Temporal and Spatial Stability on the Population Structure of Consumed and Illegally Traded Big-Headed Amazon River Turtle in the Negro River Basin, Central Amazon, Brazil
- 1Núcleo de Altos Estudos Amazônicos-NAEA, Universidade Federal do Pará (UFPA), Belém, Brazil
- 2Network on Diversity, Conservation and Use of Wildlife in Amazonia (REDEFAUNA), Belém, Brazil
- 3Amazonas Federal Institute, Manaus, Brazil
- 4National Institute of Amazon Research (INPA), Manaus, Brazil
Freshwater turtles are a valuable food resource for riverine human communities and have been historically overharvested throughout all major tropical large river basins, with consequent gradual population decreases. Even species considered to be abundant are declining, and in many cases were brought to a condition of near extinction. The collection of adult females during breeding season on nesting beaches is considered a major factor in population decline and subsequent loss of food sources for humans. There is growing consensus that adult females constitute the category which turtle populations can least afford to lose. In the Negro River Basin, the podocnemidid big-headed Amazon River turtle, Peltocephalus dumerilianus, is heavily exploited for consumption and poached for illegal trade among riverine communities and cities. Between 1997 and 2002 and in 2019, we measured live turtles and carapaces of big-headed turtles in the city of Barcelos and its surroundings, and among the riverine families living in the Jaú National Park. We compared body sizes and sex ratios between areas, periods, and between consumed and traded individuals. We found no differences between areas, even those close to Barcelos and the ones belonging to remote areas where pressure levels are lower. The individuals consumed in Jaú National Park are larger than those poached for illegal trade in both areas. There was an increase in average size between 1997 and 2002. Sex ratio was slightly skewed toward males, which were larger, and did not differ between areas and periods. Results indicate stability on size of harvested populations, which may be supporting current extraction levels. Data suggest this could be related to the absence of adult female capture during nesting for this species. We recommend protection strategies for other Amazon Podocnemidid species that focus on the protection of nesting beaches and surrounding areas where adults occupy, specifically in areas under communal protection.
Introduction
The Podocnemidid Amazon river turtles have been used for food by Amazon people long before European’s arrival in South America (Carvajal, 1543; Prestes-Carneiro et al., 2016). Peltocephalus dumerilianus (Schweigger, 1812), known as the big-headed Amazon River Turtle (hereafter big-headed), is intensively exploited in the Negro River basin, being part of the illegal regional trade (Rebêlo and Lugli, 1996; Rebêlo and Pezzuti, 2000; Rebêlo et al., 2006; Pezzuti et al., 2010; Schneider et al., 2011). This species is the second largest podocnemid in the Amazon (Figure 1), smaller only to the giant Amazon river turtle (Podocnemis expansa), and can weigh up to 16 kg (Pritchard and Trebbau, 1984; De La Ossa and Vogt, 2011).
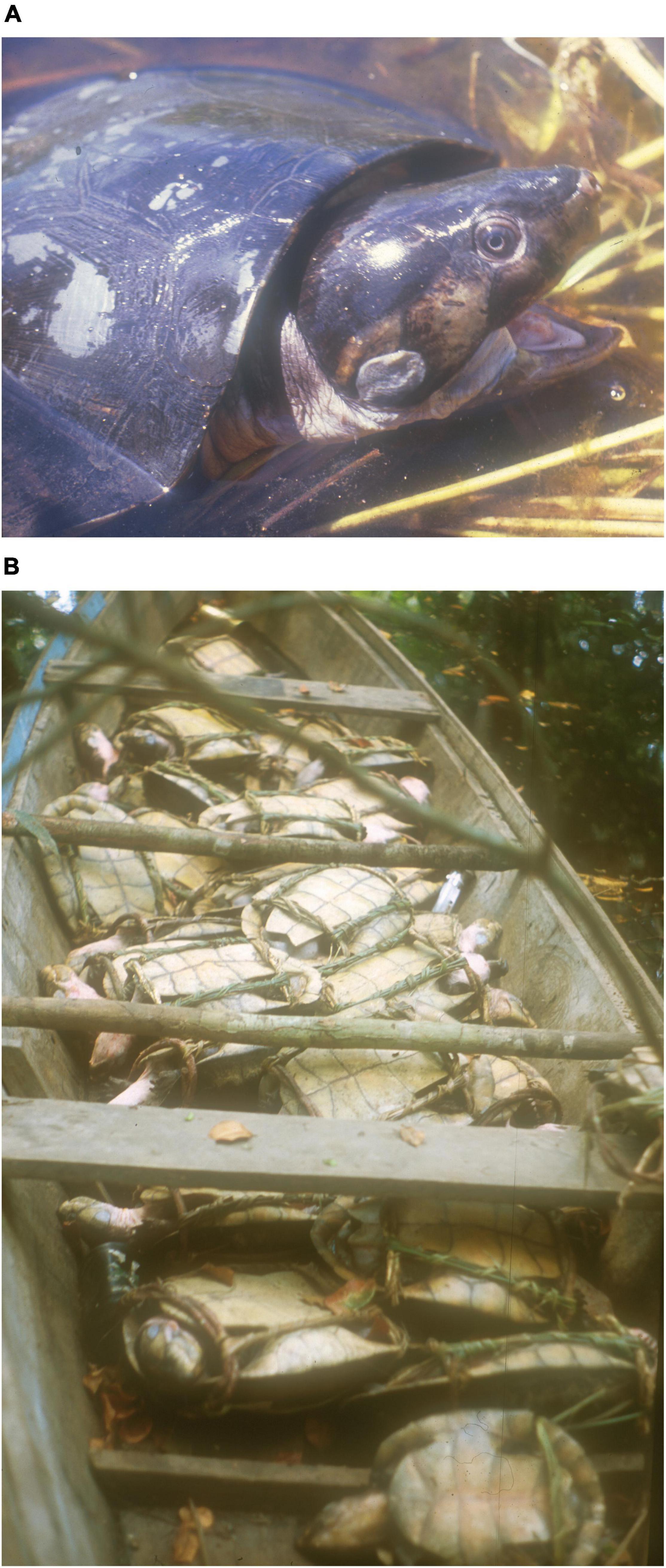
Figure 1. (A) Peltocephalus dumerilianus, the big-headed Amazon River Turtle; (B) a quantity of big-headed turtles caught in Jaú National Park and destined for the illegal trade.
The big-headed utilization of the Negro River is especially interesting due to its biological and socio-cultural characteristics. Unlike other Amazonian podocnemidids, the big-headed females nest inside the Igapó forest, in earth mounds created from fallen trees. Occasionally, they also nest on sandbanks, beaches, or ravines on the banks of water bodies (Vogt et al., 1994; Félix-Silva, 2004; Vogt, 2008). Thus, there is no capture of female big-headed during nesting (Pezzuti et al., 2004; Schneider et al., 2011), as occurs annually with the other podocnemidids (Fachín-Terán et al., 2003; Pezzuti et al., 2010; Pantoja-Lima et al., 2014). Generally, the nesting moment is the most vulnerable for chelonians and, for larger species, it represents a context in which adult females are particularly susceptible to their natural predators. Historically, the intense harvest of Amazonian podocnemidids nesting females lasted for almost 200 years, generating a large source of food, energy, and wealth for the Colonial Empire, the Brazilian Empire, and the United States of Brazil at the beginning of the Republic (Bates, 1864; Silva Coutinho, 1868; Gilmore, 1986). This likely caused the inevitable decline of the Giant Amazonian turtle populations, followed by the smaller species, until the collapse of chelonian harvesting as a relevant economic activity (Smith, 1974; Mittermeier, 1975; Johns, 1987; Pezzuti et al., 2004; Rebêlo et al., 2006).
Monitoring population structure is a crucial parameter in wildlife management (Caughley and Sinclair, 1994). The evaluation of size structure of big-headed populations through space represents an opportunity to assess the impact on Podocnemid without this key aspect of capturing of adult females during nesting. In the present study, we investigated the size distribution and sex ratio of big-headed Amazon River turtles caught for consumption and for sale in different areas along the Negro river and over two periods, with an interval of more than 20 years between samplings. Our main purpose was to detect possible variation on population size distribution and sex ratio of harvested turtles within two dimensions: over time and across geographic areas.
Materials and Methods
Study Area
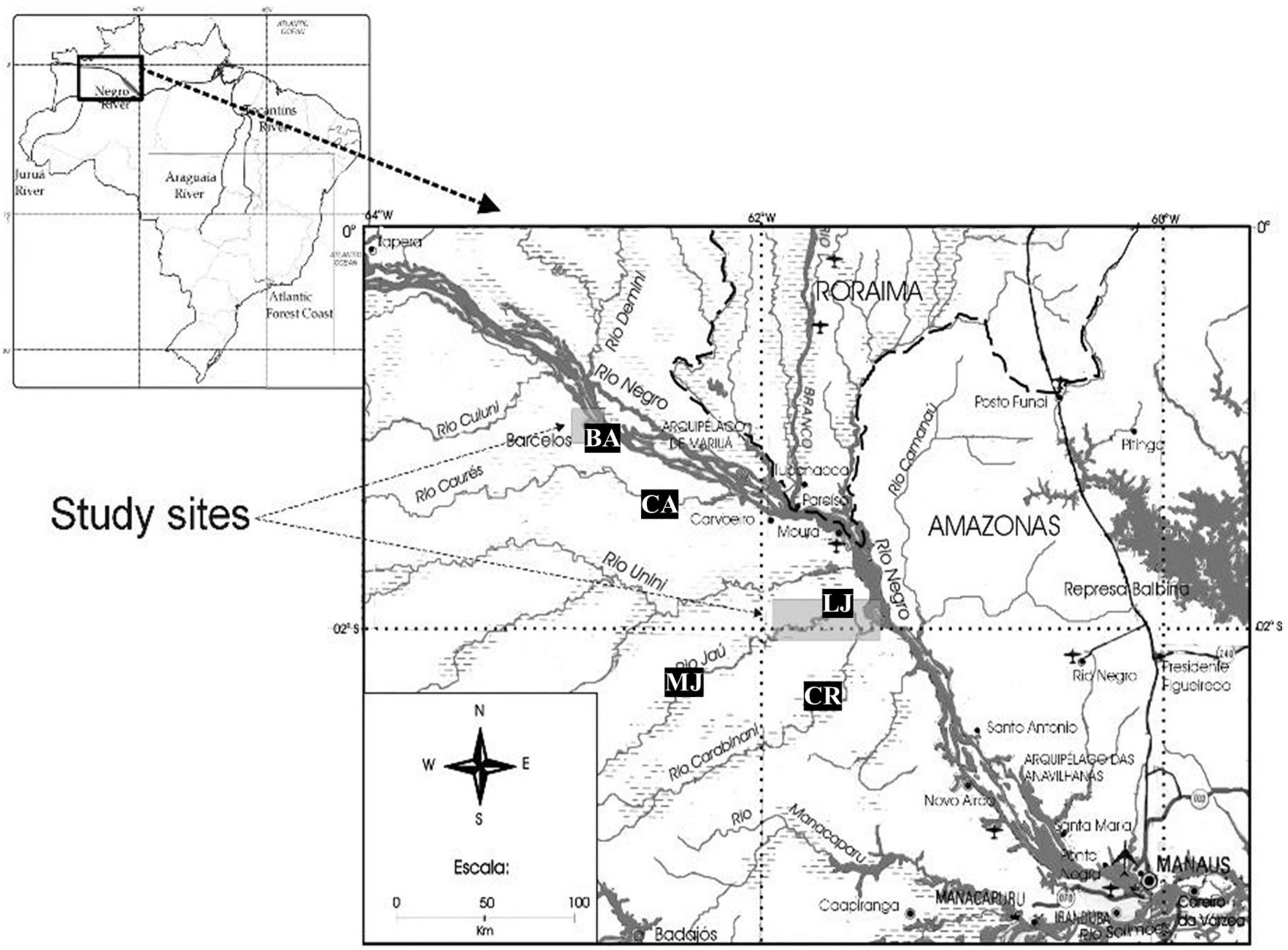
This study was carried out in two regions of the lower and middle Negro River in the Brazilian Amazon, in the city of Barcelos and in the National Park of Jaú (PNJ), in the state of Amazonas (Figure 2). Aside from the urban area of Barcelos, the area is characterized by well-preserved primary forests where basic extractive activities take place, such as gathering of non-timber forest products (particularly Brazil Nuts), subsistence hunting, and small-scale agriculture. The region has a large expanse of Igapó seasonally flooded forests that sustains a high diversity and abundance of aquatic organisms, where basic extractive activities take place, serving as the base of the regional diet (Da Silva and Begossi, 2009). The city of Barcelos, with nearly 25,000 inhabitants (IBGE, 2020), is located on the right bank of the Negro River, 405 km northwest from the city of Manaus, in the state of Amazonas. The main source of income for the local population of this city are extractive activities, especially ornamental fishing. The proximity to the Mariuá archipelago, the largest river archipelago in the world, gives this region a high biological diversity and abundance of aquatic resources (Machado, 2001; Latrubesse and Stevaux, 2015). The PNJ is located on the right bank of the lower Negro River and is equivalent to IUCN Category II Protected Area (Sistema Nacional de Unidades de Conservação [SNUC], 2000). The park includes an area of 2,272,000 ha that protects almost the entire basin of the Jaú River, a typical blackwater river whose main tributary is the Carabinani River. Both the Jaú and the Carabinani rivers are characterized by the presence of rapids that, in the dry season, separate these rivers into low (downstream of the waterfalls) and medium (upstream of the waterfalls) portions. The local inhabitants are descendants of rubber tappers and live on basic subsistence activities such as slash-and-burn agriculture, subsistence fishing and hunting, and collecting of Brazil nuts (Pezzuti et al., 2010).
Proceedings
Sampling was carried out between 1997 and 2002, and in 2019. During the first period, 18 trips were made to PNJ (June and November 1997, January, May, and October 1998, March 1999, February, April, July, and September 2000, February, June, August and November 2001, and February, June, August and December 2002) in order to conduct interviews with fishermen (Rebêlo and Pezzuti, 2000; Pezzuti et al., 2010) and participant observation (Rebêlo et al., 2006). Each fieldtrip lasted between 2–3 weeks. In November 2019, we returned to PNJ on a trip lasting 12 days. We were able to measure carapaces of live animals held captive in small pens called “currais,” and of animals eaten, whose shells were thrown along the edges of the residents’ yards. To have comparable datasets between the two periods, we visited the same communities from Lower and Middle Jaú and carefully searched for shells along the gardens and collective areas in the community to assure that most of the shells of consumed big-headeds, if not all, were measured. Thus, we were able to measure shells of individuals caught and eaten during the year of 2019. Therefore, it was possible to obtain an unprecedented series of animals measured, over more than 20 years, allowing for a robust temporal analysis of harvested big-headed individuals. Following the biometrics protocol widely used for turtles, we measured the straight carapace length and CRC (Pritchard and Trebbau, 1984; Vogt, 2008) of living animals and shells, with the aid of a large caliper (Hagloff, 1,000 m). When this tool was not available, the curved carapace length (CCC) was measured with a small measuring tape. From there, we used a Spearman Correlation to estimate the CRC (r = 0.97). Living males and females were distinguished following Rueda-Almonacid et al. (2007), with females presenting a wider opening of the cloacal scutes, and males with greater pre-cloacal tail length and thicker tail base (Rueda-Almonacid et al., 2007). Barcelos city and its surroundings were visited during 2000–2001 in the same months aforementioned, where we spent between 1 and 2 weeks carrying out the same procedures. Although prohibited, there was no enforcement to prevent turtle poaching in the city throughout the period considered, and animals are frequently encountered for sale. Big-headed turtles could be found on markets, fishermen houses, harbors, and small boats just after fishermen’s arrival at the city. Turtle carapaces were also found scattered on gardens, unoccupied grounds, areas surrounding harbors, and other city neighborhoods. In specific situations, after years of contact with the residents, we also had the opportunity to carry out biometrics of loads of animals destined for commercialization outside the PNJ, and of animals newly arrived for commercialization in Barcelos. We measured all live individuals and carapaces of eaten animals present in households’ respective gardens in the sites visited, except for Barcelos.
For the intended comparisons, the region was subdivided into five locations, two of which are located on the Negro River (Barcelos City and Caurés river) and three located within the PNJ (Carabinani river, lower Jaú river, and middle Jaú river). Below, we briefly describe the study area human population and a subjective classification of the river turtle harvesting pressure for each area. This was based on our observations and interaction with local dwellers (including poachers) and park staff, and considered the traveling distance, the presence of rapids making the journey more difficult and time consuming, and the overall perception on remoteness and harvesting intensity by local dwellers and poachers.
Middle Jaú—Inhabited by small and sparsely distributed riverside communities. Infrequently accessed by poachers, due to the rapids that make access difficult during most of the year. Low pressure.
Carabinani—Uninhabited but subject to harvest by poachers in unknown frequency. Low pressure.
Lower Jaú—Inhabited by small and sparsely distributed communities; more frequented by turtle poachers than Middle Jaú. Average pressure.
Caurés—Inhabited and frequently visited by poachers, mainly from Barcelos—average to high pressure.
Barcelos—Urban area and illegal market; receive turtles from surroundings—high pressure.
The big-headed is almost exclusively captured with a single technique, regionally called baliza. The technique consists of attaching bait made with pieces of fish (approximately 0.5–1 kg) to poles fixed to the bottom of flooded forests or close to the riverbanks. Then, the attracted turtles are harpooned at the carapace with minimum damage to the animal, since the harpoon used is small and has no barbs (Pezzuti et al., 2004). The few exceptions occur when animals are occasionally caught with other hunting techniques by residents of the PNJ. Thus, there was no methodological bias regarding differences in yield and selectivity of catches in size or sex.
To obtain a natural baseline of big-headeds for comparison, experimental turtle fisheries were conducted in April, July, and September 2000 and February 2001 in the Carabinani River region, the main tributary of the right bank of the Jaú River (Pezzuti, 2003). We chose Carabinani following local dwellers’ suggestion for an area with low harvesting pressure and greater animal abundance. Each of these turtle sampling trips lasted at least 20 days, in which three pairs formed by a biologist and senior fishermen made up a fishing unit. Fisheries were made using the baliza method and carried out for the entire day (around 10–12 h). The capture effort ranged from 54 to 58 fisheries per trip, totalizing 224 days of capture effort.
Analysis
We aimed to assess whether the size distribution and sex ratio of animals varied spatially and temporally. We hypothesized that these size distributions differ between the animals consumed, traded, and those from our experimental catches due to differences in hunting pressure and fishermen’s selectivity for larger animals for both consumption and trade. In addition, we expected that a depletion effect (Antunes et al., 2016; Tregidgo et al., 2017) from different pressure levels would lead to distinct size distributions and, thus, that the animals from Barcelos city and the closer Caurés river would be smaller and with a reduced proportion of adults. In contrast, turtles from Jaú and Carabinani, mainly those from middle Jaú, should be the larger. Similarly, we investigated to determine if sex ratios would be different between study sites or change over time considering that males grow larger in this species and are thus preferred (Pritchard and Trebbau, 1984; Pezzuti, 2003).
We performed a Permutational Multivariate Analysis of Variance (PERMANOVA) using Euclidean Distance for each treatment group using CRC as the response variable to compare size variations between years, sex, purpose (consumption, commercialization, experimental capture), seasons (rainy or dry), and localities (Barcelos, Caurés river, Carabinani river, lower Jaú river, middle Jaú river) (Clarke, 1993; Anderson, 2005). The PERMANOVA is a geometric partitioning of variation based on a chosen dissimilarity measure. This analysis allows heterogeneous dispersions among groups and unbalanced designs, avoiding the assumption of normality due to the distribution-free inferences acquired by the permutations (Anderson and Walsh, 2013; Anderson, 2014). The Euclidean Distance is commonly used to build a resemblance matrix with morphometric measurements (Velez-Zuazo et al., 2014; Miorando et al., 2015). For comparisons of sex ratio between the same parameters above, the Chi-square test of various proportions was also used. The analyzes were performed using the Bioestat software (Ayres et al., 2007) and R (R Development Core Team, 2010). For comparisons between years and periods we used just the individuals harvested within PNJ.
Results
We obtained a total of 993 carapaces measured from live or eaten individuals. Experimental catches were non-selective in size. Individuals caught ranged in size between 0.6 and 16 kg, which allowed for the intended comparisons between experimental catch size with the distribution of consumed and traded animals. The size distribution varied consistently between years (Table 1 and Figure 3), with emphasis on 2019 when we registered the largest individuals. We observed a trend of increase in body size over the 22 years (PERMANOVA, Pseudo-F = 14.13, p = 0.0001). The years 1997 and 1999 did not differ from the others, probably due to the small sample size (N = 4 and 14, respectively). The size of the animals collected in 2019 were larger than those from all other years, except for 1998, which also surpassed 2000, 2001, and 2002 (PERMANOVA pair-wise < 0.05, Supplementary Material 1). The animals from 2001 and 2002 were also larger than the ones from 2000.
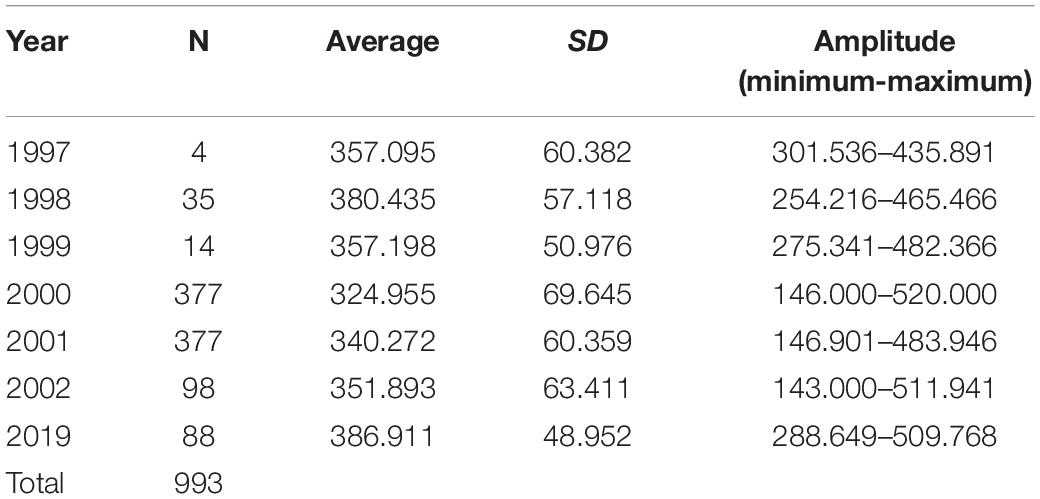
Table 1. Descriptive statistics of the distribution of measurements of the straight carapace length (SCL, mm) of big-headed (Peltocephalus dumerilianus) from the Negro River basin, Amazonas, Brazil, between 1997 and 2019 (SD = standard deviation).
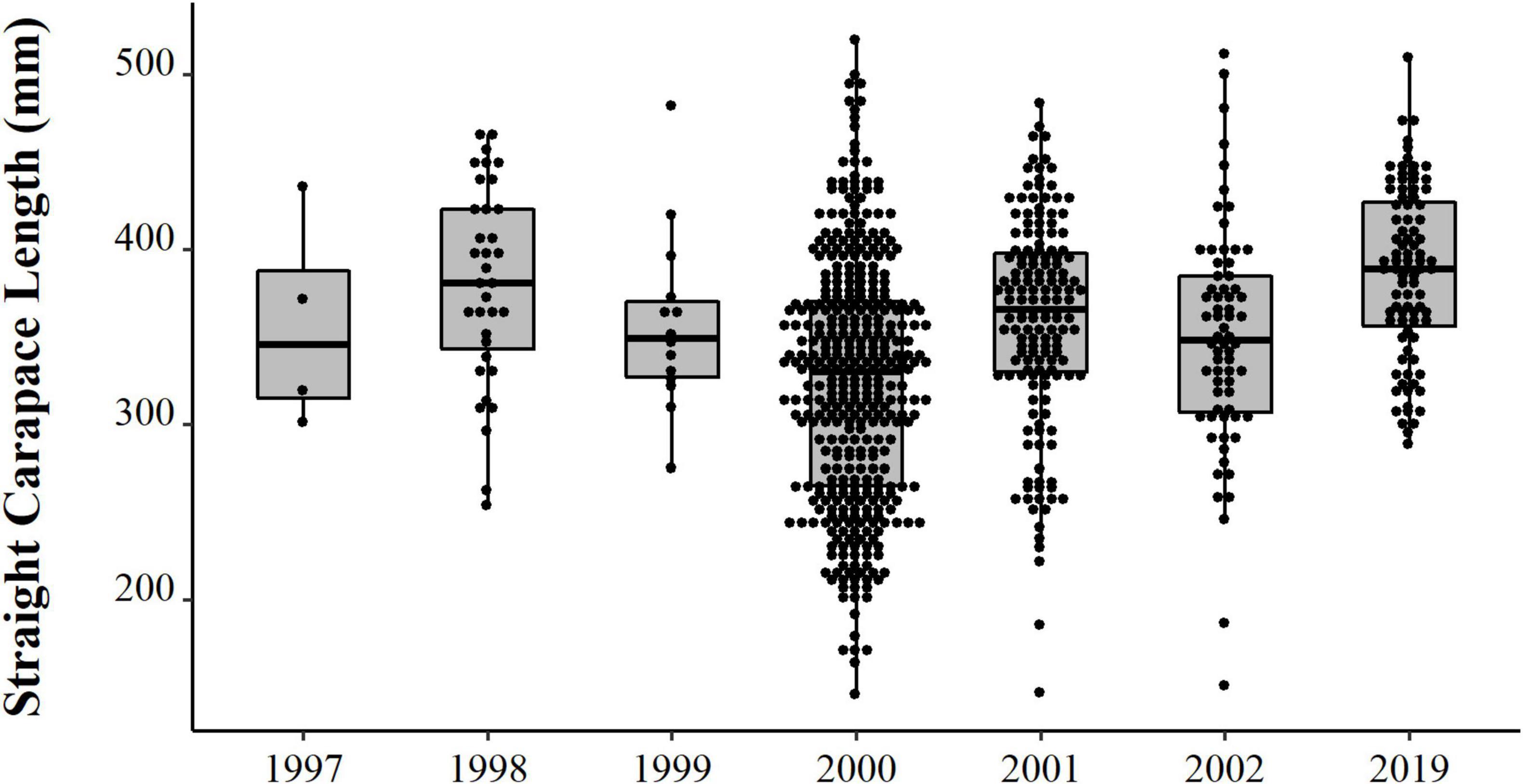
Figure 3. Size distribution (points) and descriptive statistical (boxplot) of body size (SCL, mm) of big-headed (Peltocephalus dumerilianus) from the PNJ collected between 1997 and 2019.
Comparing the data from the first collection interval (from 1997 to 2002) with the data from the second set of data collected in 2019, we observed that the animals from 2019 are significantly larger (PERMANOVA, Pseudo-F = 58.88, p = 0.0001, Figure 4). When comparing just the animals destined for commercialization, the animals from the PNJ were smaller than the animals from Barcelos (PERMANOVA, Pseudo-F = 5.61, p = 0.01, Figure 5). Males are larger than the females (PERMANOVA, Pseudo-F = 182.95, p = 0.0001, Figure 6), and individuals captured during the rainy season were larger than individuals captured in the dry season (PERMANOVA, Pseudo-F = 9.67, p = 0.02, Figure 7).
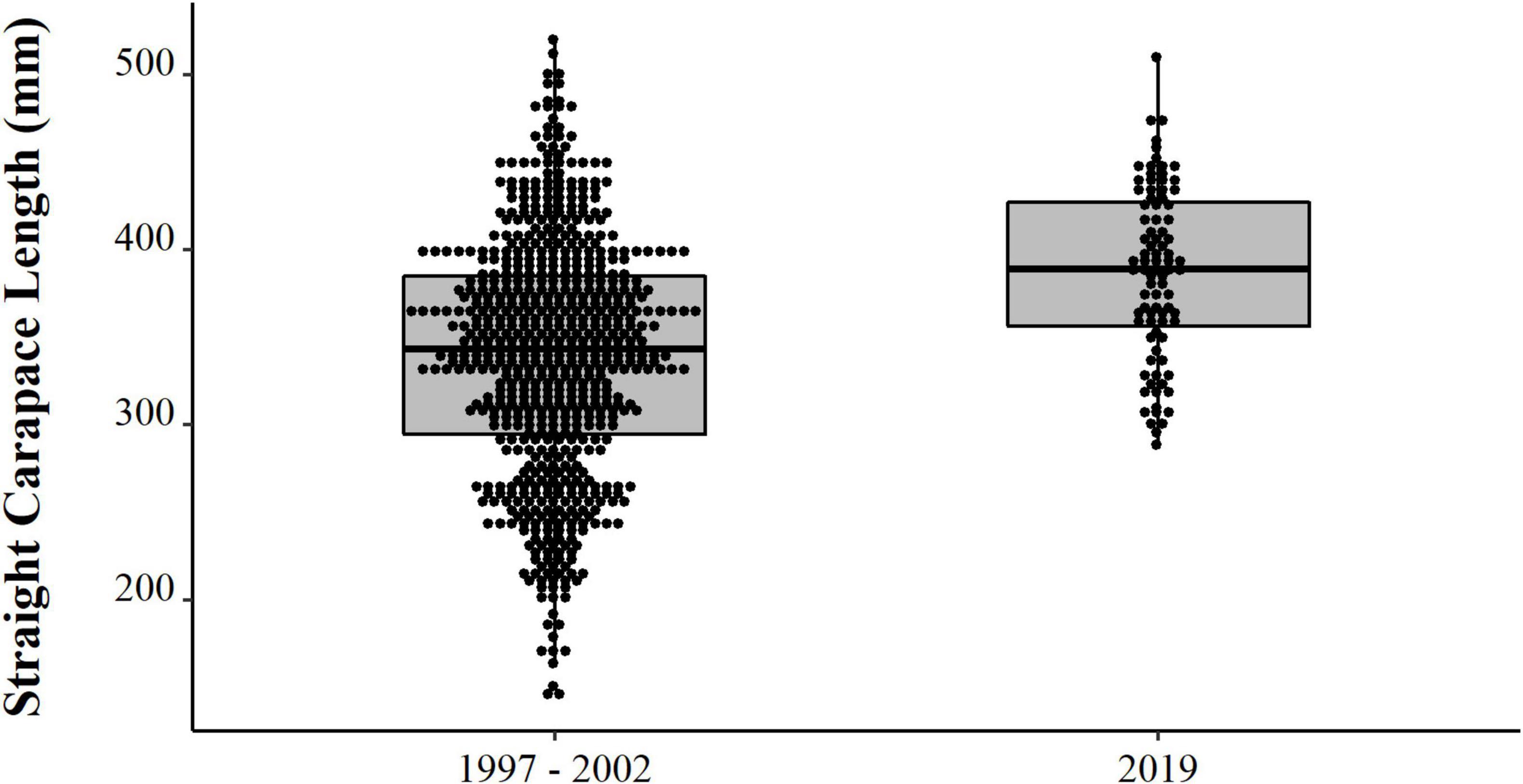
Figure 4. Temporal distribution (points) and descriptive statistical (boxplot) of body size (SCL, mm) of Big-headed (Peltocephalus dumerilianus) from the PNJ collected in both sample periods (1997–2002 and 2019).
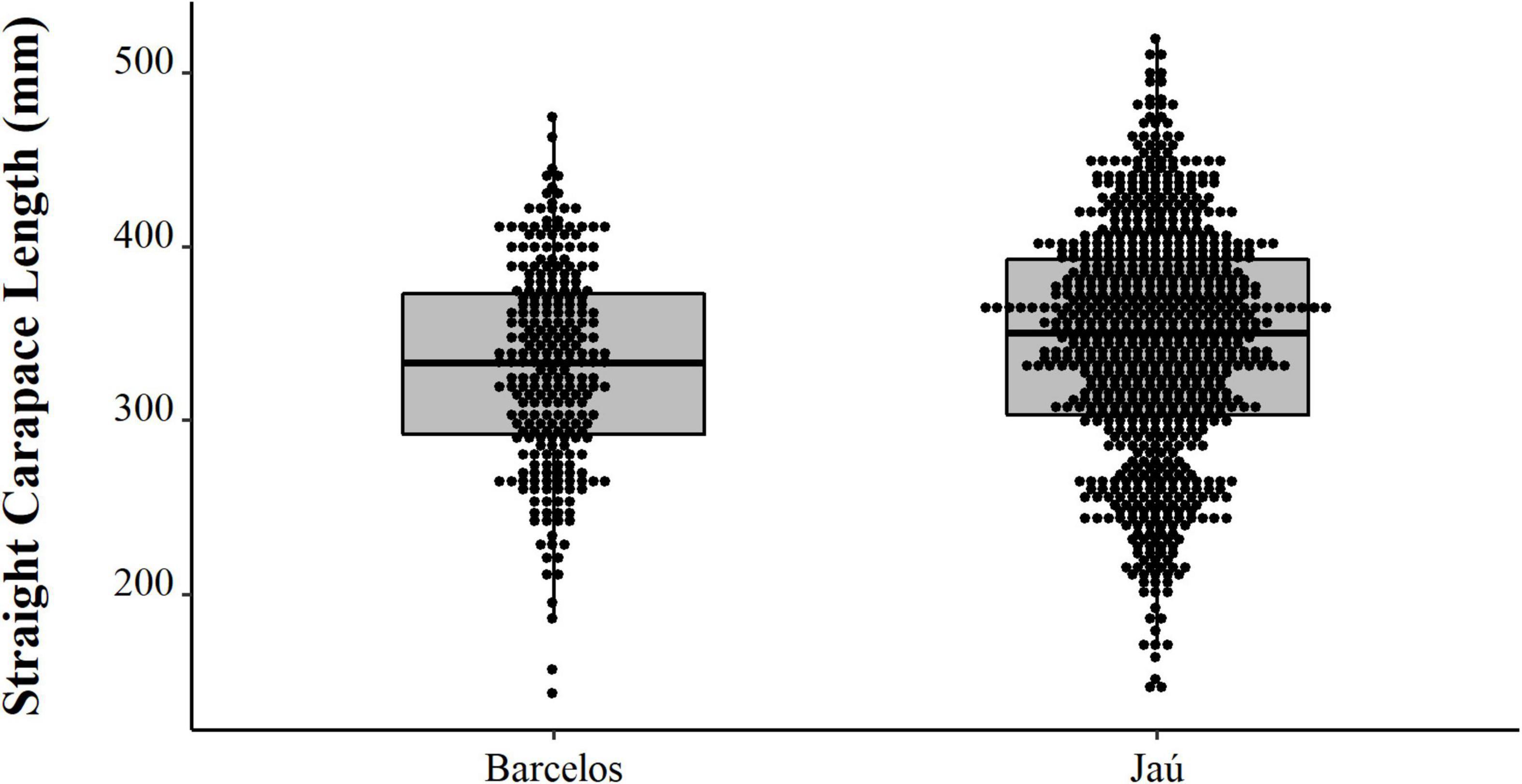
Figure 5. Spatial distribution (points) and descriptive statistics (boxplot) of body size (SCL, mm) of Big-headed (Peltocephalus dumerilianus) intended for commercialization, from the Jaú River and the city of Barcelos, Rio Negro, Amazonas between 1997 and 2002.
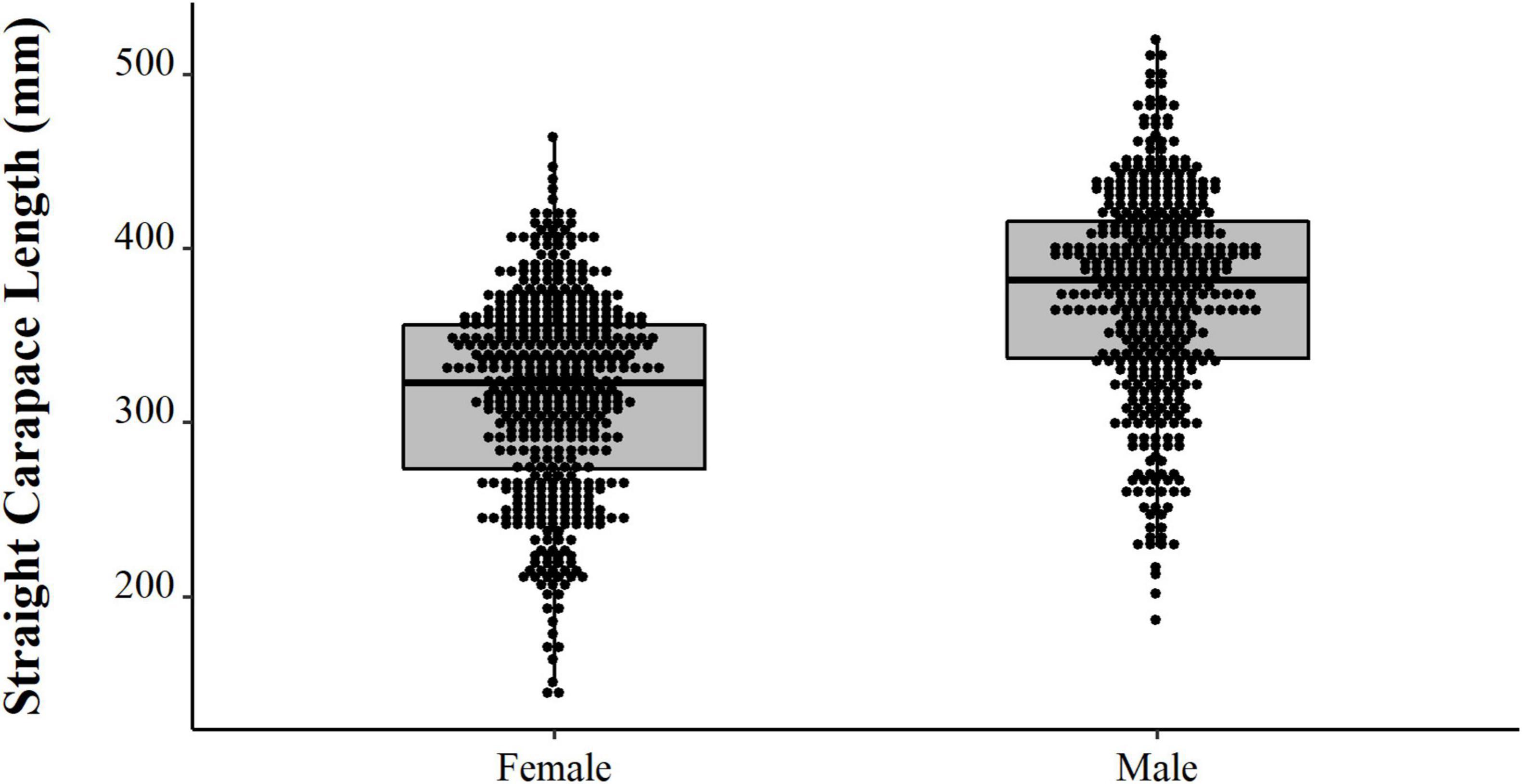
Figure 6. Temporal distribution (points) and descriptive statistical (boxplot) of body size (SCL, mm) of the male and female Big-headed (Peltocephalus dumerilianus) from the Jaú collected between 1997 and 2019.
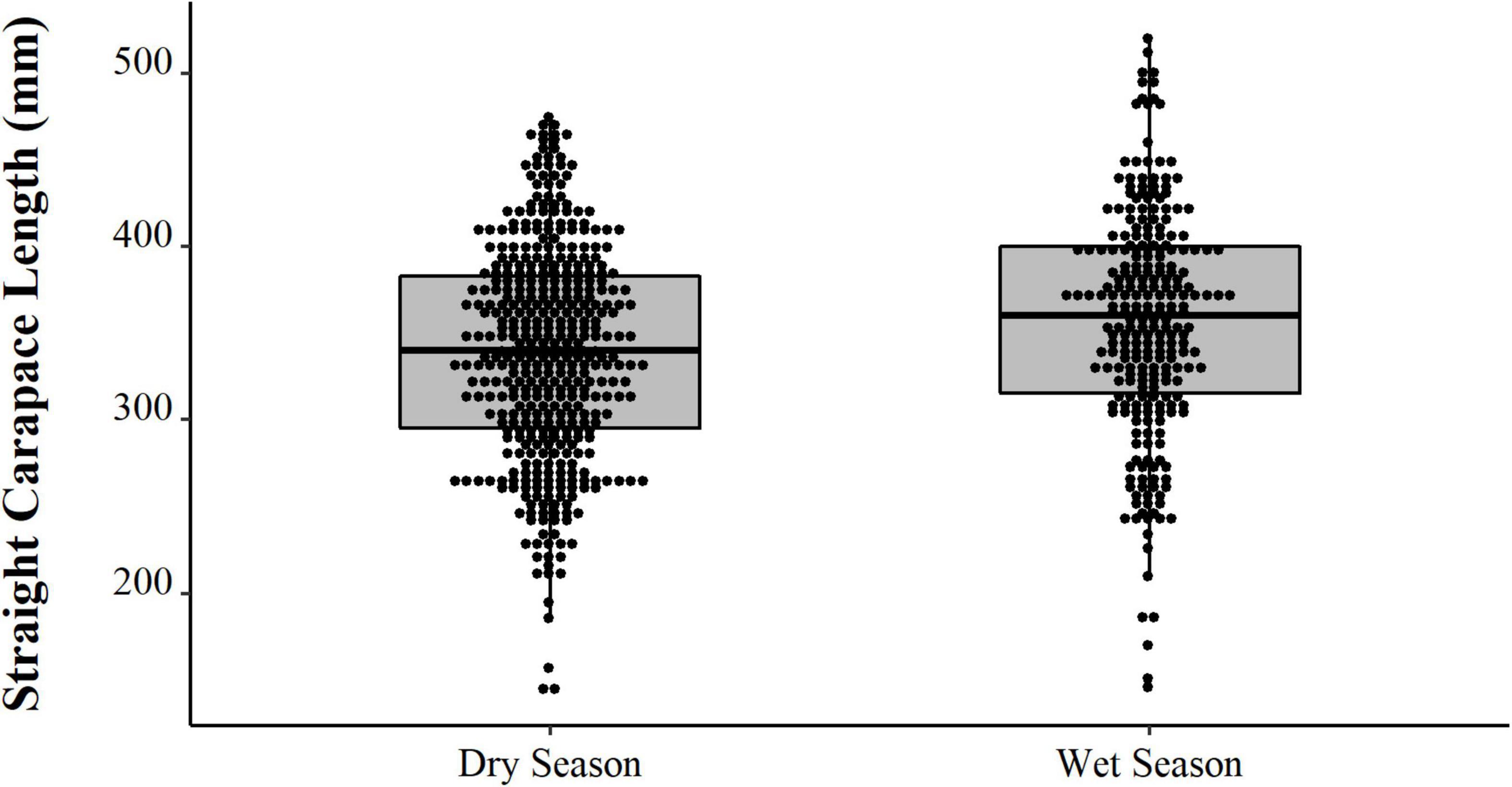
Figure 7. Distributions (points) and descriptive statistics (boxplot) of body size (SCL, mm) and Big-headed (Peltocephalus dumerilianus) from the Negro River, Amazonas, obtained between 1997 and 2019, captured in the dry and rainy seasons.
We observed contrast between the sizes of the animals captured experimentally, the animals intended for consumption by the residents, and the animals selected for sale (PERMANOVA, Pseudo-F = 36.81, p = 0.0001, Table 2 and Figure 8). The animals from the experimental fishery are smaller and those with a greater range of size distribution, followed by animals intended for commercialization, which have a lesser size range. The animals destined for consumption by the residents are larger than those caught in experimental fisheries and those destined for sale (PERMANOVA pair-wise < 0.05, Supplementary Material 1). However, they present a greater range in the distribution concerning the animals consumed. We emphasize that almost all the animals destined for consumption come from the PNJ, which is essential for interpreting these results. Comparing the sizes between the five locations, the results show substantial differences (PERMANOVA, Pseudo-F = 9.21, p = 0.0001, Figure 9). The largest animals are those from the Jaú River, especially those from the middle stretch of the river, which is the most remote area. Caurés and Barcelos individuals were significantly smaller than those from the other locations, with no difference between each other (PERMANOVA pair-wise < 0.05, Supplementary Material 1).
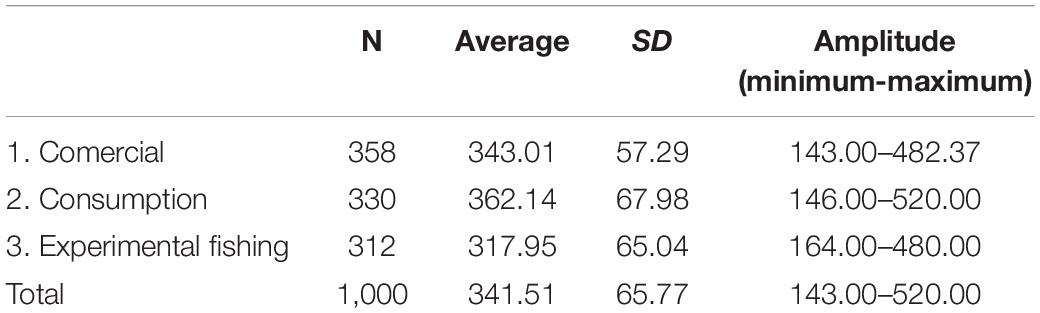
Table 2. Descriptive statistics of the rectilinear carapace length (SCL, mm) of big-headed (Peltocephalus dumerilianus) captured experimentally, intended for consumption and commercialization, between 2002 and 2019, in the Rio Negro region, Amazonas, Brazil (SD = standard deviation).
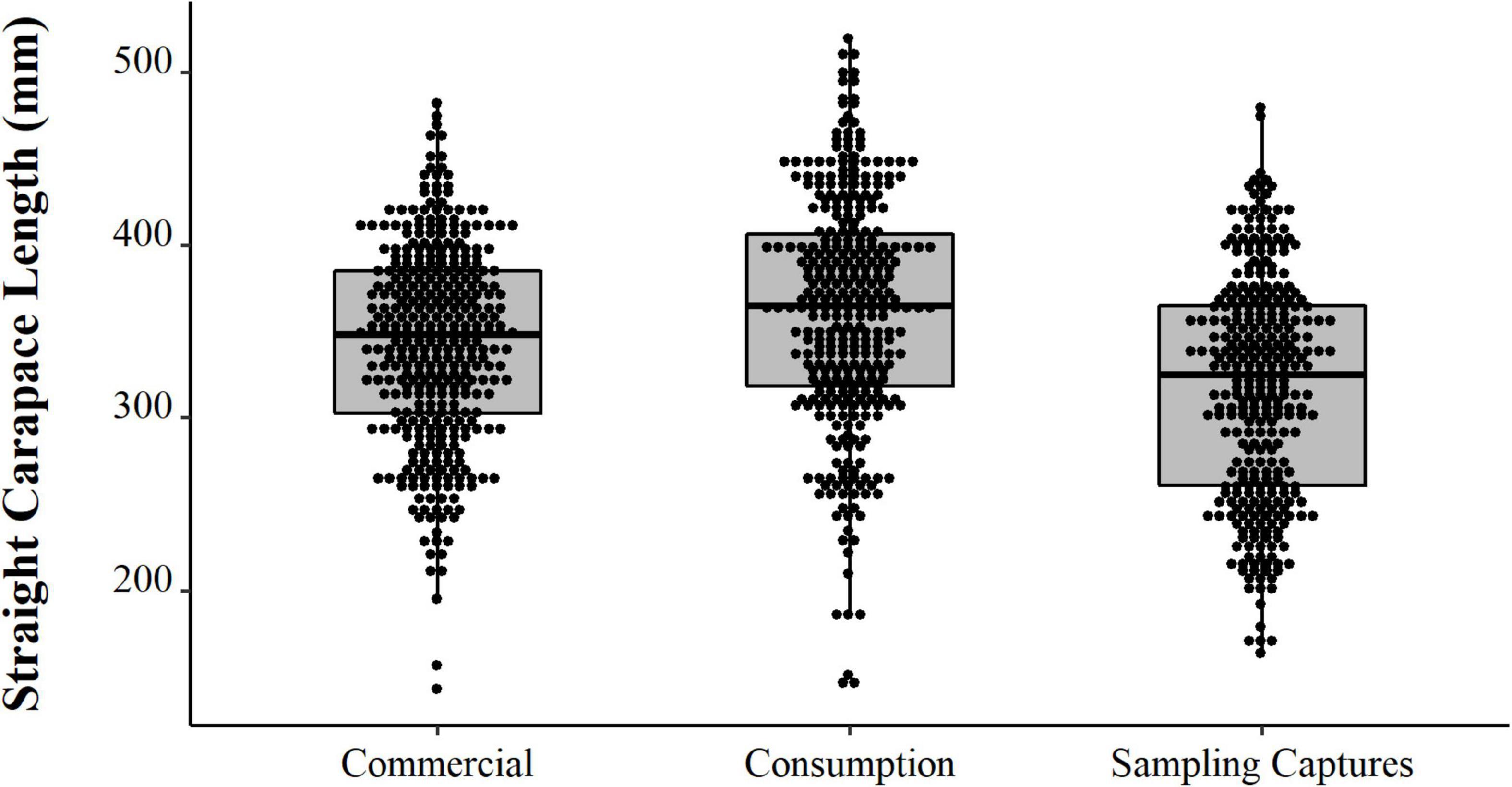
Figure 8. Distributions (points) and descriptive statistics (boxplot) of body size (SCL, mm) of Big-headed (Peltocephalus dumerilianus) captured experimentally, intended for consumption, and commercialization, between 1997 and 2002, in the region of the Negro River, Amazonas, Brazil.
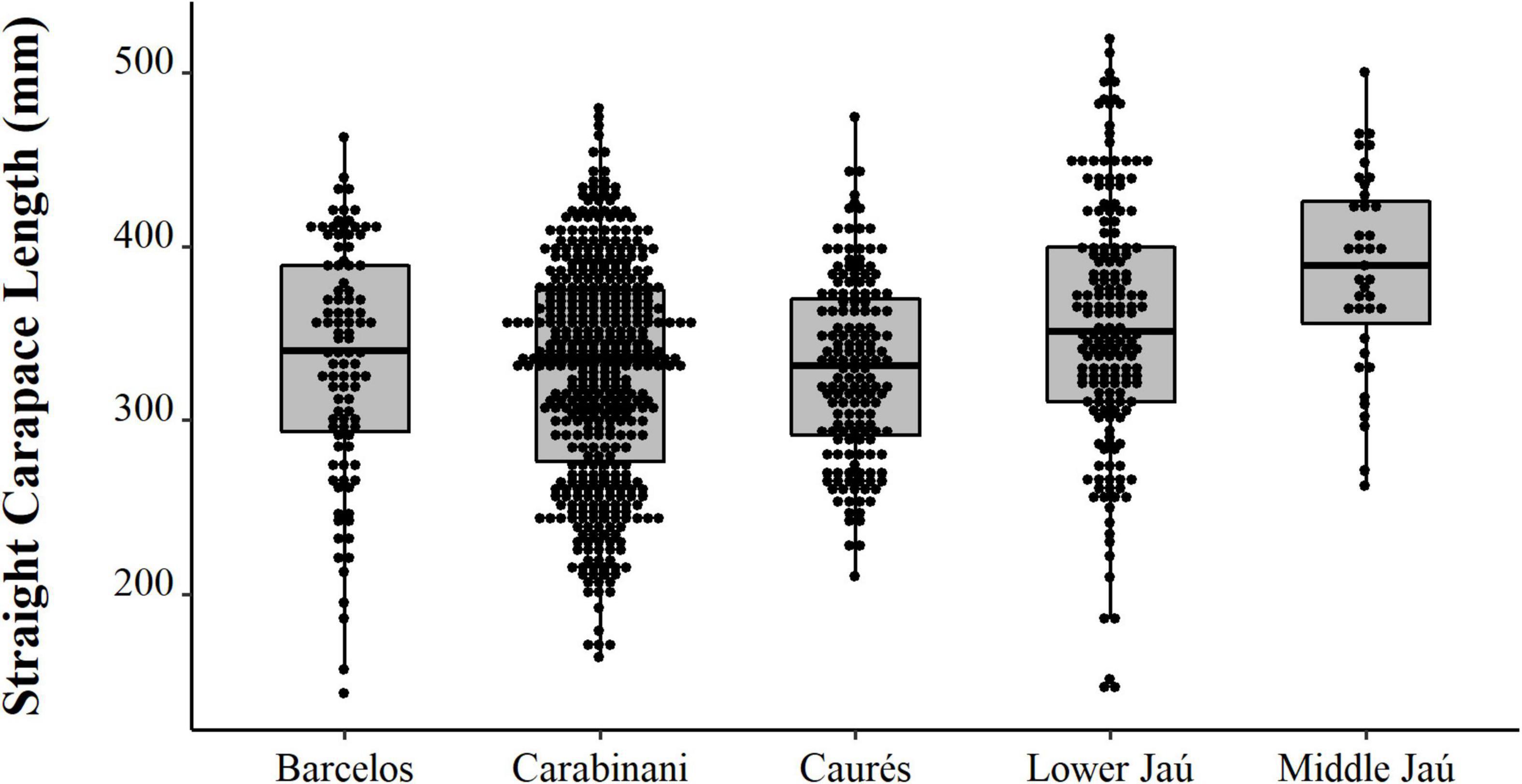
Figure 9. Spatial distribution (points) and descriptive statistics (boxplot) of body size (SCL, mm) of Big-headed (Peltocephalus dumerilianus) in different locations of the Negro River region, Amazonas, between 1997 and 2002.
When considering the animals’ sex ratio, no significant differences were detected between the animals from the Jaú and Barcelos, or between those consumed and destined for commercialization, nor between the animals captured in the dry and rainy season. When comparing the sex ratios of animals from experimental capture to animals captured for consumption and sale, we observe a higher proportion of females in the first group, while in animals intended for consumption and sale, the pattern is reversed, with a significant proportion of males (Σ2 = 33,228, p = 0.0001; Table 3).
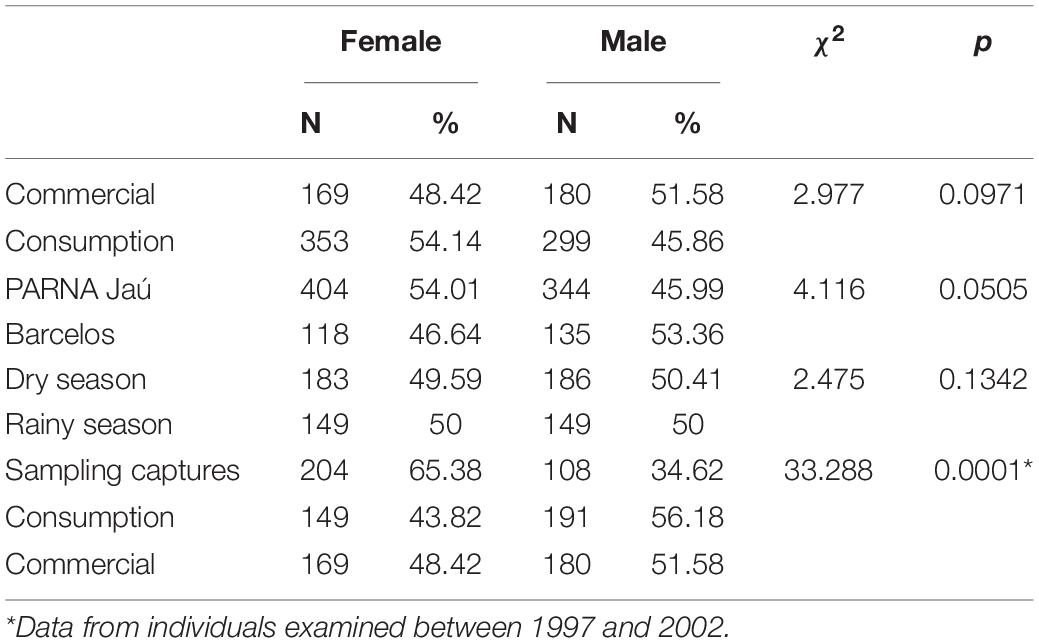
Table 3. Sex ratio of Big-headed individuals (Peltocephalus dumerilianus) examined in the Negro River basin, Amazonas, between 1997 and 2019.
Discussion
Historical records note reduction in abundance and average size of Amazon River turtle populations, especially the larger and gregarious Podocnemis expansa (Bates, 1864; Silva Coutinho, 1868; Ferreira, 1972), and a further shift to target smaller species (Johns, 1987; Rebêlo and Pezzuti, 2000). In the study area, fishermen have been selective and are consuming and trading large-sized individuals in a proportion that is different from the size composition reflected in direct captures. This poaching pattern has been maintained for at least the last two decades, and probably for a considerably longer non-monitored period.
There are extensive remote areas where harvesting is absent, and others where there is occasional and or sporadic harvesting, a central feature of a source-sink system of large proportions. In this context, the apparent resilience of the population under study could be due to the replacement of individuals migrating from source areas to the regions where harvesting takes place. However, despite observed differences within some monitored areas, harvested individuals are mainly large-sized adult individuals. In addition, while we anticipated a reduction in the size of the harvested animals over time, we observed a stable pattern both within the first 6-year monitoring period of all harvested places and in the second monitoring period 20 years later. Moreover, we observed the largest animals in 2019.
The animals sampled in Barcelos come from the Negro River and nearby tributaries. Both urban fishermen and those from surrounding riverine settlements sell turtles in town. Data indicate that it is not necessary to go very far from human population centers to capture large adult individuals. Thus, larger animals in Barcelos (a selection of animals for sale in the city) compared to animals in the PNJ (animals intended for consumption and sale) show two factors: firstly, there is a selection for larger animals, which yields more meat and reaches a higher market value; secondly, it is possible for urban fishermen to select larger animals for commercialization, even larger than those of PNJ (a National Park, with restricted access), where we expect a lower capture pressure. On the other hand, it is interesting to note that the animals consumed within the PNJ are larger than those captured for commercialization. Both have a larger size distribution than the animals from experimental fishing, confirming fishermen’s selection of larger animals for consumption and sale, but we did not expect that the animals consumed would be larger than those sold within the PNJ. On the other hand, our comparisons also clearly indicate differences between locations in congruence with our expectations, with smaller animals coming from higher pressure locations, such as Caurés and Barcelos. Considering the predominant absence of any management system nor regular enforcement effort, and the dependence of poor families to wage opportunities and other income sources, an increase in poaching levels is likely to have deeper effects on target populations.
The sex ratio recorded in experimental fishing, without any interference from selection for consumption, sale, or disposal, is the one that tends to better reflect, among the sets of animals considered here, the actual proportion of males and females in the wild. A similar result was observed by De La Ossa and Vogt (2011) in the two rivers near Barcelos. The set of harvested animals over the two periods, 1997–2002 and later in 2019, show a balance between both sexes, with a slight deviation in favor of males. In our sample, among the animals captured and selected by the fishermen for consumption and sale, there is a slight predominance of males, which can be explained by the fact that they are larger in this species. Parra-Henao et al. (2019) observed a strong predominance of males (66.7%) in a tributary of the upper river Orinoco in Colombian territory. However, the sample was small and may not reflect the sex ratio composition in the region. Even though there is a predilection for females for consumption in the region for other Podocnemidids (Pezzuti et al., 2010), the choice is for larger animals, as it is something inherent to the method. When fishing with baliza, the fisherman cannot see the animal and does not know the sex of the animal that is eating the bait before harpooning it and bringing it up. The existence of a different mobility pattern between genders, with greater travel distances and living area for females (De La Ossa and Vogt, 2011), may be one of the factors that contribute to the lower capture of females, as they move to more isolated points in the flooded forest in search of suitable places for nesting, which occurs in the ebb of the Amazon rivers (Vogt et al., 1994). The findings suggest a tendency to capture the most desired larger male adult individuals, with no observed changes over a 20-year period.
The big-headed is the least studied Podocnemidid, and there is little information about the population structure of these animals. The number of animals measured over this period far exceeds the samples presented in the few studies available. Pritchard and Trebbau (1984) provide biometric data for six individuals from the upper Rio Negro (San Carlos, Venezuela), four individuals from French Guiana, two from the Orinoco, and four from unknown sources. In a sample consisting of 165 individuals from the Itu and Cumicuri rivers, near Barcelos, De La Ossa and Vogt (2011) found an average carapace size of 376.80 and 387.60 mm for males, and 244.80 and 258.50 mm for females, respectively. Males are within our sample size range, but females are considerably smaller. In the small sample of Parra-Henao et al. (2019), the average size is 415.20 mm for males and 287.40 mm for females, which is also within the spectrum of the sample presented here. We certainly have the largest size records of the species, including an individual reaching 520 mm. Parra-Henao et al. (2019) use 260 mm of CRC for the species’ minimum sexual maturity size, mentioning Rueda-Almonacid et al. (2007). However, checking the original study, we could not confirm this information. The decision to mention it was due to the importance of this parameter.
Apparently, the pressure on the big-headed has remained, despite significant improvement in infrastructure and staffing, compared to the first period (1997–2001), when monitoring the consumption of turtles in the park was more intense. This is evident in the biometric analysis and observation of more than 90 shells found discarded and not yet decomposed and whole, indicating animals recently consumed. Our experience is that the shells decompose in a few months. According to information and evidence available about the previous period (1997–2002), in addition to new information obtained from the residents and current managers of the PNJ, the big-headeds, the tracajás (Podocnemis unifilis), and irapucas (P. erytrochephala) continue to be an important component of the traditional diet for riverine residents in the park. These animals are also clandestinely transported and traded outside the protected area. The poaching of river turtles, including big-headeds, prevailed over the 20 years of sampling interval (Fabio Osolins, personal communication).
Thus, observing a larger size structure after the 20-year interval is a positive indication of stability of average animal sizes despite continuous poaching. In the PNJ, as already mentioned, we observed stability and even a slight trend toward an increase in size of the animals consumed. On the other hand, we do not have recent data from the other locations on the Rio Negro obtained in the 1990s and early 2000s. These areas are less protected than the PNJ, and therefore, the pressure of capture may have intensified substantially.
The population of big-headed investigated occupy vast floodplains of the Negro River (Pezzuti, 2003; De La Ossa and Vogt, 2011), which are in an excellent state of conservation (Montero et al., 2014). In Barcelos, there is a huge tangle of river channels, forming the most extensive river archipelago in the world, the Mariuá, and just below the Mariua, there is a large expanse of seasonally flooded Anavilhanas forests (Latrubesse and Stevaux, 2015). In the PNJ, the environments used by animals are also extensive and complex (Ferreira, 1997). The wetlands are capable of harboring large populations of aquatic animals, especially herbivores, such as the big-headed (Perez-Emán and Paolillo, 1997; De La Ossa et al., 2011), whose trophic position at the base of the food chain may also have contributed to the biological success of the species, as well as apparent resilience in the face of the fishing pressure by riverside dwellers. This vastness of well-preserved natural areas and the landscape complexity, with a wide range of niches, may contribute to the findings of the present study.
The capture of adult females during reproduction is considered a critical factor affecting turtle populations worldwide (Klemens, 2000; Moll and Moll, 2004; Roberts, 2010), and population models indicate that conservation efforts should address measures to reduce the mortality of subadult and adult females (Crouse et al., 1987; Heppell, 1998; Crouse, 1999; Mogollones et al., 2010). For Amazonian species, there is abundant historical evidence of the unsustainability and decimation of Podocnemis populations, previously quite abundant (Bates, 1864; Silva Coutinho, 1868; Ferreira, 1972; Smith, 1974). Relevant empirical evidence of the effectiveness of protecting adult females came from communal areas along a large stretch of the Juruá River, a major whitewater Amazon tributary. After 40 years of conservation efforts aimed at protecting nesting beaches and surroundings, the population of P. expansa showed an 11.4-fold increase in the number of nests in monitored areas, with expressive increase in the number of nests of P. unifilis and P. sextuberculata as well (Campos-Silva et al., 2017).
Still, it is important to acknowledge that the resource is being used on an open-access basis. As Brazil currently lacks legislation for wildlife management and law enforcement capacity to restrain poaching, the future of this valuable resource is uncertain and deserves attention. No formal or informal rules nor management actions are taking place. Despite Brazil’s legal framework recognizing the rights of traditional communities that depend on natural resources, wildlife use is not yet regulated in the country and harvesting practices are considered illegal, except for Indigenous groups within federally demarcated lands. Local families, who have subsisted on fish and game for centuries, when found by command-and-control agents in possession of wild animals or meat, are treated as poachers and subject to fines, forfeiture of materials, or arrest (Antunes et al., 2019). This context is a major bottleneck for management procedures, such as the establishment of quotas, a zoning system, or other participatory processes. In addition, it does not allow for the opportunity of interaction between diverse stakeholders that could otherwise work together toward the construction of species management plans. There are interesting examples community-based initiatives showing recovery of Amazon River turtle populations (Campos-Silva et al., 2017; Pezzuti et al., 2018), which attest to the potential of sustainable management.
Conclusion
The size of harvested big-headed turtles in Negro River basin presented a slight trend of increase between 1997 and 2019. Size distribution between directly caught animals differed and were smaller than animals destined for consumption and trade. There were differences between areas possibly subject to different pressure, but all sites were represented by large-sized adults. Fishermen tended to select larger individuals, and to consume and trade a larger proportion of males, which are bigger than females in this species.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The project was submitted and previously approved by the Brazilian Institute of Environment and Natural Renewable Resources/IBAMA.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
Funding
This study was funded by the World Wildlife Fund (WWF, Nature and Society Program). A National Research Council (CNPq) Ph.D. scholarship was granted to JP. Vitoria Amazonica Foundation and Chico Mendes Institute for Biodiversity Conservation (ICMBIO) gave logistical support for the fieldtrips. The Brazilian Institute of Environment and Renewable Natural Resources (IBAMA) authorized this research for the first period, and ICMBIO for the second. The publication fee was provided by the University of Parà (Process No. 23073.017634/2022-67).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewers CF and PM declared a shared affiliation, with no collaboration, with the authors JP-L and GR to the handling editor at the time of review.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We are deeply thankful to Barcelos and Jaú riverine fishermen and their families, whose hospitality and confidence were essential for the data collection.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2022.640961/full#supplementary-material
References
Anderson, M. J. (2005). PERMANOVA Permutational multivariate analysis of variance. Austral Ecol. 1:24. doi: 10.1139/cjfas-58-3-626
Anderson, M. J. (2014). Permutational Multivariate Analysis of Variance (PERMANOVA). Wiley StatsRef Stat. Ref. Online.. New Jersey: John Wiley, doi: 10.1002/9781118445112.stat07841
Anderson, M. J., and Walsh, D. C. I. (2013). Permanova, Anosim, Mantel Test Face Heterogeneous Dispersions: what Null Hypothesis Are You Testing? Ecol. Monogr. 83, 557–574. doi: 10.1890/12-2010.1
Antunes, A. P., Fewster, R. M., Venticinque, E. M., Peres, C. A., Levi, T., Rohe, F., et al. (2016). Empty forest or empty rivers? Sci. Adv. 2, e1600936. doi: 10.1126/sciadv.1600936
Antunes, A. P., Pezzuti, J. C. B., Durigan, C. C., Fonseca, R., Vieira, M. A. R. M., Valsecchi, J., et al. (2019). Ongoing conspiracy of silence around hunger: subsistence hunting rights in the Brazilian Amazon. Land Use Policy 84, 1–11. doi: 10.1016/j.landusepol.2019.02.045
Ayres, M., Ayres, M. Jr., Ayres, D. L., and Santos, A. S. (2007). BioEstat 5.0. Aplicações Estatísticas nas Áreas das Ciências Biológicas e Médicas. Estado do Pará: Sociedade Civil Mamirauá/MCT/Imprensa Oficial do.
Campos-Silva, J. V., Peres, C. A., Antunes, A. P., Valsecchi, J., and Pezzuti, J. (2017). Community-based population recovery of overexploited Amazonian wildlife. Perspect. Ecol. Conserv. 15, 266–270. doi: 10.1016/j.pecon.2017.08.004
Carvajal, G. (1543). Relación del nuevo descubrimiento del famoso Rio Grande de las Amazonas. México Fondo de Cultura Econ. primera edición de 1955:157.
Caughley, G., and Sinclair, A. R. E. (1994). Wildlife Ecology and Management. New Jersey: Blackwell Science.
Clarke, K. R. (1993). Non-parametric multivariate analysis of changes in community structure. Aust. J. Ecol. 18, 117–143. doi: 10.1111/j.1442-9993.1993.tb00438.x
Crouse, D., Crowder, L. B., and Caswell, H. (1987). A stage-based model for loggerhead sea turtles and implications for conservation. Ecology 68, 1412–1423. doi: 10.2307/1939225
Crouse, D. T. (1999). Population modeling and implications for Caribbean hawksbill sea turtle management. Chelonian Conserv. Biol. 3, 185–188.
Da Silva, A. L., and Begossi, A. (2009). Biodiversity, food consumption and ecological niche dimension: a study case of the riverine populations from the Rio Negro, Amazonia, Brazil. Environ. Dev. Sustain. 11, 489–507. doi: 10.1007/s10668-007-9126-z
De La Ossa, J., and Vogt, R. C. (2011). Ecologia populacional de Peltocephalus dumerilianus Testudines. Brasil.Interciência 36, 53–58.
De La Ossa, J., Vogt, R. C., and Santos-Júnior, L. B. (2011). Feeding of Peltocephalus dumerilianus (Testudines: Podocnemididae) in a natural environment. Actual Biol. 33, 85–92.
Fachín-Terán, A., Vogt, R. C., and Thorbjarnarson, J. B. (2003). “Patterns of Use and Hunting of Turtles in the Mamirauá Sustainable Development Reserve, Amazonas, Brazil,” in People and Nature: Wildlife Conservation in South and Central America, eds K. M. Silvius, R. Bodmer, and J. M. Fragoso (New York, NY: Columbia University Press), 362–377. doi: 10.7312/silv12782-022
Félix-Silva, D. (2004). Reproductive Ecology of the big-Headed Amazon River Turtle, Peltocephalus Dumerilianus (Testudines: Pelomedusidae) at Jaú National Park, Amazonas, Brazil. [Ph D thesis]. Rio de Janeiro: State University of Rio de Janeiro.
Ferreira, A. R. (1972). Viagem Filosófica Pelas Capitanias do Grão Pará, Rio Negro, Mato Grosso e Cuiabá (1783-1792). Rio de Janeiro: Conselho Federal de cultura
Ferreira, L. V. (1997). Effects of the duration of flooding on species richness and floristic composition in three hectares in the Jaú National Park in floodplain forests in central Amazonia. Biodivers. Conserv. 6, 1353–1363.
Gilmore, R. M. (1986). “Fauna e Etnozoologia da América do Sul Tropical,” in Suma Etnológica Brasileira. Up to Data Edition of Handbook of South American Indians (1963), ed. D. Ribeiro (Maryland: Copper Square Publ. Inc), 189–233.
Heppell, S. S. (1998). Application of life-history theory and population model analysis to turtle conservation. Copeia 1998, 367–375. doi: 10.2307/1447430
Johns, A. (1987). Continuing problems for Amazonian river turtles. Oryx 21, 25–28. doi: 10.1017/S0030605300020445
Latrubesse, E. M., and Stevaux, J. C. (2015). “The anavilhanas and mariuá archipelagos: fluvial wonders from the Negro River, Amazon Basin,” in Landscapes and Landforms of Brazil, eds B. C. Vieira, A. U. R. Salgado, and L. J. C. Santos (Dordrecht: Springer), 157–169.
Machado, R. (2001). “Life and Culture on the Rio Negro,” in Conservation and Management of Ornamental Fish Resources of the Rio Negro Basin, ed. L. N. Chao (Manaus, AM: EDUA press), 245–265.
Miorando, P. S., Giarrizzo, T., and Pezzuti, J. C. B. (2015). Population structure and allometry of Podocnemis unifilis (Testudines. Brazil An. Acad. Bras. Cienc. 87, 2067–2079. doi: 10.1590/0001-3765201520140321
Mittermeier, R. A. (1975). A Turtle in Every Pot a Valuable South American Resource Going to Waste. Anim. Kingdom 78, 9–14. doi: 10.1016/0006-3207(79)90019-3
Mogollones, S. C., Rodríguez, D. J., Hernández, O., and Barreto, G. R. (2010). A demographic study of the Arrau turtle (Podocnemis expansa) in the Middle Orinoco River. Venez. Chelonian Conserv. Biol. 9, 79–89. doi: 10.2744/CCB-0778.1
Moll, D., and Moll, E. O. (2004). The Ecology, Exploitation and Conservation of River Turtles. New York, NY: Oxford University Press, 393.
Montero, J. C., Piedade, M. T. F., and Wittmann, F. (2014). Floristic variation across 600 km of inundation forests (Igapó) along the Negro River. Central Amazon. Hydrobiol. 729, 229–246. doi: 10.1007/s10750-012-1331-0
Pantoja-Lima, J., Aride, P. H., de Oliveira, A. T., Félix-Silva, D., Pezzuti, J. C., and Rebêlo, G. H. (2014). Chain of commercialization of Podocnemis spp. turtles (Testudines: Podocnemididae) in the Purus River, Amazon basin, Brazil: current status and perspectives. J. Ethnobiol. Ethnomed. 10:8. doi: 10.1186/1746-4269-10-8
Parra-Henao, K. D., Páez, V. P., Morales-Betancourt, M., and Lasso, C. A. (2019). A pilot study of habitat use and population characteristics of the big-headed Amazon river turtle. Herpetol. Notes 12, 1113–1120.
Perez-Emán, J., and Paolillo, A. (1997). Diet of the Pelomedusid Turtle Peltocephalus dumerilianus in the Venezuelan Amazon. J. Herpetol. 31, 173–179. doi: 10.2307/1565384
Pezzuti, J. C. B. (2003). Ecology and Ethnoecology of River Turtles at Jaú National Park, Brazil. [Ph.D thesis]. Campinas: State University of Campinas.
Pezzuti, J. C. B., Pantoja-Lima, J., Félix-Silva, D., and Begossi, A. (2010). Uses and taboos of turtles and tortoises at Negro River, Amazonas, Brazil. J. Ethnobiol. 30, 153–168. doi: 10.2993/0278-0771-30.1.153
Pezzuti, J. C. B., Pantoja-Lima, J., Félix-Silva, D., and Rebêlo, G. H. (2004). “A caça e a pesca no Parque Nacional do Jaú, Amazonas,” in Janelas Para a Biodiversidade, eds S. H. Borges, C. C. Durigan, and S. Iwanaga (Manaus, AM: Fundação Vitória amazônica), 213–227.
Pezzuti, J. F., Castro, D. G., McGrath, P., Saikoski Miorando, R., Sá Leitão Barboza, F., and Romagnoli, C. (2018). Commoning in dynamic environments. Ecol. Soc. 23:36. doi: 10.5751/ES-10254-230336
Prestes-Carneiro, G., Béarez, P., Bailon, S., Py-Daniel, A. R., and Neves, E. G. (2016). Subsistence fishery at Hatahara (750–1230 CE), a pre-Columbian central Amazonian village. J. Archaeol. Sci. Rep. 8, 454–462. doi: 10.1016/j.jasrep.2015.10.033
Pritchard, P. C. H., and Trebbau, P. (1984). The Turtles of Venezuela. Oxford. Ohio: Society for the Study of Amphibians and Reptiles.
R Development Core Team. (2010). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Rebêlo, G. H., and Lugli, L. (1996). “The Conservation of Freshwater and the Dwellers of the Amazonian Jaú National Park (Brazil),” in Etnobiology in Human Welfare, ed. S. K. Jain (New Delhi: Deep Publications), 253–358.
Rebêlo, G. H., and Pezzuti, J. C. B. (2000). Percepções sobre o consumo de quelônios na Amazônia: sustentabilidade e alternativas ao manejo atual. Ambient. e Soc. 6, 85–104. doi: 10.1590/S1414-753X2000000100005
Rebêlo, G. H., Pezzuti, J. C. B., Lugli, L., and Moreira, G. (2006). Pesca artesanal de quelônios no Parque Nacional do Jaú. Bol. Mus. Para. Emilio Goeldi 1, 109–125.
Rueda-Almonacid, J. V., Carr, J. L., Mittermeier, R. A., Rodriguez Mahecha, J. V., Mast, R. B., Vogt, R. C., et al. (2007). Las Tortugas y los Crocodilianos de los Países Andinos del Trópico. Bogotá: Conservación Internacional.
Schneider, L., Ferrara, C. R., Vogt, R. C., and Burguer, J. (2011). History of turtle exploitation and management techniques do conserve turtles in the Negro River basin of the Brazilian Amazon. Chelonian Conserv. Biol. 10, 149–157. doi: 10.2744/CCB-0848.1
Silva Coutinho, J. M. (1868). Sur le Tortues de l’Amazone. Bulletin de la Societé Zoologique d’Aclimatation, 2, Tome V. Paris: Bulletin de la Société Zoologique d’Aclimatation.
Sistema Nacional de Unidades de Conservação [SNUC] (2000). National System of Protected Areas. Federal Law 9985, published on 18 July 2000. Available online at: http://www.planalto.gov.br/ccivil_03/leis/l9985.htm. [Accessed on Nov 30, 2020]
Smith, N. J. J. (1974). “Destructive exploitation of the South American River Turtle,” in Yearbook of the Association of Pacific Coast Geographers, ed. R. Steiner (Corvallis: Oregon State University Press), 85–120. doi: 10.1353/pcg.1974.0000
Tregidgo, D. J., Barlow, J., Pompeu, P. S., de Almeida Rocha, M., and Parry, L. (2017). Rainforest metropolis casts 1,000-km defaunation shadow. Proc. Natl. Acad. Sci. 114, 8655–8659. doi: 10.1073/pnas.1614499114
Velez-Zuazo, X., Quiñones, J., Pacheco, A. S., Klinge, L., Paredes, E., Quispe, S., et al. (2014). Fast growing, healthy and resident green turtles (Chelonia mydas) at two neritic sites in the central and northern coast of Peru: implications for conservation. PLoS One 9:e113068. doi: 10.1371/journal.pone.0113068
Keywords: river turtle, Peltocephalus, sustainability, use, Amazon, Negro River, trade
Citation: Pezzuti JCB, Oliveira T, Pantoja-Lima J, Rebêlo GH and Félix-Silva D (2022) Temporal and Spatial Stability on the Population Structure of Consumed and Illegally Traded Big-Headed Amazon River Turtle in the Negro River Basin, Central Amazon, Brazil. Front. Ecol. Evol. 10:640961. doi: 10.3389/fevo.2022.640961
Received: 12 December 2020; Accepted: 17 March 2022;
Published: 28 April 2022.
Edited by:
Tien Ming Lee, Sun Yat-sen University, ChinaReviewed by:
Paulo Cesar Machado Andrade, Federal University of Amazonas, BrazilBenoit De Thoisy, Institut Pasteur de la Guyane, French Guiana
Carlos Freitas, Federal University of Amazonas, Brazil
Ji-Zhong Wan, Qinghai University, China
Pratheesh C. Mammen, Kerala State Disaster Management Authority (SDMA), India
Copyright © 2022 Pezzuti, Oliveira, Pantoja-Lima, Rebêlo and Félix-Silva. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Juarez C. B. Pezzuti, juarez.pezzuti@gmail.com
 Juarez C. B. Pezzuti
Juarez C. B. Pezzuti Tamires Oliveira
Tamires Oliveira Jackson Pantoja-Lima
Jackson Pantoja-Lima George Henrique Rebêlo2,4
George Henrique Rebêlo2,4