The Interplay Between Extrinsic and Intrinsic Factors in Determining Migration Decisions in Brown Trout (Salmo trutta): An Experimental Study
- 1School of Biological, Earth and Environmental Sciences, University College Cork, Cork, Ireland
- 2Environmental Research Institute, University College Cork, Cork, Ireland
- 3Food Packaging Group, School of Food and Nutritional Sciences, University College Cork, Cork, Ireland
- 4Marine Institute, Galway, Ireland
- 5Inland Fisheries Ireland, Dublin, Ireland
Many species are capable of facultative migration, but the relative roles of extrinsic vs. intrinsic factors in generating diverse migratory tactics remain unclear. Here we explore the proximate drivers of facultative migration in brown trout in an experimental laboratory setting. The effects of reduced food, as a putative environmental cue, were examined in two populations: one that exhibits high rates of anadromy (sea-migration) in nature, and one that does not exhibit anadromy in nature. Juveniles derived from wild-caught parents were reared for 2 years under four environmental treatments: low food in years 1 and 2 (Low-Low); high food in years 1 and 2 (High-High), low food in year 1 and high in year 2 (Low-High), and vice versa (High-Low). Food restriction had a significant effect on migratory tactics, with the frequency of smolts (juveniles choosing migration) highest in the Low-Low treatment in both populations. No individuals became smolts in the High-High treatment, and intermediate smolting rates were observed in the Low-High and High-Low treatments. Higher overall smolting rates in the naturally anadromous population suggested an inherited component to anadromy/migration decisions, but both populations showed variability in migratory tactics. Importantly, some fish from the naturally non-anadromous population became smolts in the experiment, implying the capacity for migration was lying “dormant,” but they exhibited lower hypo-osmoregulatory function than smolts from the naturally anadromous population. Tactic frequencies in the naturally anadromous population were more affected by food in the 2nd year, while food in the 1st year appeared more important for the naturally non-anadromous population. Migratory tactics were also related to sex, but underpinned in both sexes by growth in key periods, size, and energetic state. Collectively these results reveal how migration decisions are shaped by a complex interplay between extrinsic and intrinsic factors, informing our ability to predict how facultatively migratory populations will respond to environmental change.
Introduction
Intraspecific phenotypic variation accounts for much of the diversity of form and function in nature (Roff, 1996). Understanding the mechanisms generating and maintaining divergent phenotypes and life histories within and among populations is thus a fundamental goal of evolutionary ecology, with applied relevance to conservation and wildlife management (Naish and Hard, 2008). A particularly striking example of alternative phenotypes is the phenomenon of facultative migration, whereby individuals within a population vary in their migratory tendencies. Facultatively migratory populations can comprise a mixture of migrant and resident individuals (sometimes called “partial migration”), with migration at specific life stages occurring typically to take advantage of alterative foraging opportunities or avoid adverse abiotic (e.g., climatic) conditions (Chapman et al., 2011a). Despite its widespread occurrence across taxa and regions, fundamental gaps still exist in our understanding of proximate and ultimate drivers of facultative migration. In particular, there is a dearth of studies addressing how facultatively migratory species respond to environmental change (Doswald et al., 2009; Chapman et al., 2011b), limiting our ability to generalize about the impacts of anthropogenic factors on migratory species and to effectively manage their populations.
Polymorphisms such as facultative migration are potentially underpinned by a complex mapping between genotype and phenotype, i.e., phenotypic similarity can arise from different genotypes, or the same genotypes can produce dramatically different phenotypes through plasticity mediated by environmental cues (Roff, 1996). As such, migration and residency have often been considered as environmentally-triggered alternative phenotypes/tactics produced by an evolvable conditional strategy, where optimal tactic choice in a given context is conditional on extrinsic or intrinsic cues (Chapman et al., 2011b). This interplay between proximate and ultimate drivers of conditional strategies has been formalized as the so-called “environmentally cued threshold model” (Tomkins and Hazel, 2007). Within this framework, alternative tactics are controlled by an environmentally-sensitive status trait (e.g., physiological condition, energy state) and an inherited threshold, or “switch point,” which is assumed to be genetically variable. An individual assesses their status trait and, for example, adopts a resident tactic if it exceeds their inherited switch point, otherwise it switches to a migratory tactic. Individual physiological condition/energy state is strongly influenced by the environment, and so the assessed status trait can vary relative to the intrinsic threshold depending on external conditions; for this reason, the status trait can be thought of as an “environmental cue” and the step function relating tactic expression to cue as a “threshold reaction norm” (Tomkins and Hazel, 2007; Piche et al., 2008; Pulido, 2011; Buoro et al., 2012). There is some evidence for genetic variation in thresholds for alternative tactics, e.g., in blackcaps Sylvia atricapilla (Pulido et al., 1996) and Atlantic salmon Salmo salar (Piche et al., 2008), but detailed understanding of how external environmental variation is translated into internal physiological signals, on which migratory decisions are then based, is lacking.
Salmonine fishes (salmons, trouts, and charrs) are excellent models for disentangling causes of facultative migration as they display wide variation across a continuum of migratory strategies, coupled with obligate freshwater spawning (Klemetsen et al., 2003; Ferguson et al., 2019). Individuals can remain in freshwater post hatching for their entire life cycle, either staying in their natal stream or lake (residency tactic) or undertaking an adfluvial migration that takes them to a larger river or lake (potamodromous tactic) (Dodson et al., 2013; Ferguson et al., 2019). Facultative anadromy is an extreme form of this conditional migration strategy, where some individuals adopt the residency tactic whilst others from the same population undertake a marine migration (involving anywhere from tens to thousands of kilometers of directed movement between freshwater and saltwater). This is followed by a period of marine or estuarine feeding and growth (from months to years), before returning to spawn in natal streams (Jonsson and Jonsson, 1993). Populations can contain both resident and migratory (anadromous or potamodromous) forms, or be dominated by one life history type (Chapman et al., 2012). Both forms can breed freely in sympatry, and although offspring tend to track the tactics of their parents, either life history can be produced from a given migratory phenotype (Zimmerman and Reeves, 2000; Berejikian et al., 2014). Such flexibility indicates an interplay between genetic predisposition and environmental conditions experienced i.e., genotype by environment interactions, underpinning facultative migration (Hutchings, 2011).
The threshold reaction norm framework has been useful in understanding migratory decisions in salmonines (Hutchings and Myers, 1994; Thorpe et al., 1998; Thériault et al., 2007). If during a key decision window an individual's status trait exceeds their predetermined threshold, the fish adopts a residency tactic leading to maturation in freshwater; if not, maturation is deferred in favor of migration (Dodson et al., 2013; Kendall et al., 2014; Ferguson et al., 2017). However, the proximate factors on which individuals base the migration decision remain unclear. Previous studies have focused on a range of aspects of physiological state/energy status that may influence migratory tactics such as body size (Thériault and Dodson, 2003), lipid reserves (Jonsson and Jonsson, 2005), body condition (Hecht et al., 2015), growth (Jonsson, 1985), growth efficiency (Forseth et al., 1999; Morinville and Rasmussen, 2003), and metabolism (Sloat and Reeves, 2014). While body size is often used as a surrogate for, or argued to itself be, the status trait triggering alternative migratory tactics, the associations here have been varied and inconclusive. Larger sizes and faster growth rates have been associated with early age at migration (Jonsson, 1985), whereas others have found no size-based differences between migrants and non-migrants at a given age (Thériault and Dodson, 2003), or conversely found larger sizes (and higher lipid reserves) to be associated with freshwater maturation in lieu of anadromy (McMillan et al., 2012). These inconsistencies could reflect species' specific responses, and thus require further exploration to establish potential status traits for a given species. Studies might also be inconclusive because size is typically measured sometime after the migratory decision itself, perhaps at the parr-to-smolt transformation stage, and size at migration may not accurately reflect size when the decision was made. For example, residents may have meanwhile diverted energy into maturation and gonadal development at the expense of somatic growth (Tocher, 2003), while migrants may undergo accelerated growth as the migration itself approaches (Metcalfe, 1998).
Moreover, there may be at least two separate threshold decisions: an early one determining whether a fish will migrate per se or not, and a later one determining whether fish on a migratory trajectory actually migrate this year or defer migration to an older age (Ferguson et al., 2019). Size may be the cue used for the second decision, given that survival on entry to the sea or a lake is typically positively related to size (Klemetsen et al., 2003; Phillis et al., 2016). Yet, size at the migration point may be unrelated to, or inconsistently related to, the status trait triggering the initial migration decision, which could occur considerably earlier than the point at which migrants and resident become phenotypically distinguishable (Beakes et al., 2010). Identifying the key proximate drivers of migration is therefore complicated by the fact that the exact time windows for each of these putative decisions may not be known a priori, while correlations among physiological, energy status and growth traits may be variable across ontogeny or contexts. In the particular case of facultative anadromy, sea-migration requires a suite of adjustments in preparation for life in saltwater and therefore the physiological remodeling process, which includes changes in osmoregulation, coloration, and body shape (Tanguy et al., 1994), is likely to begin sometime in advance of the migratory period. The existence of early “decision windows” that initiate divergent life-history trajectories in salmonine fishes (Thorpe and Metcalfe, 1998; Thorpe et al., 1998) has some empirical support; for example, body condition of anadromous O. mykiss was found to be significantly lower than resident counterparts within a year of hatching and a full 12 months prior to emigration (Hecht et al., 2015).
Although the proximate drivers of migration in salmonines are unresolved, there is some consensus that potamodromous or anadromous migratory tactics are promoted by energetic limitation in natal rivers, which prevents fish reaching the inherited physiological threshold for maturation as residents (Kendall et al., 2014). Energetic limitation can arise through an interplay between environmental factors and intrinsic physiological state; for example, if freshwater food resources are insufficient to support growth rates or metabolic demands, then migration could be triggered that takes the fish to a better feeding environment such as the sea or a large lake (O'Neal and Stanford, 2011; Sloat and Reeves, 2014; Jones et al., 2015). Food limitation arising from competition at high population densities has also been shown to increase the proportion of adfluvial migratory brown trout, whereas low population densities have been associated with residency and maturation (Olsson et al., 2006; Wysujack et al., 2009). It remains largely unknown, however, during which ontogenetic stages food limitation is most important to migration decisions.
Brown trout (Salmo trutta) are an interesting model for understanding facultative migration as they exhibit highly variable strategies, with some individuals/populations remaining resident in their natal stream their entire lives, while others migrate to a larger river, a lake, an estuary, or the sea (Jonsson and Jonsson, 1993; Klemetsen et al., 2003; Cucherousset et al., 2005; Ferguson et al., 2019). Here we present the results of an experimental laboratory study of brown trout that involved F1 progeny of wild-caught parents from two populations that exhibit divergent migratory life-histories in nature. Our primary aim was to explore the interaction between intrinsic proximal factors (which may encompass both inherited and non-inherited variation) and the extrinsic environment in generating alternative migratory tactics in brown trout. Specifically, we aimed to: (i) assess the relative importance of food availability and inherited differences between populations in determining alternative migratory tactics; (ii) determine whether food restriction was more important in the first year or second year of freshwater rearing; (iii) test for differences between our two populations in their response to food restriction and its timing, which may be indicative of genotype-by-environment interactions influencing tactic frequencies, and (iv) explore associations between status traits (length, weight, condition factor) and migratory tactics. We expected that food restriction would increase the frequency of the migratory tactic overall. While we expected migratory tactic frequencies to vary overall between fish from our two population backgrounds, we also anticipated that the naturally non-anadromous stock might produce migratory phenotypes when subjected to reduced food, given that migration may only be expressed under certain environmental conditions (Roff, 1996; Pulido, 2011).
Materials and Methods
Study Populations
Wild-origin brown trout brood stock were obtained by seine netting from the Burrishoole (53° 57′ N: 09° 35′ W) and Erriff (53° 37′ 0.00″ N: 09° 40′ 17.10″ W) catchments in the west of Ireland in November 2015. Burrishoole brood stock were caught in Lough Bunaveela (46 ha, Figure S1) in the headwaters of the catchment. A local population of non-anadromous trout remain resident in Lough Bunaveela for most of their lifecycle, bar very short-distance directed movements (on the order of 10–100 s of meters) between the lake and two spawning rivers (one inflowing to the lake, the other outflowing). No obvious genetic structure at neutral microsatellite markers is evident between these spawning rivers, implying trout from Lough Bunaveela comprise a single panmictic population (R. Finlay, pers. comm.). A large run of sea trout (typically 2000+ anadromous recruits annually) occurred in the Burrishoole catchment up to 30 years ago. The Burrishoole anadromous trout run collapsed in the late 1980s, coinciding with sea-lice outbreaks following the establishment of salmon aquaculture farms in the downstream estuary. The exact spawning locations of the historic anadromous individuals within the Burrishoole catchment remain uncertain, and we cannot exclude the potential for some anadromous fish having contributed to the Bunaveela population before the anadromous population collapse. Nevertheless, despite Bunaveela spawning streams being accessible to anadromous migrants, there is little to no evidence that the Bunaveela population produced anadromous trout historically or recently (Poole et al., 2007; Magee, 2017) and we thus consider it a population that rarely, if ever, expresses anadromy.
Erriff brood stock were caught in Tawnyard Lough, a small upland lake (56 ha) on the western side of the Erriff catchment (the National Salmonid Index catchment) that is fed by a primary inflowing stream, the Glendavoch River and a number of smaller tributaries (Figure S1). The vast majority of trout spawned in the Glendavoch River are believed to disperse as fry or parr to Tawnyard Lough (a distance of a few 100 meters to a few kilometers, depending on how far up the Glendavoch River spawning occurred), although a small fraction remain permanently resident in the natal stream (P. Gargan, pers. comm.). A large run of out-migrating anadromous juveniles (in the range of 500–3,000 smolts per year over the last 30 years) is enumerated annually in a trap at the outflow of Tawnyard Lough (Gargan et al., 2016). The remaining fish never go to sea but instead spend several years growing in the lake, before returning to spawn in the Glendavoch River and smaller tributaries once mature. Brood stock from the Tawnyard population used in this experiment putatively comprised a mix of anadromous and non-anadromous fish, assumed to represent naturally occurring frequencies of anadromous and non-anadromous tactics (see Table S1 for details of brood stock), with local expertise indicating that the Tawnyard population in general shows high rates of anadromy (P. Gargan, pers comm.). In summary, we consider the Tawnward population to have a strong migratory/anadromous background, and the Bunaveela population to have essentially no (recent) anadromous background and to exhibit only limited local movements. For ease of reading, juveniles derived from Tawnyard parents are hereafter referred to simply as the “anadromous-background” population and juveniles from Bunaveela parents as the “non-anadromous background” population.
Fish Rearing
Females were stripped of eggs, and the eggs of each female were divided into two batches, each fertilized by the milt of a single male from the same source population (i.e., Tawnyard or Bunaveela; see Table S1 for full details on crossing). Fertilized eggs were then incubated in standard Heath trays in a hatchery facility located within the Burrishoole catchment. Surviving unfed fry (2–3 weeks prior to exogenous feeding) were transferred to a rearing facility at University College Cork (Aquaculture and Fisheries Development Center). While transitioning to exogenous feeding, fry were held in 100 L growth tanks on a recirculating aquaculture system (RAS) with bio filtration, and fed ad libitum to satiation using commercially available trout pellets (Skretting Ltd, Norway). The populations were kept separately in two 100 L tanks during this initial rearing phase and maintained under a natural temperature regime regulated by a single conditioning unit. Once the fry had transitioned to exogenous feeding (June 2016), they were fed ad libitum with commercial trout pellets for a period of 2 months. All fish experienced the same constant photoperiod regime (12 h of light and 12 of dark) during this initial rearing phase.
In September 2016, fish were randomly allocated into four 100 L tanks in the same RAS as described above (two tanks for Tawnyard and two tanks for Bunaveela), at which point the experimental phase began and food manipulations were initiated (see next section for experimental treatments). A random subset of fish (n = 200 per population) were given individual identifier tags using unique color combinations of visible implant elastomer tags (Northwest Marine Technology Ltd., USA). To facilitate growth, in December 2016 the fry were transferred (within their experimental groups) to 520 L growth tanks in a larger RAS in the same aquaculture facility. Continuous through flow of water prevented any waste accumulation in tanks, with returning water passed to a central holding sump and treated via mechanical filtration, protein skimming, bio filtration, and ozone and UV sterilization. Water quality in the system was monitored weekly, and levels of pH, nitrate, nitrite, and ammonia were within acceptable ranges for optimal fish health. During the experimental phase, the fish experienced a seasonally-changing photoperiod and temperature regime typical of the west of Ireland, simulated via an automated lighting system of LED lights (BioLumen, UK) above each tank and a single conditioning unit. Negligible natural mortality occurred during the experimental phase but to maintain total biomass in the RAS at acceptable levels from a water quality perspective, fish were randomly culled (n = 120 in total across all tanks) over the course of the 2 years of tank rearing, with equal fish densities maintained between food treatments. Fish that were prematurely culled were excluded from all analyses. Full details on the stripping, crossing and rearing procedures are given in Supplementary Information.
Experimental Design
The experimental phase ran for a 22 month period, from September 2016 to June 2018, with all fish humanely euthanized at the end of the experiment under license (the study and all associated procedures were carried out with ethical approval from Health Products Regulatory Authority (HPRA) Ireland, under HPRA project license AE19130/P034, and HPRA individual licenses AE19130/1087, AE19130/I200, AE19130/I201, and AE19130/I202).
To investigate the relative importance of the extrinsic environment (food supply) and intrinsic inherited factors (population-of-origin) in determining migratory tactics, juveniles from the anadromous and non-anadromous background populations were divided evenly and allocated randomly across four tanks receiving water from the same recirculating source, each experiencing a different feeding regime over the experimental phase. Populations were kept separately for the duration of the study (n = 90 per feeding treatment per population, at the beginning of the experimental phase). Great care was taken to ensure that all measured variables other than feeding regime (fish densities, temperature, photoperiod, lux, flow rates) were constant across the tanks. The four feeding regime treatments were designed to test the effects of food restriction in the early vs. late periods of this experimental phase, with each period corresponding to ~11 months [chosen because similar periods of c. 9 months have been reported to alter adfluvial migration rates in trout (Olsson et al., 2006)]. These four food regimes were as follows: (i) High-High treatment: fish fed recommended daily pellet rations for optimal growth in both periods, calculated as a percentage of their body weight and adjusted for seasonally-changing temperatures (Skretting Ltd, Norway); (ii) Low-Low treatment: fish fed 25% of recommended optimal rations in both periods; (iii) High-Low treatment: fish fed 100% of optimal daily rations in the first period and 25% of optimal daily ration in the second period; and (iv) Low-High treatment: fish fed 25% of optimal daily rations in the first period and 100% of optimal daily ration in the second period. A value of 25% of optimum levels was chosen for the Low feeding regime because similar reductions have previously been shown to reduce the frequency of the resident tactic in adfluvial brown trout (Wysujack et al., 2009). Rations were reduced down to 25% of optimal gradually over a 4-week period, to minimize stress. Within each food treatment, absolute rations were adjusted according to manufacturer's instructions (see Table S2) on a monthly basis to account for changes in body mass and temperature (i.e., there was no variation in daily rations within months, within groups).
Life History Determination and Data Collection
In the spring of 2017 and 2018 (March–June in year 1 and 2 of the experimental phase of the study), fish were routinely assessed for morphological indicators of “smoltification”: the series of morphological, physiological and behavioral changes that is generally considered a precursor to downstream migration of juvenile salmonids (Tanguy et al., 1994). Here we use “smolt” to simply mean a fish showing external morphological features consistent with preparing for a migration, and we use saltwater tolerance tests (see below) to further assess physiological aspects of smoltification. We visually assessed morphological smoltification (silvered flanks/loss of parr marks, pronounced lateral line, colorless fins and fusiform shape) according to Tanguy et al. (1994). No fish matched the morphological criteria of smolts in the spring of 2017, the very earliest point at which we expected any smoltification (Poole et al., 2007; Gargan et al., 2016). Individuals that matched the morphological criteria for smolts in spring 2018 were transferred to saltwater at 30 ppt for 24 h to assess their hypo-osmoregulation as a further indicator of anadromy capacity. We used 30 ppt salinity (following Tanguy et al., 1994) because trout often spend large amounts of time in brackish water/estuaries when migrating, hence trout smolts are typically less saltwater tolerant than other salmonids e.g., Atlantic salmon (Urke et al., 2010). After the 24-h immersion in saltwater, a period proposed to induce hypo-osmoregulation in euryhaline species (Schultz and McCormick, 2012), fish were euthanized with an overdose of MS-222 and a blood sample was taken from the caudal vasculature using a 21 G needle and a 2.6 ml heparinized syringe. Blood samples were transferred to 2 ml epindorphs and centrifuged at 8,000 rpm for 3 min. The plasma aliquot was then siphoned off and stored at −80°C before being measured for plasma chloride concentration as an indicator of hypo-osmoregulatory ability.
All fish, whether identified morphologically as smolts or non-smolts, were dissected to visually determine sex and maturation status according to gonad development. Males were classed as sexually mature if they had enlarged white testes or had running milt. Males that had visible testes that were moderately enlarged but not running milt were classed as maturing. Females were classed as mature or maturing if the body cavity contained identifiable eggs. Fish with immature gonads, or that could not be identified as either male or female by visual inspection were classed as immature at the time of sampling, and their genotypic sex was later determined using a microsatellite sex marker (P. Prodöhl, unpublished). In the wild, the natural spawning period for these brown trout populations is in late autumn/early winter, and the migratory period is in the spring (Poole et al., 2007; Gargan et al., 2016). Fish showing signs of maturity in freshwater without having first gone to sea, were considered to be on a non-anadromous trajectory, while smolts migrating to sea in a given spring were all immature. Fish in our experiment were thus classed as smolts (migratory tactic) if they were morphologically assessed as smolts and were immature, and were classed as mature (freshwater maturation tactic) if they were mature or maturing at the time of sampling. Fish that were classed as immature, but did not have morphological indicators of smoltification, were considered to have an unknown life history tactic at the time of sampling. A small number of fish (n = 12) had significant skin/fin damage at the time of sampling, and were excluded from the analysis. Whole body lipid content (%) was measured for all smolts, and for a random sample of mature fish (n = 111), using a SMART Trac 5 system (CEM GmnH, Kamp-Lintfort, Germany) of integrated microwave heating and nuclear resonance on homogenized samples.
Statistical Analysis
To assess whether food treatment and population influenced life history tactics (Aims 1 and 2), we constructed generalized linear models (GLMs) with a logit link function and binary life-history response variables. One GLM was created to predict smolt status (binary response: 1 = smolt, 0 = non-smolt) using the brglm package in R (Kosmidis, 2019) to account for separation in the data (no smolts recorded in the High-High treatment) (Heinze and Schemper, 2002). A second GLM was created to predict maturation (binary response: 1 = mature or maturing, 0 = immature). Categorical explanatory variables in both of these GLMs included food treatment (High-High, Low-High, Low-Low, High-Low), population (anadromous-background vs. non-anadromous-background), and sex (male or female) as predictors. We constructed a third GLM to test for treatment/population effects on likelihood of being classed as “unassigned” (i.e., not having expressed a migratory/resident phenotype by the end of the study (binary response: 1 = unassigned, 0 = smolt or mature). We included an interaction term between food treatment and population to determine if life history responses in each population were similar under the different food regimes (Aim 3). To test whether food restriction was more important in the early or late rearing periods (Aim 2), we conducted Tukey post-hoc tests of all possible pairwise comparisons among the levels of food treatment using the emmeans package in R (Lenth, 2019). Overall, one expects the strongest difference in life-history tactics to be found between the High-High and Low-Low treatments. If the effects of food restriction are additive and the timing of food restriction does not mater, then one expects life-history tactics in the Low-High and High-Low treatments to be intermediate between the High-High and Low-Low treatments, and not significantly different from each other. Conversely, if food restriction is more important in the first period, then one expects tactic frequencies in the Low-High treatment to be closer to those in the Low-Low treatment (and the High-Low treatment should be more similar to the High-High treatment), while if food restriction is more important in the second period, the High-Low treatment should be closer to the Low-Low treatment and the Low-High treatment to the High-High. To further explore factors influencing variation in saltwater tolerance (Aims 1–3)—a key component of life-history tactics—we constructed a linear model (normal errors) with plasma chloride concentration as the continuous response, and population, food treatment, sex, and an interaction between population and food treatment included as predictors.
To address Aim 4, we explored factors influencing variation in the length, weight and condition factor of fish at different measurement time points across the study period within a mixed-effects modeling framework [nlme package (Pinheiro et al., 2019)]. Measurement time points were September and November in 2016, February, April, June, July, September, and December in 2017, and April 2018. Condition factor was calculated as Fulton's K where:
For the subsequent analyses of status traits, we created a new categorical variable called ‘life-history tactic' with two levels: migratory (i.e., immature smolts) or mature/maturing (hereafter simply called mature). Fish which were neither classified as migratory nor mature (unassigned fish) were not included in the status trait analyses, as it could not be determined which life history trajectory they might adopt [i.e., these fish could have displayed either migratory or mature tactics the following spring (a full 3 years after hatching), but the experiment was terminated the previous spring (2 years after hatching)]. In addition to life-history tactics, month (continuous variable), population (categorical variable with two levels), food treatment (categorical variable with four levels), and sex (categorical variable with two levels) were included as fixed effects, and individual identity was included as a random effect to account for multiple measurements on some individuals. We included an interaction between life-history tactics and month (to test whether individuals on different life-history trajectories diverged through time in their length/weight/condition factor), an interaction between life-history tactics and population (to test whether average differences in length/weight/condition factor between the two tactics was similar across the two populations), and an interaction between population and food treatment (to test whether the effects of food regime were similar across populations). Temporal autocorrelation of the response variable was accounted for by modeling an autoregressive error structure as a first order lag function of month. Separate models were constructed each for length, weight, and condition factor and normal errors were assumed in each case.
We also explored factors influencing variation in final length, K and whole body lipids (i.e., the final measurements for these status traits at the end of the study) in a mixed effects modeling framework, where life-history tactics, food treatment, population, and sex were included as fixed effects, and date of terminal sample (categorical variable with 11 sampling dates) was modeled as a random effect. We included two interaction terms (life-history tactics × population, and food treatment × population), to explore whether the patterns for each population were similar across tactics and food treatments, respectively. Separate models were constructed each for length, K and whole body lipids and normal errors were assumed in each case. Marginal R2 values for mixed effect models were calculated using the MuMIn package in R (Barton, 2018).
For all of the above models, statistical significance at a 5% alpha level of predictor variables was assessed using likelihood ratio tests (LRT), and non-significant interaction terms were omitted so the main effects could be interpreted.
Finally, to assess whether variation in growth was associated with life-history tactics (Aim 4), we compared growth trajectories of migratory and mature fish by fitting three typical models of fish growth: the von-Bertelanffy growth curve, the Gompertz growth curve and a logistic growth curve. The logistic growth curve best described the data according to AIC (ΔAIC = 0), and was used for all further growth trajectory analysis. The logistic growth equation models asymptotic growth as:
Where L is fork length, L∞ is asymptotic fork length (cm), gi is the growth rate (cm/day), T is time (days) and I is the inflection point. The logistic model was fitted using non-linear least squares to length data collected on individually-identifiable fish during the experiment, with separate models fitted for smolts and mature fish. As non-linear least squares regression is sensitive to starting values of parameters, the model was fitted using the nls_multstart function from the nls.multstart package in R (Padfield and Matheson, 2018). This allowed for starting values for each parameter to be randomly selected from a bounded distribution over 1,000 iterations of the model, with the best available model then selected by AIC. To determine the fit of the most parsimonious model to our data, we bootstrapped with replacement 10,000 times and constructed 95% confidence intervals from the bootstrapped fits.
All analysis was carried out in R version 3.5.3 (R Core Team, 2019), and all statistical models were checked against assumptions of the given model (independence, non-normality of residuals, heteroscedasticity and multicollinearity).
Results
Life-History Tactics
By the end of the experimental phase, a total of 567 fish had been categorized as either smolts i.e., putatively migratory (n = 36 females and n = 18 males) or non-smolts (n = 277 females and n = 236 males). All of the smolts were by definition immature, and 15.52% of the non-smolt females and 28.39% of the non-smolt males were immature. See Table 1 for a full breakdown of life-history tactics by population background, food treatment and sex. The proportion of smolts varied according to food treatment and population (Figure 1). Highest proportions of smolts were seen in the Low-Low food treatment, in which 26.56% of the anadromous-background population, and 15.71% of the non-anadromous background population, were classified as smolts. The lowest rates of smolting were found in the High-High food treatment, in which no fish from either population were categorized as smolts. Intermediate smolting rates were observed in the other two treatments, with 6.45% of fish from the anadromous-background population and 13.75% of fish from the non-anadromous-background population classified as smolts in the Low-High treatment, and 15.87% and 1.22% of fish from each population, respectively, classified as smolts in the High-Low treatment.
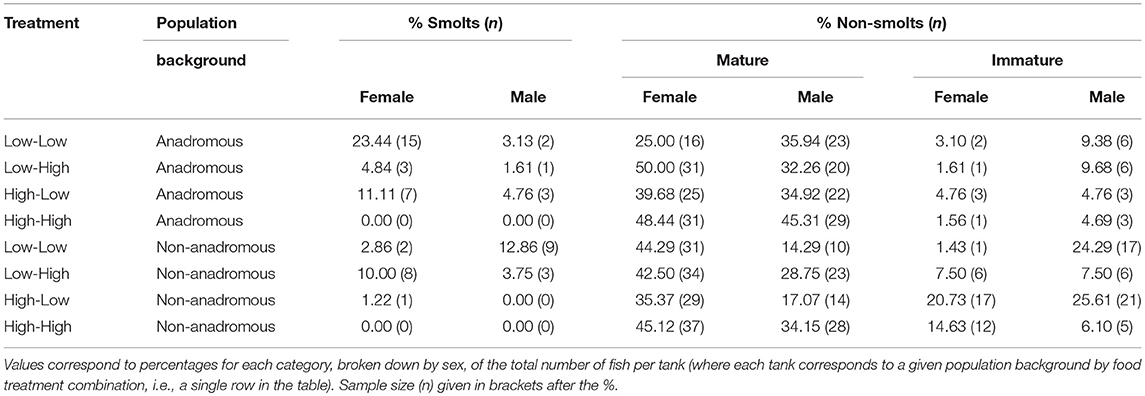
Table 1. Percentage of brown trout (n = 567, F1 offspring of wild trout from two population backgrounds) classed as smolts (i.e., migratory tactic) or non-smolts (mature or immature) after 2 years of experimental tank-rearing.
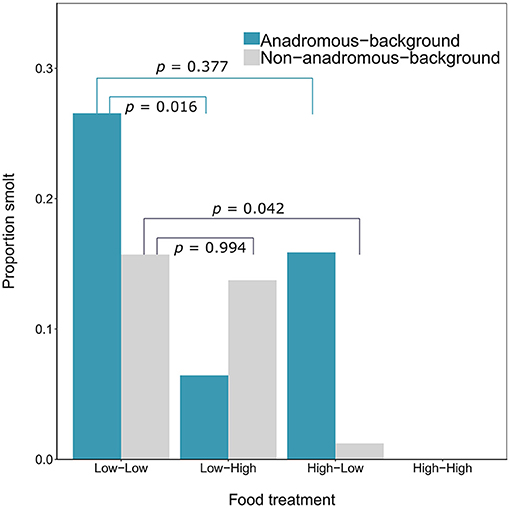
Figure 1. Proportion of brown trout (n = 567, F1 offspring of wild trout from two population backgrounds) classed as smolts after 2 years of tank rearing under varying food restriction treatments. Food treatment is denoted in the format “food in year one—food in year two,” where “high” refers to optimal food rations and “low” refers to 25% of optimal rations. P-values shown are Tukey post-hoc pairwise comparisons across all levels of food treatment for each population.
The probability of smolting was described by a GLM retaining food treatment (χ2 = 44.57, df = 3, p < 0.001), population (χ2 = 3.46, df = 1, p = 0.063), sex (χ2 = 4.40, df = 1, p = 0.036), and an interaction between food treatment and population (LRT for the model with and without interaction term: χ2 = 11.66, df = 3, p = 0.009). Overall across the two populations, there appeared to be an additive effect of food treatment on the probability of smolting — that is, the percentages of smolts in the Low-High and High-Low treatments were similar, and approximately intermediate to the percentages in the Low-Low and High-High treatments, when population was ignored (Figure 1). However, when population was taken into account, the life-history response to food treatment varied by population and appeared to be non-additive within each population (Figure 1; Table 2). Fish from the anadromous-background population exhibited a relatively high percentage of smolts (15.87%) under the High-Low treatment that was closer to the Low-Low treatment (26.56% smolts) than to the High-High treatment (0% smolts) and post-hoc comparisons of High-Low against Low-Low were not significant (p = 0.377). The opposite was true for the anadromous-background population in the Low-High treatment (6.45% smolts) with significant post-hoc comparisons of Low-Low and Low-High (p = 0.016). In contrast, fish from the non-anadromous-background population exhibited a relatively high percentage of smolts (13.75%) under the Low-High treatment that was closer to the Low-Low treatment (15.71% smolts) than to the High-High treatment (0% smolts) (post-hoc contrasts between Low-High and Low-Low were non-significant, p = 0.994), while the opposite was true for this population in the High-Low treatment (1.22% smolts) (post-hoc contrasts between High-Low and Low-Low were significant, p = 0.042). This implies that food restriction was more important in the second period for fish from the anadromous-background population, while food restriction in the first period was more important for the non-anadromous-background fish.
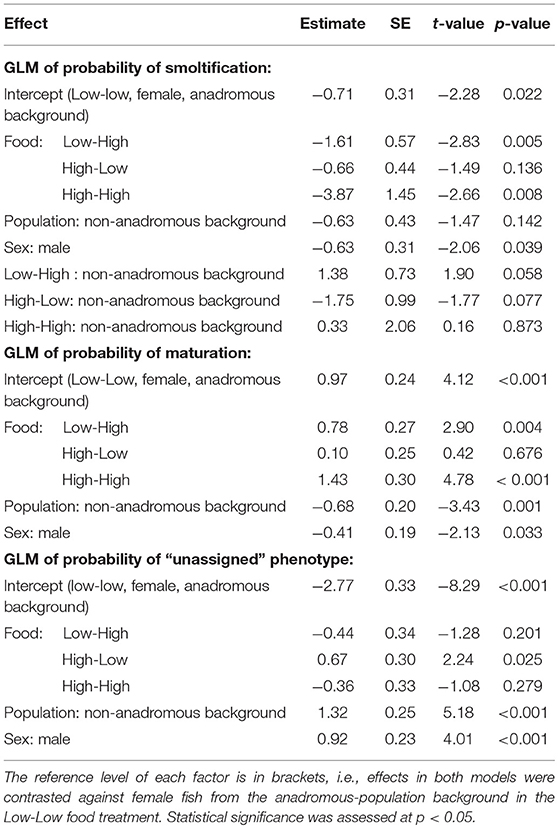
Table 2. Parameter estimates with associated standard errors (SE) for two binomial generalized linear models (GLM) predicting smolt (migratory) probability (dummy coded: smolt = 1, non-smolt = 0) and freshwater maturation (dummy coded: mature/maturing = 1, immature = 0) in brown trout (n = 567).
Maturation tactics in freshwater were also significantly affected by food treatment (χ2 = 33.03, df = 3, p < 0.001), population (χ2 = 12.14, df = 1, p < 0.001), and sex (χ2 = 4.54, df = 1, p = 0.033) but there was no significant interaction between food treatment and population (LRT for the model with and without interaction term: χ2 = 5.31, df = 3, p = 0.150). Food restriction had a negative effect on maturation probability, in direct contrast to food restriction effects on smolting rates. Fish in the Low-Low food treatment had the lowest probability of maturing (p < 0.001, Table 2), and the highest rates of maturity were observed in the High-High food treatment (p < 0.001, Table 2). Fish from the anadromous-background population were significantly more likely to mature than fish from the non-anadromous-background population in all food treatments (p = 0.001, Table 2). See Table 2 for all parameter estimates and associated standard errors. The probability of having been unassigned a life history showed similar patterns to maturation tactics, and was similarly significantly affected by food treatment (χ2 = 16.95, df = 3, p = 0.001), population (χ2 = 30.74, df = 1, p < 0.001), and sex (χ2 = 16.21, df = 1, p < 0.001), see Table 2. The interaction between food treatment and population was marginally not significant (LRT for the model with and without interaction term: χ2 = 7.75, df = 1, p = 0.052).
We found a significant effect of population on plasma chloride levels of fish classified as smolts (F = 9.47, df = 1, 48, p = 0.003), but the interaction term between population and food treatment was not significant (LRT for model with and without interaction term: F = 1.39, df = 2, p = 0.259). Fish from the anadromous-background population had significantly lower plasma chloride concentrations than non-anadromous-background fish (p = 0.003, Figure 2; Table 3). There was no significant effect of food treatment (F = 2.95, df = 2, 48, p = 0.062) or sex (F = 0.01, df = 1, 48, p = 0.991) on plasma chloride levels (Table 3).
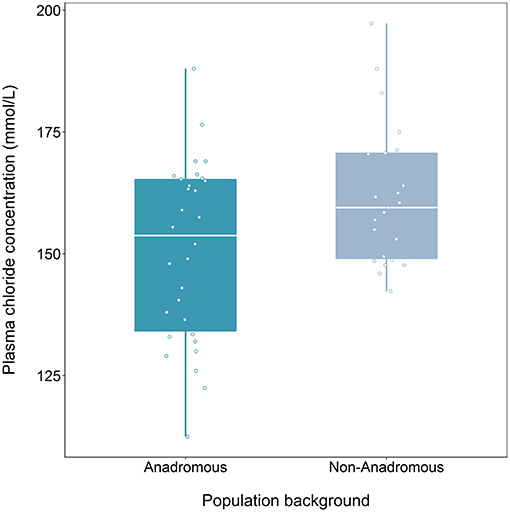
Figure 2. Plasma chloride concentration (mmol/L) after 24 h saltwater immersion of brown trout smolts (migratory tactic, n = 54) derived from two population backgrounds. The median is represented by the white horizontal lines in each box.
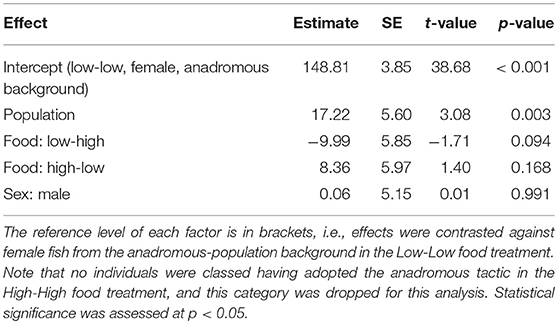
Table 3. Parameter estimates with associated standard errors (SE) for the linear model testing effects of population, sex, and food treatment on plasma chloride concentration (mmol/L) of brown trout classified as smolts (n = 54).
Factors Explaining Variation in Status Traits at Different Time Points
At the time at which the food treatments were first applied, fish from both populations were in similar condition (F = 0.41, df = 1, 137, p = 0.523), however, anadromous-background fish were heavier (F = 17.14, df = 1, 137, p < 0.001) and longer (F = 16.31, df = 1, 137, p < 0.001) than non-anadromous-background fish. A mixed model analysis indicated further divergence in these status traits over the study period that was related to life-history tactics, food treatment, and population effects (Figure 3; Table 4). The models for length (marginal R2 = 0.77), weight (marginal R2 = 0.62), and K (marginal R2 = 0.35) retained a significant interaction between food treatment and population, and a significant interaction between life-history tactics and month (Table 4). Sex did not have a significant effect on length (χ2 = 0.024, df = 1, p = 0.877), weight (χ2 = 0.050, df = 1, p = 0.823), or condition factor (χ2 = 0.082, df = 1, p = 0.774). After accounting for growth between measurement periods (i.e., the fixed effect of measurement period), smolts tended to be shorter, lighter and have lower condition than mature fish (Table S3). The differences in length, weight and K were similar for both populations (an interaction between population and life-history tactics was not retained in any of the final models, see Table 4). The significant interaction between food treatment and population indicated that fish from the anadromous-background were larger, and heavier (but in similar condition) than fish from the non-anadromous-background under both High-Low and High-High treatments (Table S3). However, in the Low-Low and Low-High treatments, there were negligible differences in length, weight and K between populations (Table S3). The significant interaction between month and life-history tactics indicated that changes in length, weight and K through time varied between smolts and mature fish. Mature fish tended to increase in length and weight quicker (Figure 3B; Table S3), while smolts tended to be in worse condition (lower K) earlier (Figure 3C; Table S3). See Table S3 for all model outputs.
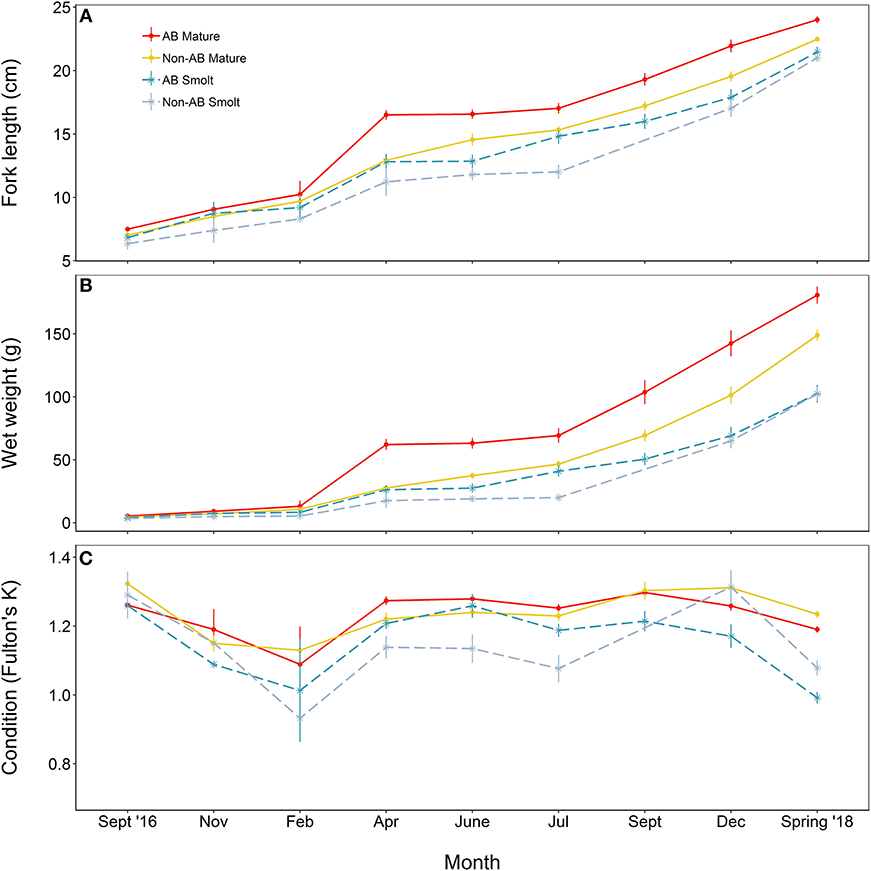
Figure 3. Trajectories of (A) length, (B) mass, and (C) condition factor (K) of brown trout offspring (derived from wild-caught parents from two populations) that were classed as either smolt (migratory tactic) or freshwater maturing (non-migratory/resident) tactic. AB, anadromous-background population; non-AB, non-anadromous-background population. Mean values (with associated standard errors) are shown for measurements taken at key time points over the course of 2 years of tank rearing.
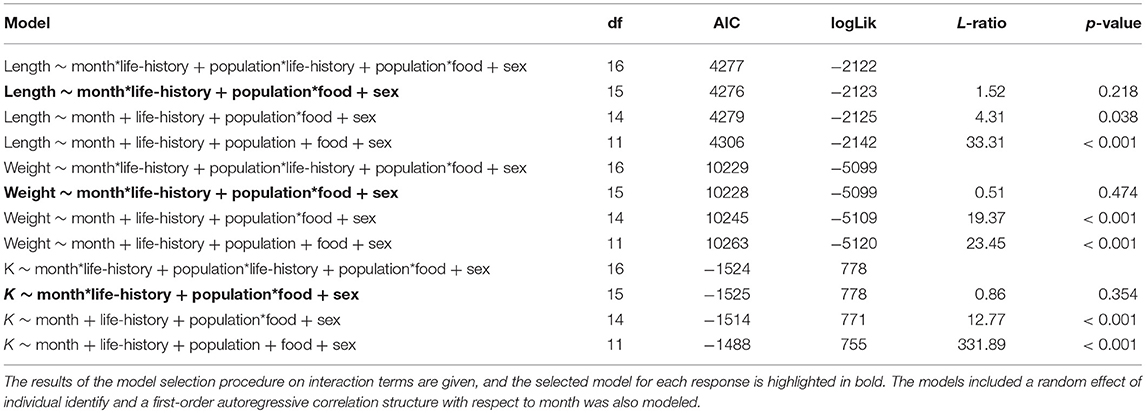
Table 4. Results of the mixed effect model analysis for length, weight and condition factor (K) trajectories of brown trout in the experiment with life-history classed as either smolts (i.e., migratory) or freshwater mature across the study period.
Factors Explaining Variation in Final Values for Status Traits
At the end of the study, fish differed in length, condition and lipid content according to food treatment, life-history tactics and population (Figure 4). The model describing length (marginal R2 = 0.50) retained a significant interaction between food treatment and population (Table 5) but did not indicate a significant effect of life-history tactics (χ2 = 2.83, df = 1, p = 0.093), or sex (χ2 = 0.005, df = 1, p = 0.947). The models describing condition (marginal R2 = 0.56) and whole body lipids (marginal R2 = 0.73, Table 5) each retained an interaction between population and food treatment (Table 5), and included a significant effect of life-history tactics on condition (χ2 = 64.58, df = 1, p < 0.001), and whole body lipids (χ2 = 7.71, df = 1, p = 0.005). Sex did not have a significant effect on condition (χ2 = 3.43, df = 1, p = 0.064) or whole body lipids (χ2 = 2.18, df = 1, p = 0.140). Overall, smolts were of similar length to mature fish at the end of study (Figure 4), but tended to be in poorer condition (p < 0.001, Table S4) and have slightly higher whole body lipids (p = 0.008, Table S4). We detected an interactive effect of food treatment and population, where fish from the anadromous-background population were larger than fish from the non-anadromous-background population, but similar under Low-Low food conditions (Table S4). However, non-anadromous-background fish were overall in better condition (p = 0.011, Table S4) and had higher whole body lipids (p < 0.001, Table S4), and these differences between populations were strongest under conditions of Low-Low food (Figure 4; Table S4). The lack of significant interactions between life-history tactics and population in the models for length, K, and whole body lipids indicated that differences between populations were similar for both mature fish and smolts (Table 5). See Table S4 for all model outputs.
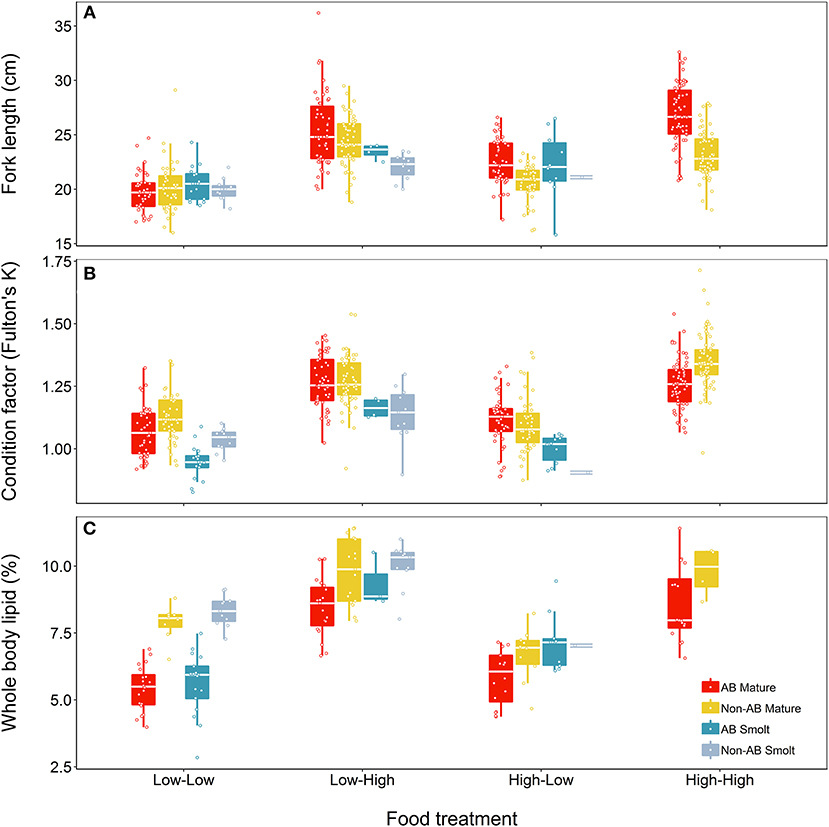
Figure 4. Effects of food treatment on final (A) length, (B) condition factor (K), and (C) whole body lipids at the end of the experimental study (Spring 2018) of brown trout offspring classed as either smolts (migratory) or freshwater maturing (non-migratory/resident). Offspring were derived from wild-caught parents from an anadromous-background population (AB) and a non-anadromous-background population (non-AB). The median is represented by the white horizontal lines in each box.
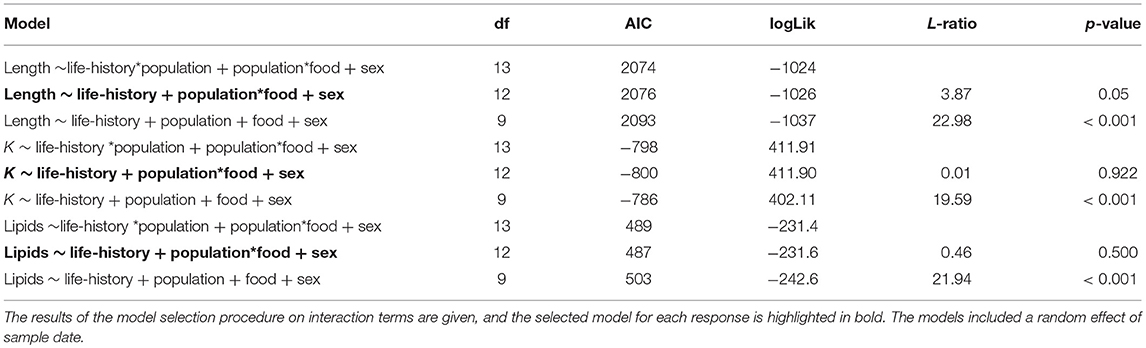
Table 5. Results of the mixed effect model analysis for length, condition factor (K), and whole body lipids of brown trout (life-history classed as either smolts or freshwater mature) at the end of the experimental study period.
Growth Rate Differences
The somatic growth of fish during the experiment was well-described by a logistic growth model. Initial model fitting indicated the most parsimonious model included separate growth parameters for smolts and mature fish. Mature fish had higher intrinsic growth rates (gi = 0.0050, SE = 0.0006, p < 0.001), a smaller asymptotic size (L∞ = 25.44, SE = 0.86, p < 0.001), and a lower point of inflection (I = 172.7, SE = 13.8, p < 0.001) than smolts, where gi = 0.0039 ± SE 0.0009 (p < 0.001), L∞ = 27.31 ± SE 4.13 (p < 0.001), and I = 305.7 ± SE 89.9 (p = 0.001). Mature individuals were relatively larger earlier in life than smolts, and had faster overall growth (Figure 5).
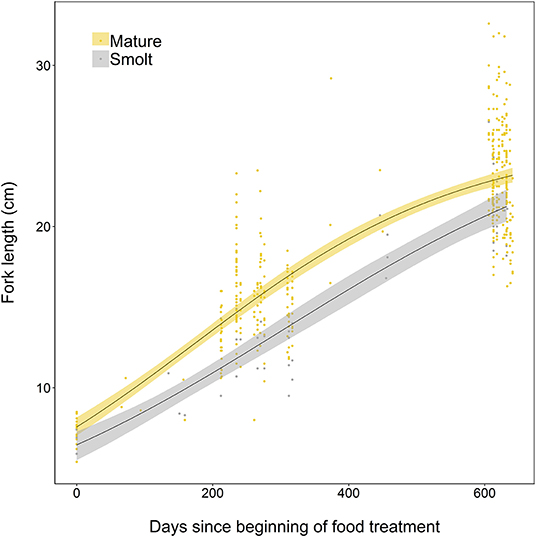
Figure 5. Growth curves, based on length measurements spanning 2 years of experimental tank-rearing, of brown trout classed as either smolt (migratory) or freshwater maturing (resident) in Spring 2018. Fitted lines are based on the best-fitting parameters from the logistic growth model, fitted using non-linear least squares regression. Shaded areas represent the 95% confidence intervals constructed by bootstrapping for 10,000 iterations.
Growth differences between the two populations were also identified, where fish from the anadromous-background population were relatively larger earlier in the study than fish from the non-anadromous-background population, and grew faster (Figure 6). Anadromous-background fish had higher intrinsic growth rates (gi = 0.0045, SE = 0.0009, p < 0.001), similar asymptotic size (L∞ = 26.83, SE = 1.68, p < 0.001), and a lower point of inflection (I = 184.1, SE = 26.9, p < 0.001) than non-anadromous-background fish, where gi = 0.0043 ± SE 0.0007 (p < 0.001), L∞ = 26.45 ± SE 1.65 (p < 0.001), and I = 236.3 ± SE 32.9 (p < 0.001).
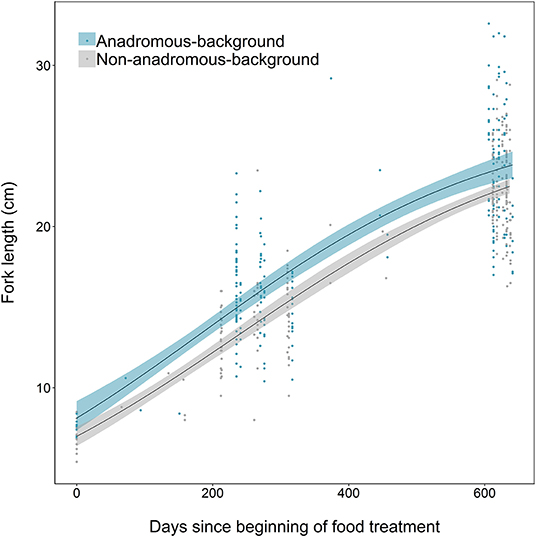
Figure 6. Growth curves, based on length measurements spanning 2 years of experimental tank rearing, of brown trout derived from two population backgrounds (anadromous or non-anadromous). Fitted lines are based on the best-fitting parameters from the logistic growth model, fitted using non-linear least squares regression. Shaded areas represent the 95% confidence intervals constructed by bootstrapping for 10,000 iterations.
Discussion
Salmonine fishes exhibit some of the most striking examples of animal migration, but uncertainty still surrounds the mechanisms by which alternative migratory tactics can be expressed, or inhibited, across salmonine populations. A principle aim of our study was to assess the importance of food availability at different time points during early ontogeny in determining migratory/life-history tactics in two populations of brown trout. Food reduction across almost 2 years led to increased rates of smolting (migratory tactic) in fish from both population backgrounds, whilst no fish were classed as having adopted the migratory tactic in either population after 2 years of experiencing high food, i.e., optimal rations (Figure 1). Migratory/life-history tactics were also influenced by population background, consistent with an inherited component to migratory/life-history decisions—fish derived from a naturally anadromous population were more often classed as smolts in our experiment, while offspring derived from a naturally non-anadromous population were more often classed as non-smolts, or having undergone freshwater maturation consistent with a residency tactic. Intriguingly, the populations responded differently to the timing of food restriction, with fish from an anadromous population background seemingly having been more affected by food restriction in their second year, whilst fish from a non-anadromous population background were more affected by food restriction in their first year. Females were more likely than males to become smolts under all food treatments. Collectively, these results indicate both extrinsic (food-driven) and intrinsic effects (related to population background, sex and other individual-level attributes) on migratory/life-history tactics in brown trout, that may interact in complex ways and influence how populations respond in the wild to changing environmental conditions.
Differences in growth and body condition were apparent from an early stage between fish adopting different life-history/migratory tactics, and were maintained across the full (almost 2-year) duration of the study. These differences were in turn also driven by both extrinsic and intrinsic effects. Extrinsic effects were evidenced by the fact that large differences in fork length, mass, body condition and whole body lipids were apparent between fish reared under different food treatments, which in turn contributed to fish adopting different life-history tactics via phenotypic plasticity. Intrinsic differences among individuals in “status traits” clearly also contributed to migratory/life-history outcomes, given that differences in body size, condition and lipids were apparent between populations, and between fish from each population that adopted different tactics within each food treatment—where the external environment was the same. Such intrinsic variation within and between populations could reflect inherited genetic effects, inherited non-genetic effects (e.g., parental effects, epigenetic inheritance), or non-inherited differences driven by early-life environmental influences that have a relatively long-lasting effect on phenotype (Burton and Metcalfe, 2014). Expanding our approach to incorporate even earlier life stages (e.g., post-hatching/fry) could further illuminate how factors in early life influence life history.
Extrinsic Factors
The observed increases in smolting in the face of food restriction, together with decreases in maturation, suggested that the reduction in food supply prevented individuals from meeting an intrinsic (e.g., genetically determined) threshold for residency and maturity in freshwater, which is in agreement with previous studies (Olsson et al., 2006; Wysujack et al., 2009; O'Neal and Stanford, 2011; Jones et al., 2015). Indeed, the absence of any smolts under conditions of high food supply was surprising, particularly within fish from the Tawnyard population (anadromous-background), which has a naturally high frequency of anadromy in the wild (Gargan et al., 2016). This suggests that, in nature, a large number of fish in the Tawnyard system must typically experience relatively low food availability as freshwater juveniles, as otherwise anadromy rates would be lower in the wild. Moreover, the balance of fitness cost and benefits of migration in the system must be such that natural selection has caused a relatively high threshold for residency to evolve (an ultimate mechanism; Hazel et al., 1990; Tomkins and Hazel, 2007; Pulido, 2011), meaning a minority of Tawnyard fish in the wild typically surpass their intrinsic freshwater maturation threshold and the anadromous tactic is more frequent.
Manipulation of the timing of food reduction revealed that life-history responses of a given population to environmental change might depend on the point during ontogeny at which the change is experienced. This could come about via two non-mutually exclusive mechanisms: populations could exhibit variation in sensitivity to cues experienced during given fixed “decision windows,” and/or the timing of the decision windows themselves may vary across populations. In our study, food restriction in the first year (Low-High treatment) was a more important driver of smolting rates than food in the second year (High-Low) for fish from the non-anadromous-background population, whereas food in the second year was more important for the anadromous-background population. This was an intriguing outcome, and hints at a complex interplay between extrinsic environment and intrinsic or population-specific factors. The apparently greater importance of food restriction in the first year for the non-anadromous-background population could perhaps be related to lower intrinsic growth rates in this population in the wild. Given their low potential growth rates, individuals in the non-anadromous-background population might be constrained to make a life-history decision (i.e., choose future migration or residency) early in life in order to divert energy intake toward meeting the associated demands of the chosen tactic. Because residents must accumulate sufficient lipid reserves to be converted into reproductive tissue before spawning (McMillan et al., 2012), in the wild, Bunaveela fish may have experienced selection for adopting a maturation trajectory relatively early in order to allow sufficient time for growth and energy accumulation, with early decision windows evolving as a consequence. In contrast, fish from the anadromous-background population with higher intrinsic growth potential may be less constrained in this regard, and may defer choice of migratory tactics to the second year of life, or indeed have flexibly reversible life-history trajectories where, for example, fish choosing residency based on high food in year one may switch to migratory tactics in response to low food in year two. There is some evidence for conditions in the second year of life being a key driver of migratory tactics in a naturally facultatively anadromous brown trout population to support this (Cucherousset et al., 2005).
Coupled with a later “decision window”/higher sensitivity to conditions in year two, a naturally high intrinsic growth propensity in the anadromous-background population could have facilitated high levels of compensatory growth when receiving optimal food resources in year two in the Low-High treatment. If growth, or some aspect of energy usage related to growth such as body condition, is used as a cue for migratory tactic choice, this may then have translated into more individuals from this population meeting their threshold for maturation in the Low-High treatment. Strong compensatory responses after periods of food restriction have been observed in salmonids in general, and interestingly, the compensatory response has often appeared to be directed toward restoring body condition, rather than size. Nicieza and Metcalfe (1997) found food restricted fish recovered similar condition to controls within a year of food supply restoration, and Alvarez and Nicieza (2005) further found a compensatory response that resulted in restoration of condition and energy status rather than skeletal growth in brown trout post food restriction.
Alternatively, we cannot rule out the presence of multiple migration vs. residency decision windows, that re-occur annually or more frequently, whereby an individual repeatedly re-assesses its status trait relative to its inherited freshwater maturation threshold and can remain “undecided” at the first or even second windows, though there is little empirical evidence for this. A simpler explanatory model is that there is a single, initial decision determining migration vs. residency, and then subsequent decision windows occur for fish on each trajectory (migrants and resident) related to the timing of expression of the adopted life-history tactic, where for example migrants must decide at what age to actually migrate (determined by pressures of size-dependent sea survival), or indeed where to migrate (Ferguson et al., 2019). Similarly, a resident individual must also decide when to mature (Thorpe and Metcalfe, 1998; Thorpe et al., 1998), a decision shown to be affected by lipid reserves in Atlantic salmon (Rowe et al., 1991; Jonsson and Jonsson, 1993, 2005) and possibly triggered by similar threshold type mechanisms in brown trout. These timing decisions could be further influenced by extrinsic environmental conditions, giving rise to a temporal continuum of migration and maturation tactics. This may explain why some fish in our study were classified as having an undetermined life-history (neither smolt nor mature) by spring of year two: these individuals may simply have been delaying expression of a migratory or freshwater maturing phenotype until the following year. These caveats must be born in mind when interpreting our experimental results, as the life-history tactic frequencies we measured in year 2 could be indicative of age-specific tactic frequencies, rather than overall rates of migration vs. residency across all ages. However, the basic conclusions were the same in the GLMs where the data were analyzed as either smolt vs. non-smolt, or immature vs. mature, giving us confidence that the patterns reflect the migration decision per se.
Variation in Status Traits Underpinning Alternative Tactics
Size-based differences between migrating individuals (those classified at the end of the study as smolts) and resident fish (those classified at the end of the study as mature) were established relatively early, with differences in weight, length, and condition that were maintained during the course of the study. The early divergence in physiological condition between migrants and residents supports the energy limitation scenario, where fish adopt migration as a result of failing to meet the necessary condition in early life to mature as residents in freshwater (Jonsson and Jonsson, 1993). Maturing fish reached an apparent size asymptote earlier than migrating fish (i.e., had smaller inflection point in Figure 5, and were larger earlier in the study). Size appears to be a potential status trait that regulates, or correlates with factors regulating early sexual maturation, as has been documented in Atlantic salmon, where anadromous males are smaller than their counterparts that mature early in freshwater as so-called “precocious parr” (Whalen and Parrish, 1999; Garant et al., 2002). However, although body size has been suggested as a major component of the status (cueing) trait for anadromy in brook trout (Thériault et al., 2007), the divergence in mass and condition we find here in our study suggests that other factors beyond size also contribute to the maturation vs. migration/anadromy decision. It seems increasingly likely that a suite of interlinked physiological components is assessed (e.g., overall energetic status or rate of change in energy), and no single trait controls the migratory/anadromy decision. Genetic covariance between life history traits such as growth, size, metabolism, and other morphological traits further suggests that migration decisions are associated with a suite of inter-linked phenotypic traits (Doctor et al., 2014; Hecht et al., 2015).
Fish on a migratory trajectory here appeared to maintain growth rates during the experiment (and had a higher inflection point), such that they were similar in length to mature fish by the end of our study. Constant, or even accelerated growth in pre-migratory fish (Metcalfe, 1998) has been explained by size-dependent survival at sea (Klemetsen et al., 2003) due to better osmoregulation ability (Finstad and Ugedal, 1998) and reduced predation on larger anadromous individuals (Dill, 1983; Jonsson et al., 2017). Interestingly here, although skeletal growth (i.e., length) was maintained, migratory fish were considerably lighter and in worse condition than mature fish at the end of study, which suggests that once on a migratory trajectory, resources were primarily allocated to meeting a size-based threshold for surviving actual migration. The maintenance of growth rates in migrants as such does not contradict the energy limitation scenario, but rather suggests that migratory fish redirect what resources they obtain into becoming large enough to survive the migration, at a cost to their overall body condition.
The diminished body condition of migratory individuals was not, however, reflected in levels of whole-body lipids at the end of the study. Contrary to our expectations, migratory fish had marginally higher levels of whole body lipids than mature fish. Lipid storage has been identified previously as an important precursor of maturation in fish (Tocher, 2003) and an indicator of a residency life history in salmonids (Tocher, 2003; Sloat and Reeves, 2014; and references therein). The unexpected trend we observed in lipids may have been a consequence of measuring lipids during the smolt migration period, at which stage fish that have initiated maturation might have already converted some of their energy stores into gonadal tissue, and hence show depleted lipids levels relative to migrants (Tocher, 2003; Sloat and Reeves, 2014). Alternatively, higher lipid levels in migrants could reflect accumulation of reserves, as either a bet-hedging strategy if resources in the migration destination are uncertain, or to fuel the migration journey itself (Stefansson et al., 2003). Pre-migratory “fattening” strategies are relatively common in migratory birds (Piersma et al., 2005) but less so in salmonines (Jonsson and Jonsson, 2005)
Intrinsic Factors
We had predicted that the two populations in our study would show variability in adopting migratory tactics across all food restriction scenarios and indeed, overall, the probability of smolting was higher in the anadromous background population than in the non-anadromous population. Moreover, higher hypo-osmoregulatory function (lower plasma chloride concentration) was documented in smolts from the former population relative to the latter, implying that smolts from the anadromous-background population were physiologically better prepared for transition to marine conditions. In contrast, although some fish from the non-anadromous-background population were classified as smolts in the experiment, these putative smolts exhibited relatively lower saltwater tolerance. A potential explanation for the reduced hypo-osmoregulatory function of non-anadromous-background smolts might be that they are poorly adapted to saltwater given their lack of (recent) evolutionary exposure to marine conditions. Relaxed selection leading to degradation of hypo-osmoregulation has similarly been observed in non-anadromous populations of landlocked Atlantic salmon (Nilsen et al., 2008; McCormick et al., 2019) and alewife Alosa pseudoharengus (Velotta et al., 2014, 2015). Alternatively, reduced saltwater tolerance could be evidence of an emerging migration continuum whereby putative smolts may have chosen a potamodromous (freshwater migratory) tactic and hence were unprepared physiologically for transitioning to saltwater. Nevertheless, the causal mechanisms underpinning anadromy and potamodromy are proposed to be similar, e.g., reduced food availability has previously been reported to increase adfluvial migration in freshwater brown trout transplanted to streams of high population density (Olsson et al., 2006). All brown trout in Ireland presumably have anadromous ancestral origins, since they would have had to recolonize the island after the Last Glacial Maximum via the sea (Ferguson et al., 2019). It thus seems more likely that the capacity for anadromy (or at least migration), albeit somewhat deteriorated in terms of saltwater tolerance, lay dormant in the Bunaveela fish, with anadromy re-expressed under experimental conditions of energy limitation.
The putative re-emergence of an anadromous life history in our Bunaveela fish is of particular interest from a fisheries management perspective, as it suggests the capacity for anadromy (or at least migration) may lie dormant within apparently resident populations. Such populations may thus have the potential to contribute to the restoration of anadromous stocks that have experienced widespread reductions, as evidenced by Gargan et al. (2006) in two formerly anadromous populations that suffered collapses. Anadromous phenotypes arising from resident genotypes have similarly been documented in O. mykiss (Kelson et al., 2019), and from common garden experiments with lake resident O. mykiss which were formally anadromous but were prevented from migrating by impassable dams or waterfalls (Thrower et al., 2004). These findings make sense within the framework of the conditional threshold model (Tomkins and Hazel, 2007), where environmental factors can affect life history tactic frequency by changing the distribution of the realized physiological state relative to inherited switch points (a proximate mechanism). Environmental factors could also drive longer term changes in tactic frequency via natural selection acting to shift the genotypic distribution of underlying switch points (an ultimate mechanism) (Hazel et al., 1990; Tomkins and Hazel, 2007; Pulido, 2011); for example, if survival or growth at sea is poor then migration may become less prevalent in the population if residents attain higher overall relative fitness than migrants. Within the Burrishoole system, the establishment of an Atlantic salmon farm in the estuary was implicated in the collapse of the anadromous life history from this catchment over a period of 30 years due to high rates of sea lice transmission (Poole et al., 1996, 2007). Reduced marine survival rates may have imposed strong selection against anadromy, and hence caused the evolution of lower mean threshold values for freshwater maturation within the Burrishoole catchment as a whole. Our current results are consistent with this evolutionary explanation, in that we demonstrated heritable differences (or at least phenotypic differences among genetically divergent populations in a common garden experiment)—a pre-requisite for evolutionary responses. However, they also show that phenotypic plasticity can drive changes in migratory tactics, which may contribute to observed life-history changes in natural populations (Gargan et al., 2006; Sandlund and Jonsson, 2016).
Early-life differences in length and mass between the two populations may proximately cause different anadromy propensities, as has been seen in brook trout, where size of juvenile fish was negatively related to probability of future residency (Thériault et al., 2007). Interestingly, though our populations differed in size early in the study (before food restriction), they were in similar condition at this time, suggesting that both populations had similar energy intake vs. output, at least initially. Higher intrinsic growth rates in the anadromous background population may have increased the likelihood of eventual energetic limitation in freshwater, thus reducing relative condition and increasing anadromy propensity (exemplified in our Low-Low food treatment). Conversely, when food resources are in ample supply, high intrinsic growth rates could hasten freshwater maturity instead of anadromy in this population (c.f. the scenario of optimal food resources in our study). Such variability in migratory tactics is a feature of salmonines in general [e.g., “retirement” from anadromy in Dolly Varden Salvelinus malma (Bond et al., 2015)] which may buffer species from increasing anthropogenic pressures in the marine environment (Russell et al., 2012).
Conclusions
Collectively, the results of this study show that the adoption of migratory tactics in brown trout involves an interplay between inherited components and environmentally cued physiological condition, in line with previous salmonines studies (Chapman et al., 2012; Dodson et al., 2013; Kendall et al., 2014). The differences we observed in population responses to food restriction and its timing suggest a complex relationship between intrinsic and extrinsic factors that may allow for a continuum of migratory tactics to exist. These population differences, together with the fact that putative anadromy emerged within offspring of a naturally non-anadromous population, emphasize that a range of life history outcomes are possible even within a single species, which can contribute to so-called portfolio effects that cushion the species as a whole from rapidly changing environmental conditions (Schindler et al., 2015). Although our study offers some important insight into how extrinsic and intrinsic factors interactively shape life-history tactics, we have only considered one element of the freshwater environment here, and future studies should expand to consider how other proximate drivers such as temperature, which influences a range of physiological and life-history traits in salmonines (Satterthwaite et al., 2010; McMillan et al., 2012; Doctor et al., 2014; Kendall et al., 2014; Sloat and Reeves, 2014), govern migratory tactics in fish from different genetic backgrounds. Moreover, it is now important to expand this approach into natural systems using, for example, common garden or reciprocal transplant experiments, to assess whether these findings hold up under real world complexities.
Finally, our results have important implications for the conservation of facultatively migratory species, which are in global decline due to in-stream barriers, habitat degradation, climate change, overfishing, and the expansion of aquaculture (Costello, 2009; Limburg and Waldman, 2009). Knowledge of how extrinsic and intrinsic factors affect fish migratory tactics may aid in successful management and restoration of facultatively migratory populations, and in doing so maintain important intraspecific biocomplexity, which offers increased resilience to effects of global change (Schindler et al., 2015).
Data Availability
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
This study was carried out in accordance with the recommendations of Health Products Regulatory Authority (HPRA) Ireland, under HPRA project license AE19130/P034, and HPRA individual licenses AE19130/I087, AE19130/I200, AE19130/I201, and AE19130/I202.
Author Contributions
TR, PM, LA, and WP conceived the study. LA, SH, TR, PG, and LH collected data and contributed to experimental design, as did MO'G and JK. LA conducted statistical analysis and led the manuscript writing. All authors contributed to interpretation of results and revisions of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank Brian Clarke, Deirdre Cotter, members of the FishEyE team at UCC, and the staff of Inland Fisheries Ireland and the Marine Institute for obtaining brood stock and for assistance in fish rearing, along with Robert Wynne, Ronan O'Sullivan, Peter Moran and Adam Kane for assistance in fish husbandry, and Jamie Coughlan for genotyping work. This research was supported by an ERC Starting Grant (639192-ALH) and an SFI ERC Support Award awarded to TER. PMcG was supported in part by grants from Science Foundation Ireland (15/IA/3028 and 16/BBSRC/3316) and by grant-in-aid (RESPI/FS/16/01) from the Marine Institute (Ireland) as part of the Marine Research Programme by the Irish Government. We thank the Associate Editor and two reviewers for comments that improved the manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2019.00222/full#supplementary-material
References
Alvarez, D., and Nicieza, A. G. (2005). Compensatory response ‘defends' energy levels but not growth trajectories in brown trout, Salmo trutta L. Proc. R. Soc. Lond. B Biol. Sci. 272, 601–607. doi: 10.1098/rspb.2004.2991
Barton, K. (2018). MuMIn: Multi-Model Inference. R package version 1.42.1. Available online at: https://CRAN.R-project.org/package=MuMIn
Beakes, M. P., Satterthwaite, W. H., Collins, E. M., Swank, D. R., Merz, J. E., Titus, R. G., et al. (2010). Smolt transformation in two california steelhead populations: effects of temporal variability in growth. Trans. Am. Fish. Soc. 139, 1263–1275. doi: 10.1577/T09-146.1
Berejikian, B. A., Bush, R. A., and Campbell, L. A. (2014). Maternal control over offspring life history in a partially anadromous species, Oncorhynchus mykiss. Trans. Am. Fish. Soc. 143, 369–379. doi: 10.1080/00028487.2013.862181
Bond, M. H., Miller, J. A., and Quinn, T. P. (2015). Beyond dichotomous life histories in partially migrating populations: cessation of anadromy in a long-lived fish. Ecology 96, 1899–1910. doi: 10.1890/14-1551.1
Buoro, M., Gimenez, O., and Prévost, E. (2012). Assessing adaptive phenotypic plasticity by means of conditional strategies from empirical data: the latent environmental threshold model. Evolution 66, 996–1009. doi: 10.1111/j.1558-5646.2011.01484.x
Burton, T., and Metcalfe, N. B. (2014). Can environmental conditions experienced in early life influence future generations? Proc. R. Soc. Lond. B Biol. Sci. 281:20140311. doi: 10.1098/rspb.2014.0311
Chapman, B. B., Brönmark, C., Nilsson, J.-Å., and Hansson, L.-A. (2011a). Partial migration: an introduction. Oikos 120, 1761–1763. doi: 10.1111/j.1600-0706.2011.20070.x
Chapman, B. B., Brönmark, C., Nilsson, J.-Å., and Hansson, L.-A. (2011b). The ecology and evolution of partial migration. Oikos 120, 1764–1775. doi: 10.1111/j.1600-0706.2011.20131.x
Chapman, B. B., Hulthén, K., Brodersen, J., Nilsson, P. A., Skov, C., Hansson, L. A., et al. (2012). Partial migration in fishes: causes and consequences. J. Fish Biol. 81, 456–478. doi: 10.1111/j.1095-8649.2012.03342.x
Costello, M. J. (2009). How sea lice from salmon farms may cause wild salmonid declines in Europe and North America and be a threat to fishes elsewhere. Proc. R. Soc. B Biol. Sci. 276, 3385–3394. doi: 10.1098/rspb.2009.0771
Cucherousset, J., Ombredane, D., Charles, K., Marchand, F., and Baglinière, J.-L. (2005). A continuum of life history tactics in a brown trout (Salmo trutta) population. Can. J. Fish. Aquat. Sci. 62, 1600–1610. doi: 10.1139/f05-057
Dill, L. M. (1983). Adaptive flexibility in the foraging behavior of fishes. Can. J. Fish. Aquat. Sci. 40, 398–408. doi: 10.1139/f83-058
Doctor, K., Berejikian, B., Hard, J. J., and VanDoornik, D. (2014). Growth-mediated life history traits of steelhead reveal phenotypic divergence and plastic response to temperature. Trans. Am. Fish. Soc. 143, 317–333. doi: 10.1080/00028487.2013.849617
Dodson, J. J., Aubin-Horth, N., Thériault, V., and Páez, D. J. (2013). The evolutionary ecology of alternative migratory tactics in salmonid fishes: alternative migratory tactics as threshold traits. Biol. Rev. 88, 602–625. doi: 10.1111/brv.12019
Doswald, N., Willis, S. G., Collingham, Y. C., Pain, D. J., Green, R. E., and Huntley, B. (2009). Potential impacts of climatic change on the breeding and non-breeding ranges and migration distance of European Sylvia warblers. J. Biogeogr. 36, 1194–1208. doi: 10.1111/j.1365-2699.2009.02086.x
Ferguson, A., Reed, T. E., Cross, T. F., Mcginnity, P., and Prodöhl, P. A. (2019). Anadromy, potamodromy and residency in brown trout Salmo trutta: the role of genes and the environment. J. Fish Biol. doi: 10.1111/jfb.14005
Ferguson, A., Reed, T. E., McGinnity, P., and Prodöhl, P. (2017). “Anadromy in brown trout (Salmo trutta): a review of the relative roles of genes and environmental factors and the implications for management and conservation,” in Sea Trout: Science and Management - Proceedings of the 2nd International Sea Trout Symposium (Leicestershire: Matador).
Finstad, B., and Ugedal, O. (1998). Smolting of sea trout Salmo trutta L./ in northern Norway. Aquaculture 168, 341–349. doi: 10.1016/S0044-8486(98)00360-3
Forseth, T., Nesje, T. F., Jonsson, B., and Hårsaker, K. (1999). Juvenile migration in brown trout: a consequence of energetic state. J. Anim. Ecol. 68, 783–793. doi: 10.1046/j.1365-2656.1999.00329.x
Garant, D., Fontaine, P.-M., Good, S. P., Dodson, J. J., and Bernatchez, L. (2002). The influence of male parental identity on growth and survival of offspring in Atlantic salmon (Salmo salar). Evol. Ecol. Res. 4, 537–549. Available online at: http://www.evolutionary-ecology.com/abstracts/v04/1352.html
Gargan, P., Kelly, F., Shephard, S., and Whelan, K. (2016). Temporal variation in sea trout Salmo trutta life history traits in the Erriff River, western Ireland. Aquacult. Environ. Interact. 8, 675–689. doi: 10.3354/aei00211
Gargan, P., Roche, W., Frode, G., and Ferguson, A. (2006). “Characteristics of sea trout (Salmo trutta L.) stocks from the Owengowla and Invermore Fisheries, Western Ireland, and Recent Trends in Marine Survival,” in Sea Trout: Biology, Conservation and Management, eds Harris, G., and Milner, N. (Oxford: Blackwells Scientific Publications), 60–75.
Hazel, W. N., Smock, R., and Johnson, M. D. (1990). A polygenic model for the evolution and maintenance of conditional strategies. Proc. R. Soc. Lond. Ser. B Biol. Sci. 242, 181–187. doi: 10.1098/rspb.1990.0122
Hecht, B. C., Hard, J. J., Thrower, F. P., and Nichols, K. M. (2015). Quantitative genetics of migration-related traits in rainbow and steelhead trout. Genes Genomes Genet. 5, 873–889. doi: 10.1534/g3.114.016469
Heinze, G., and Schemper, M. (2002). A solution to the problem of separation in logistic regression. Stat. Med. 21, 2409–2419. doi: 10.1002/sim.1047
Hutchings, J. A. (2011). Old wine in new bottles: reaction norms in salmonid fishes. Heredity 106, 421–437. doi: 10.1038/hdy.2010.166
Hutchings, J. A., and Myers, R. A. (1994). The evolution of alternative mating strategies in variable environments. Evol. Ecol. 8, 256–268. doi: 10.1007/BF01238277
Jones, D. A., Bergman, E., and Greenberg, L. (2015). Food availability in spring affects smolting in brown trout (Salmo trutta). Can. J. Fish. Aquat. Sci. 72, 1694–1699. doi: 10.1139/cjfas-2015-0106
Jonsson, B. (1985). Life history patterns of freshwater resident and sea-run migrant brown trout in Norway. Transact. Am. Fish. Soc. 114, 182–194. doi: 10.1577/1548-8659(1985)114<182:LHPOFR>2.0.CO;2
Jonsson, B., Jonsson, M., and Jonsson, N. (2017). Influences of migration phenology on survival are size-dependent in juvenile Atlantic salmon (Salmo salar). Can. J. Zool. 95, 581–587. doi: 10.1139/cjz-2016-0136
Jonsson, B., and Jonsson, N. (1993). Partial migration: niche shift versus sexual maturation in fishes. Rev Fish Biol Fisheries 3, 348–365. doi: 10.1007/BF00043384
Jonsson, B., and Jonsson, N. (2005). Lipid energy reserves influence life-history decision of Atlantic salmon (Salmo salar) and brown trout (S. trutta) in fresh water. Ecol. Freshwater Fish 14, 296–301. doi: 10.1111/j.1600-0633.2005.00098.x
Kelson, S. J., Miller, M. R., Thompson, T. Q., O'Rourke, S. M., and Carlson, S. M. (2019). Do genomics and sex predict migration in a partially migratory salmonid fish, Oncorhynchus mykiss? Can. J. Fish. Aquat. Sci. doi: 10.1139/cjfas-2018-0394
Kendall, N. W., McMillan, J. R., Sloat, M. R., Buehrens, T. W., Quinn, T. P., Pess, G. R., et al. (2014). Anadromy and residency in steelhead and rainbow trout (Oncorhynchus mykiss): a review of the processes and patterns. Can. J. Fish. Aquat. Sci. 72, 319–342. doi: 10.1139/cjfas-2014-0192
Klemetsen, A., Amundsen, P.-A., Dempson, J. B., Jonsson, B., Jonsson, N., O'Connell, M. F., et al. (2003). Atlantic salmon Salmo salar L., brown trout Salmo trutta L. and Arctic charr Salvelinus alpinus (L.): a review of aspects of their life histories. Ecol. Freshwater Fish 12, 1–59. doi: 10.1034/j.1600-0633.2003.00010.x
Kosmidis, I. (2019). brglm: Bias Reduction in Binary-Response Generalized Linear Models. R package version 0.6.2. Available online at: https://cran.r-project.org/package=brglm
Lenth, R. (2019). emmeans: Estimated Marginal Means, aka Least-Squares Means. R package version 1.3.3. Available online at: https://CRAN.R-project.org/package=emmeans
Limburg, K. E., and Waldman, J. R. (2009). Dramatic declines in north atlantic diadromous fishes. Bio Sci. 59, 955–965. doi: 10.1525/bio.2009.59.11.7
Magee, J. (2017). A Comparison of Population Structuring and Genetic Stock Identification of Brown Trout (Salmo trutta) Displaying Distinct Migratory Strategies. (Ph.D. thesis). Queen's University Belfast, Belfast, NI. p 277.
McCormick, S. D., Regish, A. M., Ardren, W. R., Björnsson, B. T., and Bernier, N. J. (2019). The evolutionary consequences for seawater performance and its hormonal control when anadromous Atlantic salmon become landlocked. Sci. Rep. 9:968. doi: 10.1038/s41598-018-37608-1
McMillan, J. R., Dunham, J. B., Reeves, G. H., Mills, J. S., and Jordan, C. E. (2012). Individual condition and stream temperature influence early maturation of rainbow and steelhead trout, Oncorhynchus mykiss. Environ. Biol. Fishes 93, 343–355. doi: 10.1007/s10641-011-9921-0
Metcalfe, N. B. (1998). The interaction between behavior and physiology in determining life history patterns in Atlantic salmon (Salmo salar). Can. J. Fish. Aquat. Sci. 55, 93–103. doi: 10.1139/d98-005
Morinville, G. R., and Rasmussen, J. B. (2003). Early juvenile bioenergetic differences between anadromous and resident brook trout (Salvelinus fontinalis). Can. J. Fish. Aquat. Sci. 60, 401–410. doi: 10.1139/f03-036
Naish, K. A., and Hard, J. J. (2008). Bridging the gap between the genotype and the phenotype: linking genetic variation, selection and adaptation in fishes. Fish Fisheries 9, 396–422. doi: 10.1111/j.1467-2979.2008.00302.x
Nicieza, A. G., and Metcalfe, N. B. (1997). Growth compensation in juvenile atlantic salmon: responses to depressed temperature and food availability. Ecology 78, 2385–2400. doi: 10.1890/0012-9658(1997)078[2385:GCIJAS]2.0.CO;2
Nilsen, T. O., Ebbesson, L. O., Kiilerich, P., Björnsson, B. T., Madsen, S. S., McCormick, S. D., et al. (2008). Endocrine systems in juvenile anadromous and landlocked Atlantic salmon (Salmo salar): seasonal development and seawater acclimation. Gen. Comp. Endocrinol. 155, 762–772. doi: 10.1016/j.ygcen.2007.08.006
Olsson, I. C., Greenberg, L. A., Bergman, E., and Wysujack, K. (2006). Environmentally induced migration: the importance of food. Ecol. Lett. 9, 645–651. doi: 10.1111/j.1461-0248.2006.00909.x
O'Neal, S. L., and Stanford, J. A. (2011). Partial migration in a robust brown trout population of a Patagonian river. Trans. Am. Fish. Soc. 140, 623–635. doi: 10.1080/00028487.2011.585577
Padfield, D., and Matheson, G. (2018). nls.Multstart: Robust Non-Linear Regression using AIC Scores. R package version 1.0.0. Available online at: https://CRAN.R-project.org/package=nls.multstart
Phillis, C. C., Moore, J. W., Buoro, M., Hayes, S. A., Garza, J. C., and Pearse, D. E. (2016). Shifting thresholds: rapid evolution of migratory life histories in steelhead/rainbow trout, Oncorhynchus mykiss. J. Hered. 107, 51–60. doi: 10.1093/jhered/esv085
Piche, J., Hutchings, J. A., and Blanchard, W. (2008). Genetic variation in threshold reaction norms for alternative reproductive tactics in male Atlantic salmon, Salmo salar. Proc. R. Soc. B Biol. Sci. 275, 1571–1575. doi: 10.1098/rspb.2008.0251
Piersma, T., Pérez-Tris, J., Mouritsen, H., Bauchinger, U., and Bairlein, F. (2005). Is there a “migratory syndrome” common to all migrant birds? Ann. N.Y. Acad. Sci. 1046, 282–293. doi: 10.1196/annals.1343.026
Pinheiro, J., Bates, D., DebRoy, S., and R Core Team (2019). nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1-140, Available online at: https://CRAN.R-project.org/package=nlme
Poole, W. R., Dillane, M., DeEyto, E., Rogan, G., Mcginnity, P., and Whelan, K. (2007). “Characteristics of the burrishoole sea trout population: census, marine survival, enhancement and stock-recruitment relationship, 1971-2003,” in Sea Trout: Biology, Conservation and Management, eds Harris, G., and Milner, N. (Oxford: Blackwells Scientific Publications), 279–306.
Poole, W. R., Whelan, K. F., Dillane, M. G., Cooke, D. J., and Mathews, M. (1996). The performance of sea trout, Salmo trutta L., stocks from the Burrishoole system western Ireland, 1970–1994. Fish. Manag. Ecol. 3, 73–92. doi: 10.1111/j.1365-2400.1996.tb00131.x
Pulido, F. (2011). Evolutionary genetics of partial migration – the threshold model of migration revis(it)ed. Oikos 120, 1776–1783. doi: 10.1111/j.1600-0706.2011.19844.x
Pulido, F., Berthold, P., and van Noordwijk, A. J. (1996). Frequency of migrants and migratory activity are genetically correlated in a bird population: evolutionary implications. Proc. Natl. Acad. Sci. U.S.A. 93, 14642–14647. doi: 10.1073/pnas.93.25.14642
R Core Team (2019). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna. Available online at: https://www.R-project.org/
Roff, D. A. (1996). The evolution of threshold traits in animals. Q. Rev. Biol. 71, 3–35. doi: 10.1086/419266
Rowe, D. K., Thorpe, J. E., and Shanks, A. M. (1991). Role of fat stores in the maturation of male atlantic salmon (Salmo salar) Parr. Can. J. Fish. Aquat. Sci. 48, 405–413. doi: 10.1139/f91-052
Russell, I. C., Aprahamian, M. W., Barry, J., Davidson, I. C., Fiske, P., Ibbotson, A. T., et al. (2012). The influence of the freshwater environment and the biological characteristics of Atlantic salmon smolts on their subsequent marine survival. ICES J Mar Sci. fsr208. 12, 1563–1573 doi: 10.1093/icesjms/fsr208
Sandlund, O. T., and Jonsson, B. (2016). Life history plasticity: migration ceased in response to environmental change? Ecol. Freshw. Fish 25, 225–233. doi: 10.1111/eff.12204
Satterthwaite, W. H., Beakes, M. P., Collins, E. M., Swank, D. R., Merz, J. E., Titus, R. G., et al. (2010). State-dependent life history models in a changing (and regulated) environment: steelhead in the California Central Valley. Evol. Appl. 3, 221–243. doi: 10.1111/j.1752-4571.2009.00103.x
Schindler, D. E., Armstrong, J. B., and Reed, T. E. (2015). The portfolio concept in ecology and evolution. Front. Ecol. Environ. 13, 257–263. doi: 10.1890/140275
Schultz, E. T., and McCormick, S. D. (2012). “Euryhalinity in an evolutionary context,” in: Fish Physiology, eds McCormick, S. D., Farrell, A. P., and Brauner, C. J. (Oxford: Elsevier), 478–534. doi: 10.1016/B978-0-12-396951-4.00010-4
Sloat, M. R., and Reeves, G. H. (2014). Individual condition, standard metabolic rate, and rearing temperature influence steelhead and rainbow trout (Oncorhynchus mykiss) life histories. Can. J. Fish. Aquat. Sci. 71, 491–501. doi: 10.1139/cjfas-2013-0366
Stefansson, S. O., Björnsson, B. T., Sundell, K., Nyhammer, G., and McCormick, S. D. (2003). Physiological characteristics of wild Atlantic salmon post-smolts during estuarine and coastal migration. J. Fish Biol. 63, 942–955. doi: 10.1046/j.1095-8649.2003.00201.x
Tanguy, J. M., Ombredane, D., Baglinière, J. L., and Prunet, P. (1994). Aspects of parr-smolt transformation in anadromous and resident forms of brown trout (Salmo trutta) in comparison with Atlantic salmon (Salmo salar). Aquaculture 121, 51–63. doi: 10.1016/0044-8486(94)90007-8
Thériault, V., and Dodson, J. J. (2003). Body size and the adoption of a migratory tactic in brook charr. J. Fish Biol. 63, 1144–1159. doi: 10.1046/j.1095-8649.2003.00233.x
Thériault, V., Garant, D., Bernatchez, L., and Dodson, J. J. (2007). Heritability of life-history tactics and genetic correlation with body size in a natural population of brook charr (Salvelinus fontinalis). J. Evol. Biol. 20, 2266–2277. doi: 10.1111/j.1420-9101.2007.01417.x
Thorpe, J. E., Mangel, M., Metcalfe, N. B., and Huntingford, F. A. (1998). Modelling the proximate basis of salmonid life-history variation, with application to Atlantic salmon, Salmo salar L. Evol. Ecol. 12, 581–599. doi: 10.1023/A:1022351814644
Thorpe, J. E., and Metcalfe, N. B. (1998). Is smolting a positive or a negative developmental decision? Aquaculture 168, 95–103. doi: 10.1016/S0044-8486(98)00342-1
Thrower, F. P., Hard, J. J., and Joyce, J. E. (2004). Genetic architecture of growth and early life-history transitions in anadromous and derived freshwater populations of steelhead. J. Fish Biol. 65, 286–307. doi: 10.1111/j.0022-1112.2004.00551.x
Tocher, D. R. (2003). Metabolism and Functions of Lipids and Fatty Acids in Teleost Fish. Rev. Fisheries Sci. 11, 107–184. doi: 10.1080/713610925
Tomkins, J. L., and Hazel, W. (2007). The status of the conditional evolutionarily stable strategy. Trends Ecol. Evol. 22, 522–528. doi: 10.1016/j.tree.2007.09.002
Urke, H. A., Koksvik, J., Arnekleiv, J. V., Hindar, K., Kroglund, F., and Kristensen, T. (2010). Seawater tolerance in Atlantic salmon, Salmo salar L., brown trout, Salmo trutta L., and S. salar × S. trutta hybrids smolt. Fish Physiol. Biochem. 36, 845–853. doi: 10.1007/s10695-009-9359-x
Velotta, J. P., McCormick, S. D., O'Neill, R. J., and Schultz, E. T. (2014). Relaxed selection causes microevolution of seawater osmoregulation and gene expression in landlocked Alewives. Oecologia 175, 1081–1092. doi: 10.1007/s00442-014-2961-3
Velotta, J. P., McCormick, S. D., and Schultz, E. T. (2015). Trade-offs in osmoregulation and parallel shifts in molecular function follow ecological transitions to freshwater in the Alewife. Evolution 69, 2676–2688. doi: 10.1111/evo.12774
Whalen, K. G., and Parrish, D. L. (1999). Effect of maturation on parr growth and smolt recruitment of Atlantic salmon. Can. J. Fish. Aquat. Sci. 56, 79–86. doi: 10.1139/f98-154
Wysujack, K., Greenberg, L. A., Bergman, E., and Olsson, I. C. (2009). The role of the environment in partial migration: food availability affects the adoption of a migratory tactic in brown trout Salmo trutta. Ecol. Freshw. Fish 18, 52–59. doi: 10.1111/j.1600-0633.2008.00322.x
Keywords: climate change, partial migration, anadromy, aquatic, brown trout, genotype by environment, Salmo trutta, proximate drivers
Citation: Archer LC, Hutton SA, Harman L, O'Grady MN, Kerry JP, Poole WR, Gargan P, McGinnity P and Reed TE (2019) The Interplay Between Extrinsic and Intrinsic Factors in Determining Migration Decisions in Brown Trout (Salmo trutta): An Experimental Study. Front. Ecol. Evol. 7:222. doi: 10.3389/fevo.2019.00222
Received: 28 March 2019; Accepted: 29 May 2019;
Published: 14 June 2019.
Edited by:
Nathan R. Senner, University of South Carolina, United StatesReviewed by:
Jonathan Paul Velotta, University of Montana, United StatesFrederick Gilbert Whoriskey, Dalhousie University, Canada
Copyright © 2019 Archer, Hutton, Harman, O'Grady, Kerry, Poole, Gargan, McGinnity and Reed. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Louise C. Archer, l.archer@umail.ucc.ie
 Louise C. Archer
Louise C. Archer Stephen A. Hutton
Stephen A. Hutton Luke Harman1,2
Luke Harman1,2  Joseph P. Kerry
Joseph P. Kerry Thomas E. Reed
Thomas E. Reed