- 1Endocrinology Unit, Department of Clinical and Experimental Medicine, Garibaldi-Nesima Medical Center, University of Catania, Catania, Italy
- 2Department of Translational and Precision Medicine, Sapienza University of Rome, Rome, Italy
- 3Division of Endocrine and Metabolic Diseases, Department of Clinical Sciences and Community Health, IRCCS Istituto Auxologico Italiano, Università degli Studi di Milano, Milan, Italy
- 4Department of Clinical and Experimental Medicine, University of Messina, Messina, Italy
- 5Endocrinology Unit, Department of Clinical and Experimental Medicine, University of Pisa, Pisa, Italy
- 6Department of Medical, Surgical and Neurological Sciences, University of Siena, Siena, Italy
Management of thyroid nodules in the era of precision medicine is continuously changing. Neck ultrasound plays a pivotal role in the diagnosis and several ultrasound stratification systems have been proposed in order to predict malignancy and help clinicians in therapeutic and follow-up decision. Ultrasound elastosonography is another powerful diagnostic technique and can be an added value to stratify the risk of malignancy of thyroid nodules. Moreover, the development of new techniques in the era of “Deep Learning,” has led to a creation of machine-learning algorithms based on ultrasound examinations that showed similar accuracy to that obtained by expert radiologists. Despite new technologies in thyroid imaging, diagnostic surgery in 50–70% of patients with indeterminate cytology is still performed. Molecular tests can increase accuracy in diagnosis when performed on “indeterminate” nodules. However, the more updated tools that can be used to this purpose in order to “rule out” (Afirma GSC) or “rule in” (Thyroseq v3) malignancy, have a main limitation: the high costs. In the last years various image-guided procedures have been proposed as alternative and less invasive approaches to surgery for symptomatic thyroid nodules. These minimally invasive techniques (laser and radio-frequency ablation, high intensity focused ultrasound and percutaneous microwave ablation) results in nodule shrinkage and improvement of local symptoms, with a lower risk of complications and minor costs compared to surgery. Finally, ultrasound-guided ablation therapy was introduced with promising results as a feasible treatment for low-risk papillary thyroid microcarcinoma or cervical lymph node metastases.
Introduction
One of the main clinical challenge in endocrine clinical practice is certainly the management of thyroid nodules disease. During the last years, new technologies have been developed and new diagnostic and therapeutic approaches have been introduced to guide clinician through the diagnosis, follow-up and therapeutic decision. This review will provide an evidence-based summary of the optimal approach to the management of thyroid nodules.
The Key Role of Ultrasound
The prevalence of nodular thyroid disease in the general population is high, reaching 60% according to ultrasound and autopsy findings (1–3), though the incidence of malignancy is relatively low, ranging 1.6% and 12% (4, 5). Thyroid ultrasonography (US) is the primary tool used for the diagnosis and the initial cancer risk stratification of thyroid nodules. Currently, it guides decision making for fine-needle aspiration biopsy (FNA), the timing of subsequent clinical evaluations during long-term follow-up (6), and the eligibility for active surveillance of suspicious nodules (7). A complete report should include a description of the whole thyroid parenchyma, nodule location, size, and sonographic features, and assessment of the lymph nodes in the neck (8–10). The US features that should be evaluated for each nodule are: echogenicity, composition (solid, cystic, mixed), margins, calcifications or other hyperechoic foci, shape, and relations with the thyroid capsule (11, 12). Ultrasound patterns associated with malignancy include: hypoechogenicity, infiltrative, irregular, or lobulated margins, micro-calcifications, taller-than-wide shape, absence of a halo. However, none of these single US pattern have sensitivity, specificity and accuracy high enough to be considered predictive for malignancy (11, 13, 14). The combination of US patterns leads to a higher specificity, but it associates to a lower sensitivity (15). Finally, it is worth to mention that the evaluation of these US features is characterized by a high interobserver variability (16, 17). In the last decade, to improve standardization of thyroid ultrasound reporting, the guidelines of the American Association of Clinical Endocrinologists/American College of Endocrinology/Associazione Medici Endocrinologi (AACE/ACE/AME) (3), the 2015 guidelines of the American Thyroid Association (ATA) (18), the guidelines of the European Thyroid Association (ETA; EU-TIRADS, European Thyroid Imaging Reporting and Data System) (8), the American College of Radiology (ACR) TIRADS (9), and the Korean Society of Thyroid Radiology's K-TIRADS system, have proposed risk stratification systems with the goal of detecting nodule at greatest risk for malignancy and then to recommend graduated size cut-offs for FNA cytology (19) (Table 1). All these risk-stratification systems are similar, but there are some differences: the endocrinological societies' systems are based on recognition of patterns, while ACR TIRADS is score-based, considering 5 US features and their sum to obtain the final classification of the nodule. Furthermore, the weight of each sonographic feature varies across various systems (e.g., echogenicity being the most important one for EU-TIRADS, and composition for K-TIRADS), and the size threshold to recommend FNA is different, too. Some of the systems have been validated in multicenter studies (20–22). Independent comparison studies (usually involving 2–3 of the systems) were mostly retrospective (23–28). Two prospective Italian studies compared the systems developed by the British Thyroid Association, the ATA, and the AACE/ACE/AME (29), or the ATA and the ETA (30) and found no significant differences between overall diagnostic accuracy. A recent study comparing the main five systems endorsed by international societies, found that four of the five (AACE/ACE/AME, ATA, ACR-, and EU-TIRADS) showed a significant diagnostic value (31). The ACR TIRADS, which classified over half of the requested biopsies as unnecessary, with a negative predictive value of 97.8%, showed the best overall performance (31). To reproduce these results in the real clinical practice, an essential prerequisite is the adoption of a uniform language and definition of suspicious features (10). Classification of thyroid nodules using any of the five classification systems results in higher interobserver agreement than evaluation of single suspicious features, and identification of nodules needing biopsy has an almost perfect agreement (32). However, a specific “training by consensus” involving joint evaluation of images can improve the reproducibility for all classifications (with significant improvements for ATA, K-TIRADS, and EU-TIRADS systems), even for trained clinicians with similar experience (32). In recent years, the useful of ultrasound patterns to stratify the risk of malignancy of indeterminate thyroid nodules, has also been evaluated (33–35). Sonographic patterns were associated with different rate of malignancy suggesting that these systems are also able to stratify the risk of malignancy in the subgroup of cytologically indeterminate thyroid. These preliminary data suggest that sonographic patterns would be useful not only to guide FNAC, but also to personalize management after an indeterminate cytological results. Recently, software applications performing automated image analysis were also proposed to extract quantitative parameters using a variety of mathematical methods. These approaches may be the basis for computer-aided diagnosis (CAD) systems to yield an automated “second opinion” (36). According to some evidence, thyroid CADs based on artificial intelligence may further improve diagnostic performance and reliability (37). The use of thyroid CAD to differentiate malignant from benign nodules showed accuracy similar to that obtained by an expert radiologist (38, 39) and may reduce intra- and inter-observer variability, that however, still remains (38). Ultrasound elastography (USE) has emerged as an additional tool in combination with B-Mode Ultrasound (US) for thyroid nodules work-up. It is a non-invasive, cost-effective, dynamic diagnostic method for the measurement of tissues elasticity (40, 41). Malignant lesions tend to be harder and firmer than the normal thyroid parenchyma or benign lesions, related to fibrosis and higher expression of Galectin-3 and Fibronectin-1 (41–43), suggesting that elastography can be useful to distinguish between benign and malignant thyroid nodule (43, 44). There are two main elastography techniques to quantify thyroid nodules stiffness currently in clinical use: strain elastography (SE), which evaluates the degree of tissue deformation induced by manual compression or acoustic forces, and in which tissue deformation is parallel to the direction of the force; and shear wave elastography (SWE), in which a push beam is created and tissue displacement is perpendicular to the direction of the force (45, 46). Many studies and meta-analyses have identified for USE some limitations and confounding factors, including nodule features (calcifications, cystic components, size, position), the operator expertise, artifacts such as carotid artery pulsation, coexistent systemic, or thyroid diseases (Hashimoto's thyroiditis, acromegaly, previous thermal ablation or radiofrequency on thyroid nodule) and pathological type of thyroid cancer (40, 41, 47–71). Therefore, USE should be performed in selected thyroid nodules by qualified operators using objective criteria provided by elastographic machines. Two clinical practice guidelines include recommendation on thyroid USE. The 2015 ATA guidelines (18) reported that USE may be a helpful tool for preoperative risk assessment in patients, although it cannot be universally recommended. The 2016 AACE/ACE/AME guidelines (3) reported that USE data are complementary to gray-scale findings, especially in nodules with indeterminate US or cytological findings. Moreover, other specialized guidelines specific for USE [European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB) (72) and World Federation of Societies for Ultrasound in Medicine and Biology (WFSUMB) guidelines (50)], provide an adequate description of the technique and its reproducibility, results and limitations. Although many reports have demonstrated that USE performed the same or better than the gray-scale US (40, 41), its diagnostic efficacy is still controversial (73). In clinical practice USE is usually performed as a complementary tool to conventional US, as the combination of the two techniques proved to have higher sensitivity (74). Recently, some studies evaluated the potential role of elastography in non-diagnostic or indeterminate nodules (43, 75), even if conventional US also has been shown to display good diagnostic results (37, 76). Utility of USE has also been explored in many studies (40, 54, 77, 78) but therefore, other authors failed to demonstrate the diagnostic utility in indeterminate nodules (75, 79) and a meta-analysis of eight studies demonstrated there was a great variability of both sensitivity and specificity in USE, with pooled estimates of 69 and 75%, respectively (77). Further studies are required concerning the supplementary role of elastography in the risk stratification of thyroid nodules.
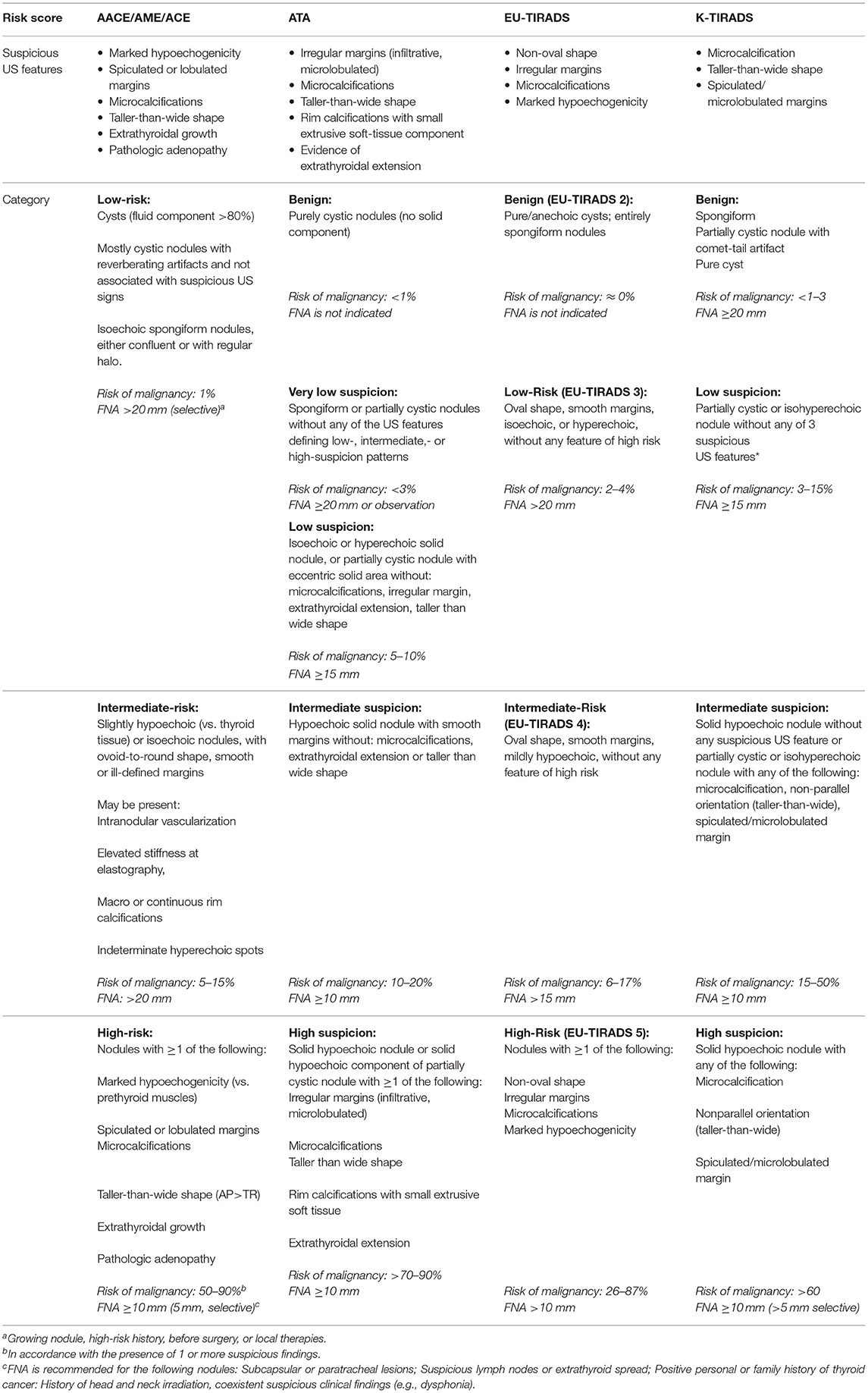
Table 1. An overview of the standardized thyroid nodule US scoring systems proposed or endorsed by international practice guidelines.
Have Molecular Analysis an Added Value?
When thyroid nodules are evaluated with fine-needle aspiration biopsy (FNAB), in ~5–20% of cases it is not possible to discriminate between benign and malignant nodules because of an indeterminate cytology (78). According to The Bethesda System for Reporting Thyroid Cytopathology (79, 80) indeterminate cytology includes two different categories: atypical or follicular lesion (Bethesda III) and follicular neoplasm/suspicious for follicular (or Hürthle cell) neoplasm (Bethesda IV). The observed rates of cancer in these categories vary widely ranging from 6 to 48% for Bethesda III and 14 to 34% for Bethesda IV (81). This wide range of cancer risk, involves that diagnostic hemithyroidectomies are still performed in order to discriminate between benign and malignant nodules. Unfortunately, in 50–70% of patients with indeterminate cytology a diagnostic surgery is performed. Moreover, surgery exposes patients to surgical risks and in the event of malignant lesions, a second-stage surgery is often indicated with additional costs and risks for patients (82–85). Molecular tests using gene expression and/or mutational analysis, have been developed to reduce the need for diagnostic surgery for indeterminate (Bethesda III/IV) thyroid nodules (86). Several molecular tests have been proposed over the years, since different gene-mutation panels has been introduced (86), with reported NPV and PPV ranging from 56 to 100% and from 19 to 100%, respectively, and the most successful are the Thyroseq and the AFIRMA Gene Expression Classifier (GEC). The first version of Thyroseq included the 7 gene panel (BRAF, H-K-N-RAS, RET/PTC1-3, PAX8/PPARγ) (87) with a reported sensitivity of about 65% (87–89). Following versions migrated to the next generation sequencing platforms (NGS) and included a 13-gene panel (ThyroSeq v1) (90) and a 56-gene panel (ThyroSeq v2) with a significant increase in sensitivity and negative predictive value (NPV) (91, 92). The last version of Thyroseq, v3, Nikiforov and Baloch (92) is a targeted NGS test that evaluates point mutations, gene fusions, copy number alterations and abnormal gene expression in 112 thyroid cancer related genes. Using the last version of Thyroseq, a recent prospective and multicenter validation study (93) on 286 cytological indeterminate nodules submitted to surgery reported a 94% of sensitivity and 82% of specificity with a NPV of 97% and a positive predictive value (PPV) of 66%. These data may obviate diagnostic surgery in up to 61% of patients with indeterminate nodules. The AFIRMA GEC is a microarray based test with a proprietary algorithm able to differentiate benign from malignant nodules based on messenger RNA expression pattern. The sensitivity is approximately of 90%, but the specificity is lower (88, 94). Moreover, a significant site to site variability in the benign call rate (range 27–53%) and in the malignancy rate (range 15.6–70%) was reported in a follow-up multicenter study (95).Very recently, the AFIRMA Genomic Sequencing Classifier (GSC) replaced the original GEC. It is a RNA sequencing based test, including 12 classifiers composed of 10,196 genes and 7 additional components in order to exclude parathyroid lesions and medullary thyroid cancer, and includes the analysis of BRAFV600E mutations, RET/PTC1 or RET/PTC3 and of specific alterations typical of Hurtle cell lesions. Compared to GEC, the GSC has a better specificity and reduces the number of histological benign samples classified as suspicious. An initial validation study showed a 36% increase in specificity compared with the GEC with a reported sensitivity of about 91% (96). Harrell et al. (97) demonstrated that GSC is able to identify less indeterminate cytology nodules as suspicious when compared to GEC, suggesting that GSC further reduces surgery by improving in specificity. In a recent independent study, Endo et al. (98) compared GEC with GSC and demonstrated that GSC had a significant higher benign call rate (76.2 vs. 48.1%), PPV (60.0 vs. 33.3%), and specificity (94.3 vs. 61.4%) than GEC in both Bethesda III and Bethesda IV categories. In particular, benign call rate of GSC was significantly higher in nodules with Hürthle cell changes (88.8 vs. 25.7%). In summary, both ThyroSeq and AFIRMA have reached a high sensitivity and enough specificity to function as rule in and rule out tests. The main problem is the limited number of validation studies and the high costs that remain a limit in their worldwide utilization. Currently, there are no data to prefer a molecular test rather than another one, and long term outcome data are needed.
Mini Invasive Treatments
Most benign thyroid nodules are asymptomatic, stable and do not require treatment, while large thyroid nodules may become responsible for pressure symptom, neck discomfort or cosmetic complaints thus resulting in decreased quality of life (99). Over the last two decades, non-surgical, minimally invasive US-guided techniques have been proposed for the treatment of symptomatic nodules. Minimally invasive procedures include percutaneous ethanol injection (PEI), laser thermal ablation (LTA), radiofrequency ablation (RFA), high intensity focused ultrasound (HIFU), and percutaneous microwave ablation (PMWA) (Table 2). PEI represents the first-line treatment for thyroid cysts and nodules with a predominant fluid component (100), while in solid nodules, LTA and RFA have proven to be very effective and safe in producing significant and stable reduction of nodule volume (101). Radiofrequency thermoablation consists in thermal ablation of the nodular tissue by exploiting the heat released by an energy source with consequent coagulation necrosis. The purpose of the treatment is to determine a volumetric reduction of the thyroid nodule, a condition that usually occurs in the weeks and months following the procedure as a consequence of the gradual replacement of the thyroid tissue with fibro-scar tissue and the procedure can be repeated after some time (102). Overall complication rate is low, about 3.5% (103). Some authors reported an higher difficulty of surgery after treatments, and exists the rare possibility of cancer spreading while treating patients with supposedly benign nodules (104). Radiofrequency thermoablation can be used for the treatment of benign nodular masses on cytological evaluation, which cause aesthetic alteration or compressive symptoms which cannot be treated surgically, for comorbidities or patient's preference. It is also recommended for the treatment of both pre-toxic and toxic nodules, when surgery or radioiodine are contraindicated or refused by the patient (3, 102, 105). Radiofrequency thermoablation has been proposed for papillary thyroid microcarcinoma and in cases of recurrence or loco-regional persistence of thyroid carcinoma when surgery is contraindicated or radiometabolic therapy has proved ineffective (18, 106). Some limitations still remain, such as the difficult to determine if cancer cells are fully eliminated even if ablation zones completely disappear on US and long-term outcomes (107). Another procedure based on the principles of hyperthermia is LTA that significantly reduce thyroid nodule volume as well as symptoms and cosmetic problems, due to coagulative necrosis into the target tissue (108, 109). A 3-year multicenter prospective randomized trial with LTA showed persistent volume reduction and local symptom improvement at 36 months after treatment (110). A systematic meta-analysis, comparing the efficacy of RFA and LTA for the treatment of benign thyroid nodules, concluded that both LTA and RFA are able to significantly decrease nodule volume, though RFA has a superior efficacy to LTA in nodule shrinkage despite minor number of treatment sessions (111). Only one study reported minor complications, as transient thyrotoxicosis and fever, after LTA (112) while no studies reported major complications such as voice change or hypothyroidism after either RFA or LA. It remains unclear if the different results are linked to the different energy delivered per ml of thyroid tissue, to the treatment time or technique. Finally, HIFU and PMWA are other promising forms of thermal ablation technique, but need further clinical testing. High intensity focused ultrasound (HIFU) have some advantages over other ablation techniques such as the ability to induce a focused thermal tissue destruction without needle puncture and seems to be less dependent on the skill of the operator. However, it produces thermal coagulation within a small volume and the ablation of a larger tissue volume may take an excessive period of time (113). The treatment efficacy (i.e., extent of nodule shrinkage at 6-month) in larger-sized benign thyroid nodules has been evaluated by Lang et al. in 63 nodules with a noticeably less efficacy for larger-sized nodules (114). HIFU is a safe treatment although transient side effects have been reported, such as pain, skin redness, mild subcutaneous swelling and transient vocal cord paralysis (115, 116). Percutaneous microwave ablation (PMWA) is a new technique that produce a rapid increase of the target tissue temperature through the rotation of molecules produced from microwave energy. Few studies analyzed the effectiveness of PMWA in the treatment of benign solid thyroid nodules. Liu et al. evaluated 474 benign thyroid nodules in 435 patients treated with PMWA showing a mean 90% decrease in volume at 1-year, with no major complications described (70). Another study by Yue et al. reported, in 110 patients treated with PMWA, a significant reduction at 1 year (ranging from 12.6 ± 15.1 to 3.2 ± 5.7 mL) (117). A retrospective, observational trial at a single institution compared the efficacy and safety of RFA in 40 patients, PMWA in 40 patients and HIFU in 14 patients with small nodules at 3 months after ablation. RFA showed a slightly better mean volume reduction of nodules (50%) than MWA (44%) and HIFU (48%). The study limitation is the short period of time (118). In conclusion, non-surgical minimally invasive approaches can be used to treat symptomatic or enlarging thyroid nodule and appear safe and effective. Currently, percutaneous ethanol injection (PEI) is recommended for symptomatic cystic or relapsing cystic lesions. Either laser thermal ablation (LTA) or radiofrequency ablation (RFA) can be used for symptomatic solid nodules. Microwave ablation (PMWA) or high intensity focused ultrasound (HIFU) are newer techniques with promising results that await further clinical evaluation.
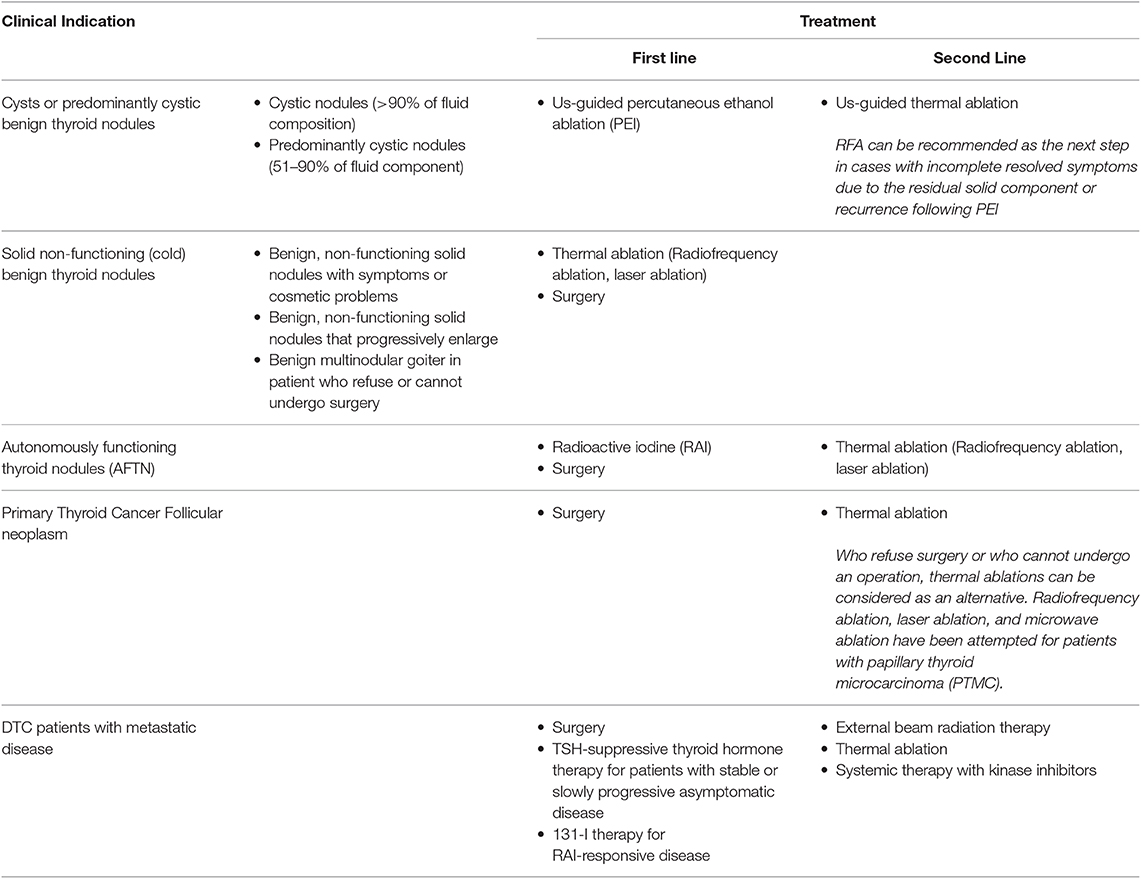
Table 2. An overview of the non-surgical, Image-Guided, Minimally Invasive Therapy for thyroid nodules or recurrent thyroid cancer.
Discussion
In the era of precision medicine, the most important landmark remains the correct identification of malignant thyroid nodules. Newer and promising imaging techniques combined with the more accurate molecular examination will be able to reduce diagnostic uncertainty. Moreover, newer therapeutic options will be able to reduce, when possible, avoidable thyroidectomy. These approaches will allow the clinician to set up a tailored management, from diagnosis to treatment, of thyroid nodule disease, according to the patient's needs.
Author Contributions
LF and MC contributed to conception and design of the review. DT, GG, MDS, MDM, MS, TR, and FM wrote the first draft of the manuscript. All authors wrote sections of the manuscript, contributed to manuscript revision and approved it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Gharib H, Papini E. Thyroid nodules: clinical importance, assessment, and treatment. Endocrinol Metab Clin North Am. (2007) 36:707–35. doi: 10.1016/j.ecl.2007.04.009
2. Burman KD, Wartofsky L. CLINICAL PRACTICE. Thyroid nodules. N Engl J Med. (2015) 373:2347–56. doi: 10.1056/NEJMcp1415786
3. Gharib H, Papini E, Garber JR, Duick DS, Harrell RM, Hegedus L, et al. American Association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi Medical Guidelines for Clinical Practice for the Diagnosis and Management of Thyroid Nodules−2016 Update. Endocr Pract. (2016) 22:622–39. doi: 10.4158/EP161208.GL
4. Smith-Bindman R, Lebda P, Feldstein VA, Sellami D, Goldstein RB, Brasic N, et al. Risk of thyroid cancer based on thyroid ultrasound imaging characteristics: results of a population-based study. JAMA Intern Med. (2013) 173:1788–96. doi: 10.1001/jamainternmed.2013.9245
5. Nam-Goong IS, Kim HY, Gong G, Lee HK, Hong SJ, Kim WB, et al. Ultrasonography-guided fine-needle aspiration of thyroid incidentaloma: correlation with pathological findings. Clin Endocrinol. (2004) 60:21–8. doi: 10.1046/j.1365-2265.2003.01912.x
6. Durante C, Grani G, Lamartina L, Filetti S, Mandel SJ, Cooper DS. The diagnosis and management of thyroid nodules: a review. JAMA. (2018) 319:914–24. doi: 10.1001/jama.2018.0898
7. Brito JP, Ito Y, Miyauchi A, Tuttle RM. A clinical framework to facilitate risk stratification when considering an active surveillance alternative to immediate biopsy and surgery in papillary microcarcinoma. Thyroid. (2016) 26:144–9. doi: 10.1089/thy.2015.0178
8. Russ G, Bonnema SJ, Erdogan MF, Durante C, Ngu R, Leenhardt L. European thyroid association guidelines for ultrasound malignancy risk stratification of thyroid nodules in adults: the EU-TIRADS. Eur Thyroid J. (2017) 6:225–37. doi: 10.1159/000478927
9. Tessler FN, Middleton WD, Grant EG, Hoang JK, Berland LL, Teefey SA, et al. ACR Thyroid Imaging, Reporting and Data System (TI-RADS): white paper of the ACR TI-RADS committee. J Am Coll Radiol. (2017) 14:587–95. doi: 10.1016/j.jacr.2017.01.046
10. Grant EG, Tessler FN, Hoang JK, Langer JE, Beland MD, Berland LL, et al. Thyroid ultrasound reporting lexicon: white paper of the ACR Thyroid Imaging, Reporting and Data System (TIRADS) committee. J Am Coll Radiol. (2015) 12(12 Pt A):1272–9. doi: 10.1016/j.jacr.2015.07.011
11. Brito JP, Gionfriddo MR, Al Nofal A, Boehmer KR, Leppin AL, Reading C, et al. The accuracy of thyroid nodule ultrasound to predict thyroid cancer: systematic review and meta-analysis. J Clin Endocrinol Metab. (2014) 99:1253–63. doi: 10.1210/jc.2013-2928
12. Campanella P, Ianni F, Rota CA, Corsello SM, Pontecorvi A. Quantification of cancer risk of each clinical and ultrasonographic suspicious feature of thyroid nodules: a systematic review and meta-analysis. Eur J Endocrinol. (2014) 170:R203–11. doi: 10.1530/EJE-13-0995
13. Razavi SA, Hadduck TA, Sadigh G, Dwamena BA. Comparative effectiveness of elastographic and B-mode ultrasound criteria for diagnostic discrimination of thyroid nodules: a meta-analysis. AJR Am J Roentgenol. (2013) 200:1317–26. doi: 10.2214/AJR.12.9215
14. Remonti LR, Kramer CK, Leitao CB, Pinto LC, Gross JL. Thyroid ultrasound features and risk of carcinoma: a systematic review and meta-analysis of observational studies. Thyroid. (2015) 25:538–50. doi: 10.1089/thy.2014.0353
15. Frates MC, Benson CB, Charboneau JW, Cibas ES, Clark OH, Coleman BG, et al. Management of thyroid nodules detected at US: Society of Radiologists in Ultrasound consensus conference statement. Radiology. (2005) 237:794–800. doi: 10.1148/radiol.2373050220
16. Grani G, D'Alessandri M, Carbotta G, Nesca A, Del Sordo M, Alessandrini S, et al. Grey-scale analysis improves the ultrasonographic evaluation of thyroid nodules. Medicine. (2015) 94:e1129. doi: 10.1097/MD.0000000000001129
17. Park SJ, Park SH, Choi YJ, Kim DW, Son EJ, Lee HS, et al. Interobserver variability and diagnostic performance in US assessment of thyroid nodule according to size. Ultraschall Med. (2012) 33:E186–90. doi: 10.1055/s-0032-1325404
18. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. (2016) 26:1–133. doi: 10.1089/thy.2015.0020
19. Shin JH, Baek JH, Chung J, Ha EJ, Kim JH, Lee YH, et al. Ultrasonography diagnosis and imaging-based management of thyroid nodules: revised korean society of thyroid radiology consensus statement and recommendations. Korean J Radiol. (2016) 17:370–95. doi: 10.3348/kjr.2016.17.3.370
20. Middleton WD, Teefey SA, Reading CC, Langer JE, Beland MD, Szabunio MM, et al. Multiinstitutional analysis of thyroid nodule risk stratification using the American college of radiology thyroid imaging reporting and data system. AJR Am J Roentgenol. (2017) 208:1331–41. doi: 10.2214/AJR.16.17613
21. Ha EJ, Moon WJ, Na DG, Lee YH, Choi N, Kim SJ, et al. A multicenter prospective validation study for the Korean thyroid imaging reporting and data system in patients with thyroid nodules. Korean J Radiol. (2016) 17:811–21. doi: 10.3348/kjr.2016.17.5.811
22. Trimboli P, Ngu R, Royer B, Giovanella L, Bigorgne C, Simo R, et al. A multicentre validation study for the EU-TIRADS using histological diagnosis as a gold standard. Clin Endocrinol. (2019) 91:340–7. doi: 10.1111/cen.13997
23. Nam SJ, Kwak JY, Moon HJ, Yoon JH, Kim EK, Koo JS. Large (>/=3cm) thyroid nodules with benign cytology: can Thyroid Imaging Reporting and Data System (TIRADS) help predict false-negative cytology? PLoS ONE. (2017) 12:e0186242. doi: 10.1371/journal.pone.0186242
24. Mendes GF, Garcia MR, Falsarella PM, Rahal A, Cavalcante FA Junior, Nery DR, et al. Fine needle aspiration biopsy of thyroid nodule smaller than 1.0 cm: accuracy of TIRADS classification system in more than 1000 nodules. Br J Radiol. (2018) 91:20170642. doi: 10.1259/bjr.20170642
25. Zheng Y, Xu S, Kang H, Zhan W. A single-center retrospective validation study of the American College of radiology thyroid imaging reporting and data system. Ultrasound Q. (2018) 34:77–83. doi: 10.1097/RUQ.0000000000000350
26. Koseoglu Atilla FD, Ozgen Saydam B, Erarslan NA, Diniz Unlu AG, Yilmaz Yasar H, Ozer M, et al. Does the ACR TI-RADS scoring allow us to safely avoid unnecessary thyroid biopsy? single center analysis in a large cohort. Endocrine. (2018) 61:398–402. doi: 10.1007/s12020-018-1620-6
27. Hoang JK, Middleton WD, Farjat AE, Langer JE, Reading CC, Teefey SA, et al. Reduction in thyroid nodule biopsies and improved accuracy with American college of radiology thyroid imaging reporting and data system. Radiology. (2018) 287:185–93. doi: 10.1148/radiol.2018172572
28. Yoon JH, Lee HS, Kim EK, Moon HJ, Kwak JY. Malignancy risk stratification of thyroid nodules: comparison between the thyroid imaging reporting and data system and the 2014 American thyroid association management guidelines. Radiology. (2016) 278:917–24. doi: 10.1148/radiol.2015150056
29. Persichetti A, Di Stasio E, Guglielmi R, Bizzarri G, Taccogna S, Misischi I, et al. Predictive value of malignancy of thyroid nodule ultrasound classification systems: a prospective study. J Clin Endocrinol Metab. (2018) 103:1359–68. doi: 10.1210/jc.2017-01708
30. Maino F, Forleo R, Martinelli M, Fralassi N, Barbato F, Pilli T, et al. Prospective validation of ATA and ETA sonographic pattern risk of thyroid nodules selected for FNAC. J Clin Endocrinol Metab. (2018) 103:2362–8. doi: 10.1210/jc.2018-00274
31. Grani G, Lamartina L, Ascoli V, Bosco D, Biffoni M, Giacomelli L, et al. Reducing the number of unnecessary thyroid biopsies while improving diagnostic accuracy: toward the “right” TIRADS. J Clin Endocrinol Metab. (2019) 104:95–102. doi: 10.1210/jc.2018-01674
32. Grani G, Lamartina L, Cantisani V, Maranghi M, Lucia P, Durante C. Interobserver agreement of various thyroid imaging reporting and data systems. Endocr Connect. (2018) 7:1–7. doi: 10.1530/EC-17-0336
33. Valderrabano P, McGettigan MJ, Lam CA, Khazai L, Thompson ZJ, Chung CH, et al. Thyroid nodules with indeterminate cytology: utility of the american thyroid association sonographic patterns for cancer risk stratification. Thyroid. (2018) 28:1004–12. doi: 10.1089/thy.2018.0085
34. Tang AL, Falciglia M, Yang H, Mark JR, Steward DL. Validation of American thyroid association ultrasound risk assessment of thyroid nodules selected for ultrasound fine-needle aspiration. Thyroid. (2017) 27:1077–82. doi: 10.1089/thy.2016.0555
35. Grani G, Lamartina L, Ascoli V, Bosco D, Nardi F, D'Ambrosio F, et al. Ultrasonography scoring systems can rule out malignancy in cytologically indeterminate thyroid nodules. Endocrine. (2017) 57:256–61. doi: 10.1007/s12020-016-1148-6
36. Sollini M, Cozzi L, Chiti A, Kirienko M. Texture analysis and machine learning to characterize suspected thyroid nodules and differentiated thyroid cancer: where do we stand? Eur J Radiol. (2018) 99:1–8. doi: 10.1016/j.ejrad.2017.12.004
37. Yoo YJ, Ha EJ, Cho YJ, Kim HL, Han M, Kang SY. Computer-aided diagnosis of thyroid nodules via ultrasonography: initial clinical experience. Korean J Radiol. (2018) 19:665–72. doi: 10.3348/kjr.2018.19.4.665
38. Jeong EY, Kim HL, Ha EJ, Park SY, Cho YJ, Han M. Computer-aided diagnosis system for thyroid nodules on ultrasonography: diagnostic performance and reproducibility based on the experience level of operators. Eur Radiol. (2019) 29:1978–85. doi: 10.1007/s00330-018-5772-9
39. Ko SY, Lee JH, Yoon JH, Na H, Hong E, Han K, et al. Deep convolutional neural network for the diagnosis of thyroid nodules on ultrasound. Head Neck. (2019) 41:885–91. doi: 10.1002/hed.25415
40. Rago T, Santini F, Scutari M, Pinchera A, Vitti P. Elastography: new developments in ultrasound for predicting malignancy in thyroid nodules. J Clin Endocrinol Metab. (2007) 92:2917–22. doi: 10.1210/jc.2007-0641
41. Asteria C, Giovanardi A, Pizzocaro A, Cozzaglio L, Morabito A, Somalvico F, et al. US-elastography in the differential diagnosis of benign and malignant thyroid nodules. Thyroid. (2008) 18:523–31. doi: 10.1089/thy.2007.0323
42. Ding J, Cheng H, Ning C, Huang J, Zhang Y. Quantitative measurement for thyroid cancer characterization based on elastography. J Ultrasound Med. (2011) 30:1259–66. doi: 10.7863/jum.2011.30.9.1259
43. Rago T, Scutari M, Santini F, Loiacono V, Piaggi P, Di Coscio G, et al. Real-time elastosonography: useful tool for refining the presurgical diagnosis in thyroid nodules with indeterminate or nondiagnostic cytology. J Clin Endocrinol Metab. (2010) 95:5274–80. doi: 10.1210/jc.2010-0901
44. Rago T, Vitti P. Role of thyroid ultrasound in the diagnostic evaluation of thyroid nodules. Best Pract Res Clin Endocrinol Metab. (2008) 22:913–28. doi: 10.1016/j.beem.2008.09.016
45. Shiina T, Nightingale KR, Palmeri ML, Hall TJ, Bamber JC, Barr RG, et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: part 1: basic principles and terminology. Ultrasound Med Biol. (2015) 41:1126–47. doi: 10.1016/j.ultrasmedbio.2015.03.009
46. Kwak JY, Kim EK. Ultrasound elastography for thyroid nodules: recent advances. Ultrasonography. (2014) 33:75–82. doi: 10.14366/usg.13025
47. Sun CY, Lei KR, Liu BJ, Bo XW, Li XL, He YP, et al. Virtual touch tissue imaging and quantification (VTIQ) in the evaluation of thyroid nodules: the associated factors leading to misdiagnosis. Sci Rep. (2017) 7:41958. doi: 10.1038/srep41958
48. Cantisani V, Lodise P, Grazhdani H, Mancuso E, Maggini E, Di Rocco G, et al. Ultrasound elastography in the evaluation of thyroid pathology. Current status. Eur J Radiol. (2014) 83:420–8. doi: 10.1016/j.ejrad.2013.05.008
49. Wang J, Li P, Sun L, Sun Y, Fang S, Liu X. Diagnostic value of strain ratio measurement in differential diagnosis of thyroid nodules coexisted with Hashimoto thyroiditis. Int J Clin Exp Med. (2015) 8:6420–6.
50. Cosgrove D, Barr R, Bojunga J, Cantisani V, Chammas MC, Dighe M, et al. WFUMB guidelines and recommendations on the clinical use of ultrasound elastography: part 4. thyroid. Ultrasound Med Biol. (2017) 43:4–26. doi: 10.1016/j.ultrasmedbio.2016.06.022
51. Veyrieres JB, Albarel F, Lombard JV, Berbis J, Sebag F, Oliver C, et al. A threshold value in Shear Wave elastography to rule out malignant thyroid nodules: a reality? Eur J Radiol. (2012) 81:3965–72. doi: 10.1016/j.ejrad.2012.09.002
52. Cho YJ, Ha EJ, Han M, Choi JW. US elastography using carotid artery pulsation may increase the diagnostic accuracy for thyroid nodules with US-pathology discordance. Ultrasound Med Biol. (2017) 43:1587–95. doi: 10.1016/j.ultrasmedbio.2017.04.007
53. Zhang YF, Xu HX, He Y, Liu C, Guo LH, Liu LN, et al. Virtual touch tissue quantification of acoustic radiation force impulse: a new ultrasound elastic imaging in the diagnosis of thyroid nodules. PLoS ONE. (2012) 7:e49094. doi: 10.1371/journal.pone.0049094
54. Zhou H, Zhou XL, Xu HX, Li DD, Liu BJ, Zhang YF, et al. Virtual touch tissue imaging and quantification in the evaluation of thyroid nodules. J Ultrasound Med. (2017) 36:251–60. doi: 10.7863/ultra.15.12070
55. Ragazzoni F, Deandrea M, Mormile A, Ramunni MJ, Garino F, Magliona G, et al. High diagnostic accuracy and interobserver reliability of real-time elastography in the evaluation of thyroid nodules. Ultrasound Med Biol. (2012) 38:1154–62. doi: 10.1016/j.ultrasmedbio.2012.02.025
56. Cantisani V, Grazhdani H, Ricci P, Mortele K, Di Segni M, D'Andrea V, et al. Q-elastosonography of solid thyroid nodules: assessment of diagnostic efficacy and interobserver variability in a large patient cohort. Eur Radiol. (2014) 24:143–50. doi: 10.1007/s00330-013-2991-y
57. Hofauer B, Mansour N, Heiser C, Wirth M, Strassen U, Loeffelbein D, et al. Reproducibility of acoustic radiation force impulse imaging in thyroid and salivary glands with experienced and inexperienced examiners. Ultrasound Med Biol. (2016) 42:2545–52. doi: 10.1016/j.ultrasmedbio.2016.06.019
58. Park SH, Kim SJ, Kim EK, Kim MJ, Son EJ, Kwak JY. Interobserver agreement in assessing the sonographic and elastographic features of malignant thyroid nodules. AJR Am J Roentgenol. (2009) 193:W416–23. doi: 10.2214/AJR.09.2541
59. Lim DJ, Luo S, Kim MH, Ko SH, Kim Y. Interobserver agreement and intraobserver reproducibility in thyroid ultrasound elastography. AJR Am J Roentgenol. (2012) 198:896–901. doi: 10.2214/AJR.11.7009
60. Kim JK, Baek JH, Lee JH, Kim JL, Ha EJ, Kim TY, et al. Ultrasound elastography for thyroid nodules: a reliable study? Ultrasound Med Biol. (2012) 38:1508–13. doi: 10.1016/j.ultrasmedbio.2012.05.017
61. Friedrich-Rust M, Romenski O, Meyer G, Dauth N, Holzer K, Grunwald F, et al. Acoustic Radiation Force Impulse-Imaging for the evaluation of the thyroid gland: a limited patient feasibility study. Ultrasonics. (2012) 52:69–74. doi: 10.1016/j.ultras.2011.06.012
62. Calvete AC, Rodriguez JM, de Dios Berna-Mestre J, Rios A, Abellan-Rivero D, Reus M. Interobserver agreement for thyroid elastography: value of the quality factor. J Ultrasound Med. (2013) 32:495–504. doi: 10.7863/jum.2013.32.3.495
63. Grazhdani H, Cantisani V, Lodise P, Di Rocco G, Proietto MC, Fioravanti E, et al. Prospective evaluation of acoustic radiation force impulse technology in the differentiation of thyroid nodules: accuracy and interobserver variability assessment. J Ultrasound. (2014) 17:13–20. doi: 10.1007/s40477-013-0062-5
64. Swan KZ, Nielsen VE, Bibby BM, Bonnema SJ. Is the reproducibility of shear wave elastography of thyroid nodules high enough for clinical use? A methodological study. Clin Endocrinol. (2017) 86:606–13. doi: 10.1111/cen.13295
65. Andrioli M, Scacchi M, Carzaniga C, Vitale G, Moro M, Poggi L, et al. Thyroid nodules in acromegaly: the role of elastography. J Ultrasound. (2010) 13:90–7. doi: 10.1016/j.jus.2010.09.008
66. Andrioli M, Valcavi R. The peculiar ultrasonographic and elastographic features of thyroid nodules after treatment with laser or radiofrequency: similarities and differences. Endocrine. (2014) 47:967–8. doi: 10.1007/s12020-014-0241-y
67. Andrioli M, Trimboli P, Amendola S, Valabrega S, Fukunari N, Mirella M, et al. Elastographic presentation of medullary thyroid carcinoma. Endocrine. (2014) 45:153–5. doi: 10.1007/s12020-013-0062-4
68. Magri F, Chytiris S, Capelli V, Alessi S, Nalon E, Rotondi M, et al. Shear wave elastography in the diagnosis of thyroid nodules: feasibility in the case of coexistent chronic autoimmune Hashimoto's thyroiditis. Clin Endocrinol. (2012) 76:137–41. doi: 10.1111/j.1365-2265.2011.04170.x
69. Li Y, Wang Y, Wu Q, Hu B. Papillary thyroid microcarcinoma co-exists with Hashimoto's thyroiditis: Is strain elastography still useful? Ultrasonics. (2016) 68:127–33. doi: 10.1016/j.ultras.2016.02.013
70. Liu YJ, Qian LX, Liu D, Zhao JF. Ultrasound-guided microwave ablation in the treatment of benign thyroid nodules in 435 patients. Exp Biol Med. (2017) 242:1515–23. doi: 10.1177/1535370217727477
71. Cappelli C, Pirola I, Gandossi E, Formenti A, Agosti B, Castellano M. Elastography evaluation of benign thyroid nodules in patients affected by hashimoto's thyroiditis. Int J Endocrinol. (2015) 2015:367054. doi: 10.1155/2015/367054
72. Cosgrove D, Piscaglia F, Bamber J, Bojunga J, Correas JM, Gilja OH, et al. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 2: Clinical applications. Ultraschall Med. (2013) 34:238–53. doi: 10.1055/s-0033-1335375
73. Moon HJ, Sung JM, Kim EK, Yoon JH, Youk JH, Kwak JY. Diagnostic performance of gray-scale US and elastography in solid thyroid nodules. Radiology. (2012) 262:1002–13. doi: 10.1148/radiol.11110839
74. Trimboli P, Guglielmi R, Monti S, Misischi I, Graziano F, Nasrollah N, et al. Ultrasound sensitivity for thyroid malignancy is increased by real-time elastography: a prospective multicenter study. J Clin Endocrinol Metab. (2012) 97:4524–30. doi: 10.1210/jc.2012-2951
75. Cappelli C, Pirola I, Gandossi E, Agosti B, Cimino E, Casella C, et al. Real-time elastography: a useful tool for predicting malignancy in thyroid nodules with nondiagnostic cytologic findings. J Ultrasound Med. (2012) 31:1777–82. doi: 10.7863/jum.2012.31.11.1777
76. Yoon JH, Moon HJ, Kim EK, Kwak JY. Inadequate cytology in thyroid nodules: should we repeat aspiration or follow-up? Ann Surg Oncol. (2011) 18:1282–9. doi: 10.1245/s10434–011-1605–7
77. Trimboli P, Treglia G, Sadeghi R, Romanelli F, Giovanella L. Reliability of real-time elastography to diagnose thyroid nodules previously read at FNAC as indeterminate: a meta-analysis. Endocrine. (2015) 50:335–43. doi: 10.1007/s12020-014-0510-9
78. Baloch ZW, Fleisher S, LiVolsi VA, Gupta PK. Diagnosis of “follicular neoplasm”: a gray zone in thyroid fine-needle aspiration cytology. Diagn Cytopathol. (2002) 26:41–4. doi: 10.1002/dc.10043
79. Baloch ZW, LiVolsi VA, Asa SL, Rosai J, Merino MJ, Randolph G, et al. Diagnostic terminology and morphologic criteria for cytologic diagnosis of thyroid lesions: a synopsis of the National Cancer Institute Thyroid Fine-Needle Aspiration State of the Science Conference. Diagn Cytopathol. (2008) 36:425–37. doi: 10.1002/dc.20830
80. Cibas ES, Ali SZ. The 2017 bethesda system for reporting thyroid cytopathology. Thyroid. (2017) 27:1341–6. doi: 10.1089/thy.2017.0500
81. Bongiovanni M, Spitale A, Faquin WC, Mazzucchelli L, Baloch ZW. The bethesda system for reporting thyroid cytopathology: a meta-analysis. Acta Cytol. (2012) 56:333–9. doi: 10.1159/000339959
82. Vriens D, Adang EM, Netea-Maier RT, Smit JW, de Wilt JH, Oyen WJ, et al. Cost-effectiveness of FDG-PET/CT for cytologically indeterminate thyroid nodules: a decision analytic approach. J Clin Endocrinol Metab. (2014) 99:3263–74. doi: 10.1210/jc.2013-3483
83. McHenry CR, Slusarczyk SJ. Hypothyroidisim following hemithyroidectomy: incidence, risk factors, and management. Surgery. (2000) 128:994–8. doi: 10.1067/msy.2000.110242
84. Rosato L, Avenia N, Bernante P, De Palma M, Gulino G, Nasi PG, et al. Complications of thyroid surgery: analysis of a multicentric study on 14,934 patients operated on in Italy over 5 years. World J Surg. (2004) 28:271–6. doi: 10.1007/s00268-003-6903-1
85. Jeannon JP, Orabi AA, Bruch GA, Abdalsalam HA, Simo R. Diagnosis of recurrent laryngeal nerve palsy after thyroidectomy: a systematic review. Int J Clin Pract. (2009) 63:624–9. doi: 10.1111/j.1742–1241.2008.01875.x
86. Paschke R, Cantara S, Crescenzi A, Jarzab B, Musholt TJ, Sobrinho Simoes M. European thyroid association guidelines regarding thyroid nodule molecular fine-needle aspiration cytology diagnostics. Eur Thyroid J. (2017) 6:115–29. doi: 10.1159/000468519
87. Nikiforov YE, Ohori NP, Hodak SP, Carty SE, LeBeau SO, Ferris RL, et al. Impact of mutational testing on the diagnosis and management of patients with cytologically indeterminate thyroid nodules: a prospective analysis of 1056 FNA samples. J Clin Endocrinol Metab. (2011) 96:3390–7. doi: 10.1210/jc.2011-1469
88. Alexander EK, Kennedy GC, Baloch ZW, Cibas ES, Chudova D, Diggans J, et al. Preoperative diagnosis of benign thyroid nodules with indeterminate cytology. N Engl J Med. (2012) 367:705–15. doi: 10.1056/NEJMoa1203208
89. Nikiforov YE, Steward DL, Robinson-Smith TM, Haugen BR, Klopper JP, Zhu Z, et al. Molecular testing for mutations in improving the fine-needle aspiration diagnosis of thyroid nodules. J Clin Endocrinol Metab. (2009) 94:2092–8. doi: 10.1210/jc.2009-0247
90. Nikiforova MN, Wald AI, Roy S, Durso MB, Nikiforov YE. Targeted next-generation sequencing panel (ThyroSeq) for detection of mutations in thyroid cancer. J Clin Endocrinol Metab. (2013) 98:E1852–60. doi: 10.1210/jc.2013-2292
91. Nikiforov YE, Carty SE, Chiosea SI, Coyne C, Duvvuri U, Ferris RL, et al. Highly accurate diagnosis of cancer in thyroid nodules with follicular neoplasm/suspicious for a follicular neoplasm cytology by ThyroSeq v2 next-generation sequencing assay. Cancer. (2014) 120:3627–34. doi: 10.1002/cncr.29038
92. Nikiforov YE, Baloch ZW. Clinical validation of the ThyroSeq v3 genomic classifier in thyroid nodules with indeterminate FNA cytology. Cancer Cytopathol. (2019) 127:225–30. doi: 10.1002/cncy.22112
93. Steward DL, Carty SE, Sippel RS, Yang SP, Sosa JA, Sipos JA, et al. Performance of a multigene genomic classifier in thyroid nodules with indeterminate cytology: a prospective blinded multicenter study. JAMA Oncol. (2019) 5:204–12. doi: 10.1001/jamaoncol.2018.4616
94. Vargas-Salas S, Martinez JR, Urra S, Dominguez JM, Mena N, Uslar T, et al. Genetic testing for indeterminate thyroid cytology: review and meta-analysis. Endocr Relat Cancer. (2018) 25:R163–77. doi: 10.1530/ERC-17-0405
95. Alexander EK, Schorr M, Klopper J, Kim C, Sipos J, Nabhan F, et al. Multicenter clinical experience with the Afirma gene expression classifier. J Clin Endocrinol Metab. (2014) 99:119–25. doi: 10.1210/jc.2013-2482
96. Patel KN, Angell TE, Babiarz J, Barth NM, Blevins T, Duh QY, et al. Performance of a genomic sequencing classifier for the preoperative diagnosis of cytologically indeterminate thyroid nodules. JAMA Surg. (2018) 153:817–24. doi: 10.1001/jamasurg.2018.1153
97. Harrell RM, Eyerly-Webb SA, Golding AC, Edwards CM, Bimston DN. Statistical comparison of afirma gsc and afirma gec outcomes in a community endocrine surgical practice: early findings. Endocr Pract. (2019) 25:161–4. doi: 10.4158/EP-2018-0395
98. Endo M, Nabhan F, Porter K, Roll K, Shirley LA, Azaryan I, et al. Afirma gene sequencing classifier compared with gene expression classifier in indeterminate thyroid nodules. Thyroid. (2019) 29:1115–24. doi: 10.1089/thy.2018.0733
99. Cesareo R, Palermo A, Pasqualini V, Cianni R, Gaspa G, Manfrini S, et al. Radiofrequency ablation for the management of thyroid nodules: A critical appraisal of the literature. Clin Endocrinol (Oxf). (2017) 87:639–48. doi: 10.1111/cen.13422
100. Baek JH, Ha EJ, Choi YJ, Sung JY, Kim JK, Shong YK. Radiofrequency versus ethanol ablation for treating predominantly cystic thyroid nodules: a randomized clinical trial. Korean J Radiol. (2015) 16:1332–40. doi: 10.3348/kjr.2015.16.6.1332
101. Papini E, Gugliemi R, Pacella CM. Laser, radiofrequency, and ethanol ablation for the management of thyroid nodules. Curr Opin Endocrinol Diabetes Obes. (2016) 23:400–6. doi: 10.1097/MED.0000000000000282
102. Papini E, Pacella CM, Solbiati LA, Achille G, Barbaro D, Bernardi S, et al. Minimally-invasive treatments for benign thyroid nodules: a Delphi-based consensus statement from the Italian minimally-invasive treatments of the thyroid (MITT) group. Int J Hyperthermia. (2019) 36:376–82. doi: 10.1080/02656736.2019.1575482
103. Kim C, Lee JH, Choi YJ, Kim WB, Sung TY, Baek JH. Complications encountered in ultrasonography-guided radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers. Eur Radiol. (2017) 27:3128–37. doi: 10.1007/s00330-016-4690-y
104. Oddo S, Spina B, Vellone VG, Giusti M. A case of thyroid cancer on the track of the radiofrequency electrode 30 months after percutaneous ablation. J Endocrinol Invest. (2017) 40:101–2. doi: 10.1007/s40618-016-0527-4
105. Garberoglio R, Aliberti C, Appetecchia M, Attard M, Boccuzzi G, Boraso F, et al. Radiofrequency ablation for thyroid nodules: which indications? The first Italian opinion statement. J Ultrasound. (2015) 18:423–30. doi: 10.1007/s40477-015-0169-y
106. Zhang M, Luo Y, Zhang Y, Tang J. Efficacy and safety of ultrasound-guided radiofrequency ablation for treating low-risk papillary thyroid microcarcinoma: a prospective study. Thyroid. (2016) 26:1581–7. doi: 10.1089/thy.2015.0471
107. Kim JH, Baek JH, Sung JY, Min HS, Kim KW, Hah JH, et al. Radiofrequency ablation of low-risk small papillary thyroidcarcinoma: preliminary results for patients ineligible for surgery. Int J Hyperthermia. (2017) 33:212–9. doi: 10.1080/02656736.2016.1230893
108. Dossing H, Bennedbaek FN, Karstrup S, Hegedus L. Benign solitary solid cold thyroid nodules: US-guided interstitial laser photocoagulation–initial experience. Radiology. (2002) 225:53–7. doi: 10.1148/radiol.2251011042
109. Pacella CM, Mauri G, Achille G, Barbaro D, Bizzarri G, De Feo P, et al. Outcomes and risk factors for complications of laser ablation for thyroid nodules: a multicenter study on 1531 patients. J Clin Endocrinol Metab. (2015) 100:3903–10. doi: 10.1210/jc.2015-1964
110. Papini E, Rago T, Gambelunghe G, Valcavi R, Bizzarri G, Vitti P, et al. Long-term efficacy of ultrasound-guided laser ablation for benign solid thyroid nodules. Results of a three-year multicenter prospective randomized trial. J Clin Endocrinol Metab. (2014) 99:3653–9. doi: 10.1210/jc.2014-1826
111. Ha EJ, Baek JH, Kim KW, Pyo J, Lee JH, Baek SH, et al. Comparative efficacy of radiofrequency and laser ablation for the treatment of benign thyroid nodules: systematic review including traditional pooling and bayesian network meta-analysis. J Clin Endocrinol Metab. (2015) 100:1903–11. doi: 10.1210/jc.2014-4077
112. Gambelunghe G, Fatone C, Ranchelli A, Fanelli C, Lucidi P, Cavaliere A, et al. A randomized controlled trial to evaluate the efficacy of ultrasound-guided laser photocoagulation for treatment of benign thyroid nodules. J Endocrinol Invest. (2006) 29:RC23–6. doi: 10.1007/BF03347368
113. Ebbini ES, ter Haar G. Ultrasound-guided therapeutic focused ultrasound: current status and future directions. Int J Hyperthermia. (2015) 31:77–89. doi: 10.3109/02656736.2014.995238
114. Lang BH, Woo YC, Chiu KW. Single-session high-intensity focused ultrasound treatment in large-sized benign thyroid nodules. Thyroid. (2017) 27:714–21. doi: 10.1089/thy.2016.0664
115. Ambrosini CE, Cianferotti L, Picone A, Torregrossa L, Segnini G, Frustaci G, et al. High-intensity focused ultrasound as an alternative to the surgical approach in primary hyperparathyroidism: a preliminary experience. J Endocrinol Invest. (2011) 34:655–9. doi: 10.1007/BF03345404
116. Lang BH, Wu ALH. High intensity focused ultrasound (HIFU) ablation of benign thyroid nodules - a systematic review. J Ther Ultrasound. (2017) 5:11. doi: 10.1186/s40349-017-0091-1
117. Yue W, Wang S, Wang B, Xu Q, Yu S, Yonglin Z, et al. Ultrasound guided percutaneous microwave ablation of benign thyroid nodules: safety and imaging follow-up in 222 patients. Eur J Radiol. (2013) 82:e11–6. doi: 10.1016/j.ejrad.2012.07.020
Keywords: microcarcinoma, thyroid nodule, ultrasound, mini invasive treatment, molecular testing
Citation: Tumino D, Grani G, Di Stefano M, Di Mauro M, Scutari M, Rago T, Fugazzola L, Castagna MG and Maino F (2020) Nodular Thyroid Disease in the Era of Precision Medicine. Front. Endocrinol. 10:907. doi: 10.3389/fendo.2019.00907
Received: 05 October 2019; Accepted: 12 December 2019;
Published: 23 January 2020.
Edited by:
Domenico Salvatore, University of Naples Federico II, ItalyReviewed by:
Rocco Bruno, Independent Researcher, Matera, ItalyMichele Minuto, University of Genoa, Italy
Copyright © 2020 Tumino, Grani, Di Stefano, Di Mauro, Scutari, Rago, Fugazzola, Castagna and Maino. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fabio Maino, fabio.maino@ao-siena.toscana.it
 Dario Tumino
Dario Tumino Giorgio Grani
Giorgio Grani Marta Di Stefano
Marta Di Stefano Maria Di Mauro
Maria Di Mauro Maria Scutari5
Maria Scutari5 Laura Fugazzola
Laura Fugazzola Fabio Maino
Fabio Maino