- NDORMS, Rheumatology Department, Nuffield Orthopaedic Centre, University of Oxford, Oxford, UK
Anti-neutrophil cytoplasm antibodies (ANCA) are associated with small vessel vasculitides (AASV) affecting the lungs and kidneys. Structured clinical assessment using the Birmingham Vasculitis Activity Score and Vasculitis Damage Index should form the basis of a treatment plan and be used to document progress, including relapse. Severe disease with organ or life threatening manifestations needs cyclophosphamide or rituximab, plus high dose glucocorticoids, followed by lower dose steroid plus azathioprine, or methotrexate. Additional plasmapheresis is effective for very severe disease, reducing dialysis dependence from 60 to 40% in the first year, but with no effect on mortality or long-term renal function, probably due to established renal damage. In milder forms of ANCA-associated vasculitis, methotrexate, leflunomide, or mycophenolate mofetil are effective. Mortality depends on initial severity: 25% in patients with renal failure or severe lung hemorrhage; 6% for generalized non-life threatening AASV but rising to 30–40% at 5 years. Mortality from GPA is four times higher than the background population. Early deaths are due to active vasculitis and infection. Subsequent deaths are more often due to cardiovascular events, infection, and cancer. We need to improve the long-term outcome, by controlling disease activity but also preventing damage and drug toxicity. By contrast, in large vessel vasculitis where mortality is much less but morbidity potentially greater, such as giant cell arteritis (GCA) and Takayasu arteritis, therapeutic options are limited. High dose glucocorticoid results in significant toxicity in over 80%. Advances in understanding the biology of the vasculitides are improving therapies. Novel, mechanism based therapies such as rituximab in AASV, mepolizumab in eosinophilic granulomatosis with polyangiitis, and tocilizumab in GCA, but the lack of reliable biomarkers remains a challenge to progress in these chronic relapsing diseases.
Introduction
The systemic vasculitides are a complex set of overlapping conditions whose natural history has been significantly modified by current therapies but continue to challenge patients and clinicians. We expect survival in over 90% (compared to over 90% mortality untreated) in the first year; about 70% with small vessel vasculitis survive up to 5 years, giving a mortality ratio of 2.6 (95% CI 2.2–3.1) compared to background (1, 2).
In large vessel vasculitis, mortality is low (3) but morbidity is high. In giant cell arteritis (GCA), visual loss occurs in up to 35% (4). In Takayasu arteritis, ischemic claudication of limbs and great vessels can require surgical reconstruction (5).
Current therapies minimize systemic and local inflammation and can preserve organ function. Immunosuppressive agents are combined with supportive management, which includes: compensating for organ dysfunction (e.g., treating hypertension or providing dialysis); dealing with or preventing comorbidity, which might arise from treatment (e.g., infection, steroid related osteoporosis, or cataract); worsening of pre-existing comorbidity (e.g., worsening of ischemic heart disease or obesity); or development of new comorbidity.
We need to ensure that we identify what we are actually treating so that we tailor the choice of treatment at the right dose and at right time for each individual.
What are We Treating?
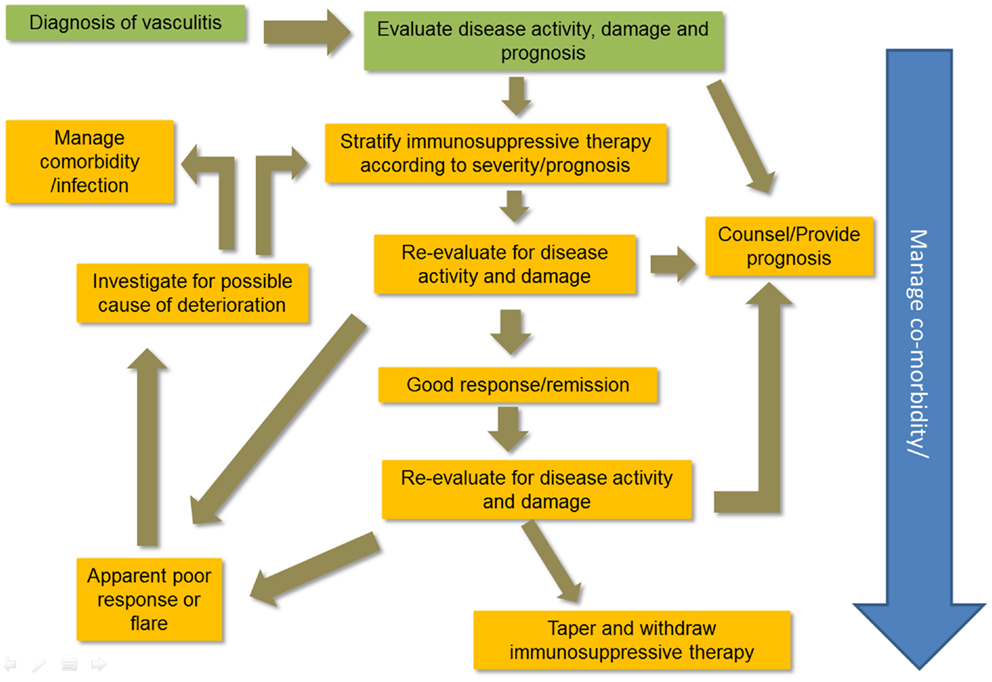
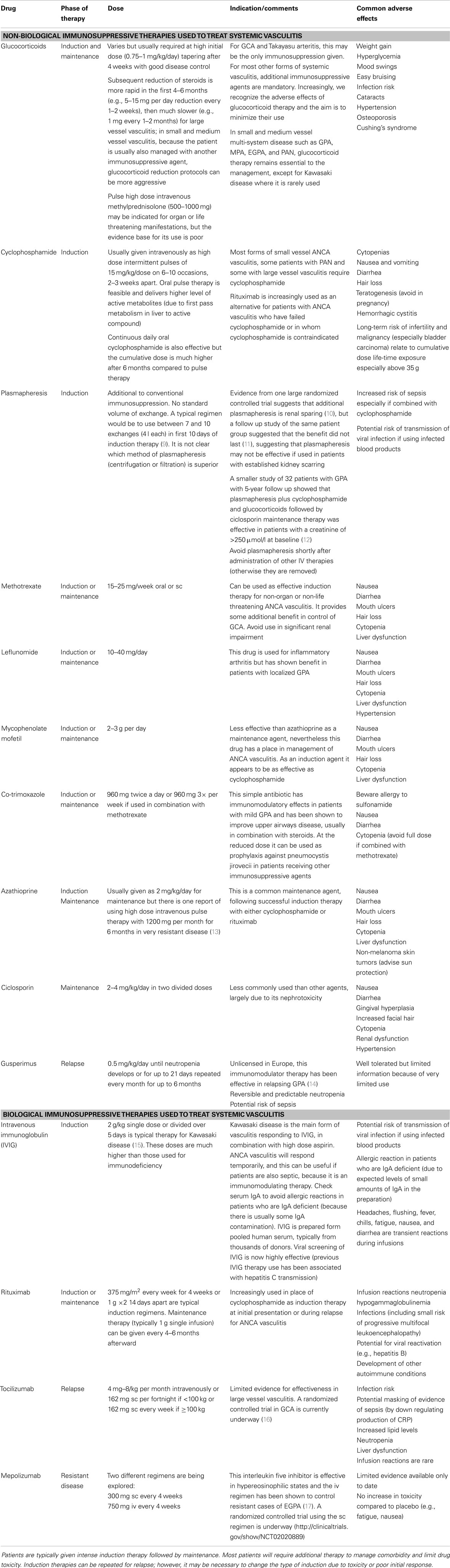
Making an accurate diagnosis of the type of vasculitis is an important part of treatment choices. Figure 1 illustrates a typical plan of management for patients with vasculitis. There are no diagnostic criteria for the vasculitides; Chapel Hill Consensus Conference definitions are widely applied (6). Classification criteria for vasculitis are currently problematic (7) and research is underway to improve them (8). However, the diagnostic label is not enough. The patient’s status should include assessment of disease severity and the context in which the disease occurs in individuals. Table 1 outlines the immunosuppressive therapies used to manage vasculitis.
The range of diseases encompassed includes small, medium, and large vessel vasculitis; small and medium vessel diseases are grouped together because the standard treatment approaches are very similar; however, they are starting to diversify as we develop more targeted agents.
For patients with a virus associated vasculitis, treatment of the virus is a prerequisite to controlling disease. Polyarteritis nodosa (PAN) related to hepatitis B (HBV-PAN), a typical form of PAN, is characterized by the absence of glomerulonephritis and the absence of anti-neutrophil cytoplasm antibodies (ANCA); relapses are rare, and never occur once viral replication has stopped and seroconversion has occurred (18). Eradication of hepatitis B is part of the management for HBV-PAN (18).Combining an anti-viral drug with plasmapheresis facilitates seroconversion and prevents the development of long-term hepatic complications of HBV. The incidence of HBV-PAN has decreased 10-fold as a result of improved blood safety and vaccination campaigns (19). In a study of 80 patients with HBV-PAN given anti-viral therapy plus immunosuppression, 5% relapsed and 30% died compared with 14.3% relapses and 48.6% deaths among 35 patients treated with immunosuppression alone. Patients who seroconverted achieved complete remission and did not relapse.
Unfortunately, the eradication of hepatitis C has been more problematic; patients with cryoglobulinaemic vasculitis may continue to require ongoing anti-viral therapy. Combination anti-viral therapy is more effective, as shown in a study of cryoglobulinaemic vasculitis; 69% of 23 cases treated with a combination of pegylated interferon alpha, ribavirin, and a protease inhibitor had achieved undetectable viral loads and a good clinical response in the majority including complete remission in 57% (20).
In small vessel vasculitis associated with ANCA, these antibodies are intricately involved in the pathogenesis (21). The role of conventional immunosuppressive agents remains important. Cyclophosphamide is the gold standard for multi-system small vessel vasculitis (22, 23); for less aggressive forms of disease, there is a potential role for leflunomide (24), methotrexate (25) or in one small series, high dose intravenous azathioprine (13). Whilst small open label studies of tumor necrosis factor (TNF) inhibition have suggested benefit (26) in disease control and improvement in abnormal endothelial dysfunction, a large randomized placebo controlled trial of etanercept, a TNF receptor protein, has shown no benefit in patients with GPA; in fact these patients had an increased risk of malignancy, which may in part have related to the inclusion of patients previously exposed to large doses of cyclophosphamide (27). Direct targeting of B cell production of antibody is an effective therapy for many but not all patients (28–30).
For patients with large vessel vasculitis such as GCA or Takayasu arteritis, the primary treatment is glucocorticoids, but as we identify disease mechanisms, we should be able to use targeted therapies, avoiding the use of high doses of steroids, which result in very significant toxicity in over 80% (31).
Mechanism Specific vs. Global Immunosuppression
Immunosuppressive therapy results in global effects on the immune system, which can be both good and bad. Glucocorticoids produce a rapid improvement in all types of vasculitis by genomic effects on the cytosolic and more rapid non-genomic effects on the membrane bound glucocorticoid receptor (32), but these effects are short lived in small vessel vasculitis. By contrast, most patients experience significant steroid toxicity (over 80% for GCA) and this relates to the total steroid load (31). It is important to tailor the dose of steroids, often used together with an immunosuppressive agent (see Table 1), to minimize the harm, while still controlling disease.
Specific targeting of inflammatory immune mechanism in vasculitis is increasingly practical as we identify the molecular pathways that are primarily responsible for the disease. The role of complement in ANCA-associated vasculitis (33) has led to the development of targeted therapy against complement 5A (C5a), which is currently being tested in clinical trials1. The involvement by ANCA itself has led to development of specific B cell ablation therapy using rituximab and now belimumab2. As newer understanding of disease mechanisms is revealed then more targets for therapy will be identified or at least we will have better recognition of how we are affecting the underlying pathways with existing therapies.
Can We Induce Remission?
The aim of managing the patients with vasculitis is to induce remission, which should be possible in the majority. However, this is a clinical remission not a cure and the majority of patients will relapse. We need to deliver treatment according to need without exposing patients to unnecessary risk whilst ensuring the maximum benefit. Conventional measurements of clinical remission are defined using disease activity scores, which are preferred to any current serological marker for small vessel and medium vessel vasculitis. In over 90% of patients with small vessel vasculitis, remission should be achieved by 6 months (22) using standard induction therapy. Further serial evaluation is important in order to detect and treat relapses.
By contrast, in large vessel vasculitis, the induction of remission is less easily documented. All the clinical symptoms and signs disappear rapidly with steroid treatment; by contrast, it is not so clear that we have adequately controlled disease at a sub-clinical level. Imaging is emerging as an effective technology to define disease activity. Unfortunately, it is expensive and can involve significant radiation exposure. The best imaging technique available is 18 fluorodeoxyglucose positron emission tomography with co-localized computerized tomography (FDG PET CT) to identify areas of abnormal glucose uptake. However, this involves exposure to an average of 14.4 mSv for females and 11.8 mSv for male patients (34) per scan. Nevertheless, it is important to quantify the presence of sub-clinical disease to find ways of preventing end stage ischemic complications or other vascular events such as thrombosis, dissection, and aneurysm. Non-invasive imaging protocols using magnetic resonance scans or ultrasound are being developed as ways of measuring change in disease state (35, 36).
How Do We Measure Remission?
Serological measurements of disease activity are not reliable in systemic vasculitis (37). ANCA testing is very useful for diagnosis, but for subsequent follow up, levels can vary independently of future disease activity (38).
The Birmingham Vasculitis Activity Score (BVAS) is the most effective validated tool to document disease activity; it can be used as to define remission, response to therapy and flare (37, 39, 40). The BVAS consists of a list of typical features of active systemic vasculitis related to each body system; each item is recorded as present only if it is judged to be due to active vasculitis. This is semi-subjective because items are derived from the patient history and physical examination and cannot always be confirmed with more objective testing. However, the BVAS is valid, reliable, and widely used in clinical trials in vasculitis to define the responsiveness to various agents including cyclophosphamide, methotrexate, mycophenolate, intravenous immunoglobulin, and rituximab. It is a valuable tool for the clinicians and strongly recommended as a routine part of disease management in small and medium vessel vasculitis (40, 41). Other versions of BVAS have been validated for use in individual forms of vasculitis, such as the BVAS/Wegener’s granulomatosis, specifically for patients with GPA (42).
The Vasculitis Damage Index (VDI) is used to assess the outcome of vasculitis, by documenting the occurrence of damage as a result of having a diagnosis of vasculitis (43, 44). VDI is recommended as a cumulative measure to define the effectiveness of therapy (by limiting or preventing the accumulation of scarring). The VDI is strongly related to mortality. The presence of VDI levels of at least five points on a scale of 0–64 items (which occurs in about a third of patients (45) when measured 6 months from diagnosis) is associated with a much higher future mortality (approximately sixfold higher) than patients with less than five items of damage (46) 6 months from diagnosis.
What Drugs Should We Use?
Each patient’s management should be based on their diagnosis and clinical state. Control of active vasculitis may be achieved using a range of therapies, depending on how rapidly and aggressively the treatment is required. The decision should be based on evidence, but interpreted for the individual to minimize harm, taking into account existing or likely co-morbidities. Some treatment protocols allow for this. For example, there are dose reductions for the dose of cyclophosphamide in older persons, those with renal impairment or with prior significant neutropenia (23).
The treatment protocol may need to be amended if unexpected changes occur in clinical status, either as a result of toxicity or if patients fail to respond to standard agents. Therapy should be withheld until inter-current infection is treated, or escalated in cases with poor initial response. Fundamental to these decisions is the regular careful clinical evaluation of patients to detect these changes. Table 1 summarizes the immunosuppressive agents commonly used as well as describing potential future therapies under investigation.
Immunosuppressive Therapies
The main drug used is cyclophosphamide, cyclic nitrogen mustard, phosphamide ester, first used as a chemotherapeutic agent in the 1950s (47). Cyclophosphamide is a cytotoxic alkylating agent capable of killing B cells and T-cells. It is life-saving in patients with small and medium vessel vasculitis and is considered the drug of choice for multi organ disease (22).
However, the toxicity has been considerable, when used as continuous daily oral therapy for up to 2.7 years, providing over 100 g life-time exposure in some patients (48, 49), mainly due to its predicted cytotoxic effects on rapidly dividing normal cells. It can cause reversible nausea, vomiting, diarrhea, and hair loss; permanent infertility and malignancy occur with increasing cumulative doses, with an incidence of 5% at 10 years and 16% after 15 years (50). There is no absolute cut-off dose to avoid toxicity, but the recent British Society for Rheumatology guidelines for management of ANCA-associated vasculitis recommend restricting total exposure to <25 g (41). Current cyclophosphamide protocols use a much lower cumulative dose and the bladder cancer incidence is not increased (51). However, in a study of male fertility risk in patients given cyclophosphamide for sarcoma (52), a total dose of >7.5 g/m2 was associated with only a 10% chance of recovery of spermatogenesis compared to 70% for those given less than this dose. The use of short courses of high dose intravenous cyclophosphamide is likely to be safer than continuous daily oral therapy (53), chiefly due to the fact that the cumulative dose is typically 30–50% less.
Cyclophosphamide is effective in reducing the mortality in ANCA-associated vasculitis (22, 23). It can be given either as a pulse intravenous high dose therapy 15 mg/kg every 2–3 weeks on 6–10 occasions or as a continuous daily oral therapy at 2 mg/kg/day (54). The latter results in much higher cumulative dose of drug over 6 months period and there is evidence of equivalent of these two regimens; although the use of pulse cyclophosphamide is associated with a higher relapse rate (23). For less aggressive forms of vasculitis, there is a potential role for leflunomide (24), methotrexate (23, 25, 55) or in one small series, high dose intravenous azathioprine (13), and mycophenolate mofetil (56). These agents are usually less toxic but also less effective than cyclophosphamide.
For patients with large vessel vasculitis such as GCA or Takayasu arteritis, the primary treatment is to suppress systemic inflammation with glucocorticoid therapy in order to prevent significant vascular complications (such as loss of sight in GCA or aortic aneurysm formation or stenosis or occlusion of peripheral arteries in Takayasu arteritis. With better understanding of disease mechanisms, we might be able to use targeted therapies, perhaps even avoiding the use of steroids, which otherwise carry very significant risk of toxicity in over 80% (31).
There is some evidence for the effectiveness of TNF inhibition in small vessel vasculitis (26), but concerns about long-term toxicity (27).In Takayasu arteritis (35), studies show improvement but are limited to small numbers and there are no randomized controlled trials. This is partly due to the problem of not having adequate end points to demonstrate a potential treatment effect as well as due to the rarity of the condition (57). Direct targeting of B cells is an effective therapy for many patients; the response (64% in complete remission by 6 months) is similar to that achieved with cyclophosphamide (53% in complete remission by 6 months) for a group of 197 patients with new or relapsing ANCA-associated vasculitis; limited evidence suggests that in 102 patients with relapsing disease previously responding to cyclophosphamide, rituximab was more effective than cyclophosphamide (67 vs. 42% in complete remission respectively, p = 0.01) (28–30).
For any form of B cell depletion therapy, it is logical to assume that reconstitution of B cells after the end of treatment will lead to recurrence (58), although this is disputed, with at least one study demonstrating that disease relapse was independent of B cell numbers (59). Nevertheless, maintenance rituximab substantially reduces the risk of recurrence of disease from 73 to 12% after 2 years follow up in retrospective cohort data (60). Another retrospective observational study of 89 patients treated with rituximab for ANCA-associated vasculitis (60) suggests that there was additional protection against future relapse by using maintenance azathioprine, methotrexate, or mycophenolate mofetil compared to no additional immunosuppressive treatment [the hazard ratio for relapse was 0.53 (95% CI 0.29–0.97) if a maintenance drug was given].
Discussion
The state of the art for therapy in vasculitis has improved, but remains unsatisfactory until we can completely control or cure the disease. We can prevent early mortality in multi-system vasculitis and have reduced the immediate effects of active vasculitis on organ function. However, our aim is to further improve the likelihood of survival and also the quality of life of those who survive, ensuring that we minimize disease activity and damage, drug toxicity, and impairment of quality of life.
With better understanding of the pathogenesis of vasculitis, we can target therapy against specific disease mechanisms. Examples of these are rituximab in ANCA-associated vasculitis; mepolizumab in eosinophilic granulomatosis with polyangiitis; complement 5a inhibition in ANCA vasculitis; and potentially Interleukin-6 (IL-6) inhibition with tocilizumab in large vessel vasculitis.
We are helped in our management of the disease by earlier diagnosis, so that treatment can be initiated before organ damage is established in most cases. Whilst the ANCA test is overused (61, 62), it has helped in earlier identification of patients with systemic vasculitis (63). Greater awareness of vasculitis as a cause of unexplained medical illness is leading to better case recognition. Imaging of arteries in large vessel vasculitis may become established as an early diagnostic test (64), which might change our current management approach.
Better management of comorbidity, particularly management of sepsis, control of hypertension, or management of renal failure have changed the outcome and potentially allowed more aggressive immunosuppression to be successful. However, in first 12 months after diagnosis of ANCA vasculitis, episodes of acute sepsis are now responsible for more deaths than vasculitis itself (49).
The role of glucocorticoids in vasculitis is being challenged. Whereas, previously they have been used at high doses for prolonged periods, we recognize their harm, coupled by the benefit from more specific therapy. We should see substantial reduction in toxicity in the coming decade, as we use lower doses or even steroid free regimens to control vasculitis.
Whilst the twentieth century has been dominated by use of the therapies for vasculitis designed to treat other conditions [e.g., cancer and rheumatoid arthritis (23, 65, 66)], drugs are now been designed specifically for vasculitis. We should see significant benefits for our patients, but we need to ensure that we measure their impact (for good and for harm). In the absence of reliable circulating biomarkers we need to use structured clinical assessment to document change in disease state in response to therapy. Development of effective, prognostic biomarkers for vasculitis would allow therapy to be targeted to disease mechanisms, tempered by safety assessments to prevent untoward harm.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Jana Vaskova for administrative support. NIHR Biomedical Research Unit in Musculoskeletal Disease at Oxford University Hospitals NHS Trust and the University of Oxford for support.
Footnotes
References
1. Flossmann O, Berden A, De Groot K, Hagen C, Harper L, Heijl C, et al. Long-term patient survival in ANCA-associated vasculitis. Ann Rheum Dis (2011) 70:488–94. doi: 10.1136/ard.2010.137778
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
2. Walton EW. Giant-cell granuloma of the respiratory tract (Wegener’s granulomatosis). Br Med J (1958) 2:265–70. doi:10.1136/bmj.2.5091.265
3. Phillip R, Luqmani R. Mortality in systemic vasculitis: a systematic review. Clin Exp Rheumatol (2008) 26(5 Suppl 51):S94–104.
4. Souza AW, Okamoto KY, Abrantes F, Schau B, Bacchiega AB, Shinjo SK. Giant cell arteritis: a multicenter observational study in Brazil. Clinics (Sao Paulo) (2013) 68(3):317–22. doi:10.6061/clinics/2013(03)OA06
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
5. Saadoun D, Lambert M, Mirault T, Resche-Rigon M, Koskas F, Cluzel P, et al. Retrospective analysis of surgery versus endovascular intervention in Takayasu arteritis: a multicenter experience. Circulation (2012) 125(6):813–9. doi:10.1161/CIRCULATIONAHA.111.058032
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
6. Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. 2012 revised international Chapel Hill consensus conference nomenclature of vasculitides. Arthritis Rheum (2013) 65(1):1–11. doi:10.1002/art.37715
7. Basu N, Watts R, Bajema I, Baslund B, Bley T, Boers M, et al. EULAR points to consider in the development of classification and diagnostic criteria in systemic vasculitis. Ann Rheum Dis (2010) 69(10):1744–50. doi:10.1136/ard.2009.119032
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
8. Craven A, Robson J, Ponte C, Grayson PC, Suppiah R, Judge A, et al. ACR/EULAR-endorsed study to develop diagnostic and classification criteria for vasculitis (DCVAS). Clin Exp Nephrol (2013) 17(5):619–21. doi:10.1007/s10157-013-0854-0
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
9. De Joode AA, Sanders JS, Smid WM, Stegeman CA. Plasmapheresis rescue therapy in progressive systemic ANCA-associated vasculitis: single-center results of stepwise escalation of immunosuppression. J Clin Apher (2014). doi:10.1002/jca.21318
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
10. Jayne DR, Gaskin G, Rasmussen N, Abramowicz D, Ferrario F, Guillevin L, et al. Randomized trial of plasma exchange or high-dosage methylprednisolone as adjunctive therapy for severe renal vasculitis. J Am Soc Nephrol (2007) 18(7):2180–8. doi:10.1681/ASN.2007010090
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
11. Walsh M, Casian A, Flossmann O, Westman K, Höglund P, Pusey C, et al. Long-term follow-up of patients with severe ANCA-associated vasculitis comparing plasma exchange to intravenous methylprednisolone treatment is unclear. Kidney Int (2013) 84(2):397–402. doi:10.1038/ki.2013.131
12. Szpirt WM, Heaf JG, Petersen J. Plasma exchange for induction and cyclosporine A for maintenance of remission in Wegener’s granulomatosis – a clinical randomized controlled trial. Nephrol Dial Transplant (2011) 26(1):206–13. doi:10.1093/ndt/gfq360
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
13. Aries PM, Hellmich B, Reinhold-Keller E, Gross WL. High-dose intravenous azathioprine pulse treatment in refractory Wegener’s granulomatosis. Rheumatology (Oxford) (2004) 43(10):1307–8. doi:10.1093/rheumatology/keh300
14. Flossmann O, Baslund B, Bruchfeld A, Tervaert JW, Hall C, Heinzel P, et al. Deoxyspergualin in relapsing and refractory Wegener’s granulomatosis. Ann Rheum Dis (2009) 68(7):1125–30. doi:10.1136/ard.2008.092429
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
15. Newburger JW, Takahashi M, Burns JC, Beiser AS, Chung KJ, Duffy CE, et al. The treatment of Kawasaki syndrome with intravenous gamma globulin. N Engl J Med (1986) 315(6):341–7. doi:10.1056/NEJM198608073150601
16. Unizony S, Arias-Urdaneta L, Miloslavsky E, Arvikar S, Khosroshahi A, Keroack B, et al. Tocilizumab for the treatment of large-vessel vasculitis (giant cell arteritis, Takayasu arteritis) and polymyalgia rheumatica. Arthritis Care Res (Hoboken) (2012) 64(11):1720–9. doi:10.1002/acr.21750
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
17. Moosig F, Gross WL, Herrmann K, Bremer JP, Hellmich B. Targeting interleukin-5 in refractory and relapsing Churg-Strauss syndrome. Ann Intern Med (2011) 155(5):341–3. doi:10.7326/0003-4819-155-5-201109060-00026
18. Guillevin L, Mahr A, Callard P, Godmer P, Pagnoux C, Leray E, et al. Hepatitis B virus-associated polyarteritis nodosa: clinical characteristics, outcome, and impact of treatment in 115 patients. Medicine (Baltimore) (2005) 84(5):313–22. doi:10.1097/01.md.0000180792.80212.5e
19. Sagnelli E, Sagnelli C, Pisaturo M, Macera M, Coppola N. Epidemiology of acute and chronic hepatitis B and delta over the last 5 decades in Italy. World J Gastroenterol (2014) 20(24):7635–43. doi:10.3748/wjg.v20.i24.7635
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
20. Saadoun D, Resche Rigon M, Thibault V, Longuet M, Pol S, Blanc F, et al. Peg-IFNα/ribavirin/protease inhibitor combination in hepatitis C virus associated mixed cryoglobulinemia vasculitis: results at week 24. Ann Rheum Dis (2014) 73(5):831–7. doi:10.1136/annrheumdis-2012-202770
21. Jennette JC, Falk RJ. Pathogenesis of antineutrophil cytoplasmic autoantibody-mediated disease. Nat Rev Rheumatol (2014) 10(8):463–73. doi:10.1038/nrrheum.2014.103
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
22. Jayne D, Rasmussen N, Andrassy K, Bacon P, Tervaert JW, Dadoniené J, et al. A randomized trial of maintenance therapy for vasculitis associated with antineutrophil cytoplasmic autoantibodies. N Engl J Med (2003) 349(1):36–44. doi:10.1056/NEJMoa020286
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
23. De Groot K, Harper L, Jayne DR, Flores Suarez LF, Gregorini G, Gross WL, et al. EUVAS (European vasculitis study group). Pulse versus daily oral cyclophosphamide for induction of remission in antineutrophil cytoplasmic antibody-associated vasculitis: a randomized trial. Ann Intern Med (2009) 150(10):670–80. doi:10.7326/0003-4819-150-10-200905190-00004
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
24. Metzler C, Miehle N, Manger K, Iking-Konert C, De Groot K, Hellmich B, et al. German network of rheumatic diseases. elevated relapse rate under oral methotrexate versus leflunomide for maintenance of remission in Wegener’s granulomatosis. Rheumatology (Oxford) (2007) 46(7):1087–91. doi:10.1093/rheumatology/kem029
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
25. De Groot K, Rasmussen N, Bacon PA, Tervaert JW, Feighery C, Gregorini G, et al. Randomized trial of cyclophosphamide versus methotrexate for induction of remission in early systemic antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum (2005) 52(8):2461–9. doi:10.1002/art.21142
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
26. Booth AD, Jayne DR, Kharbanda RK, McEniery CM, Mackenzie IS, Brown J, et al. Infliximab improves endothelial dysfunction in systemic vasculitis: a model of vascular inflammation. Circulation (2004) 109(14):1718–23. doi:10.1161/01.CIR.0000124720.18538.DD
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
27. The Wegener’s Granulomatosis Etanercept Trial Research Group. Limited versus severe Wegener’s granulomatosis: baseline data on patients in the Wegener’s granulomatosis etanercept trial. Arthritis Rheum (2003) 48:2299–309. doi:10.1002/art.11075
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
28. Stone JH, Merkel PA, Spiera R, Seo P, Langford CA, Hoffman GS, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med (2010) 363(3):221–32. doi:10.1056/NEJMoa0909905
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
29. Jones RB, Tervaert JW, Hauser T, Luqmani R, Morgan MD, Peh CA, et al. European vasculitis study group rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N Engl J Med (2010) 363(3):211–20. doi:10.1056/NEJMoa0909169
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
30. Specks U, Merkel PA, Seo P, Spiera R, Langford CA, Hoffman GS, et al. Efficacy of remission-induction regimens for ANCA-associated vasculitis. N Engl J Med (2013) 369(5):417–27. doi:10.1056/NEJMoa1213277
31. Proven A, Gabriel SE, Orces C, O’Fallon WM, Hunder GG. Glucocorticoid therapy in giant cell arteritis: duration and adverse outcomes. Arthritis Rheum (2003) 49(5):703–8. doi:10.1002/art.11388
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
32. Strehl C, Buttgereit F. Unraveling the functions of the membrane-bound glucocorticoid receptors: first clues on origin and functional activity. Ann N Y Acad Sci (2014) 1318:1–6. doi:10.1111/nyas.12364
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
33. Xiao H, Schreiber A, Heeringa P, Falk RJ, Jennette JC. Alternative complement pathway in the pathogenesis of disease mediated by anti-neutrophil cytoplasmic autoantibodies. Am J Pathol (2007) 170(1):52–64. doi:10.2353/ajpath.2007.060573
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
34. Kaushik A, Jaimini A, Tripathi M, D’Souza M, Sharma R, Mishra AK, et al. Estimation of patient dose in (18)F-FDG and (18)F-FDOPA PET/CT examinations. J Cancer Res Ther (2013) 9(3):477–83. doi:10.4103/0973-1482.119354
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
35. Youngstein T, Peters JE, Hamdulay SS, Mewar D, Price-Forbes A, Lloyd M, et al. Serial analysis of clinical and imaging indices reveals prolonged efficacy of TNF-α and IL-6 receptor targeted therapies in refractory Takayasu arteritis. Clin Exp Rheumatol (2014) 32(2 Suppl 82):S11–8.
36. Muto G, Yamashita H, Takahashi Y, Miyata Y, Morooka M, Minamimoto R, et al. Large vessel vasculitis in elderly patients: early diagnosis and steroid-response evaluation with FDG-PET/CT and contrast-enhanced CT. Rheumatol Int (2014). doi:10.1007/s00296-014-2985-3
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
37. Luqmani RA, Bacon PA, Moots RJ, Janssen BA, Pall A, Emery P, et al. Birmingham vasculitis activity score (BVAS) in systemic necrotizing vasculitis. QJM (1994) 87(11):671–8.
38. Tomasson G, Grayson PC, Mahr AD, Lavalley M, Merkel PA. Value of ANCA measurements during remission to predict a relapse of ANCA-associated vasculitis – a meta-analysis. Rheumatology (Oxford) (2012) 51(1):100–9. doi:10.1093/rheumatology/ker280
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
39. Mukhtyar C, Lee R, Brown D, Carruthers D, Dasgupta B, Dubey S, et al. Modification and validation of the Birmingham vasculitis activity score (version 3). Ann Rheum Dis (2009) 68(12):1827–32. doi:10.1136/ard.2008.101279
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
40. Mukhtyar C, Guillevin L, Cid MC, Dasgupta B, De Groot K, Gross W, et al. European vasculitis study group. EULAR recommendations for the management of primary small and medium vessel vasculitis. Ann Rheum Dis (2009) 68(3):310–7. doi:10.1136/ard.2008.088096
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
41. Ntatsaki E, Carruthers D, Chakravarty K, D’Cruz D, Harper L, Jayne D, et al. Guidelines and audit working group. BSR and BHPR guideline for the management of adults with ANCA-associated vasculitis. Rheumatology (Oxford) (2014). doi:10.1093/rheumatology/ket445
42. Stone JH, Hoffman GS, Merkel PA, Min YI, Uhlfelder ML, Hellmann DB, et al. International network for the study of the systemic vasculitides (INSSYS). A disease-specific activity index for Wegener’s granulomatosis: modification of the Birmingham vasculitis activity score. Arthritis Rheum (2001) 44(4):912–20. doi:10.1002/1529-0131(200104)44:4<912::AID-ANR148>3.0.CO;2-5
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
43. Exley AR, Bacon PA, Luqmani RA, Kitas GD, Gordon C, Savage CO, et al. Development and initial validation of the vasculitis Damage Index for the standardized clinical assessment of damage in the systemic vasculitides. Arthritis Rheum (1997) 40(2):371–80. doi:10.1002/art.1780400222
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
44. Suppiah R, Flossman O, Mukhtyar C, Alberici F, Baslund B, Brown D, et al. Measurement of damage in systemic vasculitis: a comparison of the vasculitis damage index with the combined damage assessment index. Ann Rheum Dis (2011) 70(1):80–5. doi:10.1136/ard.2009.122952
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
45. Robson J, Doll H, Suppiah R, Flossmann O, Harper L, Höglund P, et al. Damage in the anca-associated vasculitides: long-term data from the European vasculitis study group (EUVAS) therapeutic trials. Ann Rheum Dis (2013). doi:10.1136/annrheumdis-2013-203927
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
46. Exley AR, Carruthers DM, Luqmani RA, Kitas GD, Gordon C, Janssen BA, et al. Damage occurs early in systemic vasculitis and is an index of outcome. QJM (1997) 90:391–9. doi:10.1093/qjmed/90.6.391
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
47. Arnold H, Bourseaux F, Brock N. Chemotherapeutic action of a cyclic nitrogen mustard phosphamide ester (B 518-ASTA) in experimental tumours of the rat. Nature (1958) 181(4613):931. doi:10.1038/181931a0
48. Stillwell TJ, Benson RC Jr, DeRemee RA, McDonald TJ, Weiland LH. Cyclophosphamide-induced bladder toxicity in Wegener’s granulomatosis. Arthritis Rheum (1988) 31(4):465–70. doi:10.1002/art.1780310402
49. Little MA, Nightingale P, Verburgh CA, Hauser T, De Groot K, Savage C, et al. Early mortality in systemic vasculitis: relative contribution of adverse events and active vasculitis. Ann Rheum Dis (2010) 69(6):1036–43. doi:10.1136/ard.2009.109389
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
50. Talar-Williams C, Hijazi YM, Walther MM, Linehan WM, Hallahan CW, Lubensky I, et al. Cyclophosphamide-induced cystitis and bladder cancer in patients with Wegener granulomatosis. Ann Intern Med (1996) 124(5):477–8. doi:10.7326/0003-4819-124-5-199603010-00003
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
51. Heijl C, Harper L, Flossmann O, Stücker I, Scott DG, Watts RA, et al. Incidence of malignancy in patients treated for antineutrophil cytoplasm antibody-associated vasculitis: follow-up data from European vasculitis study group clinical trials. Ann Rheum Dis (2011) 70(8):1415–21. doi:10.1136/ard.2010.145250
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
52. Meistrich ML, Wilson G, Brown BW, Da Cunha MF, Lipshultz LI. Impact of cyclophosphamide on long-term reduction in sperm count in men treated with combination chemotherapy for Ewing and soft tissue sarcomas. Cancer (1992) 70(11):2703–12. doi:10.1002/1097-0142(19921201)70:11<2703::AID-CNCR2820701123>3.0.CO;2-X
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
53. Monach PA, Arnold LM, Merkel PA. Incidence and prevention of bladder toxicity from cyclophosphamide in the treatment of rheumatic diseases: a data-driven review. Arthritis Rheum (2010) 62:921. doi:10.1002/art.27674
54. Harper L, Morgan MD, Walsh M, Hoglund P, Westman K, Flossmann O, et al. Pulse versus daily oral cyclophosphamide for induction of remission in ANCA-associated vasculitis: long-term follow-up. Ann Rheum Dis (2012) 71(6):955–60. doi:10.1136/annrheumdis-2011-200477
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
55. Faurschou M, Westman K, Rasmussen N, de Groot K, Flossmann O, Höglund P, et al. Brief report: long-term outcome of a randomized clinical trial comparing methotrexate to cyclophosphamide for remission induction in early systemic antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum (2012) 64:34727. doi:10.1002/art.34547
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
56. Hiemstra TF, Walsh M, Mahr A, Savage CO, De Groot K, Harper L, et al. Mycophenolate mofetil vs azathioprine for remission maintenance in antineutrophil cytoplasmic antibody-associated vasculitis: a randomized controlled trial. JAMA (2010) 304(21):2381–8. doi:10.1001/jama.2010.1658
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
57. Direskeneli H, Aydin SZ, Kermani TA, Matteson EL, Boers M, Herlyn K, et al. Development of outcome measures for large-vessel vasculitis for use in clinical trials: opportunities, challenges, and research agenda. J Rheumatol (2011) 38(7):1471–9. doi:10.3899/jrheum.110275
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
58. Cartin-Ceba R, Golbin JM, Keogh KA, Peikert T, Sánchez-Menéndez M, Ytterberg SR, et al. Rituximab for remission induction and maintenance in refractory granulomatosis with polyangiitis (Wegener’s): ten-year experience at a single center. Arthritis Rheum (2012) 64(11):3770–8. doi:10.1002/art.34584
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
59. Smith RM, Jones RB, Guerry MJ, Laurino S, Catapano F, Chaudhry A, et al. Rituximab for remission maintenance in relapsing antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum (2012) 64(11):3760–9. doi:10.1002/art.34583
60. Azar L, Springer J, Langford CA, Hoffman GS. Rituximab with or without a conventional maintenance agent in relapsing granulomatosis with polyangiitis: a retrospective single-center study. Arthritis Rheumatol (2014) 66(10):2862–70. doi:10.1002/art.38744
61. McLaren JS, Stimson RH, McRorie ER, Coia JE, Luqmani RA. The diagnostic value of anti-neutrophil cytoplasmic antibody testing in a routine clinical setting. QJM (2001) 94(11):615–21. doi:10.1093/qjmed/94.11.615
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
62. Arnold DF, Timms A, Luqmani R, Misbah SA. Does a gating policy for ANCA overlook patients with ANCA associated vasculitis? An audit of 263 patients. J Clin Pathol (2010) 63(8):678–80. doi:10.1136/jcp.2009.072504
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
63. Edmunds M, Andrews M, Campbell A, Walls J, Feehally J. Systemic vasculitis in the 1980s – is there an increasing incidence of Wegener’s granulomatosis and microscopic polyarteritis? J R Coll Physicians Lond (1990) 24(4):284–8.
64. Schmidt WA, Kraft HE, Völker L, Vorpahl K, Gromnica-Ihle EJ. Colour Doppler sonography to diagnose temporal arteritis. Lancet (1995) 345(8953):866. doi:10.1016/S0140-6736(95)93005-1
65. Walsh M, Chaudhry A, Jayne D. Long-term follow-up of relapsing/refractory anti-neutrophil cytoplasm antibody associated vasculitis treated with the lymphocyte depleting antibody alemtuzumab (CAMPATH-1H). Ann Rheum Dis (2008) 67(9):1322–7. doi:10.1136/ard.2007.081661
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
66. Langford CA, Monach PA, Specks U, Seo P, Cuthbertson D, McAlear CA, et al. Vasculitis clinical research consortium. An open-label trial of abatacept (CTLA4-IG) in non-severe relapsing granulomatosis with polyangiitis (Wegener’s). Ann Rheum Dis (2014) 73(7):1376–9. doi:10.1136/annrheumdis-2013-204164
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Keywords: vasculitis, cyclophosphamide, rituximab, ANCA, glucocorticoid, plasmapheresis, methotrexate, azathioprine
Citation: Luqmani RA (2014) State of the art in the treatment of systemic vasculitides. Front. Immunol. 5:471. doi: 10.3389/fimmu.2014.00471
Received: 25 July 2014; Accepted: 13 September 2014;
Published online: 13 October 2014.
Edited by:
Giuseppe Alvise Ramirez, Università Vita-Salute San Raffaele, ItalyReviewed by:
Fulvio D’Acquisto, Queen Mary University of London, UKJaewoo Hong, National Institutes of Health, USA
Copyright: © 2014 Luqmani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Raashid Ahmed Luqmani, NDORMS, Rheumatology Department, Nuffield Orthopaedic Centre, University of Oxford, Windmill Road, Oxford OX3 7LD, UK e-mail: raashid.luqmani@ndorms.ox.ac.uk
 Raashid Ahmed Luqmani
Raashid Ahmed Luqmani