- Department of Cardiology, Yulin First People’s Hospital (The Sixth Affiliated Hospital of Guangxi Medical University), Yulin, China
Objective: To evaluate the efficacy of enhanced external counterpulsation (EECP) in the prevention of contrast-induced nephropathy (CIN) in patients with combined chronic kidney disease (CKD) and diabetes mellitus (DM) by comparing the changes in renal function-related indicators in patients before and after coronary angiography (CAG) or percutaneous coronary intervention (PCI).
Methods: There were 230 subjects consecutively included in the study. Of these, 30 cases with DM underwent rehydration therapy, and 200 cases underwent EECP therapy in addition to rehydration therapy, comprising 53 patients with DM and 147 patients without. All the patients were tested to measure the renal function indicators before and after CAG/PCI.
Results: The postoperative results of blood urea nitrogen (BUN), serum creatinine (Scr), estimated glomerular filtration rate (eGFR), B2 microglobulin, and high-sensitivity C-reactive protein in the three groups showed a statistically significant difference (P < 0.05). After EECP therapy, patients with DM showed a significant decrease in BUN (9.1 ± 4.2 vs. 7.2 ± 3.0, t = 3.899, P < 0.001) and a significant increase in eGFR (41.5 ± 12.7 vs. 44.0 ± 15.6, t = −2.031, P = 0.047), while the patients without DM showed a more significant difference (P < 0.001). Patients with DM showed a lower percentage of elevated Scr (66.7% vs. 43.4%, P = 0.042), a higher percentage of elevated eGFR (30.0% vs. 52.8%, P = 0.044), and a lower incidence of CIN (16.7% vs. 3.8%, P = 0.042) after EECP therapy.
Conclusion: Treatment with EECP can reduce Scr in patients with combined CKD and DM post CAG/PCI, increase eGFR, and decrease the incidence of CIN.
Introduction
Contrast-induced nephropathy (CIN) is one of the three major causes of hospital-acquired acute kidney injury (AKI) following renal hypoperfusion and drug-induced injuries. It can increase the medical costs of patients, extend the length of their hospital stay, and lead to a poor long-term prognosis. The pathogenesis of CIN is associated with ischemia and anoxia of renal medulla, direct toxicity of contrast medium, oxidative stress, apoptosis, and immunity/inflammation (1), and there are limited therapies for this disease. It is crucial to identify the high-risk population for CIN and take preventive measures. The risk factors of CIN include renal insufficiency, diabetes mellitus (DM), old age, type and dose of contrast medium, and cardio-cerebrovascular comorbidities. Patients with multiple risk factors showed a significant increase in the incidence risk (2, 3).
Enhanced external counterpulsation (EECP) is a non-invasive circulation-assisted device that is very effective in the prevention and treatment of cardiovascular diseases, cardiac rehabilitation, etc. (4) It improves the ischemia of organs and increases the quality of life of patients. Past studies have demonstrated that EECP could decrease renal vascular resistance and renin activity, increase renal blood flow by 20%–30%, and increase estimated glomerular filtration rate (eGFR) by 24% (5). Recent studies suggested possible benefits of EECP in reducing the incidence risk of CIN (6). The study evaluates the efficacy of EECP in the prevention of CIN after coronary angiography (CAG) or percutaneous coronary intervention (PCI) in the high-risk population of patients with chronic kidney disease (CKD) and DM.
Materials and methods
Demographics and methods
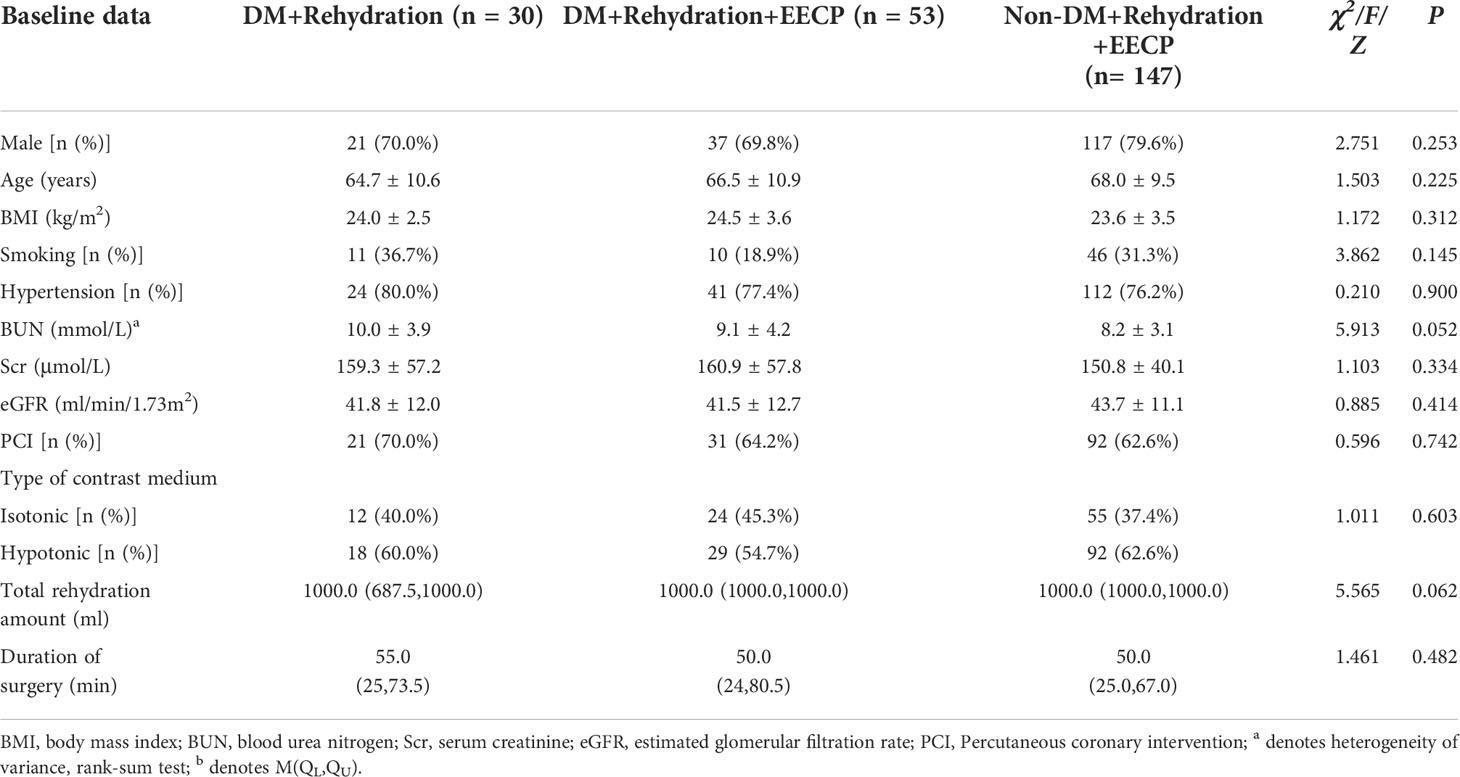
A total of 230 patients aged 67.2 ± 10.0 years with chronic renal insufficiency undergoing CAG/PCI admitted to Yulin First People’s Hospital from December 2020 to February 2022 were consecutively included as the subjects in the present study. Of these, 36.1% had DM and 62.6% underwent PCI. Thirty cases with DM underwent rehydration therapy 6–12 h before and 6–12 h after CAG/PCI (0.9% normal saline, 1.0–1.5 ml/kg/h; 0.5 ml/kg/h for patients with left ventricular ejection fraction <35%). In addition to rehydration therapy, 200 cases underwent EECP therapy, once per day, 1 h each time, 24 h before and 48–72 h after CAG/PCI; this included 53 patients with DM and 147 patients without. All patients were tested for the renal function indicators before and 48–72 h after CAG/PCI.
Criteria for diagnosis, inclusion, and exclusion
The diagnosis criteria for CIN was an absolute elevation in serum creatinine (Scr) ≥0.5 mg/dL (44.2 μmol/L) or a 25% elevation relative to baseline value (7) within 48–72 h after iodinated contrast medium was used.
To facilitate earlier identification of acute renal impairment in patients, the concept of AKI in Kidney Disease: Improving Global Outcomes was also introduced, which is defined as an absolute elevation in Scr of 0.3 mg/dL (26.5 μmol/L) (8).
The inclusion criteria were as follows: (1) age: ≥18 years old, (2) eGFR <60 ml/min/1.73 m2, (3) testing of the renal function indicators within 72 h before the operation, and (4) patient was informed and consent was obtained.
The exclusion criteria were as follows: (1) patients who had used iodinated contrast medium 30 d before inclusion, (2) patients with AKI due to other clear causes, (3) patients requesting withdrawal, (4) patients who failed to receive the re-examination of renal function indicators on time after surgery, (5) patients who underwent hemodialysis within 48 h after surgery, and (6) patients with uremia who received long-term hemodialysis.
Statistical analysis
The SPSS™ Statistics 22.0 software was used for data analysis. The enumeration data were expressed as [n (%)] and the χ2 test was used for comparison between groups. The measurement data fitting the normal distribution were expressed as ± s, one-way analysis of variance was used for comparison between groups, and the paired t-test was used for comparison within groups. The measurement data not fitting the normal distribution were expressed as median (first quartile, third quartile) [M (QL, QU)], and the rank–sum test was used for comparison between groups. A value of P < 0.05 denoted a difference was statistically significant.
Results
Baseline data
There were no statistically significant differences in gender, age, body mass index (BMI), comorbidities, preoperative renal function indicators, and type of contrast media among the three groups, and the data were comparable (Table 1).
Comparison of the postoperative renal function indicators among the three groups
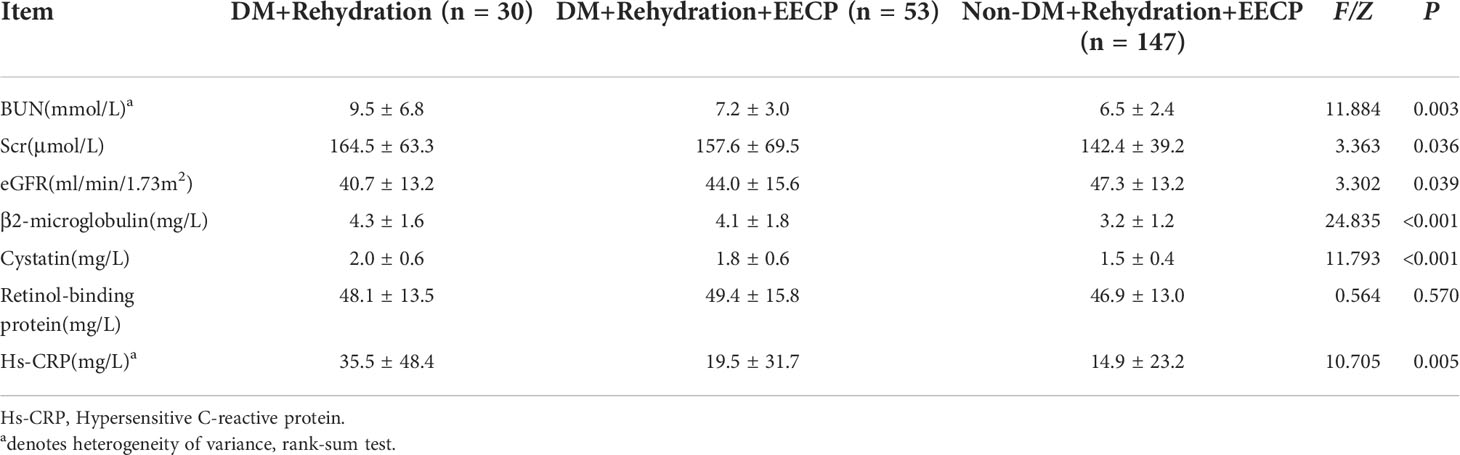
Postoperative blood urea nitrogen (BUN), Scr, eGFR, B2 microglobulin, and high-sensitivity C-reactive protein in the three groups showed statistically significant differences (P < 0.05). As shown in the pairwise comparison of the three groups, no differences were observed between DM + Rehydration and DM + Rehydration + EECP or between DM + Rehydration + EECP and non-DM + Rehydration + EECP (Table 2).
Comparison of the renal function levels in the three groups before and after the operation
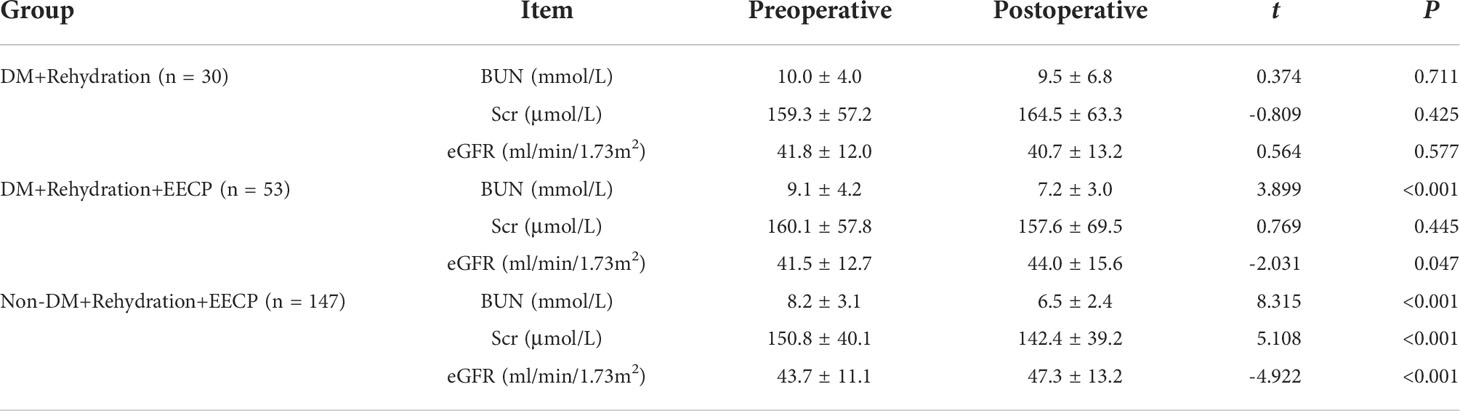
There was no significant difference in BUN, Scr, and eGFR in the DM + Rehydration group before and after the operation (P > 0.05). In the DM + Rehydration + EECP treatment group, BUN decreased significantly (9.1 ± 4.2 vs. 7.2 ± 3.0, t = 3.899, P < 0.001) and eGFR increased significantly (41.5 ± 12.7 vs. 44.0 ± 15.6, t = −2.031, P = 0.047) after the operation, and these differences were more significant between patients in the non-DM group (P < 0.001) (Table 3).
Comparison of the CIN risks in the three groups
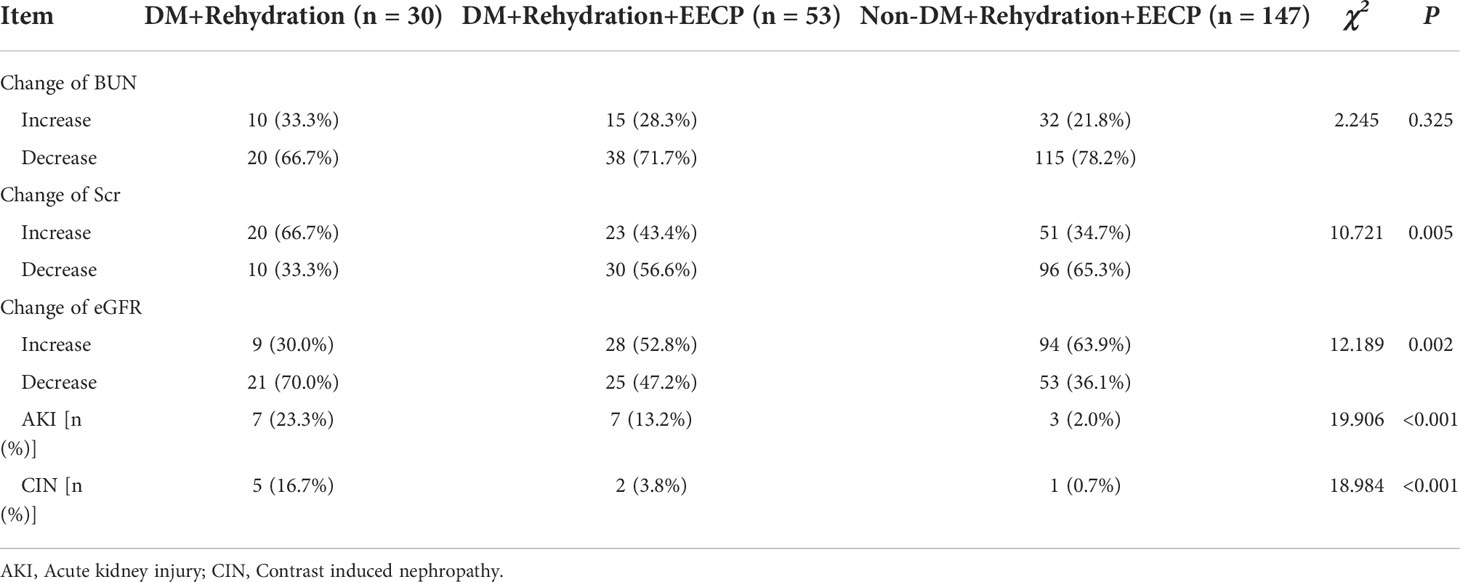
The changes of postoperative Scr and eGFR and the incidences of AKI and CIN showed a statistically significant difference in the three groups (P < 0.05). The pairwise comparison demonstrated patients with DM had a lower rate of increased Scr (66.7% vs. 43.4%, P = 0.042), a higher rate of increased eGFR (30.0% vs. 52.8%, P = 0.044), and a lower incidence of CIN (16.7% vs. 3.8%, P = 0.042) after EECP therapy. Among patients undergoing EECP therapy, the rate of AKI was higher in patients with DM (13.2% vs. 2.0%, P = 0.001) (Table 4).
Discussion
The definition of CIN is an absolute elevation in Scr ≥0.5 mg/dL (44.2 μmol/L) or a 25% elevation relative to baseline within 48–72 h after the use of iodinated contrast medium. The incidence of CIN reported in different studies is associated with the definition, potential renal impairment, type and dose of contrast medium, and other concomitant factors (9). The incidence of CIN is <3% in patients with normal renal function but up to 50% in patients with CKD. Following the use of isotonic and hypotonic contrast media, the incidence of CIN may be overestimated, but in patients adopting transarterial administration and with eGFR <30 mL/min/1.73 m2, the incidence of CIN still increased significantly (10).
A high-risk factor for CIN is DM, with the mechanism possibly associated with increased oxidative stress, forceful contraction of renal blood vessels, and signaling route induced apoptosis, under a high glucose condition (11). The incidence of CIN was only 2% in patients without DM and with normal renal function, 16% in patients with DM and normal renal function, and 38% in patients with DM and renal insufficiency (12). Up to 56% of CIN cases developed to irreversible renal failure.
Rehydration therapy is the standard care for CIN, but in the present study, the incidence of CIN was 16.7% in patients with DM after standard rehydration therapy, higher than the 6.77% from previous studies (13) and similar to the result of Liang (11.67%) (14) due to the subjects of the study having old age and a high PCI rate. However, the effect of normal saline on the prevention of CIN was contested in a study that concluded that an intravenous drip of normal saline could not reduce the risk of CIN (15). That study disrupted the existing knowledge and suggests further consideration is needed.
The data on the effect of sodium bicarbonate on preventing CIN was contradictory. The efficacy of statins in the prevention of CIN has been widely acknowledged. As suggested in a meta-analysis, rosuvastatin could decrease the incidence rate of CIN by 47% (odds ratio [OR]: 0.53, 95% confidence interval [CI]: 0.40–0.71, P < 0.0001) (16). Sulfhydryl in n-acetylcysteine is a good antioxidant and agent for removing oxygen radicals at a low cost and with no adverse reactions, so it is one option for the prevention of CIN (17). Currently, no conclusive evidence is available to prove preventive hemodialysis can prevent contrast-induced renal impairment, and the risk of invasive operation cannot be neglected, so most scholars do not recommend preventive hemodialysis (7).
Treatment with EECP is a safe, non-invasive extracorporeal circulation method that is widely used for cardiac-cerebral vascular diseases to reduce angina symptoms and the use of nitrates, increase exercise tolerance, and improve myocardial ischemia (18). It could also improve the quality of life of patients with cardiac failure (19), and have a positive effect on the hemodynamics of the coronary and carotid arteries (20, 21). However, there are few studies on the effect of EECP on renal functions. Werner reported that EECP could effectively increase renal excretion, renal blood flow, and eGFR (5). Rua discovered that serum cystatin significantly decreased from 1.00 to 0.94 mg/L (P < 0.001) and eGFR increased from 70.47 to 76.27 mL/min/1.73 m2 (P = 0.006) after EECP therapy (22).
A recent study suggested that EECP could increase the clearance rate of the contrast medium and reduce the incidence of CIN (6). First, EECP increases renal blood flow and decreases the plasma levels of renin (5). The increase in renal blood flow is due to not only an increase in renal perfusion pressure caused by increased blood return to the heart, but also an increase in nitric oxide. A previous study has shown that EECP increases the plasma concentrations of nitric oxide and decreased plasma levels of endothelin-1, and improves the renal function (23). Secondly, the increase in blood flow shear stress induced by EECP improves the function and morphology of the vascular endothelium and reduces oxidative stress and inflammatory responses. Meanwhile, increased renal blood flow shear stress induces the expression of nuclear factor erythroid-2-related factor 2 (Nrf2) in the proximal tubular epithelium (24), activates the Nrf2/Sirtuin-3 (Sirt3)/superoxide dismutase 2 (SOD2) signaling pathway, and improves the contrast-induced renal injury (25). Lastly, the increase of atrial natriuretic peptide induced by EECP has a diuretic effect (26), which improves the fluid shear stress of the kidney and reduces the viscosity of the contrast medium, thereby alleviating kidney damage caused by the contrast medium.
As demonstrated in the present study, after EECP treatment, the incidence of CIN in patients with DM decreased to 3.8%, dropping by 77.2% as compared with those treated solely with rehydration, and eGFR also increased by 2.5 ml/min/1.73m2. Among patients without DM, EECP showed a more significant effect, reducing Scr by 8.4 μmol/L and increasing eGFR by 3.6 ml/min/1.73 m2 after the operation. It may become a new choice for the prevention of CIN.
However, there are some limitations in the present study. The exact dose of the contrast medium was not recorded and was instead evaluated indirectly by the duration of the operation. In this study, not all the total amount of contrast media was recorded due to different habits of interventional surgeons in writing surgical records. Since the eGFR of the recruited patients was less than 60 mL/min/1.73m2, the risk of CIN was high. To ensure the safety of patients, only non-ionic contrast media were used, including iodixanol, omnipac, and ioversol. Lastly, the sample size was small, and the mechanism for CIN prevention by EECP was not further examined.
Conclusion
It was demonstrated that EECP can reduce the Scr level in patients with combined CKD and DM post CAG/PCI, increase eGFR, and decrease the incidence of CIN.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Yulin First People’s Hospital. The patients/participants provided their written informed consent to participate in this study.
Author contributions
Conception and design of the research: C-MZ. Acquisition of data: C-MZ, X-JZ, Z-JW, JB, and S-YQ. Analysis and interpretation of the data: C-MZ and Y-MZ. Statistical analysis: C-MZ and Y-MZ. Obtaining financing: C-MZ and Y-YL. Writing of the manuscript: C-MZ. Critical revision of the manuscript for intellectual content: Y-YL. All authors read and approved the final draft.
Funding
This work was supported by Health Committee of Guangxi (No.Z20210519).
Acknowledgments
We would like to acknowledge the hard and dedicated work of all the staff that implemented the intervention and evaluation components of the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zhang F, Lu Z, Wang F. Advances in the pathogenesis and prevention of contrast-induced nephropathy. Life Sci (2020) 259:118379. doi: 10.1016/j.lfs.2020.118379
2. Shams E, Mayrovitz HN. Contrast-induced nephropathy: A review of mechanisms and risks. Cureus (2021) 13(5):e14842. doi: 10.7759/cureus.14842
3. Zhang J, Li J, Huang JQ. Network meta-analysis of four Chinese patent medicines combined with angiotensin converting enzyme inhibitors or angiotensin receptor blockers in early diabetic nephropathy treatment. World J Tradit Chin Med (2020) 6:51–60. doi: 10.4103/wjtcm.wjtcm_41_19
4. Lin S, Xiao-Ming W, Gui-Fu W. Expert consensus on the clinical application of enhanced external counterpulsation in elderly people (2019). Aging Med (Milton). (2020) 3(1):16–24. doi: 10.1002/agm2.12097
5. Werner D, Trägner P, Wawer A, Porst H, Daniel WG, Gross P. Enhanced external counterpulsation: a new technique to augment renal function in liver cirrhosis. Nephrol Dial Transpl. (2005) 20(5):920–6. doi: 10.1093/ndt/gfh755
6. Zhang X, Yao C, Xiao Q, Wu J, Wu G. Enhanced external counterpulsation: A new method to alleviate contrast-induced acute kidney injury. Contemp. Clin Trials. (2022) 113:106653. doi: 10.1016/j.cct.2021.106653
7. Hossain MA, Costanzo E, Cosentino J, Patel C, Qaisar H, Singh V, et al. Contrast-induced nephropathy: Pathophysiology, risk factors, and prevention. Saudi J Kidney Dis Transpl. (2018) 29(1):1–9. doi: 10.4103/1319-2442.225199
8. Mercado MG, Smith DK, Guard EL. Acute kidney injury: Diagnosis and management. Am Fam. Phys. (2019) 100(11):687–94.
9. Nabi Z, Anjum N, Rashid RM, Zahideen ZU. Contrast induced nephropathy in high risk patients - myth or reality. J Ayub Med Coll Abbottabad. (2021) 33(4):568–71.
10. Muñoz de Bustillo Llorente E, de Miguel Balsa E. Radiological iodinated contrast-induced nephropathy. Rev Clin Esp (Barc). (2019) 219(7):403–10. doi: 10.1016/j.rce.2018.09.004
11. Li Y, Ren K. The mechanism of contrast-induced acute kidney injury and its association with diabetes mellitus. Contrast Media Mol Imaging. (2020) 2020:3295176. doi: 10.1155/2020/3295176
12. Lautin EM, Freeman NJ, Schoenfeld AH, Bakal CW, Haramati N, Friedman AC, et al. Radiocontrast-associated renal dysfunction: incidence and risk factors. AJR Am J Roentgenol. (1991) 157(1):49–58. doi: 10.2214/ajr.157.1.2048539
13. Wang L, Xu E, Ren S, Gu X, Zheng J, Yang J. Reduced glutathione does not further reduce contrast-induced nephropathy in elderly patients with diabetes receiving percutaneous coronary intervention. J Int Med Res (2020) 48(11):300060520964017. doi: 10.1177/0300060520964017
14. Liang M, Yang S, Fu N, Lu C, Tian F, Xing X, et al. Efficacy of alprostadil in preventing contrast-induced nephropathy in patients undergoing percutaneous coronary intervention: A multicenter prospective randomized controlled trial. Catheter. Cardiovasc Interv. (2018) 91(4):742–50. doi: 10.1002/ccd.27353
15. Nijssen EC, Rennenberg RJ, Nelemans PJ, Essers BA, Janssen MM, Vermeeren MA, et al. Prophylactic hydration to protect renal function from intravascular iodinated contrast material in patients at high risk of contrast-induced nephropathy (AMACING): a prospective, randomised, phase 3, controlled, open-label, non-inferiority trial. Lancet (2017) 389(10076):1312–22. doi: 10.1016/S0140-6736(17)30057-0
16. Zhang J, Guo Y, Jin Q, Bian L, Lin P. Meta-analysis of rosuvastatin efficacy in prevention of contrast-induced acute kidney injury. Drug Des Devel Ther (2018) 12:3685–90. doi: 10.2147/DDDT.S178020
17. Ershad M, Naji A, Vearrier D. N acetylcysteine. 2021 jun 29. in: StatPearls. In: Treasure island (FL). StatPearls Publishing (2022). Available at: https://pubmed.ncbi.nlm.nih.gov/30725868/.
18. Caceres J, Atal P, Arora R, Yee D. Enhanced external counterpulsation: A unique treatment for the "No-option" refractory angina patient. J Clin Pharm Ther (2021) 46(2):295–303. doi: 10.1111/jcpt.13330
19. Zhou ZF, Wang DJ, Li XM, Zhang CL, Wu CY. Effects of enhanced external counterpulsation on exercise capacity and quality of life in patients with chronic heart failure: A meta-analysis. Med (Baltimore). (2021) 100(27):e26536. doi: 10.1097/MD.0000000000026536
20. Xu L, Chen X, Cui M, Ren C, Yu H, Gao W, et al. The improvement of the shear stress and oscillatory shear index of coronary arteries during enhanced external counterpulsation in patients with coronary heart disease. PloS One (2020) 15(3):e0230144. doi: 10.1371/journal.pone.0230144
21. Tian S, Pan W, Peng J, Wang H, Deng B, Liang Y, et al. Hemodynamic responses in carotid bifurcation induced by enhanced external counterpulsation stimulation in healthy controls and patients with neurological disorders. Front Physiol (2021) 12:717080. doi: 10.3389/fphys.2021.717080
22. Ruangkanchanasetr P, Mahanonda N, Raungratanaamporn O, Ruckpanich P, Kitiyakara C, Chaiprasert A, et al. Effect of enhanced external counterpulsation treatment on renal function in cardiac patients. BMC Nephrol. (2013) 14::193. doi: 10.1186/1471-2369-14-193
23. Akhtar M, Wu GF, Du ZM, Zheng ZS, Michaels AD. Effect of external counterpulsation on plasma nitric oxide and endothelin-1 levels. Am J Cardiol (2006) 98(1):28–30. doi: 10.1016/j.amjcard.2006.01.053
24. Fukuda Y, Kaishima M, Ohnishi T, Tohyama K, Chisaki I, Nakayama Y, et al. Fluid shear stress stimulates MATE2-K expression via Nrf2 pathway activation. Biochem Biophys Res Commun (2017) 484(2):358–64. doi: 10.1016/j.bbrc.2017.01.124
25. Zhou Q, Wang X, Shao X, Wang H, Liu X, Ke X, et al. tert-butylhydroquinone treatment alleviates contrast-induced nephropathy in rats by activating the Nrf2/Sirt3/SOD2 signaling pathway. Oxid Med Cell Longev (2019) 2019:4657651. doi: 10.1155/2019/4657651
Keywords: enhanced external counterpulsation, chronic renal disease, diabetes mellitus, CAG/PCI, contrast-induced nephropathy
Citation: Zeng C-M, Zhao Y-M, Zhong X-J, Wu Z-J, Bai J, Qiu S-Y and Li Y-Y (2022) Reduction in risk of contrast-induced nephropathy in patients with chronic kidney disease and diabetes mellitus by enhanced external counterpulsation. Front. Endocrinol. 13:973452. doi: 10.3389/fendo.2022.973452
Received: 20 June 2022; Accepted: 14 September 2022;
Published: 17 October 2022.
Edited by:
Sonia Q. Doi, Uniformed Services University of the Health Sciences, United StatesReviewed by:
Akshyaya Pradhan, King George Medical Univeirsty, IndiaAhmet Karabulut, Istanbul Medicine Hospital, Turkey
Copyright © 2022 Zeng, Zhao, Zhong, Wu, Bai, Qiu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi-Yi Li, liyiyiloyy93@126.com
†These authors have contributed equally to this work
 Chun-Mei Zeng†
Chun-Mei Zeng† Yi-Yi Li
Yi-Yi Li