- 1Department of Genetics, University of Texas MD Anderson Cancer Center, Houston, TX, United States
- 2Département AVIV, Physiologie Moléculaire et Adaptation, CNRS UMR7221, Muséum National d’Histoire Naturelle, Paris, France
Dlx5 and Dlx6 encode distal-less homeodomain transcription factors that are present in the genome as a linked pair at a single locus. Dlx5 and Dlx6 have redundant roles in craniofacial, skeletal, and uterine development. Previously, we performed a transcriptome comparison for anti-Müllerian hormone (AMH)-induced genes expressed in the Müllerian duct mesenchyme of male and female mouse embryos. In that study, we found that Dlx5 transcripts were nearly seven-fold higher in males compared to females and Dlx6 transcripts were found only in males, suggesting they may be AMH-induced genes. Therefore, we investigated the role of Dlx5 and Dlx6 during AMH-induced Müllerian duct regression. We found that Dlx5 was detected in the male Müllerian duct mesenchyme from E14.5 to E16.5. In contrast, in female embryos Dlx5 was detected in the Müllerian duct epithelium. Dlx6 expression in Müllerian duct mesenchyme was restricted to males. Dlx6 expression was not detected in female Müllerian duct mesenchyme or epithelium. Genetic experiments showed that AMH signaling is necessary for Dlx5 and Dlx6 expression. Müllerian duct regression was variable in Dlx5 homozygous mutant males at E16.5, ranging from regression like controls to a block in Müllerian duct regression. In E16.5 Dlx6 homozygous mutants, Müllerian duct tissue persisted primarily in the region adjacent to the testes. In Dlx5-6 double homozygous mutant males Müllerian duct regression was also found to be incomplete but more severe than either single mutant. These studies suggest that Dlx5 and Dlx6 act redundantly to mediate AMH-induced Müllerian duct regression during male differentiation.
Introduction
The male reproductive tract organs include the vas deferentia, epididymides, and seminal vesicles. These structures provide the conduit for movement and maturation of spermatozoa from the testes for sexual reproduction. While formation and differentiation of these male reproductive tract organs are essential for reproduction, another process which eliminates a progenitor organ system termed Müllerian duct regression is also required for male development (1). These developmental processes are regulated by the presence or absence of fetal hormones (2).
The reproductive tract organs of mammals are derived from two pairs of epithelial tubes surrounded by mesenchyme called the Wolffian ducts (progenitor male reproductive tract) and Müllerian ducts (progenitor female reproductive tract). The Wolffian ducts differentiate into the vas deferentia, epididymides, and seminal vesicles. The oviducts, uterus and upper vagina are derived from the Müllerian ducts. Interestingly, both the Wolffian and Müllerian ducts are formed regardless of the genetic sex of the embryo. Differential hormone signaling after the formation of these genital ducts results in Wolffian duct loss in female embryos and Müllerian duct regression in male embryos.
Müllerian duct regression is mediated by the TGF-β family member anti-Müllerian hormone (AMH) secreted by Sertoli cells of the fetal testes (3). AMH signaling acts in the Müllerian duct mesenchyme through ACVR1 or BMPR1A type 1 receptors (shared with the bone morphogenetic protein (BMP) signaling pathway) and a sole anti-Müllerian hormone type 2 receptor (AMHR2) (4, 5). Amhr2 is expressed in the Müllerian duct mesenchyme and in the somatic cells of the gonads (6). In the Mullerian duct mesenchyme, AMH binds AMHR2 which then activates its type 1 receptors. This AMH ligand receptor complex subsequently phosphorylates R-SMAD1, 5 and 8 (also shared with the BMP signaling pathway) (4). These redundant phosphorylated R-SMADs then presumably activate downstream effectors of AMH signaling.
Amh and Amhr2 are each required for Müllerian duct regression. Male mice homozygous for either Amh or Amhr2 null alleles retain Müllerian duct derived structures, including a complete uterus and oviducts (7, 8). Further, mutations of either the AMH or AMHR2 genes in human males result in Persistent Mullerian Duct Syndrome (PMDS). Like mouse models with Amh and Amhr2 loss, patients with PMDS have testes and male secondary sex characteristics but retain female reproductive tract organs associated with their male reproductive tract (9–11). A mutation in Amhr2 in dogs is also known to result in PMDS (12). Males of these species with loss of either AMH or AMHR2 also have subfertility likely due to a combination of the effects of cryptorchidism (observed in humans and dogs) and the presence of superimposed Müllerian duct-derived tissues impeding sperm passage (mouse, human and dog) (9, 12). AMH alone is also sufficient for Müllerian duct regression in female embryos. Transgenic female mice with widespread expression of human AMH have complete regression of the Müllerian ducts and lack a uterus and oviducts (13).
Few downstream effectors of AMH have been identified. In mice, loss of the signaling molecule beta-catenin encoded by Ctnnb1 in the Müllerian duct mesenchyme results in the complete retention of the Müllerian duct in newborn males (14). In a recent RNA-seq study we compared male and female mouse Müllerian duct mesenchyme transcriptomes soon after the initiation of AMH signaling in males to identify potential AMH-induced target genes. In that study we identified the BMP target Osterix/Sp7 (Osx) (15, 16). We found that AMH signaling is necessary and sufficient for Osx expression. Osx null male mice have a delay in Müllerian duct regression but later Müllerian duct derived tissues are eliminated (16).
This transcriptome analysis also revealed differential upregulation of BMP target genes Dlx5 and Dlx6 in the Müllerian mesenchyme of males in comparison to females. Based on this transcriptome data and that the BMP pathway shares multiple signaling components with the AMH signaling pathway, we further characterize Dlx5 and Dlx6 expression and function during Müllerian regression. We show that AMH signaling is necessary for Dlx5 and Dlx6 expression in Müllerian duct mesenchyme. Loss of Dlx5 or Dlx6 results in partial retention of the Müllerian ducts in male mice, whereas double mutant males consistently retain more Müllerian tissue. This study identifies Dlx5 and Dlx6 as redundant downstream effectors of AMH signaling for Müllerian regression.
Materials and methods
Mice
Swiss outbred mice were obtained from Taconic Biosciences. C57BL/6J mice were obtained from the Jackson Laboratory. Dlx6tm1Jlr mice were obtained from the MMRRC (Mutant Mouse Resource and Research Center). Amhr2tm3(cre)Bhr [Amhr2-Cre (17),], Amhr2tm2Bhr (Amhr2-lacZ (18), Dlx5tm1Levi [Dlx5-lacZ (19),], Dlx6tm1Jlr (Dlx6-lacZ (20), Dlx5/Dlx6tm1Levi [Dlx5-6 (21),] mice were maintained on a predominantly C57BL/6J genetic background. Amhr2-Cre, Amhr2-lacZ, Dlx5-lacZ, Dlx6-lacZ, and Dlx5-6 mice were genotyped by PCR as described previously. All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Texas MD Anderson Cancer Center. Studies were performed consistent with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
β-galactosidase staining
Dlx5-lacZ and Dlx6-lacZ heterozygous males were bred with Swiss females to establish timed matings for β-galactosidase (β-gal) staining as previously described (16).
Immunofluorescence
Immunofluorescence was performed as previously described (22). Rabbit anti-DLX5 polyclonal antibody (Sigma-Aldrich Cat# HPA005670, RRID : AB_1078681, 1:50). Rabbit anti-PAX2 polyclonal antibody (Thermo Fisher Scientific Cat# 71-6000, RRID : AB_2533990, 1:100). Primary antibodies were detected with goat anti-rabbit IgG (H+L) Alexa Fluor Plus 488 rabbit (Thermo Fisher Scientific Cat# A32731TR, RRID : AB_2866491, 1:200). At least three specimens of each genotype were analyzed.
Microscopy and image analyses
Z-stack images of wholemount immunofluorescent staining for PAX2 were acquired using an A1 Nikon confocal microscope. Linear length in microns of Wolffian duct epithelium and retained Müllerian duct epithelium marked by PAX2 immunofluorescent staining in 3D volume rendered confocal images were measured in Imaris (Bitplane). Length of Wolffian duct epithelium was measured from point of fusion with seminal vesicle to start of coiling in epididymis on left and right sides. Retained Müllerian duct epithelium lengths were measured along the Wolffian duct epithelium on left and right sides beginning at the point of Müllerian duct fusion at the urogenital sinus.
Statistical analyses
Measurements of retained Müllerian duct epithelium length to Wolffian duct epithelium length ratios in Dlx5-6 homozygous mutant and control males were subjected to a Welch’s t-test (t-test; two-sample assuming unequal variances) using Microsoft Excel. A P value of less than .05 was considered statistically significant.
Results
Dlx5 and Dlx6 expression in the developing reproductive organs is sexually dimorphic
Previously, we generated transcriptomes by RNA-seq of FACS-purified Müllerian duct mesenchyme cells from E14.5 male and female embryos to identify candidate genes induced by AMH that mediate Müllerian duct regression (16). At this stage of development, male mesenchymal cells are responding to AMH secreted by the testes, whereas in female embryos these cells are naïve to AMH because the fetal ovaries do not express AMH. Analysis of the bulk RNA-seq results showed that Dlx5 transcripts were 6.7-fold higher in males compared to females and Dlx6 transcripts were found only in males, suggesting these genes may respond to AMH signaling to mediate Müllerian duct regression (Table 1).
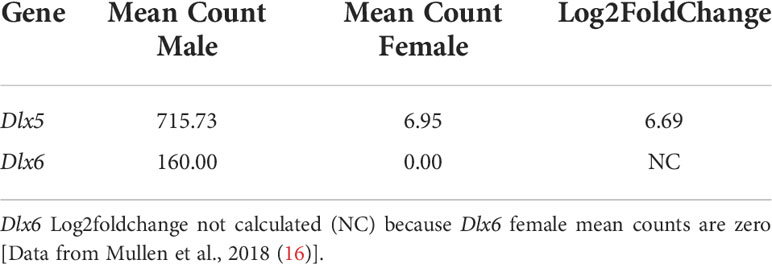
Table 1 Dlx5 and Dlx6 RNA-Seq mean of counts normalized for sequencing depth and Dlx5 Log2foldchange.
Previous studies in the mouse showed that Dlx5 is expressed in the epithelium of the E18.5 uterus and subsequently in the luminal and glandular epithelia of the postnatal and adult uterus (23). In that study, expression was also detected earlier in the Müllerian ducts at E15.5 and E16.5. We examined DLX5 expression in the Müllerian ducts of E14.5 male and female embryos by immunofluorescence (Figure 1). In males, DLX5 was detected in the mesenchymal cells surrounding the Müllerian duct epithelium (Figure 1A). In contrast, but consistent with previous studies (23), DLX5 expression was detected in the epithelial cells of the Müllerian duct of E14.5 female embryos (Figure 1B).
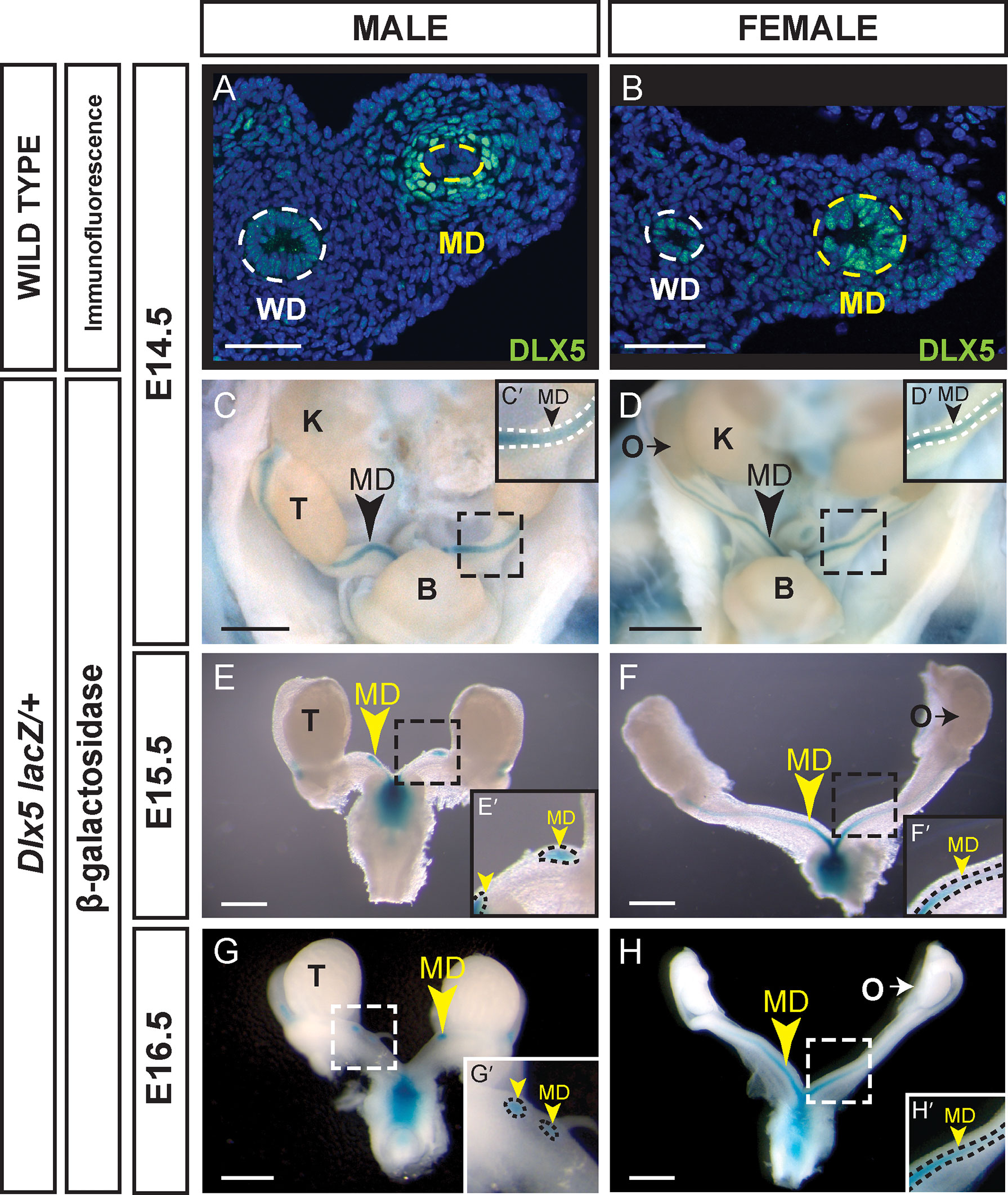
Figure 1 Sexually dimorphic expression of DLX5 in the male Müllerian duct mesenchyme and female Müllerian duct epithelium. Immunofluorescent staining of DLX5 in cross sections of the mesonephros from E14.5 male (A) and female (B) embryos. Dotted lines surround MD and Wolffian duct (WD) epithelium. Scale bars = 50 µm. Dlx5-lacZ expression in male MD is lost as MD regresses. β-gal-stained urogenital organs from E14.5 to 16.5 Dlx5-lacZ heterozygous male (C, E, G) and female (D, F, H) embryos. Dashed square boxes show location of higher magnification insets. Arrowheads, Müllerian ducts. MD, Müllerian duct; WD, Wolffian duct; B, bladder; K, kidney; O, ovary; T, testis. Scale bars = 500 µm. (C’- H’) Higher magnification insets. Arrowheads, Müllerian ducts. MD. Dotted lines surround MD. Each sex and genotype analyzed n≥3.
We also used a Dlx5-lacZ knock-in allele to examine Dlx5 expression in the developing reproductive organs (19, 20). Dlx5-lacZ expression was detected at E14.5 in the Müllerian ducts of both male and female embryos (Figures 1C, D). In male and female embryos, β-gal staining was observed throughout the entire length of the Müllerian ducts. At E15.5 and E16.5, Dlx5-lacZ expression persisted in the residual Müllerian ducts of male embryos (Figures 1E, G). Consistent with earlier studies, in Dlx5-lacZ female embryos at E15.5 and E16.5, β-gal staining was detected throughout the entire length of the Müllerian ducts with strongest staining caudally (Figures 1E, G) (23). These data indicate that Dlx5 is expressed in the male Müllerian duct mesenchyme and female Müllerian duct epithelium. Thus, Dlx5 exhibits a sexually dimorphic pattern of expression in the developing Müllerian system.
We used a Dlx6-lacZ knock-in allele (20) to examine Dlx6 expression in the developing reproductive organs (Figure 2). Dlx6-lacZ expression in male embryos was detected at E14.5 and E15.5 throughout the entire length of the Müllerian ducts and later at E16.5 as the Müllerian duct was regressing (Figures 2A-C). Histological analysis showed that β-gal expression was localized to the Müllerian duct mesenchyme (Figure 2B’). No β-gal staining was detected in the Müllerian ducts of female embryos at each time point examined (E14.5 to E16.5) (Figures 2D-F). These results show that Dlx6 is expressed in the male Müllerian duct mesenchyme. The male-specific expression of Dlx6 in the developing reproductive organs demonstrates that it is expressed in a sexually dimorphic pattern. These findings also show that Dlx5 and Dlx6 are co-expressed in the male Müllerian duct mesenchyme at the initiation and during ductal regression.
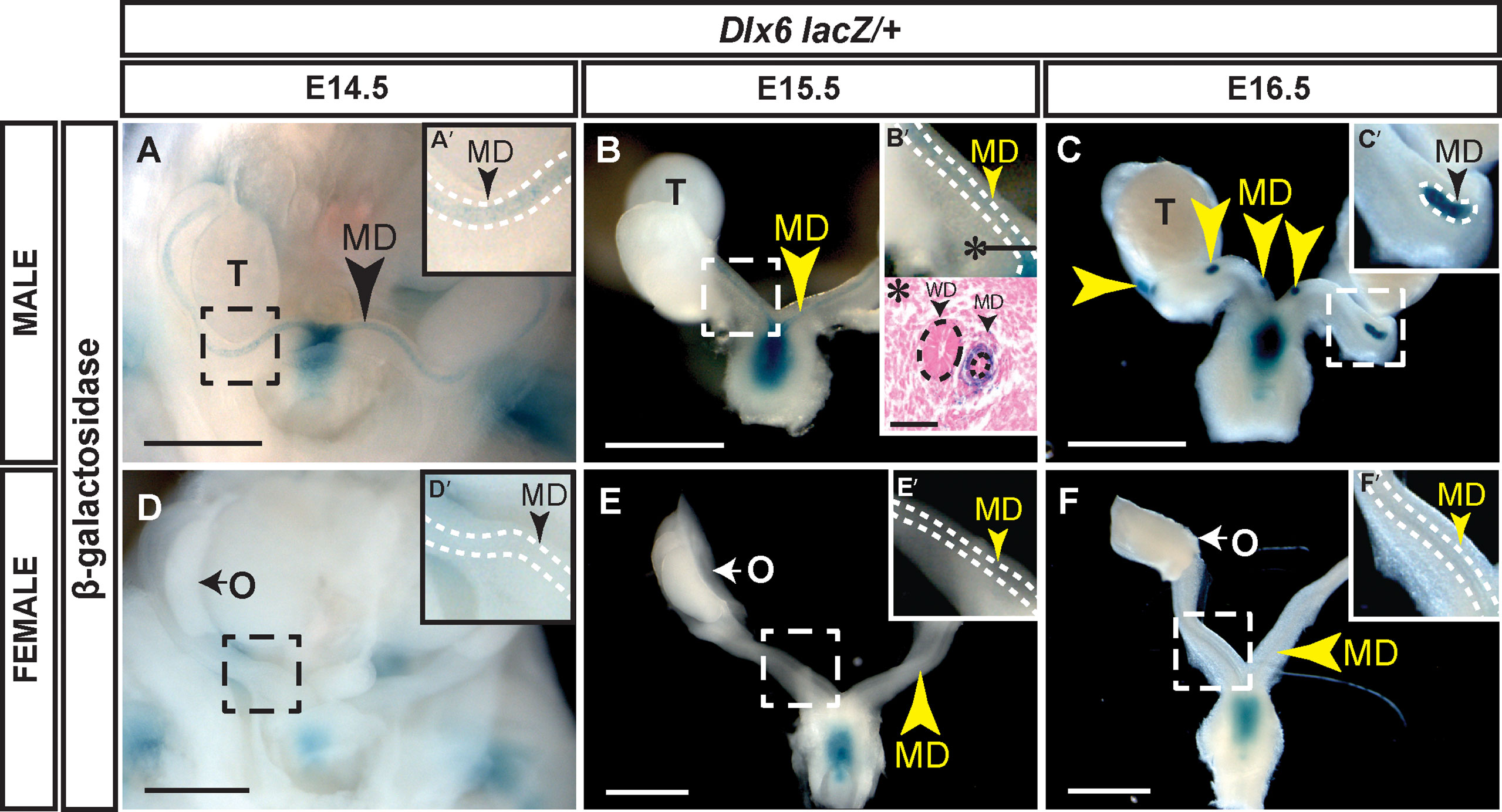
Figure 2 Dlx6-lacZ expression in male Müllerian duct mesenchyme but not in female Müllerian ducts. Wholemount β-gal-stained urogenital organs from E14.5 to 16.5 Dlx6-lacZ heterozygous male (A-C) and female (D-F) embryos. Arrowheads, Müllerian ducts. MD, Müllerian ducts; O, ovary; T, testis. Dashed square boxes show location of higher magnification insets. Scale bars = 500 µm. (A’-F’) Higher magnification insets. Arrowheads, Müllerian ducts. MD, Müllerian duct. Dotted white lines surround MD. (B’*) Eosin counterstained paraffin section (10 µm) of wholemount β-gal-stained E15.5 Dlx6-lacZ heterozygous male. Asterisk denotes location of paraffin cross section. Arrowheads, Müllerian ducts. MD; Wolffian ducts, WD. Dotted lines surround MD and WD epithelium. Scale bars = 50 µm. Each sex and genotype analyzed n≥3.
AMH is necessary for Dlx5 and Dlx6 expression in the Müllerian duct mesenchyme
The temporal and spatial patterns of expression for Dlx5 and Dlx6 in the developing male Müllerian duct mesenchyme are consistent with the idea that they are induced by AMH signaling. AMH is expressed in the fetal testis at ~E12.0 (24). At ~E13.5 Amhr2 expression is activated in the Müllerian duct mesenchyme initiating AMH signaling (18). We first observe Dlx5 and Dlx6 expression in the Müllerian duct mesenchyme at ~E14.5, a timing consistent with their activation by the AMH-signaling pathway. To test this idea, we performed genetic experiments examining Dlx5 and Dlx6 expression in the absence of AMH signaling in male embryos.
To determine if AMH signaling is required for Dlx5 expression, we performed DLX5 immunostaining of the Müllerian ducts of Amhr2 Cre/lacZ mutant males at E14.5 (Figure 3). Amhr2-Cre and Amhr2-lacZ are knock-in alleles that are also loss-of-function alleles (17, 18). Thus, Amhr2 Cre/lacZ mutants lack Amhr2 function, blocking AMH signaling, resulting in males with a retained and fully developed Müllerian system (8). DLX5 positive Müllerian duct mesenchyme cells were detected in wild-type male controls (Figure 3A). No DLX5 immunostaining was detected in the Müllerian duct mesenchyme of Amhr2 Cre/lacZ mutant males (Figure 3B). This suggests that DLX5 expression requires AMH signaling.
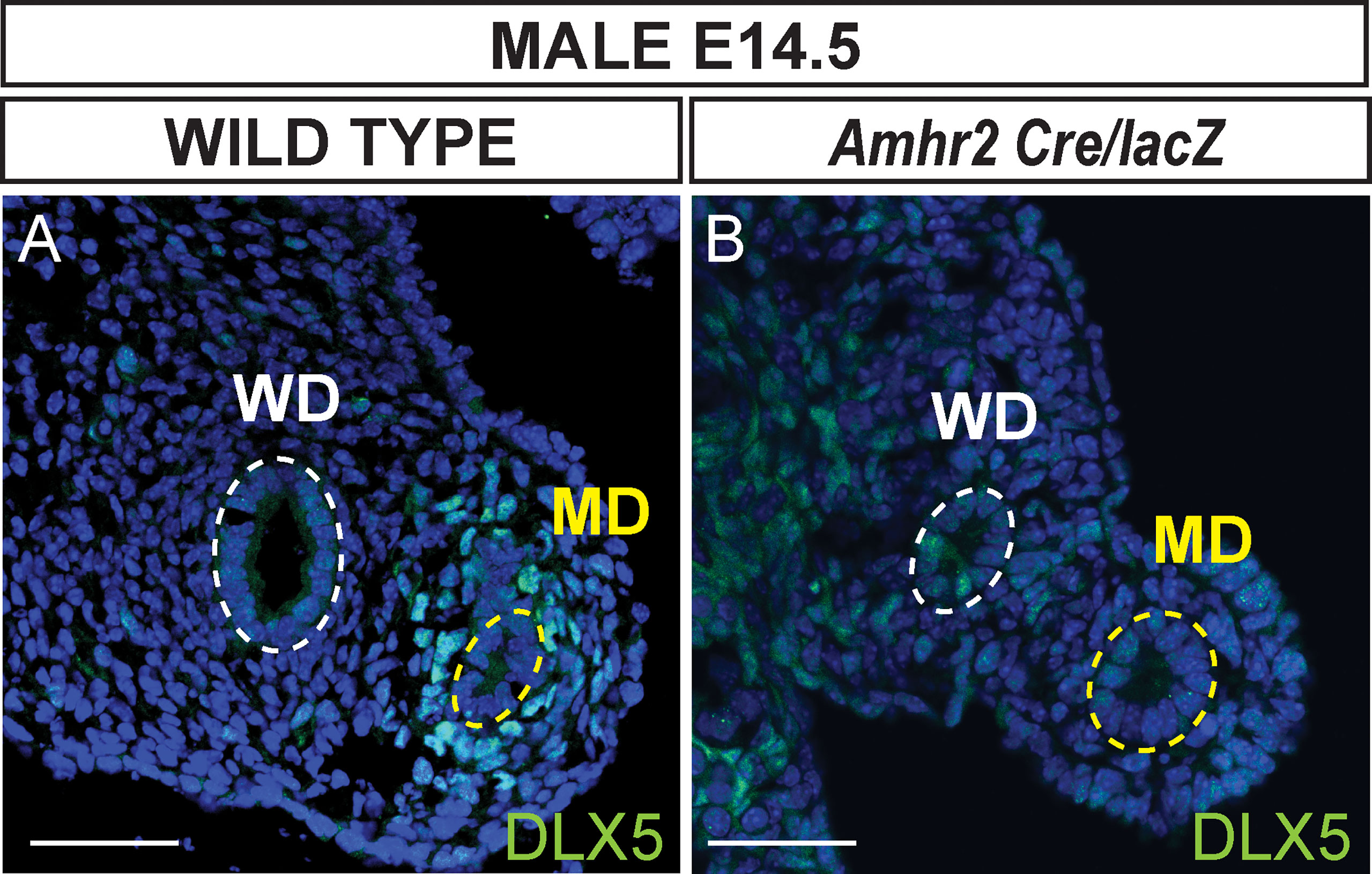
Figure 3 AMH signaling is required for DLX5 expression. Immunofluorescent staining of DLX5 in cross sections of the mesonephros from E14.5 wild-type (A) and Dlx5-lacZ/+; Amhr2-Cre/lacZ (B) male embryos. DLX5 is not expressed in Amhr2-Cre/lacZ males, indicating AMH signaling is required for DLX5 expression in the male Müllerian duct (MD). Yellow dotted lines surround the MD epithelium; white dotted lines surround the Wolffian duct epithelium (WD). MD, Müllerian duct; WD, Wolffian duct. Scale bars = 50 µm. Each genotype analyzed n=3.
To determine if AMH signaling was required for Dlx6 expression, we generated E15.5 Dlx6-lacZ/+; Amhr2-Cre/Cre male embryos (Figure 4). β-gal staining was detected in the residual Müllerian duct tissue of Dlx6-lacZ/+ embryos (Figure 4A). However, no β-gal staining was observed in the Müllerian ducts of E15.5 Dlx6-lacZ/+; Amhr2-Cre/Cre male embryos that retained a complete Müllerian system due to the block in AMH signaling (Figure 4B). This suggests that Dlx6-lacZ expression requires AMH signaling.
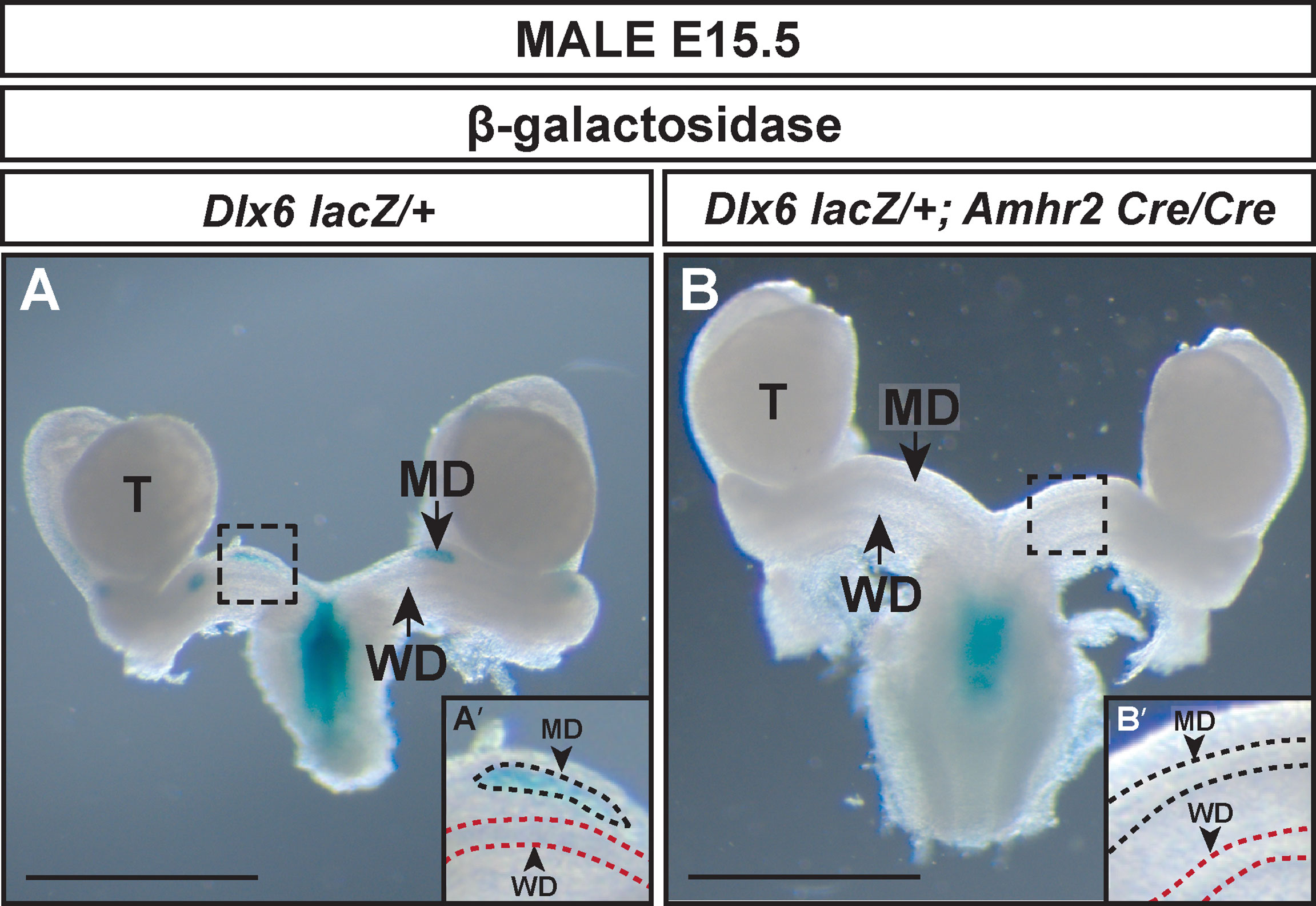
Figure 4 AMH signaling is required for Dlx6-lacZ expression in the Müllerian duct. Wholemount β-gal-stained urogenital organs from E15.5 Dlx6-lacZ/+ (A) and Dlx6-lacZ/+; Amhr2-Cre/Cre (B) male embryos. Arrow in (A), residual β-gal-stained Müllerian duct (MD) tissue. Arrow in (B), absence of β-gal-stained Müllerian duct tissue in fully retained Müllerian duct caused by loss of Amhr2. Dashed square boxes show location of higher magnification insets. MD, Müllerian duct; T, testis; WD, Wolffian duct. Scale bars=1000 µm. (A’, B’) Higher magnification insets. Arrowheads, Müllerian ducts, MD; Wolffian ducts, WD. Dotted lines surround MD (black) and WD (red). Each genotype analyzed n=3.
Dlx5 and Dlx6 regulate Müllerian duct regression
Dlx5-lacZ homozygotes die shortly after birth with craniofacial defects (19). In wild-type male embryos, most of the Müllerian duct is regressed at E16.5 and regression is complete by E18.5. We initially screened for the presence of residual Müllerian duct-derived tissues (uterus) by histological analysis of Dlx5-lacZ homozygous mutants at E18.5 (Figures 5A, B). None of the 4 Dlx5-lacZ homozygous mutant males screened by histological sectioning had uterine tissue (Figure 5B). We next examined Müllerian duct regression in Dlx5-lacZ homozygous mutant males and controls at E16.5, using lacZ expression to mark the Müllerian duct. Five of 6 Dlx5-lacZ homozygous mutant males analyzed had Müllerian duct regression like controls (Figure 5C). However, one of the mutants retained a significant amount of Müllerian duct (Figure 5D). These results indicate that Dlx5 contributes to Müllerian duct regression.
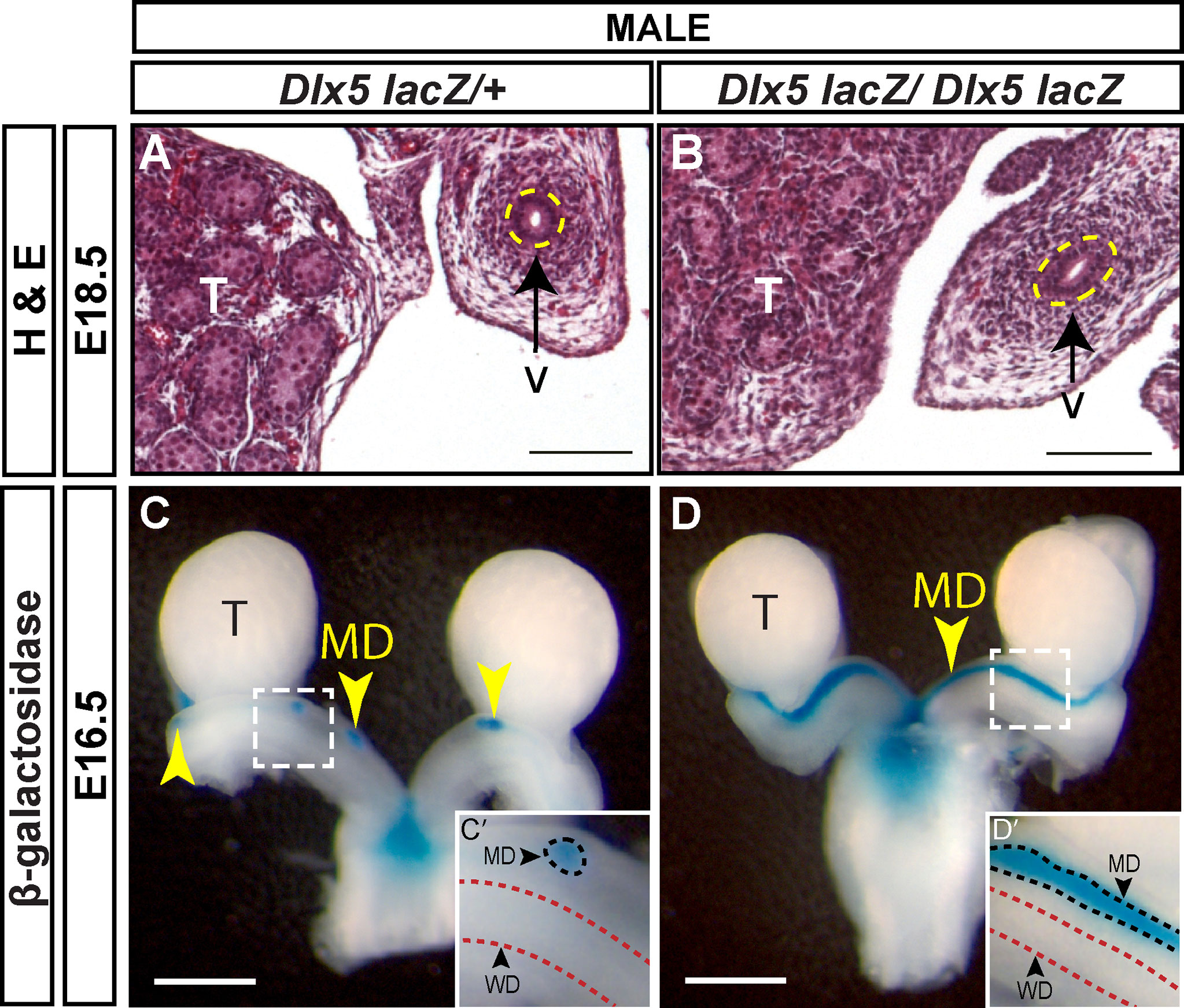
Figure 5 Defects in Müllerian regression are observed in some Dlx5 mutants at E16.5 but are resolved by E18.5. Hematoxylin (H) and Eosin (E) stained cross sections of the vas deferens (v) adjacent to the testis (T) from E18.5 Dlx5-lacZ/+ (A) and Dlx5-lacZ/Dlx5-lacZ (B) male embryos. Yellow dotted lines surround the vas deferens epithelium. Scale = 100 µm. Wholemount β-gal-stained urogenital organs from E16.5 Dlx5-lacZ/+ (C) and Dlx5-lacZ/Dlx5-lacZ (D) male embryos. Yellow arrowheads, Müllerian ducts. T, testis; MD, Müllerian duct. Scale bars = 500 µm. (C’, D’) Higher magnification insets. Arrowheads, Müllerian ducts, MD; Wolffian ducts, WD. Dotted lines surround MD (black) and WD (red). E18.5 each genotype analyzed (n=4); E16.5 Dlx5-lacZ/+ (n=5); E16.5 Dlx5-lacZ/lacZ (n=6).
Dlx6-lacZ homozygous mutants die within a day after birth also with craniofacial abnormalities (20). Like the Dlx5-lacZ mutants, we examined Müllerian duct regression by lacZ expression in mutants and controls at E16.5 and E18.5. At E16.5, control males showed very small β-gal positive regions, indicating residual Müllerian duct tissue (Figures 6A, C). In contrast, in E16.5 Dlx6-lacZ homozygous mutant male embryos there were still significant stretches of β-gal staining notably in the Müllerian ducts adjacent to the testes (Figures 6B, D). However, by E18.5, Müllerian duct regression in Dlx6-lacZ homozygous mutant male embryos was comparable to control males (Figures 6E, F). These results suggest that loss of Dlx6 results in a delay in Müllerian duct regression and Dlx6 contributes to Müllerian duct regression.
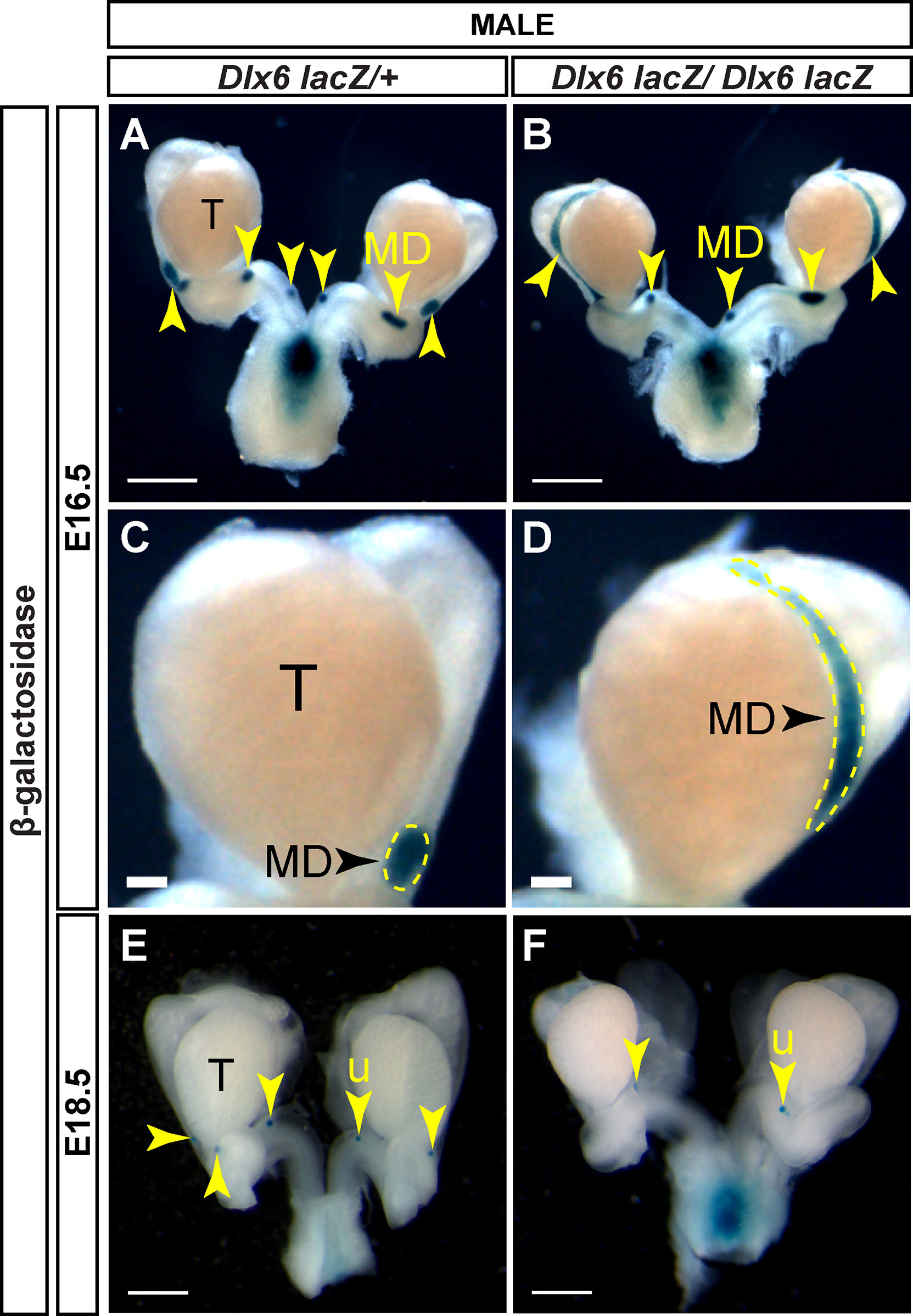
Figure 6 Loss of Dlx6 results in a delay in Müllerian duct regression. Wholemount β-gal-stained urogenital organs from E16.5 Dlx6-lacZ/+ (A) and Dlx6-lacZ/Dlx6-lacZ (B) male embryos. Higher magnification of testis and adjacent Müllerian duct from E16.5 Dlx6-lacZ/+ (C) and Dlx6-lacZ/Dlx6-lacZ (D) male embryos. Wholemount β-gal-stained urogenital organs from E18.5 Dlx6-lacZ/+ (E) and Dlx6-lacZ/Dlx6-lacZ (F) male embryos. Arrowheads, residual Müllerian ducts (A-D) and uterus (E, F). Yellow dotted lines surround Müllerian ducts (C,D). T, testis; MD, Müllerian duct; u, uterus. E16.5 Dlx6-lacZ/+ (n=5); E16.5 Dlx6-lacZ/lacZ (n=4); E18.5 Dlx6-lacZ/+ (n=5); E18.5 Dlx6-lacZ/lacZ (n=4). Scale bars = 500 µm (A, B, E, F), 100 µm (C,D).
Müllerian duct regression in Dlx5 male homozygous mutants was variable, whereas in Dlx6 male homozygous mutants it was delayed. Dlx5 and Dlx6 are co-expressed in the male Müllerian duct mesenchyme, suggesting that they may act together for Müllerian duct regression. Therefore, we examined Müllerian duct regression in Dlx5-6 homozygous mutant males. The Dlx5-6 allele (Dlx5/Dlx6tm1Levi) is a deletion of both Dlx5 and Dlx6 coding regions that also contains a lacZ reporter (21). However, the lacZ reporter in this allele is not functional. Pax2 is expressed in the developing Müllerian duct epithelium (25). Therefore, we followed Müllerian duct regression by wholemount immunostaining for PAX2. At E16.5, much like the Dlx5 male homozygous mutants, one Dlx5-6 homozygous mutants had near complete retention of the Müllerian duct epithelium while others were comparable to wild type (Figures 7A, B). To quantify the portion of Müllerian duct epithelium retained in Dlx5-6 homozygous mutants and controls we calculated the ratio of the length of retained Müllerian duct epithelium to Wolffian duct epithelium from the whole mount images (Figure 8). Ratios for Dlx5-6 homozygous mutants were 0.70 (Figure 7B), 0.32 and 0.27 in comparison to ratios of controls 0.38, 0.22, 0.24 and 0.19 (Figure 7A). At E18.5 ratios of retained uterine epithelium to vas deferens epithelium for Dlx5-6 homozygous mutants were significantly increased in comparison to controls (Figure 7C (ratio 0.03), Figure 7D (ratio 0.35) and Figure 8). Consistent with these results, initial histology at E18.5 detected retained uterine tissue in 1 of 4 Dlx5-6 homozygous mutant males examined (Figures 7E, F). These results suggest that Dlx5 and Dlx6 contribute to Müllerian duct regression and may be functioning redundantly.

Figure 7 Dlx5-6 mutant males retain significant portions of uterine tissue at E18.5 compared to controls. Wholemount PAX2 immunofluorescent staining of E16.5 Dlx5-6/+ (A) and Dlx5-6/Dlx5-6 (B) male embryos and E18.5 Dlx5-6/+ (C) and Dlx5-6/Dlx5-6 (D) male embryos. Yellow arrowheads, Müllerian ducts (A, B) and uterus (C, D). White arrowheads, Wolffian ducts (A, B) and vas deferens (C, D). MD, Müllerian duct; WD, Wolffian duct; T, testis; v, vas deferens; u, uterus. Scale bars = 500 µm. Hematoxylin (H) and Eosin (E) stained cross sections of the vas deferens (v) adjacent to the testis (T) from E18.5 Dlx5-6/+ (E) and Dlx5-6/Dlx5-6 (F) male embryos. u, uterus. Scale bars =100 µm. E16.5 Dlx5-6/+ (n=4); E16.5 Dlx5-6/Dlx5-6 (n=3); E18.5 Dlx5-6/+ (whole mount n=6, histology n=4); E18.5 Dlx5-6/Dlx5-6 (whole mount n=3, histology n=3).
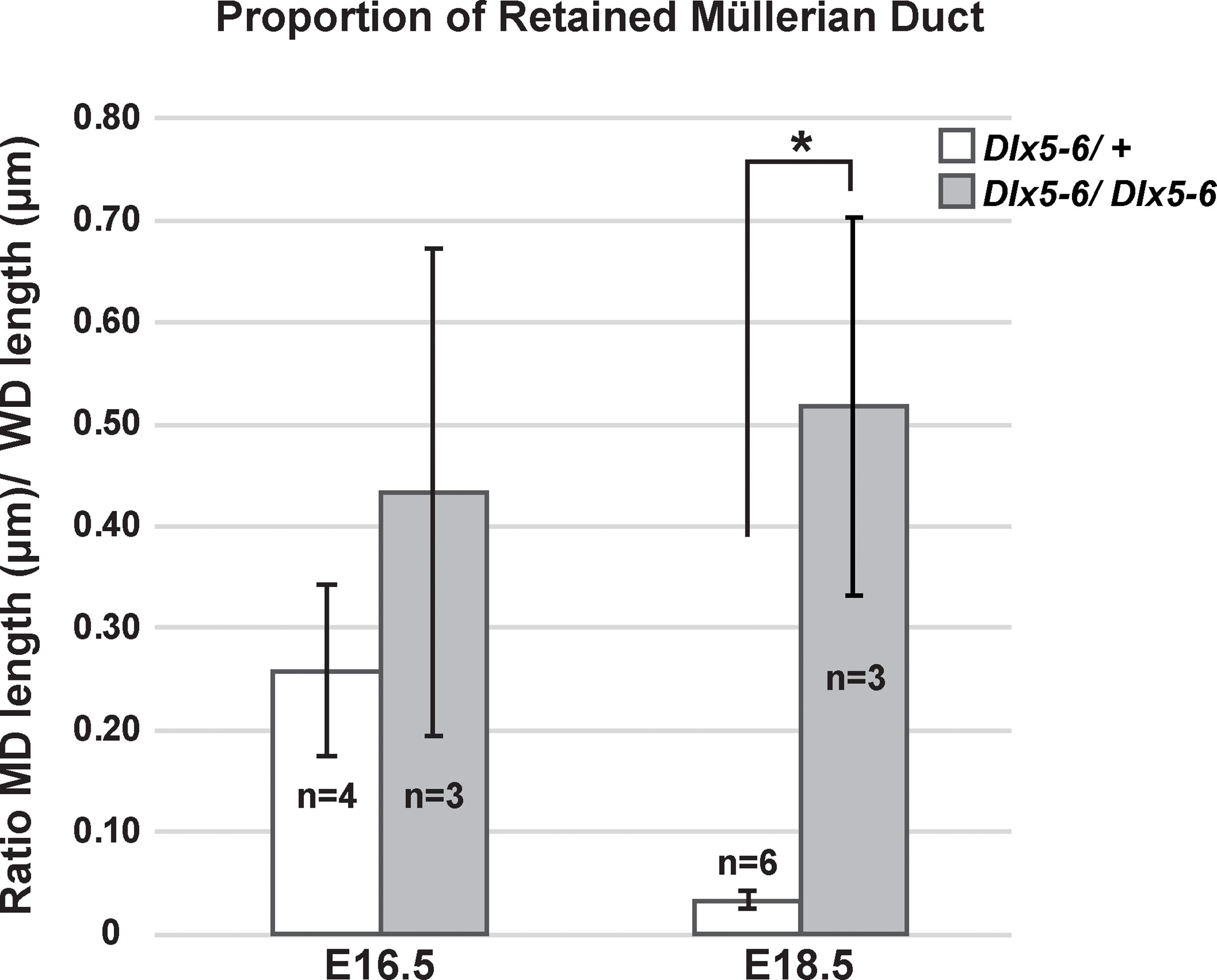
Figure 8 Proportion of retained Müllerian duct in Dlx5-6 mutant males is increased at E18.5 in comparison to controls. Quantification of the ratio of retained Müllerian duct epithelium length to Wolffian duct epithelium length in Dlx5-6/+ and Dlx5-6/Dlx5-6 males at E16.5 and E18.5. Data are presented as mean ± standard deviation. Asterisk (*) indicates statistically significant difference (p ≤ 0.05).
Discussion
Previously, we compared the transcriptomes of FACS-isolated E14.5 male and female Müllerian duct mesenchyme cells in a screen for AMH-induced genes that mediate Müllerian duct regression during male differentiation (16). In our initial analysis, we identified Osx as an AMH-induced gene that is expressed in the male Müllerian duct mesenchyme to regulate Müllerian duct regression. Upon further analysis of the transcriptomes, we found that the distal-less homeobox genes Dlx5 and Dlx6 were upregulated in males relative to females. We found that both genes are expressed in the male Müllerian duct mesenchyme and are dependent on AMH signaling. The Dlx5-null and Dlx6-null male embryos (19, 20) showed variable retention of the Müllerian ducts and a delay in Müllerian duct regression, whereas the Dlx5-6-null male embryos (21) retained more Müllerian duct tissue compared to each single mutant. These findings place a new pair of transcription factors that act together in the gene regulatory network to mediate AMH-induced Müllerian duct regression during male differentiation (26).
AMH signaling is required for Dlx5 and Dlx6 expression in the male Müllerian duct mesenchyme
We found that both Dlx5 and Dlx6 are expressed in the male Müllerian duct mesenchyme during the embryonic stages coinciding with the initiation and progression of Müllerian duct regression. Dlx genes are organized as linked pairs in the mammalian genome (27). These Dlx gene pairs are typically co-expressed in various tissues, suggesting common cis regulation. Perhaps Dlx5 and Dlx6 share Müllerian duct mesenchyme-specific regulatory elements that respond to AMH signaling.
Previously, we discovered that Osx was expressed in the male but not in female Müllerian duct mesenchyme (16). Our observations of Dlx5 and Dlx6 expression add to a growing list of genes that exhibit sexually dimorphic patterns of expression in the Müllerian ducts. We found that the expression of Dlx5, and Dlx6 in the Müllerian duct mesenchyme was dependent upon AMH signaling through AMHR2. Similar results were found for Osterix expression (16). Amhr2 is expressed in the Müllerian duct mesenchyme of both male and female embryos (6, 17, 18). The female Müllerian duct mesenchyme is competent to respond to AMH because ectopic AMH can induce Müllerian duct regression, eliminating the development of the uterus and oviducts (13). Thus, the sexually dimorphic expression of Osx, Dlx5, and Dlx6 expression in the Müllerian duct mesenchyme mirrors the sexually dimorphic expression of fetal AMH.
Interestingly, Dlx5 is also expressed in the Müllerian duct of female embryos. However, in contrast to mesenchymal expression in male embryos, expression in female embryos was localized to the Müllerian duct epithelium. This epithelial expression persists at later stages of embryogenesis and postnatally in the luminal and glandular epithelium of the uterus (23). We found that Dlx6 expression in the Müllerian duct was only in male embryos; no expression was detected in female embryos. However, Dlx6 expression is detected later in the postnatal uterine epithelium (23). These observations suggest that the expression of Dlx5 in the epithelium of the female Müllerian duct is independent of AMH signaling, suggesting that other factors direct expression in this tissue compartment.
Dlx5 and Dlx6 mediate Müllerian duct regression
Simultaneous deletion of Dlx5 and Dlx6 in the postnatal uterus leads to alterations in uterine adenogenesis and infertility, suggesting that they act redundantly for epithelial morphogenesis in the uterus (23). In addition to the postnatal uterus, redundant functions for Dlx5 and Dlx6 have also been reported for limb and craniofacial development (19–21, 28, 29). The overlapping expression patterns of Dlx5 and Dlx6 suggested that they functioned redundantly in the male Müllerian duct mesenchyme. Male homozygotes for each single mutation retain variable amounts of Müllerian tissue and male homozygous double mutants retain more Müllerian tissue. Interestingly, the Dlx5-6 double mutant males did not show complete retention and differentiation of the Müllerian system like Amh and Amhr2 mutant males (7, 8). The incompletely penetrant Müllerian duct regression phenotypes in the Dlx5-6 double mutant male embryos suggests that there are other mediators of AMH-induced Müllerian duct regression that act together with Dlx5-6 (e.g. Osx). Previous studies suggest that there are significantly excess levels of AMH produced for Müllerian duct regression because Amh levels must be reduced to ~10% of wild type to uncover partial Müllerian duct regression phenotypes (18). Perhaps downstream target genes of AMH signaling must be collectively inactivated for a complete block to Müllerian duct regression (30,31).
Dlx genes are known targets of BMP signaling (26). Interestingly, AMH signal transduction shares receptors (BMPR1A and ACVR1) and downstream R-SMAD proteins (SMAD1, 5, 8) with BMP signaling (4, 5). Considering the temporal and spatial expression of Dlx5 and Dlx6 in the Müllerian duct mesenchyme requires AMH signaling it is possible that they are direct transcriptional targets. Osx was discovered as a BMP-induced gene (15). Osx, Dlx5, and Dlx6 each have roles in skeleton development and Müllerian duct regression (15, 16, 19–21, 28, 29). Do these transcription factor genes regulate the same downstream effectors in skeleton-forming and Müllerian duct mesenchyme cells, or do they regulate different sets of target genes? This question can be addressed by identifying tissue specific cis regulatory elements that are bound by OSX, DLX5, and DLX6 and regulate transcription.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was reviewed and approved by Institutional Animal Care and Use Committee of the University of Texas MD Anderson Cancer Center.
Author contributions
RM and RB conceived the study and designed experiments. RM performed experiments and analyzed data. BB and GL generated mutant embryos. RM and RB wrote the paper with input from all authors. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by National Institutes of Health (NIH) grant HD030284 and the Ben F. Love Endowment to R.R.B. Confocal microscopy was supported by NIH Shared Instrumentation Grant (1S10OD024976-01). Veterinary resources were supported by NIH Grant CA16672.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgements
We thank Dr. Ying Wang for advice on immunofluorescent studies, Kazi Hossain for technical assistance with microscopy and the members of our labs for helpful discussions.
References
1. Mullen RD, Behringer RR. Molecular genetics of mullerian duct formation, regression and differentiation. Sex Dev (2014) 8:281–96. doi: 10.1159/000364935
2. Josso N, Cate RL, Picard JY, Vigier B, di Clemente N, Wilson C, et al. Anti-mullerian hormone: the jost factor. Recent Prog Horm Res (1993) 48:1–59. doi: 10.1016/b978-0-12-571148-7.50005-1
3. Josso N, Picard JY. Genetics of anti-mullerian hormone and its signaling pathway. Best Pract Res Clin Endocrinol Metab (2022) 36:101634. doi: 10.1016/j.beem.2022.101634
4. Orvis GD, Jamin SP, Kwan KM, Mishina Y, Kaartinen VM, Huang S, et al. Functional redundancy of TGF-beta family type I receptors and receptor-smads in mediating anti-mullerian hormone-induced mullerian duct regression in the mouse. Biol Reprod (2008) 78:994–1001. doi: 10.1095/biolreprod.107.066605
5. Jamin SP, Arango NA, Mishina Y, Hanks MC, Behringer RR. Requirement of Bmpr1a for mullerian duct regression during male sexual development. Nat Genet (2002) 32:408–10. doi: 10.1038/ng1003
6. Baarends WM, van Helmond MJ, Post M, van der Schoot PJ, Hoogerbrugge JW, de Winter JP, et al. A novel member of the transmembrane serine/threonine kinase receptor family is specifically expressed in the gonads and in mesenchymal cells adjacent to the mullerian duct. Development (1994) 120:189–97. doi: 10.1242/dev.120.1.189
7. Behringer RR, Finegold MJ, Cate RL. Mullerian-inhibiting substance function during mammalian sexual development. Cell (1994) 79:415–25. doi: 10.1016/0092-8674(94)90251-8
8. Mishina Y, Rey R, Finegold MJ, Matzuk MM, Josso N, Cate RL, et al. Genetic analysis of the mullerian-inhibiting substance signal transduction pathway in mammalian sexual differentiation. Genes Dev (1996) 10:2577–87. doi: 10.1101/gad.10.20.2577
9. Picard JY, Josso N. Persistent mullerian duct syndrome: an update. Reprod Fertil Dev (2019) 31:1240–5. doi: 10.1071/RD17501
10. Imbeaud S, Faure E, Lamarre I, Mattei MG, di Clemente N, Tizard R, et al. Insensitivity to anti-mullerian hormone due to a mutation in the human anti-mullerian hormone receptor. Nat Genet (1995) 11:382–8. doi: 10.1038/ng1295-382
11. Knebelmann B, Boussin L, Guerrier D, Legeai L, Kahn A, Josso N, et al. Anti-mullerian hormone bruxelles: a nonsense mutation associated with the persistent mullerian duct syndrome. Proc Natl Acad Sci USA (1991) 88:3767–71. doi: 10.1073/pnas.88.9.3767
12. Wu HN, Li HX. Adaptive neural control design for nonlinear distributed parameter systems with persistent bounded disturbances. IEEE Trans Neural Netw (2009) 20:1630–44. doi: 10.1109/TNN.2009.2028887
13. Behringer RR, Cate RL, Froelick GJ, Palmiter RD, Brinster RL. Abnormal sexual development in transgenic mice chronically expressing mullerian inhibiting substance. Nature (1990) 345:167–70. doi: 10.1038/345167a0
14. Kobayashi A, Stewart CA, Wang Y, Fujioka K, Thomas NC, Jamin SP, et al. Beta-catenin is essential for mullerian duct regression during male sexual differentiation. Development (2011) 138:1967–75. doi: 10.1242/dev.056143
15. Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, et al. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell (2002) 108:17–29. doi: 10.1016/s0092-8674(01)00622-5
16. Mullen RD, Wang Y, Liu B, Moore EL, Behringer RR. Osterix functions downstream of anti-mullerian hormone signaling to regulate mullerian duct regression. Proc Natl Acad Sci USA (2018) 115:8382–7. doi: 10.1073/pnas.1721793115
17. Jamin SP, Arango NA, Mishina Y, Behringer RR. Genetic studies of the AMH/MIS signaling pathway for Müllerian duct regression. Mol Cell Endocrinol (2003) 211(1–2):15–9. doi: 10.1016/j.mce.2003.09.006
18. Arango NA, Kobayashi A, Wang Y, Jamin SP, Lee HH, Orvis GD, et al. A mesenchymal perspective of mullerian duct differentiation and regression in Amhr2-lacZ mice. Mol Reprod Dev (2008) 75:1154–62. doi: 10.1002/mrd.20858
19. Acampora D, Merlo GR, Paleari L, Zerega B, Postiglione MP, Mantero S, et al. Craniofacial, vestibular and bone defects in mice lacking the distal-less-related gene Dlx5. Development (1999) 126:3795–809. doi: 10.1242/dev.126.17.3795
20. Jeong J, Li X, McEvilly RJ, Rosenfeld MG, Lufkin T, Rubenstein JL. Dlx genes pattern mammalian jaw primordium by regulating both lower jaw-specific and upper jaw-specific genetic programs. Development (2008) 135:2905–16. doi: 10.1242/dev.019778
21. Merlo GR, Paleari L, Mantero S, Genova F, Beverdam A, Palmisano GL, et al. Mouse model of split hand/foot malformation type I. Genesis (2002) 33:97–101. doi: 10.1002/gene.10098
22. Stewart CA, Wang Y, Bonilla-Claudio M, Martin JF, Gonzalez G, Taketo MM, et al. CTNNB1 in mesenchyme regulates epithelial cell differentiation during mullerian duct and postnatal uterine development. Mol Endocrinol (2013) 27:1442–54. doi: 10.1210/me.2012-1126
23. Bellessort B, Le Cardinal M, Bachelot A, Narboux-Neme N, Garagnani P, Pirazzini C, et al. Dlx5 and Dlx6 control uterine adenogenesis during post-natal maturation: possible consequences for endometriosis. Hum Mol Genet (2016) 25:97–108. doi: 10.1093/hmg/ddv452
24. Munsterberg A, Lovell-Badge R. Expression of the mouse anti-mullerian hormone gene suggests a role in both male and female sexual differentiation. Development (1991) 113:613–24. doi: 10.1242/dev.113.2.613
25. Torres M, Gomez-Pardo E, Dressler GR, Gruss P. Pax-2 controls multiple steps of urogenital development. Development (1995) 121:4057–65. doi: 10.1242/dev.121.12.4057
26. Moses MM, Behringer RR. A gene regulatory network for mullerian duct regression. Environ Epigenet (2019) 5:dvz017. doi: 10.1093/eep/dvz017
27. Panganiban G, Rubenstein JL. Developmental functions of the distal-less/Dlx homeobox genes. Development (2002) 129:4371–86. doi: 10.1242/dev.129.19.4371
28. Beverdam A, Merlo GR, Paleari L, Mantero S, Genova F, Barbieri O, et al. Jaw transformation with gain of symmetry after Dlx5/Dlx6 inactivation: mirror of the past? Genesis (2002) 34:221–7. doi: 10.1002/gene.10156
29. Robledo RF, Rajan L, Li X, Lufkin T. The Dlx5 and Dlx6 homeobox genes are essential for craniofacial, axial, and appendicular skeletal development. Genes Dev (2002) 16:1089–101. doi: 10.1101/gad.988402
30. Roberts LM, Visser JA, Ingraham HA. Involvement of a matrix metalloproteinase in MIS-induced cell death during urogenital development. Development (2002) 129:1487–96. doi: 10.1242/dev.129.6.1487
31. Park JH, Tanaka Y, Arango NA, Zhang L, Benedict LA, Roh MI, et al. Induction of WNT inhibitory factor 1 expression by mullerian inhibiting substance/antiMullerian hormone in the mullerian duct mesenchyme is linked to mullerian duct regression. Dev Biol (2014) 386:227–36. doi: 10.1016/j.ydbio.2013.12.015
Keywords: sex differentiation, anti-Müllerian hormone, reproductive tract development, Dlx5, Dlx6, Amhr2
Citation: Mullen RD, Bellessort B, Levi G and Behringer RR (2022) Distal-less homeobox genes Dlx5/6 regulate Müllerian duct regression. Front. Endocrinol. 13:916173. doi: 10.3389/fendo.2022.916173
Received: 08 April 2022; Accepted: 29 June 2022;
Published: 15 July 2022.
Edited by:
Andrew Pask, The University of Melbourne, AustraliaReviewed by:
Abir Mukherjee, Royal Veterinary College (RVC), United KingdomCraig Smith, Monash University, Australia
Patricia Donahoe, Harvard Medical School, United States
Copyright © 2022 Mullen, Bellessort, Levi and Behringer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Richard R. Behringer, rrb@mdanderson.org
 Rachel D. Mullen
Rachel D. Mullen Brice Bellessort
Brice Bellessort Giovanni Levi2
Giovanni Levi2 Richard R. Behringer
Richard R. Behringer