Developmental Plasticity in Primate Coordinated Song: Parallels and Divergences With Duetting Songbirds
- Museo de Historia Natural Noel Kempff Mercado, Santa Cruz de la Sierra, Bolivia
Homeothermic animals (birds and mammals) are prime model systems for investigating the developmental plasticity and neural mechanisms of vocal duetting, a cooperative acoustic signal that prevails in family-living and pair-bonded species including humans. This review focuses on the nature of this trait and its nurturing during ontogeny and extending into adulthood. I begin by outlining the underpinning concepts of duet codes and pair-specific answering rules as used by birds to develop their learned coordinated song, driven by a complex interaction between self-generated and socially mediated auditory feedback. The more tractable avian model of duetting helps identify research gaps in singing primates that also use duetting as a type of intraspecific vocal interaction. Nevertheless, it has become clear that primate coordinated song—whether overlapping or antiphonal—is subject to some degree of vocal flexibility. This is reflected in the ability of lesser apes, titi monkeys, tarsiers, and lemurs to adjust the structure and timing of their calls through (1) social influence, (2) coordinated duetting both before and after mating, (3) the repair of vocal mistakes, (4) the production of heterosexual song early in life, (5) vocal accommodation in call rhythm, (6) conditioning, and (7) innovation. Furthermore, experimental work on the neural underpinnings of avian and mammalian antiphonal duets point to a hierarchical (cortico-subcortical) control mechanism that regulates, via inhibition, the temporal segregation of rapid vocal exchanges. I discuss some weaknesses in this growing field of research and highlight prospective avenues for future investigation.
Introduction
“The development of communication is fundamentally embedded in social interactions across individual brains (Hasson et al., 2012).” Duetting, the coordinated sequences of acoustic signals exchanged between two individuals, has emerged as a remarkable phenotype of two brains wired to either cooperate or mitigate conflict (Fortune et al., 2011; Hoffmann et al., 2019; Okobi et al., 2019; Coleman et al., 2021). Whether this is a matter of hard or soft wiring remains an open question, but the diversity of mammalian and avian song duets holds great research promise for exploring how dyadic vocal interactions are shaped during ontogeny.
Here, I review the evidence for developmental plasticity in singing non-human primates1, highlighting parallels and divergences with research on duetting songbirds. Collectively, these two phyla encompass tropical species that share similar socio-ecological characteristics, including putative sexual monogamy, family-living, and year-round territoriality with robust arboreal adaptations (Tobias et al., 2016; De Gregorio et al., 2022). However, they also differ in one key aspect, namely “vocal production learning,” which is the ability to produce novel sounds from auditory experience (Janik and Slater, 2000; Vernes et al., 2021). While oscine songbirds (passerines) stand out as fine vocal learners, evidence of this is limited in non-human primates [Snowdon, 2017a; Janik and Knörnschild, 2021; but see Lameira (2017) who makes a strong case of vocal production learning in the voiceless calls of great apes].
The Nuts and Bolts of Songbird Duetting
The considerable progress in research on avian duetting is marked by several influential reviews (Farabaugh, 1982; Hall, 2009; Dahlin and Benedict, 2014). Duetting patterns in songbirds range from loosely coordinated song (Benedict and McEntee, 2009; Tobias and Seddon, 2009) to synchronized or antiphonal song2 uttered with exquisite temporal precision (Wickler and Seibt, 1980; Templeton et al., 2013; Kovach et al., 2014) and combining alternation and synchrony (Mann et al., 2006).
Duet Codes and Answering Rules
Duetting behavior occurs at both the individual and pair levels (Levin, 1996), while Logue (2006) studied duetting from an operational perspective in which two individuals establish a shared set of rules. This led to the notion of a “duet code”—a set of answering rules one individual uses to answer its mate’s song (Logue, 2006; Logue et al., 2008). While a duet is a pair-level property, a duet code is an individual attribute, and answers according to a duet code “adhere” to that code (Logue and Krupp, 2016). At its simplest, a single pairing rule, such as ‘‘answer F1 to M1,’’3 generates the cyclical duet [i-n(M1-F1)]4 produced by many songbirds (Levin, 1996; Rogers, 2005). A more complex duet code, such as “answer F1 to M1, F2 to M2, and F3 to M3,” generates a non-repeated duet [i-(M1-F1-M2-F2-M3-F3)], as produced by an African weaver bird endowed with such a large syllable repertoire that both partners constantly switch between syllable types (Voigt et al., 2006; Lemazina et al., 2021). Logue’s duet code concept opened up new avenues for measuring how code complexity and adherence vary across species (Logue and Krupp, 2016), whether duet codes are pair-specific (Mennill and Vehrencamp, 2005; Templeton et al., 2013), whether one sex or both adhere to these codes (Mann et al., 2003; Rivera-Cáceres, 2015), and whether duet codes emerge spontaneously in newly formed adult pairs or require vocal practice (Levin, 1996; Rivera-Cáceres et al., 2016). This begs the question: do young birds learn duet codes from their elders?
Duet Code Learning
Evidence that duet codes are learned from adults comes from observations of juveniles singing alongside their parents (Farabaugh, 1982; Hall, 2009). Such collective singing presumably allows juveniles to gain duetting experience, which not only requires learning what to answer and when but also mastering the duet rhythm in coordination with breathing given the rapid alternation (2–5 Hz) of male and female syllables (Hoffmann et al., 2019; Coleman et al., 2021). For example, song coordination in juvenile canebrake wrens improves over time via parental influence and independently of maturational effects, indicating a learning process (Rivera-Cáceres et al., 2018). Whether song acquisition results from copying a same-sex parent or integrating auditory information from both parental “tutors” remains unknown. There may also be alternative modes of code development with age. For example, a code might be retained throughout life (“close-ended”), whereby phrase-pairing rules remain constant regardless of partner identity (Levin, 1996); alternatively, mature individuals might re-learn a code each time they acquire a new mate (“open-ended”; Wickler, 1980). In the case of canebrake wrens, different pairs have distinct duetting rules, suggesting that learning in adulthood is likely. Indeed, removing and translocating individuals of well-established pairs confirmed that adult wrens re-learn pair-specific duet codes after re-mating, with males showing more flexibility in phrase-pairing rules than females (Rivera-Cáceres et al., 2016). Consequently, Rivera-Cáceres et al. (2018) proposed a three-step model for duet learning: (1) memorizing song material from auditory exposure, (2) rehearsing duet songs with both parents, and (3) re-learning to coordinate songs with a breeding partner (Figure 1A). Whether these two latter forms of sensorimotor learning share the same neural connections is the subject of future research (Nieder and Mooney, 2019).
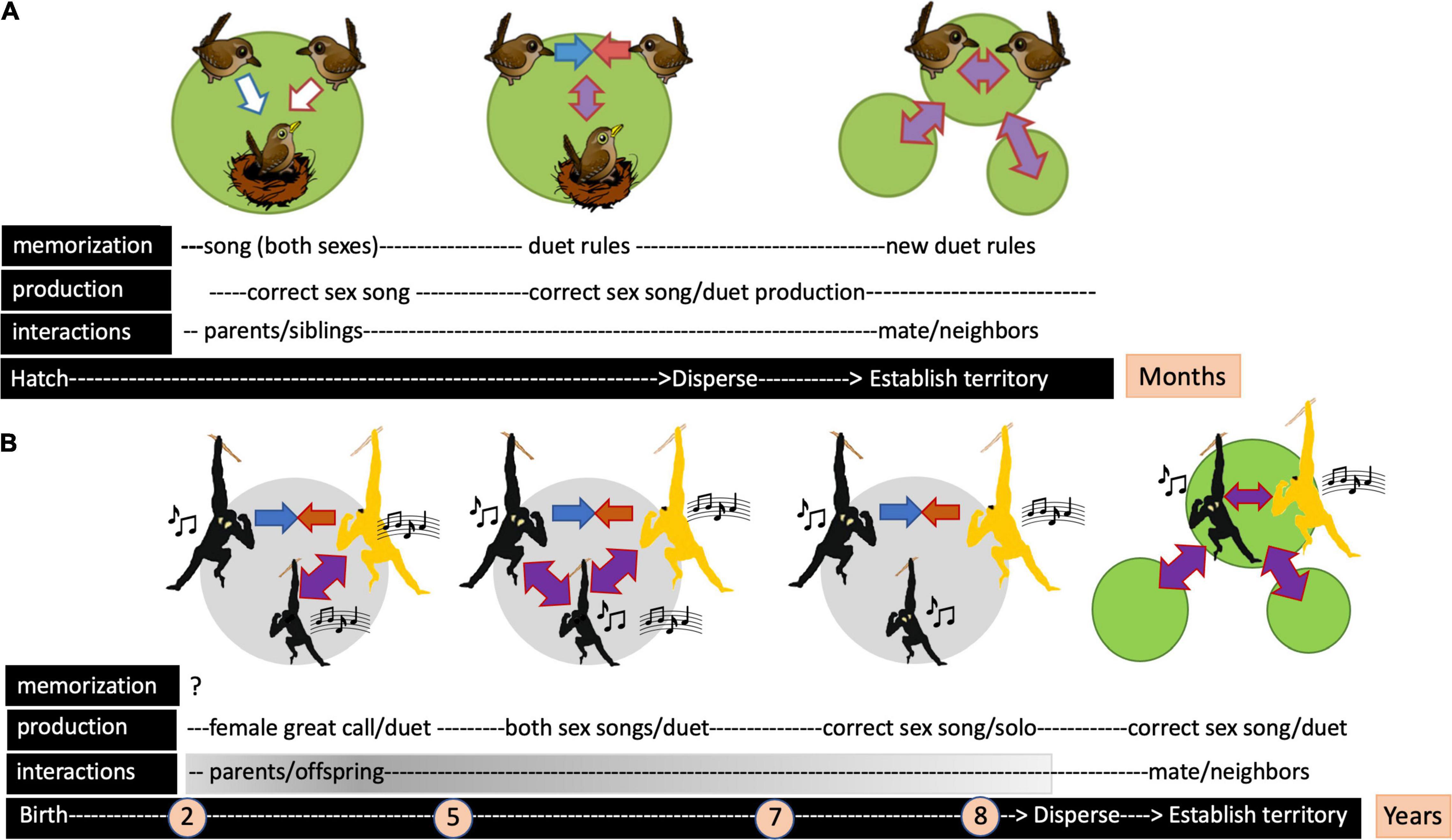
Figure 1. Diagram illustrating the timing of song acquisition and the nature of social interactions that characterize the development of antiphonal duets in a songbird (canebrake wren) and a lesser ape (southern yellow-cheeked gibbon). (A) The songbird model of duet acquisition in which young birds first memorize sounds heard from both parents acting as tutors, then “learn” duet codes during mutual singing sessions with a same-sex parent; following post-natal dispersion, mature individuals “re-learn” to combine song elements with a new mate, performing a duet which is used to advertise territory ownership and/or pair bond strength. (B) The lesser ape model of duet acquisition in which a young male first develops a female-like great call while co-singing with his mother until reaching sexual maturity (3–5 years). Sexually mature daughters – not shown in the diagram – have acquired the basic pattern of the maternal song (Merker and Cox, 1999), which is then perfected during co-singing sessions with the mother until leaving the parental group (Koda et al., 2013). Mother-son vocal interactions continue at a decreasing rate (gray gradient) until adolescence. From 5 to 7 years, sons utter both male and female song elements and subsequently discard the female-like great call from their repertoire, retaining only male song (coda). The male coda consists of a multi-modulation note and a staccato note that develop in that sequence until at least 8 years of age (Hradec et al., 2021). In the absence of experimental evidence, gibbons are not considered vocal learners, but the memorization phase remains questionable. Arrows denote vocal interactions. Note the difference in the timeline between the two model systems. Green circles refer to observations made in the wild; gray circles depict events observed in zoo animals [adapted from Rivera-Cáceres and Templeton (2019), Hradec et al. (2021)].
Many songbirds co-sing in rapid turn on a syllable-to-syllable basis with sub-second latencies (Mann et al., 2009; Fortune et al., 2011; Rivera-Cáceres, 2015). To achieve such tight coordination, individuals rely on sensory information originating from two sources of auditory feedback—one generated by the bird’s own voice (autogenous) and the other from its singing partner (heterogeneous). Owing to the velocity of sound, the longer the distance between the duetters the longer the delay for both receivers. Duetting songbirds adapt to these delays by altering the timing of their singing (Fortune et al., 2011) or by using visual cues in open habitats (Rek and Magrath, 2016, 2020). How, then, is auditory feedback encoded in the brain?
Neural Mechanisms
Neuroanatomical studies of duetting songbirds reveal the presence of well-developed brain nuclei dedicated to song production learning in both sexes, which contrasts with the females of species in which only males sing (Nottebohm and Arnold, 1976; Brenowitz and Arnold, 1986; Deng et al., 2001; but see Lobato et al., 2015). Research into the neural underpinnings of antiphonal duetting targets the HVC (used as a proper name), a high-order forebrain song nucleus involved in sensorimotor learning (Nieder and Mooney, 2019). Contrary to neurophysiological data obtained for songbirds in which only males sing, extracellular recordings in the HVC of anesthetized wrens show strong responses to auditory presentations of both male and female song when played in isolation (Fortune et al., 2011). Furthermore, experimental manipulation of song stimuli shows a sensitivity of HVC neurons to inter-syllable intervals. Importantly, the response strength of HVC neurons to duet stimuli exceeds the sum of neuronal responses to each individual’s song. This suggests that each participant not only knows what to sing but also develops an internal representation of the pair-specific duet (Fortune et al., 2011). Groundbreaking work in free-ranging African weavers further demonstrates the alternation of neuronal activity in each partner’s HVC, with bursts temporally locked to syllable onsets (Hoffmann et al., 2019). This “on-off” pattern appears to be regulated by heterogeneous auditory feedback that reciprocally inhibits HVC premotor activity (Coleman et al., 2021). Such brain-to-brain coupling mechanisms ensure precise timing of dyadic vocal interactions, most likely through gamma-aminobutyric acid-ergic inhibition (Benichov and Vallentin, 2020). For comprehensive reviews on this topic, see Elie et al. (2019) and Rivera-Cáceres and Templeton (2019).
Duetting Styles in Singing Primates
Worldwide, singing primates comprise 72 species, some of which are nocturnal and others diurnal; most share a family-living and territorial social system mediated by loud, coordinated calls emitted at predictable times, usually around dawn and/or dusk (De Gregorio et al., 2022). The gibbons’ “great-call sequence” combines the female great call and male coda, often repeated alternately [i-n(F1-M1)], with a pronounced sexual divocalism (Marshall and Marshall, 1976; Geissmann, 2002). Sexually dimorphic species duet antiphonally, whereas in monomorphic taxa, singers tend to overlap (Deputte, 1982). The duet songs of lemurs, tarsiers, and the Mentawai langur overlap, except in Lepilemur edwardsi (Méndez-Cárdenas and Zimmermann, 2009) and Tarsius niemitzi (Shekelle et al., 2019). Sexually monomorphic indris advertise with duets and choruses5 in which the paired males and females overlap more than any other dyad while dominant and non-dominant individuals avoid overlapping (Gamba et al., 2016). In each of these lineages, sex-differentiated calls often occupy a different frequency register, making them readily distinguishable on spectrograms (Tilson and Tenaza, 1976; Nietsch, 1999; Torti et al., 2013). In contrast, Neotropical titi monkey duets overlap extensively, both in the time and frequency domains, with male and female contributions exhibiting an anti-phase-locked pattern of phrase coordination devoid of discrete turns (Robinson, 1979; Müller and Anzenberger, 2002; Caselli et al., 2014; Adret et al., 2018a; Clink et al., 2019, 2022). In each of these primate lineages, there is increasing evidence of vocal malleability for this trait, long thought to be subject to strong genetic constraints (Brockelman and Schilling, 1984; Tenaza, 1985; Hammerschmidt and Fischer, 2008).
Flexibility in the Coordinated Song of Singing Primates
Vocal flexibility, the capacity for modifying vocalizations according to context, can affect call structure, amplitude, timing, duration, and rhythm. For duetting animals, this includes individuals adjusting their singing to either their partner’s or neighbors’ vocal outputs.
Interactive Group Singing
Neighboring groups of singing primates often call antiphonally (Kinzey et al., 1977; Marler and Tenaza, 1977; Raemaekers and Raemaekers, 1985) and counter-sung solos and duets are longer than solos and duets sung alone (Tenaza, 1976; Mitani, 1985). In support of the flexible timing of vocal output, active counter-singing and singing motivation have been experimentally corroborated (Chivers and MacKinnon, 1977; Mitani, 1988; Dooley and Judge, 2007). Studies of communication networks showing that siamangs are sensitive to their neighbors’ group disruption (Morino et al., 2021) are likely to unveil further instances of vocal flexibility in the future.
Within-Pair Vocal Coordination and Repair
Individual gibbons flexibly time their contributions relative to their mates’ during the great-call sequence. Guided by subtle changes in female introductory notes that signal an impending great call, the male suspends phonation; cued by her post-climax descending notes, he resumes singing with a coda phrase according to a precise turn-taking pattern (Terleph et al., 2018a). Flexibility is needed given individual variability in the female great call (Terleph et al., 2015, 2016). Adjustment made by hylobatids in response to a mate’s vocal “mistakes” are termed “repairs,” a universal principle of human conversation (Schegloff et al., 1977; Dingemanse et al., 2015). Repairs have been scrutinized for self-corrected, stalled, and aborted great calls (Haimoff, 1988; Haraway and Maples, 1998; Terleph et al., 2018a). Such studies confirm the existence of duet codes and answering rules in lesser apes. Non-adherence to the duet code (e.g., production of atypical notes or unexpected call timing) may result in duet interruption and song reset by the mate.
Vocal Accommodation in Call Rhythm
Coordinated singing and rhythm dynamics are not necessarily tied (Ravignani et al., 2014). For example, inter-onset call intervals extracted at each level of the indri’s song organization (i.e., units and phrases), reveal music-like categorical rhythmicity (De Gregorio et al., 2021a). Both in adults and young individuals, females exhibit more flexibility than males, with a sensitivity to chorus size (Gamba et al., 2016; De Gregorio et al., 2019, 2021b). Sex-related “divergence” in indri song rhythm contrasts with titis and tarsiers. In a cross-sectional study of duetting pairs of titi monkeys, partners were found to adjust pulse rate and phrase duration to one another, showing call “convergence” (Clink et al., 2019). A longitudinal study with newly formed pairs of titis might establish whether vocal learning is involved through convergence in the spectral features of calls, as reported in marmosets (Elowson and Snowdon, 1994; Snowdon and Elowson, 1999; Zürcher et al., 2021). Likewise, male and female tarsiers flexibly adjust call rhythm relative to their partner through simultaneous accelerations and decelerations (Clink et al., 2020). Within-pair convergence in duet tempo might be achieved by entrainment, i.e., spontaneous responsiveness to a perceived rhythmic signal (de Reus et al., 2021).
Parental Influence
Immature individuals singing jointly with their elders have long sparked research attention (Deputte, 1982; Raemaekers et al., 1984; Pollock, 1986; Reichard, 2003). A longitudinal study of mother-daughter vocal interactions in gibbons revealed the acquisition of correct note sequencing over time (5–30 months; Merker and Cox, 1999). In a cross-sectional study of free-ranging family groups, an inverse relationship was found between mother-daughter co-singing rates and call synchronization; less proficient daughters co-sang at higher rates. Interestingly, mothers adjusted their song to a more stereotyped pattern when co-singing than when singing alone, suggesting a “teaching role” of mothers (Koda et al., 2013). While sexually mature females sing an adult-like maternal song (Brockelman and Schilling, 1984; Merker and Cox, 1999; Koda et al., 2013), males master the multi-part coda phrase years later (Hradec et al., 2021) via an intriguing developmental trajectory (Figure 1B).
Production of Heterosexual Song
Spontaneous production of a female-like great call by immature males has been reported in several gibbon species (Koda et al., 2014; Hradec et al., 2016, 2017, 2021). A triggering role of the maternal call in young males, possibly associated with low androgen levels, has also been invoked (Koda et al., 2014). Immature individuals producing male calls potentially face aggression from the father (Hradec et al., 2021) and there is evidence that the stress hormone cortisol may negatively interact with testosterone in influencing the expression of secondary sexual traits (Puts et al., 2016). Close monitoring of hormone levels would be worthwhile in order to determine the impact of parent–offspring relationships on gibbon song development (Burns and Judge, 2016).
Acquisition of a Pair-Specific Duet Code
To reproduce outside their natal groups, mature individuals must coordinate their song with a prospective mate “having both different genetic parentage and a different history of developmental experience than their own” (Haraway and Maples, 1998). In indris, spectral-temporal features of descending phrases correlate with genetic distance in males, whereas females are less constrained (Torti et al., 2017). Thus, indri choruses may inform conspecifics about individuals’ genetic relatedness. Such an effect is less apparent in titi duets (Clink et al., 2022). Consistent with vocal flexibility and duet code learning, the duets of long-term mates are better coordinated than those of newly formed pairs (Geissmann, 1986, 1999; Maples et al., 1989; Müller and Anzenberger, 2002).
Conditioning
Robust conditioned responses are obtained in lesser apes via reinforcement and extinction procedures in which song presentation is contingent upon an individual’s own vocalization (Haraway et al., 1981; Maples and Haraway, 1982; Maples et al., 1988). Moreover, both in lemurs and gibbons, phonation can be brought under volitional control in response to an arbitrary visual signal (Wilson, 1975; Koda et al., 2007), thus demonstrating voluntary control over call timing.
Innovation
Captive siamangs can alter their calls using various “tricks,” including the production of hand- modulated and echoing calls (Badraun et al., 1998). Geissmann (2009) observed one female gibbon who amplified her duet contribution by slamming the sliding door of her sleeping quarters at the climax of her great call.
Causal Mechanisms
As renowned “soprano singers” (Koda et al., 2012), gibbons produce pure-tonal melodious song that requires appropriate hormonal and neural machinery for pitch control. Contrasting with humans, however, higher androgen levels result in calls with a higher pitch (Barelli et al., 2013; Puts et al., 2016). Experiments in a helium-oxygen atmosphere revealed that the unshifted call fundamental frequency is strongly attenuated and the first harmonic is emphasized, suggesting that the sound source (larynx) operates independently of the supralaryngeal vocal tract (Koda et al., 2012). Thus, call flexibility can be achieved by controlling laryngeal function and/or the resonance filter configuration (Gamba et al., 2011, 2017; Fitch et al., 2016), but the challenge is to account for the larynx development (Zhang et al., 2020). Importantly, bipolar excitation in the inferior portion of the precentral gyrus in the left hemisphere yields adduction of the vocal folds (Mott et al., 1911). This suggests that, in the gibbon brain, a direct pathway exists from the laryngeal representation in the primary motor cortex to the laryngeal motoneurons of the nucleus ambiguus, which controls the muscles of the larynx for vocal production (Simonyan, 2014). This might explain why gibbons can be trained to call on command (Koda et al., 2007; but see Hage and Nieder, 2013).
Discussion and Future Directions
From strepsirrhines to lesser apes, the duetting patterns of singing primates provide compelling evidence of developmental plasticity extending into adulthood. This is consistent with the view that non-human primates exhibit more flexibility in their vocal behavior than is generally acknowledged (Snowdon, 2009, 2017a,2017b,2018). Promising areas of ongoing research include (1) vocal convergence as a learning process, linked to pair-bond strength (Clink et al., 2019, 2020), (2) sex-dependent mechanisms regulating “acquisition” of categorical duet rhythms (De Gregorio et al., 2021a), and (3) the potential for parental tutoring and vocal production learning in gibbons (Koda et al., 2013; Koda, 2016; Terleph et al., 2018b; Hradec et al., 2021).
Striking similarities have emerged in duet acquisition between songbirds and singing primates (Table 1). In both phyla, young individuals co-sing extensively with their elders, although timescales can widely differ (Figure 1). Furthermore, in species with sex-specific repertoires, males and females can produce heterosexual song (Geissmann, 1983; Chen et al., 2008; Rivera-Cáceres and Templeton, 2019; Hradec et al., 2021). The production of heterosexual song early in life suggests a pre-existing or learned auditory template (Adret, 2004; Cheyne et al., 2007), possibly engaging a mirror-neuron system (Newman, 2014).
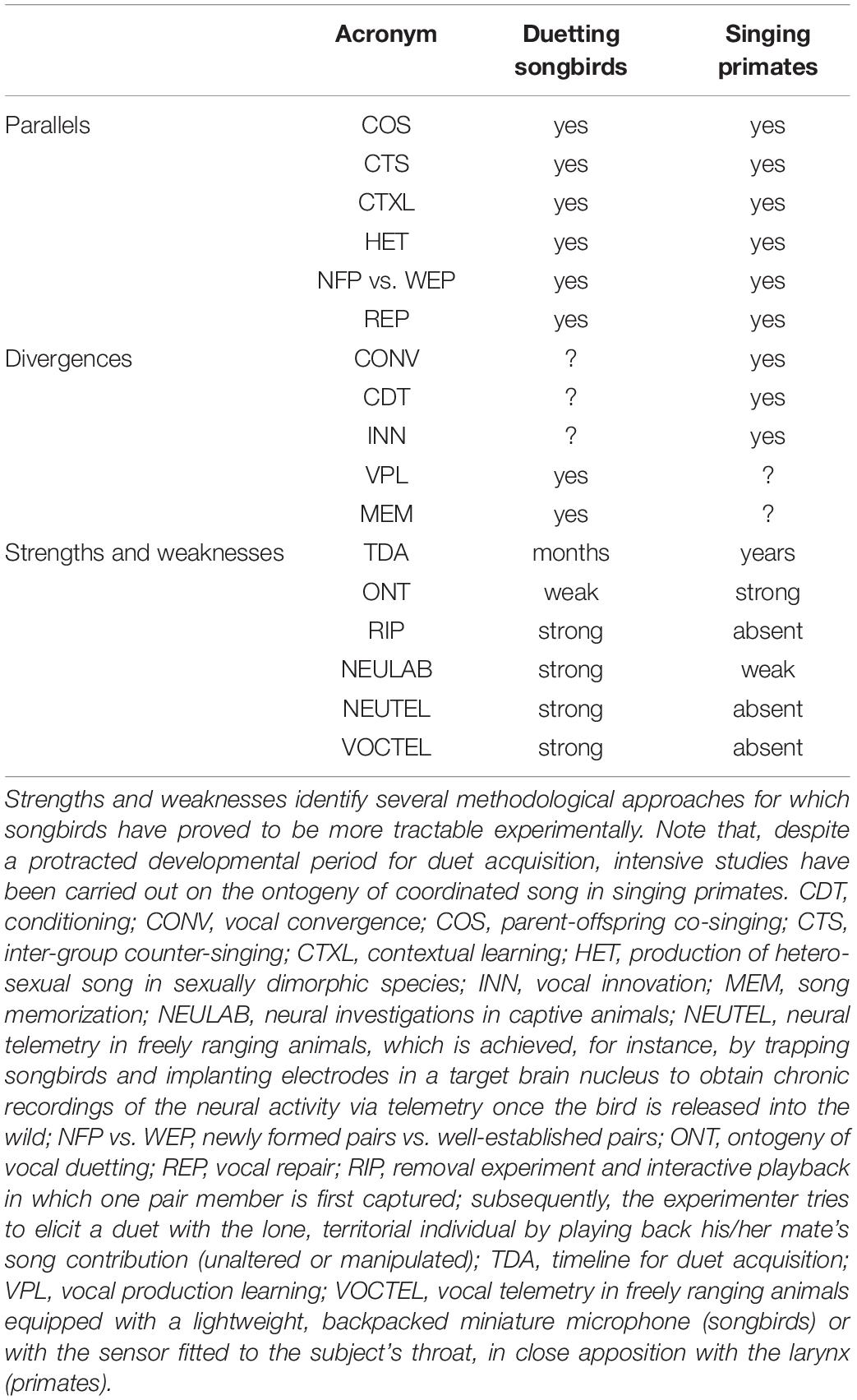
Table 1. Parallels and divergences in vocal plasticity between duetting songbirds and singing primates.
Research currently tends to focus on antiphonal duets, given their potential as precursors of turn-taking conversations in humans (Levinson, 2016). At the same time, bio-acoustics research in titi monkey duets has been hampered by extensive call overlap (Caselli et al., 2014; Adret et al., 2018a; Clink et al., 2019); cracking the code will require radio-tracking calls with miniature voice detectors (Adret et al., 2018b), as has been elegantly demonstrated in songbirds (Hoffmann et al., 2019; Lemazina et al., 2021). Another solution is conducting studies in captive (or wild) populations for which high speed video of vocalizing animals can be paired with high quality audio to ensure caller identity (Haimoff, 1981). Performant computational methods also allow effective clustering of acoustic signatures at multiple levels within animal vocal repertoires (Sainburg et al., 2020). A machine-learning approach to acoustic stream segregation might further help resolve the “cocktail party problem” (Elhilali, 2017). Developmental studies of duet acquisition in singing primates are also needed to investigate vocal flexibility in response to anthropogenic noise (Duarte et al., 2017).
While the neuroscience of pair-bonding in socially monogamous mammals is well documented (Bales et al., 2017; Potretzke and Ryabinin, 2019), a significant gap in knowledge concerns the neural mechanisms of duetting in singing primates. Integrating respiratory functions associated with coordinated song is also necessary to account for the generation of rhythmic patterns (Laje and Mindlin, 2003; Amador et al., 2005). Neuroimaging studies provide a powerful, non-invasive approach to mapping brain areas activated by antiphonal calling (Takahashi et al., 2021). Singing rodents, which offer a genetically tractable model system, produce antiphonal duets, which, much like duetting songbirds, reveals a hierarchical (cortico-subcortical) control mechanism that regulates the temporal segregation of rapid vocal exchanges via inhibition (Okobi et al., 2019). Emergence, deep in the evolutionary past, of an interlocking mechanism derived from sender-listener brain coupling (Hasson et al., 2012) may have been a key step in the evolution of human conversation.
Author Contributions
PA conceived and wrote the article and approved the final version of the manuscript.
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
I would like to thank both reviewers for insightful comments and Editage (www.editage.com) for English language editing.
Footnotes
- ^ Singing primates are distributed in Southeast Asia (e.g., gibbons, tarsiers, and the Mentawai langur), Madagascar (e.g., indri and Milne Edwards’ sportive lemurs), and South America (e.g., titi monkeys).
- ^ A series of notes of different types, uttered following a hierarchical structure, and characterized by a frequency variation.
- ^ Where F1 and M1 stand for female and male syllable types, respectively.
- ^ Where “i” stands for the introductory notes, with n > 1.
- ^ Coordinated song uttered by more than two individuals within a family group.
References
Adret, P. (2004). In search of the song template. N. Y. Acad. Sci. 1016, 303–324. doi: 10.1196/annals.1298.005
Adret, P., Dingess, K. A., Caselli, C. B., Vermeer, J., Martínez, J., Luna Amancio, J. C., et al. (2018a). Duetting patterns of titi monkeys (Primates: Callicebinae) and relationships with phylogeny. Animals 8, 178–211. doi: 10.3390/ani8100178
Adret, P., Cochran, J. S., and Suarez Rado, M. (2018b). Airborne vs. radio-transmitted vocalizations in two primates: a technical report. Bioacoustics 27, 273–294. doi: 10.1080/09524622.2017.1335617
Amador, A., Trevisan, M. A., and Mindlin, G. B. (2005). Simple neural substrate predicts complex rhythmic structure in duetting birds. Phys. Rev. E 72:031905. doi: 10.1103/PhysRevE.72.031905
Badraun, J. C., Mootnick, A. R., Deaner, R. O., Agoramoorthy, G., and McNeese, K. M. (1998). Hand modulation of vocalization in Siamangs, Hylobates syndactylus. lnt. Zoo Yb 36, 84–89. doi: 10.1111/j.1748-1090.1998.tb02888.x
Bales, K. L., Arias, del Razoa, R., Conklina, Q. A., Hartman, S., Mayera, H. S., et al. (2017). Titi monkeys as a novel non-human primate model for the neurobiology of pair bonding. Yale J. Biol. Med. 90, 373–387.
Barelli, C., Mundry, R., Heistermann, M., and Hammerschmidt, K. (2013). Cues to androgens and quality in male gibbon songs. PLoS One 8:e82748. doi: 10.1371/journal.pone.0082748
Benedict, L., and McEntee, J. P. (2009). Context, structural variability and distinctiveness of California towhee (Pipilo crissalis) vocal duets. Ethology 115, 77–86. doi: 10.1111/j.1439-0310.2008.01583.x
Benichov, J. I., and Vallentin, D. (2020). Inhibition within a premotor circuit controls the timing of vocal turn-taking in zebra finches. Nat. Commun. 11:221. doi: 10.1038/s41467-019-13938-0
Brenowitz, E. A., and Arnold, A. (1986). Interspecific comparisons of the size of neural song control regions and song complexity in duetting birds: evolutionary implications. J. Neurosci. 6, 2875–2879. doi: 10.1523/JNEUROSCI.06-10-02875.1986
Brockelman, W. Y., and Schilling, D. (1984). Inheritance of stereotyped gibbon calls. Nature 312:634. doi: 10.1038/312634a0
Burns, B. L., and Judge, D. S. (2016). The varied path to adulthood: plasticity in developmental timing in hylobatids. Am. J. Primatol. 78, 610–625. doi: 10.1002/ajp.22523
Caselli, C. B., Mennill, D. J., Bicca-Marques, J. C., and Setz, E. Z. F. (2014). Vocal behavior of black-fronted titi monkeys (Callicebus nigrifrons): Acoustic properties and behavioral contexts of loud calls. Am. J. Primatol. 76, 788–800. doi: 10.1002/ajp.22270
Chen, H.-C., Kamolnorranath, S., and Kaplan, G. (2008). Female crested gibbons (genus Nomascus) sing male song. Vietnamese J. Primatol. 2, 47–53.
Cheyne, S., Chivers, D. J., and Sugardjito, J. (2007). Covariation in the great calls of rehabilitant and wild gibbons (Hylobates albibarbis). Raffles Bull. Zool. 55, 201–207.
Chivers, D. J., and MacKinnon, J. P. (1977). On the behavior of siamang after playback of their calls. Primates 18, 943–948. doi: 10.1007/BF02382944
Clink, D. J., Lau, A. R., and Bales, K. L. (2019). Age-related changes and vocal convergence in titi monkey duet pulses. Behaviour 156, 1–24. doi: 10.1163/1568539X-00003575
Clink, D. J., Lau, A. R., Kanthaswamy, S., Johnson, L. M., and Bales, K. L. (2022). Moderate evidence for heritability in the duet contributions of a South American primate. J. Evol. Biol. 35, 51–63. doi: 10.1111/jeb.13962
Clink, D. J., Tasirin, J. S., and Klinck, H. (2020). Vocal individuality and rhythm in male and female duet contributions of a nonhuman primate. Curr. Zool. 66, 173–186. doi: 10.1093/cz/zoz035
Coleman, M. J., Day, N. F., Rivera-Parrac, P., and Fortune, E. S. (2021). Neurophysiological coordination of duet singing. PNAS 118:e2018188118. doi: 10.1073/pnas.2018188118
Dahlin, C. R., and Benedict, L. (2014). Angry birds need not apply: a perspective on the flexible form and multifunctionality of avian vocal duets. Ethology 120, 1–10. doi: 10.1111/eth.12182
De Gregorio, C., Daria Valente, Raimondi, T., Torti, V., Miaretsoa, L., Friard, O., et al. (2021a). Categorical rhythms in a singing primate. Curr. Biol. 31, R1379–R1380. doi: 10.1016/j.cub.2021.09.032
De Gregorio, C., Carugati, F., Estienne, V., Valente, D., Raimondi, T., Torti, V., et al. (2021b). Born to sing! Song development in a singing primate. Curr. Zool. 67, 585–596. doi: 10.1093/cz/zoab018
De Gregorio, C., Carugati, F., Valente, D., Raimondi, T., Torti, V., Miaretsoa, L., et al. (2022). Notes on a tree: reframing the relevance of primate choruses, duets, and solo songs. Ethol. Ecol. Evol. doi: 10.1080/03949370.2021.2015451
De Gregorio, C., Zanoli, A., Valente, D., Torti, V., Bonadonna, G., Randrianarison, R. M., et al. (2019). Female indris determine the rhythmic structure of the song and sustain a higher cost when the chorus size increases. Curr. Zool. 65, 89–97. doi: 10.1093/cz/zoy058
de Reus, K., Soma, M., Anichini, M., Gamba, M., de Heer Kloots, M., Lense, M., et al. (2021). Rhythm in dyadic interactions. Phil. Trans. R. Soc. B 376:20200337. doi: 10.1098/rstb.2020.0337
Deng, C., Kaplan, G., and Rogers, L. J. (2001). Similarity of the song nuclei of male and female Australian magpies (Gymnorhina tibicen). Behav. Brain Res. 123, 89–102. doi: 10.1016/s0166-4328(01)00200-5
Deputte, B. (1982). “Duetting in male and female songs of the white-cheeked gibbon (Hylobates concolor leucogenys),” in Primate Communication, eds C. T. Snowdon, C. H. Brown, and M. R. Petersen (Cambridge: Cambridge University Press), 67–93.
Dingemanse, M., Roberts, S. G., Baranova, J., Blythe, J., Drew, P., Floyd, S., et al. (2015). Universal principles in the repair of communication problems. PLoS One 10:e0136100. doi: 10.1371/journal.pone.0136100
Dooley, H., and Judge, D. (2007). Vocal responses of captive gibbon groups to a mate change in a pair of white-cheeked gibbons (Nomascus leucogenys). Folia Primatol. 78, 228–239. doi: 10.1159/000102318
Duarte, M., Kaizer, M., Young, R., Rodrigues, M., and Sousa-Lima, R. (2017). Mining noise affects loud call structures and emission patterns of wild black-fronted titi monkeys. Primates 59, 89–97. doi: 10.1007/s10329-017-0629-624
Elhilali, M. (2017). “Modelling the cocktail party problem,” in The Auditory System at the Cocktail Party, eds J. C. Middlebrooks, J. Z. Simon, A. N. Popper, and R. R. Fay (Berlin: Springer Handbook of Auditory Research), doi: 10.1007/978-3-319-51662-2_5
Elie, J. E., Hoffmann, S., Dunning, J. L., Coleman, M. J., Fortune, E. S., and Prather, J. F. (2019). From perception to action: the role of auditory input in shaping vocal communication and social behaviors in birds. Brain Behav. Evol. 94, 51–60. doi: 10.1159/000504380
Elowson, A. M., and Snowdon, C. T. (1994). Pygmy marmosets, Cebuella pygmaea, modify vocal structure in response to changed social environment. Anim. Behav. 47, 1267–1277. doi: 10.1006/anbe.1994.1175
Farabaugh, S. M. (1982). “The ecological and social significance of duetting,” in Acoustic Communication in Birds, eds D. E. Kroodsma and E. H. Miller (New York, NY: Academic press), 85–124.
Fitch, W. T., de Boer, B., Mathur, N., and Ghazanfar, A. A. (2016). Monkey vocal tracts are speech-ready. Sci. Adv. 2:e1600723. doi: 10.1126/sciadv.1600723
Fortune, E. S., Rodríguez, C., Li, D., Ball, G. F., and Coleman, M. J. (2011). Neural Mechanisms for the coordination of duet singing in wrens. Science 334, 666–670. doi: 10.1126/science.1209867
Gamba, M., Favaro, L., Araldi, A., Matteucci, V., Giacoma, C., and Friard, O. (2017). Modeling individual vocal differences in group-living lemurs using vocal tract morphology. Curr. Zool. 63, 467–475. doi: 10.1093/cz/zox023
Gamba, M., Favaro, L., Torti, V., Sorrentino, V., and Giacoma, C. (2011). Vocal tract flexibility and variation in the vocal output in wild indris. Bioacoustics 20, 251–265. doi: 10.1080/09524622.2011.9753649
Gamba, M., Torti, V., Estienne, V., Randrianarison, R. M., Valente, D., Rovara, P., et al. (2016). The Indris have got rhythm! timing and pitch variation of a primate song examined between sexes and age classes. Front. Neurosci. 10:249. doi: 10.3389/fnins.2016.00249
Geissmann, T. (1983). Female capped gibbon (Hylobates pileatus Gray 1891) sings male song. J. Human Evol. 12, 667–671. doi: 10.1016/S0047-2484(83)80006-2
Geissmann, T. (1986). Mate change enhances duetting activity in the Siamang gibbon (Hylobates syndactylus). Behaviour 96, 17–27. doi: 10.1163/156853986x00199
Geissmann, T. (1999). Duet songs of the siamang, Hylobates syndactylus: ii. testing the pair-bonding hypothesis during a partner exchange. Behaviour 136, 1005–1039. doi: 10.1163/156853999501694
Geissmann, T. (2002). Duet-splitting and the evolution of gibbon songs. Biol. Rev. 77, 57–76. doi: 10.1017/s1464793101005826
Geissmann, T. (2009). Door slamming: tool-use by a captive white-handed gibbon (Hylobates lar). Gibbon J. 5, 53–60. doi: 10.5167/UZH-20113
Hage, S. R., and Nieder, A. (2013). Single neurons in monkey prefrontal cortex encode volitional initiation of vocalizations. Nat. Com. 4:2409. doi: 10.1038/ncomms3409
Haimoff, E. H. (1981). Video analysis of siamang (Hylobates syndactylus) songs. Behaviour 76, 128–151. doi: 10.2307/4534093
Haimoff, E. H. (1988). The organization of repair in the songs of gibbons. Semiotica 68, 89–120. doi: 10.1515/semi.1988.68.1-2.89
Hall, M. L. (2009). A review of vocal duetting in birds. Adv. Stud. Behav. 40, 67–121. doi: 10.1016/S0065-3454(09)40003-2
Hammerschmidt, K., and Fischer, J. (2008). “Constraints in primate vocal production,” in Evolution of Communicative Flexibility: Complexity, Creativity, and Adaptability, eds D. K. Oller and U. Griebel (Cambridge, MA: MIT Press), 93–120. doi: 10.7551/mitpress/9780262151214.003.0005
Haraway, M. M., and Maples, E. G. (1998). Flexibility in the species-typical songs of gibbons. Primates 39, 1–12. doi: 10.1007/BF02557739
Haraway, M. M., Maples, E. G., and Tolson, S. (1981). Taped vocalization as a reinforcer of vocal behavior in a Siamang gibbon (Symphalangus syndactylus). Psychol. Rep. 49, 995–999. doi: 10.2466/pr0.1981.49.3.995
Hasson, U., Ghazanfar, A. A., Galantucci, B., Garrod, S., and Keysers, C. (2012). Brain-to-Brain coupling: a mechanism for creating and sharing a social world. Trends Cogn. Sci. 16, 114–121. doi: 10.1016/j.tics.2011.12.007
Hoffmann, S., Trost, L., Voigt, C., Leitner, S., Lemazina, A., Sagunsky, H., et al. (2019). Duets recorded in the wild reveal that interindividually coordinated motor control enables cooperative behavior. Nat. Com. 10:2577. doi: 10.1038/s41467-019-10593-3
Hradec, M., Bolechová, P., and Svobodova, I. (2016). Production of a female-specific great call in an immature male gibbon, the Nomascus genus. Primates 57, 445–448. doi: 10.1007/s10329-016-0569-564
Hradec, M., Illmann, G., Bartoš, L., and Bolechová, P. (2021). The transition from the female–like great calls to male calls during ontogeny in southern yellow–cheeked gibbon males (Nomascus gabriellae). Sci. Rep. 11:22040. doi: 10.1038/s41598-021-01648-x
Hradec, M., Linhart, P., Bartoš, L., and Bolechová, P. (2017). The traits of the great calls in the juvenile and adolescent gibbon males Nomascus gabriellae. PLoS One 12:e0173959. doi: 10.1371/journal.pone.0173959
Janik, V. M., and Knörnschild, M. (2021). Vocal production learning in mammals revisited. Phil. Trans. R. Soc. B 376:20200244. doi: 10.1098/rstb.2020.0244
Janik, V. M., and Slater, P. J. B. (2000). The different roles of social learning in communication. Anim. Behav. 60, 1–11. doi: 10.1006/anbe.2000.1410
Kinzey, W. G., Rosenberger, A. L., Heisler, P. S., Prowse, D. L., and Trilling, J. S. (1977). A preliminary field investigation of the Yellow handed titi monkey, Callicebus torquatus torquatus, in Northern Peru. Primates 18, 159–181. doi: 10.1007/bf02382957
Koda, H. (2016). “Gibbon songs: understanding the evolution and development of this unique form of vocal communication,” in Evolution of Gibbons and Siamang. Developments in Primatology: Progress and Prospects, eds U. Reichard, H. Hirai, and C. Barelli (New York: Springer), 347–357. doi: 10.1007/978-1-4939-5614-2_15
Koda, H., Lemasson, A., Oyakawa, C., Rizaldi, Pamungkas, J., and Masataka, N. (2013). Possible role of mother-daughter vocal interactions on the development of species-specific song in gibbons. PLoS One 8:e71432. doi: 10.1371/journal.pone.0071432
Koda, H., Masataka, N., Kato, A., and Oyakawa, C. (2007). Experimental evidence for the volitional control of vocal production in an immature gibbon. Behaviour 144, 681–692. doi: 10.1163/156853907781347817
Koda, H., Nishimura, T., Tokuda, I. T., Oyakawa, C., Nihonmatsu, T., and Masataka, N. (2012). Soprano singing in gibbons. Am. J. Phys. Anthropol. 149, 347–355. doi: 10.1002/ajpa.22124
Koda, H., Oyakawa, C., Kato, A., Shimizu, D., Rizaldi, Koyama, Y., et al. (2014). Immature male gibbons produce female-specific songs. Primates 55, 13–17. doi: 10.1007/s10329-013-0390-2
Kovach, C., Hall, M. L., Verhencamp, S. L., and Mennill, D. J. (2014). Timing isn’t everything: responses of tropical wrens to coordinated duets, uncoordinated duets and alternating solos. Anim. Behav. 95, 101–109. doi: 10.1016/j.anbehav.2014.06.012
Laje, R., and Mindlin, G. B. (2003). Highly structured duets in the song of the South American Hornero. Phys. Rev. Lett. 91:258104. doi: 10.1103/PhysRevLett.91.258104
Lameira, A. R. (2017). Bidding evidence for primate vocal learning and the cultural substrate for speech evolution. Neurosci. Biobehav. Rev. 83, 429–439. doi: 10.1016/j.neubiorev.2017.09.021
Lemazina, A., Trost, L., Gahr, M., and Hoffmann, S. (2021). The multifaceted vocal duets of white-browed sparrow weavers are based on complex duetting rules. J. Avian Biol. 52:e02703. doi: 10.1111/jav.02703
Levin, R. N. (1996). Song behaviour and reproductive strategies in a duetting wren, Thryothorus nigricapillus: I. removal experiments. Anim. Behav. 52, 1093–1106.
Levinson, S. C. (2016). Turn-taking in human communication-origins and implications for language processing. Trends Cogn. Sci. 20, 6–14. doi: 10.1016/j.tics.2015.10.010
Lobato, M., Vellema, M., Gahr, C., Leitão, A., de Lima, S. M. A., Geberzahn, N., et al. (2015). Mismatch in sexual dimorphism of developing song and song control system in blue-capped cordon-bleus, a songbird species with singing females and males. Front. Ecol. Evol. 3:117. doi: 10.3389/fevo.2015.00117
Logue, D. M. (2006). The duet code of the female black-bellied wren. Condor 108, 326–335. doi: 10.1093/condor/108.2.326
Logue, D. M., and Krupp, D. B. (2016). Duetting as a collective behavior. Front. Ecol. Evol. 4:7. doi: 10.3389/fevo.2016.00007
Logue, D. M., Chalmers, C., and Gowland, H. (2008). The behavioural mechanisms underlying temporal coordination in black-bellied wren duets. Anim. Behav. 75, 1803–1808. doi: 10.1016/j.anbehav.2007.10.036
Mann, N. I., Dingess, K. A., and Slater, P. J. B. (2006). Antiphonal four-part synchronized chorusing in a neotropical wren. Biol. Lett. 2, 1–4. doi: 10.1098/rsbl.2005.0373
Mann, N. I., Dingess, K. A., Barker, F. K., Graves, J. A., and Slater, P. J. B. (2009). A comparative study of song form and duetting in neotropical Thryothorus wrens. Behaviour 146, 1–43. doi: 10.1163/156853908X390913
Mann, N. I., Marshall-Ball, L., and Slater, P. J. B. (2003). The complex song duet of the plain wren. Condor 105, 672–682. doi: 10.1650/7208
Maples, E. G. Jr., and Haraway, M. M. (1982). Taped vocalization as a reinforcer of vocal behavior in a female agile gibbon (Hylobates agilis). Psychol. Rep. 493, 995–999. doi: 10.2466/pr0.1982.51.1.95
Maples, E. G. Jr., Haraway, M. M., and Hutto, C. W. (1989). Development of coordinated singing in a newly formed siamang pair (Hylobates syndactylus). Zoo Biol. 8, 367–378. doi: 10.1002/zoo.1430080407
Maples, E. G., Haraway, M. M., and Collie, L. (1988). Interactive singing of a male Mueller’s gibbon with a simulated neighbor. Zoo Biol. 7, 115–122. doi: 10.1002/zoo.1430070204
Marler, P., and Tenaza (1977). “Signaling behavior of apes with special reference to vocalization,” in How Animals Communicate, ed. T. A. Sebeok (Bloomington: Indiana University Press).
Marshall, J., and Marshall, E. (1976). Gibbons and their territorial songs. Science 193, 235–237. doi: 10.1126/science.193.4249.235
Méndez-Cárdenas, M. G., and Zimmermann, E. (2009). Duetting—a mechanism to strengthen pair bonds in a dispersed pair-living primate (Lepilemur edwardsi)? Am. J. Phys. Anthropol. 139, 523–532. doi: 10.1002/ajpa.21017
Mennill, D. J., and Vehrencamp, S. L. (2005). Sex differences in singing and duetting behavior of neotropical rufous-and-white wrens (Thryothorus rufalbus). Auk 122, 175–186. doi: 10.1093/auk/122.1.175
Merker, B., and Cox, C. (1999). Development of the female great call in Hylobates gabriellae: a case study. Folia Primatol. 70, 97–106. doi: 10.1159/000021680
Mitani, J. C. (1985). Gibbon song duets and intergroup spacing. Behaviour 92, 59–96. doi: 10.1163/156853985X00389
Mitani, J. C. (1988). Male gibbon (Hylobates agilis) singing behavior: natural history, song variations, and function. Ethology 79, 177–194. doi: 10.1111/j.1439-0310.1988.tb00710.x
Morino, L., Pasquaretta, C., Sueur, C., and MacIntosh, A. J. J. (2021). Communication network reflects social instability in a wild siamang (Symphalangus syndactylus) population. Int. J. Primatol. 42, 618–639. doi: 10.1007/s10764-021-00227-1
Mott, F. W., Schuster, E., and Sherrington, C. S. (1911). Motor localisation in the brain of the gibbon, correlated with a histological examination. Proc. Roy. Soc. B London 84, 67–74. doi: 10.1098/rspb.1911.0047
Müller, A. E., and Anzenberger, G. (2002). Duetting in the titi monkey Callicebus cupreus: structure, pair specificity and development of duets. Folia Primatol. 73, 104–115. doi: 10.1159/000064788
Newman, J. D. (2014). Vocal coordination and vocal imitation: a role for mirror neurons? Behav. Brain Sci. 37, 211–212. doi: 10.1017/S0140525X13002410
Nieder, A., and Mooney, R. (2019). The neurobiology of innate, volitional and learned vocalizations in mammals and birds. Phil. Trans. R. Soc. B 375:20190054. doi: 10.1098/rstb.2019.0054
Nietsch, A. (1999). Duet vocalizations among different populations of Sulawesi tarsiers. Int. J. Primatol. 20, 567–583. doi: 10.1023/A:1020342807709
Nottebohm, F., and Arnold, A. P. (1976). Sexual dimorphism in vocal control areas of the songbird brain. Science 194, 211–213. doi: 10.1126/science.959852
Okobi, D. E. Jr., Banerjee, A., Matheson, A. M. M., Phelps, S. M., and Long, M. A. (2019). Motor cortical control of vocal interaction in neotropical singing mice. Science 363, 983–988. doi: 10.1126/science.aau9480
Pollock, J. I. (1986). The song of the indris (Indri indri; Primates: Lemuroidea): natural history, form, and function. Int. J. Primatol. 7, 225–264. doi: 10.1007/BF02736391
Potretzke, S., and Ryabinin, A. E. (2019). The prairie vole model of pair-bonding and its sensitivity to addictive substances. Front. Psychol. 10:2477. doi: 10.3389/fpsyg.2019.02477
Puts, D. A., Hill, A. K., Bailey, D. H., Walker, R. S., Rendall, D., Wheatley, J. R., et al. (2016). Sexual selection on male vocal fundamental frequency in humans and other anthropoids. Proc. R. Soc. B 283:20152830. doi: 10.1098/rspb.2015.2830
Raemaekers, J. J., and Raemaekers, P. M. (1985). Long-range vocal interactions between groups of gibbons (Hylobates lar). Behaviour 95, 26–44. doi: 10.1163/156853985X00037
Raemaekers, J. J., Raemaekers, P. M., and Haimoff, E. H. (1984). Loud calls of the gibbon repertoire, (Hylobates lar): organization and context. Behaviour 91, 146–189. doi: 10.1163/156853984X00263
Ravignani, A., Bowling, D. L., and Fitch, W. T. (2014). Chorusing, synchrony, and the evolutionary functions of rhythm. Front. Psychol. 5:1118. doi: 10.3389/fpsyg.2014.01118
Reichard, U. H. (2003). “Social monogamy in gibbons: the male perspective,” in Monogamy. Mating Strategies and Partnerships in Birds, Humans and Other Mammals, eds U. H. Reichard and C. Boesch (Cambridge: Cambridge University), 190–213. doi: 10.1017/cbo9781139087247.013
Rek, P., and Magrath, R. D. (2016). Multimodal duetting in magpie-larks: how do vocal and visual components contribute to a cooperative signal’s function? Anim. Behav. 117, 35–42. doi: 10.1016/j.anbehav.2016.04.024
Rek, P., and Magrath, R. D. (2020). Visual displays enhance vocal duet production and the perception of coordination despite spatial separation of partners. Anim. Behav. 168, 231–241. doi: 10.1016/j.anbehav.2020.08.002
Rivera-Cáceres, K. D. (2015). Plain wrens Cantorchilus modestus zeledoni adjust their singing tempo based on self and partner’s cues to perform precisely coordinated duets. J. Avian Biol. 46, 361–368. doi: 10.1111/jav.00575
Rivera-Cáceres, K. D., and Templeton, C. N. (2019). A duetting perspective on avian song learning. Behav. Processes 163, 71–80.
Rivera-Cáceres, K. D., Quirós-Guerrero, E., Araya-Salas, M., and Searcy, W. A. (2016). Neotropical wrens learn new duet rules as adults. Proc. R. Soc. B 283:20161819. doi: 10.1098/rspb.2016.1819
Rivera-Cáceres, K. D., Quirós-Guerrero, E., Araya-Salas, M., Templeton, C. N., and Searcy, W. A. (2018). Early development of vocal interaction rules in a duetting songbird. R. Soc. Open Sci. 5:171791. doi: 10.1098/rsos.171791
Robinson, J. G. (1979). An analysis of the organization of vocal communication in the titi monkey, Callicebus moloch. Z. Tierpsychol. 49, 381–403. doi: 10.1111/j.1439-0310.1979.tb00300.x
Rogers, A. C. (2005). Male and female song structure and singing behavior in the duetting eastern whipbird, Psophodes olivaceus. Aust. J. Zool. 53, 157–166. doi: 10.1071/ZO04083
Sainburg, T., Thielk, M., and Gentner, T. Q. (2020). Finding, visualizing, and quantifying latent structure across diverse animal vocal repertoires. PLoS Comput. Biol. 16:e1008228. doi: 10.1371/journal.pcbi.1008228
Schegloff, E., Jefferson, G., and Sacks, H. (1977). The preference for self-correction in the organization of repair in conversation. Language 53, 362–382. doi: 10.2307/413107
Shekelle, M., Groves, C. P., Maryanto, I., Mittermeier, R. A., Salim, A., and Springer, M. S. (2019). A new tarsier species from the Togean islands of central Sulawesi, Indonesia, with references to Wallacea and conservation on Sulawesi. Primate Conserv. 33, 65–73.
Simonyan, K. (2014). The laryngeal motor cortex: its organization and connectivity. Curr. Opin. Neurobiol. 28, 15–21. doi: 10.1016/j.conb.2014.05.006
Snowdon, C. T. (2009). Plasticity of communication in nonhuman primates. Adv. Stu. Behav. 40, 239–276. doi: 10.1016/S0065-3454(09)40007-X
Snowdon, C. T. (2017a). Learning from monkey “talk”. Science 355, 1120–1122. doi: 10.1126/science.aam7443
Snowdon, C. T. (2017b). “Vocal communication in family living and pair-bonded primates,” in Primate Hearing and Communication, eds R. M. Quam, M. A. Ramsier, R. R. Fay, and A. N. Popper (Berlin: Springer), 141–174. doi: 10.1007/978-3-319-59478-1_6
Snowdon, C. T. (2018). Cognitive components of vocal communication: a case study. Animals 8:126. doi: 10.3390/ani8070126
Snowdon, C. T., and Elowson, A. M. (1999). Pygmy marmosets modify vocal structure when paired. Ethology 105, 893–908. doi: 10.1046/j.1439-0310.1999.00483.x
Takahashi, D. Y., El Hady, A., Zhang, Y. S., Liao, D. A., Montaldo, G., Urban, A., et al. (2021). Social-vocal brain networks in a non-human primate. bioRxiv [Preprint] doi: 10.1101/2021.12.01.470701
Templeton, C. N., Mann, N. I., Ríos-Chelén, A. A., Quiros-Guerrero, E., Macías Garcia, C., and Slater, P. J. B. (2013). An experimental study of duet integration in the happy wren, Pheugopedius felix. Anim. Behav. 86, 821–827. doi: 10.1016/j.anbehav.2013.07.022
Tenaza, R. R. (1976). Songs, choruses, and countersinging of Kloss’ gibbons (Hylobates klossii) in Siberut Island, Indonesia. Z. Tierpsychol. 40, 37–52. doi: 10.1111/j.1439-0310.1976.tb00924.x
Tenaza, R. R. (1985). Songs of hybrid gibbons (Hylobates lar x N. muelleri). Am. J. Primatol. 8, 249–253. doi: 10.1002/ajp.1350080307
Terleph, T. A., Malaivijitnond, S., and Reichard, U. H. (2015). Lar gibbon (Hylobates lar) great call reveals individual caller identity. Am. J. Primatol. 77, 811–821. doi: 10.1002/ajp.22406
Terleph, T. A., Malaivijitnond, S., and Reichard, U. H. (2016). Age related decline in female lar gibbon great call performance suggests that call features correlate with physical condition. BMC Evol. Biol. 16:4. doi: 10.1186/s12862-015-0578-8.
Terleph, T. A., Malaivijitnond, S., and Reichard, U. H. (2018a). Male white-handed gibbons flexibly time duet contributions. Behav. Ecol. Sociobiol. 72:16. doi: 10.1007/s00265-017-2432-z
Terleph, T. A., Malaivijitnond, S., and Reichard, U. H. (2018b). An analysis of white-handed gibbon male song reveals speech-like phrases. Am. J. Phys. Anthropol. 2018, 1–12. doi: 10.1002/ajpa.23451
Tilson, R. L., and Tenaza, R. R. (1976). Monogamy and duetting in an Old World monkey. Nature 263, 320–321. doi: 10.1038/263320a0
Tobias, J. A., and Seddon, N. (2009). Signal jamming mediates sexual conflict in a duetting bird. Curr. Biol. 19, 577–582. doi: 10.1016/j.cub.2009.02.036
Tobias, J. A., Sheard, C., Seddon, N., Meade, A., Cotton, A. J., and Nakagawa, S. (2016). Territoriality, social bonds, and the evolution of communal signaling in birds. Front. Ecol. Evol. 4:74. doi: 10.3389/fevo.2016.00074
Torti, V., Bonadonna, G., De Gregorio, C., Valente, D., Randrianarison, R. M., Friard, O., et al. (2017). An intra-population analysis of the indris’ song dissimilarity in the light of genetic distance. Sci. Rep. 7:10140. doi: 10.1038/s41598-017-10656-9
Torti, V., Gamba, M., Rabemananjara, Z. H., and Giacoma, C. (2013). The songs of the indris (Mammalia: Primates: Indridae): contextual variation in the long-distance calls of a lemur. Ital. J. Zool. 80, 596–607. doi: 10.1080/11250003.2013.845261
Vernes, S. C., Kriengwatana, B. P., Beeck, V. C., Fischer, J., Tyack, P. L., ten Cate, C., et al. (2021). The multi-dimensional nature of vocal learning. Phil. Trans. R. Soc. B 376:20200236. doi: 10.1098/rstb.2020.0236
Voigt, C., Leitner, S., and Gahr, M. (2006). Repertoire and structure of duet and solo songs in cooperatively breeding white-browed sparrow weavers. Behaviour 143, 159–182. doi: 10.1163/156853906775900739
Wickler, W. (1980). Vocal dueting and the pair bond. I. Coyness and partner commitment: a hypothesis. Z. Tierpsychol. 52, 201–209.
Wickler, W., and Seibt, U. (1980). Vocal dueting and the pair bond. II. Unisono dueting in the African forest weaver, Symplectes bicolor. Z. Tierpsychol. 52, 217–226. doi: 10.1111/j.1439-0310.1980.tb00713.x
Wilson. (1975). Discriminative conditioning of vocalizations in Lemur catta. Anim. Behav. 23, 432–436. doi: 10.1016/0003-3472(75)90091-3
Zhang, Y. S., Takahashi, D. Y., Liao, D. A., Ghazanfar, A. A., and Elemans, C. P. H. (2020). Vocal state change through laryngeal development. Nat. Comm. 10:4592. doi: 10.1038/s41467-019-12588-
Keywords: antiphonal, brain-to-brain coupling, development, duet code, singing primates, songbirds, vocal flexibility
Citation: Adret P (2022) Developmental Plasticity in Primate Coordinated Song: Parallels and Divergences With Duetting Songbirds. Front. Ecol. Evol. 10:862196. doi: 10.3389/fevo.2022.862196
Received: 25 January 2022; Accepted: 28 February 2022;
Published: 25 March 2022.
Edited by:
Andrew James Jonathan MacIntosh, Kyoto University, JapanReviewed by:
Allison Lau, University of California, Davis, United StatesMarco Gamba, University of Turin, Italy
Copyright © 2022 Adret. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Patrice Adret, patrice.adret@gmail.com
 Patrice Adret
Patrice Adret