- 1Department of Cardiovascular Center, The First Hospital of Jilin University, Changchun, Jilin, China
- 2School of Basic Medical Sciences, Changchun University of Chinese Medicine, Changchun, Jilin, China
- 3Department of Cardiology, Nepal Armed Police Force Hospital, Kathmandu, Nepal
- 4Department of Echocardiography, Cardiovascular Center, The First Hospital of Jilin University, Changchun, Jilin, China
- 5Department of Radiology, The First Hospital of Jilin University, Jilin Provincial Key Laboratory of Medical Imaging and Big Data, Changchun, Jilin, China
In recent decades, the epicardial adipose tissue (EAT) has been at the forefront of scientific research because of its diverse role in the pathogenesis of cardiovascular diseases (CVDs). EAT lies between the myocardium and the visceral pericardium. The same microcirculation exists both in the epicardial fat and the myocardium. Under physiological circumstances, EAT serves as cushion and protects coronary arteries and myocardium from violent distortion and impact. In addition, EAT acts as an energy lipid source, thermoregulator, and endocrine organ. Under pathological conditions, EAT dysfunction promotes various CVDs progression in several ways. It seems that various secretions of the epicardial fat are responsible for myocardial metabolic disturbances and, finally, leads to CVDs. Therefore, EAT might be an early predictor of CVDs. Furthermore, different non-invasive imaging techniques have been proposed to identify and assess EAT as an important parameter to stratify the CVD risk. We also present the potential therapeutic possibilities aiming at modifying the function of EAT. This paper aims to provide overview of the potential role of EAT in CVDs, discuss different imaging techniques to assess EAT, and provide potential therapeutic options for EAT. Hence, EAT may represent as a potential predictor and a novel therapeutic target for management of CVDs in the future.
1 Introduction
Cardiovascular disease (CVD) is a considerable health condition that affects millions of individuals all over the world. To date, many risk factors are associated with the increasing incidence of CVDs. Among them, obesity has gained wide scientific interest. Obesity is closely associated with many other cardiovascular disease risk factors such as hypertension, dyslipidemia, metabolic syndrome, and diabetes mellitus. It is well-established that increased adiposity releases plenty of inflammatory cytokines that lead to a low-grade inflammatory microenvironment, endothelial dysfunction, and oxidative stress, and finally results in several CVDs (1, 2). The epicardial adipose tissue (EAT) is the visceral fat that deposits between the visceral pericardium and the myocardium and has direct contact with the myocardium and coronary artery (3). It usually presents as a white adipose tissue, but it also displays brown or beige fat-like features (4). Physiologically, EAT serves as thermoregulator and provides energy to the myocardium. Furthermore, EAT displays as an endocrine organ with metabolic activities and secretes bioactive molecules that affect the heart and coronary arteries via paracrine or vasocrine effects (5, 6). In recent years, evidence has shown that EAT is associated with CVDs. Therefore, different non-invasive imaging techniques have been proposed to identify and assess EAT to evaluate the risk of CVDs.
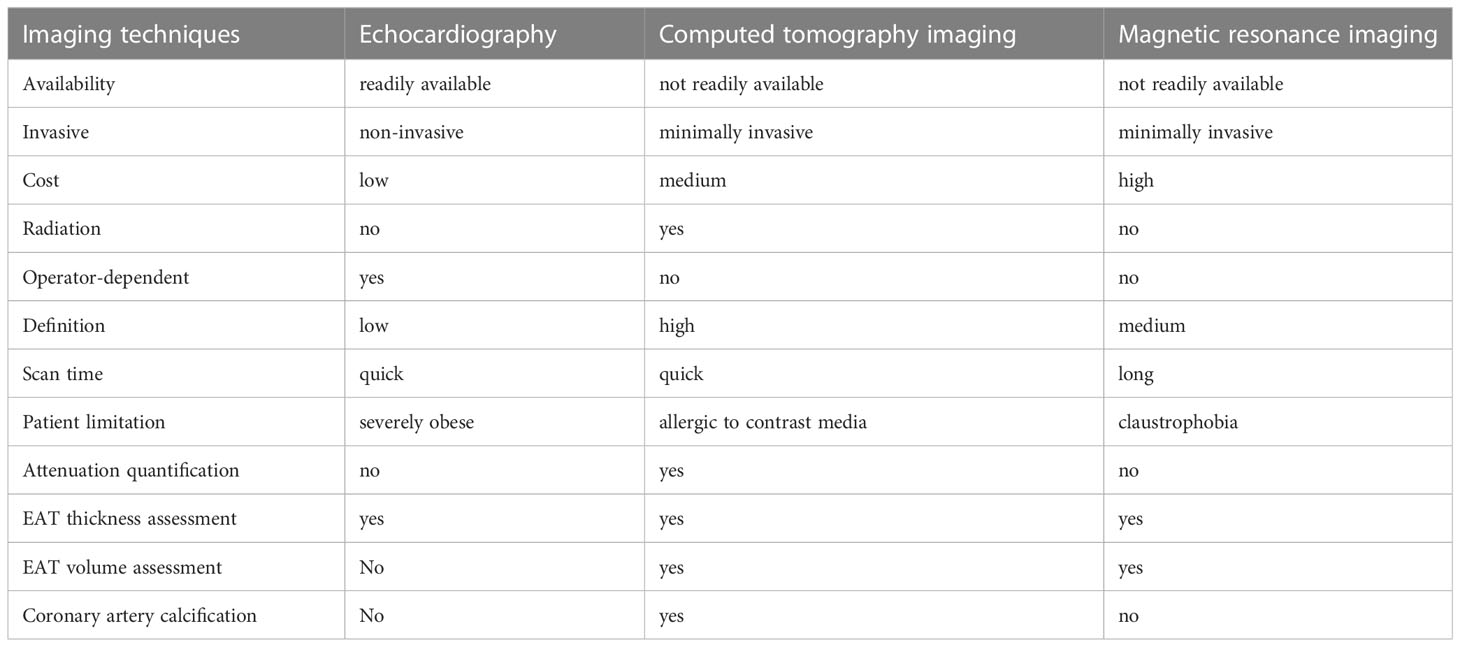
There are mainly three non-invasive imaging techniques that are used to evaluate EAT. First, echocardiography is used to evaluate EAT, which measures two-dimensional EAT thickness. It is an inexpensive, readily available, fairly accurate, and reproducible technique. Cardiac computed tomography (CCT) and cardiac magnetic resonance (CMR) imaging allow for three-dimensional EAT estimation. The former has higher space resolution and reproducibility for fat quantification, but it has limitations of radiation exposure and complex manual segmentation. However, the latter has no radiation exposure, but it is limited by space resolution, reproducibility, and higher cost. CMR is also difficult to perform in obese patients.
In this review, we summarize anatomical, physiological, and pathophysiological characteristics of EAT and focus on the potential role of EAT in CVDs and discuss different imaging techniques to assess EAT. In recent years, several papers have shown that EAT measurement via non-invasive imaging techniques serves as an important diagnostic tool to assess cardiovascular risks. Therefore, EAT may be a potential biomarker to monitor CVDs and their complications.
2 Epicardial adipose tissue: anatomy and physiology
2.1 Anatomy
The adipose tissue surrounding the heart can be divided into EAT, pericardial adipose tissue, paracardial adipose tissue, and perivascular adipose tissue (Figure 1). EAT lies between the myocardium and visceral pericardium and is made up of adipocytes, ganglia, nerves, and inflammatory, stromovascular, and immune cell (6). The pericardial adipose tissue (PAT) consists of epicardial and paracardial fat depots (7). The EAT and PAT are the entire pericardial fat. They have different embryological origins but share similar morphological features. EAT is derived from the splanchnopleuric mesoderm, and PAT is derived from the thoracic mesoderm (7).
EAT makes up 20% of the cardiac mass and covers 80% of the cardiac surface under normal physiological condition. It is non-homogeneously distributed around the heart (6, 8). EAT is mostly localized at the cardiac base and apex, in the atrioventricular and interventricular grooves, and around the coronary arteries. It is thicker around the right ventricle than around the left ventricle. In general, EAT can be differentiated into peri-coronary and peri-myocardial EAT. The former is located directly around or on the coronary artery adventitia; the latter is located just over the myocardium and is in direct contact with the myocardium (9). Vascular supply by coronary arteries in EAT forms part of the perivascular adventitia (10). It is thought to play a protective mechanical role against the tension and twist of an arterial pulse (11, 12). The increased EAT might result in cardiac disorders with increased arrhythmogenicity (13). It is hypothesized that EAT increases fatty infiltrates in the proximity of myocytes that leads to structural remodeling and abnormal impulse generation, which contributes to cardiac arrhythmias (13).
2.2 Physiology
2.2.1 Physical protective barrier and energy fat source
The epicardial fat surrounds the coronary arteries and myocardium; hence, EAT is considered to act as a buffering system in normal physiological conditions. It protects the heart and coronary arteries from mechanical deformation and facilitates vascular remodeling (14). In addition, EAT has thermogenic function that protects the heart from hypothermia. The increased thermogenic potential is due to brown adipocytes in EAT. Since EAT is more lipolytic than other adipose tissue depots, it releases abundant free fatty acids (FFAs) during high energy demand period, which is the main source of energy for the myocardium (14–16). In addition, EAT is present close to the myocardium and acts as a buffer to protect the heart from exposure to excessively high FFAs and lipotoxicity (17, 18).
2.2.2 Adipose tissue properties
Based on embryological, histological, and functional aspects, adipose tissue can be divided into two major groups: white and brown adipose tissue. The former has relatively few mitochondria and a single big lipid droplet, while the latter has multiple small lipid droplets and abundant mitochondria (19). EAT is basically a white adipose tissue but also has brown and beige fat-like features. It releases many mediators through expressing thermogenic genes related to brown and beige adipose tissues, such as tumor necrosis factor alpha (TNF-α), interleukin (IL)-1β, IL-1 receptor antagonist, IL-6, IL-8, IL-10, C-reactive protein (CRP), and plasminogen active inhibitor 1 (20). These factors may be involved in the communication between EAT and myocardial tissue through endocrine effect because they share the same capillary circulation (19). It is reported that EAT is twice as metabolically active as normal white adipose tissue, which is related to lipolysis and free fatty acid release. In addition, EAT has an increased capacity to release free fatty acids into the blood circulation and decreased glucose consumption compared to other adipose tissues (18). It also alters the bioavailability of adipokines and leads to adipocyte hypertrophy, tissue hypoxia, inflammation, and oxidative stress (7, 21). Brown fat generates heat in response to cold temperature and autonomic nervous system activation. Like brown fat, EAT also protects the myocardium from hypothermia.
2.2.3 Endocrine organ
Besides acting as energy depot, EAT also serves as an endocrine organ that regulates the heart homeostasis. There are two classical interaction mechanisms between the myocardium and the EAT: vasocrine and paracrine. On the one hand, adipokines and FFAs, as vasocrine signaling molecules, are released from EAT that enters the vasa vasorum directly and are transported downstream into the arterial wall. On the other hand, EAT-derived adipokines diffuse in interstitial fluid that cross the vascular wall (adventitia, media, and intima), and finally interact with vasa vasorum, endothelia, and vascular smooth muscle cells of the coronary arteries (18, 22). However, extracellular vesicles, containing cytokines and microRNAs have been confirmed as new communication modes (23). FFAs are the main energy source of the heart. EAT secretes vasoactive products that regulate coronary arterial tone to facilitate the FFA influx. In addition, fatty acid binding protein-4, expressed by EAT, may participate in the intracellular transport of FFAs from EAT into the myocardium (24).
3 Cardiac imaging of EAT
3.1 Echocardiography
The advantages of echocardiography to measure EAT thickness include low cost and more convenient, accessible, and reproducible (Table 1). However, there are few limitations. It is operator dependent. EAT is located in some areas of the heart that cannot be visualized with the ultrasound. In addition, obese patients have poor acoustic window. Although EAT thickness is considered as a useful diagnostic tool, the normal value of EAT is still undetermined. Iacobellis et al. (25, 26) reported a transthoracic echocardiographic method of evaluating EAT thickness on the free wall of the right ventricle from both parasternal long- and short-axis views. They choose the right ventricle to measure EAT because it is considered as the thickest absolute epicardial fat layer (27), and parasternal long- and short-axis views allow the most accurate measurement of EAT on the right ventricle with optimal cursor beam orientation. In addition, they reported an average epicardial fat thickness of 7 mm in men and 6.5 mm in women for standard clinical references (28). Another study that enrolled 459 patients with Grade I and II essential hypertension demonstrated that patients with EAT thickness >7 mm exhibited higher left ventricular mass index, diastolic dysfunction, and increased carotid stiffness and intima-media thickness (29). In addition, Islas et al. reported that acute myocardial infarction patients with EAT >4 mm have worse left ventricular systolic function and have large infarct size. EAT >4 mm is an independent predictor of major adverse cardiovascular events at 5-year follow-up (30).
In addition, Parisi et al. presented a novel method to measure EAT thickness at the level of the fold of Rindfleisch, a pericardial recess where the parietal pericardium does not exert a mechanical compression on visceral fat (31). Moreover, echo-EAT thickness showed a significant correlation with the CMR-EAT thickness, both measured at the Rindfleisch fold. Although echocardiography is convenient and reproducible, it cannot reflect the variability in EAT thickness or EAT volume accurately. Multidetector CT and cardiac MRI can provide a more accurate and volumetric quantification of EAT.
3.2 Cardiac computed tomography
CCT is another imaging modality used to measure EAT. Although CCT has high spatial resolution and provides three-dimensional view of the heart, it is costly and requires radiation exposure (Table 1). Currently, coronary CT angiography (CTA) provides an optimal method that enables the characterization of morphological changes in the pericoronary adipose tissue (PCAT) and simultaneous assessment of coronary atherosclerosis (32). CT attenuation of the adipose tissue reflects morphological derangements of adipocytes that are exposed to the effects of local vascular inflammation. EAT volume and density are considered as independent markers of adverse cardiometabolic risk that can be measured by CCT (33, 34). Additionally, increased EAT volume is a predictor of CVDs that include obstructive coronary stenosis, myocardial ischemia, and coronary syndromes (33, 35–37). However, the upper cutoff CT value of normal EAT remains undermined. Dey et al. reviewed literatures that reported different inclusion criteria with CT value varying between 125.0 and 139.4 cm3 for men and 119.0–125.0 cm3 for women (38). In addition, EAT density or attenuation has also been associated with CVDs. It is reported that EAT attenuation is associated with coronary artery calcification, acute myocardial infarction, and coronary adverse events (34, 39–41). PCAT is considered as a metric of local vascular inflammation. The widely accepted definition of PCAT by coronary CTA is voxels ranging from −190 to −30 Hounsfield units, with volume of interest that extends to an orthogonal distance equivalent to the diameter of the target vessel (42). Antonopoulos et al. presented a method to detect coronary inflammation by characterizing the changes in PCAT CT attenuation (43). They have demonstrated that the average attenuation of EAT is inversely related to adipogenic gene expression and adipocyte size in a large cohort of patients who have undergone cardiac surgery.
Nowadays, although manual segmentation of EAT quantification is the method of choice, it is operator dependent, time consuming, and not suitable for routine clinical practice. Thus, artificial intelligence that includes machine and deep learning received more attention to obtain fast, automatic, and reliable measures of EAT by CCT.
3.3 Cardiac magnetic resonance
CMR is now considered as the gold standard for measuring visceral adipose tissue (44–46). CMR provides excellent visualization of visceral and parietal pericardia. Cardiac imaging is not affected in patients with excess subcutaneous fat. It enables easy assessment and volumetric quantification of EAT. Although there is no use of radiation and contrast agents, CMR is expensive and time consuming. It is also difficult to perform in patients with claustrophobia (Table 1). Additionally, CMR can differentiate cardiac fat into EAT and paracardial fat.
MRI provides explicit parameters about EAT volume and mass by using the spin-echo sequence technique (2, 47). Manual contouring of EAT area at end-diastole during cardiac cycles provides precise quantification of EAT volume (47). A 3D-Dixon sequence (an electrocardiography triggered and respiratory navigator gated 3D-gradient echo pulse sequence was used for cardiac Dixon imaging) has been shown to be a reliable method for EAT quantification in studies (48). Dixon method separates fat and water signal via voxel intensity differences present between in- and opposed-phase MR images (48). Rami Homsi et al. (49) enrolled 34 healthy volunteers (22 men; BMI range, 14–42 kg/m(2); age range, 21–79 years) and measured parameters of pericardial and epicardial adipose volume (PAV, EAV) using a 3D-Dixon-based CMR approach. They found that the average EAV was 77.0± 55.3 ml, and PAV was 158.0 ± 126.4 ml; both were highly correlated. Therefore, they proposed a 3D-Dixon-based method that allows accurate measurement of cardiac fat volume and provides a valuable tool for cardiovascular risk stratification.
Moreover, CMR measures EAV, left ventricular compliance, pulse wave velocity, and other indicators simultaneously, which can evaluate aortic stiffness, myocardial strain, and fibrosis (50, 51). A combined measurement by CMR may support the evaluation of risk and prognosis of CVDs.
4 Epicardial adipose tissue: a new biomarker for cardiovascular disease risk assessment
Evidence strongly supports the role of structural and functional changes of EAT in the pathogenesis of various cardiovascular diseases (Figure 2).
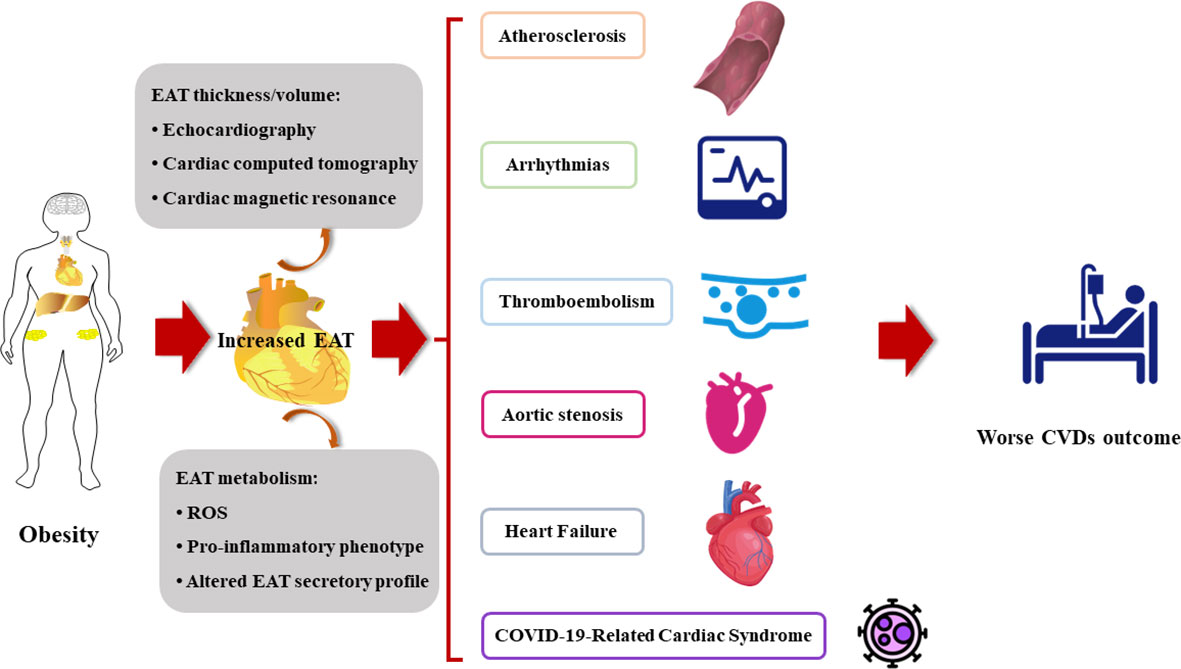
Figure 2 The increased EAT contributes to the onset and the poor prognosis of cardiovascular diseases. CVDs, cardiovascular diseases; EAT, epicardial adipose tissue; ROS, reactive oxygen species.
4.1 Atherosclerosis
Atherosclerosis is characterized by deposition of immune cells and cholesterol in the subendothelial space of arteries. As EAT is a metabolically endocrine tissue with abundant proinflammatory cytokines, it is considered to be associated with atherosclerosis. It is reported that EAT leads to CVDs by involving in the mechanism of inflammation, insulin resistance, and oxidative stress. C1q TNF-related protein 3 (CTRP3) is an adipokine with anti-inflammatory and cardioprotective properties. It has also been demonstrated to activate nuclear factor kappa B (NF-κB) signaling and PI3K/Akt/eNOS pathway to attenuate obesity-related inflammation responses and insulin resistance and thus regresses atherosclerosis (52, 53). A study involving 34 patients with elective post-coronary artery by-pass graft (post-CABG) showed that EAT with lower CTRP3 mRNA level is closely associated with coronary atherosclerosis and cardiac dysfunction (54). IL-1β and angiopoietin-like-4 (an inhibitor of lipoprotein lipase secreted by adipose tissue) were highly expressed in EAT patients with coronary artery diseases (55). Adipocyte oxidative stress, characterized by the imbalance of ROS and redox signaling, is related to metabolic CVDs. It has been demonstrated that EAT can produce more ROS compared to subcutaneous adipose tissue because it has higher expression of NADPH components gp91phox and p47 phox (56). The hyperglycemia and insulin resistance accelerate adipocyte oxidative stress (57–59). A recent study showed that patients with severe coronary atherosclerosis, glucose and insulin metabolic disorder, and serum adiponectin reduction are significantly linked with higher oxidative stress in EAT adipocytes (60). In addition, EAT thickness was related with endothelial dysfunction (61), and it was concluded that EAT may predict the early reversible stages of atherosclerosis. The data from different studies showed that increased EAT volume is involved in high-risk coronary plaque formation. In addition, patients with high-risk coronary plaque have quantitatively higher EAT volume (35, 62). A prospective cohort study found that EAT and plasma IL-8 level are associated with elevated coronary artery calcium score, which is an independent predictor of coronary atherosclerosis (63).
EAT functions as an endocrine organ. Recent studies have focused on the signaling molecules released by EAT. A novel mechanism for EAT-induced CVDs is the secretion of exosomes that contain non-coding RNAs, especially microRNAs (miRNAs), which are subsequently absorbed by endothelial cells or cardiomyocytes (64–66). A previous study verified that increased has-miRNA-34a is associated with coronary artery diseases (67). A recent micro- and lncRNA microarrays followed by GO-KEGG functional enrichment analysis demonstrated a sex-dependent unique mi/lncRNAs. They are involved in inflammation, adipogenesis, and cardiomyocyte apoptosis. They are also modified in human epicardial fat in both patients with and without coronary artery disease. Examples include has-miR-320 family, hsa-miR-21, hsa-24-3p, hsa-miR-378, and hsa-miR-33 (68).
4.2 Arrhythmias
Atrial fibrillation (AF), the most common arrhythmia, is the major cause of ischemic stroke, heart failure, and cardiovascular mortality. Atrial electrophysiological and structural remodeling is the underlying mechanism of AF, which is characterized by myocardial fibrosis, and the underlying mechanism is heterogeneous (69). The mechanism of arrhythmias includes adipocyte infiltration, pro-fibrotic, and pro-inflammatory paracrine effects, oxidative stress, and other pathways (69–71). A study in 215 acute embolic stroke patients showed that increased periatrial EAT thickness on the left side is associated with AF (72). In patients with AF who have undergone pulmonary vein isolation, EAT volume is associated with AF recurrence (73). In another study, most of the persistent AF patients who are not anticoagulated and with increased periatrial EAT thickness were also associated with an increased risk of cardiovascular events (74). Julia and colleagues found that patients with lone AF have larger volume and higher attenuation of EAT compared with patients without cardiac arrhythmias (75). Moreover, non-uniformity of EAT radiomic gray level is the only independent predictor of post-ablation AF recurrence within 12 months follow-up (75).
EAT was found to be significantly higher in the patients with nephrotic syndrome and in patients with ECG showing the atrial depolarization and ventricular repolarization (76). Another study demonstrated that EAT volume exerts reverse relationship with heart rate recovery that indicates the potential adverse effects of EAT on cardiac autonomic function (77). It may result from the pathogenic effect of local inflammatory cytokines secreted from nearby visceral fats. As an endocrine organ, EAT influences adjacent myocardium by secreting a series of bioactive molecules, such as exosomes carrying circular RNAs (circRNAs), and regulates atrial electrical and structural remodeling. Zheng and colleagues identified differently expressed circRNA in EAT via RNA sequencing, such as hsa_circRNA_000932 and hsa_circ_0078619, which may work as endogenous RNAs to capture various miRNAs miR-103a-2-5p and miR-199a-5p, and subsequently regulate the expression of cardiovascular disorders-related protein-coding genes (78).
4.3 Aortic stenosis
With the global epidemiological increase in elderly population, AS becomes a challenging disease, representing an important cause of morbidity, hospitalization, and death in aged population. Generally, AS is considered as the result of a complex process, driven by inflammation and involving multifactorial pathological mechanisms promoting valvular calcification and valvular bone deposition (79). Importantly, obesity-related chronic systemic inflammation is associated with a significant increase in the amount of EAT, the cardiac visceral fat, which is considered a transducer of the adverse effects of systemic inflammation and metabolic disorders on the heart (80). As EAT can mediate the deleterious effects of systemic inflammation on the myocardium, it may contribute to the pathogenesis of calcific AS. Parisi et al. hypothesized that EAT may participate in the inflammatory burden of aortic stenosis (81). Mahabadi et al. found that EAT thickness, quantified using transthoracic echocardiography, was significantly associated with severe aortic stenosis, independent of traditional risk factors (82). Moreover, Arangalagea et al. showed that EAT volume was independently associated with LV mass in a prospective cohort of patients with aortic stenosis (83). These results support the hypothesis of a potent proinflammatory activation of EAT in patients with AS and suggest the involvement of cardiac visceral fat in inflammatory and atherogenic phenomena occurring in the AV and promoting its degeneration and calcification (79, 81).
4.4 Thromboembolism
The relationship between AF and thromboembolism is well established. Recently, preliminary investigations demonstrate the possible relationship between EAT and AF-related thromboembolism. In a recent study in AF patients who developed stroke, EAT volume was significantly increased. Hence, total EAT is considered as an independent predictor of higher risk of stroke occurrence after AF diagnosis (84). In addition, EAT thickness was higher in non-valvular AF patients compared to healthy subjects. EAT thickness was also related with the CHA2DS2−VASc score in patients with non-valvular AF (85). A multicenter study in Korea enrolled 3,464 individuals and showed that larger peri-atrial EAT volume was independently associated with post-ablation embolic stroke regardless of AF recurrence and CHA2DS2-VASc score (86). Patients with post-ablation embolic stroke had a greater prevalence of prior thromboembolism, lower creatinine clearance, larger left atrial diameter, frequent AF recurrence, and abundant total and peri-atrial EAT (86).
However, another study showed that EAT thickness was directly related with CHA2DS2-VASc scores in patients with sinus rhythm (87). A single-center retrospective study enrolled 202 patients and showed that a thickened EAT was associated with low left atrial appendage flow velocity and had increased risk of thromboembolic phenomena in the presence of AF (88). The mechanism of correlation between EAT and embolic stroke might be explained by EAT-mediated atrial cardiomyopathy, which is characterized by LA enlargement, increased wall stiffness, hypercontractility, endothelial dysfunction, and impaired reservoir function that lead to atrial prothrombotic milieu (84, 86, 89, 90).
4.5 Heart failure
EAT is associated with risk factors for HF, such as obesity, metabolic syndrome, hypertension, and diabetes. Numerous studies focused on the relationship between EAT and HF. A study enrolled 72 type-2 diabetes subjects with normal cardiac function and verified that subjects with higher EAT thickness showed a lower cardiac workload, worse cardiopulmonary function and subclinical cardiac systolic dysfunction after maximal cardiopulmonary exercise test with similar duration of exercise (91). Another study found that HF patients have higher EAT than the control group. Hence, EAT can be considered as a prognostic predictor of HF with preserved ejection fraction (HFpEF) (92). In a prospective multinational PROMIS-HfpEF cohort, increased EAT has been shown to be associated with cardiac structural alterations, adiposity, inflammation, lower insulin sensitivity, and endothelial dysfunction related to HFpEF pathology (93). In addition, a proteomic analysis of EAT from 2,416 HFpEF patients found that EAT proteins such as CD36, POSTN, and TRAP1 were differentially expressed in HFpEF (94). In another study, increased EAT thickness was found closely related with brachial–ankle pulse-wave velocity in HFpEF patients and indicated that thicker EAT may be independently associated with arterial stiffness (95).
Interestingly, in a multicenter cohort study, EAT thickness was found to be greater in patients with HFpEF than HFrEF/HFmrEF. In addition, the EAT thickness is associated with reduced left atrial and left ventricle function in HFpEF, but with better function in HFrEF/HFmrEF (96). Similar results were also found in post-ablation AF patients (97). However, in patients with non-ischemic cardiomyopathy, EAT volume was found to be greater in the LV reverse remodeling group than in the non-remodeling group, which suggests that EAT volume is an independent predictor of LV reverse remodeling in patients with non-ischemic cardiomyopathy (98). Findings by Hao and colleagues indicate that EAT mediates cardiomyocyte apoptosis after acute myocardial infarction through secretion of complement factor D and activation of poly ADP-ribosepolymerase-1 (99), which may subsequently result in heart failure. A recent study in mice model with preserved ejection fraction found that inflammasome-mediated pyroptosis pathway was activated in the EAT. Moreover, suppression of pyroptosis-related protein gasdermin D in cultured EAT could lower cardiomyocyte inflammation and autophagy (100).
4.6 COVID-19-related cardiac syndrome
The coronavirus disease 2019 (COVID-19) pandemic has spread worldwide with more than 6 million deaths recorded globally (101). Besides pneumonia, myocardial injury is a typical COVID-19-related complication and is present in 20%–30% of patients that contributed to 40% of deaths (102). Patients with larger EAT seem to get higher cardiac risk in COVID-19 patients (103, 104), with worse outcomes (105). Moreover, the type of adipose tissue and its distribution seems to play a crucial role in COVID-19 severity (101). It is well-known that EAT has direct anatomical and functional contiguity with the myocardium, and these two tissues share the same microcirculation, which support the pathophysiology of COVID-19-related cardiac injury. Therefore, clinical studies and practice on COVID-19-related CVDs have focused on cardiac adipose tissue. Recent studies have shown that in patients with COVID-19, higher EAT volume and lower EAT density may be independent predictors of an unfavorable disease prognosis, including cardiovascular complications and death (104, 106, 107). It is reported that EAT is like a highly inflammatory region with dense macrophage infiltrates and highly enriched proinflammatory cytokines, which are overexpressed in COVID-19 patients with CVDs that facilitates viral spread and augments immune response (18, 108, 109). COVID-19-related cardiac injury is characterized by decreased angiotensin-converting enzyme 2 (ACE2) and entry ligand receptor, with pathogenetic role (20, 108). Previous studies indicated that ACE2 deficiency mediates myocardial inflammation, and ACE2 reduction is associated with EAT inflammation (110). In addition, ACE2 downregulation leads to the proinflammatory polarization of M1 macrophages in EAT and results in the dysregulation of the inflammatory response, which is highly observed in COVID-19. Moreover, ICU patients with a higher EAT volume had a higher risk of developing pulmonary embolism compared to those with lower EAT volume (111). Therefore, EAT plays a role in COVID-19-related CVDs and has potential to become a clinically measurable and modifiable therapeutic target.
5 Therapeutic options in EAT
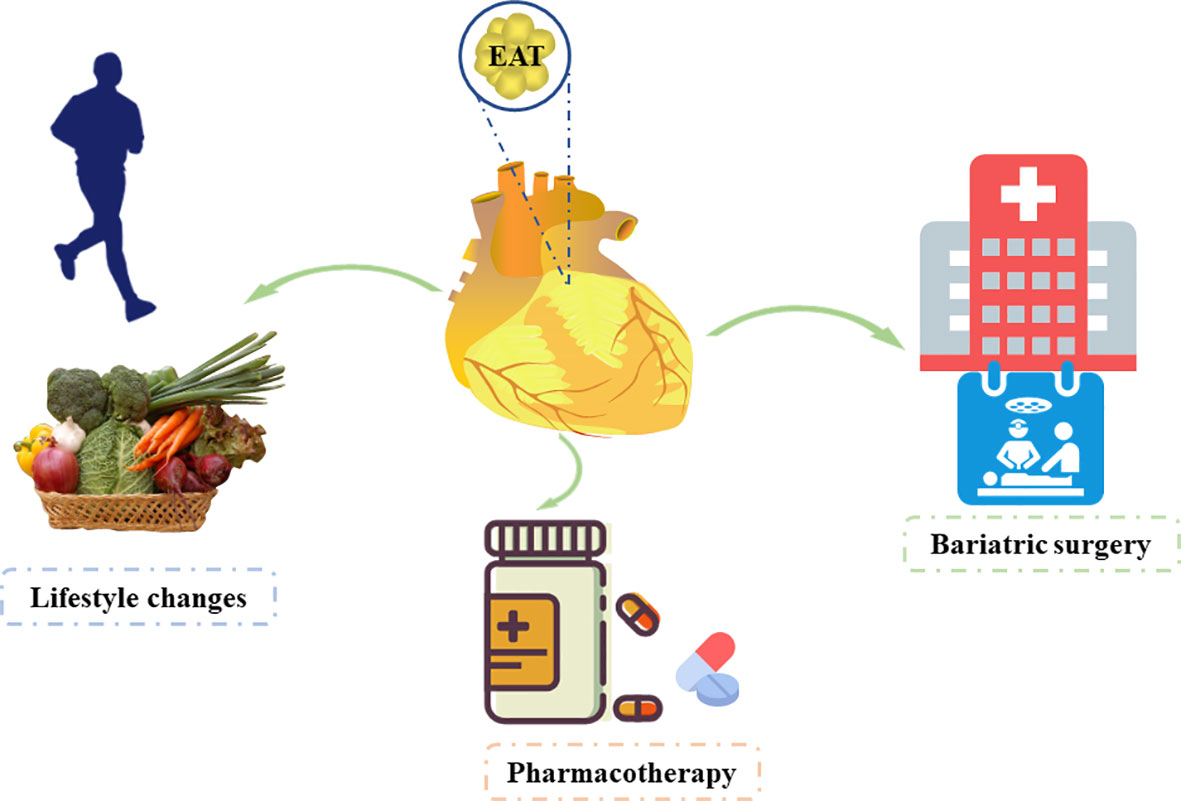
We have discussed that EAT is an independent risk factor and has potential to become a therapeutic target for CVDs. Hence, studies have focused on reducing EAT (Figure 3).
5.1 Lifestyle intervention
EAT is exacerbated by several unhealthy life styles, such as sedentary life, weight gain, and an unbalanced diet (112, 113). Lifestyle intervention based on dietary control and regular physical activity is an essential “first-line” strategy for the clinical management of obesity. Physical exercise and strict diet control reduce visceral fat, including EAT, and improve cardiac function (114, 115). A recent study showed that bad childhood experience is associated with increased EAT in children with depression and reduced physical exercise (116). Studies have indicated that regular physical activity is an effective non-invasive strategy for reducing EAT that may provide beneficial effects on the cardiovascular system (113, 117, 118). Several studies showed that aerobic exercise decreases EAT thickness significantly in obese men (118, 119). Another study from India showed that the 12-week regular Taekwondo training reduces the EAT thickness significantly in elderly women with hypertension (120). Another study from Turkey enrolled 74 obese women and found that long-term, sustained weight loss reduces epicardial fat thickness significantly as assessed by echocardiography, which can be used as an indicator of metabolic profile for weight reduction in obese women (121). Moreover, the decrease in epicardial fat thickness was significantly higher in patients who reversed their metabolic syndrome diagnosis with weight loss than in those whose metabolic syndrome status was unchanged. Iacobellis et al. reported significant reduction in epicardial fat in severely obese patients after 6 months of low-calorie diet (122). However, a systematic review and meta-analysis conducted by Rabkin and Campbell (123) showed that diet and bariatric surgery markedly reduced EAT, but this was not achieved with exercise. Moreover, a reduction in body mass index was significantly associated with reduced EAT by diet-based interventions.
5.2 Medical treatment
The impact of medical treatment on EAT is worth investigating. The use of statin is associated with decreased adipokine release from visceral EAT.
Parisi et al. (124) reported that statin therapy was significantly associated with lower EAT thickness and with lower levels of EAT-secreted inflammatory mediators. Of note, there was a significant correlation between EAT thickness and its proinflammatory status. Among lipid-lowering agents, atorvastatin has more significant effect than simvastatin and ezetimibe (125). Other studies also demonstrated that statins reduce EAT volume (124, 126). Additionally, antidiabetic drugs such as GLP-1A (127, 128), metformin (129, 130), and SGLT2 inhibitors (131, 132) were also proved to reduce EAT. For individuals with severe obesity, bariatric surgery is the most reliable treatment. It is well-known that different depots of adipose tissue and visceral fat change after bariatric surgery. Weight loss following bariatric surgery is associated with EAT reduction (133). Hunt et al. reported that severely obese subjects have lower EAT during a 14-year follow-up after bariatric surgery (134).
6 Conclusion
The cardiovascular system is widely affected by EAT. The expansion and remodeling of EAT contributes to vascular dysfunction and CVDs significantly. The evolving field of non-invasive imaging technique-based EAT composition analysis showed great potential for the stratification of CVD risk. Therefore, it is critical to identify strategies that are capable of reducing cardiovascular risk by modulating EAT mass, distribution, and function. At present, there is growing interest regarding EAT. In the future, the assessment of EAT may become part of the clinical practice to help clinicians identify patients at great risk of developing CVDs and to provide information on their clinical and therapeutic prognosis.
Author contributions
CL reviewed the literature and drafted this review. XL reviewed the literature and corrected the figures. BA, LC, and WL reviewed the literature, gave critical comments, and revised the manuscript. YW gave critical comments and revised the manuscript. YW and HZ reviewed the literature, gave critical comments, revised the manuscript, and took charge of project supervision and administration. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (CL, grant no. 82000347; HMZ, grant no. 12226003; YW, grant number 82170362), the Jilin Province Science and Technology Project (CL, grant no. YDZJ202301ZYTS441; HMZ, grant no. 2YDZJ202201ZYTS679), and Jilin Province Science and Technology Department Science and Technology Innovation Talents Cultivation Program (HMZ, grant no. 20180519008JH).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Inciardi RM, Chandra A. Epicardial adipose tissue in heart failure: risk factor or mediator? Eur J Heart Fail (2022) 24(8):1357–8. doi: 10.1002/ejhf.2577
2. Karampetsou N, Alexopoulos L, Minia A, Pliaka V, Tsolakos N, Kontzoglou K, et al. Epicardial adipose tissue as an independent cardiometabolic risk factor for coronary artery disease. Cureus (2022) 14(6):e25578. doi: 10.7759/cureus.25578
3. Tay JCK, Yap J. Epicardial adipose tissue: more than meets the eye. Int J Cardiol (2022) 362:174–5. doi: 10.1016/j.ijcard.2022.05.001
4. Iacobellis G. Aging effects on epicardial adipose tissue. Front Aging (2021) 2:666260. doi: 10.3389/fragi.2021.666260
5. Iacobellis G, Corradi D, Sharma AM. Epicardial adipose tissue: anatomic, biomolecular and clinical relationships with the heart. Nat Clin Pract Cardiovasc Med (2005) 2(10):536–43. doi: 10.1038/ncpcardio0319
6. Iacobellis G, Bianco AC. Epicardial adipose tissue: emerging physiological, pathophysiological and clinical features. Trends Endocrinol Metab (2011) 22(11):450–7. doi: 10.1016/j.tem.2011.07.003
7. Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H, et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation (2003) 108(20):2460–6. doi: 10.1161/01.CIR.0000099542.57313.C5
8. Shirani J, Berezowski K, Roberts WC. Quantitative measurement of normal and excessive (cor adiposum) subepicardial adipose tissue, its clinical significance, and its effect on electrocardiographic QRS voltage. Am J Cardiol (1995) 76(5):414–8. doi: 10.1016/S0002-9149(99)80116-7
9. Iacobellis G. Epicardial adipose tissue in contemporary cardiology. Nat Rev Cardiol (2022) 19(9):593–606. doi: 10.1038/s41569-022-00679-9
10. Krishnan A, Sharma H, Yuan D, Trollope AF, Chilton L. The role of epicardial adipose tissue in the development of atrial fibrillation, coronary artery disease and chronic heart failure in the context of obesity and type 2 diabetes mellitus: a narrative review. J Cardiovasc Dev Dis (2022) 9(7):217. doi: 10.3390/jcdd9070217
11. Antonopoulos AS, Antoniades C. The role of epicardial adipose tissue in cardiac biology: classic concepts and emerging roles. J Physiol (2017) 595(12):3907–17. doi: 10.1113/JP273049
12. Selthofer-Relatic K, Bosnjak I. Myocardial fat as a part of cardiac visceral adipose tissue: physiological and pathophysiological view. J Endocrinol Invest (2015) 38(9):933–9. doi: 10.1007/s40618-015-0258-y
13. Pabon MA, Manocha K, Cheung JW, Lo JC. Linking arrhythmias and adipocytes: insights, mechanisms, and future directions. Front Physiol (2018) 9:1752. doi: 10.3389/fphys.2018.01752
14. Rabkin SW. Epicardial fat: properties, function and relationship to obesity. Obes Rev (2007) 8(3):253–61. doi: 10.1111/j.1467-789X.2006.00293.x
15. Chechi K, Richard D. Thermogenic potential and physiological relevance of human epicardial adipose tissue. Int J Obes Suppl (2015) 5(Suppl 1):S28–34. doi: 10.1038/ijosup.2015.8
16. Sacks HS, Fain JN, Holman B, Cheema P, Chary A, Parks F, et al. Uncoupling protein-1 and related messenger ribonucleic acids in human epicardial and other adipose tissues: epicardial fat functioning as brown fat. J Clin Endocrinol Metab (2009) 94(9):3611–5. doi: 10.1210/jc.2009-0571
17. Marchington JM, Mattacks CA, Pond CM. Adipose tissue in the mammalian heart and pericardium: structure, foetal development and biochemical properties. Comp Biochem Physiol B (1989) 94(2):225–32. doi: 10.1016/0305-0491(89)90337-4
18. Iacobellis G, Malavazos AE, Corsi MM. Epicardial fat: from the biomolecular aspects to the clinical practice. Int J Biochem Cell Biol (2011) 43(12):1651–4. doi: 10.1016/j.biocel.2011.09.006
19. Zoico E, Rubele S, De Caro A, Nori N, Mazzali G, Fantin F, et al. Brown and beige adipose tissue and aging. Front Endocrinol (2019) 10:368. doi: 10.3389/fendo.2019.00368
20. Rossi AP, Muollo V, Dalla Valle Z, Urbani S, Pellegrini M, Ghoch M, et al. The role of obesity, body composition, and nutrition in COVID-19 pandemia: a narrative review. Nutrients (2022) 14(17):3493. doi: 10.3390/nu14173493
21. Baker AR, Silva NF, Quinn DW, Harte AL, Pagano D, Bonser RS, et al. Human epicardial adipose tissue expresses a pathogenic profile of adipocytokines in patients with cardiovascular disease. Cardiovasc Diabetol (2006) 5:1. doi: 10.1186/1475-2840-5-1
22. Doukbi E, Soghomonian A, Sengenes C, Ahmed S, Ancel P, Dutour A, et al. Browning epicardial adipose tissue: friend or foe? Cells (2022) 11(6):991. doi: 10.3390/cells11060991
23. Shaihov-Teper O, Ram E, Ballan N, Brzezinski RY, Naftali-Shani N, Masoud R, et al. Extracellular vesicles from epicardial fat facilitate atrial fibrillation. Circulation (2021) 143(25):2475–93. doi: 10.1161/CIRCULATIONAHA.120.052009
24. Vural B, Atalar F, Ciftci C, Demirkan A, Susleyici-Duman B, Gunay D, et al. Presence of fatty-acid-binding protein 4 expression in human epicardial adipose tissue in metabolic syndrome. Cardiovasc Pathol (2008) 17(6):392–8. doi: 10.1016/j.carpath.2008.02.006
25. Iacobellis G, Ribaudo MC, Assael F, Vecci E, Tiberti C, Zappaterreno A, et al. Echocardiographic epicardial adipose tissue is related to anthropometric and clinical parameters of metabolic syndrome: a new indicator of cardiovascular risk. J Clin Endocrinol Metab (2003) 88(11):5163–8. doi: 10.1210/jc.2003-030698
26. Iacobellis G, Assael F, Ribaudo MC, Zappaterreno A, Alessi G, Mario U, et al. Epicardial fat from echocardiography: a new method for visceral adipose tissue prediction. Obes Res (2003) 11(2):304–10. doi: 10.1038/oby.2003.45
27. Schejbal V. [Epicardial fatty tissue of the right ventricle–morphology, morphometry and functional significance]. Pneumologie (1989) 43(9):490–9.
28. Iacobellis G, Willens HJ. Echocardiographic epicardial fat: a review of research and clinical applications. J Am Soc Echocardiogr (2009) 22(12):1311–9. doi: 10.1016/j.echo.2009.10.013
29. Natale F, Tedesco MA, Mocerino R, de Simone V, Di Marco GM, Aronne L, et al. Visceral adiposity and arterial stiffness: echocardiographic epicardial fat thickness reflects, better than waist circumference, carotid arterial stiffness in a large population of hypertensives. Eur J Echocardiogr (2009) 10(4):549–55. doi: 10.1093/ejechocard/jep002
30. Islas F, Gutierrez E, Cachofeiro V, Martinez-Martinez E, Marin G, Olmos C, et al. Importance of cardiac imaging assessment of epicardial adipose tissue after a first episode of myocardial infarction. Front Cardiovasc Med (2022) 9:995367. doi: 10.3389/fcvm.2022.995367
31. Parisi V, Petraglia L, Formisano R, Caruso A, Grimaldi MG, Bruzzese D, et al. Validation of the echocardiographic assessment of epicardial adipose tissue thickness at the rindfleisch fold for the prediction of coronary artery disease. Nutr Metab Cardiovasc Dis (2020) 30(1):99–105. doi: 10.1016/j.numecd.2019.08.007
32. Lin A, Dey D, Wong DTL, Nerlekar N. Perivascular adipose tissue and coronary atherosclerosis: from biology to imaging phenotyping. Curr Atheroscler Rep (2019) 21(12):47. doi: 10.1007/s11883-019-0817-3
33. Xu Y, Cheng X, Hong K, Huang C, Wan L. How to interpret epicardial adipose tissue as a cause of coronary artery disease: a meta-analysis. Coron Artery Dis (2012) 23(4):227–33. doi: 10.1097/MCA.0b013e328351ab2c
34. Mahabadi AA, Balcer B, Dykun I, Forsting M, Schlosser T, Heusch G, et al. Cardiac computed tomography-derived epicardial fat volume and attenuation independently distinguish patients with and without myocardial infarction. PloS One (2017) 12(8):e0183514. doi: 10.1371/journal.pone.0183514
35. Nerlekar N, Brown AJ, Muthalaly RG, Talman A, Hettige T, Cameron JD, et al. Association of epicardial adipose tissue and high-risk plaque characteristics: a systematic review and meta-analysis. J Am Heart Assoc (2017) 6(8):e006379.
36. Mahabadi AA, Berg MH, Lehmann N, Kalsch H, Bauer M, Kara K, et al. Association of epicardial fat with cardiovascular risk factors and incident myocardial infarction in the general population: the Heinz nixdorf recall study. J Am Coll Cardiol (2013) 61(13):1388–95. doi: 10.1016/j.jacc.2012.11.062
37. Mancio J, Azevedo D, Saraiva F, Azevedo AI, Pires-Morais G, Leite-Moreira A, et al. Epicardial adipose tissue volume assessed by computed tomography and coronary artery disease: a systematic review and meta-analysis. Eur Heart J Cardiovasc Imaging (2018) 19(5):490–7. doi: 10.1093/ehjci/jex314
38. Dey D, Nakazato R, Li D, Berman DS. Epicardial and thoracic fat - noninvasive measurement and clinical implications. Cardiovasc Diagn Ther (2012) 2(2):85–93.
39. Pracon R, Kruk M, Kepka C, Pregowski J, Opolski MP, Dzielinska Z, et al. Epicardial adipose tissue radiodensity is independently related to coronary atherosclerosis. a multidetector computed tomography study. Circ J (2011) 75(2):391–7.
40. Liu Z, Wang S, Wang Y, Zhou N, Shu J, Stamm C, et al. Association of epicardial adipose tissue attenuation with coronary atherosclerosis in patients with a high risk of coronary artery disease. Atherosclerosis (2019) 284:230–6. doi: 10.1016/j.atherosclerosis.2019.01.033
41. Goeller M, Achenbach S, Marwan M, Doris MK, Cadet S, Commandeur F, et al. Epicardial adipose tissue density and volume are related to subclinical atherosclerosis, inflammation and major adverse cardiac events in asymptomatic subjects. J Cardiovasc Comput Tomogr (2018) 12(1):67–73. doi: 10.1016/j.jcct.2017.11.007
42. Yuvaraj J, Cheng K, Lin A, Psaltis PJ, Nicholls SJ, Wong DTL. The emerging role of CT-based imaging in adipose tissue and coronary inflammation. Cells (2021) 10(5):1196. doi: 10.3390/cells10051196
43. Antonopoulos AS, Sanna F, Sabharwal N, Thomas S, Oikonomou EK, Herdman L, et al. Detecting human coronary inflammation by imaging perivascular fat. Sci Transl Med (2017) 9:398. doi: 10.1126/scitranslmed.aal2658
44. Ross R, Shaw KD, Martel Y, de Guise J, Hudson R, Avruch L. Determination of total and regional adipose tissue distribution by magnetic resonance imaging in android women. Basic Life Sci (1993), 60:177–80. doi: 10.1007/978-1-4899-1268-8_40
45. Ross R, Leger L, Morris D, Guise J, Guardo R. Quantification of adipose tissue by MRI: relationship with anthropometric variables. J Appl Physiol (1985) 72(2):787–95.
46. Sharma RC, Kramsch DM, Lee PL, Colletti P, Jiao Q. Quantitation and localization of regional body fat distribution–a comparison between magnetic resonance imaging and somatometry. Obes Res (1996) 4(2):167–78. doi: 10.1002/j.1550-8528.1996.tb00530.x
47. Guglielmo M, Lin A, Dey D, Baggiano A, Fusini L, Muscogiuri G, et al. Epicardial fat and coronary artery disease: role of cardiac imaging. Atherosclerosis (2021) 321:30–8. doi: 10.1016/j.atherosclerosis.2021.02.008
48. Salari R, Ballard DH, Hoegger MJ, Young D, Shetty AS. Fat-only Dixon: how to use it in body MRI. Abdom Radiol (2022) 47(7):2527–44. doi: 10.1007/s00261-022-03546-w
49. Homsi R, Meier-Schroers M, Gieseke J, Dabir D, Luetkens JA, Kuetting DL, et al. 3D-Dixon MRI based volumetry of peri- and epicardial fat. Int J Cardiovasc Imaging (2016) 32(2):291–9. doi: 10.1007/s10554-015-0778-8
50. Skoda I, Henningsson M, Stenberg S, Sundin J, Carlhall CJ. Simultaneous assessment of left atrial fibrosis and epicardial adipose tissue using 3D late gadolinium enhanced Dixon MRI. J Magn Reson Imaging (2022) 56(5):1393–403. doi: 10.1002/jmri.28100
51. Homsi R, Thomas D, Gieseke J, Meier-Schroers M, Dabir D, Kuetting D, et al. Epicardial fat volume and aortic stiffness in healthy individuals: a quantitative cardiac magnetic resonance study. Rofo (2016) 188(9):853–8. doi: 10.1055/s-0042-110098
52. Chen L, Qin L, Liu X, Meng X. CTRP3 alleviates ox-LDL-Induced inflammatory response and endothelial dysfunction in mouse aortic endothelial cells by activating the PI3K/Akt/eNOS pathway. Inflammation (2019) 42(4):1350–9. doi: 10.1007/s10753-019-00996-1
53. Schmid A, Kopp A, Hanses F, Karrasch T, Schaffler A. TNF-related protein-3 (CTRP-3) attenuates lipopolysaccharide (LPS)-induced systemic inflammation and adipose tissue erk-1/-2 phosphorylation in mice in vivo. Biochem Biophys Res Commun (2014) 452(1):8–13. doi: 10.1016/j.bbrc.2014.06.054
54. Matloch Z, Mraz M, Kasperova BJ, Kratochvilova H, Svoboda P, Pleyerova I, et al. Decreased epicardial CTRP3 mRNA levels in patients with type 2 diabetes mellitus and coronary artery disease undergoing elective cardiac surgery: a possible association with coronary atherosclerosis. Int J Mol Sci (2022) 23(17):9988. doi: 10.3390/ijms23179988
55. Katanasaka Y, Saito A, Sunagawa Y, Sari N, Funamoto M, Shimizu S, et al. ANGPTL4 expression is increased in epicardial adipose tissue of patients with coronary artery disease. J Clin Med (2022) 11(9):2449. doi: 10.3390/jcm11092449
56. Sacks HS, Fain JN, Cheema P, Bahouth SW, Garrett E, Wolf RY, et al. Depot-specific overexpression of proinflammatory, redox, endothelial cell, and angiogenic genes in epicardial fat adjacent to severe stable coronary atherosclerosis. Metab Syndr Relat Disord (2011) 9(6):433–9. doi: 10.1089/met.2011.0024
57. Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest (2004) 114(12):1752–61. doi: 10.1172/JCI21625
58. Berezin AE, Berezin AA, Lichtenauer M. Emerging role of adipocyte dysfunction in inducing heart failure among obese patients with prediabetes and known diabetes mellitus. Front Cardiovasc Med (2020) 7:583175. doi: 10.3389/fcvm.2020.583175
59. Maslov LN, Naryzhnaya NV, Boshchenko AA, Popov SV, Ivanov VV, Oeltgen PR. Is oxidative stress of adipocytes a cause or a consequence of the metabolic syndrome? J Clin Transl Endocrinol (2019) 15:1–5. doi: 10.1016/j.jcte.2018.11.001
60. Naryzhnaya NV, Koshelskaya OA, Kologrivova IV, Suslova TE, Kharitonova OA, Andreev SL, et al. Production of reactive oxygen species by epicardial adipocytes is associated with an increase in postprandial glycemia, postprandial insulin, and a decrease in serum adiponectin in patients with severe coronary atherosclerosis. Biomedicines (2022) 10(8):2054. doi: 10.3390/biomedicines10082054
61. Tekaya AB, Mehmli T, Mrad IB, Fendri A, Boukriba S, Bouden S, et al. Increased epicardial adipose tissue thickness correlates with endothelial dysfunction in spondyloarthritis. Clin Rheumatol (2022) 41(10):3017–25. doi: 10.1007/s10067-022-06261-5
62. Aprigliano G, Scuteri L, Iafelice I, Li Volsi L, Cuko B, Palloshi A, et al. Epicardial adipose tissue thickness and acute coronary syndrome: a matter of how much or how? Int J Cardiol (2015) 199:8–9. doi: 10.1016/j.ijcard.2015.06.168
63. Christensen RH, von Scholten BJ, Hansen CS, Heywood SE, Rosenmeier JB, Andersen UB, et al. Epicardial, pericardial and total cardiac fat and cardiovascular disease in type 2 diabetic patients with elevated urinary albumin excretion rate. Eur J Prev Cardiol (2017) 24(14):1517–24. doi: 10.1177/2047487317717820
64. Patel VB, Shah S, Verma S, Oudit GY. Epicardial adipose tissue as a metabolic transducer: role in heart failure and coronary artery disease. Heart Fail Rev (2017) 22(6):889–902. doi: 10.1007/s10741-017-9644-1
65. Pellegrini L, Foglio E, Pontemezzo E, Germani A, Russo MA, Limana F. Cardiac repair: the intricate crosstalk between the epicardium and the myocardium. Curr Stem Cell Res Ther (2020) 15(8):661–73. doi: 10.2174/1574888X15666200219105448
66. Thomou T, Mori MA, Dreyfuss JM, Konishi M, Sakaguchi M, Wolfrum C, et al. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature (2017) 542(7642):450–5. doi: 10.1038/nature21365
67. Mari-Alexandre J, Barcelo-Molina M, Sanz-Sanchez J, Molina P, Sancho J, Abellan Y, et al. Thickness and an altered miRNA expression in the epicardial adipose tissue is associated with coronary heart disease in sudden death victims. Rev Esp Cardiol (2019) 72(1):30–9. doi: 10.1016/j.rec.2017.12.007
68. Flinn B, Adams C, Chowdhury N, Gress T, Santanam N. Profiling of non-coding regulators and their targets in epicardial fat from patients with coronary artery disease. Int J Mol Sci (2022) 23(10):5297. doi: 10.3390/ijms23105297
69. Wong CX, Ganesan AN, Selvanayagam JB. Epicardial fat and atrial fibrillation: current evidence, potential mechanisms, clinical implications, and future directions. Eur Heart J (2017) 38(17):1294–302.
70. Conte M, Petraglia L, Cabaro S, Valerio V, Poggio P, Pilato E, et al. Epicardial adipose tissue and cardiac arrhythmias: focus on atrial fibrillation. Front Cardiovasc Med (2022) 9:932262. doi: 10.3389/fcvm.2022.932262
71. Scarano Pereira JP, Owen E, Martinino A, Akmal K, Abouelazayem M, Graham Y, et al. Epicardial adipose tissue, obesity, and the occurrence of atrial fibrillation: an overview of pathophysiology and treatment methods. Expert Rev Cardiovasc Ther (2022) 20(4):307–22. doi: 10.1080/14779072.2022.2067144
72. Edsen F, Habib P, Matz O, Nikoubashman O, Wiesmann M, Frick M, et al. Epicardial adipose tissue thickness assessed by CT is a marker of atrial fibrillation in stroke patients. Ann Clin Transl Neurol (2022) 9(10):1668–72. doi: 10.1002/acn3.51617
73. Beyer C, Tokarska L, Stuhlinger M, Feuchtner G, Hintringer F, Honold S, et al. Structural cardiac remodeling in atrial fibrillation. JACC Cardiovasc Imaging (2021) 14(11):2199–208. doi: 10.1016/j.jcmg.2021.04.027
74. Chu CY, Lee WH, Hsu PC, Lee MK, Lee HH, Chiu CA, et al. Association of increased epicardial adipose tissue thickness with adverse cardiovascular outcomes in patients with atrial fibrillation. Medicine (2016) 95(11):e2874. doi: 10.1097/MD.0000000000002874
75. Ilyushenkova J, Sazonova S, Popov E, Zavadovsky K, Batalov R, Archakov E, et al. Radiomic phenotype of epicardial adipose tissue in the prognosis of atrial fibrillation recurrence after catheter ablation in patients with lone atrial fibrillation. J Arrhythm (2022) 38(5):682–93. doi: 10.1002/joa3.12760
76. Simsek OO, Demircan T, Erfidan G, Emir B, Basaran C, Alparslan C, et al. Epicardial adipose tissue and risk of arrhythmia in nephrotic syndrome. Pediatr Int (2022) 64(1):e15323.
77. Chang SH, Chu PH, Tsai CT, Kuo JY, Tsai JP, Hung TC, et al. Both epicardial and peri-aortic adipose tissue blunt heart rate recovery beyond body fat mass. Front Cardiovasc Med (2022) 9:939515. doi: 10.3389/fcvm.2022.939515
78. Zheng H, Peng Y, Wang P, Su P, Zhao L. The integrative network of circRNA, miRNA and mRNA of epicardial adipose tissue in patients with atrial fibrillation. Am J Transl Res (2022) 14(9):6550–62.
79. Conte M, Petraglia L, Campana P, Gerundo G, Caruso A, Grimaldi MG, et al. The role of inflammation and metabolic risk factors in the pathogenesis of calcific aortic valve stenosis. Aging Clin Exp Res (2021) 33(7):1765–70. doi: 10.1007/s40520-020-01681-2
80. Packer M. Epicardial adipose tissue may mediate deleterious effects of obesity and inflammation on the myocardium. J Am Coll Cardiol (2018) 71(20):2360–72. doi: 10.1016/j.jacc.2018.03.509
81. Parisi V, Rengo G, Pagano G, D’Esposito V, Passaretti F, Caruso A, et al. Epicardial adipose tissue has an increased thickness and is a source of inflammatory mediators in patients with calcific aortic stenosis. Int J Cardiol (2015) 186:167–9. doi: 10.1016/j.ijcard.2015.03.201
82. Mahabadi AA, Kahlert HA, Dykun I, Balcer B, Kahlert P, Rassaf T. Epicardial adipose tissue thickness independently predicts severe aortic valve stenosis. J Heart Valve Dis (2017) 26(3):262–7.
83. Arangalage D, Mathieu T, Nguyen V, Cimadevilla C, Kerneis C, Duval X, et al. Epicardial adipose tissue volume is associated with left ventricular remodelling in calcific aortic valve stenosis. Arch Cardiovasc Dis (2019) 112(10):594–603. doi: 10.1016/j.acvd.2019.06.005
84. Tsao HM, Hu WC, Tsai PH, Lee CL, Liu FC, Wang HH, et al. The abundance of epicardial adipose tissue surrounding left atrium is associated with the occurrence of stroke in patients with atrial fibrillation. Medicine (2016) 95(14):e3260. doi: 10.1097/MD.0000000000003260
85. Akdag S, Simsek H, Sahin M, Akyol A, Duz R, Babat N. Association of epicardial adipose tissue thickness and inflammation parameters with CHA2DS2-VASASc score in patients with nonvalvular atrial fibrillation. Ther Clin Risk Manag (2015) 11:1675–81. doi: 10.2147/TCRM.S94955
86. Ahn J, Shin SY, Shim J, Kim YH, Han SJ, Choi EK, et al. Association between epicardial adipose tissue and embolic stroke after catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol (2019) 30(11):2209–16. doi: 10.1111/jce.14154
87. Aksoy F, Guler S, Kahraman F, Oskay T, Varol E. The relation between echocardiographic epicardial fat thickness and CHA2DS2-VASc score in patients with sinus rhythm. Braz J Cardiovasc Surg (2019) 34(1):41–7. doi: 10.21470/1678-9741-2018-0230
88. Yamaguchi S, Otaki Y, Tamarappoo B, Yoshida J, Ikenaga H, Friedman J, et al. The association between epicardial adipose tissue thickness around the right ventricular free wall evaluated by transthoracic echocardiography and left atrial appendage function. Int J Cardiovasc Imaging (2020) 36(4):585–93. doi: 10.1007/s10554-019-01748-w
89. Acampa M, Lazzerini PE, Martini G. Atrial cardiopathy and sympatho-vagal imbalance in cryptogenic stroke: pathogenic mechanisms and effects on electrocardiographic markers. Front Neurol (2018) 9:469. doi: 10.3389/fneur.2018.00469
90. Cho KI, Kim BJ, Cho SH, Lee JH, Kim MK, Yoo BG. Epicardial fat thickness and free fatty acid level are predictors of acute ischemic stroke with atrial fibrillation. J Cardiovasc Imaging (2018) 26(2):65–74. doi: 10.4250/jcvi.2018.26.e1
91. Nesti L, Pugliese NR, Chiriaco M, Trico D, Baldi S, Natali A. Epicardial adipose tissue thickness is associated with reduced peak oxygen consumption and systolic reserve in patients with type 2 diabetes and normal heart function. Diabetes Obes Metab (2022) 25(1):177–188.
92. Ates K, Demir M. Importance of epicardial adipose tissue as a predictor of heart failure with preserved ejection fraction. Rev Assoc Med Bras (1992) 68(9):1178–84.
93. Venkateshvaran A, Faxen UL, Hage C, Michaelsson E, Svedlund S, Saraste A, et al. Association of epicardial adipose tissue with proteomics, coronary flow reserve, cardiac structure and function, and quality of life in heart failure with preserved ejection fraction: insights from the PROMIS-HFpEF study. Eur J Heart Fail (2022) 24(12):2251–60. doi: 10.1002/ejhf.2709
94. He S, Zhu H, Zhang J, Wu X, Zhao L, Yang X. Proteomic analysis of epicardial adipose tissue from heart disease patients with concomitant heart failure with preserved ejection fraction. Int J Cardiol (2022) 362:118–25. doi: 10.1016/j.ijcard.2022.05.067
95. Liu Z, Hu W, Zhang H, Tao H, Lei P, Liu J, et al. EAT thickness as a predominant feature for evaluating arterial stiffness in patients with heart failure with preserved ejection fraction. Diabetes Metab Syndr Obes (2022) 15:1217–26. doi: 10.2147/DMSO.S356001
96. Jin X, Hung CL, Tay WT, Soon D, Sim D, Sung KT, et al. Epicardial adipose tissue related to left atrial and ventricular function in heart failure with preserved versus reduced and mildly reduced ejection fraction. Eur J Heart Fail (2022) 24(8):1346–56. doi: 10.1002/ejhf.2513
97. Wang X, Butcher SC, Kuneman JH, Lustosa RP, Fortuni F, Ajmone Marsan N, et al. The quantity of epicardial adipose tissue in patients having ablation for atrial fibrillation with and without heart failure. Am J Cardiol (2022) 172:54–61. doi: 10.1016/j.amjcard.2022.02.021
98. Yamaguchi Y, Shibata A, Yoshida T, Tanihata A, Hayashi H, Kitada R, et al. Epicardial adipose tissue volume is an independent predictor of left ventricular reverse remodeling in patients with non-ischemic cardiomyopathy. Int J Cardiol (2022) 356:60–5. doi: 10.1016/j.ijcard.2022.03.051
99. Hao S, Zhang J, Pei Y, Guo L, Liang Z. Complement factor d derived from epicardial adipose tissue participates in cardiomyocyte apoptosis after myocardial infarction by mediating PARP-1 activity. Cell Signal (2022) 101:110518. doi: 10.1016/j.cellsig.2022.110518
100. Xia YY, Shi Y, Li Z, Li H, Wu LD, Zhou WY, et al. Involvement of pyroptosis pathway in epicardial adipose tissue - myocardium axis in experimental heart failure with preserved ejection fraction. Biochem Biophys Res Commun (2022) 636(Pt 2):62–70. doi: 10.1016/j.bbrc.2022.10.109
101. Chen JM. Novel statistics predict the COVID-19 pandemic could terminate in 2022. J Med Virol (2022) 94(6):2845–8. doi: 10.1002/jmv.27661
102. Akhmerov A, Marban E. COVID-19 and the heart. Circ Res (2020) 126(10):1443–55. doi: 10.1161/CIRCRESAHA.120.317055
103. Slipczuk L, Castagna F, Schonberger A, Novogrodsky E, Sekerak R, Dey D, et al. Coronary artery calcification and epicardial adipose tissue as independent predictors of mortality in COVID-19. Int J Cardiovasc Imaging (2021) 37(10):3093–100. doi: 10.1007/s10554-021-02276-2
104. Deng M, Qi Y, Deng L, Wang H, Xu Y, Li Z, et al. Obesity as a potential predictor of disease severity in young COVID-19 patients: a retrospective study. Obesity (2020) 28(10):1815–25. doi: 10.1002/oby.22943
105. Grodecki K, Lin A, Razipour A, Cadet S, McElhinney PA, Chan C, et al. Epicardial adipose tissue is associated with extent of pneumonia and adverse outcomes in patients with COVID-19. Metabolism (2021) 115:154436. doi: 10.1016/j.metabol.2020.154436
106. Abrishami A, Eslami V, Baharvand Z, Khalili N, Saghamanesh S, Zarei E, et al. Epicardial adipose tissue, inflammatory biomarkers and COVID-19: is there a possible relationship? Int Immunopharmacol (2021) 90:107174. doi: 10.1016/j.intimp.2020.107174
107. Gasecka A, Pruc M, Kukula K, Gilis-Malinowska N, Filipiak KJ, Jaguszewski MJ, et al. Post-COVID-19 heart syndrome. Cardiol J (2021) 28(2):353–4. doi: 10.5603/CJ.a2021.0028
108. Malavazos AE, Goldberger JJ, Iacobellis G. Does epicardial fat contribute to COVID-19 myocardial inflammation? Eur Heart J (2020) 41(24):2333. doi: 10.1093/eurheartj/ehaa471
109. Ryan PM, Caplice NM. Is adipose tissue a reservoir for viral spread, immune activation, and cytokine amplification in coronavirus disease 2019? Obesity (2020) 28(7):1191–4. doi: 10.1002/oby.22843
110. Patel VB, Mori J, McLean BA, Basu R, Das SK, Ramprasath T, et al. ACE2 deficiency worsens epicardial adipose tissue inflammation and cardiac dysfunction in response to diet-induced obesity. Diabetes (2016) 65(1):85–95. doi: 10.2337/db15-0399
111. Rossi AP, Donadello K, Schweiger V, Zamboni GA, Valle ZD, Zamboni M, et al. Epicardial adipose tissue volume and CT-attenuation as prognostic factors for pulmonary embolism and mortality in critically ill patients affected by COVID-19. Eur J Clin Nutr (2022) 77(1):105–11.
112. Christensen RH, von Scholten BJ, Lehrskov LL, Rossing P, Jorgensen PG. Epicardial adipose tissue: an emerging biomarker of cardiovascular complications in type 2 diabetes? Ther Adv Endocrinol Metab (2020) 11:2042018820928824. doi: 10.1177/2042018820928824
113. Launbo N, Zobel EH, von Scholten BJ, Faerch K, Jorgensen PG, Christensen RH. Targeting epicardial adipose tissue with exercise, diet, bariatric surgery or pharmaceutical interventions: a systematic review and meta-analysis. Obes Rev (2021) 22(1):e13136. doi: 10.1111/obr.13136
114. Colonetti T, Grande AJ, Amaral MC, Colonetti L, Uggioni ML, da Rosa MI, et al. Effect of exercise on epicardial adipose tissue in adults: a systematic review and meta-analyses. Heart Fail Rev (2021) 26(6):1399–411. doi: 10.1007/s10741-020-09965-5
115. Saco-Ledo G, Valenzuela PL, Castillo-Garcia A, Arenas J, Leon-Sanz M, Ruilope LM, et al. Physical exercise and epicardial adipose tissue: a systematic review and meta-analysis of randomized controlled trials. Obes Rev (2021) 22(1):e13103. doi: 10.1111/obr.13103
116. Bertele S, Heitland I, Fraccarollo D, Stapel B, Bauersachs J, Westhoff-Bleck M, et al. Behavioral pathway to a broken heart: the link between adverse childhood experiences, depression, physical exercise and cardiovascular health. Front Psychiatry (2022) 13:1002143. doi: 10.3389/fpsyt.2022.1002143
117. Nyawo TA, Pheiffer C, Mazibuko-Mbeje SE, Mthembu SXH, Nyambuya TM, Nkambule BB, et al. Physical exercise potentially targets epicardial adipose tissue to reduce cardiovascular disease risk in patients with metabolic diseases: oxidative stress and inflammation emerge as major therapeutic targets. Antioxidants (2021) 10(11):1758. doi: 10.3390/antiox10111758
118. Bairapareddy KC, Maiya AG, Kumar P, Nayak K, Guddattu V, Nayak V. Effect of aerobic exercise on echocardiographic epicardial adipose tissue thickness in overweight individuals. Diabetes Metab Syndr Obes (2018) 11:303–12. doi: 10.2147/DMSO.S145862
119. Kim MK, Tomita T, Kim MJ, Sasai H, Maeda S, Tanaka K. Aerobic exercise training reduces epicardial fat in obese men. J Appl Physiol (1985) 106(1):5–11.
120. Kim YH, Jeong MK, Park H, Park SK. Effects of regular taekwondo intervention on health-related physical fitness, cardiovascular disease risk factors and epicardial adipose tissue in elderly women with hypertension. Int J Environ Res Public Health (2021) 18(6):2935. doi: 10.3390/ijerph18062935
121. Ersan Demirci D, Demirci D, Eke RN. Reversal of metabolic syndrome with weight loss decreases epicardial fat more than weight loss alone in women with obesity. Turk Kardiyol Dern Ars (2022) 50(1):45–56. doi: 10.5543/tkda.2022.21063
122. Iacobellis G, Singh N, Wharton S, Sharma AM. Substantial changes in epicardial fat thickness after weight loss in severely obese subjects. Obesity (2008) 16(7):1693–7. doi: 10.1038/oby.2008.251
123. Rabkin SW, Campbell H. Comparison of reducing epicardial fat by exercise, diet or bariatric surgery weight loss strategies: a systematic review and meta-analysis. Obes Rev (2015) 16(5):406–15. doi: 10.1111/obr.12270
124. Parisi V, Petraglia L, D’Esposito V, Cabaro S, Rengo G, Caruso A, et al. Statin therapy modulates thickness and inflammatory profile of human epicardial adipose tissue. Int J Cardiol (2019) 274:326–30. doi: 10.1016/j.ijcard.2018.06.106
125. Park JH, Park YS, Kim YJ, Lee IS, Kim JH, Lee JH, et al. Effects of statins on the epicardial fat thickness in patients with coronary artery stenosis underwent percutaneous coronary intervention: comparison of atorvastatin with simvastatin/ezetimibe. J Cardiovasc Ultrasound (2010) 18(4):121–6. doi: 10.4250/jcu.2010.18.4.121
126. Raggi P, Davidson M, Callister TQ, Welty FK, Bachmann GA, Hecht H, et al. Aggressive versus moderate lipid-lowering therapy in hypercholesterolemic postmenopausal women: beyond endorsed lipid lowering with EBT scanning (BELLES). Circulation (2005) 112(4):563–71. doi: 10.1161/CIRCULATIONAHA.104.512681
127. Iacobellis G, Villasante Fricke AC. Effects of semaglutide versus dulaglutide on epicardial fat thickness in subjects with type 2 diabetes and obesity. J Endocr Soc (2020) 4(4):bvz042. doi: 10.1210/jendso/bvz042
128. Dozio E, Vianello E, Malavazos AE, Tacchini L, Schmitz G, Iacobellis G, et al. Epicardial adipose tissue GLP-1 receptor is associated with genes involved in fatty acid oxidation and white-to-brown fat differentiation: a target to modulate cardiovascular risk? Int J Cardiol (2019) 292:218–24. doi: 10.1016/j.ijcard.2019.04.039
129. Ziyrek M, Kahraman S, Ozdemir E, Dogan A. Metformin monotherapy significantly decreases epicardial adipose tissue thickness in newly diagnosed type 2 diabetes patients. Rev Port Cardiol (2019) 38(6):419–23. doi: 10.1016/j.repc.2018.08.010
130. Gunes H, Gunes H, Ozmen S, Celik E, Temiz F. Effects of metformin on epicardial adipose tissue and atrial electromechanical delay of obese children with insulin resistance. Cardiol Young (2020) 30(10):1429–32. doi: 10.1017/S1047951120002103
131. Yagi S, Hirata Y, Ise T, Kusunose K, Yamada H, Fukuda D, et al. Canagliflozin reduces epicardial fat in patients with type 2 diabetes mellitus. Diabetol Metab Syndr (2017) 9:78. doi: 10.1186/s13098-017-0275-4
132. Iacobellis G, Gra-Menendez S. Effects of dapagliflozin on epicardial fat thickness in patients with type 2 diabetes and obesity. Obesity (2020) 28(6):1068–74. doi: 10.1002/oby.22798
133. Sorimachi H, Obokata M, Omote K, Reddy YNV, Takahashi N, Koepp KE, et al. Long-term changes in cardiac structure and function following bariatric surgery. J Am Coll Cardiol (2022) 80(16):1501–12. doi: 10.1016/j.jacc.2022.08.738
Keywords: epicardial adipose tissue, obesity, cardiovascular diseases, cardiac imaging, management
Citation: Li C, Liu X, Adhikari BK, Chen L, Liu W, Wang Y and Zhang H (2023) The role of epicardial adipose tissue dysfunction in cardiovascular diseases: an overview of pathophysiology, evaluation, and management. Front. Endocrinol. 14:1167952. doi: 10.3389/fendo.2023.1167952
Received: 17 February 2023; Accepted: 21 April 2023;
Published: 16 May 2023.
Edited by:
Xiaodong Sun, Affiliated Hospital of Weifang Medical University, ChinaReviewed by:
Hui Liu, Guangdong Provincial People’s Hospital, ChinaLaura Petraglia, University of Naples Federico II, Italy
Yulia Dyleva, Research Institute for Complex Issues of Cardiovascular Diseases, Russia
Marit Granér, Helsinki University Central Hospital, Finland
Copyright © 2023 Li, Liu, Adhikari, Chen, Liu, Wang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yonggang Wang, wangyg1982@jlu.edu.cn; Huimao Zhang, huimao@jlu.edu.cn
 Cheng Li
Cheng Li Xinyu Liu2
Xinyu Liu2 Binay Kumar Adhikari
Binay Kumar Adhikari Yonggang Wang
Yonggang Wang Huimao Zhang
Huimao Zhang