- 1Experimental Therapeutics Laboratory, School of Pharmacy and Medical Science, Hanson Institute and Sansom Institute for Health Research, University of South Australia, Adelaide, SA, Australia
- 2Robinson Research Institute and Adelaide Medical School, University of Adelaide, Adelaide, SA, Australia
The success of pregnancy is contingent on the maternal immune system recognizing and accommodating a growing semi-allogeneic fetus. Specialized subsets of lymphocytes capable of negative regulation are fundamental in this process, and include the regulatory T cells (Tregs) and potentially, regulatory B cells (Bregs). Most of our current understanding of the immune regulatory role of Bregs comes from studies in the fields of autoimmunity, transplantation tolerance, and cancer biology. Bregs control autoimmune diseases and can elicit graft tolerance by inhibiting the differentiation of effector T cells and dendritic cells (DCs), and activating Tregs. Furthermore, in cancer, Bregs are hijacked by neoplastic cells to promote tumorigenesis. Pregnancy therefore represents a condition that reconciles these fields—mechanisms must be in place to ensure maternal immunological tolerance throughout gravidity to allow the semi-allogeneic fetus to grow within. Thus, the mechanisms underlying Breg activities in autoimmune diseases, transplantation tolerance, and cancer may take place during pregnancy as well. In this review, we discuss the potential role of Bregs as guardians of pregnancy and propose an endocrine-modulated feedback loop highlighting the Breg–Treg–tolerogenic DC interface essential for the induction of maternal immune tolerance.
Introduction
Pregnancy is a remarkable biological process wherein the mother carries an offspring that is semi-allogenic, that is, half of its genome is derived from the foreign paternal parent. It imposes significant physiological stress, requiring the mother’s body to undergo immense functional changes. Pregnancy in mammals represents a unique compromise in the context of immunity—the maternal immune system must accommodate the semi-allogenic fetus by dampening its immune responses to maintain a state of immunological tolerance throughout gravidity, while retaining the capacity to identify pathogens and destroy them with appropriate control of resultant inflammation. This dichotomy necessarily requires a fine and highly regulated balance between immune tolerance and immune activation.
In pregnancy, the immune profiles of dendritic cells (DCs), natural killer cells, macrophages, and T cells are by necessity modified (1–4). Recently, it has been shown that the B cell profile is also modified in response to the immunological needs of the mother during pregnancy (5). The role of B cells in pregnancy holds much interest, especially in light of the recent identification of a B cell subset capable of negative regulation, that is, can suppress immune inflammation. Due to their unique role amongst the B cell types, this subset is aptly designated as ‘regulatory B cells’ or ‘Bregs.’
Regulatory B cells are being extensively studied in three major fields: autoimmunity, transplantation tolerance, and cancer. Seemingly different, conditions in these fields are in fact outcomes of immune dysfunction. Whereas autoimmune diseases reflect an inadequate ability to control an overactive immune response to self, development of cancer results from the enthusiastic suppression of the immune system thus allowing a mutated self to survive and proliferate. The role of Bregs as negative regulators of the immune system varies in these different contexts. As mediators of immune suppression, they have been implicated in the cessation of autoimmune diseases and graft tolerance as the expansion of the Breg population is highly correlated with amelioration of the disease and maintenance of tolerance (6). On the other hand, Bregs have been tagged as pro-tumorigenic due to their capacity to inhibit the action of effector immune cells whose purpose is to identify and eliminate cancer cells, hence providing a favorable environment for the tumor to grow (7, 8).
As pregnancy requires selective immunological tolerance, this condition may be viewed as a reconciliation of the two extreme paradigms. The mechanisms that inhibit immune flares or chronic inflammation in autoimmune diseases and transplantation may be at play in the induction of maternal immune tolerance during pregnancy. Similarly, the pro-tumorigenic mechanisms that allow cancer development to take place may also be responsible for allowing the growth and development of a semi-allogeneic fetus in the mother’s womb. In this review, we investigate the possible mechanisms by which Bregs confer protection to the semi-allogeneic fetus during pregnancy by assessing their role in autoimmune diseases, graft tolerance, and cancer. We also examine how the maternal endocrine system sets the stage for pregnancy, particularly how it endeavors to create an environment conducive for the expansion of Bregs.
Regulatory B Cells
B cells are best known to organize immune responses by production of antibodies. Representing the humoral arm of adaptive immunity, they are the primary facilitators of antigen-specific immune responses via antibody production and differentiation into memory cells that provide long-lasting immunity. However, reports over the past 40 years indicate that not all B cells function for that purpose. The earliest studies (1974) found that B cells could suppress delayed-type hypersensitivity reactions in guinea pigs, implying an inhibitory effect of B cells on T cell function (9, 10). Further evidence of this B cell regulatory phenotype eventuated more than two decades later, with the observation in a murine autoimmune model that inflammation was exacerbated in the absence of B cells (11). While this suggested that B cells may play a down-modulating role in the inflammatory response, it was only in 2000 that Mizoguchi et al. formally described and reported a subset of B cells that inhibited, rather than promoted, the inflammatory response in a mouse model of inflammatory bowel disease (12). This peculiarly suppressive B cell subset was classified as ‘regulatory B cells’ or ‘Bregs.’ Since then, defective Breg function or deficiency in Breg levels have been implicated in conditions involving uncontrolled pro-inflammatory immune responses; most extensively in autoimmune diseases and renal transplantation cases (13–16).
Breg Phenotypic Identification
Defining a specific Breg phenotype has proven to be a difficult as multiple B cell “subsets” have been reported to function as negative regulators of the immune response. While there is no unifying characteristics with respect to cell surface activation and lineage markers as of yet, initial reports indicated that the regulative properties of these unique B cells were attributed exclusively to the production of the anti-inflammatory cytokine interleukin-10 (IL-10) (13, 17, 18). However, more recent studies have revealed B cell subsets with IL-10-independent regulatory functions, indicating that some Bregs employ a multi-mechanistic, and possibly cooperative, approach for regulating immune responses. Given the lack of a unified approach and as IL-10 production is the most reported mechanism of suppressive action; IL-10 production remains the defining feature of Bregs.
Different B-cell subsets that have been attributed with regulatory function in mice include the transitional 2 marginal-zone precursor (T2-MZP) cells, CD5+CD1dhiIL-10+ B (B10) cells, follicular (FO) B cells, marginal-zone (MZ) B cells, CD5+B-1a cells, CD5+CD178+ killer B cells, GIFT-15 B cells, plasma cells, plasmablasts, TIM-1+ B cells, and PD-L1hi B cells (19, 20). In humans, immature B cells, IL-10+ B cells (B10), GrB+ B cells, Br1 cells, and plasmablasts are reported to play immunosuppressive roles (19). Despite the diversity in phenotype, most B cell subsets that carry out negative regulation produce anti-inflammatory cytokines, with the majority of the cell surface marker-defined subsets enriched with IL-10-producing cells. In mice, the suppressive IL-10-producing Bregs, also known as B10 cells are characterized by the CD1dhiCD5+ phenotype (21). Among the splenic B10 cells, both marginal-zone B (MZ B) cells and T2-MZP B cells have been shown to have a protective effect in mouse models of lupus and autoimmune arthritis due to their IL-10 competency (22, 23). The peritoneal cavity contains B-1a cells that are also a major source of IL-10 (24). In humans, CD19+CD24hiCD38hi B cells isolated from human peripheral blood are classified as Bregs due to their ability to suppress inflammation by a combination of IL-10 production and CD80 and CD86 costimulation (25), while the IL-10-competent CD24hiCD27+ B cells are proposed as the Breg subset analogous to the mouse regulatory B10 cells (26). The heterogeneity of these subsets suggests that Bregs are not derived from one specific lineage; rather they may acquire their regulatory ability through exposure to environmental stimuli.
Since surface markers identifying these subsets are varied, there are currently ongoing attempts to identify a unique global indicator for Bregs, analogous to the transcription factor FOXP3, a defining feature of regulatory T cells (Tregs). Evidence suggests that the T cell immunoglobulin and mucin domain 1 (TIM-1) may be an inclusive marker for Bregs as it identifies about 70% of all IL-10+ B cells in all subsets mentioned (27). However, CD9 has also been reported as a marker for murine IL-10-competent Bregs by virtue of transcriptomic analysis which strongly associated its expression with Breg biogenesis and function (28). In principle, identification of a unique marker signifying a direct regulatory function in B cells would reduce the reliance on functional assays and increase our understanding of the role that Bregs may play in different diseases and allow exploration of therapeutic options. To date, elucidating the Breg phenotype and underlying ontology continues to be an active research endeavor.
Bregs’ Suppression Mechanisms
Since formal recognition, Bregs have been described mainly in the context of autoimmunity, transplantation tolerance, and cancer. The best studied mechanism for negative regulation of Bregs is their capacity to produce anti-inflammatory cytokines, especially IL-10. The principal function of IL-10 is to repress excessive inflammation and maintain homeostasis through numerous mechanisms, which includes hampering antigen presentation and expression of costimulatory molecules CD80, CD86, and MHC class II by macrophages and DCs, as well as limiting the activation of and cytokine release by T cells in immune responses and promoting the differentiation of Tregs (29).
While IL-10 production remains the hallmark of Breg’s negative regulatory function, other independent suppressive mechanisms have come to light in recent years and include transforming growth factor beta (TGF-β), IL-35, and IL-17. Production of TGF-β by B cells leads to promotion of Treg development in diabetes, transplant tolerance, allergic diseases, and colitis (30). Moreover, about 60% of IL-10-producing B cells in mice also express IL-35, a cytokine identified as a key player in the recovery phase of experimental autoimmune encephalomyelitis (EAE), and necessary for improved resistance to Salmonella infection (31). Secretion of IL-17 by activated B cells has been reported to occur upon contact with parasite-derived trans-sialidase, a feature shown to be critical in the control and resolution of infection (32). This result emphasizes the importance of Breg function in a disease that has a high potential to develop into chronic inflammation.
Various surface molecules on B cells have also been identified as the main instigators of negative regulation upon cognate interaction with pro-inflammatory cells. Among the most studied are programmed death receptor ligand 1 (PD-L1), granzyme B, and Fas ligand (FasL). Upregulated PD-L1 expression on B cells is critical in down-modulating T cell activity. In EAE, adoptive transfer studies demonstrate that PD-L1+ B cells confer protection and reduce disease severity via restriction of helper T (Th) cells inflammatory role and induction of Treg activity (33–35). Furthermore, PD-L1+ B cells have been shown to regulate cytotoxic T cells in both Salmonella infection and prostate cancer (36, 37), with high expression shown to directly suppress the FO Th response and differentiation via attenuation of downstream signaling pathways concomitant to programmed death receptor 1 (PD-1) ligation (38). Similarly, B cells expressing FasL were found to be critical in preventing graft rejection in mice and modulating the induction of autoimmune disease through a myriad of mechanisms including promotion of apoptosis of autoreactive T cells and the generation of Tregs (39, 40). Lastly, IL-21-induced granzyme B expression in B cells was shown to impart a regulatory function against effector T cells in the tumor microenvironment (41).
Breg Detection and Expansion
Due to the inconsistencies in specific surface markers used to classify Breg subsets, the most common approach for Breg identification remains a combination of surface marker staining and intracellular IL-10 staining. In mice, isolated Bregs do not readily produce IL-10 ex vivo, therefore to identify this specific subset of B cells, cells are subjected to acute B cell stimulation—5 h of culture with a cocktail of lipopolysaccharide (LPS), phorbol 12-myristate 13-acetate, and ionomycin to enhance the translation of cytokine genes, and thus detectable IL-10 production (42). In the spleen and other lymphoid tissues, Breg progenitor cells have also been identified (43). These are defined as B cells that do not express IL-10 following the short-term stimulation but may be induced to Breg maturation and IL-10 competency by culture with agonistic CD40 monoclonal antibody for 48 h. This technique has proven beneficial for expanding this rare subset for subsequent in vivo applications such as adoptive transfer experiments. Together, active and progenitor Bregs account for 3–8% of basal B cell levels in the murine spleen, of which 1–3% are IL-10-competent Bregs. Lower percentages are found in the blood, lymph nodes, intestinal tissues, Peyer’s patches, and the central nervous system (21, 44, 45). In humans, IL-10-producing B cells isolated from the peripheral blood are found to express increased levels of CD19, IgD, CD27, CD48, and/or CD148 at varying proportions and phenotypic combinations. IL-10-competent Bregs have also been identified in peripheral tissues such as spleen, tonsils, and umbilical cord blood at less than 1% of the total B cell population (26).
Bregs in Autoimmunity
In the last decade, numerous studies have been conducted to determine the operating mechanisms that impart Breg-mediated suppressive function. The majority of what is currently known stems from the multitude of studies focusing on autoimmune diseases where it has been demonstrated that Bregs interact and regulate the function of T cells, DCs, monocytes, macrophages, and natural killer cells.
The regulatory role of B cells in EAE, a mouse model for multiple sclerosis (MS), was first apparent when exacerbation of disease was observed in B cell-deficient B10.PLμMT mice (11). The current model posits that Bregs regulate EAE severity through IL-10 production and expression of costimulatory molecules CD40, CD80, and CD86, thus limiting the type 1 cytokine response via cross talk between B cells and T cells, and recruitment of FOXP3+ Tregs into the central nervous system (6, 14). B cells expressing toll-like receptor (TLR)-2 and TLR-4 also suppressed inflammatory T cell responses (Th1 and Th17) and stimulated the recovery phase of EAE (46). Other mechanisms such as the presence of glucocorticoid-induced TNF ligand and elevated levels of B and T lymphocyte attenuator on B cells also play a role in inducing and maintaining the Treg pool in the central nervous system during EAE, a process which ultimately facilitates the protection against or amelioration of the disease (47, 48). Interestingly, recent evidence indicates that Bregs can also directly access the central nervous system (CNS). Bone marrow cells transiently stimulated with the TLR-9 agonist CpG generate proB (CpG-proB) cells, which upon transfer to recipient mice at the onset of EAE symptoms differentiate into mature B cells with regulatory function. Some of these mature B cells home to the inflamed CNS for local production of IL-10 thus instigating a switch of the host cytokine profile from inflammatory to immunoregulatory, while others enter reactive lymph nodes and restrain encephalitogenic T cells via CCL19 expression. Together these functions cooperatively enhanced amelioration of active EAE (49). Evidence of the regulatory function of B cells in human disease is also accumulating, with B cells from MS patients observed to have diminished capacity for IL-10 production on activation as opposed to healthy controls. This suggests that dysregulation of this pathway contributes to MS pathogenesis (50–52).
Type 1 diabetes (T1D) is characterized by the autoimmune-mediated destruction of pancreatic β cells. Circulating CD19+CD27−CD24hiCD38hi Bregs have been found to decrease with age and as such are hypothesized to be a major contributing factor to the prevalence of T1D in older age groups (53). Patients with T1D also exhibit the lowest frequency of B10 cells compared with patients with type 2 diabetes, latent autoimmune diabetes in adults, and healthy controls (54). These results highlight the importance of B cells in the immunopathogenesis of autoimmune diabetes and suggest that antigen-activated B10 cells may have a role to play in inhibiting autoreactive T cell responses to islet-specific antigen in healthy individuals, as the absence of this population leads to hyperglycemia in T1D patients. In non-obese diabetic (NOD) mice, adoptive transfer of CD1dhiCD5+IL-10+ Bregs prevented T1D development. Further, the addition of tolerogenic DCs to the transferred therapeutic mix reversed the onset of T1D by augmenting the frequency of IL-10+ Bregs (55). In the same manner, adoptive transfer of CpG-proB cells, either directly or indirectly after maturation into mature Bregs, protected against the pathogenesis of T1D in NOD mice via suppression of IL-21 and induction of apoptosis in effector T cell populations, including specific diabetogenic T cells (56). The DC- and CpG-mediated suppression of T1D through Breg activity reiterates the notion that B cells have an intrinsic suppressive potential, which is only activated when the environmental cues are appropriate.
In systemic lupus erythematosus (SLE), Bregs are emerging as vital players during disease initiation. Adoptive transfer of splenic CD5+CD1dhi Bregs from wild-type mice extended the survival period of CD19−/− mice in this spontaneous lupus model (57). In humans, SLE patients exhibited similar frequencies of circulating CD24hiCD38hi B cells but had significantly lower IL-10+ percentages compared to normal healthy controls (58). Further, there is evidence of functional impairment in Bregs from SLE patients. Isolated Bregs were unresponsive to CD40 ligation and exhibited lower IL-10 production compared to Bregs obtained from normal patients, which resulted in inadequate suppression of T cell activation and proliferation (59). Compromised cross talk between IL-10-producing CD24+CD38hi Bregs and interferon alpha-producing plasmacytoid dendritic cells has also been documented as a key contributor to the pathogenesis of SLE (60).
Studies of rheumatoid arthritis (RA) suggest that Bregs are responsible for the primary induction of Tregs. Specifically, IL-10 from B cells aids in the establishment of the Treg to inflammatory Th1 and Th17 cell ratio as seen in chimeric (IL-10−/− B cell) mice which exhibit a significant decrease in FOXP3 expressing CD4+ T cells; the low number of Tregs occurred in parallel with an increase in Th1 and Th17 cell populations in the draining lymph nodes of the inflamed joints (61). Furthermore, the adoptive transfer of Bregs into these chimeric mice restored the Treg population to normal levels and decreased inflammation by inhibiting Th1 and Th17 differentiation, thus decreasing IFNγ and IL-17 cytokine levels, and reinstating a balanced Th1/Th2 response (62). This notion of Bregs influencing T cell plasticity was similarly demonstrated by injection of exogenous B10 cells which effectively suppressed the development of arthritis by suppressing Th17 cell generation (63). Clinical studies report decreased frequency of CD24hiCD38hi Bregs in RA patients compared to unaffected individuals; it was shown that normal levels of Bregs effectively inhibited CD4+CD25− T cell differentiation into Th1 and Th17 cells and promoted the differentiation of T cells into Tregs via IL-10 production (64). Moreover, it has been demonstrated that many RA patients achieve remission during pregnancy, presumably due to a shift in T cell function from a Th1 to a Th2 phenotype, but relapse postpartum, a phenomenon possibly correlated to the increase of Breg levels during pregnancy and consequent decrease upon parturition (65–67).
The regulatory function of human B cells in autoimmune diseases is realized in case studies where complete B cell depletion therapy with rituximab (anti-CD20 monoclonal antibody) has resulted in aggravation of the disease or onset of new immune-mediated pathologies. For instance, a patient with intractable ulcerative colitis experienced severe clinical aggravation of disease upon treatment with rituximab, a result ascribed to the depletion of both the intestinal and systemic suppressive B cell population (68). Similarly, rituximab was an effective treatment in a patient with Graves’ disease; however, development of ulcerative colitis and arthritis was observed shortly thereafter. Again, the depletion of colonic B cells was implicated, with symptoms alleviated upon repopulation (69).
Therefore, in autoimmune disease, the suppressive role of Bregs in the framework of T cell function occurs at two levels: (1) control of T cell plasticity by invoking the production of Tregs, inhibiting activation of naïve T cells, and suppressing Th differentiation; and (2) manipulation of the production of proinflammatory and anti-inflammatory cytokines and their balance thereof.
Bregs in Transplantation Tolerance
Graft tolerance has been best studied in liver and kidney transplantations. Postoperative care involves continuous treatment with immunosuppressive drugs to prevent the immune response to the alloantigen, which may lead to organ rejection. Some recipients require immunosuppression medication for their entire lifetime; however there are reports of operationally tolerant recipients maintaining graft function despite immunosuppressive drug withdrawal (70). The containment of the inflammatory response that typically ensues without immunosuppressive drugs points to the valuable function of regulatory immune cells—the Tregs which have an established role in graft tolerance, and the Bregs, whose role in transplantation has recently emerged.
Initial reports implicating a role for B cells as facilitators of graft tolerance resulted from studies investigating the effect of CD40 blockade on resting B cells. Permanent survival of mouse pancreatic islet allografts was demonstrated upon pretreatment with allogeneic non-T cell small lymphocytes (elutriated from spleen cell suspensions) plus blocking antibody to CD40L (71). Moreover, pretreatment of resting B cells with anti-CD40 monoclonal antibody prolonged fully allogeneic mouse cardiac allografts and augmented the hyporesponsiveness to MHC molecules in vivo (72), suggesting a dominant role of the CD40/CD40L pathway in tolerance induction. The mechanism by which anti-CD40 antibodies can elicit a tolerogenic response was elucidated a decade later when it was demonstrated that CD40 ligation resulted in the differentiation of IL-10+ transitional-2 (T2) Bregs, the rescue of B cells, and transitional B cell subsets from apoptosis, as well as prevention of differentiation into mature FO B cells. Specifically, anti-CD40 Ab administration induced the differentiation of T2 and MZ B cells and expanded the population of IL-10+ Bregs in vitro and in vivo, resulting in significantly improved renal disease and controlled progression of lupus in mice (73). This was also reflected in tolerant kidney and cord blood transplant patients where increased CD40 ligation potentiated IL-10 production by naïve and transitional B cells (74–76).
In a rat model of kidney allografts, intravenous injection of bulk donor B cells to the recipient at the time of transplantation was by itself proven to be effective in inducing long-term acceptance (more than 300 days) of the graft. This was a significantly longer time period than when donor T cells were administered 17 days after transplantation (77). Studies of islet allografts in mice identified TIM-1 as an inclusive marker for IL-10 and IL-4 expressing Bregs in all major B cell subpopulations including the transitional, MZ, FO, and CD5+CD1dhi cells. More importantly, ligation by anti-TIM-1 induced the regulatory function of these B cells constitutively expressing TIM-1 via IL-10 production. Adoptive transfer of TIM-1+ B cells from untreated BALB/c allograft recipients into chemically diabetic B cell-deficient allograft recipients prolonged graft survival by activation of transferred IL-10-producing TIM-1+ B cells and augmented the frequency of FOXP3+ Tregs (27). Moreover, the mucin domain of TIM-1 has been identified as being primarily responsible for Breg induction and maintenance in prolonged allograft survival (78). Aside from TIM-1+ Bregs, tolerance in a mouse model of MHC class I mismatched skin transplantation was associated with a transient expansion in T2 B cells in mice tolerized by donor splenocyte transfusion and ligation with CD40L. These tolerized B cells were characterized by expression of downregulated levels of CD86 and upregulated levels of TIM-1, and prolonged skin allograft survival in vivo, as well as suppressed T cell activation in vitro. Moreover, it was seen in this study that tolerized B cells and graft-specific Tregs worked synergistically in prolonging graft survival, albeit in an IL-10-independent fashion (79). Similarly, the adoptive transfer of TGF-β-producing Bregs induced the expression of FOXP3 in CD4+CD25− T cells, thus activating and expanding the Treg population which contributed to protracted graft survival (80). These reports reinforce the multi-mechanistic mode of suppression by Bregs in that through IL-10, TGF-β, CD86 costimulation, and other surface molecules, antigen-specific tolerized B cells no longer stimulate allospecific T cell activation, but instead support the induction of Treg function to enact a tolerogenic response.
In humans, research has also indicated a critical role for B cells in regulating alloimmunity. For operationally tolerant renal transplant recipients, renal allograft tolerance was strongly associated with a B cell signature consisting of an increased expression of multiple B cell differentiation and activation genes over that observed in non-tolerant patients receiving immunosuppression and non-transplanted controls. The three genes identified as reliable predictors of operational tolerance—IGKV4-1, IGLL1, and IGKV1D-13—are genes that are upregulated during transition from pre-B to mature B cells and during class switching post-antigen stimulation, suggesting that transitioning B cells may be highly involved in tolerance induction and/or maintenance (15). Moreover, operationally tolerant patients displayed higher transitional (CD24hiCD38hi) and naïve B cell frequencies, lower numbers of memory B cells, and an enriched IL-10+ B cell population—all phenotypes that were strongly associated with reduced allograft rejection rates (81–84). Together these recent findings suggest assigning a prospective biomarker status on transitional B cells. Additionally, operationally tolerant patients retain the capacity to activate the CD40 pathway essential to Breg activation (16), and although IL-10-producing Bregs are higher in these patients, other mechanisms such as granzyme B+ and TGF-β production may also account for the inhibitory effect on T cells essential to maintaining transplantation tolerance (80, 85).
Bregs in Cancer
Oncogenesis is primarily a battle between the immune system and the cancer stem cells. Tumor development and progression is dependent on the lenience of the microenvironment, to which leukocytes play a critical role. Both anti-tumorigenic and pro-tumorigenic immune mechanisms transpire in the early stages of cancer development, with the net effect dictating whether the tumor develops or not (86). In particular, the induction of immune tolerance within the tumor environment has been shown to curtail immune-enhancing anti-tumorigenic efforts and encourage tumor progression and subsequent metastases. Immunosuppressive cells such as Bregs and Tregs have been implicated in facilitating immune tolerance, and ultimately, cancer escape.
For instance, tumor growth is contingent on the immune profile within the local microenvironment. Strong infiltration of antitumor CD8+ T cells and natural killer cells and decreased levels of regulatory B and T cells and myeloid-derived suppressor cells (MDSCs) all result in tumor destruction, whereas reversed proportions support tumor growth. In the tumor-draining lymph nodes, increased accumulation of B and T cells resulted in lymphangiogenesis and increased lymph flow (87), which contributed to and accelerated the rate of metastasis (88). Moreover, B cell accumulation in lymph nodes of preneoplastic Eμ-c-myc mice was positively correlated with expansion of the lymphatic sinuses and enhanced tumor growth, as opposed to preneoplastic B cell-deficient μMT mice which did not undergo lymphangiogenesis and exhibited minimal tumor growth (89). However, tumor growth was accelerated in the B cell-deficient μMT mice upon adoptive transfer of naïve T2-MZP cells, highlighting the role of transitional and suppressive B cells in cancer escape. Furthermore, B cells bearing downregulated MHC class II and CD86 expression and upregulated Ly6A/E, PD-L1, and CD39 selectively accumulated in the draining lymph nodes of mice with HPV-related cancer. The presence of PD-L1 and CD39 on the B cell surface confers regulative properties via direct inhibition of T cell function upon cognate interaction. The depletion of B cells in this model resulted in a robust Th1 cytokine response and decline in recruitment of Tregs, which was accompanied by a strong infiltration into the tumor of CD8+ T cells and ultimately tumor rejection (90). Interestingly, although primary colorectal tumors contain a significant memory and plasma B cell infiltrate suggestive of an active antitumor response, CD24hiCD38hi Bregs were significantly increased in metastatic tissue indicating a shift toward active immunosuppression in the local microenvironment (91). Thus the current literature suggests that B cell infiltration and accumulation in the tumor site and draining lymph nodes establishes an immunosuppressive microenvironment that leads to inhibition of antitumor immune responses and expansion of the critical lymphatic network, thereby facilitating tumor growth and metastasis.
Investigations into the specific role of Bregs in cancer have recently increased following the discovery of a unique B cell subset termed tumor-evoked Bregs (tBregs) in Balb/c mice harboring 4T1 carcinoma cells (8). In this model it was shown that cancer-eradicating B cells were ‘hijacked’ by resident cancer cells within the tumor microenvironment and converted into immunosuppressive Bregs. These tBregs do not conform to any of the established Breg phenotypes; instead they resemble activated mature B2 cells (CD19+CD25hiCD69hi and B7-H1hiCD81hiCD86hiCD62LloIgMint). Incubation of non-regulatory CD4+ T cells with tBregs bearing high expression levels of CD40, CD80, CD86, MHC class I and II molecules, and TGF-β production capacity led to the significant generation of CD4+CD25+FOXP3+ Tregs which in turn inhibited CD8+ T cell proliferation—thereby facilitating breast cancer escape and metastasis (8). More recently, it has been demonstrated that B cells infiltrating the mammary tumor bed are ‘educated’ through cognate interactions with resident tumor cells. These tumor-infiltrating B cells acquired PD-L1 expression and TGF-β competency which contributed to an immunosuppressive phenotype characterized by an enhanced inhibitory capacity against CD4+CD25− T cells, CD8+ T cells, and CD49b+NK cells, as well as promotion of Treg expansion (92). Therefore, infiltrating B cells subverted into Bregs by the tumor microenvironment facilitates the necessary critical changes in the local immune profile to support a pro-tumorigenic outcome.
In humans, studies reveal that a higher frequency of Bregs is indicative of enhanced tumor aggressiveness and poorer prognosis. For instance, hepatocellular carcinoma patients harbor significantly more peripheral Bregs compared to healthy controls (93); malignancy in non-small cell lung cancer is associated with an increased frequency of IL-10-producing Bregs, CD4+CD25+/highCD127low/− Tregs, and MDSCs (94); patients with tongue squamous cell carcinoma, gastric cancer, and colorectal cancer also exhibit a higher frequency of Bregs within the tumor itself compared to unaffected neighboring tissues, with numbers positively correlated with an increased frequency of Tregs (91, 95, 96). Moreover, in vitro studies of lung cancer cells demonstrated their direct capacity to upregulate Treg and Breg phenotypes in lymphocyte cocultures (94). As for the mechanism, IL-10 and TGF-β competency appear to be the primary facilitators of suppression. For example, peripheral blood CD19+CD24hiCD38hi Bregs isolated from gastric cancer patients converted CD4+CD25− effector T cells into CD4+FOXP3+ Tregs via expression of TGF-β (95). Moreover, IL-10 facilitates cross talk between CD19+IL-10+ Bregs and CD4+CD25− T cells in tongue squamous cell carcinoma patients, resulting in the conversion of these resting T cells into Tregs (96). Likewise, IL-10 has been shown to induce the expansion of Tregs in peripheral lymphoid organs in gastric cancer patients (95).
Bregs and Female Sex Hormones
The two major sex hormones involved in pregnancy are estrogen and progesterone, with each hormone playing a central role at different time points during gravidity. Progesterone promotes endometrial decidualization for the implantation of the embryo and maintains uterine relaxation throughout pregnancy (97). Estrogen on the other hand, plays a central role in angiogenesis which is needed for placentation and sustenance of the fetus (98). High estrogen levels and the withdrawal of progesterone promote the onset of labor, and ultimately, human parturition. Female sex hormones were found to have a significant influence on inflammatory responses when women with autoimmune conditions were reported to experience a change in their disease activity upon pregnancy. For instance, women that acquired SLE during pregnancy generally experienced a more severe affliction compared to non-pregnant patients (99, 100). Existing RA is often ameliorated by the surge of immunoinhibitory estrogen during pregnancy, which relapses postpartum upon an increase in the immunostimulatory hormone prolactin (101, 102). There is also evidence that female sex hormones are capable of modifying decidual immune cells, such as DCs and uterine natural killer cells, from being actively pro-inflammatory to being tolerogenic (103). Considering that pregnancy outcomes essentially rely on the effective control of the inflammatory response, essential pregnancy hormones should be investigated in any study of immune cell changes during pregnancy.
Estrogen regulation of immune cells, whether innate or adaptive, has been established in recent years. Its presence can increase splenic neutrophil numbers, alter the phagocytic capacity of macrophages, and enhance the maturation of DCs (2). It has also been shown to regulate the activities of CD4+ and CD8+ T cells and promote the expansion and activity of Tregs by increasing FOXP3, PD-1, and cytotoxic T-lymphocyte-associated protein 4 expression (34, 104, 105). RA and MS can both be ameliorated by estrogen-induced Treg expansion and activation which leads to immune suppression (104, 106). B cells are likewise affected by the presence of estrogen. During lymphopoiesis, a rise in estrogen levels leads to a reduction of B cell precursors and an expansion of mature B cells. Estrogen activation therefore affects B cell maturation, and ensures that the immune system is equipped to defend the body against pathogens if required (5).
Two receptors are responsible for estrogen-mediated signaling—estrogen receptor α (ERα) and estrogen receptor β and upon activation act as transcription factors for a range of estrogen-sensitive genes, with ERα more predominant on B cells (107). Studies of EAE demonstrate that the protective effect of 17β-estradiol (E2) comes from its interaction with ERα on immune cells, including B cells (106). While many studies have explored the protective effect of estrogen in autoimmune diseases, primarily by focusing on effects on Tregs (34, 108), others have highlighted the role of B cells by showing that E2 conferred protection against the induction of EAE in mice even after ablation of Treg; a result unsuccessful in B cell-deficient mice (6, 109). Specifically, E2 augmented the frequency of splenic IL-10+ Bregs via the PD-1/PD-L1 mechanism (110, 111) which led to a decline in the frequency of CD11b+CD45hi-activated macrophages, DCs, and infiltrating CD4+ cells in the spinal cord (112). Together, these results suggest that Bregs may have carried out this protective effect via their responsiveness to estrogen. This was confirmed by studies which demonstrated that estrogen-receptor positive B cells upregulate Treg function in EAE (34).
The effect of progesterone on innate immune cells, mainly monocytes, DCs, and natural killer cells, has also been elucidated (103), and in a manner similar to estrogen, depends on the presence of progesterone receptors. These receptors also act as nuclear transcription factors upon activation and may either be progesterone receptor A (PR-A) or progesterone receptor B (PR-B) (113). The specific actions of progesterone relevant to myometrial function are mediated by the combined and distinct genomic actions of PR-A and PR-B. Myometrial cells express PR-B for most of the pregnancy, facilitating progesterone-mediated Th2-like maternal immune responses, resulting in reduced production of pro-inflammatory cytokines, and increased secretion of IL-10 (97, 114). At the latter part of pregnancy nearing the onset of parturition, PR-A gene expression becomes more predominant, blocking the transcriptional anti-inflammatory activity of PR-B and consequently increasing pro-inflammatory gene expression and promoting labor (97). Progesterone function in cancer and autoimmune diseases occurs comparably to that in pregnancy, in that progesterone inhibits T cell proliferation and pro-inflammatory cytokines IL-2, IL-17, and IFNγ expression while enhancing IL-4, IL-5, IL-6, and IL-10 cytokine production (98, 115). Moreover, there is a concurrent expansion of B cells associated with the increase in IL-10, and a lack of effect of progesterone on Tregs (116). Collectively, this suggests a possible signaling pathway wherein progesterone elicits IL-10 augmentation by activating Bregs.
The molecular mechanisms by which estrogen and progesterone operate appear to be distinct from one another. Whereas estrogen elicits a strong effect on Tregs, progesterone does not. However, both have been documented to affect the B cell immune profile. The current literature gives insights as to how the female sex hormones shape the immune response in various clinical conditions, and overall indicate that estrogen and progesterone foster a tolerant environment within the pregnancy milieu by expanding all regulatory cells. Changes in the circulating amount and distribution of these cells during pregnancy indicates that adaptations are in place to ensure that the mother’s body is well equipped to conceive and grow a semi-allogeneic fetus.
Bregs in Pregnancy
Previous studies of Bregs in the context of autoimmune disease, graft transplantation, and cancer all indicate one thing—Bregs dampen the inflammatory response and foster a stable tolerant immune profile within the local microenvironment. Based on the current literature, it is therefore reasonable to speculate that Bregs may be a key player in pregnancy. Their anti-inflammatory role in autoimmunity and graft tolerance suggest relevance to the induction of maternal immunological tolerance, while their pro-tumorigenic role in cancer may be applicable in pregnancy, i.e., maternal–fetal cognate interactions may hijack naïve B cells and educate them to become Bregs thus ensuring the fetus’ unperturbed growth. The pregnancy hormones estrogen and progesterone are likewise critically involved in the establishment, maintenance, and termination of pregnancy.
During pregnancy, as the mother’s womb houses and protects the semi-allogeneic fetus, the response of the maternal immune system is the key determinant to pregnancy success, with failure to induce selective immune tolerance toward the fetus resultant in pregnancy loss. Modification of the maternal immune response is orchestrated by multiple cytokines that influence the nature and abundance of leukocyte subsets in the uterus and placenta and includes the reduction of antigen-presenting function of monocytes, macrophages, and DCs; inhibition of natural killer cells, T cells, and B cells; proliferation of uterine killer cells; maintenance of tolerogenic DCs; and the induction of Tregs (117). These immunological adaptations during early pregnancy pave the way for two main objectives—to protect the fetus from immune rejection and to facilitate the tissue remodeling processes needed for placental development (118).
Primarily, Bregs are considered highly relevant in the pregnancy milieu as they are a major cellular source of the potent anti-inflammatory cytokine IL-10. This cytokine is vital for optimal pregnancy outcomes, with IL-10 deficiency associated with fetal resorption, growth restriction, and even death (118–120). IL-10 is found in abundant amounts in the uterus and placenta during pregnancy and has been identified as a critical player for counteracting the pro-inflammatory cytokine response. Even at the initial conception stage, inflammation resulting from the recognition of paternal antigens is thwarted by the presence of IL-10 (121). Furthermore, administration of a sub-clinical dose of LPS to pregnant IL-10−/− mice resulted in a 10-fold reduction in viability of semi-allogeneic fetuses compared to unaffected controls. Analysis of maternal serum and uterine tissue in these LPS challenged IL-10−/− mice demonstrated high levels of the pro-inflammatory cytokines TNFα, IL-6, IL-1A, and IL-12p40, a result which could be abrogated by administration of exogenous IL-10 (118, 122, 123). Therefore, IL-10 confers pregnancy protection by multiple mechanisms including inhibition of pro-inflammatory cells such as macrophages and monocytes, and suppression of pro-inflammatory cytokine and chemokine production such as TNFα, IL-6, IL-1a, IL-8, and IL-12 (118, 124, 125). These findings demonstrate the capacity of IL-10 to regulate the inflammatory response in the uterus and placenta during gestation.
Evidence of a direct role for Bregs was indicated in comparative mouse studies of normal and abortion-prone pregnancies which showed a diminished frequency of B10 cells in the maternal spleen of abortion-prone mice, whereas normal pregnancies harbored an increased number of B10 cells. Furthermore, adoptive transfer of B10 cells rescued pregnancies in abortion-prone mice, by inhibition of DC maturation and expansion of the Treg population (126). Another Breg phenotype reported as critical to pregnancy success are the MZ B cells that are found expanded in the spleen of normal pregnant mice, but lacking in mice undergoing pregnancy complications. Additionally, successful pregnancy was correlated with the capacity of MZ B cells to produce enhanced levels of immunoglobulin (IgM and IgA) which likely shifts the immune response from a Th1-like to a Th2-like profile (127). Although also IL-10 competent (22), there is no evidence yet linking MZ B cell cytokine production to their role in pregnancy well-being. In humans, IL-10-producing CD19+CD24hiCD27+ B cells in the peripheral blood have been shown to be significantly higher in women undergoing normal pregnancies as opposed to non-pregnant women, or women who had suffered spontaneous abortions (128). Bregs isolated from peripheral blood of pregnant women taken during the first trimester successfully inhibited TNFα secretion by activated T effector cells ex vivo (128). Interestingly, CD24hiCD38hi Bregs were found to be significantly lower during the third trimester of pregnancy and on delivery day compared to non-pregnant and postpartum controls, possibly as a result of a drop in female sex hormones that influence B cell activation (129). Although limited in number, these studies on Breg function in pregnancy, and the underpinning Breg-mediated immunosuppression, parallels that observed in autoimmune disease, graft tolerance, and cancer. In all conditions, Bregs impose a suppressive environment via induction and maintenance of Tregs, modification of the Th response, and inhibition of effector cell responses including cytolytic T cells, NK cells, and DCs.
As Breg function in pregnancy appears to be interlinked with that of Treg and DCs, defining the working mechanisms is requisite. Both Tregs and DCs are critical players in determining pregnancy outcomes. In normal pregnancy, the majority of decidual DCs remain immature which contributes to the maintenance of a fetal-tolerant local environment. These immature DCs are thus referred to as tolerogenic DCs (130). Tregs are likewise crucial contributors in achieving and maintaining maternal–fetal immune tolerance. In mice, a natural periodic accumulation of Tregs during the estrus stage is seen, purportedly in preparation for the implantation of a semi-allogeneic fetus (131). At the onset of embryo implantation, Tregs are recruited to the para-aortic lymph nodes draining the uterus (132). Expansion of the Treg population is critical in improving the rates of successful pregnancy as deficiency or harboring insufficient numbers of Tregs in the vicinity of the uterus at any time during pregnancy leads to abortion or miscarriage (4, 133). In the context of pregnancy, tolerogenic DCs and Tregs foster a fetal-tolerant environment via production of anti-inflammatory IL-10 and TGF-β (134).
During pregnancy, if an anti-inflammatory signal such as IL-10 or TGF-β is lacking, the DC phenotype remains such that it prevents the activation of T cells that protect the semi-allogeneic fetus (135). It is thus pertinent that DCs retain their immature state to acquire the tolerogenic phenotype. IL-10 and or TGF-β must then be initially present in the pregnancy milieu to either set off the induction of tolerogenic DCs followed by the induction of Tregs, or directly drive the induction of Tregs. However, given that Bregs are (1) established sources of both IL-10 and TGF-β, (2) significantly increased in numbers during normal pregnancy, and (3) are responsive to endocrine modulation, it is plausible that Bregs also play a critical role in pregnancy, especially in the initial induction of maternal immunological tolerance. In support, the findings of Jensen et al. (126) suggest that IL-10 production from B10 cells likely influences the non-maturation of IL-10-receptor rich DCs which then expand the Treg population in pregnant mice. Based on the current literature indicating similar immunosuppressive roles and direct interface among these three types of immune cells, a Breg–Treg–tolerogenic DC feedback loop is proposed for the maintenance of a tolerant pregnancy milieu (illustrated in Figure 1).
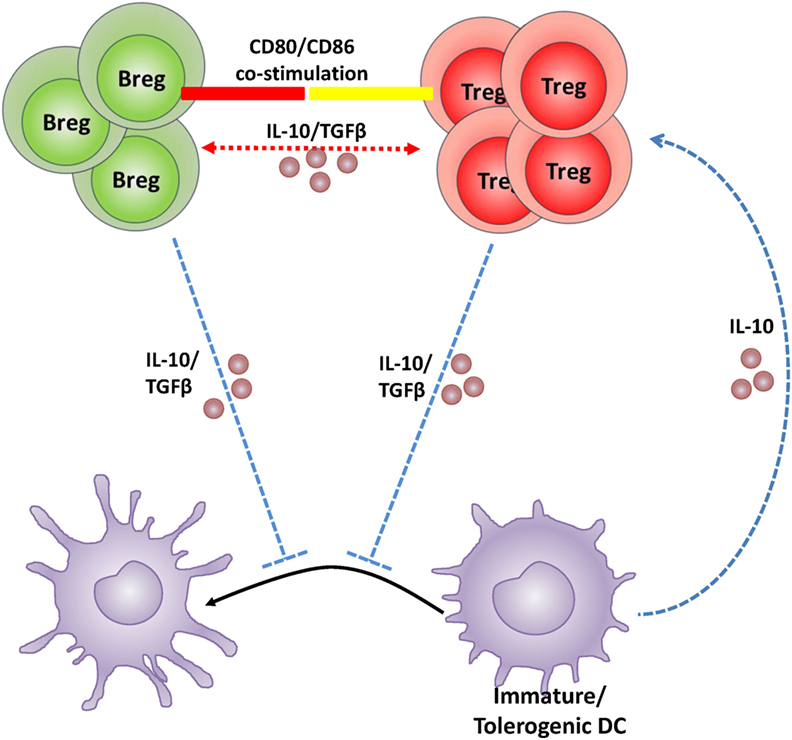
Figure 1. Schematic illustration of the hypothetical feedback loop between regulatory B cell (Breg), regulatory T cell (Treg), and dendritic cells (DCs) during pregnancy. Bregs create a tolerant pregnancy milieu either by direct induction and expansion of the Treg population via CD80/CD86 costimulation and/or IL-10 and TGFβ production (depicted in red); or by inhibition of DC maturation causing retention of a tolerogenic phenotype and in turn expansion of the Treg population via IL-10 (depicted in blue). Tregs and tolerogenic DCs produce IL-10 and may maintain the feedback loop responsible for negative regulation within the pregnancy milieu. The exact mechanism by which Bregs recruit Tregs and inhibit DC maturation in the pregnancy context is, however, yet to be established.
Conclusion
The function of Bregs in autoimmune diseases, graft tolerance, and cancer sheds light as to their emerging role in pregnancy. Studies of autoimmune disease and graft tolerance indicate that the immune response attempts to regain homeostasis by Breg-mediated negative regulation. In cancer, tumor-educated Bregs represent an adaptation that encourages a mutated self to survive and proliferate. Integrating these fields, it is reasonable to suggest that pregnancy represents a predicament common to both—the need for constant immune tolerance for the duration of the pregnancy, and the permission to allow a semi-allogeneic fetus to grow within. However to date, the direct role that Bregs play in maternal tolerance in pregnancy has not been fully investigated, and is the subject of ongoing investigations in our laboratory. Supporting studies suggest that the pregnancy sex hormones, estrogen and progesterone, may also be critical prerequisites for Breg activation and function, with a Breg–Treg–tolerogenic DC feedback loop potentially underpinning the induction and maintenance of a tolerant environment necessary for a successful pregnancy. Bregs therefore, are potential guardians of pregnancy well-being, and require further exploration as to their role in this context.
Author Contributions
RG-G researched and wrote the manuscript. KD conceptualized the investigation and edited the manuscript.
Conflict of Interest Statement
The authors declare that review of the literature was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
This work was supported by NHMRC Project (APP1020984) and Fellowship (APP1012386) grants to KD; and University of South Australia President’s Scholarship to RG-G.
References
1. Parham P. NK cells and trophoblasts: partners in pregnancy. J Exp Med (2004) 200(8):951–5. doi:10.1084/jem.20041783
2. Segerer SE, Staib C, Kaemmerer U, Frambach T, Honig A, Dietl J, et al. Dendritic cells: elegant arbiters in human reproduction. Curr Pharm Biotechnol (2012) 13(8):1378–84. doi:10.2174/138920112800784916
3. Faas MM, Spaans F, De Vos P. Monocytes and macrophages in pregnancy and pre-eclampsia. Front Immunol (2014) 5:298. doi:10.3389/fimmu.2014.00298
4. Ruocco MG, Chaouat G, Florez L, Bensussan A, Klatzmann D. Regulatory T-cells in pregnancy: historical perspective, state of the art, and burning questions. Front Immunol (2014) 5:389. doi:10.3389/fimmu.2014.00389
5. Muzzio DO, Soldati R, Ehrhardt J, Utpatel K, Evert M, Zenclussen AC, et al. B cell development undergoes profound modifications and adaptations during pregnancy in mice. Biol Reprod (2014) 91(5):115. doi:10.1095/biolreprod.114.122366
6. Mann MK, Maresz K, Shriver LP, Tan Y, Dittel BN. B cell regulation of CD4+CD25+ T regulatory cells and IL-10 via B7 is essential for recovery from experimental autoimmune encephalomyelitis. J Immunol (2007) 178(6):3447–56. doi:10.4049/jimmunol.178.6.3447
7. Inoue S, Leitner WW, Golding B, Scott D. Inhibitory effects of B cells on antitumor immunity. Cancer Res (2006) 66(15):7741–7. doi:10.1158/0008-5472.CAN-05-3766
8. Olkhanud PB, Damdinsuren B, Bodogai M, Gress RE, Sen R, Wejksza K, et al. Tumor-evoked regulatory B cells promote breast cancer metastasis by converting resting CD4(+) T cells to T-regulatory cells. Cancer Res (2011) 71(10):3505–15. doi:10.1158/0008-5472.CAN-10-4316
9. Katz SI, Parker D, Turk JL. B-cell suppression of delayed hypersensitivity reactions. Nature (1974) 251(5475):550–1.
10. Neta R, Salvin SB. Specific suppression of delayed hypersensitivity: the possible presence of a suppressor B cell in the regulation of delayed hypersensitivity. J Immunol (1974) 113(6):1716–25.
11. Wolf SD, Dittel BN, Hardardottir F, Janeway CA Jr. Experimental autoimmune encephalomyelitis induction in genetically B cell-deficient mice. J Exp Med (1996) 184(6):2271–8.
12. Mizoguchi E, Mizoguchi A, Preffer FI, Bhan AK. Regulatory role of mature B cells in a murine model of inflammatory bowel disease. Int Immunol (2000) 12(5):597–605. doi:10.1093/intimm/12.5.597
13. Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol (2002) 3(10):944–50. doi:10.1038/ni833
14. Matsushita T, Yanaba K, Bouaziz JD, Fujimoto M, Tedder TF. Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J Clin Invest (2008) 118(10):3420–30. doi:10.1172/JCI36030
15. Newell KA, Asare A, Kirk AD, Gisler TD, Bourcier K, Suthanthiran M, et al. Identification of a B cell signature associated with renal transplant tolerance in humans. J Clin Invest (2010) 120(6):1836–47. doi:10.1172/JCI39933
16. Silva HM, Takenaka MC, Moraes-Vieira PM, Monteiro SM, Hernandez MO, Chaara W, et al. Preserving the B-cell compartment favors operational tolerance in human renal transplantation. Mol Med (2012) 18:733–43. doi:10.2119/molmed.2011.00281
17. Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity (2002) 16(2):219–30. doi:10.1016/S1074-7613(02)00274-1
18. Mauri C, Gray D, Mushtaq N, Londei M. Prevention of arthritis by interleukin 10-producing B cells. J Exp Med (2003) 197(4):489–501. doi:10.1084/jem.20021293
19. Mauri C, Menon M. The expanding family of regulatory B cells. Int Immunol (2015) 27(10):479–86. doi:10.1093/intimm/dxv038
20. Rosser EC, Mauri C. Regulatory B cells: origin, phenotype, and function. Immunity (2015) 42(4):607–12. doi:10.1016/j.immuni.2015.04.005
21. Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity (2008) 28(5):639–50. doi:10.1016/j.immuni.2008.03.017
22. Lenert P, Brummel R, Field EH, Ashman RF. TLR-9 activation of marginal zone B cells in lupus mice regulates immunity through increased IL-10 production. J Clin Immunol (2005) 25(1):29–40. doi:10.1007/s10875-005-0355-6
23. Evans JG, Chavez-Rueda KA, Eddaoudi A, Meyer-Bahlburg A, Rawlings DJ, Ehrenstein MR, et al. Novel suppressive function of transitional 2 B cells in experimental arthritis. J Immunol (2007) 178(12):7868–78. doi:10.4049/jimmunol.178.12.7868
24. O’Garra A, Chang R, Go N, Hastings R, Haughton G, Howard M. Ly-1 B (B-1) cells are the main source of B cell-derived interleukin 10. Eur J Immunol (1992) 22(3):711–7. doi:10.1002/eji.1830220314
25. Blair PA, Norena LY, Flores-Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR, et al. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic lupus erythematosus patients. Immunity (2010) 32(1):129–40. doi:10.1016/j.immuni.2009.11.009
26. Iwata Y, Matsushita T, Horikawa M, Dilillo DJ, Yanaba K, Venturi GM, et al. Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood (2011) 117(2):530–41. doi:10.1182/blood-2010-07-294249
27. Ding Q, Yeung M, Camirand G, Zeng Q, Akiba H, Yagita H, et al. Regulatory B cells are identified by expression of TIM-1 and can be induced through TIM-1 ligation to promote tolerance in mice. J Clin Invest (2011) 121(9):3645–56. doi:10.1172/JCI46274
28. Sun J, Wang J, Pefanis E, Chao J, Rothschild G, Tachibana I, et al. Transcriptomics identify CD9 as a marker of murine IL-10-competent regulatory B cells. Cell Rep (2015) 13(6):1110–7. doi:10.1016/j.celrep.2015.09.070
29. Pestka S, Krause CD, Sarkar D, Walter MR, Shi Y, Fisher PB. Interleukin-10 and related cytokines and receptors. Annu Rev Immunol (2004) 22:929–79. doi:10.1146/annurev.immunol.22.012703.104622
30. Ray A, Wang L, Dittel BN. IL-10-independent regulatory B-cell subsets and mechanisms of action. Int Immunol (2015) 27(10):531–6. doi:10.1093/intimm/dxv033
31. Shen P, Roch T, Lampropoulou V, O’Connor RA, Stervbo U, Hilgenberg E, et al. IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases. Nature (2014) 507(7492):366–70. doi:10.1038/nature12979
32. Bermejo DA, Jackson SW, Gorosito-Serran M, Acosta-Rodriguez EV, Amezcua-Vesely MC, Sather BD, et al. Trypanosoma cruzi trans-sialidase initiates a program independent of the transcription factors RORgammat and Ahr that leads to IL-17 production by activated B cells. Nat Immunol (2013) 14(5):514–22. doi:10.1038/ni.2569
33. Bodhankar S, Wang C, Vandenbark AA, Offner H. Estrogen-induced protection against experimental autoimmune encephalomyelitis is abrogated in the absence of B cells. Eur J Immunol (2011) 41(4):1165–75. doi:10.1002/eji.201040992
34. Bodhankar S, Vandenbark AA, Offner H. Oestrogen treatment of experimental autoimmune encephalomyelitis requires 17beta-oestradiol-receptor-positive B cells that up-regulate PD-1 on CD4+ Foxp3+ regulatory T cells. Immunology (2012) 137(4):282–93. doi:10.1111/imm.12013
35. Bodhankar S, Galipeau D, Vandenbark AA, Offner H. PD-1 interaction with PD-L1 but not PD-L2 on B-cells mediates protective effects of estrogen against EAE. J Clin Cell Immunol (2013) 4(3):143. doi:10.4172/2155-9899.1000143
36. Lopez-Medina M, Carrillo-Martin I, Leyva-Rangel J, Alpuche-Aranda C, Ortiz-Navarrete V. Salmonella impairs CD8 T cell response through PD-1: PD-L axis. Immunobiology (2015) 220(12):1369–80. doi:10.1016/j.imbio.2015.07.005
37. Shalapour S, Font-Burgada J, Di Caro G, Zhong Z, Sanchez-Lopez E, Dhar D, et al. Immunosuppressive plasma cells impede T-cell-dependent immunogenic chemotherapy. Nature (2015) 521(7550):94–8. doi:10.1038/nature14395
38. Khan AR, Hams E, Floudas A, Sparwasser T, Weaver CT, Fallon PG. PD-L1hi B cells are critical regulators of humoral immunity. Nat Commun (2015) 6:5997. doi:10.1038/ncomms6997
39. Minagawa R, Okano S, Tomita Y, Kishihara K, Yamada H, Nomoto K, et al. The critical role of Fas-Fas ligand interaction in donor-specific transfusion-induced tolerance to H-Y antigen. Transplantation (2004) 78(6):799–806. doi:10.1097/01.TP.0000129799.96439.6F
40. Lundy SK, Fox DA. Reduced Fas ligand-expressing splenic CD5+ B lymphocytes in severe collagen-induced arthritis. Arthritis Res Ther (2009) 11(4):R128. doi:10.1186/ar2795
41. Lindner S, Dahlke K, Sontheimer K, Hagn M, Kaltenmeier C, Barth TF, et al. Interleukin 21-induced granzyme B-expressing B cells infiltrate tumors and regulate T cells. Cancer Res (2013) 73(8):2468–79. doi:10.1158/0008-5472.CAN-12-3450
42. Matsushita T, Tedder TF. Identifying regulatory B cells (B10 cells) that produce IL-10 in mice. Methods Mol Biol (2011) 677:99–111. doi:10.1007/978-1-60761-869-0_7
43. Yanaba K, Bouaziz JD, Matsushita T, Tsubata T, Tedder TF. The development and function of regulatory B cells expressing IL-10 (B10 cells) requires antigen receptor diversity and TLR signals. J Immunol (2009) 182(12):7459–72. doi:10.4049/jimmunol.0900270
44. Matsushita T, Horikawa M, Iwata Y, Tedder TF. Regulatory B cells (B10 cells) and regulatory T cells have independent roles in controlling experimental autoimmune encephalomyelitis initiation and late-phase immunopathogenesis. J Immunol (2010) 185(4):2240–52. doi:10.4049/jimmunol.1001307
45. Maseda D, Candando KM, Smith SH, Kalampokis I, Weaver CT, Plevy SE, et al. Peritoneal cavity regulatory B cells (B10 cells) modulate IFN-gamma+CD4+ T cell numbers during colitis development in mice. J Immunol (2013) 191(5):2780–95. doi:10.4049/jimmunol.1300649
46. Lampropoulou V, Hoehlig K, Roch T, Neves P, Gomez EC, Sweenie CH, et al. TLR-activated B cells suppress T cell-mediated autoimmunity. J Immunol (2008) 180(7):4763–73. doi:10.4049/jimmunol.180.7.4763
47. Ray A, Basu S, Williams CB, Salzman NH, Dittel BN. A novel IL-10-independent regulatory role for B cells in suppressing autoimmunity by maintenance of regulatory T cells via GITR ligand. J Immunol (2012) 188(7):3188–98. doi:10.4049/jimmunol.1103354
48. Huarte E, Jun S, Rynda-Apple A, Golden S, Jackiw L, Hoffman C, et al. Regulatory T cell dysfunction acquiesces to BTLA+ regulatory B cells subsequent to oral intervention in experimental autoimmune encephalomyelitis. J Immunol (2016) 196(12):5036–46. doi:10.4049/jimmunol.1501973
49. Korniotis S, Gras C, Letscher H, Montandon R, Megret J, Siegert S, et al. Treatment of ongoing autoimmune encephalomyelitis with activated B-cell progenitors maturing into regulatory B cells. Nat Commun (2016) 7:12134. doi:10.1038/ncomms12134
50. Duddy M, Niino M, Adatia F, Hebert S, Freedman M, Atkins H, et al. Distinct effector cytokine profiles of memory and naive human B cell subsets and implication in multiple sclerosis. J Immunol (2007) 178(10):6092–9. doi:10.4049/jimmunol.178.10.6092
51. Hirotani M, Niino M, Fukazawa T, Kikuchi S, Yabe I, Hamada S, et al. Decreased IL-10 production mediated by toll-like receptor 9 in B cells in multiple sclerosis. J Neuroimmunol (2010) 221(1–2):95–100. doi:10.1016/j.jneuroim.2010.02.012
52. Knippenberg S, Peelen E, Smolders J, Thewissen M, Menheere P, Cohen Tervaert JW, et al. Reduction in IL-10 producing B cells (Breg) in multiple sclerosis is accompanied by a reduced naive/memory Breg ratio during a relapse but not in remission. J Neuroimmunol (2011) 239(1–2):80–6. doi:10.1016/j.jneuroim.2011.08.019
53. Thompson WS, Pekalski ML, Simons HZ, Smyth DJ, Castro-Dopico X, Guo H, et al. Multi-parametric flow cytometric and genetic investigation of the peripheral B cell compartment in human type 1 diabetes. Clin Exp Immunol (2014) 177(3):571–85. doi:10.1111/cei.12362
54. Deng C, Xiang Y, Tan T, Ren Z, Cao C, Huang G, et al. Altered peripheral B-lymphocyte subsets in type 1 diabetes and latent autoimmune diabetes in adults. Diabetes Care (2016) 39(3):434–40. doi:10.2337/dc15-1765
55. Di Caro V, Phillips B, Engman C, Harnaha J, Trucco M, Giannoukakis N. Involvement of suppressive B-lymphocytes in the mechanism of tolerogenic dendritic cell reversal of type 1 diabetes in NOD mice. PLoS One (2014) 9(1):e83575. doi:10.1371/journal.pone.0083575
56. Montandon R, Korniotis S, Layseca-Espinosa E, Gras C, Megret J, Ezine S, et al. Innate pro-B-cell progenitors protect against type 1 diabetes by regulating autoimmune effector T cells. Proc Natl Acad Sci U S A (2013) 110(24):E2199–208. doi:10.1073/pnas.1222446110
57. Watanabe R, Ishiura N, Nakashima H, Kuwano Y, Okochi H, Tamaki K, et al. Regulatory B cells (B10 cells) have a suppressive role in murine lupus: CD19 and B10 cell deficiency exacerbates systemic autoimmunity. J Immunol (2010) 184(9):4801–9. doi:10.4049/jimmunol.0902385
58. Heinemann K, Wilde B, Hoerning A, Tebbe B, Kribben A, Witzke O, et al. Decreased IL-10(+) regulatory B cells (Bregs) in lupus nephritis patients. Scand J Rheumatol (2016) 45(4):312–6. doi:10.3109/03009742.2015.1126346
59. Gao N, Dresel J, Eckstein V, Gellert R, Storch H, Venigalla RK, et al. Impaired suppressive capacity of activation-induced regulatory B cells in systemic lupus erythematosus. Arthritis Rheumatol (2014) 66(10):2849–61. doi:10.1002/art.38742
60. Menon M, Blair PA, Isenberg DA, Mauri C. A regulatory feedback between plasmacytoid dendritic cells and regulatory B cells is aberrant in systemic lupus erythematosus. Immunity (2016) 44(3):683–97. doi:10.1016/j.immuni.2016.02.012
61. Carter NA, Vasconcellos R, Rosser EC, Tulone C, Munoz-Suano A, Kamanaka M, et al. Mice lacking endogenous IL-10-producing regulatory B cells develop exacerbated disease and present with an increased frequency of Th1/Th17 but a decrease in regulatory T cells. J Immunol (2011) 186(10):5569–79. doi:10.4049/jimmunol.1100284
62. Carter NA, Rosser EC, Mauri C. Interleukin-10 produced by B cells is crucial for the suppression of Th17/Th1 responses, induction of T regulatory type 1 cells and reduction of collagen-induced arthritis. Arthritis Res Ther (2012) 14(1):R32. doi:10.1186/ar3736
63. Yang M, Deng J, Liu Y, Ko KH, Wang X, Jiao Z, et al. IL-10-producing regulatory B10 cells ameliorate collagen-induced arthritis via suppressing Th17 cell generation. Am J Pathol (2012) 180(6):2375–85. doi:10.1016/j.ajpath.2012.03.010
64. Flores-Borja F, Bosma A, Ng D, Reddy V, Ehrenstein MR, Isenberg DA, et al. CD19+CD24hiCD38hi B cells maintain regulatory T cells while limiting TH1 and TH17 differentiation. Sci Transl Med (2013) 5(173):173ra123. doi:10.1126/scitranslmed.3005407
65. Ostensen M, Forger F, Nelson JL, Schuhmacher A, Hebisch G, Villiger PM. Pregnancy in patients with rheumatic disease: anti-inflammatory cytokines increase in pregnancy and decrease post partum. Ann Rheum Dis (2005) 64(6):839–44. doi:10.1136/ard.2004.029538
66. de Man YA, Dolhain RJ, van de Geijn FE, Willemsen SP, Hazes JM. Disease activity of rheumatoid arthritis during pregnancy: results from a nationwide prospective study. Arthritis Rheum (2008) 59(9):1241–8. doi:10.1002/art.24003
67. Rolle L, Memarzadeh M, Ignatov TI, Karsten F, Costa SD, Zenclussen AC, et al. Regulatory B-cells expand in peripheral blood of pregnant women having normal pregnancies but are very low in women suffering from spontaneous abortions. J Reprod Immunol (2012) 94(1):35–35. doi:10.1016/j.jri.2012.03.301
68. Goetz M, Atreya R, Ghalibafian M, Galle PR, Neurath MF. Exacerbation of ulcerative colitis after rituximab salvage therapy. Inflamm Bowel Dis (2007) 13(11):1365–8. doi:10.1002/ibd.20215
69. El Fassi D, Nielsen CH, Kjeldsen J, Clemmensen O, Hegedus L. Ulcerative colitis following B lymphocyte depletion with rituximab in a patient with Graves’ disease. Gut (2008) 57(5):714–5. doi:10.1136/gut.2007.138305
70. Roussey-Kesler G, Giral M, Moreau A, Subra JF, Legendre C, Noel C, et al. Clinical operational tolerance after kidney transplantation. Am J Transplant (2006) 6(4):736–46. doi:10.1111/j.1600-6143.2006.01280.x
71. Parker DC, Greiner DL, Phillips NE, Appel MC, Steele AW, Durie FH, et al. Survival of mouse pancreatic islet allografts in recipients treated with allogeneic small lymphocytes and antibody to CD40 ligand. Proc Natl Acad Sci U S A (1995) 92(21):9560–4.
72. Niimi M, Pearson TC, Larsen CP, Alexander DZ, Hollenbaugh D, Aruffo A, et al. The role of the CD40 pathway in alloantigen-induced hyporesponsiveness in vivo. J Immunol (1998) 161(10):5331–7.
73. Blair PA, Chavez-Rueda KA, Evans JG, Shlomchik MJ, Eddaoudi A, Isenberg DA, et al. Selective targeting of B cells with agonistic anti-CD40 is an efficacious strategy for the generation of induced regulatory T2-like B cells and for the suppression of lupus in MRL/lpr mice. J Immunol (2009) 182(6):3492–502. doi:10.4049/jimmunol.0803052
74. Nova-Lamperti E, Chana P, Mobillo P, Runglall M, Kamra Y, McGregor R, et al. Increased CD40 ligation and reduced BCR signalling leads to higher IL-10 production in B cells from tolerant kidney transplant patients. Transplantation (2016). doi:10.1097/TP.0000000000001341
75. Nova-Lamperti E, Fanelli G, Becker PD, Chana P, Elgueta R, Dodd PC, et al. IL-10-produced by human transitional B-cells down-regulates CD86 expression on B-cells leading to inhibition of CD4+T-cell responses. Sci Rep (2016) 6:20044. doi:10.1038/srep20044
76. Sarvaria A, Basar R, Mehta RS, Shaim H, Muftuoglu M, Khoder A, et al. IL-10+ regulatory B cells are enriched in cord blood and may protect against cGVHD after cord blood transplantation. Blood (2016) 128(10):1346–61. doi:10.1182/blood-2016-01-695122
77. Yan Y, van der Putten K, Bowen DG, Painter DM, Kohar J, Sharland AF, et al. Postoperative administration of donor B cells induces rat kidney allograft acceptance: lack of association with Th2 cytokine expression in long-term accepted grafts. Transplantation (2002) 73(7):1123–30.
78. Yeung MY, Ding Q, Brooks CR, Xiao S, Workman CJ, Vignali DA, et al. TIM-1 signaling is required for maintenance and induction of regulatory B cells. Am J Transplant (2015) 15(4):942–53. doi:10.1111/ajt.13087
79. Moreau A, Blair PA, Chai JG, Ratnasothy K, Stolarczyk E, Alhabbab R, et al. Transitional-2 B cells acquire regulatory function during tolerance induction and contribute to allograft survival. Eur J Immunol (2015) 45(3):843–53. doi:10.1002/eji.201445082
80. Lee KM, Stott RT, Zhao G, SooHoo J, Xiong W, Lian MM, et al. TGF-beta-producing regulatory B cells induce regulatory T cells and promote transplantation tolerance. Eur J Immunol (2014) 44(6):1728–36. doi:10.1002/eji.201344062
81. Leibler C, Matignon M, Pilon C, Montespan F, Bigot J, Lang P, et al. Kidney transplant recipients treated with belatacept exhibit increased naive and transitional B cells. Am J Transplant (2014) 14(5):1173–82. doi:10.1111/ajt.12721
82. Newell KA, Asare A, Sanz I, Wei C, Rosenberg A, Gao Z, et al. Longitudinal studies of a B cell-derived signature of tolerance in renal transplant recipients. Am J Transplant (2015) 15(11):2908–20. doi:10.1111/ajt.13480
83. Shabir S, Girdlestone J, Briggs D, Kaul B, Smith H, Daga S, et al. Transitional B lymphocytes are associated with protection from kidney allograft rejection: a prospective study. Am J Transplant (2015) 15(5):1384–91. doi:10.1111/ajt.13122
84. Bigot J, Pilon C, Matignon M, Grondin C, Leibler C, Aissat A, et al. Transcriptomic signature of the CD24hi CD38hi transitional B cells associated with an immunoregulatory phenotype in renal transplant recipients. Am J Transplant (2016) 16(12):3430–42. doi:10.1111/ajt.13904
85. Chesneau M, Michel L, Dugast E, Chenouard A, Baron D, Pallier A, et al. Tolerant kidney transplant patients produce B cells with regulatory properties. J Am Soc Nephrol (2015) 26(10):2588–98. doi:10.1681/ASN.2014040404
86. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell (2010) 140(6):883–99. doi:10.1016/j.cell.2010.01.025
87. Harrell MI, Iritani BM, Ruddell A. Tumor-induced sentinel lymph node lymphangiogenesis and increased lymph flow precede melanoma metastasis. Am J Pathol (2007) 170(2):774–86. doi:10.2353/ajpath.2007.060761
88. Ruddell A, Harrell MI, Furuya M, Kirschbaum SB, Iritani BM. B lymphocytes promote lymphogenous metastasis of lymphoma and melanoma. Neoplasia (2011) 13(8):748–57. doi:10.1593/neo.11756
89. Ganti SN, Albershardt TC, Iritani BM, Ruddell A. Regulatory B cells preferentially accumulate in tumor-draining lymph nodes and promote tumor growth. Sci Rep (2015) 5:12255. doi:10.1038/srep12255
90. Tang A, Dadaglio G, Oberkampf M, Di Carlo S, Peduto L, Laubreton D, et al. B cells promote tumor progression in a mouse model of HPV-mediated cervical cancer. Int J Cancer (2016) 139(6):1358–71. doi:10.1002/ijc.30169
91. Shimabukuro-Vornhagen A, Schlosser HA, Gryschok L, Malcher J, Wennhold K, Garcia-Marquez M, et al. Characterization of tumor-associated B-cell subsets in patients with colorectal cancer. Oncotarget (2014) 5(13):4651–64. doi:10.18632/oncotarget.1701
92. Zhang Y, Morgan R, Chen C, Cai Y, Clark E, Khan WN, et al. Mammary-tumor-educated B cells acquire LAP/TGF-beta and PD-L1 expression and suppress anti-tumor immune responses. Int Immunol (2016) 28(9):423–33. doi:10.1093/intimm/dxw007
93. Shao Y, Lo CM, Ling CC, Liu XB, Ng KT, Chu AC, et al. Regulatory B cells accelerate hepatocellular carcinoma progression via CD40/CD154 signaling pathway. Cancer Lett (2014) 355(2):264–72. doi:10.1016/j.canlet.2014.09.026
94. Zhou J, Min Z, Zhang D, Wang W, Marincola F, Wang X. Enhanced frequency and potential mechanism of B regulatory cells in patients with lung cancer. J Transl Med (2014) 12:304. doi:10.1186/s12967-014-0304-0
95. Wang WW, Yuan XL, Chen H, Xie GH, Ma YH, Zheng YX, et al. CD19+CD24hiCD38hiBregs involved in downregulate helper T cells and upregulate regulatory T cells in gastric cancer. Oncotarget (2015) 6(32):33486–99. doi:10.18632/oncotarget.5588
96. Zhou X, Su YX, Lao XM, Liang YJ, Liao GQ. CD19(+)IL-10(+) regulatory B cells affect survival of tongue squamous cell carcinoma patients and induce resting CD4(+) T cells to CD4(+)Foxp3(+) regulatory T cells. Oral Oncol (2016) 53:27–35. doi:10.1016/j.oraloncology.2015.11.003
97. Tan H, Yi L, Rote NS, Hurd WW, Mesiano S. Progesterone receptor-A and -B have opposite effects on proinflammatory gene expression in human myometrial cells: implications for progesterone actions in human pregnancy and parturition. J Clin Endocrinol Metab (2012) 97(5):E719–30. doi:10.1210/jc.2011-3251
98. Enninga EA, Holtan SG, Creedon DJ, Dronca RS, Nevala WK, Ognjanovic S, et al. Immunomodulatory effects of sex hormones: requirements for pregnancy and relevance in melanoma. Mayo Clin Proc (2014) 89(4):520–35. doi:10.1016/j.mayocp.2014.01.006
99. Wei Q, Ouyang Y, Zeng W, Duan L, Ge J, Liao H. Pregnancy complicating systemic lupus erythematosus: a series of 86 cases. Arch Gynecol Obstet (2011) 284(5):1067–71. doi:10.1007/s00404-010-1786-5
100. Zhao C, Zhao J, Huang Y, Wang Z, Wang H, Zhang H, et al. New-onset systemic lupus erythematosus during pregnancy. Clin Rheumatol (2013) 32(6):815–22. doi:10.1007/s10067-013-2180-z
101. Jara LJ, Lavalle C, Fraga A, Gomez-Sanchez C, Silveira LH, Martinez-Osuna P, et al. Prolactin, immunoregulation, and autoimmune diseases. Semin Arthritis Rheum (1991) 20(5):273–84.
102. Jorgensen C, Sany J. Modulation of the immune response by the neuro-endocrine axis in rheumatoid arthritis. Clin Exp Rheumatol (1994) 12(4):435–41.
103. Kyurkchiev D, Ivanova-Todorova E, Kyurkchiev SD. New target cells of the immunomodulatory effects of progesterone. Reprod Biomed Online (2010) 21(3):304–11. doi:10.1016/j.rbmo.2010.04.014
104. Polanczyk MJ, Hopke C, Huan J, Vandenbark AA, Offner H. Enhanced FoxP3 expression and Treg cell function in pregnant and estrogen-treated mice. J Neuroimmunol (2005) 170(1–2):85–92. doi:10.1016/j.jneuroim.2005.08.023
105. Tai P, Wang J, Jin H, Song X, Yan J, Kang Y, et al. Induction of regulatory T cells by physiological level estrogen. J Cell Physiol (2008) 214(2):456–64. doi:10.1002/jcp.21221
106. Polanczyk M, Zamora A, Subramanian S, Matejuk A, Hess DL, Blankenhorn EP, et al. The protective effect of 17β-estradiol on experimental autoimmune encephalomyelitis is mediated through estrogen receptor-α. Am J Pathol (2003) 163(4):1599–605. doi:10.1016/s0002-9440(10)63516-x
107. Phiel KL, Henderson RA, Adelman SJ, Elloso MM. Differential estrogen receptor gene expression in human peripheral blood mononuclear cell populations. Immunol Lett (2005) 97(1):107–13. doi:10.1016/j.imlet.2004.10.007
108. Polanczyk M, Yellayi S, Zamora A, Subramanian S, Tovey M, Vandenbark AA, et al. Estrogen receptor-1 (Esr1) and -2 (Esr2) regulate the severity of clinical experimental allergic encephalomyelitis in male mice. Am J Pathol (2004) 164(6):1915–24. doi:10.1016/s0002-9440(10)63752-2
109. Subramanian S, Yates M, Vandenbark AA, Offner H. Oestrogen-mediated protection of experimental autoimmune encephalomyelitis in the absence of Foxp3+ regulatory T cells implicates compensatory pathways including regulatory B cells. Immunology (2011) 132(3):340–7. doi:10.1111/j.1365-2567.2010.03380.x
110. Bodhankar S, Chen Y, Lapato A, Vandenbark AA, Murphy SJ, Saugstad JA, et al. Regulatory CD8(+)CD122 (+) T-cells predominate in CNS after treatment of experimental stroke in male mice with IL-10-secreting B-cells. Metab Brain Dis (2015) 30(4):911–24. doi:10.1007/s11011-014-9639-8
111. Zhang J, Benedek G, Bodhankar S, Lapato A, Vandenbark AA, Offner H. IL-10 producing B cells partially restore E2-mediated protection against EAE in PD-L1 deficient mice. J Neuroimmunol (2015) 285:129–36. doi:10.1016/j.jneuroim.2015.06.002
112. Zhang J, Lapato A, Bodhankar S, Vandenbark AA, Offner H. Treatment with IL-10 producing B cells in combination with E2 ameliorates EAE severity and decreases CNS inflammation in B cell-deficient mice. Metab Brain Dis (2015) 30(5):1117–27. doi:10.1007/s11011-015-9661-5
113. Szekeres-Bartho J, Halasz M, Palkovics T. Progesterone in pregnancy; receptor-ligand interaction and signaling pathways. J Reprod Immunol (2009) 83(1–2):60–4. doi:10.1016/j.jri.2009.06.262
114. Lin H, Mosmann TR, Guilbert L, Tuntipopipat S, Wegmann TG. Synthesis of T helper 2-type cytokines at the maternal-fetal interface. J Immunol (1993) 151(9):4562–73.
115. Garay L, Gonzalez Deniselle MC, Meyer M, Costa JJ, Lima A, Roig P, et al. Protective effects of progesterone administration on axonal pathology in mice with experimental autoimmune encephalomyelitis. Brain Res (2009) 1283:177–85. doi:10.1016/j.brainres.2009.04.057
116. Yates MA, Li Y, Chlebeck P, Proctor T, Vandenbark AA, Offner H. Progesterone treatment reduces disease severity and increases IL-10 in experimental autoimmune encephalomyelitis. J Neuroimmunol (2010) 220(1–2):136–9. doi:10.1016/j.jneuroim.2010.01.013
117. Schumacher A, Costa SD, Zenclussen AC. Endocrine factors modulating immune responses in pregnancy. Front Immunol (2014) 5:196. doi:10.3389/fimmu.2014.00196
118. Robertson SA, Care AS, Skinner RJ. Interleukin 10 regulates inflammatory cytokine synthesis to protect against lipopolysaccharide-induced abortion and fetal growth restriction in mice. Biol Reprod (2007) 76(5):738–48. doi:10.1095/biolreprod.106.056143
119. Chaouat G, Assal Meliani A, Martal J, Raghupathy R, Elliott JF, Mosmann T, et al. IL-10 prevents naturally occurring fetal loss in the CBA x DBA/2 mating combination, and local defect in IL-10 production in this abortion-prone combination is corrected by in vivo injection of IFN-tau. J Immunol (1995) 154(9):4261–8.
120. Murphy SP, Fast LD, Hanna NN, Sharma S. Uterine NK cells mediate inflammation-induced fetal demise in IL-10-Null mice. J Immunol (2005) 175(6):4084–90. doi:10.4049/jimmunol.175.6.4084
121. White CA, Johansson M, Roberts CT, Ramsay AJ, Robertson SA. Effect of interleukin-10 null mutation on maternal immune response and reproductive outcome in mice. Biol Reprod (2004) 70(1):123–31. doi:10.1095/biolreprod.103.018754
122. Terrone DA, Rinehart BK, Granger JP, Barrilleaux PS, Martin JN Jr, Bennett WA. Interleukin-10 administration and bacterial endotoxin-induced preterm birth in a rat model. Obstet Gynecol (2001) 98(3):476–80. doi:10.1016/S0029-7844(01)01424-7
123. Robertson SA, Skinner RJ, Care AS. Essential role for IL-10 in resistance to lipopolysaccharide-induced preterm labor in mice. J Immunol (2006) 177(7):4888–96. doi:10.4049/jimmunol.177.7.4888
124. de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med (1991) 174(5):1209–20.
125. Fiorentino DF, Zlotnik A, Mosmann TR, Howard M, O’Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol (1991) 147(11):3815–22.
126. Jensen F, Muzzio D, Soldati R, Fest S, Zenclussen AC. Regulatory B10 cells restore pregnancy tolerance in a mouse model. Biol Reprod (2013) 89(4):90. doi:10.1095/biolreprod.113.110791
127. Muzzio DO, Ziegler KB, Ehrhardt J, Zygmunt M, Jensen F. Marginal zone B cells emerge as a critical component of pregnancy well-being. Reproduction (2016) 151(1):29–37. doi:10.1530/REP-15-0274
128. Rolle L, Memarzadeh Tehran M, Morell-Garcia A, Raeva Y, Schumacher A, Hartig R, et al. Cutting edge: IL-10-producing regulatory B cells in early human pregnancy. Am J Reprod Immunol (2013) 70(6):448–53. doi:10.1111/aji.12157
129. Lima J, Martins C, Leandro MJ, Nunes G, Sousa MJ, Branco JC, et al. Characterization of B cells in healthy pregnant women from late pregnancy to post-partum: a prospective observational study. BMC Pregnancy Childbirth (2016) 16(1):139. doi:10.1186/s12884-016-0927-7
130. Blois SM, Alba Soto CD, Tometten M, Klapp BF, Margni RA, Arck PC. Lineage, maturity, and phenotype of uterine murine dendritic cells throughout gestation indicate a protective role in maintaining pregnancy. Biol Reprod (2004) 70(4):1018–23. doi:10.1095/biolreprod.103.022640
131. Kallikourdis M, Betz AG. Periodic accumulation of regulatory T cells in the uterus: preparation for the implantation of a semi-allogeneic fetus? PLoS One (2007) 2(4):e382. doi:10.1371/journal.pone.0000382
132. Chen T, Darrasse-Jeze G, Bergot AS, Courau T, Churlaud G, Valdivia K, et al. Self-specific memory regulatory T cells protect embryos at implantation in mice. J Immunol (2013) 191(5):2273–81. doi:10.4049/jimmunol.1202413
133. Zenclussen AC. Regulatory T cells in pregnancy. Springer Semin Immunopathol (2006) 28(1):31–9. doi:10.1007/s00281-006-0023-6
134. Alijotas-Reig J, Llurba E, Gris JM. Potentiating maternal immune tolerance in pregnancy: a new challenging role for regulatory T cells. Placenta (2014) 35(4):241–8. doi:10.1016/j.placenta.2014.02.004
Keywords: regulatory B cells, regulatory T cells, autoimmunity, cancer, pregnancy, tolerance, estrogen, progesterone
Citation: Guzman-Genuino RM and Diener KR (2017) Regulatory B Cells in Pregnancy: Lessons from Autoimmunity, Graft Tolerance, and Cancer. Front. Immunol. 8:172. doi: 10.3389/fimmu.2017.00172
Received: 02 November 2016; Accepted: 03 February 2017;
Published: 17 February 2017
Edited by:
Song Guo Zheng, Penn State Milton S. Hershey Medical Center, USAReviewed by:
Abdelhadi Saoudi, Institut national de la santé et de la recherche médicale (INSERM), FranceTerry Barton Strom, Harvard Medical School, USA
Copyright: © 2017 Guzman-Genuino and Diener. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kerrilyn R. Diener, kerrilyn.diener@adelaide.edu.au
 Ruth Marian Guzman-Genuino
Ruth Marian Guzman-Genuino Kerrilyn R. Diener
Kerrilyn R. Diener