Potential of RFID telemetry for monitoring ground-dwelling beetle movements: A Mediterranean dry grassland study
- Avignon University, Aix-Marseille University, CNRS, IRD, IMBE, Avignon, France
Better understanding insects’ movements could help preserve and restore the insect communities that are key to the functioning of grasslands. Recent technological advances have led to spectacular achievements in movement ecology, making it possible to track the individual movements of a wide variety of organisms, including the smallest. However, monitoring systems such as RFID tags may negatively impact an organism’s life history, with potential consequences on the reliability of data and conclusions. This study explored the potential of passive RFID tags to track the movements of three small ground-dwelling beetle species, a predator (Poecilus sericeus, Carabidae), a detritivore (Asida sericea, Tenebrionidae) and a granivore (Acinopus picipes, Carabidae), in a Mediterranean dry grassland degraded by years of cultivation. First, we tested whether carrying tags might impact individuals’ behaviour, using a before-and-after design under laboratory conditions. Despite a trend toward shorter displacements, we found no significant short-term effect of the tags on individuals’ movements. Second, we tracked a total of 25 tagged beetles in their natural environment every 4 h for 48 h. We highlight the principal limitation of using passive tags with small terrestrial beetles: the antenna has to pass over the tags to detect them, which restricts tracking to a few consecutive days after which the probability of locating an individual is low. However, the data obtained sheds light on the biological rhythms and daily movement capabilities of our target species: A. sericea is more mobile and P. sericeus less mobile than expected. Such knowledge could help predict the species’ ability to recolonise degraded areas, enabling appropriate restoration actions to be designed based on landscape ecology principles.
1. Introduction
The global decline of grasslands is making conservation and restoration actions a priority (Buisson et al., 2022). Usually, it is plant species that are targetted in restoration actions, under the general assumption that insect populations will naturally follow the restoration of plant communities (Hilderbrand et al., 2005). However, this assumption neglects key processes that underpin the establishment and persistence of insect populations in sites to be restored, such as their ability to naturally reach these areas.
Ensuring that species can move between habitats or from preserved to degraded areas is vital for their maintenance and for ecosystem functioning and restoration (Massol et al., 2011; Poli et al., 2020). Considering species movements should therefore be a prerequisite when planning species conservation and habitat restoration (Katzner and Arlettaz, 2020). Currently, however, movement data are too often neglected when implementing conservation plans (Allen and Singh, 2016), reducing the effectiveness of traditional approaches such as protected areas given the spatial scale of species’ movements (Thirgood et al., 2004). One of the challenges in species conservation is therefore to determine how, where, when and why species move.
The recent emergence of the science of movement that provides detailed spatio-temporal data is a significant advance in species conservation (Allen and Singh, 2016). Movement processes inform the foraging ecology and dispersal capacity of organisms (Ramos-Fernández et al., 2004). Research in movement ecology is generating knowledge of factors like the extent and use of geographical ranges, migratory pathways, phenology and activity period, the knowledge required for flexible conservation strategies in space and time (Le Gouar et al., 2015; Bérces and Růžičková, 2019). For example, to estimate the migratory range of stag beetles (Lucanus cervus) as a measure of connectivity among neighbouring populations for future conservation measures, Rink and Sinsch (2007) equipped individuals with 350 mg transmitters. They observed that about 1% of males are capable of maintaining gene flux among nest sites within a radius of about 3 km. Thus, isolated populations more than 3 km apart have an increased probability of local extinction.
Movement ecology has evolved thanks to recent technological advances such as radio frequency identification, hereafter RFID (e.g., Moreau et al., 2011), harmonic radar (e.g., Milanesio et al., 2016) or radio telemetry tags also called very high frequency (VHF) radio telemetry (e.g., Růžičková and Veselý, 2016). These technologies have been widely used to study the space use and movement behaviour of vertebrates (Holyoak et al., 2008). In comparison, insect movement ecology remains relatively neglected, despite the recent miniaturisation of tracking devices that has enabled accurate estimation of movements, even for small organisms like insects (Reynolds and Riley, 2002; Milanesio et al., 2016). Riecken and Raths (1996) were the first to use small radio-transmitters (0.6–0.7 g) to study the dispersal and habitat use of a terrestrial ground-dwelling insect, Carabus coriaceus, a large beetle about 40 mm long. Since then, a wide array of ground and flying arthropods has been tracked (Kissling et al., 2014), from tarantulas (e.g., Janowski-Bell and Horner, 1999) and beetles such as Carabidae (e.g., Negro et al., 2008) to hymenopterans (Henry et al., 2012) and dragonflies (Moskowitz and May, 2017).
When used on insects, tracking devices are commonly glued either directly on their external tegument (e.g., Batsleer et al., 2020) or to a line that is itself fixed onto the insects’ back (e.g., Vinatier et al., 2010). However, this equipment may constrain insect behaviour and affect different aspects of an arthropod’s life history (e.g., energy, movement, foraging, mating), potentially reducing the reliability of data and conclusions (Henry et al., 2012). Batsleer et al. (2020) recently called for more systematic documentation of potential effects of tags on arthropods’ behaviour. Their review found that only 12% of the papers quantified the impacts of tags on insects. Most of the remaining papers claimed no effect, based on tag/body weight rules. The 5% rule—developed for flying or swimming vertebrates—considers that a tag/body weight ratio above 5% induces significant impacts (Kenward, 2001). Boiteau and Colpitts (2001) recommended that tags should weigh no more than 23–33% of the beetle’s acceptable extra loading to limit impacts on the number and quality of flights. However, such general rules do not make much sense, as tag impacts are most likely dependent on study species, tag type, sex and environmental context (Jepsen et al., 2005). For example, predator and scavenger beetles of similar size and weight are unlikely to be affected in the same way by the tag. Unlike scavengers, predators often have to move quickly and sometimes over large areas to detect and catch their prey, which may make them more sensitive to additional weight.
Each of the available technologies has its own advantages and drawbacks for the study of arthropod movements (Kissling et al., 2014; Batsleer et al., 2020). Active radio transmitters allow individuals to be tracked over distances of more than 300 m for ground-dwelling insects (Negro et al., 2008); however, the problem is the weight of the transmitter, sometimes as heavy as a given arthropod. Passive tags (harmonic radar and RFID) have been applied to a broader range of arthropods, but the short detection distance complicates the monitoring of arthropods in the field. How much of a drawback this is depends, however, on the structure of the habitat; passive tags may be more appropriate in open habitats such as grasslands, where tagged individuals are easier to detect. For smaller arthropod species, where tag weight makes passive tags the only option, current challenges in monitoring movements include the limited detection distance and potential impacts on the animals’ behaviour.
This study investigated the potential of passive RFID transmitters to track the movements of three small ground-dwelling beetle species typical of the Plaine de la Crau (Southern France) dry grassland: a predator (Poecilus sericeus, Fischer von Waldheim, 1824, Carabidae), a detritivore [Asida sericea (Olivier, 1795), Tenebrionidae] and a granivore [Acinopus picipes (Olivier, 1795), Carabidae]. We first explored how the tags impacted beetle behaviour, using a before-and-after design under laboratory conditions. Then we tracked tagged individuals in their natural environment, located in a protected nature reserve. Our findings are discussed in terms of their relevance to the conservation of beetle species in a protected dry grassland.
2. Materials and methods
2.1. Study site
La Crau is a plain dominated by dry grasslands located in the former delta of the Durance river (Southern France), to the east of the Rhône valley and the Camargue. This region is characterised by a Mediterranean climate, with low annual precipitation (400–500 mm per year), long hot summers and mild winters (mean annual temperature: 14° C). On average, the strong predominant wind (“mistral”) blows from the North–West 334 days per year and the sun shines 3,000 h per year, generating very high levels of evapotranspiration (Devaux et al., 1983). In La Crau dry grasslands, about 50% of the soil surface is covered with stones transported by the Durance river 650,000 to 30,000 years ago. The substratum is an impermeable conglomerate bedrock, 40 cm deep, which limits the accessibility of groundwater for vegetation. Our field experiment took place in the centre of the sheepfold “Peau de Meau” (43.571525°N, 4.831853°E, elevation 10 m, 160 ha). The site is representative of the remaining 11,500 ha of dry grassland vegetation typical of the area, i.e., dominated by Brachypodium retusum P. Beauv. L., Poaceae and Thymus vulgaris L., Lamiaceae (Buisson and Dutoit, 2004; Römermann et al., 2005).
2.2. Study species
We focussed on three apterous beetle species: Asida (polasida) sericea (Olivier 1795), Poecilus sericeus (Fischer von Waldheim, 1824), Acinopus (acinopus) picipes (Olivier, 1795) (Figure 1). They are among the most abundant non-flying beetle species on the La Crau dry grassland, A. sericea and P. sericeus often jointly accounting for nearly 40% of the individuals caught (Blight et al., 2011). The three species are of similar size, with average body length from 11 to 14 mm (Jeannel, 1942; Soldati, 2006), but belong to three different feeding guilds. As a Tenebrionidae, A. sericea is a detritivore species. Acinopus picipes is an omnivorous-granivorous species with a large head and stout mandibles (Talarico et al., 2016), whereas P. sericeus is a predator with an elongated body and long mandibles (Jeannel, 1942). Their life cycles also differ: P. sericeus overwinters in adult stage, A. picipes overwinters in larval stages (Talarico et al., 2016) and A. sericea’s imago can be found all year long (Soldati, 2006).

Figure 1. Study species and study area (A) the dry grasslands of la Crau and marking of release points, (B) Asida sericea, (C) Acinopus picipes, (D) Poecilus sericeus equipped with an RFID-tag on its back, (E) Asida sericea equipped with an RFID-tag and released in the field.
2.3. Monitoring devices
Based on preliminary tests with harmonic radar technology (RH, unpublished results), we decided to use radio frequency identification (RFID) to monitor the beetles’ movement. The major issue with harmonic radar is individuals’ movements being hindered by the long wire antennas getting caught in vegetation, as described in O’Neal et al. (2004). Despite a shorter detection range, RFID eliminates the problem of the protruding antenna and allows tagged individuals to be distinguished according to unique signals. We used miniature passive RFID tags (Mini HPT8 PIT Tag, Biomark Inc., Boise, ID, USA) 8 mm long by 1.4 mm wide, i.e., shorter and narrower than our study species. These tags weigh about 30 mg, which means that individuals equipped with glued diodes carried roughly 30% of their body mass. Tagging our study individuals added 26% extra weight to A. picipes, which weighs on average 0.12 g, 35% to A. sericea, which weighs on average 0.14 g, and 26% to P. sericeus, which weighs on average 0.12 g (Table 1).
Tags were glued to the insect’s elytra with cyanoacrylate glue (Super glue 3 power flex mini trio, Loctite, Henkel, Germ). We tracked the tagged insects with a hand-held battery-powered transponder reading device (HPR Plus Reader, Biomark) equipped with a 40 cm diameter portable antenna (BP Plus Antenna, Biomark). The antenna had to pass over a tag to detect it. Detection height was also limited to a few centimetres (up to 30 cm, depending on the presence of stones under which beetles can hide).
2.4. Lab experiments
We first conducted an experiment to assess the impact of laboratory conditions and tags on the movements of the three species. A control group (N = 6 for P. sericeus and N = 5 for A. picipes and A. sericea) was tested for signs of familiarisation with an arena where they were repeatedly released. We hypothesised that their exploratory behaviour might be reduced by repeated release in the arena, through habituation to this new context. Individuals were released three times in the centre of a 42 cm × 27 cm plastic box (hereafter the arena), its bottom covered with white blotting paper to increase contrast with the dark insects. They were filmed for 4:30 min using a camera (alternatively 2 cameras: Nikon Coolpix P100, 10 megapixels and Nikon Coolpix P7700, 12 megapixel) attached to a copy stand. A second group of individuals (N = 10 for A. sericea, N = 14 for P. sericeus and N = 7 for A. picipes) was tested for the effect of the tags on behaviour and movements. Each individual was released three times in the arena and their behaviour recorded. The first time, the individual was “undisturbed”; the second time, a drop of glue was placed on it; the third time, the tag was glued onto its back. With or without tags, the glue was allowed to dry for 20 min before the individual’s movements were filmed for 4:30 min using the device described above. We hypothesised that small tags glued to their elytra would not alter the movement capability of these ground-dwelling non-flying beetles.
Temperature in the experimental room was about 23°C. Filming was done in daylight and additional artificial light was used to improve image quality and avoid shading. Note that if an insect did not move for the first 2 min, we switched to the next individual.
All the individuals used in these experiments were hand-caught in the field and stored in boxes with soil and rocks retrieved from the field. Boxes were kept moist and oxygenated. Lab experiments were conducted in the days following their capture.
Data for each individual was treated in three steps. First, pictures (1920 × 1088 px) were extracted from the films (30 frames per sec.) at regular 5-s intervals with VLC© (scene filter on and recording ratio of 150), yielding 54 images per film. Second, the individual’s spatial coordinates in the arena were calculated via automated image analysis, as in Mallard et al. (2013). Third, two variables related to the individual’s activity in the arena were calculated: (1) average distance travelled in 5 s. time steps (pixels), and (2) total area explored, defined by number of pixels visited in an arena divided into 200 square pixels.
The average behaviours of individuals from the different species and treatments were compared via repeated measures ANOVA (hereafter anova), which allows for the same individuals to be measured on the same outcome variables under different conditions (three consecutive runs with or without equipment), using the function anova_test from the package rstatix (Kassambara, 2022). We accounted for multiple comparisons with Holm’s adjustment, less conservative than the Bonferroni method (Holm, 1979). Non-normal variables were transformed using the function bestNormalize (Peterson, 2021).
To investigate how different individuals responded to our treatments, we used the intraclass correlation coefficient (ICC) with the function icc from package irr (Gamer et al., 2019). The ICC measures the reliability of ratings in inter-raters or in test–retest designs by comparing the variability of different ratings for the same individuals to the total variation across all ratings and all individuals (Shrout and Fleiss, 1979). Both agreement and consistency were measured. An ICC value above 0.9 means agreement (or consistency) is excellent, a value between 0.75 and 0.9 good, a value between 0.5 and 0.75 moderate and below 0.5 poor (Koo and Li, 2016). Non-normal variables were transformed using the function bestNormalize (Peterson, 2021).
2.5. Field experiment
The movements of individual beetles in the field were monitored every 4 h for 48 h (17–19 May 2021). Individuals were hand-caught in the morning of day 1 and kept in plastic boxes before their release. They were equipped with the same RFID tags as in the laboratory experiment (see above) and observed for 30 min minimum to confirm successful tag attachment. Individuals were released on day 1 at 14h30. Release points were located 10 m apart to facilitate detection procedures, reduce interference among tagged individuals and maximise the heterogeneity of stone and vegetation cover encountered in the dry grassland. Release points were located in a 40 m diameter circle to facilitate logistics and were marked with a labelled and coloured pole (Figure 1).
Initially, we released 25 individuals (N = 7 for A. sericea and N = 9 for P. sericeus and A. picipes), the number of individuals per species reflecting capture success. Every 4 h, all individuals were detected and their new position marked with a labelled pole. We recorded the time, the distance travelled since release with a decametre and the direction taken since release (deviation from compass direction, distance-bearing approach (Rùžièková and Elek, 2021) with the compass application of a smartphone (using a single iPhone 5S to avoid bias). This provided two daily time steps per time of day: afternoon (10:30–14:30, 14:30–18:30), night (18:30–22:30, 22:30–2:30), morning (2:30–6:30, 6:30–10:30).
When the individual appeared motionless after three consecutive detections, its physical condition was checked. Twelve hours after starting the experiment, there were two dead individuals and seven lost their tag. Nine additional individuals were released at 14h30 on day 2 (N = 3 for each species). One week later, an exhaustive field search was conducted over a circle of 6 m around the release points. The distance and angle to release point were recorded for each detected individual; undetected individuals were considered to have left these search areas.
This experiment took place under typical spring weather conditions, mainly sunny days with 30–50 km/h northerly wind and light rain (< 0.5 mm for 2 h). The temperatures of the soil (10 cm below soil surface) and air (at 1.2 m above soil in the shade) were recorded with two loggers (Hobbo®, Pendant temp logger 64K). There was a clear night-day temperature contrast, with an average temperature of 18.8°C in the soil and 19.0°C in the air, a minimum temperature of 14.1°C in the soil and 8.6°C in the air and a maximum temperature of 27.0°C in the soil and 31.0°C in the air.
To test for differences between sessions in distance travelled for each species (morning, day and night), we used the non-parametric Kruskal-Wallis test from the package rstatix (Kassambara, 2022), as the data did not follow a normal distribution, even after transformation. Pairwise comparisons were performed using the pairwise.wilcox.test function with Holm’s correction from the package rstatix (Kassambara, 2022).
All statistical analyses were performed using R Statistical Software (v4.1.2 R Core Team, 2022).
3. Results
3.1. Lab experiments
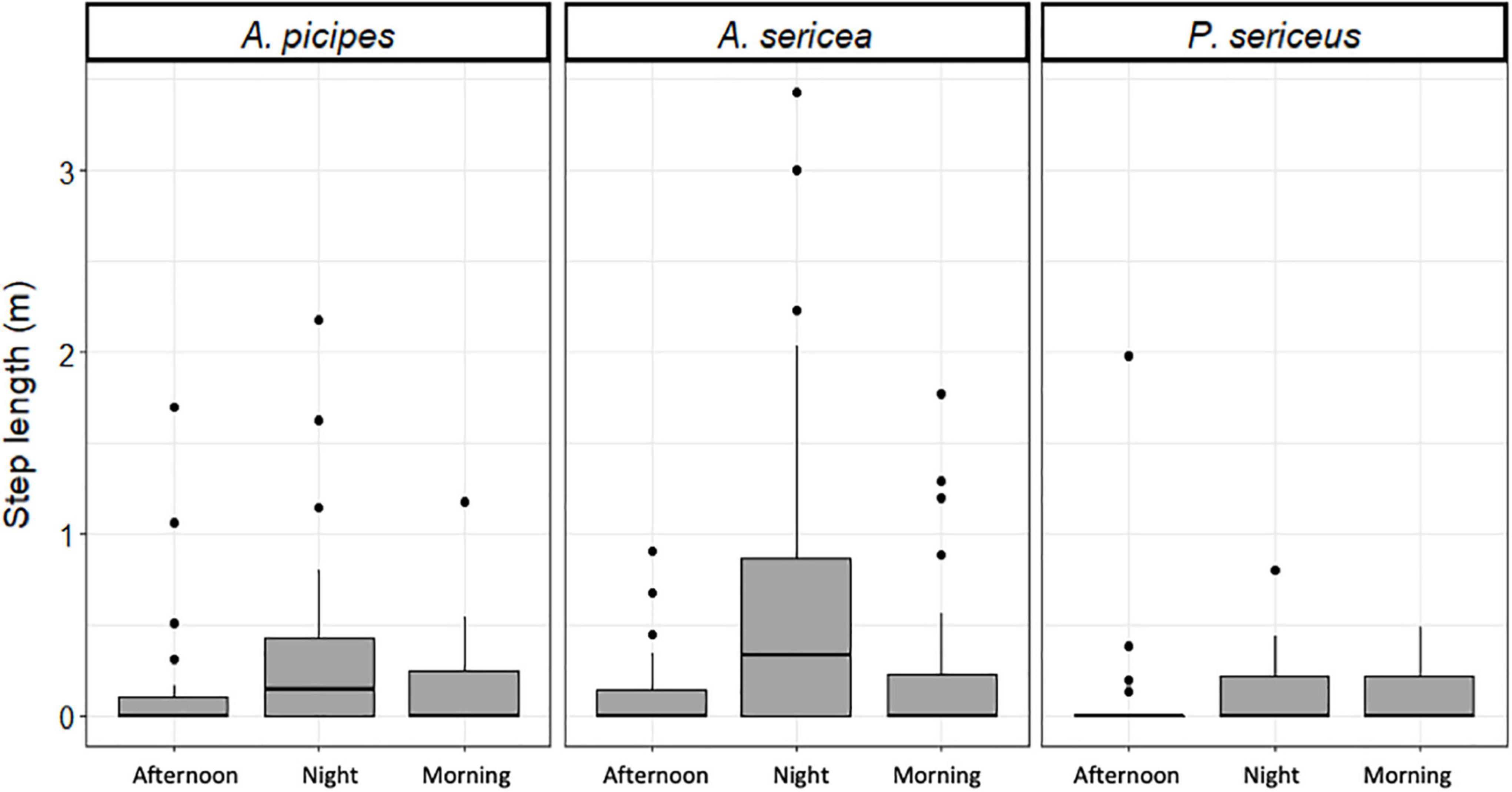
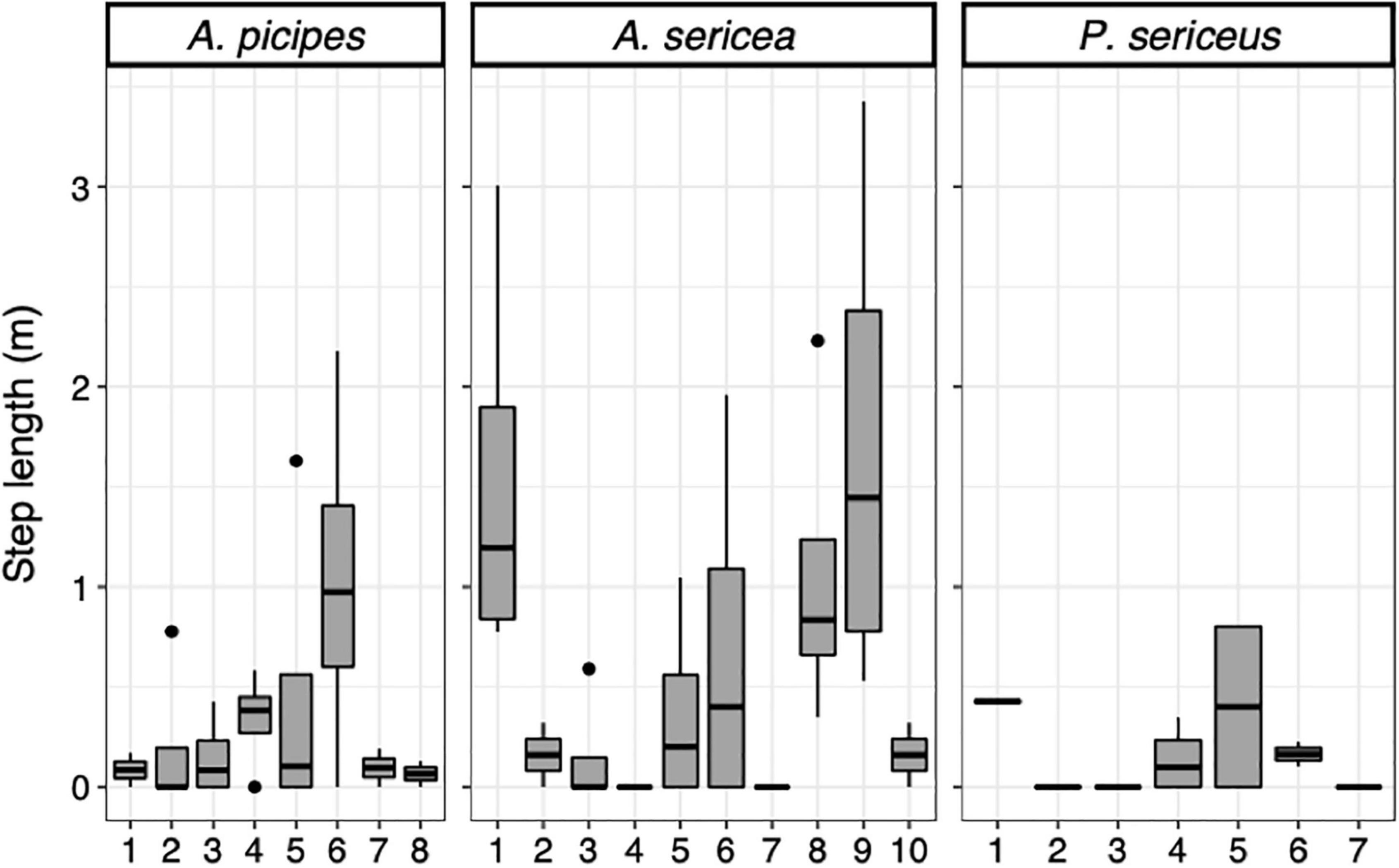
We observed strong individual variations in the behaviour of beetles in the arena, with some individuals circling along the edges, some widely exploring the surface and some remaining still in a corner (Figure 2). No clear sign of habituation to the arena was found, none of the analysis being significant (Figure 3). Moreover, the area explored by individuals differed in a completely idiosyncratic manner between arena sessions (ICC < 0.5) (Figure 3B). The distance travelled was moderately consistent (ICC ∼ 0.6) for A. sericea and A. picipes across the three runs, with some individuals travelling fast while others remained slow.
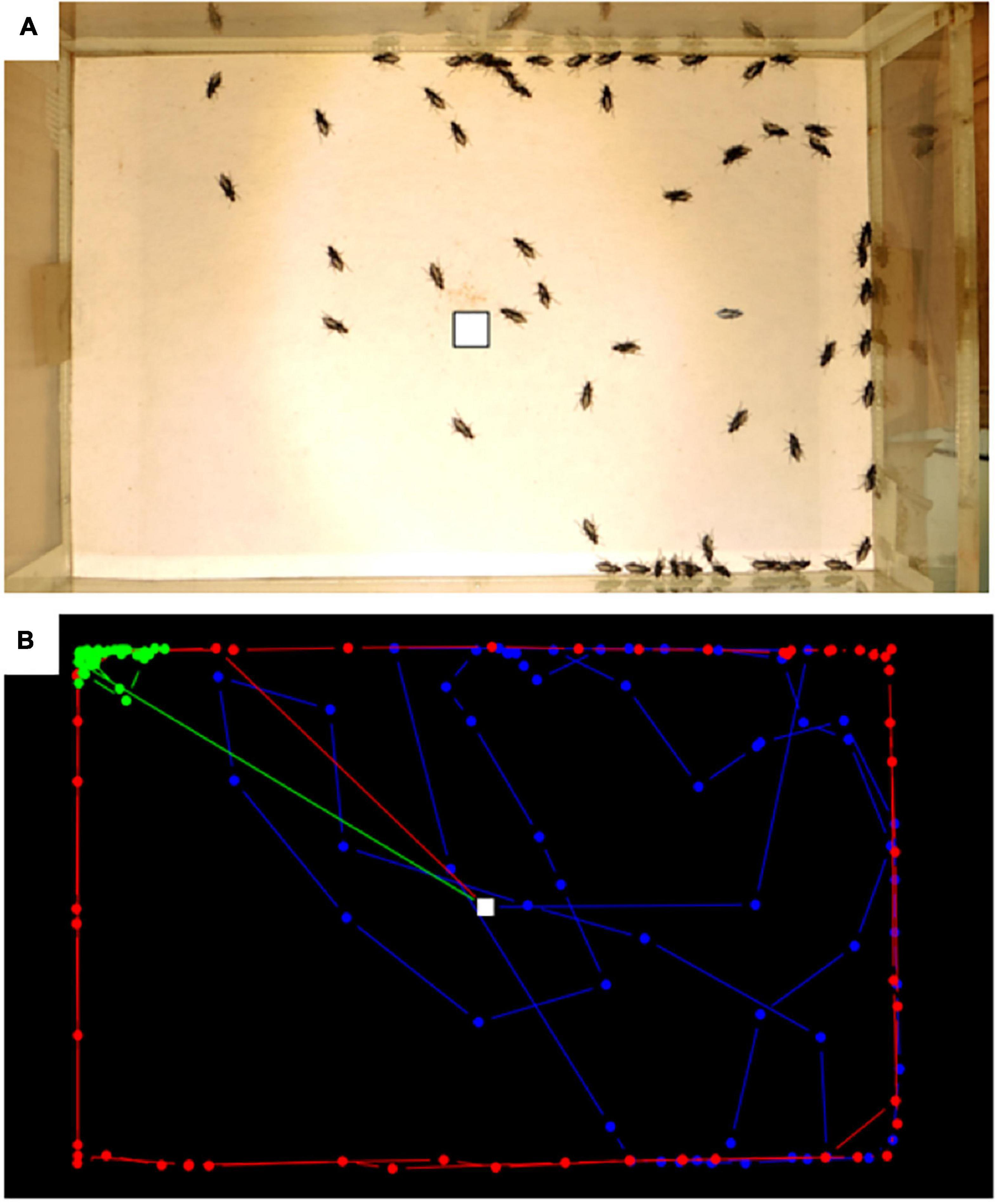
Figure 2. Monitoring beetle movements in the lab, (A) example of an experimental run with all-time steps overlapped, (B) example of three contrasting trajectories in blue, green and red. White square correspond to the release point.
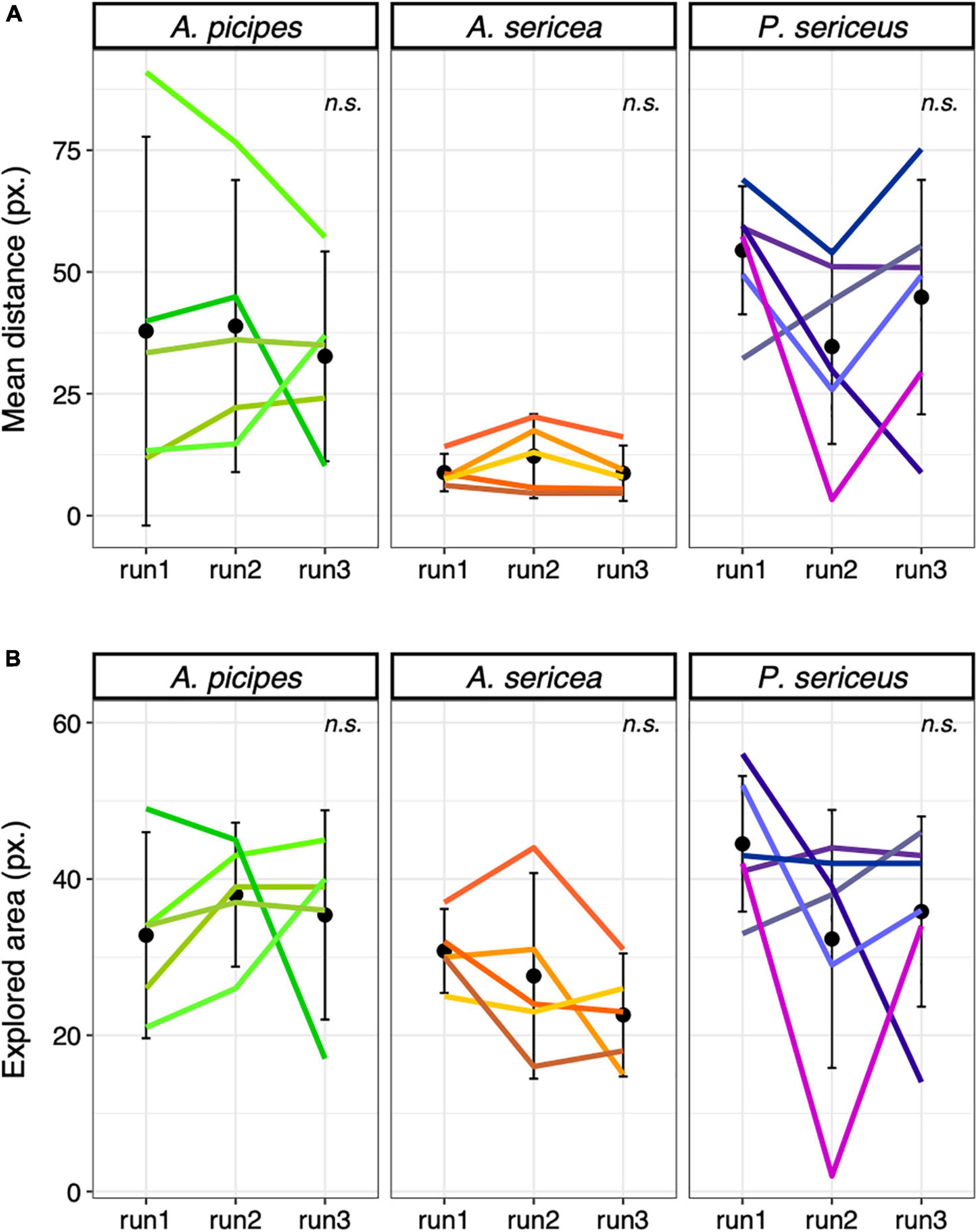
Figure 3. Control test, (A) Mean distance ( ± SE) travelled per time step among species and runs in the arena in pixels and (B) Mean explored area ( ± SE) among species and runs in the arena. n.s. = non-significant. Each coloured line represents an individual.
In contrast, for the tag-effect test, we observed an overall effect on A. sericea [F(2, 18) = 15.15; p < 0.001]. Individuals covered significantly shorter distances per time step with the diode on their back than when carrying nothing (df = 2; p = 0.004) (Figure 4A), and also tended to cover shorter distances when they had glue alone on their backs compared to nothing (df = 2; p = 0.067) (Figure 4B). An overall effect was also observed on A. picipes [F(2, 12) = 4.71; p = 0.031] and P. sericeus, [F(1.31, 17.02) = 5.22; p = 0.028], but none of the comparisons were significant after applying Holm’s correction. Neither carrying the glue alone nor the diode affected the area explored. None of the ICC values were above 0.5, indicating poor consistency in the individuals’ behaviour and poor agreement in their ranking among treatments.
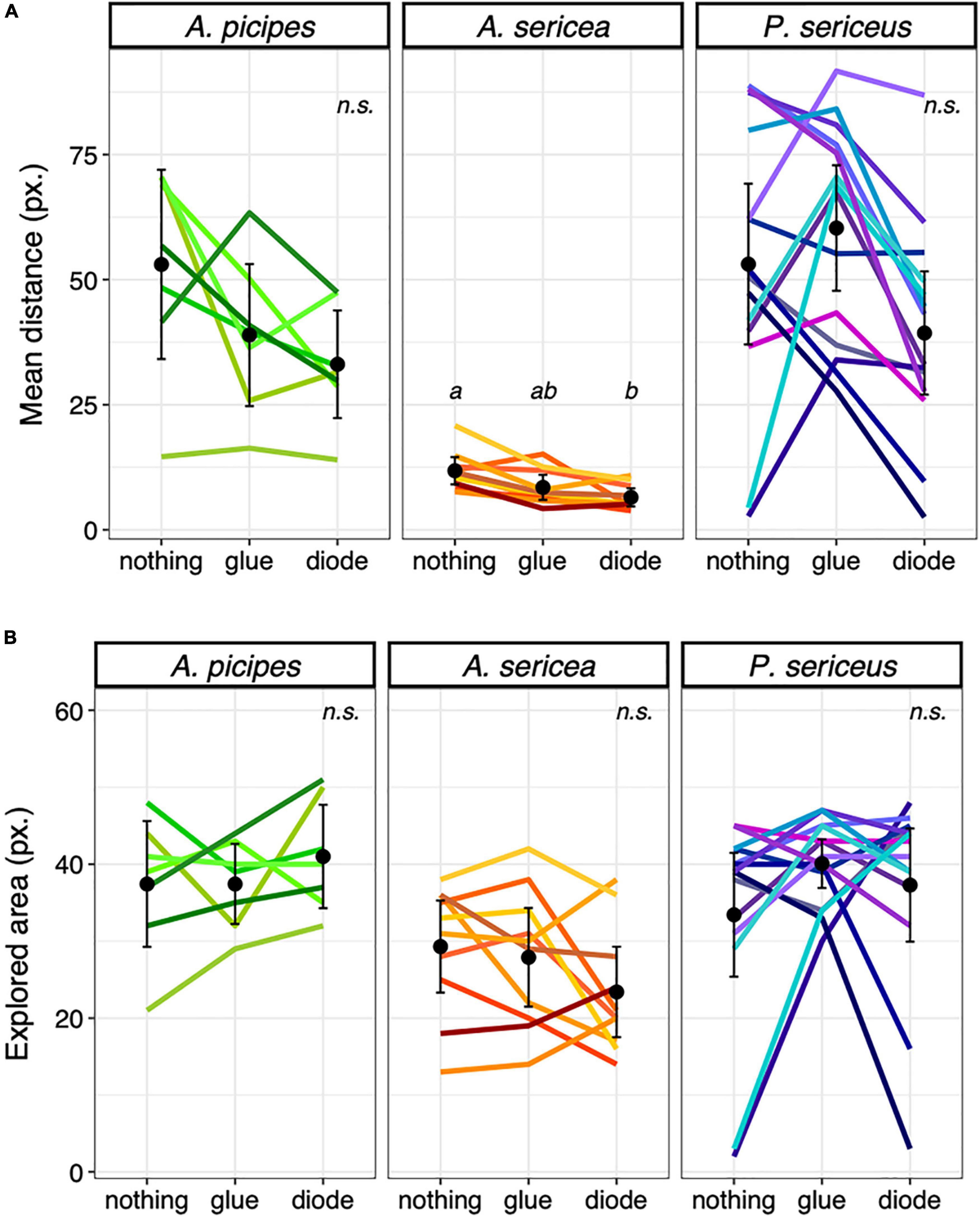
Figure 4. Effect of the diode, (A) Mean distance ( ± SE) travelled per time step among species and runs in the arena in pixels and (B) Mean explored area ( ± SE) among species and runs in the arena. Different letters indicate significant differences between runs (P < 0.05), n.s. = non-significant. Each coloured line represents an individual.
3.2. Field experiment
In the end, movement data was obtained on 25 individuals over 24 to 48 h: 8 A. picipes (out of 12 released), 10 A. sericea (out of 10 released) and 7 P. sericeus (out of 12 released). The other individuals were preyed on (2) or lost their diodes (7). One week later, within a 6 m radius of the release points, the field search identified 11 individuals that had moved farther than a week earlier (2 A. picipes, 5 A. sericea, 4 P. sericeus) and five that had not moved (due to territoriality, death or lost diode); the 9 other individuals were not found (Table 2, see Appendix 1 for trajectory examples).
The distances travelled by the tagged individuals did not significantly differ between species due to high individual variability (all the metrics yielded the same results). After 24 h, A. picipes individuals were found approximately 0.81 metres from their starting point (max = 2.63 m), having travelled an average of 0.22 metre in 4 h (max = 2.17). Asida sericea individuals were found approximately 1.03 metres from their starting point (max = 3.62 m), having travelled an average of 0.33 metre in 4 h (max = 3.42). Poecilus sericeus individuals tended to travel less and were found approximately 0.5 metres from their starting point (max = 1.91 m), having travelled an average of 0.13 metre in 4 h (max = 1.98). On average, individuals started to move after 3.5-time steps for A. picipes, 4.6 for A. sericea and 5.8 for P. sericeus.
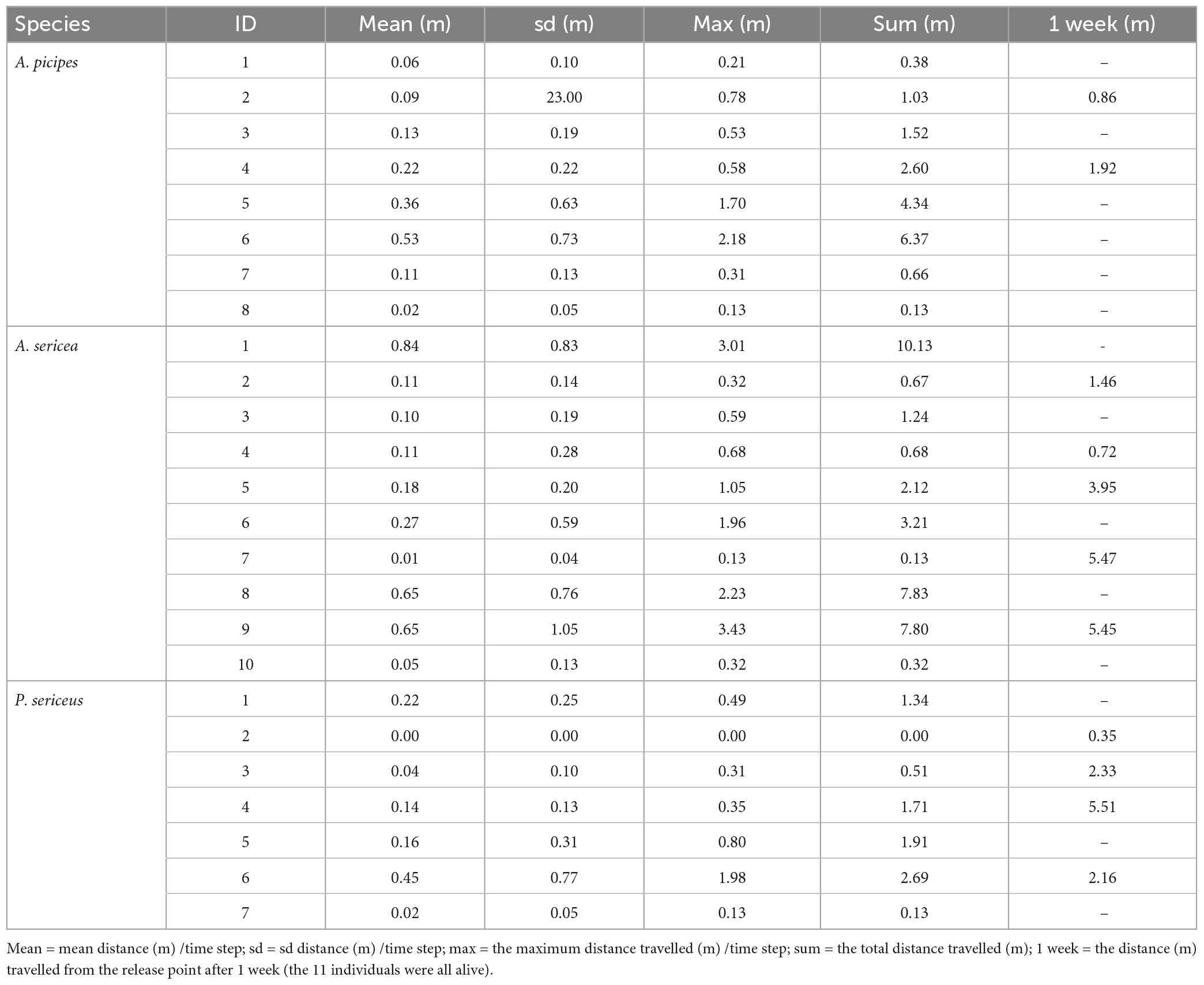
We observed a significant effect of time of day on movement patterns (df = 2; χ2 = 12.78; p < 0.01) (Figure 5). Individuals moved more at night than in the morning (df = 2; p = 0.026) and substantially more at night than in the afternoon (df = 2; p = 0.003), with no significant difference between morning and afternoon (df = 2; p = 0.226). The effect was, however, not equally strong for all three species. Time of day significantly affected the movements of Asida sericea (df = 2; χ2 = 8.47; p = 0.015), individuals moving significantly more at night than in the afternoon (df = 2; p = 0.022). In contrast, no significant effect was observed for A. picipes (df = 2; χ2 = 4.6; p = 0.1) or P. sericeus (df = 2; χ2 = 1.27; p = 0.53). Mobility was largely idiosyncratic, with some individuals moving more during the different time steps than others (Figure 6).
4. Discussion
Assessing the movement capability of insects is essential for the implementation of effective conservation and restoration measures. While the recent miniaturisation of tags is making it easier to track animals, including insects, there remain constraints such as the impact of tag weight on individual movement and the short detection distance. Here, we found weak effects of the tag on individual behaviours of three small ground-dwelling beetles in the laboratory, effects that were limited to the detritivore species. We obtained data on their daily behaviour by closely monitoring individuals in their habitat for 48 h and then a week later, but with a significant number of losses. In the light of these results, we discuss the implications of these beetle species’ daily movements for the recovery of their populations in degraded areas of the Plaine de la Crau dry grassland.
4.1. Validation of the tracking technique
Previous radio tracking using passive tags was limited to beetles of more than 20 mm in length and a 0.4 g body mass (Kissling et al., 2014; Testud et al., 2019). Here, we demonstrated the validity of this technique even for smaller beetles (15 mm and 0.12 g). Despite a tendency toward shorter displacements, we observed few significant changes in the behaviour of tagged individuals under laboratory conditions. Only A. sericea covered significantly shorter distances per time step with the diode on. However, caution is required in interpreting these results; although we did not observe strong short-term behavioural effects, the tags could have impacts over several days or weeks.
Because insects are known to be able to carry large extra loads for feeding or building activities, studies on insects’ movements are generally conducted using a tag-to-body-mass ratio greater than the 5% body-mass rule established for vertebrates. Here, the full tagging system did not seem to strongly affect the beetles’ movements, despite adding 26% to the body weight of A. picipes, 26% to P. sericeus and 35% to A. sericea. Actually, despite the growing use of telemetry techniques for tracking insects, their impacts are rarely assessed. Only 12% of the 173 studies reviewed by Batsleer et al. (2020) quantified the effect of tags on arthropods. In one example, the activity of the flightless cricket, Gryllus locorojo, reduced as tag weight increased from 18 to 127% of the individual’s body mass (Kaláb et al., 2021). Boiteau et al. (2010), studying the beetle Conotrachelus nenuphar, reported that a tag weighing around 15% of its body mass did not reduce its mean walking speed. Another approach is to consider that tag weight must not exceed the maximum mass of an item the species can lift (Batsleer et al., 2020). This would, however, be difficult to determine for our studied species, which does not have to grab and carry food before eating it.
Our field experiment successfully used RFID to track small ground-dwelling beetle movements in the species’ habitat. Despite the short detection distance (the antenna having to pass over the tag to detect it), we were able to locate all individuals except those that lost their tag or were preyed on. This meant we could track a reasonable proportion of the total tagged individuals (25 out of 34). However, our ability to locate individuals decreased greatly after 7 days, with only 16 out of 25 found after thoroughly searching an area 6 metres around the release point. It is therefore unlikely that we could track individuals over several weeks or months without close monitoring, which would be time-consuming and uncertain to succeed. The nature of the data collected is therefore limited to species’ daily movements and foraging behaviour, without covering dispersal sensu stricto (Silcox et al., 2011). The method’s applicability is also limited to species moving over short distances.
4.2. Species and individual-level data acquisition
Although limited in time, the data we collected provide useful knowledge on the behaviour and ecology of the three studied species, such as differences according to diet and daily activity. We observed differences between species in the distances travelled. Unexpectedly, Asida sericea travelled the most and farthest in the field, whereas it was the least active and most impacted by the tag in the laboratory. Poecilus sericeus, the predator needing to search for prey, could have been expected to move around more than a detritivore or a granivorous species with easy access to food resources. Two non-exclusive explanations can be put forward. Firstly, while it appeared to be unaffected by the tag in the laboratory, P. sericeus may suffer more from the extra weight in a more structured environment of stones and dense vegetation, the species being thinner and lighter than the detritivore A. sericea. Secondly, P. sericeus might prefer to remain hidden from its prey, rather than actively hunting. Our experimental setup, limited in time and space, did not allow us to properly assess distance travelled and our arenas contained no hiding places.
Using both day and night sessions provided fundamental data on the phenology of the three species. They were all more active at night, which is relatively common in beetles, especially dark species. Dark nocturnal species have evolved as an interspecific adaptation to avoid predation (Hernández, 2002), and being nocturnal also allows them to avoid the warmer and drier daytime conditions.
Radio frequency identification tracking also provided useful data at the individual level. Confirming our observations in the laboratory, we recorded high within-species variability in behaviour. The individuals that were the least active the first day remained less active the following day, regardless of species. These consistent inter-individual differences might suggest the presence of personality traits (Sih et al., 2004) in these three beetles; however, this question will need to be more thoroughly investigated based on a larger number of individuals. From a conservation perspective, animal personality has important implications. Dispersal is often phenotype-biassed, with bolder and more exploratory individuals showing a higher dispersal tendency (Cote and Clobert, 2007; Cote et al., 2010). Such data could help explain colonisation dynamics (Cote et al., 2010) and the degree of success of species re-introduction (de Azevedo and Young, 2021).
4.3. Implications for the conservation of grassland beetle populations
This study highlights the limitations of using passive tags to track the movements of small terrestrial beetles. The fact that detection requires the antenna to pass over the tag makes it impossible to track individuals that cover several metres per day over several weeks, and thus to accurately identify their dispersal capabilities and home range. However, the data collected here provide valuable information on the biology of the three species, such as their nocturnal activity.
We were also able to determine their daily movements, which might have implications for their conservation and restoration in the study grassland. This dry grassland has been degraded by years of cultivation requiring the removal of the original stone cover (Buisson and Dutoit, 2006). Stones are a structuring factor for beetle communities (Blight et al., 2011), probably creating better microclimatic conditions (havens of moisture and low temperatures in summer, Lamb and Chapman, 1943; Nobel et al., 1992). These daily movement data shed light on the dynamics of natural colonisation of degraded areas from the original habitat. Asida sericea and P. sericeus, the two most abundant species in the original ecosystem, remain present in the formerly cultivated areas but in significantly lower abundance (Fadda et al., 2007; Blight et al., 2011). Although both ground-dwelling species move relatively short distances in the course of a day, they do move, confirming the hypothesis put forward by Fadda et al. (2008) of a possible natural colonisation of degraded areas from the nearby original habitat. Indeed, in the study area, degraded grasslands are contiguous with preserved grassland and there are no physical barriers such as concrete roads. The lower population densities found in formerly cultivated areas are therefore more likely due to less suitable habitats rather than to limited movement.
Knowing the rate of daily movement is also relevant for the implementation of effective restoration actions. One such proposed action is the restoration of stone cover, key for beetle communities. Even small stone patches dispersed randomly appear to be an effective way to increase beetle richness and abundance on formerly cultivated fields, as suggested by Blight et al. (2011) “several stone patches dispersed over a wide area maximises edge effects between the ecosystem to be restored and the restored habitat patches.” However, the spatial distribution of the patches that will ensure the most efficient management depends on the beetles’ movement capability. Our RFID monitoring suggests that this distance should not exceed 6 m, as 58% of individuals were found within 6 m of the release point 1 week later.
5. Conclusion
While we confirm the limitations of using RFID technology to track small ground-dwelling beetles over a long period in a dry Mediterranean grassland, the data collected provide interesting data on their biology, that may help in the development of restoration actions. Further work should address the challenge of tracking individuals’ movements between restored habitat patches.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
OB, BG, and CA conceived the ideas. OB, BG, LM, and CA designed the experiment and collected the data. LM and CA analysed the data. OB and CA wrote the first draft. All authors contributed critically to the drafts.
Funding
This study was supported by the European Research Council, project ERC STG SCALED no. 949812.
Acknowledgments
We thank Nicolas Bachelier, Paul Guerra, Marine Rousseau, Hélène De Meringo, Victorine Demiralp, and Karolina Argote for their help in the field. We also thank Axel Wolf and the Eco-Musée de Crau for allowing us access to the Nature Reserve of Peau de Meau.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2023.1040931/full#supplementary-material
References
Allen, A. M., and Singh, N. J. (2016). Linking movement ecology with wildlife management and conservation. Front. Ecol. Evol. 3:155. doi: 10.3389/FEVO.2015.00155
Batsleer, F., Bonte, D., Dekeukeleire, D., Goossens, S., Poelmans, W., Van der Cruyssen, E., et al. (2020). The neglected impact of tracking devices on terrestrial arthropods. Methods Ecol. Evol. 11, 350–361. doi: 10.1111/2041-210X.13356
Bérces, S., and Růžičková, J. (2019). Hungarian natural history museum. Budapest Acta Zool. Acad. Sci. Hungaricae 65, 335–348.
Blight, O., Fadda, S., Orgeas, J., Ponel, P., Buisson, E., and Dutoit, T. (2011). Using stone cover patches and grazing exclusion to restore ground-active beetle communities in a degraded pseudo-steppe. J. Insect Conserv. 15, 561–572. doi: 10.1007/s10841-010-9358-3
Boiteau, G., and Colpitts, B. (2001). Electronic tags for the tracking of insects in flight: effect of weight on flight performance of adult Colorado potato beetles. Entomol. Exp. Appl. 100, 187–193. doi: 10.1046/j.1570-7458.2001.00863.x
Boiteau, G., Vincent, C., Meloche, F., and Leskey, T. C. (2010). Harmonic radar: Assessing the impact of tag weight on walking activity of Colorado potato beetle, plum curculio, and western corn rootworm. J. Econ. Entomol. 103, 63–69. doi: 10.1603/EC09113
Buisson, E., Archibald, S., Fidelis, A., and Suding, K. N. (2022). Ancient grasslands guide ambitious goals in grassland restoration. Science 377, 594–598. doi: 10.1126/science.abo4605
Buisson, E., and Dutoit, T. (2004). Colonisation by native species of abandoned farmland adjacent to a remnant patch of Mediterranean steppe. Plant Ecol. 174, 371–384.
Buisson, E., and Dutoit, T. (2006). Creation of the Natural Reserve of la Crau: Implications for the creation and management of protected areas. J. Environ. Manag. 80, 318–326. doi: 10.1016/j.jenvman.2005.09.013
Cote, J., and Clobert, J. (2007). Social information and emigration: lessons from immigrants. Ecol. Lett. 10, 411–417.
Cote, J., Fogarty, S., Weinersmith, K., Brodin, T., and Sih, A. (2010). Personality traits and dispersal tendency in the invasive mosquitofish (Gambusia affinis). Proc. Biol. Sci. 277, 1571–1579. doi: 10.1098/rspb.2009.2128
de Azevedo, C. S., and Young, R. J. (2021). Animal personality and conservation: basics for inspiring new research. Animals 11:1019. doi: 10.3390/ani11041019
Devaux, J. P., Archiloque, A., BorelL, L., Bourrelly, M., and Louis-Palluel. (1983). Notice de la carte phyto-écologique de la Crau (Bouches du Rhône). Biol. Écologie Méditerranéenne 10, 5–54.
Fadda, S., Orgeas, J., Ponel, P., Buisson, E., and Dutoit, T. (2008). Conservation of grassland patches failed to enhance colonization of ground-active beetles on formerly cultivated plots. Environ. Conserv. 35, 109–116.
Fadda, S., Orgeas, J., Ponel, P., Buisson, E., Torre, F., and Dutoit, T. (2007). Past cultivation is a factor driving organization of dry grassland ground-active beetle communities. Environ. Conserv. 34, 132–139.
Gamer, M., Lemon, J., Fellows, I., and Singh, P. (2019). irr: Various Coefficients of Interrater Reliability and Agreement. R package version 0.84.1.
Henry, M., Béguin, M., Requier, F., Rollin, O., Odoux, J. F., Aupinel, P., et al. (2012). A common pesticide decreases foraging success and survival in honey bees. Science 336, 348–350.
Hernández, M. I. M. (2002). The night and day of dung beetles (Coleoptera, Scarabaeidae) in the Serra do Japi, Brazil: elytra colour related to daily activity. Rev. Bras. Entomol. 46, 597–600.
Hilderbrand, R. H., Watts, A. C., Randle, A. M., Hilderbrand, R. H., Watts, A. C., and Randle, A. M. (2005). The myths of restoration ecology. Ecol. Soc. 10:19.
Holyoak, M., Casagrandi, R., Nathan, R., Revilla, E., and Spiegel, O. (2008). Trends and missing parts in the study of movement ecology. PNAS 105, 19060–19065. doi: 10.1073/pnas.0800483105
Janowski-Bell, M. E., and Horner, N. V. (1999). Movement of the male brown tarantula, Aphonopelma hentzi (Araneae, Theraphosidae), using radio telemetry. J. Arachnol. 27, 503–512.
Jeannel, R. (1942). Faune de France Coléoptères Carabiques. Fédération franc̨aise des sociétés de sciences naturelles, Paris: Lechevalier et fils, 573–1173.
Jepsen, N., Schreck, C., Clements, S., and Thorstad, E. B. (2005). “A brief discussion on the 2% tag/bodymass rule of thumb. Aquatic telemetry: advances and applications,” in Proceedings of the Fifth Conference on Fish Telemetry held in Europe, (Ustica).
Kaláb, O., Musiolek, D., Rusnok, P., Hurtik, P., Tomis, M., and Koèárek, P. (2021). Estimating the effect of tracking tag weight on insect movement using video analysis: a case study with a flightless orthopteran. PLoS One 16:e0255117. doi: 10.1371/journal.pone.0255117
Kassambara, A. (2022). Rstatix: Pipe-Friendly Framework for Basic Statistical Tests. R package version 0.7.1.
Katzner, T. E., and Arlettaz, R. (2020). Evaluating contributions of recent tracking-based animal movement ecology to conservation management. Front. Ecol. Evol. 7:519. doi: 10.3389/FEVO.2019.00519/BIBTEX
Kissling, D. W., Pattemore, D. E., and Hagen, M. (2014). Challenges and prospects in the telemetry of insects. Biol. Rev. 89, 511–530. doi: 10.1111/brv.12065
Koo, T. K., and Li, M. Y. (2016). Cracking the code: providing insight into the fundamentals of research and evidence-based practice a guideline of selecting and reporting intraclass correlation coefficients for reliability research. J. Chiopr Med. 15, 155–163.
Lamb, J., and Chapman, J. E. (1943). Effect of surface stones on erosion, evaporation, soil temperature, and soil moisture1. Agron. J. 35, 567–578.
Le Gouar, P. J., Dubois, G. F., Vignon, V., Brustel, H., and Vernon, P. (2015). Demographic parameters of sexes in an elusive insect: implications for monitoring methods. Popul. Ecol. 57, 227–236.
Mallard, F., Le Bourlot, V., and Tully, T. (2013). An automated image analysis system to measure and count organisms in laboratory microcosms. PloS One 8:e64387. doi: 10.1371/journal.pone.0064387
Massol, F., Gravel, D., Mouquet, N., Cadotte, M. W., Fukami, T., and Leibold, M. A. (2011). Linking community and ecosystem dynamics through spatial ecology. Ecol. Lett. 14, 313–323.
Milanesio, D., Saccani, M., Maggiora, R., Laurino, D., and Porporato, M. (2016). Design of an harmonic radar for the tracking of the Asian yellow-legged hornet. Ecol. Evol. 6, 2170–2178. doi: 10.1002/ece3.2011
Moreau, M., Arrufat, P., Latil, G., and Jeanson, R. (2011). Use of radio-tagging to map spatial organization and social interactions in insects. J. Exp. Biol. 214, 17–21. doi: 10.1242/jeb.050526
Moskowitz, D., and May, M. (2017). Adult tiger spiketail (Cordulegaster erronea Hagen) habitat use and home range observed via radio-telemetry with conservation recommendations. J. Insect Conserv. 21, 885–895.
Negro, M. A., Casale, A. C., Migliore, L. U. C. A., Palestrini, C. L., and Rolando, A. N. (2008). Habitat use and movement patterns Carabus olympiae. Eur. J. Entomol. 5759, 105–112.
Nobel, P. S., Miller, P. M., and Graham, E. A. (1992). Influence of rocks on soil temperature, soil water potential, and rooting patterns for desert succulents. Oecologia 92, 90–96. doi: 10.1007/BF00317267
O’Neal, M. E., Landis, D. A., Rothwell, E., Kempel, L., and Reinhard, D. (2004). Tracking insects with harmonic radar: A case study. Am. Entomol. 50, 212–218. doi: 10.1093/ae/50.4.212
Peterson, R. A. (2021). Finding optimal normalizing transformations via bestnormalize. R J. 13, 310–329. doi: 10.32614/RJ-2021-041
Poli, C., Hightower, J., and Fletcher, R. J. (2020). Validating network connectivity with observed movement in experimental landscapes undergoing habitat destruction. J. Appl. Ecol. 57, 1426–1437.
R Core Team (2022). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Ramos-Fernández, G., Mateos, J. L., Miramontes, O., Cocho, G., Larralde, H., and Ayala-Orozco, B. (2004). Lévy walk patterns in the foraging movements of spider monkeys (Ateles geoffroyi). Behav. Ecol. Sociobiol. 55, 223–230.
Reynolds, D. R., and Riley, J. R. (2002). Remote-sensing, telemetric and computer-based technologies for investigating insect movement: a survey of existing and potential techniques. Comput. Electron. Agric. 35, 271–307.
Riecken, U., and Raths, U. (1996). Use of radio telemetry for studying dispersal and habitat use of Carabus coriaceus L. Ann. Zool. Fennici 33, 109–116.
Rink, M., and Sinsch, U. (2007). Radio-telemetric monitoring of dispersing stag beetles: implications for conservation. J. Zool. 272, 235–243.
Römermann, C., Dutoit, T., Poschlod, P., and Buisson, E. (2005). Influence of former cultivation on the unique Mediterranean steppe of France and consequences for conservation management. Biol. Conserv. 121, 21–33.
Rùžièková, J., and Elek, Z. (2021). Recording fine-scale movement of ground beetles by two methods: potentials and methodological pitfalls. Ecol. Evol. 11, 8562–8572. doi: 10.1002/ece3.7670
Růžičková, J., and Veselý, M. (2016). Using radio telemetry to track ground beetles: movement of Carabus ullrichii. Biologia 71, 924–930.
Shrout, P. E., and Fleiss, J. L. (1979). Intraclass correlations: uses in assessing rater reliability. Psychol. Bull. 86, 420–428.
Sih, A., Bell, A., and Johnson, J. C. (2004). Behavioral syndromes: An ecological and evolutionary overview. Trends Ecol. Evol. 19, 372–378. doi: 10.1016/j.tree.2004.04.009
Silcox, D. E., Doskocil, J. P., Sorenson, C. E., and Brandenburg, R. L. (2011). Radio frequency identification tagging: a novel approach to monitoring surface and subterranean insects. Am. Entomol. 57, 86–93.
Soldati, F. (2006). Les asida latreille 1802 de la faune de France (col. tenebrionidae) rutilans, ed. Les Presses Littéraires. Saint-Estéve: Les Presses Littéraires.
Talarico, F., Giglio, A., Pizzolotto, R., and Brandmayr, P. (2016). A synthesis of feeding habits and reproduction rhythm in Italian seed-feeding ground beetles (Coleoptera: Carabidae). Eur. J. Entomol. 113, 325–336.
Testud, G., Vergnes, A., Cordier, P., Labarraque, D., and Miaud, C. (2019). Automatic detection of small PIT-tagged animals using wildlife crossings. Anim. Biotelemetry 7:21. doi: 10.1186/s40317-019-0183-5
Thirgood, S., Mosser, A., Tham, S., Hopcraft, G., Mwangomo, E., Mlengeya, T., et al. (2004). Can parks protect migratory ungulates? the case of the Serengeti wildebeest. Anim. Conserv. 7, 113–120.
Vinatier, F., Chailleux, A., Duyck, P.-F., Salmon, F., Lescourret, F., and Tixier, P. (2010). Radiotelemetry unravels movements of a walking insect species in heterogeneous environments. Anim. Behav. 80, 221–229. doi: 10.1016/j.anbehav.2010.04.022
Appendix 1
Trajectories in the field for individuals that moved at least 1 m from their release point. Release points have been overlapped. Each individual is represented by a given colour. The white square shows their release point. Dots represent the position of the individuals during the 48 h and open circles the position after 1 week (when known). Dotted circles represent distances of 1 m and 6 m from the release point.
Keywords: movement ecology, conservation, grassland, insects, radio-tracking
Citation: Blight O, Geslin B, Mottet L and Albert CH (2023) Potential of RFID telemetry for monitoring ground-dwelling beetle movements: A Mediterranean dry grassland study. Front. Ecol. Evol. 11:1040931. doi: 10.3389/fevo.2023.1040931
Received: 09 September 2022; Accepted: 15 March 2023;
Published: 17 April 2023.
Edited by:
Péter Török, University of Debrecen, HungaryReviewed by:
Jana Růžičková, ELKH-ELTE-MTM Integrative Ecology Research Group, HungaryBéla Tóthmérész, University of Debrecen, Hungary
Copyright © 2023 Blight, Geslin, Mottet and Albert. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Olivier Blight, olivier.blight@imbe.fr
†These authors have contributed equally to this work
 Olivier Blight
Olivier Blight Benoît Geslin
Benoît Geslin Lola Mottet
Lola Mottet Cécile H. Albert†
Cécile H. Albert†