- Section of Investigative Medicine, Department of Metabolism, Digestion and Reproduction, Imperial College School of Medicine, Imperial College London, London, United Kingdom
Feedback from oestradiol (E2) plays a critical role in the regulation of major events in the physiological menstrual cycle including the release of gonadotrophins to stimulate follicular growth, and the mid-cycle luteinising hormone (LH) surge that leads to ovulation. E2 predominantly exerts its action via oestrogen receptor-alpha (ERα), however, as gonadotrophin releasing hormone (GnRH) neurons lack ERα, E2-feedback is posited to be indirectly mediated via upstream neurons. Kisspeptin (KP) is a neuropeptide expressed in hypothalamic KP-neurons that control GnRH secretion and plays a key role in the central mechanism regulating the hypothalamic-pituitary-gonadal (HPG) axis. In the rodent arcuate (ARC) nucleus, KP is co-expressed with Neurokinin B and Dynorphin; and thus, these neurons are termed ‘Kisspeptin-Neurokinin B-Dynorphin’ (KNDy) neurons. ARC KP-neurons function as the ‘GnRH pulse generator’ to regulate GnRH pulsatility, as well as mediating negative feedback from E2. A second KP neuronal population is present in the rostral periventricular area of the third ventricle (RP3V), which includes anteroventral periventricular (AVPV) nucleus and preoptic area neurons. These RP3V KP-neurons mediate positive feedback to induce the mid-cycle luteinising hormone (LH) surge and subsequent ovulation. Here, we describe the role of KP-neurons in these two regions in mediating this differential feedback from oestrogens. We conclude by considering reproductive diseases for which exploitation of these mechanisms could yield future therapies.
Introduction
Major events in the physiological menstrual cycle including follicular development and ovulation are tightly regulated by intricate negative and positive feedback mechanisms in response to sex-steroids that underpin the hypothalamic-pituitary-gonadal (HPG) axis (1). Pulsatile secretion of hypothalamic gonadotrophin releasing hormone (GnRH) stimulates gonadotrophin secretion from the anterior pituitary gland, and subsequent folliculogenesis and oestradiol (E2) secretion from the ovaries (1). During follicular development, pulsatile GnRH secretion is modulated by negative feedback from circulating E2 (1). In contrast, at the preovulatory stage, high E2 concentrations exert positive feedback to result in the mid-cycle LH surge and ovulation (1). Both negative and positive feedback from E2 are mediated via oestrogen receptor-alpha (ERα) (2). Formerly, given the lack of ERα on GnRH neurons, the mechanism by which E2 exerts its feedback on GnRH neurons was unclear, but consistent with E2-feedback being mediated indirectly via upstream neurons (2). A putative mediator of this E2-feedback are hypothalamic neurons expressing the neuropeptide kisspeptin (KP).
KP is a key regulator of hypothalamic GnRH secretion and the HPG axis (3). Inactivating variants of the gene encoding KP (KISS1) or its receptor KISS1R (KISS1R) result in congenital hypogonadotrophic hypogonadism and a failure to proceed through puberty in humans and murine models (3–5). KP-neurons are located in two discrete hypothalamic neuronal populations in rodents; the arcuate nucleus (ARC) (which is equivalent to the infundibular nucleus in humans) and the rostral periventricular area of the third ventricle (RP3V) comprising of KP-neurons in the anteroventral periventricular (AVPV) nucleus and preoptic area (6). In particular, KP-neurons in the ARC (ARC KP-neurons) co-express Neurokinin B (NKB) and Dynorphin, and hence are termed Kisspeptin-Neurokinin B-Dynorphin (KNDy) neurons (7).
In this review, we explore evidence to support ARC KP-neurons as mediators of E2-induced negative feedback to regulate GnRH pulsatility, and RP3V KP-neurons as mediators of positive feedback in response to higher E2 levels to induce the mid-cycle LH surge/ovulation. The input of neuropeptides such as glutamate, as well as metabolic signals such as leptin are also considered. Finally, reproductive diseases that result in perturbation of LH secretion are considered, for which exploitation of these mechanisms may yield future therapies.
Kisspeptin neurons and feedback by E2
The activity of kisspeptin neurons varies throughout the menstrual cycle and is tightly regulated by oestradiol (E2) (1). During the follicular and majority of the luteal phases of the menstrual cycle, the presence of low E2 results in inhibition of ARC KP-neurons (negative feedback) and thus maintains pulsatile secretion of GnRH (1, 2). In the mid-luteal phase, high E2 stimulates RP3V KP-neurons (positive feedback) which results in the GnRH/LH surge responsible for triggering ovulation (1, 2). This differential feedback response of KP-neurons by low and high E2 levels has been observed in multiple species. For instance, studies in rodents have demonstrated increased expression of KISS1 mRNA levels in both the ARC during metestrus and dioestrus (low E2 state) and the RP3V during proestrus (high E2 state) (8). Furthermore, postmenopausal women (low E2 state) have increased KISS1 mRNA levels in KP-neurons of the infundibular nucleus (equivalent to ARC in rodents) (9).
The mechanisms underlying the divergent feedback of E2 on KP-neurons are complex. ERα (encoded for by Esr1) is responsible for mediating both negative and positive feedback from E2 (10, 11). ERα can signal either by translocation to the nucleus and recruitment of cofactors to oestrogen response element (ERE) (classical pathway), or by recruitment of other transcription factors not via the ERE (non-classical pathway) (10, 11). Notably, E2-induced positive feedback occurs via the classical pathway, whereas negative feedback is mediated via the non-classical pathway (10, 11). In AVPV KP-neurons in the RP3V, E2 increases recruitment of ERα to the Kiss1 promoter region which results in enhanced histone acetylation (12). In turn, histone acetylation induces chromatin loop formation between the Kiss1 promoter and Kiss1 gene enhancer, leading to an increase in AVPV-specific Kiss1 gene expression (12). The opposite effect is observed in ARC KP-neurons whereby the Kiss1 promoter region undergoes histone deacetylation and subsequent reduced gene expression following E2 (12).
A recent murine study shed light on how KP-neurons respond divergently to high and low E2 concentrations, by revealing differential RNA transcriptional responses between KP-neurons in the ARC and the RP3V (13). They identified 1583 oestrogen-responsive genes (majority suppressed) within the ARC, and 222 genes (majority upregulated) in the RP3V, thus showing that there are more oestrogen-responsive genes in the ARC than RP3V (13). Interestingly, whilst Esr1 (which encodes ERα) expression was increased in both RP3V and ARC KP-neurons, no differences in Esr2 (which encodes ERβ) or Gper1 (which encodes G-protein coupled oestrogen receptor) were observed (13). Furthermore, ERα interacted with 8 of 70 E2-dependent transcription factors within the ARC, but 0 of 10 E2-dependent transcription factors within RP3V (13). Despite disparate E2-regulation, RP3V and ARC KP-neurons displayed 96 overlapping genes, with changes in 62 genes being analogous (e.g. Ghsr, Pgr, Npr2, Gad2, Calm1, Pcp4), and 34 genes being regulated in a contrasting manner (e.g. Kiss1, Vgf, Chrna7, Tmem35a) (13). Overall, these data indicate that the effects of oestrogens are predominantly mediated by ERα in both the ARC and RP3V neurons, and that there are more oestrogen responsive genes in the ARC (mediating negative feedback) than in the RP3V (mediating positive feedback) (13).
Whilst ERα is the major mediator of E2 feedback on kisspeptin neurons, ERβ has been shown to potentiate E2 positive feedback in the RP3V. For instance, OVX rats have a two-fold increase in the Esr1/Esr2 ratio in the ARC compared to the RP3V, irrespective of E2 replacement, consistent with greater presence of ERβ in the RP3V (14). ERβ regulates E2-induced positive feedback by increasing transcriptional activity of ERα and enhancing the responsiveness of RP3V neurons to high concentrations of E2 (14). In the presence of low E2 levels, ERβ has an inhibitory effect on ERα in the RP3V and thus it could have an indirect role in mediating E2 negative feedback (14) (Figure 1).
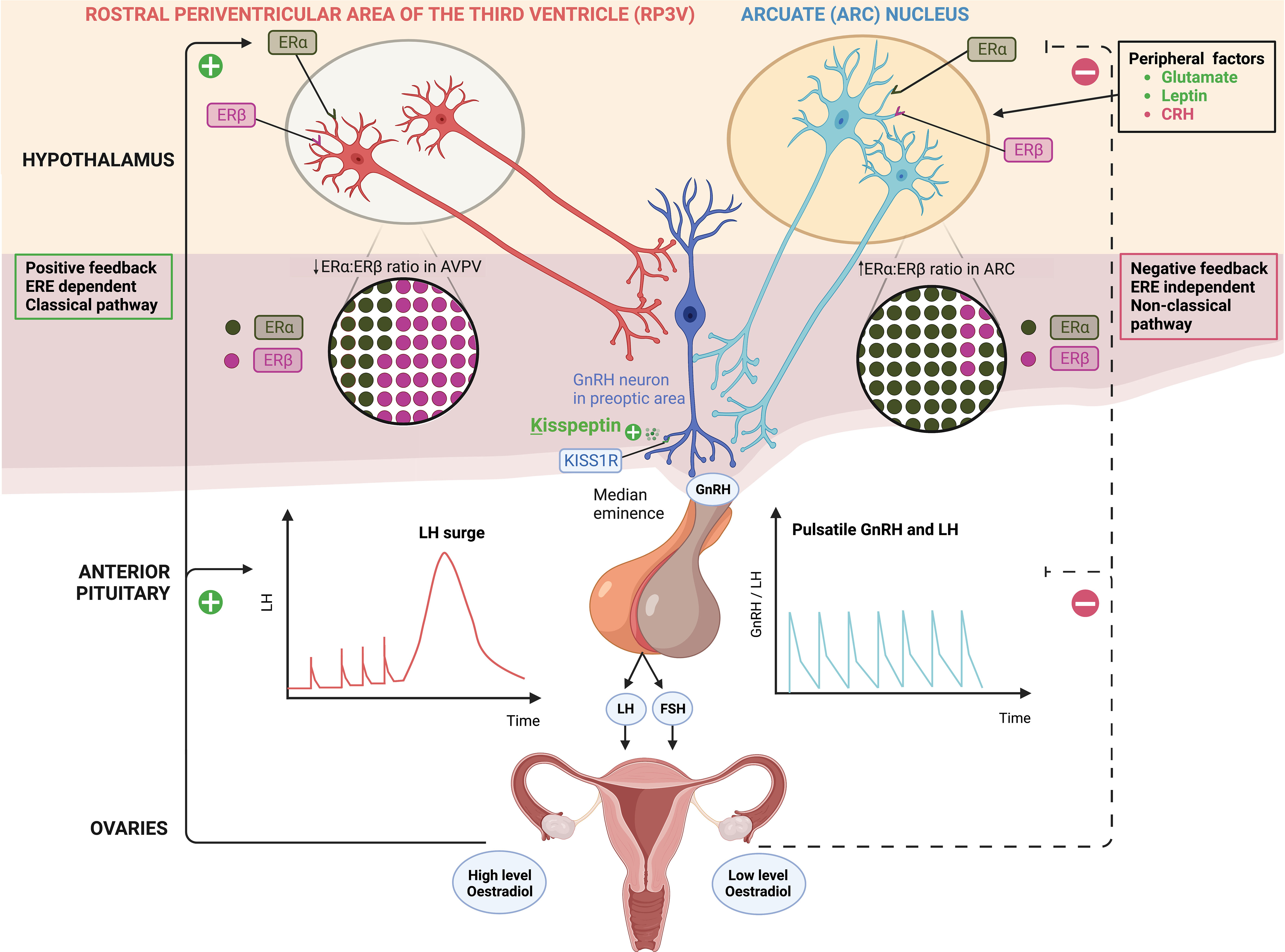
Figure 1 Distinct populations of kisspeptin neurons in the hypothalamus; kisspeptin neurons in the arcuate nucleus (ARC KP-neurons) and rostral periventricular area of the third ventricle (RP3V KP-neurons) which includes anteroventral periventricular (AVPV) nucleus. ARC KP-neurons exhibit episodic activity that induce pulsatile GnRH and LH secretion through oestradiol (E2) mediated negative-feedback and are known as the ‘GnRH pulse generator’ depicted on the right. Synchronisation of these neurons is driven by NKB and dynorphin; NKB stimulates whereas dynorphin inhibits kisspeptin release (not shown). Peripheral factors are depicted [glutamate (stimulatory), leptin (stimulatory) and CRH (inhibitory)] acting at the level of the brain to regulate kisspeptin output. By contrast RP3V KP-neurons induce an LH surge through E2 mediated positive-feedback and ovulation depicted on the left. Oestrogen receptor α (ERα) is responsible for mediating both negative and positive feedback from E2. ERα can signal either by translocation to the nucleus and recruitment of cofactors to oestrogen response element (ERE) (classical pathway), or by recruitment of other transcription factors not via the ERE (non-classical pathway). Notably, E2-induced positive feedback occurs via the classical pathway, whereas negative feedback is mediated via the non-classical pathway. Oestrogen receptor β (ERβ) has been shown to potentiate E2 positive feedback in the RP3V in the presence of high E2 levels and has an inhibitory effect on ERα in the AVPV in the presence of low E2 levels. Regardless of E2 levels, the ratio of ERα:ERβ is twice as high in the ARC as in the AVPV.ARC, arcuate nucleus; AVPV, anteroventral periventricular nucleus; CRH, corticotrophin-releasing hormone; E2, oestradiol; ERα, oestrogen receptor α; ERβ, oestrogen receptor β; ERE, oestrogen response element; FSH, follicle-stimulating hormone; GnRH, gonadotrophin releasing hormone; KISS1R, kisspeptin receptor; LH, luteinising hormone; NKB, neurokinin B; RP3V, rostral periventricular area of the third ventricle.
The feedback mechanism of E2 is particularly intricate within ARC KP-neurons. KP-neurons in the ARC co-express Neurokinin B (NKB) and Dynorphin and hence are termed KNDy neurons. E2 modulates pulsatile KP release by adjusting the stimulatory (NKB) and inhibitory (dynorphin) inputs on ARC KNDy neurons (15, 16). Whilst E2 treatment reduces NKB gene expression, dynorphin (Pdyn) gene expression levels remain unchanged (15, 16). This suggests that the E2 negative feedback on ARC KP-neurons is predominantly mediated through NKB suppression.
Kisspeptin neurons in arcuate nucleus regulate GnRH pulsatility
ARC KP-neurons regulate GnRH pulsatility, and are regarded as the ‘GnRH pulse generator’ (17). ARC KP-neurons innervate GnRH neurons at their distal dendrons in the median eminence of the hypothalamus and release KP (13). The activity of KP-neurons is episodic as indicated by studies measuring multiple unit activity (MUA) near ARC KP-neurons in goats (18, 19) and rats (20), as well as calcium activity in ARC KP-neurons in female mice (21). KP is the main output signal of ARC KNDy neurons to induce pulsatile GnRH, and in turn LH, secretion (22). Optogenetic studies reveal that activation of channel rhodopsin expressing ARC KP-neurons, induces pulsatile LH secretion in Kiss1-Cre mice; whereas inhibition of ARC KP-neurons suppresses pulsatile LH secretion (both frequency and amplitude) (22, 23). Indeed, global knockout of the Kiss1 gene (Kiss1-/-) in ovariectomised female rats leads to complete loss of LH pulses (17, 24) and specifically, knock out of greater than 90% of ARC Kiss1-neurons results in marked suppression of LH pulses (17). Interestingly, rescuing a minimum of 20% of KNDy neurons is sufficient to restore pulsatile LH secretion (17). Collectively, these data suggest that ARC KP-neurons act as the intrinsic GnRH pulse generator and that there is redundancy in the number of ARC KP-neurons needed to maintain GnRH pulsatility.
NKB and dynorphin modulate GnRH and LH pulsatility
Kisspeptin is co-expressed with NKB and Dynorphin in ARC KNDy neurons and this expression pattern is consistent across several species including the mouse (25), rat (24, 26), goat (19), monkey (9), and sheep (27). Whilst the output of ARC KNDy neurons is KP, the synchronisation of these neurons is driven by NKB and dynorphin. NKB initiates whereas dynorphin terminates KNDy neuronal activity in order to induce pulsatile secretion of KP (18, 19, 28, 29). The effects of NKB and dynorphin on ARC KP neuronal pulsatility are mediated via neurokinin-receptor 3 (NK3R) and κ-opioid receptor (KOR), respectively (19, 30).
Neurokinin B and NK3R agonists have been shown to stimulate ARC KP-neurons and downstream GnRH/LH pulsatile secretion in various species. For instance, in ovariectomised (OVX) goats, central administration of NKB (direct injection into the ARC) (31) as well as peripheral administration of NK3R agonist (intravenous injection of senktide) increases multiple-unit activity (MUA, a measure of GnRH/LH pulsatility) (19). Notably, serum LH levels remain unchanged in OVX goats with E2 replacement, thus indicating that NKB action on ARC-KP-neurons is more sensitive to low rather than high E2 concentrations (19). Similar findings have also been observed in OVX ewes (32) and mice (30); however adult rats demonstrate reduced LH pulse frequency following senktide, thus suggesting species variation (33, 34). LH pulse frequency is reduced following NK3R antagonists (32, 35), and specifically MRK-08 has been shown to lead to complete abolishment of LH pulses (27). Activation of KOR (by dynorphin/KOR agonists) decreases MUA frequency in OVX goats (18) and mice (30); whilst inhibition of KOR (by KOR antagonists: nor-binaltorphimine, PF-4455242, naloxone) increases MUA in OVX goats (18, 36), ewes (32), mice (37) but blocks MUA/LH pulses in OVX rats (34). Interestingly, in gonadal intact prepubertal rats, NKB-induced LH pulses are unaffected by Dynorphin (Dyn) but blocked by a KP receptor antagonist (38). This supports the notion that NKB related LH secretion is dependent on KP, but not on Dyn signalling (38). Overall, these data indicate that NKB activity is critical for the activation of ARC KP-neurons and generating the GnRH pulse.
External factors and ARC-KNDy-neuronal firing
The frequency and amplitude of ARC KP neuronal firing is influenced by several positive and negative modulators in addition to E2 (39). Glutamate (24) and leptin (40) are positive modulators that have stimulatory effects on ARC KP-neurons. Glutamate induces LH pulses via KP-neurons as demonstrated in Kiss1-KO rats that fail to increase LH secretion following monosodium glutamate/NMDA (glutamatergic agonist) injections (24). Furthermore, optogenetic stimulation of ARC-KNDy neurons mitigates upstream glutamate-induced signalling, thus highlighting the importance of ARC-KP-neurons in mediating responses to glutamate (41).
Leptin, a key mediator of energy availability, modulates ARC KP neuronal firing frequency (40), although the action of leptin on KP-neurons is not thought to be direct (42). In leptin-resistant states (due to high leptin concentrations as may occur in obesity), ARC KP-neurons are quiescent inhibiting reproduction (40). Leptin-resistant mice have reduced ARC Kiss1 expression, and are infertile (40). Thus, one mechanism by which obesity causes hypogonadism is via the induction of leptin resistance, leading to decreased action of KP-neurons via interneurons.
Padilla et al. explored how starvation can negatively modulate KNDy neuron firing by investigating Agouti-related peptide (AgRP) neurons, which project to ARC KP-neurons (43). AgRP is a neuropeptide that potently stimulates appetite and reduces energy expenditure in response to starvation (44). Ablation of AgRP neurons in neonatal mice resulted in less inhibitory input to ARC KP-neurons (43). Using optogenetic techniques, AgRP neurons were shown to inhibit both ARC KP-neurons and KP-expressing neurons in the AVPV (43). Using chemogenetic techniques, chronic AgRP signalling was shown to impair fertility in mice (43). Indeed, Wu et al. demonstrated that fertility is restored in leptin-deficient mice when AgRP neurons are ablated (44). Since there appears to be no direct signalling between AgRP and GnRH neurons (43), ARC KP-neurons could provide a credible link between nutrition and fertility.
Emotional stress can also impair fertility through GnRH suppression (45). Lin et al. demonstrate that the central amygdala (CeA) suppresses the GnRH pulse generator in female mice in response to psychogenic and immunological stressors (45).The amygdala releases corticotrophin-releasing hormone (CRH), subsequently activating the hypothalamic-pituitary-adrenal-axis, in response to stress (45). Kinsey-Jones et al. found that high CRH suppresses ARC KP-neurons, leading to reduced LH pulsatility (46). Whilst the CeA exerts inhibitory effects on ovulation, the medial amygdala (MeA) stimulates it, since lesioning of the MeA blocks ovulation, whilst stimulation advances the time of the LH surge (45). Together, these data highlight how Kiss1 neurons can integrate signals to modulate GnRH pulsatility and impair fertility in times of stress or metabolic disturbance.
Kisspeptin neurons in RP3V induce an LH surge to trigger ovulation
Kisspeptin neurons in RP3V expressing ERα are present across mammalian species and induce an LH surge through E2-mediated positive-feedback (26, 47). Elevated E2 levels can initiate a pre-ovulatory LH surge across all studied mammalian species (48), however the mechanism downstream of this signal varies between species, with the hypothalamus identified as the site of primary action in rodents and sheep, whereas the pituitary is of higher relative importance in primates (49). More recently, neural progesterone (P4) has been identified as a key player in the generation of LH surges downstream of E2 signalling pathway (50, 51). Total P4 receptor knockout studies saw the absence of an LH surge, and reduced c-Fos (a marker of neuronal activation) in RP3V KP-neurons. Reintroduction of P4 receptors solely in the AVPV re-established the LH surge (50, 51). In vitro studies highlighted that P4 also augments kisspeptin expression and release from RP3V KP-neurons neurons in conjunction with E2 (52). Together, astrocytes local to the AVPV seem to be the source of the P4, induced by E2-moderated positive-feedback (53). Pre-ovulatory levels of E2 upregulate Kiss1 expression via histone acetylation in the Kiss1 promoter region (11), with preoptic/AVPV Kiss1 neurons being fundamental to the generation of a pre-ovulatory LH surge in rodents (54).
Further to relaying positive steroidal feedback to GnRH neurons, RP3V KP-neurons also act as an integrative hub for neural afferents involved in ovulation (55). LH surges in mice occur consistently at midday of the proestrus stage of their oestrous cycle (56) suggesting that importance of circadian rhythm to the LH surge. Neurotracing studies reveal that the suprachiasmatic nucleus (SCN) provides an afferent input to RP3V KP-neurons neurons through vasopressin (57). Unilateral lesion studies in the SCN reveal that Kiss1 mRNA expression in RP3V KP-neurons neurons are regulated by an oscillator in the dorsomedial SCN (58). As such the dorsomedial SCN regulates GnRH activity during ovulation through upregulating expression of kisspeptin in RP3V KP-neurons neurons. Whether there is circadian control over ovulation in humans remains unclear (59, 60) although it seems possible given that shift workers are at risk of reduced fertility and irregular menstrual cycles (61, 62). However, any effects of shift work on menstrual cycles could also be due to high-stress conditions, which as previously described inhibit activity of KP-neurons.
Additionally, ERα-expressing noradrenergic cell groups may be involved in LH surge generation through modulating KP release from AVPV neurons (63). It is known that noradrenaline facilitates LH surge generation (64, 65), however a link to KP has only recently been posited. Administration of an α1-blocker reduces Kiss1 expression in the preoptic area (POA) and ultimately LH release, suggesting that noradrenaline release facilitates KP synthesis prior to an LH surge (66). Interestingly α1-receptors have not been identified on RP3V KP-neurons neurons (66), indicating that noradrenaline modulates KP-neurons upstream of the POA. Due to the expression of α1-receptors in the SCN (67), it has been proposed that noradrenaline modulates vasopressinergic inputs from the SCN to RP3V KP-neurons neurons to mediate KP release in the median eminence (68).
RP3V KP-neurons neurons receive afferents from ARC KP-neurons via glutamate suggesting that ARC KP-neurons play a role in LH surge generation in addition to their role in pulse generation (29). Furthermore, optogenetic stimulation can spontaneously provoke LH surges in an E2-dependent manner (68). In ARC KP-neurons, glutamate expression is increased by high E2 levels (69). Thus, E2 may positively feedback on the ARC to increase glutamatergic inputs to the AVPV at the time of ovulation to facilitate LH surge generation. To what extent glutamatergic inputs play a role in LH surge generation remains unclear; glutamatergic inputs to RP3V KP-neurons neurons from ARC KP-neurons are not essential to the LH surge, as knocking out glutamate in all KP-neurons had no effect on ability of mice to undergo oestrous cycles (69).
Kisspeptin neurons may be implicated in disorders of reproduction
Hypogonadotrophic hypogonadism is a deficiency of hypothalamic GnRH leading to decreased adenohypophyseal secretion of LH and FSH, resulting in failure to undergo puberty and infertility (3, 4). Two groups almost simultaneously identified an autosomal recessive cause of hypogonadotrophic hypogonadism in consanguineous families, caused by inactivating variants in KISS1R (3, 4). Seminara et al. developed a Kiss1r-deficient mouse-model, which had small prepubertal ovaries and absent follicular maturation, replicating the hypogonadotrophic hypogonadism phenotype observed in humans (4). Conversely, an autosomal dominant activating variant of the KISS1R results in precocious puberty caused by early maturation of the HPG-axis (70).
Functional hypothalamic amenorrhoea (FHA) is a common cause of amenorrhoea in women of reproductive age (71). It is caused by reduced GnRH pulsatility, leading to deficient LH pulsatility, in the absence of structural abnormality (71, 72). Weight-loss and intense exercise regimens limiting energy availability, as well as psychological stress are the most common causes of FHA (73). Changes in activity of hypothalamic ARC KP-neurons in relation to such inputs modulates the activity of GnRH pulsatility and subsequently fertility (43, 74). Restoring fertility requires reestablishment of gonadotrophin secretion, rather than by sex-steroid replacement alone, which can be achieved by administration of KP (72). Animal models suggest that undernutrition is associated with decreased hypothalamic Kiss1 expression in the ARC, and thus conceptually, KP administration is an attractive approach to restore reproductive health. Unfortunately, stimulation of gonadotropin production was not sustained after high dose chronic stimulation in women, which was attributed to tachyphylaxis (72). In order for replacing KP to represent a future therapy for FHA, more developing treatment protocols that can induce sustained stimulation of the HPG axis is much needed.
Another common cause of secondary amenorrhoea is polycystic ovary syndrome (PCOS) (71). GnRH and LH pulse frequency are increased in PCOS, which results in increased ovarian androgen production (a cardinal feature of PCOS). Indeed, LH pulse frequency is useful for distinguishing between FHA and PCOS, as the two commonest pathological causes of secondary amenorrhoea (73). Possible mechanisms contributing to increased LH pulse frequency include loss of progesterone negative feedback (due to the opposing effects of androgens) and enhanced GABAergic drive to GnRH neurons (75–77). Indeed, LH production is increased in PNA mice subject to chemo- and opto-genetic activation of GABA neurons in the ARC, highlighting the stimulatory role of GABAergic neurons have on GnRH neurons (77). However, there is evidence that KNDy neuronal activity is key to the increased LH pulse frequency and amplitude seen in PCOS. A study using constant, chronic letrozole delivery to PCOS mouse models found that Kiss and Tac2 (encoding NKB in rodent species) gene expression were strongly upregulated in the ARC (78). Further, cfos expression was upregulated in ARC KP-neurons, indicating greater neuronal activation (78). However, not all features of PCOS are replicated in animal models of PCOS, as a recent study using PNA mice did not show that KNDy neuronal firing frequency was increased (75).
Women with PCOS and oligomenorrhoea had an increase in circulating KP pulse frequency (79), and whilst pulses in circulating KP and LH levels were coupled in eumenorrheic women, this coupling was lost in oligomenorrheic women (79). A randomised, placebo-controlled trial in women with PCOS showed that AZD4901, a NK3R antagonist, reduced LH pulse frequency, serum LH and testosterone concentrations in women with PCOS (80). This indicates that NKB action on KNDy neurons, may be enhanced in women with PCOS and their activity could represent a target for treatment.
These conditions highlight how relative inhibition or stimulation of KNDy neurons affects the HPG axis, with detrimental effects fertility. Importantly, manipulation of these circuits could be useful in developing future therapies.
Conclusion
LH pulsatility and the midcycle LH surge are necessary for normal follicular development and ovulation, respectively. Hypothalamic KNDy neurons release KP to stimulate GnRH neurons and induce pulsatile secretion of GnRH and subsequently gonadotrophins. Inhibition of the activity of KNDy neurons in states of stress, or over/under nutrition can result in inhibition of the reproductive axis. Manipulation of these neuronal circuits may be useful in developing future therapies for diseases caused by derangements in KNDy neuronal firing, including PCOS and functional hypogonadotrophic hypogonadism.
Author contributions
HS, SB, MC, SM, TP, AP, OV, IA, AL, KK, and BP wrote sections of the manuscript and contributed to manuscript revision. AA contributed to revision and editing of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
All illustrations are original and were created with BioRender.com. This article presents independent research. The Section of Endocrinology and Investigative Medicine is funded by grants from the MRC, NIHR and is supported by the NIHR Biomedical Research Centre Funding Scheme and the NIHR/ Imperial Clinical Research Facility. KK is supported by NIHR Academic Clinical Fellowship Award ACF-2021-21-001 and acknowledges infrastructure support for this research from the NIHR Imperial Biomedical Research Centre (BRC). BP is supported by an MRC Clinical Research Training Fellowship (No. MR/W024144/1). AA is supported by an NIHR Clinician Scientist award (CS-2018-18-ST2-002).
Conflict of interest
AA has undertaken consultancy work for Myovant Sciences Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Tsutsumi R, Webster NJ. GnRH pulsatility, the pituitary response and reproductive dysfunction. Endocr J (2009) 56:729–37. doi: 10.1507/endocrj.k09e-185
2. Herbison AE, Theodosis DT. Localization of oestrogen receptors in preoptic neurons containing neurotensin but not tyrosine hydroxylase, cholecystokinin or luteinizing hormone-releasing hormone in the male and female rat. Neuroscience (1992) 50:283–98. doi: 10.1016/0306-4522(92)90423-y
3. de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E, Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA (2003) 100:10972–6. doi: 10.1073/pnas.1834399100
4. Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS Jr, Shagoury JK, et al. The GPR54 gene as a regulator of puberty. N Engl J Med (2003) 349:1614–27. doi: 10.1056/NEJMoa035322
5. Topaloglu AK, Tello JA, Kotan LD, Ozbek MN, Yilmaz MB, Erdogan S, et al. Inactivating KISS1 mutation and hypogonadotropic hypogonadism. N Engl J Med (2012) 366:629–35. doi: 10.1056/NEJMoa1111184
6. Ikegami K, Minabe S, Ieda N, Goto T, Sugimoto A, Nakamura S, et al. Evidence of involvement of neurone-glia/neurone-neurone communications via gap junctions in synchronised activity of KNDy neurones. J Neuroendocrinol (2017) 29(6):1–14. doi: 10.1111/jne.12480
7. Ruka KA, Burger LL, Moenter SM. Regulation of arcuate neurons coexpressing kisspeptin, neurokinin b, and dynorphin by modulators of neurokinin 3 and kappa-opioid receptors in adult male mice. Endocrinology (2013) 154:2761–71. doi: 10.1210/en.2013-1268
8. Maeda K, Adachi S, Inoue K, Ohkura S, Tsukamura H. Metastin/kisspeptin and control of estrous cycle in rats. Rev Endocr Metab Disord (2007) 8:21–9. doi: 10.1007/s11154-007-9032-6
9. Rometo AM, Krajewski SJ, Voytko ML, Rance NE. Hypertrophy and increased kisspeptin gene expression in the hypothalamic infundibular nucleus of postmenopausal women and ovariectomized monkeys. J Clin Endocrinol Metab (2007) 92:2744–50. doi: 10.1210/jc.2007-0553
10. Yasar P, Ayaz G, User SD, Gupur G, Muyan M. Molecular mechanism of estrogen-estrogen receptor signaling. Reprod Med Biol (2017) 16:4–20. doi: 10.1002/rmb2.12006
11. Tomikawa J, Uenoyama Y, Ozawa M, Fukanuma T, Takase K, Goto T, et al. Epigenetic regulation of Kiss1 gene expression mediating estrogen-positive feedback action in the mouse brain. Proc Natl Acad Sci USA (2012) 109:E1294–1301. doi: 10.1073/pnas.1114245109
12. Sterner DE, Berger SL. Acetylation of histones and transcription-related factors. Microbiol Mol Biol Rev (2000) 64:435–59. doi: 10.1128/MMBR.64.2.435-459.2000
13. Gocz B, Takacs S, Skrapits K, Rumpler E, Solymosi N, Poliska S, et al. Estrogen differentially regulates transcriptional landscapes of preoptic and arcuate kisspeptin neuron populations. Front Endocrinol (Lausanne) (2022) 13:960769. doi: 10.3389/fendo.2022.960769
14. Henriques PC, Aquino NSS, Campideli-Santana AC, Silva JF, Araujo-Lopes R, Franci CR, et al. Hypothalamic expression of estrogen receptor isoforms underlies estradiol control of luteinizing hormone in female rats. Endocrinology (2022) 163(8):1–13. doi: 10.1210/endocr/bqac101
15. Mostari P, Ieda N, Deura C, Minabe S, Yamada S, Uenoyama Y, et al. Dynorphin-kappa opioid receptor signaling partly mediates estrogen negative feedback effect on LH pulses in female rats. J Reprod Dev (2013) 59:266–72. doi: 10.1262/jrd.2012-193
16. Dellovade TL, Merchenthaler I. Estrogen regulation of neurokinin b gene expression in the mouse arcuate nucleus is mediated by estrogen receptor alpha. Endocrinology (2004) 145:736–42. doi: 10.1210/en.2003-0894
17. Nagae M, Uenoyama Y, Okamoto S, Tsuchida H, Ikegami K, Goto T, et al. Direct evidence that KNDy neurons maintain gonadotropin pulses and folliculogenesis as the GnRH pulse generator. Proc Natl Acad Sci USA (2021) 118(5):e2009156118. doi: 10.1073/pnas.2009156118
18. Wakabayashi Y, Nakada T, Murata K, Ohkura S, Mogi K, Navarro VM, et al. Neurokinin b and dynorphin a in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci (2010) 30:3124–32. doi: 10.1523/JNEUROSCI.5848-09.2010
19. Yamamura T, Wakabayashi Y, Ohkura S, Navarro VM, Okamura H. Effects of intravenous administration of neurokinin receptor subtype-selective agonists on gonadotropin-releasing hormone pulse generator activity and luteinizing hormone secretion in goats. J Reprod Dev (2015) 61:20–9. doi: 10.1262/jrd.2014-109
20. Kinsey-Jones JS, Li XF, Luckman SM, O’Byrne KT. Effects of kisspeptin-10 on the electrophysiological manifestation of gonadotropin-releasing hormone pulse generator activity in the female rat. Endocrinology (2008) 149:1004–8. doi: 10.1210/en.2007-1505
21. McQuillan HJ, Han SY, Cheong I, Herbison AE. GnRH pulse generator activity across the estrous cycle of female mice. Endocrinology (2019) 160:1480–91. doi: 10.1210/en.2019-00193
22. Clarkson J, Han SY, Piet R, McLennan T, Kane GM, Ng J, et al. Definition of the hypothalamic GnRH pulse generator in mice. Proc Natl Acad Sci USA (2017) 114:E10216–23. doi: 10.1073/pnas.1713897114
23. Han SY, McLennan T, Czieselsky K, Herbison AE. Selective optogenetic activation of arcuate kisspeptin neurons generates pulsatile luteinizing hormone secretion. Proc Natl Acad Sci USA (2015) 112:13109–14. doi: 10.1073/pnas.1512243112
24. Uenoyama Y, Nakamura S, Hayakawa Y, Ikegami K, Watanabe Y, Deura C, et al. Lack of pulse and surge modes and glutamatergic stimulation of luteinising hormone release in Kiss1 knockout rats. J Neuroendocrinol (2015) 27:187–97. doi: 10.1111/jne.12257
25. Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, et al. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology (2004) 145:4073–7. doi: 10.1210/en.2004-0431
26. Adachi S, Yamada S, Takatsu Y, Matsui H, Kinoshita M, Takase K, et al. Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J Reprod Dev (2007) 53:367–78. doi: 10.1262/jrd.18146
27. Li Q, Millar RP, Clarke IJ, Smith JT. Evidence that neurokinin b controls basal gonadotropin-releasing hormone secretion but is not critical for estrogen-positive feedback in sheep. Neuroendocrinology (2015) 101:161–74. doi: 10.1159/000377702
28. Goodman RL, Lehman MN, Smith JT, Coolen LM, de Oliveira CV, Jafarzadehshirazi MR, et al. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin a and neurokinin b. Endocrinology (2007) 148:5752–60. doi: 10.1210/en.2007-0961
29. Qiu J, Nestor CC, Zhang C, Padilla SL, Palmiter RD, Kelly MJ, et al. High-frequency stimulation-induced peptide release synchronizes arcuate kisspeptin neurons and excites GnRH neurons. Elife (2016) 5:e16246. doi: 10.7554/eLife.16246
30. Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA, Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin b neurons in the arcuate nucleus of the mouse. J Neurosci (2009) 29:11859–66. doi: 10.1523/JNEUROSCI.1569-09.2009
31. Wakabayashi Y, Okamura H, Yamamura T. Local administration of neurokinin b in the arcuate nucleus accelerates the neural activity of the GnRH pulse generator in goats. J Reprod Dev (2021) 67:352–8. doi: 10.1262/jrd.2021-055
32. Goodman RL, Hileman SM, Nestor CC, Porter KL, Connors JM, Hardy SL, et al. Kisspeptin, neurokinin b, and dynorphin act in the arcuate nucleus to control activity of the GnRH pulse generator in ewes. Endocrinology (2013) 154:4259–69. doi: 10.1210/en.2013-1331
33. Grachev P, Li XF, Kinsey-Jones JS, di Domenico AL, Millar RP, Lightman SL, et al. Suppression of the GnRH pulse generator by neurokinin b involves a kappa-opioid receptor-dependent mechanism. Endocrinology (2012) 153:4894–904. doi: 10.1210/en.2012-1574
34. Kinsey-Jones JS, Grachev P, Li XF, Lin YS, Milligan SR, Lightman SL, et al. The inhibitory effects of neurokinin b on GnRH pulse generator frequency in the female rat. Endocrinology (2012) 153:307–15. doi: 10.1210/en.2011-1641
35. Sasaki T, Sonoda T, Tatebayashi R, Kitagawa Y, Oishi S, Yamamoto K, et al. Peripheral administration of SB223412, a selective neurokinin-3 receptor antagonist, suppresses pulsatile luteinizing hormone secretion by acting on the gonadotropin-releasing hormone pulse generator in estrogen-treated ovariectomized female goats. J Reprod Dev (2020) 66:351–7. doi: 10.1262/jrd.2019-145
36. Sasaki T, Ito D, Sonoda T, Morita Y, Wakabayashi Y, Yamamura T, et al. Peripheral administration of kappa-opioid receptor antagonist stimulates gonadotropin-releasing hormone pulse generator activity in ovariectomized, estrogen-treated female goats. Domest Anim Endocrinol (2019) 68:83–91. doi: 10.1016/j.domaniend.2018.12.011
37. Lippincott MF, Leon S, Chan YM, Fergani C, Talbi R, Farooqi IS, et al. Hypothalamic reproductive endocrine pulse generator activity independent of neurokinin b and dynorphin signaling. J Clin Endocrinol Metab (2019) 104:4304–18. doi: 10.1210/jc.2019-00146
38. Grachev P, Li XF, Lin YS, Hu MH, Elsamani L, Paterson SJ, et al. GPR54-dependent stimulation of luteinizing hormone secretion by neurokinin b in prepubertal rats. PloS One (2012) 7:e44344. doi: 10.1371/journal.pone.0044344
39. Ikegami K, Watanabe Y, Nakamura S, Goto T, Inoue N, Uenoyama Y, et al. Cellular and molecular mechanisms regulating the KNDy neuronal activities to generate and modulate GnRH pulse in mammals. Front Neuroendocrinol (2022) 64:100968. doi: 10.1016/j.yfrne.2021.100968
40. Quennell JH, Howell CS, Roa J, Augustine RA, Grattan DR, Anderson GM. Leptin deficiency and diet-induced obesity reduce hypothalamic kisspeptin expression in mice. Endocrinology (2011) 152:1541–50. doi: 10.1210/en.2010-1100
41. Voliotis M, Li XF, De Burgh RA, Lass G, Ivanova D, McIntyre C, et al. Modulation of pulsatile GnRH dynamics across the ovarian cycle via changes in the network excitability and basal activity of the arcuate kisspeptin network. Elife (2021) 10:e71252. doi: 10.7554/eLife.71252
42. Cravo RM, Frazao R, Perello M, Osborne-Lawrence S, Williams KW, Zigman JM, et al. Leptin signaling in Kiss1 neurons arises after pubertal development. PloS One (2013) 8:e58698. doi: 10.1371/journal.pone.0058698
43. Padilla SL, Qiu J, Nestor CC, Zhang C, Smith AW, Whiddon BB, et al. AgRP to Kiss1 neuron signaling links nutritional state and fertility. Proc Natl Acad Sci USA (2017) 114:2413–8. doi: 10.1073/pnas.1621065114
44. Wu Q, Whiddon BB, Palmiter RD. Ablation of neurons expressing agouti-related protein, but not melanin concentrating hormone, in leptin-deficient mice restores metabolic functions and fertility. Proc Natl Acad Sci USA (2012) 109:3155–60. doi: 10.1073/pnas.1120501109
45. Lin Y, Li X, Lupi M, Kinsey-Jones JS, Shao B, Lightman SL, et al. The role of the medial and central amygdala in stress-induced suppression of pulsatile LH secretion in female rats. Endocrinology (2011) 152:545–55. doi: 10.1210/en.2010-1003
46. Kinsey-Jones JS, Li XF, Knox AM, Wilkinson ES, Zhu XL, Chaudhary AA, et al. Down-regulation of hypothalamic kisspeptin and its receptor, Kiss1r, mRNA expression is associated with stress-induced suppression of luteinising hormone secretion in the female rat. J Neuroendocrinol (2009) 21:20–9. doi: 10.1111/j.1365-2826.2008.01807.x
47. Kinoshita M, Tsukamura H, Adachi S, Matsui H, Uenoyama Y, Iwata K, et al. Involvement of central metastin in the regulation of preovulatory luteinizing hormone surge and estrous cyclicity in female rats. Endocrinology (2005) 146:4431–6. doi: 10.1210/en.2005-0195
48. Herbison AE. Control of puberty onset and fertility by gonadotropin-releasing hormone neurons. Nat Rev Endocrinol (2016) 12:452–66. doi: 10.1038/nrendo.2016.70
49. Plant TM. A comparison of the neuroendocrine mechanisms underlying the initiation of the preovulatory LH surge in the human, old world monkey and rodent. Front Neuroendocrinol (2012) 33:160–8. doi: 10.1016/j.yfrne.2012.02.002
50. Stephens SB, Tolson KP, Rouse ML Jr, Poling MC, Hashimoto-Partyka MK, Mellon PL, et al. Absent progesterone signaling in kisspeptin neurons disrupts the LH surge and impairs fertility in female mice. Endocrinology (2015) 156:3091–7. doi: 10.1210/en.2015-1300
51. Mohr MA, Esparza LA, Steffen P, Micevych PE, Kauffman AS. Progesterone receptors in AVPV kisspeptin neurons are sufficient for positive feedback induction of the LH surge. Endocrinology (2021) 162(11):bqab161. doi: 10.1210/endocr/bqab161
52. Mittelman-Smith MA, Wong AM, Micevych PE. Estrogen and progesterone integration in an in vitro model of RP3V kisspeptin neurons. Neuroendocrinology (2018) 106:101–15. doi: 10.1159/000471878
53. Sinchak K, Mohr MA, Micevych PE. Hypothalamic astrocyte development and physiology for neuroprogesterone induction of the luteinizing hormone surge. Front Endocrinol (Lausanne) (2020) 11:420. doi: 10.3389/fendo.2020.00420
54. Wiegand SJ, Terasawa E. Discrete lesions reveal functional heterogeneity of suprachiasmatic structures in regulation of gonadotropin secretion in the female rat. Neuroendocrinology (1982) 34:395–404. doi: 10.1159/000123335
55. Hrabovszky E, Ciofi P, Vida B, Horvath MC, Keller E, Caraty A, et al. The kisspeptin system of the human hypothalamus: sexual dimorphism and relationship with gonadotropin-releasing hormone and neurokinin b neurons. Eur J Neurosci (2010) 31:1984–98. doi: 10.1111/j.1460-9568.2010.07239.x
56. Bingel AS, Schwartz NB. Timing of LH release and ovulation in the post partum mouse. J Reprod Fertil (1969) 19:231–7. doi: 10.1530/jrf.0.0190231
57. Vida B, Deli L, Hrabovszky E, Kalamatianos T, Caraty A, Coen CW, et al. Evidence for suprachiasmatic vasopressin neurones innervating kisspeptin neurones in the rostral periventricular area of the mouse brain: regulation by oestrogen. J Neuroendocrinol (2010) 22:1032–9. doi: 10.1111/j.1365-2826.2010.02045.x
58. Smarr BL, Morris E, de la Iglesia HO. The dorsomedial suprachiasmatic nucleus times circadian expression of Kiss1 and the luteinizing hormone surge. Endocrinology (2012) 153:2839–50. doi: 10.1210/en.2011-1857
59. Klingman KM, Marsh EE, Klerman EB, Anderson EJ, Hall JE. Absence of circadian rhythms of gonadotropin secretion in women. J Clin Endocrinol Metab (2011) 96:1456–61. doi: 10.1210/jc.2010-2739
60. Kerdelhue B, Brown S, Lenoir V, Queenan JT Jr, Jones GS, Scholler R, et al. Timing of initiation of the preovulatory luteinizing hormone surge and its relationship with the circadian cortisol rhythm in the human. Neuroendocrinology (2002) 75:158–63. doi: 10.1159/000048233
61. Knutsson A. Health disorders of shift workers. Occup Med (Lond) (2003) 53:103–8. doi: 10.1093/occmed/kqg048
62. Labyak S, Lava S, Turek F, Zee P. Effects of shiftwork on sleep and menstrual function in nurses. Health Care Women Int (2002) 23:703–14. doi: 10.1080/07399330290107449
63. Haywood SA, Simonian SX, van der Beek EM, Bicknell RJ, Herbison AE. Fluctuating estrogen and progesterone receptor expression in brainstem norepinephrine neurons through the rat estrous cycle. Endocrinology (1999) 140:3255–63. doi: 10.1210/endo.140.7.6869
64. Le WW, Berghorn KA, Smith MS, Hoffman GE. Alpha1-adrenergic receptor blockade blocks LH secretion but not LHRH cFos activation. Brain Res (1997) 747:236–45. doi: 10.1016/s0006-8993(96)01269-3
65. Weick RF. Acute effects of adrenergic receptor blocking drugs and neuroleptic agents on pulsatile discharges of luteinizing hormone in the ovariectomized rat. Neuroendocrinology (1978) 26:108–17. doi: 10.1159/000122774
66. Kalil B, Ribeiro AB, Leite CM, Uchoa ET, Carolino RO, Cardoso TS, et al. The increase in signaling by kisspeptin neurons in the preoptic area and associated changes in clock gene expression that trigger the LH surge in female rats are dependent on the facilitatory action of a noradrenaline input. Endocrinology (2016) 157:323–35. doi: 10.1210/en.2015-1323
67. Weiland NG, Wise PM. Estrogen alters the diurnal rhythm of alpha 1-adrenergic receptor densities in selected brain regions. Endocrinology (1987) 121:1751–8. doi: 10.1210/endo-121-5-1751
68. Lin XH, Lass G, Kong LS, Wang H, Li XF, Huang HF, et al. Optogenetic activation of arcuate kisspeptin neurons generates a luteinizing hormone surge-like secretion in an estradiol-dependent manner. Front Endocrinol (Lausanne) (2021) 12:775233. doi: 10.3389/fendo.2021.775233
69. Qiu J, Rivera HM, Bosch MA, Padilla SL, Stincic TL, Palmiter RD, et al. Estrogenic-dependent glutamatergic neurotransmission from kisspeptin neurons governs feeding circuits in females. Elife (2018) 7:e35656. doi: 10.7554/eLife.35656
70. Teles MG, Bianco SD, Brito VN, Trarbach EB, Kuohung W, Xu S, et al. A GPR54-activating mutation in a patient with central precocious puberty. N Engl J Med (2008) 358:709–15. doi: 10.1056/NEJMoa073443
71. Roberts RE, Farahani L, Webber L, Jayasena C. Current understanding of hypothalamic amenorrhoea. Ther Adv Endocrinol Metab (2020) 11:2042018820945854. doi: 10.1177/2042018820945854
72. Jayasena CN, Abbara A, Veldhuis JD, Comninos AN, Ratnasabapathy R, De Silva A, et al. Increasing LH pulsatility in women with hypothalamic amenorrhoea using intravenous infusion of kisspeptin-54. J Clin Endocrinol Metab (2014) 99:E953–961. doi: 10.1210/jc.2013-1569
73. Phylactou M, Clarke SA, Patel B, Baggaley C, Jayasena CN, Kelsey TW, et al. Clinical and biochemical discriminants between functional hypothalamic amenorrhoea (FHA) and polycystic ovary syndrome (PCOS). Clin Endocrinol (Oxf) (2021) 95:239–52. doi: 10.1111/cen.14402
74. Li XF, Hu MH, Li SY, Geach C, Hikima A, Rose S, et al. Overexpression of corticotropin releasing factor in the central nucleus of the amygdala advances puberty and disrupts reproductive cycles in female rats. Endocrinology (2014) 155:3934–44. doi: 10.1210/en.2014-1339
75. Gibson AG, Jaime J, Burger LL, Moenter SM. Prenatal androgen treatment does not alter the firing activity of hypothalamic arcuate kisspeptin neurons in female mice. eNeuro (2021) 8(5):eneuro.0306-21.2021. doi: 10.1523/ENEURO.0306-21.2021
76. Coyle C, Campbell RE. Pathological pulses in PCOS. Mol Cell Endocrinol (2019) 498:110561. doi: 10.1016/j.mce.2019.110561
77. Silva MSB, Desroziers E, Hessler S, Prescott M, Coyle C, Herbison AE, et al. Activation of arcuate nucleus GABA neurons promotes luteinizing hormone secretion and reproductive dysfunction: Implications for polycystic ovary syndrome. EBioMedicine (2019) 44:582–96. doi: 10.1016/j.ebiom.2019.05.065
78. Esparza LA, Schafer D, Ho BS, Thackray VG, Kauffman AS. Hyperactive LH pulses and elevated kisspeptin and NKB gene expression in the arcuate nucleus of a PCOS mouse model. Endocrinology (2020) 161(4):bqaa018. doi: 10.1210/endocr/bqaa018
79. Katulski K, Podfigurna A, Czyzyk A, Meczekalski B, Genazzani AD. Kisspeptin and LH pulsatile temporal coupling in PCOS patients. Endocrine (2018) 61:149–57. doi: 10.1007/s12020-018-1609-1
Keywords: kisspeptin, ovulation, oestrogen receptor, KNDy neurons, arcuate nucleus, anteroventral periventricular nucleus, gonadotrophin releasing hormone, luteinising hormone
Citation: Stevenson H, Bartram S, Charalambides MM, Murthy S, Petitt T, Pradeep A, Vineall O, Abaraonye I, Lancaster A, Koysombat K, Patel B and Abbara A (2022) Kisspeptin-neuron control of LH pulsatility and ovulation. Front. Endocrinol. 13:951938. doi: 10.3389/fendo.2022.951938
Received: 24 May 2022; Accepted: 01 November 2022;
Published: 21 November 2022.
Edited by:
Takashi Yazawa, Asahikawa Medical University, JapanReviewed by:
Haruhiko Kanasaki, Shimane University, JapanRichard Anthony DeFazio, University of Michigan, United States
Copyright © 2022 Stevenson, Bartram, Charalambides, Murthy, Petitt, Pradeep, Vineall, Abaraonye, Lancaster, Koysombat, Patel and Abbara. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ali Abbara, ali.abbara@imperial.ac.uk
†These authors have contributed equally to this work
 Harvey Stevenson
Harvey Stevenson Samuel Bartram†
Samuel Bartram† Mikaela Maria Charalambides
Mikaela Maria Charalambides Kanyada Koysombat
Kanyada Koysombat Ali Abbara
Ali Abbara