- 1Longgang Ear-Nose-Throat (ENT) Hospital, Institute of Ear-Nose-Throat (ENT) and Shenzhen Key Laboratory of Ear-Nose-Throat (ENT), Shenzhen, China
- 2Shenzhen Longgang Institute of Stomatology, Longgang Ear-Nose-Throat (ENT) Hospital, Shenzhen, China
- 3Department of Laboratory Medicine, Shenzhen Hospital, Peking University, Shenzhen, China
- 4School of Biomedical Sciences, Faculty of Medicine, The Chinese University of Hong Kong, Shatin, China
It has been well documented that there is a two-way relationship between diabetes mellitus and periodontitis. Diabetes mellitus represents an established risk factor for chronic periodontitis. Conversely, chronic periodontitis adversely modulates serum glucose levels in diabetic patients. Activated immune and inflammatory responses are noted during diabetes and periodontitis, under the modulation of similar biological mediators. These activated responses result in increased activity of certain immune-inflammatory mediators including adipokines and microRNAs in diabetic patients with periodontal disease. Notably, certain microbes in the oral cavity were identified to be involved in the occurrence of diabetes and periodontitis. In other words, these immune-inflammatory mediators and microbes may potentially serve as biomarkers for risk assessment and therapy selection in diabetes and periodontitis. In this review, we briefly provide an updated overview on different potential biomarkers, providing novel diagnostic and therapeutic insights on periodontal complications and diabetes mellitus.
Introduction
Diabetes mellitus is clinically and genetically a heterogeneous group of disorders characterized by dysregulated nutrient metabolism, resulting from defects in insulin secretion and action (1). Hyperglycemia, the hallmark of diabetes mellitus, can lead to a range of chronic complications associated with long-term damage and dysfunction in various organs and body systems (2). Importantly, diabetic patients is more likely to develop chronic periodontitis (3), where a two-way relationship has been previously documented between diabetes and periodontitis (4, 5) (Figure 1). However, the detailed mechanisms underlying the bidirectional relationship remain largely unknown. Pathologically, the hyperactive inflammatory response plays a contributory role in the progression of these two diseases (6). Particularly, diabetes causes the activation of immune and inflammatory responses in periodontal tissues, increasing the risk of periodontitis. The activated responses subsequently result in increased secretion of cytokines, amplified oxidative damage, and disruption of receptor-mediated signaling. Altogether, these events accelerate the breakdown of periodontal connective tissues and resorption of alveolar bone, thus exacerbating periodontitis. In the other direction, periodontitis may cause dysregulated glycemic control in diabetic patients. Periodontal bacteria and their metabolic products, together with locally produced inflammatory cytokines and mediators in the inflamed periodontal tissues, enter the circulation to trigger systemic inflammation, further worsening glucose tolerance and insulin resistance (7). Certain pathogenic microbes in the oral cavity even aggravate the progression of periodontitis and diabetes mellitus by triggering host inflammation and causing burden on host immunity. Biomarkers serve as useful indicators during the onset and development of inflammatory and systemic diseases. In addition to the screening and risk assessment of diseases, biomarkers could also be applied in staging, grading, and selection of therapies (8). It has been reported that the levels of certain molecular biomarkers, such as adipokines and microRNAs, vary significantly in saliva, serum and gingival crevicular fluid (GCF) of individuals with both diabetes mellitus and periodontitis (9). Moreover, several strategies have been developed to safely and conveniently collect GCF from individuals, such as extracrevicular and intracrevicular GCF collection techniques (10). Hence, saliva, serum and GCF represent feasible sources of biomarkers for diagnostic purposes. This article aims to review the potential biomarkers in diabetes and periodontitis, providing novel diagnostic and therapeutic insights on periodontal complications in associated with diabetes mellitus.
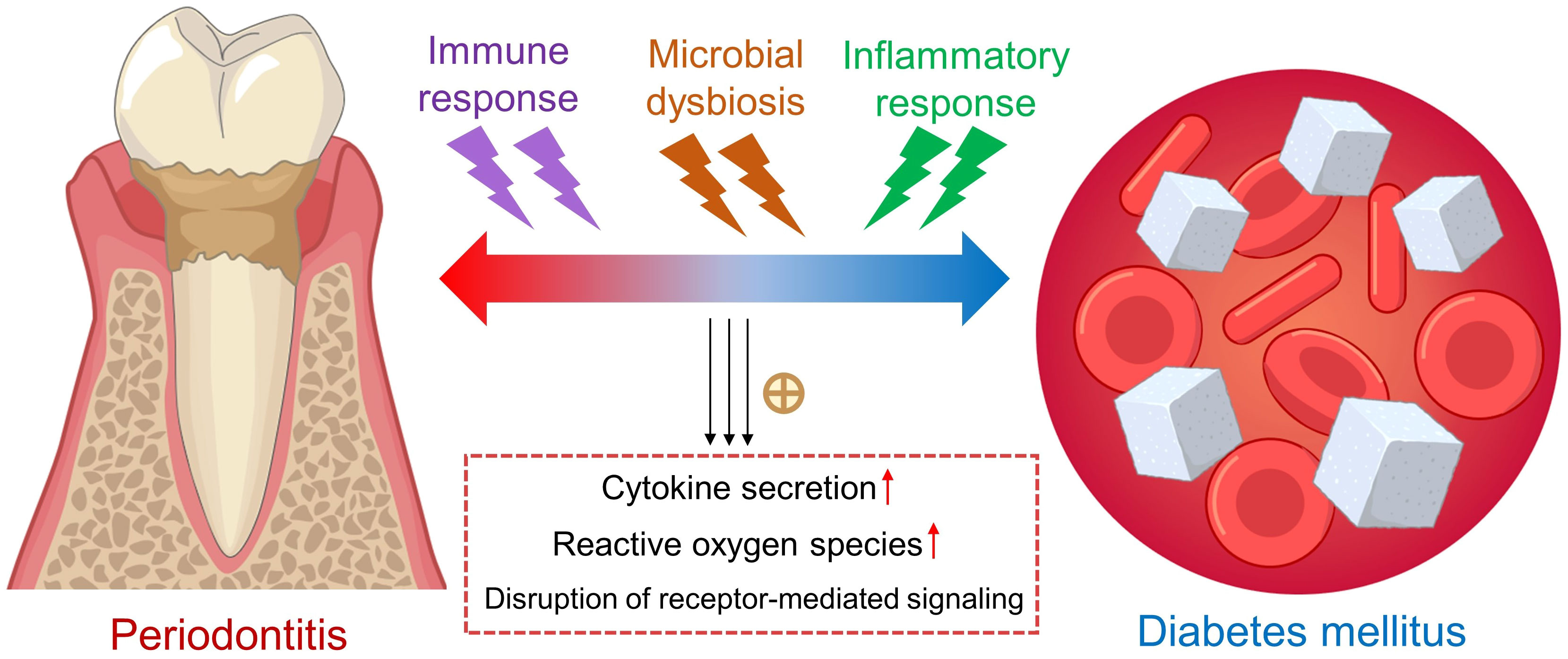
Figure 1 The bidirectional relationship between periodontitis and diabetes mellitus. Hyperactive immune and inflammatory responses participate in the vicious cycle between periodontitis and type 2 diabetes mellitus, associated with increased secretion of proinflammatory cytokines, higher oxidative stress and disruption of signaling pathways. Yellow cross: promotion. Up red arrows: increase.
Adipokines
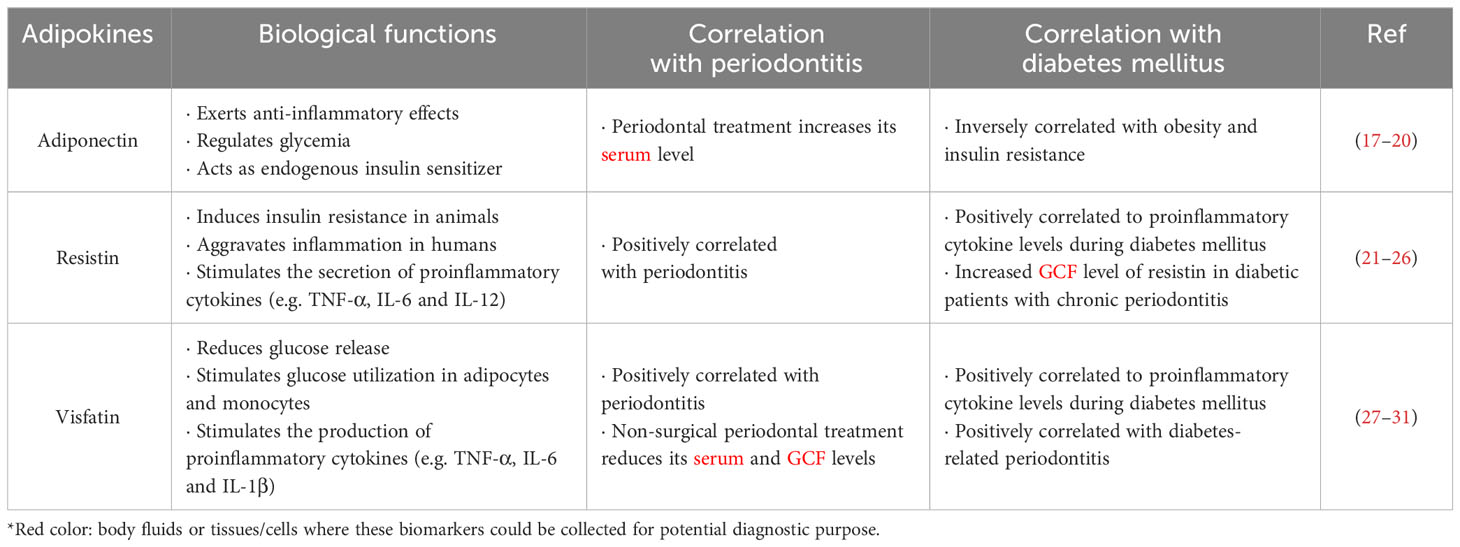
Adipokines are a group of secretory proteins mainly released by an active endocrine organ, particularly adipose tissue, into the systemic circulation. Adipokines are believed to be tightly associated with energy control, insulin sensitivity, and immune-inflammatory responses (11, 12). During diabetes mellitus, abnormal metabolism in adipose tissue may affect various organs via adipokine production (13). Periodontitis can lead to a proinflammatory state and affect adipokine levels in serum, tear fluid and GCF of obese patients (14, 15). Furthermore, diabetes mellitus can promote dyslipidemia and inflammation via regulation of adipokines (16). It has been postulated that the concentration of adipokines might be indicative to chronic metabolic disorders and pathological processes in local tissues. Therefore, any variations of adipokine levels in body fluids might be indicative to the severity of diabetes mellitus and chronic periodontitis (11) (Table 1).
Adiponectin
A bidirectional relationship exists between diabetes mellitus and periodontitis (32). It has been reported that diabetic subjects with insulin resistance are more likely to develop severe periodontitis (33, 34). Adiponectin, an adipokine mainly secreted by adipocytes, exerts anti-inflammatory effects and plays a pivotal role in regulating glycemia (17). Adiponectin acts as an endogenous insulin sensitizer in the regulation of insulin sensitivity, and its level is inversely correlated with obesity and insulin resistance (18). Some intervention studies have suggested that periodontal treatment could significantly increase serum adiponectin levels in type 2 diabetic patients with periodontitis (19, 20). Furthermore, periodontal therapy has been shown to be associated with improved glycemic control and insulin resistance in diabetic patients (35). More importantly, another clinical trial stated that periodontal intervention also improved lipid profile, reduced inflammatory cytokines in serum, and elevated levels of serum adiponectin in diabetic patients (34). Adiponectin profoundly improves insulin sensitivity by inhibiting glucose output from the liver, and limiting glucose uptake by adipose tissue and muscle (36).
Resistin
Named after its apparent ability to ‘resist insulin’, resistin is another adipokine first discovered in murine. Resistin is an 11 kDa protein encoded on chromosome 8, which was once classified as a unique signaling molecule in-between obesity and type 2 diabetes mellitus (21, 26). In multiple obese animal models, resistin was shown to induce insulin resistance, where hyper-resistinemia remarkably impairs insulin sensitivity. However, the exact role of resistin in obesity and type 2 diabetes mellitus in humans have not been comprehensively defined (37–39). In contrast, human resistin is predominantly expressed in peripheral-blood mononuclear cells. It aggravates inflammation which has been conclusively associated with the development of obesity and insulin resistance (23). It has been reported that resistin acts as a proinflammatory molecule by stimulating the secretion of tumor necrosis factor α (TNF-α), interleukin (IL)-6 and IL-12, thereby inducing its own production via a positive feedback cycle during periodontitis (22). Of note, the elevated levels of resistin in periodontal disease may highlight its role as a specific and sensitive biomarker in the early detection and intervention of diabetes-related periodontitis. Joshi et al. has reported that GCF level of resistin was significantly higher in diabetic patients with chronic periodontitis, where its level showed no correlation with glycated hemoglobin (HbA1c) value (24). Such finding indicated that resistin is more closely related to the inflammatory condition instead of the glycemic state of the individual, suggesting resistin as an inflammatory biomarker in two diseases. Another study has shown that single-nucleotide polymorphism of resistin gene is correlated to the resistin levels in serum and GCF of diabetic patients with chronic periodontitis (25). Moreover, in vitro clues are present that release of inflammatory cytokines, such as IL-6 and TNF-α, and maturation of monocytes into macrophages could alter resistin levels. Therefore, it is reasonable to postulate that periodontal inflammation, possibly via cytokine secretion and macrophage maturation, might influence resistin expression (25). However, more long-term interventional studies in larger sample sizes are needed to fully uncover the cause-effect relationships between resistin levels and diabetes-related periodontitis (8).
Visfatin
Visfatin, also known as pre-B-cell colony enhancing factor, is a 52 kDa adipokine secreted by visceral adipose tissues (28). By binding to insulin receptor at a site distinct from that of insulin, visfatin exerts insulin-like effects to reduce glucose release and stimulate glucose utilization in adipocytes and myocytes (40). Furthermore, visfatin has been reported to induce the productions of proinflammatory cytokines, like IL-6, TNF-α and IL-1β, during infection and inflammation phases (29). During periodontal inflammation, periodontopathogens trigger local expressions of IL-6 and TNF-α in periodontal tissues. These proinflammatory cytokines, in turn, trigger visfatin production in periodontal tissues. Bahammam and Attia have found significantly elevated levels of IL-6, TNF-α, and visfatin in GCF of diabetic patients afflicted with chronic periodontitis (27). Clinically, compared to periodontally healthy individuals and diabetic patients, the mean visfatin levels remained the highest in both serum and GCF of diabetic patients afflicted with chronic periodontitis. Meanwhile, visfatin concentrations in both serum and GCF were shown positively correlated with the severity of periodontal disease (30). Importantly, non-surgical periodontal treatment was reported to remarkably reduce visfatin levels in serum and GCF of diabetic patients with periodontitis (31). These clues suggested that visfatin might serve as a potential predictor and therapeutic target in the management of diabetes mellitus and periodontitis (41).
Proinflammatory cytokines
It is well established that diabetes is a disorder of inflammation and metabolic dysregulation, associated with increased production of cytokines, including IL-6, IL-1β, and TNF-α (42, 43). It has been suggested that periodontal therapy could reduce systemic inflammation in diabetic patients by targeting intraoral bacteria and reducing periodontal inflammation (44). Poor glycemic control in type 2 diabetic patients is clinically associated with poor prognosis of periodontal tissues (45). Levels of these inflammatory cytokines are significantly higher in patients with periodontitis (46). These cytokines stimulate bone resorption by inducing osteoclast progenitor proliferation, as well as the production of chemokines, extracellular matrix metalloproteinases (MMPs), cytokines, collagenases, and prostaglandins (47). Besides, increased levels of these cytokines further alter insulin sensitivity through direct and indirect mechanisms (48), resulting in a vicious cycle between diabetes progression and periodontal damage (49). Interestingly, periodontal therapy could elicit beneficial effects on glycemic control via suppressing these cytokines, facilitating a less-pronounced inflammatory state (43, 50). Moreover, IL-6, IL-1β, and TNF-α are confirmed important modulators in bone metabolism within oral cavity. Altogether, these cytokines in serum and GCF can be potentially considered as biomarkers in the prediction, intervention and treatment of chronic periodontitis afflicted with type 2 diabetes mellitus (43, 49, 51).
Another important mediator in chronic inflammatory diseases, soluble tumor necrosis factor-like weak inducer of apoptosis (sTWEAK), is gaining increasing attentions recently (52). sTWEAK belongs to the TNF superfamily cytokines and elicits an immunoregulatory role in periodontitis and diabetes mellitus (53, 54). Serum sTWEAK level was shown to be significantly lower in patients with chronic periodontitis, and lowest in patients with concomitant chronic periodontitis and type 2 diabetes mellitus (55). However, another recent clinical study found that significantly higher levels of circulating sTWEAK were observed in severe periodontal patients when compared to those without periodontitis (56). More extensive studies are still needed to correlate the temporal change in circulating sTWEAK level with the progression of concomitant periodontitis and diabetes mellitus.
Oxidative stress markers
Increasing oxidative stress can be considered as a critical contributing factor during the pathogenesis of both diabetes mellitus and periodontitis (57). Some previous clinical studies have reported the alterations of oxidative stress markers in different body fluids of patients with concomitant periodontitis and diabetes mellitus. For instance, the salivary levels of the free radical marker malondialdehyde were higher in patients with chronic periodontitis when compared to healthy individuals, where the levels became even higher in patients with concomitant periodontitis and diabetes mellitus (58). Superoxide dismutase (SOD) is an antioxidant enzyme which protects against deleterious effects of high oxidative stress. Another clinical study has shown that the serum levels of superoxide dismutase were the highest in patients with concomitant periodontitis and diabetes mellitus when compared to those of periodontal patients and healthy individuals (59). Catalase is one the central antioxidant enzymes to constitute the primary defense against oxidative damage. A previous clinical trial found that the serum levels of catalase in patients with concomitant periodontitis and diabetes mellitus were lower than individuals with and without periodontitis (60). Furthermore, the serum and GCF levels of 4-hydroxy-2-nonenal, a product of lipid peroxidation, were shown to be higher in patients with both diabetes mellitus and periodontitis (61). These clinical findings suggested oxidative stress as a link between the two diseases.
MicroRNAs
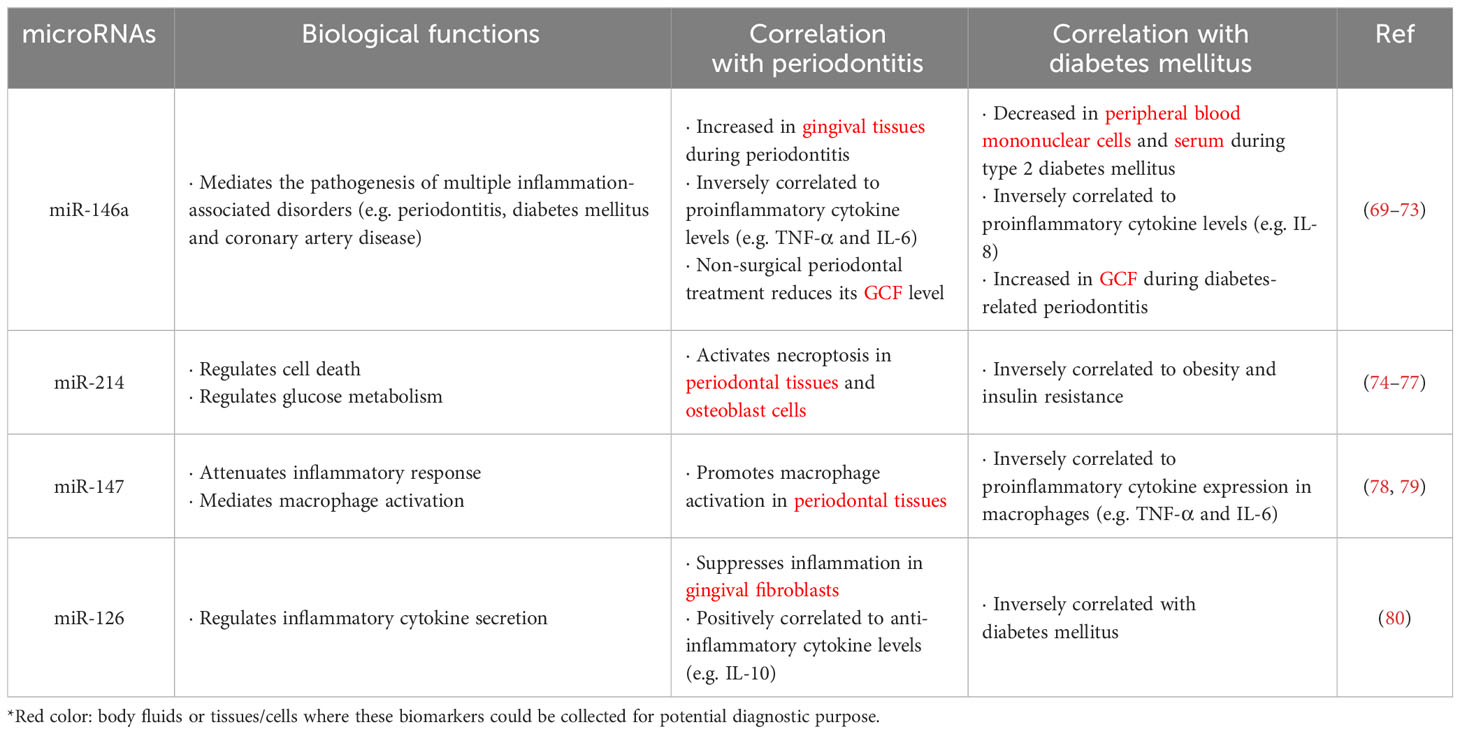
MicroRNAs represent a group of small non-coding regulatory RNAs (~22 nucleotides) which post-transcriptionally lower stability and suppress gene expression (62). In mammalian cells, more than 2,500 microRNAs have been reported to regulate >60% of protein-coding genes (63). Revealed by extensive studies, microRNAs have been confirmed to regulate various physiological and pathological processes in various human diseases, including autoimmune disorders, cancers and inflammatory disorders (64). Of note, microRNAs play a regulatory role in the pathogenesis of periodontitis, where the microRNA profiles in healthy and inflamed gingival tissues significantly vary (65, 66). Meanwhile, microRNAs also play a critical role in the mediation of glucose homeostasis and progression of diabetes mellitus. Nowadays, more and more studies have recognized microRNAs as biomarkers in clinical medicine, where microRNAs can potentially be prognostic and predictive biomarkers in the treatment of chronic periodontitis and diabetes mellitus (67–69) (Table 2).
Various microRNAs have been demonstrated to be key regulators in inflammation. A substantial literature indicated that miR-146a is involved in the pathogenesis of multiple inflammatory disorders, such as chronic periodontitis, diabetes mellitus and coronary artery disease (70). miR-146a levels have been found significantly lower in peripheral blood mononuclear cells from patients with type 2 diabetes mellitus (71). Notably, serum levels of miR-146a remarkably decrease in type 2 diabetic patients, which is inversely correlated to that of the proinflammatory cytokine IL-8 (72). Moreover, the miR-146a expression also changes during chronic periodontitis. Motedayyen et al. demonstrated that higher levels of miR-146a, whereas lower expressions of inflammatory cytokines, particularly IL-6 and TNF-α, were observed in the gingival tissues of periodontitis patients (73). These findings suggested miR-146a as a negative regulator for immune response (81). Since miR-146a plays crucial roles in both diabetes mellitus and periodontitis, it is reasonable to hypothesize that miR-146a may participate in the bidirectional relationship of two diseases. A recent study has revealed that expressions of inflammatory cytokines diminished upon transfection of miR-146a into lipopolysaccharides (LPS)-stimulated adipocytes and gingival fibroblasts, when co-cultured with macrophages. Similarly, transfection of miR-146a into macrophages down-regulated TNF-α expression in the presence of inflammatory stimuli. In vivo findings suggested that intravenous injection of miR-146a protected C57BL/6 mice from high-fat diet-induced inflammatory insults in adipose and gingival tissues. These findings implied a protective role of miR-146a against inflammation-related obesity and periodontal disease (82). Clinically, miR-146a level was reported to be significantly higher in GCF of type 2 diabetic patients afflicted with periodontitis, but decreased upon non-surgical periodontal treatment (69). These preclinical and clinical studies hinted that miR-146a might be a potential biomarker and therapeutic target for the treatment of periodontitis and diabetes mellitus.
Other microRNAs, including miR-214, miR-147 and miR-126, have been reported to play pivotal roles in both diabetes and periodontitis. Necroptosis, a newly discovered mode of programmed cell death, is a highly proinflammatory event involved in the pathogenesis of periodontitis and diabetes (83, 84). Previous studies have shown that miR-214 is responsible for the regulation of cell death and glucose metabolism (74–76). Ou et al. has found that miR-214 regulates necroptosis through targeting activating transcription factor 4 (ATF4) in periodontal tissues and osteoblast cells under co-stimulation by high glucose and LPS (77). In rats, experimental periodontitis was shown to promote systemic insulin resistance by inducing macrophage activation and hence inflammation in adipose tissue (85). Notably, miR-147 was reported to act as a negative regulator in attenuating inflammatory response in murine macrophages (78). However, another study showed that miR-147 seems to promote M1 polarization, the classical activation of macrophages, in periodontal tissues of obese rats (79). Further verification and mechanistic study are required to uncover the conflicting role of miR-147 in periodontitis and diabetes mellitus, especially in human subjects. Another microRNA, miR-126, has been shown to play a protective role against high glucose-induced inflammation in human gingival fibroblasts. Mechanistically, miR-126 promotes the secretion of the anti-inflammatory cytokine IL-10 via targeting tumor necrosis factor (TNF) receptor associated factor 6 (TRAF6) (80). Taken together, the emerging roles of microRNAs, including miR-146a, miR-214, miR-147 and miR-126, may provide new insights into the diagnostic and therapeutic strategies on the treatment of concomitant periodontitis and type 2 diabetes mellitus.
Glycoproteins
A chitin- and heparin-binding glycoprotein, YKL-40, is secreted by activated neutrophils and macrophages during acute or chronic inflammatory diseases (86). Among patients with chronic periodontitis, GCF levels of YKL-40 in patients with type 2 diabetes mellitus were often higher (87–89). Another acidic glycoprotein, Chromogranin A (CgA), is identified in the extracellular vesicles secreted by neurons and endocrine cells. The concentration of CgA is known to be increased in response to psychological stress, the risk factor for periodontal disease (90, 91). Zhang et al. indicated that CgA values in saliva samples of chronic periodontitis patients with or without type 2 diabetes mellitus were significantly higher than those of control groups. These findings suggested that salivary CgA could be a potential biomarker for periodontitis and diabetes mellitus (92).
Oral microbes
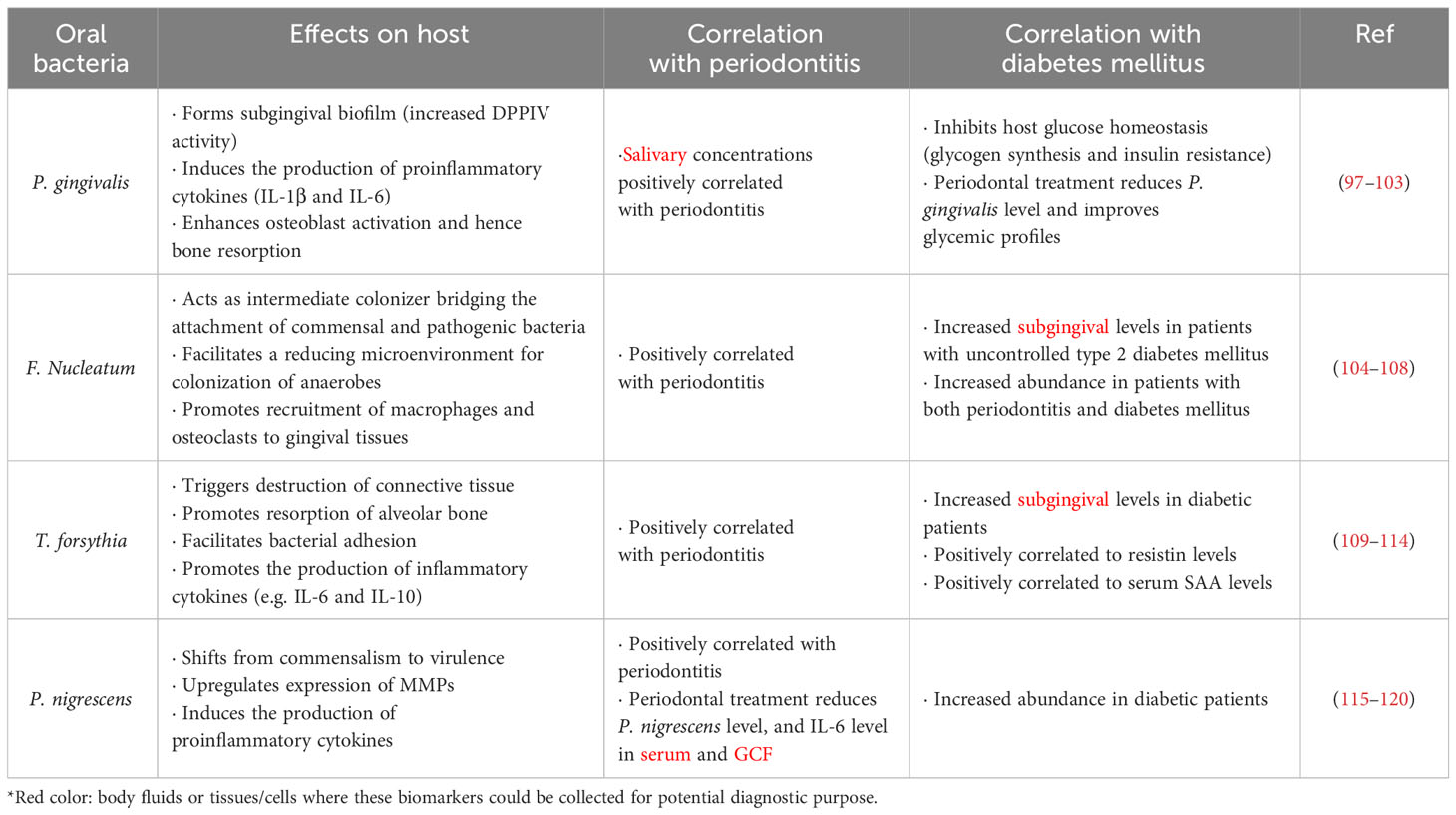
Our oral cavity is inhabited by diverse microbes including bacteria, fungi and protozoa, where over 700 bacterial species have been identified (93). Disruption of balance between commensal and harmful microbes in the oral cavity is associated with the pathogenesis of certain diseases, including periodontitis (94), diabetes mellitus (95), and cancers (96). In addition to the endogenous biomarkers mentioned, such as adipokines and microRNAs, the microbes in the oral cavity can also be potential biomarkers of multiple diseases. Notably, alteration in the abundance of certain bacterial species in the oral cavity may be indicative to the severity of periodontitis and diabetes mellitus (Table 3).
Porphyromonas gingivalis
Porphyromonas gingivalis (P. gingivalis) is a Gram-negative anaerobe in the oral cavity, and is considered as the major pathogenic bacterium for periodontitis (121). The capacity of P. gingivalis to form subgingival biofilm, associated with increased DPPIV activity, greatly contribute to the pathogenesis of periodontitis (98). Additionally, P. gingivalis promotes pathogenesis of aggressive periodontitis by inducing the production of proinflammatory cytokines, such as IL-1β and IL-6, from peripheral T helper cells (99). Meanwhile, P. gingivalis can enhance osteoclast activation and Th1-Th17-response, which further aggravate bone resorption during the pathogenesis of destructive periodontitis (103). Clinically, the salivary concentrations of P. gingivalis, IL-1β and matrix metalloproteinase (MMP)-8 are associated with the severity of periodontitis (102). On the other hand, P. gingivalis overgrowth in the oral cavity can affect host glucose homeostasis. Once oral P. gingivalis is translocated to the liver, it can inhibit glycogen synthesis via the Akt/GSK-3β signaling, resulting in a higher glucose level (100). Oral colonization with periodontal pathogens, particularly P. gingivalis, impaired insulin resistance in high fat diet-fed mice (97). In a previous clinical study, periodontal treatment improved glycemic profiles and reduced detection rate of subgingival P. gingivalis in type 2 diabetic patients (101). These preclinical and clinical findings suggested P. gingivalis as a potential microbial biomarker for periodontitis and diabetes mellitus.
Fusobacterium nucleatum
Another periodontal pathogen, Fusobacterium nucleatum (F. Nucleatum) is also correlated to the occurrence of diabetes mellitus. F. Nucleatum is a Gram-negative anaerobe, predominantly found in biofilms of dental plaques (122). F. Nucleatum acts as an intermediate colonizer bridging the attachment of commensal and pathogenic bacteria on tooth and epithelial surfaces. Moreover, F. Nucleatum contributes to the reducing microenvironment that facilitates the colonization of oxygen-intolerant microbes (106). Coherently, F. Nucleatum significantly enhanced the invasion of human gingival epithelial cells by P. gingivalis (108). Oral infection with F. Nucleatum promotes recruitment of macrophages and osteoclasts towards gingival tissues, driving inflammation and bone resorption (105). Clinically, among patients suffering from chronic periodontitis, higher subgingival levels of F. Nucleatum were observed in those with uncontrolled type 2 diabetes mellitus (104). Meanwhile, higher F. Nucleatum levels were associated with poorer glycemic control in patients with both chronic periodontitis and type 2 diabetes mellitus (107). However, the detailed mechanism on how F. Nucleatum is related to the progression of diabetes mellitus remains elusive.
Tannerella forsythia
Tannerella forsythia (T. forsythia), another Gram-negative anaerobe, is also considered as a major contributor to the development of periodontitis. T. forsythia triggers destruction of connective tissue and resorption of alveolar bone during periodontitis progression (110). Higher subgingival T. forsythia levels were observed in obese individuals than those with normal BMIs (111), implying a higher risk of periodontitis in obese individuals. The leucine-rich-repeat protein (BspA) expressed by T. forsythia has been shown to play a contributory role in bacterial adhesion and inflammation in dental tissues. T. forsythia can stimulate the secretion of proinflammatory cytokines and chemokines from monocytes and osteoblasts respectively, driving inflammation and bone resorption (113). Additionally, T. forsythia enhances the expression of inflammatory cytokines (e.g. IL-6 and IL-10) in macrophages and dendritic cells, in a toll-like receptor 2 (TLR2)-dependent manner (112). In type 2 diabetic patients, the abundance of T. forsythia in subgingival plaque was found higher than that of non-diabetic individuals (114). Interestingly, higher levels of periodontal pathogens, including P. gingivalis and T. forsythia, were observed along with higher resistin levels in saliva of obese type 2 diabetic patients (109). In mice, oral infection with T. forsythia remarkably increased the serum levels of serum amyloid A (SAA), the subclinical inflammatory biomarker in multiple diseases like diabetes mellitus, atherosclerosis and rheumatic diseases (110).
Prevotella nigrescens
Prevotella nigrescens (P. nigrescens) is another Gram-negative and non-spore forming anaerobe commonly found in the dental plaques of periodontitis patients (118). During the progression of periodontitis, P. nigrescens shifts from commensalism to virulence via the upregulation of MMPs (120). High levels of MMPs (e.g. MMP-8 and MMP-9) often reflect periodontal inflammation (115). P. nigrescens can induce IL-1β production in dendritic cells through the activation of TLR2 and nucleotide-binding oligomerization domain like receptor pyrin domain containing 3 (NLRP3) inflammasome (117). In type 2 diabetic patients, higher abundance of P. nigrescens was also noted in periodontitis sites when compared with those of non-diabetic individuals (116). In non-diabetic pregnant women, periodontal therapy remarkably reduced P. nigrescens abundance in dental plaque, and IL-6 levels in serum and GCF (119). Therefore, it is reasonable to postulate that periodontal treatment may elicit similar anti-inflammatory effects in diabetic patients by decreasing P. nigrescens abundance.
Due to the ease of obtaining samples from saliva and dental plaques, microbes in the oral cavity might be efficient and potential biomarkers for disease diagnosis and evaluation of therapeutic outcomes in periodontitis and diabetes mellitus. However, selection of a single bacterial strain may not most accurately evaluate the severity of diseases. In contrast, selection of multiple microbial biomarkers, or even in combination with other endogenous biomarkers, may further improve the accuracy and consistency of disease prediction and evaluation.
Therapeutic insights and future perspectives
Notably, some of the mentioned biomarkers might also serve as therapeutic targets for the treatment of diabetes mellitus and periodontitis. In other words, therapeutic strategies that could suppress the levels of certain biomarkers might alleviate the progression of the two diseases, particularly when certain biomarkers are involved in the pathogenic mechanisms of both diseases. For instance, non-surgical periodontal treatment could reduce serum and GCF levels of visfatin and improve glucose homeostasis in patients with concomitant periodontitis and diabetes mellitus (22). Of note, visfatin can alter glucose metabolism and promote inflammation in gingival tissues (123). Furthermore, periodontal treatment was shown to improve glycemic profiles along with reduced persistence of P. gingivalis in type 2 diabetic patients (101), where P. gingivalis is the major pathogenic bacterium for periodontitis and can alter glucose level once translocated to liver (100). Future studies shall investigate whether other biomarkers participate in the pathogenic processes of both diseases for more therapeutic insights.
The above studies also indicate that periodontal treatment is possible to alter glycemic control in patients. On the other hand, treatment that improves glycemic control might promote periodontal health. A previous clinical study showed that effective glycemic control without periodontal treatment could also improve bleeding on probing in patients (124). It is therefore interesting to investigate whether glycemic control alone could alter biomarker levels in oral cavity (e.g. GCF) of patients in future study. Besides, further efforts are needed to clarify whether a certain biomarker is a cause or consequence of diabetes mellitus and periodontitis. Therapeutic strategies that alter the consequent biomarker might not necessarily alleviate lesions and disease progressions of both diseases.
Conclusions
Epidemiologically, periodontitis is now considered as a risk factor for diabetes mellitus, and has been designated as the sixth complication of diabetes mellitus (125, 126). It is also reasonable to consider periodontitis as a co-morbidity of diabetes mellitus (127). Increased periodontal breakdown in patients with diabetes can attribute to the activation of immune and inflammatory responses, and increased susceptibility to infection. Certain endogenous biomarkers, including adipokines, microRNAs, inflammatory mediators, oxidative stress markers, and glycoproteins have been reported to play important roles in initiating and regulating different effector stages of immune and inflammatory responses (69, 128). Potentially, these low-molecular-weight proteins or non-coding RNAs are not only therapeutic targets, but also clinical predictors for earlier diagnosis and intervention for periodontitis and diabetes mellitus (20, 129). Importantly, overgrowth of certain bacterial strains in the oral cavity might be indicative to both periodontitis and diabetes mellitus. Further biomarker research would be a worthwhile endeavor to deepen our understanding towards the bidirectional relationship between type 2 diabetes mellitus and periodontitis.
Author contributions
SL: Writing – original draft. HL: Investigation, Validation, Writing – review & editing. HK: Writing – original draft. SYW: Investigation, Validation, Writing – review & editing. CKC: Conceptualization, Writing – review & editing. JX: Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Guangdong Basic and Applied Basic Research Foundation (2020A1515010237 and 2019A1515110128); Shenzhen Key Medical Discipline Construction Fund (No.SZXK039); Special Fund for Science and Technology Development of Longgang District, Shenzhen (LGKCYLWS2021000031 and LGWJ2021-116).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1292596/full#supplementary-material
Abbreviations
ATF4, activating transcription factor 4; BspA, leucine-rich-repeat protein; CgA, Chromogranin A; GCF, gingival crevicular fluid; HbA1c, hemoglobin; IL-6, interleukin-6; LPS, lipopolysaccharides; MMP, matrix metalloproteinase; NLRP3, nucleotide-binding oligomerization domain like receptor pyrin domain containing 3; SAA, serum amyloid A; SOD, superoxide dismutase; sTWEAK, soluble tumor necrosis factor-like weak inducer of apoptosis; TNF-α, tumor necrosis factor α; TLR2, toll-like receptor 2; TRAF6, tumor necrosis factor (TNF) receptor associated factor 6.
References
1. Arya S, Duhan J, Tewari S, Sangwan P, Ghalaut V, Aggarwal S. Healing of apical periodontitis after nonsurgical treatment in patients with type 2 diabetes. J Endod (2017) 43:1623–7. doi: 10.1016/j.joen.2017.05.013
2. Lalla E, Papapanou PN. Diabetes mellitus and periodontitis: a tale of two common interrelated diseases. Nat Rev Endocrinol (2011) 7:738–48. doi: 10.1038/nrendo.2011.106
3. Winning L, Patterson CC, Neville CE, Kee F, Linden GJ. Periodontitis and incident type 2 diabetes: a prospective cohort study. J Clin Periodontol (2017) 44:266–74. doi: 10.1111/jcpe.12691
4. Genco RJ. Current view of risk factors for periodontal diseases. J periodontol (1996) 67:1041–9. doi: 10.1902/jop.1996.67.10.1041
5. Kinane DF, Chestnutt IG. Relationship of diabetes to periodontitis. Curr Opin Periodontol (1997) 4:29–34.
6. Preshaw PM, Bissett SM. Periodontitis and diabetes. Br Dent J (2019) 227:577–84. doi: 10.1038/s41415-019-0794-5
7. Zhou X, Zhang W, Liu X, Li Y. Interrelationship between diabetes and periodontitis: role of hyperlipidemia. Arch Oral Biol (2015) 60:667–74. doi: 10.1016/j.archoralbio.2014.11.008
8. Devanoorkar A, Kathariya R, Guttiganur N, Gopalakrishnan D, Bagchi P. Resistin: a potential biomarker for periodontitis influenced diabetes mellitus and diabetes induced periodontitis. Dis markers (2014) 2014:930206. doi: 10.1155/2014/930206
9. Bazyar H, Maghsoumi-Norouzabad L, Yarahmadi M, Gholinezhad H, Moradi L, Salehi P, et al. The impacts of synbiotic supplementation on periodontal indices and biomarkers of oxidative stress in type 2 diabetes mellitus patients with chronic periodontitis under non-surgical periodontal therapy. A Double-Blind Placebo-Controlled Trial Diabetes Metab Syndr Obes (2020) 13:19–29. doi: 10.2147/DMSO.S230060
10. Nazar Majeed Z, Philip K, Alabsi AM, Pushparajan S, Swaminathan D. Identification of gingival crevicular fluid sampling, analytical methods, and oral biomarkers for the diagnosis and monitoring of periodontal diseases: A systematic review. Dis Markers (2016) 2016:1804727. doi: 10.1155/2016/1804727
11. Duffles LF, Hermont AP, Abreu LG, Pordeus IA, Silva TA. Association between obesity and adipokines levels in saliva and gingival crevicular fluid: A systematic review and meta-analysis. J Evidence-Based Med (2019) 12:313–24. doi: 10.1111/jebm.12363
12. Li Y, Ding L, Hassan W, Abdelkader D, Shang J. Adipokines and hepatic insulin resistance. J Diabetes Res (2013) 2013:170532. doi: 10.1155/2013/170532
13. Luo S, Yang X, Wang D, Ni J, Wu J, Xu Z, et al. Periodontitis contributes to aberrant metabolism in type 2 diabetes mellitus rats by stimulating the expression of adipokines. J Periodontal Res (2016) 51:453–61. doi: 10.1111/jre.12322
14. Andrukhov O, Ulm C, Reischl H, Nguyen PQ, Matejka M, Rausch-Fan X. Serum cytokine levels in periodontitis patients in relation to the bacterial load. J Periodontol (2011) 82:885–92. doi: 10.1902/jop.2010.100425
15. Pradeep AR, Nagpal K, Karvekar S, Patnaik K. Levels of lipocalin-2 in crevicular fluid and tear fluid in chronic periodontitis and obesity subjects. J Invest Clin dentistry (2016) 7:376–82. doi: 10.1111/jicd.12165
16. Mooradian AD. Dyslipidemia in type 2 diabetes mellitus. Nat Clin Pract Endocrinol Metab (2009) 5:150–9. doi: 10.1038/ncpendmet1066
17. Davies RC, Jaedicke KM, Barksby HE, Jitprasertwong P, Al-Shahwani RM, Taylor JJ, et al. Do patients with aggressive periodontitis have evidence of diabetes? A pilot study. J Periodontal Res (2011) 46:663–72. doi: 10.1111/j.1600-0765.2011.01388.x
18. Mantzoros CS, Li T, Manson JE, Meigs JB, Hu FB. Circulating adiponectin levels are associated with better glycemic control, more favorable lipid profile, and reduced inflammation in women with type 2 diabetes. J Clin Endocrinol Metab (2005) 90:4542–8. doi: 10.1210/jc.2005-0372
19. Matsumoto S, Ogawa H, Soda S, Hirayama S, Amarasena N, Aizawa Y, et al. Effect of antimicrobial periodontal treatment and maintenance on serum adiponectin in type 2 diabetes mellitus. J Clin Periodontol (2009) 36:142–8. doi: 10.1111/j.1600-051X.2008.01359.x
20. Sun WL, Chen LL, Zhang SZ, Ren YZ, Qin GM. Changes of adiponectin and inflammatory cytokines after periodontal intervention in type 2 diabetes patients with periodontitis. Arch Oral Biol (2010) 55:970–4. doi: 10.1016/j.archoralbio.2010.08.001
21. Cobbold C. Type 2 diabetes mellitus risk and exercise: is resistin involved? J Sports Med Phys Fitness (2019) 59:290–7. doi: 10.23736/S0022-4707.18.08258-0
22. Gokhale NH, Acharya AB, Patil VS, Trivedi DJ, Setty S, Thakur SL. Resistin levels in gingival crevicular fluid of patients with chronic periodontitis and type 2 diabetes mellitus. J Periodontol (2014) 85:610–7. doi: 10.1902/jop.2013.130092
23. Huang X, Yang Z. Resistin’s, obesity and insulin resistance: the continuing disconnect between rodents and humans. J Endocrinological Invest (2016) 39:607–15. doi: 10.1007/s40618-015-0408-2
24. Joshi A, Maddipati S, Chatterjee A, Lihala R, Gupta A. Gingival crevicular fluid resistin levels in chronic periodontitis with type 2 diabetes before and after non-surgical periodontal therapy: A clinico-biochemical study. Indian J Dental Res (2019) 30:47–51. doi: 10.4103/ijdr.IJDR_215_17
25. Rode PA, Kolte RA, Kolte AP, Purohit HJ, Ahuja CR. Relevance of single-nucleotide polymorphism to the expression of resistin gene affecting serum and gingival crevicular fluid resistin levels in chronic periodontitis and type 2 diabetes mellitus: A randomized control clinical trial. J Indian Soc Periodontol (2019) 23:131–6. doi: 10.4103/jisp.jisp_361_18
26. Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, et al. The hormone resistin links obesity to diabetes. Nature (2001) 409:307–12. doi: 10.1038/35053000
27. Bahammam MA, Attia MS. Effects of systemic simvastatin on the concentrations of visfatin, tumor necrosis factor-alpha, and interleukin-6 in gingival crevicular fluid in patients with type 2 diabetes and chronic periodontitis. J Immunol Res (2018) 2018:8481735. doi: 10.1155/2018/8481735
28. Fukuhara A, Matsuda M, Nishizawa M, Segawa K, Tanaka M, Kishimoto K, et al. Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science (2005) 307:426–30. doi: 10.1126/science.1097243
29. Moschen AR, Kaser A, Enrich B, Mosheimer B, Theurl M, Niederegger H, et al. Visfatin, an adipocytokine with proinflammatory and immunomodulating properties. J Immunol (2007) 178:1748–58. doi: 10.4049/jimmunol.178.3.1748
30. Pradeep AR, Raghavendra NM, Sharma A, Patel SP, Raju A, Kathariya R, et al. Association of serum and crevicular visfatin levels in periodontal health and disease with type 2 diabetes mellitus. J Periodontol (2012) 83:629–34. doi: 10.1902/jop.2011.110272
31. Wu Y, Chen L, Wei B, Luo K, Yan F. Effect of non-surgical periodontal treatment on visfatin concentrations in serum and gingival crevicular fluid of patients with chronic periodontitis and type 2 diabetes mellitus. J Periodontol (2015) 86:795–800. doi: 10.1902/jop.2015.140476
32. Taylor GW, Borgnakke WS. Periodontal disease: associations with diabetes, glycemic control and complications. Oral Diseases (2008) 14:191–203. doi: 10.1111/j.1601-0825.2008.01442.x
33. Genco RJ, Grossi SG, Ho A, Nishimura F, Murayama Y. A proposed model linking inflammation to obesity, diabetes, and periodontal infections. J Periodontol (2005) 76 Suppl 11S:2075–84. doi: 10.1902/jop.2005.76.11-S.2075
34. Sun WL, Chen LL, Zhang SZ, Wu YM, Ren YZ, Qin GM. Inflammatory cytokines, adiponectin, insulin resistance and metabolic control after periodontal intervention in patients with type 2 diabetes and chronic periodontitis. Intern Med (2011) 50:1569–74. doi: 10.2169/internalmedicine.50.5166
35. Bharti P, Katagiri S, Nitta H, Nagasawa T, Kobayashi H, Takeuchi Y, et al. Periodontal treatment with topical antibiotics improves glycemic control in association with elevated serum adiponectin in patients with type 2 diabetes mellitus. Obes Res Clin practice (2013) 7:e129–e38. doi: 10.1016/j.orcp.2011.11.005
36. Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med (2002) 8:1288–95. doi: 10.1038/nm788
37. Jaganathan R, Ravindran R, Dhanasekaran S. Emerging role of adipocytokines in type 2 diabetes as mediators of insulin resistance and cardiovascular disease. Can J Diabetes (2018) 42:446–56 e1. doi: 10.1016/j.jcjd.2017.10.040
38. Shuldiner AR, Yang R, Gong DW. Resistin, obesity, and insulin resistance–the emerging role of the adipocyte as an endocrine organ. New Engl J Med (2001) 345:1345–6. doi: 10.1056/NEJM200111013451814
39. Ukkola O. Resistin - a mediator of obesity-associated insulin resistance or an innocent bystander? Eur J Endocrinol (2002) 147:571–4. doi: 10.1530/eje.0.1470571
40. Adeghate E. Visfatin: structure, function and relation to diabetes mellitus and other dysfunctions. Curr Medicinal Chem (2008) 15:1851–62. doi: 10.2174/092986708785133004
41. Ogawa H, Damrongrungruang T, Hori S, Nouno K, Minagawa K, Sato M, et al. Effect of periodontal treatment on adipokines in type 2 diabetes. World J Diabetes (2014) 5:924–31. doi: 10.4239/wjd.v5.i6.924
42. Cutando A, Montero J, Gomez-de Diego R, Ferrera MJ, Lopez-Valverde A. Effect of topical application of melatonin on serum levels of C-reactive protein (CRP), interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-alpha) in patients with type 1 or type 2 diabetes and periodontal disease. J Clin Exp Dentistry (2015) 7:e628–33. doi: 10.4317/jced.52604
43. Jiang ZL, Cui YQ, Gao R, Li Y, Fu ZC, Zhang B, et al. Study of TNF-alpha, IL-1beta and LPS levels in the gingival crevicular fluid of a rat model of diabetes mellitus and periodontitis. Dis Markers (2013) 34:295–304. doi: 10.1155/2013/156798
44. Kardesler L, Buduneli N, Cetinkalp S, Kinane DF. Adipokines and inflammatory mediators after initial periodontal treatment in patients with type 2 diabetes and chronic periodontitis. J Periodontol (2010) 81:24–33. doi: 10.1902/jop.2009.090267
45. Preshaw PM, Alba AL, Herrera D, Jepsen S, Konstantinidis A, Makrilakis K, et al. Periodontitis and diabetes: a two-way relationship. Diabetologia (2012) 55:21–31. doi: 10.1007/s00125-011-2342-y
46. Javed F, Al-Askar M, Al-Hezaimi K. Cytokine profile in the gingival crevicular fluid of periodontitis patients with and without type 2 diabetes: a literature review. J Periodontol (2012) 83:156–61. doi: 10.1902/jop.2011.110207
47. Martinez-Aguilar VM, Carrillo-Avila BA, Sauri-Esquivel EA, Guzman-Marin E, Jimenez-Coello M, Escobar-Garcia DM, et al. Quantification of TNF-alpha in patients with periodontitis and type 2 diabetes. BioMed Res Int (2019) 2019:7984891. doi: 10.1155/2019/7984891
48. Gorska R, Gregorek H, Kowalski J, Laskus-Perendyk A, Syczewska M, Madalinski K. Relationship between clinical parameters and cytokine profiles in inflamed gingival tissue and serum samples from patients with chronic periodontitis. J Clin Periodontol (2003) 30:1046–52. doi: 10.1046/j.0303-6979.2003.00425.x
49. Singhal S, Pradeep AR, Kanoriya D, Garg V. Human soluble receptor for advanced glycation end products and tumor necrosis factor-alpha as gingival crevicular fluid and serum markers of inflammation in chronic periodontitis and type 2 diabetes. J Oral Sci (2016) 58:547–53. doi: 10.2334/josnusd.16-0017
50. Nishimura F, Iwamoto Y, Mineshiba J, Shimizu A, Soga Y, Murayama Y. Periodontal disease and diabetes mellitus: the role of tumor necrosis factor-alpha in a 2-way relationship. J Periodontol (2003) 74:97–102. doi: 10.1902/jop.2003.74.1.97
51. Bakshi D, Kaur G, Singh D, Sahota J, Thakur A, Grover S. Estimation of plasma levels of tumor necrosis factor-a, interleukin-4 and 6 in patients with chronic periodontitis and type II diabetes mellitus. J Contemp Dental Practice (2018) 19:166–9. doi: 10.5005/jp-journals-10024-2231
52. Kaplan M, Yuksel M, Ates I, Yaln Kilic ZM, Kilic H, Ates H, et al. Are sTWEAK and IL-17A levels in inflammatory bowel disease associated with disease activity and etiopathogenesis? Inflammatory Bowel Dis (2016) 22:615–22. doi: 10.1097/MIB.0000000000000632
53. Diaz-Lopez A, Chacon MR, Bullo M, Maymo-Masip E, Martinez-Gonzalez MA, Estruch R, et al. Serum sTWEAK concentrations and risk of developing type 2 diabetes in a high cardiovascular risk population: a nested case-control study. J Clin Endocrinol Metab (2013) 98:3482–90. doi: 10.1210/jc.2013-1848
54. Leira Y, Iglesias-Rey R, Gomez-Lado N, Aguiar P, Sobrino T, D’Aiuto F, et al. Periodontitis and vascular inflammatory biomarkers: an experimental in vivo study in rats. Odontology (2020) 108:202–12. doi: 10.1007/s10266-019-00461-3
55. Acharya AB, Chandrashekar A, Acharya S, Shettar L, Thakur S. Serum sTWEAK levels in chronic periodontitis and type 2 diabetes mellitus. Diabetes Metab Syndrome (2019) 13:1609–13. doi: 10.1016/j.dsx.2019.03.027
56. Leira Y, Ameijeira P, Dominguez C, Lopez-Arias E, Avila-Gomez P, Perez-Mato M, et al. Severe periodontitis is linked with increased peripheral levels of sTWEAK and PTX3 in chronic migraineurs. Clin Oral investigations (2020) 24:597–606. doi: 10.1007/s00784-019-02950-9
58. Trivedi S, Lal N, Mahdi AA, Mittal M, Singh B, Pandey S. Evaluation of antioxidant enzymes activity and malondialdehyde levels in patients with chronic periodontitis and diabetes mellitus. J Periodontol (2014) 85:713–20. doi: 10.1902/jop.2013.130066
59. Thomas B, Rao A, Prasad BR, Kumari S. Serum levels of antioxidants and superoxide dismutase in periodontitis patients with diabetes type 2. J Indian Soc Periodontol (2014) 18:451–5. doi: 10.4103/0972-124X.138686
60. Thomas B, Ramesh A, Suresh S, Prasad BR. A comparative evaluation of antioxidant enzymes and selenium in the serum of periodontitis patients with diabetes mellitus type 2. Contemp Clin Dent (2013) 4:176–80. doi: 10.4103/0976-237X.114867
61. Pradeep AR, Agarwal E, Bajaj P, Rao NS. 4-Hydroxy-2-nonenal, an oxidative stress marker in crevicular fluid and serum in type 2 diabetes with chronic periodontitis. Contemp Clin Dent (2013) 4:281–5. doi: 10.4103/0976-237X.118342
62. Fisher K, Lin J. MicroRNA in inflammatory bowel disease: Translational research and clinical implication. World J Gastroenterol (2015) 21:12274–82. doi: 10.3748/wjg.v21.i43.12274
63. Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res (2009) 19:92–105. doi: 10.1101/gr.082701.108
64. Nayar G, Gauna A, Chukkapalli S, Velsko I, Kesavalu L, Cha S. Polymicrobial infection alter inflammatory microRNA in rat salivary glands during periodontal disease. Anaerobe (2016) 38:70–5. doi: 10.1016/j.anaerobe.2015.10.005
65. Stoecklin-Wasmer C, Guarnieri P, Celenti R, Demmer RT, Kebschull M, Papapanou PN. MicroRNAs and their target genes in gingival tissues. J Dental Res (2012) 91:934–40. doi: 10.1177/0022034512456551
66. Xie YF, Shu R, Jiang SY, Liu DL, Zhang XL. Comparison of microRNA profiles of human periodontal diseased and healthy gingival tissues. Int J Oral sci (2011) 3:125–34. doi: 10.4248/IJOS11046
67. Huang W. MicroRNAs: biomarkers, diagnostics, and therapeutics. Methods Mol Biol (2017) 1617:57–67. doi: 10.1007/978-1-4939-7046-9_4
68. Li B, Fan J, Chen N. A novel regulator of type II diabetes: microRNA-143. Trends Endocrinol metabolism: TEM (2018) 29:380–8. doi: 10.1016/j.tem.2018.03.019
69. Radovic N, Nikolic Jakoba N, Petrovic N, Milosavljevic A, Brkovic B, Roganovic J. MicroRNA-146a and microRNA-155 as novel crevicular fluid biomarkers for periodontitis in non-diabetic and type 2 diabetic patients. J Clin periodontol (2018) 45:663–71. doi: 10.1111/jcpe.12888
70. Bagavad Gita J, George AV, Pavithra N, Chandrasekaran SC, Latchumanadhas K, Gnanamani A. Dysregulation of miR-146a by periodontal pathogens: A risk for acute coronary syndrome. J periodontol (2019) 90:756–65. doi: 10.1002/JPER.18-0466
71. Balasubramanyam M, Aravind S, Gokulakrishnan K, Prabu P, Sathishkumar C, Ranjani H, et al. Impaired miR-146a expression links subclinical inflammation and insulin resistance in Type 2 diabetes. Mol Cell Biochem (2011) 351:197–205. doi: 10.1007/s11010-011-0727-3
72. Baldeon RL, Weigelt K, de Wit H, Ozcan B, van Oudenaren A, Sempertegui F, et al. Decreased serum level of miR-146a as sign of chronic inflammation in type 2 diabetic patients. PloS One (2014) 9:e115209. doi: 10.1371/journal.pone.0115209
73. Motedayyen H, Ghotloo S, Saffari M, Sattari M, Amid R. Evaluation of microRNA-146a and its targets in gingival tissues of patients with chronic periodontitis. J periodontol (2015) 86:1380–5. doi: 10.1902/jop.2015.150319
74. Fan Y, Wu Y. Tetramethylpyrazine alleviates neural apoptosis in injured spinal cord via the downregulation of miR-214-3p. Biomed pharmacother = Biomed pharmacotherapie (2017) 94:827–33. doi: 10.1016/j.biopha.2017.07.162
75. Li K, Zhang J, Yu J, Liu B, Guo Y, Deng J, et al. MicroRNA-214 suppresses gluconeogenesis by targeting activating transcriptional factor 4. J Biol Chem (2015) 290:8185–95. doi: 10.1074/jbc.M114.633990
76. Maity S, Das F, Ghosh-Choudhury N, Kasinath BS, Ghosh Choudhury G. High glucose increases miR-214 to power a feedback loop involving PTEN and the Akt/mTORC1 signaling axis. FEBS letters (2019) 593:2261–72. doi: 10.1002/1873-3468.13505
77. Ou L, Sun T, Cheng Y, Huang L, Zhan X, Zhang P, et al. MicroRNA-214 contributes to regulation of necroptosis via targeting ATF4 in diabetes-associated periodontitis. J Cell Biochem (2019) 120:14791–803. doi: 10.1002/jcb.28740
78. Liu G, Friggeri A, Yang Y, Park YJ, Tsuruta Y, Abraham E. miR-147, a microRNA that is induced upon Toll-like receptor stimulation, regulates murine macrophage inflammatory responses. Proc Natl Acad Sci United States America (2009) 106:15819–24. doi: 10.1073/pnas.0901216106
79. Xu R, Zeng G, Wang S, Tao H, Ren L, Zhang Z, et al. Periodontitis promotes the diabetic development of obese rat via miR-147 induced classical macrophage activation. Biomed pharmacother = Biomed pharmacotherapie (2016) 83:892–7. doi: 10.1016/j.biopha.2016.07.030
80. Wu Y, Song LT, Li JS, Zhu DW, Jiang SY, Deng JY. MicroRNA-126 regulates inflammatory cytokine secretion in human gingival fibroblasts under high glucose via targeting tumor necrosis factor receptor associated factor 6. J periodontol (2017) 88:e179–e87. doi: 10.1902/jop.2017.170091
81. Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci United States America (2006) 103:12481–6. doi: 10.1073/pnas.0605298103
82. Sanada T, Sano T, Sotomaru Y, Alshargabi R, Yamawaki Y, Yamashita A, et al. Anti-inflammatory effects of miRNA-146a induced in adipose and periodontal tissues. Biochem biophysics Rep (2020) 22:100757. doi: 10.1016/j.bbrep.2020.100757
83. Ke X, Lei L, Li H, Yan F. Manipulation of necroptosis by Porphyromonas gingivalis in periodontitis development. Mol Immunol (2016) 77:8–13. doi: 10.1016/j.molimm.2016.07.010
84. Xu Y, Gao H, Hu Y, Fang Y, Qi C, Huang J, et al. High glucose-induced apoptosis and necroptosis in podocytes is regulated by UCHL1 via RIPK1/RIPK3 pathway. Exp Cell Res (2019) 382:111463. doi: 10.1016/j.yexcr.2019.06.008
85. Su Y, Wang D, Xuan D, Ni J, Luo S, Xie B, et al. Periodontitis as a novel contributor of adipose tissue inflammation promotes insulin resistance in a rat model. J periodontol (2013) 84:1617–26. doi: 10.1902/jop.2013.120442
86. Keles ZP, Keles GC, Avci B, Cetinkaya BO, Emingil G. Analysis of YKL-40 acute-phase protein and interleukin-6 levels in periodontal disease. J periodontol (2014) 85:1240–6. doi: 10.1902/jop.2014.130631
87. Damodar S, Mehta DS. Effect of scaling and root planing on gingival crevicular fluid level of YKL-40 acute phase protein in chronic periodontitis patients with or without type 2 diabetes mellitus: A clinico-biochemical study. J Indian Soc Periodontol (2018) 22:40–4. doi: 10.4103/jisp.jisp_95_17
88. Kido J, Bando Y, Bando M, Kajiura Y, Hiroshima Y, Inagaki Y, et al. YKL-40 level in gingival crevicular fluid from patients with periodontitis and type 2 diabetes. Oral diseases (2015) 21:667–73. doi: 10.1111/odi.12334
89. Kumar PA, Kripal K, Chandrasekaran K, Bhavanam SR. Estimation of YKL-40 levels in serum and gingival crevicular fluid in chronic periodontitis and type 2 diabetes patients among south Indian population: A clinical study. Contemp Clin dentistry (2019) 10:304–10. doi: 10.4103/ccd.ccd_629_18
90. Kogawa EM, Grisi DC, Falcao DP, Amorim IA, Rezende TM, da Silva IC, et al. Impact of glycemic control on oral health status in type 2 diabetes individuals and its association with salivary and plasma levels of chromogranin A. Arch Oral Biol (2016) 62:10–9. doi: 10.1016/j.archoralbio.2015.11.005
91. Zheng A, Moritani T. Effect of the combination of ginseng, oriental bezoar and glycyrrhiza on autonomic nervous activity and immune system under mental arithmetic stress. J Nutr Sci vitaminol (2008) 54:244–9. doi: 10.3177/jnsv.54.244
92. Zhang T, Jiang H, Wang T, Liu S, Rausch-Fan X, Yang P. Salivary chromogranin A and myeloid-related protein-8/14 from periodontitis patients with type 2 diabetes. Quintessence Int (2019) 50:808–14. doi: 10.3290/j.qi.a43233
93. Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol (2005) 43:5721–32. doi: 10.1128/JCM.43.11.5721-5732.2005
94. Curtis MA, Zenobia C, Darveau RP. The relationship of the oral microbiotia to periodontal health and disease. Cell Host Microbe (2011) 10:302–6. doi: 10.1016/j.chom.2011.09.008
95. Graves DT, Correa JD, Silva TA. The oral microbiota is modified by systemic diseases. J Dent Res (2019) 98:148–56. doi: 10.1177/0022034518805739
96. Karpinski TM. Role of oral microbiota in cancer development. Microorganisms (2019) 7(1):20. doi: 10.3390/microorganisms7010020
97. Blasco-Baque V, Garidou L, Pomie C, Escoula Q, Loubieres P, Le Gall-David S, et al. Periodontitis induced by Porphyromonas gingivalis drives periodontal microbiota dysbiosis and insulin resistance via an impaired adaptive immune response. Gut (2017) 66:872–85. doi: 10.1136/gutjnl-2015-309897
98. Clais S, Boulet G, Kerstens M, Horemans T, Teughels W, Quirynen M, et al. Importance of biofilm formation and dipeptidyl peptidase IV for the pathogenicity of clinical Porphyromonas gingivalis isolates. Pathog Dis (2014) 70:408–13. doi: 10.1111/2049-632X.12156
99. Gonzales JR, Groeger S, Johansson A, Meyle J. T helper cells from aggressive periodontitis patients produce higher levels of interleukin-1 beta and interleukin-6 in interaction with Porphyromonas gingivalis. Clin Oral Investig (2014) 18:1835–43. doi: 10.1007/s00784-013-1162-5
100. Ishikawa M, Yoshida K, Okamura H, Ochiai K, Takamura H, Fujiwara N, et al. Oral Porphyromonas gingivalis translocates to the liver and regulates hepatic glycogen synthesis through the Akt/GSK-3beta signaling pathway. Biochim Biophys Acta (2013) 1832:2035–43. doi: 10.1016/j.bbadis.2013.07.012
101. Makiura N, Ojima M, Kou Y, Furuta N, Okahashi N, Shizukuishi S, et al. Relationship of Porphyromonas gingivalis with glycemic level in patients with type 2 diabetes following periodontal treatment. Oral Microbiol Immunol (2008) 23:348–51. doi: 10.1111/j.1399-302X.2007.00426.x
102. Salminen A, Gursoy UK, Paju S, Hyvarinen K, Mantyla P, Buhlin K, et al. Salivary biomarkers of bacterial burden, inflammatory response, and tissue destruction in periodontitis. J Clin Periodontol (2014) 41:442–50. doi: 10.1111/jcpe.12234
103. Vernal R, Diaz-Zuniga J, Melgar-Rodriguez S, Pujol M, Diaz-Guerra E, Silva A, et al. Activation of RANKL-induced osteoclasts and memory T lymphocytes by Porphyromonas gingivalis is serotype dependant. J Clin Periodontol (2014) 41:451–9. doi: 10.1111/jcpe.12236
104. Casarin RC, Barbagallo A, Meulman T, Santos VR, Sallum EA, Nociti FH, et al. Subgingival biodiversity in subjects with uncontrolled type-2 diabetes and chronic periodontitis. J Periodontal Res (2013) 48:30–6. doi: 10.1111/j.1600-0765.2012.01498.x
105. Johnson L, Almeida-da-Silva CLC, Takiya CM, Figliuolo V, Rocha GM, Weissmuller G, et al. Oral infection of mice with Fusobacterium nucleatum results in macrophage recruitment to the dental pulp and bone resorption. BioMed J (2018) 41:184–93. doi: 10.1016/j.bj.2018.05.001
106. Kolenbrander PE, Andersen RN, Blehert DS, Egland PG, Foster JS, Palmer RJ Jr. Communication among oral bacteria. Microbiol Mol Biol Rev (2002) 66:486–505. doi: 10.1128/MMBR.66.3.486-505.2002
107. Miranda TS, Feres M, Retamal-Valdes B, Perez-Chaparro PJ, Maciel SS, Duarte PM. Influence of glycemic control on the levels of subgingival periodontal pathogens in patients with generalized chronic periodontitis and type 2 diabetes. J Appl Oral Sci (2017) 25:82–9. doi: 10.1590/1678-77572016-0302
108. Saito A, Inagaki S, Kimizuka R, Okuda K, Hosaka Y, Nakagawa T, et al. Fusobacterium nucleatum enhances invasion of human gingival epithelial and aortic endothelial cells by Porphyromonas gingivalis. FEMS Immunol Med Microbiol (2008) 54:349–55. doi: 10.1111/j.1574-695X.2008.00481.x
109. Al-Rawi N, Al-Marzooq F. The relation between periodontopathogenic bacterial levels and resistin in the saliva of obese type 2 diabetic patients. J Diabetes Res (2017) 2017:2643079. doi: 10.1155/2017/2643079
110. Chukkapalli SS, Rivera-Kweh MF, Velsko IM, Chen H, Zheng D, Bhattacharyya I, et al. Chronic oral infection with major periodontal bacteria Tannerella forsythia modulates systemic atherosclerosis risk factors and inflammatory markers. Pathog Dis (2015) 73(3):ftv009. doi: 10.1093/femspd/ftv009
111. Haffajee AD, Socransky SS. Relation of body mass index, periodontitis and Tannerella forsythia. J Clin Periodontol (2009) 36:89–99. doi: 10.1111/j.1600-051X.2008.01356.x
112. Myneni SR, Settem RP, Connell TD, Keegan AD, Gaffen SL, Sharma A. TLR2 signaling and Th2 responses drive Tannerella forsythia-induced periodontal bone loss. J Immunol (2011) 187:501–9. doi: 10.4049/jimmunol.1100683
113. Sharma A, Inagaki S, Honma K, Sfintescu C, Baker PJ, Evans RT. Tannerella forsythia-induced alveolar bone loss in mice involves leucine-rich-repeat BspA protein. J Dent Res (2005) 84:462–7. doi: 10.1177/154405910508400512
114. Zhou M, Rong R, Munro D, Zhu C, Gao X, Zhang Q, et al. Investigation of the effect of type 2 diabetes mellitus on subgingival plaque microbiota by high-throughput 16S rDNA pyrosequencing. PloS One (2013) 8:e61516. doi: 10.1371/journal.pone.0061516
115. Al-Majid A, Alassiri S, Rathnayake N, Tervahartiala T, Gieselmann DR, Sorsa T. Matrix metalloproteinase-8 as an inflammatory and prevention biomarker in periodontal and peri-implant diseases. Int J Dent (2018) 2018:7891323. doi: 10.1155/2018/7891323
116. Ebersole JL, Holt SC, Hansard R, Novak MJ. Microbiologic and immunologic characteristics of periodontal disease in Hispanic americans with type 2 diabetes. J Periodontol (2008) 79:637–46. doi: 10.1902/jop.2008.070455
117. Jang HM, Park JY, Lee YJ, Kang MJ, Jo SG, Jeong YJ, et al. TLR2 and the NLRP3 inflammasome mediate IL-1beta production in Prevotella nigrescens-infected dendritic cells. Int J Med Sci (2021) 18:432–40. doi: 10.7150/ijms.47197
118. Maeda N, Okamoto M, Kondo K, Ishikawa H, Osada R, Tsurumoto A, et al. Incidence of Prevotella intermedia and Prevotella nigrescens in periodontal health and disease. Microbiol Immunol (1998) 42:583–9. doi: 10.1111/j.1348-0421.1998.tb02328.x
119. Offenbacher S, Lin D, Strauss R, McKaig R, Irving J, Barros SP, et al. Effects of periodontal therapy during pregnancy on periodontal status, biologic parameters, and pregnancy outcomes: a pilot study. J Periodontol (2006) 77:2011–24. doi: 10.1902/jop.2006.060047
120. Szafranski SP, Deng ZL, Tomasch J, Jarek M, Bhuju S, Meisinger C, et al. Functional biomarkers for chronic periodontitis and insights into the roles of Prevotella nigrescens and Fusobacterium nucleatum; a metatranscriptome analysis. NPJ Biofilms Microbiomes (2015) 1:15017. doi: 10.1038/npjbiofilms.2015.17
121. Mysak J, Podzimek S, Sommerova P, Lyuya-Mi Y, Bartova J, Janatova T, et al. Porphyromonas gingivalis: major periodontopathic pathogen overview. J Immunol Res (2014) 2014:476068. doi: 10.1155/2014/476068
122. Signat B, Roques C, Poulet P, Duffaut D. Fusobacterium nucleatum in periodontal health and disease. Curr Issues Mol Biol (2011) 13:25–36.
123. Ozcan E, Saygun NI, Ilikci R, Karslioglu Y, Musabak U, Yesillik S. Increased visfatin expression is associated with nuclear factor-kappa B and phosphatidylinositol 3-kinase in periodontal inflammation. Clin Oral Investig (2017) 21:1113–21. doi: 10.1007/s00784-016-1871-7
124. Katagiri S, Nitta H, Nagasawa T, Izumi Y, Kanazawa M, Matsuo A, et al. Effect of glycemic control on periodontitis in type 2 diabetic patients with periodontal disease. J Diabetes Investig (2013) 4:320–5. doi: 10.1111/jdi.12026
125. Bascones-Martinez A, Munoz-Corcuera M, Bascones-Ilundain J. [Diabetes and periodontitis: A bidirectional relationship]. Medicina clinica (2015) 145:31–5. doi: 10.1016/j.medcle.2015.12.048
126. Loe H. Periodontal disease. The sixth complication of diabetes mellitus. Diabetes Care (1993) 16:329–34. doi: 10.2337/diacare.16.1.329
127. Luong A, Tawfik AN, Islamoglu H, Gobriel HS, Ali N, Ansari P, et al. Periodontitis and diabetes mellitus co-morbidity: A molecular dialogue. J Oral Biosci (2021) 63:360–9. doi: 10.1016/j.job.2021.10.006
128. Atieh MA, Faggion CM Jr., Seymour GJ. Cytokines in patients with type 2 diabetes and chronic periodontitis: A systematic review and meta-analysis. Diabetes Res Clin practice (2014) 104:e38–45. doi: 10.1016/j.diabres.2014.02.002
Keywords: biomarkers, periodontitis, diabetes mellitus, adipokines, microRNAs, oral microbiome
Citation: Li S, Li H, Kong H, Wu SY, Cheng CK and Xu J (2023) Endogenous and microbial biomarkers for periodontitis and type 2 diabetes mellitus. Front. Endocrinol. 14:1292596. doi: 10.3389/fendo.2023.1292596
Received: 11 September 2023; Accepted: 20 November 2023;
Published: 05 December 2023.
Edited by:
Jian Ma, Harbin Medical University, ChinaReviewed by:
Yiqian Li, Stony Brook University, United StatesKai Song, University of California, Los Angeles, United States
Copyright © 2023 Li, Li, Kong, Wu, Cheng and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian Xu, xj-sz@hotmail.com
 Songjun Li1
Songjun Li1 Shang Ying Wu
Shang Ying Wu Chak Kwong Cheng
Chak Kwong Cheng Jian Xu
Jian Xu