- 1Department of Biomedical Informatics, The Ohio State University College of Medicine, Columbus, OH, United States
- 2Division of Clinical Cancer Genomics, City of Hope National Medical Center, Duarte, CA, United States
- 3Division of Human Genetics, The Ohio State University Wexner Medical Center, Columbus, OH, United States
- 4Epidemiology and Genomics Research Program, Division of Cancer Control and Population Sciences, National Cancer Institute, Bethesda, MD, United States
- 5Division of Cancer Prevention and Control, Department of Internal Medicine, College of Medicine, The Ohio State University, Columbus, OH, United States
- 6Division of Epidemiology, College of Public Health, The Ohio State University, Columbus, OH, United States
Background: A national priority in the United States is to promote patient engagement in cancer genomics research, especially among diverse and understudied populations. Several cancer genomics research programs have emerged to accomplish this priority, yet questions remain about the meaning and methods of patient engagement. This study explored how cancer genomics research programs define engagement and what strategies they use to engage patients across stages in the conduct of research.
Methods: An environmental scan was conducted of cancer genomics research programs focused on patient engagement. Research programs were identified and characterized using materials identified from publicly available sources (e.g., websites), a targeted literature review, and interviews with key informants. Descriptive information about the programs and their definitions of engagement, were synthesized using thematic analysis. The engagement strategies were synthesized and mapped to different stages in the conduct of research, including recruitment, consent, data collection, sharing results, and retention.
Results: Ten research programs were identified, examples of which include the Cancer Moonshot Biobank, the MyPART Network, NCI-CONNECT, and the Participant Engagement and Cancer Genome Sequencing (PE-CGS) Network. All programs aimed to include understudied or underrepresented populations. Based on publicly available information, four programs explicitly defined engagement. These definitions similarly characterized engagement as being interpersonal, reciprocal, and continuous. Five general strategies of engagement were identified across the programs: 1) digital (such as websites) and 2) non-digital communications (such as radio broadcasts, or printed brochures); 3) partnering with community organizations; 4) providing incentives; and 5) affiliating with non-academic medical centers. Digital communications were the only strategy used across all stages of the conduct of research. Programs tailored these strategies to their study goals, including overcoming barriers to research participation among diverse populations.
Conclusion: Programs studying cancer genomics are deeply committed to increasing research participation among diverse populations through patient engagement. Yet, the field needs to reach a consensus on the meaning of patient engagement, develop a taxonomy of patient engagement measures in cancer genomics research, and identify optimal strategies to engage patients in cancer genomics. Addressing these needs could enable patient engagement to fulfill its potential and accelerate the pace of cancer genomic discoveries.
1 Introduction
Rapid improvements in genome sequencing technology have revolutionized our ability to molecularly characterize cancer tumors and have, in turn, transformed our understanding of cancer biology (Wang et al., 2016; Wang et al., 2020). The understanding of cancer biology holds promise for improving the diagnosis and treatment of cancer (Hutter and Zenklusen, 2018), but that promise has yet to be realized for many patient populations who have not been adequately included in research. Hundreds of different cancers lack the molecular characterization of tumors required to guide patient therapy (National Cancer Institute, 2016). Similarly, past cancer genomics research inadequately include patients from underrepresented racial and ethnic minority groups and other underserved populations (Spratt et al., 2016). Evidence indicates that large genomic sequencing efforts such as The Cancer Genome Atlas overrepresent non-Hispanic white patients compared to the United States population and underrepresent patients from diverse racial and ethnic backgrounds, especially Asian and Hispanic patients (Spratt et al., 2016; Stein et al., 2021). These shortcomings result in inequities in the development of cancer therapies and the receipt of cancer care.
Several factors contribute to the inadequate characterization of all cancers and the underrepresentation of diverse patient populations in cancer genomics research. Logistical barriers impacting both patients and researchers can hinder access to research (Seruga et al., 2014). Rare cancers are difficult to study due to low disease incidence and patients’ receipt of treatment at geographically dispersed institutions, including community hospitals (Painter et al., 2020). Patients can face additional logistical barriers such as language differences, competing demands for time, and limited access to transportation (George et al., 2014; Seruga et al., 2014). Studying genetics and genomics can add further complications due to information complexity, familial and community relationships, and education needs regarding cancer progression as well as genomics (Rebbeck et al., 2022). Many structural and historical barriers also prevent underrepresented groups from participating in research. Willingness to participate can be dampened by experiences of injustices in the healthcare system, poor communication with researchers or healthcare providers, and distrust based on legacies of exploitive research (Gamble, 1997; Corbie-Smith et al., 2002; Woodahl et al., 2014; Sutton et al., 2019; Geneviève et al., 2020). More specifically, concerns about privacy, unauthorized use of biospecimens, and stigmatizing interpretations of research results can pose barriers to participating in genetics research. These types of misgivings are exemplified by two cases that involved the unauthorized use of samples from the Havasupai in Arizona (Arizona, United States) (Bommersbach, 2008) and the Nuu-Chah-Nulth in British Columbia (Canada), and resulted in a resolution of the National Congress of American Indians affirming tribal ownership of health-related data (National Congress of American Indians, 2022).
In recent years patient engagement has received increasing attention for its potential to democratize the research process and improve the value of research (Cancer Research UK; Deverka et al., 2018; Freeman-Daily et al., 2018; Anampa-Guzmán et al., 2022). Across the world prominent initiatives and national-level infrastructure supports patient engagement in research broadly and genomics research specifically (Cancer Research UK; Candadian Institutes of Health Research; Patient Centered Outcomes Research Institute (PCORI); Patient Focused Medicine; Lough, 2015; I.H.R., 2020; Health, 2022). Despite the attention given to patient engagement in research, the meaning of the term varies, especially across international settings (Cancer Research UK; Murphy et al., 2021; Schuster et al., 2022a). In the United States, the term patient engagement in research has been used to characterize patients’ contributions to research via roles that range from “passive” study participants to “active” patients involved in all phases of the research process (Domecq et al., 2014; Katz and Paskett, 2015; Shippee et al., 2015; Fergusson et al., 2018; Hemphill et al., 2020; Rebbeck et al., 2022), which have been previously defined as preparatory, execution, and translational phases of research. Recent work suggests ways to broaden the conceptualization of engaging patients in cancer research in both passive and active roles (Schuster et al., 2022b).
A prominent national initiative in the United States—the Cancer MoonshotSM–funds a network of research programs for direct patient engagement (National Cancer Institute, 2016). With funding from the United States National Cancer Institute (NCI), this network aims to directly engage patients to contribute their comprehensive tumor profile data to expand knowledge about what therapies work, in whom, and in which types of cancer (National Cancer Institute, 2022b). The meaning of the term “direct patient engagement” is not defined explicitly, but implicitly entails increasing research participation, especially among understudied and historically underrepresented populations (National Cancer Institute, 2016; 2022b). The purpose of engaging a greater diversity of study participants is to ensure that research and clinical trials can benefit people from all communities. There is, however, a lack of knowledge about how research programs studying cancer genomics operationalize engagement of patients as study participants.
This study sought to: identify relevant cancer genomics research programs in the United States that are focused on increasing the diversity of study participants; describe how they define patient engagement; and characterize the strategies they use to engage study participants in cancer genomics research. Additionally, we sought to identify future directions for research on patient engagement in cancer genomics research. We expect the findings will be of interest to researchers seeking to understand the status of patient engagement in research programs studying cancer genomics. It is also likely to be of interest to researchers seeking more information on the conduct of patient engagement and to funders seeking to advance the science of patient engagement.
2 Methods and materials
An environmental scan was conducted (Rowel et al., 2005; Krok-Schoen et al., 2019) of research programs engaging patients in cancer genomics research. For the purposes of this paper, research programs can include research networks comprised of multiple institutions as well as large, centralized genomics data collection initiatives. Environmental scans can describe the context in which decisions and processes are currently and have historically been made. They are an adaptive approach to identifying and synthesizing large and diverse forms of information (Rowel et al., 2005; Krok-Schoen et al., 2019). Additionally, they can inform strategic planning, decision making, and future areas of inquiry (Wilburn et al., 2016). An environmental scan is well-suited to characterize patient engagement in research programs studying cancer genomics given the nascency of the field and the potential lack of information in any one data source.
Research programs were identified via publicly available information, a targeted literature review, and interviews with key informants. Research programs were included in the environmental scan if they made explicit reference to engaging patients as study participants in cancer genomics research; were focused on enrolling patients from the United States; and expressed interest in expanding the inclusion of study participants from a diversity of populations across the United States. Programs were excluded if they were solely focused on engaging patients as active contributors to the research process, including the preparation, execution, and translation of research.
Publicly available materials such as program websites, research protocols, abstracts, and grey literature were identified via Google searches, forward/backward referencing, and clinicaltrial.gov. Descriptive data were extracted for each program using a priori developed tools on: research program description (e.g., target cancer types, target population, enrollment start date(s), study design(s), and key goals); definition of engagement; and strategies for engagement. Descriptive data was also extracted from program websites using a priori identified categories related to content, visual design, user engagement principles (Beaunoyer et al., 2017; Mac et al., 2020; W3 Web Accessibility Iniative, 2022) and the strategies used to engage study participants and the stage of research that the strategy corresponds to (e.g., recruitment, consent, data collection, sharing results, retention) (Rebbeck et al., 2022).
The targeted literature review was conducted by searching databases including PubMed, Google Scholar, Embase, and Web of Science. The search strategy for all databases included three general concepts: 1) patient engagement, 2) cancer, and 3) genomics. Search strategies were tailored to databases using appropriate terminology, truncations, and operators. Information was limited to English-language and the years 2010 to present (2022). Articles were selected if they pertained to research programs using patient engagement to study cancer genomics. Data were extracted following an a priori defined extraction template that included documenting information such as background information (e.g., title, year); engagement definitions; and the strategies used to engage study participants and the corresponding study activities (Rebbeck et al., 2022).
Interviews were conducted with 18 key informants, who were identified purposively through publicly available information; word-of-mouth; and snowball sampling, whereby key informants directly referred study team members to new potential key informants. Two key informants per included research program were invited to participate in the interviews. Key informants from all but one research program participated in the interviews. Interviews were completed via Zoom following a semi-structured interview guide with questions on topics such as the program’s: conceptualizations of engagement, types of engagement strategies used, and perceived strengths or limitations of engagement strategies. These questions directly pertained to the aim of this environmental scan and were intended to accompany and contextualize findings from the other sources of information. Information was recorded using a combination of video recordings, transcriptions, and field notes. On average, the interviews lasted 65 min.
Thematic analysis was used to narratively synthesize the research programs’ written definitions of engagement and to categorize their engagement strategies. The categories of patient engagement strategies for each program were then mapped to different study activities in the conduct of cancer genomics research, including recruitment, consent, data collection, sharing results, and retention. Brief case examples were gathered to illustrate the use of each strategy. This environmental scan was deemed non-human subject research by the Ohio State University College of Medicine Institutional Review Board (OSU IRB #2021E0565).
3 Results
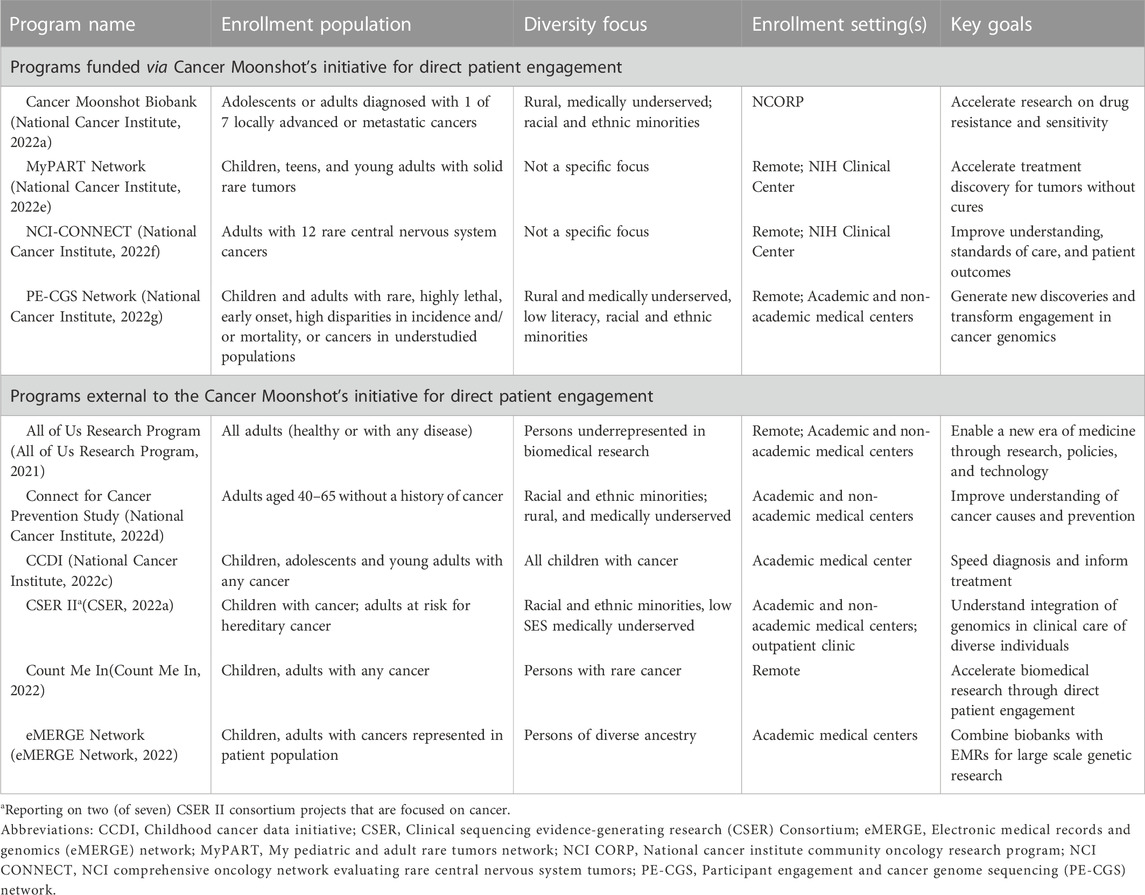
In total ten research programs were identified that are engaging patients in cancer genomics research. Table 1 describes the goals and enrollment populations and settings of each research program. The estimated cohort size for studies conducted by each research program ranges from the hundreds to over one million. The cohorts represent a diversity of populations that include individuals across age groups, racial and ethnic identities, geographic locations, and with different types of cancers. Four of the ten programs are funded through the Cancer Moonshot initiative to “Establish A Network for Direct Patient Engagement” (National Cancer Institute, 2022b). They focus on cancers among populations whose data on cancer health risks and outcomes are currently limited, including cancers that are rare; early onset; highly lethal; locally advanced or metastatic; or have high disparities in incidence/mortality. Two of these four programs are also focused on enrolling participants who are medically underserved and/or from diverse geographic and racial and ethnic backgrounds.
The six programs not funded by the Cancer Moonshot are broader in their disease focuses, recruiting patients with many different cancer types. For example, the Clinical Sequencing Evidence-Generating Research (CSER) Consortium includes a project that is studying a range of hereditary cancer in adults among diverse populations. The All of Us Research Program is unique among the programs included in this scan because it does not focus specifically on cancer, but rather collects data from any adults, including healthy adults or those with any disease. Five of the six programs not funded by the Cancer Moonshot focus on enrolling underrepresented or medically underserved populations.
3.1 Definitions of engagement
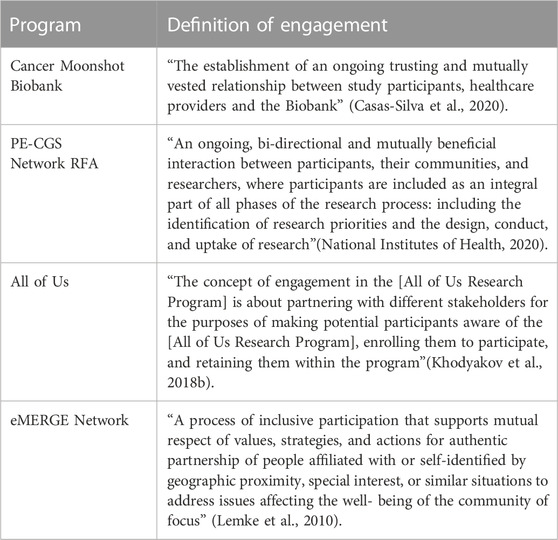
Four research programs explicitly defined engagement (Table 2). Key themes reflected the idea that engagement should be: 1) interpersonal, 2) reciprocal, and 3) continuous. The interpersonal theme appears in all four definitions and is reflected in the use of terms such as “trusting” (Casas-Silva et al., 2020), “relationship”(Casas-Silva et al., 2020), and “authentic partnership” (Lemke et al., 2010) between study participants, communities, and researchers. Reciprocity is captured in three of the four definitions with terms such as “mutual respect”(Lemke et al., 2010), “mutually vested” (Casas-Silva et al., 2020), “mutually beneficial” (National Institutes of Health, 2020) and “bi-directional” (National Institutes of Health, 2020) interactions. The continuous theme related to “retaining” (Khodyakov et al., 2018b) participants and “ongoing” (Casas-Silva et al., 2020) interactions throughout “all phases” (National Institutes of Health, 2020) of the research process. However, the definitions focused on different phases of the research process with only one focused on all phases of the research process, including the design, conduct, and uptake of research (National Institutes of Health, 2020). It was most common to focus on the continuous nature of interactions with patients who are study participants.
3.2 Strategies to engage study participants
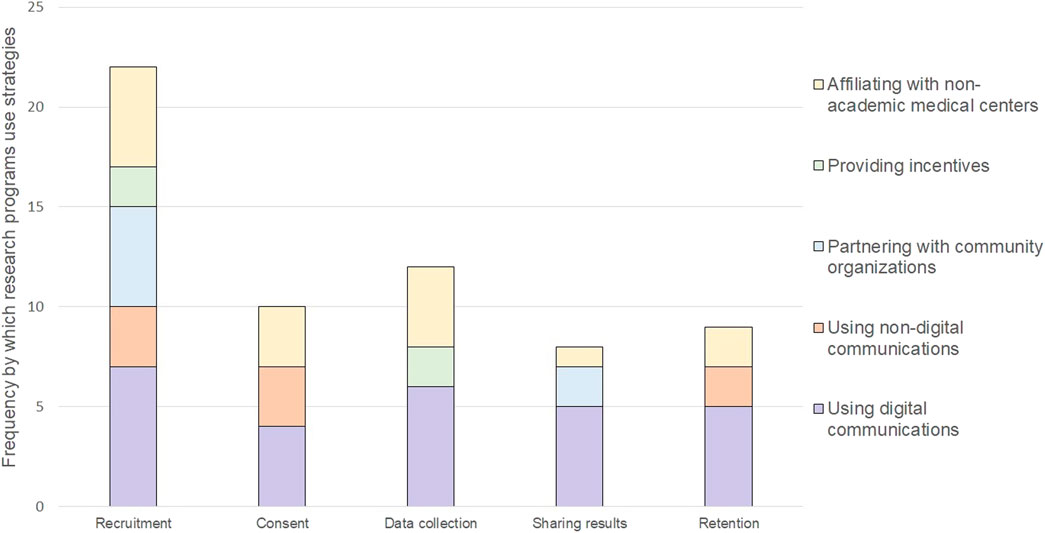
Each research program carried out different study participant engagement strategies to meet enrollment goals, align with their conceptualization of engagement (if described), and overcome known barriers to participation in cancer genomics research. Every research program described opportunities for patients to be involved in the design of their program and its research, including the use of advisory boards, community champions, townhall meetings, deliberative democracy, one-on-one feedback sessions; and human centered-design to guide the strategies they use to engage study participants (Lemke et al., 2010; McCarty et al., 2011; Khodyakov et al., 2018a; Casas-Silva et al., 2020; All of Us Research Program, 2021; O’Daniel et al., 2022). We identified five categories of strategies that programs use to engage patients as study participants. These categories included: 1) using digital communications, 2) using non-digital communications, 3) partnering with community organizations, 4) providing incentives, and 5) affiliating with non-academic medical organizations. We mapped these categories of engagement strategies to different opportunities for engaging study participants during the conduct of research (Figure 1). The strategies are described in more detail below along with brief case examples to describe how and why a strategy was used.
3.2.1 Using digital communications
Using digital communications were relevant to multiple points of engagement with study participants and was the most frequently used strategy. Digital communications, such as websites, videos, electronic newsletters, or social media, offer the opportunity to reach large numbers of prospective participants across broad geographic locations and can enable patients to participate remotely. The programs used digital communications to share information about cancer, genomics, and cancer research; visually depict the steps entailed with participating and explain how long each step should take; and help participants enroll in their study, complete electronic consents, share electronic health records, complete health surveys, and receive individual-level results and aggregate-level study findings. As an example, the Cancer Moonshot Biobank uses its website to support recruitment, post study and research updates, and provide access to a secure patient portal for participants’ receipt of individual biomarker reports (Casas-Silva et al., 2020; Casas-Silva et al., 2021). Some programs tailored their digital communications to reach diverse racial and ethnic populations, including by providing a Spanish version of the website (All of Us Research Program, 2022; Count Me In, 2022; National Cancer Institute, 2022f; National Cancer Institute, 2022a), including images that reflect a diversity of demographics (All of Us Research Program, 2022; Count Me In, 2022; National Cancer Institute, 2022f; National Cancer Institute, 2022a; National Cancer Institute, 2022e; National Cancer Institute, 2022g), and explicitly stating their commitment to promoting diversity and inclusion in research (CSER, 2022b; CSER, 2022a). The use of Frequently Asked Question (FAQs) and infographics were also frequently used to communicate what participation entails (All of Us Research Program, 2022; Count Me In, 2022; National Cancer Institute, 2022a; National Cancer Institute, 2022f; National Cancer Institute, 2022g). Even though digital communications were components of research programs’ study protocols before the COVID-19 pandemic emerged, the pandemic accelerated programs use of it.
3.2.2 Using non-digital communications
Digital communications were reported in the literature and by key informants as being insufficient to promote recruitment or sustain high levels of retention. Non-digital communications were viewed as supporting the needs of those with limited access to technology as well as those who may be less comfortable using it. Non-digital communications were used most commonly to promote recruitment, obtain consent, and promote retention. For recruitment, programs reported using print brochures, radio advertisements, community events, and press coverage (All of Us Research Program, 2021). Their non-digital communications to support retention included support call centers and other strategies successfully implemented by other long-term cohort studies such as reminder calls to participants to keep them engaged in the project; birthday cards to participants from the program; outreach telephone calls to a participant-designated friend or family member (if consented to contact); referral cards for family and friends; and enrollment certificates for recognition (All of Us Research Program, 2021). Print newsletters were also reported to support retention and sharing of overall results. To reach Spanish speaking populations, the studies in the CSER Consortium developed non-digital educational content, informational brochures, and informed consent documents in Spanish (Amendola et al., 2018; CSER, 2022b).
3.2.3 Partnering with community organizations
Partnering with community organizations was viewed as providing a bridge between patients and researchers. Community organizations included patient advocacy organizations, community outreach organizations, and social change organizations. Programs described establishing collaborative relationships with community organizations to facilitate dissemination of information and materials to their respective communities and to serve as cultural brokers who could advocate for the needs and concerns of their community as well as build bridges between their communities and researchers. In some cases, community organizations were also viewed as partners who could support enrollment in programs’ studies. As such, community organizations were valuable partners in promoting recruitment as well as in disseminating aggregate study findings to the community. The MyPART Network, for example, partners with non-profit organizations to work together in numerous ways, including to disseminate research opportunities and findings to relevant audiences and to ensure that the research is centered on the needs of people with rare tumors (National Cancer Institute, 2022e). Additionally, the PE-CGS Network identified partnerships with patient-centered advocacy and community organizations as an important way to engage patients, optimize recruitment, and seamlessly return results (National Cancer Institute, 2022g). Moreover, these partnerships were seen as a vital way to gather input and feedback from those affected by the research, including patients and their families and communities (National Cancer Institute, 2022g).
3.2.4 Providing incentives
Extrinsic incentives were described as a way to demonstrate respect for study participation. They were also viewed as a mechanism to help address barriers to participation, such as competing demands for time or limited access to transportation. They often took the form of direct payment, gift cards, and/or parking validation. For example, in the Connect for Cancer Prevention Study, after participants complete their first survey and donate their first blood sample, they will receive $25 in cash or as a gift card (depending on the healthcare system they’re affiliated with) (National Cancer Institute, 2022d). The study also covers parking validation when donating samples in person (National Cancer Institute, 2022d). In some instances incentives also included providing access to experts who often have more experience with particular types of cancer than patients’ personal medical team and may be able to provide advice on cancer treatments and/or identify relevant treatment trials. As part of NCI-CONNECT, for instance, study participants can visit with experts associated with study’s neuro-oncology care team for an evaluation and consultation (National Cancer Institute, 2022f). The consultation is free and provides participants with accurate diagnosis and treatment guidance. Generally, incentives were considered as one way to support the participation of traditionally underrepresented populations and, in a small way, rectify past negative and harmful experiences with research (All of Us Research Program, 2021).
3.2.5 Affiliating with non-academic medical centers
Affiliating with non-academic medical centers was considered an opportunity to reach a larger and more diverse patient population treated in a variety of healthcare delivery settings. Programs indicated that affiliating with non-academic medical centers could accelerate accrual, facilitate the collection of data and biospecimens, increase the diversity of participants, and enhance the relevance of study findings. Non-academic medical centers included regional medical centers, federally qualified health centers, the Veterans Health Administration, integrated healthcare systems, and institutions affiliated with the NCI Community Oncology Research Program (NCORP) (All of Us Research Program, 2021; National Cancer Institute, 2022a; National Cancer Institute, 2022d). As one example, the Cancer Moonshot Biobank aims to collect and distribute longitudinal cancer biospecimens from study participants receiving standard of care therapy at participating NCI Community Oncology Research Program (NCORP) institutions (The International Society for Biological and Environmental Repositories, 2022). Affiliating with NCORP sites and making funding available for them to implement local engagement strategies is designed to support the goal of increasing participation among rural and other medically underserved communities as well as enrolling participants who represent the racial and ethnic diversity of the United States (The International Society for Biological and Environmental Repositories, 2022).
3.3 Questions about current state of patient engagement in cancer genomics research
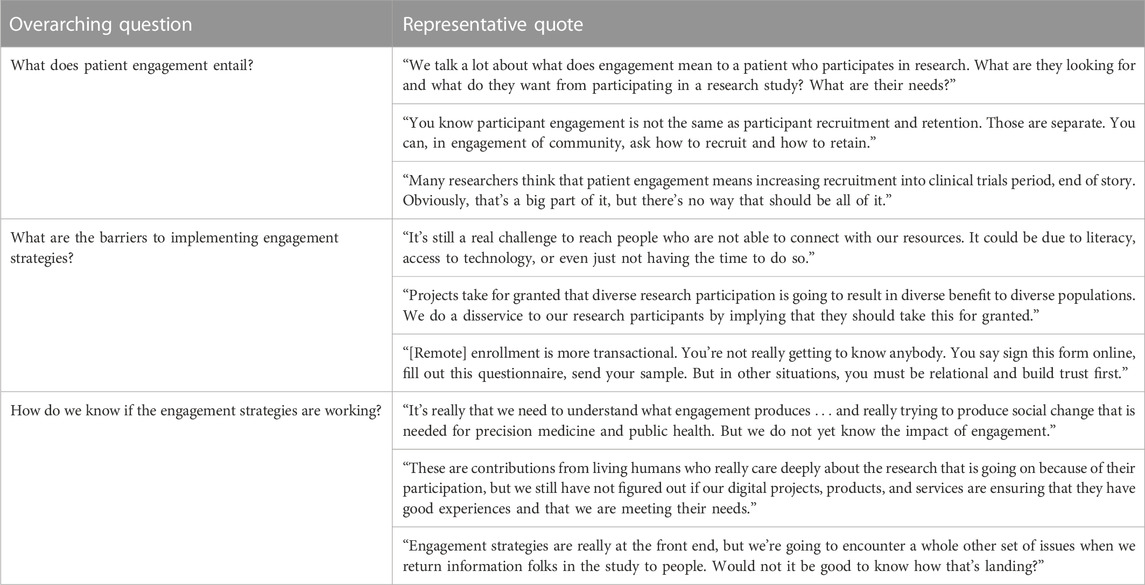
Key informants raised a number of questions about the current state of patient engagement in cancer genomics research that fell into three broad categories: 1) what does patient engagement entail? 2) what are the barriers to implementing engagement strategies? and 3) how do we know if the engagement strategies are working? These categories are summarized in Table 3 and accompanied by representative quotations from our key informants.
The questions raised about what patient engagement entails highlighted discordance about the relationship between engagement and recruitment. The comments revealed that many activities used for engagement may also be used for recruitment and figuring out how to separate these ideas is difficult. For some key informants’ the term patient engagement should only be used to refer to patients’ informing the design and conduct of research, but not activities for patients as study participants, in spite of current practice. Additionally, among key informants who applied the term patient engagement to patients in their role as study participants, they explicitly wondered what patients were seeking from their participation in research.
Related to the questions about the barriers to implementing engagement strategies, there were concerns about digital communication’s ability to overcome technological barriers to participation as well as barriers related to distrust. There was doubt that digital communications alone are sufficient for developing trust and mutually respectful relationships, which in line with the definitions of engagement, were perceived to be crucial components of engaging diverse study participants. Building relationships with patients’ and their communities before asking them to participate in a study was an important consideration. One concern was whether researchers are misrepresenting the benefits of study participation, and the inadvertent consequences of over-promising and under-delivering on the benefits of participating in genomics research.
In terms of questions about how do we know if engagement is working, key informants noted the importance of studying the effectiveness of patient engagement from the short-term to the long-term. In the short-term key informants expressed questions about process measures such as how well programs were meeting study participants’ expectations and needs. In the long-term, key informants wanted evidence on which engagement strategies are meeting program goals of reaching diverse participants and how engagement strategies are achieving health outcomes such as transforming cancer therapy and cancer care delivery. They noted, however, that such evidence is lacking at this point.
4 Discussion
Patient engagement in cancer genomics research is supported by national priorities such as the Cancer Moonshot to reflect the full diversity of the United States (National Cancer Institute, 2016; The All of Us Research Program Investigators, 2019). The findings of this environmental scan demonstrate the intent to increase the representation of diverse populations in these ten cancer genomics research programs. They strive to include study participants who represent broad and diverse populations, including across age, race, ethnic group, geographic location, and types of cancers. Additionally, they aim to increase study participation among populations with understudied cancers and those historically exploited or excluded in cancer genomics research. As highlighted by the goals of the Cancer Moonshot, the representation of diverse populations in cancer research is critical for rapidly advancing our understanding of cancer and improving patient care.
The meaning of engaging patients as study participants was generally broad. We cataloged research programs existing written definitions of engagement in cancer genomics research, and in doing so, identified a shift in the meaning of patient engagement. In particular, as reported in Table 2, we found that engagement of patients as study participants includes being interpersonal and reciprocal on an ongoing basis, which is more expansive than categorizing study participation as passive engagement. This understanding of engagement contradicts a historical emphasis of engagement on recruitment. In support of engagement as an ongoing process, we identified that patient engagement in cancer genomics research entails interactions between participants and researchers across five stages of conducting a study—recruitment, consent, data collection, return of results, and retention—using five types of strategies. The most prominent of these strategies was digital communications, which was the only strategy used across all five stages of conducting a study.
Through this review we found three existing needs to meaningfully advance the science of patient engagement in cancer genomics research and serve as a baseline from which we can track our progress on addressing those needs. The three needs are to: 1) reach an agreement on the meaning of patient engagement; 2) develop a clear taxonomy of measures to be able to assess the quality and comparative effectiveness of engagement strategies; and 3) identify the comparative effectiveness of engagement strategies. Addressing these three needs would facilitate the use of engagement strategies in cancer genomics research and would enable a better understanding of how to tailor different engagement strategies to different groups.
First, the field of cancer genomics research needs to reach an agreement on the meaning of patient engagement because ambiguity remains. While the spirit of the definition of engagement was arguably similar, only four of the ten programs had an explicit, written definition of engagement as shown in Table 2. Of these definitions, three of the four referred to patient engagement as occurring, at least in part, between researchers and patients as study participants from recruitment to retention through ongoing, reciprocal relationships. It is not clear if the remaining programs would have defined engagement in concordant ways because key informants raised conflicting viewpoints about the meaning of engagement. As indicated in Table 3, some key informants were adamant that engagement is different from recruitment and others continued to think of recruitment as an important part of engagement. Regardless, in synthesizing the written definitions from Table 2, we observe that the emphasis on engaging patients as study participants in ongoing, reciprocal relationships expands upon previous conceptualizations; namely, where engaging study participants was classified as having a passive level of involvement (Domecq et al., 2014; Shippee et al., 2015). While a broad sense of engagement might enable reaching specific communities, developing a consistent definition would address key questions about the purpose and priorities of patient engagement as identified by the key informants and would also help operationalize and assess patient engagement efforts. Recent work synthesizes definitions of engagement across diverse academic disciplines (Schuster et al., 2022a), including engagement marketing (Lewis et al., 2022), and could be a foundation for operationally defining engagement in cancer genomics research.
Second, the field ought to develop a clear taxonomy of engagement measures to assess the comparative effectiveness of patient engagement strategies in cancer genomics research. Currently, there is a lack of available measures and measures that could be appropriate for different populations. The purpose of an evaluation informs the metrics to be used. The taxonomy could be grounded in a Donabedian model that classifies measures of engagement according to structure, process, and outcomes. The type of measure would inform the methods—quantitative, qualitative or mixed-methods—needed to assess them as well as the necessity of demonstrating appropriate instrument development and testing (e.g., validity, reliability, feasibility, acceptability, responsiveness, interpretability, appropriateness, precision). Developing a taxonomy of engagement measures would support clear reporting of patient engagement so that researchers could replicate similar approaches and also fulfill study reporting guidelines known as the ‘Guidance for Reporting Involvement of Patients and the Public (GRIPP2) (Staniszewska et al., 2017). The development of such a taxonomy could address several questions raised by the key informants. For instance, a taxonomy could ensure that measures exist to understand what patients perceive as transactional. It could also ensure that measures exist to appropriately assess engagement across all five stages of conducting a study–recruitment, consent, data collection, return of results, and retention–not just at the start of the study.
Third, the field lacks empirical research testing and comparing the effectiveness of engagement strategies in cancer genomics research. At the present time, it is not possible to recommend best practices on which of the engagement strategies are optimal for any given study activity. Key informants indicated the importance of understanding the value of engagement, and not overstating the assumed value of it. Over-promising the benefits of research participation to diverse populations could have the downstream effect of eroding trust and ongoing, long-term engagement. As such studying engagement strategies is a key need for future research. Some literature suggests the importance of studying engagement through the lens of adaptive learning systems that are initially informed by existing strategies and enabled to be carried out through iterative effectiveness testing (All of Us Research Program, 2021). With this perspective, and given that challenges will inevitably arise, there is a need for programs to be able to nimbly make modifications to their study protocols and materials. Committing to research that empirically tests the comparative effectiveness of engagement strategies raises a potentially difficult task. Researchers, funders, and journals will need to be willing to report and publish on failed efforts. This will be particularly important if research programs are going to in fact design and implement strategies that effectively support the inclusion of all participants.
This research is limited in several ways. First, we only briefly address the role of patient engagement in the research programs during the preparatory phase of research, as defined previously (Domecq et al., 2014; Shippee et al., 2015), and we did not address its role during the translational phases of research. These are, however, important aspects of patient engagement where engagement could inform developing research questions, setting priorities, developing protocols, guiding enrollment, analyzing data, and disseminating key findings. There are many strategies for this type of engagement and evidence of their use came up during our scan. However, this was beyond the scope of this environmental scan and future research should assess programs’ use of patient engagement across these additional phases of research. Another major limitation is that we cannot be sure we captured all cancer genetic research programs that are doing engagement. Similarly, we cannot be sure that we captured all types of engagement strategies being used by the research programs nor could we determine the motives driving use of the different strategies. While environmental scans focus on the phenomenon being studied, this highlights a key opportunity for deeper qualitative research to understand the motivations for patient engagement and to compare how engagement is being done and measured within and across research programs (Schuster et al., 2022b). That said, our environmental scan was thorough and was meant to represent a snapshot of the time in which we reviewed them. Additionally, the content we report in was verified as of finalizing the manuscript, so findings are as up to date as possible. Finally, we did not directly engage patients as a data source in this environmental scan because we sought to characterize programs’ current definitions and practices of engagement. Future work is still needed because the involvement and opinions of patients are critical to advancing the field of engagement in cancer genomics research and several research programs noted how they strive to embed patients throughout their work.
The study provides a contemporary look at patient engagement in the context of research programs studying cancer genomics in the US. We utilized a comprehensive environmental scan to select and characterize the evidence. The findings signal a deep commitment to increasing research participation among diverse populations and a conceptualization of engagement that is responsive to overcoming barriers to participation. Yet, the field has clear needs to inform its practice of patient engagement, which entails advancing the science of patient engagement in cancer genomics research. The field must define what engagement entails, identify how to measure engagement, and generate evidence on the effectiveness of engagement strategies. Addressing these needs could enable patient engagement to fulfill its potential and accelerate the pace of cancer genomic discoveries.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
All authors contributed to the manuscript’s conception. Material preparation and literature review were performed by AS, NC, and JP. Key informant interviews were performed by AS. Evidence synthesis was performed by AS, NC, and JP. The first draft of the manuscript was written by AS and all authors edited and commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This work was funded by the Ohio State University Comprehensive Cancer Center and, in part, from a grant from the National Cancer Institute (U24 CA252977-01).
Conflict of interest
EP has grants to the institution from Merck Foundation, Pfizer and Genentech for work outside this project. HH is on the scientific advisory boards for Genome Medical, Invitae Genetics, and Promega, is a consultant for 23andMe, AIM Specialty Health, and GI OnDemand, and has stock/stock options in GI OnDemand and Genome Medical.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2023.1053613/full#supplementary-material
References
All of Us Research Program (2022). Join all of us. [Online]. Available at: https://www.joinallofus.org/(Accessed Sept 1, 2022).
All of Us Research Program (2021). Operational protocol. Available at: https://allofus.nih.gov/about/all-us-research-program-protocol (Accessed Sept 13, 2022).
Amendola, L. M., Berg, J. S., Horowitz, C. R., Angelo, F., Bensen, J. T., Biesecker, B. B., et al. (2018). The clinical sequencing evidence-generating research consortium: Integrating genomic sequencing in diverse and medically underserved populations. Am. J. Hum. Genet. 103 (3), 319–327. doi:10.1016/j.ajhg.2018.08.007
Anampa-Guzmán, A., Freeman-Daily, J., Fisch, M., Lou, E., Pennell, N. A., Painter, C. A., et al. (2022). The rise of the expert patient in cancer: From backseat passenger to Co-navigator. JCO Oncol. Pract. 18 (8), 578–583. doi:10.1200/op.21.00763
Beaunoyer, E., Arsenault, M., Lomanowska, A. M., and Guitton, M. J. (2017). Understanding online health information: Evaluation, tools, and strategies. Patient Educ. Couns. 100 (2), 183–189. doi:10.1016/j.pec.2016.08.028
Bommersbach, J. (2008). Arizona’s broken arrow: Did Arizona state university genetically rape the Havasupai tribe? Phoenix magazine.
Cancer Research Uk, Patient Involvement toolkit for researchers. [Online]. Available at: https://www.cancerresearchuk.org/funding-for-researchers/patient-involvement-toolkit-for-researchers (Accessed September 15, 2022).
Candadian Institutes of Health Research Strategy for patient-oriented research. [Online]. Available: https://cihr-irsc.gc.ca/e/41204.html (Accessed September 9, 2022).
Casas-Silva, E., Agrawal, L., Ellis, H. J., Gopalakrishnan, V., Guan, P., Jensen, M., et al. (2021). Abstract 2632: Fostering engagement in biobanking and research through the NCI Cancer Moonshot Biobank patient and provider engagement website. Cancer Res. 81 (13), 2632. doi:10.1158/1538-7445.Am2021-2632
Casas-Silva, E., Ellis, H., Gopalakrishnan, V., Weil, C., Rao, A., Agrawal, L., et al. (2020). Abstract 4352: Fostering research participation through NCI's Cancer Moonshot Biobank engagement portal.
Corbie-Smith, G., Thomas, S. B., and St George, D. M. (2002). Distrust, race, and research. Arch. Intern Med. 162 (21), 2458–2463. doi:10.1001/archinte.162.21.2458
Count Me In (2022). Join Count me in. [Online]. Available: https://joincountmein.org/(Accessed Sept 22, 2022).
CSER (2022a). Clinical sequencing evidence-generating research (CSER). Consortium [Online]. Available at: https://cser-consortium.org/(Accessed May 3, 2022).
CSER (2022b). CSER consortium research materials. [Online]. Available: https://cser-consortium.org/cser-research-materials (Accessed Sept 22, 2022).
The All of Us Research Program Investigators Denny, J. C., Rutter, J. L., Goldstein, D. B., Philippakis, A., Smoller, J. W., et al. (2019). The “all of us” research program. N. Engl. J. Med. 381 (7), 668–676. doi:10.1056/NEJMsr1809937
Deverka, P. A., Bangs, R., Kreizenbeck, K., Delaney, D. M., Hershman, D. L., Blanke, C. D., et al. (2018). “A new framework for patient engagement in cancer clinical trials cooperative group studies,” in Journal of the national cancer Institute (Oxford University Press).
Domecq, J. P., Prutsky, G., Elraiyah, T., Wang, Z., Nabhan, M., Shippee, N., et al. (2014). Patient engagement in research: A systematic review. BMC Health Serv. Res. 14 (1), 89. doi:10.1186/1472-6963-14-89
eMERGE Network (2022). Electronic MEdical records and genomics (eMERGE) network. [Online]. Available: https://emerge-network.org/(Accessed Sept 22, 2022).
Fergusson, D., Monfaredi, Z., Pussegoda, K., Garritty, C., Lyddiatt, A., Shea, B., et al. (2018). The prevalence of patient engagement in published trials: A systematic review. Res. Involv Engagem. 4, 17. doi:10.1186/s40900-018-0099-x
Freeman-Daily, J., Elkins, I., Greco, L., Horn, M., Addario, B., Leduc, D., et al. (2018). OA10.02 oncogene-driven patient groups: A new era for research partnerships. J. Thorac. Oncol. 13 (10), S343. doi:10.1016/j.jtho.2018.08.289
Gamble, V. N. (1997). Under the shadow of Tuskegee: African Americans and health care. Am. J. Public Health 87 (11), 1773–1778. doi:10.2105/ajph.87.11.1773
Geneviève, L. D., Martani, A., Shaw, D., Elger, B. S., and Wangmo, T. (2020). Structural racism in precision medicine: Leaving no one behind. BMC Med. Ethics 21 (1), 17. doi:10.1186/s12910-020-0457-8
George, S., Duran, N., and Norris, K. (2014). A systematic review of barriers and facilitators to minority research participation among african Americans, latinos, asian Americans, and pacific islanders. Am. J. Public Health 104 (2), e16–e31. doi:10.2105/ajph.2013.301706
Health, G. A. f. G. (2022). The Engagement Framework: Building inclusive engagement practices in genomics. [Online]. Available at: https://www.ga4gh.org/news/the-engagement-framework-building-inclusive-engagement-practices-in-genomics/(Accessed December 12, 2022).
Hemphill, R., Forsythe, L. P., Heckert, A. L., Amolegbe, A., Maurer, M., Carman, K. L., et al. (2020). What motivates patients and caregivers to engage in health research and how engagement affects their lives: Qualitative survey findings. Health Expect. 23 (2), 328–336. doi:10.1111/hex.12979
Hutter, C., and Zenklusen, J. C. (2018). The cancer genome Atlas: Creating lasting value beyond its data. Cell. 173 (2), 283–285. doi:10.1016/j.cell.2018.03.042
I.H.R. (2020). Advancing genomics research in Ireland. [Online]. Available at: https://hrci.ie/wp-content/uploads/2021/03/IHRF-2020-Advancing-Genomics-Research.pdf (Accessed December, 2022).
Katz, M. L., and Paskett, E. D. (2015). The process of engaging members from two underserved populations in the development of interventions to promote the uptake of the HPV vaccine. Health Promot. Pract. 16 (3), 443–453. doi:10.1177/1524839914559776
Khodyakov, D., Bromley, E., Evans, S. K., and Sieck, K. (2018b). Best practices for participant and stakeholder engagement in the all of us research program. Santa Monica, CA: RAND Corporation.
Khodyakov, D., Bromley, E., Evans, S., and Sieck, K. (2018a). Best practices for participant and stakeholder engagement in the all of us research program.
Krok-Schoen, J. L., Bernardo, B. M., Elena, J. W., Green, P. A., Hoover, E., Peng, J., et al. (2019). An environmental scan of biopsychosocial and clinical variables in cohort studies of cancer survivors. Cancer Epidemiol. Biomarkers Prev. 28 (10), 1621–1641. doi:10.1158/1055-9965.Epi-18-0541
Lemke, A. A., Wu, J. T., Waudby, C., Pulley, J., Somkin, C. P., and Trinidad, S. B. (2010). Community engagement in biobanking: Experiences from the eMERGE Network. Genomics, Soc. policy 6 (3), 50. doi:10.1186/1746-5354-6-3-50
Lewis, M. A., Uhrig, J. D., Adams, E. T., Brown, J. A., Sanders, A., and o.U., t. R. I. A. I. T. (2022). Engagement marketing for social good: Application to the all of us research program. Front. Genet. 13. doi:10.3389/fgene.2022.889195
Lough, S. (2015). Need to define patient engagement in research. Cmaj 187 (12), E385–E386. doi:10.1503/cmaj.109-5109
Mac, O. A., Thayre, A., Tan, S., and Dodd, R. H. (2020). Web-based health information following the renewal of the cervical screening program in Australia: Evaluation of readability, understandability, and credibility. J. Med. Internet Res. 22 (6), e16701. doi:10.2196/16701
McCarty, C. A., Chisholm, R. L., Chute, C. G., Kullo, I. J., Jarvik, G. P., Larson, E. B., et al. (2011). The eMERGE network: A consortium of biorepositories linked to electronic medical records data for conducting genomic studies. BMC Med. Genomics 4, 13. doi:10.1186/1755-8794-4-13
Murphy, A., Bere, N., Vamvakas, S., and Mavris, M. (2021). The added value of patient engagement in early dialogue at EMA: Scientific advice as a case study. Front. Med. (Lausanne) 8, 811855. doi:10.3389/fmed.2021.811855
National Cancer Institute (2022f). NCI-CONNECT: Clinical Trials. Available at: https://www.cancer.gov/rare-brain-spine-tumor/refer-participate/clinical-studies (Accessed Sept 22, 2022).
National Cancer Institute (2022b). Cancer Moonshot research initiatives: Establish a network for direct patient engagement. [Online]. Available at: https://www.cancer.gov/research/key-initiatives/moonshot-cancer-initiative/implementation/patient-engagement (Accessed Sept 2, 2022).
National Cancer Institute (2022c). Childhood cancer data initiative (CCDI). [Online]. Available at: https://www.cancer.gov/research/areas/childhood/childhood-cancer-data-initiative (Accessed May 3, 2022).
National Cancer Institute (2022d). Connect for cancer prevention study. [Online]. Available at: https://www.cancer.gov/connect-prevention-study/(Accessed May 3, 2022).
National Cancer Institute (2022e). MyPART - my pediatric and adult rare tumor network. [Online]. Available: https://www.cancer.gov/pediatric-adult-rare-tumor/(Accessed May 3, 2022).
National Cancer Institute (2022g). Participant Engagement and cancer genome sequencing (PE-CGS) network [online]. [Accessed Sept 2 2022].
National Cancer Institute (2022a). The cancer Moonshot Biobank. [Online]. Available at: https://moonshotbiobank.cancer.gov/(Accessed Sept 22, 2022).
National Congress of American Indians (2022). Tribal ownership of health-related data. Available at: https://www.ncai.org/resources/resolutions/tribal-ownership-of-health-related-data.
National Institutes of Health (2020). Participant Engagement and cancer genome sequencing (PE-CGS): Research centers (U2C clinical trial optional). RFA-CA-19-045. [Online]. Available at: https://grants.nih.gov/grants/guide/rfa-files/rfa-ca-19-045.html.
O’Daniel, J. M., Ackerman, S., Desrosiers, L. R., Rego, S., Knight, S. J., Mollison, L., et al. (2022). Integration of stakeholder engagement from development to dissemination in genomic medicine research: Approaches and outcomes from the CSER Consortium. Genet. Med. 24 (5), 1108–1119. doi:10.1016/j.gim.2022.01.008
Painter, C. A., Jain, E., Tomson, B. N., Dunphy, M., Stoddard, R. E., Thomas, B. S., et al. (2020). The angiosarcoma project: Enabling genomic and clinical discoveries in a rare cancer through patient-partnered research. Nat. Med. 26 (2), 181–187. doi:10.1038/s41591-019-0749-z
Patient Centered Outcomes Research Institute (PCORI) Science of engagement PCORI funding announcement. [Online]. Available at: https://www.pcori.org/funding-opportunities/announcement/science-engagement-pcori-funding-announcement (Accessed May 13, 2022).
Patient Focused Medicine (2022). Framework for patient engagement. Available at: https://patientfocusedmedicine.org/framework-for-patient-engagement/(Accessed September 9, 2022).
Rebbeck, T. R., Bridges, J. F. P., Mack, J. W., Gray, S. W., Trent, J. M., George, S., et al. (2022). A framework for promoting diversity, equity, and inclusion in genetics and genomics research. JAMA Health Forum 3 (4), e220603. doi:10.1001/jamahealthforum.2022.0603
Rowel, R., Moore, N. D., Nowrojee, S., Memiah, P., and Bronner, Y. (2005). The utility of the environmental scan for public health practice: Lessons from an urban program to increase cancer screening. J. Natl. Med. Assoc. 97 (4), 527–534.
Schuster, A. L. R., Crossnohere, N. L., Bachini, M., Blair, C. K., Carpten, J. D., Claus, E. B., et al. (2022a). Priorities to promote participant engagement in the participant engagement and cancer genome sequencing (PE-CGS) network. Cancer Epidemiol Biomarkers Prev Forthcoming.
Schuster, A. L. R., Hampel, H., Paskett, E. D., and Bridges, J. F. P. (2022b). Rethinking patient engagement in cancer research. The Patient - Patient-Centered Outcomes Research. doi:10.1007/s40271-022-00604-9
Seruga, B., Sadikov, A., Cazap, E. L., Delgado, L. B., Digumarti, R., Leighl, N. B., et al. (2014). Barriers and challenges to global clinical cancer research. Oncologist 19 (1), 61–67. doi:10.1634/theoncologist.2013-0290
Shippee, N. D., Domecq Garces, J. P., Prutsky Lopez, G. J., Wang, Z., Elraiyah, T. A., Nabhan, M., et al. (2015). Patient and service user engagement in research: A systematic review and synthesized framework. Health expect. 18 (5), 1151–1166. doi:10.1111/hex.12090
Spratt, D. E., Chan, T., Waldron, L., Speers, C., Feng, F. Y., Ogunwobi, O. O., et al. (2016). Racial/ethnic disparities in genomic sequencing. JAMA Oncol. 2 (8), 1070–1074. doi:10.1001/jamaoncol.2016.1854
Staniszewska, S., Brett, J., Simera, I., Seers, K., Mockford, C., Goodlad, S., et al. (2017). GRIPP2 reporting checklists: Tools to improve reporting of patient and public involvement in research. BMJ 358, 3453. doi:10.1136/bmj.j3453
Stein, J. N., Charlot, M., and Cykert, S. (2021). Building toward antiracist cancer research and practice: The case of precision medicine. JCO Oncol. Pract. 17 (5), 273–277. doi:10.1200/OP.20.01070
Sutton, A. L., He, J., Tanner, E., Edmonds, M. C., Henderson, A., Hurtado de Mendoza, A., et al. (2019). Understanding medical mistrust in black women at risk of BRCA 1/2 mutations. J. Health Dispar. Res. Pract. 12 (3), 35–47.
The future of cancer genomics lies in the clinic (2020). The era of massive cancer sequencing projects has reached a turning point. Nature 578 (7793), 7–8. doi:10.1038/d41586-020-00308-w
The International Society for Biological and Environmental Repositories (2022). Biobanking: Shaping the scientific journey. O-07 NCI's cancer Moonshot Biobank: Supporting diversity through local patient engagement. Biopreservation Biobanking 0 (0). null. doi:10.1089/bio.2022.29104.abstracts
W3 Web Accessibility Iniative (2022). WCAG 2 overview. [Online]. Available: https://www.w3.org/WAI/standards-guidelines/wcag/(Accessed Sept 22, 2022).
Wang, Z., Jensen, M. A., and Zenklusen, J. C. (2016). A practical guide to the cancer genome Atlas (TCGA). Methods Mol. Biol. 1418, 111–141. doi:10.1007/978-1-4939-3578-9_6
Wilburn, A., Vanderpool, R. C., and Knight, J. R. (2016). Environmental scanning as a public health tool: Kentucky's human papillomavirus vaccination project. Prev. Chronic Dis. 13, E109. doi:10.5888/pcd13.160165
Keywords: Cancer Moonshot, patient engagement, patient participation, health equity, genomics
Citation: Schuster ALR, Crossnohere NL, Paskett J, Thomas N, Hampel H, Ma Q, Tiner JC, Paskett ED and Bridges JFP (2023) Promoting patient engagement in cancer genomics research programs: An environmental scan. Front. Genet. 14:1053613. doi: 10.3389/fgene.2023.1053613
Received: 26 September 2022; Accepted: 06 January 2023;
Published: 18 January 2023.
Edited by:
Hauke Thomsen, ProtaGene CGT GmbH, GermanyReviewed by:
Michelle Dow Keawphalouk Narangajavana, Massachusetts Institute of Technology, United StatesJared C. Roach, Institute for Systems Biology (ISB), United States
Copyright © 2023 Schuster, Crossnohere, Paskett, Thomas, Hampel, Ma, Tiner, Paskett and Bridges. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anne L. R. Schuster, anne.schuster@osumc.edu
 Anne L. R. Schuster
Anne L. R. Schuster Norah L. Crossnohere1
Norah L. Crossnohere1 Jonathan Paskett
Jonathan Paskett Qin Ma
Qin Ma Electra D. Paskett
Electra D. Paskett