Consumption and Preferences for Wild and Domestic Meat in Indigenous Communities in the Brazilian Atlantic Forest
- 1Department of Ecology and Systematics, Universidade Federal da Paraíba, João Pessoa, Brazil
- 2Rede de Pesquisa para Estudos Sobre Diversidade, Conservação e Uso da Fauna na Amazônia (REDEFAUNA), Manaus, Brazil
- 3Programa de Pós-graduação em Etnobiologia e Conservação da Natureza, Universidade Federal Rural de Pernambuco, Recife, Brazil
- 4Centre for International Forestry Research (CIFOR), Bogor, Indonesia
- 5Laboratory of Ethnobiology, Universidade Estadual da Paraíba, Campina Grande, Brazil
Wild animals have traditionally been the main sources of protein available, if not the only, to numerous indigenous populations worldwide. However, greater access to markets, reduced availability or access to wildlife, and policies in support of agricultural development, have shifted food habits toward domestic and industrial sources of protein. In this study, we evaluated consumption patterns and preferences/avoidances for wild animals (wildmeat, crustaceans, and fish) in comparison to domestic sources of protein among the Potiguara living on the Brazilian coast. Using data from 843 semi-structured interviews applied to students from 28 indigenous villages, we found that domestic meats were more consumed and preferred as compared to wild animals (aquatic and game animals), despite the high abundance of fish and crustacean resources in the surveyed area. Consumption and preference for game were higher among male students while avoidance was higher among female students. The avoidance of domestic meats and fish was low for both genders. The occupation of the fathers affected students’ food habits, in those nature-related occupations (farmer, fisherman/woman, sugarcane worker) conditioned greater consumption of wildmeat and fish, while non-nature related occupations lead to greater consumption of protein from domestic sources. The consumption of protein from all sources increased with the distance between villages and a protected area. Our results indicate that the younger generation of Potiguaras does not regularly consume wildmeat and fish and their preference for domestic sources of protein is shaped by the socio-environmental context, access to different types of meat, and taste preferences.
Introduction
The use of wild animals for food is a millennial practice across diverse cultures (Alves et al., 2018; Anderson, 2020; Braga-Pereira et al., 2021a) and continues to contribute to diet diversification around the world (Hanazaki et al., 2009; Powell et al., 2015; Alves and van Vliet, 2018; Donders and Barriocanal, 2020; Oliveira et al., 2022). Wild animals harvested to meet nutritional needs include numerous taxa, but vertebrates make the bulk of the animals sought after for their meat, fat, bones, and eggs (Bodmer et al., 1997). Between 230 and 833 million people rely on wild meat (the meat from wild mammals, reptiles, amphibians, and birds) as a source of protein (Nielsen et al., 2018). Insects, mollusks, crustacea, and fish are also sought after as a source of protein, particularly in coastal or riverine ecosystems (Vallega, 2013). In Brazil, for example, shellfish catching is a common extractive activity concentrated close to estuaries (Nishida et al., 2006, 2008) and land crabs (Brachyura crabs) represent one of the major groups in terms of economic relevance in mangrove ecosystems (Alves and Nishida, 2002, 2003; Alves et al., 2005; Nishida et al., 2006; Nordi et al., 2009; Capistrano and Lopes, 2012; Nascimento et al., 2012, 2016, 2017). The traditional knowledge of indigenous people across the globe has played a key role in identifying living organisms that are endowed with nutritional and medicinal values important for humans. These traditional human societies have accumulated a vast store of knowledge about animals through the centuries, and this zoological knowledge is an important part of our human cultural heritage (Kendie et al., 2018; Braga-Pereira et al., 2021b).
Nowadays, while indigenous communities continue to represent this legacy through the continuation of practices that involve the use of wild animals for food, several factors linked to globalization, such as access to markets, migration, urbanization, and changes in lifestyles, have induced the rapid replacement of wild animals (van Vliet et al., 2014; Donders and Barriocanal, 2020; Leyva-Trinidad et al., 2021) to the advantage of domestic and industrial sources of protein, often incentivized by the market and governmental programs (Sarti et al., 2015; Bellinger and Andrade, 2016). For example, in the Bolivian Amazon, the Tsimane’s diet is characterized by an increase in market foods (in particular pasta, refined sugar, salt, and oil) and a decreased consumption of game (Kraft et al., 2018). Chicken and eggs are now the most frequently consumed source of protein among Ticuna, Cocama, and Yagua children in Puerto Nariño, Colombia, far beyond fish and game (van Vliet et al., 2015a). Recent studies in Brazil suggest that particularly in the North-Eastern region contemporary Indigenous diets are lacking in quality, and are characterized by a high prevalence of purchased foods (Welch et al., 2021).
While most of the recent literature looking at nutritional transitions in South America has focused on the Amazon region (Coimbra and Santos, 1991; Santos and Coimbra, 2003; Tavares et al., 2003; Lourenço et al., 2008; Welch et al., 2009; Nardoto et al., 2011; van Vliet et al., 2015a; Donders and Barriocanal, 2020) and the Andes (Walrod et al., 2018; Chee et al., 2019), less is known about the changes observed in nutritional patterns in the Atlantic forest biome. This forest represents the second-largest rainforest of the American continent. Being home to 30% of the Brazilian indigenous lands (ISA, 2021), but also coincides with the most urbanized areas of Brazil (Tabarelli et al., 2005). Even though it has been largely destroyed, the Atlantic forest biome is still home to more than 8,000 endemic species of vascular plants, amphibians, reptiles, birds, and mammals (Myers et al., 2000) and includes several key formations such as mangroves, restingas (coastal forest and scrub on sandy soils), high-elevation grassland (campo rupestre), and brejos (humid forests resulting from orographic rainfall in otherwise semidesert scrub in the northeast of Brazil) (Câmara, 2003).
In the current study, we investigated the consumption of wild animals and domesticated animals among the Potiguara living in the Atlantic Forest. The Potiguara have traditionally based their livelihoods on the use of marine wild animals (such as shellfish, crabs, and fish) and terrestrial wild vertebrates as main sources of food (Rocha et al., 2008). Based on semi-structured interviews conducted among Potiguara teenagers, we assessed the frequencies of consumption of wild and domesticated animals and preferences or aversion for different animal species. We analyzed the influence of gender, socio-economic background of the household, and access to market and natural resources as predictors for consumption and preference behaviors.
Methods
Study Area and the Potiguara Indigenous
The research was carried out in indigenous schools of the Potiguara Indigenous Land (Potiguara IL) (33,757 ha). The Potiguara IL is in the Atlantic Forest biome and spreads over three municipalities, Baia da Traição, Marcação and Rio Tinto, in the State of Paraíba, Brazil (Figure 1). It was demarcated in 1983, homologated in 1991, and its population is divided among 32 villages. The Potiguara IL partially overlaps with two designated protected areas, the Area of Relevant Ecological Interest Manguezais da Foz do Rio Mamanguape and the Environmental Protection Area Barra do Rio Mamanguape (EPA BRM) (Rodrigues et al., 2008). This region includes the Mamanguape River estuary which harbors ca. 6,000 ha of reasonably preserved mangrove ecosystem, the largest of its kind in the State of Paraíba. The mangrove forest at Mamanguape is typically well preserved although certain sites show signs of anthropogenic disturbance. Main land use types in the Potiguara IL are sugar cane fields (ca. 10,000 ha), crops and pastures (ca. 5,100 ha), fallows and carrasco (ca. 4,800 ha), (a shrubby phytophysiognomy typical of North-eastern Brazil) gardens and small-scale farmings (about 1,300 ha), forest remnants and open arboreal savanna, named “Tabuleiro” (ca. 8,400 ha) (Cardoso and Guimarães, 2012).
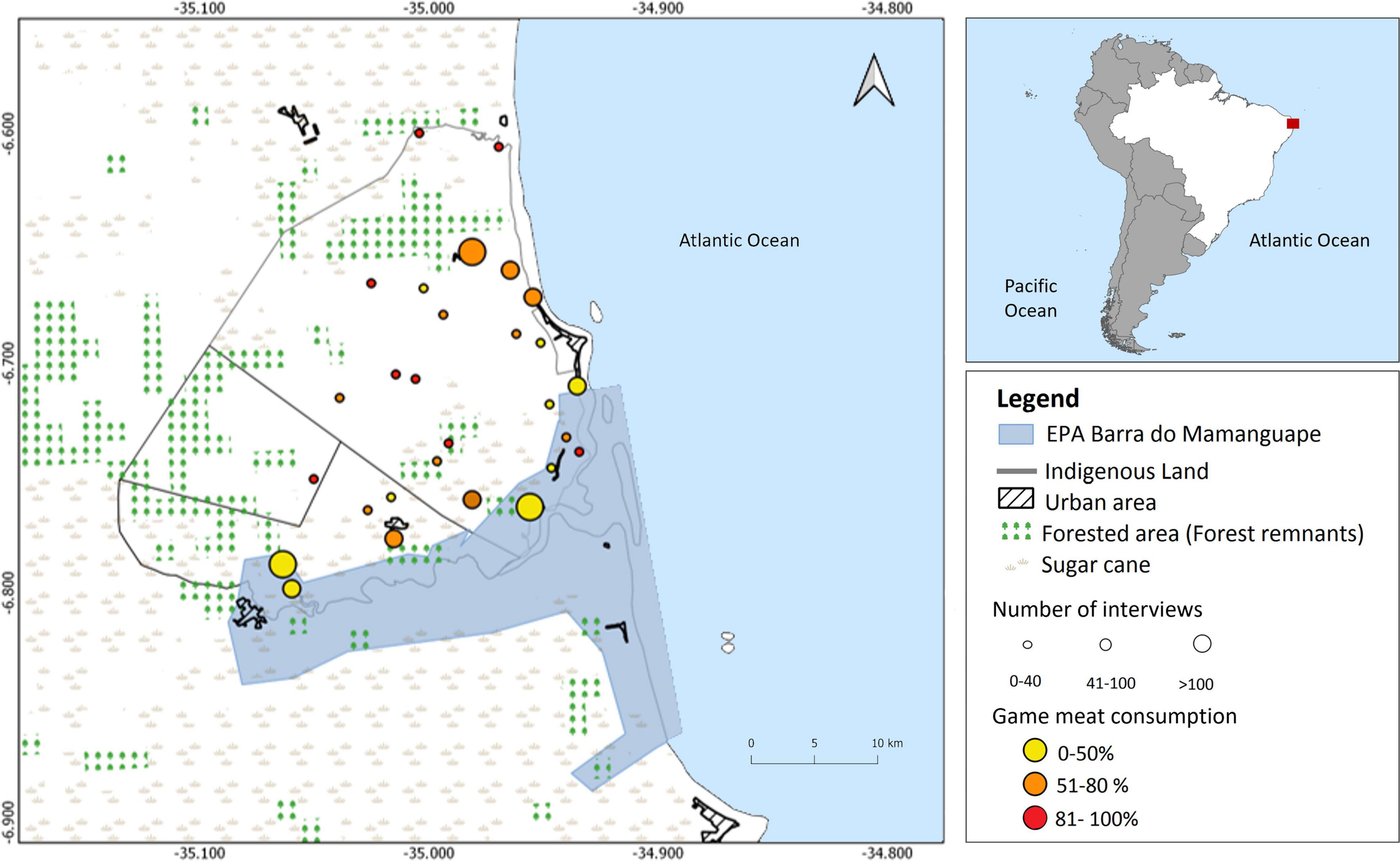
Figure 1. Map of the study area portraying the 28 indigenous villages in the Potiguara territory where the study was carried out. Solid dots indicate the villages of the interviewed students. Dot sizes are proportional to the number of interviewed students from each village. Circle colors denote the number of students that have consumed game at least once in their lifetime in each village, ranging from yellow (smaller number) to red (greater number). Map generated using QGIS; Datum: SIRGAS2000. Source: IBGE, Editedin Adobe Photoshop and Elaborated in March 2022.
The mangrove is an economically profitable environment for the Potiguara. It is where fish, crustaceans, and mollusks are obtained, and a space upon which many of the inhabitants of the area depend for a living (Nishida et al., 2008; Rocha et al., 2008). Fishermen and women have a very keen knowledge of the mangrove and are hence able to locate aquatic animals and develop efficient techniques for capturing them (Nishida et al., 2006; Rocha et al., 2012). Part of the fish is consumed or given to kinspeople and neighbors with whom one is on friendly terms, and part is sold in the village or the local market when the catch is bigger (Araújo, 2019).
During the eighteenth century, deforestation for sugar cane plantations transformed the physical scenery and the social and technical relations among the Potiguaras. The expropriation of lands and the expansion of sugar cane cultivation led to widespread discontent, requiring the Potiguara to seek the demarcation of their lands as early as the 1980s. Intense deforestation did not simply result in the drastic reduction of animal and vegetal populations (both in individual numbers and in variety); the phenomenon also caused significant modifications in hydrography. With the mills, sugarcane stretched into the productive space of the Potiguara, ravaging existing areas of vegetation and restricting agricultural and fishing activities (Araújo, 2015).
As of 1993, the use of wild resources by the Potiguara was further restricted by the creation of the EPA BRM, which was created primarily to protect manatee (Trichechus manatus) and other aquatic species (Federal decree No. 924, 10 September 1993). Currently, fisheries (fishes, crustaceans, and mollusks), aquaculture (shrimp farming), and agriculture (crops and ranching) are regulated inside the EPA BRM (Mourão and Nordi, 2003; Araujo and Nishida, 2007; Rodrigues et al., 2008; Cardoso and Guimarães, 2012; Nascimento et al., 2016). Over time, conflicts have arisen between distinct modalities of management or access to and use of resources, and by the socially unequal display of the environmental damages and risks caused by industrial endeavors during the historical process. It is precisely against the conditions imposed by dominant policies that the Potiguara seek to impose their power, to access and control the resources present in their territory—through the household ecology, where they build domanial spaces and define mobilities (Araújo, 2019).
The villages are scattered throughout the indigenous territory, connected by unpaved roads and a paved road that connects them to the nearest urban centers of the Mamanguape, Baía da Traição, Rio Tinto, and Marcação cities in less than 30 min drive. In these neighboring towns, industrialized foods of animal and vegetable origin are readily available in the supermarkets (Cardoso and Guimarães, 2012). In the villages themselves, there are small grocery stores that also supply industrialized products, meat from domestic animals, and processed meats.
Ethics Statement
This research was authorized by the Brazilian Agency for Indigenous Affairs (FUNAI) (No. 94/AAEP/PRES/2017) and indigenous leaders in compliance with CNS Resolution No. 304 of 2000. The research project was approved by the Research Ethics Committee and the National Research Ethics Commission (CEP/CONEP) number CAAE 7695941790008069, following Resolution No. 466/2012 of the National Health Council (CNS) of the Ministry of Health (no. 2,491,305). Authorizations to conduct the research in schools and free and informed consent forms were obtained from school principals, teachers, legal age students (18 plus), and parents or legal guardians in the case of underage students. The research process was thoroughly explained before each classroom and the interviewees were assured that their identities would not be disclosed. Still, some students refused to participate in the study, as well as 1 of the 10 Potiguara IL schools within the educational levels targeted by the study.
Data Collection
We conducted data collection between February and August 2018 in nine public schools (State and municipally managed, 8 and 1, respectively) of the Potiguara IL, located in the municipalities of Baia da Traição (three schools), Rio Tinto (two schools) and Marcação (four schools). These schools were attended by students from 28 indigenous villages, seven of which are located inside the EPA BRM. Before the study, a pilot was carried out with 83 random students from three schools to verify if students understood the proposed questions and to verify the need for adjustments in the questionnaire. Subsequently, visits were made to all public schools (n = 10) within the Potiguara IL to explain the research goals and to request authorization from the schools’ directors to carry out the research.
Nine schools granted us the authorization requested. Out of the 84 classrooms present in those 9 schools, we used a sub-sample of 64 classrooms (about 7 classrooms per school) trying to cover different school levels. For the elementary school, we covered an average of 1.4 classrooms per scholar level (SD = 0.11) and for high school, we covered an average of 1.14 classrooms per scholar level (SD = 0.13). Authorization from the teacher was also requested before applying the questionnaire to the students.
Questionnaires were applied individually to 843 students out of 1,850 (45.6%) enrolled in Potiguara IL public schools. Interviewed students were 55% (n = 463) female and 45% male (n = 380), aged 10 to 73 years old (94.4% between 10 and 20 years, 4.1% between 21 and 30, and 1.5% between 31 and 73) and attending Middle and high school. Interviewees lived in villages located inside or outside the EPA BRM (321 and 522 students, respectively). To reduce any risks of potential bias in responses, due to the restrictions of hunting within the PA, the objectives of this study were framed around nutrition and food security, not around conservation or environmental education. The teachers in each classroom, who are trusted and respected by the students, were in charge of explaining the research goals to the class and reading out loud a consent form guaranteeing the confidentiality of the student’s identity in the classroom. Moreover, the teacher explained that the children should not feel any shame or reservations to share their nutritional preferences and habits and that no judgment would be made on their choices, which would remain anonymous. In each classroom, a researcher distributed the questionnaires among the students and, after the classroom teacher read the questionnaire aloud, we asked the students to answer the questions. A researcher and a teacher in each classroom clarified possible doubts of the students about the questions. The questionnaire was divided into the following sections:
(1) Social information: The first section of the questionnaire inquired about the socioeconomic background of the respondent’s household, such as the student’s parents’ occupation, age, and gender.
(2) Meat consumption: The students were asked to list the type of animals they consumed, the frequency of consumption, and the last time they had consumed that animal. As a follow-up, a 24-h recall was used to assess recent consumption of animal protein (from domestic or wild origin). The 24-h recall method is a low-cost and effective tool for rapid evaluations, collecting information from several respondents in a minimum amount of time (van Vliet et al., 2014).
(3) Meat taste preference and meat avoidance: Students were asked to select three types of preferred sources of protein-based on a multiple-choice list derived from all available sources of protein in the region. The question asked: “Among the sources of protein listed below, please list those that you like eating the most in terms of taste?” Students were also asked to freely mention animals protein that they would avoid eating if offered.
Data Compilation
The occupation of the students’ fathers and mothers was independently categorized as (1) nature-related (fisher, farmer, or sugarcane plantation worker) and (2) not nature-related (service industry worker, public or private employee, business owner, merchant, unemployed or retired).
Spatial information was downloaded from the Brazilian Institute of Geography and Statistics (IBGE, 2010). The shortest distance from each village to the EPA BRM boundary was manually estimated using the Measure Distance tool in Google Earth. For villages located within the EPA, BRM distance was set to zero.
Meat protein classification: Wild animals were categorized as aquatic animals (mollusks, crustaceans, and fish) and game (terrestrial mammals, birds, reptiles, and amphibians). Species identification was determined through visits to local communities and by consulting recent ethnotaxonomic and ethnozoological studies conducted in the study region (Mourão and Nordi, 2003; Araujo et al., 2006; Feijó and Langguth, 2013; Nascimento et al., 2016). For non-wild meat, we considered the domestic source of protein (eggs, the meat of cow and pig, chicken, etc.) and processed meat (canned meat/fish, etc.).
Data Analysis
Consumption and Taste Preference for Domestic or Wild Animals
We performed a linear model to investigate the relationship between species consumed in the last 24 h and preferred species.
Effect of Social Variables on Consumption and Preference
We used generalized linear mixed models (GLMMs) to verify if social factors influenced on the:
(1) Consumption of domestic, fish, and game meat in the day before the interview (24-h recall);
(2) preference for these different protein sources;
(3) consumption of game in previous weeks.
Predictor variables included: (i) distance from the students’ villages in relation to the environmental protection area; (ii) students’ sex; (iii) students’ age; (iv) mothers’ occupation, and (v) fathers’ occupation. We considered the student and the student village predictor variables of random effect, while other predictor variables were considered as fixed effects. We tested and found no collinearity (p > 0.06) between all predictor variables.
We used residual checks to verify whether our models were, in principle, suitable or otherwise. We used the Akaike information criterion to select models of interest if ΔAIC values > 6 (ΔAIC obtain from the difference between a null and complete model AIC values) (Harrison et al., 2018). To evaluate the performance of each model we used the receiver operating characteristic (ROC) curve and its area under the curve (ROC AUC). The ROC AUC scores can vary from 0.5 (predictive ability expected by chance) to 1 (perfect predictive ability), with values higher than 0.7 indicating good predictive capabilities of the models (Swets, 1988; Stringham et al., 2021). All inferential analyses were performed in R ver. 3.5.3 (R Core Team, 2019) using packages MuMin, lme4 (Oksanen et al., 2013), and pROC (Robin et al., 2021).
Factors Related to Rejection of Domestic and Wild Species
We performed Principal Component Analysis (PCA) to find out how the causes used by students to explain rejection (feelings) were related to different species and students’ gender. We interpreted the results when the first two components of the PCA (PC1 and PC2) represented at least 70% of the variation in the data.
The data matrix was transformed using the Hellinger method so that the PCA could properly represent our data. We used an Anosim permutation test a posteriori to verify the existence of groupings of cited species and the sex of the interviewee.
Results
The interviewed Potiguara students (n = 843) reported consuming a variety of vertebrate and invertebrate species, both domestic and wild. Among vertebrates, the most representative group were mammals (n = 14 species) and fish (n = 13 species). Among invertebrates, there were five species of crustaceans and one of mollusk (Supplementary Material). A total of 51% of the students consumed game meat from 22 taxa at least once in their lifetime. Of those, 46% had consumed game meat during the year of the interview (the year 2018) and 54% in past years. The game species consumed by most students were armadillos (Dasypodidae) (n = 237; 28%), capybara (Hydrochoerus hydrochaeris) (n = 166; 20%) and iguana (Iguana iguana) (n = 128; 15%).
The 24 h-recall survey yielded 40 species of animals consumed belonging to 32 genera distributed among aquatic animals (n = 24), game (n = 9), and domestic (n = 5) species. Despite the lower number of domestic species consumed, 70% of the 1,945 reported events of animal consumption were domestic meat sources (n = 1,359). From this, beef (n = 377; 28%) and chicken (n = 263; 19%) were the most consumed sources of domestic meat. Wild animals were only consumed by 40% of the respondents in the 24 h recall (n = 586). Of those, most had consumed aquatic animals (95%), and 5% had consumed game. Armadillos (Dasypodidae) was the game taxon consumed by most students (n = 11), followed by capybara (n = 5) (Figure 2).
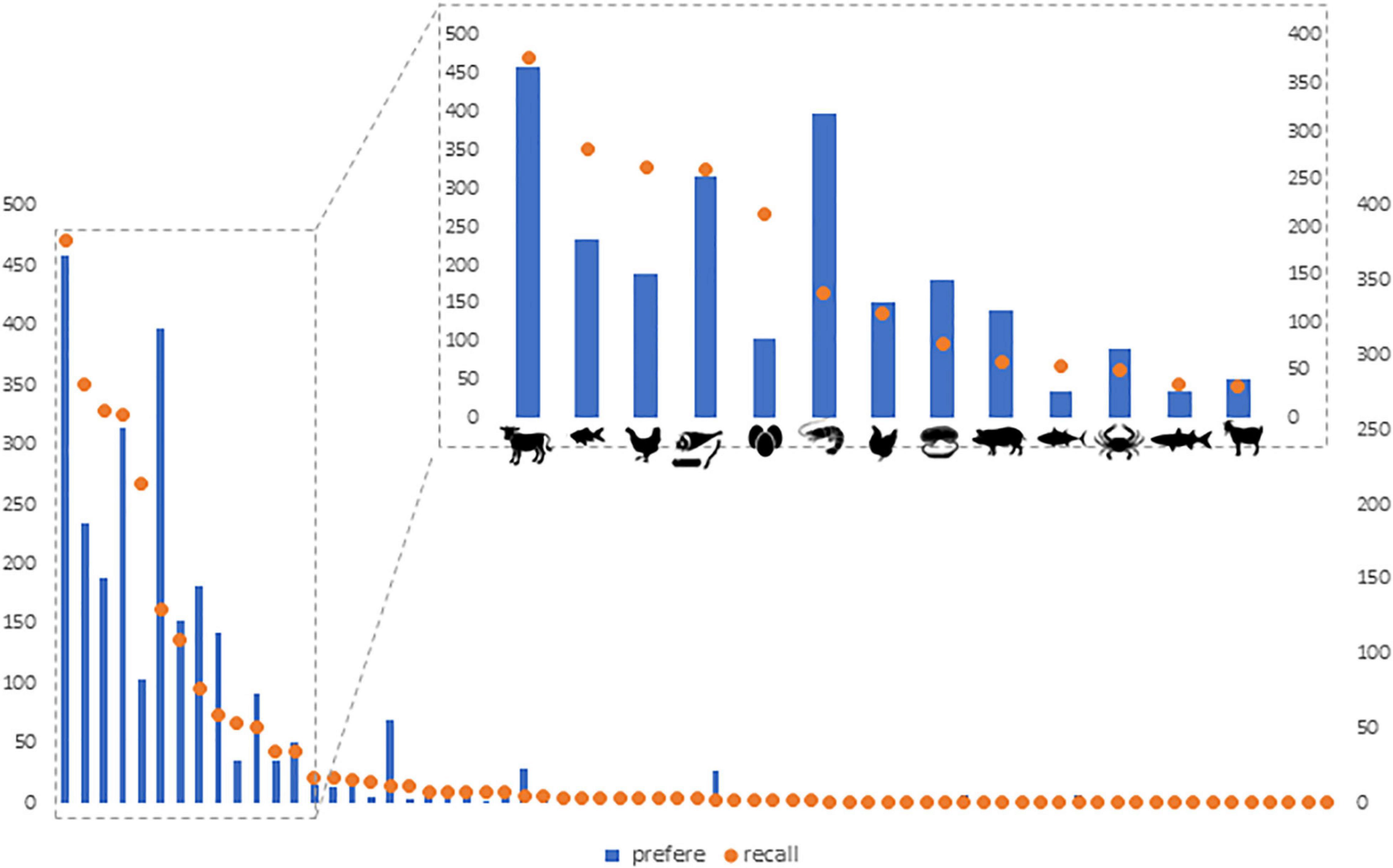
Figure 2. Potiguara students’ consumption (24-h recall) (orange circle) and preference (blue bars) for sources of animal protein. The left Y-axis is the number of consumption events reported per species and the right Y-axis is the number of times the species was mentioned as preferred. Zoomed area shows the 13 most consumed types of meat the day before the interview.
Preference
Potiguara students cited a total of 45 taxa as preferred meat divided among aquatic wildlife (24 taxa and 12 identified species), game animals (15 taxa and 9 identified species), and six domestic species1. However, from a total of 2,559 citations of preferred meat, the percentage of domestic sources (1,459; 57%) was significantly higher than that of wild sources (1,110; 43%) (p = 6.932e-16). The preferred items among domestic sources were beef (n = 458 citations, 31%) and processed meats (n = 315 citations, 28%).
Considering only wild animals, preference for aquatic animals was significantly higher than that for game (p < 2.2e-16) (Table 1). The most cited items were crustaceans (n = 521 citations, 46%), fish (n = 234, 22%) and mollusks (16%). Across all taxa, preference and consumption on the previous day were positively correlated (p < 2.2e-16) (Figure 2).
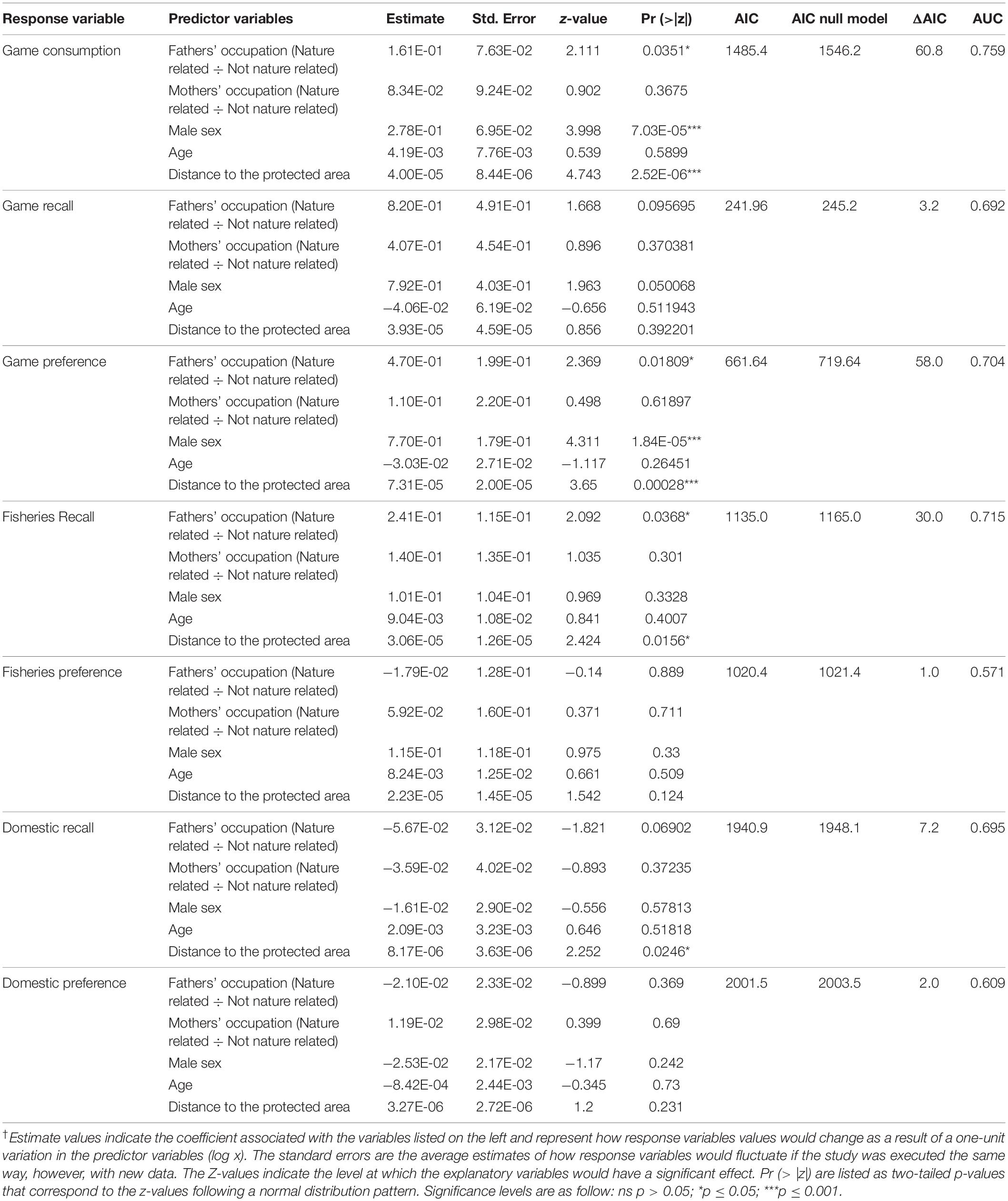
Table 1. Estimates of generalized linear mixed models selected based on the Akaike Information Criterion (AIC) and the area under the curve (AUC) of the receiver operating characteristic (ROC).†
Social Factors Affecting Consumption and Preference for Different Sources of Meat
A total of 321 students in the study’s cohort lived in villages within the protected area (EPA BRM) and 116 (36%) of them had consumed game at least once. The proportion of students from outside the protected area (n = 522) who had consumed game meat was almost double (60%, n = 314) that of students living inside the protected area (Figure 1). Our analyses show that consumption of game in previous weeks (Figure 3A) and preference (Figure 3C) increased with the distance from the protected area. The consumption of fish and domestic meat (but not the preference) also increased with the distance from the protected area (Figures 3D,F). We found that male students and students whose fathers are involved in nature-related work eat game more often (24 h survey and overall) and show a higher preference for it (Figures 3A–C). Fish consumption was higher for students whose fathers are involved in nature-related work (Figure 3D), while a non-significant trend indicates that students whose parents do not develop nature-related work consume more domestic meat (Figure 3F). We did not find any influence of the variables analyzed on the preference for aquatic animals and domestic resources (Figures 3E,G).
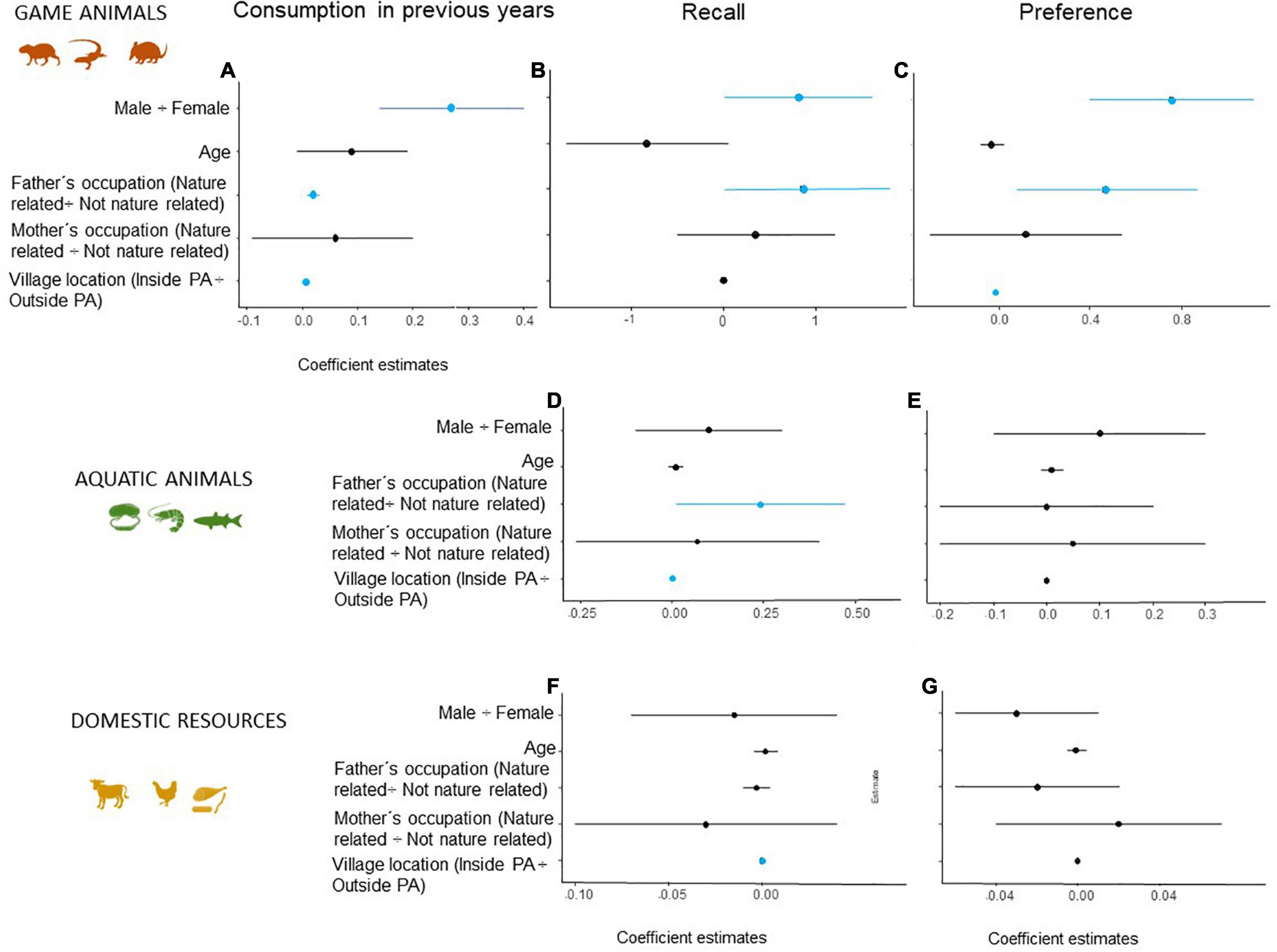
Figure 3. Generalized linear mixed models (GLMMs) to evaluate consumption in previous weeks, consumption the day before the interview (24-h recall), and preference for game resources (A–C, respectively), aquatic wildlife (D,E), and domestic sources of meat (F,G). Coefficient estimates (±95% confidence interval) show how social factors modulate (direction and magnitude) consumption of game. Black and blue dots represent, respectively, the absence of significant effect and significant positive effect in the response variable.
Avoidances
Most of the students expressed rejection to some type of meat (n = 677; 80.3%). Among those students, 77% cited game animals (n = 524 students), 20% domestic animals (n = 133) and 3% aquatic wildlife (n = 20). A total of 42 taxa were mentioned, being 34 wild and 8 domestics. Taxa rejected by more students were snakes (n = 114) and armadillos (n = 61). Disgust (14%) and pity (13%) were the most common causes for rejecting different types of meat.
The PCA analysis described a total of 77% of the data variance and shows how different causes for rejection (feelings) are related to the most rejected animal species and the gender of the students (Figure 4). Rejection toward the meat of some species was shown to be related to people’s feelings and their perception of its visual appearance. For male and female students, the feeling of pity was associated with the sloth (Bradypus variegatus) and the Brazilian rabbit (Sylvilagus brasiliensis), while certain qualities of the meat, like smell, taste, and appearance, motivated the rejection of caiman (Caiman latirostris). The rejection of pork and snake meat by students of both genders was strongly related to them being considered harmful if eaten.
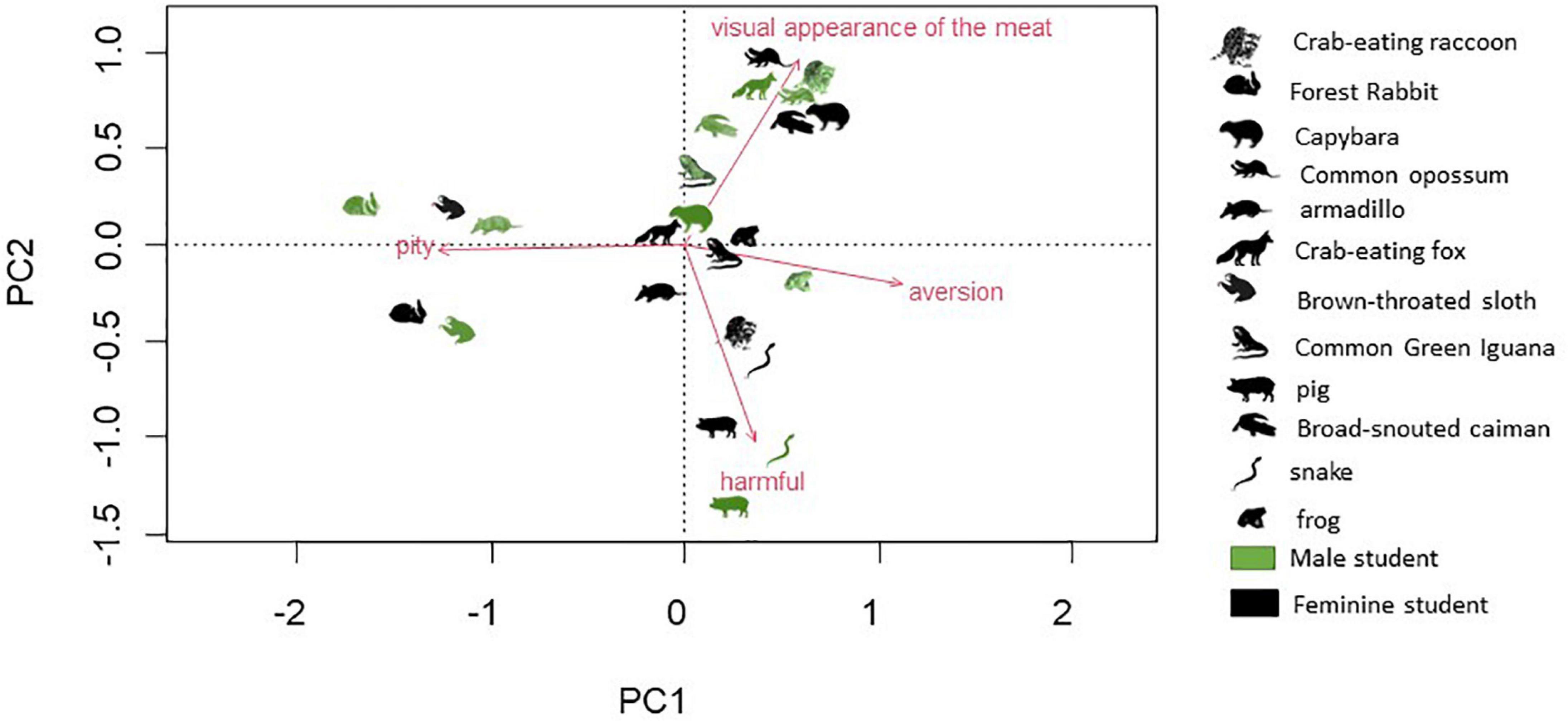
Figure 4. Description of reject species in terms of motivations for rejection by female and male students obtained from a Principal Component Analysis (PCA).
Discussion
In this paper, we assessed the dietary patterns among the Potiguara, particularly the consumption of animal-based sources of protein. Our study confirms trends observed in other parts of South America where industrial meats bypass the consumption of wild-sourced proteins among the younger generations of indigenous peoples (Murrieta and Dufour, 2004; Nardoto et al., 2011; van Vliet et al., 2015b). This transition is particularly common as urbanization progresses, domestic and industrialized sources of animal protein become readily available, and wildlife decreases as a result of habitat fragmentation and water pollution (Nardoto et al., 2011; Prist et al., 2012; van Vliet et al., 2014, van Vliet et al., 2015b,c; Vanegas et al., 2016). In this context, our study shows that young Potiguara does not consume game animals frequently and that they prefer to eat beef or industrial meats.
The higher consumption of domestic sources of animal protein outside the protected area might be associated with the dense road network and easier access to food markets from neighboring urban centers. Connection to markets via roads has been pointed out in several places as an important determinant of household food consumption (Kramer et al., 2009). This is because the roads allow access to industrialized products, thus providing a diet shift from traditional to market-based foods. Conversely, the low frequency of game consumption found can probably be explained by low wildlife abundance. In our study site, the landscape is composed of small forest remnants immersed in a matrix of agricultural fields, sugarcane plantations, and shrimp farming ponds. Most villages are connected and nearby urban centers through a network of highways and dirt roads (Cardoso and Guimarães, 2012).
Wildlife abundance in the Potiguara IL has dramatically decreased since the agricultural and real estate expansion in the 1980s, a general pattern across the Atlantic Forest of Northeastern Brazil, which has led to the highest defaunation rates of terrestrial vertebrates in the country (Bogoni et al., 2018). These changes have a marked effect on the consumption of game animals in the study area.
In contrast, aquatic wildlife represents a considerable portion of the Potiguara diet, the majority of the Potiguara population depends on aquatic wildlife harvested at the Mamanguape river estuary and mangrove, especially fish and crustaceans (Mourão and Nordi, 2003; Araujo et al., 2006; Araujo and Nishida, 2007). For instance, the third most consumed animal recorded by the 24-h recall method was crab Ucides cordatus which for decades has been a key source of income in the region. Other species widely available in the region are commonly consumed by the young Potiguara, such as clams, “marisco” Anomalocardia brasiliana, and Mugil curema (e.g., mullet/tainha). Rocha et al. (2008) recorded 68 species of fish, crustaceans, and mollusks used for food or income in the estuary of the Mamanguape River, many of which were also recorded in this study (e.g., mullet/tainha Mugil curema, snook/camurim Centropomus sp.). Aquatic wildlife has a prominent status as part of the Potiguara identity and is the main staple in Potiguara’s traditional feasts, such as during the “shrimp festival” when fish and crustaceans are shared among villages (Cardoso and Guimarães, 2012).
The high consumption of aquatic wildlife recorded in this study could be related to the numerous rivers and abundant aquatic resources (Rocha et al., 2008; Cardoso and Guimarães, 2012) which provide enough food for Potiguara households. As observed in Amazonia, greater fish availability induces lower reliance on hunting (Sarti et al., 2015; Endo et al., 2016) and this is yet another reason for the low game consumption. The healthy estuary is in stark contrast with the highly fragmented forest remnants of the region (Alves and Nishida, 2002). Villages situated in the coastal areas have access to marine environments with a variety of plant and animal species that are not available to inland communities (Turner, 1995; Mitchell and Donald, 2001; Mulrennan and Bussières, 2018), therefore, they are expected to be incorporated into the local diet. As remarked by Alves and Rosa (2006), the use of more accessible resources possibly is related to historic aspects, for example, the knowledge focusing on species familiar to the locals, reflecting the transmission of knowledge through generations.
We found that both consumptions of aquatic animals and game increased with the distance from the protected area. This might be because, inside the protected area, the use and management of aquatic resources are allowed, but hunting is forbidden (Brasil, 2000). On the other hand, indigenous peoples have rights to their land and their natural resources, game species included, warranted by the Brazilian constitution (Brasil, 1988). Since the Potiguara IL and the EPA BRM (the protected area) partially overlap, the Potiguara must constantly navigate a legal limbo of conflicting rules. Hunting is portrayed as a negative or criminal activity by the environmental agencies in charge of the EPA BRM and this contributes to further marginalizing this activity. Fears of being penalized by law enforcement jeopardize the role that hunting used to play in the protein complementation of the Potiguara living inside the protected area. Although we found a low consumption of game meat as compared to aquatic resources and domestic sources of animal protein, this consumption still occurs and deserves attention, both from the point of view of food security discussed previously, and in relation to the target species conservation. Regarding this, we emphasize that in the studied areas where hunting is allowed there is no management and monitoring program of game fauna. These programs could reduce potential impacts of hunting on game, in addition to promoting a better understanding of the current consumption and importance of game animals for the Potiguara population. Regarding on that, successful initiatives have integrated scientific and local knowledge into local hunting rules and sustainable harvest of target species, while promoting biodiversity conservation and empowering local people (Campos-Silva et al., 2018).
In our study, the consumption of wild animals (game and fisheries) was higher among children whose fathers have nature-related occupations. This pattern might result from their higher exposure to wild resources during worktime, especially among those fathers whose income comes from exploiting wild resources (e.g., fishers). Opportunistic hunting upon chance encounters during agricultural or other activities has also been reported in Madagascar (Gardner and Davies, 2014). In the studied region women play an important role in supporting families by harvesting wild species for food, such as “camurim”/snook Centropomus sp., and for traditional medicine use, such as seahorse Hippocampus reidi and tarpon Megalops atlanticus (Rocha et al., 2012). Nevertheless, a statistically significant relationship was not found between fisher mothers and students’ consumption of fishing resources, maybe due to the low proportion of fisher mothers among the study’s cohort (ca. 4%).
The lack of effect of age on the consumption of game meat by the Potiguara might be explained by the small age range of the cohort of students in the study (94.4% aged 10–20 years). Possibly, younger people consider consuming game meat an outdated tradition, especially in more urbanized areas. Studies covering a wider age range have shown that game meat consumption is lower among younger people, who often regard it as not socially acceptable (Luiselli et al., 2019). We found a greater preference for game meat among male students, who also consumed it more often when compared to female students. In a study developed in Cameroon based on 1 year of weekly recall, data men reported eating game meat more often than women (149 women, 117 men, aged 16–75 years). However, this pattern was not stable across all age groups. Consumption of game meat was more frequent among younger men than among younger women (<35 years of age), whereas older women ate game meat more often than older men (aged 35–54 years) (Duda et al., 2018).
Nearly one-fourth of the students (n = 205) deemed wild meat of certain species as repulsive, such as snake, frog, and the lizard S. merianae. The main reasons for students to reject the meat of certain animals were related to feelings of pity and disgust, considering its consumption harmful, and to specific qualities of the meat, like smell, taste, and appearance. A similar pattern has been recorded in traditional communities elsewhere and is often related to food taboos (Barboza et al., 2016), animals’ morphology or smell (Figueiredo and Barros, 2016), and the feeding habits of scavenger species (Souza and Alves, 2014). Prevalent rejection of sloths might result from peoples’ affection since they are regularly observed in green spaces near the communities or urban areas, and the species is often subject to awareness campaigns developed by conservation projects in the area. We postulate that the greater preference for domestic meat and aquatic resources among Potiguara students is mainly due to the greater students’ familiarity with those sources of protein.
The nutritional transition evidenced in our study area among the young Potiguara leads to concerns already raised in other studies that have recorded the rapid changes taking place in the diets of Indigenous peoples (Kuhnlein, 2009; Welch et al., 2009; van Vliet et al., 2015a). These transitions are sensitive indicators of the ecological and cultural costs that the changes brought about by modernity imply for indigenous people (Dounias and Froment, 2011; van Vliet et al., 2015a). The replacement of game meat or fish for industrialized meats and the change to a more sedentary lifestyle leads to increasing rates of overweight, obesity, and associated chronic diseases such as cancer, heart disease, and diabetes (Popkin, 1994; Popkin and Gordon-Larsen, 2004; Parrotta et al., 2015). The transformations of landscapes, economies, and lifestyles of these populations represent threats to their sovereignty and food security, which go beyond that since the right to healthy food is interconnected with ethnic identity, traditional knowledge systems, sustainability, and wellbeing (van Vliet et al., 2018, 2022; Jacob et al., 2021; Welch et al., 2021). In this sense, nutritional transitions and their implications for indigenous peoples must be seriously taken into consideration in public policies, especially those focused on biodiversity conservation, food, nutrition, and health.
Despite its growing dietary importance, information on animal protein consumption by indigenous groups from Brazil is scarce, emphasizing the need for further research on the topic. To our knowledge, this is the first study to address the role that wild animals and domestic and processed meat/fish play in the food security of coastal indigenous populations from Brazil.
Although we focused our interviews mainly on young people, we believe that our results can extrapolate this scope, since students’ eating habits are a reflect of the daily eating behaviors of the homes where these students are inserted. This study illustrates that wild animal’s consumption and preference by Potiguara students are modulated by a set of environmental and social factors, such as students’ father’s occupation, policies, and regulations, deforestation, and urbanization that shape the use of wildlife as a source of food and determine their role in securing food sufficiency among traditional populations.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The research project was approved by the Research Ethics Committee and the National Research Ethics Commission (CEP/CONEP), in accordance with Resolution No. 466/2012 of the National Health Council (CNS) of the Ministry of Health (No. 2,491,305). This research was authorized by the Brazilian Agency for Indian Affairs (FUNAI) (No. 94/AAEP/PRES/2017) and indigenous leaders in compliance with CNS Resolution No. 304 of 2000. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author Contributions
CS, NV, and RA conceived the ideas and designed the methodology. CS collected the data. CS, FB-P, and AB compiled and analyzed the data. CS and FB-P wrote the original draft. CS, FB-P, AB, NV, and RA edited the manuscript. All authors gave final approval for publication.
Funding
RA was supported by a productivity research grant from the National Council for Scientific and Technological Development (CNPq). This study was supported in part by Brazilian National Council for Scientific and Technological Development (CNPq; grant 422041/2018-1) and Center for International Forestry Research (CIFOR).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We sincerely thank the Potiguara students who participated in the study and the school’s staff. Without them, this work would not be possible. We would like to thank FUNAI and all schools’ principals for authorizing and supporting this research. We thank the researcher Daniel Pereira Munari for his help in editing and translating the manuscript. We thank the reviewers WC and GR for their important contributions to this manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2022.900398/full#supplementary-material
Footnotes
References
Alves, R. R. N., Nishida, A. K., and Hernández, M. I. (2005). Environmental perception of gatherers of the crab ’caranguejo-uçá′ (Ucides cordatus, Decapoda, Brachyura) affecting their collection attitudes. J. Ethnobiol. Ethnomed. 1:10. doi: 10.1186/1746-4269-1-10
Alves, R. R. N., and Rosa, I. L. (2006). From cnidarians to mammals: the use of animals as remedies in fishing communities in NE Brazil. J. Ethnopharmacol. 107, 259–276. doi: 10.1016/j.jep.2006.03.007
Alves, R. R. N., and Nishida, A. K. (2002). A ecdise do caranguejo-uçá, Ucides cordatus L. (Decapoda, Brachyura) na visão dos caranguejeiros. Interciencia 27, 110–117.
Alves, R. R. N., and Nishida, A. K. (2003). Aspectos socioeconômicos e percepção ambiental dos catadores de caranguejo-uçá Ucides cordatus cordatus (L. 1763) (Decapoda, Brachyura) do estuário do Rio Mamanguape, Nordeste do Brasil. Interciencia 28, 36–43.
Alves, R. R. N., Souto, W. M. S., Fernandes-Ferreira, H., Bezerra, D. M. M., Barboza, R. R. D., and Vieira, W. L. S. (2018). “The importance of hunting in human societies,” in Ethnozoology: Animals in Our Lives, eds R. R. N. Alves and U. P. Albuquerque (Cambridge, MA: Academic Press), 95–118. doi: 10.1016/B978-0-12-809913-1.00007-7
Alves, R. R. N., and van Vliet, N. (2018). “Wild fauna on the menu,” in Ethnozoology: Animals in Our Lives, eds R. R. N. Alves and U. P. Albuquerque (Cambridge, MA: Academic Press), 167–194. doi: 10.1016/B978-0-12-809913-1.00010-7
Anderson, J. K. (2020). Hunting in the Ancient World. Available Online at: https://ixtheo.de/Record/272807982 [accessed January 21, 2021].
Araujo, H. F. P., and Nishida, A. K. (2007). Conhecimento de pescadores artesanais sobre a composição da avifauna em estuários paraibanos: uma contribuição para a conservação. Sitientibus 7, 67–77. doi: 10.13102/scb8133
Araujo, H. F. P., Rodrigues, R. C., and Nishida, A. K. (2006). Composição da avifauna em complexos estuarinos no estado da Paraíba, Brasil. Rev. Bras. Ornitol. 14, 249–259.
Araújo, M. Q. (2015). Entre Terreiros, Roçados e Marés: Um Estudo Sobre a Organização Doméstica Entre Os Potiguara do Litoral Norte da Paraíba. Rio Tinto: Universidade Federal da Paraíba.
Araújo, M. Q. (2019). Household ecology, environments and technical processes among the Potiguara of Jaraguá village (Paraíba, Brazil). Vibrant Virtual Braz. Anthropol. 16, 1–23. doi: 10.1590/1809-43412019v16d502
Barboza, R. R. D., Lopes, S. F., Souto, W. M., Fernandes-Ferreira, H., and Alves, R. R. N. (2016). The role of game mammals as bushmeat in the Caatinga, northeast Brazil. Ecol. Soc. 21:2. doi: 10.5751/ES-08358-210202
Bellinger, C., and Andrade, L. (2016). Alimentação nas Escolas Indígenas: Desafios Para Incorporar Práticas e Saberes. Available Online at: https://cpisp.org.br/wp-content/uploads/2016/05/Alimentacao_Escolas_Indigenas.pdf [accessed January 21, 2021].
Bodmer, R. E., Eisenberg, J. F., and Redford, K. H. (1997). Hunting and the likelihood of extinction of Amazonian mammals: caza y probabilidad de extinción de mamiferos Amazónicos. Conserv. Biol. 11, 460–466. doi: 10.1046/j.1523-1739.1997.96022.x
Bogoni, J. A., Pires, J. S. R., Graipel, M. E., Peroni, N., and Peres, C. A. (2018). Wish you were here: how defaunated is the Atlantic Forest biome of its medium-to large-bodied mammal fauna? PLoS One 13:e0204515. doi: 10.1371/journal.pone.0204515
Braga-Pereira, F., Morcatty, T. Q., El Bizri, H. R., Tavares, A. S., Mere-Roncal, C., González-Crespo, C., et al. (2021b). Congruence of local ecological knowledge (LEK) based methods and line-transect surveys in estimating wildlife abundance in tropical forests. Methods Ecol. Evol. 13, 743–756. doi: 10.1111/2041-210X.13773
Braga-Pereira, F., Peres, C. A., Alves, R. R. N., and Van-Dúnem Santos, C. (2021a). Intrinsic and extrinsic motivations governing prey choice by hunters in a post-war African forest-savannah macromosaic. PLoS One 16:e0261198. doi: 10.1371/journal.pone.0261198
Brasil (1988). Artigo 231 da Constituição Federal de 1988. Available Online at: http://www.planalto.gov.br/ccivil_03/constituicao/constituicao.htm [accessed December 4, 2021].
Brasil (2000). Lei n° 9.98, de 18 de Julho de 2000. Law Establishing the National System of Nature Conservation Units. Available Online at: http://www.planalto.gov.br/ccivil_03/leis/l9985.htm [accessed December 4, 2021].
Câmara, I. G. (2003). “Brief history of conservation in the Atlantic Forest,” in The Atlantic Forest of South America: Biodiversity Status, Threats, and Outlook, eds C. GalindoLeal and I. G. Câmara (Washington, DC: Center for Applied Biodiversity Science and Island Press), 31–42.
Campos-Silva, J. V., Hawes, J. E., Andrade, P. C., and Peres, C. (2018). Unintended multispecies co-benefits of an Amazonian community-based conservation programme. Nat. Sustain. 1, 650–656. doi: 10.1038/s41893-018-0170-5
Capistrano, J., and Lopes, P. (2012). Crab gatherers perceive concrete changes in the life history traits of Ucides cordatus (Linnaeus, 1763), but overestimate their past and current catches. Ethnobiol. Conserv. 1, 1–21. doi: 10.15451/ec2012-8-1.7-1-21
Chee, V. A., Teran, E., Hernandez, I., Wright, L., Izurieta, R., Reina-Ortiz, M., et al. (2019). ‘Desculturización,’ urbanization, and nutrition transition among urban Kichwas indigenous communities residing in the Andes highlands of Ecuador. Public Health 176, 21–28. doi: 10.1016/j.puhe.2019.07.015
Coimbra, C. E., and Santos, R. V. (1991). Avaliação do estado nutricional num contexto de mudança sócio-econômica: o grupo indígena Suruí do Estado de Rondônia, Brasil. Cad. Saúde Pública 7, 538–562. doi: 10.1590/S0102-311X1991000400006
Donders, I., and Barriocanal, C. (2020). The influence of markets on the nutrition transition of hunter-gatherers: lessons from the western Amazon. Int. J. Environ. Res. Public Health 17:6307. doi: 10.3390/ijerph17176307
Dounias, E., and Froment, A. (2011). From foraging to farming among present-day forest hunter-gatherers: consequences on diet and health. Int. For. Rev. 13, 294–304. doi: 10.1505/146554811798293818
Duda, R., Gallois, S., and Reyes-García, V. (2018). Ethnozoology of bushmeat. Importance of wildlife in diet, food avoidances and perception of health among the Baka (Cameroon). Rev. Ethnoecol. 14, 1–41. doi: 10.4000/ethnoecologie.3976
Endo, W., Peres, C. A., and Haugaasen, T. (2016). Flood pulse dynamics affects exploitation of both aquatic and terrestrial prey by Amazonian floodplain settlements. Biol. Conserv. 201, 129–136. doi: 10.1016/j.biocon.2016.07.006
Feijó, A., and Langguth, A. (2013). Mamíferos de médio e grande porte do Nordeste do Brasil: distribuição e taxonomia, com descrição de novas espécies. Rev. Nordestina Biol. 22, 3–225.
Figueiredo, R. A. A. D., and Barros, F. B. (2016). Hunting, preparing, and eating the ‘bicho do mato’: feeding practices among quilombolas in the Ipaú-Anilzinho extractive reserve (Pará State). Bol. Mus. Para. Emílio Goeldi 11, 691–713. doi: 10.1590/1981.81222016000300009
Gardner, C. J., and Davies, Z. G. (2014). Rural bushmeat consumption within multiple use protected areas: qualitative evidence from southwest Madagascar. Hum. Ecol. 42, 21–34. doi: 10.1007/s10745-013-9629-1
Hanazaki, N., Alves, R. R. N., and Begossi, A. (2009). Hunting and use of terrestrial fauna used by Caiçaras from the Atlantic Forest coast (Brazil). J. Ethnobiol. Ethnomed. 5:36. doi: 10.1186/1746-4269-5-36
Harrison, X. A., Donaldson, L., Correa-Cano, M. E., Evans, J., Fisher, D. N., Goodwin, C. E., et al. (2018). A brief introduction to mixed effects modelling and multi-model inference in ecology. PeerJ 6:e4794. doi: 10.7717/peerj.4794
IBGE (2010). Instituto Brasileiro de Geografia e Estatística. Available Online at: https://censo2010.ibge.gov.br/ [accessed December 4, 2021].
ISA (2021). Terras Indígenas. Available Online at: https://terrasindigenas.org.br/pt-br/brasil [accessed December 4, 2021].
Jacob, M. C. M., Teixeira, C. D., Bautista, D. A., and Ramos, V. A. N. (2021). Ethnonutrition. Ethnobiol. Conserv. 10, 1–8.
Kendie, F. A., Mekuriaw, S. A., and Dagnew, M. A. (2018). Ethnozoological study of traditional medicinal appreciation of animals and their products among the indigenous people of Metema Woreda, North-Western Ethiopia. J. Ethnobiol. Ethnomed. 14:37. doi: 10.1186/s13002-018-0234-7
Kraft, T. S., Stieglitz, J., Trumble, B. C., Martin, M., Kaplan, H., and Gurven, M. (2018). Nutrition transition in 2 lowland Bolivian subsistence populations. Am. J. Clin. Nutr. 108, 1183–1195. doi: 10.1093/ajcn/nqy250
Kramer, D. B., Urquhart, G., and Schmitt, K. (2009). Globalization and the connection of remote communities: a review of household effects and their biodiversity implications. Ecol. Econ. 68, 2897–2909. doi: 10.1016/j.ecolecon.2009.06.026
Kuhnlein, H. V. (2009). “Introduction: why are indigenous peoples’ food systems important and why do they need documentation?,” in Indigenous Peoples’ Food Systems: the Many Dimensions of Culture, Diversity and Environment for Nutrition and Health, eds H. V. Kuhnlein, B. Erasmus, and D. Spigelski (Rome: FAO), 1–4.
Leyva-Trinidad, D. A., Pérez-Vázquez, A., Newell, G. E., Albarado, J. C. G., and Jácome, A. G. (2021). Food security strategies of an indigenous community in Veracruz, Mexico. Ethnobiol. Conserv. 10:41. doi: 10.15451/ec2021-11-10.41-1-16
Lourenço, A. E. P., Santos, R. V., Orellan, J. D. Y., and Coimbra, C. E. A. (2008). Nutrition transition in Amazonia: obesity and socioeconomic change in the Suruí Indians from Brazil. Am. J. Hum. Biol. 20, 564–571. doi: 10.1002/ajhb.20781
Luiselli, L., Hema, E. M., Segniagbeto, G. H., Ouattara, V., Eniang, E. A., Di Vittorio, M., et al. (2019). Understanding the influence of non-wealth factors in determining bushmeat consumption: results from four West African countries. Acta Oecol. 94, 47–56. doi: 10.1016/j.actao.2017.10.002
Mitchell, D., and Donald, L. (2001). Sharing resources on the North Pacific coast of North America: the case of the eulachon fishery. Anthropologica 43, 19–35.
Mourão, J., and Nordi, N. (2003). Ethnoichthyology of artisanal fishermen from the estuary of Mamanguape River, Paraíba, Brazil. Bol. Inst. Pesca 29, 9–17.
Mulrennan, M. E., and Bussières, V. (2018). Social-ecological resilience in indigenous coastal edge contexts. Ecol. Soc. 23:18. doi: 10.5751/ES-10341-230318
Murrieta, R. S. S., and Dufour, D. L. (2004). Fish and Farinha: protein and energy consumption in Amazonian rural communities on Ituqui Island, Brazil. Ecol. Food. Nutr. 43, 231–255. doi: 10.1080/03670240490447550
Myers, N., Mittermeier, R. A., Mittermeier, C. G., Da Fonseca, G. A., and Kent, J. (2000). Biodiversity hotspots for conservation priorities. Nature 403, 853–858. doi: 10.1038/35002501
Nardoto, G. B., Murrieta, R. S. S., Prates, L. E. G., Adams, C., Garavello, M. E. P., Schor, T., et al. (2011). Frozen chicken for wild fish: nutritional transition in the Brazilian Amazon region determined by carbon and nitrogen stable isotope ratios in fingernails. Am. J. Hum. Biol. 23, 642–650. doi: 10.1002/ajhb.21192
Nascimento, D. M., Alves, Â. G. C., Alves, R. R. N., Barboza, R. R. D., Diele, K., and Mourao, J. S. (2016). An examination of the techniques used to capture mangrove crabs, Ucides cordatus, in the Mamanguape River estuary, Northeastern Brazil, with implications for management. Ocean Coast. Manag. 130, 50–57. doi: 10.1016/j.ocecoaman.2016.05.010
Nascimento, D. M., Alves, R. R. N., Barboza, R. R. D., Schmidt, A. J., Diele, K., and Mourão, J. S. (2017). Commercial relationships between intermediaries and harvesters of the mangrove crab Ucides cordatus (Linnaeus, 1763) in the Mamanguape River estuary, Brazil, and their socio-ecological implications. Ecol. Econ. 131, 44–51. doi: 10.1016/j.ecolecon.2016.08.017
Nascimento, D. M., Ferreira, E. N., Bezerra, D. M., Rocha, P. D., Alves, R. R. N., and Mourão, J. S. (2012). Capture techniques’ use of Caranguejo-uçá crabs (Ucides cordatus) in Paraíba state (Northeastern Brazil) and its socio-environmental implications. Natl. Acad. Bras. Cienc. 84, 1051–1064. doi: 10.1590/s0001-37652012005000066
Nielsen, M. R., Meilby, H., Smith-Hall, C., Pouliot, M., and Treue, T. (2018). The importance of wild meat in the global south. Ecol. Econ. 146, 696–705. doi: 10.1016/j.ecolecon.2017.12.018
Nishida, A. K., Nordi, N., and Alves, R. R. N. (2006). Mollusc gathering in Northeast Brazil: an ethnoecological approach. Hum. Ecol. 34, 133–145. doi: 10.1007/s10745-005-9005-x
Nishida, A. K., Nordi, N., and Alves, R. R. N. (2008). Aspectos socioeconômicos dos catadores de moluscos do litoral paraibano, Nordeste do Brasil. Rev. Biol. Ciênc. Terra 8, 207–215.
Nordi, N., Nishida, A. K., and Alves, R. R. N. (2009). Effectiveness of two gathering techniques for Ucides cordatus in Northeast Brazil: implications for the sustainability of mangrove ecosystems. Hum. Ecol. 37, 121–127. doi: 10.1007/s10745-009-9214-9
Oksanen, J., Blanchet, F. G., Kindt, R., Legendre, P., Minchin, P. R., O’hara, R. B., et al. (2013). Community Ecology Package. R Package Version 2(0).
Oliveira, M. A., El Bizri, H. R., Morcatty, T. Q., Messias, M. R., and Costa Doria, C. R. (2022). Freelisting as a suitable method to estimate the composition and harvest rates of hunted species in tropical forests. Ethnobiol. Conserv. 11, 1–9. doi: 10.15451/ec2022-03-11.08-1-9
Parrotta, J. A., Dey de Pryck, J., Darko Obiri, B., Padoch, C., Powell, B., Sandbrook, C., et al. (2015). “The historical, environmental and socio-economic context of forests and tree-based systems for food security and nutrition,” in Forests, Trees and Landscapes for Food Security and Nutrition, eds B. Vira, C. Wildburger, and S. Mansourian (Vienna: IUFRO), 51–86. doi: 10.11647/OBP.0085.03
Popkin, B. (1994). The nutrition transition in low-income countries: an emerging crisis. Nutr. Rev. 52, 285–298. doi: 10.1111/j.1753-4887.1994.tb01460.x
Popkin, B. M., and Gordon-Larsen, P. (2004). The nutrition transition: worldwide obesity dynamics and their determinants. Int. J. Obes. 28, S2–S9. doi: 10.1038/sj.ijo.0802804
Powell, B., Thilsted, S. H., Ickowitz, A., Termote, C., Sunderland, T., and Herforth, A. (2015). Improving diets with wild and cultivated biodiversity from across the landscape. Food Secur. 7, 535–554.
Prist, P. R., Michalski, F., and Metzger, J. P. (2012). How deforestation pattern in the Amazon influences vertebrate richness and community composition. Landsc. Ecol. 27, 799–812. doi: 10.1007/s10980-012-9729-0
R Core Team (2019). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Robin, X., Turck, N., Hainard, A., Tiberti, N., Lisacek, F., Sanchez, J. C., et al. (2021). “Package ‘pROC”’, in R Package Version 1.17.0.1.
Rocha, M. S., Santiago, I. M., Cortez, C. S., Trindade, P. M., and Mourão, J. S. (2012). Use of fishing resources by women in the Mamanguape River Estuary, Paraíba state, Brazil. An. Acad. Bras. Cienc. 84, 1189–1199. doi: 10.1590/s0001-37652012005000060
Rocha, M. S. P., Mourão, J. S., Souto, W. M. S., Barboza, R. R. D., and Alves, R. R. N. (2008). Uso dos recursos pesqueiros no Estuário do Rio Mamanguape, Estado da Paraíba, Brasil. Interciencia 33, 903–909.
Rodrigues, G. S., Rodrigues, I. A., Buschinelli, C. D. A., Queiroz, J. F., Frighetto, R. T. S., Antunes, L. R., et al. (2008). Gestão Ambiental Territorial na Área de Proteção Ambiental da Barra do Rio Mamanguape (PB). Available Online at: https://agris.fao.org/agris-search/search.do?recordID=BR2008115335 [accessed December 4, 2021].
Santos, R. V., and Coimbra, C. E. A. (2003). “Cenários e tendências da saúde e da epidemiologia dos povos indígenas no Brasil,” in Epidemiologia e Saúde dos Povos Indígenas no Brasil, eds C. E. A. Coimbra, R. V. Santos, and A. L. Escobar (Rio de Janeiro: Editora Fiocruz), 13–48.
Sarti, F. M., Adams, C., Morsello, C., Van Vliet, N., Schor, T., Yagüe, B., et al. (2015). Beyond protein intake: bushmeat as source of micronutrients in the Amazon. Ecol. Soc. 20:22. doi: 10.5751/ES-07934-200422
Souza, J. B., and Alves, R. R. N. (2014). Hunting and wildlife use in an Atlantic Forest remnant of Northeastern Brazil. Trop. Conserv. Sci. 7, 145–160.
Stringham, O. C., García-Díaz, P., Toomes, A., Mitchell, L., Ross, J., and Cassey, P. (2021). Reptile smuggling is predicted by trends in the legal exotic pet trade. EcoEvoRxiv [Preprint] doi: 10.32942/osf.io/t42fd
Tabarelli, M., Pinto, L. P., Silva, J. M. C., Hirota, M. M., and Bedê, L. C. (2005). Desafios e oportunidades para a conservação da biodiversidade na Mata Atlântica brasileira. Megadiversidade 1, 132–138.
Tavares, E. F., Vieira-Filho, J. P. B., Andriolo, A., Sanudo, A., Gimeno, S. G. A., and Franco, L. J. (2003). Metabolic profile and cardiovascular risk patterns of an Indian tribe living in the Amazon Region of Brazil. Hum. Biol. 75, 31–46. doi: 10.1353/hub.2003.0028
Vallega, A. (2013). Fundamentals of Integrated Coastal Management. Berlin: Springer Science & Business Media.
van Vliet, N., Gonzalez, A., Nyumu, J., Muhindo, J., Paemelaere, E., Cerutti, P., et al. (2022). Reducing wild meat sales and promoting local food security: lessons learnt from a behavior change campaign in Yangambi, democratic republic of Congo. Ethnobiol. Conserv. 11:9. doi: 10.15451/ec2022-04-11.09-1-14
van Vliet, N., Quiceno-Mesa, M. P., Cruz-Antia, D., and Yagüe, B. (2014). Carne de Caça e Segurança Alimentar na Zona da Tríplice Fronteira Amazônica (Colômbia, Peru e Brasil). Available Online at: http://www.cifor.org/publications/pdf_files/Books/BVanVliet1401P.pdf [accessed December 4, 2021].
van Vliet, N., Quiceno-Mesa, M. P., Cruz-Antia, D., Tellez, L., Martins, C., Haiden, E., et al. (2015a). From fish and bushmeat to chicken nuggets: the nutrition transition in a continuum from rural to urban settings in the Tri frontier Amazon region. Ethnobiol. Conserv. 4:6. doi: 10.15451/ec2015-7-4.6-1-12
van Vliet, N., Nebesse, C., and Nasi, R. (2015b). Bushmeat consumption among rural and urban children from Province Orientale, Democratic Republic of Congo. Oryx 49, 165–174. doi: 10.1017/S0030605313000549
van Vliet, N., Quiceno, M. P., Cruz, D., de Aquino, L. J. N., Yagüe, B., Schor, T., et al. (2015c). Bushmeat networks link the forest to urban areas in the trifrontier region between Brazil, Colombia, and Peru. Ecol. Soc. 20:21. doi: 10.5751/ES-07782-200321
van Vliet, N., Schulte-Herbruggen, B., Vanegas, L., Yair-Cuesta, E., Sandrin, F., and Nasi, R. (2018). Wild animals (fish and wildmeat) contribute to dietary diversity among food insecure urban teenagers the case of Quibdó, Colombia. Ethnobiol. Conserv. 7:2. doi: 10.15451/ec2018-01-7.02-1-15
Vanegas, L., van Vliet, N., Cruz, D., and Sandrin, F. (2016). Contribución proteica de animales silvestres y domésticos a losmenús de los contextos rurales, peri-urbanos y urbanos de variasregiones de Colombia. Biota Colomb 17, 26–43. doi: 10.21068/C2016v17r01a03
Walrod, J., Seccareccia, E., Sarmiento, I., Pimentel, J. P., Misra, S., Morales, J., et al. (2018). Community factors associated with stunting, overweight and food insecurity: a community-based mixed-method study in four Andean indigenous communities in Ecuador. BMJ Open 8:e020760. doi: 10.1136/bmjopen-2017-020760
Welch, J. R., Ferreira, A. A., Santos, R., Gugelmin, S., Werneck, G., and Coimbra, C. (2009). Nutrition transition, socioeconomic differentiation, and gender among adult Xavante Indians, Brazilian Amazon. Hum. Ecol. 37, 13–26. doi: 10.1007/s10745-009-9216-7
Keywords: wildlife, game, fish, domestic protein, diet, ethnozoology, nutrition, protect area
Citation: Santos CP, Braga-Pereira F, Borges AKM, Van Vliet N and Alves RRN (2022) Consumption and Preferences for Wild and Domestic Meat in Indigenous Communities in the Brazilian Atlantic Forest. Front. Ecol. Evol. 10:900398. doi: 10.3389/fevo.2022.900398
Received: 20 March 2022; Accepted: 13 June 2022;
Published: 12 July 2022.
Edited by:
Umesh Srinivasan, Indian Institute of Science (IISc), IndiaReviewed by:
Willandia Chaves, Virginia Tech, United StatesGabriel Augusto Marques Rossi, Vila Velha University, Brazil
Copyright © 2022 Santos, Braga-Pereira, Borges, Van Vliet and Alves. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Claudileide Pereira dos Santos, claupsantoss@gmail.com; Franciany Braga-Pereira, franbraga83@yahoo.com.br
 Claudileide Pereira dos Santos
Claudileide Pereira dos Santos Franciany Braga-Pereira
Franciany Braga-Pereira Anna Karolina Martins Borges
Anna Karolina Martins Borges Nathalie Van Vliet
Nathalie Van Vliet Rômulo Romeu Nóbrega Alves
Rômulo Romeu Nóbrega Alves