- 1Department of Pediatrics, Toranomon Hospital, Tokyo, Japan
- 2Department of Pediatrics, Teikyo University School of Medicine, Tokyo, Japan
- 3Fukushima Global Medical Science Center, Fukushima Medical University, Fukushima, Japan
Turner syndrome (TS) is a chromosomal disorder affecting females characterized by short stature and gonadal dysgenesis. Untreated girls with TS reportedly are approximately 20-cm shorter than normal girls within their respective populations. The growth patterns of girls with TS also differ from those of the general population. They are born a little smaller than the normal population possibly due to a mild developmental delay in the uterus. After birth, their growth velocity declines sharply until 2 years of age, then continues to decline gradually until the pubertal age of normal children and then drops drastically around the pubertal period of normal children because of the lack of a pubertal spurt. After puberty, their growth velocity increases a little because of the lack of epiphyseal closure. A secular trend in height growth has been observed in girls with TS so growth in excess of the secular trend should be used wherever available in evaluating the growth in these girls. Growth hormone (GH) has been used to accelerate growth and is known to increase adult height. Estrogen replacement treatment is also necessary for most girls with TS because of hypergonadotropic hypogonadism. Therefore, both GH therapy and estrogen replacement treatment are essential in girls with TS. An optimal treatment should be determined considering both GH treatment and age-appropriate induction of puberty. In this review, we discuss the growth in girls with TS, including overall growth, pubertal growth, the secular trend, growth-promoting treatment, and sex hormone replacement treatment.
1. Introduction
Turner syndrome (TS) is a chromosomal disorder characterized by short stature and gonadal dysgenesis. TS affects 1 in 2,000–2,500 live female births and is the most common chromosomal disorder. Diagnosis is confirmed by chromosomal examination (G-band analysis) and is defined as having a karyotype that contains a cell line of monosomy lacking at least a distal major part in the short arm of the X chromosome (1–3). One of the most significant features of the syndrome is short stature. Untreated girls are reported to be approximately 20-cm shorter than normal girls within their respective populations (4). In addition to their short stature, the growth patterns of girls with TS differ from those of the general population mainly because of the short stature homeobox-containing gene on the X chromosome haploinsufficiency and their ovarian insufficiency. Growth hormone (GH) has been used to accelerate growth and is known to increase adult height (5).
Disease-specific growth charts have been developed for understanding growth patterns of individuals with TS and detecting their deviations from typical growth patterns (6, 7). These charts are very useful for evaluating the effects of growth-promoting treatment among patients as well. At present, disease-specific growth charts are available for several syndromes, such as TS, Prader–Willi syndrome, Down syndrome, Williams syndrome, and Noonan syndrome (8–14). TS-specific growth charts have been constructed in many countries worldwide (8, 9, 15–26) and are widely used for following up patients and evaluating the effect of growth-promoting treatment. In addition, TS-specific growth charts have had a role in revealing the overall growth patterns of girls with TS. We previously discussed our experiences in establishing disease-specific growth charts (7), and the main characteristics of several TS-specific growth charts are summarized in Table 1.
In this review, we discuss the growth in girls with TS, including overall growth, pubertal growth, secular trend, growth-promoting treatment, and sex hormone replacement treatment.
1.1. Overall growth in girls with TS
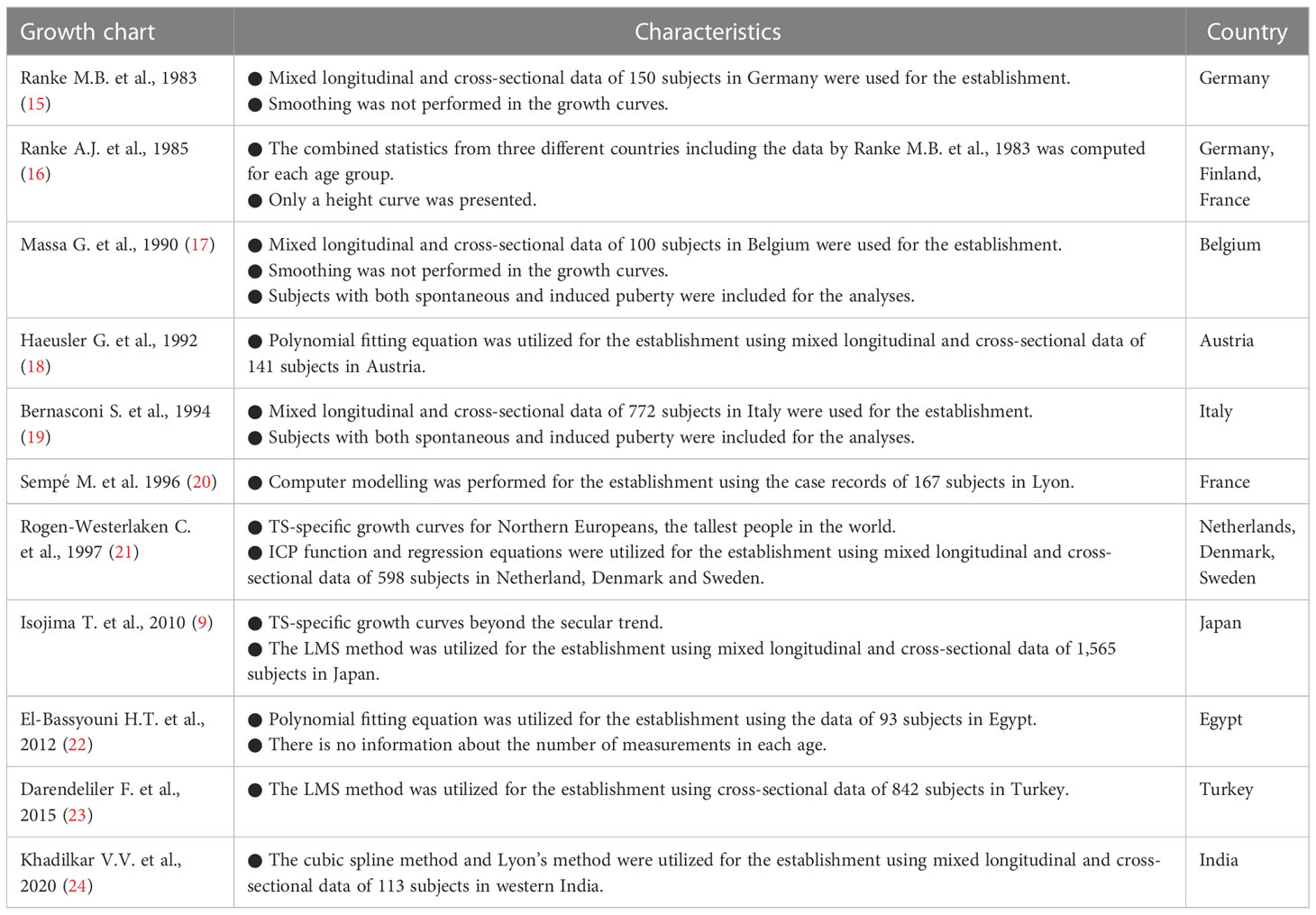
To visualize overall growth in girls with TS, median standard deviation scores (SDSs) of height, weight, body mass index (BMI), and weight for height (WFH) of Japanese girls with TS for Japanese normal population are shown in Figure 1A (height, weight, and BMI) and Figure 1B (WFH) (9, 27). The population of Japan is generally considered ethnically homogeneous and the growth patterns of girls with TS are similar among various ethnicities, judging from reported TS-specific growth charts (8, 9, 15–26). Therefore, we think that the visualization could help our understanding of overall growth in girls with TS not only in Japan but also in other countries.
Figure 1 (A) Median height (black line), weight (black dotted line), and body mass index (BMI: gray line) standard deviation scores (SDS) of girls with Turner syndrome (TS) in the normal population. (B) Comparisons of weight for height (WFH) median lines between girls with TS (black dotted line) and girls of the normal population (gray line).
Growth patterns of height in girls with TS differ from those of the normal population (Figure 1A). The median height SDS is smaller for Japanese girls with TS at birth (−0.76) than the median for the Japanese normal population, which is probably caused by a mild developmental delay in the uterus. This intrauterine growth retardation is also reported in several populations (15, 17–20). After birth, it declines sharply until 2 years of age. Then it continues to decline gradually until the pubertal age of normal children. Therefore, the height of most girls with TS is often within the normal range during infancy but usually falls below the fifth percentile of the general population ≤5 years of age (1). The height then drops drastically around the pubertal period of normal children because of the lack of pubertal spurt. This distinct growth pattern often leads to identification of growth problems during the period. After puberty, height recovers a little because of the lack of epiphyseal closure. These differences result in a median adult height SDS of −3.30 for Japanese girls with TS at age 20 years in a normal population, which is similar to overseas data (−2.54 to −4.15 SDS) (4). It is generally thought that untreated adults with TS are approximately 20-cm shorter than normal female adults (4), although ethnic background may affect adult height in girls with TS.
Girls with TS reportedly often become overweight as they grow up (15, 19, 21, 26), and many problems of females with TS in adult life are compounded by obesity (28). Weight-gain patterns in girls with TS are not as distinctive as height patterns, but are also different from normal-population weight-gain patterns, judging from median SDS changes during growth (Figure 1A). This weight-gain pattern declines between 1 and 6 years of age from −1.2 to −1.8 SDS, then increases to −1.2 SDS until 10 years of age. Subsequently, it gradually declines until 15 years of age, then increases again. When we evaluate weight gain in clinical settings, it is vital to consider the balance between height and weight gain, and the BMI and WFH are typically used for that purpose. Growth patterns of BMI in girls with TS differ from those in the normal population as well and resemble the weight-gain pattern except that the values are between 0 and 1 SDS and the increase after age 15 years can be negligible (Figure 1A). Considering the short stature in girls with TS, the positive value in BMI SDS should mean that girls with TS tend to be plump, which is consistent with the reported fact that girls with TS often become overweight as they grow up. WFH in girls with TS is almost the same as that in normal girls <100 cm tall, but more rapid increase in the WFH is evident in girls with TS ≥100 cm tall (Figure 1B). Considering that the median height at 6 years of age in Japanese girls with TS is 99.8 cm, the difference in body composition between girls with TS and the normal population might become evident from approximately 6 years of age. It is notable that the growth pattern of the median-weight SDS and -BMI SDS changes from a decrease to an increase at age 6 years when the BMI rebound occurs in the normal population on average. Accordingly, the growth patterns for BMI and WFH are totally different between girls with TS and the normal population, so careful attention is needed when assessing overweight or obesity status when employing the BMI and WFH as surrogate indices. We previously reported that there is a ≥30% discrepancy in the determination of overweight status in girls with TS aged ≥10 years when they were judged by two indices for the general population (29), although further investigation is essential to properly interpret this discrepancy.
1.2. Pubertal growth in girls with TS
Ovarian failure is one of the cardinal characteristics in girls with TS, but some of these girls undergo spontaneous puberty. It is unlikely that GH treatment affects the age of onset or the prevalence of spontaneous pubertal development in girls with TS (30, 31). Spontaneous thelarche has been reported in approximately one-third of girls with TS, which occurs most often in those with mosaicism, from many countries (17, 30–33), but only ≤6% of them have regular menstrual cycles, and spontaneous pregnancies are rare (30). Therefore, many girls with TS require sex hormone replacement therapy because of incomplete ovarian function (30, 31).
TS accompanied by spontaneous puberty should show a slightly different growth pattern from those without puberty. In fact, girls with TS and spontaneous puberty reportedly are significantly taller than those without spontaneous puberty at ≥12 years old, but the spontaneous pubertal development does not influence their adult height (17). Additionally, a slight premenarcheal growth spurt has been observed in girls with TS who experienced genital bleeding (16, 26). Therefore, when constructing TS-specific charts, two types of growth charts should be necessary during the pubertal period. However, for practical reasons, such as a limited number of pubertal subjects, only one type of TS-specific growth chart for girls without spontaneous puberty has been developed in many countries. It is vital for physicians to keep this fact in mind when they follow girls with TS and spontaneous puberty.
1.3. Secular trend of growth in girls with TS
A secular trend of growth has been observed both in the normal population (34, 35) and girls with TS (8, 36). Regarding girls with TS, we previously reported the finding of a secular trend of height (8, 9). To investigate the secular trend of height in girls with TS in Japan, we plotted the data of 1,867 subjects compiled in the Foundation for Growth Science (FGS; a nationwide cohort of patients before and after GH treatment) from 1991 to 2004 for evaluation of eligibility for GH treatment on the previous TS-specific growth chart developed by Suwa in 1992 (26) (Figure 2). The data were distributed in the upper part of the growth charts in older ages, which we thought should be due to the secular trend of height. Considering that the previous TS-specific growth charts were reported in 1992 based on the data of 704 girls with TS (6,255 measurements) born from 1955 to 1989 (median: unknown) obtained from a questionnaire survey of hospitals, most of the subjects for the growth charts should have been born before the secular trend of height reached a plateau because the increase in height in Japan plateaued around 1990. Therefore, TS-specific charts should be updated, and we revised them for Japanese girls in 2010 using semi-longitudinal data (5,772 measurements) from both the FGS and two major hospitals for TS care. We found that the height at 20 years of age was 3.1-cm higher in the revised chart (141.3 cm) than in the previous chart (138.2 cm), which was nearly equivalent to the difference in mean female adult heights in the general population between 1970 (155.6 cm) and 1990 (157.9 cm) (8, 9). The secular trends of birth weight and height at the start of GH treatment in girls with TS also have been reported (36). Using anthropometric measurements in KIGS (Pfizer International Growth Database), girls with TS who were registered in the database between 1987 and 2012 and born between 1975 and 2004 were analyzed by comparing their data across 5-year birth-year groups. A significantly positive secular trend for birth weight (+0.18 SD) and height at the start of GH treatment (+0.38 SD) was detected in the study (36). In summary, there is a secular trend of growth in girls with TS, so growth references beyond the secular trend when evaluating growth should be used wherever available.

Figure 2 Scatter plots of Turner syndrome (TS) height data compiled by the Foundation of Growth Science, Japan, on the previous TS growth charts 26.
1.4. Growth-promoting treatment for girls with TS
GH has been shown to be effective for increasing adult height of 5–8 cm at a dosage of 42–50 μg/kg/day (5, 32, 37–41) and has been approved for growth-promoting treatment for girls with TS in many countries. On the other hand, a large interindividual variation in growth response to GH treatment has been reported (5, 32, 37–40), and the response of patients with TS was relatively poor and almost equal to that of achondroplasia and lower than that of GH deficiency or Noonan syndrome judging from the first year of GH treatment in clinical trials (32). In addition, several factors, such as younger age at initiation of GH treatment or timing of pubertal induction, affect treatment efficacy (5, 32, 37–40). To date, the optimal age for commencement of GH treatment has not been established, but ≥4 years of treatment prior to puberty has been associated with greater treatment effect (41). Evaluating the treatment effect is essential in clinical practice, and TS-specific growth charts are widely used for that purpose. An optimal treatment for girls with TS should be determined in each girl considering both GH treatment and an age-appropriate induction of puberty.
The existing literature shows that addition of oxandrolone, a nonaromatizable androgen (anabolic steroid), to GH has a beneficial effect on adult height in girls with TS (42, 43). Although the magnitude of the positive effect reportedly differs for different treatment regimens and subject characteristics (43), a recent systematic review showed that oxandrolone plus GH treatment was better than GH in terms of adult height by a mean difference of 2.7 cm (42). Oxandrolone is not available worldwide, so other anabolic steroids might be considered. For example, methenolone acetate is currently used in Japan (32). However, there is the potential for unwanted effects in a nonaromatizable androgen treatment, such as delayed breast development and dose-dependent virilization (including clitoromegaly, voice deepening, hirsutism, and acne). Therefore, caution is needed when administering this treatment. The consensus guideline from the 2016 Cincinnati International Turner Syndrome meeting suggested that concomitant treatment with oxandrolone from ≥10 years of age at 0.03 mg/kg/day and maintained at <0.05 mg/kg/day, if the diagnosis of TS and GH treatment initiation is delayed, and/or AH outcome is likely to be unsatisfactory with the standard GH dose alone (41).
1.5. Estrogen replacement treatment for girls with TS to increase growth
Estrogen replacement treatment is necessary for most girls with TS because of hypergonadotropic hypogonadism. Although the optimal procedure of the treatment has not yet been determined, the consensus guideline recommended that it should start between 11 and 12 years of age and then increase to adult dose over 2–3 years to mimic the physiology of healthy girls and improve psychological quality of life (41). Although the optimal regimen is still being elucidated, transdermal estradiol is preferable at this stage, because it is more physiological than other available drugs. On the other hand, estrogen replacement advances skeletal maturation and eventually causes epiphyseal fusion (32, 44, 45), which results in cessation of linear growth and lower adult height. For this reason, the timing of estrogen replacement induction has often been delayed until the mid-teen years, but it was recently reported that delaying pubertal induction beyond 12 years did not significantly improve adult height (43). Therefore, in recent clinical practice, a low dose of estrogen is thought to be preferable at treatment initiation, and a dose increase is usually determined by using TS-specific growth charts and evaluating each patient in terms of clinical symptoms, age, remaining growth potential, and patient satisfaction (41). Recently, ultra-low dose oral ethinyl estradiol initiation (25 ng/kg/day) during the prepubertal period combined with GH was reported to be effective for a modest synergistic increase in adult height (38, 46). In addition, there is the promising report that gradual increasing oral ethinyl estradiol from an extremely low dose of 1-5 ng/kg/day may produce good adult height (47). However, this treatment is not routinely recommended at this stage, because an optimal estrogen replacement treatment has not been determined and its long-term safety has not been assessed (41).
2. Concluding remarks
Girls with TS have short stature and hypergonadotropic hypogonadism, which should be treated in childhood. Both GH treatment and estrogen replacement therapy are essential in girls with TS. Since the growth pattern of these girls is very different from that of the normal population, understanding their growth pattern is vital for optimizing both their follow-up and treatment. TS-specific growth charts should be used for those purposes.
Author contributions
TI conceived of the article, performed the literature search, and drafted the initial manuscript. TI and SY critically revised the work; both approve of the final submission. All authors contributed to the article and approved the submitted version.
Funding
Tsuyoshi Isojima was supported by Grant-in-Aid from the Ministry of Education, Science, Sports, and Culture of Japan (21K07827).
Acknowledgments
We would like to thank Enago (www.enago.jp) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
2. Ranke MB, Saenger P. Turner's syndrome. Lancet (2001) 358:309–14. doi: 10.1016/S0140-6736(01)05487-3
3. Sybert VP, McCauley E. Turner's syndrome. N Engl J Med (2004) 351:1227–38. doi: 10.1056/NEJMra030360
4. Ranke MB, Grauer ML. Adult height in turner syndrome: Results of a multinational survey 1993. Horm Res (1994) 42:90–4. doi: 10.1159/000184154
5. Baxter L, Bryant J, Cave CB, Milne R. Recombinant growth hormone for children and adolescents with turner syndrome. Cochrane Database Syst Rev (2007) 1:CD003887. doi: 10.1002/14651858.CD003887.pub2
6. Ranke MB. Disease-specific standards in congenital syndromes. Horm Res (1996) 45 Suppl 2:35–41. doi: 10.1159/000184845
7. Isojima T, Yokoya S. Development of disease-specific growth charts in turner syndrome and noonan syndrome. Ann Pediatr Endocrinol Metab (2017) 22:240–6. doi: 10.6065/apem.2017.22.4.240
8. Isojima T, Yokoya S, Ito J, Horikawa R, Tanaka T. New reference growth charts for Japanese girls with turner syndrome. Pediatr Int (2009) 51:709–14. doi: 10.1111/j.1442-200X.2009.02838.x
9. Isojima T, Yokoya S, Ito J, Naiki Y, Horikawa R, Tanaka T. Proposal of new auxological standards for Japanese girls with turner syndrome. Clin Pediatr Endocrinol (2010) 19:69–82. doi: 10.1297/cpe.19.69
10. Nagai T, Matsuo N, Kayanuma Y, Tonoki H, Fukushima Y, Ohashi H, et al. Standard growth curves for Japanese patients with prader-willi syndrome. Am J Med Genet (2000) 95:130–4. doi: 10.1002/1096-8628(20001113)95:2<130::AID-AJMG7>3.0.CO;2-R
11. Styles ME, Cole TJ, Dennis J, Preece MA. New cross sectional stature, weight, and head circumference references for down's syndrome in the UK and republic of Ireland. Arch Dis Child (2002) 87:104–8. doi: 10.1136/adc.87.2.104
12. Martin ND, Smith WR, Cole TJ, Preece MA. New height, weight and head circumference charts for British children with williams syndrome. Arch Dis Child (2007) 92:598–601. doi: 10.1136/adc.2006.107946
13. Isojima T, Sakazume S, Hasegawa T, Ogata T, Nakanishi T, Nagai T, et al. Growth references for Japanese individuals with noonan syndrome. Pediatr Res (2016) 79:543–8. doi: 10.1038/pr.2015.254
14. Isojima T, Sakazume S, Hasegawa T, Ogata T, Nakanishi T, Nagai T, et al. Validation of auxological reference values for Japanese children with noonan syndrome and comparison with growth in children with turner syndrome. Clin Pediatr Endocrinol (2017) 26:153–64. doi: 10.1297/cpe.26.153
15. Ranke MB. Turner syndrome: Spontaneous growth in 150 cases and review of the literature. Eur J Pediatr (1983) 141:81–8. doi: 10.1007/BF00496795
16. Lyon AJ, Preece MA, Grant DB. Growth curve for girls with turner syndrome. Arch Dis Child (1985) 60:932–5. doi: 10.1136/adc.60.10.932
17. Massa G, Vanderschueren-Lodeweyckx M, Malvaux P. Linear growth in patients with turner syndrome: Influence of spontaneous puberty and parental height. Eur J Pediatr (1990) 149:246–50. doi: 10.1007/BF02106283
18. Haeusler G, Schemper M, Frisch H, Blümel P, Schmitt K, Plöchl E. Spontaneous growth in turner syndrome: Evidence for a minor pubertal growth spurt. Eur J Pediatr (1992) 151:283–7. doi: 10.1007/BF02072230
19. Bernasconi S, Larizza D, Benso L, Volta C, Vannelli S, Milani S, et al. Turner's syndrome in Italy: Familial characteristics, neonatal data, standards for birth weight and for height and weight from infancy to adulthood. Acta Paediatr (1994) 83:292–8. doi: 10.1111/j.1651-2227.1994.tb18097.x
20. Sempé M, Hansson-Bondallaz C, Limoni C. Growth curves in untreated ullrich-turner syndrome: French reference standards 1-22 years. Eur J Pediatr (1996) 155:862–9. doi: 10.1007/BF02282835
21. Rongen-Westerlaken C, Corel L, van den Broeck J, Massa G, Karlberg J, Albertsson-Wikland K, et al. Reference values for height, height velocity and weight in turner's syndrome. Swedish study group for GH treatment. Acta Paediatr (1997) 86:937–42. doi: 10.1111/j.1651-2227.1997.tb15174.x
22. El-Bassyouni HT, Afifi HH, Aglan MS, Mahmoud WM, Zaki ME. Growth curves of Egyptian patients with turner syndrome. Am J Med Genet Part A (2012) 158A:2687–91. doi: 10.1002/ajmg.a.35518
23. Darendeliler F, Yeşilkaya E, Bereket A, Baş F, Bundak R, Sarı E, et al. Growth curves for Turkish girls with turner syndrome: Results of the Turkish turner syndrome study group. J Clin Res Pediatr Endocrinol (2015) 7:183–91. doi: 10.4274/jcrpe.2023
24. Khadilkar VV, Karguppikar MB, Ekbote VH, Khadilkar AN. Turner syndrome growth charts: A western India experience. Indian J Endocr Metab (2020) 24:333–7. doi: 10.4103/ijem.IJEM_123_20
25. Bertapelli F, Barros-Filho A, Antonio M, Barbeta CJ, de Lemos-Marini SH, Guerra-Junior G. Growth curves for girls with turner syndrome. Biomed Res Int (2014) 2014:687978. doi: 10.1155/2014/687978
26. Suwa S. Standards for growth and growth velocity in turner's syndrome. Acta Paediatr Jpn (1992) 34:206–20; discussion 221. doi: 10.1111/j.1442-200x.1992.tb00951.x
27. Isojima T, Kato N, Ito Y, Kanzaki S, Murata M. Growth standard charts for Japanese children with mean and standard deviation (SD) values based on the year 2000 national survey. Clin Pediatr Endocrinol (2016) 25:71–6. doi: 10.1297/cpe.25.71
28. Bondy CA, Group TSS. Care of girls and women with turner syndrome: A guideline of the turner syndrome study group. J Clin Endocrinol Metab (2007) 92:10–25. doi: 10.1210/jc.2006-1374
29. Isojima T, Yokoya S, Ito J, Horikawa R, Tanaka T. Inconsistent determination of overweight by two anthropometric indices in girls with turner syndrome. Acta Paediatr (2009) 98:513–8. doi: 10.1111/j.1651-2227.2008.01132.x
30. Pasquino AM, Passeri F, Pucarelli I, Segni M, Municchi G. Spontaneous pubertal development in turner's syndrome. Italian study group for turner's syndrome. J Clin Endocrinol Metab (1997) 82:1810–3. doi: 10.1210/jcem.82.6.3970
31. Tanaka T, Igarashi Y, Ozono K, Ohyama K, Ogawa M, Osada H, et al. Frequencies of spontaneous breast development and spontaneous menarche in turner syndrome in Japan. Clin Pediatr Endocrinol (2015) 24:167–73. doi: 10.1297/cpe.24.167
32. Tanaka T. Perspectives on growth promoting treatment for patients with turner syndrome in Japan. Clin Pediatr Endocrinol (2020) 29:91–7. doi: 10.1297/cpe.29.91
33. Negreiros LP, Bolina ER, Guimarães MM. Pubertal development profile in patients with turner syndrome. J Pediatr Endocrinol Metab (2014) 27:845–9. doi: 10.1515/jpem-2013-0256
34. Takaishi M. Secular changes in growth of Japanese children. J Pediatr Endocrinol (1994) 7:163–73. doi: 10.1515/JPEM.1994.7.2.163
35. Cole TJ. Secular trends in growth. Proc Nutr Soc (2000) 59:317–24. doi: 10.1017/S0029665100000355
36. Woelfle J, Lindberg A, Aydin F, Ong KK, Camacho-Hubner C, Gohlke B. Secular trends on birth parameters, growth, and pubertal timing in girls with turner syndrome. Front Endocrinol (Lausanne) (2018) 9:54. doi: 10.3389/fendo.2018.00054
37. Stephure DK, C. Canadian Growth Hormone Advisory. Impact of growth hormone supplementation on adult height in turner syndrome: Results of the Canadian randomized controlled trial. J Clin Endocrinol Metab (2005) 90:3360–6. doi: 10.1210/jc.2004-2187
38. Ross JL, Quigley CA, Cao D, Feuillan P, Kowal K, Chipman JJ, et al. Growth hormone plus childhood low-dose estrogen in turner's syndrome. N Engl J Med (2011) 364:1230–42. doi: 10.1056/NEJMoa1005669
39. Cleemann Wang A, Hagen CP, Nedaeifard L, Juul A, Jensen RB. Growth and adult height in girls with turner syndrome following IGF-1 titrated growth hormone treatment. J Clin Endocrinol Metab (2020) 105:2566–74. doi: 10.1210/clinem/dgaa274
40. Blum WF, Ross JL, Zimmermann AG, Quigley CA, Child CJ, Kalifa G, et al. GH treatment to final height produces similar height gains in patients with SHOX deficiency and turner syndrome: Results of a multicenter trial. J Clin Endocrinol Metab (2013) 98:E1383–92. doi: 10.1210/jc.2013-1222
41. Gravholt CH, Andersen NH, Conway GS, Dekkers OM, Geffner ME, Klein KO, et al. Clinical practice guidelines for the care of girls and women with turner syndrome: Proceedings from the 2016 Cincinnati international turner syndrome meeting. Eur J Endocrinol (2017) 177:G1–G70. doi: 10.1530/EJE-17-0430
42. Mohamed S, Alkofide H, Adi YA, Amer YS, AlFaleh K. Oxandrolone for growth hormone-treated girls aged up to 18 years with turner syndrome. Cochrane Database Syst Rev (2019) 10:CD010736. doi: 10.1002/14651858.CD010736.pub2
43. Gault EJ, Cole TJ, Casey S, Hindmarsh PC, Betts P, Dunger DB, et al. Effect of oxandrolone and timing of pubertal induction on final height in turner syndrome: Final analysis of the UK randomised placebo-controlled trial. Arch Dis Child (2021) 106:74–6. doi: 10.1136/archdischild-2019-317695
44. Morishima A, Grumbach MM, Simpson ER, Fisher C, Qin K. Aromatase deficiency in male and female siblings caused by a novel mutation and the physiological role of estrogens. J Clin Endocrinol Metab (1995) 80:3689–98. doi: 10.1210/jcem.80.12.8530621
45. Smith EP, Boyd J, Frank GR, Takahashi H, Cohen RM, Specker B, et al. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med (1994) 331:1056–61. doi: 10.1056/NEJM199410203311604
46. Quigley CA, Wan X, Garg S, Kowal K, Cutler GB Jr., Ross JL. Effects of low-dose estrogen replacement during childhood on pubertal development and gonadotropin concentrations in patients with turner syndrome: Results of a randomized, double-blind, placebo-controlled clinical trial. J Clin Endocrinol Metab (2014) 99:E1754–64. doi: 10.1210/jc.2013-4518
Keywords: Turner syndrome, growth, growth chart, secular trend, growth hormone, estrogen
Citation: Isojima T and Yokoya S (2023) Growth in girls with Turner syndrome. Front. Endocrinol. 13:1068128. doi: 10.3389/fendo.2022.1068128
Received: 12 October 2022; Accepted: 14 December 2022;
Published: 12 January 2023.
Edited by:
Yukihiro Hasegawa, Tokyo Metropolitan Children’s Medical Center, JapanReviewed by:
Hideya Sakakibara, Yokohama City University Medical Center, JapanDaisuke Ariyasu, Kawasaki Municipal Hospital, Japan
Copyright © 2023 Isojima and Yokoya. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tsuyoshi Isojima, t-isojima@toranomon.gr.jp
 Tsuyoshi Isojima
Tsuyoshi Isojima Susumu Yokoya3
Susumu Yokoya3