Clinical Guidelines for Dentistry in China During the Coronavirus Disease 2019 Pandemic
- 1Department of Stomatology, The First Affiliated Hospital of Hainan Medical University, Haikou, China
- 2State Key Laboratory of Oral Diseases & National Clinical Research Center for Oral Diseases, West China Hospital of Stomatology, Sichuan University, Chengdu, China
Preventing the spread of Coronavirus Disease 2019 (COVID-19) has become the focus of epidemiologists as the highly infectious respiratory disease spreads primarily by close, person-to-person contact via droplets or the skin. Aerosol dissemination may occur in a closed, high-aerosol environment. The aerosols generated in dental procedures can pollute surrounding air and device surfaces. In this paper, we summarize prevention and control measures relating to dentistry. We focus on the relationship between COVID-19 and dental disease prevention and control in dental treatment procedures and imaging examinations, oral health education and perspectives, and guidance for the practice of dentistry during the COVID-19 pandemic to provide a consistent and broadly endorsed standard for dental hospital and clinics.
Introduction
By January 2020, COVID-2019 had broken out and spread around the world. The highly infectious respiratory disease caused by the 2019 novel coronavirus (2019-nCoV), officially known as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is an enveloped, positive-stranded RNA virus that is highly infectious and has a high incidence rate, particularly in crowds (1). Complications of COVID-19 include acute respiratory distress syndrome (ARDS), respiratory failure, acute myocardial injury, liver injury, septic shock, and even multiple organ failure. Immune-mediated injury may play a critical role in the pathogenesis of COVID-19. Viral infection of pneumocytes induces local inflammatory responses and promotes the release of cytokines, including TGF-β1, IL-6, IL-1β, TNF-α, and numerous chemokines that serve to recruit circulating leukocytes. In severe forms of COVID-19, the ensuing inflammatory cascades may lead to a cytokine storm, as observed in a recent study that documented elevated serum cytokine levels including, TNF-α, IL-2, IL-10, G-CSF and MCP (2). The cytokine storm is believed to be a key factor driving both ARDS and extra-pulmonary organ failure (3).
As the oral cavity is one of the first interfaces between the exterior and body, this pathway of viral colonization and infection is critical for the onset of COVID-19 (4, 5). A decrease in the oral viral load would diminish the amount of virus expelled and reduce the risk of transmission (6). SARS-CoV-2 is highly contagious, and most individuals within the population are susceptible to infection. While the majority of cases present with mild symptoms, a minority progress to acute respiratory illness and hypoxia requiring hospitalization, and a subset develop acute respiratory distress syndrome, multi-organ failure, or have a fatal outcome (7). SARS-CoV-2 has the characteristics of strong transmission, long incubation time (8, 9), and certain variability. In the central database Global Influenza Data Sharing Program (GISAID), more than 16,000 SARS-CoV-2 gene sequences have been collected. However, whether these genetic mutations strengthen or weaken their infectivity or lethality remains uncertain. Viral shedding occurs from the respiratory tract, saliva, feces, and urine, resulting in other sources of virus spread (10, 11). Furthermore, there have been reports of infection transmission from asymptomatic contact, implying that COVID-19 is also contagious during the incubation period (12). It has also been confirmed that those without symptoms can spread the virus (13, 14). Currently, no specific antiviral measures are available to treat COVID-19. Although several vaccines have been approved for clinical trials in China, the United States, and beyond, it will still take time to vaccinate the critical number of people needed to stop COVID-19 progression in a country. The key problem of prevention and control is how to decrease cross-infection in clinics to reduce the risk of COVID-19 outbreak.
At present, the global economy is beginning to recover, and medical institutions are also seeking to return to work under the premise of good prevention and control of COVID-19. However, owing to the infectious nature of the COVID-19 and the clinical characteristics of dentistry, the risk of infection of oral medical staff and related staff has increased significantly since the start of the outbreak, which has brought great challenges to disease prevention and control in dental hospitals during the pandemic. In order to reduce transmission risk in dental departments, the key links and risk points of COVID-2019 and dentistry should be considered to increase efforts to prevent and control disease transmission, strengthen regular management, and reconstruct diagnosis and treatment procedures.
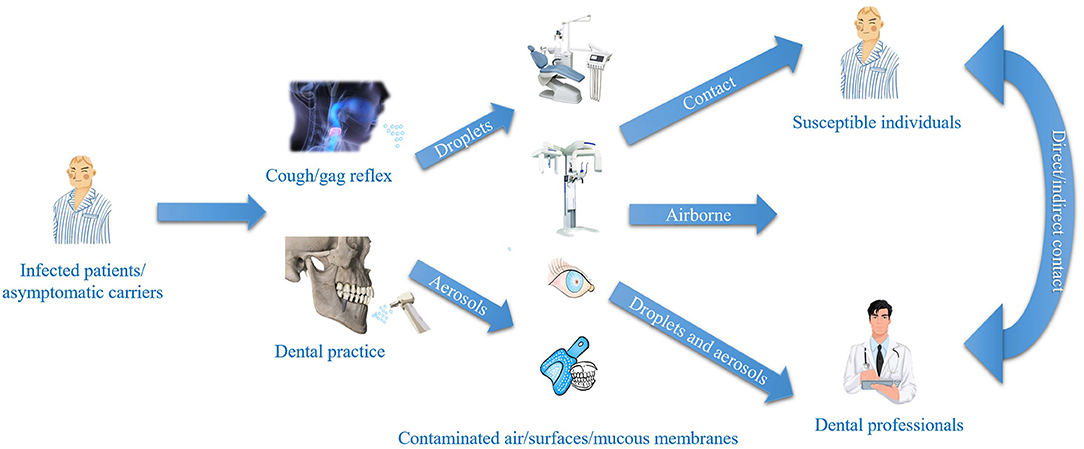
Among the medical professionals, dentists are with the greatest exposure to the COVID-19 pandemic because the work environment and the oral cavity represent a high potential source for the rapid spread of SARS-CoV-2 (15). Owing to the characteristics of dentistry, significant amounts of droplets and aerosols are generated in most treatment procedures, which create a high risk of infection to dental patients and professionals (16), as shown in Figure 1.
Aerosols mixed with the blood and saliva of patients are produced in dental practices. Moreover, live viruses are present in the blood and saliva of infected individuals (17). Shivakumar et al. considered that atmospheric microbial contamination (CFU/plate) was four times higher during working sessions than the levels before working sessions (18). Hans et al. reported that in 1 min, a high-speed handpiece produced 1,000 CFU of bacteria, 95% of which were <5 μm in diameter (19). Droplets <5 μm in diameter are suspended in the air and dehydrated into droplets, which flow with the air and pollute the diagnosis and treatment area environment. And human coronaviruses including Middle East respiratory syndrome-coronavirus (MERS-CoV) and SARS-CoV can survive for up to several days when suspended in human secretions, and then undergo onward transmission (20). Moreover, large-diameter droplets settle quickly near the droplet source, and if not treated in time, are suspended in the air again, resulting in secondary pollution (21). The contamination produced by different dental procedures is also different. In terms of contamination, blood-contaminated splatter was found to be significantly higher for procedures (n = 22) using high-speed air-turbine handpieces (77.3% ± 74.9%−79.7%; p = 0.002) than for surgical cases (n = 136) using low-speed handpieces (45.6% ± 42.2%−49.0%; p = 0.002) (22). Ishihama et al. found that both the surgical gown and visor mask showed evidence of higher invisible blood contamination (88 and 75%, respectively) compared with visible stains (64 and 60%, respectively), and surgical procedures lasting 20 min or more produced more evidence of blood contamination on a surgeon's personal protective equipment (PPE) (23). And bacterial aerosols were able to spread into areas where there was no dental activity, indicating that dental treatments significantly increase the levels of bacterial air contamination in both a multi-chair dental clinic and a closed dental operatory. Grenier et al. used a biological air sampler to sample the air, 3 h after the beginning of dental treatments, there was noticeable contamination in the inactive dental treatment area (42 CFU/m3) (24). In addition, during a dentist's routine operation, they can often come into contact with a patient's saliva, oral secretions, blood, and gingival crevicular fluid. Doctors are exposed to various pathogenic microorganisms on the surface of oral materials or instruments by hand. Although imaging examinations do not produce a large number of aerosols, the foreign body sensation caused by intraoral film in the mouth may lead to violent coughing, which generates aerosol. The instruments that come into direct contact with saliva have also made it difficult to prevent and control the pandemic.
The outbreak of COVID-19 has clearly placed health professionals at risk (25). The oral cavity and the work environment represent a high potential source for transmissibility and susceptibility to this and other etiological agents (4, 15, 26). Ensuring personal and health safety for healthcare workers (HCWs) is essential, as an infected HCW may also act as a source of cross-transmission (10).
Therefore, in order to prevent the spread of the COVID-2019 epidemic effectively, minimize the risk of COVID-2019 transmission in dentistry, and ensure safety in the diagnosis and treatment of patients and in the routines of medical personnel, several detailed strategies to block virus transmission are recommended according to the characteristics of COVID-2019 and dentistry. The purpose of the clinical guidelines is to provide a consistent and broadly endorsed standard of appropriate dental practice to assist dentists and patients during the COVID-19 pandemic.
Prevention and Control for Dentistry
Medical Treatment Procedures
Prognostics and Triage
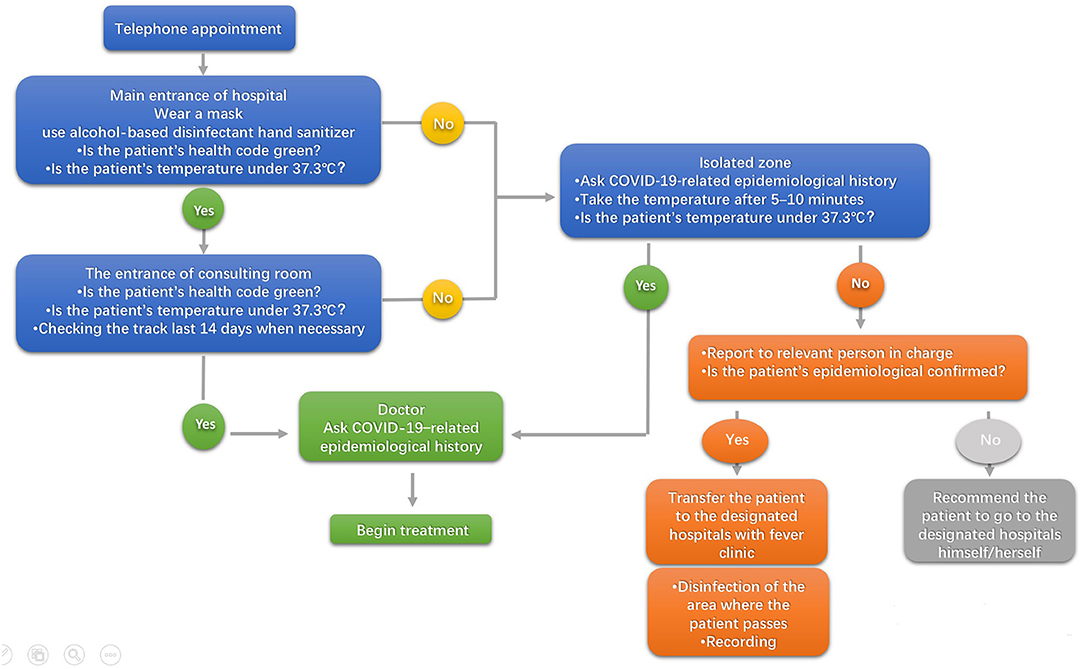
The risks of oral diagnosis and treatment, precautions, admission process and department consultation service hotline, should be introduced on the clinic or hospital's official website and public platform. The online hospital appointment register system should also provide more convenient services and improve the health care environment. Before entering the hospital, the health codes should be checked. Only patients who take the green code may enter the clinic or hospital. The measures of time-interval appointment and three-level prognostics and triage should also be implemented. Patients are required to wear a mask and use alcohol-based disinfectant hand sanitizer (75%) in the room's triage. At the first level prognostics and triage, the health code and temperature of the patient are checked at the reception desk of the hospital. A contact-free forehead thermometer is strongly recommended. At the second level prognostics and triage, the health code, temperature, and appointment should be checked again at the reception desk of the dental implant department. At the third level of prognostics and triage, the epidemiological contact history, clinical manifestations, and a thorough clinical examination should be taken by the dentist to ensure that the patient is not a suspected COVID-19 case. An isolation area for suspected cases are set up in each triage area. Well-ventilated rooms and negatively pressured rooms or Isolated are available for suspected cases with COVID-19 outside the department. The procedure for prognostics and triage is shown in Figure 2.
Protection for Dental Professionals and Control of the Environment
All patients should be treated as suspected COVID-19 patients during the COVID-2019 pandemic. The use of PPE, including disposable working caps, disposable surgical masks, medical protective masks (N95/N99), goggles/face shields, shoe covers, work clothes (white gowns or hand-washing clothes), disposable isolation clothes, and one or two disposable latex gloves, is recommended to protect mucosa and skin from saliva secretion or potentially infected blood (27).
Hand Hygiene
Hand hygiene is considered the most critical measure for reducing the risk of transmitting pathogenic microorganisms to patients. SARS-CoV-2 can persist in aerosols for up to 3 h and on plastic and stainless steel surfaces for up to 3 days, depending on the temperature, the humidity of the environment, or the type of surface (28). This reinforces the need for good hand hygiene and the importance of thorough disinfection of all surfaces within the dental clinic, especially the treatment equipment. A six-step hand-washing technique advocated by the World Health Organization has been found to be the most effective for reducing bacteria. Hands should be cleaned immediately before direct patient contact and immediately after. Hand washing should take 40–60 s. Follow these six steps every time: (1) Wet hands with clean, running water (warm or cold) and apply soap to cover all hand surfaces. Rub hands palm to palm. Rub hands palm with fingers interlaced. (2) Rub back of each hand with palm of other hand with fingers interlaced. (3) Rub with back of fingers to opposing palms with fingers interlocked. (4) Rub each thumb clasped in opposite hand using a rotational movement. (5) Rub tips of fingers in opposite palm in a circular motion. (6) Rub each wrist with opposite hand. Rinse hands with water. Dry thoroughly with a single-use towel (28). A quick hand disinfection solution is also recommended for dentists to disinfect their hands at any moment. Moreover, dental staff must pay more attention to their own health conditions and report them to the clinic or hospital every day.
Air Sterilization
Isolation and organized disinfection have been established as important methods for the control and prevention of further spread of SARS-CoV-2. Ultraviolet (UV) irradiation has been recommended for air disinfection because SARS-CoV-2 is sensitive to UV light and is deactivated within 30 min of irradiation. However, UV irradiation must only be performed when no one is present. In a clinic, patients come in randomly throughout the day. In addition, the air disinfection effect decreases over time. Air flow sterilization is convenient and effective for air disinfection. Plasma air sterilizers are commonly used in most CT rooms for air disinfection. Plasma air sterilizers can be operated when people are around and are not affected by the movement of people. They perform air disinfection dynamically and continuously, making up for the shortcomings of UV germicidal irradiation and achieving a better disinfection effect (29).
To ensure the persistence of air disinfection effect and safety, we recommend the use of plasma air disinfection machine combined with a ultraviolet disinfection lamps to disinfect the air in the dental clinic and imaging examination room to avoid the cross-infection of doctors and patients.
Isolation and Disinfection of the Medical Environment
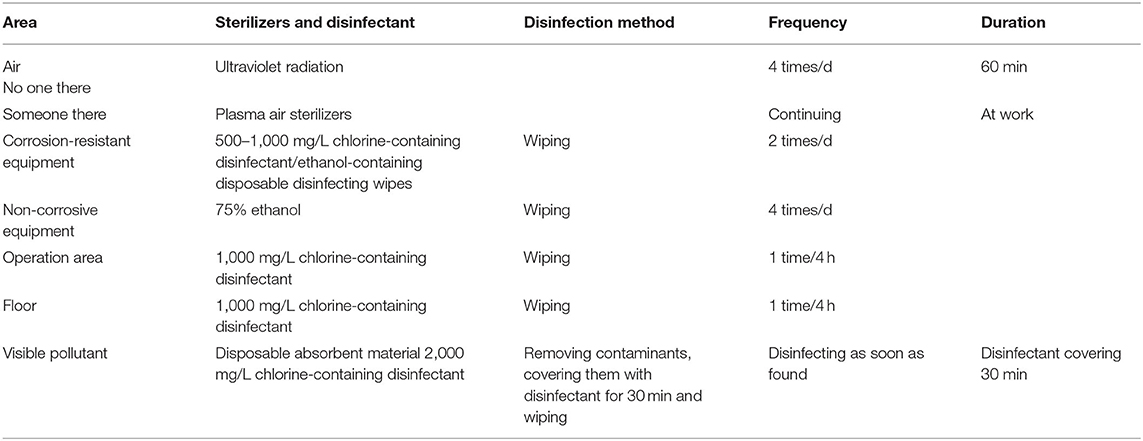
It is important to increase the distance between dental units or a distance of at least one dental unit. Before and after treatment, disinfection procedures and environmental cleaning should be followed. The selection and guidance of the sterilizers and disinfectant are shown in Table 1. Alternatively, suspected COVID-19 cases may be treated in a negatively pressured room or a well-ventilated, isolated room, if available.
Mouth Rinse Before the Treatment
Some oral antiseptics used as a pre-procedure rinse have shown efficacy in significantly reducing the risk of cross-infection, reducing the amount of bacteria in aerosols (30).
Before every treatment, patients use a mouth rinse with 0.23% povidone-iodine, 0.05% cetylpyridinium chloride, or 1.5% hydrogen peroxide and wear goggles and a bib throughout the procedure (31). Povidone-iodine (0.23%) is used as a mouthwash for in vitro rapidly inactivated (15 s of exposure) SARS-CoV (32). To minimize aerosol production, dentists are suggested to use a dental dam, hand instrumentation, and a high-volume saliva ejector during the treatment and not use a 3-in-1 syringe. The four-handed technique is beneficial for controlling infection.
Medical-Treatment Room Classification
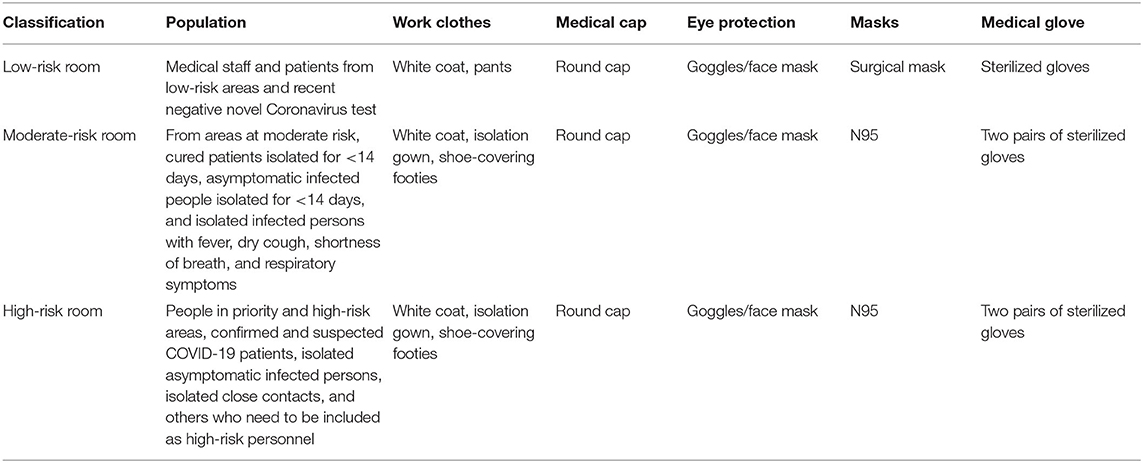
Interim guidance on infection control and prevention during healthcare is recommended by the Chinese State Council when COVID-19 infection is suspected. The criteria for the different risk rooms and levels of protection are shown in Table 2.
Impression
Disinfection of dental impressions is an essential routine that aims to protect dental personnel who handle casts or impressions against exposure to potentially pathogenic microorganisms (33). Washing of dental impressions under tap water was the first recommended procedure for disinfection. However, the microbial reduction associated with water washing was found to be considerably lower than the 40–90% reduction in microbial load stated in some literature (33). Chemical disinfection through immersion by a glutaraldehyde-based disinfectant was shown to be effective in eliminating all microbial forms for both silicone and alginate without modifying the dimensional stability. Moreover, 75% alcohol-based spray disinfectants are also suitable for impression materials (34). An intraoral digital scan for implant-level impression may be a better technique to decrease the risk of transmission of diseases in the dental clinic and laboratory in the future.
Imaging Examination and COVID-19 Pandemic
The most commonly used techniques in dentistry are intraoral radiography, cone beam computed tomography (CBCT) and dental panoramic radiographs (DPRs) (35). However, such imaging examinations should be limited to one patient at a time whenever possible. The constraints that COVID-19 has imposed upon dental practice might limit the hitherto free use of intraoral radiography, mainly owing to the potentially increased risk of producing a virus-laden aerosol. Potential sources of aerosol production in intraoral radiography include gagging and coughing. In one study, the overall frequency of gagging during intraoral radiography was 13% (36). These problems have prompted dentists to consider different technologies, such as DPRs and CBCT, to accomplish the goals of intraoral radiography.
The Centers for Disease Control and Prevention (CDC) recommends that this should be done in accordance with the CDC's 2003 guidelines (37). Radiology equipment and accessories should be prepared so that only the instruments and sterile supplies needed for the dental procedures are readily accessible. Any equipment that is exposed during the procedure should be considered contaminated and should be sterilized properly after completion of the procedure. To clean and disinfect the radiology operatory after a patient without suspected or confirmed COVID-19, it is recommended that the clinician wait 20–30 min after completion of clinical care and the exit of each patient to begin cleaning and disinfecting room surfaces to allow for droplets to fall from the air (37). Delaying entry into the operatory until a sufficient time has elapsed allows enough air changes to remove potentially infectious particles (38). Air disinfection and changes per hour represent the ratio of the volume of air flowing through a space in a certain period of time (the airflow rate) to the volume of that space (the room volume). A minimum of 6 is acceptable. Furthermore, a standard antiseptic (alcohol impregnated wipes or 75% alcohol sprinkling can) and lead/lead-equivalent aprons should be used to wipe down equipment.
Oral Health Education
Preoperative Guidance
Patients with chronic diseases, such as hypertension, diabetes and heart disease, need more attention to prevent infection, fever, and other postoperative complications.
Postoperative Care
Some patients develop a mild fever not exceeding 38°C 24 h after surgery. However, a normal temperature can usually be obtained after 24–48 h. Fever can not only cause worries, but also cause inconvenience during the COVID-19 pandemic. Therefore, it is recommended to use anti-inflammatory and analgesic drugs for prevention before surgery. In addition, patients need to know about the possibility of postoperative fever. Once fever symptoms appear, symptomatic treatment and close observation at home should be recommended in 48 h. If there is no significant improvement after 48 h, a timely follow-up visit should be taken. Blood routine and C-reactive protein tests should be performed in addition to clinical examination. If the patient's condition is diagnosed as a bacterial infection, antibiotic therapy is needed. If the patient's fever is not associated with the surgery, patients should be registered and referred to designated hospitals or established fever clinics. If a patient has been to epidemic regions within the past 14 d, a quarantine of at least 14 d is suggested (35).
Dental care and epidemic protection information are advertised in official accounts, the WeChat app, and other technology platforms, especially during the COVID-19 pandemic. Anti-bacterial mouthwash, such as compound chlorhexidine taken by gargling, should be used three times a day for at least 1 week. This can decrease the infection rate of a wound more effectively. Patients treated with maxillary sinus augmentation are not recommended to blow their noses for 1 month, and they are told that sinus symptoms may develop. Tough foods are not recommended within 3 months postoperatively. Patients should also pay attention to keep warm and avoid cold. Regular return visit, regular exercise, a well-balanced diet, sleep, and the cultivation of a good mood can all improve immunity, as can frequent hand washing, regular ventilation, and daily temperature measurements. Most ordinary people are suggested to take rigorous personal preventive measures including wearing medical surgical masks in public places together with social distancing and frequent hand washing (39). If patient have fever, cough, sore throat, fatigue, or other suspicious symptoms, they are recommended to go to the designated fever hospital immediately.
Perspectives
As the COVID-19 continues to spread in many countries, prevention and control of SARS-CoV-2 infections are more important than in 2020. A health and economic crisis of unprecedented proportions is in progress. The paralysis of economic activities has had devastating effects that is expected to cause a long-lasting recession globally because pandemics often result in global recessions (40). Moreover, there is an evident scarcity of literature related to financial education for dental offices. Emergency financial reserves and long-term investments should be part of the recommendations to protect the incomes of the self-employed or financially beleaguered dentist.
Vaccines will be required to achieve sufficient herd immunity to SARS-CoV-2 infection to ultimately control the COVID-19 pandemic (41). The World Health Organization (WHO) has listed more than 200 COVID-19 vaccines as under development, some of which have come into clinical application (42). The hope that preventive vaccines will control COVID-19 is justified by the impact that other vaccines have had on preventing disability and death. COVID-19 vaccines may provide effective control of the pandemic's spread.
Teledentistry is an emerging trend for dental professionals during the pandemic and a viable option to offering off-site direct dental services. It involves the use of telecommunications and information technology for dental consultation, education, care and public awareness in the same manner as telemedicine and telehealth. Infected patients and asymptomatic patients who do not show any signs and symptoms are considered by dental offices as major sources of cross-infection among dentists and patients. By 2025, over 60% of the population all over the world will be using mobile internet (43). Thus, remote treatment via the WeChat app, video conversation, phone calls, and other technologies have given rise to a new look at the dentist-patient relationship. Until the pandemic is brought under control, teledentistry represents a promising tool to maintain contact with patients without putting them or dentists at a high risk of infection.
Conclusions
This article offers a clinical guideline for the practice of dentistry during the COVID-19 pandemic to provide a consistent and broadly endorsed standard for dental hospital and clinics. As the current COVID-19 pandemic continues to unfold, so too will the need to develop and reconfigure the current strategy of dentists. A large number of patients have access to dental hospitals and clinics, and prevention and control are essential to blocking the spread of the COVID-19 pandemic. In the diagnosis and treatment of conditions requiring dentistry, reducing the exposure risk when performing aerosol-generating procedures during the use of low-/high-speed dental handpieces is the focus of disease prevention and control. Reducing the risk of cross-infection and providing humanistic care are important parts of treatment from clinical reception to postoperative care and follow-up visit. Stopping the spread of the COVID-19 pandemic will require the coordination of hospitals, dentists, and patients.
Author Contributions
WL, JW, and YW drafted the manuscript. All authors contributed to manuscript revision and approved the submitted version.
Funding
This study was funded by the grant: Hainan Natural Science Foundation of China (No. 818MS142).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
References
1. Corman VM, Muth D, Niemeyer D, and Drosten C. Hosts and sources of endemic human coronaviruses. Adv Virus Res. (2018) 100:163–88. doi: 10.1016/bs.aivir.2018.01.001
2. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. (2020) 395:497–506. doi: 10.1016/S0140-6736(20)30183-5
3. Li C, and Xu XN. Host immune responses to SARS coronavirus in humans. Mol Biol SARS-Coronavirus. (2009) 16:259–78. doi: 10.1007/978-3-642-03683-5_16
4. Xu H, Zhong L, Deng J, Peng J, Dan H, Zeng X, et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. (2020) 12:8. doi: 10.1038/s41368-020-0074-x
5. Xu J, Li Y, Gan F, Du Y, and Yao Y. Salivary glands: potential reservoirs for COVID-19 asymptomatic infection. J Dent Res. (2020) 99:989. doi: 10.1177/0022034520918518
6. Herrera D, Serrano J, Roldán S, and Sanz M. Is the oral cavity relevant in SARS-CoV-2 pandemic? Clin Oral Investig. (2020) 24:2925–30. doi: 10.1007/s00784-020-03413-2
7. Hu Y, Sun J, Dai Z, Deng H, Li X, Huang Q, et al. Prevalence and severity of coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. J Clin Virol. (2020) 127:104371. doi: 10.1016/j.jcv.2020.104371
8. de Wit E, van Doremalen N, and Falzarano D. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. (2016) 14:523–34. doi: 10.1038/nrmicro.2016.81
9. Wu YC, Chen CS, and Chan YJ. The outbreak of COVID-19: an overview. J Chin Med Assoc. (2020) 83:217–20. doi: 10.1097/JCMA.0000000000000270
10. Li Q, Guan X, Wu P, Wang X, Zhou L, Tong YQ, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. (2020) 382:1199–207. doi: 10.1056/NEJMoa2001316
11. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. (2020) 395:507–13. doi: 10.1016/S0140-6736(20)30211-7
12. Rothe C, Schunk M, Sothmann P, Bretzel G, Froeschl G, Wallrauch C, et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med. (2020) 382:970–1. doi: 10.1056/NEJMc2001468
13. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of 2019 novel coronavirus infection in China. N Engl J Med. (2020) 382:1708–20. doi: 10.1101/2020.02.06.20020974
14. Backer JA, Klinkenberg D, and Wallinga J. Incubation period of 2019 novel coronavirus (2019-nCoV) infections among travellers from Wuhan, China. Euro Surveill. (2020) 25:2000062. doi: 10.1101/2020.01.27.20018986
15. Sabino-Silva R, Jardim AC, and Siqueira WL. Coronavirus COVID-19 impacts to dentistry and potential salivary diagnosis. Clin Oral Investig. (2020) 24:1619–21. doi: 10.1007/s00784-020-03248-x
16. Lu CW, Liu XF, and Jia ZF. 2019-nCoV transmission through the ocular surface must not be ignored. Lancet. (2020) 395:e39. doi: 10.1016/S0140-6736(20)30313-5
17. To KK, Tsang OT, Yip CC, Chan KH, Wu TC, Chan JM, et al. Consistent detection of 2019 novel coronavirus in Saliva. Clin Infect Dis. (2020) 71:841–3. doi: 10.1093/cid/ciaa149
18. Shivakumar KM, Prashant GM, Madhu Shankari GS, Subba Reddy VV, and Chandu GN. Assessment of atmospheric microbial contamination in a mobile dental unit. Indian J Dent Res. (2007) 18:177–80. doi: 10.4103/0970-9290.35828
19. Andersen HK, Fiehn NE, and Larsen T. Effect of steam sterilization inside the turbine chambers of dental turbines. Oral Surg Oral Med Oral Radiol Endod. (1999) 87:184–8. doi: 10.1016/S1079-2104(99)70271-4
20. Otter JA, Yezli S, Salkeld JAG, and French GL. Evidence that contaminated surfaces contribute to the transmission of hospital pathogens and an overview of strategies to address contaminated surfaces in hospital settings. Am J Infect Control. (2013) 41:S6–11. doi: 10.1016/j.ajic.2012.12.004
21. Liu Y, Ning Z, Chen Y, Gali NK, Sun L, Duan Y, et al. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. (2020) 582:557–60. doi: 10.1038/s41586-020-2271-3
22. Aguilar-Duran L, Bara-Casaus JJ, Aguilar-Duran S, Valmaseda-Castellón E, and Figueiredo R. Blood spatter in oral surgery prevalence and risk factors. J Am Dent Assoc. (2020) 151:438–43. doi: 10.1016/j.adaj.2020.02.026
23. Ishihama K, Iida S, Koizumi H, Wada T, Adachi T, Isomura-Tanaka E, et al. High incidence of blood exposure due to imperceptible contaminated splatters during oral surgery. J Oral Maxillofac Surg. (2008) 66:704–10. doi: 10.1016/j.joms.2007.06.663
24. Grenier D. Quantitative analysis of bacterial aerosols in two different dental clinic environments. Appl Environ Microbiol. (1995) 61:3165–8. doi: 10.1128/aem.61.8.3165-3168.1995
25. Nejatidanesh F, Khosravi Z, Goroohi H, Badrian H, and Savabi O. Risk of contamination of different areas of dentist's face during dental practices. Int J Prev Med. (2013) 4:611–5.
26. Harrel SK, and Molinari J. Aerosols and splatter in dentistry: a brief review of the literature and infection control implications. J Am Dent Assoc. (2004) 135:429–37. doi: 10.14219/jada.archive.2004.0207
27. Bahl P, Doolan C, de Silva C, Chughtai AA, Bourouiba L, MacIntyre CR, et al. Airborne or droplet precautions for health workers treating coronavirus disease 2019? J Infect Dis. (2020) jiaa189:1–8. doi: 10.1093/infdis/jiaa189
28. World Health Organization. Infection Prevention and Control of Epidemicand Pandemic-Prone Acute Respiratory Infections in Health Care. Available online at: https://www.who.int/publications/i/item/infection-prevention-and-control-of-epidemic-and-pandemic-prone-acute-respiratory-infections-in-health-care (accessed April 7, 2014).
29. Cheng Y, Hu J, Chen H, Wu L, Liao J, Cheng L, et al. Effects of different methods of air disinfection of computed tomography rooms dedicated to COVID-19 cases. Biomed Res Int. (2020) 5302910:1–5. doi: 10.1155/2020/5302910
30. Logothetis DD, and Martinez-Welles JM. Reducing bacterial aerosol contamination with a chlorhexidine gluconate pre-rinse. J Am Dent Assoc. (1995) 126:1634–9. doi: 10.14219/jada.archive.1995.0111
31. American Dentistry Association. ADA Interim Guidance for Minimizing Risk of COVID-19 Transmission Risk When Treating Dental Emergencies. Available online at: https://www.ada.org/en/publications/ada-news/2020-archive/april/ada-releases-interim-guidance-on-minimizing-covid-19-transmission-risk-when-treating-emergencies (accessed April 1, 2020).
32. Kelly N, Nic Iomhair A, and McKenna G. Can oral rinses play a role in preventing transmission of COVID 19 infection? Evid Based Dent. (2020) 21:42–3. doi: 10.1038/s41432-020-0099-1
33. Azevedo MJ, Correia I, Portela A, and Sampaio-Maia B. A simple and effective method for addition silicone impression disinfection. J Adv Prosthodont. (2019) 11:155–61. doi: 10.4047/jap.2019.11.3.155
34. Demajo JK, Cassar V, Farrugia C, Millan-Sango D, Sammut C, Valdramidis V, et al. Effectiveness of disinfectants on antimicrobial and physical properties of dental impression materials. Int J Prosthodont. (2016) 29:63–7. doi: 10.11607/ijp.4358
35. MacDonald DS, Colosi DC, Mupparapu M, Kumar V, Shintaku WH, Ahmad M, et al. Guidelines for oral and maxillofacial imaging: COVID-19 considerations. Oral Surg Oral Med Oral Pathol Oral Radiol. (2020) 131:99–110. doi: 10.1016/j.oooo.2020.10.017
36. Sewerin I. Gagging in dental radiography. Oral Surg Oral Med Oral Pathol. (1984) 58:725–8. doi: 10.1016/0030-4220(84)90043-4
37. Kohn WG, Collins AS, Cleveland JL, Harte JA, Eklund KJ, Malvitz DM, et al. Guidelines for infection control in dental health-care settings-2003. MMWR Recomm Rep. (2003) 52:1–61.
38. Mossa-Basha M, Meltzer CC, Kim DC, Tuite MJ, Kolli KP, Tan BS, et al. Radiology department preparedness for COVID-19: radiology scientific expert panel. Radiology. (2020) 296:E106–12. doi: 10.1148/radiol.2020200988
39. Shi Y, Wang G, Cai XP, Deng JW, Zheng L, Zhu HH, et al. An overview of COVID-19. J Zhejiang Univ Sci B. (2020) 21:343–60. doi: 10.1631/jzus.B2000083
40. Açikgöz Ö, and Günay A. The early impact of the COVID-19 pandemic on the global and Turkish economy. Turk J Med Sci. (2020) 50:520–6. doi: 10.3906/sag-2004-6
41. Graham BS. Rapid COVID-19 vaccine development. Science. (2020) 368:945–6. doi: 10.1126/science.abb8923
42. Haynes BF, Corey L, Fernandes P, Gilbert PB, Hotez PJ, Rao S, et al. Prospects for a safe COVID-19 vaccine. Sci Transl Med. (2020) 12:eabe0948. doi: 10.1126/scitranslmed.abe0948
43. GSMA. Themobileeconomy. London. Available online at: https://www.gsma.com/mobileeconomy/ (accessed March, 2020).
Keywords: COVID-19, pandemic, aerosol, infection control, dentistry
Citation: Luo W, Wang J, Tang M, Peng J, Ma W and Wu Y (2021) Clinical Guidelines for Dentistry in China During the Coronavirus Disease 2019 Pandemic. Front. Dent. Med. 2:704393. doi: 10.3389/fdmed.2021.704393
Received: 02 May 2021; Accepted: 27 May 2021;
Published: 24 June 2021.
Edited by:
Sukumaran Anil, Hamad Medical Corporation, QatarReviewed by:
Raissa Micaella Marcello-Machado, Campinas State University, BrazilAntoine Nicolas Berberi, Lebanese University, Lebanon
Copyright © 2021 Luo, Wang, Tang, Peng, Ma and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yingying Wu, 364662698@qq.com
†These authors have contributed equally to this work and share first authorship
 Wen Luo
Wen Luo Jing Wang
Jing Wang Maoxue Tang
Maoxue Tang Jiaming Peng
Jiaming Peng Wenmin Ma1
Wenmin Ma1  Yingying Wu
Yingying Wu