- Health Research Institute, Physical Education and Sport Sciences, University of Limerick, Limerick, Ireland
Skeletal muscle represents the largest organ in the body, comprises 36–42% of body weight, and has recently been recognized as having an endocrine function. Proteins expressed and released by muscle that have autocrine, paracrine, and endocrine bioactivities have been termed myokines. It is likely that muscle contraction represents the primary stimulus for the synthesis and secretion of myokines to enable communication with other organs such as the liver, adipose tissue, brain, and auto-regulation of muscle metabolism. To date, several hundred myokines in the muscle secretome have been identified, a sub-population of which are specifically induced by skeletal muscle contraction. However, the bioactivity of many of these myokines and the mechanism through which they act has either not yet been characterized or remains poorly understood. Physical activity and exercise are recognized as a central tenet in both the prevention and treatment of type 2 diabetes (T2D). Recent data suggest humoral factors such as muscle-derived secretory proteins may mediate the beneficial effects of exercise in the treatment of metabolic diseases. This mini-review aims to summarize our current knowledge on the role of contraction-induced myokines in mediating the beneficial effects of physical activity and exercise in the prevention and treatment of T2D, specifically glucose and lipid metabolism. Future directions as to how we can optimize contraction-induced myokine secretion to inform exercise protocols for the prevention and treatment of T2D will also be discussed.
Introduction
Skeletal muscle has recently been identified as an endocrine organ that synthesizes and secretes proteins known as myokines (1). These myokines are involved in autocrine regulation of metabolism in the muscle itself and paracrine/endocrine regulation of other tissues and organs such as the liver, adipose, and brain.
As skeletal muscle represents the largest organ in the body, the influence of myokines on whole-body metabolism is potentially significant (2, 3). As skeletal muscle contraction is likely the primary stimulus for myokine synthesis and secretion, it is plausible that myokines mediate, in part at least, beneficial adaptations to tissues in response to exercise. Recent research has identified several hundred myokines, a large sub-population of which are specifically induced by contraction (4). However, the specific bioactivity of a vast number of myokines remains largely undescribed and poorly understood. Furthermore, little is known about the role of type, intensity, or frequency of contraction in regulating myokine production and release.
Exercise has long been established as a central tenet to both the prevention and treatment of type 2 diabetes (T2D) (5). Though a number of mechanisms through which exercise confers these metabolic benefits have been well characterized (5), the pluripotency of exercise is not yet fully understood. One such mechanism is via cross-talk between tissues stimulated by contraction and release of myokines regulating tissue function. This creates a clear link between exercise and the regulation of whole-body metabolism. There have been several examples of this in recent research, most notably, the role of the contraction-induced myokine IL-6 in mediating skeletal muscle glucose uptake (6–8). These findings generated excitement as to the potential roles of contraction-induced myokines in the prevention of insulin resistance and metabolic diseases such as obesity and T2D. To date, a number of contraction-induced myokines have been identified which play a role in regulating glucose uptake, insulin sensitivity, and fat metabolism, leading factors in the development of T2D (9).
The purpose of this mini-review is to discuss known metabolic roles for contraction-induced myokines that aid in the prevention/treatment of T2D. Future directions in optimizing exercise protocols to maximize the potential of contraction-induced myokines by the type and intensity of exercise and how this informs exercise prescription will also be discussed.
Myokines and Metabolism
Contraction-induced myokines have been shown to have autocrine, paracrine, and endocrine effects on numerous tissues. In this section, the evidence of contraction as a stimulus for myokine secretion, based on electrical pulse stimulation (EPS) models and/or an increase in circulating concentrations immediately post-exercise, and their effect on metabolic functions affecting the development of T2D in muscle, adipose, and liver will be discussed.
Myokines Regulating Glucose Metabolism
IL-6
Evidence exists for a number of contraction-induced myokines with roles for glucose uptake and insulin sensitivity. IL-6 is most prominent in the literature and has been the focus since the early 2000s of those trying to identify the “exercise factor” through which skeletal muscles communicate to central and peripheral organs (10). IL-6 transcription in skeletal muscle and release to circulation in large volumes in response to contraction was first characterized in 2002 (11). Increased circulating concentrations of IL-6 are known to be affected by both the intensity and duration of contraction in humans (8, 12). Higher intensity and longer duration exercise result in increased circulating concentrations of IL-6 in humans (8, 12). IL-6 release in response to exercise is also dependent on the energy status of the cell, determined by pre-exercise glycogen content, whereby low glycogen content results in a greater release of IL-6 to the energy crisis in the muscle cell during contraction (6). In vitro studies demonstrate that IL-6 treatment increases glucose uptake through AMP-activated protein kinase [adenosine monophosphate kinase (AMPK)] and phosphatidylinosotol 3-kinase (PI3K) pathways (13). Carey et al. (7) reported increased insulin-dependent glucose uptake in vivo in response to IL-6 infusion. By contrast, Harder-Lauridsen et al. (14) found no increase in glucose uptake during euglycemic hyperinsulinemic clamp with IL-6 infusion in T2D individuals, though there was a reduction in the plasma insulin suggesting increased insulin sensitivity (14). Jiang et al. (15) found a differential effect of IL-6 treatment on primary myotubes from normal glucose tolerant and T2D, suggesting a blunted role of IL-6 on T2D muscle. IL-6 treatment upregulated both insulin-dependent and -independent glucose uptake and glycogen synthesis in healthy myotubes, but this effect was lost in T2D myotubes. This suggests that from a glucose control perspective, the contraction-induced myokine IL-6 is effective in the prevention of T2D but may be ineffective for glucose uptake in patients with existing T2D.
IL-13
IL-13 is released from human primary myotubes in vitro and has been demonstrated to have an “insulin-like” effect on glucose metabolism in human muscle by increasing glucose uptake, glycogen synthesis, and glucose oxidation in normal and T2D primary myotubes (15). This “insulin-like” effect is mediated through activation of Akt and PI3K pathways. IL-13 expression is increased in response to strength training in human skeletal muscle (16), but no evidence exists for an increase in plasma IL-13. This suggests the influence of IL-13 on glucose metabolism is localized to the muscle in an autocrine/paracrine manner.
Follistatin-Like-1 (FSTL-1)
Follistatin-like-1 is a secretory myokine of the follistatin family, known to be secreted in vitro by C2C12s (murine cell line) (17). Furthermore, Görgens et al. (17) demonstrated FSTL-1 expression and release from human primary myotubes. Interestingly, contraction of primary myotubes by EPS did not induce the secretion of FSTL-1; however, an increase in circulating plasma FSTL-1 in humans is observed following an acute bout of aerobic exercise. In vitro incubation of L6 myotubes (rat cell line) in FSTL-1 has been shown to increase glucose uptake in an AMPK- and calcium–calmodulin kinase-dependent manner (18), resulting in increased GLUT4 mRNA expression and translocation to the plasma membrane mediating enhanced glucose control.
Chitinase-3-Like-1 Protein (CHI3L1)
Electrical pulse stimulation of primary human skeletal muscle cells increases CHI3L1 expression and secretion (19). Acute aerobic and resistance exercise increase circulating CHI3L1; however, combined training had no effect, suggesting a transient exercise response. Evidence indicates that CHI3L1 regulates myoblast proliferation, suggesting a role in muscle growth thus affecting the size and volume of this organ as a “sink” for blood glucose (19). Furthermore, though CHI3L1 is induced by inflammation as well as contraction, it improves glucose uptake and insulin action under pro-inflammatory conditions in human primary skeletal muscle cells through activation of its receptor protease-activated receptor 2 (19). This suggests that CHI3L1 could regulate skeletal muscle glucose uptake under pro-inflammatory conditions observed in obesity and T2D.
IL-15
IL-15 is a known contraction-induced myokine secreted in humans post both aerobic and resistance exercise (20, 21) with similar responses between lean and obese participants (22). IL-15 has an effect on glucose uptake in C2C12 skeletal muscle cells (murine cell line) via activation of AMPK (23). Krolopp et al. (24) found a similar increase in glucose uptake with IL-15 treatment, mediated by an enhanced GLUT4 translocation to the plasma membrane. However, in contrast to the findings of Gray and colleagues, GLUT4 translocation was not initiated by activation of AMPK, but rather through the Janus kinase–signal transducer and activation of transcription protein 3 (STAT3) pathway. It is not entirely clear why there is no increase in phosphorylation of AMPK in this study, when using a higher dose of IL-15 (100 vs 1 ng/ml).
IL-8
IL-8 is secreted by primary human myotubes following EPS (25) and circulating IL-8 increases in response to endurance exercise in humans (26, 27). IL-8 is primarily associated with inflammation and angiogenesis; however, Gray and Kamolrat (23) demonstrated in vitro an increase in glucose uptake in C2C12s in response to treatment with IL-8 via phosphorylation of AMPK. A role for IL-8 in glucose uptake in vivo is less clear but may be mediated by increased vascularization, an effect which is lost in muscle from T2D (28).
Fibroblast Growth Factor-21 (FGF-21)
Fibroblast growth factor-21 treatment improves glucose tolerance and insulin sensitivity in the liver of obese Zucker rats (29). FGF-21 treatment has also been demonstrated to lower blood glucose and enhance insulin sensitivity in a diabetic mouse model (30). FGF-21, mediated by activation of Akt, improves glucose uptake in primary human adipocytes, which is enhanced when combined with insulin, reducing the required level of insulin to achieve the same glucose uptake (31). Muise et al. (32) confirmed reduced plasma glucose in WT, HFD, and diabetic mouse models treated with FGF-21 perfusion and identified upregulation of genes associated with several pathways such as glucose uptake, and insulin receptor signaling regulated by FGF-21 in brown and white adipose tissue (WAT) and adipocytes in vitro. FGF-21 also increased basal and insulin-stimulated glucose uptake in primary human myotubes by increasing GLUT1 mRNA and translocation to the plasma membrane (33). Circulating concentrations of FGF-21 are increased after an acute bout of endurance exercise in humans (34) and enhanced by higher intensity exercise (35). Short-term training also resulted in increased circulating FGF-21, which was associated with lower fasting glucose (36). Conversely, 3 weeks of sprint interval training results in reduced circulating FGF-21 (37). Similarly, 3 months of combined resistance and aerobic training resulted in a modest decrease in serum FGF-21 in obese women (38). This suggests that an acute bout of exercise leads to a transient increase in FGF-21 but the effect of chronic training is equivocal. Circulating FGF-21 is increased in T2Ds compared to normal glucose tolerant individuals and correlated with fasting insulin and BMI (33). Perhaps, the effect of chronic training is to decrease fasting insulin and adipose mass and thereby reduce circulating FGF-21. The acute increase in FGF-21 post-exercise is likely from muscle with the action of sensitizing muscle, adipose, and liver to insulin to facilitate glucose uptake.
Irisin
Irisin is a controversial candidate, primarily thought to be secreted not only by muscle but also in small amounts by adipose tissue. The main point of contention has been the detection of this myokine in its glycosylated and deglycosylated forms [for review, see Ref. (39)]. Future research should focus on detection by mass spectrometry as per (40); however, the in vivo data reported here use the best validated ELISA technique (39). Circulating irisin increases in response to high-intensity interval exercise, resistance exercise, and continuous moderate exercise in both healthy and metabolic syndrome patients (41). Some data suggest a greater increase following resistance compared with aerobic exercise (42). Serum irisin is regulated by exercise intensity, with greater increases following high-intensity exercise (43, 44). By contrast, other research reports an increase in the expression of FNDC5 in human skeletal muscle following 12 weeks of training but a paradoxical decrease in circulating irisin (45). Though synthesized in muscle, it is not clear if irisin is secreted from muscle directly either in vitro or in vivo. Incubation of L6 myotubes in irisin in vitro results in increased glucose uptake in a dose-dependent manner and is mediated by activation of AMPK and ACC (46). Irisin treatment also upregulates expression of PGC-1α4, a specific isoform associated with muscle hypertrophy, in primary myocytes (47). This was accompanied by increased IGF-1 and decreased myostatin expression, suggesting it a role in regulation of muscle growth, thus providing a larger muscle mass to act as a sink for blood glucose. Irisin perfusion in HFD mice resulted in decreased fasting blood glucose and improved glucose and insulin tolerance (48). Furthermore, FNDC5 overexpression in obese and HFD mice led to increased serum irisin resulting in decreased serum fasting glucose and improved glucose tolerance and insulin sensitivity in HFD mice (48).
Brain-Derived Neurotrophic Factor (BDNF)
The effect of resistance training on circulating BDNF remains equivocal. Several studies report no change in BDNF after either acute or chronic resistance training (49–53). By contrast, Yarrow et al. (54) and Coelho et al. (55) report increased plasma BDNF after acute and chronic resistance training. Circulating BDNF increases after both acute and chronic aerobic exercise in healthy participants [for review, see Ref. (56)]. Though a dose response is not apparent, there is evidence to support a greater increase in circulating BDNF following high-intensity exercise (57, 58), although whether muscle was the direct source of BDNF remains unclear. BDNF mRNA expression is increased by contraction of skeletal muscle cells; however, there is no evidence to show BDNF is secreted by muscle cells following contraction (59). BDNF treatment reduces blood glucose in a diabetic rodent model (60). Yamanaka et al. (61) also found that chronic BDNF infusion improved glucose uptake and metabolism in BAT and muscle of rodents.
Myokines Regulating Fat Metabolism
IL-6
IL-6 infusion stimulates lipolysis and whole-body fatty acid (FA) oxidation in healthy males (62). Similarly, IL-6 treatment in humans results in elevated FA oxidation measured by palmitate oxidation and disappearance rates and a decreased respiratory quotient, peaking 60 min post-infusion (63). Increased whole-body lipolysis is mediated by STAT3 signaling to upregulate skeletal muscle but not adipose tissue lipolysis. Similarly, Petersen et al.(64) found that IL-6 infusion resulted in an increased rate of palmitate appearance and disappearance in human serum of both normal glucose tolerant and T2D patients. In vitro experiments confirmed increased lipolysis in adipocytes and FA oxidation in L6 myotubes (60). These data suggest IL-6 plays a beneficial role in fat metabolism through the upregulation of lipolysis in skeletal muscle and an increase in FA oxidation that is maintained in T2D.
IL-15
IL-15 administration to rodents resulted in a 35% decrease in WAT and a 20% decrease in circulating triglycerides, suggesting a role for IL-15 in lipid metabolism (65). Overexpression and oversecretion of IL-15 in a transgenic mouse model resulted in decreased total body and visceral fat (66). Treatment of adipocytes with IL-15 resulted in decreased deposition of lipids (67). Pierce et al. (68) perfused human subcutaneous adipose tissue with IL-15 via a microdialysis probe and observed an increase in adipose tissue lipolysis of lean participants. However, this effect was lost in obese participants, whereby, IL-15 perfusion suppressed lipolysis. Interestingly, muscle-derived IL-15, induced by exercise did not have an effect on adipose tissue lipolysis in either lean or obese (68). Therefore, the role of IL-15 in regulating lipolysis in humans remains equivocal and requires further investigation. Little information exists on a role for IL-15 in lipid oxidation; however, Almendro et al. (69) demonstrated an effect of chronic IL-15 administration to rodents on the fate of an exogenous lipid bolus. IL-15 reduced de novo lipogenesis in adipose tissue in response to an exogenous lipid load and favored oxidation in muscle and liver via the upregulation of FA transport genes. Further evidence for IL-15 and lipid oxidation in both healthy and T2Ds is required.
Brain-Derived Neurotrophic Factor
Brain-derived neurotrophic factor treatment of L6 myotubes and intact ex vivo muscle results in increased FA oxidation via activation of AMPK (59). Chronic BDNF treatment reduces circulating FAs, total cholesterol, and phospholipids in a diabetic rodent model (60). Chronic intracerebroventricular BDNF administration is also shown to decrease body weight, fat mass, adipocyte size, and serum triglycerides and promote lipolysis (70). Exercise induced increases in plasma BDNF are equivalent in obese and non-obese individuals but are not associated with increases in either whole-body glucose or FA oxidation (71). Further work is required to determine the effect of contraction-induced BDNF on fat metabolism in muscle, adipose, and liver.
Irisin
Irisin treatment of 3T3-L1 adipocytes in vitro induces increased gene expression of lipolysis-related genes including adipose triglyceride lipase, hormone-sensitive lipase (HSL), and protein expression of fatty acid-binding protein 4, suggesting irisin has potential to increase lipolysis (72). By contrast, Wang et al. (73) found no effect of irisin on HSL or ATGL protein expression or expression of lipolysis-related genes in 3T3L-1 adipocytes. Irisin perfusion in HFD mice resulted in decreased serum cholesterol, triglycerides, and free FAs (48). FNDC5 overexpression in obese and HFD mice led to increased serum irisin resulting in decreased serum triglycerides and free FAs in obese and HFD mice (48). Irisin treatment of adipocytes resulted in increased expression of UCP-1 and increased energy expenditure. Irisin also induced expression of metabolic genes (CPT-1, PPARα, HSL) and prevented lipid accumulation (74). Irisin treatment of myocytes also elevated FA oxidation suggesting a protective effect against progression of T2D (75).
Myonectin
Myonectin, a member of the C1q/TNF-related protein family, is expressed in skeletal muscle and released to the circulation in response to exercise in animal studies (76). In vivo myonectin administration reduced circulating levels of free FAs without altering adipose tissue lipolysis in mice. This reduction in circulating free FAs is purported to occur by an increase in FA uptake upregulated by increased expression of FA transport genes such as CD36, FATP1, Fabp1, and Fabp4 (76).
Fibroblast Growth Factor-21
Fibroblast growth factor-21 treatment of 3T3L-1 adipocytes attenuates lipolysis and expression of perilipin (77) and has also been shown to increase hepatic FA oxidation (78). Chronic FGF21 treatment reduces serum and hepatic triglyceride levels in diet-induced obese mice (79). These data suggest an influential role for FGF-21 in regulation of lipid metabolism.
Optimizing the Myokine Response for the Prevention and Treatment of T2D
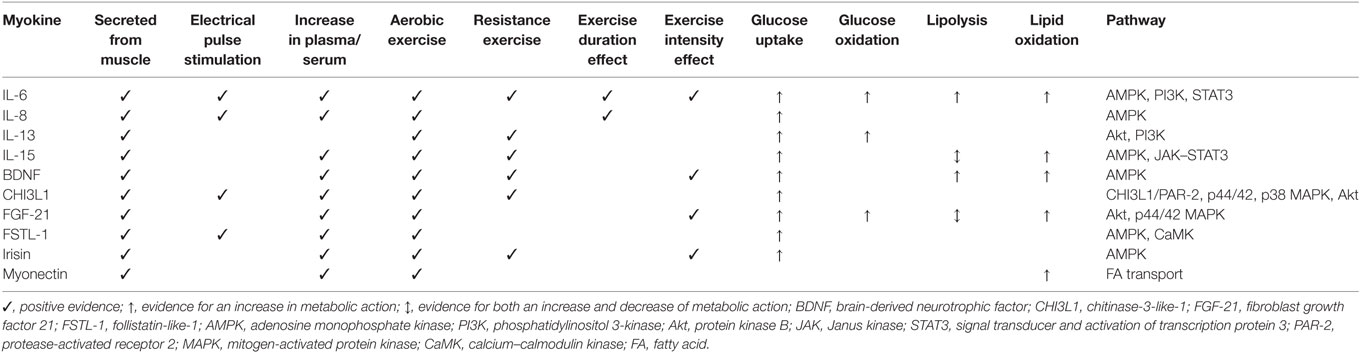
This review has outlined the role of myokines in regulating glucose and fat metabolism as potential mechanisms through which exercise can protect against the onset or progression of T2D. To harness the actions of contraction-induced myokines, we must establish the types, intensity, and volume of contraction required to maximize these regulators to inform future exercise protocols for the prevention and treatment of T2D. Table 1 summarizes what we currently know with respect to the contraction-induced myokines discussed, in terms of how they are modulated by exercise and their actions in metabolic regulation. The role for aerobic exercise is clear, with evidence for an increase in circulating concentrations post-exercise for all myokines discussed, except IL-13, which appears to be acting in an auto/paracrine manner in response to resistance training only. This aligns with current recommendations that aerobic exercise is the primary component of any regimen in the prevention/treatment of T2D (80). It is logical to expect a dose response to contraction; but so far, few studies have demonstrated an effect or a minimum duration of exercise (12, 27). Similarly, few studies have demonstrated an effect for intensity, with higher intensity exercise generally eliciting a greater increase in circulating myokines (8, 35, 41, 43, 44, 57, 58). Resistance exercise effectively enhances circulating concentrations of the majority of myokines discussed (Il-6, IL-15, BDNF, CHI3L1, irisin) confirming the rationale for inclusion in prevention/treatment protocols.
In order to optimize future exercise prescription and policy to maximize the response and effect of myokines on metabolism, it is clear from this mini-review that there is a need to definitively characterize the following in both healthy and T2D participants: (i) the myokine response to an acute bout of aerobic exercise of varying durations (as low as 10 min); (ii) the myokine response to aerobic exercise of varying intensities; and (iii) the myokine response to resistance exercise of varying volume and intensities. To date, much of the evidence describing the mechanism through which recently identified myokines modulate metabolic function have been characterized using in vitro cell models which do not necessarily translate to the in vivo human situation. Though this is a necessary preliminary approach, it is important to acknowledge this as a significant limitation when interpreting the findings of the current literature.
Finally, this review has focused predominantly on tissue crosstalk by myokines released to the circulation; however, it is likely that more myokines are secreted post-exercise exclusively to the interstitium where they are exerting a local effect. More work is required to identify the entire in vivo contraction-induced secretome by techniques such as interstitial microdialysis. Furthermore, there is a need to establish the bioactivity of contraction-induced myokines for both local and systemic tissues.
Author Contributions
BC conceptualized the paper, reviewed the literature, and wrote the manuscript in its entirety.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Febbraio MA, Pedersen BK. Contraction-induced myokine production and release: is skeletal muscle an endocrine organ? Exerc Sport Sci Rev (2005) 33(3):114–9. doi:10.1097/00003677-200507000-00003
2. Pedersen BK. Muscles and their myokines. J Exp Biol (2011) 214(Pt 2):337–46. doi:10.1242/jeb.048074
3. Whitham M, Febbraio MA. The ever-expanding myokinome: discovery challenges and therapeutic implications. Nat Rev Drug Discov (2016) 15(10):719–29. doi:10.1038/nrd.2016.153
4. Raschke S, Eckardt K, Bjørklund Holven K, Jensen J, Eckel J. Identification and validation of novel contraction-regulated myokines released from primary human skeletal muscle cells. PLoS One (2013) 8(4):e62008. doi:10.1371/journal.pone.0062008
5. Fiuza-Luces C, Garatachea N, Berger NA, Lucia A. Exercise is the real polypill. Physiology (Bethesda) (2013) 28(5):330–58. doi:10.1152/physiol.00019.2013
6. Steensberg A, Febbraio MA, Osada T, Schjerling P, van Hall G, Saltin B, et al. Interleukin-6 production in contracting human skeletal muscle is influenced by pre-exercise muscle glycogen content. J Physiol (2001) 537(Pt 2):633–9. doi:10.1111/j.1469-7793.2001.00633.x
7. Carey AL, Steinberg GR, Macaulay SL, Thomas WG, Holmes AG, Ramm G, et al. Interleukin-6 increases insulin-stimulated glucose disposal in humans and glucose uptake and fatty acid oxidation in vitro via AMP-activated protein kinase. Diabetes (2006) 55(10):2688–97. doi:10.2337/db05-1404
8. Helge JW, Stallknecht B, Pedersen BK, Galbo H, Kiens B, Richter EA. The effect of graded exercise on IL-6 release and glucose uptake in human skeletal muscle. J Physiol (2003) 546(Pt 1):299–305. doi:10.1113/jphysiol.2002.030437
9. Eckardt K, Görgens SW, Raschke S, Eckel J. Myokines in insulin resistance and type 2 diabetes. Diabetologia (2014) 57(6):1087–99. doi:10.1007/s00125-014-3224-x
10. Pedersen BK, Steensberg A, Fischer C, Keller C, Keller P, Plomgaard P, et al. Searching for the exercise factor: is IL-6 a candidate? J Muscle Res Cell Motil (2003) 24(2–3):113–9. doi:10.1023/A:1026070911202
11. Steensberg A, Keller C, Starkie RL, Osada T, Febbraio MA, Pedersen BK. IL-6 and TNF-alpha expression in, and release from, contracting human skeletal muscle. Am J Physiol Endocrinol Metab (2002) 283(6):E1272–8. doi:10.1152/ajpendo.00255.2002
12. Steensberg A, van Hall G, Osada T, Sacchetti M, Saltin B, Klarlund Pedersen B. Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J Physiol (2000) 529(Pt 1):237–42. doi:10.1111/j.1469-7793.2000.00237.x
13. Al-Khalili L, Bouzakri K, Glund S, Lönnqvist F, Koistinen HA, Krook A. Signaling specificity of interleukin-6 action on glucose and lipid metabolism in skeletal muscle. Mol Endocrinol (2006) 20(12):3364–75. doi:10.1210/me.2005-0490
14. Harder-Lauridsen NM, Krogh-Madsen R, Holst JJ, Plomgaard P, Leick L, Pedersen BK, et al. Effect of IL-6 on the insulin sensitivity in patients with type 2 diabetes. Am J Physiol Endocrinol Metab (2014) 306(7):E769–78. doi:10.1152/ajpendo.00571.2013
15. Jiang LQ, Duque-Guimaraes DE, Machado UF, Zierath JR, Krook A. Altered response of skeletal muscle to IL-6 in type 2 diabetic patients. Diabetes (2013) 62(2):355–61. doi:10.2337/db11-1790
16. Prokopchuk O, Liu Y, Wang L, Wirth K, Schmidtbleicher D, Steinacker JM. Skeletal muscle IL-4, IL-4Ralpha, IL-13 and IL-13Ralpha1 expression and response to strength training. Exerc Immunol Rev (2007) 13:67–75.
17. Görgens SW, Raschke S, Holven KB, Jensen J, Eckardt K, Eckel J. Regulation of follistatin-like protein 1 expression and secretion in primary human skeletal muscle cells. Arch Physiol Biochem (2013) 119(2):75–80. doi:10.3109/13813455.2013.768270
18. Lee HJ, Lee JO, Lee YW, Kim SA, Park SH, Kim HS. Kalirin, a GEF for Rac1, plays an important role in FSTL-1-mediated glucose uptake in skeletal muscle cells. Cell Signal (2016) 29:150–7. doi:10.1016/j.cellsig.2016.10.013
19. Görgens SW, Hjorth M, Eckardt K, Wichert S, Norheim F, Holen T, et al. The exercise-regulated myokine chitinase-3-like protein 1 stimulates human myocyte proliferation. Acta Physiol (Oxf) (2016) 216(3):330–45. doi:10.1111/apha.12579
20. Brunelli DT, Chacon-Mikahil MP, Gáspari AF, Lopes WA, Bonganha V, Bonfante IL, et al. Combined training reduces subclinical inflammation in obese middle-age men. Med Sci Sports Exerc (2015) 47(10):2207–15. doi:10.1249/MSS.0000000000000658
21. Tamura Y, Watanabe K, Kantani T, Hayashi J, Ishida N, Kaneki M. Upregulation of circulating IL-15 by treadmill running in healthy individuals: is IL-15 an endocrine mediator of the beneficial effects of endurance exercise? Endocr J (2011) 58(3):211–5. doi:10.1507/endocrj.K10E-400
22. Christiansen T, Bruun JM, Paulsen SK, Olholm J, Overgaard K, Pedersen SB, et al. Acute exercise increases circulating inflammatory markers in overweight and obese compared with lean subjects. Eur J Appl Physiol (2013) 113(6):1635–42. doi:10.1007/s00421-013-2592-0
23. Gray SR, Kamolrat T. The effect of exercise induced cytokines on insulin stimulated glucose transport in C2C12 cells. Cytokine (2011) 55(2):221–8. doi:10.1016/j.cyto.2011.04.019
24. Krolopp JE, Thornton SM, Abbott MJ. IL-15 activates the Jak3/STAT3 signaling pathway to mediate glucose uptake in skeletal muscle cells. Front Physiol (2016) 7:626. doi:10.3389/fphys.2016.00626
25. Scheler M, Irmler M, Lehr S, Hartwig S, Staiger H, Al-Hasani H, et al. Cytokine response of primary human myotubes in an in vitro exercise model. Am J Physiol Cell Physiol (2013) 305(8):C877–86. doi:10.1152/ajpcell.00043.2013
26. Landers-Ramos RQ, Jenkins NT, Spangenburg EE, Hagberg JM, Prior SJ. Circulating angiogenic and inflammatory cytokine responses to acute aerobic exercise in trained and sedentary young men. Eur J Appl Physiol (2014) 114(7):1377–84. doi:10.1007/s00421-014-2861-6
27. Nieman DC, Henson DA, Smith LL, Utter AC, Vinci DM, Davis JM, et al. Cytokine changes after a marathon race. J Appl Physiol (1985) (2001) 91(1):109–14.
28. Amir Levy Y, Ciaraldi TP, Mudaliar SR, Phillips SA, Henry RR. Excessive secretion of IL-8 by skeletal muscle in type 2 diabetes impairs tube growth: potential role of PI3K and the Tie2 receptor. Am J Physiol Endocrinol Metab (2015) 309(1):E22–34. doi:10.1152/ajpendo.00513.2014
29. Bernardo B, Lu M, Bandyopadhyay G, Li P, Zhou Y, Huang J, et al. FGF21 does not require interscapular brown adipose tissue and improves liver metabolic profile in animal models of obesity and insulin-resistance. Sci Rep (2015) 5:11382. doi:10.1038/srep11382
30. Xu J, Stanislaus S, Chinookoswong N, Lau YY, Hager T, Patel J, et al. Acute glucose-lowering and insulin-sensitizing action of FGF21 in insulin-resistant mouse models-association with liver and adipose tissue effects. Am J Physiol Endocrinol Metab (2009) 297(5):E1105–14. doi:10.1152/ajpendo.00348.2009
31. Lee DV, Li D, Yan Q, Zhu Y, Goodwin B, Calle R, et al. Fibroblast growth factor 21 improves insulin sensitivity and synergizes with insulin in human adipose stem cell-derived (hASC) adipocytes. PLoS One (2014) 9(11):e111767. doi:10.1371/journal.pone.0111767
32. Muise ES, Souza S, Chi A, Tan Y, Zhao X, Liu F, et al. Downstream signaling pathways in mouse adipose tissues following acute in vivo administration of fibroblast growth factor 21. PLoS One (2013) 8(9):e73011. doi:10.1371/journal.pone.0073011
33. Mashili FL, Austin RL, Deshmukh AS, Fritz T, Caidahl K, Bergdahl K, et al. Direct effects of FGF21 on glucose uptake in human skeletal muscle: implications for type 2 diabetes and obesity. Diabetes Metab Res Rev (2011) 27(3):286–97. doi:10.1002/dmrr.1177
34. Tanimura Y, Aoi W, Takanami Y, Kawai Y, Mizushima K, Naito Y, et al. Acute exercise increases fibroblast growth factor 21 in metabolic organs and circulation. Physiol Rep (2016) 4(12):e12828. doi:10.14814/phy2.12828
35. Kim KH, Kim SH, Min YK, Yang HM, Lee JB, Lee MS. Acute exercise induces FGF21 expression in mice and in healthy humans. PLoS One (2013) 8(5):e63517. doi:10.1371/journal.pone.0063517
36. Cuevas-Ramos D, Almeda-Valdés P, Meza-Arana CE, Brito-Córdova G, Gómez-Pérez FJ, Mehta R, et al. Exercise increases serum fibroblast growth factor 21 (FGF21) levels. PLoS One (2012) 7(5):e38022. doi:10.1371/journal.pone.0038022
37. Scalzo RL, Peltonen GL, Giordano GR, Binns SE, Klochak AL, Paris HL, et al. Regulators of human white adipose browning: evidence for sympathetic control and sexual dimorphic responses to sprint interval training. PLoS One (2014) 9(6):e90696. doi:10.1371/journal.pone.0090696
38. Yang M, Dong J, Liu H, Li L, Yang G. Effects of short-term continuous subcutaneous insulin infusion on fasting plasma fibroblast growth factor-21 levels in patients with newly diagnosed type 2 diabetes mellitus. PLoS One (2011) 6(10):e26359. doi:10.1371/journal.pone.0026359
39. Perakakis N, Triantafyllou GA, Fernández-Real JM, Huh JY, Park KH, Seufert J, et al. Physiology and role of irisin in glucose homeostasis. Nat Rev Endocrinol (2017). doi:10.1038/nrendo.2016.221
40. Jedrychowski MP, Wrann CD, Paulo JA, Gerber KK, Szpyt J, Robinson MM, et al. Detection and quantitation of circulating human irisin by tandem mass spectrometry. Cell Metab (2015) 22(4):734–40. doi:10.1016/j.cmet.2015.08.001
41. Huh JY, Siopi A, Mougios V, Park KH, Mantzoros CS. Irisin in response to exercise in humans with and without metabolic syndrome. J Clin Endocrinol Metab (2015) 100(3):E453–7. doi:10.1210/jc.2014-2416
42. Tsuchiya Y, Ando D, Takamatsu K, Goto K. Resistance exercise induces a greater irisin response than endurance exercise. Metabolism (2015) 64(9):1042–50. doi:10.1016/j.metabol.2015.05.010
43. Löffler D, Müller U, Scheuermann K, Friebe D, Gesing J, Bielitz J, et al. Serum irisin levels are regulated by acute strenuous exercise. J Clin Endocrinol Metab (2015) 100(4):1289–99. doi:10.1210/jc.2014-2932
44. Tsuchiya Y, Ando D, Goto K, Kiuchi M, Yamakita M, Koyama K. High-intensity exercise causes greater irisin response compared with low-intensity exercise under similar energy consumption. Tohoku J Exp Med (2014) 233(2):135–40. doi:10.1620/tjem.233.135
45. Norheim F, Langleite TM, Hjorth M, Holen T, Kielland A, Stadheim HK, et al. The effects of acute and chronic exercise on PGC-1alpha, irisin and browning of subcutaneous adipose tissue in humans. FEBS J (2014) 281(3):739–49. doi:10.1111/febs.12619
46. Lee HJ, Lee JO, Kim N, Kim JK, Kim HI, Lee YW, et al. Irisin, a novel myokine, regulates glucose uptake in skeletal muscle cells via AMPK. Mol Endocrinol (2015) 29(6):873–81. doi:10.1210/me.2014-1353
47. Huh JY, Mougios V, Kabasakalis A, Fatouros I, Siopi A, Douroudos II, et al. Exercise-induced irisin secretion is independent of age or fitness level and increased irisin may directly modulate muscle metabolism through AMPK activation. J Clin Endocrinol Metab (2014) 99(11):E2154–61. doi:10.1210/jc.2014-1437
48. Xiong XQ, Chen D, Sun HJ, Ding L, Wang JJ, Chen Q, et al. FNDC5 overexpression and irisin ameliorate glucose/lipid metabolic derangements and enhance lipolysis in obesity. Biochim Biophys Acta (2015) 1852(9):1867–75. doi:10.1016/j.bbadis.2015.06.017
49. Goekint M, De Pauw K, Roelands B, Njemini R, Bautmans I, Mets T, et al. Strength training does not influence serum brain-derived neurotrophic factor. Eur J Appl Physiol (2010) 110(2):285–93. doi:10.1007/s00421-010-1461-3
50. Rojas Vega S, Knicker A, Hollmann W, Bloch W, Strüder HK. Effect of resistance exercise on serum levels of growth factors in humans. Horm Metab Res (2010) 42(13):982–6. doi:10.1055/s-0030-1267950
51. Correia PR, Pansani A, Machado F, Andrade M, Carlos da Silva A, Scorza FA, et al. Acute strength exercise and the involvement of small or large muscle mass on plasma brain-derived neurotrophic factor levels. Clinics (Sao Paulo) (2010) 65(11):1123–6. doi:10.1590/S1807-59322010001100012
52. Schiffer T, Schulte S, Hollmann W, Bloch W, Strüder HK. Effects of strength and endurance training on brain-derived neurotrophic factor and insulin-like growth factor 1 in humans. Horm Metab Res (2009) 41(3):250–4. doi:10.1055/s-0028-1093322
53. Levinger I, Goodman C, Matthews V, Hare DL, Jerums G, Garnham A, et al. BDNF, metabolic risk factors, and resistance training in middle-aged individuals. Med Sci Sports Exerc (2008) 40(3):535–41. doi:10.1249/MSS.0b013e31815dd057
54. Yarrow JF, White LJ, McCoy SC, Borst SE. Training augments resistance exercise induced elevation of circulating brain derived neurotrophic factor (BDNF). Neurosci Lett (2010) 479(2):161–5. doi:10.1016/j.neulet.2010.05.058
55. Coelho FG, Gobbi S, Andreatto CA, Corazza DI, Pedroso RV, Santos-Galduróz RF. Physical exercise modulates peripheral levels of brain-derived neurotrophic factor (BDNF): a systematic review of experimental studies in the elderly. Arch Gerontol Geriatr (2013) 56(1):10–5. doi:10.1016/j.archger.2012.06.003
56. Huang T, Larsen KT, Ried-Larsen M, Møller NC, Andersen LB. The effects of physical activity and exercise on brain-derived neurotrophic factor in healthy humans: a review. Scand J Med Sci Sports (2014) 24(1):1–10. doi:10.1111/sms.12069
57. Winter B, Breitenstein C, Mooren FC, Voelker K, Fobker M, Lechtermann A, et al. High impact running improves learning. Neurobiol Learn Mem (2007) 87(4):597–609. doi:10.1016/j.nlm.2006.11.003
58. Ferris LT, Williams JS, Shen CL. The effect of acute exercise on serum brain-derived neurotrophic factor levels and cognitive function. Med Sci Sports Exerc (2007) 39(4):728–34. doi:10.1249/mss.0b013e31802f04c7
59. Matthews VB, Aström MB, Chan MH, Bruce CR, Krabbe KS, Prelovsek O, et al. Brain-derived neurotrophic factor is produced by skeletal muscle cells in response to contraction and enhances fat oxidation via activation of AMP-activated protein kinase. Diabetologia (2009) 52(7):1409–18. doi:10.1007/s00125-009-1364-1
60. Tsuchida A, Nonomura T, Nakagawa T, Itakura Y, Ono-Kishino M, Yamanaka M, et al. Brain-derived neurotrophic factor ameliorates lipid metabolism in diabetic mice. Diabetes Obes Metab (2002) 4(4):262–9. doi:10.1046/j.1463-1326.2002.00206.x
61. Yamanaka M, Tsuchida A, Nakagawa T, Nonomura T, Ono-Kishino M, Sugaru E, et al. Brain-derived neurotrophic factor enhances glucose utilization in peripheral tissues of diabetic mice. Diabetes Obes Metab (2007) 9(1):59–64. doi:10.1111/j.1463-1326.2006.00572.x
62. van Hall G, Steensberg A, Sacchetti M, Fischer C, Keller C, Schjerling P, et al. Interleukin-6 stimulates lipolysis and fat oxidation in humans. J Clin Endocrinol Metab (2003) 88(7):3005–10. doi:10.1210/jc.2002-021687
63. Wolsk E, Mygind H, Grøndahl TS, Pedersen BK, van Hall G. IL-6 selectively stimulates fat metabolism in human skeletal muscle. Am J Physiol Endocrinol Metab (2010) 299(5):E832–40. doi:10.1152/ajpendo.00328.2010
64. Petersen EW, Carey AL, Sacchetti M, Steinberg GR, Macaulay SL, Febbraio MA, et al. Acute IL-6 treatment increases fatty acid turnover in elderly humans in vivo and in tissue culture in vitro. Am J Physiol Endocrinol Metab (2005) 288(1):E155–62. doi:10.1152/ajpendo.00257.2004
65. Carbó N, López-Soriano J, Costelli P, Alvarez B, Busquets S, Baccino FM, et al. Interleukin-15 mediates reciprocal regulation of adipose and muscle mass: a potential role in body weight control. Biochim Biophys Acta (2001) 1526(1):17–24. doi:10.1016/S0304-4165(00)00188-4
66. Quinn LS, Anderson BG, Strait-Bodey L, Stroud AM, Argilés JM. Oversecretion of interleukin-15 from skeletal muscle reduces adiposity. Am J Physiol Endocrinol Metab (2009) 296(1):E191–202. doi:10.1152/ajpendo.90506.2008
67. Barra NG, Reid S, MacKenzie R, Werstuck G, Trigatti BL, Richards C, et al. Interleukin-15 contributes to the regulation of murine adipose tissue and human adipocytes. Obesity (Silver Spring) (2010) 18(8):1601–7. doi:10.1038/oby.2009.445
68. Pierce JR, Maples JM, Hickner RC. IL-15 concentrations in skeletal muscle and subcutaneous adipose tissue in lean and obese humans: local effects of IL-15 on adipose tissue lipolysis. Am J Physiol Endocrinol Metab (2015) 308(12):E1131–9. doi:10.1152/ajpendo.00575.2014
69. Almendro V, Fuster G, Busquets S, Ametller E, Figueras M, Argilés JM, et al. Effects of IL-15 on rat brown adipose tissue: uncoupling proteins and PPARs. Obesity (Silver Spring) (2008) 16(2):285–9. doi:10.1038/oby.2007.47
70. Toriya M, Maekawa F, Maejima Y, Onaka T, Fujiwara K, Nakagawa T, et al. Long-term infusion of brain-derived neurotrophic factor reduces food intake and body weight via a corticotrophin-releasing hormone pathway in the paraventricular nucleus of the hypothalamus. J Neuroendocrinol (2010) 22(9):987–95. doi:10.1111/j.1365-2826.2010.02039.x
71. Slusher AL, Whitehurst M, Zoeller RF, Mock JT, Maharaj A, Huang CJ. Brain-derived neurotrophic factor and substrate utilization following acute aerobic exercise in obese individuals. J Neuroendocrinol (2015) 27(5):370–6. doi:10.1111/jne.12275
72. Gao S, Li F, Li H, Huang Y, Liu Y, Chen Y. Effects and molecular mechanism of GST-irisin on lipolysis and autocrine function in 3T3-L1 adipocytes. PLoS One (2016) 11(1):e0147480. doi:10.1371/journal.pone.0147480
73. Wang C, Wang L, Li W, Yan F, Tian M, Wu C, et al. Irisin has no effect on lipolysis in 3T3-L1 adipocytes or fatty acid metabolism in HepG2 hepatocytes. Endocrine (2015) 49(1):90–6. doi:10.1007/s12020-014-0458-9
74. Huh JY, Dincer F, Mesfum E, Mantzoros CS. Irisin stimulates muscle growth-related genes and regulates adipocyte differentiation and metabolism in humans. Int J Obes (Lond) (2014) 38(12):1538–44. doi:10.1038/ijo.2014.42
75. Xin C, Liu J, Zhang J, Zhu D, Wang H, Xiong L, et al. Irisin improves fatty acid oxidation and glucose utilization in type 2 diabetes by regulating the AMPK signaling pathway. Int J Obes (Lond) (2016) 40(3):443–51. doi:10.1038/ijo.2015.199
76. Seldin MM, Peterson JM, Byerly MS, Wei Z, Wong GW. Myonectin (CTRP15), a novel myokine that links skeletal muscle to systemic lipid homeostasis. J Biol Chem (2012) 287(15):11968–80. doi:10.1074/jbc.M111.336834
77. Arner P, Pettersson A, Mitchell PJ, Dunbar JD, Kharitonenkov A, Rydén M. FGF21 attenuates lipolysis in human adipocytes – a possible link to improved insulin sensitivity. FEBS Lett (2008) 582(12):1725–30. doi:10.1016/j.febslet.2008.04.038
78. Potthoff MJ, Inagaki T, Satapati S, Ding X, He T, Goetz R, et al. FGF21 induces PGC-1alpha and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proc Natl Acad Sci U S A (2009) 106(26):10853–8. doi:10.1073/pnas.0904187106
79. Xu J, Lloyd DJ, Hale C, Stanislaus S, Chen M, Sivits G, et al. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes (2009) 58(1):250–9. doi:10.2337/db08-0392
Keywords: exercise, myokines, muscle, endocrine, diabetes
Citation: Carson BP (2017) The Potential Role of Contraction-Induced Myokines in the Regulation of Metabolic Function for the Prevention and Treatment of Type 2 Diabetes. Front. Endocrinol. 8:97. doi: 10.3389/fendo.2017.00097
Received: 24 February 2017; Accepted: 18 April 2017;
Published: 02 May 2017
Edited by:
Kristian Karstoft, University of Copenhagen, DenmarkReviewed by:
Martin Whitham, Garvan Institute of Medical Research, AustraliaAshraf Al Madhoun, Dasman Diabetes Institute, Kuwait
Copyright: © 2017 Carson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Brian P. Carson, brian.carson@ul.ie
 Brian P. Carson
Brian P. Carson