- 1Gastroenterology and Interventional Endoscopy, Azienda Unità Sanitaria Locale di Bologna (AUSL Bologna), Bologna, Italy
- 2Digestive Endoscopy Unit, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome, Italy
- 3Centre for Endoscopic Research Therapeutics and Training (CERTT), Università Cattolica del Sacro Cuore, Rome, Italy
- 4Digestive Endoscopy, Evangelisches Krankenhaus, Düsseldorf, Germany
- 5Department of General Surgery, Gastroenterology and Interventional Endoscopy, Azienda Unità Sanitaria Locale di Bologna (AUSL Bologna), Bologna, Italy
Nowadays, stenting malignant biliary stenosis (extrahepatic or hilar), benign biliary stenosis, and pancreatic duct stenosis in chronic pancreatitis as well as stenting for prophylaxis of post- endoscopic retrograde cholangiopancreatography pancreatitis and for failed extraction of biliary stones or endoscopic papillectomy are the many common challenges for a bilio-pancreatic endoscopist. The purpose of this review is to provide a practical approach to bilio-pancreatic stenting indications and techniques. Having a thorough understanding of stenting indications and techniques, for a bilio-pancreatic endoscopist means being able to develop a tailored approach for each clinical scenario depending on the type of stent used. Biliary stents, in fact, vary in diameter, length, and composition, making it possible to give each patient personalized treatment.
1. Introduction
The bilio-pancreatic stenting performed to drain the malignant stenoses of the common bile duct, has several indications for malignant and benign diseases involving the common bile duct, the hepatic hilum, and the pancreatic duct in the present day.
A bilio-pancreatic endoscopist must have a thorough understanding of stenting indications and techniques to develop a tailored approach for each clinical scenario. The choice regarding the type of stent to be used is an important step. Biliary stents vary in diameter, length, and composition, making it possible to give each patient personalized treatment.
The purpose of this review is to provide a practical approach to bilio-pancreatic stenting indications and techniques.
2. Malignant extrahepatic biliary stenosis
Malignant stenoses of the extrahepatic biliary tract are most frequently caused by pancreatic tumors, predominantly located at the level of the head or uncinate process, and by cholangiocarcinoma. Other causes of malignant stenosis of the extrahepatic biliary tract include ampullary/duodenal tumors, gallbladder tumors, and metastases of other tumors infiltrating the head of the pancreas and the main biliary duct (1).
Before endoscopic drainage, the diagnosis and stage of the disease must be established (2, 3) by cross-sectional imaging and tissue acquisition.
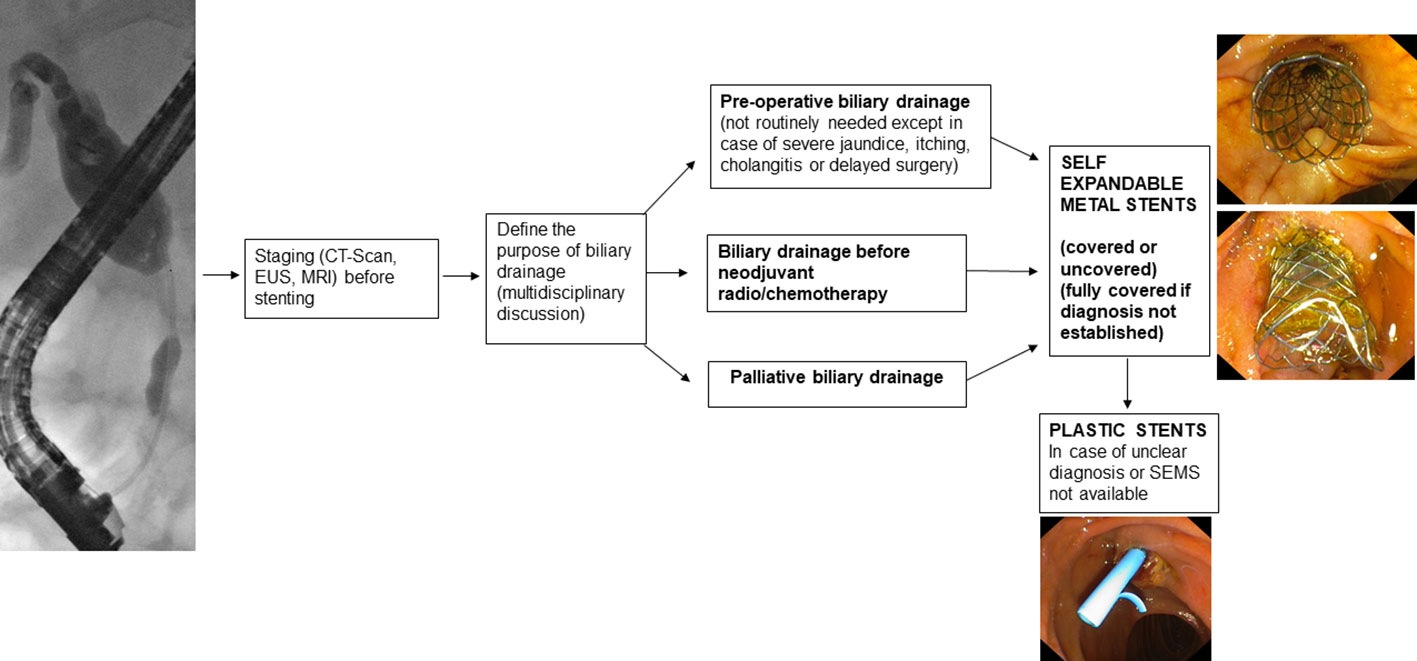
2.1. Preoperative drainage
Preoperative drainage should not be routinely performed. In fact, early surgery leads to fewer complications than preoperative biliary drainage (4, 5). Preoperative drainage should be reserved for patients with cholangitis, severe jaundice, itching, patients who are candidates for neoadjuvant therapy, or patients whose surgery is expected to be delayed by more than 2 weeks (2).
If a decision is made to place a preoperative biliary drain, the endoscopic route should be preferred over the percutaneous route (6) because percutaneous drainage, in addition to having higher morbidity (7), may lead to the development of tumor seeding (8) thus compromising the curability of the disease.
Regarding the type of stent to be used, Self-Expandable Metal Stents (SEMS) have a lower obstruction rate than Plastic Stents (PS) (9–11) and maintain patency longer (12).
In addition, it has been seen that, in patients who need to undergo neoadjuvant therapy, the use of SEMS compared to the use of PS leads to a lower incidence of delay in the performance of neoadjuvant therapy while maintaining similar overall treatment costs (13). The cost-effectiveness analysis by Almadi et al. (14) also shows that placement of SEMS compared with PS is not only cost-effective but also associated with a higher likelihood of achieving the oncologic outcomes needed to perform surgery sooner. In the case of preoperative drain placement, therefore, the use of 10-mm SEMS is recommended (2).
Regarding the type of SEMS, Fully Covered Self-Expandable Metal Stents (FCSEMS) show a lower complication rate than PS (15, 16). Acute pancreatitis occurred more frequently when the FCSEMS is place but it does not affect the waiting time for surgery (15). The FCSEMS should be preferred if the diagnosis is not histologically confirmed (Figure 1).
In patients with biliary obstruction due to pancreatic adenocarcinoma who are candidates for neoadjuvant therapy, several randomized controlled trials (RCTs) have been performed: in the RCT conducted by Gardner et al. (17), in which the use of FCSEMS, Uncovered Self-Expandable Metal Stents (USEMS), and PS were compared, FCSEMS were seen to lead to a shorter delay in the performance of neoadjuvant therapy. In the RCT conducted by Seo et al. (18), FCSEMS and USEMS were shown to maintain equal drainage function in the two groups (72.2% vs 72.9%). The reasons for stent failure were mainly tumor ingrowth for USEMS and stent migration for FCSEMS. The incidence of cholecystitis was similar in the two groups. However, in a recent meta-analysis (19), including 2358 patients, the FCSEMS were superior to the USEMS with respect to prevent recurrent biliary obstruction. Although there was no significant difference in total procedure-related adverse events between the two types of SEMS.
2.2. Palloatove drainage
In reference to palliative drainage of malignant stenosis of the extrahepatic biliary tract, the transpapillary endoscopic approach with placement of SEMS is recommended (2, 20). In RCTs (21–24) performed on patients with unresectable distal malignant biliary obstruction, SEMS placement, as compared to PS, demonstrated a higher patency rate, lower incidence of complications, and greater cost-effectiveness. Even in patients with fewer than 3 months of survival and with metastatic disease in whom SEMS or PS had been placed, costs were similar (25) and better quality of life was shown in SEMS patients at follow-up (26). The most recent meta-analysis available in the literature on the drainage of unresectable distal biliary duct malignant stenosis (27), confirmed a higher patency rate with SEMS, a lower incidence of stent dysfunction, and a lower incidence of requiring reintervention in SEMS-bearing patients compared to PS-bearing patients. In addition, in the subgroup analysis, the study showed that placement of Partially Covered Self-Expandable Metal Stents (PCSEMS) or FCSEMS compared with PS placement leads to increased mean survival.
Nevertheless, the choice of SEMS type remains controversial because both USEMS and FCSEMS placement have advantages and disadvantages. The most recent meta-analysis (28) confirms that in patients with FCSEMS versus USEMS there is no difference in the incidence of stent failure, mortality, or development of complications. Likewise, in the RCT by Conio et al. (29), there was no significant difference in the incidence of stent malfunction or survival of patients with FCSEMS or USEMS. The causes of FCSEMS malfunction are stent migration (7% vs 0% in the USEMS group) and stent obstruction by sludge (8% in the FCSEMS group) or tumor overgrowth (4% in the FCSEMS group), while the leading cause of USEMS malfunction is tumor ingrowth (10% in the USEMS group). However, in cases where the diagnosis is not definite, a USEMS should not be placed because tissue ingrowth reduces its long-term patency and compromises its possibility of removal, which, even using the “stent-in-stent” technique (30, 31) may be difficult (32) or impossible.
In a recent RCT (33) the use of USEMS was compared with the use of PCSEMS and showed a higher incidence (p=0.0467) of occlusion in USEMS (43.8%) compared with PCSEMS (22.7%) with a significantly longer mean stent patency (p=0.0112) in the PCSEMS-carrying group (455 days) compared with the USEMS-carrying group (301 days).
Regarding the incidence of cholecystitis, an increased risk of cholecystitis in patients carrying FCSEMS was not found in the literature (34–37). However, in some studies (38), in patients with gallbladder in situ, the proximal end of the FCSEMS was placed below the orifice of the cystic duct. Risk factors for the development of cholecystitis (39, 40) appear to be tumor involvement in the cystic duct, the presence of cholelithiasis, and opacification with contrast medium of the gallbladder before stent placement.
In single stent placement, there is no need for routine sphincterotomy if PCSEMS or USEMS are placed. The RCT conducted by Hayashi et al. (41) on 200 patients with distal malignant stenosis from unresectable pancreatic cancer showed that performing sphincterotomy before PCSEMS placement does not lead to a reduction in the risk of post-endoscopic retrograde cholangiopancreatography (ERCP) pancreatitis and other adverse events. The most recent meta-analysis available in the literature (42) indeed shows that performing a sphincterotomy before placement of a stent, leads to an increased incidence of the risk of bleeding and cholangitis, and the risk of developing post-procedure pancreatitis is unchanged. Regarding the placement of FCSEMS, it remains controversial whether a sphincterotomy should be performed because there is a hypothetical risk that FCSEMS will close the pancreatic duct outlet, increasing the risk of post-ERCP pancreatitis (2).
In the case of malfunctioning SEMS placed in patients with distal malignant biliary stenosis, a PS or new SEMS should be placed inside the previous SEMS (2, 43). One possible advantage of FCSEMS is that covered stents can be removed and subsequent replaced with a new SEMS (43).
Several new types of stents are in development, with the aim of prolonging the function of FCSEMS. For example, FCSEMS with a chemotherapeutic agent incorporated into the stent covering material, have been tested on animals and small cohorts of patients with malignant stenosis of the distal biliary duct. To date, available studies do not show an advantage in terms of clinical outcomes over standard SEMS placement (44, 45). FCSEMS with antireflux valves (46–48) and FCSEMS with anti-migration systems (49, 50) have also been studied with promising results. Studies with larger patient samples and longer-term follow-ups are needed to validate the present results.
3. Malignant hilar biliary stenosis
Malignant hilar biliary stenoses can occur by intraductal development of primary biliary tract tumors or local extension of tumors, such as gallbladder cancer, or by ab extrinsic compression in the case of metastatic lymph node involvement at the hilar level (51). The indications for endoscopic stent placement to drain malignant hilar biliary stenoses are preoperative drainage and palliation.
Before intervention to decompress the biliary tract, the patient should be studied using all necessary imaging techniques, because the accuracy of Computed Tomography (CT) and Magnetic resonance imaging (MRI) disease staging decreases after biliary stent placement due to ductal decompression and imaging artifacts (2, 52). Magnetic resonance cholangiopancreatography (MRCP) can provide a detailed “road-map” to perform optimal biliary drainage avoiding the scenario of “opacified and undrained bile ducts” and thus reducing the rate of infectious complications. In addition, imaging techniques such as CT/MRI, in cases of portal vein thrombosis, can assess the presence and extent of hepatic atrophy. Drainage of an atrophic liver segment should be avoided because it increases the risk of developing cholangitis (53) and does not lead to increased clinical success and survival (54).
The approach to drain malignant hilar stenosis should be established by a multidisciplinary team in high-volume referral centers and each case should be considered unique (55). In the multidisciplinary setting, the actual malignancy of the stenosis, its extent, and the most appropriate therapeutic approach should be ascertained to achieve adequate drainage of intrahepatic biliary branches of functioning liver parenchyma (56). Suboptimal drainage of intrahepatic bile ducts is the main reason for the high rate of cholangitis secondary to drainage of malignant hilar stenosis (57) and can turn a patient with a potentially resectable tumor into an inoperable one.
Bismuth and Corlette’s classification (58) divides malignant hilar stenosis into 4 types based on the involvement of the main hepatic duct and central segmental ducts. Depending on the type of stenosis, the number of stents to be inserted can be roughly estimated. Type I is a non-hilar stenosis located in the proximal hepatic duct more than 2 cm below the hilar confluence so placement of a single stent can ensure complete drainage. In type II, the confluence of the main hepatic ducts is involved, so two stents are needed to drain the left and right hemisystems. In type IIIa and IIIb, the confluence and the right (anterior/posterior) or left (IV/II and III segments) secondary ducts are involved; in this case, 3 stents would be needed to drain all the intrahepatic branches. Type IV reflects an advanced disease state in which both left and right main and secondary ducts are involved and theoretically 4 stents would be needed.
Because hilar stenoses are often complex, as in type IIIa for example, to achieve complete drainage, it must not only be decided whether a unilateral or bilateral stent should be placed but the need for multisectoral stent placement must be considered as well (59).
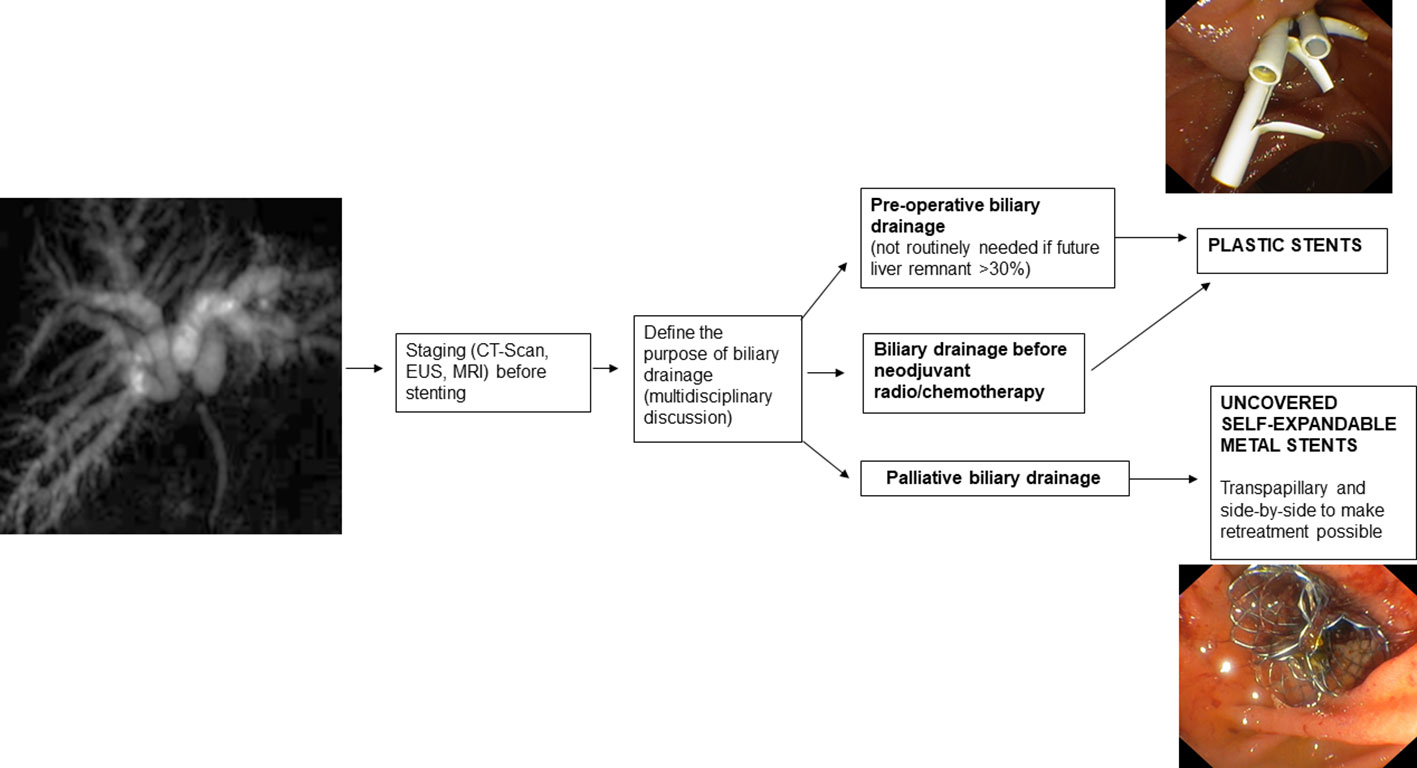
3.1. Preoperative drainage
The indication and type of preoperative biliary drainage should be decided by a multidisciplinary team based on patient characteristics and institutional experience (2).
Placement of a preoperative biliary drain is associated with an increased risk of postoperative infection (60, 61), and in some surgeries, such as left hepatectomy, it is generally not indicated (61, 62). However, there are conditions associated with a high risk of postoperative liver failure, such as an estimated hepatic residual volume ≤ 30% after surgery, for which preoperative drain placement is indicated (63).
In patients who are candidates for major hepatic resection with bilirubinemia > 3 mg/dL, the preoperative biliary drainage, limited to the future liver remnant has been suggested.
Regarding the choice between endoscopic and percutaneous approaches for placement of a preoperative biliary drain, ASGE guidelines (64) suggest the first-line use of endoscopic drainage over percutaneous drainage based on the evidence of a higher incidence of tumor metastasis in patients treated with percutaneous drainage than those treated with endoscopic drainage (65, 66).
Generally, percutaneous drainage is preferred when the patient has altered gastro-duodenal anatomy, when the bile ducts to be drained are not accessible with ERCP, and when adequate endoscopic biliary drainage has not been obtained.
If preoperative endoscopic drainage of hepatic hilum stenosis is performed, plastic stents (PS) or nasobiliary tubes are indicated (67).
Although less comfortable for the patient, nasobiliary drainage is found to lead to a lower incidence of cholangitis, postoperative fistulas, and stent malfunction (68). In fact, a lower incidence of occlusion with the nasobiliary tube than with the PS has been reported (69).
PS placement is indicated preoperatively and when a decision on the type of treatment, curative or palliative, has not yet been established. They are preferred in these cases because they are easily removable and do not prevent further therapeutic procedures. In addition, their diameter and length can be adapted to the intrahepatic biliary tree, and when sufficient drainage is not achieved, multiple PSs can be inserted to improve bile outflow.
PS insertion in malignant hilar stenoses should be performed by (1) selective cannulation with a guide wire of the intrahepatic ducts upstream of the hilar stenosis; (2) selective anterograde injection of contrast into the selected bile duct to define the anatomy of the stenosis; (3) balloon dilation (4-6 mm) of the stenosis, if necessary; (4) insertion of plastic stents to drain the opacified duct, thus avoiding the scenario of “injected and undrained ducts”.
3.2. Palliative drainage
The goals of palliative biliary drainage are used to relieve jaundice and prevent cholangitis improving quality of life and allowing possible radiation and chemotherapy treatments (70).
Guidelines (2, 64) recommend that the choice of drainage type should be determined based on stenosis characteristics, local expertise, and patient preference. Bismuth type I and II stenoses should first be addressed endoscopically whereas, with the Bismuth type III and IV stenoses an endoscopic, percutaneous, or combined approach can be used, depending on the type of stenosis. In fact, in cases of complex hilar stenosis, the placement of a percutaneous drain and an endoscopic drain should not be considered exclusive of each other. For example, it could be initially placed a percutaneous drain, which could then be used as a guide to place a stent endoscopically (Rendez-vous technique) (71).
Regardless of the technique used, biliary tract drainage should involve at least 50% of the liver volume reducing the risk of infection and liver failure. The minimum portion of the liver volume, excluding tumor volume, that should be drained has been analysed in several studies (53, 72, 73) which conclude that drainage of more than 50 percent of the liver volume is an independent factor for a greater decrease in bilirubin levels, a lower incidence of cholangitis, and a longer survival rate.
In the case of established malignancy and palliative purpose of hilar stenosis drainage, USEMS are recommended because they allow, compared with other types of SEMS, drainage of the lateral bile ducts through the uncovered meshes (74). In addition, compared with PSs, USEMS improve survival and ensure longer-lasting drainage patency (75). While USEMS have a higher cost than PS, they also present potential cost savings due to the higher patency rate leading to the requirement for fewer re-interventions (76, 77). However, it must be kept in mind that once placed, the USEMS are difficult or even impossible to remove. This may jeopardize possible subsequent surgical treatments so the USEMS should only be placed in cases of established palliative treatment of malignant hilar stenosis. Furthermore, regarding the placement of the USEMS, adequate stent release must be studied because inadequate stent placement can lead to significant difficulties in subsequent reinterventions when placing additional drains between the stent meshes. Therefore, in cases where there is uncertainty about the diagnosis and/or inability to obtain adequate endoscopic drainage, PS placement is recommended (64).
Placement of USEMS should be done only after an adequate cannulation of intrahepatic ducts. To avoid contrast opacification of ducts that cannot be subsequently drained, opacification with contrast medium should be avoided before performing guidewire cannulation of intrahepatic ducts (78–80). In this technique, once the stenosis is crossed with a guidewire, contrast medium is injected before stent insertion. Some authors have also proposed not using contrast medium at this stage either, keeping the narrowing of the stent at the level of the stenosis as the only reference point for stent placement during the release of the proximal end of the stent (81).
When more than one stent needs to be placed, guidewires should be placed first, possibly several, over all the stenoses and they should be dilated to 6 mm to facilitate subsequent stent placement. It is usually preferred to place the stent in the left hepatic duct first, which is generally more angled. In case of difficulties in placing the stent due to the stenosis being very angulated, it is suggested to switch from a “floppy” guidewire to a more rigid one.
The choice of USEMS depends on the ductal diameter, the number of USEMS required, and the length of the stenosis. There are different USEMS available on the market today (82). Their length varies from 4 to 12 cm and their diameter from 6 to 10 mm. Commonly for hilar strictures 8-10 cm long and 8-10 mm diameter USEMS are needed.
However, placement of multiple USEMS to drain intrahepatic ducts remains a complex procedure since it is often difficult to advance other metallic stents into the biliary duct after the first metallic stent has been released. In case of difficulties, a stiffer guidewire can be used to change the insertion angle of the metallic stent to be inserted, alternatively the technique described by Hookey et al. (83) can be used, in which an 8.5 F straight plastic stent, with the proximal flange removed, is placed at the level of the main biliary duct to facilitate the passage of a second metal stent after the release of a first metal stent.
An alternative technique to the placement of multiple side-by-side USEMS (SBS) is the stent-in-stent (SIS) placement technique, also known as Y-stent. In this technique, one stent is released, often into the left hepatic duct for greater angulation, and a second stent is then released into the other hepatic duct through the meshes of the first stent. The SIS technique is most widely used in Japan and Korea (84) because it does not expose the main biliary duct to over-dilatation. Many USEMS dedicated to the SIS approach have been developed: in most of these, only the central part of the stent can be used to insert another stent between the meshes while in some of these, the uniform mesh size allows the insertion of another stent in each region of the USEMS (85, 86). The SBS technique is preferred in the West because, in the event of stent obstruction, endoscopic reintervention is usually successful, unlike the SIS technique where reintervention may be prohibitive (87).
The distal margin of the USEMS in the SIS configuration should be placed at the transpapillary (88),”shotgun” level to facilitate possible future reintervention in case of stent failure. However, some authors disagree and believe that avoiding transpapillary placement could potentially keep Oddi’s sphincter intact leading to a possible reduction in the risk of developing cholangitis (89).
After the USEMS release, the main problem that can develop in the long term is stent occlusion. Stent occlusion can occur by sludge deposition or by tissue ingrowth and/or overgrowth. Sludge can be removed with a Fogarty balloon, while tissue ingrowth and/or overgrowth can be resolved by inserting a second USEMS or a plastic stent, depending on life expectancy.
The patency of USEMS can be increased by applying photodynamic therapy (90) or radiofrequency ablation (91, 92), prior to stent insertion. These techniques seem promising, but they are not widely used, are expensive and data to date are limited (93).
4. Benign biliary stenosis
Benign biliary tract stenoses can occur post-surgically or be correlated to bilio-pancreatic diseases. Chronic pancreatitis is the disease that most frequently leads to benign biliary tract stenosis. Post-surgical stenoses, on the other hand, are mainly associated with laparoscopic cholecystectomy surgeries and liver transplant surgeries (94).
While in malignant stenoses endoscopic treatment is limited to providing drainage of the biliary tract, in benign stenoses endoscopic treatment with stent placement is used for therapeutic purposes and is directed at the regression of the stenosis. After stent placement in malignant biliary stenoses, an endoscopic re-intervention is performed only on demand, whereas in benign biliary stenoses the scheduling of subsequent endoscopic follow-up appointments for removal/replacement of placed stents is recommended (2). In this regard, in the endoscopic treatment of benign biliary stenoses, a sphincterotomy is recommended to facilitate retreatment with repeated stent replacements.
Endoscopy is the first-line treatment in the treatment of benign biliary stenoses (95) and involves the placement of PS or FCSEMS that are selected according to the type of stenosis to be approached (2).
If a PS is used, placement of multiple parallel plastic stents (MPS) is recommended (96), inserting as many stents as possible every 3-4 months for 1 year (97). Recent studies propose prolonging plastic stent replacement to every 6 months in selected categories of patients (98, 99).
To insert MPS first it is often necessary to dilate the stenosis at the first treatment with a 4-6 mm balloon depending on the size of the bile duct upstream of the stenosis. After insertion of the first PS, the bile duct is again cannulated with a 6F catheter on a hydrophilic guidewire and another stent is placed parallel to the previous one. The procedure is repeated until the maximum number of stents (preferably 10F in diameter) are placed, according to the degree of stenosis and the diameter of the biliary duct upstream and downstream of the stenosis. During the procedure, good coordination between operator and assistant is required to avoid intrahepatic migration of the stents (100).
In contrast, in the case of FCSEMS placement, it is generally not necessary to perform dilatation of the stenosis before stent insertion. However, when releasing FCSEMS it should be considered that some FCSEMS shorten significantly after release and that the distal part of the FCSEMS should be placed 5-10 mm downstream of the papilla to facilitate its future removal. Replacement of FCSEMS is recommended every 6 months to prevent degradation of the membrane covering the metal mesh of the stent, although there is little evidence for this (101, 102).
A recent meta-analysis comparing the use of MPS with the use of FCSEMS in benign biliary stenosis (103) found no differences in the incidence of stenosis resolution, stenosis recurrence, and adverse events in the two groups. Another meta-analysis (104) reported that a significantly lower number of procedures in the FCSEMS group was required. Nevertheless, long-time follow-up is available for MPS only.
FCSEMS have the advantage of having a longer patency than MPS (105) but have a migration risk of about 9%, which varies according to the etiology of the stenosis on which the stent was applied (106). Indeed, it has been seen that in patients with chronic pancreatitis, migration of FCSEMS occurs less frequently than in biliary tract stenosis with other etiology [4.6% versus 14% (p = 0.006)] (107). To reduce the risk of FCSEMS migration, a double pigtail plastic stent can be placed within the FCSEMS (108).
Placement of FCSEMS also presents the risk of stenosis development at the proximal margin of the stent especially if the diameter of the placed stent is larger than the diameter of the biliary duct upstream of the stenosis (109). Therefore, it seems appropriate to consider using FCSEMS with a diameter of 8 mm rather than the more commonly used 10 mm.
It is crucial to select the right stent on a case-by-case basis depending on the length of the stenosis, its location, and its etiology (102).
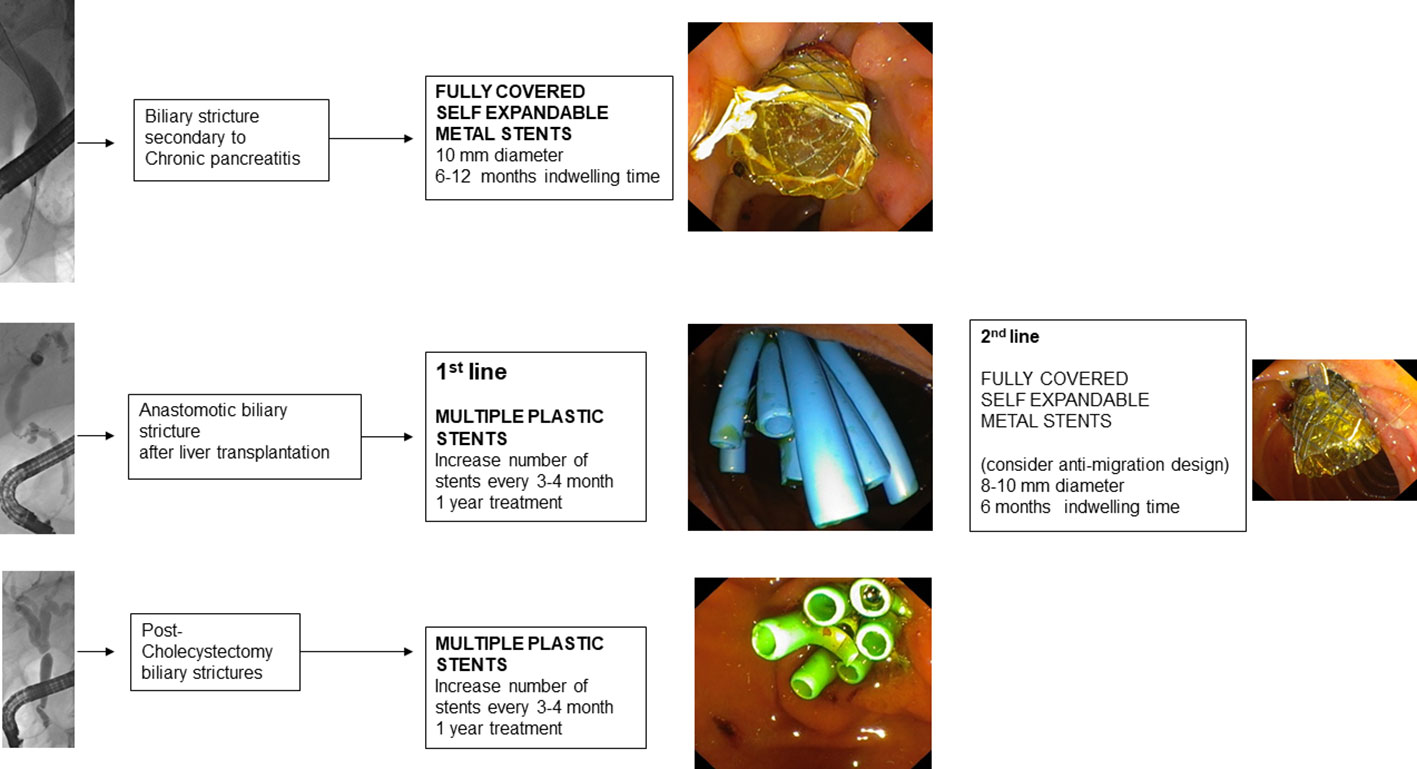
4.1. Benign biliary tract stenoses related to chronic pancreatitis
Biliary tract stenoses from chronic pancreatitis may have different etiology: they may be secondary to scarring of the pancreatic parenchyma following repeated inflammatory episodes (110), secondary to acute inflammation of the pancreatic parenchyma, or secondary to compression of pancreatic pseudocysts (111). In the latter two conditions, obstruction resolves with resolution of the acute inflammatory process or with drainage of the pseudocyst.
Stenoses of the distal biliary duct secondary to a scarring process of the pancreatic parenchyma, especially if pancreatic calcifications are present (112), tend to be refractory to endoscopic treatment more than other benign stenoses of the biliary tract (113) because they have a greater extension and are located at the level of the calcific fibrotic tissue of the head of the pancreas.
Before determining the treatment of biliary stenosis secondary to chronic pancreatitis, it is mandatory to exclude the presence of a tumor with cross-sectional imaging and echoendoscopy. In cases of benign scarring stenosis of the biliary duct, endoscopic treatment represents the first line of treatment, being less invasive than surgical treatment (95), and is indicated in symptomatic patients and in cases of persistent elevation of alkaline phosphatase and/or bilirubin values for more than one month (114).
Endoscopic treatment of scarring stenosis of the biliary duct from chronic pancreatitis involves the temporary insertion of MPS or FCSEMS (114).
The use of MPS involves cannulating the biliary duct, performing sphincterotomy, dilating the stenosis, and inserting multiple 10 F PSs. The procedure should be repeated every 3-4 months until the incision of the stenosis is no longer visible on a balloon cholangiography and the contrast medium flows freely into the duodenum. Placement of MPS has demonstrated clinical success ranging from 44% to 92% (97).
Treatment with FCSEMS, on the other hand, involves the insertion of an FCSEMS at the level of the distal biliary duct and generally does not require endoscopic reintervention before 6 months. FCSEMS are often preferred because they have a larger diameter than multiple plastic stents (a 10 mm FCSEMS has the equivalent diameter of 7 10 F PSs) and require less endoscopic reintervention (115).
Two RCTs (116, 117), performed in patients with benign distal stenosis of the biliary duct secondary to chronic pancreatitis, demonstrated equal clinical success and equal adverse events in patients treated with MPS and patients treated with FCSEMS. The RCT conducted by Ramchandani et al. (117) with a 2-year follow-up, showed 77.1% (54/70) stenosis resolution in the group with MPS and 75.8% (47/62) in the group with FCSEMS. The group of patients with MPS was treated with placement of at least two 8.5 or 10 F PS side-by-side. Patients were then endoscopically re-evaluated at 4 and 8 months for replacement and addition of more PSs. At 12 months, the PSs were removed, and the therapy outcome was evaluated using cholangiography. In the group of patients carrying FCSEMS, an 8- or 10-mm diameter FCSEMS was placed, and the endoscopic removal procedure was scheduled after 12 months in which, as in the other group, the outcome of treatment was also evaluated with cholangiography. This study brought an important technical contribution: a reasonable safety profile was demonstrated in the removal of in the removal of FCSEMS at 12 months, rather than at 6 months as in other studies (118). Based on this evidence, a cost-effective analysis was recently conducted that demonstrated that treatment with FCSEMS with stent removal after 12 months is cost-effective compared with treatment with MPS (119). However, further studies with more prolonged follow-up are needed because there may also be recurrences of late stenosis (120).
4.2. Benign biliary tract stenosis post liver transplantation
Post-liver transplantation biliary tract stenoses arise more frequently after living donor transplantation than after deceased donor transplantation (121) and can be distinguished into anastomotic biliary stenosis (ABS) and non-anastomotic biliary stenosis (NABS) (122).
ABS are the most common and can occur at both duct-to-duct biliary anastomoses and hepaticojejunal anastomoses. They are usually single, short stenoses and are located, in the case of deceased donor transplantation, at the level of the middle choledochus or, in the case of living donor transplantation, near the hepatic hilum. ABS are secondary to the fibrotic healing process of the biliary epithelium and local tissue ischemia (123).
NABS, on the other hand, are localized intra- and extrahepatically at least 5 mm proximal to the anastomosis. Their etiopathogenesis is multifactorial (124) but is generally associated with underlying tissue ischemia (125).
A peculiar feature of post-transplant stenoses is that, even in cases of tight stenosis, the bile ducts upstream of the stenosis, probably due to the presence of fibrosis, do not show the same degree of dilatation as would occur in non-transplanted liver ducts (126).
Persistent elevation of cholestasis indices after transplantation, even in asymptomatic patients, should raise suspicion of biliary stenosis (127, 128). Suspected biliary stenosis should therefore be investigated by inserting contrast medium into the T-tube if still in place, or if not, by an MRCP scan (129).
The therapeutic approach to post-transplant stenosis should be discussed in multidisciplinary settings in highly specialized centers (130).
For stenoses of duct-to-duct anastomoses the first-line approach is endoscopic, whereas for stenoses on hepaticojejunal anastomoses the percutaneous approach is generally preferred. Although, with the improvement of enteroscopic techniques (131, 132) endoscopic access to the papilla is now possible in 68-93% of cases (133, 134).
Endoscopic treatment usually involves performing sphincterotomy, balloon dilatation (4-6 mm) of the stenosis, and placement of MPS. Because of the small diameter of the donor ducts, it is sometimes necessary to insert small caliber MPS (7 or 8.5 F). Every 3 months the procedure is repeated with additional stents added until the stenosis is resolved (135). The response time to endoscopic therapy varies according to the time of presentation of stenosis after the liver transplantation. In fact, ABS arising in the first 30 days postoperatively are associated with a good response within 6 months of treatment (136), while late presentation ABS (>90 days) generally need prolonged treatment to avoid recurrence (137).
In the recent prospective study by Tarantino et al. (138) of 87 patients with post-transplant ABS stenosis, treatment with MPS demonstrated 98.9% clinical success in an average treatment time of 8 months with an average of 4.7 endoscopic procedures and an average placement of 3.7 PS.
However, treated patients need to be followed up over time because recurrence of stenosis can occur even years after treatment. In fact, long-term follow-up of MPS-treated patients demonstrated recurrence of stenosis in about 6 percent of patients with an average follow-up of 5.8 years (139). In the case of recurrence of stenosis, endoscopic treatment with dilatation and placement of MPS is effective (140).
To prevent the need to repeat endoscopic procedures every 3 months to replace and add PS, the use of FCSEMS was also studied in patients with ABS. The use of FCSEMS would allow stent replacement every 6 months resulting in only two endoscopic procedures per year. Three RCTs were performed (141–143) that did not demonstrate superiority of treatment with FCSEMS over treatment with MPS. The high migration rate is the main limiting factor for the use of FCSEMS in ABS (144). A recent systematic review of studies of endoscopically treated ABS patients reported a FCSEMS migration rate of 16% (145). To reduce the risk of FCSEMS migration, an FCSEMS with an antimigration waist can be used that can be fully released intraductally due to the presence of a long wire that reaches the duodenum and allows easy subsequent removal. This stent has demonstrated a migration rate reduced to 0-3% (146, 147). FCSEMS with antimigration fins have also been developed and have shown promising results (145).
Treatment of NABS is more complicated, especially if stenoses arise more than 1 year after transplantation (148), and long-term therapeutic success is limited to 50-75% of cases with up to 25-50% of patients eventually requiring retransplantation or dying (149).
4.3. Post-cholecystectomy biliary tract injuries
Post-laparoscopic cholecystectomy biliary tract injuries are usually the result of direct surgical trauma and may be related to a leak from the cystic stump, lesions of the main biliary duct, and/or lesions of the aberrant right sectoral hepatic ducts.
The clinical presentation varies depending on the type of lesion found and whether a leak is present or not. The MRCP is important in planning the therapeutic strategy (150).
For postoperative stenosis of the main biliary duct, the endoscopic approach is the first line of treatment and involves MPS insertion with reintervention every 3-4 months, increasing the number of stents until the resolution of the stenosis has been achieved (151). FCSEMS stents may be considered if the stenosis is located at least 2 cm below the hepatic confluence (2).
After treatment of stenosis with MPS, patients with an average follow-up of 13 years had no recurrence of stenosis in 88.6% (31/35) of cases, and the recurrences were all successfully treated endoscopically (152).
A recent cohort study (153) of 154 patients with post-cholecystectomy biliary tract stenosis treated with MPS reported a resolution of stenosis in 96.7% of patients. Patients underwent an average of 4.2 ± 1.5 endoscopic procedures over an average treatment period of 11.8 ± 6.4 months with the placement of an average number of 4.3 ± 1.6 stents.
The use of FCSEMS in post-cholecystectomy stenosis was recently evaluated in a subgroup analysis of an international study on the evaluation of the efficacy of FCSEMS in benign biliary stenosis (154). It showed significantly inferior results compared to treatment with MPS. In fact, after a mean permanence time of 10.9 months, the resolution of stenosis occurred in only 72% (13/18) of patients and at 5-year follow-up, only 61% of these patients had no recurrence of stenosis.
In the case of leakage from the cystic duct or the Luschka’s duct, the goal is to eliminate the transpapillary pressure gradient to better drain the bile. This can be achieved by placing a PS in the main biliary duct with or without the performance of sphincterotomy (155). Two recent meta-analyses (156, 157) compared the treatment of biliary duct leaks with sphincterotomy alone, with PS placement alone, or with PS placement associated with the performance of sphincterotomy. They revealed that combined treatment is associated with a higher therapeutic success rate than the other treatments.
In case of refractory leaks, MPS or FCSEMS placement should be a strategy for rescue endotherapy (158).
Regarding aberrant segmental bile duct lesions (159), if an aberrant duct stenosis is found, treatment with MPS is effective. However, for the remaining types of damage, the indication and outcome of endoscopic treatment vary depending on the type of lesion reported according to Strasberg’s classification (160). In fact, for lesions in which there is complete transection of the aberrant duct with a leak, cannulation of the aberrant duct from the cystic stump or the main biliary duct may be attempted. If successful, a plastic stent may be placed to create an “endoscopic anastomosis” and thus achieve closure of the fistula. After 4-8 weeks after placement of the plastic stent, removal of the stent and subsequent dilatation of the aberrant duct with the placement of MPS can be performed. In contrast, in the case of complete occlusion of the aberrant duct without a leak, the “wait and see” strategy is preferred considering the high endoscopic failure rate and possible good outcome without any specific treatment.
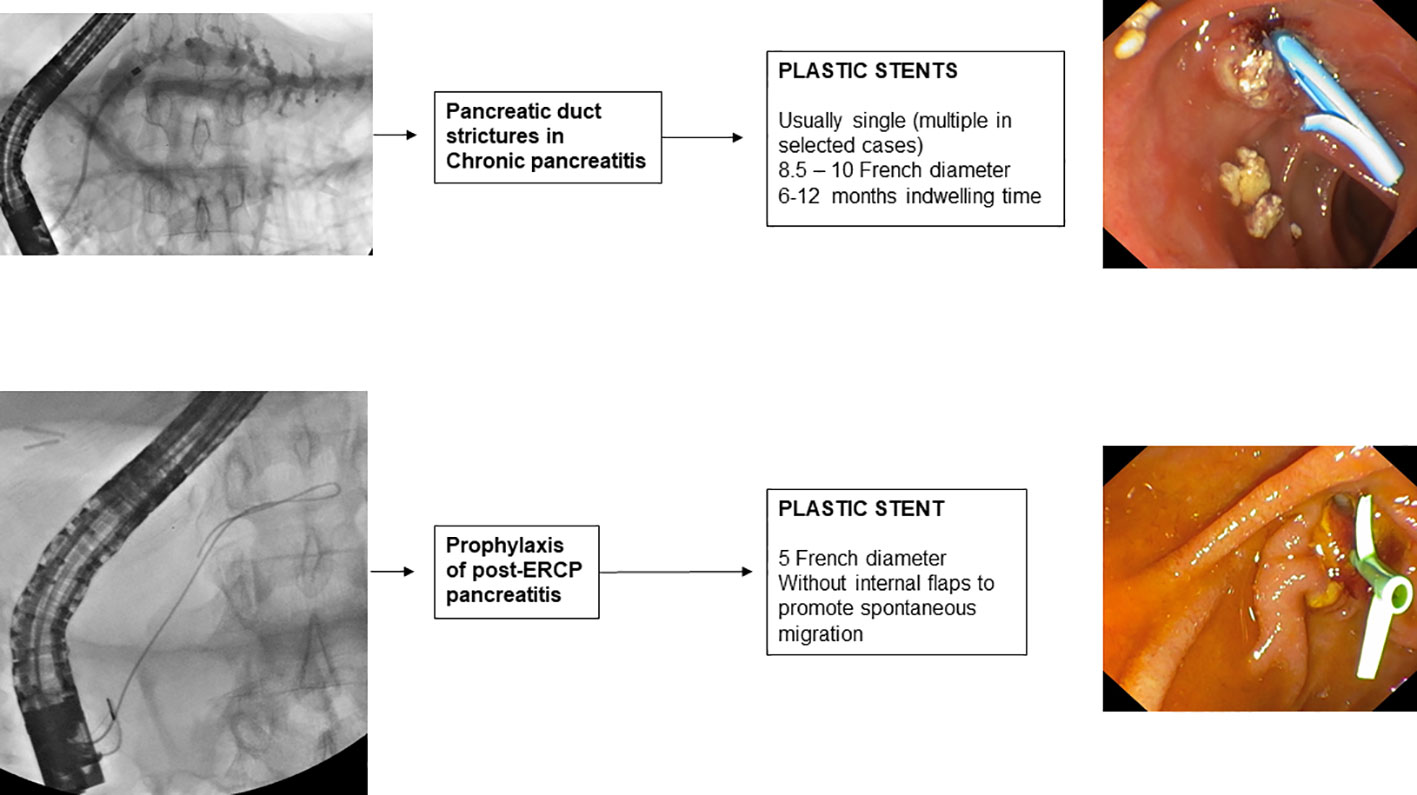
5. Pancreatic duct stenosis in chronic pancreatitis
The main symptom of chronic pancreatitis is pain. Pain is exacerbated by alcohol intake, by cigarette smoking and by a diet inadequate for pancreatic function. Ductal hypertension, a condition that develops as a result of the presence of pancreatolithiasis and/or pancreatic duct stenosis, plays a major role in its etiopathogenesis.
The treatment of a patient with chronic pancreatitis with a pancreatic duct stenosis who presents with pain symptoms unresponsive to medical therapy should be discussed in a multidisciplinary setting. The patient should be studied by imaging techniques, such as CT and MR with MRCP, to rule out an underlying malignancy. MRCP is the most accurate imaging technique to visualize pancreatic duct stenosis and assess the extent of dilatation upstream of the duct (161).
Guidelines (114, 162) recommend as the first-line endoscopic treatment of pancreatic stenosis, the insertion at the level of the stenosis of a single 10 F straight plastic stent, which can be left in place for 1 year if pain symptoms are in remission. The endoscopic procedure involves cannulation of the main pancreatic duct, sphincterotomy, pneumatic dilatation of the stenosis, and subsequent placement of a plastic stent of 8.5 or 10 F with a variable length, depending on the size of the stenosis. The procedure leads to pain remission in 70-94% of patients (163).
However, about one-third of patients require prolonged stenting beyond 1 year (164). In this group of patients, more aggressive treatment may improve the clinical outcome, which is why the guidelines suggest considering MPS insertion increasing the radial force of dilatation. Treatment with MPS in patients with refractory pancreatic stenosis was proposed in 2006 by Costamagna et al. (165) who reported the treatment of 19 patients in whom an average of 3 8.5-11.5 F PSs were placed and were removed after 6-12 months. This treatment resulted in the resolution of pain symptoms in 84% of patients and no major complications were reported. Prior to MPS placement, the endoscopic procedure involves removal of the previously placed single pancreatic plastic stent, cannulation with a 0.035-inch hydrophilic guidewire of the pancreatic duct, and subsequent pneumatic dilation of the stenosis with a dilation balloon selected according to the diameter (6-10 mm) and length (2 ± 4 cm) of the stenosis. In the placement of multiple PSs, the stent of the largest extension and size (10 or 11.5 F) is inserted first to reduce the risk of stent migration into the pancreatic duct during the release of subsequent PSs. After the first stent, as many stents of the same or smaller size as possible are inserted into the stenosis. After 6 to 12 months of treatment, all PSs are removed endoscopically with forceps or a loop, dilatation of the stenosis is verified by passing a 12 mm balloon, and, upon completion, a 6 F nasopancreatic drainage tube is placed with the tip in the tail of the pancreas. Temporary placement of a nasopancreatic drainage tube allows the outflow of contrast medium from the pancreatic duct into the duodenum to be checked and any onset of pain during forced injection to be noted.
In a subsequent 2019 follow-up study of the same group (166), in which 48 patients with refractory pancreatic stenosis were included, after treatment with MPS, the stenosis was resolved in 83.3% (40/48) of patients. The 8 patients that were refractory to MPS treatment underwent a second MPS treatment with resolution of stenosis occurring in 3 patients. The 5 patients who did not show resolution of stenosis even after the second treatment refused surgery and continued to be treated endoscopically with a single plastic stent replacement annually or on-demand (167). During an average follow-up of 9.5 years, 74.4% (32/43) of treated patients that had resolution of stenosis remained asymptomatic. 25.6% (11/43) of the patients had a recurrence of pain after a mean time of 26.4 months and underwent endoscopic follow-up: in 3 of these patients a recurrence of pancreatic stenosis was evidenced requiring re-stenting while in the remaining 8 no stenosis was evidenced and the pancreatic duct was successfully drained after plugs extraction. No major complications were reported in all treatments performed.
The major drawback of this approach is the need for multiple sessions of endotherapy, which may affect patient compliance (168). Therefore, in patients with refractory pancreatic stenosis, the use of FCSEMS has been studied (169). In fact, specific FCSEMS have been developed that can be applied in pancreatic stenosis. These stents have a central portion with irregular cells that impart a different radial force that reduces their migration. In the prospective study with a long follow-up (170), this type of FCSEMS was placed in 15 patients, and after treatment, 90% of the patients remained asymptomatic after 3 years. Prior to insertion of the FCSEMS at the level of the pancreatic stenosis, removal of the previously placed plastic stent and a mechanical dilatation with an 8.5 F Soehendra dilator or, if the mechanical dilatation cannot be performed, a pneumatic dilatation with a 4 mm balloon should be performed. A 6 or 8 mm FCSEMS is then placed, depending on the diameter of the pancreatic duct upstream of the stenosis and varying in length depending on the stenosis. Finally, the FCSEMS is removed 6 months after placement.
In a recent meta-analysis of studies of patients with refractory pancreatic stenosis treated endoscopically (171), treatment with FCSEMS appears to have similar efficacy to treatment with MPS but has a higher risk of adverse events (38.6% vs 14.3%). The main adverse events reported with FCSEMS are risk of migration (12.8%), risk of bile duct occlusion (7.7%), and development of de novo pancreatic duct stenosis (up to 27%%). In contrast, the latter complication has never been reported in MPS treatment. The occurrence of abdominal pain such that the FCSEMS had to be removed was also reported in patients in whom a 10 mm FCSEMS, rather than an 8 mm FCSEMS, had been placed (172).
Pancreatic stenting is also an option to “by-pass” pancreatic stones obtaining ductal decompression and asses symptoms relief; pancreatic stenting in this setting is complex and need the integration with extracorporeal and intraductal lithotripsy.
6. Prophylactic pancreatic duct stenting
The risk of post-ERCP pancreatitis (PEP) increases if, inadvertently, the main pancreatic duct is opacified or a guidewire is inserted into it. A recent RCT (173), conducted in 167 patients undergoing ERCP for the first time in whom the pancreatic duct was inadvertently cannulated, compared placement of a 5 F pancreatic plastic stent with no stent placement. The study showed that prophylactic placement of a pancreatic plastic stent significantly reduced the risk of post-ERCP pancreatitis (12.6% vs. 25%). The most recent meta-analyses available in the literature (174, 175) also confirm this evidence. Therefore, guidelines (176) recommend that in case of inadvertent cannulation or opacification of the pancreatic duct, a prophylactic pancreatic stent should be placed. The use of a 3 or 5 cm 5 F plastic (177) pancreatic stent without an internal flange (178), but with a duodenal flange or pigtail is preferred to prevent intraductal migration of the stent (179). Spontaneous distal migration of the pancreatic stent should be confirmed by direct abdominal imaging after 7 to 15 days, and if the stent is still in place, endoscopic removal is necessary. Biodegradable stents are under evaluation for PEP prophylaxis avoiding the need for radiological control and eventual endoscopic procedure to remove the stent; lack of data and high cost limt the use of biodegradable bilio-pancreatic stents in clinical practice.
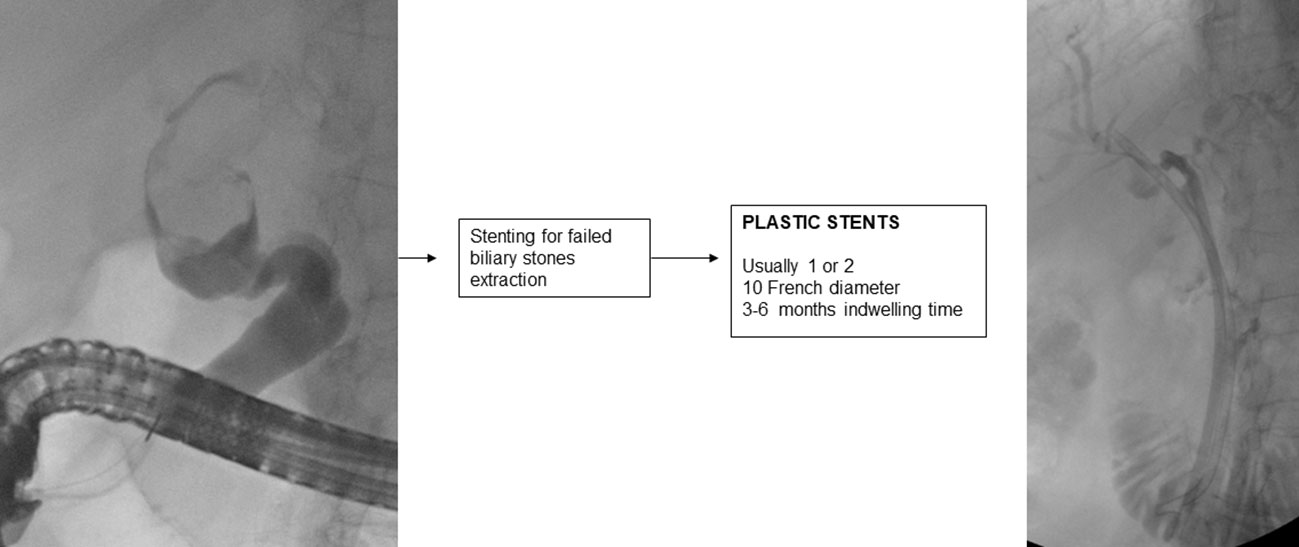
7. Stenting for failed extraction of biliary stones
If biliary lithiasis cannot be treated endoscopically because of a failure of available treatments and/or the patient’s clinical condition, temporary placement of a plastic stent is recommended (2).
A 10 F straight or double-pigtail plastic stent is usually placed, which allows passage of bile into the space created by the stent between the stone and that part of the biliary tract causing continuous friction of the stone, which aided by respiratory movement, reduces its size in 3-6 months in about half of the cases (180–182).
It is important to endoscopically re-evaluate the patient within 3 (183) to 6 months, because if left in place for longer, the plastic stent may promote the development of severe and even fatal cholangitis (184).
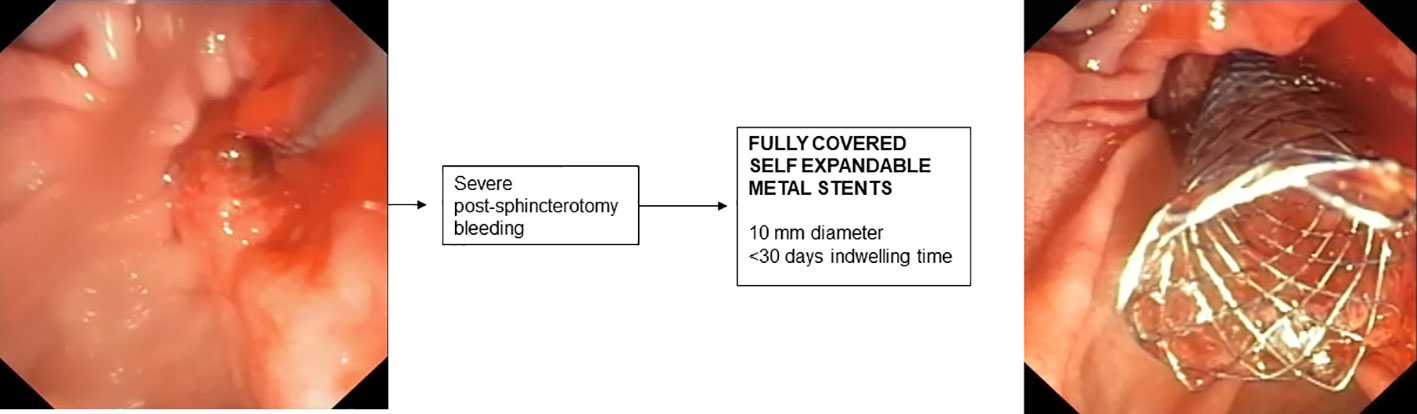
8. Stenting for post-sphincterotomy bleeding
In most cases, post-sphincterotomy bleeding resolves spontaneously (185). In cases of massive bleeding requiring urgent endoscopic treatment, placement of an FCSEMS is recommended when bleeding is refractory to standard hemostatic treatments (176). In retrospective studies, treatment with FCSEMS appears effective and safe (186, 187) and early placement of FCSEMS appears to reduce the incidence of recurrent bleeding (188). The optimal time for removal of FCSEMS after bleeding has stopped has not been established and is generally removed after a few days depending on the patient’s clinical condition. However, removal within 4 weeks is recommended to avoid the risk of complications related to “forgotten SEMS.”
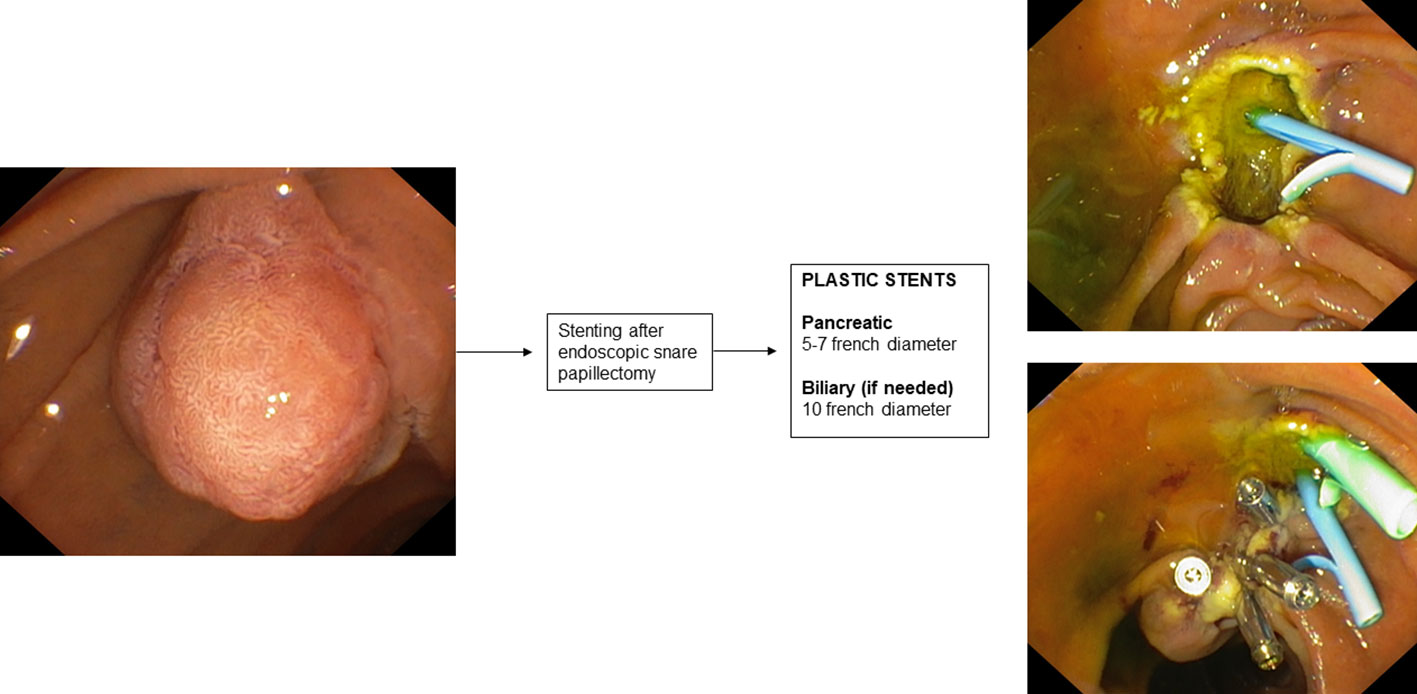
9. Stenting during endoscopic papillectomy
Mucosal cautery during endoscopic papillectomy leads to edema of the pancreatic orifice resulting in duct occlusion and increased risk of developing post-ERCP pancreatitis (189).
There is only one RCT in the literature (190) aimed at evaluating the prophylaxis of post-papillectomy pancreatitis by placement of a pancreatic plastic stent. This RCT involved 19 patients who underwent endoscopic papillectomy and were randomized into two groups according to whether or not they underwent pancreatic plastic stent placement after ampullary adenoma resection. In patients who underwent pancreatic stent placement, a 3- or 5 cm-long single-flange 5 F straight pancreatic plastic stent was placed. The study reported a significantly higher incidence of post-procedure pancreatitis in the group without a pancreatic stent (33% vs 0%). A recent systematic review with pooled analysis (191) also showed that the incidence of post-procedure pancreatitis varied significantly in subgroup analysis based on whether or not a pancreatic stent was placed after endoscopic papillectomy.
Therefore, according to this evidence, the guidelines (189) recommend prophylactic placement of a plastic pancreatic stent after endoscopic papillectomy. The use of biodegradable stents may also be a promising indication (192).
Clearly, patients with pancreas divisum do not fall under this indication, thus it is important to define the patient’s anatomy by performing MRCP before the procedure. Biliary stenting after endoscopic papillectomy is not routinely indicated. In some cases, such as suspected endobiliary growth of the ampullary adenoma or bleeding, placement of a plastic biliary stent may also be considered.
10. Conclusions
Endoscopic bilio-pancreatic stenting is a first-line therapeutic option for palliation, pre-operative drainage and treatment of several bilio-pancreatic diseases. Correct choice of the stent is essential for a “tailored” treatment of such complex diseases, avoiding serious problems related to the so called “forgotten stent”, especially in benign diseases. Figures 1–7 summarizes current indications and type of stents for an endoscopic approach to bilio-pancreatic strictures.
Author contributions
AC, RL, and AT contributed to conception and design of the study. AC wrote the first draft of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The Authors declare the following Competing Financial Interests: GC is a consultant for Boston Scientific, Cook Medical and Olympus. CG is a consultant for Microtech Europe GmbH and Boston Scientific. AT is a consultant for Boston Scientific and Olympus.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Singh A, Gelrud A, Agarwal B. Biliary strictures: diagnostic considerations and approach. Gastroenterol Rep (2015) 3(1):22–31. doi: 10.1093/gastro/gou072
2. Dumonceau JM, Tringali A, Papanikolaou IS, Blero D, Mangiavillano B, Schmidt A, et al. Endoscopic biliary stenting: indications, choice of stents, and results: European society of gastrointestinal endoscopy (ESGE) clinical guideline - updated October 2017. Endosc vol (2018) 50(9):910–30. doi: 10.1055/a-0659-9864
3. Viesca MFY, Arvanitakis M. Early diagnosis and management of malignant distal biliary obstruction: A review on current recommendations and guidelines. Clin Exp Gastroenterol (20192019) 12:415–32. doi: 10.2147/CEG.S195714
4. van der Gaag NA, Rauws EA, van Eijck CH, Bruno MJ, van der Harst E, Kubben FJ, et al. Preoperative biliary drainage for cancer of the head of the pancreas. N Engl J Med (2010) 362(2):129–37. doi: 10.1056/NEJMoa0903230
5. Lee PJ, Podugu A, Wu D, Lee AC, Stevens T, Windsor JA. Preoperative biliary drainage in resectable pancreatic cancer: a systematic review and network meta-analysis. HPB (2018) 20(6):477–86. doi: 10.1016/j.hpb.2017.12.007
6. Tanisaka Y, Mizuide M, Fujita A, Ogawa T, Katsuda H, Saito Y, et al. Current status of endoscopic biliary drainage in patients with distal malignant biliary obstruction. J Clin Med (2021) 10(19):4619. doi: 10.3390/jcm10194619
7. Miura F, Sano K, Wada K, Shibuya M, Ikeda Y, Takahashi K, et al. Prognostic impact of type of preoperative biliary drainage in patients with distal cholangiocarcinoma. Am J Surg (2017) 214:256–61. doi: 10.1016/j.amjsurg.2017.01.010
8. Takahashi Y, Nagino M, Nishio H, Ebata T, Igami T, Nimura Y. Percutaneous transhepatic biliary drainage catheter tract recurrence in cholangiocarcinoma. Br J Surg (2010) 97(12):1860–6. doi: 10.1002/bjs.7228
9. Kuwatani M, Nakamura T, Hayashi T, Kimura Y, Ono M, Motoya M, et al. Clinical outcomes of biliary drainage during a neoadjuvant therapy for pancreatic cancer: Metal versus plastic stents. Gut Liver (2020) 14:269–73. doi: 10.5009/gnl18573
10. Tamura T, Itonaga M, Ashida R, Yamashita Y, Hatamaru K, Kawaji Y, et al. Covered self-expandable metal stents versus plastic stents for preoperative biliary drainage in patients receiving neo-adjuvant chemotherapy for borderline resectable pancreatic cancer: Prospective randomized study. Dig Endosc (2021) 33(7):1170–8. doi: 10.1111/den.13926
11. Hasegawa S, Kubota K, Yagi S, Kurita Y, Sato T, Hosono K, et al. Covered metallic stent placement for biliary drainage could be promising in the coming era of neoadjuvant chemo-radiation therapy for all pancreatic cancer. J Hepatobiliary Pancreat Sci (2021) 28(7):617–24. doi: 10.1002/jhbp.958
12. Nakamura K, Sho M, Akahori T, Nagai M, Nishiwada S, Nakagawa K, et al. A comparison between plastic and metallic biliary stent placement in patients receiving preoperative neoadjuvant chemoradiotherapy for resectable pancreatic cancer. World J Surg (2019) 43:642–8. doi: 10.1007/s00268-018-4820-6
13. Adams MA, Anderson MA, Myles JD, Khalatbari S, Scheiman JM. Self-expanding metal stents (SEMS) provide superior outcomes compared to plastic stents for pancreatic cancer patients undergoing neoadjuvant therapy. J Gastrointest Oncol (2012) 3(4):309–13. doi: 10.3978/j.issn.2078-6891.2011.050
14. Almadi MA, Gardner TB, Chen YI, Adam V, Barkun J, Barkun A. Use of stents in patients undergoing chemotherapy for borderline resectable pancreatic cancer-causing biliary obstruction while awaiting surgery: A cost-effectiveness analysis. Endosc Int Open (2021) 9(9):E1413–20. doi: 10.1055/a-1497-1562
15. Tol JAMG, van Hooft JE, Timmer R, Kubben FJ, van der Harst E, de Hingh IH, et al. Metal or plastic stents for preoperative biliary drainage in resectable pancreatic cancer. Gut (2016) 65(12):1981–7. doi: 10.1136/gutjnl-2014-308762
16. Tsuboi T, Sasaki T, Serikawa M, Ishii Y, Mouri T, Shimizu A, et al. Preoperative biliary drainage in cases of borderline resectable pancreatic cancer treated with neoadjuvant chemotherapy and surgery. Gastroenterol Res Pract (2016) 2016:7968201. doi: 10.1155/2016/7968201
17. Gardner TB, Spangler CC, Byanova KL, Ripple GH, Rockacy MJ, Levenick JM, et al. Cost-effectiveness and clinical efficacy of biliary stents in patients undergoing neoadjuvant therapy for pancreatic adenocarcinoma in a randomized controlled trial. Gastrointest Endosc (2016) 84:460–6. doi: 10.1016/j.gie.2016.02.047
18. Seo DW, Sherman S, Dua KS, Slivka A, Roy A, Costamagna G, et al. Covered and uncovered biliary metal stents provide similar relief of biliary obstruction during neoadjuvant therapy in pancreatic cancer: a randomized trial. Gastrointest Endosc (2019) 90(4):602–612.e4. doi: 10.1016/j.gie.2019.06.032
19. Yamashita Y, Tachikawa A, Shimokawa T, Yamazaki H, Itonaga M, Sakai Y, et al. Covered versus uncovered metal stent for endoscopic drainage of a malignant distal biliary obstruction: Meta-analysis. Dig Endosc (2022) 34(5):938–51. doi: 10.1111/den.14260
20. Nakai Y, Isayama H, Wang HP, Rerknimitr R, Khor C, Yasuda I, et al. International consensus statements for endoscopic management of distal biliary stricture. J Gastroenterol Hepatol (2020) 35:6. doi: 10.1111/jgh.14955
21. Nakai Y, Isayama H, Wang HP, Rerknimitr R, Khor C, Yasuda I, et al. Randomized trial of self-expanding metal stents versus polyethylene stents for distal malignant biliary obstruction. Lancet (1992) 340:1488–92. doi: 10.1016/0140-6736(92)92752-2
22. Knyrim K, Wagner HJ, Pausch J, Vakil N. A prospective, randomized, controlled trial of metal stents for malignant obstruction of the common bile duct. Endoscopy (1993) 25:207–12. doi: 10.1055/s-2007-1010294
23. Prat F, Chapat O, Ducot B, Ponchon T, Pelletier G, Fritsch J, et al. A randomized trial of endoscopic drainage methods for inoperable malignant strictures of the common bile duct. Gastrointest Endosc (1998) 47:1–7. doi: 10.1016/S0016-5107(98)70291-3
24. Isayama H, Yasuda I, Ryozawa S, Maguchi H, Igarashi Y, Matsuyama Y, et al. Results of a Japanese multicenter, randomized trial of endoscopic stenting for non-resectable pancreatic head cancer (JM-test): Covered wallstent versus double layer stent. Dig Endosc (2011) 23:310–5. doi: 10.1111/j.1443-1661.2011.01124.x
25. Walter D, van Boeckel PGA, Groenen MJ, Weusten BL, Witteman BJ, Tan G, et al. Cost efficacy of metal stents for palliation of extrahepatic bile duct obstruction in a randomized controlled trial. Gastroenterology (2015) 149:130–8. doi: 10.1053/j.gastro.2015.03.012
26. Walter D, van Boeckel PG, Groenen MJ, Weusten BL, Witteman BJ, Tan G, Brink MA, et al. Higher quality of life after metal stent placement compared with plastic stent placement for malignant extrahepatic bile duct obstruction: a randomized controlled trial. Eur J Gastroenterol Hepatol (2017) 29:231–237. doi: 10.1097/MEG.0000000000000762
27. Scatimburgo MVCV, Ribeiro IB, de Moura DTH, Sagae VMT, Hirsch BS, Boghossian MB, et al. Biliary drainage in inoperable malignant biliary distal obstruction: A systematic review and meta-analysis. World J gastrointest Surg vol (2021) 13:5. doi: 10.4240/wjgs.v13.i5.493
28. Tringali A, Hassan C, Rota M, Rossi M, Mutignani M, Aabakken L. Covered vs. uncovered self-expandable metal stents for malignant distal biliary strictures: a systematic review and meta-analysis. Endoscopy (2018) 50(06):631–41. doi: 10.1055/s-0043-125062
29. Conio M, Mangiavillano B, Caruso A, Filiberti RA, Baron TH, De Luca L, et al. Covered versus uncovered self-expandable metal stent for palliation of primary malignant extrahepatic biliary strictures: a randomized multicentre study. Gastrointest Endosc (2018) 88(2):283–91.e3. doi: 10.1016/j.gie.2018.03.029
30. Arias Dachary FJ, Chioccioli C, Deprez PH. Application of the “covered stent-in-uncovered-stent” technique for easy and safe removal of embedded biliary uncovered SEMS with tissue ingrowth. Endoscopy (2010) 42(Suppl. 02):E304 – 305. doi: 10.1055/s-0030-1255792
31. Tan DM, Lillemoe KD, Fogel EL. A new technique for endoscopic removal of uncovered biliary self-expandable metal stents: stent-in-stent technique with a fully covered biliary stent. Gastrointest Endosc (2012) 75:923–925. doi: 10.1016/j.gie.2011.05.008
32. Lahlal M, Gigot JF, Annet L, Deprez PH. Successful endoscopic extraction of a double uncovered expandable metal stent. Endoscopy (2009) 41 Suppl 2:E98–9. doi: 10.1055/s-2008-1077770
33. Sakai Y, Sugiyama H, Kawaguchi Y, Kawashima Y, Hirata N, Nakaji S, et al. Uncovered versus covered metallic stents for the management of unresectable malignant distal biliary obstruction: a randomized multicenter trial. Scandinavian J Gastroenterol (2021) 56(10):1229–35. doi: 10.1080/00365521.2021.1938207
34. Saleem A, Leggett CL, Murad MH, Baron TH. Meta-analysis of randomized trials comparing the patency of covered and uncovered self-expandable metal stents for palliation of distal malignant bile duct obstruction. Gastrointest Endosc (2011) 74(2):321–7.e1-3. doi: 10.1016/j.gie.2011.03.1249
35. Almadi MA, Barkun AN, Martel M. No benefit of covered vs uncovered self-expandable metal stents in patients with malignant distal biliary obstruction: a meta-analysis. Clin Gastroenterol Hepatol (2013) 11(1):27–37.e1. doi: 10.1016/j.cgh.2012.10.019
36. Li J, Li T, Sun P, Yu Q, Wang K, Chang W, et al. Covered versus uncovered self-expandable metal stents for managing malignant distal biliary obstruction: a meta-analysis. PLoS One (2016) 11(2):e0149066. doi: 10.1371/journal.pone.0149066
37. Alastal Y, Hammad TA, Khan MA, Khalilet BW, Khan S, Ismail MK, et al. Risk of post-ERCP pancreatitis after placement of covered versus uncovered selfexpandable biliary metal stents: a systematic review and metaanalysis. JOP (2015) 16:452–8.
38. Isayama H, Komatsu Y, Tsujino T, Sasahira N, Hirano K, Toda N, et al. A prospective randomised study of “covered” versus “uncovered” diamond stents for the management of distal malignant biliary obstruction. Gut (2004) 53:729 –734. doi: 10.1136/gut.2003.018945
39. Suk KT, Kim HS, Kim JW, Baik SK, Kwon SO, Kim HG, et al. Risk factors for cholecystitis after metal stent placement in malignant biliary obstruction. Gastrointest Endosc (2006) 64(4):522–9. doi: 10.1016/j.gie.2006.06.022
40. Jung JH, Park SW, Hyun B, Lee J, Koh DH, Chung D. Identification of risk factors for obstructive cholecystitis following placement of biliary stent in unresectable malignant biliary obstruction: a 5-year retrospective analysis in single center. Surg endosc (2021) 35(6):2679–89. doi: 10.1007/s00464-020-07694-2
41. Hayashi T, Kawakami H, Osanai M, Ishiwatari H, Naruse H, Hisai H, et al. No benefit of endoscopic sphincterotomy before biliary placement of self-expandable metal stents for unresectable pancreatic cancer. Clin Gastroenterol Hepatol (2015) 13:1151–8. doi: 10.1016/j.cgh.2015.01.008
42. Mangiavillano B, Montale A, Frazzoni L, Bianchetti M, Sethi A, Repici A, et al. Endoscopic biliary self-expandable metallic stent in malignant biliary obstruction with or without sphincterotomy: systematic review and meta-analysis. Endosc Int Open vol (2019) 7(1):E26–35. doi: 10.1055/a-0752-9956
43. Takenaka M, Masatoshi K. Endoscopic reintervention for recurrence of malignant biliary obstruction: Developing the best strategy. Gut liver (2022) 16(4):525–34. doi: 10.5009/gnl210228
44. Jang SI, Lee KT, Choi JS, Jeong S, Lee DH, Kim YT, et al. Efficacy of a paclitaxel-eluting biliary metal stent with sodium caprate in malignant biliary obstruction: a prospective randomized comparative study. Endosc vol (2019) 51(9):843–51. doi: 10.1055/a-0754-5763
45. Mohan BP, Canakis A, Khan SR, Chandan S, Ponnada S, McDonough S, et al. Drug eluting versus covered metal stents in malignant biliary strictures-is there a clinical benefit?: A systematic review and meta-analysis. J Clin Gastroenterol (2021) 55(3):271–7. doi: 10.1097/MCG.0000000000001377
46. Lee YN, Moon JH, Choi HJ, Choi MH, Lee TH, Cha SW, et al. Effectiveness of a newly designed antireflux valve metal stent to reduce duodenobiliary reflux in patients with unresectable distal malignant biliary obstruction: a randomized, controlled pilot study (with videos). Gastrointest Endosc (2016) 83(2):404–12. doi: 10.1016/j.gie.2015.08.084
47. Kin T, Ishii K, Okabe Y, Itoi T, Katanuma A. Feasibility of biliary stenting to distal malignant biliary obstruction using a novel designed metal stent with duckbill-shaped anti-reflux valve. Dig Endosc (2021) 33:648–55. doi: 10.1111/den.13827
48. Yamada Y, Sasaki T, Takeda T, Mie T, Furukawa T, Kasuga A, et al. A novel laser-cut fully covered metal stent with anti-reflux valve in patients with malignant distal biliary obstruction refractory to conventional covered metal stent. J Hepatobiliary Pancreat Sci (2021) 28:563–71. doi: 10.1002/jhbp.966
49. Park DH, Lee SS, Lee TH, Ryu CH, Kim HJ, Seo DW, et al. Anchoring flap versus flared end, fully covered self-expandable metal stents to prevent migration in patients with benign biliary strictures: a multicenter, prospective, comparative pilot study (with videos). Gastrointest Endosc (2011) 73(1):64–70. doi: 10.1016/j.gie.2010.09.039
50. Minaga K, Kitano M, Imai H, Harwani Y, Yamao K, Kamata K, et al. Evaluation of anti-migration properties of biliary covered self-expandable metal stents. World J Gastroenterol (2016) 22(30):6917–24. doi: 10.3748/wjg.v22.i30.6917
51. Gerges C, Schumacher B, Terheggen G, Neuhaus H. Expandable metal stents for malignant hilar biliary obstruction. Gastrointest endosc Clinics North America (2011) 21(3):481–97. doi: 10.1016/j.giec.2011.04.004
52. Mansour JC, Aloia TA, Crane CH, Heimbach JK, Nagino M, Vauthey JN. Hilar cholangiocarcinoma: expert consensus statement. HPB (Oxford) (2015) 17:691–9. doi: 10.1111/hpb.12450
53. Vienne A, Hobeika E, Gouya H, Lapidus N, Fritsch J, Choury AD, et al. Prediction of drainage effectiveness during endoscopic stenting of malignant hilar strictures: the role of liver volume assessment. Gastrointest Endosc (2010) 72(4):728–35. doi: 10.1016/j.gie.2010.06.040
54. Gwon DI, Ko GY, Sung KB, Yoon HK, Shin JH, Hyoung Kim J, et al. Percutaneous biliary metallic stent placement in patients with unilobar portal vein occlusion caused by advanced hilar malignancy: outcome of unilateral versus bilateral stenting. Am J Roentgenol (2011) 197(4):795–801. doi: 10.2214/AJR.11.6424
55. Boškoski I, Schepis T, Tringali A, Familiari P, Bove V, Attili F, et al. Personalized endoscopy in complex malignant hilar biliary strictures. J personalized Med (2021) 11(2):78. doi: 10.3390/jpm11020078
56. Larghi A, Tringali A, Lecca PG, Giordano M, Costamagna G. Management of hilar biliary strictures. Am J Gastroenterol (2008) 103(2):458–73. doi: 10.1111/j.1572-0241.2007.01645.x
57. Moole H, Dharmapuri S, Duvvuri A, Dharmapuri S, Boddireddy R, Moole V, et al. Endoscopic versus percutaneous biliary drainage in palliation of advanced malignant hilar obstruction: a meta-analysis and systematic review. Can J Gastroenterol Hepatol (2016) 2016:8. article 4726078. doi: 10.1155/2016/4726078
58. Bismuth H, Majno PE. Biliary strictures: classification based on the principles of surgical treatment. World J Surg (2001) 25(10):1241–4. doi: 10.1007/s00268-001-0102-8
59. Tringali A, Boškoski I, Costamagna G. Endoscopic stenting in hilar cholangiocarcinoma: When, how, and how much to drain? Gastroenterol Res Pract (2019) 2019:5161350. doi: 10.1155/2019/5161350
60. Liu F, Li Y, Wei Y, Li B. Preoperative biliary drainage before resection for hilar cholangiocarcinoma: whether or not? A systematic Rev Dig Dis Sci (2011) 56:663–672. doi: 10.1007/s10620-010-1338-7
61. Celotti A, Solaini L, Montori G, Coccolini F, Tognali D, Baiocchi G. Preoperative biliary drainage in hilar cholangiocarcinoma: systematic review and meta-analysis. Eur J Surg Oncol (2017) 43(9):1628–35. doi: 10.1016/j.ejso.2017.04.001
62. Liu F, Li Y, Wei Y, Li B. Preoperative biliary drainage before resection for hilar cholangiocarcinoma: whether or not? a systematic review. Digestive Dis Sci (2011) 56(3):663–72. doi: 10.1007/s10620-010-1338-7
63. Wiggers JK, Groot Koerkamp B, Cieslak KP, Doussot A, van Klaveren D, Allen PJ, et al. Postoperative mortality after liver resection for perihilar cholangiocarcinoma: development of a risk score and importance of biliary drainage of the future liver remnant. J Am Coll Surg (2016) 223(2):321–331.e321. doi: 10.1016/j.jamcollsurg.2016.03.035
64. Qumseya BJ, Jamil LH, Elmunzer BJ, Riaz A, Ceppa EP, Thosani NC, et al. ASGE guideline on the role of endoscopy in the management of malignant hilar obstruction. Gastrointest endosc (2021) 94(2):222–34.e22. doi: 10.1016/j.gie.2020.12.035
65. Wang L, Lin N, Xin F, Ke Q, Zeng Y, Liu J. A systematic review of the comparison of the incidence of seeding metastasis between endoscopic biliary drainage and percutaneous transhepatic biliary drainage for resectable malignant biliary obstruction. World J Surg Oncol (2019) 17:116. doi: 10.1186/s12957-019-1656-y
66. Hirano S, Tanaka E, Tsuchikawa T, Matsumoto J, Kawakami H, Nakamura T, et al. Oncological benefit of preoperative endoscopic biliary drainage in patients with hilar cholangiocarcinoma. J Hepatobil Pancreat Sci (2014) 21:533–40. doi: 10.1002/jhbp.76
67. Kawashima H, Itoh A, Ohno E, Itoh Y, Ebata T, Nagino M, et al. Preoperative endoscopic nasobiliary drainage in 164 consecutive patients with suspected perihilar cholangiocarcinoma: a retrospective study of efficacy and risk factors related to complications. Ann Surg (2013) 257:121–127. doi: 10.1097/SLA.0b013e318262b2e9
68. Lin H, Pezzilli R, Liu X. The safety and efficacy of nasobiliary drainage versus biliary stenting in malignant biliary obstruction: A systematic review and meta-analysis. Medicine (2016) 95:e5253. doi: 10.1097/MD.0000000000005253
69. Kawakami H, Kuwatani M, Onodera M, Haba S, Eto K, Ehira N, et al. Endoscopic nasobiliary drainage is the most suitable preoperative biliary drainage method in the management of patients with hilar cholangiocarcinoma. J Gastroenterol (2011) 46:242–248. doi: 10.1007/s00535-010-0298-1
70. Squadroni M, Tondulli L, Gatta G, Mosconi S, Beretta G, Labianca R. Cholangiocarcinoma. Crit Rev Oncol (2017) 116:11–31. doi: 10.1016/j.critrevonc.2016.11.012
71. Neu B, Nennstiel S, Von Delius S, Abdelhafez M, Bajbouj M, Schmid R, et al. Endoscopic rendez-vous reconstruction of complete biliary obstruction. Dig Liver Dis (2017) 49:769–72. doi: 10.1016/j.dld.2017.01.170
72. Takahashi E, Fukasawa M, Sato T, Takano S, Kadokura M, Shindo H, et al. Biliary drainage strategy of unresectable malignant hilar strictures by computed tomography volumetry. World J Gastroenterol (2015) 21(16):4946–53. doi: 10.3748/wjg.v21.i16.4946
73. Bulajic M, Panic N, Radunovic M, Scepanovic R, Perunovic R, Stevanovic P, et al. Clinical outcome in patients with hilar malignant strictures type II bismuth-corlette treated by minimally invasive unilateral versus bilateral endoscopic biliary drainage. Hepatobiliary Pancreatic Dis Int (2012) 11(2):209–14. doi: 10.1016/S1499-3872(12)60150-7
74. Costamagna G, Tringali A. Can we insert a covered stent, partially or not, in case of hilar biliary stenosis? Endosc Int Open (2017) 5(12):E1218–9. doi: 10.1055/s-0043-120665
75. Sangchan A, Kongkasame W, Pugkhem A, Jenwitheesuk K, Mairiang P. Efficacy of metal and plastic stents in unresectable complex hilar cholangiocarcinoma: a randomized controlled trial. Gastrointest Endosc (2012) 76:93–9. doi: 10.1016/j.gie.2012.02.048
76. Jarnagin WR, Fong Y, DeMatteo RP, Gonen M, Burke EC, Bodniewicz BS J, et al. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg (2001) 234:507–17. doi: 10.1097/00000658-200110000-00010
77. Wagner HJ, Knyrim K, Vakil N, Klose KJ. Plastic endoprostheses versus metal stents in the palliative treatment of malignant hilar biliary obstruction. a prospective and randomized trial. Endoscopy (1993) 25:213–8. doi: 10.1055/s-2007-1010295
78. Chang WH, Kortan P, Haber GB. Outcome in patients with bifurcation tumors who undergo unilateral versus bilateral hepatic duct drainage. Gastrointest Endosc (1998) 47:354–62. doi: 10.1016/S0016-5107(98)70218-4
79. Deviere J, Baize M, de Toeuf J, Cremer M. Long-term follow-up of patients with hilar malignant stricture treated by endoscopic internal biliary drainage. Gastrointest Endosc (1988) 34:95–101. doi: 10.1016/S0016-5107(88)71271-7
80. Yasuda I, Mukai T, Moriwaki H. Unilateral versus bilateral endoscopic biliary stenting for malignant hilar biliary strictures. Dig Endosc (2013) 25(Suppl. 02):81–85. doi: 10.1111/den.12060
81. Singh V, Singh G, Verma GR, Singh K, Gulati M. Contrast-free unilateral endoscopic palliation in malignant hilar biliary obstruction: new method. J Gastroenterol Hepatol (2004) 19:589–92. doi: 10.1111/j.1440-1746.2003.03313.x
82. Fugazza A, Capogreco A, Repici A. Endoprosthetics for luminal obstruction. Techniques Innov Gastrointest Endosc (2022) 22(4):192–9. doi: 10.1016/j.tige.2020.06.003
83. Hookey LC, Le Moine O, Deviere J. Use of a temporary plastic stent to facilitate the placement of multiple self-expanding metal stents in malignant biliary hilar strictures. Gastrointest endosc (2005) 62(4):605–9. doi: 10.1016/j.gie.2005.04.051
84. Isayama H, Fujisawa T, Ishii S, Saito H, Suzuki A, Takasaki Y, et al. SEMS insertion for hilar stricture: Which stent, how and why? In: Lee D, editor. Advanced ERCP for complicated and refractory biliary and pancreatic diseases. Singapore: Springer (2020).
85. Kogure H, Isayama H, Kawakubo K, Sasaki T, Yamamoto N, Hirano K, et al. Endoscopic bilateral metallic stenting for malignant hilar obstruction using newly designed stents. J Hepatobiliary Pancreat Sci (2011) 18(5):653–7. doi: 10.1007/s00534-011-0407-4
86. Kogure H, Isayama H, Nakai Y, Tsujino T, Matsubara S, Yashima Y, et al. High single-session success rate of endoscopic bilateral stent-in-stent placement with modified large cell niti-s stents for malignant hilar biliary obstruction. Dig Endosc (2014) 26(1):93–9. doi: 10.1111/den.12055
87. Lee TH, Moon JH, Park S. Biliary stenting for hilar malignant biliary obstruction. Dig Endosc (2020) 32:275–86. doi: 10.1111/den.13549
88. Boškoski I, Tringali A, Familiari P, Bove V, Landi R, Attili F, et al. A 17 years retrospective study on multiple metal stents for complex malignant hilar biliary strictures: Survival, stents patency and outcomes of re- interventions for occluded metal stents. Digestive Liver Dis (2019) 51(9):1287–93. doi: 10.1016/j.dld.2019.03.032
89. Okamoto T, Fujioka S, Yanagisawa S, Yanaga K, Kakutani H, Tajiri H, et al. Placement of a metallic stent across the main duodenal papilla may predispose to cholangitis. Gastrointest Endosc (2006) 63(6):792–6. doi: 10.1016/j.gie.2005.05.015
90. Gerhardt T, Rings D, Höblinger A, Heller J, Sauerbruch T, Schepke M, et al. Combination of bilateral metal stenting and trans-stent photodynamic therapy for palliative treatment of hilar cholangiocarcinoma. Z für Gastroenterologie (2010) 48(1):28–32. doi: 10.1055/s-0028-1109983
91. Inoue T, Ibusuki M, Kitano R, Kobayashi Y, Ohashi T, Nakade Y, et al. Endobiliary radiofrequency ablation combined with bilateral metal stent placement for malignant hilar biliary obstruction. Endoscopy (2020) 52:595–9. doi: 10.1055/a-1133-4448
92. Kadayifci A, Atar M, Forcione DG, Casey BW, Kelsey PB, Brugge WR. Radiofrequency ablation for the management of occluded biliary metal stents. Endoscopy (2016) 48(12):1096–101. doi: 10.1055/s-0042-115938
93. Tringali A. Endobiliary radiofrequency ablation: promising but not fully standardized. ” Endosc (2020) 52(7):535–6. doi: 10.1055/a-1167-2502
94. Schepis T, Boškoski I, Tringali A, Costamagna G. Role of endoscopic retrograde cholangiopancreatography in benign biliary strictures. Gastrointest Endosc Clinics North America (2022) 32(3):455–75. doi: 10.1016/j.giec.2022.01.006
95. Hu B, Sun B, Cai Q, Wong Lau JY, Ma S, Itoi T, et al. Asia-Pacific consensus guidelines for endoscopic management of benign biliary strictures. Gastrointest endosc vol (2017) 86:1. doi: 10.1016/j.gie.2017.02.031
96. Costamagna G, Pandolfi M, Mutignani M, Spada C, Perri V. Long-term results of endoscopic management of postoperative bile duct strictures with increasing numbers of stents. Gastrointest Endosc (2001) 54(2):162–8. doi: 10.1067/mge.2001.116876
97. Draganov P, Hoffman B, Marsh W, Cotton P, Cunningham J. Long-term outcome in patients with benign biliary strictures treated endoscopically with multiple stents. Gastrointest Endosc (2002) 55(6):680–6. doi: 10.1067/mge.2002.122955
98. Chiba M, Kato M, Kinoshita Y, Shimamoto N, Tomita Y, Abe T, et al. Best period to replace or change plastic stents with self-expandable metallic stents using multivariate competing risk regression analysis. Sci Rep (2020) 10(1):13080. doi: 10.1038/s41598-020-70081-3
99. Lawrence C, Romagnuolo J, Payne KM, Hawes RH, Cotton PB. Low symptomatic premature stent occlusion of multiple plastic stents for benign biliary strictures: comparing standard and prolonged stent change intervals. Gastrointest Endosc (2010) 72:558–63. doi: 10.1016/j.gie.2010.05.029
100. Tringali A, Bove V, Costamagna G. Endoscopic approach to benign biliary obstruction. Gastrointest Intervention (2015) 4(Issue 1):1–8. doi: 10.1016/j.gii.2015.04.001
101. Devière J, Nageshwar Reddy D, Püspök A, Ponchon T, Bruno MJ, Bourke MJ, et al. Successful management of benign biliary strictures with fully covered self-expanding metal stents. Gastroenterology (2014) 147(2):385–95. doi: 10.1053/j.gastro.2014.04.043
102. Wong MY, Saxena P, Kaffes AJ. Benign biliary strictures: A systematic review on endoscopic treatment options. Diagnostics (Basel) (2020) 10(4):221. doi: 10.3390/diagnostics10040221
103. Kamal F, Ali Khan M, Lee-Smith W, Sharma S, Acharya A, Imam Z, et al. Metal versus plastic stents in the management of benign biliary strictures: systematic review and meta-analysis of randomized controlled trials. Eur J Gastroenterol Hepatol (2022) 34(5):478–87. doi: 10.1097/MEG.0000000000002352
104. Khan MA, Baron TH, Kamal F, Ali B, Nollan R, Ismail MK, et al. Efficacy of self expandable metal stents in management of benign biliary strictures and comparison with multiple plastic stents: a meta-analysis. Endoscopy (2017) 49:682–94. doi: 10.1055/s-0043-109865
105. Bartel MJ, Higa JT, Tokar JL. The status of SEMS versus plastic stents for benign biliary strictures. Curr Gastroenterol Rep (2019) 21(7):29. doi: 10.1007/s11894-019-0696-3
106. Bill JG, Mullady DK. Stenting for benign and malignant biliary strictures. Gastrointest endosc Clinics North America (2019) 29(2):215–35. doi: 10.1016/j.giec.2018.12.001
107. Siiki A, Helminen M, Sand J, Laukkarinen J. Covered self-expanding metal stents may be preferable to plastic stents in the treatment of chronic pancreatitis-related biliary strictures: a systematic review comparing 2 methods of stent therapy in benign biliary strictures. J Clin Gastroenterol (2014) 48(7):635–43. doi: 10.1097/MCG.0000000000000020
108. Park JK, Moon JH, Choi HJ, Min SK, Lee TH, Cheon GJ, et al. Anchoring of a fully covered self-expandable metal stent with a 5F double-pigtail plastic stent to prevent migration in the management of benign biliary strictures. Am J Gastroenterol (2011) 106:1761. doi: 10.1038/ajg.2011.212
109. Kasher JA, Corasanti JG, Tarnasky PR, McHenry L, Fogel E, Cunningham J. A multicenter analysis of safety and outcome of removal of a fully covered self-expandable metal stent during ERCP. Gastrointest Endosc (2011) 73(6):1292–7. doi: 10.1016/j.gie.2011.01.043
110. Sarles H, Sahel J. Cholestasis and lesions of the biliary tract in chronic pancreatitis. Gut (1978) 19:851–7. doi: 10.1136/gut.19.9.851
111. Delhaye M, Arvanitakis M, Bali M, Matos C, Devière J. Endoscopic therapy for chronic pancreatitis. Scand J Surg (2005) 94:143–53. doi: 10.1177/145749690509400211
112. Ma MX, Jayasekeran V, Chong AK. Benign biliary strictures: prevalence, impact, and management strategies. Clin Exp Gastroenterol (2019) 12:83–92. doi: 10.2147/CEG.S165016
113. Familiari P, Boškoski I, Bove V, Costamagna G. ERCP for biliary strictures associated with chronic pancreatitis. Gastrointest Endosc Clin N Am (2013) 23(4):833–45. doi: 10.1016/j.giec.2013.06.007
114. Dumonceau JM, Delhaye M, Tringali A, Arvanitakis M, Sanchez-Yague A, Vaysse T, et al. Endoscopic treatment of chronic pancreatitis: European society of gastrointestinal endoscopy (ESGE) guideline- updated august 2018. Endoscopy (2019) 51(2):179–93. doi: 10.1055/a-0822-0832
115. Perri V, Boškoski I, Tringali A, Familiari P, Mutignani M, Marmo R, et al. Fully covered self-expandable metal stents in biliary strictures caused by chronic pancreatitis not responding to plastic stenting: a prospective study with 2 years of follow-up. Gastrointest Endosc (2012) 75:1271–7. doi: 10.1016/j.gie.2012.02.002
116. Haapamäki C, Kylänpää L, Udd M, Lindström O, Grönroos J, Saarela A, et al. Randomized multicenter study of multiple plastic stents vs. covered self-expandable metallic stent in the treatment of biliary stricture in chronic pancreatitis. Endoscopy (2015) 47:605–10. doi: 10.1055/s-0034-1391331
117. Ramchandani M, Lakhtakia S, Costamagna G, Tringali A, Püspöek A, Tribl B, et al. Fully covered selfexpanding metal stent vs multiple plastic stents to treat benign biliary strictures secondary to chronic pancreatitis: a multicenter randomized trial. Gastroenterology (2021) 161(1):185–95. doi: 10.1053/j.gastro.2021.03.015
118. Ertem FU, Adam S. The race to resolution of benign biliary strictures: Slow and steady vs pedal to the covered metal? Gastroenterology (2021) 161(1):31–3. doi: 10.1053/j.gastro.2021.04.032
119. Thiruvengadam NR, Saumoy M, Schneider Y, Kochman ML. Fully covered self-expanding stents are cost-effective at remediating biliary strictures in patients with chronic pancreatitis. Clin Gastroenterol Hepatol (2022) S1542–3565(22):00145–8. doi: 10.1016/j.cgh.2022.02.019
120. Lakhtakia S, Reddy N, Dolak W, Ponchon T, Bruno MJ, Bourke MJ, et al. Long-term outcomes after temporary placement of a self-expanding fully covered metal stent for benign biliary strictures secondary to chronic pancreatitis. Gastrointest Endosc (2020) 91:361–369.e3n. doi: 10.1016/j.gie.2019.08.037
121. Wan P, Yu X, Xia Q. Operative outcomes of adult living donor liver transplantation and deceased donor liver transplantation: a systematic review and meta-analysis. Liver Transpl (2014) 20:425–36. doi: 10.1002/lt.23836
122. Kochhar G, Parungao JM, Hanouneh IA, Parsi MA. Biliary complications following liver transplantation. World J Gastroenterol (2013) 19(19):2841–6. doi: 10.3748/wjg.v19.i19.2841
123. Sharma S, Gurakar A, Jabbour N. Biliary strictures following liver transplantation: past, present and preventive strategies. Liver Transpl (2008) 14:759–69. doi: 10.1002/lt.21509
124. Guichelaar MM, Benson JT, Malinchoc M, Krom RA, Wiesner RH, Charlton MR. Risk factors for and clinical course of non-anastomotic biliary strictures after livertransplantation. Am J Transplant (2003) 3:885–90. doi: 10.1034/j.1600-6143.2003.00165.x
125. Ito K, Siegelman ES, Stolpen AH, Mitchell DG. MR imaging of complications after liver transplantation. AJR Am J Roentgenol (2000) 175(4):1145–9. doi: 10.2214/ajr.175.4.1751145
126. St Peter S, Rodriquez-Davalos MI, Rodriguez-Luna HM, Harrison EM, Moss AA, Mulligan DC. Significance of proximal biliary dilatation in patients with anastomotic strictures after liver transplantation. Dig Dis Sci (2004) 49:1207–11. doi: 10.1023/B:DDAS.0000037814.96308.7a
127. Venu M, Brown RD, Lepe R, Berkes J, Cotler SJ, Benedetti E, et al. Laboratory diagnosis and nonoperative management of biliary complications in living donor liver transplant patients. J Clin Gastroenterol (2007) 41:501–6. doi: 10.1097/01.mcg.0000247986.95053.2a
128. Woo HY, Lee IS, Chang JH, Youn SB, Bae SH, Choi JY, et al. Outcome of donor biliary complications following living donor liver transplantation. Korean J Intern Med (2018) 33(4):705–15. doi: 10.1016/S0168-8278(18)30989-9
129. Xu YB, Min ZG, Jiang HX, Qin SY, Hu BL. Diagnostic value of magnetic resonance cholangiopancreatography for biliary complications in orthotopic liver transplantation: a meta-analysis. Transplant Proc (2013) 45:2341–6. doi: 10.1016/j.transproceed.2013.03.031
130. Larghi A, Tringali A, Rimbaş M, Barbaro F, Perri V, Rizzatti G, et al. Endoscopic management of benign biliary strictures after liver transplantation. Liver Transplant (2019) 25(2):323–35. doi: 10.1002/lt.25358
131. Sato T, Kogure H, Nakai Y, Ishigaki K, Hakuta R, Saito K, et al. Double-balloon endoscopy-assisted treatment of hepaticojejunostomy anastomotic strictures and predictive factors for treatment success. Surg Endosc (2019) 34:1612–20. doi: 10.1007/s00464-019-06924-6
132. Sato T, Kogure H, Nakai Y, Kanai S, Ishigaki K, Hakuta R, et al. Endoscopic treatment of hepaticojejunostomy anastomotic strictures using fully-covered metal stents. Digestive endosc (2021) 33(3):451–7. doi: 10.1111/den.13773
133. Tsujino T, Isayama H, Kogure H, Sato T, Nakai Y, Koike K. Endoscopic management of biliary strictures after living donor liver transplantation. Clin J Gastroenterol (2017) 10:297–311. doi: 10.1007/s12328-017-0754-z
134. Inamdar S, Slattery E, Sejpal DV, Miller LS, Pleskow DK, Berzin TM, et al. Systematic review and meta-analysis of single-balloon enteroscopy-assisted ERCP in patients with surgically altered GI anatomy. Gastrointest Endosc (2015) 82:9–19. doi: 10.1016/j.gie.2015.02.013
135. Arain MA, Attam R, Freeman ML. Advances in endoscopic management of biliary tract complications after liver transplantation. Liver Transpl (2013) 19:482–98. doi: 10.1002/lt.23624
136. Graziadei IW, Schwaighofer H, Koch R, Nachbaur K, Koenigsrainer A, Margreiter R, et al. Long-term outcome of endoscopic treatment of biliary strictures after liver transplantation. Liver Transpl (2006) 12(5):718–25. doi: 10.1002/lt.20644
137. Alazmi WM, Fogel EL, Watkins JL, McHenry L, Tector JA, Fridell J, et al. Recurrence rate of anastomotic biliary strictures in patients who have had previous successful endoscopic therapy for anastomotic narrowing after orthotopic liver transplantation. Endoscopy (2006) 38(6):571–4. doi: 10.1055/s-2006-925027
138. Tarantino I, Amata M, Cicchese N, Ligresti D, Barresi L, Granata A, et al. Sequential multistenting protocol in biliary stenosis after liver transplantation: a prospective analysis. Endoscopy (2019) 51(12):1130–5. doi: 10.1055/a-0977-3158
139. Tringali A, Barbaro F, Pizzicannella M, Boškoski I, Familiari P, Perri V, et al. Endoscopic management with multiple plastic stents of anastomotic biliary stricture following liver transplantation: long-term results. Endoscopy (2016) 48:546–51. doi: 10.1055/s-0042-100277
140. Dai SC, Goldberg D, Agarwal A, Ma GK, Yam C, Ahmad NA, et al. Endoscopic therapy is effective for recurrent anastomotic biliary strictures after orthotopic liver transplantation. Ann Hepatol (2017) 16:924–31. doi: 10.5604/01.3001.0010.5284
141. Martins FP, De Paulo GA, Contini MLC, Ferrari AP. Metal versus plastic stents for anastomotic biliary strictures after liver transplantation: a randomized controlled trial. Gastrointest Endosc (2018) 87:131. doi: 10.1016/j.gie.2017.04.013
142. Tal AO, Finkelmeier F, Filmann N, Kylänpää L, Udd M, Parzanese I, et al. Multiple plastic stents versus covered metal stent for treatment of anastomotic biliary strictures after liver transplantation: a prospective, randomized, multicenter trial. Gastrointest Endosc (2017) 86(6):1038–45. doi: 10.1016/j.gie.2017.03.009
143. Kaffes A, Griffin S, Vaughan R, James M, Chua T, Tee H, et al. A randomized trial of a fully covered self-expandable metallic stent versus plastic stents in anastomotic biliary strictures after liver transplantation. Therap Adv Gastroenterol (2014) 7:64–71. doi: 10.1177/1756283X13503614
144. Conigliaro R, Pigò F, Bertani H, BASALT Group, Greco S, Burti C, et al. Migration rate using fully covered metal stent in anastomotic strictures after liver transplantation: Results from the BASALT study group. Liver Int (2022) 42(8):1861–71. doi: 10.1111/liv.15246
145. Kao D, Zepeda-Gomez S, Tandon P, Bain VG. Managing the post-liver transplantation anastomotic biliary stricture: multiple plastic versus metal stents: a systematic review. Gastrointest Endosc (2013) 77:679–91. doi: 10.1016/j.gie.2013.01.015
146. Aepli P, St John A, Gupta S, Hourigan LF, Vaughan R, Efthymiou M, et al. Success and complications of an intra-ductal fully covered self-expanding metal stent (ID-FCSEMS) to treat anastomotic biliary strictures (AS) after orthotopic liver transplantation (OLT). Surg Endosc (2017) 31:1558–63. doi: 10.1007/s00464-016-5138-9
147. Joshi D, Warner B, Harrison P, Devlin J, Reffitt D, El-Sherif Y, et al. PTH-113 the success of kaffes stent insertions for post liver transplant anastomotic strictures. Gut (2018) 67:A135. doi: 10.1136/gutjnl-2018-BSGAbstracts.269
148. Barbaro F, Tringali A, Baldan A, Boskoski I, Familiari P, Onder G, et al. Endoscopic management with plastic stents of nonanastomotic biliary strictures following liver transplantation: a single center experience. Endoscopy (2018) 50:S71. doi: 10.1055/s-0038-1637237
149. Rustagi T, Jamidar PA. Endoscopic management of benign biliary strictures. Curr Gastroenterol Rep (2015) 17(1):422. doi: 10.1007/s11894-014-0422-0
150. Perri V, Familiari P, Tringali A, Boskoski I, Costamagna G. Plastic biliary stents for benign biliary diseases. Gastrointest endosc Clinics North America (2011) 21(3):405–33. doi: 10.1016/j.giec.2011.04.012
151. Kuzela L, Oltman M, Sutka J, Hrcka R, Novotna T, Vavrecka A. Prospective follow-up of patients with bile duct strictures secondary to laparoscopic cholecystectomy, treated endoscopically with multiple stents. Hepatogastroenterology (2005) 52(65):1357–61.
152. Costamagna G, Tringali A, Mutignani M, Perri V, Spada C, Pandolfi M, et al. Endotherapy of postoperative biliary strictures with multiple stents: results after more than 10 years of follow up. Gastrointest Endosc (2010) 72:551–7. doi: 10.1016/j.gie.2010.04.052
153. Costamagna G, Tringali A, Perri V, Familiari P, Boškoski I, Barbaro F, et al. Endotherapy of postcholecystectomy biliary strictures with multiple plastic stents: long-term results in a large cohort of patients. Gastrointest endosc (2020) 91(1):81–9. doi: 10.1016/j.gie.2019.05.042
154. Tringali A, Reddy DN, Ponchon T, Neuhaus H, Lladó FG, Navarrete C, et al. Treatment of post-cholecystectomy biliary strictures with fully-covered self-expanding metal stents - results after 5 years of follow-up. BMC Gastroenterol (2019) 19(1):214. doi: 10.1186/s12876-019-1129-3
155. Gawlik C, Carneval M. A review of the management of bile leaks. Cureus (2021) 13(5):e14937. doi: 10.7759/cureus.14937
156. Vlaemynck K, Lahousse L, Vanlander A, Piessevaux H, Hindryckx P. Endoscopic management of biliary leaks: a systematic review with meta-analysis. Endosc vol (2019) 51:11. doi: 10.1055/a-0835-5940
157. Nagra N, Klair JS, Jayaraj M, Murali AR, Singh D, Law J, et al. Biliary sphincterotomy alone vs biliary stent with or without biliary sphincterotomy for the management of post-cholecystectomy bile leak: a sytematic review and meta-analysis. Digestive Dis (Basel Switzerland) (2022) 40(6):810–5. doi: 10.1159/000522328
158. Canena J, Horta D, Coimbra J, Meireles L, Russo P, Marques I, et al. Outcomes of endoscopic management of primary and refractory postcholecystectomy biliary leaks in a multicentre review of 178 patients. BMC Gastroenterol (2015) 15:105. doi: 10.1186/s12876-015-0334-y
159. Tringali A, Massinha P, Schepis T, Landi R, Boškoski I, Perri V, et al. Long-term outcomes of endoscopic treatment of aberrant hepatic duct injuries after cholecystectomy. Gastrointest Endosc (2020) 91(3):584–92. doi: 10.1016/j.gie.2019.09.043
160. Strasberg SM, Hertl M, Soper NJ. An analysis of the problem of biliary injury during laparoscopic cholecystectomy. J Am Coll Surg (1995) 180(1):101–25.
161. Maurea S, Caleo O, Mollica C, Imbriaco M, Mainenti PP, Palumbo C, et al. Comparative diagnostic evaluation with MR cholangiopancreatography, ultrasonography and CT in patients with pancreatobiliary disease. Radiol Med (2009) 114:390–402. doi: 10.1007/s11547-009-0374-x
162. Kitano M, Gress TM, Garg PK, Itoi T, Irisawa A, Isayama H, et al. International consensus guidelines on interventional endoscopy in chronic pancreatitis. recommendations from the working group for the international consensus guidelines for chronic pancreatitis in collaboration with the international association of pancreatology, the American pancreatic association, the Japan pancreas society, and European pancreatic club. Pancreatology (2020) 20(6):1045–55. doi: 10.1016/j.pan.2020.05.022
163. Nguyen-Tang T, Dumonceau JM. Endoscopic treatment in chronic pancreatitis, timing, duration and type of intervention. Best Pract Res Clin Gastroenterol (2010) 24:281–98. doi: 10.1016/j.bpg.2010.03.002
164. Costamagna G. Multiple stents in pancreatic strictures of chronic pancreatitis: is it worth the effort? Endosc Int Open (2019) 7(12):E1699–700. doi: 10.1055/a-1006-2708
165. Costamagna G, Bulajic M, Tringali A, Pandolfi M, Gabbrielli A, Spada C, et al. Multiple stenting of refractory pancreatic duct strictures in severe chronic pancreatitis: Long-term results. Endoscopy (2006) 38:254–9. doi: 10.1055/s-2005-921069
166. Tringali A, Bove V, Vadala di Prampero SF, Boškoski I, Familiari P, Perri V, et al. Long-term follow-up after multiple plastic stenting for refractory pancreatic duct strictures in chronic pancreatitis. Endoscopy (2019) 51:930–5. doi: 10.1055/a-0959-6163
167. Lohr JM, Dominguez-Munoz E, Rosendahl J, Besselink M, Mayerle J, Lerch MM, et al. United European gastroenterology evidence based guidelines for the diagnosis and therapy of chronic pancreatitis (HaPanEU). U Eur Gastroenterol J (2017) 2:153–99. doi: 10.1177/2050640616684695
168. Nabi Z, Lakhtakia S. Endoscopic management of chronic pancreatitis. Digestive endosc (2021) 33(7):1059–72. doi: 10.1111/den.13968
169. Alkhiari R, Kahaleh M. Management of benign pancreatic strictures. In: Lee D, editor. Advanced ERCP for complicated and refractory biliary and pancreatic diseases. Singapore: Springer (2020).
170. Tringali A, Vadala di Prampero SF, Landi R, Bove V, Familiari P, Hamanaka J, et al. Fully covered self-expandable metal stents to dilate persistent pancreatic strictures in chronic pancreatitis: long-term follow-up from a prospective study. Gastrointest Endosc (2018) 88:939. doi: 10.1016/j.gie.2018.08.019
171. Sofi AA, Khan MA, Ahmad S, Khan Z, Peerzada MM, Sunguk J, et al. Comparison of clinical outcomes of multiple plastic stents and covered metal stent in refractory pancreatic ductal strictures in chronic pancreatitis- a systematic review and meta-analysis. Pancreatology (2021) 21(5):854–61. doi: 10.1016/j.pan.2021.03.017.
172. Sharaiha RZ, Novikov A, Weaver K, Marfatia P, Buscaglia JM, DiMaio CJ, et al. Fully covered self-expanding metal stents for refractory pancreatic duct strictures in symptomatic chronic pancreatitis, US experience. Endosc Int Open (2019) 7(11):E1419–23. doi: 10.1055/a-0858-2169
173. Phillip V, Pukitis A, Epstein A, Hapfelmeier A, Haf D, Schwab M, et al. Pancreatic stenting to prevent post-ERCP pancreatitis: a randomized multicenter trial. Endosc Int Open (2019) 07:E860–8. doi: 10.1055/a-0886-6384
174. Sugimoto M, Takagi T, Suzuki R, Konno N, Asama H, Sato Y, et al. Pancreatic stents to prevent post-endoscopic retrograde cholangiopancreatography pancreatitis: A meta-analysis. World J Meta-Anal (2019) 7:249–58. doi: 10.13105/wjma.v7.i5.249
175. Da Cruz Portela J, Marques Bernardo W, de Moura D, Pronze Franzini TM, de Azeredo Coutinho LM, Ottoboni Brunaldi V, et al. Pancreatic stent placement for prevention of post-ERCP pancreatitis in high risk patients: a systematic review and meta-analysis. JOP (2019) 20:16–23.
176. Dumonceau JM, Kapral C, Aabakken L, Papanikolaou IS, Tringali A, Vanbiervliet G, et al. ERCP-related adverse events: European society of gastrointestinal endoscopy (ESGE) guideline. Endoscopy (2020) 52(2):127–49. doi: 10.1055/a-1075-4080
177. Afghani E, Akshintala VS, Khashab MA, Law JK, Hutfless SM, Kim KJ, et al. 5-fr vs. 3-fr pancreatic stents for the prevention of post-ERCP pancreatitis in high-risk patients: a systematic review and network meta-analysis. Endoscopy (2014) 46:573–80. doi: 10.1055/s-0034-1365701
178. He Q, Wang L, Peng C, Zou X, Zhan Q, Xu Y, et al. Modified prophylactic 5-fr pancreatic duct stent enhances the rate of spontaneous dislodgement: A multicentre randomized controlled trial. U Eur Gastroenterol J (2018) 6:1519–26. doi: 10.1177/2050640618804729
179. Matsumoto K, Katanuma A, Maguchi H. Endoscopic removal technique of migrated pancreatic plastic stents. J Hepato-Biliary-Pancreat Sci (2014) 21:E34–40. doi: 10.1002/jhbp.94
180. Mohammed N, Pinder M, Harris K. Endoscopic biliary stenting in irretrievable common bile duct stones: stent exchange or expectant management – tertiary-centre experience and systematic review. Frontline Gastroenterol (2016) 7:176–86. doi: 10.1136/flgastro-2015-100566
181. Bergman JJ, Rauws EA, Tijssen JG, Tytgat GN, Huibregtse K. Biliary endoprostheses in elderly patients with endoscopically irretrievable common bile duct stones: report on 117 patients. Gastrointest Endosc (1995) 42:195–201. doi: 10.1016/S0016-5107(95)70091-9
182. Chan AC, Ng EK, Chung SC, Lai CW, Lau JY, Sung JJ, et al. Common bile duct stones become smaller after endoscopic biliary stenting. Endoscopy (1998) 30:356–9. doi: 10.1055/s-2007-1001282
183. Di Giorgio P, Manes G, Grimaldi E, Schettino M, D'Alessandro A, Di Giorgio A, et al. Endoscopic plastic stenting for bile duct stones: stent changing on demand or every 3 months. a prospective comparison study. Endoscopy (2013) 45:1014–7. doi: 10.1055/s-0033-1344556
184. Pisello F, Geraci G, Li VF, Modica G, Sciumè C. Permanent stenting in “unextractable” common bile duct stones in high risk patients. a prospective randomized study comparing two different stents. Langenbecks Arch Surg (2008) 393:857–63. doi: 10.1007/s00423-008-0388-1
185. Ferreira LE, Baron TH. Post-sphincterotomy bleeding: who, what, when, and how. Am J Gastroenterol (2007) 102:2850–8. doi: 10.1111/j.1572-0241.2007.01563.x
186. Cochrane J, Schlepp G. Comparing endoscopic intervention against fully covered self-expanding metal stent placement for post-endoscopic sphincterotomy bleed (CEASE study). Endosc Int Open (2016) 4:E1261–4. doi: 10.1055/s-0042-118227
187. Bilal M, Chandnani M, McDonald NM, Miller CS, Saperia J, Wadhwa V, et al. Use of fully covered self-expanding metal biliary stents for managing endoscopic biliary sphincterotomy related bleeding. Endosc Int Open (2021) 9(5):E667–73. doi: 10.1055/a-1380-3268.
188. Inoue T, Ibusuki M, Kitano R, Kobayashi Y, Ohashi T, Nakade Y, et al. Early Covered Self-Expandable Metal Stent Placement Is Effective for Massive Post-endoscopic Sphincterotomy Bleeding. Dig Dis Sci (2020) 65(11):3324–31. doi: 10.1007/s10620-020-06057-0.
189. Vanbiervliet G, Strijker M, Arvanitakis M, Aelvoet A, Arnelo U, Beyna T, et al. Endoscopic management of ampullary tumors: European society of gastrointestinal endoscopy (ESGE) guideline. Endoscopy (2021) 53(4):429–48. doi: 10.1055/a-1397-3198
190. Harewood GC, Pochron NL, Gostout CJ. Prospective, randomized, controlled trial of prophylactic pancreatic stent placement for endoscopic snare excision of the duodenal ampulla. Gastrointest Endosc (2005) 62:367–70. doi: 10.1016/j.gie.2005.04.020
191. Spadaccini M, Fugazza A, Frazzoni L, Leo MD, Auriemma F, Carrara S, et al. Endoscopic papillectomy for neoplastic ampullary lesions: A systematic review with pooled analysis. U Eur Gastroenterol J (2020) 8:44–51. doi: 10.1177/2050640619868367
Keywords: endoscopic retrograd colangiopancreatography, stent, malignant biliary stenosis, benign biliary stenosis, chronic panceatitis, post ERCP pancreatitis, transpapillary stenting, SEMS (self-expandable metallic stent)
Citation: Cappello A, Landi R, Gerges C, Cennamo V, Costamagna G and Tringali A (2023) Trans-papillary bilio-pancreatic stenting: When how and which stent. Front. Gastroenterol. 1:1092263. doi: 10.3389/fgstr.2022.1092263
Received: 07 November 2022; Accepted: 06 December 2022;
Published: 05 January 2023.
Edited by:
Sumant Inamdar, University of Arkansas System, United StatesReviewed by:
Jayanta Samanta, Post Graduate Institute of Medical Education and Research (PGIMER), IndiaYousuke Nakai, The University of Tokyo, Japan
Ioannis (Jiannis) Anastasiou, University of Arkansas for Medical Sciences, United States
Copyright © 2023 Cappello, Landi, Gerges, Cennamo, Costamagna and Tringali. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rosario Landi, rosario.landi@policlinicogemelli.it
 Annalisa Cappello1
Annalisa Cappello1 Rosario Landi
Rosario Landi Christian Gerges
Christian Gerges Guido Costamagna
Guido Costamagna Andrea Tringali
Andrea Tringali