- 1Department of Endocrinology and Metabology, The First Affiliated Hospital of Shandong First Medical University and Shandong Provincial Qianfoshan Hospital, Jinan, China
- 2Center for Big Data Research in Health and Medicine, The First Affiliated Hospital of Shandong First Medical University and Shandong Provincial Qianfoshan Hospital, Jinan, China
- 3Organization and Personnel Section, Weifang Municipal Center for Disease Control and Prevention, Weifang, China
- 4School of Public Health, Shandong First Medical University & Shandong Academy of Medical Sciences, Taian, China
- 5Department of Quality Control, Anqiu City People's Hospital, Weifang, China
- 6Department of Clinical Pharmacy, The First Affiliated Hospital of Shandong First Medical University & Shandong Provincial Qianfoshan Hospital, Jinan, China
- 7Department of Clinical Pharmacy, Shandong Provincial Qianfoshan Hospital, Shandong University, Jinan, China
- 8School of Public Health, Weifang Medical University, Weifang, China
- 9Shandong Provincial Qianfoshan Hospital, Cheeloo College of Medicine, Shandong University, Jinan, China
Background: Subacute thyroiditis (SAT) is a self-limited inflammatory thyroid disease with recurring episodes. However, the results regarding the recurrence rate and risk factors for SAT are inconsistent. This meta-analysis aimed to summarize the evidence of the recurrence rate and the risk factors for SAT.
Methods: The present study involved the performance of a systematic literature search of all English studies published in PubMed, Embase, Web of Science, and The Cochrane Library from inception to August 20, 2021. Cohort studies that reported the SAT recurrence rate and risk factors for recurrence were included. Two independent investigators extracted relevant information. Fixed- and random-effects models were used to pool effect sizes based on study heterogeneity.
Results: Eighteen cohort studies were identified. The pooled findings showed that the recurrence rate was 12.0% (95% CI: 8.2%, 17.1%). The risk of recurrence in the glucocorticoids group was higher than that in the NSAIDs group (RR = 1.84, 95% CI: 1.04, 3.24). However, there was no significant difference in age or sex between the recurrence group and the non-recurrence group. Findings from one or two cohort studies also indicated that the copresence of HLA-B*18:01 and -B*35, the number of days required to taper prednisolone (PSL) to 5 mg/day, the duration of disease before treatment less than 30 days, the sialic acid level, or the TSH level at the termination of treatment and further extension of the hypoechoic area and increase in thyroid volume were related to the recurrence of SAT.
Conclusion: Recurrence was common in SAT patients. The present study indicated that glucocorticoid treatment was associated with a higher recurrence rate of SAT than NSAIDs treatment. The clinical implications of this association should be interpreted with caution, and further clinical trials on the long-term effects of different treatment strategies are needed.
Introduction
Subacute thyroiditis (SAT), also known as granulomatous thyroiditis, giant cell thyroiditis, and de Quervain thyroiditis, accounts for 5% of all clinical thyroid abnormalities (1, 2). The peak incidence occurs at 30 – 50 years, and women are affected three times more frequently than men (3, 4). It is generally believed that the occurrence of SAT is related to viral infection or autoimmune response, and susceptibility is related to human leukocyte antigen (HLA), mainly related to HLA-B*35, HLA-B*18:01, DRB1*01, and C*04:01 (3, 5–7).
SAT is a self-limited inflammatory thyroid disease, that usually has three phase course. The first phase of the acute inflammatory process destroys the thyroid follicles and releases thyroid hormones into the circulatory system, resulting in thyrotoxicosis. Then, the thyroid is depleted of stored thyroid hormone, and a phase of hypothyroidism typically occurs. Finally, thyroid hormone and thyroid-stimulating hormone (TSH) levels return to normal as the disease subsides, usually within 12 months (8, 9). However, some patients may experience recurrence or permanent hypothyroidism during follow-up (5, 10). The incidence rate of SAT has maintained an upward trend in recent years (11). Recurrence and prolonged treatment time have become severe problems for the treatment of SAT (12, 13).
Recurrence is usually defined as the relapse of episodes of pain with elevated laboratory parameters erythrocyte sedimentation rate (ESR) or C-reactive protein (CRP) and ultrasonographic findings (14). Many studies have reported recurrence rates, but depending on the studied population, discrepancies in the SAT recurrence rate vary significantly between studied groups, ranging from 0% to over 30% (15, 16). Recurrence occurs when the PSL dose is gradually reduced during treatment and even many years after the first attack (17, 18). SAT recurrence can seriously affect the lives of patients and create psychological troubles for them. Thus, the determination of the risk groups for recurrent SAT can guide clinicians in preventing early recurrence and provide early diagnosis and proper treatment. Although studies have investigated the risk factors for the recurrence of SAT in certain areas, endocrinologists’ knowledge gap on SAT relapse remains to be addressed. To our knowledge, no study has systematically and comprehensively reviewed the SAT recurrence rate and risk factors for SAT recurrence through meta-analysis. This study aimed to conduct a systematic review and meta-analysis of cohort studies to estimate SAT recurrence rates and summarize the risk factors for SAT recurrence.
Materials and Methods
This study followed the Meta-Analysis of Observational Studies in Epidemiology (MOOSE) (Supplementary Table 1) and the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines (Supplementary Table 2) (19, 20).
Literature Search
We used a comprehensive search strategy to identify relevant English language literature in the following electronic databases: PubMed, Embase, Web of Science, and The Cochrane Library (up to August 20, 2021). The full search strategy is shown in Supplementary Table 3, and includes Medical Subject Headings (MeSH) headings and free term searches for “subacute thyroiditis”, “de Quervain thyroiditis”, “recurrence” and “cohort study”. The subjects of the studies were defined as humans, and the language of the articles was limited to English. We also manually searched reference lists from the included studies to identify potential additional eligible studies.
Study Selection
The inclusion criteria were as follows: (1) cohort study; (2) patients diagnosed with SAT based on their clinical diagnosis (12, 21, 22); (3) baseline and follow-up number of patients ≥ 10; and (4) the study reported the SAT recurrence rates or odds ratios (ORs), relative risks (RRs), hazard ratios (HRs) with 95% confidence intervals (CIs) of risk factors, and equivalent data. If multiple articles were published from the same cohort, the most informative report was included. Articles that did not meet the eligibility criteria were excluded.
Two authors (JZ and JL) independently screened titles and abstracts initially, and full-text articles were evaluated to ensure that they met the eligible inclusion criteria. If there were disagreements that could not be resolved through discussion, another author (FT) was invited to make a decision.
Data Extraction and Quality Assessment
Data were extracted from each of the included studies. The extracted data included the first author of the study, publication year, country, sample characteristics (e.g., sample size, mean age or range, the number of females), duration of follow-up, recurrence rate, treatment, risk factors investigated, significant risk factors and related effect size (e.g., ORs, RRs, HRs) with 95% CIs. Risk factors for recurrence included general characteristics (e.g., age, sex), therapy, HLA haplotype, laboratory parameters and ultrasonography.
The Newcastle-Ottawa Quality Scale (NOS) (23) was used to assess the quality of the included cohort studies. It consists of eight items and three components: selection, comparability and outcome. Additionally, the total stars range from 0 to 9. Studies with ≥ 7 stars were regarded as high-quality.
Data extraction and quality assessment were performed by two independent investigators (JZ and JL). Any disagreement was settled by discussion.
Statistical Analysis
We performed a meta-analysis of the recurrence rate and risk factors associated with SAT recurrence. Heterogeneity between studies was assessed using Cochran’s Q statistic and I2 values. I2 described the percentage of total change due to heterogeneity between studies rather than chance. If the heterogeneity was high (I2 > 50%), the random-effects model was adopted as the pooled method. Otherwise, the fixed-effects model was used. As the recurrence rate of SAT did not follow a normal distribution, logit transformation was adopted to transform the recurrence rate of SAT. For high heterogeneity, we used univariate meta-regression to explore the possible sources of between-study heterogeneity. Sensitivity analysis was used to assess the stability of the merger effect. A funnel plot and Egger’s test were used to estimate publication bias. Data were analyzed using R (R version 3.5.2; The R Foundation for Statistical Computing; Mathsoft, Cambridge, MA, USA). All tests were 2-sided, and p < 0.05 was considered statistically significant.
Results
Characteristics of Included Studies
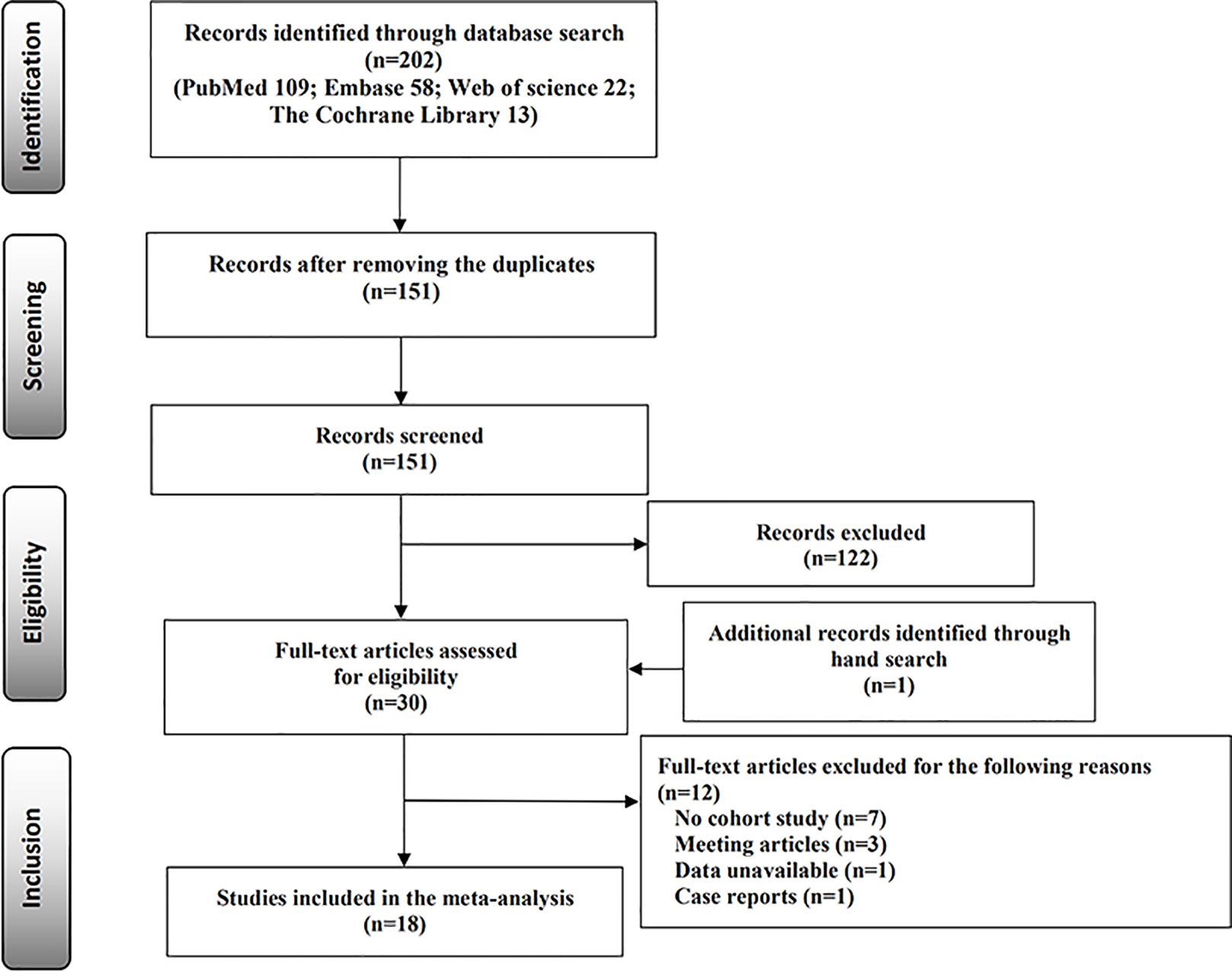
A total of 202 published studies were identified through the electronic database search, including PubMed (n = 109), Embase (n = 58), Web of Science (n = 22), and The Cochrane Library (n = 13). After removing duplicate publications (n = 51) and reviewing the titles and abstracts, 122 studies were excluded. One study was identified through the reference lists of the existing relevant studies. We carefully read the full text and excluded 12 studies that did not meet the two criteria (Supplementary Table 4). Finally, 18 cohort studies were identified that met the inclusion criteria (11, 12, 14–18, 21, 22, 24–32). The selection process of the studies is displayed in the flow diagram (Figure 1).
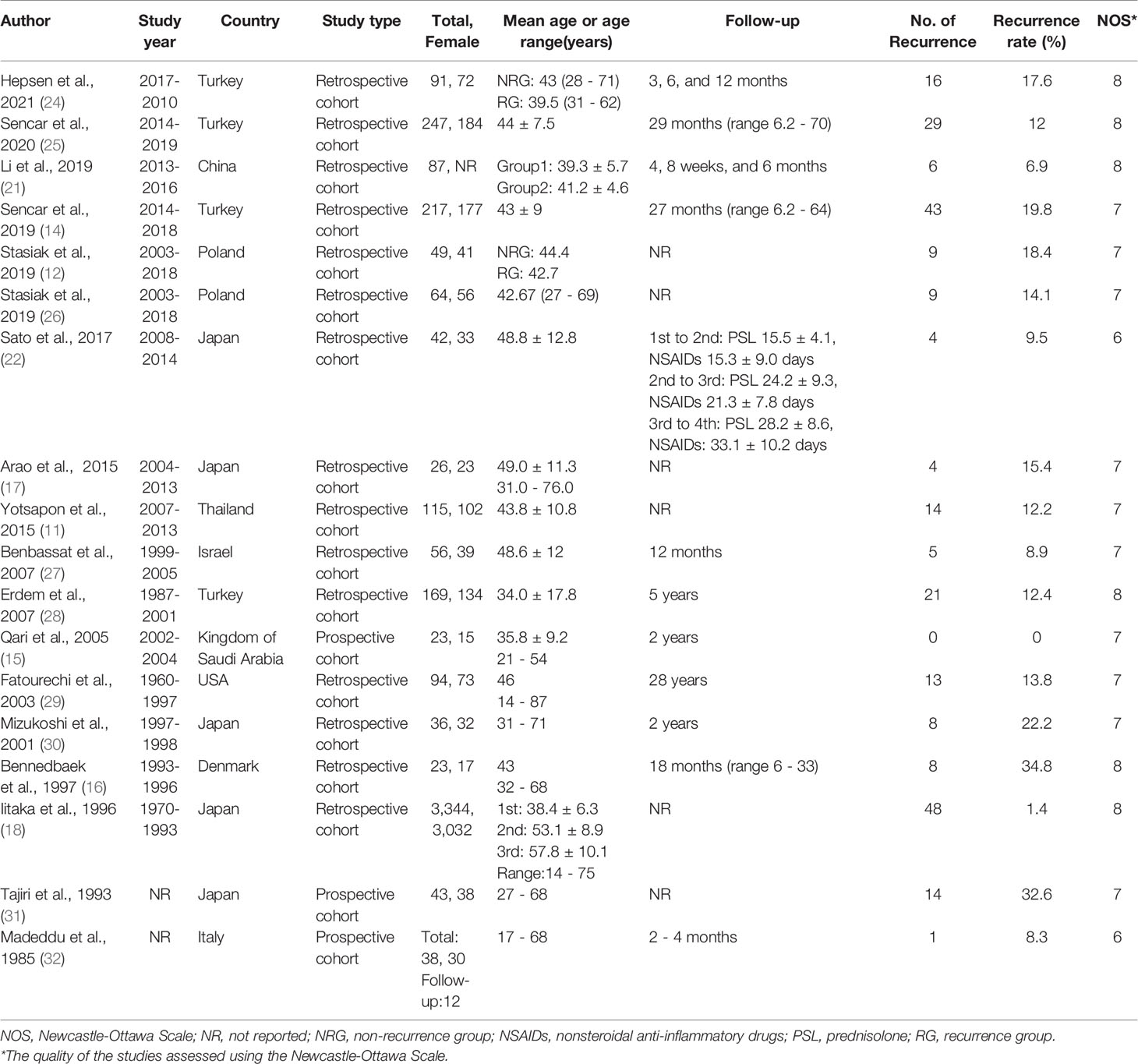
Tables 1, 2 provide the characteristics, quality and main risk factors for all studies included in the review. The included studies were published between 1985 and 2021 among ten countries: Japan (n = 5) (17, 18, 22, 30, 31), Turkey (n = 4) (14, 24, 25, 28), Poland (n = 2) (12, 26), China (n = 1) (21), Denmark (n = 1) (16), Italy (n = 1) (32), Israel (n = 1) (27), the Kingdom of Saudi Arabia (n = 1) (15), Thailand (n = 1) (11), and the USA (n = 1) (29). The sample size of the included studies varied from 23 (15, 16) to 3,344 (18), and the duration of mean follow-up varied from 2 months (22) to 5 years (28). Sixteen studies (11, 12, 14–18, 21, 24–31) were of high quality and 2 studies (22, 32) were of moderate quality. The details of critical appraisal according to the NOS are presented in Supplementary Table 5.
Recurrence Rate
A total of 18 studies reported the recurrence rate of SAT. The recurrence rates varied from 0% (15) to 34.8% (16). The pooled recurrence rate was 12.0% (95% CI: 8.2%, 17.1%), with significant heterogeneity (I2 = 87.9%, p < 0.01) (Figure 2A). The results of univariate meta-regression indicated that publication year (coefficient = 0.14, p = 0.80), sample (coefficient = -0.53, p = 0.21), country (coefficient = 0.52, p = 0.27), follow-up period (coefficient = 0.05, p = 0.86), and study type (coefficient = 0.03, p = 0.96) were not sources of heterogeneity (Supplementary Table 6).
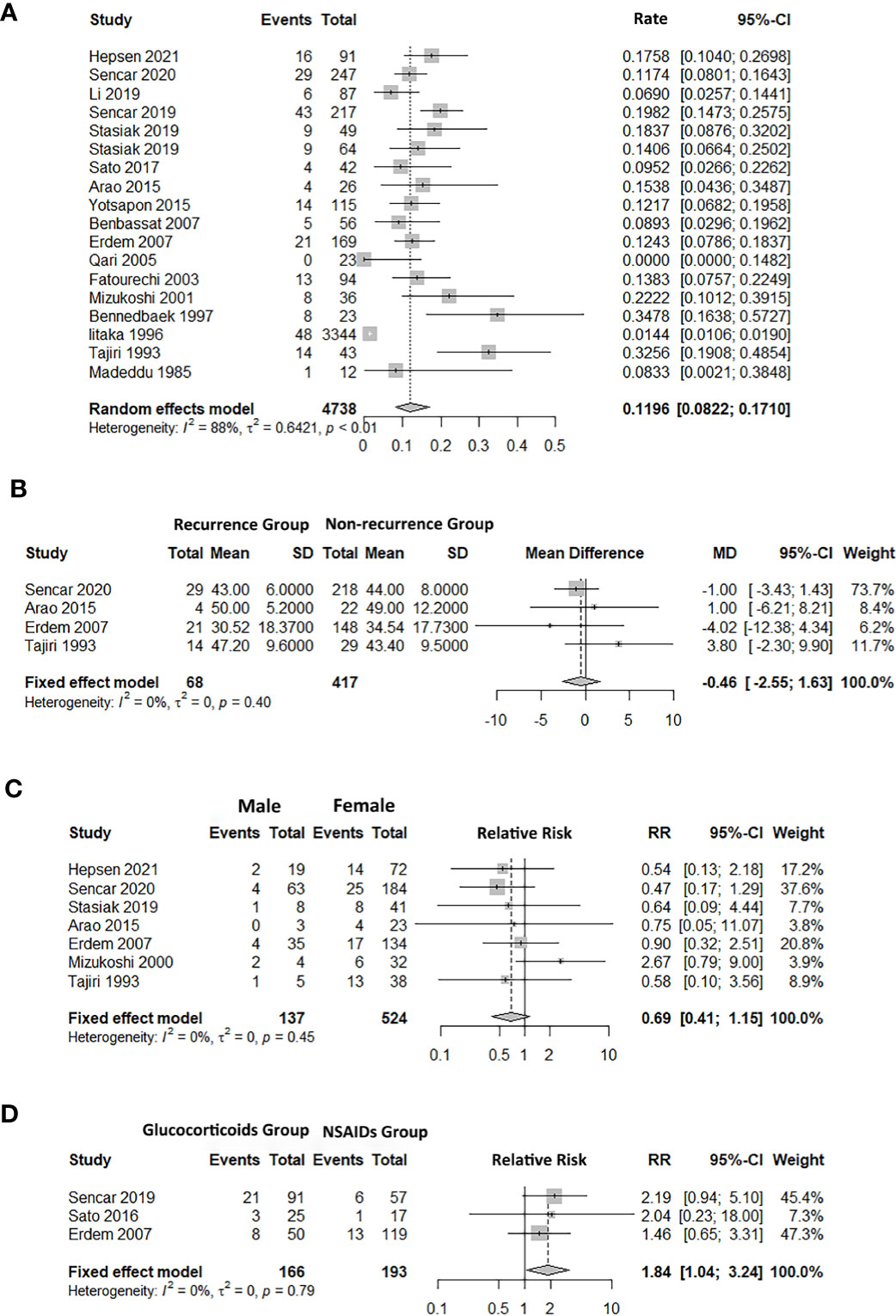
Figure 2 Forest plot of SAT recurrence rate and the association between factors and subacute thyroiditis (SAT) recurrence. (A) recurrence rate. (B) age. (C) sex. (D) treatment (glucocorticoids/NSAIDs). 95% CI, confidence interval.
Risk Factors for the SAT Recurrence
General Characteristics
Five studies reported the age difference for recurrence (17, 18, 25, 28, 31). The pooled finding suggested that there was no significant difference in age between the recurrence group (RG) and the non-recurrence group (NRG) (Age MD = -0.46, 95% CI: -2.55, 1.63; Figure 2B).
Seven studies referred to the recurrence and non-recurrence situation of males and females, and the number of recurrence of males was less than that of females (12, 17, 24, 25, 28, 30, 31). However, the pooled recurrence rate showed that there was no significant difference in sex between the RG and NRG groups (RR = 0.69, 95% CI: 0.41, 1.15; Figure 2C).
Therapy
Hepsen et al. compared low- and high-dose steroids in the treatment of SAT, and the findings showed that high-dose steroids had a higher SAT recurrence rate than low-dose steroids (24). Five studies focused on the effects of different treatments (14, 18, 21, 22, 28), and three of them reported the recurrence outcome between glucocorticoids and NSAIDs (14, 22, 28). The pooled result showed that the risk of recurrence in the glucocorticoids group was higher than the NSAIDs group (RR = 1.84, 95% CI: 1.04, 3.24; Figure 2D). The number of days required to taper the PSL dose to 5 mg/day (NRG: 44.3 ± 15.3, RG: 19.0 ± 11.9, p = 0.012) and the duration of the disease before therapy less than 30 days were also associated with the recurrence rate (17, 31).
HLA Haplotype
Stasiak et al. explored the relationship between the HLA haplotype and recurrence (12). The findings showed that the risk of SAT recurrence depended on HLA, and the determining factor was the copresence of HLA-B*18:01 and -B*35.
Laboratory Parameters, Ultrasonography
Hepsen et al. reported that the TSH level at the end of the treatment was a predictor of recurrence (24). Stasiak et al. found that TSH, free triiodothyronine (FT3), and free thyroxine (FT4) were significantly different between RG and NRG and that elevated anti-thyroid peroxidase antibody (aTPO) concentration at the first SAT episode was a protective factor (Table 2) (12). Moreover, sialic acid levels at the termination of treatment were an important risk factor (31). In addition, Bennedbaek et al. indicated that further extension of the hypoechoic area and an increase in thyroid volume were risk factors for SAT recurrence (16).
Sensitivity Analysis and Publication Bias
In the sensitivity analysis, no individual study substantially influenced the pooled recurrence rate of SAT (Supplementary Figure 1). The funnel plot can visually assess publication bias, and the horizontal line represents summary effect estimates. The points on the funnel plot of the recurrence rate did not fall onto the line (Figure 3A). Egger’s test of recurrence rate indicated no publication bias (p = 0.1137). The funnel plots and Egger’s tests of factors indicated no statistically significant potential for publication bias in the assessment of recurrence risk factors: age (p = 0.7066), sex (p = 0.8735), and treatment (p = 0.8632) (Figures 3B–D).
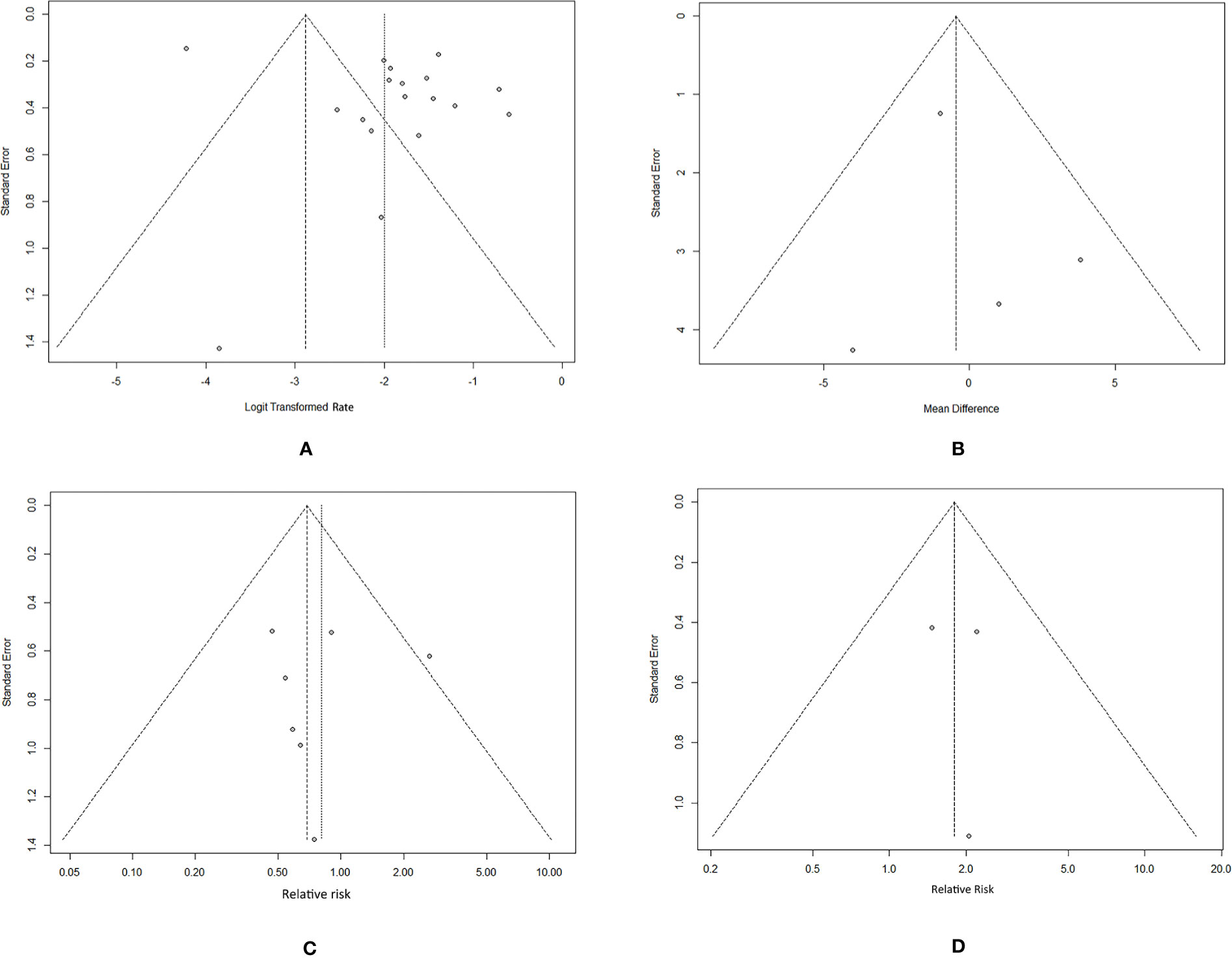
Figure 3 Funnel plots of the SAT recurrence rate and risk factors. (A) recurrence rate. (B) age. (C) sex. (D) treatment (glucocorticoids/NSAIDs).
Discussion
To the best of our knowledge, this is the first meta-analysis examining the recurrence rates and risk factors for SAT more comprehensively using data from cohort studies. Our study confirmed that 12.0% of SAT patients experienced recurrence. Previous studies reported that the occurrence of SAT is common in middle-aged women, but the current results of this study indicated that age and sex are not statistically significantly associated with SAT recurrence (27, 33).
For SAT therapy, these included studies mainly reported two different treatments, glucocorticoids (PSL and methylprednisolone)/NSAIDs and PSL + PV (Prunella vulgaris)/PSL. The current main purpose of SAT treatment is to relieve symptoms. The American Thyroid Association recommends corticosteroids to treat severe patients, but it does not provide objective criteria defining severe cases. The standard recommendation is to use prednisone 40 mg/day for 1 - 2 weeks, and then gradually taper the dose (34). Medication is generally based on clinical experience. As all included studies were retrospective cohort studies, and there was no significant difference in clinical background between the glucocorticoids group and the NSAIDs group, we cannot find the basis for treatment options (14, 22, 28). In terms of symptom relief, the view that corticosteroid treatment is superior to NSAIDs has been widely accepted, especially considering the aspect of the quicker effect, faster pain relief, etc. (22, 29, 35). Nevertheless, side effects and SAT recurrence are prone to occur in association with corticosteroid treatment (11, 16, 29, 30). The pooled findings of this study also suggested that the risk of recurrence caused among glucocorticoid-treated group is higher than that in the NSAIDs-treated group (RR = 1.84, 95% CI: 1.04, 3.24). This finding is consistent with those of previous studies and the underlying cause of recurrence may be that glucocorticoids are used to treat severe patients or patients who do not respond to NSAIDs. These patients are more likely to have a recurrence, and premature discontinuation of glucocorticoids can also lead to recurrence (35–37). Hepsen et al. showed that the recurrence rate of high-dose steroids is higher than that of low-dose steroids, which may be related to high-dose steroids that may promote virus replication and are more likely to cause recurrence (24). Kubota et al. believed that 15 mg/day PSL can be applied to Japanese people (38). Koirala et al. treated SAT with an initial dose of 20 mg/day and observed no adverse effects (39). Although these studies were not randomized controlled trials, they still suggested that low-dose PSL may reduce the recurrence rate and have an excellent therapeutic effect. Similarly, Soltani et al. reviewed latest studies regarding the most appropriate dosage of prednisolone with the lowest recurrence rate in the treatment of patients with subacute granulomatous thyroiditis, suggest that 15 - 20 mg/day of prednisolone is the best choice (40).
In addition, the duration of PSL administration is a potential risk factor. During PSL treatment, when tapering PSL from 10 mg/day to 5 mg/day, SAT recurrence is most likely to occur. Meanwhile, if the PSL is stopped too early, the pain may be more likely to recur. Therefore, at least six weeks for a therapeutic period before tapering PSL to 5 mg/day is highly suggested by clinicians to prevent recurrences, and extending the duration of PSL treatment at 10 mg/day may decrease the recurrence rate (17, 30). In addition, Tajiri et al. indicated that the time to be treated is a crucial factor for recurrence. In particular, patients who initiated PSL treatment within 30 days of onset had a higher recurrence rate than those who experienced a longer duration of illness prior to the treatment because inflammation of the thyroid gland may be improved in the natural course of the disease (31). In the future, we need to pay attention to the treatment dose and treatment duration to obtain more evidence that affects the high recurrence rate of PSL treatment. We cannot draw conclusions about the prognosis of PSL combined with PV in SAT treatment because only one included study explored the recurrence of PSL + PV/PSL treatment, and there was no significant difference between the two groups. This study suggested that we can focus on combination drugs in the future to enhance the therapeutic effect and reduce the recurrence rate.
Yamamoto et al. reported recurrence after ten years in three cases and suggested that HLA-A26 may be related to the predisposition to SAT recurrence (41). However, this was a case report and no other studies have proven that HLA-A26 is a risk factor for recurrence thus far. Stasiak et al. confirmed that the copresence of HLA-B*18:01 and -B*35 is the decisive factor of recurrence through a high resolution HLA haplotype (12). Patients with this HLA haplotype are more likely to experience recurrence, and high-risk patients with recurrence can be screened by identifying HLA haplotypes. The latest case report reported that three siblings with SAT lacked the copresence of HLA-B*18:01 and -B*35, but one of them had three episodes of recurrence, which may be related to the existence of some other HLA alleles. The coexistence of these HLA alleles may increase or decrease the susceptibility to recurrence (42). These results indicated that the impact of the HLA genotype on recurrence is complex and important. We need research on the potential relationship between more HLA haplotypes and recurrence to support these conclusions.
Hepsen et al. suggested that the TSH level at the end of treatment was associated with recurrence (24). Stasiak et al. found that TSH, FT3, and FT4 were significantly different between RG and NRG (12). Moreover, the increase in aTPO concentration during the first episode of SAT is a protective factor (12). In addition, Tajiri et al. suggested that the sialic acid level at the termination of treatment is a risk factor (31). Due to the inconsistent measurement time of laboratory parameters, we cannot obtain more reliable evidence. Three included studies focused on the ultrasonography of SAT recurrence (16, 25, 28). The finding of Bennedbaek et al. showed that recurrence is related to the further extension of hypoechoic areas and an increase in thyroid volume and has nothing to do with the extension of hypoechogenicity or initial thyroid function (16). Both Sencar et al. and Bennedbeak et al. mentioned that the initial thyroid volume is not related to recurrence (16, 25). Similarly, there was no difference in the type of nodules shown by ultrasound between the RG and NRG (28).
Recurrences of SAT may occur soon after the initial therapy, but they also happen even many years after the first attack (41, 43). In the included studies, Iitaka et al. also reported that 48 out of 3,344 SAT patients (1.4%) had multiple recurrences over 24 years (18). The data showed that symptoms during recurrence were generally milder than those in the first episode and the incubation period seemed to shorten as patients aged. The faster response of the immune system may make the symptoms of recurrent reactions milder (18).
This meta-analysis has several strengths. First, we conducted a comprehensive literature search, and the included studies were cohort studies that could provide more convincing results than case-control studies, cross-sectional studies or sporadic case reports. Second, the sample size was large, with a total of 4,764 SAT patients, and the follow-up time was long, with an average of 4 years. Third, accurate estimation of the recurrence rate and comprehensive research on risk factors can enable clinicians to provide better consultations for patients who have experienced the first SAT. Fourth, it compared the two main SAT treatments in the clinic, which can provide clinical guidance for clinicians. However, the potential limitations of this meta-analysis should be considered. First, most of the included studies were retrospective cohort studies, and the quality of historical clinical data may not be guaranteed. Second, because SAT is a rare type of thyroiditis, most of the included studies reported fewer than 100 patients and limited information on the risk factors for SAT recurrence. These limitations may impose a modest constraint on interpreting these findings, but they should not substantively undermine the internal validity of our study. Third, our main purpose was to explore the recurrence rate and the risk factors for SAT, therefore, we did not pay attention to the long-term prognosis of the disease.
In summary, our study demonstrated that 12.0% of patients might develop SAT recurrence. Moreover, the risk of recurrence in patients treated with glucocorticoids is higher than of patients treated with of NSAIDs. Treatment-related factors, HLA haplotype, the sialic acid level, or the TSH level at the termination of treatment, further extension of the hypoechoic area and increase in thyroid volume were all potential predictors for recurrence of SAT. Further randomized controlled trials, prospective cohort studies, and studies on the molecular and cellular mechanisms are needed to explore the association between these factors and SAT recurrence. Moreover, the choice of treatment should also consider the impact on the long-term prognosis of patients, such as thyroid function. It is recommended to carry out more clinical studies of different therapies to observe the prognosis and long-term effects of SAT patients. Our findings might shed light on the choice of therapeutic optimization for clinicians to reduce recurrence and have important implications for improving the quality of life of SAT patients in the future.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author Contributions
Study concept and design: FT. Acquisition, analysis, interpretation of data: JZ, GD, XiaoL, JL, and LD. Drafting of the manuscript: JZ. Critical revision of the manuscript for important intellectual content: FT, GD, and LD. Statistical analysis: JZ, GD, XianL, and JL. Administrative, technical, or material support: All authors. All authors have approved the final draft of the manuscript.
Funding
This study was supported by grants from National Natural Science Foundation of China (71804093), Academic Promotion Programme of Shandong First Medical University (2019LJ005), Project of Priority Research from Department of Science and Technology of Shandong Province (2020RKB14114), and Science and Technology development Plan of Traditional Chinese Medicine in Shandong Province (2019-0374).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2021.783439/full#supplementary-material
References
1. Jameson JL, Mandel SJ, Weetman AP. Disorders of the Thyroid Gland. In: Kasper D, Fauci A, Hauser S, editors. Harrisons Principles of Internal Medicine. 2. 19th Ed. New York: McGraw Hill Education/Medical (2015). p. 2298.
2. Effraimidis G, Feldt-Rasmussen U. Thyroiditis, Infectious and Subacute. In: Huhtaniemi I, Luciano Martini L, editors. Encyclopedia of Endocrine Diseases. 4. 2 Ed. The United StatesElsevier-Saunders (2018). p. 737–45.
3. Pearce EN, Farwell AP, Braverman LE. Thyroiditis. N Engl J Med (2003) 348:2646–55. doi: 10.1056/NEJMra021194
4. Crile G, Rumsey E. Subacute Thyroiditis. J Am Med Assoc (1950) 142:458–62. doi: 10.1001/jama.1950.02910250006002
5. Alfadda AA, Sallam RM, Elawad GE, Aldhukair H, Alyahya MM. Subacute Thyroiditis: Clinical Presentation and Long Term Outcome. Int J Endocrinol (2014) 2014:794943. doi: 10.1155/2014/794943
6. Nyulassy S, Hnilica P, Buc M, Guman M, Hirschová V, Stefanovic J. Subacute (De Quervain’s) Thyroiditis: Association With HLA-Bw35 Antigen and Abnormalities of the Complement System, Immunoglobulins and Other Serum Proteins. J Clin Endocrinol Metab (1977) 45:270–4. doi: 10.1210/jcem-45-2-270
7. Stasiak M, Tymoniuk B, Michalak R, Stasiak B, Kowalski ML, Lewinski A. Subacute Thyroiditis Is Associated With HLA-B*18:01, -DRB1*01 and -C*04:01-the Significance of the New Molecular Background. J Clin Med (2020) 9:534. doi: 10.3390/jcm9020534
8. Gorges J, Ulrich J, Keck C, Muller-Wieland D, Diederich S, Janssen O. Long-Term Outcome of Subacute Thyroiditis. Exp Clin Endocrinol Diabetes (2019) 128:703–8. doi: 10.1055/a-0998-8035
10. Lio S, Pontecorvi A, Caruso M, Monaco F, D’Armiento M. Transitory Subclinical and Permanent Hypothyroidism in the Course of Subacute Thyroiditis (De Quervain). Acta Endocrinol (Copenh) (1984) 106:67–70. doi: 10.1530/acta.0.1060067
11. Yotsapon T, Sirinate K, Siriwan B, Soontaree N, Thep H. Clinical Features and Outcomes of Subacute Thyroiditis in Thai Patients. J ASEAN Fed Endocrine Soc (2015) 30:125–8. doi: 10.15605/jafes.030.02.03
12. Stasiak M, Tymoniuk B, Stasiak B, Lewinski A. The Risk of Recurrence of Subacute Thyroiditis Is HLA-Dependent. Int J Mol Sci (2019) 20:1089. doi: 10.3390/ijms20051089
13. Saklamaz A. Is There a Drug Effect on the Development of Permanent Hypothyroidism in Subacute Thyroiditis? Acta Endocrinol (Buchar) (2017) 13:119–23. doi: 10.4183/aeb.2017.119
14. Sencar ME, Calapkulu M, Sakiz D, Hepsen S, Kus A, Akhanli P, et al. An Evaluation of the Results of the Steroid and non-Steroidal Anti-Inflammatory Drug Treatments in Subacute Thyroiditis in Relation to Persistent Hypothyroidism and Recurrence. Sci Rep (2019) 9:16899. doi: 10.1038/s41598-019-53475-w
16. Bennedbaek FN, Hegedüs L. The Value of Ultrasonography in the Diagnosis and Follow-Up of Subacute Thyroiditis. Thyroid (1997) 7:45–50. doi: 10.1089/thy.1997.7.45
17. Arao T, Okada Y, Torimoto K, Kurozumi A, Narisawa M, Yamamoto S, et al. Prednisolone Dosing Regimen for Treatment of Subacute Thyroiditis. J UOEH (2015) 37:103–10. doi: 10.7888/juoeh.37.103
18. Iitaka M, Momotani N, Ishii JKI. Incidence of Subacute Thyroiditis Recurrences After a Prolonged Latency: 24-Year Survey. J Clin Endocrinol Metab (1996) 81:466–9. doi: 10.1210/jcem.81.2.8636251
19. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-Analysis of Observational Studies in Epidemiology: A Proposal for Reporting. Meta-Analysis of Observational Studies in Epidemiology (MOOSE) Group. JAMA (2000) 283:2008–12. doi: 10.1001/jama.283.15.2008
20. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 Statement. Syst Rev (2015) 4:1. doi: 10.1186/2046-4053-4-1
21. Li F, Wu Y, Chen L, Hu L, Liu X. Initial Treatment Combined With Prunella Vulgaris Reduced Prednisolone Consumption for Patients With Subacute Thyroiditis. Ann Transl Med (2019) 7(3):45. doi: 10.21037/atm.2019.01.07
22. Sato J, Uchida T, Komiya K, Goto H, Takeno K, Suzuki R, et al. Comparison of the Therapeutic Effects of Prednisolone and Nonsteroidal Anti-Inflammatory Drugs in Patients With Subacute Thyroiditis. Endocrine (2017) 55:209–14. doi: 10.1007/s12020-016-1122-3
23. Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/nosgen.pdf (Accessed May 10, 2021).
24. Hepsen S, Akhanli P, Sencar ME, Duger H, Sakiz D, Kizilgul M, et al. The Evaluation of Low- and High-Dose Steroid Treatments in Subacute Thyroiditis: A Retrospective Observational Study. Endocr Pract (2021) 27:594–600. doi: 10.1016/j.eprac.2020.11.009
25. Sencar ME, Calapkulu M, Sakiz D, Akhanli P, Hepsen S, Duger H, et al. The Contribution of Ultrasonographic Findings to the Prognosis of Subacute Thyroiditis. Arch Endocrinol Metab (2020) 64:306–11. doi: 10.20945/2359-3997000000253
26. Stasiak M, Michalak R, Stasiak B, Lewinski A. Clinical Characteristics of Subacute Thyroiditis is Different Than it Used to be - Current State Based on 15 Years Own Material. Neuro Endocrinol Lett (2019) 39:489–95.
27. Benbassat CA, Olchovsky D, Tsvetov G, Shimon I. Subacute Thyroiditis: Clinical Characteristics and Treatment Outcome in Fifty-Six Consecutive Patients Diagnosed Between 1999 and 2005. J Endocrinol Invest (2007) 30:631–5. doi: 10.1007/BF03347442
28. Erdem N, Erdogan M, Ozbek M, Karadeniz M, Cetinkalp S, Gokhan Ozgen A, et al. Demographic and Clinical Features of Patients With Subacute Thyroiditis: Results of 169 Patients From a Single University Center in Turkey. J Endocrinol Invest (2007) 30:546–50. doi: 10.1007/BF03346347
29. Fatourechi V, Aniszewski JP, Fatourechi GZ, Atkinson EJ, Jacobsen SJ. Clinical Features and Outcome of Subacute Thyroiditis in an Incidence Cohort: Olmsted County, Minnesota, Study. J Clin Endocrinol Metab (2003) 88:2100–5. doi: 10.1210/jc.2002-021799
30. Mizukoshi T, Noguchi S, Murakami T, Futata T, Yamashita H. Evaluation of Recurrence in 36 Subacute Thyroiditis Patients Managed With Prednisolone. Intern Med (2001) 40:292–5. doi: 10.2169/internalmedicine.40.292
31. Tajiri J, Noguchi S, Morita M, Tamaru M, Murakami T, Murakami N. Serum Sialic Acid Levels in the Diagnosis and Follow-Up of Subacute Granulomatous Thyroiditis. Endocr J (1993) 40:83–7. doi: 10.1507/endocrj.40.83
32. Madeddu G, Casu AR, Costanza C, Marras G, Arrasl ML, Marrosu A, et al. Serum Thyroglobulin Levels in the Diagnosis and Follow-Up of Subacute ‘Painful’ Thyroiditis. A Sequential Study. Arch Intern Med (1985) 145:243–7. doi: 10.1001/archinte.145.2.243
34. Ross DS, Burch HB, Cooper DS, Greenlee MC, Laurberg P, Maia AL, et al. 2016American Thyroid Association Guidelines for Diagnosis and Management of Hyperthyroidism and Other Causes of Thyrotoxicosis. Thyroid (2016) 26:1343–421. doi: 10.1089/thy.2016.0229
35. Volpé R. The Management of Subacute (Dequervain’s) Thyroiditis. Thyroid (1993) 3:253–5. doi: 10.1089/thy.1993.3.253
36. Kim M, Ladenson PW. Subacute (De Quervain’s) Thyroiditis. In: Goldman L, Schafer AI, editors. Goldman’s Cecil Medicine. 1. 25th Ed. United States: Elsevier-Saunders (2015). p. 1509.
37. Samuels MH. Subacute, Silent, and Postpartum Thyroiditis. Med Clinics North America (2012) 96:223–33. doi: 10.1016/j.mcna.2012.01.003
38. Kubota S, Nishihara E, Kudo T, Ito M, Amino N, Miyauchi A. Initial Treatment With 15 Mg of Prednisolone Daily Is Sufficient for Most Patients With Subacute Thyroiditis in Japan. Thyroid (2013) 23:269–72. doi: 10.1089/thy.2012.0459
39. Koirala KP, Sharma V. Treatment of Acute Painful Thyroiditis With Low Dose Prednisolone: A Study on Patients From Western Nepal. J Clin Diagn Res (2015) 9:MC01–3. doi: 10.7860/JCDR/2015/14893.6427
40. Soltani A, Nourani F, Roudsari SB, Jouybari L, Fathi M, Haghighat S, et al. Identifying the Lowest Effective Initial Dose of Prednisolone for the Treatment of Subacute Granulomatous Thyroiditis a Systematic Review and Meta-Analysis. Curr Rev Clin Exp Pharmacol (2021). doi: 10.2174/2772432816666211012092112
41. Yamamoto M, Saito S, Sakurada T, Tamura M, Kudo T, Yoshida K, et al. Recurrence of Subacute Thyroiditis Over 10 Years After the First Attack in Three Cases. Endocrinol Jpn (1988) 35:833–9. doi: 10.1507/endocrj1954.35.833
42. Stasiak M, Lewinski A. Strong Correlation Between HLA and Clinical Course of Subacute Thyroiditis-a Report of the Three Siblings. Genes (Basel) (2020) 11:1282. doi: 10.3390/genes11111282
Keywords: subacute thyroiditis, recurrence rate, risk factors, meta-analysis, cohort study
Citation: Zhang J, Ding G, Li J, Li X, Ding L, Li X, Yang S and Tang F (2021) Risk Factors for Subacute Thyroiditis Recurrence: A Systematic Review and Meta-Analysis of Cohort Studies. Front. Endocrinol. 12:783439. doi: 10.3389/fendo.2021.783439
Received: 29 September 2021; Accepted: 30 November 2021;
Published: 23 December 2021.
Edited by:
Loredana Pagano, University of Turin, ItalyReviewed by:
Sara Garberoglio, Humanitas Cellini Clinic, ItalyMagdalena Stasiak, Polish Mother’s Memorial Hospital Research Institute, Poland
Copyright © 2021 Zhang, Ding, Li, Li, Ding, Li, Yang and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fang Tang, tangfangsdu@126.com
†These authors have contributed equally to this work
 Jing Zhang
Jing Zhang Guoyong Ding
Guoyong Ding Jingru Li1,2,5
Jingru Li1,2,5 Xiao Li
Xiao Li Fang Tang
Fang Tang