- Pharmakologisches Institut, Universität Heidelberg, Heidelberg, Germany
Transient receptor potential (TRP) proteins form cation channels that are regulated through strikingly diverse mechanisms including multiple cell surface receptors, changes in temperature, in pH and osmolarity, in cytosolic free Ca2+ concentration ([Ca2+]i), and by phosphoinositides which makes them polymodal sensors for fine tuning of many cellular and systemic processes in the body. The 28 TRP proteins identified in mammals are classified into six subfamilies: TRPC, TRPV, TRPM, TRPA, TRPML, and TRPP. When activated, they contribute to cell depolarization and Ca2+ entry. In mast cells, the increase of [Ca2+]i is fundamental for their biological activity, and several entry pathways for Ca2+ and other cations were described including Ca2+ release activated Ca2+ (CRAC) channels. Like in other non-excitable cells, TRP channels could directly contribute to Ca2+ influx via the plasma membrane as constituents of Ca2+ conducting channel complexes or indirectly by shifting the membrane potential and regulation of the driving force for Ca2+ entry through independent Ca2+ entry channels. Here, we summarize the current knowledge about the expression of individual Trp genes with the majority of the 28 members being yet identified in different mast cell models, and we highlight mechanisms how they can regulate mast cell functions. Since specific agonists or blockers are still lacking for most members of the TRP family, studies to unravel their function and activation mode still rely on experiments using genetic approaches and transgenic animals. RNAi approaches suggest a functional role for TRPC1, TRPC5, and TRPM7 in mast cell derived cell lines or primary mast cells, and studies using Trp gene knock-out mice reveal a critical role for TRPM4 in mast cell activation and for mast cell mediated cutaneous anaphylaxis, whereas a direct role of cold- and menthol-activated TRPM8 channels seems to be unlikely for the development of cold urticaria at least in mice.
Ca2+ Entry Pathways and Mast Cell Activation
In mast cells, the increase of free cytosolic Ca2+ regulates a variety of cellular processes including degranulation with release of preformed inflammatory mediators (Ozawa et al., 1993), production of eicosanoids such as leukotrienes (Chang et al., 2006), activation of transcription factors including the nuclear factor of activated T cells (NFAT; Kar et al., 2011), and synthesis of cytokines (Plaut et al., 1989) as well as cytoskeletal rearrangements required for migration of mast cells and chemotaxis (Hartmann et al., 1997). Mast cell derived mediators can evoke both pathological inflammatory responses in allergic or autoimmune diseases but they can also have protective functions, e.g., by induction of innate immune responses leading to clearance of pathogens or by degrading endogenous and exogenous toxins (Marshall, 2004; Galli et al., 2005; Metz and Maurer, 2007). The importance of extracellular Ca2+ as a requirement for anaphylactic release of histamine from rat peritoneal mast cells was already shown in 1972 (Foreman and Mongar, 1972), and it could be shown that Ca2+ could be replaced by Sr2+ or Ba2+ to achieve mast cell degranulation (Foreman et al., 1977). Later, it was shown that substantial amounts of Ca2+ in the external medium (i.e., >50 μM) was absolutely required for degranulation in rat basophilic leukemia (RBL)-2H3 cells (Beaven et al., 1984) leading to the concept that antigen-mediated mast cells degranulation is dependent on Ca2+ influx through Ca2+ channels that are permeable for Sr2+/Ba2+ (Ma and Beaven, 2009, 2011). Distinct temporal and spatial patterns of the increase in intracellular Ca2+ concentration triggered by individual Ca2+ mobilizing mast cell activators may explain the specificity of mast cell responses. For example, differences in the amplitude and duration of Ca2+ signals in response to different stimulants differentially influence histamine secretion or production of eicosanoids (van Haaster et al., 1995). Also, it was shown that, under certain conditions, mast cells can be stimulated to undergo chemotaxis without degranulation (Taub et al., 1995). More recent work has revealed that a range of mast cell responses are activated by spatially restricted Ca2+ signals just below the plasma membrane (Di Capite and Parekh, 2009; Kar et al., 2011).
Although mast cells have numerous functions beyond the development of allergies, studies of mast cell signal transduction have mainly been driven by the central role of these cells in allergic inflammatory responses (Metcalfe et al., 1997). The manifestations of mast cell-driven allergic reactions are considered to be mainly a consequence of the release of pro-inflammatory mediators following antigen-induced aggregation of high-affinity receptors for IgE (FcεRI), expressed at the mast cell surface. FcεRI cross-linking activates a large number of signaling molecules (Blank and Rivera, 2004; Gilfillan and Tkaczyk, 2006). A major downstream target is phospholipase Cγ1 (PLCγ1), which catalyzes the hydrolysis of phosphatidylinositol (4,5) bisphosphate (PIP2) to diacylglycerol (DAG) and inositol (1,4,5) trisphosphate (IP3). DAG and IP3 promote protein kinase C (PKC) activation and release of Ca2+ from intracellular stores, respectively, followed by an influx of Ca2+ from the extracellular space. Also in other non-excitable cells the depletion of intracellular Ca2+ stores correlates with influx of Ca2+ from the extracellular space suggesting that the amount of Ca2+ in the stores controls the extent of Ca2+ influx, a process that was called store-operated Ca2+ entry (SOCE) or capacitative Ca2+ entry (CCE; Putney, 1986; Parekh and Putney, 2005) and described later also in mast cells (Putney, 1986; Ali et al., 1994; Falcone and Fewtrell, 1995). Ionic currents mediating this Ca2+ influx were first characterized by Hoth and Penner(1992, 1993) in RBL cells and rat peritoneal mast cells. The physiological hallmark of the underlying channel is a high selectivity for Ca2+ over other cations and its activation by depletion of intracellular Ca2+ stores, e.g., by IP3 and chelation of cytosolic Ca2+. The Ca2+ selectivity of Ca2+ release activated Ca2+ (CRAC) channels is similar to that of voltage-activated Ca2+ channels (VDCC) which were shown to be expressed and to modulate mast cell activation (Yoshimaru et al., 2009), but the conductance of CRAC channels is much lower. Also, replacement of external Ca2+ with Sr2+ or Ba2+ resulted in a decline in ICRAC activity (Zweifach and Lewis, 1996) indicating that CRAC channels cannot represent the exclusive Ca2+ entry channel mediating Ca2+ dependent mast cell degranulation. The desire to identify the molecular nature of SOCE was and still is the motivation for many workers to characterize mammalian transient receptor potential (TRP) channels. However, the pore properties of most TRP channels that have been studied in detail, including TRPV6, which was identified in primary murine and human mast cells (Turner et al., 2007), and TRPV5, appeared not to match the pore properties of CRAC channels (see below and Owsianik et al., 2006b). Recently, Orai1 (also known as CRACM1) was identified as the pore-forming CRAC channel subunit (Feske et al., 2006; Vig et al., 2006; Lewis, 2007) and another protein called stromal interaction molecule 1 (Stim1) was shown to represent the Ca2+ sensor coupling the process of depletion of intracellular Ca2+ stores with Ca2+ influx across the plasma membrane through CRAC channels (Lewis, 2007). Orai1 and its homologs Orai2 and Orai3 were shown to be expressed in RBL-2H3 cells and bone marrow-derived mast cells (BMMCs; Gross et al., 2007). SOCE is substantially decreased in Orai1-deficient BMMCs (Gwack et al., 2008), and the FcεRI-mediated Ca2+ entry is reduced by 70%, while the cells completely lack detectable Ca2+ (CRAC) currents (Vig et al., 2008). Interestingly, in this study it was concluded that Ca2+ release from intracellular stores is unchanged in Orai1-deficient BMMCs (Vig et al., 2008) suggesting that ORAI1 proteins are non-critical components for the refilling of these Ca2+ stores. The relative contribution of Orai2 and Orai3 for Ca2+ entry in mast cells is still unclear. Although these results support the conclusion that ORAI1 (CRACM1)-proteins build the Ca2+-selective channels responsible for the majority of Ca2+ entry into mast cells, there is still some residual Ca2+ entry in Orai1-deficient BMMCs after stimulation of the FcεRI or store depletion. Ca2+ entry in Orai1-deficient mast cells following activation with other Ca2+ mobilizing mast cell activators has not been analysed so far. This suggests that other channel proteins – especially members of the TRP family such as TRPC5 (Ma et al., 2008) – might contribute to Ca2+ entry either as part of an Orai/TRP channel complex (Liu et al., 2003, 2007; Liao et al., 2007; Yuan et al., 2007) or independently.
In addition to activation of FcεRI and depletion of intracellular Ca2+ stores there is increasing evidence that receptors for other ligands such as adenosine, endothelin-1, stem cell factor (SCF), lysophosphatidylcholine (LPC), sphingosine-1 phosphate (S1P), substance P, and others, which markedly influence mast cell activation in a physiological setting, do also induce elevations of [Ca2+]i. These receptors can either potentiate FcεRI-mediated mast cell activation or, by themselves, stimulate the release of mast cell mediators. The pathways leading to elevation of [Ca2+]i following stimulation of these receptors are still poorly characterized, and particularly it is not known whether or to which degree the resultant receptor-activated Ca2+ entry is mediated by store depletion. Receptor stimulation using substance P or compound 48/80 leads to a Ca2+ influx through non-selective cation channels in rat peritoneal mast cells (Penner et al., 1988; Kuno and Kimura, 1992; Fasolato et al., 1993). Recently, an additional mechanism for regulation of Ca2+ entry in mast cells has been discovered that does not rely on mast cell intrinsic mechanisms such as receptor stimulation by soluble mediators or regulation of the driving force for Ca2+ entry but on the interaction with another cell type: it could be shown that binding of OX40 expressed on regulatory T cells to the mast cell based receptor OX40L inhibits Ca2+ entry by an increase of intracellular cAMP levels in mast cells (Gri et al., 2008). The molecular constituents of the Ca2+ conducting channels regulated by this new pathway are unknown like those channels activated by the numerous Ca2+ mobilizing agonists described above, but members of the TRP family are potential candidates also for these Ca2+ entry pathways.
TRP Proteins Form Cation Channels
Transient receptor potential channels are a large and functionally heterogeneous family of cation-conducting channel proteins, which are activated and regulated through strikingly diverse mechanisms. The first TRP channel gene was discovered in Drosophila melanogaster (Montell and Rubin, 1989) in the analysis of a fly mutant whose photoreceptors failed to retain a sustained response to maintained light stimuli. In mammals, TRP proteins were identified in most cases by their sequence homology. Nevertheless, some TRPs were identified by expression cloning, e.g., TRPV1 as the receptor for vanilloids such as capsaicin, or by positional cloning efforts to identify genes disrupted in human diseases, e.g., TRPML1 (also designated as mucolipin, MCOLN1, ML1) as a gene that is mutated in mucolipidosis type IV or TRPP2 (also designated as polycystin-2, PKD2, PC2) in autosomal polycystic kidney disease.
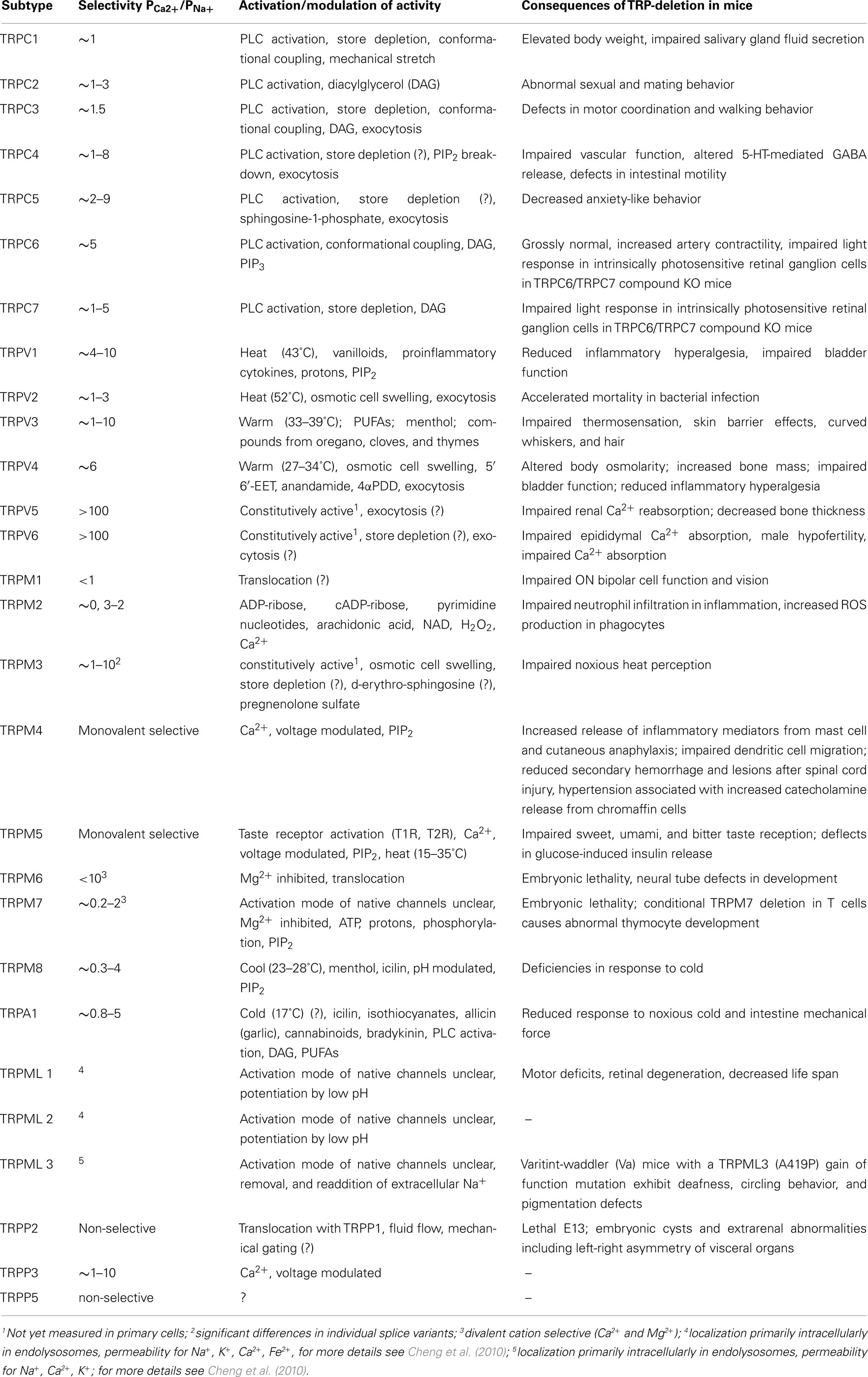
The mammalian 28 TRP proteins are classified according to structural homology into six subfamilies: TRPC (canonical), TRPV (vanilloid), TRPM (melastatin), TRPA (ankyrin), TRPML (mucolipin), and TRPP (polycystin; Montell et al., 2002; Clapham et al., 2005; Wu et al., 2010). All TRP channels are assumed to have six-transmembrane (6TM) polypeptide subunits that assemble as tetramers to form cation-permeable pores (Owsianik et al., 2006a). Most TRP channels show little voltage dependence and are non-selective with a permeability for Ca2+ over Na+ (ratio PCa/PNa) below 10. Therefore, TRP channels are not only important for Ca2+ entry via the plasma membrane but play also an important role in electrogenesis regulating the driving forces for Ca2+ entry via other Ca2+-permeable channels. Table 1 (Venkatachalam and Montell, 2007; Gees et al., 2010) gives an overview about the permeability of channels formed by individual TRP proteins, but it has to be emphasized that most of these information is based on studies of heterologously expressed channel proteins and the characteristics of those channels may differ significantly from native channel complexes existing in primary cells since the ectopically expressed channel proteins do not necessarily act in accordance with the native cellular context as in primary cells.
Transient receptor potentials are expressed in many (if not in all) excitable and non-excitable cells and are involved in sensing of a variety of environmental stimuli such as temperature, pH, osmolarity, pheromones, taste, and plant compounds. TRP channels participate in numerous cellular processes to determine organ and integrative body functions, and several mutations in Trp genes appear to be causative factors in rare heritable channelopathies (Freichel and Flockerzi, 2007; Nilius and Owsianik, 2010). Although the search for natural ligands and chemical modulators of TRP channels as therapeutics has been intensified in the last years, specific agonists or blockers are still lacking for most members of the TRP family until now. Therefore, genetic approaches are still required to advance a causal understanding of their physiological functions in primary cells, in organs, for systemic functions of organisms and for disease states. These approaches include over-expression of dominant-negative variants, antisense oligonucleotides, and RNAi as well as targeted deletion of the gene of interest using homologous recombination (Freichel et al., 2011). Another obstacle that hinders the analysis of endogenous TRP channels is that specific antibodies are rare for most TRP proteins (Meissner et al., 2011) and, accordingly, this impedes investigations of the assembly and localization of TRP channels, but also the control of the effectiveness of RNAi approaches.
Expression and Postulated Functions of TRP Proteins in Mast Cells
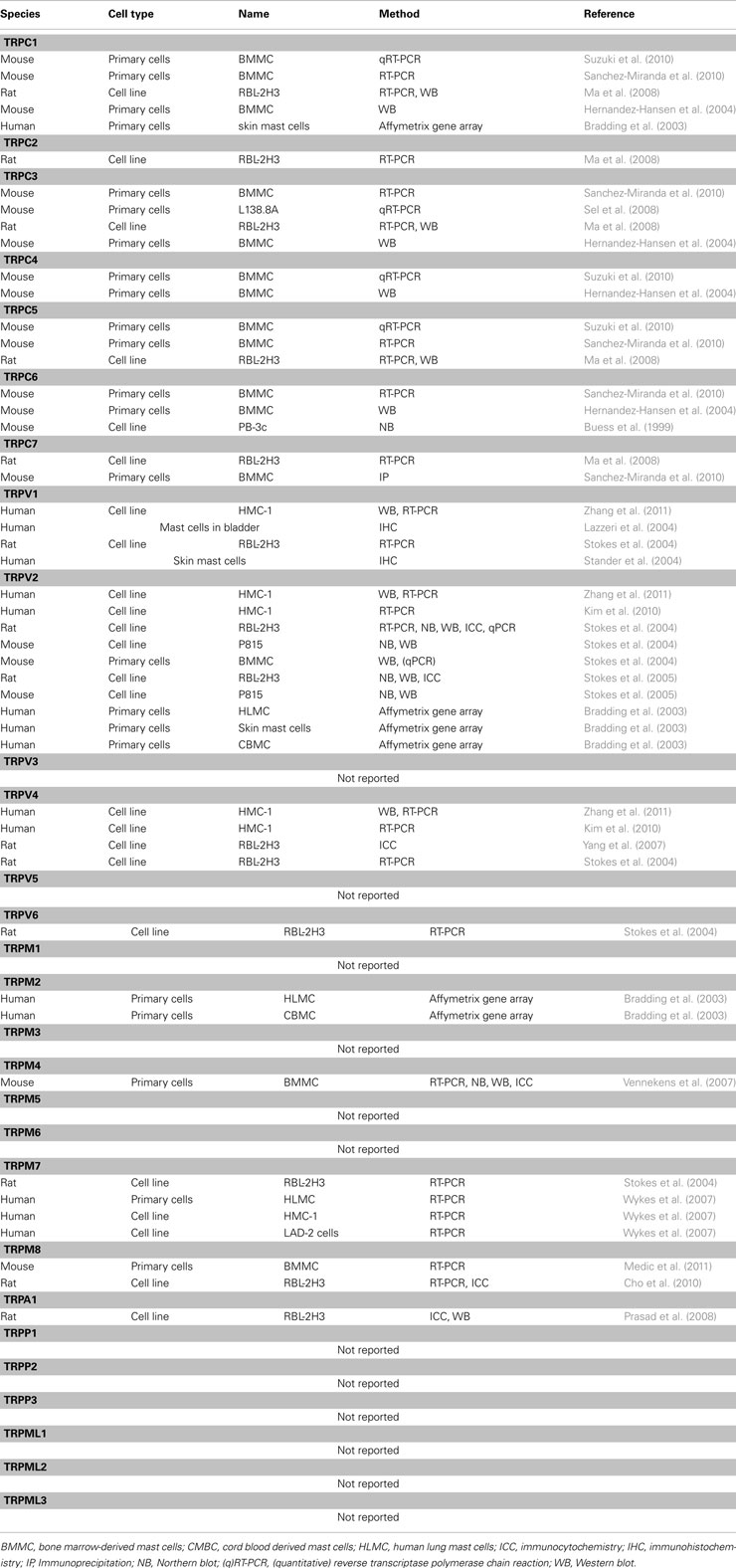
In the following we highlight key features regarding activation and functions of individual members of the TRP subfamilies, concerning their expression in mast cell models and established or postulated mast cell functions. Table 1 systematically summarizes information about the permeability, mode of activation, and the biological functions in native systems as revealed by mouse models for the individual mammalian TRP proteins. For more detailed information regarding structure, gating, special functional aspects of splice variants, TRP channelopathies, and citations of the wealth of original manuscripts about mammalian TRP proteins since their first description and functional characterization in 1995/1996 (Wes et al., 1995; Zhu et al., 1995; Philipp et al., 1996), we refer to various excellent recent review articles (Venkatachalam and Montell, 2007; Gees et al., 2010; Nilius and Owsianik, 2010; Wu et al., 2010). The current knowledge about the expression of TRP proteins in different cell lines and primary mast cell models (Table 2) is based on analysis using RT-PCR, gene arrays or quantitative PCR approaches as well as Western Blot analysis or immunocytochemistry for which commercial anti-TRP antibodies were used in most cases. For the latter approach, it has to be mentioned that many commercial suppliers pass the burden of antibody validation to the end user and that the specificity of the antibodies used was rarely tested rigorously in preparations of cells/tissues that do not express the target protein at all (Meissner et al., 2011). The functional role of individual TRP proteins in mast cells was analysed using TRP channel antagonists, RNAi approaches, and TRP deficient mouse models. Regarding RNAi approaches it needs to be noted that – despite the merits of this technology – off-target effects are not unusual, and were also described in a study where transfection of a Trpm7-specific siRNA achieved complete suppression of Trpm7 mRNA in other primary cells but also significantly reduced TRPM2 expression levels (Aarts et al., 2003). Secondly, the control of the effectiveness of RNAi approaches requires specific antibodies against the target TRP protein to avoid the situation that at the same time a poorly characterized RNAi experiment is controlled by non-validated antibodies and vice versa. Reports using mast cells derived from mice with targeted deletion of Trp genes are restricted until now to cells from mice with ubiquitous inactivation of individual Trp genes, in which the observed phenotype may be affected by compensatory mechanisms.
TRPC Channels
The mammalian members of the TRPC family can be divided into three subfamilies on the basis of functional similarities and sequence homology: TRPC1/TRPC4/TRPC5, TRPC3/TRPC6/TRPC7, and TRPC2. Physical interaction between the TRPC family members was studied by co-immunoprecipitation and FRET experiments with heterologously expressed proteins and using immunoprecipitation from brain protein fraction using antibodies directed against peptides derived from TRPC proteins and revealed that TRPC1, TRPC4, and TRPC5 may coassemble as well as TRPC3, TRPC6, and TRPC7 (Lintschinger et al., 2000; Strubing et al., 2001; Goel et al., 2002; Hofmann et al., 2002). In embryonic rat brain, it was found that TRPC1, TRPC4, and TRPC5 interact with TRPC3 and TRPC6 (Strubing et al., 2003). All TRPC proteins seem to be activated by stimulation of G-protein-coupled receptors or receptor-tyrosine kinases. The activation of the PLC pathway leads to the opening of TRPC channels but in parallel to stimulation of IP3 receptors and subsequent depletion of intracellular Ca2+ stores and, hence, SOCE. This raised the issue whether TRPC proteins are constituents of cation channels mediating SOCE, and a contribution of TRPC proteins to SOCE has been suggested very early after their first discovery (Hardie and Minke, 1993). In principle, over-expressed mammalian TRPCs have all been reported to be activated by store depletion (for review see, e.g., Venkatachalam et al., 2002). However, the dependence of TRPCs on store depletion requires special conditions. For instance, TRPC3 channels, which are activated directly by PLC-derived DAG in many expression systems, were also reported to be activated by reduction of the filling state of intracellular Ca2+ stores (Kiselyov et al., 1998; Vazquez et al., 2003), but the ability of TRPC3 to contribute to SOCE was only found when it was expressed at low densities (Vazquez et al., 2003; Yildirim et al., 2005). The underlying cause for this phenomenon has not been identified, but one explanation is that at high expression levels the transfected TRPCs titrate regulatory subunits that confer SOCE characteristics to TRPCs. Although numerous studies show that Orai1 is the pore-forming subunit of store-operated channels (Lewis, 2007), a model with Orai as a regulatory subunit of SOCE channels composed of pore-forming TRPC subunits has recently been proposed (Liao et al., 2008). Additionally, Stim1 was found to hetermultimerize TRPC proteins either directly (TRPC1, TRPC4, TRPC5) or indirectly (TRPC3, TRPC6) to determine their store-operated activation mode (Yuan et al., 2007, 2009). Taken together, the question of whether and how TRPC proteins contribute to SOCE remains highly controversial (Parekh, 2010) and cannot be generally answered but may depend on the relative expression of the above mentioned and/or not yet identified constituents that make up SOC channel complexes in individual cell types.
The permeability (PCa/PNa) of channels formed by heterologously expressed TRPC proteins varies between 1 and 10 (Table 1 and Venkatachalam and Montell, 2007; Gees et al., 2010). Whether TRPC1 can form functional homomeric channels by itself still remains debatable. TRPC1 forms heteromeric channels with TRPC4 or TRPC5, which have properties distinct from those of homomultimers (Strubing et al., 2001). TRPC4 and TRPC5 share many structural and functional characteristics such as the activation by Gq/11-coupled receptors. On the other hand there are considerable discrepancies regarding the precise activation mechanism in response to PLC stimulation and with respect to their channel properties (e.g., permeability), even if homomeric TRPC proteins are analyzed (Cavalie, 2007). The differences may result from the expression system used and thereby emphasize the need to analyze the action of individual TRPC proteins in the context of their native environment. This can be exemplified by studies in mice with inactivation of the Trpc4 gene. Depending on the cell type studied, channels with completely different characteristics are impaired in Trpc4-deficient mice: deletion of the Trpc4 gene results in 95% reduction of acetylcholine (ACh)-triggered store-operated channels with a Ca2+ over Na+ selectivity of >100 in endothelial cells whereas in ileal smooth muscle cells the loss of TRPC4 leads to an 80% reduction of ACh-evoked currents which are predominantly carried by Na+ and activated by PIP2 breakdown rather than by store depletion (Freichel et al., 2001; Tsvilovskyy et al., 2009). These results show that TRPC gating and function cannot necessarily be extrapolated across cell types.
TRPC3, TRPC6, and TRPC7 when expressed in heterologous systems are potentiated by Gq/11-coupled receptors or by direct application of diacylglycerol (DAG) analogs (Hofmann et al., 1999). Interestingly, the density of inward currents evoked by a DAG derivate were significantly higher in smooth muscle cells isolated from Trpc6−/− mice. This was explained by formation of TRPC3 homo-oligomeric channel complexes in TRPC6-deficient smooth muscle cells, because mRNA expression of Trpc3 appears to be up-regulated two- to threefold in cells of Trpc6−/− mice (Dietrich et al., 2005). This example demonstrates that the analysis of cation channels consisting of TRPC proteins is further aggravated by the fact that inactivation of a given TRP protein may be compensated by up- or down-regulation of other genes including physically or functionally interacting TRP genes. Time-dependent inactivation strategies may be used in such cases, or compound knock-out mice in which all redundant TRP proteins are inactivated simultaneously.
Trpc2 is a pseudogene in humans, but its rodent ortholog encodes a functional TRPC2 channel important to pheromone sensing in vomeronasal organ, where it can be directly activated by DAG (Lucas et al., 2003).
Expression of Trpc genes was found in various types of mast cells (Table 2). TRPC1 has been detected in murine BMMC on mRNA (Sanchez-Miranda et al., 2010; Suzuki et al., 2010) and protein (Hernandez-Hansen et al., 2004) level, also in the rat cell line RBL-2H3 (Ma et al., 2008) and using a microarray expression analysis in human skin mast cells (Bradding et al., 2003). In RBL-2H3 cells, knockdown of TRPC1 and TRPC3 proteins through expression of corresponding shRNAs decreased the cells sensitivity to antigen-stimulation and shifted the Ca2+ wave initiation site from the tips of extended cell protrusions to the cell body (Cohen et al., 2009). Interestingly, in mice deficient for the Src family kinase Fyn expression of TRPC1 proteins was reduced by ∼30%, and cation currents, depolymerization of cortical F-actin and degranulation triggered by FcεRI-stimulation was significantly reduced whereas mast cell degranulation evoked by ATP, substance P, or thrombin was unaffected. Similar effects were observed by downregulation of TRPC1 expression using siRNAs against TRPC1 and the deficits in FcεRI-triggered mast cell activation could be rescued by exogenous expression of TRPC1 (Suzuki et al., 2010). Trpc2 mRNA has been reported in mouse BMMC using RT-PCR (Ma et al., 2008). Expression of TRPC3 was reported in mouse BMMC via Western Blot (Hernandez-Hansen et al., 2004) and RT-PCR (Sanchez-Miranda et al., 2010) and with both methods in the cell lines L138.8A and RBL-2H3 (Ma et al., 2008). In BMMCs, transcripts of Trpc4 and Trpc5 (Suzuki et al., 2010) and TRPC4 proteins (Hernandez-Hansen et al., 2004) were found. TRPC5 transcript and protein expression was reported in RBL-2H3 cells (Ma et al., 2008). shRNA-mediated knock down of TRPC5 in RBL-2H3 cells was associated with a reduced Ca2+ entry following depletion of intracellular Ca2+ stores and based on over-expression experiments in these cells it was proposed that TRPC5 associates with Stim1 and Orai1 in a stoichiometric manner to build Ca2+ and Sr2+ permeable channels that can be discriminated from channels made by Orai1 and Stim1 (Ma et al., 2008). However, receptor-mediated Ca2+ entry or membrane currents have not been analysed in this study. Trpc6 and Trpc7 transcripts and proteins were also found in mouse BMMC (Hernandez-Hansen et al., 2004; Sanchez-Miranda et al., 2010) and based on immunoprecipitation experiments using an antibody that was designed to detect all three members of the TRPC3/TRPC6/TRPC7 subfamily it was proposed that TRPC3/TRPC6/TRPC7, like TRPC1 (Suzuki et al., 2010), interact with fyn kinase during FcεRI-mediated mast cell activation (Sanchez-Miranda et al., 2010).
TRPV Channels
Similar to the TRPC family, the TRPV (vanilloid) family can be divided into two subfamilies on the basis of structure, function and Ca2+ selectivity: TRPV1-4, and TRPV5/6. In the TRPV subfamily, TRPV5 and TRPV6 can form heteromeric channel complexes (Hoenderop et al., 2003b; Hellwig et al., 2005; Schaefer, 2005). Furthermore, TRPV1 can associate with TRPV2 and TRPV3 (Cheng et al., 2007) and widespread interaction has been shown for TRPV1-TRPV4 (Cheng et al., 2007).
TRPV1, TRPV2, TRPV3, and TRPV4 are non-selective cation channels that are activated by a different range in temperatures, respectively, and by numerous other stimuli (Table 1). In addition, TRPV1 could be activated by low pH (Caterina et al., 1997; Jordt et al., 2000) and by vanilloid compounds, such as capsaicin and capsinate found in hot (chili) and non-pungent (bell) peppers, respectively (Caterina et al., 1997; Iida et al., 2003).
TRPV2 (like TRPV4) is activated by osmotic cell swelling and has a critical role in macrophage particle binding and phagocytosis (Link et al., 2010). TRPV3 is activated by a variety of botanical compounds (Moqrich et al., 2005). TRPV4 is sensitive to osmotic and mechanical stimuli, such as cell swelling or fluid flow. It could be activated by arachidonic acid metabolite 5′, 6′-epoxyeicosatrienoic acid (5′, 6′-EET; Vriens et al., 2005) or by 4α-Phorbol 12,13-Didecanoate (Watanabe et al., 2002).
TRPV5 and TRPV6 are the only highly Ca2+-selective channels in the TRP channel family. They are not heat-sensitive and tend to be active at low [Ca2+]i concentrations and physiological membrane potentials (Vennekens et al., 2000). Both proteins form constitutively active channels when heterologously expressed in different cell lines, but similar channel activity has never been recorded in the primary cell types that express TRPV5 or TRPV6, respectively. TRPV5 is essential for Ca2+ reabsorption in the kidney (Hoenderop et al., 2003a), and TRPV6 proteins determine Ca2+-absorption in the epididymal epithelium and, thereby, sperm function and male fertility (Weissgerber et al., 2011).
All members of the TRPV proteins were reported to be expressed in various mast cell models except TRPV3 and TRPV5. For example, transcripts of Trpv1, Trpv2, Trpv4, and Trpv6 were identified in RBL-2H3 cells (Stokes et al., 2004), Trpv1, Trpv2, and Trpv6 in the human cell line HMC-1 (Zhang et al., 2011) and TRPV1 proteins in mast cells of the human bladder (Lazzeri et al., 2004) and skin (Stander et al., 2004). The TRPV1 specific agonists capsaicin or resiniferatoxin induced calcium uptake in several mouse mast cell lines, in BMMC, but not in PMC (Biro et al., 1998).
Treatment of BMMCs with ruthenium red, that is used as non-specific inhibitor of TRPV channels (Vriens et al., 2009), inhibited FcεeRI-mediated increase of cytosolic Ca2+ concentration (Lam et al., 2008). Mast cells are not only activated by specific allergens but also by various physical stimuli, e.g., rubbing, pressure, cold, heat which induce physical urticaria (Grabbe, 2001). These processes might be mediated through mechano- or thermosensitive TRP channels. Since ruthenium red inhibited the elevation of [Ca2+]i and subsequent histamine release in RBL-2H3 cells induced by shear stress (Yang et al., 2009) and temperature increase (Stokes et al., 2004) or after irradiation with soft power lasers (Yang et al., 2007), a role of TRPV proteins was proposed in the above mast cell activating pathways. As Ruthenium Red inhibits several other channels in addition to TRPV channels, this concept requires validation by independent approaches.
Transcripts of Trpv6 (formerly designated as Ca2+ transport protein 1, CaT1) have been detected in RBL-2H3 cells (Stokes et al., 2004). It had been proposed that TRPV6 comprises all or part of the CRAC pore (Yue et al., 2001) but later it was shown that TRPV6 expressed in HEK cells and CRAC in RBL-2H3 cells exhibit many differences in biophysical properties demonstrating that the pores of TRPV6 and CRAC are not identical (Voets et al., 2001). In this line, treatment of RBL-2H3 cells with antisense and siRNA probes directed against Trpv6 transcripts, respectively, does not affect endogenous CRAC currents corroborating that TRPV6 is not a component of the native CRAC current in RBL mast cells. Nevertheless, expression of amino-terminal TRPV6 fragments, which are able to suppress currents through over-expressed TRPV6 channels, substantially suppressed activation of endogenous CRAC (Kahr et al., 2004).
TRPM Channels
The members of the TRPM (melastatin) family are divided into four groups: TRPM1/3, TRPM2/8, TRPM4/5, and TRPM 6/7. For TRPM channels, heteromerization has only been reported for TRPM6 and TRPM7 so far (Chubanov et al., 2004, 2005; Li et al., 2006; Jiang, 2007). The Ca2+ permeability of channels formed by TRPM proteins ranges from monovalent selective (TRPM4 and TRPM5) to highly Ca2+ permeable (TRPM3α2, TRPM6, and TRPM7).
The founding member of this subfamily, Trpm1 (initially termed melastatin), was initially identified as a gene that is downregulated in highly metastatic melanoma cells (Duncan et al., 1998). TRPM1 proteins reside in intracellular organelles and do not reach the plasma membrane upon heterologous expression, but form non-selective currents when expressed in SK-Mel22a melanoma cells (Oancea et al., 2009). TRPM1 is activated by the mGluR6 signaling cascade and thus is required for the depolarizing light response in ON bipolar cells (Morgans et al., 2009) and mutations in the Trpm1 gene are associated with congenital stationary night blindness (CSNB) disease in humans (Li et al., 2009).
TRPM3 proteins are able to form constitutively active cation channels. Various splice variants are expressed from the Trpm3 gene with TRPM3α1 being poorly permeable for divalent cations, whereas TRPM3α2-induced channels conduct Ca2+ and Mg2+. The steroid hormone pregnenolone sulfate may act as endogenous ligand for TRPM3 (Wagner et al., 2008).
TRPM2 is activated by ADP-ribose (EC50, 100 μM) and activated by H2O2 and under conditions of ROS production (Hara et al., 2002; Perraud et al., 2005), and deletion of Trpm2 leads to decreased reactive oxygen species-induced chemokine production in monocytes (Yamamoto et al., 2008). Recently, Di et al. (2011) showed that deletion of Trpm2 increases ROS production in phagocytes and introduced the concept that TRPM2-mediated cation entry and subsequent membrane depolarization functions as an inhibitory feedback mechanism for ROS production in these cells.
TRPM4 and TRPM5 are (together with TRPM3α1) the only monovalent-selective ion channels of the TRP family (Launay et al., 2002; Hofmann et al., 2003). TRPM4, but not TRPM5, is inhibited by intracellular ATP, whereas TRPM5 is inhibited by intracellular acidic pH. Both are activated by an increase in Ca2+ levels in the cytosol, but the sensitivity to [Ca2+]i as determined by different research groups varies greatly (Vennekens and Nilius, 2007). Studies in Trpm4−/− mice reveal a critical role for TRPM4 proteins in mast cell activation (see below). Moreover, these mice exhibited high blood pressure due to elevated release of catecholamines (Mathar et al., 2010). Trpm5−/− mice show abolished sweet, umami, and bitter taste reception (Zhang et al., 2003) and impaired glucose-induced insulin secretion (Colsoul et al., 2010).
TRPM6 and TRPM7 are unique among ion channels because they possess both ion channel and protein kinase activities. Channels formed by these proteins allow Mg2+ and Ca2+ entry into the cell and are inhibited by intracellular Mg2+ (0.3–1.0 mM; Nadler et al., 2001; Voets et al., 2004b). TRPM6 is primarily expressed in kidney and intestine, where it has been suggested to be responsible for epithelial Mg2+ reabsorption (Schlingmann et al., 2002). TRPM7 is ubiquitously expressed and deletion of the Trpm7 gene leads to embryonic lethality (Jin et al., 2008; Weissgerber et al., 2008).
Like TRPM1, TRPM8 was originally identified in a screen of cancer-related genes (Tsavaler et al., 2001). It can be activated by cold (8–28°C) and enhanced by cooling compounds such as menthol and icilin (McKemy et al., 2002) and Trpm8−/− mice show deficits in their ability to discriminate between cold and warm surfaces (Bautista et al., 2007). Temperature modulates the voltage dependence of the channel, and menthol and icilin mimick this effect (Voets et al., 2004a).
Expression of TRPM1, TRPM3, TRPM5, and TRPM6 has not been reported in mast cell models, while the other members of TRPM subfamily play important roles in mast cell functions. Trpm2 transcripts were identified in human lung mast cells and cord blood derived mast cells in a microarray expression analysis (Bradding et al., 2003). In mouse BMMC, Trpm4 transcripts were detected using Northern blot analysis and the 138 kDa TRPM4 proteins were identified in BMMCs of wild type but not of Trpm4−/− mice (Vennekens et al., 2007). After preabsorbtion of the same anti-TRPM4 antibody using microsomal membrane protein fractions from BMMCs of TRPM4−/− mice a specific staining of TRPM4 proteins in connective tissue mast cells in skin sections could be achieved. TRPM4 proteins, similarly like TRPM5 proteins, act as Ca2+-activated non-selective cation channels and critically determine the driving force for Ca2+ influx into cells (Launay et al., 2004). It could be shown that TRPM4 channels depolarize the membrane following adenosine- and FcεRI-stimulation and, thereby, critically decrease the Ca2+ influx in BMMC’s via CRAC channels. Accordingly, activated Trpm4−/− BMMCs release excessive histamine, leukotrienes and tumor necrosis factor (TNF), and Trpm4−/− animals display a more severe acute anaphylactic response in the skin compared to wild-type controls indicating that TRPM4 channel activation is an efficient mechanism for limiting antigen-induced mast cell activation (Vennekens et al., 2007). Additionally, antigen- and SCF-induced migration of BMMCs is largely diminished in the absence of TRPM4, and F-actin formation is reduced in DNP-HSA-stimulated BMMCs from Trpm4−/− mice (Shimizu et al., 2009). At this point it has to be mentioned that the increased [Ca2+]i elevation observed upon FcεRI-stimulation in TRPM4-deficient mice led to increased release of TNF-α but not of IL-6. It is known, that release of both mast cell mediators is calcium-dependent, but an explanation for this difference may be that transcription and/or production of IL-6 may already be saturated under these conditions of FcεRI-stimulation and could not be further stimulated by the additional increase of [Ca2+]i in TRPM4-deficient BMMCs.
Expression of TRPM7 has been found in human lung mast cells and the human cell line LAD-2 (Wykes et al., 2007) as well as in RBL-2H3 cells (Stokes et al., 2004) and Mg2+-inhibited currents (MIC), which can be mediated by TRPM7 proteins, were identified in RBL-2H3 cells, HMC-1 cells, and human lung mast cells. Downregulation of TRPM7 expression in HMC-1 cells and human lung mast cells resulted in significant reduction of MIC currents and mast cell survival which could not be rescued by an increase in the extracellular Mg2+ concentration (Wykes et al., 2007). In addition, TRPM7 currents in RBL-2H3 cells might be involved in Ca2+ and Mg2+ entry during cell cycle regulation since it could be shown that MIC currents were strongly upregulated specifically in the G1 phase of the cell cycle to meet cellular demands for Ca2+ and/or Mg2+ fluxes during this stage of cell division (Tani et al., 2007). Trpm7-deficient mice as well as mice homozygous for an allele lacking the kinase domain of TRPM7 (Trpm7Δkinase/Δkinase) die early during development (Jin et al., 2008; Weissgerber et al., 2008; Ryazanova et al., 2010), but Trpm7+/Δkinase are viable and MIC currents are significantly reduced in PMCs of these mice (Ryazanova et al., 2010). However, the consequences of MIC current reduction for mast cell activation have not been reported so far.
TRPM8 proteins, which form Ca2+-conducting cation channels activated by menthol or cold, have also been proposed as mediators of mast cell activation, e.g., in the development of cold urticaria. However, Medic et al. (2011) found no deficits in mast cell activation using Ca2+ imaging, mast cell degranulation and development of passive anaphylaxis in Trpm8−/− mice, which makes a role of TRPM8 for mast cell activation in cold urticaria unlikely.
TRPA1 Channels
TRPA1 is the only member of the TRPA (ankyrin) family characterized by the 14 amino-terminal ankyrin repeats (Story et al., 2003). Its expression in hair cells led to the hypothesis that it forms an auditory mechanotransduction channel (Corey et al., 2004), but this concept could not be supported sufficiently as, e.g., Trpa1−/− mice exhibit no overt vestibular deficits and auditory responses are completely normal (Bautista et al., 2006; Kwan et al., 2006). TRPA1 was reported to be activated by noxious cold but the thermosensitivity (Story et al., 2003; Corey et al., 2004) of TRPA1 is also debated. TRPA1 is activated by various chemicals (Bandell et al., 2004) including allyl isothiocyanate (the pungent compound in mustard oil), allicin (from garlic), cinnamaldehyde (from cinnamon), menthol (from mint), tetrahydrocannabinol (from marijuana), nicotine (from tobacco), and bradykinin (see Table 1). Recently it was reported that protein kinase A/phospholipase C-mediated trafficking to the plasma membrane contributes to TRPA1 activation (Schmidt et al., 2009).
TRPA1 has been detected in mast cells so far, but in resting mast cells it was predominantly localized in intracellular vesicular structures and interacts with secretogranin III, a protein involved in secretory granule biogenesis in mast cells, implicating that TRPA1 might play an alternative role to the regulation of cation entry across the plasma membrane in mast cells compared to other cell types (Turner et al., 2007; Prasad et al., 2008).
TRPML and TRPP Channels
The TRPML (mucolipin) family contains three mammalian members: TRPML1, TRPML2, and TRPML3. The TRPML proteins show only low homology with the other TRP channels and are comparatively shorter. Heterologously expressed TRPML proteins can interact with each other (Venkatachalam et al., 2006; Curcio-Morelli et al., 2010). Trpml1 was first identified by a positional cloning strategy as the gene mutated in patients suffering from Mucolipidosis type IV (MLIV; Bargal et al., 2000), TRPML3 was discovered as the channel mutated in varitint-waddler mice, characterized by a variegated coat color, vestibular defects, hyperactivity, and embryonic lethality (Xu et al., 2007). TRPML1 and TRPML2 are ubiquitously expressed and localize primarily to the lysosomal and late endosomal compartments (Manzoni et al., 2004; Puertollano and Kiselyov, 2009). Recently, Dong et al. developed a method allowing the measurement of ion currents directly in endolysosomes. This was achieved by treatment of the cells with Vacuolin-1, leading to an increase of diameter of the endolysosomes from 0.1–0.5 to 2–3 μM which makes them accessible for patch-clamp measurements. In this way, it could be shown that expressed TRPML1 proteins form constitutively active cation channels which, besides Na+ and Ca2+, can conduct several other cations, e.g., Mn2+, Zn2+, and Fe2+ out of the lumen of the organelles into the cytosol. The mechanism leading to activation of TRPML1 is still unclear (Dong et al., 2008, 2010).
The TRPP (polycystin) family comprises eight members, from which only PKD2 (TRPP2, PC2, polycystin-2), PKD2-L1 (TRPP3, polycystin-L), and PKD2-L2 (TRPP5, polycystin-L2) are shown to be channels. TRPP2 is reported to form a Ca2+-permeable cation channel in the plasma membrane that can be activated by downstream of G protein-coupled receptor and/or receptor-tyrosine kinase at the cell (Ma et al., 2005), but was also shown to form a Ca2+ release channel in the ER (Wegierski et al., 2009). TRPP3 is reported to form Ca2+-permeable non-selective cation channels with a large single channel conductance modulated by pH (Chen et al., 1999; Huang et al., 2006). TRPP5 is thought to form a Ca2+-permeable non-selective cation channel (Guo et al., 2000). As indicated above, individual members of TRP subfamilies are able to interact. However, TRPP2 was the first example showing that heteromultimerization cannot only occur between members of the same subfamily since interaction of TRPP2 was reported with TRPC1 (Tsiokas et al., 1999; Bai et al., 2008; Kobori et al., 2009; Zhang et al., 2009) or with TRPV4 (Kottgen et al., 2008; Stewart et al., 2010). Recently, also another example of interaction of TRP proteins of different subfamilies, i.e., TRPV4 with TRPC1, has been described (Ma et al., 2010). It has to be mentioned that the nomenclature of TRPP proteins was changed recently (Clapham et al., 2012), and TRPP2 is now designated TRPP1, TRPP3 is now TRPP2, and TRPP5 is now TRPP3. Until now, members of the TRPML- or TRPP-channel subfamily were not identified in mast cells.
Mechanisms: How TRP Channels Regulate Changes in [Ca2+]i and Mast Cell Activation
Taken together, TRP channels were found to influence cellular Ca2+ signaling by several mechanisms. First, by conducting Ca2+ ions to various degrees TRP channels directly contribute to Ca2+ influx via the plasma membrane. TRP channels with high calcium selectivity are TRPV5 and TRPV6 (Vennekens et al., 2000), but also non-selective TRP channels composed of, e.g., TRPC1 (Suzuki et al., 2010) or TRPC5 (Ma et al., 2008) may contribute in this way. Second, by conducting Na+ TRP channels mediate electrogenic effects through plasma membrane depolarization which may have opposite consequence in different cell types: in contrast to excitable cells, where TRP-mediated Na+ entry and depolarization can enhance Ca2+ entry by gating of voltage-operated Ca2+ channels (Tsvilovskyy et al., 2009), they can do the opposite in non-excitable cells such as mast cells; here a significant part of stimulated Ca2+ entry enters the cell via inwardly rectifying CRAC channels, and TRP-mediated Na+ entry and subsequent depolarization reduces the driving force for Ca2+ entry by shifting the membrane potential toward more positive potentials (Vennekens et al., 2007). A counteracting mechanism by a Ca2+ activated K+ channel leading to membrane hyperpolarization and an increase of the driving force for Ca2+ entry following FcεRI-stimulation was shown for SK4 (KCa3.1) proteins (Shumilina et al., 2008) emphasizing the relevance of this regulatory principle for adjusting [Ca2+]i and activation of mast cells. Third, TRP channels can be activated or inhibited by themselves by Ca2+ which further contributes to the complexity of regulation of [Ca2+]i. TRP channels that are activated by Ca2+ include TRPC1, TRPC4, TRPC5, TRPC6, TRPV4, TRPM2, TRPA1, TRPM4, and TRPM5. Also many TRP channels are modulated by Ca2+ via complex signaling cascades including Ca2+/calmodulin binding, Ca2+-dependent modulation of phospholipase C, and Ca2+-dependent activation of PKC. Fourth, there is emerging evidence that several TRP channels are also located in the membrane of the sarco/endoplasmic reticulum (SR/ER), endosomes, lysosomes, or other intracellular vesicles where they can serve as Ca2+ release channels and conduct Ca2+ from the luminal stores into the cytoplasm which may affect specific mast cell functions; for instance, it was reported that TRPV1, TRPM8, and TRPP2 can form Ca2+-conducting channels in the SR/ER, and TRPML1, TRPM2, and TRPV2 may function as Ca2+-release channels in endo-/lysosomes (for more details see Dong et al., 2010; Gees et al., 2010). Finally, it has to be considered that many studies about Ca2+-dependent mast cell functions analysed measurements of global cytoplasmic Ca2+ concentration rather than the nature of the Ca2+ signals, e.g., differences in the frequency of transient episodic Ca2+ elevations (Ca2+ spikes/oscillations) and subcellular localization and direction of Ca2+ signals such as Ca2+ waves which may be crucial for distinct mast cell functions. In this line, a threshold of Ca2+ elevation in the vicinity of store-operated Ca2+ channels is apparently necessary to induce nuclear NFAT translocation as a parameter for activation of gene expression (Kar et al., 2011, 2012). In this case the signal triggered by this localized Ca2+ entry pathway might be achieved by spatially sequestered calmodulin molecules as mediators. Likewise, it would not be surprising if also a specific localization pattern of certain TRP channel proteins and their dynamic change during mast cell activation could be identified in the future as a mechanism that explains a specific pattern of mast cell activation such as activation of individual transcription factors or release of a defined set of mast cell mediators.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft including the Schwerpunktprogramm 1394 (“Mast cells – promotors of health and modulators of disease,” SPP 1394).
References
Aarts, M., Iihara, K., Wei, W. L., Xiong, Z. G., Arundine, M., Cerwinski, W., Macdonald, J. F., and Tymianski, M. (2003). A key role for TRPM7 channels in anoxic neuronal death. Cell 115, 863–877.
Ali, H., Maeyama, K., Sagi-Eisenberg, R., and Beaven, M. A. (1994). Antigen and thapsigargin promote influx of Ca2+ in rat basophilic RBL-2H3 cells by ostensibly similar mechanisms that allow filling of inositol 1,4,5-trisphosphate-sensitive and mitochondrial Ca2+ stores. Biochem. J. 304(Pt 2), 431–440.
Bai, C. X., Giamarchi, A., Rodat-Despoix, L., Padilla, F., Downs, T., Tsiokas, L., and Delmas, P. (2008). Formation of a new receptor-operated channel by heteromeric assembly of TRPP2 and TRPC1 subunits. EMBO Rep. 9, 472–479.
Bandell, M., Story, G. M., Hwang, S. W., Viswanath, V., Eid, S. R., Petrus, M. J., Earley, T. J., and Patapoutian, A. (2004). Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 41, 849–857.
Bargal, R., Avidan, N., Ben-Asher, E., Olender, Z., Zeigler, M., Frumkin, A., Raas-Rothschild, A., Glusman, G., Lancet, D., and Bach, G. (2000). Identification of the gene causing mucolipidosis type IV. Nat. Genet. 26, 118–123.
Bautista, D. M., Jordt, S. E., Nikai, T., Tsuruda, P. R., Read, A. J., Poblete, J., Yamoah, E. N., Basbaum, A. I., and Julius, D. (2006). TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 124, 1269–1282.
Bautista, D. M., Siemens, J., Glazer, J. M., Tsuruda, P. R., Basbaum, A. I., Stucky, C. L., Jordt, S. E., and Julius, D. (2007). The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 448, 204–208.
Beaven, M. A., Rogers, J., Moore, J. P., Hesketh, T. R., Smith, G. A., and Metcalfe, J. C. (1984). The mechanism of the calcium signal and correlation with histamine release in 2H3 cells. J. Biol. Chem. 259, 7129–7136.
Biro, T., Maurer, M., Modarres, S., Lewin, N. E., Brodie, C., Acs, G., Acs, P., Paus, R., and Blumberg, P. M. (1998). Characterization of functional vanilloid receptors expressed by mast cells. Blood 91, 1332–1340.
Blank, U., and Rivera, J. (2004). The ins and outs of IgE-dependent mast-cell exocytosis. Trends Immunol. 25, 266–273.
Bradding, P., Okayama, Y., Kambe, N., and Saito, H. (2003). Ion channel gene expression in human lung, skin, and cord blood-derived mast cells. J. Leukoc. Biol. 73, 614–620.
Buess, M., Engler, O., Hirsch, H. H., and Moroni, C. (1999). Search for oncogenic regulators in an autocrine tumor model using differential display PCR: identification of novel candidate genes including the calcium channel mtrp6. Oncogene 18, 1487–1494.
Caterina, M. J., Schumacher, M. A., Tominaga, M., Rosen, T. A., Levine, J. D., and Julius, D. (1997). The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389, 816–824.
Chang, W. C., Nelson, C., and Parekh, A. B. (2006). Ca2+ influx through CRAC channels activates cytosolic phospholipase A2, leukotriene C4 secretion, and expression of c-fos through ERK-dependent and -independent pathways in mast cells. FASEB J. 20, 2381–2383.
Chen, X. Z., Vassilev, P. M., Basora, N., Peng, J. B., Nomura, H., Segal, Y., Brown, E. M., Reeders, S. T., Hediger, M. A., and Zhou, J. (1999). Polycystin-L is a calcium-regulated cation channel permeable to calcium ions. Nature 401, 383–386.
Cheng, W., Yang, F., Takanishi, C. L., and Zheng, J. (2007). Thermosensitive TRPV channel subunits coassemble into heteromeric channels with intermediate conductance and gating properties. J. Gen. Physiol. 129, 191–207.
Cheng, X., Shen, D., Samie, M., and Xu, H. (2010). Mucolipins: intracellular TRPML1-3 channels. FEBS Lett. 584, 2013–2021.
Cho, Y., Jang, Y., Yang, Y. D., Lee, C. H., Lee, Y., and Oh, U. (2010). TRPM8 mediates cold and menthol allergies associated with mast cell activation. Cell Calcium 48, 202–208.
Chubanov, V., Mederos, Y., Schnitzler, M., Waring, J., Plank, A., and Gudermann, T. (2005). Emerging roles of TRPM6/TRPM7 channel kinase signal transduction complexes. Naunyn Schmiedebergs Arch. Pharmacol. 371, 334–341.
Chubanov, V., Waldegger, S., Mederos, Y., Schnitzler, M., Vitzthum, H., Sassen, M. C., Seyberth, H. W., Konrad, M., and Gudermann, T. (2004). Disruption of TRPM6/TRPM7 complex formation by a mutation in the TRPM6 gene causes hypomagnesemia with secondary hypocalcemia. Proc. Natl. Acad. Sci. U.S.A. 101, 2894–2899.
Clapham, D., Nilius, B., and Owsianik, G. (2012). Transient Receptor Potential Channels [Online]. [accessed March 5, 2012]. Available at: http://www.iuphar-db.org/DATABASE/FamilyMenuForward?familyId=78
Clapham, D. E., Julius, D., Montell, C., and Schultz, G. (2005). International union of pharmacology. XLIX. Nomenclature and structure-function relationships of transient receptor potential channels. Pharmacol. Rev. 57, 427–450.
Cohen, R., Torres, A., Ma, H. T., Holowka, D., and Baird, B. (2009). Ca2+ waves initiate antigen-stimulated Ca2+ responses in mast cells. J. Immunol. 183, 6478–6488.
Colsoul, B., Schraenen, A., Lemaire, K., Quintens, R., Van Lommel, L., Segal, A., Owsianik, G., Talavera, K., Voets, T., Margolskee, R. F., Kokrashvili, Z., Gilon, P., Nilius, B., Schuit, F. C., and Vennekens, R. (2010). Loss of high-frequency glucose-induced Ca2+ oscillations in pancreatic islets correlates with impaired glucose tolerance in Trpm5-/- mice. Proc. Natl. Acad. Sci. U.S.A. 107, 5208–5213.
Corey, D. P., Garcia-Anoveros, J., Holt, J. R., Kwan, K. Y., Lin, S. Y., Vollrath, M. A., Amalfitano, A., Cheung, E. L., Derfler, B. H., Duggan, A., Geleoc, G. S., Gray, P. A., Hoffman, M. P., Rehm, H. L., Tamasauskas, D., and Zhang, D. S. (2004). TRPA1 is a candidate for the mechanosensitive transduction channel of vertebrate hair cells. Nature 432, 723–730.
Curcio-Morelli, C., Zhang, P., Venugopal, B., Charles, F. A., Browning, M. F., Cantiello, H. F., and Slaugenhaupt, S. A. (2010). Functional multimerization of mucolipin channel proteins. J. Cell. Physiol. 222, 328–335.
Di, A., Gao, X. P., Qian, F., Kawamura, T., Han, J., Hecquet, C., Ye, R. D., Vogel, S. M., and Malik, A. B. (2011). The redox-sensitive cation channel TRPM2 modulates phagocyte ROS production and inflammation. Nat. Immunol. 13, 29–34.
Di Capite, J., and Parekh, A. B. (2009). CRAC channels and Ca2+ signaling in mast cells. Immunol. Rev. 231, 45–58.
Dietrich, A., Mederos, Y. S. M., Gollasch, M., Gross, V., Storch, U., Dubrovska, G., Obst, M., Yildirim, E., Salanova, B., Kalwa, H., Essin, K., Pinkenburg, O., Luft, F. C., Gudermann, T., and Birnbaumer, L. (2005). Increased vascular smooth muscle contractility in TRPC6-/- mice. Mol. Cell. Biol. 25, 6980–6989.
Dong, X. P., Cheng, X., Mills, E., Delling, M., Wang, F., Kurz, T., and Xu, H. (2008). The type IV mucolipidosis-associated protein TRPML1 is an endolysosomal iron release channel. Nature 455, 992–996.
Dong, X. P., Wang, X., and Xu, H. (2010). TRP channels of intracellular membranes. J. Neurochem. 113, 313–328.
Duncan, L. M., Deeds, J., Hunter, J., Shao, J., Holmgren, L. M., Woolf, E. A., Tepper, R. I., and Shyjan, A. W. (1998). Down-regulation of the novel gene melastatin correlates with potential for melanoma metastasis. Cancer Res. 58, 1515–1520.
Falcone, D., and Fewtrell, C. (1995). Ca(2+)-ATPase inhibitor, cyclopiazonic acid, releases Ca2+ from intracellular stores in RBL-2H3 mast cells and activates a Ca2+ influx pathway that is permeable to sodium and manganese. J. Cell. Physiol. 164, 205–213.
Fasolato, C., Hoth, M., Matthews, G., and Penner, R. (1993). Ca2+ and Mn2+ influx through receptor-mediated activation of nonspecific cation channels in mast cells. Proc. Natl. Acad. Sci. U.S.A. 90, 3068–3072.
Feske, S., Gwack, Y., Prakriya, M., Srikanth, S., Puppel, S. H., Tanasa, B., Hogan, P. G., Lewis, R. S., Daly, M., and Rao, A. (2006). A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature 441, 179–185.
Foreman, J. C., Hallett, M. B., and Mongar, J. L. (1977). Movement of strontium ions into mast cells and its relationship to the secretory response. J. Physiol. (Lond.) 271, 233–251.
Foreman, J. C., and Mongar, J. L. (1972). The role of the alkaline earth ions in anaphylactic histamine secretion. J. Physiol. (Lond.) 224, 753–769.
Freichel, M., and Flockerzi, V. (2007). Biological functions of TRPs unravelled by spontaneous mutations and transgenic animals. Biochem. Soc. Trans. 35, 120–123.
Freichel, M., Kriebs, U., Vogt, D., Mannebach, S., and Weißgerber, P. (2011). “Strategies and protocols to generate mouse models with targeted mutations to analyze TRP channel functions,” in TRP Channels, ed. M. Zhu (Boca Raton: CRC Press), 167–193.
Freichel, M., Suh, S. H., Pfeifer, A., Schweig, U., Trost, C., Weissgerber, P., Biel, M., Philipp, S., Freise, D., Droogmans, G., Hofmann, F., Flockerzi, V., and Nilius, B. (2001). Lack of an endothelial store-operated Ca2+ current impairs agonist-dependent vasorelaxation in TRP4-/- mice. Nat. Cell Biol. 3, 121–127.
Galli, S. J., Nakae, S., and Tsai, M. (2005). Mast cells in the development of adaptive immune responses. Nat. Immunol. 6, 135–142.
Gees, M., Colsoul, B., and Nilius, B. (2010). The role of transient receptor potential cation channels in Ca2+ signaling. Cold Spring Harb. Perspect. Biol. 2, a003962.
Gilfillan, A. M., and Tkaczyk, C. (2006). Integrated signalling pathways for mast-cell activation. Nat. Rev. Immunol. 6, 218–230.
Goel, M., Sinkins, W. G., and Schilling, W. P. (2002). Selective association of TRPC channel subunits in rat brain synaptosomes. J. Biol. Chem. 277, 48303–48310.
Grabbe, J. (2001). Pathomechanisms in physical urticaria. J. Investig. Dermatol. Symp. Proc. 6, 135–136.
Gri, G., Piconese, S., Frossi, B., Manfroi, V., Merluzzi, S., Tripodo, C., Viola, A., Odom, S., Rivera, J., Colombo, M. P., and Pucillo, C. E. (2008). CD4+CD25+ regulatory T cells suppress mast cell degranulation and allergic responses through OX40-OX40L interaction. Immunity 29, 771–781.
Gross, S. A., Wissenbach, U., Philipp, S. E., Freichel, M., Cavalie, A., and Flockerzi, V. (2007). Murine ORAI2 splice variants form functional Ca2+ release-activated Ca2+ (CRAC) channels. J. Biol. Chem. 282, 19375–19384.
Guo, L., Schreiber, T. H., Weremowicz, S., Morton, C. C., Lee, C., and Zhou, J. (2000). Identification and characterization of a novel polycystin family member, polycystin-L2, in mouse and human: sequence, expression, alternative splicing, and chromosomal localization. Genomics 64, 241–251.
Gwack, Y., Srikanth, S., Oh-Hora, M., Hogan, P. G., Lamperti, E. D., Yamashita, M., Gelinas, C., Neems, D. S., Sasaki, Y., Feske, S., Prakriya, M., Rajewsky, K., and Rao, A. (2008). Hair loss and defective T- and B-cell function in mice lacking ORAI1. Mol. Cell. Biol. 28, 5209–5222.
Hara, Y., Wakamori, M., Ishii, M., Maeno, E., Nishida, M., Yoshida, T., Yamada, H., Shimizu, S., Mori, E., Kudoh, J., Shimizu, N., Kurose, H., Okada, Y., Imoto, K., and Mori, Y. (2002). LTRPC2 Ca2+-permeable channel activated by changes in redox status confers susceptibility to cell death. Mol. Cell 9, 163–173.
Hardie, R. C., and Minke, B. (1993). Novel Ca2+ channels underlying transduction in Drosophila photoreceptors: implications for phosphoinositide-mediated Ca2+ mobilization. Trends Neurosci. 16, 371–376.
Hartmann, K., Henz, B. M., Kruger-Krasagakes, S., Kohl, J., Burger, R., Guhl, S., Haase, I., Lippert, U., and Zuberbier, T. (1997). C3a and C5a stimulate chemotaxis of human mast cells. Blood 89, 2863–2870.
Hellwig, N., Albrecht, N., Harteneck, C., Schultz, G., and Schaefer, M. (2005). Homo- and heteromeric assembly of TRPV channel subunits. J. Cell Sci. 118, 917–928.
Hernandez-Hansen, V., Smith, A. J., Surviladze, Z., Chigaev, A., Mazel, T., Kalesnikoff, J., Lowell, C. A., Krystal, G., Sklar, L. A., Wilson, B. S., and Oliver, J. M. (2004). Dysregulated FcepsilonRI signaling and altered Fyn and SHIP activities in Lyn-deficient mast cells. J. Immunol. 173, 100–112.
Hoenderop, J. G., Van Leeuwen, J. P., Van Der Eerden, B. C., Kersten, F. F., Van Der Kemp, A. W., Merillat, A. M., Waarsing, J. H., Rossier, B. C., Vallon, V., Hummler, E., and Bindels, R. J. (2003a). Renal Ca2+ wasting, hyperabsorption, and reduced bone thickness in mice lacking TRPV5. J. Clin. Invest. 112, 1906–1914.
Hoenderop, J. G., Voets, T., Hoefs, S., Weidema, F., Prenen, J., Nilius, B., and Bindels, R. J. (2003b). Homo- and heterotetrameric architecture of the epithelial Ca2+ channels TRPV5 and TRPV6. EMBO J. 22, 776–785.
Hofmann, T., Chubanov, V., Gudermann, T., and Montell, C. (2003). TRPM5 is a voltage-modulated and Ca(2+)-activated monovalent selective cation channel. Curr. Biol. 13, 1153–1158.
Hofmann, T., Obukhov, A. G., Schaefer, M., Harteneck, C., Gudermann, T., and Schultz, G. (1999). Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature 397, 259–263.
Hofmann, T., Schaefer, M., Schultz, G., and Gudermann, T. (2002). Subunit composition of mammalian transient receptor potential channels in living cells. Proc. Natl. Acad. Sci. U.S.A. 99, 7461–7466.
Hoth, M., and Penner, R. (1992). Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature 355, 353–356.
Hoth, M., and Penner, R. (1993). Calcium release-activated calcium current in rat mast cells. J. Physiol. (Lond.) 465, 359–386.
Huang, A. L., Chen, X., Hoon, M. A., Chandrashekar, J., Guo, W., Trankner, D., Ryba, N. J., and Zuker, C. S. (2006). The cells and logic for mammalian sour taste detection. Nature 442, 934–938.
Iida, T., Moriyama, T., Kobata, K., Morita, A., Murayama, N., Hashizume, S., Fushiki, T., Yazawa, S., Watanabe, T., and Tominaga, M. (2003). TRPV1 activation and induction of nociceptive response by a non-pungent capsaicin-like compound, capsiate. Neuropharmacology 44, 958–967.
Jiang, L. H. (2007). Subunit interaction in channel assembly and functional regulation of transient receptor potential melastatin (TRPM) channels. Biochem. Soc. Trans. 35, 86–88.
Jin, J., Desai, B. N., Navarro, B., Donovan, A., Andrews, N. C., and Clapham, D. E. (2008). Deletion of Trpm7 disrupts embryonic development and thymopoiesis without altering Mg2+ homeostasis. Science 322, 756–760.
Jordt, S. E., Tominaga, M., and Julius, D. (2000). Acid potentiation of the capsaicin receptor determined by a key extracellular site. Proc. Natl. Acad. Sci. U.S.A. 97, 8134–8139.
Kahr, H., Schindl, R., Fritsch, R., Heinze, B., Hofbauer, M., Hack, M. E., Mortelmaier, M. A., Groschner, K., Peng, J. B., Takanaga, H., Hediger, M. A., and Romanin, C. (2004). CaT1 knock-down strategies fail to affect CRAC channels in mucosal-type mast cells. J. Physiol. (Lond.) 557, 121–132.
Kar, P., Nelson, C., and Parekh, A. B. (2011). Selective activation of the transcription factor NFAT1 by calcium microdomains near Ca2+ release-activated Ca2+ (CRAC) channels. J. Biol. Chem. 286, 14795–14803.
Kar, P., Nelson, C., and Parekh, A. B. (2012). CRAC channels drive digital activation and provide analog control and synergy to Ca(2+)-dependent gene regulation. Curr. Biol. 22, 242–247.
Kim, K. S., Shin, D. H., Nam, J. H., Park, K. S., Zhang, Y. H., Kim, W. K., and Kim, S. J. (2010). Functional expression of TRPV4 cation channels in human mast cell line (HMC-1). Korean J. Physiol. Pharmacol. 14, 419–425.
Kiselyov, K., Xu, X., Mozhayeva, G., Kuo, T., Pessah, I., Mignery, G., Zhu, X., Birnbaumer, L., and Muallem, S. (1998). Functional interaction between InsP3 receptors and store-operated Htrp3 channels. Nature 396, 478–482.
Kobori, T., Smith, G. D., Sandford, R., and Edwardson, J. M. (2009). The transient receptor potential channels TRPP2 and TRPC1 form a heterotetramer with a 2:2 stoichiometry and an alternating subunit arrangement. J. Biol. Chem. 284, 35507–35513.
Kottgen, M., Buchholz, B., Garcia-Gonzalez, M. A., Kotsis, F., Fu, X., Doerken, M., Boehlke, C., Steffl, D., Tauber, R., Wegierski, T., Nitschke, R., Suzuki, M., Kramer-Zucker, A., Germino, G. G., Watnick, T., Prenen, J., Nilius, B., Kuehn, E. W., and Walz, G. (2008). TRPP2 and TRPV4 form a polymodal sensory channel complex. J. Cell Biol. 182, 437–447.
Kuno, M., and Kimura, M. (1992). Noise of secretagogue-induced inward currents dependent on extracellular calcium in rat mast cells. J. Membr. Biol. 128, 53–61.
Kwan, K. Y., Allchorne, A. J., Vollrath, M. A., Christensen, A. P., Zhang, D. S., Woolf, C. J., and Corey, D. P. (2006). TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron 50, 277–289.
Lam, R. S., Shumilina, E., Matzner, N., Zemtsova, I. M., Sobiesiak, M., Lang, C., Felder, E., Dietl, P., Huber, S. M., and Lang, F. (2008). Phosphatidylinositol-3-kinase regulates mast cell ion channel activity. Cell. Physiol. Biochem. 22, 169–176.
Launay, P., Cheng, H., Srivatsan, S., Penner, R., Fleig, A., and Kinet, J. P. (2004). TRPM4 regulates calcium oscillations after T cell activation. Science 306, 1374–1377.
Launay, P., Fleig, A., Perraud, A. L., Scharenberg, A. M., Penner, R., and Kinet, J. P. (2002). TRPM4 is a Ca2+-activated nonselective cation channel mediating cell membrane depolarization. Cell 109, 397–407.
Lazzeri, M., Vannucchi, M. G., Zardo, C., Spinelli, M., Beneforti, P., Turini, D., and Faussone-Pellegrini, M. S. (2004). Immunohistochemical evidence of vanilloid receptor 1 in normal human urinary bladder. Eur. Urol. 46, 792–798.
Lewis, R. S. (2007). The molecular choreography of a store-operated calcium channel. Nature 446, 284–287.
Li, M., Jiang, J., and Yue, L. (2006). Functional characterization of homo- and heteromeric channel kinases TRPM6 and TRPM7. J. Gen. Physiol. 127, 525–537.
Li, Z., Sergouniotis, P. I., Michaelides, M., Mackay, D. S., Wright, G. A., Devery, S., Moore, A. T., Holder, G. E., Robson, A. G., and Webster, A. R. (2009). Recessive mutations of the gene TRPM1 abrogate ON bipolar cell function and cause complete congenital stationary night blindness in humans. Am. J. Hum. Genet. 85, 711–719.
Liao, Y., Erxleben, C., Abramowitz, J., Flockerzi, V., Zhu, M. X., Armstrong, D. L., and Birnbaumer, L. (2008). Functional interactions among Orai1, TRPCs, and STIM1 suggest a STIM-regulated heteromeric Orai/TRPC model for SOCE/Icrac channels. Proc. Natl. Acad. Sci. U.S.A. 105, 2895–2900.
Liao, Y., Erxleben, C., Yildirim, E., Abramowitz, J., Armstrong, D. L., and Birnbaumer, L. (2007). Orai proteins interact with TRPC channels and confer responsiveness to store depletion. Proc. Natl. Acad. Sci. U.S.A. 104, 4682–4687.
Link, T. M., Park, U., Vonakis, B. M., Raben, D. M., Soloski, M. J., and Caterina, M. J. (2010). TRPV2 has a pivotal role in macrophage particle binding and phagocytosis. Nat. Immunol. 11, 232–239.
Lintschinger, B., Balzer-Geldsetzer, M., Baskaran, T., Graier, W. F., Romanin, C., Zhu, M. X., and Groschner, K. (2000). Coassembly of Trp1 and Trp3 proteins generates diacylglycerol- and Ca2+-sensitive cation channels. J. Biol. Chem. 275, 27799–27805.
Liu, C. H., Wang, T., Postma, M., Obukhov, A. G., Montell, C., and Hardie, R. C. (2007). In vivo identification and manipulation of the Ca2 + selectivity filter in the Drosophila transient receptor potential channel. J. Neurosci. 27, 604–615.
Liu, X., Singh, B. B., and Ambudkar, I. S. (2003). TRPC1 is required for functional store-operated Ca2+ channels. Role of acidic amino acid residues in the S5-S6 region. J. Biol. Chem. 278, 11337–11343.
Lucas, P., Ukhanov, K., Leinders-Zufall, T., and Zufall, F. (2003). A diacylglycerol-gated cation channel in vomeronasal neuron dendrites is impaired in TRPC2 mutant mice: mechanism of pheromone transduction. Neuron 40, 551–561.
Ma, H. T., and Beaven, M. A. (2009). Regulation of Ca2+ signaling with particular focus on mast cells. Crit. Rev. Immunol. 29, 155–186.
Ma, H. T., and Beaven, M. A. (2011). Regulators of Ca(2+) signaling in mast cells: potential targets for treatment of mast cell-related diseases? Adv. Exp. Med. Biol. 716, 62–90.
Ma, H. T., Peng, Z., Hiragun, T., Iwaki, S., Gilfillan, A. M., and Beaven, M. A. (2008). Canonical transient receptor potential 5 channel in conjunction with Orai1 and STIM1 allows Sr2+ entry, optimal influx of Ca2+, and degranulation in a rat mast cell line. J. Immunol. 180, 2233–2239.
Ma, R., Li, W. P., Rundle, D., Kong, J., Akbarali, H. I., and Tsiokas, L. (2005). PKD2 functions as an epidermal growth factor-activated plasma membrane channel. Mol. Cell. Biol. 25, 8285–8298.
Ma, X., Cao, J., Luo, J., Nilius, B., Huang, Y., Ambudkar, I. S., and Yao, X. (2010). Depletion of intracellular Ca2+ stores stimulates the translocation of vanilloid transient receptor potential 4-c1 heteromeric channels to the plasma membrane. Arterioscler. Thromb. Vasc. Biol. 30, 2249–2255.
Manzoni, M., Monti, E., Bresciani, R., Bozzato, A., Barlati, S., Bassi, M. T., and Borsani, G. (2004). Overexpression of wild-type and mutant mucolipin proteins in mammalian cells: effects on the late endocytic compartment organization. FEBS Lett. 567, 219–224.
Mathar, I., Vennekens, R., Meissner, M., Kees, F., Van Der Mieren, G., Camacho Londono, J. E., Uhl, S., Voets, T., Hummel, B., Van Den Bergh, A., Herijgers, P., Nilius, B., Flockerzi, V., Schweda, F., and Freichel, M. (2010). Increased catecholamine secretion contributes to hypertension in TRPM4-deficient mice. J. Clin. Invest. 120, 3267–3279.
McKemy, D. D., Neuhausser, W. M., and Julius, D. (2002). Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 416, 52–58.
Medic, N., Desai, A., Komarow, H., Burch, L. H., Bandara, G., Beaven, M. A., Metcalfe, D. D., and Gilfillan, A. M. (2011). Examination of the role of TRPM8 in human mast cell activation and its relevance to the etiology of cold-induced urticaria. Cell Calcium 50, 473–480.
Meissner, M., Obmann, V., Hoschke, M., Link, S., Jung, M., Held, G., Philipp, S., Zimmermann, R., and Flockerzi, V. (2011). “Lessons of studying TRP channels with antibodies,” in TRP Channels, ed. M. Zhu (Boca Raton: CRC Press), 135–148.
Metz, M., and Maurer, M. (2007). Mast cells – key effector cells in immune responses. Trends Immunol. 28, 234–241.
Montell, C., Birnbaumer, L., and Flockerzi, V. (2002). The TRP channels, a remarkably functional family. Cell 108, 595–598.
Montell, C., and Rubin, G. M. (1989). Molecular characterization of the Drosophila trp locus: a putative integral membrane protein required for phototransduction. Neuron 2, 1313–1323.
Moqrich, A., Hwang, S. W., Earley, T. J., Petrus, M. J., Murray, A. N., Spencer, K. S., Andahazy, M., Story, G. M., and Patapoutian, A. (2005). Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science 307, 1468–1472.
Morgans, C. W., Zhang, J., Jeffrey, B. G., Nelson, S. M., Burke, N. S., Duvoisin, R. M., and Brown, R. L. (2009). TRPM1 is required for the depolarizing light response in retinal ON-bipolar cells. Proc. Natl. Acad. Sci. U.S.A. 106, 19174–19178.
Nadler, M. J., Hermosura, M. C., Inabe, K., Perraud, A. L., Zhu, Q., Stokes, A. J., Kurosaki, T., Kinet, J. P., Penner, R., Scharenberg, A. M., and Fleig, A. (2001). LTRPC7 is a Mg.ATP-regulated divalent cation channel required for cell viability. Nature 411, 590–595.
Nilius, B., and Owsianik, G. (2010). Transient receptor potential channelopathies. Pflugers Arch. 460, 437–450.
Oancea, E., Vriens, J., Brauchi, S., Jun, J., Splawski, I., and Clapham, D. E. (2009). TRPM1 forms ion channels associated with melanin content in melanocytes. Sci. Signal. 2, ra21.
Owsianik, G., D’Hoedt, D., Voets, T., and Nilius, B. (2006a). Structure-function relationship of the TRP channel superfamily. Rev. Physiol. Biochem. Pharmacol. 156, 61–90.
Owsianik, G., Talavera, K., Voets, T., and Nilius, B. (2006b). Permeation and selectivity of TRP channels. Annu. Rev. Physiol. 68, 685–717.
Ozawa, K., Szallasi, Z., Kazanietz, M. G., Blumberg, P. M., Mischak, H., Mushinski, J. F., and Beaven, M. A. (1993). Ca(2+)-dependent and Ca(2+)-independent isozymes of protein kinase C mediate exocytosis in antigen-stimulated rat basophilic RBL-2H3 cells. Reconstitution of secretory responses with Ca2+ and purified isozymes in washed permeabilized cells. J. Biol. Chem. 268, 1749–1756.
Parekh, A. B. (2010). Store-operated CRAC channels: function in health and disease. Nat. Rev. Drug Discov. 9, 399–410.
Parekh, A. B., and Putney, J. W. Jr. (2005). Store-operated calcium channels. Physiol. Rev. 85, 757–810.
Penner, R., Matthews, G., and Neher, E. (1988). Regulation of calcium influx by second messengers in rat mast cells. Nature 334, 499–504.
Perraud, A. L., Takanishi, C. L., Shen, B., Kang, S., Smith, M. K., Schmitz, C., Knowles, H. M., Ferraris, D., Li, W., Zhang, J., Stoddard, B. L., and Scharenberg, A. M. (2005). Accumulation of free ADP-ribose from mitochondria mediates oxidative stress-induced gating of TRPM2 cation channels. J. Biol. Chem. 280, 6138–6148.
Philipp, S., Cavalie, A., Freichel, M., Wissenbach, U., Zimmer, S., Trost, C., Marquart, A., Murakami, M., and Flockerzi, V. (1996). A mammalian capacitative calcium entry channel homologous to Drosophila TRP and TRPL. EMBO J. 15, 6166–6171.
Plaut, M., Pierce, J. H., Watson, C. J., Hanley-Hyde, J., Nordan, R. P., and Paul, W. E. (1989). Mast cell lines produce lymphokines in response to cross-linkage of Fc epsilon RI or to calcium ionophores. Nature 339, 64–67.
Prasad, P., Yanagihara, A. A., Small-Howard, A. L., Turner, H., and Stokes, A. J. (2008). Secretogranin III directs secretory vesicle biogenesis in mast cells in a manner dependent upon interaction with chromogranin A. J. Immunol. 181, 5024–5034.
Puertollano, R., and Kiselyov, K. (2009). TRPMLs: in sickness and in health. Am. J. Physiol. Renal Physiol. 296, F1245–F1254.
Ryazanova, L. V., Rondon, L. J., Zierler, S., Hu, Z., Galli, J., Yamaguchi, T. P., Mazur, A., Fleig, A., and Ryazanov, A. G. (2010). TRPM7 is essential for Mg(2+) homeostasis in mammals. Nat. Commun. 1, 109.
Sanchez-Miranda, E., Ibarra-Sanchez, A., and Gonzalez-Espinosa, C. (2010). Fyn kinase controls FcepsilonRI receptor-operated calcium entry necessary for full degranulation in mast cells. Biochem. Biophys. Res. Commun. 391, 1714–1720.
Schaefer, M. (2005). Homo- and heteromeric assembly of TRP channel subunits. Pflugers Arch. 451, 35–42.
Schlingmann, K. P., Weber, S., Peters, M., Niemann Nejsum, L., Vitzthum, H., Klingel, K., Kratz, M., Haddad, E., Ristoff, E., Dinour, D., Syrrou, M., Nielsen, S., Sassen, M., Waldegger, S., Seyberth, H. W., and Konrad, M. (2002). Hypomagnesemia with secondary hypocalcemia is caused by mutations in TRPM6, a new member of the TRPM gene family. Nat. Genet. 31, 166–170.
Schmidt, M., Dubin, A. E., Petrus, M. J., Earley, T. J., and Patapoutian, A. (2009). Nociceptive signals induce trafficking of TRPA1 to the plasma membrane. Neuron 64, 498–509.
Sel, S., Rost, B. R., Yildirim, A. O., Sel, B., Kalwa, H., Fehrenbach, H., Renz, H., Gudermann, T., and Dietrich, A. (2008). Loss of classical transient receptor potential 6 channel reduces allergic airway response. Clin. Exp. Allergy 38, 1548–1558.
Shimizu, T., Owsianik, G., Freichel, M., Flockerzi, V., Nilius, B., and Vennekens, R. (2009). TRPM4 regulates migration of mast cells in mice. Cell Calcium 45, 226–232.
Shumilina, E., Lam, R. S., Wolbing, F., Matzner, N., Zemtsova, I. M., Sobiesiak, M., Mahmud, H., Sausbier, U., Biedermann, T., Ruth, P., Sausbier, M., and Lang, F. (2008). Blunted IgE-mediated activation of mast cells in mice lacking the Ca2+-activated K+ channel KCa3.1. J. Immunol. 180, 8040–8047.
Stander, S., Moormann, C., Schumacher, M., Buddenkotte, J., Artuc, M., Shpacovitch, V., Brzoska, T., Lippert, U., Henz, B. M., Luger, T. A., Metze, D., and Steinhoff, M. (2004). Expression of vanilloid receptor subtype 1 in cutaneous sensory nerve fibers, mast cells, and epithelial cells of appendage structures. Exp. Dermatol. 13, 129–139.
Stewart, A. P., Smith, G. D., Sandford, R. N., and Edwardson, J. M. (2010). Atomic force microscopy reveals the alternating subunit arrangement of the TRPP2-TRPV4 heterotetramer. Biophys. J. 99, 790–797.
Stokes, A. J., Shimoda, L. M., Koblan-Huberson, M., Adra, C. N., and Turner, H. (2004). A TRPV2-PKA signaling module for transduction of physical stimuli in mast cells. J. Exp. Med. 200, 137–147.
Stokes, A. J., Wakano, C., Del Carmen, K. A., Koblan-Huberson, M., and Turner, H. (2005). Formation of a physiological complex between TRPV2 and RGA protein promotes cell surface expression of TRPV2. J. Cell. Biochem. 94, 669–683.
Story, G. M., Peier, A. M., Reeve, A. J., Eid, S. R., Mosbacher, J., Hricik, T. R., Earley, T. J., Hergarden, A. C., Andersson, D. A., Hwang, S. W., Mcintyre, P., Jegla, T., Bevan, S., and Patapoutian, A. (2003). ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 112, 819–829.
Strubing, C., Krapivinsky, G., Krapivinsky, L., and Clapham, D. E. (2001). TRPC1 and TRPC5 form a novel cation channel in mammalian brain. Neuron 29, 645–655.
Strubing, C., Krapivinsky, G., Krapivinsky, L., and Clapham, D. E. (2003). Formation of novel TRPC channels by complex subunit interactions in embryonic brain. J. Biol. Chem. 278, 39014–39019.
Suzuki, R., Liu, X., Olivera, A., Aguiniga, L., Yamashita, Y., Blank, U., Ambudkar, I., and Rivera, J. (2010). Loss of TRPC1-mediated Ca2+ influx contributes to impaired degranulation in Fyn-deficient mouse bone marrow-derived mast cells. J. Leukoc. Biol. 88, 863–875.
Tani, D., Monteilh-Zoller, M. K., Fleig, A., and Penner, R. (2007). Cell cycle-dependent regulation of store-operated I(CRAC) and Mg2+-nucleotide-regulated MagNuM (TRPM7) currents. Cell Calcium 41, 249–260.
Taub, D., Dastych, J., Inamura, N., Upton, J., Kelvin, D., Metcalfe, D., and Oppenheim, J. (1995). Bone marrow-derived murine mast cells migrate, but do not degranulate, in response to chemokines. J. Immunol. 154, 2393–2402.
Tsavaler, L., Shapero, M. H., Morkowski, S., and Laus, R. (2001). Trp-p8, a novel prostate-specific gene, is up-regulated in prostate cancer and other malignancies and shares high homology with transient receptor potential calcium channel proteins. Cancer Res. 61, 3760–3769.
Tsiokas, L., Arnould, T., Zhu, C., Kim, E., Walz, G., and Sukhatme, V. P. (1999). Specific association of the gene product of PKD2 with the TRPC1 channel. Proc. Natl. Acad. Sci. U.S.A. 96, 3934–3939.
Tsvilovskyy, V. V., Zholos, A. V., Aberle, T., Philipp, S. E., Dietrich, A., Zhu, M. X., Birnbaumer, L., Freichel, M., and Flockerzi, V. (2009). Deletion of TRPC4 and TRPC6 in mice impairs smooth muscle contraction and intestinal motility in vivo. Gastroenterology 137, 1415–1424.
Turner, H., Del Carmen, K. A., and Stokes, A. (2007). Link between TRPV channels and mast cell function. Handb. Exp. Pharmacol. 179, 457–471.
van Haaster, C. M., Engels, W., Lemmens, P. J., Hornstra, G., Van Der Vusse, G. J., and Heemskerk, J. W. (1995). Differential release of histamine and prostaglandin D2 in rat peritoneal mast cells: roles of cytosolic calcium and protein tyrosine kinases. Biochim. Biophys. Acta 1265, 79–88.
Vazquez, G., Wedel, B. J., Trebak, M., St. John Bird, G., and Putney, J. W. Jr. (2003). Expression level of the canonical transient receptor potential 3 (TRPC3) channel determines its mechanism of activation. J. Biol. Chem. 278, 21649–21654.
Venkatachalam, K., Hofmann, T., and Montell, C. (2006). Lysosomal localization of TRPML3 depends on TRPML2 and the mucolipidosis-associated protein TRPML1. J. Biol. Chem. 281, 17517–17527.
Venkatachalam, K., Van Rossum, D. B., Patterson, R. L., Ma, H. T., and Gill, D. L. (2002). The cellular and molecular basis of store-operated calcium entry. Nat. Cell Biol. 4, E263–E272.
Vennekens, R., Hoenderop, J. G., Prenen, J., Stuiver, M., Willems, P. H., Droogmans, G., Nilius, B., and Bindels, R. J. (2000). Permeation and gating properties of the novel epithelial Ca(2+) channel. J. Biol. Chem. 275, 3963–3969.
Vennekens, R., and Nilius, B. (2007). Insights into TRPM4 function, regulation and physiological role. Handb. Exp. Pharmacol. 179, 269–285.
Vennekens, R., Olausson, J., Meissner, M., Bloch, W., Mathar, I., Philipp, S. E., Schmitz, F., Weissgerber, P., Nilius, B., Flockerzi, V., and Freichel, M. (2007). Increased IgE-dependent mast cell activation and anaphylactic responses in mice lacking the calcium-activated nonselective cation channel TRPM4. Nat. Immunol. 8, 312–320.
Vig, M., Dehaven, W. I., Bird, G. S., Billingsley, J. M., Wang, H., Rao, P. E., Hutchings, A. B., Jouvin, M. H., Putney, J. W., and Kinet, J. P. (2008). Defective mast cell effector functions in mice lacking the CRACM1 pore subunit of store-operated calcium release-activated calcium channels. Nat. Immunol. 9, 89–96.
Vig, M., Peinelt, C., Beck, A., Koomoa, D. L., Rabah, D., Koblan-Huberson, M., Kraft, S., Turner, H., Fleig, A., Penner, R., and Kinet, J. P. (2006). CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science 312, 1220–1223.
Voets, T., Droogmans, G., Wissenbach, U., Janssens, A., Flockerzi, V., and Nilius, B. (2004a). The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature 430, 748–754.
Voets, T., Nilius, B., Hoefs, S., Van Der Kemp, A. W., Droogmans, G., Bindels, R. J., and Hoenderop, J. G. (2004b). TRPM6 forms the Mg2+influx channel involved in intestinal and renal Mg2+absorption. J. Biol. Chem. 279, 19–25.
Voets, T., Prenen, J., Fleig, A., Vennekens, R., Watanabe, H., Hoenderop, J. G., Bindels, R. J., Droogmans, G., Penner, R., and Nilius, B. (2001). CaT1 and the calcium release-activated calcium channel manifest distinct pore properties. J. Biol. Chem. 276, 47767–47770.
Vriens, J., Appendino, G., and Nilius, B. (2009). Pharmacology of vanilloid transient receptor potential cation channels. Mol. Pharmacol. 75, 1262–1279.
Vriens, J., Owsianik, G., Fisslthaler, B., Suzuki, M., Janssens, A., Voets, T., Morisseau, C., Hammock, B. D., Fleming, I., Busse, R., and Nilius, B. (2005). Modulation of the Ca2 permeable cation channel TRPV4 by cytochrome P450 epoxygenases in vascular endothelium. Circ. Res. 97, 908–915.
Wagner, T. F., Loch, S., Lambert, S., Straub, I., Mannebach, S., Mathar, I., Dufer, M., Lis, A., Flockerzi, V., Philipp, S. E., and Oberwinkler, J. (2008). Transient receptor potential M3 channels are ionotropic steroid receptors in pancreatic beta cells. Nat. Cell Biol. 10, 1421–1430.
Watanabe, H., Davis, J. B., Smart, D., Jerman, J. C., Smith, G. D., Hayes, P., Vriens, J., Cairns, W., Wissenbach, U., Prenen, J., Flockerzi, V., Droogmans, G., Benham, C. D., and Nilius, B. (2002). Activation of TRPV4 channels (hVRL-2/mTRP12) by phorbol derivatives. J. Biol. Chem. 277, 13569–13577.
Wegierski, T., Steffl, D., Kopp, C., Tauber, R., Buchholz, B., Nitschke, R., Kuehn, E. W., Walz, G., and Kottgen, M. (2009). TRPP2 channels regulate apoptosis through the Ca2+ concentration in the endoplasmic reticulum. EMBO J. 28, 490–499.
Weissgerber, P., Cavalie, A., Buchholz, S., Flockerzi, V., and Freichel, M. (2008). Early embryonic lethality following inactivation of the TRPM7 gene in mice. Naunyn Schmiedebergs Arch. Pharmacol. 377, 35.
Weissgerber, P., Kriebs, U., Tsvilovskyy, V., Olausson, J., Kretz, O., Stoerger, C., Vennekens, R., Wissenbach, U., Middendorff, R., Flockerzi, V., and Freichel, M. (2011). Male fertility depends on Ca(2)+ absorption by TRPV6 in epididymal epithelia. Sci. Signal. 4, ra27.
Wes, P. D., Chevesich, J., Jeromin, A., Rosenberg, C., Stetten, G., and Montell, C. (1995). TRPC1, a human homolog of a Drosophila store-operated channel. Proc. Natl. Acad. Sci. U.S.A. 92, 9652–9656.
Wu, L. J., Sweet, T. B., and Clapham, D. E. (2010). International union of basic and clinical pharmacology. LXXVI. Current progress in the mammalian TRP ion channel family. Pharmacol. Rev. 62, 381–404.
Wykes, R. C., Lee, M., Duffy, S. M., Yang, W., Seward, E. P., and Bradding, P. (2007). Functional transient receptor potential melastatin 7 channels are critical for human mast cell survival. J. Immunol. 179, 4045–4052.
Xu, H., Delling, M., Li, L., Dong, X., and Clapham, D. E. (2007). Activating mutation in a mucolipin transient receptor potential channel leads to melanocyte loss in varitint-waddler mice. Proc. Natl. Acad. Sci. U.S.A. 104, 18321–18326.
Yamamoto, S., Shimizu, S., Kiyonaka, S., Takahashi, N., Wajima, T., Hara, Y., Negoro, T., Hiroi, T., Kiuchi, Y., Okada, T., Kaneko, S., Lange, I., Fleig, A., Penner, R., Nishi, M., Takeshima, H., and Mori, Y. (2008). TRPM2-mediated Ca2+influx induces chemokine production in monocytes that aggravates inflammatory neutrophil infiltration. Nat. Med. 14, 738–747.
Yang, W., Chen, J., and Zhou, L. (2009). Effects of shear stress on intracellular calcium change and histamine release in rat basophilic leukemia (RBL-2H3) cells. J. Environ. Pathol. Toxicol. Oncol. 28, 223–230.
Yang, W. Z., Chen, J. Y., Yu, J. T., and Zhou, L. W. (2007). Effects of low power laser irradiation on intracellular calcium and histamine release in RBL-2H3 mast cells. Photochem. Photobiol. 83, 979–984.
Yildirim, E., Kawasaki, B. T., and Birnbaumer, L. (2005). Molecular cloning of TRPC3a, an N-terminally extended, store-operated variant of the human C3 transient receptor potential channel. Proc. Natl. Acad. Sci. U.S.A. 102, 3307–3311.
Yoshimaru, T., Suzuki, Y., Inoue, T., and Ra, C. (2009). L-type Ca2+ channels in mast cells: activation by membrane depolarization and distinct roles in regulating mediator release from store-operated Ca2+ channels. Mol. Immunol. 46, 1267–1277.
Yuan, J. P., Kim, M. S., Zeng, W., Shin, D. M., Huang, G., Worley, P. F., and Muallem, S. (2009). TRPC channels as STIM1-regulated SOCs. Channels (Austin) 3, 221–225.
Yuan, J. P., Zeng, W., Huang, G. N., Worley, P. F., and Muallem, S. (2007). STIM1 heteromultimerizes TRPC channels to determine their function as store-operated channels. Nat. Cell Biol. 9, 636–645.
Yue, L., Peng, J. B., Hediger, M. A., and Clapham, D. E. (2001). CaT1 manifests the pore properties of the calcium-release-activated calcium channel. Nature 410, 705–709.
Zhang, D., Spielmann, A., Wang, L., Ding, G., Huang, F., Gu, Q., and Schwarz, W. (2011). Mast-cell degranulation induced by physical stimuli involves the activation of transient-receptor-potential channel TRPV2. Physiol. Res. 61, 113–124.
Zhang, P., Luo, Y., Chasan, B., Gonzalez-Perrett, S., Montalbetti, N., Timpanaro, G. A., Cantero Mdel, R., Ramos, A. J., Goldmann, W. H., Zhou, J., and Cantiello, H. F. (2009). The multimeric structure of polycystin-2 (TRPP2): structural-functional correlates of homo- and hetero-multimers with TRPC1. Hum. Mol. Genet. 18, 1238–1251.
Zhang, Y., Hoon, M. A., Chandrashekar, J., Mueller, K. L., Cook, B., Wu, D., Zuker, C. S., and Ryba, N. J. (2003). Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell 112, 293–301.
Zhu, X., Chu, P. B., Peyton, M., and Birnbaumer, L. (1995). Molecular cloning of a widely expressed human homologue for the Drosophila trp gene. FEBS Lett. 373, 193–198.
Keywords: TRP proteins, cation channels, Ca2+ signaling, mast cell activation
Citation: Freichel M, Almering J and Tsvilovskyy V (2012) The role of TRP proteins in mast cells. Front. Immun. 3:150. doi: 10.3389/fimmu.2012.00150
Received: 14 March 2012; Accepted: 22 May 2012;
Published online: 12 June 2012.
Edited by:
Ulrich Blank, Université Paris-Diderot Paris 7, FranceReviewed by:
Marc Benhamou, Institut National de la Santé et de la Recherche Médicale, FrancePierre Launay, Institut National de la Santé et de la Recherche Médicale, France
Copyright: © 2012 Freichel, Almering and Tsvilovskyy. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: Marc Freichel, Pharmakologisches Institut, Universität Heidelberg, Im Neuenheimer Feld 366, 69120 Heidelberg, Germany. e-mail: marc.freichel@pharma.uni-heidelberg.de
 Marc Freichel*
Marc Freichel*