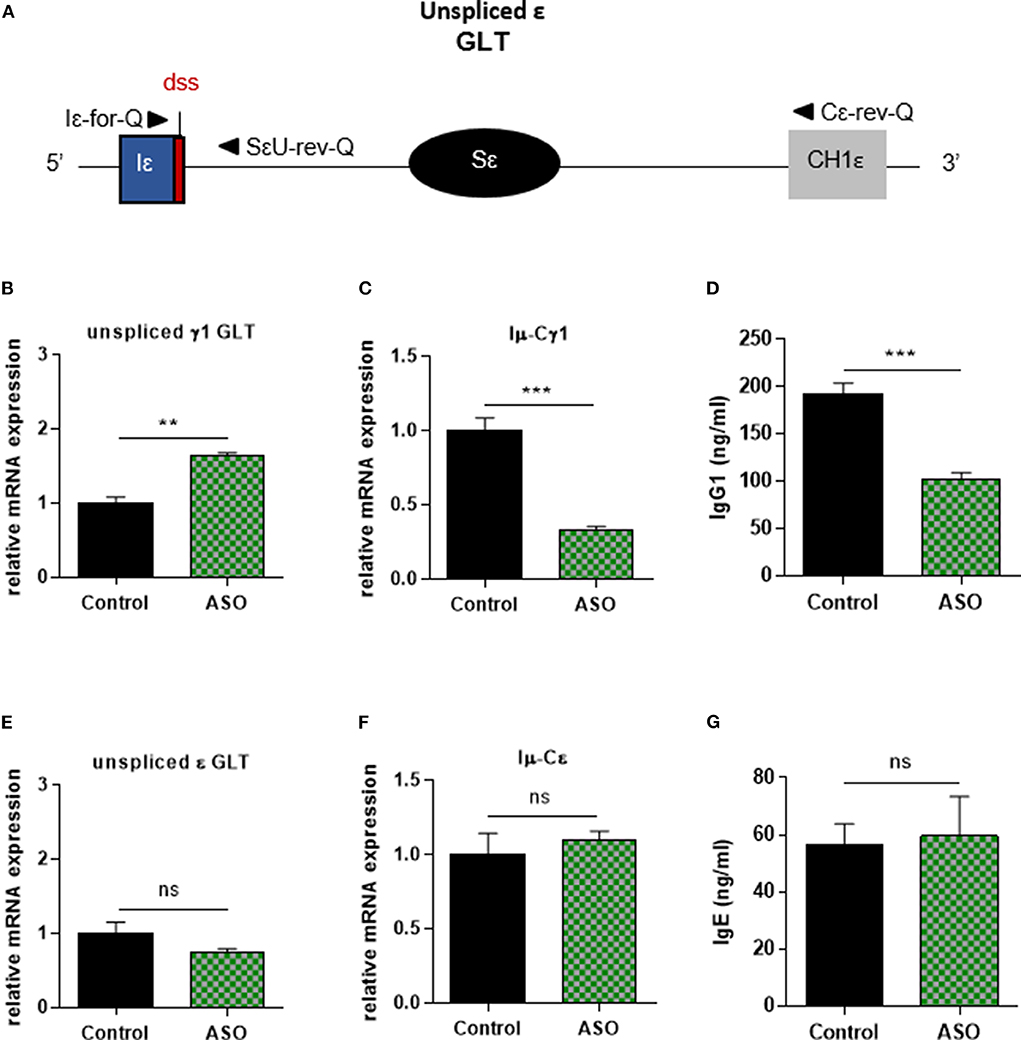
Figure 3. Specific IgG1 class switching inhibition in B cells treated by Iγ1 exon dss ASO. Splenic B cells were isolated from C57BL/6 mice, stimulated with anti-CD40 + IL4 and treated with 2 μM Iγ1 dss ASO (ASO) or an irrelevant ASO (control) for 2 days (B,E) or 4 days (C,D,F,G). (A) Schematic representation of unspliced ε GLT. Iε, ε I exon; Sε, ε switch region; CH1ε, ε constant exon 1; dss, donor splice site. (B,E) Unspliced γ1 (B) and ε (E) GLT expression monitored as described in Figure 2. Iε-for-Q and SεU-rev-Q primers, described in schema A, were used for ε GLT expression determination. (C,F) Post-switch Iμ-Cγ1 and Iμ-Cε mRNA expressions monitored as described in Figure 2. (D,G) Quantification of IgG1 and IgE in culture supernatants by ELISA. (B–G) Data are means ± SEM of two independent experiments, n = 3–4 for each group. Unpaired two-tailed Student's t test was used to determine significance. ns: non-significant, **P <0.01, ***P <0.001.
Collectively, these data indicate that splicing of γ1 GLTs is necessary for CSR to IgG1 and strongly suggest that the use of the constitutive Iγ1 exon dss during splicing is required for efficient CSR.
Treatment With an ASO Targeting the Constitutive I exon dss in the Donor Sμ Region Inhibits IgG Class Switching
Our results showed that the ASO strategy targeting the constitutive I exon dss from the Sγ1 acceptor region inhibited CSR to IgG1. We next developed a similar approach to determine whether specifically masking the constitutive I exon dss from the donor Sμ region could also inhibit CSR to IgG1. Splenic B cells isolated from WT mice were stimulated by LPS + IL4 and treated with an ASO targeting the constitutive Iμ exon dss (Iμ dss ASO) (Figure 4A). In order to study the consequences of Iμ dss ASO on spliced Iμ GLTs, we performed PCR using the Iμ-Cμ primer pair (Iμ-for and Cμ-rev, described in Figure 4A and Supplementary Table 1) on cDNA from stimulated splenic B cells treated with 2 μM ASO for 2 days. A band corresponding to the constitutively spliced Iμ transcript (involving the constitutive Iμ exon dss) was detected in control but slightly detected after Iμ dss ASO treatment (Figure 4B). Similar to what was observed in stimulated splenic B cells treated with Iγ1 dss ASO, we detected the presence of alternatively spliced Iμ transcripts in cells treated with Iμ dss ASO. These alternative transcripts were indicative of the use of alternative Iμ dss present in the intronic S region previously described by Kuzin and collaborators (28). In addition, after 3 days of culture with LPS + IL4 and 2 μM ASO, CSR to IgG1 was significantly diminished in B cells treated with Iμ dss ASO compared to B cells treated with an irrelevant control ASO (Figures 4C,D). After 2 days of 2 μM ASO treatment, expression of unspliced γ1 GLTs was similar in B cells treated with an irrelevant control or Iμ dss ASO (Figure 4E). This showed that the defect in IgG1 class switching observed after treatment by Iμ dss ASO was not due to an inhibition of γ1 GLT splicing but to the splicing decrease of Iμ GLTs. Therefore, correct splicing of GLTs produced in both the donor Sμ and the acceptor Sγ1 regions is required for efficient IgG1 class switching.
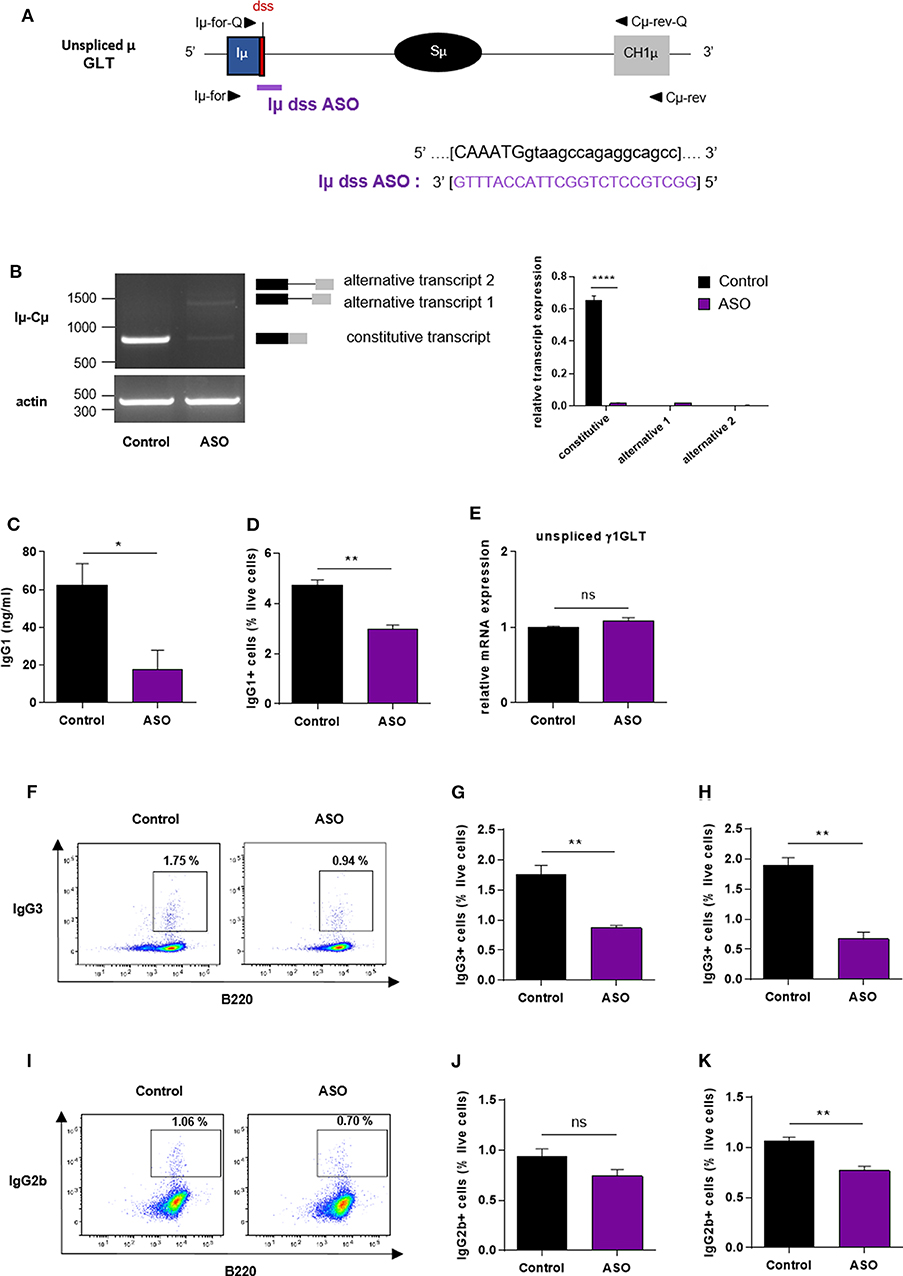
Figure 4. Impaired antibody class switching in B cells treated by Iμ exon dss ASO. (A) Antisense oligonucleotide targeting the donor splice site of Iμ exon (Iμ dss ASO) was designed and synthetized as “vivo-morpholino ASO” permitting passive administration of ASO in the cells (Gene Tools, LLC). Targeted μ GLT (uppercase: exon sequence; lowercase: intron sequence) and Iμ dss ASO sequences are indicated. Iμ, μ I exon; Sμ, μ switch region; CH1μ, μ constant exon 1; dss, donor splice site. (B–K) Splenic B cells were isolated from C57BL/6 mice, stimulated with LPS + IL4 (B–E) or LPS (F–K) and treated with 2 μM (B–E), 3 μM (F,G,J) or 4 μM (H,I,K) Iμ dss ASO or an irrelevant control ASO for 2 days (B,E) or 3 days (C,D,F–K). (B) Constitutively and alternatively spliced μ transcripts analyzed as described in Figure 2 using Iμ-for and Cμ-rev primers, described in schema A. One experiment out of three is shown. Relative quantification of amplification products was done using ImageJ software and expressed after normalization to actin band intensity. (C) Quantification of IgG1 in culture supernatants by ELISA. (D) Percentage of IgG1-positive cells determined by flow cytometry as described in Figure 2. (E) Unspliced γ1 GLT expression monitored as described in Figure 2. (F,I) Flow cytometry analysis of purified B-cell populations using the indicated cell surface markers. Plots are gated on live cells. The percentage of B220+IgG3+ (F) or B220+IgG2b+ (I) cells is indicated. One experiment out of three is shown. (G,H,J,K) Percentage of IgG3-positive (G,H) or IgG2b-positive (J,K) cells determined by flow cytometry. (B–K) Data are means ± SEM, n = 3 for each group. Unpaired two-tailed Student's t test was used to determine significance. ns: non-significant, *P <0.05, **P <0.01, ****P <0.0001.
We next investigated whether masking the constitutive Iμ dss could also inhibit class switching to other isotypes than IgG1. Splenic B cells isolated from WT mice were stimulated by LPS and treated with Iμ dss ASO. After 3 days of culture with LPS and 3 or 4 μM ASO, the percentage of IgG3-positive B cells was significantly diminished in B cells treated with Iμ dss ASO compared to B cells treated with an irrelevant control ASO (Figures 4F–H). Moreover, treatment of B cells with Iμ dss ASO strongly decreased the level of γ3 heavy-chain protein (Supplementary Figure 4). After 3 days of culture with LPS, the percentage of IgG2b-positive B cells was significantly diminished only in B cells treated with 4 μM Iμ dss ASO compared to B cells treated with an irrelevant control ASO (Figures 4I–K).
These results indicated that treatment with ASO masking the constitutive Iμ dss on the donor Sμ region globally reduced CSR, albeit with variable efficiency depending on the switched isotypes.
Discussion
S region transcription per se promotes basal CSR, but for optimal efficiency, the process requires the presence of the intact I region, implicating factors beyond transcription through the S region in the regulation of class switching (29). Transcription and pre-mRNA processing are functionally coupled. In our study, through the experimental uncoupling of splicing from transcription, we identified distinct functions of I exon dss that control antibody class switching at transcriptional and post-transcriptional levels. During transcription, we provide evidence that deletion of the Iγ1 exon dss decreased accumulation of Ser5P RNA pol II and chromatin accessibility in the promoter–Sγ1 region. Moreover, our data demonstrated that, in S regions primed for CSR, GLT splicing involving the constitutive I exon dss is an essential step for efficient CSR.
Regarding the impact of I exon dss on transcription, our comparative analysis of s-hMT and hMT B cells indicated that, in the absence of Iγ1 dss, GL transcription and RNA pol II loading were very weak at the γ1 locus. Interestingly, RNA pol II binding was strongly diminished at the hMT promoter, suggesting that the presence of Iγ1 exon dss enhanced the initiation of GL transcription. These data are consistent with the poor transcription observed upon deletion of a large portion of Iγ2b exon including dss (30). Although the function of I exon dss in transcription regulation has been overlooked, such intron-mediated enhancement of gene expression has long been described in a wide range of organisms, including mammals (31). Indeed, it has been demonstrated that the presence of a dss facilitates the transcription preinitiation complex assembly and stimulates transcription even in the absence of splicing (32, 33). Whether the recognition of I exon dss promotes the formation of pre-initiation complex (32) and involves a gene looping interaction between promoter and dss, as described in yeast cells (34), remains to be investigated. Our ChIP analysis further indicated that, in the absence of Iγ1 exon dss, RNA pol II pausing is markedly decreased in the promoter–Sγ1 region, whereas the rate of RNA pol II elongation remains mostly unchanged. It is tempting to speculate that the I exon dss acts as an anchor for the co-transcriptional machinery and is required for appropriate associations of critical factors, like Spt5, with RNA pol II to enable recruitment of AID to S regions. After B cell stimulation, transcribed I–S regions are a focus for increased modified histones, such as H3ac, H3K9ac, or H3K4me3, and chromatin accessibility (13, 16). Reinforcing the idea that GL transcription, RNA pol II pausing, and the establishment of active chromatin marks in S regions are interconnected (10), we found a global reduction of active H3ac marks in the promoter–Sγ1 region of stimulated B cells from hMT mice, compared to s-hMT. The H3K9ac and H3K4me3 enrichment specifically detected upstream the Sγ1 region was also lost in hMT mice. Interestingly, it has been proposed that H3K4me3 serves as a mark for recruiting the recombinase machinery for CSR independently of its function in transcription (15, 35). These studies have shown that Spt5 and the histone chaperone FAcilitates Chromatin Transcription (FACT) are not required for transcription of S regions but regulate H3K4me3 modification and DNA cleavage in CSR (15, 35).
Iγ1 exon dss recognition is necessary for the regulation of GL transcription. Consequently, using classical I exon deletion and/or replacement mouse models neither allow distinguishing the roles of I exon dss in the interconnected processes of transcription and splicing nor permit distinguishing requirements of splicing per se from that of stable S GLTs (36). Several studies including ours have shown the efficacy of ASO-mediated approaches to modify RNA splicing in B-lineage cells (26, 37, 38). Here, we used ASOs targeting a short sequence (23–25 nucleotides) spanning the Iγ1 or Iμ exon dss on pre-mRNA to analyze the consequences of GLT splicing defect on CSR in splenic B cells from WT mice. We showed that, in addition to its positive effect on transcription, I exon dss recognition is required at the post-transcriptional level for efficient CSR. Indeed, we demonstrated that masking Iγ1 exon dss inhibited CSR to IgG1 and masking Iμ exon dss decreased CSR to IgG1, IgG3, and, to a lesser extent, IgG2b isotypes. Further investigations in both mouse and human B cells will be necessary to check whether masking Iμ exon dss could decrease CSR to all isotypes. ASOs masking I exon dss in other acceptor S regions than Sγ1 could also be used to verify whether the recognition of I exon dss is required for efficient CSR toward each switched Ig isotype.
Our results strongly suggest that splicing of GLTs per se is not sufficient to induce CSR. Indeed, we found a marked reduction of CSR to IgG1 upon treatment with ASOs masking constitutive I exon dss on GLTs produced in the donor Sμ or the acceptor Sγ1 regions, even though alternative splicing events could be readily detected. By contrast, Kuzin and collaborators showed normal surface Ig expression and serum Ig levels in ΔIμ-s−/− mice harboring a deletion of 236 bp spanning the constitutive Iμ dss (28). As expected, there were no detectable spliced Iμ transcripts in these mice. Nevertheless, alternative “Iμ-like” GLTs driven by regulatory elements other than Eμ were detected at low levels in B cells from ΔIμ-s−/− mice. The authors suggested that such alternative “Iμ-like” GLTs directly contribute to CSR activity (28). However, our results showed that these alternative “Iμ-like” GLTs might not be sufficient for efficient CSR activity in vitro. Indeed, after treatment of B cells with Iμ dss ASO, we detected Iμ-Cμ alternative transcripts indicative of the use of the same cryptic Sμ dss than described by Kuzin and collaborators whereas a drastic inhibition of CSR was observed. The regulation of μ locus is complex, and further investigations are needed to understand the discrepancies concerning the impact of Iμ dss absence at the DNA level, in genetically modified mouse model, and at the RNA level, with our ASO strategy, on CSR. Nonetheless, our data are in agreement with a study of Ruminy and collaborators (39). They detected recurrent acquired mutations at the Iμ dss on the functional IgH allele of t(14;18) positive lymphomas cases presenting restricted IgM expression and proposed that disruption of Iμ constitutive dss, inducing the expression of abnormal GLTs, may be involved in the perturbation of CSR observed in these lymphomas. Moreover, Spt4 depletion by siRNA in the CH12F3-2A B cell line severely impaired CSR to IgA despite a dramatically increased expression of cryptic transcripts initiating from the Sμ intronic region (35). As observed in the donor Sμ region, masking the constitutive I exon dss on GLTs produced in the acceptor Sγ1 region with Iγ1 dss ASO strongly inhibited CSR to IgG1 despite Iγ1–Cγ1 alternative transcripts being detectable. Additional molecular studies will be required to delineate the mechanism involved in the ASO-mediated inhibition of CSR. Several scenarios could explain the inhibition of CSR by I exon dss ASOs. First, RNA-binding proteins have been shown to interact with AID (22, 40–42), and the ASO, attached to the GLTs, could avoid fixation of such proteins necessary for CSR. Second, it has been described that, when the accumulation of intronic switch RNAs was prevented from the onset of CH12F3-2A B cell stimulation, R-loop levels were decreased by half in S regions (24). In our study, after I exon dss ASO treatment, the accumulation of unspliced GLTs indicated a strong splicing inhibition. Thus, even though low levels of constitutive and alternative spliced GLTs are detected after ASO treatment, the lariat abundance may be too weak to induce R-loop formation at S regions and efficient CSR. Third, even if alternatively spliced GLTs were detected after ASO treatments, the sequence of intronic lariat generated after alternative splicing could prevent efficient CSR. For example, alternative lariat sequence could impede the lariat debranching by DBR1 and the subsequent generation of G-quadruplex RNA structures that participate in guiding AID to specific S regions through RNA:DNA base pairing (23). Alternative lariat sequences could also avoid the fixation of DDX1 to G-quadruplex switch RNAs and consequently impair AID binding to S region DNA (24). These explanations are consistent with a model whereby processed S region transcripts serve as a guide for RNAs to target AID, in a sequence-dependent manner, to the S region DNA from which they were transcribed (36).
In summary, our study highlighted a dual role for I exon dss during CSR, at the DNA and RNA levels, and further paves the way for antisense strategies in the modulation of antibody class switching and immune response efficiency.
Data Availability Statement
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
Ethics Statement
The animal study was reviewed and approved by the 'Ministère de l'Education Nationale, de l'Enseignement Supérieur et de la Recherche'. APAFIS#15279-2018052915087229 v3.
Author Contributions
AM, MA, and J-ML performed experiments and analyzed data. CC, SL, and NS performed experiments. LD conceived the project, designed experiments, analyzed the data, and wrote the manuscript. SP conceived the project, designed experiments, performed experiments, analyzed the data, and wrote the manuscript.
Funding
This work was supported by grants from Fondation ARC (PJA 20161204724), INCa (PLBIO15-256), ANR (2017-CE15-0024-01), Ligue Contre le Cancer (CD87, CD19, and CD23) and Fondation Française pour la Recherche contre le Myélome et les Gammapathies monoclonales (FFRMG).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the staff of our animal facility. We also thank J. Cook-Moreau and E. Pinaud (UMR CNRS 7276, INSERM 1262, Limoges, France) for critical reading of the manuscript and A. Radbruch (Leibniz Institute, Berlin, Germany) for providing hMT and s-hMT mice. This manuscript has been released as a preprint at bioRxiv (43).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2020.00780/full#supplementary-material
References
1. Arakawa H. Requirement of the activation-induced deaminase (AID) gene for immunoglobulin gene conversion. Science. (2002) 295:1301–6. doi: 10.1126/science.1067308
2. Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. (2000) 102:553–63. doi: 10.1016/S0092-8674(00)00078-7
3. Matthews AJ, Zheng S, DiMenna LJ, Chaudhuri J. Regulation of immunoglobulin class-switch recombination. In: Alt F, editor. Advances in Immunology. (2014) 122:1–57. doi: 10.1016/B978-0-12-800267-4.00001-8
4. Lennon GG, Perry RP. C mu-containing transcripts initiate heterogeneously within the IgH enhancer region and contain a novel 5'-nontranslatable exon. Nature. (1985) 318:475–8. doi: 10.1038/318475a0
5. Lutzker S, Alt FW. Structure and expression of germ line immunoglobulin gamma 2b transcripts. Mol Cell Biol. (1988) 8:1849–52. doi: 10.1128/MCB.8.4.1849
6. Tian M, Alt FW. Transcription-induced cleavage of immunoglobulin switch regions by nucleotide excision repair nucleases in vitro. J Biol Chem. (2000) 275:24163–72. doi: 10.1074/jbc.M003343200
7. Yu K, Chedin F, Hsieh CL, Wilson TE, Lieber MR. R-loops at immunoglobulin class switch regions in the chromosomes of stimulated B cells. Nat Immunol. (2003) 4:442–51. doi: 10.1038/ni919
8. Chaudhuri J, Tian M, Khuong C, Chua K, Pinaud E, Alt FW. Transcription-targeted DNA deamination by the AID antibody diversification enzyme. Nature. (2003) 422:726–30. doi: 10.1038/nature01574
9. Huertas P, Aguilera A. Cotranscriptionally formed DNA:RNA hybrids mediate transcription elongation impairment and transcription-associated recombination. Mol Cell. (2003) 12:711–21. doi: 10.1016/j.molcel.2003.08.010
10. Kenter AL. AID targeting is dependent on RNA polymerase II pausing. Semin Immunol. (2012) 24:281–6. doi: 10.1016/j.smim.2012.06.001
11. Pavri R, Nussenzweig MC. AID targeting in antibody diversity. Adv Immunol. (2011) 110:1–26. doi: 10.1016/B978-0-12-387663-8.00005-3
12. Daniel JA, Nussenzweig A. The AID-induced DNA damage response in chromatin. Mol Cell. (2013) 50:309–21. doi: 10.1016/j.molcel.2013.04.017
13. Jeevan-Raj BP, Robert I, Heyer V, Page A, Wang JH, Cammas F, et al. Epigenetic tethering of AID to the donor switch region during immunoglobulin class switch recombination. J Exp Med. (2011) 208:1649–60. doi: 10.1084/jem.20110118
14. Kuang FL, Luo Z, Scharff MD. H3 trimethyl K9 and H3 acetyl K9 chromatin modifications are associated with class switch recombination. Proc Natl Acad Sci USA. (2009) 106:5288–93. doi: 10.1073/pnas.0901368106
15. Stanlie A, Aida M, Muramatsu M, Honjo T, Begum NA. Histone3 lysine4 trimethylation regulated by the facilitates chromatin transcription complex is critical for DNA cleavage in class switch recombination. Proc Natl Acad Sci USA. (2010) 107:22190–5. doi: 10.1073/pnas.1016923108
16. Wang L, Wuerffel R, Feldman S, Khamlichi AA, Kenter AL. S region sequence, RNA polymerase II, and histone modifications create chromatin accessibility during class switch recombination. J Exp Med. (2009) 206:1817–30. doi: 10.1084/jem.20081678
17. Basu U, Meng FL, Keim C, Grinstein V, Pefanis E, Eccleston J, et al. The RNA exosome targets the AID cytidine deaminase to both strands of transcribed duplex DNA substrates. Cell. (2011) 144:353–63. doi: 10.1016/j.cell.2011.01.001
18. Pavri R, Gazumyan A, Jankovic M, Di Virgilio M, Klein I, Ansarah-Sobrinho C, et al. Activation-induced cytidine deaminase targets DNA at sites of RNA polymerase II stalling by interaction with Spt5. Cell. (2010) 143:122–33. doi: 10.1016/j.cell.2010.09.017
19. Hein K, Lorenz MGO, Siebenkotten G, Petry K, Christine R, Radbruch A. Processing of switch transcripts is required for targeting of antibody class switch recombination. J Exp Med. (1998) 188:2369–74. doi: 10.1084/jem.188.12.2369
20. Lorenz M, Jung S, Radbruch A. Switch transcripts in immunoglobulin class switching. Science. (1995) 267:1825–8. doi: 10.1126/science.7892607
21. Conticello SG, Ganesh K, Xue K, Lu M, Rada C, Neuberger MS. Interaction between antibody-diversification enzyme AID and spliceosome-associated factor CTNNBL1. Mol Cell. (2008) 31:474–84. doi: 10.1016/j.molcel.2008.07.009
22. Nowak U, Matthews AJ, Zheng S, Chaudhuri J. The splicing regulator PTBP2 interacts with the cytidine deaminase AID and promotes binding of AID to switch-region DNA. Nat Immunol. (2011) 12:160–6. doi: 10.1038/ni.1977
23. Zheng S, Vuong BQ, Vaidyanathan B, Lin JY, Huang FT, Chaudhuri J. Non-coding RNA generated following lariat debranching mediates targeting of AID to DNA. Cell. (2015) 161:762–73. doi: 10.1016/j.cell.2015.03.020
24. Ribeiro de Almeida C, Dhir S, Dhir A, Moghaddam AE, Sattentau Q, Meinhart A, et al. RNA Helicase DDX1 converts RNA G-Quadruplex structures into R-Loops to promote IgH class switch recombination. Mol Cell. (2018) 70:650–62.e8. doi: 10.1016/j.molcel.2018.04.001
25. Rouaud P, Vincent-Fabert C, Saintamand A, Fiancette R, Marquet M, Robert I, et al. The IgH 3′ regulatory region controls somatic hypermutation in germinal center B cells. J Exp Med. (2013) 210:1501–7. doi: 10.1084/jem.20130072
26. Ashi MO, Srour N, Lambert JM, Marchalot A, Martin O, Le Noir S, et al. Physiological and druggable skipping of immunoglobulin variable exons in plasma cells. Cell Mol Immunol. (2019) 16:810–9. doi: 10.1038/s41423-018-0160-6
27. Desmet FO, Hamroun D, Lalande M, Collod-Béroud G, Claustres M, Béroud C. Human splicing finder: an online bioinformatics tool to predict splicing signals. Nucleic Acids Res. (2009) 37:e67. doi: 10.1093/nar/gkp215
28. Kuzin II, Ugine GD, Wu D, Young F, Chen J, Bottaro A. Normal isotype switching in B cells lacking the Iμ exon splice donor site: evidence for multiple Iμ-like germline transcripts. J Immunol. (2000) 164:1451–7. doi: 10.4049/jimmunol.164.3.1451
29. Bottaro A, Lansford R, Xu L, Zhang J, Rothman P, Alt FW. S region transcription per se promotes basal IgE class switch recombination but additional factors regulate the efficiency of the process. EMBO J. (1994) 13:665–74. doi: 10.1002/j.1460-2075.1994.tb06305.x
30. Seidl KJ, Bottaro A, Vo A, Zhang J, Davidson L, Alt FW. An expressed neo(r) cassette provides required functions of the 1gamma2b exon for class switching. Int Immunol. (1998) 10:1683–92. doi: 10.1093/intimm/10.11.1683
31. Shaul O. How introns enhance gene expression. Int J Biochem Cell Biol. (2017) 91:145–55. doi: 10.1016/j.biocel.2017.06.016
32. Damgaard CK, Kahns S, Lykke-Andersen S, Nielsen AL, Jensen TH, Kjems J. A 5′ splice site enhances the recruitment of basal transcription initiation factors in vivo. Mol Cell. (2008) 29:271–8. doi: 10.1016/j.molcel.2007.11.035
33. Furger A. Promoter proximal splice sites enhance transcription. Genes Dev. (2002) 16:2792–9. doi: 10.1101/gad.983602
34. Moabbi AM, Agarwal N, El Kaderi B, Ansari A. Role for gene looping in intron-mediated enhancement of transcription. Proc Natl Acad Sci USA. (2012) 109:8505–10. doi: 10.1073/pnas.1112400109
35. Stanlie A, Begum NA, Akiyama H, Honjo T. The DSIF Subunits Spt4 and Spt5 have distinct roles at various phases of immunoglobulin class switch recombination. PLoS Genet. (2012) 8:e1002675. doi: 10.1371/journal.pgen.1002675
36. Yewdell WT, Chaudhuri J. A transcriptional serenAID: the role of noncoding RNAs in class switch recombination. Int Immunol. (2017) 29:183–96. doi: 10.1093/intimm/dxx027
37. Bestas B, Moreno PMD, Blomberg KEM, Mohammad DK, Saleh AF, Sutlu T, et al. Splice-correcting oligonucleotides restore BTK function in X-linked agammaglobulinemia model. J Clin Invest. (2014) 124:4067–81. doi: 10.1172/JCI76175
38. Dewaele M, Tabaglio T, Willekens K, Bezzi M, Teo SX, Low DHP, et al. Antisense oligonucleotide–mediated MDM4 exon 6 skipping impairs tumor growth. J Clin Invest. (2015) 126:68–84. doi: 10.1172/JCI82534
39. Ruminy P, Jardin F, Penther D, Picquenot JM, Parmentier F, Buchonnet G, et al. Recurrent disruption of the Iμ splice donor site in t(14;18) positive lymphomas : a potential molecular basis for aberrant downstream class switch recombination. Genes Chromosomes Cancer. (2007) 46:735–44. doi: 10.1002/gcc.20453
40. Chen J, Cai Z, Bai M, Yu X, Zhang C, Cao C, et al. The RNA-binding protein ROD1/PTBP3 cotranscriptionally defines AID-loading sites to mediate antibody class switch in mammalian genomes. Cell Res. (2018) 28:981–95. doi: 10.1038/s41422-018-0076-9
41. Hu W, Begum NA, Mondal S, Stanlie A, Honjo T. Identification of DNA cleavage- and recombination-specific hnRNP cofactors for activation-induced cytidine deaminase. Proc Natl Acad Sci USA. (2015) 112:5791–6. doi: 10.1073/pnas.1506167112
42. Mondal S, Begum NA, Hu W, Honjo T. Functional requirements of AID's higher order structures and their interaction with RNA-binding proteins. Proc Natl Acad Sci USA. (2016) 113:E1545–54. doi: 10.1073/pnas.1601678113
Keywords: B cells, class switch recombination, splicing, germline transcripts, I exon, antisense oligonucleotides
Citation: Marchalot A, Ashi MO, Lambert J-M, Carrion C, Lecardeur S, Srour N, Delpy L and Le Pennec S (2020) Uncoupling Splicing From Transcription Using Antisense Oligonucleotides Reveals a Dual Role for I Exon Donor Splice Sites in Antibody Class Switching. Front. Immunol. 11:780. doi: 10.3389/fimmu.2020.00780
Received: 15 January 2020; Accepted: 06 April 2020;
Published: 08 May 2020.
Edited by:
Deborah K Dunn-Walters, University of Surrey, United KingdomReviewed by:
David Jonathan Fear, King's College London, United KingdomPaolo Casali, University of Texas Health Science Center San Antonio, United States
Copyright © 2020 Marchalot, Ashi, Lambert, Carrion, Lecardeur, Srour, Delpy and Le Pennec. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Laurent Delpy, laurent.delpy@unilim.fr; Soazig Le Pennec, soazig.le-pennec@unilim.fr
†These authors have contributed equally to this work and share first authorship
 Anne Marchalot1†
Anne Marchalot1† Mohamad Omar Ashi
Mohamad Omar Ashi Laurent Delpy
Laurent Delpy Soazig Le Pennec
Soazig Le Pennec