Standard Operating Protocol for Ion-Exchange Capacity of Anion Exchange Membranes
- 1Versogen, Wilmington, DE, United States
- 2Center for Catalytic Science and Technology, Department of Chemical and Biomolecular Engineering, University of Delaware, Newark, DE, United States
- 3Nel Hydrogen, Wallingford, CT, United States
Ion-exchange capacity (IEC) is the measure of a material’s capability to displace ions formerly incorporated within its structure. IEC is a key feature of anion-exchange membranes (AEM), as it determines the AEM’s ability to conduct the ions required to sustain the electrochemical reactions where they are utilized. As an intrinsic property, measuring the IEC accurately is essential to study AEMs and understand their performance within devices. In this method article, a facile and accurate standard operating procedure (SOP) to measure the IEC of AEMs is proposed. When compared to conventional acid-base back-titration or Mohr titration, the proposed method combines the fast reaction between silver and halide ions and the accuracy of the potentiometric titration, providing a convenient and precise protocol for researchers in the field.
1 Introduction
Ion-exchange capacity (IEC) is a key material property of anion-exchange membranes (AEM). The IEC value is a measure of the concentration of ion-conducting functional groups as milliequivalent per gram of the AEM and generally has a proportional relationship with many other AEM properties such as anion conductivity, swelling ratio, water uptake, and water/gas permeance. Coupled with other characterization techniques, changes in the IEC can provide insights on active sites—associated with durability of anion conductivity—cation degradation (NMR), carboxylic acid formation (NMR), hydroxyl group formation (NMR), and undesired ion contamination (XRF). This information provides critical insights towards the efficient design and diagnostic of the AEM utilized in electrochemical systems and provides guidance to researchers on chemical structure design, morphology control, applications, and device troubleshooting. Therefore, a standard operating procedure (SOP) to accurately measure IEC on AEMs is essential.
Conventional methods such as acid-base back-titration (Si et al., 2014a; Si et al., 2014b; Mohanty et al., 2015; Liu et al., 2018; You et al., 2018; Han et al., 2019; Yang et al., 2019; You et al., 2019; Zhu et al., 2019) and Mohr titration (Mohanty et al., 2015; Vandiver et al., 2016; Liu and Kohl, 2018; Allushi et al., 2019; Divekar et al., 2019; Mandal et al., 2019; Pham et al., 2019; Ziv et al., 2019; Buggy et al., 2020; Mondal et al., 2020; Yang et al., 2020) are widely applied in AEM research. The acid-base back-titration utilizes the molar difference between the basic titrant and the acid analyte when testing an OH− form AEM to calculate the sample IEC value. The endpoint is determined with a phenolphthalein indicator. The Mohr titration is based on the fast reaction of a silver ion titrant and halide analyte, with potassium chromate (K2CrO4) as colorimetric indicator. Both methods have the advantages of widely accessible supplies and short learning curves. However, the acid-base back-titration requires precise measurement of the concentration and volume for both the titrant and analyte. The Mohr titration, on the other hand, requires multiple ion-exchanges with sodium nitrate (NaNO3) and transfers of the rinsate for analyte preparation. Additionally, for both methods, the titration endpoint is determined by the color change of the indicators, which is prone to operator errors. These factors often introduce artificial errors and lead to erroneously measured IEC values, sometimes being as high as -65% error versus theoretical value (Figure 1). Here we focus on comparing the new method with acid-base back-titration and Mohr titration due to their domination in recent AEM publications. Other colorimetric titrations such as Volhard titration reported by Ramani and coworkers are valuable progress in the AEM field (Arges et al., 2012). However, they are not widely used in the AEM field and thus they are not included in this discussion.
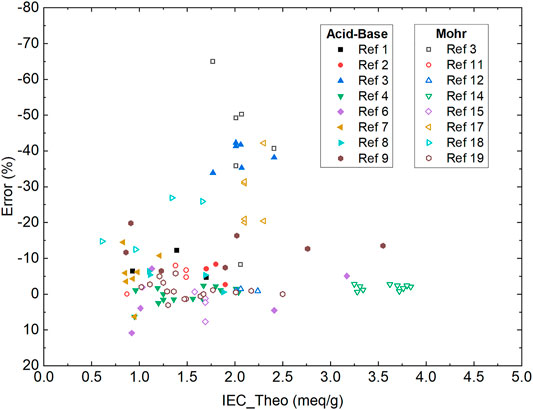
FIGURE 1. Summary of Errors versus Theoretical IEC values from typical aggregated from the AEMs reported (Si et al., 2014a; Si et al., 2014b; Mohanty et al., 2015; Vandiver et al., 2016; Liu et al., 2018; Liu and Kohl, 2018; You et al., 2018; Allushi et al., 2019; Divekar et al., 2019; Han et al., 2019; Mandal et al., 2019; Pham et al., 2019; Yang et al., 2019; You et al., 2019; Zhu et al., 2019; Ziv et al., 2019; Buggy et al., 2020; Mondal et al., 2020; Yang et al., 2020). The error was calculated as (IEC_Meseaured - IEC_Theoretical)/(IEC_Theoretical) × 100%.
Herein, we propose a facile and precise method to measure the IEC of an AEM sample by utilizing the fast reaction of the silver ion with bromide coupled with the high accuracy of the potentiometric titration for endpoint determination. The proposed method encompasses four steps: 1. Ion-exchange to Br− form; 2. Dry weight measurement; 3. Sample pretreatment; and 4. Potentiometric titration. No liquid transfer, manual titration, or indicators are involved in the procedure. Lithium triflate (LiOTf) is added to the analyte to drive the ion-exchange to completion—getting all the bromide ions to the anolyte—and the auto titrator equipped with the silver selective electrode is used to measure the endpoint with high accuracy. Sample dry weight is the only input data obtained manually. The new method overcomes the errors that were introduced in the conventional methods due to incomplete ion-exchange, long procedure, manual titration, and difficulties in determining the titration endpoint.
2 Protocol Scope
2.1 Scope and Applicability
The purpose of this SOP is to describe the method for measuring the IEC of an AEM. The membrane sample must have a mass greater than 50 mg.
2.2 Summary of Method
An AEM sample (50–100 mg) in hydroxide, bicarbonate, chloride, or bromide form is exchanged to the bromide form by soaking in a solution (15–40 ml) composed of 4 M KBr and 0.02 M KOH. After each exchange, the sample is rinsed in DI water (50–100 ml). This procedure is repeated three times. The same procedure can be applied to AEM samples in any mixed form of hydroxide, carbonate, and/or bicarbonate as will occur when hydroxide form samples are exposed to air. If the sample is in an unknown or any other anion form, such as iodide, it is recommended to repeat the ion-exchange procedure 10 times. It is optional to perform extra ion-exchange with bromide form samples but recommended to prevent undesired anion contamination. A small amount of KOH is added to ensure that any primary, secondary, and tertiary amines (including any other types of acid acceptors such as imidazole, tertiary phosphine, and pyridine) in the sample are not protonated and do not contribute to the measured IEC. Based on the relative affinity of bromide and hydroxide ions, the error caused by the exchange of hydroxide for bromide in the membrane is estimated to be less than 0.1%. The membrane is then rinsed with DI water until the conductivity of the rinsate is within 10% of that of DI water. The dry weight of the membrane sample is obtained. Then, the AEM sample is exchanged with LiOTf (lithium triflate) by mixing an equal weight of LiOTf salt and 150 ml of DI water with the sample. About 1 ml of 2% HNO3 is added to adjust the pH to ca. 3–4. The Hanna HI901 automatic titrator is used for titration with a 0.02N AgNO3 standard solution. A silver/sulfide combination ion-selective electrode (HI 4115) is used to monitor the titration.
2.3 Personnel Qualifications/Responsibilities
Operators should have basic laboratory knowledge on titration processes and should be trained on the operation of the Hanna HI901 potentiostatic titrator and accompanying software before performing this experiment.
2.4 Health and Safety Warning
All solutions should be handled with care, using appropriate PPE. The operator should review the safety data sheets for the chemicals involved, especially the ones of nitric acid and lithium triflate. The membrane should never be handled without gloves, as the chloride ions in the sweat can interfere with the measurement. Additionally, the silver nitrate standard solution should be kept away from light, as it will hydrolyze and affect the measurements. Discard if you see brown deposits of silver oxide.
2.5 Equipment and Supplies
Potassium bromide (KBr), deionized (DI) water, 0.02 M silver nitrate standard solution (AgNO3, 0.02 M), lithium triflate (LiOTf), nitric acid (HNO3), potassium hydroxide (KOH), Hanna HI901, and sensor HI 4115.
2.6 Nomenclature and Definitions
Ion-exchange capacity (IEC); anion-exchange membrane (AEM),Potassium bromide (KBr);Deionized (DI);Silver nitrate (AgNO3), lithium triflate (LiOTf), potassium hydroxide (KOH);Nitric acid (HNO3).
2.7 Recommended Reading
Kenkel, J. (2003). Analytical Chemistry for Technicians. 1 (3 ed.). CRC Press. pp. 108–109.
Yoder, L. (1919). “Adaptation of the Mohr Volumetric Method to General Determinations of Chlorine”. Industrial and Engineering Chemistry. 11 (8): 755.
Hulanicki, A. Głąb, S. (2013) Encyclopedia of Analytical Science. (2 ed.). Elsevier. pp. 114–121.
3 Procedure
3.1 Step-by-Step Procedure
3.1.1 Ion-Exchange to Br− Form
• The AEM sample (50 ± 10 mg is recommended), originally in bicarbonate form, for example, is exchanged to the bromide form by soaking in ∼15 ml 4 M KBr and 0.02 M KOH solution in a 20 ml glass vial then rinsing with ∼50 ml of DI water for 30 min for each soaking. This is completed on a shaker table at room temperature and repeated three times with fresh solutions. For anion-exchange resins or thicker AEM (≥100 µm), longer soaking time (3 h), vigorous shaking, elevated temperature (50°C), and extra exchange times (10 times) are recommended to prevent incomplete ion-exchange. When testing thick resins, an initial baseline should be established by testing ion exchange over 3, 9, and 24 h and measuring the IEC. If different IEC values are measured, the ion exchange time will need to be increased until no difference is measured. Increments of 12 h can be used after the 24 h mark is reached. Grade II or ASTM Type II DI water (conductivity ≤1 μS/cm) is required in all the processes.
• The membrane is then rinsed with DI water and completely immersed in ∼50 ml DI water at room temperature for 30 min while placing it again on a shaker table. This is repeated at least three times with fresh DI water until the conductivity of the rinsate is comparable to DI water (within 10%).
3.1.2 Dry Weight Measurement
• The membrane sample is dried overnight at 80°C on a glass vial using a convection oven. The dry mass of the membrane sample is obtained using an analytical balance with a resolution of 0.1 mg. 50 mg sample weight is recommended. The weight measurement should be made promptly after removing the sample from the oven, as the membrane will absorb water from the atmosphere. The membrane does not need to be kept dry after weight measurement.
• Alternatively, the bromide form membrane can be also dried in a sealed vial with an inlet/outlet of dry nitrogen flow overnight at room temperature. After removing the nitrogen flow and resealing, the vial with the sample inside it was transferred to the analytical balance. The sample dry weight measurement should be completed promptly within 5 min.
3.1.3 Sample Pre-Treatment
• A Hanna HI901 charged with a 0.02N AgNO3 solution is used to titrate the sample. The AgNO3 solution must be kept in a dark or amber bottle. Verify the expiration date of this analytical standard.
• The sensor HI 4115 must be charged with enough 1 M KNO3 so that the electrode solution is above the ceramic junction. In the case of a low electrode solution, 1M KNO3 standard solution should be purchased and filled.
• The Burette tip of Hanna HI901 must be primed through dispensing increasingly smaller amounts of titrant into the waste solution until it can reliably dispense the smallest solution increment.
• The AEM sample is exchanged with LiOTf by mixing ∼50 mg of dry LiOTf salt and ∼150 ml of DI water in a 250 ml beaker with 1,000 RPM agitation at room temperature for 30 min. Adjust the beaker position to prevent any splash, which will cause a lower IEC value. The sample cup must be stirred while titrating. The HI901 has a built-in impeller, but for small-volume sample cups, a magnetic stir bar can be used. LiOTf should be stored before and after use in a desiccator due to its hygroscopic nature.
• Add about 1 ml of 2% HNO3 to tune the pH to ca. 3–4 required to ensure silver ions are completely immersed in the solution. Adjust the beaker position and solution level with DI water so that all the electrodes are fully immersed.
3.1.4 Potentiometric Titration
• Select the IEC titration program on the auto-titrator. The mass of the AEM sample is entered into the software and the titration begins with 0.05 ml of 0.02 N AgNO3 per increment.
• The solution is continuously stirred and automatically titrated with sensor HI 4115 recording the potential of the silver ion-selective electrode, which is determined by the solubility product of silver bromide and the remaining free bromide concentration.
• IEC, potential, and volume dispensed results are displayed.
• Sample cup is removed and stir bar is rinsed with DI water. Sensor HI 4115 should also be cleaned by dabbing with a Kimwipe that has been wetted with DI water.
3.2 Sample Preparation and Analysis
A sample of 50 ± 10 mg should be anion exchanged with KBr, washed with DI water, dried overnight, and weighed carefully.
3.3 Sample Handling and Preservation
Before and after the experiment, AEM samples should be stored in clean sample containers (e.g. Petri dish).
3.4 Computer Hardware and Software
Origin or an equivalent program can be used to find the maximum of the first derivative of the measured Ag ISE potential over volume. Data files can be moved to the computer from the Hanna HI901 using a thumb drive.
3.5 Data Collection, Analysis, and Records Management
Sample ID, polymer dry weight, number of ion-exchange repeats, calculation of IEC, measured IEC, and theoretical IEC (if applicable) should be recorded elsewhere both electronically and in a lab notebook by the user.
4 Results
The measured IEC value in bromide form will be displayed on the auto titrator screen at the end of the titration. Calculations of IEC are performed by finding the maximum of the first derivative of the measured Ag ISE potential over volume (Figure 2). This gives the equivalence point of the titration. The IEC value can be calculated with Eq. 1.
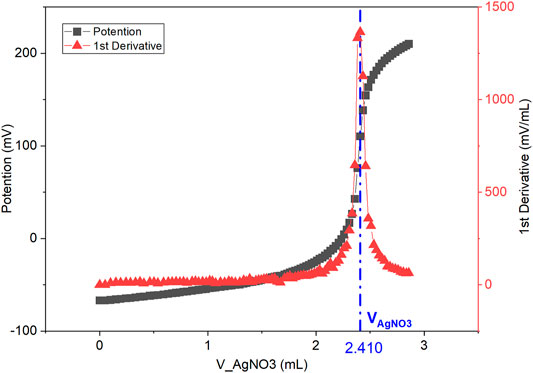
FIGURE 2. Titration curve of Ag ISE potential (mV) over AgNO3 titrant volume (ml) is denoted as black squares. First derivative of potential over volume, mV/mL vs. mL is denoted as red triangles. The maximum at VAgNO3 (2.410 ml) is the titration endpoint. AEM sample dry weight is measured as mdry = 24.4 mg.
The IEC in bromide form is determined as follows (meq/g):
where VAgNO3 is the titrated volume of AgNO3 titrant (ml); CAgNO3 is the normality of AgNO3 (mmol/mL or meq/mL); and mdry is the weight of dry AEM sample (g).
The measured IEC value (1.97 meq/g) of the AEM sample (i.e. PAP-TP-85) is very close to the theoretical value of 1.94 meq/g in Br− form with only a 2% error (Wang et al., 2019).
Converting the IEC into other forms can be calculated with Eq. 2. Take AEM, (A)m(B)n, for example.
where FWA and FWB are the formula weights of monomer units A and B without anions (g/mol); FWBr and FWX are the formula weight of anions (g/mol, e.g. X = OH−, Cl−); and m and n are the monomer ratio (%) on the polymer structure.
5 Quality Control and Quality Assurance
5.1 Instrument or Method Calibration and Standardization
Hanna HI901 calibration check should be performed before experiments. This calibration is performed by dispensing bromide standard solution into a sample cup at a known volume (e.g., 2 ml, 10 ml), and running the IEC test. The IEC test results should show the consumption matches the volume dispensed and the IEC should match the bromide standard concentration within 2%. Additionally, the IEC of each AEM should be measured in triplicate for verification, as well as having a sample weight of ∼50 mg to minimize error.
5.2 Cautions
Clean equipment after each use. Solutions used are not generally corrosive to the system, but they should still be removed immediately after an experiment for best practices. Keep silver nitrate in an amber bottle, as it is light-sensitive. Keep lithium triflate in a dry environment and well-sealed as it is hygroscopic. The titration needs to be conducted in slightly acid conditions to avoid the formation of silver oxides. Never touch the membrane with bare hands, as it will lead to interfering ions.
5.3 Common Issues
• Membrane dry weight is too low (less than 50 mg).
• Ion-exchange solution is not refreshed after each exchange.
• The conductivity/salt content of DI water rinsate is not measured.
• Sample is not fully dried before weighing.
• Weight measurement is not conducted in a dry environment.
• Sample is not measured timely after moving out of the oven/dryer.
• Sample has static electricity (very common in a dry environment).
• Auto titrator is not calibrated before titration.
• Burette and pipe are not primed.
• Splash from vigorous stirring.
• End tips of electrodes are not fully immersed in the analyte.
• The electrode standard solution is low.
5.4 Interferences
• Incomplete ion exchange—May lead to lower IEC value when nonhalide anions are left in the AEM sample.
• Insufficient DI water rinse—Leads to higher IEC value when extra Br− is left in AEM sample.
• Inaccurate membrane weight—Leads to lower IEC value due to falsely high membrane weight with extra water.
• Degraded AgNO3 standard solution—Leads to higher IEC value due to extra volume needed with lower Ag− concentration. AgNO3 decomposes when exposed to light.
• Faulty sensor—Unable to determine the titration endpoint due to malfunctional sensor.
• Unprimed burette—Leads to lower IEC value due to air trapped inside the burette or pumping line.
• Violent stirring—Leads to lower IEC value due to analyte loss from splash.
• Poor lab hygiene—Leads to lower IEC value from AgNO3 residue or higher IEC value from Br-residue due to lack of clean up after experiments. Contact of the membrane with bare hands introduces chloride ions from the sweat.
5.5 Troubleshooting
Lower measured IEC value than theoretical.
• Instrument calibration.
• Clean impeller, burette, and electrodes with DI water.
• Increase exchange repeats in the “ion-exchange to Br-form” step.
• Prolong drying time and weigh dry membrane promptly after removing from the dry environment.
• Use an anti-static gun such as Zerostat when measuring membrane dry weight.
• Prime burette to remove air bubbles.
• Increase minimum dispensing amount until the burette can reliably dispense the smallest solution increment.
• Adjust analyte level so that impeller and electrodes are fully immersed.
5.5.1 Higher Measured IEC Value Than Theoretical
• Instrument calibration.
• Clean impeller, burette, and electrodes with DI water.
• Increase DI water rinse repeats and verify rinsate conductivity.
• Verify the normality of the standard AgNO3 solution. Replace with a fresh standard solution if the AgNO3 degraded.
5.5.2 Multiple Equivalent Points on the Titration Curve
• Increase exchange repeats to 15 or even 20 times in the “ion-exchange to Br-form” step.
• Check LiOTf and HNO3 solutions for halide contamination by titration of diluted samples of each.
5.5.3 Unable to Obtain Titration Curve
• Charge the sensor HI 4115 with enough 1M KNO3 so that the electrode solution is above the ceramic junction.
• Adjust analyte level so that the impeller and electrodes are fully immersed.
• Replace sensors if necessary.
5.6 Error Analysis
Inevitably, most four-place analytical balances are accurate to ±0.0001 g. Therefore, for the sample of 24.4 mg dry weight, in an ideal case, it consists of a relative error of 0.4% (= 0.1/24.4 mg × 100%) from weight measurement. The purchased 0.02 N silver nitrate standard solution (Hanna HI70448) does not list the concentration error which is also usually too small to take into consideration. We can assume that there is no concentration error of fresh standard 0.02N AgNO3 solution. Despite the fast reaction of silver with halide and the high sensitivity of electrodes, the endpoint volume of AgNO3 may deviate from the true volume by one minimum dispensing amount which is 0.02 ml in this case. The relative error from titration is calculated as 0.8% (= 0.02/2.41 ml × 100 %).
Based on Eq. 1, the relative error of IEC shall be calculated with Eq. 3.
where e%,IEC is the relative error of IEC, e%,V is the relative error of endpoint volume, and e%,m is the relative error of sample dry weight.
In the case study, a relatively light sample (<25 mg) was selected to showcase the accuracy of the proposed method. The e%,V and e%,m will decrease to half if the recommended sample dry weight (50 mg) is applied and the e%,IEC will be as low as 0.4%. To minimize the relative error, a proper sample dry weight and multiple weight measurements are recommended.
6 Discussion
The results show the significant improvement of the Silver–Halide potentiometric method over the other two conventional colorimetric methods. The acid-base back-titration has the advantage of simplicity. However, the requirements of standard solutions for both acid and base, as well as the colorimetric endpoint determination, inevitably introduce more artificial errors. Mohr titration, on the other hand, suffers from the poor sensitivity of potassium chromate indicator.
Additionally, the auto-metering capability of the auto-titrator notably decreases the concerns of operator error, as manual operations are significantly minimized. The improvement in simplicity and accuracy is due to the fast reaction of silver-bromide, sensitive reading of electrode, precise metering of auto-titrator, and well-designed procedure.
Finally, it is worth noting that after addressing the titration endpoint issue, the measurement of sample dry weight becomes the major source of errors. Even with just a few water molecules coordinated to each cation group, errors of 2%–5% are easily introduced. Compared to other anion forms, bromide from AEM is usually less hygroscopic. Thus, it loses water faster and absorbs water slower. When weighing is conducted correctly according to the Step-by-Step Procedure, the operator can obtain accurate sample dry weight easily.
In summary, a facile and accurate standard operating procedure (SOP) for measuring the IEC of AEM samples is described. The switch from using two standard solutions in acid-base back-titration to using a single AgNO3 standard solution in the Silver–Halide system greatly simplifies the sample preparation process. The implementation of the potentiometric method with silver selective electrode overcomes the difficulties of determining the titration endpoint, which exists in the Mohr titration where the colorimetric indicator is applied.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
LW developed the standard operating protocol. SR-C and KH set up the apparatus and performed measurements of ion-exchange capacity. BS and AM provided guidance on electrochemistry. MU performed sample preparation. LW, SR-C, KH, and YY conceived the ideas and wrote the manuscript with support from other coauthros.
Funding
The information, data, or work presented herein was funded in part by the Advanced Research Projects Agency-Energy (ARPA-E), U.S. Department of Energy, under Award Number DE-AR0000771 and DE-AR0001149. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof.
Conflict of Interest
LW, SR-C, KH, and YY were employed by Versogen; AM is employed by Nel Hydrogen.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Allushi, A., Pham, T. H., Olsson, J. S., and Jannasch, P. (2019). Ether-free Polyfluorenes Tethered with Quinuclidinium Cations as Hydroxide Exchange Membranes. J. Mater. Chem. A. 7 (47), 27164–27174. doi:10.1039/c9ta09213g
Arges, C. G., Parrondo, J., Johnson, G., Nadhan, A., and Ramani, V. (2012). Assessing the Influence of Different Cation Chemistries on Ionic Conductivity and Alkaline Stability of Anion Exchange Membranes. J. Mater. Chem. 22 (9), 3733. doi:10.1039/c2jm14898f
Buggy, N. C., Du, Y., Kuo, M.-C., Ahrens, K. A., Wilkinson, J. S., Seifert, S., et al. (2020). A Polyethylene-Based Triblock Copolymer Anion Exchange Membrane with High Conductivity and Practical Mechanical Properties. ACS Appl. Polym. Mater. 2 (3), 1294–1303. doi:10.1021/acsapm.9b01182
Divekar, A. G., Kuo, M. C., Park, A. M., Motz, A. R., Page‐Belknap, Z. S., Owczarczyk, Z., et al. (2019). The Impact of Alkyl Tri‐methyl Ammonium Side Chains on Perfluorinated Ionic Membranes for Electrochemical Applications. J. Polym. Sci. Part. B: Polym. Phys. 57 (11), 700–712. doi:10.1002/polb.24825
Han, J., Peng, Y., Lin, B., Zhu, Y., Ren, Z., Xiao, L., et al. (2019). Hydrophobic Side-Chain Attached Polyarylether-Based Anion Exchange Membranes with Enhanced Alkaline Stability. ACS Appl. Energ. Mater. 2 (11), 8052–8059. doi:10.1021/acsaem.9b01553
Liu, L., Chu, X., Liao, J., Huang, Y., Li, Y., Ge, Z., et al. (2018). Tuning the Properties of Poly(2,6-Dimethyl-1,4-Phenylene Oxide) Anion Exchange Membranes and Their Performance in H2/O2 Fuel Cells. Energy Environ. Sci. 11 (2), 435–446. doi:10.1039/c7ee02468a
Liu, L., and Kohl, P. A. (2018). Anion Conducting Multiblock Copolymers with Different Tethered Cations. J. Polym. Sci. Part. A: Polym. Chem. 56 (13), 1395–1403. doi:10.1002/pola.29020
Mandal, M., Huang, G., Hassan, N. U., Peng, X., Gu, T., Brooks-Starks, A. H., et al. (2019). The Importance of Water Transport in High Conductivity and High-Power Alkaline Fuel Cells. J. Electrochem. Soc. 167 (5), 054501. doi:10.1149/2.0022005jes
Mohanty, A. D., Ryu, C. Y., Kim, Y. S., and Bae, C. (2015). Stable Elastomeric Anion Exchange Membranes Based on Quaternary Ammonium-Tethered Polystyrene-B-Poly(ethylene-Co-Butylene)-B-Polystyrene Triblock Copolymers. Macromolecules 48 (19), 7085–7095. doi:10.1021/acs.macromol.5b01382
Mondal, A. N., Hou, J., He, Y., Wu, L., Ge, L., and Xu, T. (2020). Preparation of Click-Driven Cross-Linked Anion Exchange Membranes with Low Water Uptake. Particuology 48, 65–73. doi:10.1016/j.partic.2018.08.012
Pham, T. H., Olsson, J. S., and Jannasch, P. (2019). Effects of the N-Alicyclic Cation and Backbone Structures on the Performance of Poly(terphenyl)-Based Hydroxide Exchange Membranes. J. Mater. Chem. A. 7 (26), 15895–15906. doi:10.1039/c9ta05531b
Si, Z., Qiu, L., Dong, H., Gu, F., Li, Y., and Yan, F. (2014). Effects of Substituents and Substitution Positions on Alkaline Stability of Imidazolium Cations and Their Corresponding Anion-Exchange Membranes. ACS Appl. Mater. Inter. 6 (6), 4346–4355. doi:10.1021/am500022c
Si, Z., Sun, Z., Gu, F., Qiu, L., and Yan, F. (2014). Alkaline Stable Imidazolium-Based Ionomers Containing Poly(arylene Ether Sulfone) Side Chains for Alkaline Anion Exchange Membranes. J. Mater. Chem. A. 2 (12), 4413–4421. doi:10.1039/c3ta15178f
Vandiver, M. A., Caire, B. R., Pandey, T. P., Li, Y., Seifert, S., Kusoglu, A., et al. (2016). Effect of Hydration on the Mechanical Properties and Ion Conduction in a Polyethylene-B-Poly(vinylbenzyl Trimethylammonium) Anion Exchange Membrane. J. Membr. Sci. 497, 67–76. doi:10.1016/j.memsci.2015.09.034
Wang, J., Zhao, Y., Setzler, B. P., Rojas-Carbonell, S., Ben Yehuda, C., Amel, A., et al. (2019). Poly(aryl Piperidinium) Membranes and Ionomers for Hydroxide Exchange Membrane Fuel Cells. Nat. Energ. 4 (5), 392–398. doi:10.1038/s41560-019-0372-8
Yang, C., Liu, L., Huang, Y., Dong, J., and Li, N. (2019). Anion-conductive Poly(2,6-Dimethyl-1,4-Phenylene Oxide) Grafted with Tailored Polystyrene Chains for Alkaline Fuel Cells. J. Membr. Sci. 573, 247–256. doi:10.1016/j.memsci.2018.12.013
Yang, K., Chu, X., Zhang, X., Li, X., Zheng, J., Li, S., et al. (2020). The Effect of Polymer Backbones and Cation Functional Groups on Properties of Anion Exchange Membranes for Fuel Cells. J. Membr. Sci. 603, 118025. doi:10.1016/j.memsci.2020.118025
You, W., Hugar, K. M., and Coates, G. W. (2018). Synthesis of Alkaline Anion Exchange Membranes with Chemically Stable Imidazolium Cations: Unexpected Cross-Linked Macrocycles from Ring-Fused ROMP Monomers. Macromolecules 51 (8), 3212–3218. doi:10.1021/acs.macromol.8b00209
You, W., Padgett, E., MacMillan, S. N., Muller, D. A., and Coates, G. W. (2019). Highly Conductive and Chemically Stable Alkaline Anion Exchange Membranes via ROMP of Trans -cyclooctene Derivatives. Proc. Natl. Acad. Sci. U.S.A. 116 (20), 9729–9734. doi:10.1073/pnas.1900988116
Zhu, L., Yu, X., Peng, X., Zimudzi, T. J., Saikia, N., Kwasny, M. T., et al. (2019). Poly(olefin)-Based Anion Exchange Membranes Prepared Using Ziegler-Natta Polymerization. Macromolecules 52 (11), 4030–4041. doi:10.1021/acs.macromol.8b02756
Keywords: SOP, AEM, IEC, protocol, ion exchange, membrane
Citation: Wang L, Rojas-Carbonell S, Hu K, Setzler BP, Motz AR, Ueckermann ME and Yan Y (2022) Standard Operating Protocol for Ion-Exchange Capacity of Anion Exchange Membranes. Front. Energy Res. 10:887893. doi: 10.3389/fenrg.2022.887893
Received: 02 March 2022; Accepted: 28 March 2022;
Published: 03 May 2022.
Edited by:
Olga A. Marina, Pacific Northwest National Laboratory (DOE), United StatesReviewed by:
Christopher George Arges, Louisiana State University, United StatesEnrico Negro, University of Padua, Italy
Copyright © 2022 Wang, Rojas-Carbonell, Hu, Setzler, Motz, Ueckermann and Yan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yushan Yan, Yushan@Versogen.com
 Lan Wang
Lan Wang Santiago Rojas-Carbonell
Santiago Rojas-Carbonell Keda Hu1
Keda Hu1 