- 1Department of Gynecology, Longhua Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai, China
- 2Department of Reproductive Center, Shuguang Hospital Affiliated to Shanghai University of Traditional Chinese Medicine, Shanghai, China
Background: To determine whether progestin-primed ovarian stimulation (PPOS) is more effective for women with diminished ovarian reserve (DOR) than clomiphene citrate (CC)/letrozole (LE) plus gonadotropin in IVF or ICSI treatment.
Methods: Nine databases were searched until May 24, 2023, to identify relevant studies. Forest plots were used to present the results of this meta-analysis. Begg’s and Egger’s tests were applied to estimate publication bias. Subgroup and sensitivity analysis were performed to check the potential sources of heterogeneity and verify the robustness of the pooled results, respectively.
Results: A total of 14 studies with 4182 participants were included for meta-analysis. There was evidence of a statistically notable increase in clinical pregnancy rate (OR = 1.39, 95%CI [1.01, 1.91], p = 0.05), optimal embryos rate (OR = 1.50, 95%CI [1.20, 1.88], p = 0.0004), and cumulative pregnancy rate (OR = 1.73, 95%CI [1.14, 2.60], p = 0.009), the duration and the amount of gonadotropin required (MD = 1.56, 95%CI [0.47, 2.66], p = 0.005; SMD = 1.51, 95%CI [0.90, 2.12], p < 0.00001), along with decrease cycle cancellation rate (OR = 0.78, 95%CI [0.64, 0.95], p = 0.02), luteinizing hormone (LH) level on the day of hCG (SMD = -0.81, 95%CI [-1.10, -0.53], p < 0.00001), and premature LH surge rate (OR = 0.10, 95%CI [0.07, 0.15], p < 0.00001) when PPOS was used. No evidence for publication bias within results was revealed.
Conclusions: Based on evidence-based results, PPOS protocol seems to improve IVF/ICSI outcomes for women with DOR. More research with larger sample sizes and rigorous designs are required to further explore the value of PPOS among women diagnosed with DOR.
Systematic review registration: www.crd.york.ac.uk, identifier CRD42023430202.
1 Introduction
Infertility is a severe health problem and affects 9% reproductive-aged women globally (1). The incidence of infertility has grown substantially, and it is estimated to impact 186 million people in the 21st century (2). One of the primary causes of infertility is diminished ovarian reserve (DOR) (3). A recent statistic based on 181,536 assisted reproductive technology (ART) cycles demonstrated that the overall prevalence of DOR is estimated to be 19 to 26% in the US (4). Furthermore, DOR, characterized as decreased oocyte quality and quantity, is significantly associated with poor reproductive outcomes, which is still a serious clinical challenge for ART treatment (5, 6). Numerous studies indicate that infertile women with DOR experienced higher miscarriage rates, lower chance of possessing at least one euploid blastocyst, increased risk of cycle cancellation and poor ovarian response in in-vitro fertilization (IVF) (7–10). Therefore, it is imperative to explore appropriate ovarian stimulation protocols to improve the outcomes for women with DOR undergoing ART.
Currently, no guideline or consensus recommends an applicable ovarian stimulation protocol for women with DOR. In clinical practice, pituitary suppression and gonadotropins are widely employed to prevent premature luteinizing hormone (LH) surge and ovulation in the course of IVF or intracytoplasmic sperm injection (ICSI) cycles. Despite their overall effectiveness, high-dose gonadotropins stimulation is often accompanied with ovarian hyperstimulation syndrome, reduced live birth rate, worse oocyte quality, and higher medication costs (11–13). In addition, gonadotropin-releasing hormone (GnRH) applied in pituitary suppression had been proven that 0.34% to 8.0% fail to manage premature LH surge (14). Moreover, GnRH antagonist protocol in IVF cycles could increase uterine natural killer cells and tumour necrosis factor α, which negatively affects endometrial receptivity (15). Clomiphene citrate (CC) and letrozole (LE) are often administered in IVF treatment for ovarian stimulation as well. A retrospective cohort study has revealed that the live birth rate was significantly lower in CC cycles compared to that with natural cycles (p = 0.01), whose underlying mechanism might be that CC influenced uterine receptivity by reducing endometrial thickness through antiestrogenic effects (16). Besides, the use of LE during inducing ovulation has been reported to be correlated with a notable risk of elevating progesterone levels, which has an adverse effect on the pregnancy rate (17). Hence, over the past few years, an alternative approach known as progestin-primed ovarian stimulation (PPOS) in controlling the LH surge has attracted lots of clinicians. Observational studies have demonstrated that PPOS generated a similar formation of euploid blastocysts per oocyte, live birth rate, cumulative ongoing pregnancy, and metaphase II oocytes (MII), along with 2 pronuclear fertilized oocytes (2PN) with GnRH antagonist protocol (18–20). Simultaneously, a retrospective cohort study involving 3556 infants revealed that PPOS resulted in similar neonatal outcomes, including the early neonatal death, preterm birth, rates of low birthweight and large/small-for-gestational age, when compared with GnRH agonist short protocol (21).
However, several clinical studies investigating the value of PPOS protocol for women with DOR undergoing IVF or ICSI produced conflicting results. For example, Liu et al. (22) included 108 cases and showed that PPOS protocol during IVF has the same clinical pregnancy rate, optimal embryos rate and cycle cancellation rate compared with CC plus gonadotropin stimulation, which is contrary to Zhao’s study (23). Meanwhile, Fu et al. (24) demonstrated that the number of oocytes retrieved, optimal embryos rate and cycle cancellation rate were not improved with PPOS protocol compared with CC plus LE stimulation. Still, Zhang’s study (25) confirmed that the PPOS protocol group achieved more oocytes retrieved, optimal embryos rate and lower cycle cancellation rate than CC plus LE group. The divergent conclusions above may be insufficiently estimated because of the limited sample sizes from single clinical research. Therefore, we performed this meta-analysis to summarize the existing evidence quantitatively and inform clinical practice. The study’s specific concern was as follows: Does PPOS improve the outcomes for women with DOR undergoing IVF or ICSI compared with CC/LE plus gonadotropin stimulation?
2 Materials and methods
This study (PROSPERO registration No. CRD42023430202) was conducted following the preferred reporting program of the systematic review and meta-analysis (PRISMA) (26).
2.1 Search strategy
We thoroughly searched nine databases, including English-language databases Cochrane Library, Sinomed, EBSCO, Web of Science, Scopus, PubMed, and Chinese-language databases Wanfang, VIP Information and China National Knowledge Infrastructure (CNKI) from inception up to May 24, 2023. We included use different combinations of the following search terms: “decreased ovarian reserve”, “declined ovarian reserve”, “diminished ovarian reserve”, and “assisted reproduction technology”, “ICSI”, “IVF”, “mild stimulation”, “microstimulation”, “progestin primed ovarian stimulation”, “clomiphene plus gonadotropin”, “letrozole plus gonadotropin”. The first two authors (G.Y.L. and X.F.Z) independently screened the articles through titles, abstracts, and full texts to identify the eligibility of the studies. In addition, we carefully checked the references from retrieved studies to obtain more relevant research as much as possible.
2.2 Inclusion and exclusion criteria
The studies met the following criteria would be included (1): studies of patients who were diagnosed with DOR (AFC < 5~7 or FSH ≥ 10IU/L or AMH < 1.1ng/mL) (27, 28); (2) all patients received ART treatment, including IVF and ICSI; (3) studies divided patients into two groups in term of ovarian stimulation protocols (PPOS versus CC/LE plus gonadotropin) regardless of the types of progestin; (4) studies provided the diagnostic criteria for DOR and basal characteristics (i.e., age, duration of infertility) of patients with sufficient data; (5) types of study were randomized controlled trials, observational studies and cross sectional studies; (6) there were no ethnic and geographical restrictions.
The exclusion criteria included: (1) researches were self-controlled study; (2) patients with polycystic ovary syndrome, abnormal endometrium, intrauterine adhesion, uterine malformation, reproductive tumors and chromosomal abnormalities; (3) meta-analysis, study protocol, duplicate publications, reviews, animal experiments and conference papers; (4) studies that were not published in either Chinese or English.
2.3 Data extraction and quality assessment
A standardized form was adopted by the first two authors (GL and XZ) to perform data extraction independently. The following data were retrieved: study population characteristics (i.e., age, duration of infertility, body mass index), details of the treatments (i.e., type of gonadotropin, intervention of ovarian stimulation protocol) and outcomes in each group. The primary outcomes were cycle cancellation rate, clinical pregnancy rate, the number of oocytes retrieved, and premature LH surge rate. The secondary outcomes were optimal embryos rate, fertilization rate, live birth rate, cleavage rate, embryo implantation rate, estradiol (E2) and LH on the day of hCG, duration of gonadotropin used, total dose of gonadotropin, cumulative pregnancy rate and early miscarriage rate. Meanwhile, the Newcastle-Ottawa scale (NOS) was utilized by two independent reviewers (GL and XZ) to evaluate the quality of the included articles. Studies were considered to be of high quality with scores of ≥ 6 (29). Any discrepancies were determined by discussing with the corresponding author (LX).
2.4 Statistical analysis
Analysis of the data was conducted with Review Manager 5.3 and Stata 15.1 software. The continuous variables (for example, the number of oocytes retrieved) were presented with a standardized mean difference (SMD) or mean difference (MD) with 95% confidence intervals (CIs). For dichotomous variables (for example, cycle cancellation rate), odds ratios (OR) with 95% Cls were shown. The heterogeneity in the meta-analysis was estimated by utilizing the I2 statistic. An I2 ≥ 50% was considered massive heterogeneity; then, the random-effects model was used. Otherwise, the fixed-effects model was adopted. Besides, the potential sources of heterogeneity were checked by subgroup analysis. p ≤ 0.05 was deemed statistically significant. Furthermore, Begg’s and Egger’s tests were applied by evaluating the P value to explore publication bias if at least ten studies were involved. When the p value was > 0.05, it is considered that there was no publication bias existing. If at least five studies were included, a sensitivity analysis was adopted by excluding individual articles to appraise the robustness of the pooled results.
3 Results
3.1 Included articles
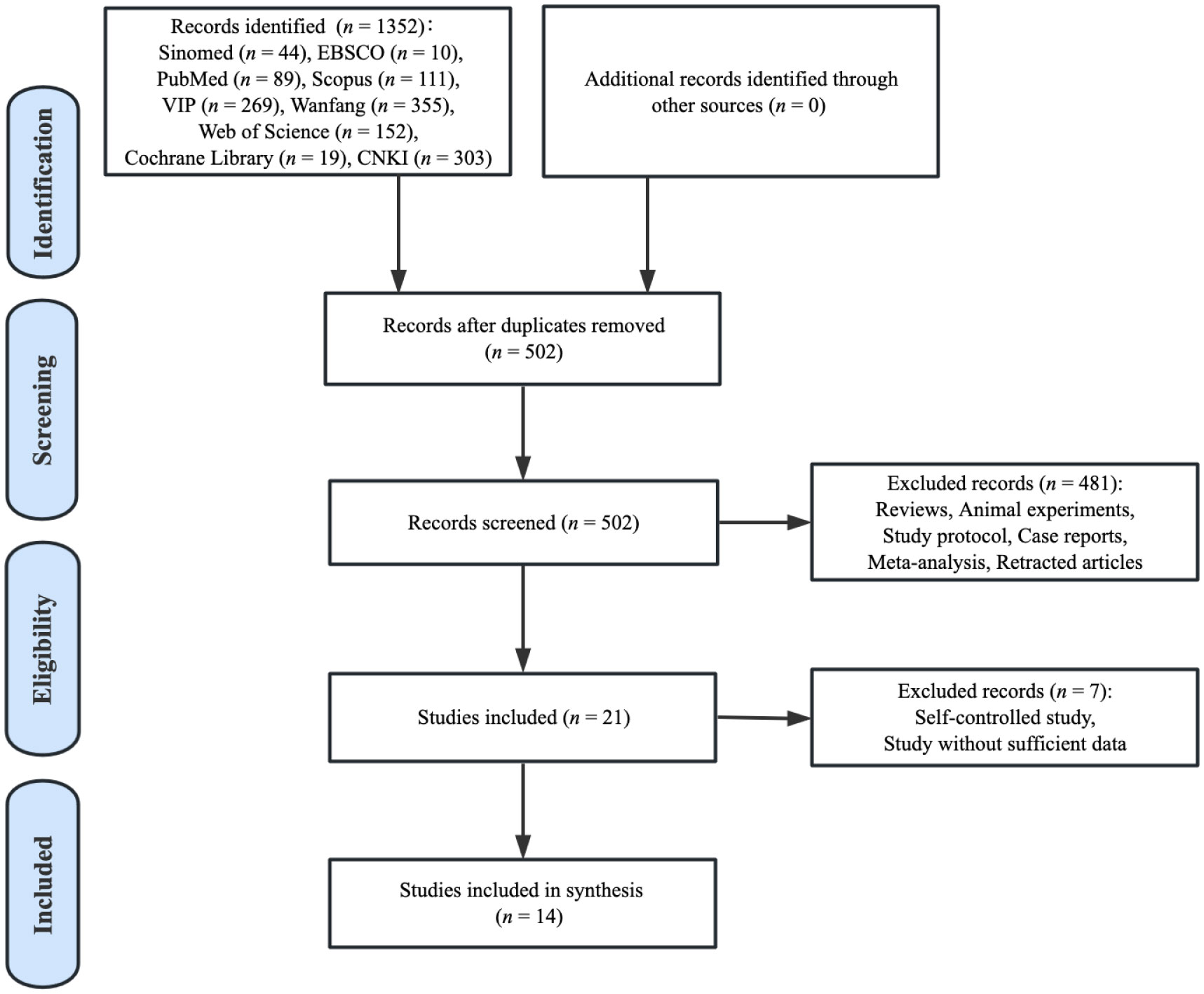
The flow chart exhibited in the PRISMA figure (Figure 1) shows the selection of records included. The search strategy identified 1352 articles via database searching. After removing duplicates, 850 papers were excluded. Of the 502 studies identified, 481 studies were removed because they met the basic exclusion criteria when going through the titles and abstracts. After the full-text screening, seven relevant articles were further excluded as they were self-controlled studies or without sufficient data. Ultimately, a total of 14 studies were included in the analysis of this review.
3.2 Study characteristics
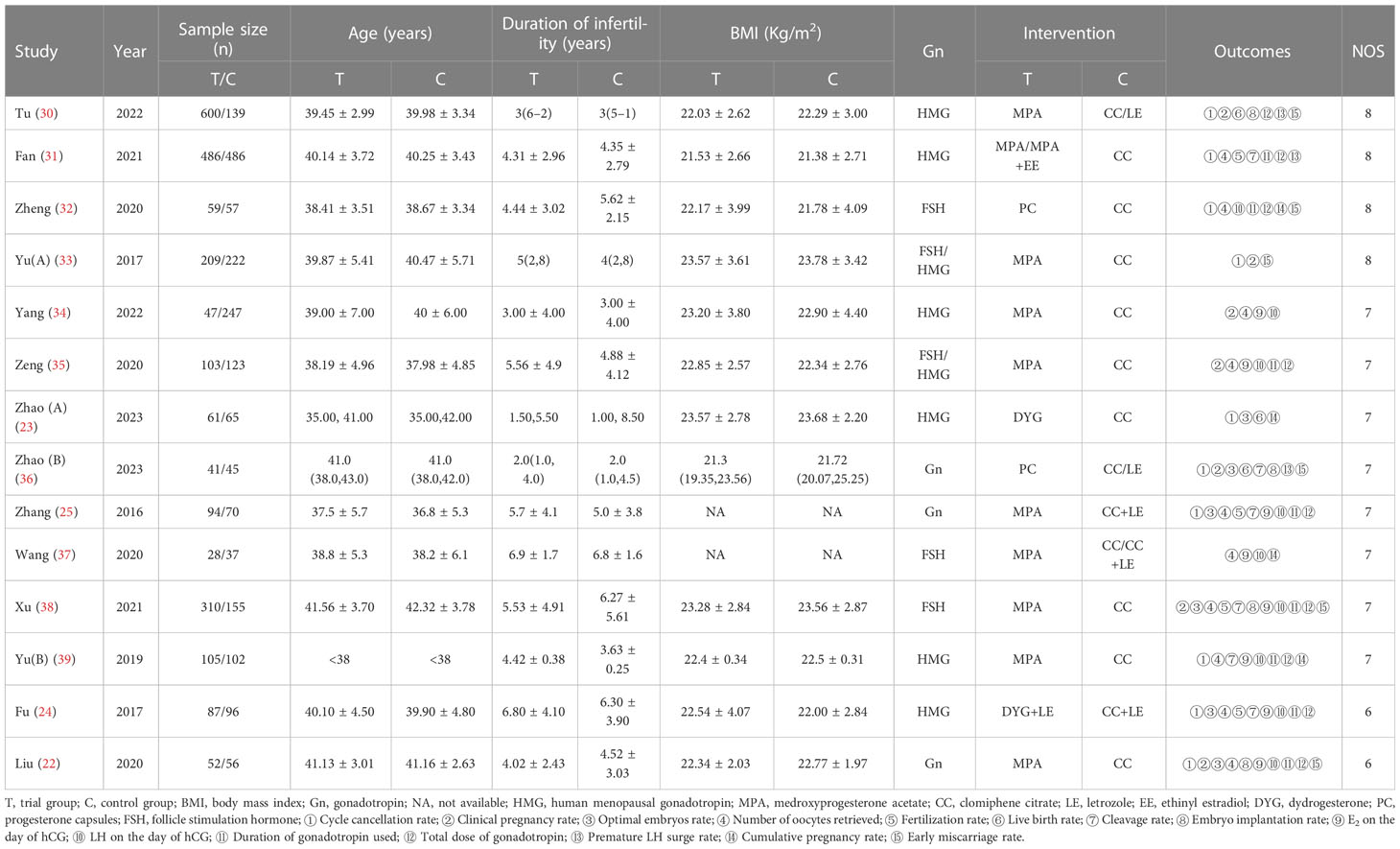
Table 1 shows the main characteristics of all included studies. We included 14 studies involving 4182 women with DOR undergoing IVF/ICSI treatment. The sample sizes in each trial varied from 65 to 972, and the publication year ranged from 2016 to 2023. All the patients included were from China and were categorized into trial group treated with PPOS and control group treated with CC/LE plus gonadotropin. The trial group and the control group comprised 2282 and 1900 cases, respectively. Four studies (23, 30, 36, 39) applied the median and 25th - 75th percentiles for continuous variables with skewed distributions. In the control group, nine studies reported patients treated with CC, two studies treated with CC plus LE, one study received CC or CC plus LE, and the remaining two studies were CC or LE. Further, the characteristics of sex hormones level and AFC were also presented in Supplementary Table 1.
3.3 Quality assessment
All researches were retrospective cohort studies, and the quality assessment were estimated in accordance with the NOS. Four of them were rated eight scores. Eight studies obtained seven scores, and two were evaluated as six scores. Although all studies assessed were of high quality, a common reason attributed to score low on study quality assessment was lack of sufficient detail in outcomes assessment procedures. Table 1 presents the NOS score of each study included.
3.4 Outcome measurements
3.4.1 The primary outcomes
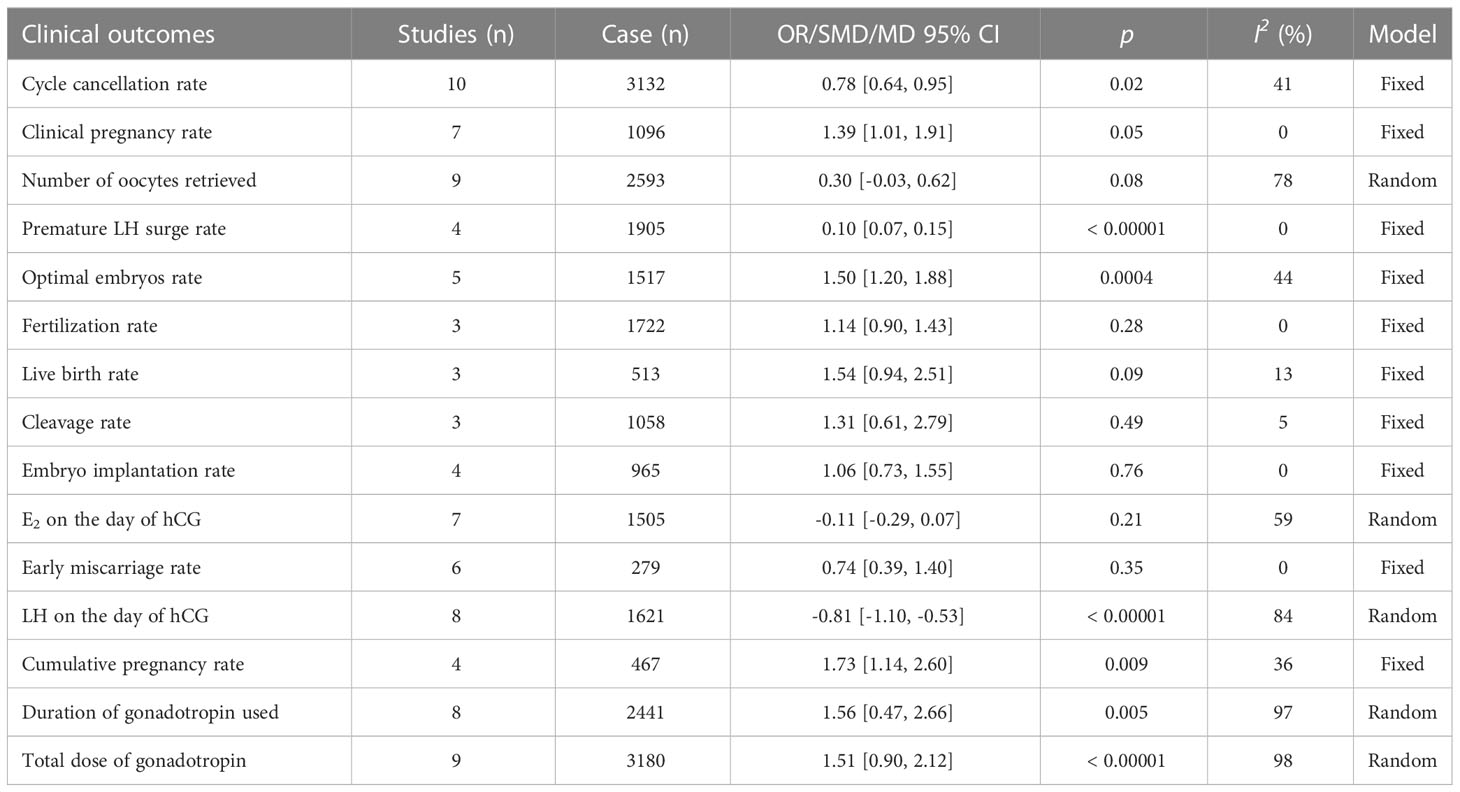
Ten studies investigated the association between PPOS and cycle cancellation rate. PPOS demonstrated a favourable result for cycle cancellation rate with the pooled OR being 0.78, (95% CI: 0.64, 0.95), I2 = 41%, p = 0.02 when compared with women treated with CC/LE plus gonadotropin stimulation. Seven studies reported clinical pregnancy rate in patients with PPOS intervention. The result showed that the pooled OR was 1.39 (95% CI: 1.01, 1.91), I2 = 0%, p = 0.05, revealing a higher rate of clinical pregnancy rate for women treated with PPOS compared with the control group. Furthermore, ten studies estimated the association of PPOS with the number of oocytes retrieved; after excluding Yu’s study (39) by sensitivity analysis, the heterogeneity decreased from 99% to 78%; therefore, the pooled MD was 0.30 (95% CI: -0.03, 0.62), I2 = 78%, p = 0.08, suggesting that PPOS nearly yielded the same the number of oocytes retrieved with the control group. In addition, four studies with 1905 cases reported premature LH surge rate. There was evidence of a notable decrease in premature LH surge rate when PPOS was used (OR 0.10, [95% CI: 0.07, 0.15], I2 = 0%, p < 0.00001) (Table 2; Figure 2).
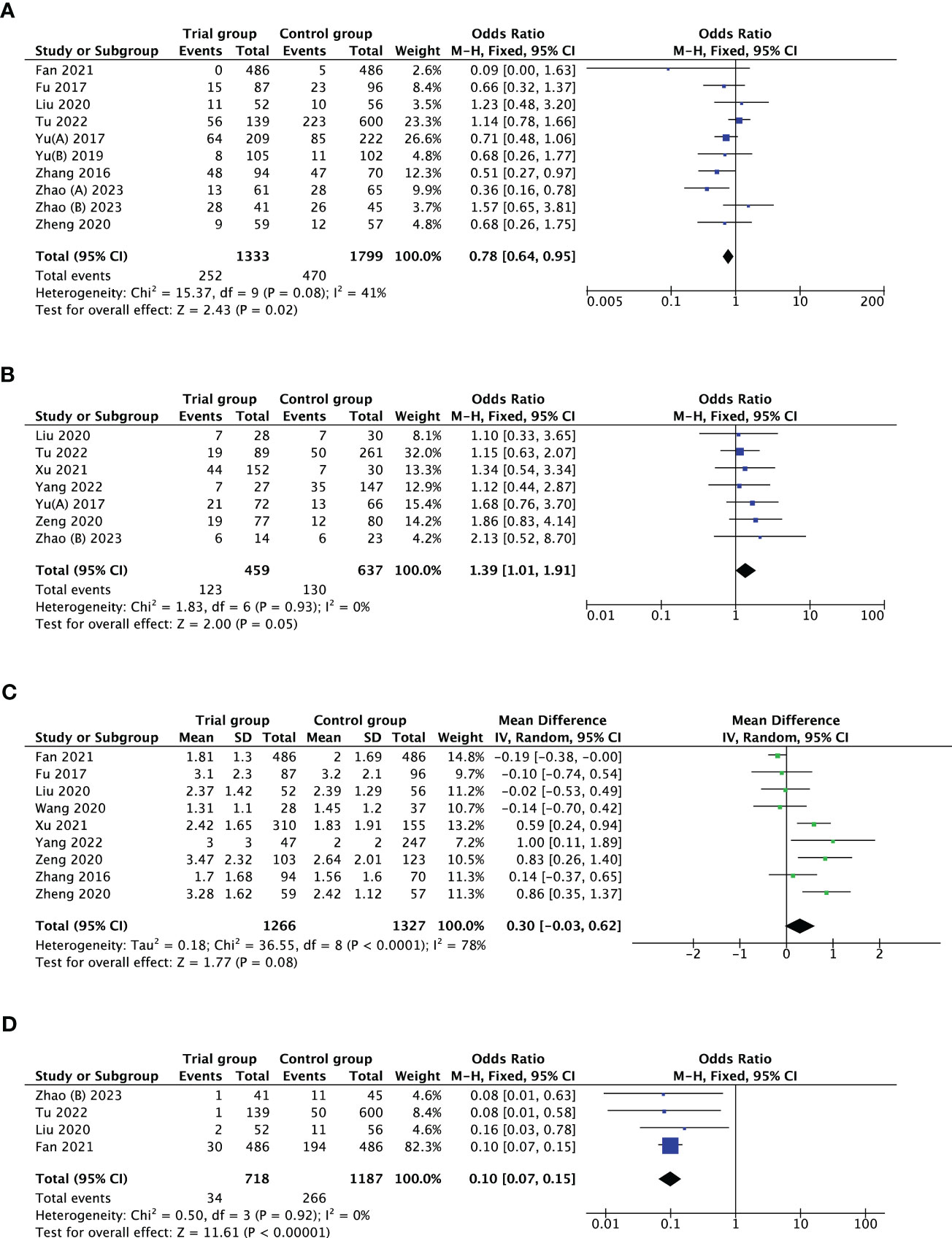
Figure 2 Forest plot of studies evaluating cycle cancellation rate (A); clinical pregnancy rate (B); number of oocytes retrieved (C); premature LH surge rate (D).
3.4.2 The secondary outcomes
The optimal embryos rate was reported by six studies. After removing Zhao’s study (36) by sensitivity analysis, the heterogeneity declined from 58% to 44%; thus, there was evidence of a substantial increase in optimal embryos rate with PPOS compared to CC/LE plus gonadotropin (OR 1.50, [95% CI: 1.20, 1.88], I2 = 44%, p = 0.0004). However, it was noteworthy that there was no evidence of a statistically striking difference in fertilization rate, live birth rate, cleavage rate, embryo implantation rate, E2 on the day of hCG and early miscarriage rate between the groups (p > 0.05). Furthermore, nine studies investigated the association between PPOS with LH on the day of hCG, after removing Yu’s study (39) through sensitivity analysis, the heterogeneity reduced from 98% to 84%; among the women with DOR, PPOS was also shown to be an advantageous outcome (SMD -0.81, [95% CI: -1.10, -0.53], I2 = 84%, p < 0.00001) (Figure 3).
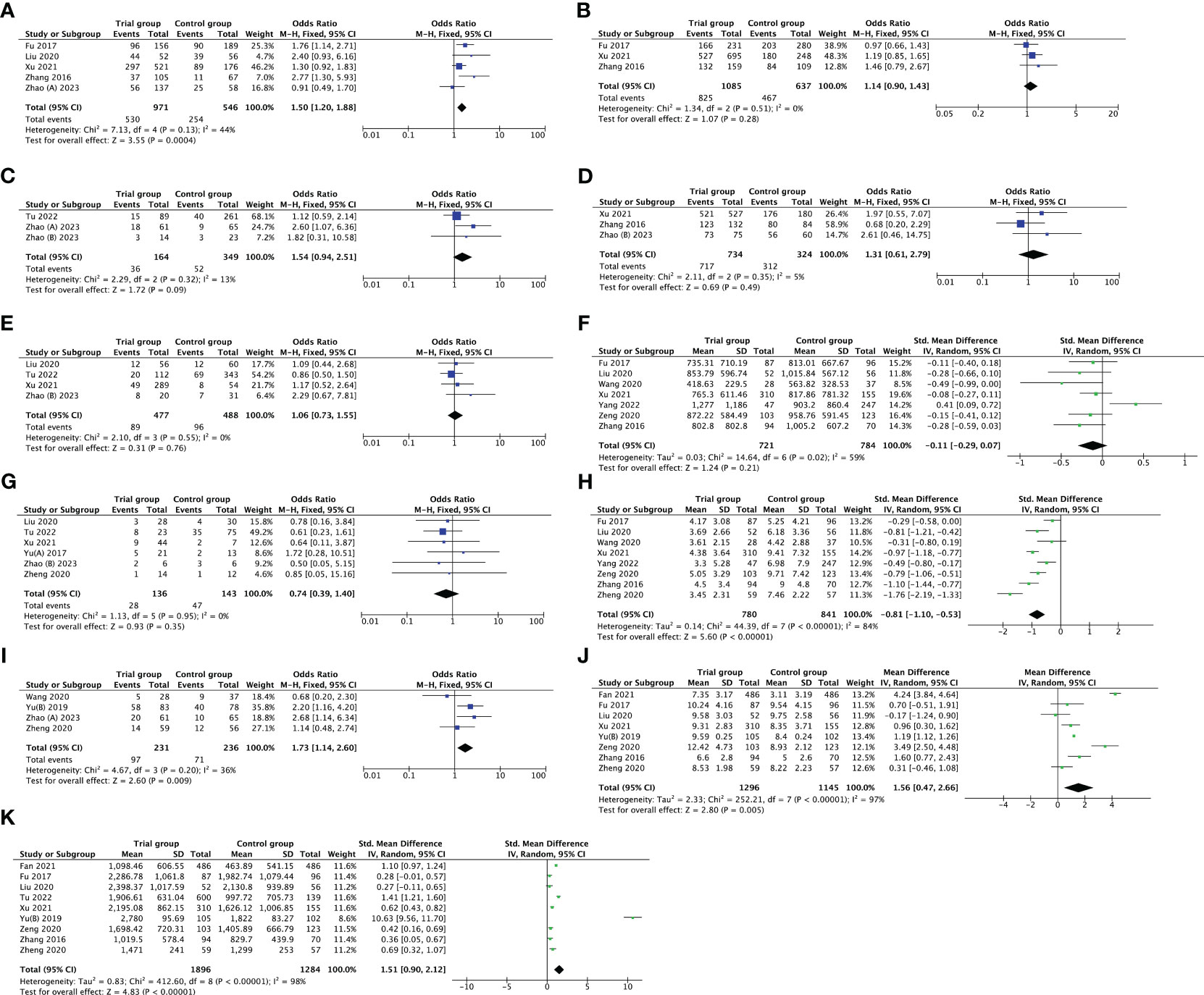
Figure 3 Forest plot of studies evaluating optimal embryos rate (A); fertilization rate (B); live birth rate (C); cleavage rate (D); embryo implantation rate (E); E2 on the day of hCG (F); early miscarriage rate (G); LH on the day of hCG (H); cumulative pregnancy rate (I); duration of gonadotropin (J); total dose of gonadotropin (K).
In addition, the duration of gonadotropin used was measured in eight trials, and the total dose of gonadotropin was recorded in nine studies. The pooled results indicated that the PPOS protocol may statistically increase the duration of gonadotropin used (MD 1.56, [95% CI: 0.47, 2.66], I2 = 97%, p = 0.005), and total dose of gonadotropin required (SMD 1.51, [95% CI: 0.90, 2.12], I2 = 98%, p < 0.00001). Although sensitivity analysis was utilized, no individual research impacted the pooled results. Simultaneously, subgroup analysis based on different types of gonadotropin (HMG vs. FSH) explored the potential heterogeneity, but the heterogeneity did not modify (Supplementary Figure 1). What’s more, four studies reported cumulative pregnancy rate, and the pooled result showed that PPOS was more superior to CC/LE plus gonadotropin in increasing cumulative pregnancy rate (OR 1.73, [95% CI: 1.14, 2.60], I2 = 36%, p = 0.009) (Figure 3). All the results above are listed in Table 2.
3.5 Publication bias
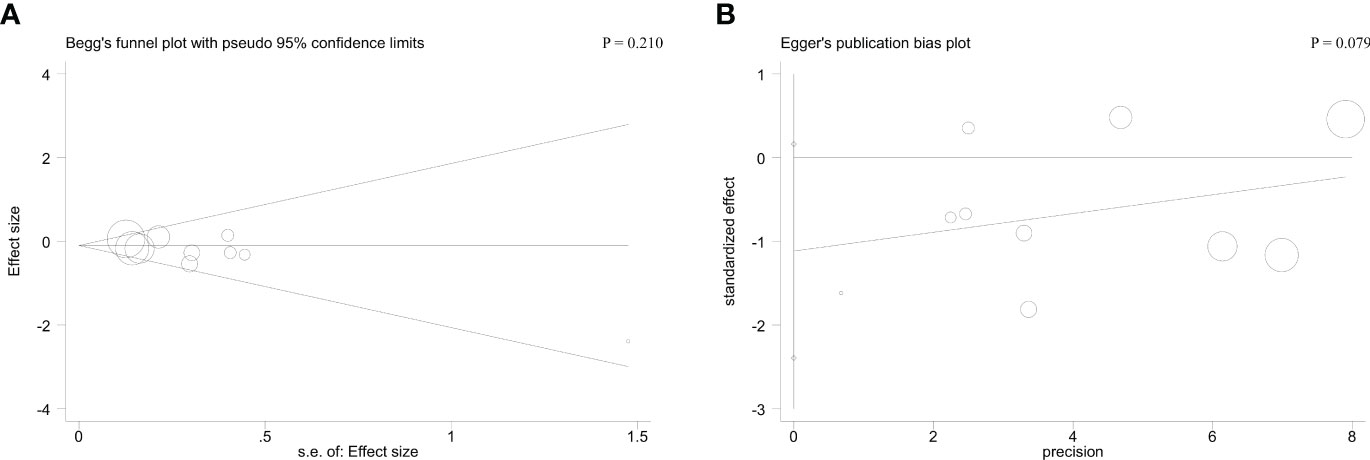
Begg’s and Egger’s tests were applied to detect hidden publication bias. Regarding cycle cancellation rate, the form of the funnel plots with a symmetrical appearance was checked. The P value of Begg’s and Egger’s tests were 0.210 and 0.079, respectively, for cycle cancellation rate. Therefore, there was no meaningful publication bias in this meta-analysis (Figure 4).
4 Discussion
Women with DOR are always likely to experience a premature LH surge since they usually possess fewer antral follicles which develop and mature rapidly and are vulnerable to premature luteinization (40). Clinically, conventional IVF protocols often provide unsatisfying results, such as disappointing embryo quality, the number of oocytes retrieved, and total embryos (41–43). Meanwhile, patients with DOR were more difficult to obtain an expected result in managing LH surge than those with normal ovarian reserve (43). Therefore, it is essential to identify an effective ovarian stimulation protocol to better the outcome of DOR women with ART treatment. Currently, PPOS, first proposed by Kuang et al. (44) in 2015 to compare pregnancy outcomes for women undergoing IVF/ICSI with frozen embryo transfer, has drawn our interest since it is more superior to short protocol in preventing premature LH surges and as effective as short protocol in improving IVF/ICSI outcomes. The main administration of exogenous progesterone used in PPOS is dydrogesterone (DYG), progesterone capsules (PC), and medroxyprogesterone acetate (MPA) (45). In recent years, PPOS has been widely recognized as a vital protocol for ovarian stimulation, especially in women with DOR (43). For example, a previous self-controlled study enrolled infertile patients with DOR proved that MPA substantially suppressed LH surge and facilitated pregnancy rate, high-grade embryos, MII oocytes, normal fertilized oocytes, and live birth rate compared to CC protocol, which might involve complex molecular mechanisms (46). Substantial evidence has implied that the PPOS protocol could influence the follicular microenvironment by regulating miR-4261 and miR-6869-5p expression in granulosa cells (47). Meanwhile, MPA might increase the ovulation rate by ameliorating the mRNA expression of GJA1 and VEGF in follicles (48). Additionally, DYG could stimulate oocyte maturation and ovulation by boosting the concentrations of acylcarnitines, lysophospholipids, urea, putrescine, and free amino acids via the purinergic signaling and arachidonic acid metabolic pathway in ovary (49). Still, the mechanism underlying progestin that ameliorates outcomes for women with DOR is not elaborated clearly. Consequently, further research is required to investigate the exact mechanism.
However, so far, there is no evidence-based medical support to inform the use of PPOS in patients with DOR undergoing IVF/ICSI. Thus, this meta-analysis was performed to explore the value of PPOS on patients with DOR. In this study, we included 14 articles involving 4182 women with DOR. According to the pooled results of the study, forest plots distinctly presented that the use of PPOS could notably reduce the incidence of cycle cancellation rate and increase clinical pregnancy rate. On the other hand, our result without heterogeneity demonstrated that there was a significant value in preventing premature LH surge when PPOS applied. Regarding the secondary outcomes, women with DOR treated with PPOS were significantly associated with superior optimal embryos rate, lower LH on the day of hCG, increase in the duration and the amount of gonadotropins required, and a higher incidence of cumulative pregnancy rate. Nonetheless, there was absence of evidence to proof that PPOS protocol is correlated with a considerable difference in the number of oocytes retrieved, fertilization rate, live birth rate, cleavage rate, embryo implantation rate, E2 on the day of hCG, and early miscarriage rate compared with CC/LE plus gonadotropin protocols. In addition, according to Begg’s and Egger’s tests, no publication bias existed among the studies, and each result was also estimated by sensitivity analysis, which indicated that our results are robust and reliable. Taken together, we consider PPOS to be an effective protocol for patients with DOR, based on the high-quality evidence above, which might be valuable for clinicians to choose ovarian stimulation strategies.
A previous meta-analysis focusing on PPOS for patients in ART demonstrated that PPOS is profitable for women with different ovarian reserve (43). However, they only searched four databases and included nine articles published before 2020. Among the nine studies included, solely two studies with 544 cases compared the difference of PPOS with natural cycle or antagonist protocol. Therefore, their results ought to be interpreted with caution as the different control protocols and small sample sizes were included. To our knowledge, this is the first meta-analysis to investigate the clinical value of PPOS for women with DOR undergoing IVF or ICSI compared to CC/LE plus gonadotropin stimulation. Our study has several strengths. First, this meta-analysis included only women with DOR receiving PPOS or CC/LE plus gonadotropin stimulation during IVF or ICSI, thereby offering a more specific reflection on the value of PPOS in this unique population. Second, we carefully screened nine databases and included 4182 women with DOR in this analysis. The databases enrolled were more comprehensive, and the sample sizes were larger than in the previous study as well. Third, the earlier meta-analysis (43) failed to check publication bias and stability of their conclusions. Instead, we used Begg’s and Egger’s tests, along with sensitivity analysis to verify our results. Hence, we are convinced that our conclusions are more suitable for clinical practice.
However, several limitations should be acknowledged. First of all, the 14 articles selected were retrospective studies, which may exist certain biases and, to some extent, generate a weak evidence grade compared to randomized controlled trials (RCTs). Whereas the NOS results presented that all studies included were high-quality. Second, all studies failed to report the adverse effects, such as ovarian hyperstimulation syndrome and deep vein thrombosis during the use of PPOS; thus, the safety of PPOS cannot be estimated by meta-analysis, which might be an inherent deficiency of this study. Third, significant heterogeneities could still be noticed in some outcomes, like the duration and total dose of gonadotropin used, although we performed subgroup analysis. We consider the heterogeneity may be explained by the different amounts of gonadotropin applied for each patient according to the concentrations of sexual hormones and the size and quantity of developing follicles. Lastly, four studies utilized the median and 25th - 75th percentiles for continuous variables with skewed distributions, which enabled us cannot pool their related data into our analysis. Hence, more high-quality multicenter RCTs are required to further confirm the value of PPOS for patients with DOR.
5 Conclusion
In summary, the results of this systematic review and meta-analysis confirmed that PPOS might improve clinical pregnancy rate, optimal embryos rate, and cumulative pregnancy rate for women with DOR who are undergoing IVF/ICSI. In addition, PPOS might decrease cycle cancellation rate, LH level on the day of hCG, and premature LH surge rate for DOR patients. This would benefit clinicians in adjusting ovarian stimulation strategy. However, the duration and the amount of gonadotropins required were higher with the PPOS protocol. Therefore, we suggest that women with DOR undergoing IVF/ICSI should be appropriately evaluated before receiving the PPOS protocol.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
GL, XZ, SL, and LX: literature search, screening, and data extraction. GL and XL: data analysis and results visualization. GL and LX: manuscript draft. LX: manuscript modification. All authors reviewed the final version of the manuscript and approved it for publication. All authors contributed to the article and approved the submitted version.
Funding
The Three-Year Action Plan for Further Accelerating the Development of Traditional Chinese Medicine Inheritance and Innovation in Shanghai (2021-2023) [grant numbers ZY(2021- 2023)-0209-01].
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1232935/full#supplementary-material
References
1. Boivin J, Bunting L, Collins JA, Nygren KG. International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Hum Reprod (2007) 22(6):1506–12. doi: 10.1093/humrep/dem046
2. Inhorn MC, Patrizio P. Infertility around the globe: new thinking on gender, reproductive technologies and global movements in the 21st century. Hum Reprod Update (2015) 21(4):411–26. doi: 10.1093/humupd/dmv016
3. Carson SA, Kallen AN. Diagnosis and management of infertility: A review. JAMA (2021) 326(1):65–76. doi: 10.1001/jama.2021.4788
4. Devine K, Mumford SL, Wu M, DeCherney AH, Hill MJ, Propst A. Diminished ovarian reserve in the United States assisted reproductive technology population: diagnostic trends among 181,536 cycles from the Society for Assisted Reproductive Technology Clinic Outcomes Reporting System. Fertil Steril (2015) 104(3):612–19.e3. doi: 10.1016/j.fertnstert.2015.05.017
5. Jaswa EG, McCulloch CE, Simbulan R, Cedars MI, Rosen MP. Diminished ovarian reserve is associated with reduced euploid rates via preimplantation genetic testing for aneuploidy independently from age: evidence for concomitant reduction in oocyte quality with quantity. Fertil Steril (2021) 115(4):966–73. doi: 10.1016/j.fertnstert.2020.10.051
6. Christodoulaki A, Boel A, Tang M, De Roo C, Stoop D, Heindryckx B. Prospects of germline nuclear transfer in women with diminished ovarian reserve. Front Endocrinol (Lausanne) (2021) 12:635370. doi: 10.3389/fendo.2021.635370
7. Zhang W, Zhang L, Liu Y, Li J, Xu X, Niu W, et al. Higher chromosomal aberration frequency in products of conception from women older than 32 years old with diminished ovarian reserve undergoing IVF/ICSI. Aging (Albany NY) (2021) 13(7):10128–40. doi: 10.18632/aging.202772
8. Arnanz A, Bayram A, Elkhatib I, Abdala A, El-Damen A, Patel R, et al. Antimüllerian hormone (AMH) and age as predictors of preimplantation genetic testing for aneuploidies (PGT-A) cycle outcomes and blastocyst quality on day 5 in women undergoing in vitro fertilization (IVF). J Assist Reprod Genet (2023) 40(6):1467–77. doi: 10.1007/s10815-023-02805-z
9. Kawwass JF, Hipp HS, Session DR, Kissin DM, Jamieson DJ; , National ART Surveillance System Group. Severity of diminished ovarian reserve and chance of success with assisted reproductive technology. J Reprod Med (2017) 62(3-4):153–60.
10. Ferraretti AP, La Marca A, Fauser BC, Tarlatzis B, Nargund G, Gianaroli L. ESHRE consensus on the definition of 'poor response' to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod (2011) 26(7):1616–24. doi: 10.1093/humrep/der092
11. Singh R, Kaur S, Yadav S, Bhatia S. Gonadotropins as pharmacological agents in assisted reproductive technology and polycystic ovary syndrome. Trends Endocrinol Metab (2023) 34(4):194–215. doi: 10.1016/j.tem.2023.02.002
12. Baker VL, Brown MB, Luke B, Smith GW, Ireland JJ. Gonadotropin dose is negatively correlated with live birth rate: analysis of more than 650,000 assisted reproductive technology cycles. Fertil Steril (2015) 104(5):1145–52.e525. doi: 10.1016/j.fertnstert.2015.07.1151
13. Moffat R, Hansali C, Schoetzau A, Ahler A, Gobrecht U, Beutler S, et al. Randomised controlled trial on the effect of clomiphene citrate and gonadotropin dose on ovarian response markers and IVF outcomes in poor responders. Hum Reprod (2021) 36(4):987–97. doi: 10.1093/humrep/deaa336
14. Chen Q, Chai W, Wang Y, Cai R, Zhang S, Lu X, et al. Progestin vs. Gonadotropin-Releasing Hormone Antagonist for the Prevention of Premature Luteinizing Hormone Surges in Poor Responders Undergoing in vitro Fertilization Treatment: A Randomized Controlled Trial. Front Endocrinol (Lausanne) (2019) 10:796. doi: 10.3389/fendo.2019.00796
15. Xu B, Wang J, Xia L, Zhang D, Wu X, Zhang A. Increased Uterine NK cell numbers and perforin expression during the implantation phase in IVF Cycles with GnRH Antagonist Protocol. Sci Rep (2017) 7:39912. doi: 10.1038/srep39912
16. Kato K, Ezoe K, Yabuuchi A, Fukuda J, Kuroda T, Ueno S, et al. Comparison of pregnancy outcomes following fresh and electively frozen single blastocyst transfer in natural cycle and clomiphene-stimulated IVF cycles. Hum Reprod Open (2018) 2018(3):hoy006. doi: 10.1093/hropen/hoy006
17. Liu R, Zhou L, Chen X, He H, Cai Z. Letrozole supplementation and the increased risk of elevated progesterone levels on trigger day. Front Endocrinol (Lausanne) (2022) 13:904089. doi: 10.3389/fendo.2022.904089
18. La Marca A, Capuzzo M, Sacchi S, Imbrogno MG, Spinella F, Varricchio MT, et al. Comparison of euploidy rates of blastocysts in women treated with progestins or GnRH antagonist to prevent the luteinizing hormone surge during ovarian stimulation. Hum Reprod (2020) 35(6):1325–31. doi: 10.1093/humrep/deaa068
19. Kalafat E, Turkgeldi E, Yıldız S, Dizdar M, Keles I, Ata B. Outcomes of a gnRH agonist trigger following a gnRH antagonist or flexible progestin-primed ovarian stimulation cycle. Front Endocrinol (Lausanne) (2022) 13:837880. doi: 10.3389/fendo.2022.837880
20. Zhou R, Dong M, Huang L, Wang S, Fan L, Liang X, et al. Comparison of cumulative live birth rates between progestin-primed ovarian stimulation protocol and gonadotropin-releasing hormone antagonist protocol in different populations. Front Endocrinol (Lausanne) (2023) 14:1117513. doi: 10.3389/fendo.2023.1117513
21. Huang J, Xie Q, Lin J, Lu X, Wang N, Gao H, et al. Neonatal outcomes and congenital malformations in children born after dydrogesterone application in progestin-primed ovarian stimulation protocol for IVF: a retrospective cohort study. Drug Des Devel Ther (2019) 26:13:2553–2563. doi: 10.2147/DDDT.S210228
22. Liu Y, Nan Y, Chen F, Wang H, Li YJ. Application effect of different ovarian stimulation protocols in elderly patients with diminished ovarian reserve. J Xinxiang Med University (2020) 37(5):465–70. doi: 10.7683/xxyxyxb.2020.05.016
23. Zhao HJ, Zhang XH, Chen J, Lu J, Zhang HF, Chang WL. Comparison of three controlled ovarian hyperstimulation protocols in advanced-age infertile patients with diminished ovarian reserve. J Int Reprod Health/Family Planning (2023) 42(1):13–7. doi: 10.12280/gjszjk.20220213
24. Fu YH, Yang TC, Xi HT, Zhao JZ. Effect of progestin-primed ovarian stimulation scheme in patients with diminished ovarian reserve. J Wenzhou Med University (2017) 47(4):258–62. doi: 10.3969/j.issn.2095-9400.2017.04.006
25. Zhang C, Zhu P, WQ Y, Zhang X, Song XM, Lv L, et al. Progestinprimed ovarian stimulation protocol in women with poor ovarian reserve undergone IVF/ICSI programmes. Chin J Birth Health Heredity (2016) 24(10):111–4.
26. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (2021) 372:n71. doi: 10.1136/bmj.n71
27. Cohen J, Chabbert-Buffet N, Darai E. Diminished ovarian reserve, premature ovarian failure, poor ovarian responder–a plea for universal definitions. J Assist Reprod Genet (2015) 32(12):1709–12. doi: 10.1007/s10815-015-0595-y
28. Expert Group of Consensus on Clinical Diagnosis & Management of Diminished Ovarian Reserve and Reproductive Endocrinology & Fertility Preservation Section of Chinese Society on Fertility Preservation under Chinese Preventive Medicine Association. Consensus on clinical diagnosis and management of diminished ovarian reserve. J Reprod Med (2022) 31(4):425–34. doi: 10.3969/j.issn.1004-3845.2022.04.001
29. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol (2010) 25(9):603–5. doi: 10.1007/s10654-010-9491-z
30. Tu X, You B, Jing M, Lin C, Zhang R. Progestin-primed ovarian stimulation versus mild stimulation protocol in advanced age women with diminished ovarian reserve undergoing their first in vitro fertilization cycle: A retrospective cohort study. Front Endocrinol (Lausanne) (2021) 12:801026. doi: 10.3389/fendo.2021.801026
31. Fan XY, Yu S, Wang Y, Kuang YP. Controllability comparison between progestin-primed ovarian stimulation and mild stimulation protocols in patients with diminished ovarian reserve in oocytes retrieval cycle. Chin J Clin Med (2021) 28(4):539–43. doi: 10.12025/j.issn.1008-6358.2021.20210409
32. Zheng J, Zhou LM, Sun YT, Xia AL, Li M, Liang K. Comparison of the clinical outcomes of three ovulation induction protocols in elderly patients with decline in ovarian reserve. Chin J Reprod Contraception (2020) 40(3):194–200. doi: 10.3760/cma.j.cn101441-20190325-00121
33. Yu G, Xiang YG, Li Y, Wan LJ, Tan L. Application of progestin-primed ovarian stimulation or mild stimulation protocols in patients with diminished ovarian reserve. Chin J Pract Gynecology Obstetrics (2017) 33(5):502–6. doi: 10.19538/j.fk2017050116
34. Yang ZX, Wen Y, Su CM, Wang HW, Rao M, Zheng YH, et al. Comparison the clinical outcomes of progestin-primed ovarian stimulation during follicular period and mild-stimulation in patients with diminished ovarian reserve. J Kunming Med University (2022) 43(12):53–7. doi: 10.12259/j.issn.2095-610X.S20221211
35. Zeng QL, Yan Y, Li YP, Zhao J. Comparison of clinical outcomes and economic benefits between progestin-primed ovarian stimulation and micro-stimulation in patients with diminished ovarian reserve. Chin J Clin Res (2020) 33(7):908–12. doi: 10.13429/j.cnki.cjcr.2020.07.010
36. Zhao SS, Peng Z, Tu ZY, Zhao YH, Zou L. Clinical analysis of IVF/ICSI-ET assisted pregnancy therapy in elder patients with diminished ovarian reserve. J Reprod Med (2023) 32(3):350–6. doi: 10.3969/j.issn.1004-3845.2023.03.008
37. Wang CL, Xu J. Clinical efficacy of modified naturalcycle for treating women with ovarian reserve dysfunction. Chin J Family Planning (2020) 28(11):1864–7. doi: 10.3969/j.issn.1004-8189.2020.11.033
38. Xu XY. Analysis of clinical outcomes of different ovulation stimulation protocols in elderly patients with diminished ovarian reserve. Henan, China: Zhengzhou University (2021).
39. Yu CM, Dai XL, Wang YF, Gao TT, Xia XY, Chen L. Effect of PPOS protocol on IVF-ET outcomes in different ages patients with DOR. Maternal Child Health Care China (2019) 34(17):4043–5. doi: 10.7620/zgfybj.j.issn.1001-4411.2019.17.53
40. Wu Y-G, Barad DH, Kushnir VA, Lazzaroni E, Wang Q, Albertini DF, et al. Aging-related premature luteinization of granulosa cells is avoided by early oocyte retrieval. J Endocrinol (2015) 226(3):167–80. doi: 10.1530/JOE-15-0246
41. Datta AK, Maheshwari A, Felix N, Campbell S, Nargund G. Mild versus conventional ovarian stimulation for IVF in poor, normal and hyper-responders: a systematic review and meta-analysis. Hum Reprod Update (2021) 27(2):229–53. doi: 10.1093/humupd/dmaa035
42. Mizrachi Y, Horowitz E, Farhi J, Raziel A, Weissman A. Ovarian stimulation for freeze-all IVF cycles: a systematic review. Hum Reprod Update (2020) 26(1):118–35. doi: 10.1093/humupd/dmz037
43. Guan S, Feng Y, Huang Y, Huang J. Progestin-primed ovarian stimulation protocol for patients in assisted reproductive technology: A meta-analysis of randomized controlled trials. Front Endocrinol (Lausanne) (2021) 12:702558. doi: 10.3389/fendo.2021.702558
44. Kuang Y, Chen Q, Fu Y, Wang Y, Hong Q, Lyu Q, et al. Medroxyprogesterone acetate is an effective oral alternative for preventing premature luteinizing hormone surges in women undergoing controlled ovarian hyperstimulation for in vitro fertilization. Fertil Steril (2015) 104(1):62–70.e3. doi: 10.1016/j.fertnstert.2015.03.022
45. Alexandru P, Cekic SG, Yildiz S, Turkgeldi E, Ata B. Progestins versus GnRH analogues for pituitary suppression during ovarian stimulation for assisted reproductive technology: a systematic review and meta-analysis. Reprod BioMed Online (2020) 40(6):894–903. doi: 10.1016/j.rbmo.2020.01.027
46. Yu C-M, Dai X-L, Wang Y-F, Gao TT, Cao F, Xia XY, et al. Progestin-primed ovarian stimulation improves the outcomes of IVF/ICSI cycles in infertile women with diminished ovarian reserve. J Chin Med Assoc (2019) 82(11):845–8. doi: 10.1097/JCMA.0000000000000177
47. Yu J, Zhu D, Zeng C, Zhang Y, Yang H, Xu Y. MicroRNA expression profiles in the granulosa cells of infertile patients undergoing progestin primed ovarian stimulation. Eur J Obstet Gynecol Reprod Biol (2022) 276:228–35. doi: 10.1016/j.ejogrb.2022.08.001
48. Seekallu SV, Toosi BM, Grazul-Bilska AT, Rawlings NC. Markers of ovarian antral follicular development in sheep: comparison of follicles destined to ovulate from the final or penultimate follicular wave of the estrous cycle. Reproduction (2010) 140(4):559–68. doi: 10.1530/REP-10-0064
Keywords: diminished ovarian reserve, progestin-primed ovarian stimulation, clomiphene, letrozole, in vitro fertilization, intracytoplasmic sperm injection
Citation: Lin G, Zhong X, Li S, Liu X and Xu L (2023) The clinical value of progestin-primed ovarian stimulation protocol for women with diminished ovarian reserve undergoing IVF/ICSI: a systematic review and meta-analysis. Front. Endocrinol. 14:1232935. doi: 10.3389/fendo.2023.1232935
Received: 01 June 2023; Accepted: 26 July 2023;
Published: 21 August 2023.
Edited by:
Alexander N. Comninos, Imperial College Healthcare NHS Trust, United KingdomReviewed by:
Akanksha Garg, Imperial College London, United KingdomPei Chia Eng, National University Hospital, Singapore
Arthur Yeung, Imperial College London, United Kingdom
Copyright © 2023 Lin, Zhong, Li, Liu and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lianwei Xu, xu_lianwei2800@shutcm.edu.cn
 Guangyao Lin
Guangyao Lin Xiufang Zhong2
Xiufang Zhong2 Xiyu Liu
Xiyu Liu Lianwei Xu
Lianwei Xu