- 1Department of Health Management & Engineering Laboratory for Health Management, The First Affiliated Hospital of Shandong First Medical University & Shandong Provincial Qianfoshan Hospital, Jinan, China
- 2Department of Medical Record Management and Statistics, Shandong Provincial Qianfoshan Hospital & The First Affiliated Hospital of Shandong First Medical University, Jinan, China
- 3Department of Nursing, Center for Mental Health of Jinan City, Jinan, China
- 4Department of Gastroenterology, Shandong Rongjun General Hospital, Jinan, China
Background: Low klotho is associated with aging-related traits. However, no study has assessed the association between klotho and oral health in a large sample of population. This study aimed to explore the association between serum α-klotho and oral health in US Adults.
Methods: Data were from the National Health and Nutrition Examination Survey. Oral health parameters included periodontitis, self-rated oral health, and tooth loss. Logistic regression and restricted cubic spline models were adopted to evaluate the associations.
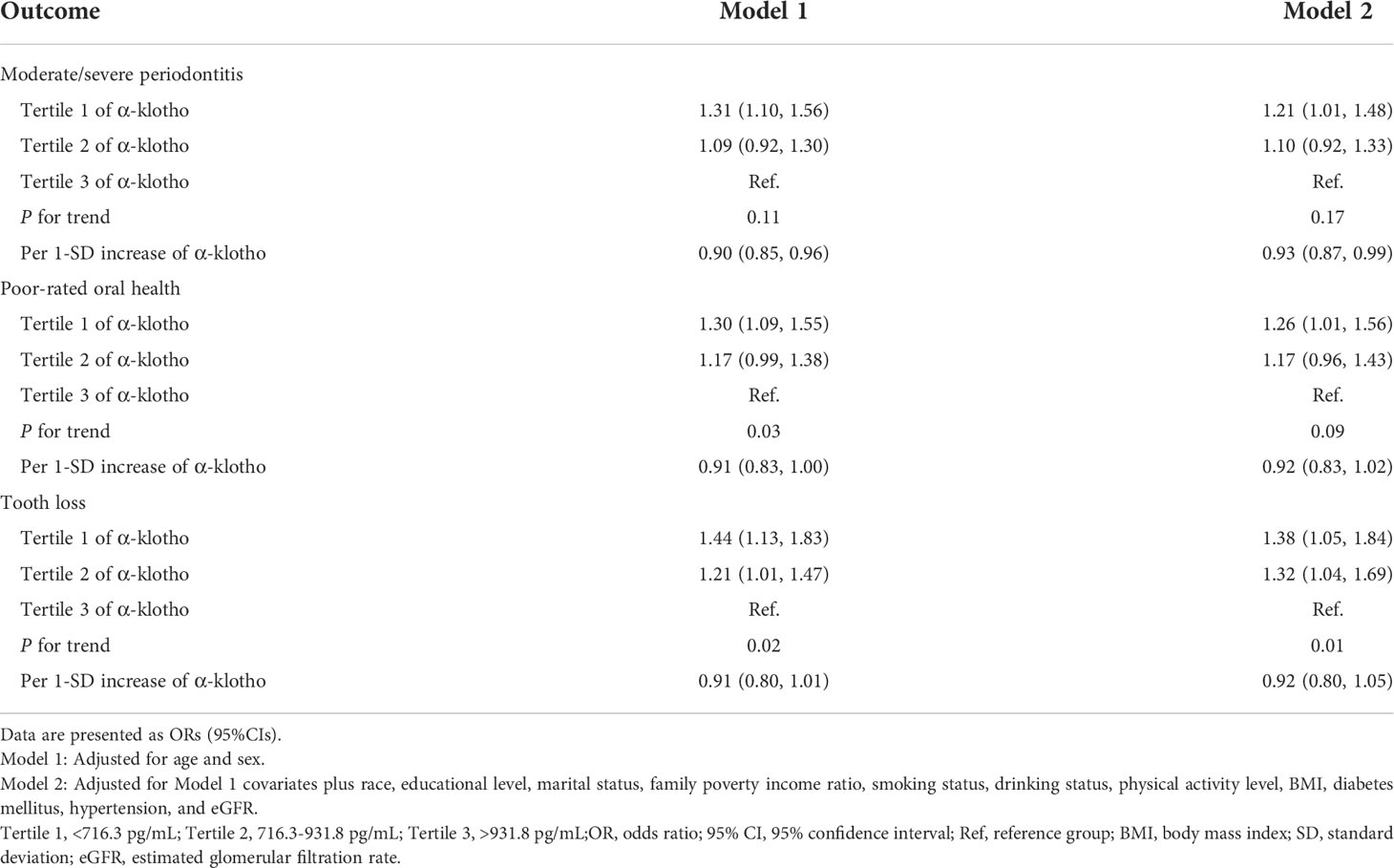
Results: A total of 6187 participants were included in the study. The median of the α-klotho level was 815.2 pg/mL. Serum α-Klotho was significantly lower in participants with poor oral health (all P <0.01). Compared with the highest tertile, the lowest tertile of α-klotho was associated with moderate/severe periodontitis, poor-rated oral health, and tooth loss, with OR (95% CI) being 1.21 (1.01, 1.48), 1.26 (1.01, 1.56) and 1.38 (1.05, 1.84), respectively. An increment of per 1 standard deviation in the α-klotho concentration was associated with lower odds of moderate/severe periodontitis (OR: 0.93; 95% CI: 0.87, 0.99). Linear dose-response relationships were found between α-klotho and the odds of moderate/severe periodontitis (P for non-linearity=0.88) and poor-rated oral health (P for non-linearity=0.66). An L-shaped dose-response relationship was found between levels of α-klotho and the odds of tooth loss (P for non-linearity=0.04).
Conclusions: Serum α-klotho was associated with oral health. Further studies are necessary to clarify the potential mechanisms and demonstrate the predictive ability of klotho in oral diseases.
Introduction
Oral disease has become a serious public health issue worldwide (1, 2). The Global Burden of Disease Study in 2017 has shown that nearly 3.47 billion global people suffered from oral disorders (3), which were more common in socio-economically disadvantaged individuals (4). Oral health, enabling natural eating, speaking, smiling, and socializing, plays a vital role in overall health (5, 6). Deterioration of oral health can lead to various adverse health outcomes, such as cardiovascular diseases (7), cognitive impairment (8), disability (9), and all-cause mortality (10). However, studies have suggested that dental diseases could be prevented and reversed (11, 12). Cohort studies have also suggested that the improvement of oral health might decrease the risk of multiple diseases, including dyslipidemia (13), stroke (14), gastrointestinal cancer (15), new-onset diabetes (16), atrial fibrillation and heart failure (17), and chronic kidney disease (18). Considering the importance of oral health, it is imperative to identify the potential predictive markers and risk factors to improve risk assessment and allow targeted prevention of oral disorders in the early stage.
Klotho protein, a multifunctional protein with antiaging properties, is predominantly expressed in the kidney, parathyroid glands, and choroid plexus of the brain. Secreted klotho is involved in a variety of physiological processes through various mechanisms, such as regulation of serum levels of phosphate and vitamin D, acceleration of oxidative stress (19, 20), etc. Animal models have suggested that mice with lower expression of klotho had shortened lifespan (21), increased inflammatory response, degenerated cognition (22), and delayed skeletal muscle regeneration (23). Epidemiological evidence has suggested the role of low circulating klotho in the risk of various chronic diseases and aging-related traits, including metabolic disorders (19, 24), diminished ovarian reserve (25), and poor physical performance (26, 27). Moreover, klotho was proved to be associated with cerebral small vessel disease (28) and be a biomarker of good functional outcome in patients with acute ischemic stroke (29). These findings have provided insight into the implication of circulating klotho in multiple diseases, that klotho deficiency may not only serve as a potential biomarker but also be a risk factor for developing diseases. Given that oral health could deteriorate with senescence (30, 31), it is crucial to determine the association between serum klotho and oral health, to provide a guide for oral condition prevention. Although abnormal histology and morphology of oral structures were observed in both human (32, 33) and animal specimens (34, 35), it is still unclear whether klotho is associated with oral health in a large population-based study.
Thus, we used data from the National Health and Nutrition Examination Surveys (NHANES) to examine the association between serum α-klotho and oral health and further explore the strength of association in stratified analyses across related characteristics among a nationally representative sample of adults from the United States.
Null hypotheses:
1. There are non-significant associations of low serum α-klotho with moderate/severe periodontitis, poor-rated oral health, and tooth loss.
2. The dose-responses relationships of serum α-klotho with the risk of moderate/severe periodontitis, poor-rated oral health, and tooth loss are linear.
Methods
Sample population
The NHANES is a national program to assess the health and nutritional status of the US sample. A multistage and stratified sampling method was used to identify the participants with good representativeness. Recruited participants were invited to complete standardized questionnaires and undergo a physical examination by laboratory testing after providing written informed consents. Socio-demographic characteristics, physical examinations (including height, weight, blood pressure, clinical periodontal attachment loss, probing pocket depths, and the assessments of remaining tooth), dietary information, and laboratory data were collected. The study protocol was approved by the National Center for Health Statistics Research Ethics Review Board (Protocol #2005-06 and Protocol #2011-17).
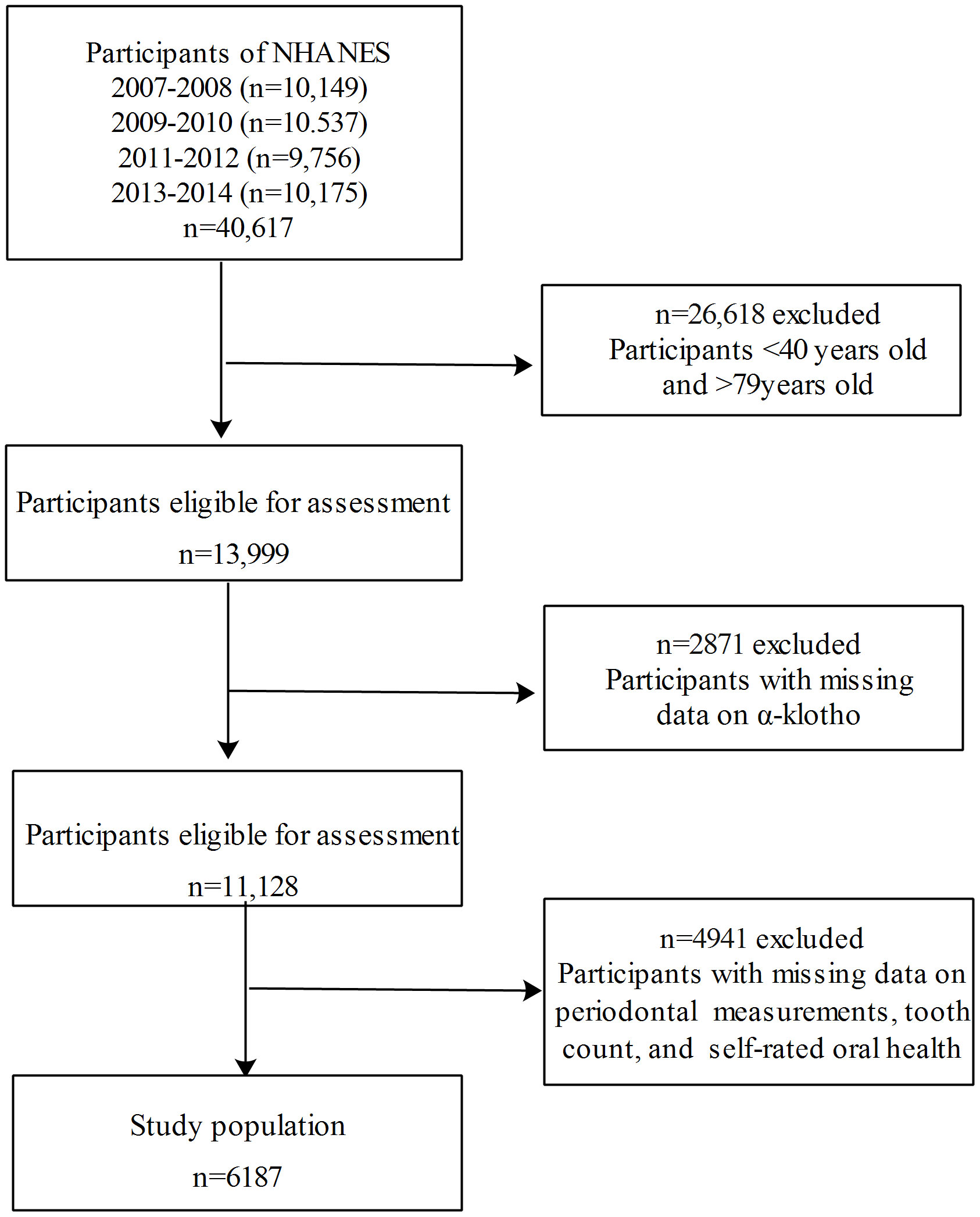
Data were combined across 4 continuous NHANES cycles: 2007-2008, 2009-2010, 2011-2012, 2013-2014. Stepwisely excluded those aged <40 or >79 years (n=26618), those with missing data on α-klotho (n=2871), and those with missing data on oral measures (including periodontal examination, self-rated oral health investigation, and assessment of remaining teeth; n=4941), a total of 6187 participants aged 40-79 years with complete information on α-klotho and oral measures were included in the final analysis. The inclusion and exclusion flowchart of the study population is presented in Figure 1.
Serum α-Klotho concentrations
Blood samples were stored at -80°C and serum α-klotho was measured among participants aged from 40 to 79 years old. Strictly following the manufacturer’s protocol, serum α-klotho was assayed using a solid-phase sandwich enzyme-linked immunosorbent assay (ELISA 27998, IBL International, Japan). This assay is reported to have a sensitivity of 6 pg/mL. All sample analyses were performed twice and the average of the two values was calculated as the final value of serum α-klotho. More details regarding the laboratory methodology of α-klotho concentrations determination can be available on the NHANES website. In our analyses, α-klotho was categorized by tertiles.
Assessment of oral health
Oral measures included periodontal examination, investigation of self-rated oral health, and assessment of remaining teeth. A full-mouth periodontal examination, including clinical attachment loss and probing pocket depths, was carried out at six sites per tooth. The periodontal disease diagnosis and staging were carried out following the Center for Disease Control and Prevention and Prevention-American Academy of Periodontology consensus for epidemiologic studies recommendation (36). Periodontitis was categorized as moderate/severe and non/mild periodontitis. Self-reported oral health was assessed via responses to the single item: “how would you rate the health of your teeth and gums?” The participants chose one of the following responses: “excellent”, “very good”, “good”, “fair”, or “poor”. Self-rated oral health was categorized as good-rated oral health (excellent/very good/good), and poor-rated oral health (fair/poor). The number of teeth was categorized as a 2-level category, and tooth loss was defined as the number of teeth remaining <21 based on the literature review (37).
Covariate information
Covariates concerning social demography, lifestyle, and health status adjusted in models were collected, including sex, age, race, educational level, marital status, family poverty income ratio, smoking status, drinking status, physical activity level, body mass index (BMI), diabetes mellitus, and hypertension. Educational level was categorized into two groups (below high school, high school or above). Marital status was categorized into two groups (married/living with a partner, widowed/divorced/separated/never married). Drinking status was defined as <12 or ≥12 alcohol drinks per year (36). Physical activity was asses by the Global Physical Activity Questionnaire (GPAQ) subdivided as high and low/moderate levels. Diabetes mellitus was defined by self-reported physician diagnosis or glucose measures (fasting blood glucose ≥126 mg/dL or glycated hemoglobin ≥6.5%) or current use of glucose-lowering medications. Hypertension was defined as having clinically diagnosed hypertension and/or systolic blood pressure ≥140 mmHg and/or diastolic blood pressure≥90 mmHg. To evaluate the kidney function of the participants, the estimated glomerular filtration rate (eGFR) was estimated using the Chronic Kidney Disease Epidemiology Collaboration equation (38).
Statistical analyses
The differences in sample characteristics across the tertiles of α-klotho were compared using ANOVA for continuous variables and chi-square tests for categorical variables, respectively. Logistic regression models were used to estimate the odds ratios (ORs) and 95% corresponding confidence intervals (CIs) for oral health parameters. α-Klotho was examined as both a continuous variable and a categorized variable (the highest tertile group was used as the reference group). Model 1 initially adjusted for age and sex. Model 2 adjusted for race, educational level, marital status, family poverty income ratio, smoking status, drinking status, physical activity level, BMI, diabetes mellitus, hypertension, and eGFR, additionally. We also conducted stratified analyses by potential factors, including sex, age (<60 and ≥60 years), BMI (underweight/normal: <24.9 kg/m2 and overweight/obese: ≥24.9 kg/m2), physical activity (high or low/moderate), the status of hypertension (yes or no), and the status of diabetes mellitus (yes or no). In addition, the shape of the dose-response relationships was explored using restricted cubic splines (RCS) with three knots at the 25th, 50th, and 75th percentiles. All statistical analyses were performed using Stata v14.0.
Results
Sample characteristics
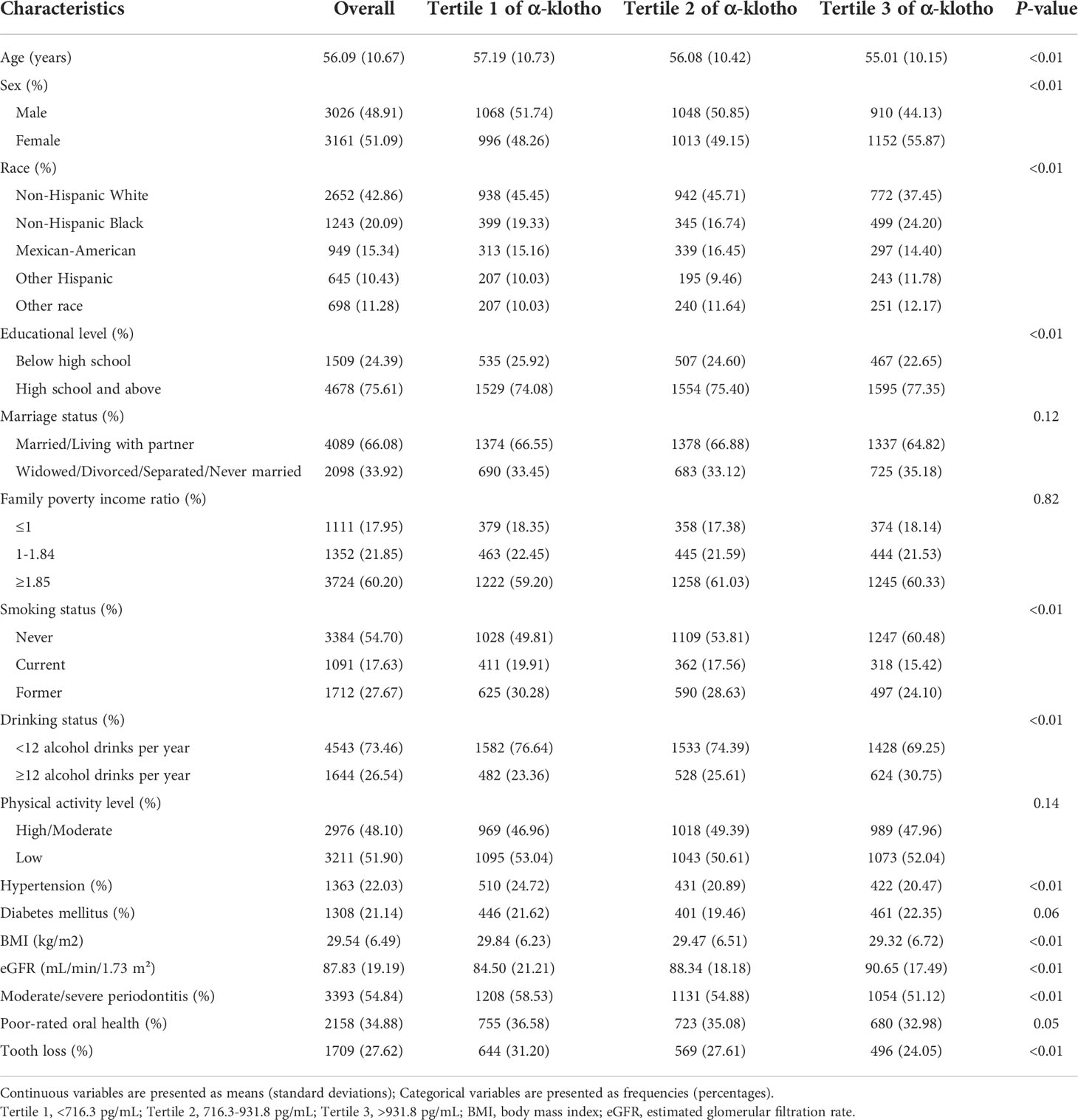
The characteristics of the study participants are shown in Table 1. Of 6178 participants, the prevalence of moderate/severe periodontitis, poor-rated oral health, and tooth loss were 54.84%, 34.88%, and 27.62%, respectively, and the median serum α-klotho level was 815.2 pg/mL (25th-75th percentiles: 665.9-1010.7 pg/mL). Participants in the lowest tertile group (<716.3 pg/mL) were older, more likely to be male and had lower eGFR, higher BMI and higher prevalence of hypertension. The prevalence of moderate/severe periodontitis (58.53%), poor-rated oral health (36.58%), and tooth loss (31.20%) were the highest in the lowest tertile group. Figure 2 shows the serum α-klotho level by oral health status. α-Klotho levels were significantly lower in participants with poorer oral health, with the medians being 800.5, 804.7, and 790.4 pg/mL for moderate/severe periodontitis, poor-rated oral health, and tooth loss, respectively (all P<0.01).
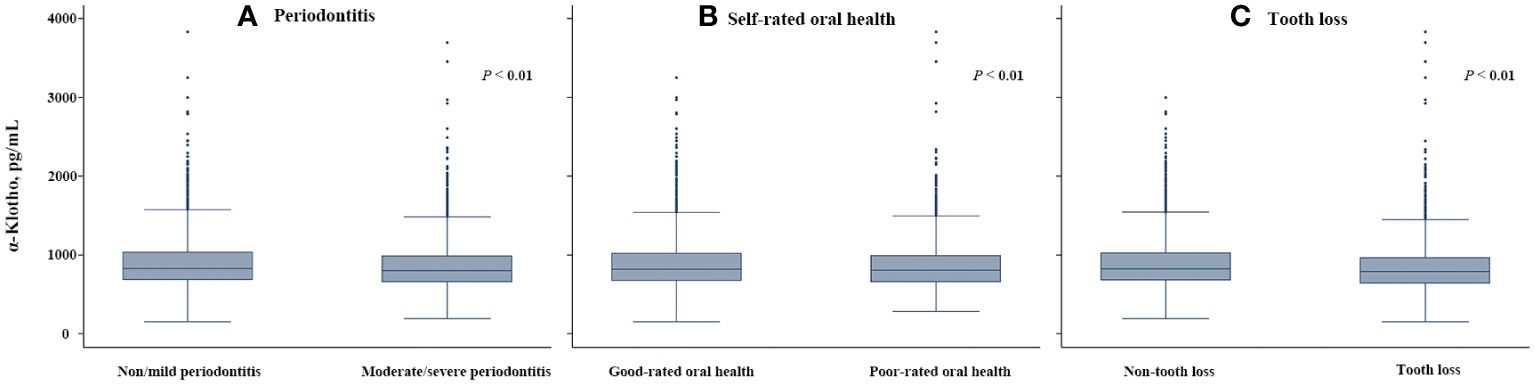
Figure 2 Serum α-klotho levels by oral health status compared by Mann-Whitney U tests: (A) Moderate/severe periodontitis, (B) Poor-rated oral health, and (C) Tooth loss.
Associations of α-klotho with oral health parameters
The associations of α-klotho with oral health parameters are presented in Table 2. Compared with the highest tertile, the lowest tertile of α-klotho was associated with moderate/severe periodontitis, poor-rated oral health, and tooth loss, with OR (95% CI) being 1.21 (1.01, 1.48), 1.26 (1.01, 1.56) and 1.38 (1.05, 1.84), respectively, after adjusting for potential confounders. The trend test also showed that with the decrease of α-klotho, the odds of tooth loss increased (P for trend =0.01). An increment of per 1 standard deviation in α-klotho was associated with lower odds of moderate/severe periodontitis (OR: 0.93; 95% CI: 0.87, 0.99).
Dose-response relationship of serum α-klotho level with oral health
The dose-response relationship of serum α-klotho level with oral health is presented in Figure 3. Linear dose-response relationships were found between serum α-klotho and the odds of moderate/severe periodontitis (P for non-linearity =0.88) and poor-rated oral health (P for non-linearity =0.66). An L-shaped dose-response relationship was found between α-klotho and the odds of tooth loss (P for non-linearity =0.04), suggesting that under the highest tertile of α-klotho concentration, the odds of tooth loss increase with the decline of the serum α-klotho concentration.
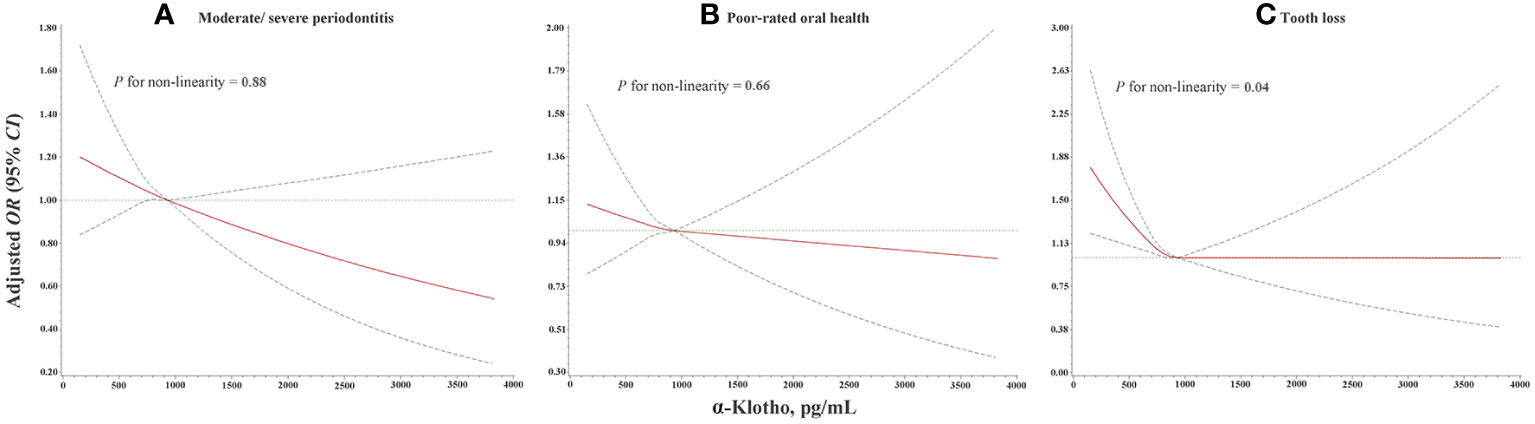
Figure 3 Dose-response relationship of α-klotho with (A) moderate/severe periodontitis, (B) poor-rated oral health, and (C) tooth loss from RCS analysis with three knots at the 25th, 50th, and 75th percentiles with adjustment for age, sex, race, educational level, marital status, family poverty income ratio, smoking status, drinking status, physical activity level, BMI, diabetes mellitus, hypertension, and eGFR. OR, odds ratio; 95% CI, 95% confidence interval; RCS, restricted cubic splines; BMI, body mass index; eGFR, estimated glomerular filtration rate.
Stratified analysis of the association between α-klotho and oral health
The stratified analysis of the association between α-klotho and oral health is shown in Table 3. Compared with the highest tertile, the lowest tertile of α-klotho was associated with poor-rated oral health and tooth loss in females (OR: 1.46, 95% CI: 1.10, 1.93 for poor-rated oral health; OR: 1.57, 95% CI: 1.16, 2.13 for tooth loss), adults with low physical activity (OR: 1.53, 95% CI: 1.16, 2.02 for poor-rated oral health; OR: 1.49, 95% CI: 1.05, 2.10 for tooth loss), and adults who were overweight or obese (OR: 1.38, 95% CI: 1.10, 1.72 for poor-rated oral health; OR: 1.55, 95% CI: 1.10, 2.16 for tooth loss).
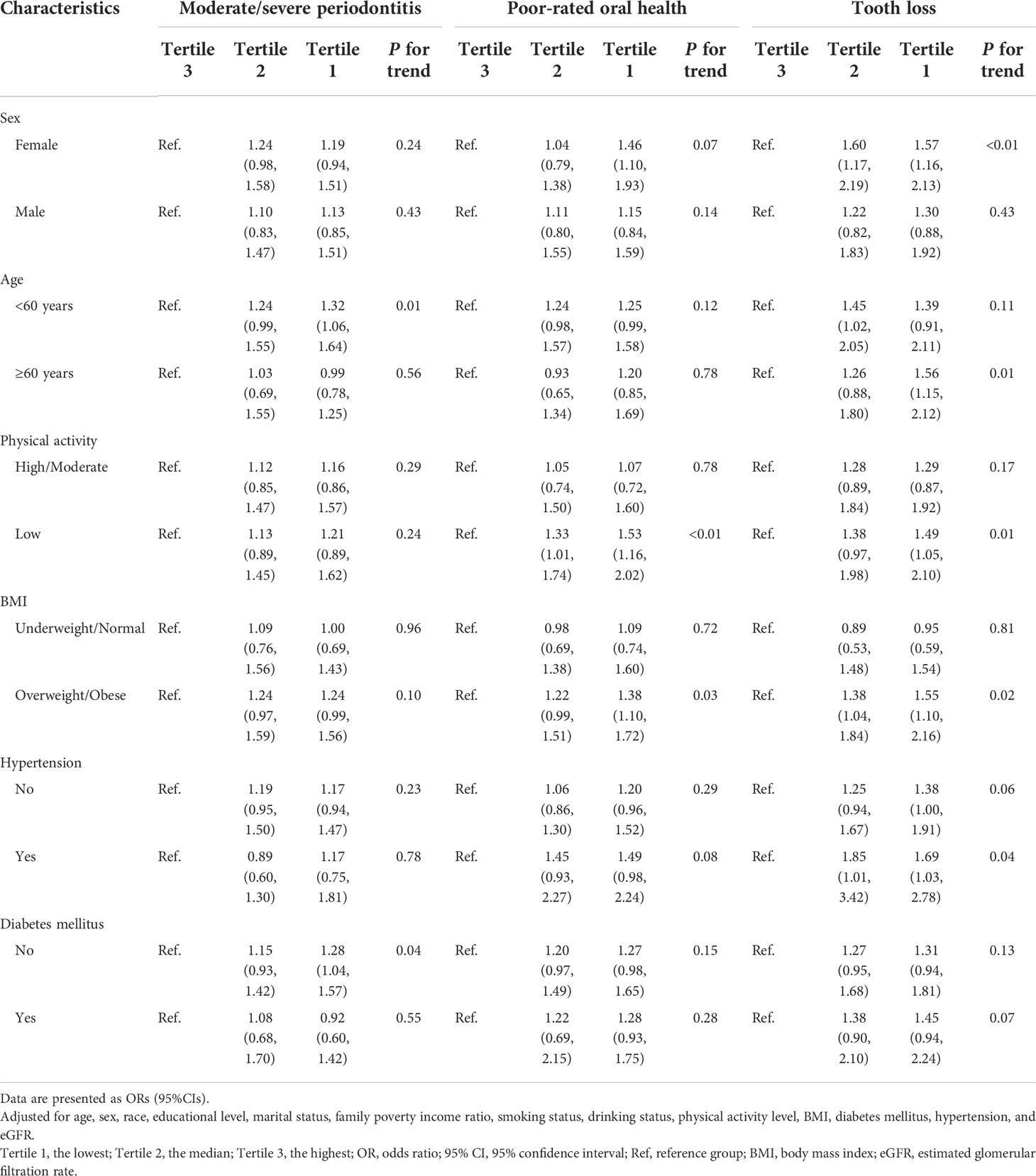
Table 3 Weighted ORs (95%CIs) of the association between α-klotho and oral health in stratified analyses.
Discussion
In the present study, we explored the association between α-klotho and oral health among a nationally representative sample of U.S. adults. The levels of α-klotho were significantly lower in participants with poorer oral health than those in their counterparts. Meanwhile, low α-klotho was associated with increased odds of moderate/severe periodontitis, poor-rated oral health, and tooth loss. And, the associations between α-klotho and oral health parameters were presented in dose-response manners. Furthermore, stratified analyses demonstrated the association of α-klotho with oral health across diverse subgroups.
The observed association between klotho and oral health in our study was consistent with previous studies. A cross-sectional study conducted in China showed that compared with those in the healthy counterparts, the levels of klotho in the gingival crevicular fluid and gingival tissues of chronic periodontitis patients were significantly lower (32). Another cross-sectional study conducted in the US demonstrated that increased tooth pulp density was observed in a hyperphosphatemic familial tumoral calcinosis patient caused by mutations in klotho (33). Moreover, a meta-analysis including 18 studies conducted in 2018 showed that lower klotho was associated with oral malignancy (OR: 0.034; 95% CI: 0.002, 0.630), indicating its function as a biomarker in oral cancer (39). Similar findings were also found in animal models. A study conducted in Japan showed that compared with the phenotype of wild-type, abnormal histology of the periodontal tissue caused by the disrupted signal of αklotho/fibroblast growth factor (FGF) 23 pathway, including narrowed periodontal spaces, amorphous structures in the periodontal ligament, and irregular distribution of periodontal fibers, was observed in klotho-deficient mice (34). Likewise, another study demonstrated that klotho-deficient mice presented abnormal distribution and morphology of odontoblasts and dentin matrix of the mandibular incisors, meanwhile, the apoptosis reaction was detected in the embedded odontoblast-like cells and pulpal cells only among klotho-deficient mice, compared with wild-type counterparts (35). Our study extended existing evidence by demonstrating the association in a sample of large population. Notably, an L-shaped relationship was found between α-klotho level and the odds of tooth loss, suggesting that the odds of tooth loss increase with the decline of the serum α-klotho concentration. The observed associations suggested that serum α-klotho levels may be a potential biomarker of oral diseases and deficiency in α-klotho may play a part in their progression, which can expand our understanding of the potential roles of klotho in oral health and provide a novel insight into preventing the development of oral diseases.
Several possible mechanisms may explain the association between klotho and oral health. First, klotho could inhibit oxidative stress and down-regulate cell apoptosis in human periodontal ligament stem cells (hPLSCs) (32, 40), which were considered a potential mechanism underlying oral conditions (41). Previous studies have shown that under a simulated oxidative stress circumstance by H2O2, the level of reactive oxygen species (ROS) and malondialdehyde were both reduced, meanwhile, the activity of antioxidant enzymes (including superoxide dismutase, glutathione peroxidase, and catalase) was restored, by the treatment of klotho protein in hPLSC. Moreover, the expressions of apoptotic-related proteins (including BAX and Caspase-3) in hPLSCs were also reduced by the addiction of klotho (32, 40). Thus, a deficiency of klotho may attribute to the accumulation of ROS and the acceleration of cell apoptosis, leading to the deterioration of oral health eventually. Second, lack of klotho may induce the abnormal synthesis of alkaline phosphatase (ALP) and bone matrix proteins (including dentin matrix protein-1 [DMP-1] and osteopontin [OPN]) in tooth (34, 35), which are essential for dentin formation and mineralization (42). Animal studies have shown that a weak reaction for ALP immune-staining and irregular distributions of DMP-1 and OPN were observed in oral tissues of klotho-deficient mice (34, 35). Third, α-klotho regulates the metabolism of main mineral components (including calcium [Ca] and phosphorus [P]) of tooth (19). Lower concentrations of Ca and P in incisors of klotho-deficient mice were observed in a previous study (35). Therefore, the reduction of Ca and P in dental tissue by the deficiency of klotho may result in oral conditions. Besides anti-oxidative stress, dentin proteins synthesis, and mineral homeostasis, klotho may also take part in substance metabolism (glucose, fatty acid, and bile acid), responses to feeding and fasting, energy expenditure, hypothalamic–pituitary–adrenal axis activity, and sympathetic activity, underlining its potential endocrine role in various human disorders, including cancers (liver, colon, breast and prostate), bile acid diarrhea, chronic kidney disease, type 2 diabetes mellitus, obesity, nonalcoholic fatty liver disease, cardiovascular disease, anorexia nervosa and tumor-induced osteomalacia (19).
In stratified analyses, we found that low α-klotho was associated with poor-rated oral health and tooth loss only in females. This might be explained by the effect of sex hormone change in menopause among females. A longitudinal study of 1341 postmenopausal women with a mean follow-up of 5.1-year showed that 28.7% of participants lost at least one tooth during follow-up (43). Besides, it is reported that bone loss was at an accelerated rate of 2% per year during menopause and lasted for six years thereafter (44). Of note, osteoporosis was considered a sign of periodontal diseases in postmenopausal women (45). Moreover, this finding was only pronounced in adults with low or moderate physical activity and those who were overweight or obese, compared with their counterparts. The preference for health-compromising behaviors in those two subgroups may explain part of the observed discrepancies, such as a diet rich in sugars (46), consumption of beverages (47, 48), use of tobacco (49), poor oral hygiene (47, 50), suggesting that oral health education programs aimed at establishing favorable health-related behaviors should be implemented among these two groups of people. We also found that the association between low α-klotho and tooth loss was stronger in those with hypertension. One potential reason may be that hypertension is a risk factor for tooth loss (51), indicating a cumulative effect of hypertension and low α-klotho on tooth loss.
Our study examined the association between α-klotho and oral health based on a nationally representative sample, which facilitates the generalization of the findings. Second, the dose-response relationships in our findings help us to evaluate how the odds of oral conditions change with the continuous decrease of α-klotho. However, several limitations of our study should be noted. First, the cross-sectional design does not allow us to infer causation between α-klotho and oral diseases or conditions and to examine whether the klotho level could be corrected to improve oral health. Second, confounding factors, including dental visits, and history of head and neck radiotherapy were not assessed in the present study,which might have influenced our findings. Third, due to the constraints of the NHANES database, the parameters of oral health were not defined comprehensively: 1. Periodontitis was defined by clinical attachment loss and probing pocket depths without consideration of saliva or gingival crevicular fluid in our study. 2. Tooth loss was simply defined by the number of remaining teeth, overlooking the cause of the tooth loss in the present study. Fourth, as shown in Supplementary Table 1, Some characteristics between included and excluded participants were different, which might bring about bias in estimating the associations. Thus, prospective studies and randomized controlled trials incorporating a more extensive investigation of oral health markers and other confounding factors are needed to fully clarify the causal relationship.
Conclusions
In conclusion, we found that serum α-klotho is associated with oral health in US adults. And the association presents in a dose-response manner. It may be of significant public health and clinical importance that provide new insights into the implication of klotho in oral diseases. Further studies are necessary to clarify the potential mechanisms underlying the association and demonstrate the predictive ability of α-klotho in oral diseases.
Data availability statement
The datasets presented in this article are not readily available because of privacy or ethical restrictions. The data used and analyzed during the present study are available from the corresponding author on reasonable request.
Ethics statement
The study protocol was approved by the National Center for Health Statistics Research Ethics Review Board. The patients/participants provided their written informed consent to participate in this study.
Author contributions
X-LY and G-QC conceived and designed the study. G-QC analyzed the data and wrote the manuscript. YL, J-FW and YD provided comments and technical advice. All authors revised the manuscript critically for important intellectual content, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This work was supported by Shandong Provincial Key Research and Development Program (grant no. 2019GSF108196), Center of China–US Sports Economics and Health Engineering of Shandong (grant no. SDCA20191013), Academic Promotion Programme of Shandong First Medical University (grant no. 2019QL013).
Acknowledgments
We gratefully acknowledge all of the study participants and staff for their assistance during the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.970575/full#supplementary-material
References
1. Dye BA. The global burden of oral disease: Research and public health significance. J Dent Res (2017) 96(4):361–63. doi: 10.1177/0022034517693567
2. Ahmadi S, Klingelhofer D, Erbe C, Holzgreve F, Groneberg DA, Ohlendorf D. Oral health: Global research performance under changing regional health burdens. Int J Environ Res Public Health (2021) 18(11):5743–55. doi: 10.3390/ijerph18115743
3. James SL, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: A systematic analysis for the global burden of disease study 2017. Lancet (2018) 392(10159):1789–858. doi: 10.1016/s0140-6736(18)32279-7
4. Cianetti S, Valenti C, Orso M, Lomurno G, Nardone M, Lomurno AP, et al. Systematic review of the literature on dental caries and periodontal disease in socio-economically disadvantaged individuals. Int J Environ Res Public Health (2021) 18(23):12360–81. doi: 10.3390/ijerph182312360
5. Joseph BK, Kullman L, Sharma PN. The oral-systemic disease connection: a retrospective study. Clin Oral Investig (2016) 20(8):2267–73. doi: 10.1007/s00784-016-1725-3
6. Dörfer C, Benz C, Aida J, Campard G. The relationship of oral health with general health and NCDs: a brief review. Int Dent J (2017) 67 Suppl 2:14–8. doi: 10.1111/idj.12360
7. Gianos E, Jackson EA, Tejpal A, Aspry K, O’Keefe J, Aggarwal M, et al. Oral health and atherosclerotic cardiovascular disease: A review. Am J Prev Cardiol (2021) 7:100179. doi: 10.1016/j.ajpc.2021.100179
8. Naorungroj S, Schoenbach VJ, Wruck L, Mosley TH, Gottesman RF, Alonso A, et al. Tooth loss, periodontal disease, and cognitive decline in the atherosclerosis risk in communities (ARIC) study. Community Dent Oral Epidemiol (2015) 43(1):47–57. doi: 10.1111/cdoe.12128
9. Kotronia E, Wannamethee SG, Papacosta AO, Whincup PH, Lennon LT, Visser M, et al. Oral health, disability and physical function: Results from studies of older people in the united kingdom and united states of America. J Am Med Dir Assoc (2019) 20(12):1654. doi: 10.1016/j.jamda.2019.06.010
10. Li A, Chen Y, Visser A, Marks LAM, Tjakkes GE. Combined association of cognitive impairment and poor oral health on mortality risk in older adults: Results from the NHANES with 15 years of follow-up. J Periodontol (2021) 93(6):888–900. doi: 10.1002/jper.21-0292
11. Axelsson P, Lindhe J, Nyström B. On the prevention of caries and periodontal disease. results of a 15-year longitudinal study in adults. J Clin Periodontol (1991) 18(3):182–9. doi: 10.1111/j.1600-051x.1991.tb01131.x
12. Axelsson P, Nystrom B, Lindhe J. The long-term effect of a plaque control program on tooth mortality, caries and periodontal disease in adults. results after 30 years of maintenance. J Clin Periodontol (2004) 31(9):749–57. doi: 10.1111/j.1600-051X.2004.00563.x
13. Song TJ, Kim JW, Kim J. Oral health and changes in lipid profile: A nationwide cohort study. J Clin Periodontol (2020) 47(12):1437–45. doi: 10.1111/jcpe.13373
14. Chang Y, Woo HG, Lee JS, Song TJ. Better oral hygiene is associated with lower risk of stroke. J Periodontol (2021) 92(1):87–94. doi: 10.1002/jper.20-0053
15. Lee K, Lee JS, Kim J, Lee H, Chang Y, Woo HG, et al. Oral health and gastrointestinal cancer: A nationwide cohort study. J Clin Periodontol (2020) 47(7):796–808. doi: 10.1111/jcpe.13304
16. Chang Y, Lee JS, Lee KJ, Woo HG, Song TJ. Improved oral hygiene is associated with decreased risk of new-onset diabetes: a nationwide population-based cohort study. Diabetologia (2020) 63(5):924–33. doi: 10.1007/s00125-020-05112-9
17. Chang Y, Woo HG, Park J, Lee JS, Song TJ. Improved oral hygiene care is associated with decreased risk of occurrence for atrial fibrillation and heart failure: A nationwide population-based cohort study. Eur J Prev Cardiol (2020) 27(17):1835–45. doi: 10.1177/2047487319886018
18. Chang Y, Lee JS, Woo HG, Ryu DR, Kim JW, Song TJ. Improved oral hygiene care and chronic kidney disease occurrence: A nationwide population-based retrospective cohort study. Med (Baltimore) (2021) 100(47):e27845. doi: 10.1097/md.0000000000027845
19. Kuro OM. The klotho proteins in health and disease. Nat Rev Nephrol (2019) 15(1):27–44. doi: 10.1038/s41581-018-0078-3
20. Typiak M, Piwkowska A. Antiinflammatory actions of klotho: Implications for therapy of diabetic nephropathy. Int J Mol Sci (2021) 22(2):956–70. doi: 10.3390/ijms22020956
21. Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, et al. Suppression of aging in mice by the hormone klotho. Science (2005) 309(5742):1829–33. doi: 10.1126/science.1112766
22. Kundu P, Zimmerman B, Quinn JF, Kaye J, Mattek N, Westaway SK, et al. Serum levels of α-klotho are correlated with cerebrospinal fluid levels and predict measures of cognitive function. J Alzheimers Dis (2022) 86(3):1471–81. doi: 10.3233/jad-215719
23. Sahu A, Mamiya H, Shinde SN, Cheikhi A, Winter LL, Vo NV, et al. Age-related declines in α-klotho drive progenitor cell mitochondrial dysfunction and impaired muscle regeneration. Nat Commun (2018) 9(1):4859. doi: 10.1038/s41467-018-07253-3
24. Landry T, Li P, Shookster D, Jiang Z, Li H, Laing BT, et al. Centrally circulating alpha-klotho inversely correlates with human obesity and modulates arcuate cell populations in mice. Mol Metab (2021) 44:101136. doi: 10.1016/j.molmet.2020.101136
25. Xu X, Hao Y, Zhong Q, Hang J, Zhao Y, Qiao J. Low KLOTHO level related to aging is associated with diminished ovarian reserve. Fertil Steril (2020) 114(6):1250–55. doi: 10.1016/j.fertnstert.2020.06.035
26. Semba RD, Cappola AR, Sun K, Bandinelli S, Dalal M, Crasto C, et al. Relationship of low plasma klotho with poor grip strength in older community-dwelling adults: the InCHIANTI study. Eur J Appl Physiol (2012) 112(4):1215–20. doi: 10.1007/s00421-011-2072-3
27. Semba RD, Ferrucci L, Sun K, Simonsick E, Turner R, Miljkovic I, et al. Low plasma klotho concentrations and decline of knee strength in older adults. J Gerontol A Biol Sci Med Sci (2016) 71(1):103–8. doi: 10.1093/gerona/glv077
28. Woo HG, Chang Y, Ryu DR, Song TJ. Plasma klotho concentration is associated with the presence, burden and progression of cerebral small vessel disease in patients with acute ischaemic stroke. PloS One (2019) 14(8):e0220796. doi: 10.1371/journal.pone.0220796
29. Lee JB, Woo HG, Chang Y, Jin YM, Jo I, Kim J, et al. Plasma klotho concentrations predict functional outcome at three months after acute ischemic stroke patients. Ann Med (2019) 51(3-4):262–69. doi: 10.1080/07853890.2019.1617434
30. Haresaku S, Nakashima F, Hara Y, Kuroki M, Aoki H, Kubota K, et al. Associations of oral health-related quality of life with age, oral status, and oral function among psychiatric inpatients in Japan: a cross-sectional study. BMC Oral Health (2020) 20(1):361. doi: 10.1186/s12903-020-01355-5
31. Raphael C. Oral health and aging. Am J Public Health (2017) 107(S1):S44–s45. doi: 10.2105/ajph.2017.303835
32. Zhu L, Xie H, Liu Q, Ma F, Wu H. Klotho inhibits H2 O2 -induced oxidative stress and apoptosis in periodontal ligament stem cells by regulating UCP2 expression. Clin Exp Pharmacol Physiol (2021) 48(10):1412–20. doi: 10.1111/1440-1681.13547
33. Lee AE, Chu EY, Gardner PJ, Duverger O, Saikali A, Wang SK, et al. A cross-sectional cohort study of the effects of FGF23 deficiency and hyperphosphatemia on dental structures in hyperphosphatemic familial tumoral calcinosis. JBMR Plus (2021) 5(5):e10470. doi: 10.1002/jbm4.10470
34. Hikone K, Hasegawa T, Tsuchiya E, Hongo H, Sasaki M, Yamamoto T, et al. Histochemical examination on periodontal tissues of klotho-deficient mice fed with phosphate-insufficient diet. J Histochem Cytochem (2017) 65(4):207–21. doi: 10.1369/0022155416689670
35. Suzuki H, Amizuka N, Oda K, Noda M, Ohshima H, Maeda T. Involvement of the klotho protein in dentin formation and mineralization. Anat Rec (Hoboken) (2008) 291(2):183–90. doi: 10.1002/ar.20630
36. Yin S, Wang J, Bai Y, Yang Z, Cui J, Xiao Y, et al. Association between healthy eating index-2015 and kidney stones in American adults: A cross-sectional analysis of NHANES 2007-2018. Front Nutr (2022) 9:820190. doi: 10.3389/fnut.2022.820190
37. Kang J, Wu B, Bunce D, Ide M, Pavitt S, Wu J. Cognitive function and oral health among ageing adults. Community Dent Oral Epidemiol (2019) 47(3):259–66. doi: 10.1111/cdoe.12452
38. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med (2009) 150(9):604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
39. Mao S, Wang X, Wu L, Zang D, Shi W. Association between klotho expression and malignancies risk and progression: A meta-analysis. Clin Chim Acta (2018) 484:14–20. doi: 10.1016/j.cca.2018.05.033
40. Chen H, Huang X, Fu C, Wu X, Peng Y, Lin X, et al. Recombinant klotho protects human periodontal ligament stem cells by regulating mitochondrial function and the antioxidant system during H2O2-induced oxidative stress. Oxid Med Cell Longev (2019) 2019:9261565. doi: 10.1155/2019/9261565
41. Chen M, Cai W, Zhao S, Shi L, Chen Y, Li X, et al. Oxidative stress-related biomarkers in saliva and gingival crevicular fluid associated with chronic periodontitis: A systematic review and meta-analysis. J Clin Periodontol (2019) 46(6):608–22. doi: 10.1111/jcpe.13112
42. Qin C, D’Souza R, Feng JQ. Dentin matrix protein 1 (DMP1): new and important roles for biomineralization and phosphate homeostasis. J Dent Res (2007) 86(12):1134–41. doi: 10.1177/154405910708601202
43. Bole C, Wactawski-Wende J, Hovey KM, Genco RJ, Hausmann E. Clinical and community risk models of incident tooth loss in postmenopausal women from the buffalo osteo perio study. Community Dent Oral Epidemiol (2010) 38(6):487–97. doi: 10.1111/j.1600-0528.2010.00555.x
44. Dobbs MB, Buckwalter J, Saltzman C. Osteoporosis: the increasing role of the orthopaedist. Iowa Orthop J (1999) 19:43–52.
45. Richa RY, Puranik MP, Shrivastava A. Association between osteoporosis and periodontal disease among postmenopausal Indian women. J Investig Clin Dent (2017) 8(3):e12223. doi: 10.1111/jicd.12223
46. da Silva Gasparotto G, Pereira da Silva M, Miranda Medeiros Cruz R, de Campos W. Overweight and physical activity practice associated with eating behavior of Brazilian college students. Nutr Hosp (2015) 32(2):616–21. doi: 10.3305/nh.2015.32.2.9159
47. Nihtila A, West N, Lussi A, Bouchard P, Ottolenghi L, Senekola E, et al. Oral health behavior and lifestyle factors among overweight and non-overweight young adults in Europe: A cross-sectional questionnaire study. Healthcare (2016) 4(2):21–30. doi: 10.3390/healthcare4020021
48. Nissinen K, Mikkila V, Mannisto S, Lahti-Koski M, Rasanen L, Viikari J, et al. Sweets and sugar-sweetened soft drink intake in childhood in relation to adult BMI and overweight. the cardiovascular risk in young finns study. Public Health Nutr (2009) 12(11):2018–26. doi: 10.1017/S1368980009005849
49. Großschädl F, Titze S, Burkert N, Stronegger WJ. Moderate- and vigorous-intensity exercise behaviour according to the transtheoretical model: associations with smoking and BMI among Austrian adults. Wiener klinische Wochenschrift (2013) 125(9-10):270–78. doi: 10.1007/s00508-013-0361-z
50. Virtanen JI, Muikku T, Simila T, Cinar AB, Pohjola V. Physical activity, BMI and oral health behaviour among adolescents: Finnish school health promotion study. Eur J Public Health (2019) 29(2):296–302. doi: 10.1093/eurpub/cky193
Keywords: klotho, oral health, periodontitis, tooth loss, dose-response relationship
Citation: Chen G-Q, Duan Y, Wang J-F, Lian Y and Yin X-L (2022) Serum α-Klotho associated with oral health among a nationally representative sample of US adults. Front. Endocrinol. 13:970575. doi: 10.3389/fendo.2022.970575
Received: 16 June 2022; Accepted: 30 August 2022;
Published: 20 September 2022.
Edited by:
Rizwan Ahmed, Johns Hopkins University, United StatesReviewed by:
Salman Shahid, University of Maryland, United StatesYi Zhu, Mayo Clinic, United States
Tae-Jin Song, Ewha Womans University, South Korea
Jie Lee, MacKay Memorial Hospital, Taiwan
Copyright © 2022 Chen, Duan, Wang, Lian and Yin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiu-Li Yin, yinxiuli001@126.com
 Guo-Qiang Chen
Guo-Qiang Chen Yao Duan2
Yao Duan2 Ying Lian
Ying Lian