- 1Department of Nutrition, Xiangya Hospital, Central South University, Changsha, China
- 2Department of Nephrology, The Second Xiangya Hospital of Central South University, Changsha, China
- 3Department of Reproduction and Genetics, The First Affiliated Hospital of Kunming Medical University, Kunming, China
- 4National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha, China
Diabetic nephropathy (DN) involves serious lipid metabolism disorder, and renal ectopic lipid deposition aggravates DN progression. However, the molecular mechanism of renal lipid deposition in DN remains unclear. Lipid droplets (LDs) are lipid pools in cells that change dynamically in response to the cellular energy needs. The LDs and mitochondria are connected through a part of the mitochondria known as the peridroplet mitochondria (PDM). In this review, we summarize the definition, detection methods, and function of the PDM. Finally, we discuss the research status of PDM in DN and the possibility of its use as a therapeutic target.
1 Introduction
Presently, the number of diabetes patients is increasing worldwide. As a systemic metabolic disease, diabetes may cause serious microvascular complications such as diabetic nephropathy (DN) (1). DN has gradually become the leading cause of end-stage renal disease (ESRD) in developed countries. However, specific drugs for DN are not available till date; therefore, DN pathogenesis should be explored urgently to develop new therapeutic drugs (2–4). Lipid metabolism disorder is a key factor in DN progression. Ectopic lipid deposition is aggravated in DN, which further promotes tubule cell inflammation and apoptosis and ultimately aggravates the pathological changes of DN (5–7). However, the mechanism of abnormal lipid deposition in DN remains unclear.
The cell is an organic entity and the organelles are not completely separated; in particular, organelles such as mitochondria-associated endoplasmic reticulum membranes (MAMs) are connected (8, 9). MAM integrity maintenance is essential for signal communication and cell homeostasis (10–12). Lipid droplets (LDs), as energy-storing organelles in cells, are also closely linked to the mitochondria. The mitochondria part closely connected to the LDs is also called peridroplet mitochondria (PDM) (13–15). The PDM plays a key role in maintaining lipid homeostasis and energy metabolism, and its abnormality can cause metabolic disorders (16, 17). In this review, we systematically summarized the progress of studies on PDM and discussed its potential role in DN.
2 LD structure
LD is a dynamic organelle, and this dynamic behavior reflects the overall cellular metabolic level (18–21). LD are also energy storing organelles that rapidly mobilize lipids to release fatty acids to provide cellular energy through β-oxidation. Moreover, LDs inhibit lipotoxicity caused by free fatty acids by isolating lipids (22–24). LDs are ubiquitous in cells and have a single phospholipid bilayer that insulates neutral lipids from the cytoplasm, protecting cells from the toxicity of free fatty acids (25). Furthermore, various proteins are embedded in the phospholipid bilayer to mediate different functions of the LDs (26–28). Approximately 200 proteins located on LDs have been identified (29), such as Rab GTPase (30, 31), PNPLA family (32, 33), and LXRα (34), with the development of biological techniques. These proteins play a key role in maintaining the stability of LD structure and timely response to nutritional status. There are two main mechanisms of LD decomposition: lipolysis (35, 36) and lipophagy (37–39). Lipolysis is the release of free fatty acids from triacylglycerol under the sequential action of adipose triglyceride lipase (ATGL), hormone-sensitive lipase (HSL), and monacylglycerol lipase (MAGL), whereas lipophagy is a form of selective autophagy in which lipid droplets are swallowed by the autophagosome membrane and fused with lysosomes, which are degraded by hydrolases (40). Several studies have indicated that LDs dynamically interact with other organelles, particularly the mitochondria, endoplasmic reticulum, endosomes, and peroxisomes, which is essential for maintaining cellular energy metabolism homeostasis.
3 β-Oxidation in mitochondria
Mitochondria are the center of cellular energy metabolism, where fatty acids can undergo β-oxidation to provide energy for cells. Fatty acid oxidation pathway can be divided into α-oxidation, β-oxidation and ω-oxidation, among which α-oxidation and ω-oxidation only exist in eukaryotes, while β-oxidation is the main fatty acid degradation pathway in eukaryotes or prokaryotes (41, 42). The fatty acids are activated by combining with coenzyme A (CoA) to form fatty acid acyl-CoA esters to participate in metabolic pathways. In the presence of ATP and Mg2+, the acyl portion of fatty acids is linked to the sulfur atoms in CoA through thioester bonds to form fatty acyl-CoA derivatives, pyrophosphate (PPi), and adenosine monophosphate (AMP) (43). Subsequently, the fatty acyl-CoA ester is converted to acyl-carnitine in the presence of L-carnitine, which enters the outer mitochondrial membrane. L-carnitine acyl-transferases subtypes, carnitine palmitoyltransferase-1 (CPT1) and carnitine palmitoyltransferase-2 (CPT2), have been detected in humans. Acyl-carnitine crosses the inner mitochondrial membrane under the action of carnitine acyl-carnitine translocase (CACT). Then, CPT2 regenerates the fatty acyl-CoA ester, which is successively dehydrogenated, hydrated, dehydrogenated, and thiolyzed to form acetyl-CoA by the fatty acid β-oxidase system in the mitochondrial matrix, to participate in energy generation (43).
4 What is the PDM?
In cells, mitochondria are the energy factories and LDs are energy pools. The latest studies have suggested connections between the mitochondria and LD, which were first observed in 1959 (44). With the development of biotechnology, several studies have revealed the link between LDs and mitochondria, and the LD-associated mitochondrial part is called PDM (45, 46). In fact, the biological functions, proteins, crest structure, and dynamics of PDM and cytoplasmic mitochondria are different (45). Recently, as the PDM has attracted attention, the quantifiable parameters and definition of PDM has also been proposed: a. Mitochondria in direct contact with LD that can be observed by electron microscopy; b. This part of the mitochondria is tightly linked to the LD, even after mechanical cell disruption or purification (45).
5 Molecular linkage mechanism and function of PDM
5.1 Proteins mediating PDM integrity
As dynamic organelle junctions, PDM integrity adjusts itself according to the cellular energy requirements, which is precisely regulated by certain proteins. Till date, some proteins involved in the coupling between LDs and mitochondria have been identified, and their loss destroys PDM integrity.
5.1.1 SNAP23
SNAP23 belongs to the family of soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) proteins that share a unique SNARE motif in the form of conserved sequences of 60–70 amino acids (47). The SNARE motifs mediate SNARE complexes formation and, thus, play a key role in intimal fusion in many cells. SNAP23 is also involved in maintaining PMD integrity. Lim et al. demonstrated that ADP-ribosylation factor (ARF)-related protein 1 (ARFRP1) recruits SNAP23 to a site close to LD, thereby promoting LD growth in hepatitis C virus (HCV) infected cells (48). This suggested that SNAP23 is essential for LD expansion. Furthermore, Sadh et al. reported that SNAP23 level was higher in LDs in the fasted mice liver than in the control mice liver, which is accompanied by increased LD-mitochondria interactions (49). Similarly, Strauss et al. showed that SNAP23 co-localizes mainly with the mitochondria in healthy trained lean human muscles (50). These studies strongly suggest that SNAP23 is closely related to LDs and mitochondria. Moreover, Jagerstrom et al. demonstrated a direct relationship between SNAP23 and PDM, further suggesting that SNAP23 downregulation reduces mitochondrial β-oxidation, which is accompanied by PDM integrity destruction (51).
5.1.2 Perilipin 5 (PLIN5)
PLIN5 is a 463-residue protein that belongs to the perilipin protein family, which is expressed in highly oxidized tissues such as the heart and oxidized skeletal muscle. In high fat-fed PLIN5−/− mice, the liver TAG content and expression of FA synthesis-related enzymes decreased (52). Moreover, PLIN5 plays an important role in regulating lipolysis by influencing the protein-protein interaction between ATGL and 1-acylglycerol-3-phosphate O-acyltransferase (ABHD5), an ATGL activator (53–55). Interestingly, PLIN5 is also closely related to mitochondrial homeostasis. Immunogold electron microscopy and western blots of isolated mitochondria have indicated that PLIN5 is also expressed in not only LDs but also the mitochondria (56). Moreover, PLIN5 expression correlates with mitochondrial respiration rate for lipid-derived substrates in rat muscle (56). Interestingly, fatty acid respiration did not increase in mitochondria isolated from PLIN5-overexpressed muscles, while lipid oxidation increased in the homogenate containing PLIN5-coated LDs (56). Similarly, Andersson et al. showed that PLIN5 deficiency significantly damages the oxidative capacity of mitochondria in cardiomyocytes, is accompanied by the changes in lipid acyl composition of mitochondrial membrane phospholipids, and significantly reduces mitochondrial membrane depolarization (57). Thus, PLIN5 is involved in fatty acid oxidation from LDs to mitochondria. Furthermore, LD accumulation, mitochondrial dysfunction, and apoptosis increased, while PLIN5 expression substantially decreased in palmitic acid-treated cardiomyocytes (58). Acetylcholine treatment significantly upregulated PLIN5 expression, improved LD lipolysis, and increased LD-mitochondrial connection integrity, whereas PLIN5 knockout destroyed the protective effect of acetylcholine (58). Similarly, PLIN5 mRNA and protein levels were notably upregulated in hydrogen peroxide or lipopolysaccharide (LPS)-treated HepG2 cells, which increased LD-mitochondrial contact and downregulated intracellular ROS levels (59). Further studies determined that PLIN5 is localized at the LD-mitochondrial junction in human skeletal muscles through stimulated emission depletion (STED) microscopy and correlative light-electron microscopy (CLEM) performed (60). Although PLIN5 is involved in LD-mitochondria connection formation, the specific molecular mechanism is still unclear. An unidentified mitochondrial outer membrane protein may be involved in this process, which should be verified in further studies.
Some studies revealed that in addition to SNAP23 and PLIN5, other proteins mediate PDM integrity. Caveolin-1 (Cav-1), a multifunctional membrane protein, is the main component of caveolae and plays an important role in regulating endocytosis, stress response, and signal transduction (61). Meanwhile, it is also a LD envelope protein involved in lipid metabolism and LD formation. Kuo et al. showed impaired LD formation in Cav-1-deficient endothelial cells (62). Interestingly, its expression is also closely related to mitochondrial function. Wang et al. demonstrated that hippocampal overexpression of neuron-targeted Cav-1 significantly reduces mitochondrial damage and enhances mitochondrial respiration (Wang et al., 2021). Similarly, Cav-1 deficiency notably reduced mitochondrial respiration, decreased oxidative phosphorylated complex I activity, and downregulated the NAD+/NADH ratio, ultimately inducing premature aging (63). In addition, the high-quality electron micrographs of intact LDs and other adipocyte components by high pressure and rapid tissue freezing indicated LD-mitochondria connection, while the absence of Cav-1 resulted in a significant and almost complete absence of mitochondria in contact with LDs (64). Moreover, acyl-CoA: diacylglycerol acyltransferase 2 (DGAT2), a key enzyme that catalyzes the synthesis of triacylglycerol, is primarily located in the endoplasmic reticulum (ER) under basic conditions, but using oleic acid promotes TG synthesis, which transfers DGAT2 to the LD surface, where it co-locates with the mitochondria (65). Mechanistically, DGAT2 interacts with the mitochondria through the 67 N-terminal amino acids of DGAT2, which could guide a red fluorescent protein to the mitochondria (65). Furthermore, mitoguardin-2 (MIGA2) (66), an outer mitochondrial membrane protein, and VPS13D are also involved in LD-mitochondria coupling (Figure 1). Although the structural proteins of mitochondria and LDs have been partially revealed, further studies should focus on the proteins involved in PDM integrity maintenance and the molecular mechanisms involved.
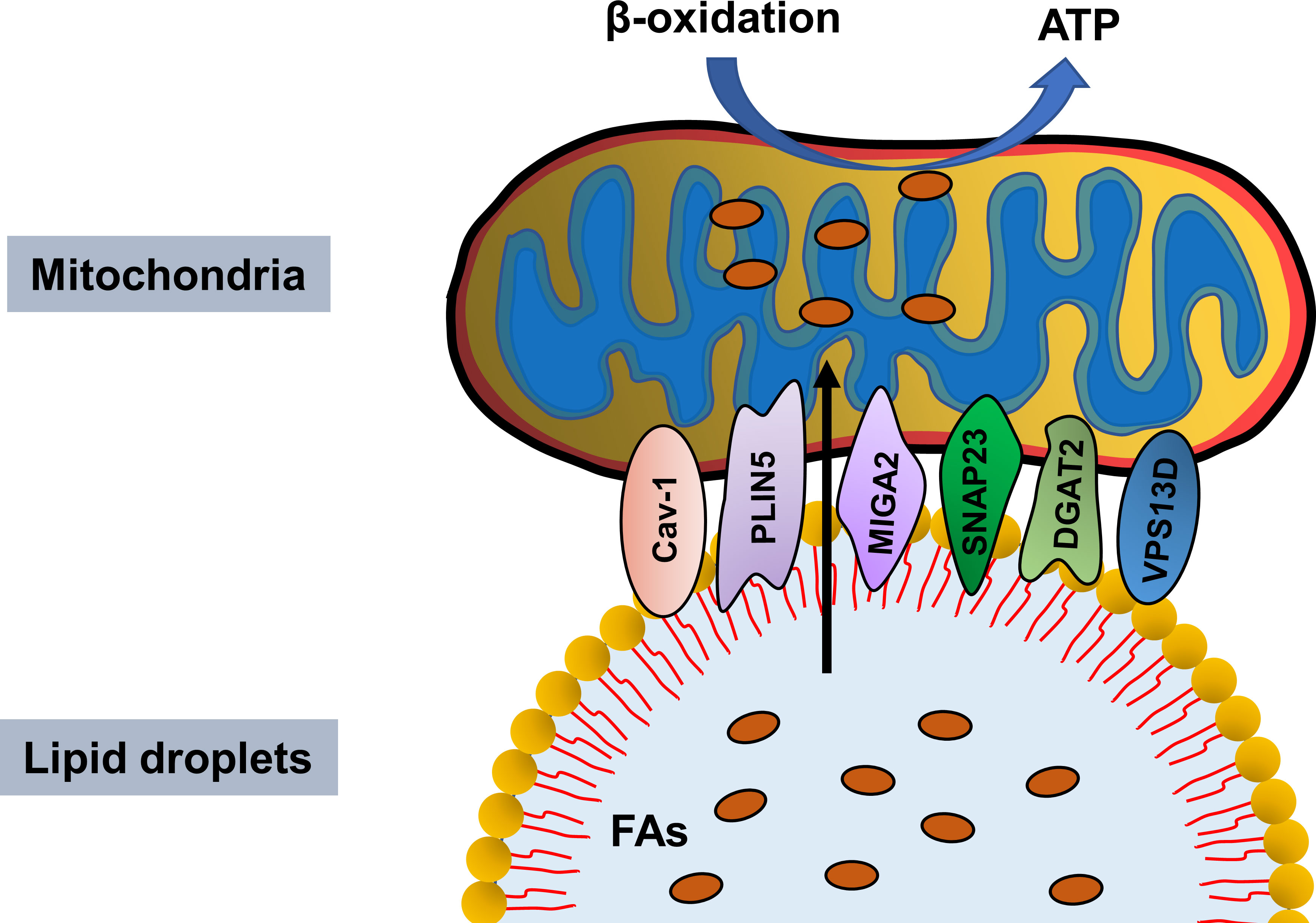
Figure 1 Proteins mediating lipid droplet and mitochondria integrity. Cav-1, PLIN5, MIGA2, SNAP23, DGAT2 and VPS13D maintain lipid droplet–mitochondria connection, thus facilitating fatty acids transfer in from lipid droplet to mitochondria for β-oxidation and ATP generation.
5.2 Function of PDM
Currently, the function of PDM is mainly focused on lipid metabolism. In cells, LDs are the storage organelles and mitochondria are the energy factories. Thus, as a link between them, PDM regulation is indispensable in dealing with cellular energy disturbances. On the one hand, PDM directly connect LDs with the mitochondria, which can efficiently promote fatty acids transfer into the mitochondria for oxidation, thus providing cells with sufficient energy to resist external stimuli; on the other hand, it also confines free fatty acids to a small range, thereby reducing the toxicity of free fatty acids to cells. Rambold et al. demonstrated that labeled fatty acids are stored in the LDs of nutrient-rich cells, while fatty acids are transferred from the LDs in starving cells mitochondria for oxidation. Interestingly, fatty acids are not released from free cytoplasmic pools, but from LD sources and this process required the LDs to be close to the mitochondria (67). These findings suggest that increased PDM integrity significantly promotes fatty acid translocation from LDs to mitochondria for energy production. In addition to fatty acid oxidation, PDM is also involved in LD expansion through providing ATP for TAG synthesis. Benador et al. showed that the purified PDM showed stronger pyruvate oxidation, electron transfer, and ATP synthesis than cytoplasmic mitochondria; moreover, the PLIN5-induced LD-mitochondrial integrity upregulated the ATP-synthase-dependent triacylglyceride synthesis (45). This seemingly contradictory result may be because PDM plays different roles in different nutritional states. In nutrient-rich cells, PDM could mobilize the mitochondria to supply ATP to speed up LDs synthesis; When exposed to external stress, PDM ensures rapid fatty acid transfer from LDs to mitochondria for oxidative energy production.
6 PDM detection methods
Transmission electron microscopy (TEM) is the most direct detection method of PDM, a sub-organelle structure (45, 66, 68, 69). The high resolution of TEM allows the direct observation of LD and mitochondria connection. However, quantifying PDM integrity is difficult and requires instrument precision. In addition, double staining of living cells is another method of detecting PDM by direct observation. PDM (colocalization area) can be observed under confocal microscopy by co-staining living cells with LD markers (BODIPY) and mitochondrial dye (Mito-tracker) (45). The advantage of this method is that the interaction between LDs and mitochondria can be directly observed, but PDM function and protein expression cannot be studied. The optimal method for functional studies is to extract PDM, and Benador et al. have developed a method to isolate PDM according to degree of PDM attachment to LD (70). This method allowed the observation of differences in PDM protein expression under different nutritional or disease states, but the purity of the extracted PDM is related to many factors. However, further work is needed to optimize this separation scheme to accommodate organizational differences. In addition to these methods, in situ proximity ligation assay (PLA) may also be a potential way to detect PDM and is an effective method to detect the connection between ER and mitochondria using marker proteins (71).
7 Potential role of PDM in DN
DN, as a metabolic disease, is often accompanied by lipid metabolism disorder and several studies have revealed that lipid metabolism disorder aggravates DN progression. Edelstein et al. demonstrated increased lipid deposition and enlarged intracellular LDs in DN patients undergoing renal biopsy than those in the control (72). Furthermore, the increased lipid deposition in DN is associated with the expression of lipid metabolism disorder-associated genes. In DN state, the expression of key proteins in the fatty acid β-oxidation pathway (PPAR-α, carnitine palmitoyltransferase 1, acyl-CoA oxidase, and L-FABP) and those mediating cholesterol efflux (ABCA1, ABCG1, and apoE) notably decreased, while the expression of lipid uptake receptors, such as LDL, oxidized LDL, and acetylated LDL receptors, are significantly upregulated (72). Moreover, the degree of lipid disturbance was closely related to renal inflammation and glomerular filtration rate (72). Similarly, we have also showed that renal lipid deposition aggravates with the progression of pathological stages of DN and is associated with renal tubular interstitial damage (Yang et al., 2021). However, liraglutide, a novel hypoglycemic agent, improves renal outcomes in type 2 diabetes patients by inhibiting lipid synthesis and promoting lipid lipolysis in DN rats (73). These findings strongly suggest that lipid metabolism disorders are a risk factor for DN progression, and that DN patients may benefit from ameliorating lipid metabolism disorders.
As an organ with high oxygen consumption, adequate energy supply helps maintain the steady state of kidney function. Renal proximal tubule cells utilize non-esterified fatty acids to maximize ATP production through β-oxidation (74). During DN, increased ROS and hyperglycemia changes the electron transport chain, thus reducing ATP production and increasing apoptosis (75). Moreover, Rossi et al. compared the renal proteomic data and blood metabolic profiles of Timp3-knockout and control mice, showing abnormal fatty acid β-oxidation, which is further aggravated after STZ induced DN (76). Similarly, high glucose treatment induced fatty acid deposition and downregulated β-oxidation rate in cultured human proximal tubule cells, thus changing in the phenotype of epithelial-to-mesenchymal transition (EMT); moreover, acetyl-CoA carboxylase 2 (ACC2) siRNA treatment accelerated β-oxidation rate, thus alleviating the adverse effects of high glucose (77). In fact, the impaired lipids of mitochondrial β-oxidation could be used as DN progression markers (78). These studies suggest abnormal fatty acid β-oxidation in the kidney during DN. Furthermore, accelerated fatty acids β-oxidation could ameliorate diabetic kidney injury (74, 79). Fenofibrate, a commonly used drug for DN, plays a protective role by promoting fatty acid β-oxidation (80, 81) (Figure 2).
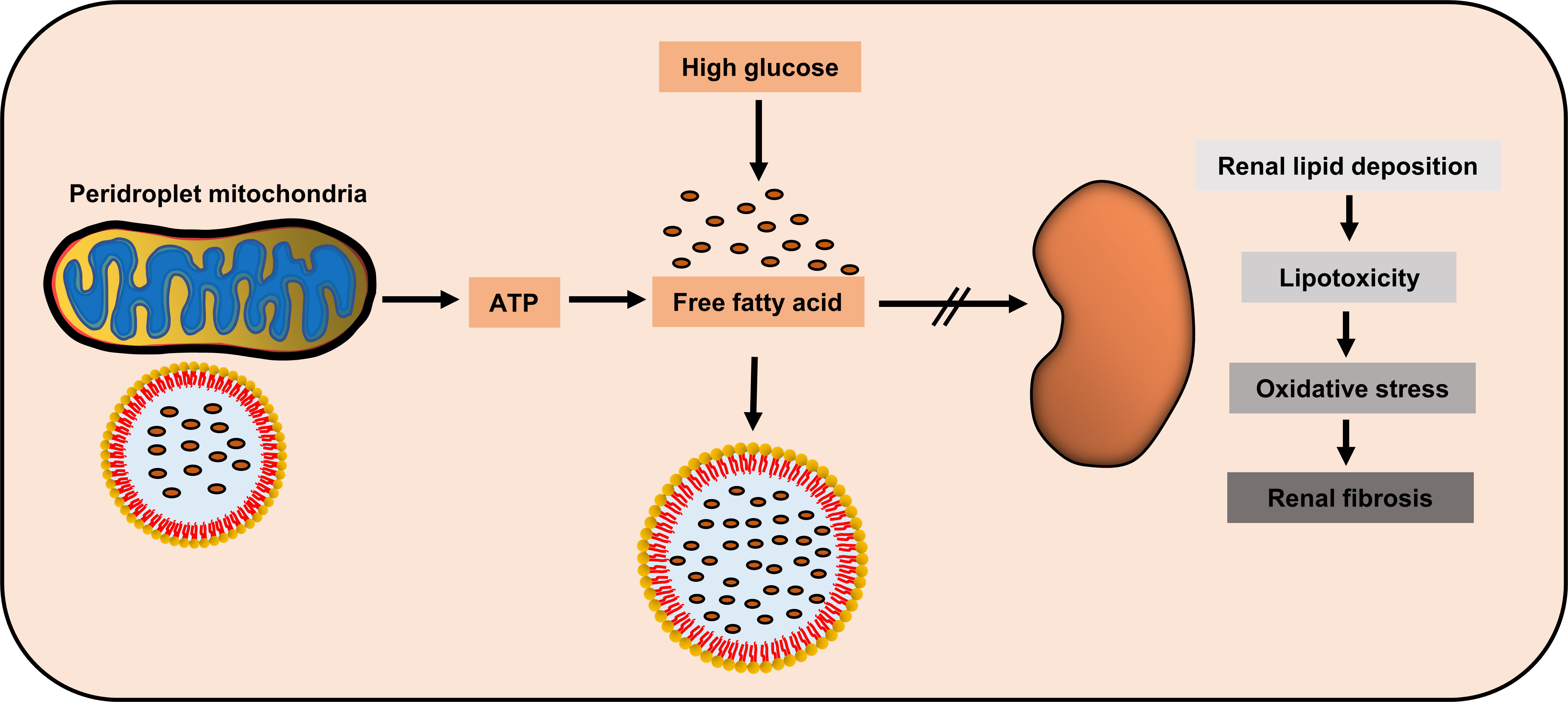
Figure 2 Potential renal protection of peridroplet mitochondria (PDM) in diabetic nephropathy. High glucose could induce the kidney to produce high amount of free fatty acids, while PDM generate ATP to promote lipotoxic fatty acids transfer into lipid droplets to be isolated, to protect kidney cells.
Although few studies have focused on PDM in diseases and the relationship between PDM and DN is unclear, PDM may have some potential link with DN. On one hand, PDM can promote fatty acids β-oxidation, which protects against DN. On the other hand, PDM can also promote LD synthesis, which restricts the free fatty acids in the cytoplasm to LDs, thus, greatly reducing the lipotoxicity. This needs to be verified by further studies, but as an emerging research hotspot, the role of PDM in metabolic diseases, particularly diabetic kidney diseases, deserves further discussion.
8 Conclusion
LDs and mitochondria are important energy storage and productivity factories in cells and the interaction between LDs and mitochondria are involved in maintaining of cell energy metabolism homeostasis. Very few studies have focused on their interaction till date. DN is a metabolic disorder and lipotoxicity and mitochondrial energy imbalance play a key role in its progression. Interestingly, PDM is critical in reducing lipotoxicity and accelerating energy generation. However, no studies have focused on the interaction between PDM and DN. In this review, we have summarized the definition, detection, and function of PDM and explores the potential protective role of PDM in DN. However, many questions remain unsolved. In addition to lipid metabolism and LD synthesis, does PDM maintain intracellular mitochondrial homeostasis? Besides lipid deposition, does PDM integrity change in the kidney tissue of DN patients? What are the molecular mechanisms mediating PDM integrity destruction? Are there compounds that specifically regulate PDM integrity? Although many questions to be addressed in future research, PDM provides a new perspective for us to further understand and prevent DN progression.
Author contributions
MY and XW wrote the manuscript, SL, JY, WC, LH, DL, and LZ edited the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (81900069, 82000697).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Samsu N. Diabetic nephropathy: Challenges in pathogenesis, diagnosis, and treatment. BioMed Res Int (2021) 2021:1497449. doi: 10.1155/2021/1497449
2. Wada J, Makino H. Inflammation and the pathogenesis of diabetic nephropathy. Clin Sci (Lond) (2013) 124:139–52. doi: 10.1042/CS20120198
3. Sagoo MK, Gnudi L. Diabetic nephropathy: An overview. Methods Mol Biol (2020) 2067:3–7. doi: 10.1007/978-1-4939-9841-8_1
4. Selby NM, Taal MW. An updated overview of diabetic nephropathy: Diagnosis, prognosis, treatment goals and latest guidelines. Diabetes Obes Metab (2020) 22 Suppl 1:3–15. doi: 10.1111/dom.14007
5. Thongnak L, Pongchaidecha A, Lungkaphin A. Renal lipid metabolism andlipotoxicity in diabetes. Am J Med Sci (2020) 359:84–99. doi: 10.1016/j.amjms.2019.11.004
6. Zhao YH, Fan YJ. Resveratrol improves lipid metabolism in diabetic nephropathy rats. Front Biosci (Landmark Ed) (2020) 25:1913–24. doi: 10.2741/4885
7. Han Y, Xiong S, Zhao H, Yang S, Yang M, Zhu X, et al. Lipophagy deficiency exacerbates ectopic lipid accumulation and tubular cells injury in diabetic nephropathy. Cell Death Dis (2021) 12:1031. doi: 10.1038/s41419-021-04326-y
8. Han JM, Periwal V. A mathematical model of calcium dynamics: Obesity and mitochondria-associated ER membranes. PloS Comput Biol (2019) 15:e1006661. doi: 10.1371/journal.pcbi.1006661
9. Yu W, Jin H, Huang Y. Mitochondria-associated membranes (MAMs): A potential therapeutic target for treating alzheimer's disease. Clin Sci (Lond) (2021) 135:109–26. doi: 10.1042/CS20200844
10. Hayashi T, Rizzuto R, Hajnoczky G, Su TP. MAM: More than just a housekeeper. Trends Cell Biol (2009) 19:81–8. doi: 10.1016/j.tcb.2008.12.002
11. Agrawal RR, Montesinos J, Larrea D, Area-Gomez E, Pera M. The silence of the fats: A MAM's story about Alzheimer. Neurobiol Dis (2020) 145:105062. doi: 10.1016/j.nbd.2020.105062
12. Pera M, Montesinos J, Larrea D, Agrawal RR, Velasco KR, Stavrovskaya IG, et al. MAM and C99, key players in the pathogenesis of alzheimer's disease. Int Rev Neurobiol (2020) 154:235–78. doi: 10.1016/bs.irn.2020.03.016
13. Symonds ME, Farhat G, Aldiss P, Pope M, Budge H. Brown adipose tissue and glucose homeostasis - the link between climate change and the global rise in obesity and diabetes. Adipocyte (2019) 8:46–50. doi: 10.1080/21623945.2018.1551689
14. Miller N, Wolf D, Alsabeeh N, Mahdaviani K, Segawa M, Liesa M, et al. High-throughput image analysis of lipid-Droplet-Bound mitochondria. Methods Mol Biol (2021) 2276:285–303. doi: 10.1007/978-1-0716-1266-8_22
15. Ngo J, Benador IY, Brownstein AJ, Vergnes L, Veliova M, Shum M, et al. Isolation and functional analysis of peridroplet mitochondria from murine brown adipose tissue. STAR Protoc (2021) 2:100243. doi: 10.1016/j.xpro.2020.100243
16. Cui L, Liu P. Two types of contact between lipid droplets and mitochondria. Front Cell Dev Biol (2020) 8:618322. doi: 10.3389/fcell.2020.618322
17. Eynaudi A, Diaz-Castro F, Borquez JC, Bravo-Sagua R, Parra V, Troncoso R, et al. Differential effects of oleic and palmitic acids on lipid droplet-mitochondria interaction in the hepatic cell line HepG2. Front Nutr (2021) 8:775382. doi: 10.3389/fnut.2021.775382
18. Petan T, Jarc E, Jusovic M. Lipid droplets in cancer: Guardians of fat in a stressful world. Molecules (2018) 23:1941. doi: 10.3390/molecules23081941
19. Brink J, Fourie R, Sebolai O, Albertyn J, Pohl CH. The role of lipid droplets in microbial pathogenesis. J Med Microbiol (2021) 70:001383. doi: 10.1099/jmm.0.001383
20. Li-Beisson Y, Kong F, Wang P, Lee Y, Kang BH. The disassembly of lipid droplets in chlamydomonas. New Phytol (2021) 231:1359–64. doi: 10.1111/nph.17505
21. Ralhan I, Chang CL, Lippincott-Schwartz J, Ioannou MS. Lipid droplets in the nervous system. J Cell Biol (2021) 220:e202102136. doi: 10.1083/jcb.202102136
22. Wang CW. Lipid droplets, lipophagy, and beyond. Biochim Biophys Acta (2016) 1861:793–805. doi: 10.1016/j.bbalip.2015.12.010
23. Nguyen TB, Olzmann JA. Lipid droplets and lipotoxicity during autophagy. Autophagy (2017) 13:2002–3. doi: 10.1080/15548627.2017.1359451
24. Tong X, Stein R. Lipid droplets protect human beta-cells from lipotoxicity-induced stress and cell identity changes. Diabetes (2021) 70:2595–607. doi: 10.2337/db21-0261
25. Wilfling F, Haas JT, Walther TC, Farese RJ. Lipid droplet biogenesis. Curr Opin Cell Biol (2014) 29:39–45. doi: 10.1016/j.ceb.2014.03.008
26. Carr RM, Ahima RS. Pathophysiology of lipid droplet proteins in liver diseases. Exp Cell Res (2016) 340:187–92. doi: 10.1016/j.yexcr.2015.10.021
27. Schneider MR. Lipid droplets and associated proteins in sebocytes. Exp Cell Res (2016) 340:205–8. doi: 10.1016/j.yexcr.2015.11.008
28. Huang A. Plant lipid droplets and their associated proteins: Potential for rapid advances. Plant Physiol (2018) 176:1894–918. doi: 10.1104/pp.17.01677
29. Sztalryd C, Brasaemle DL. The perilipin family of lipid droplet proteins: Gatekeepers of intracellular lipolysis. Biochim Biophys Acta Mol Cell Biol Lipids (2017) 1862:1221–32. doi: 10.1016/j.bbalip.2017.07.009
30. Li C, Yu SS. Rab proteins as regulators of lipid droplet formation and lipolysis. Cell Biol Int (2016) 40:1026–32. doi: 10.1002/cbin.10650
31. Xu D, Li Y, Wu L, Li Y, Zhao D, Yu J, et al. Rab18 promotes lipid droplet (LD) growth by tethering the ER to LDs through SNARE and NRZ interactions. J Cell Biol (2018) 217:975–95. doi: 10.1083/jcb.201704184
32. Murugesan S, Goldberg EB, Dou E, Brown WJ. Identification of diverse lipid droplet targeting motifs in the PNPLA family of triglyceride lipases. PloS One (2013) 8:e64950. doi: 10.1371/journal.pone.0064950
33. Chang P, Sun T, Heier C, Gao H, Xu H, Huang F. Interaction of the lysophospholipase PNPLA7 with lipid droplets through the catalytic region. Mol Cells (2020) 43:286–97. doi: 10.14348/molcells.2020.2283
34. Su W, Peng J, Li S, Dai YB, Wang CJ, Xu H, et al. Liver X receptor alpha induces 17beta-hydroxysteroid dehydrogenase-13 expression through SREBP-1c. Am J Physiol Endocrinol Metab (2017) 312:E357–67. doi: 10.1152/ajpendo.00310.2016
35. Birnbaum MJ. Lipolysis: More than just a lipase. J Cell Biol (2003) 161:1011–2. doi: 10.1083/jcb.200306008
36. Bolsoni-Lopes A, Alonso-Vale MI. Lipolysis and lipases in white adipose tissue - an update. Arch Endocrinol Metab (2015) 59:335–42. doi: 10.1590/2359-3997000000067
37. Liu Q, Wang YM, Gu HF. Lipophagy in atherosclerosis. Clin Chim Acta (2020) 511:208–14. doi: 10.1016/j.cca.2020.10.025
38. Shin DW. Lipophagy: Molecular mechanisms and implications in metabolic disorders. Mol Cells (2020) 43:686–93. doi: 10.14348/molcells.2020.0046
39. Zhao T, Wu K, Hogstrand C, Xu YH, Chen GH, Wei CC, et al. Lipophagy mediated carbohydrate-induced changes of lipid metabolism via oxidative stress, endoplasmic reticulum (ER) stress and ChREBP/PPARgamma pathways. Cell Mol Life Sci (2020) 77:1987–2003. doi: 10.1007/s00018-019-03263-6
40. Jarc E, Petan T. Lipid droplets and the management of cellular stress. Yale J Biol Med (2019) 92:435–52.
41. Wu L, Lin S, Li D. Comparative inhibition studies of enoyl-CoA hydratase 1 and enoyl-CoA hydratase 2 in long-chain fatty acid oxidation. Org Lett (2008) 10:3355–8. doi: 10.1021/ol801267e
42. Cintolesi A, Rodriguez-Moya M, Gonzalez R. Fatty acid oxidation: Systems analysis and applications. Wiley Interdiscip Rev Syst Biol Med (2013) 5:575–85. doi: 10.1002/wsbm.1226
43. Adeva-Andany MM, Carneiro-Freire N, Seco-Filgueira M, Fernandez-Fernandez C, Mourino-Bayolo D. Mitochondrial beta-oxidation of saturated fatty acids in humans. Mitochondrion (2019) 46:73–90. doi: 10.1016/j.mito.2018.02.009
44. Benador IY, Veliova M, Liesa M, Shirihai OS. Mitochondria bound to lipid droplets: Where mitochondrial dynamics regulate lipid storage and utilization. Cell Metab (2019) 29:827–35. doi: 10.1016/j.cmet.2019.02.011
45. Benador IY, Veliova M, Mahdaviani K, Petcherski A, Wikstrom JD, Assali EA, et al. Mitochondria bound to lipid droplets have unique bioenergetics, composition, and dynamics that support lipid droplet expansion. Cell Metab (2018) 27:869–85. doi: 10.1016/j.cmet.2018.03.003
46. Acin-Perez R, Petcherski A, Veliova M, Benador IY, Assali EA, Colleluori G, et al. Recruitment and remodeling of peridroplet mitochondria in human adipose tissue. Redox Biol (2021) 46:102087. doi: 10.1016/j.redox.2021.102087
47. Hatsuzawa K, Sakurai C. Regulatory mechanism of SNAP23 in phagosome formation and maturation. Yonago Acta Med (2020) 63:135–45. doi: 10.33160/yam.2020.08.001
48. Lim YS, Ngo HT, Lee J, Son K, Park EM, Hwang S. ADP-ribosylation factor-related protein 1 interacts with NS5A and regulates hepatitis c virus propagation. Sci Rep (2016) 6:31211. doi: 10.1038/srep31211
49. Sadh K, Rai P, Mallik R. Feeding-fasting dependent recruitment of membrane microdomain proteins to lipid droplets purified from the liver. PloS One (2017) 12:e183022. doi: 10.1371/journal.pone.0183022
50. Strauss JA, Shaw CS, Bradley H, Wilson OJ, Dorval T, Pilling J, et al. Immunofluorescence microscopy of SNAP23 in human skeletal muscle reveals colocalization with plasma membrane, lipid droplets, and mitochondria. Physiol Rep (2016) 4:e12662. doi: 10.14814/phy2.12662
51. Jagerstrom S, Polesie S, Wickstrom Y, Johansson BR, Schroder HD, Højlund K, et al. Lipid droplets interact with mitochondria using SNAP23. Cell Biol Int (2009) 33:934–40. doi: 10.1016/j.cellbi.2009.06.011
52. Keenan SN, Meex RC, Lo J, Ryan A, Nie S, Montgomery MK, et al. Perilipin 5 deletion in hepatocytes remodels lipid metabolism and causes hepatic insulin resistance in mice. Diabetes (2019) 68:543–55. doi: 10.2337/db18-0670
53. Granneman JG, Moore HP, Mottillo EP, Zhu Z, Zhou L. Interactions of perilipin-5 (Plin5) with adipose triglyceride lipase. J Biol Chem (2011) 286:5126–35. doi: 10.1074/jbc.M110.180711
54. Wang H, Bell M, Sreenivasan U, Sreenevasan U, Hu H, Liu J, et al. Unique regulation of adipose triglyceride lipase (ATGL) by perilipin 5, a lipid droplet-associated protein. J Biol Chem (2011) 286:15707–15. doi: 10.1074/jbc.M110.207779
55. Gallardo-Montejano VI, Yang C, Hahner L, McAfee JL, Johnson JA, Holland WL, et al. Perilipin 5 links mitochondrial uncoupled respiration in brown fat to healthy white fat remodeling and systemic glucose tolerance. Nat Commun (2021) 12:3320. doi: 10.1038/s41467-021-23601-2
56. Bosma M, Minnaard R, Sparks LM, Schaart G, Losen M, Baets MH, et al. The lipid droplet coat protein perilipin 5 also localizes to muscle mitochondria. Histochem Cell Biol (2012) 137:205–16. doi: 10.1007/s00418-011-0888-x
57. Andersson L, Drevinge C, Mardani I, Dalen KT, Stahlman M, Klevstig M, et al. Deficiency in perilipin 5 reduces mitochondrial function and membrane depolarization in mouse hearts. Int J Biochem Cell Biol (2017) 91:9–13. doi: 10.1016/j.biocel.2017.07.021
58. Wu Q, Zhao M, He X, Xue R, Li D, Yu X, et al. Acetylcholine reduces palmitate-induced cardiomyocyte apoptosis by promoting lipid droplet lipolysis and perilipin 5-mediated lipid droplet-mitochondria interaction. Cell Cycle (2021) 20:1890–906. doi: 10.1080/15384101.2021.1965734
59. Tan Y, Jin Y, Wang Q, Huang J, Wu X, Ren Z. Perilipin 5 protects against cellular oxidative stress by enhancing mitochondrial function in HepG2 cells. Cells (2019) 8:1241. doi: 10.3390/cells8101241
60. Gemmink A, Daemen S, Kuijpers H, Schaart G, Duimel H, Iglesias C, et al. Super-resolution microscopy localizes perilipin 5 at lipid droplet-mitochondria interaction sites and at lipid droplets juxtaposing to perilipin 2. Biochim Biophys Acta Mol Cell Biol Lipids (2018) 1863:1423–32. doi: 10.1016/j.bbalip.2018.08.016
61. Luo S, Yang M, Zhao H, Han Y, Jiang N, Yang J, et al. Caveolin-1 regulates cellular metabolism: A potential therapeutic target in kidney disease. Front Pharmacol (2021) 12:768100. doi: 10.3389/fphar.2021.768100
62. Kuo A, Lee MY, Yang K, Gross RW, Sessa WC. Caveolin-1 regulates lipid droplet metabolism in endothelial cells via autocrine prostacyclin-stimulated, cAMP-mediated lipolysis. J Biol Chem (2018) 293:973–83. doi: 10.1074/jbc.RA117.000980
63. Yu DM, Jung SH, An HT, Lee S, Hong J, Park JS, et al. Caveolin-1 deficiency induces premature senescence with mitochondrial dysfunction. Aging Cell (2017) 16:773–84. doi: 10.1111/acel.12606
64. Cohen AW, Razani B, Schubert W, Williams TM, Wang XB, Lyengar P, et al. Role of caveolin-1 in the modulation of lipolysis and lipid droplet formation. Diabetes (2004) 53:1261–70. doi: 10.2337/diabetes.53.5.1261
65. Stone SJ, Levin MC, Zhou P, Han J, Walther TC, Farese RV, et al. The endoplasmic reticulum enzyme DGAT2 is found in mitochondria-associated membranes and has a mitochondrial targeting signal that promotes its association with mitochondria. J Biol Chem (2009) 284:5352–61. doi: 10.1074/jbc.M805768200
66. Freyre C, Rauher PC, Ejsing CS, Klemm RW. MIGA2 links mitochondria, the ER, and lipid droplets and promotes de novo lipogenesis in adipocytes. Mol Cell (2019) 76:811–25. doi: 10.1016/j.molcel.2019.09.011
67. Rambold AS, Cohen S, Lippincott-Schwartz J. Fatty acid trafficking in starved cells: Regulation by lipid droplet lipolysis, autophagy, and mitochondrial fusion dynamics. Dev Cell (2015) 32:678–92. doi: 10.1016/j.devcel.2015.01.029
68. Tarnopolsky MA, Rennie CD, Robertshaw HA, Fedak-Tarnopolsky SN, Devries MC, Hamadeh MJ. Influence of endurance exercise training and sex on intramyocellular lipid and mitochondrial ultrastructure, substrate use, and mitochondrial enzyme activity. Am J Physiol Regul Integr Comp Physiol (2007) 292:R1271–8. doi: 10.1152/ajpregu.00472.2006
69. Bleck C, Kim Y, Willingham TB, Glancy B. Subcellular connectomic analyses of energy networks in striated muscle. Nat Commun (2018) 9:5111. doi: 10.1038/s41467-018-07676-y
70. Veliova M, Petcherski A, Liesa M, Shirihai OS. The biology of lipid droplet-bound mitochondria. Semin Cell Dev Biol (2020) 108:55–64. doi: 10.1016/j.semcdb.2020.04.013
71. Yang M, Zhao L, Gao P, Zhu X, Han Y, Chen X, et al. DsbA-l ameliorates high glucose induced tubular damage through maintaining MAM integrity. EBioMedicine (2019) 43:607–19. doi: 10.1016/j.ebiom.2019.04.044
72. Herman-Edelstein M, Scherzer P, Tobar A, Levi M, Gafter U. Altered renal lipid metabolism and renal lipid accumulation in human diabetic nephropathy. J Lipid Res (2014) 55:561–72. doi: 10.1194/jlr.P040501
73. Su K, Yi B, Yao BQ, Xia T, Yang YF, Zhang ZH, et al. Liraglutide attenuates renal tubular ectopic lipid deposition in rats with diabetic nephropathy by inhibiting lipid synthesis and promoting lipolysis. Pharmacol Res (2020) 156:104778. doi: 10.1016/j.phrs.2020.104778
74. Bhargava P, Schnellmann RG. Mitochondrial energetics in the kidney. Nat Rev Nephrol (2017) 13:629–46. doi: 10.1038/nrneph.2017.107
75. Flemming NB, Gallo LA, Ward MS, Forbes JM. Tapping into mitochondria to find novel targets for diabetes complications. Curr Drug Targets (2016) 17:1341–9. doi: 10.2174/1389450116666150727114410
76. Rossi C, Marzano V, Consalvo A, Zucchelli M, Levi MS, Casagrande V, et al. Proteomic and metabolomic characterization of streptozotocin-induced diabetic nephropathy in TIMP3-deficient mice. Acta Diabetol (2018) 55:121–9. doi: 10.1007/s00592-017-1074-y
77. Xu Y, Huang J, Xin W, Chen L, Zhao X, Lv Z, et al. Lipid accumulation is ahead of epithelial-to-mesenchymal transition and therapeutic intervention by acetyl-CoA carboxylase 2 silence in diabetic nephropathy. Metabolism (2014) 63:716–26. doi: 10.1016/j.metabol.2014.02.010
78. Afshinnia F, Nair V, Lin J, Rajendiran TM, Soni T, Byun J, et al. Increased lipogenesis and impaired beta-oxidation predict type 2 diabetic kidney disease progression in American indians. JCI Insight (2019) 4:e130317. doi: 10.1172/jci.insight.130317
79. Szeto HH, Liu S, Soong Y, Alam N, Prusky GT, Seshan SV. Protection of mitochondria prevents high-fat diet-induced glomerulopathy and proximal tubular injury. Kidney Int (2016) 90:997–1011. doi: 10.1016/j.kint.2016.06.013
80. Lakhia R, Yheskel M, Flaten A, Quittner-Strom EB, Holland WL, Patel V. PPARalpha agonist fenofibrate enhances fatty acid beta-oxidation and attenuates polycystic kidney and liver disease in mice. Am J Physiol Renal Physiol (2018) 314:F122–31. doi: 10.1152/ajprenal.00352.2017
Keywords: peridroplet mitochondria, lipid droplets, diabetic nephropathy, mitochondria, β-oxidation
Citation: Yang M, Luo S, Yang J, Chen W, He L, Liu D, Zhao L and Wang X (2022) Lipid droplet - mitochondria coupling: A novel lipid metabolism regulatory hub in diabetic nephropathy. Front. Endocrinol. 13:1017387. doi: 10.3389/fendo.2022.1017387
Received: 12 August 2022; Accepted: 04 October 2022;
Published: 25 October 2022.
Edited by:
Yao-Wu Liu, Xuzhou Medical University, ChinaReviewed by:
Ismail Syed, Beth Israel Deaconess Medical Center and Harvard Medical School, United StatesXiaodong Chen, Huazhong Agricultural University, China
Copyright © 2022 Yang, Luo, Yang, Chen, He, Liu, Zhao and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xi Wang, 4011183@csu.edu.cn
 Ming Yang
Ming Yang Shilu Luo
Shilu Luo Jinfei Yang2
Jinfei Yang2 Wei Chen
Wei Chen Liyu He
Liyu He Di Liu
Di Liu Li Zhao
Li Zhao Xi Wang
Xi Wang