Genetic deficiency of protein inhibitor of activated STAT3 suppresses experimental abdominal aortic aneurysms
- 1Institute of Cardiovascular Science, Translational Medicine Institute, Xi'an Jiaotong University Health Science Center, Xi’an, China
- 2Department of Vascular Surgery, The Second Hospital of Tianjin Medical University, Tianjin, China
- 3Laboratory Animal Center, Xi'an Jiaotong University Health Science Center, Xi’an, China
- 4Department of Surgery, Stanford University School of Medicine, Stanford, CA, United States
- 5School of Health Science, Faculty of Medicine, Kagoshima University, Kagoshima, Japan
Aim: Signal transducer and activator of transcription (STAT) signaling is critical for the pathogenesis of abdominal aortic aneurysms (AAAs). Though protein inhibitor of activated STAT3 (PIAS3) negatively modulates STAT3 activity, but its role in AAA disease remains undefined.
Method: AAAs were induced in PIAS3 deficient (PIAS3−/−) and wild type (PIAS3+/+) male mice via transient intra-aortic elastase infusion. AAAs were assessed by in situ measurements of infrarenal aortic external diameters prior to (day 0) and 14 days after elastase infusion. Characteristic aneurysmal pathologies were evaluated by histopathology.
Results: Fourteen days following elastase infusion, aneurysmal aortic diameter was reduced by an approximately 50% in PIAS3−/− as compared to PIAS3+/+ mice. On histological analyses, PIAS3−/− mice showed less medial elastin degradation (media score: 2.5) and smooth muscle cell loss (media score: 3.0) than those in PIAS3+/+ mice (media score: 4 for both elastin and SMC destruction). Aortic wall leukocyte accumulation including macrophages, CD4+ T cells, CD8+ T cells and B cells as well as mural neovessel formation were significantly reduced in PIAS3−/− as compared to PIAS3+/+ mice. Additionally, PIAS3 deficiency also downregulated the expression levels of matrix metalloproteinases 2 and 9 by 61% and 70%, respectively, in aneurysmal lesion.
Conclusion: PIAS3 deficiency ameliorated experimental AAAs in conjunction with reduced medial elastin degradation and smooth muscle cell depletion, mural leukocyte accumulation and angiogenesis.
Introduction
Abdominal aortic aneurysm (AAA) is a lethal degenerative disease that is prevalent in older smoker men (1, 2). Inflammation is one of well-established pathophysiological mechanisms in the genesis of AAAs. For example, the circulating levels of inflammatory cytokines such as interleukin (IL)-6, IL-1β, tumor necrosis factor (TNF)-α and other mediators were elevated in patients with AAAs and may mediate AAA formation and progression (3–5). Inhibition of inflammatory cytokines effectively attenuated experimental AAA formation (6–8).
Janus kinase (JAK)/signal transducer and activator of transcription (STAT) signaling critically regulates inflammatory responses (9, 10). It is involved in cytokine production, immune cell recruitment, and initiation of adaptive responses (9, 11). Four JAKs (JAK1-JAK3 and tyrosine kinase 2) and seven STATs (STAT1-STAT4, STAT5a, STAT5b, and STAT6) are present in mammalian cells (12). JAK2/STAT3 signaling modulates cell proliferation as well as cell survival. Additionally, enhanced JAK2/STAT3 signaling activity has been reported in clinical AAA specimens (13).
Excessively activating JAK/STAT signaling leads to dysregulated immune responses and thus tissue damage. Several endogenous inhibitory proteins, such as protein inhibitor of activated STAT (PIAS) and suppressor of cytokine signaling (SOCS), are evolved to limit overwhelming inflammation due to augmented JAK/STAT activity. We previously showed that PIAS3 was reversely associated with atherosclerotic progression and inhibited inflammatory responses and smooth muscle cell (SMC) proliferation (14). AAA as a chronic inflammatory disease may share some common pathogenic pathways with atherosclerosis. The role PIAS3 plays in AAAs has not been clarified. Therefore, the present study was to investigate the influence of PIAS3 deficiency on experimental AAAs in the elastase-induced AAA model.
Materials and methods
Animals
PIAS3 gene (NM_001165949) is located on mouse chromosome 3 with fourteen exons (15). PIAS3 deficient (PIAS3−/−) mice on C57BL/6 genetic background were created in Cyagen Biosciences (Suzhou) Inc (Taicang, Jiangsu, China) using the CRISPR/CAS9 technique. Briefly, the exons of 2–9 were selected as target sites, and two gRNAs with Cas9 mRNA were microinjected into zygotes for PIAS3 deficient mouse generation (Figure 1A). F0 founders were identified via PCR genotyping followed by DNA sequencing. Homozygotes (PIAS3−/−) and wild type (PIAS3+/+) littermates were screened and used for all experiments. The use and care of animals as well as all experimental procedures were reviewed and approved by the Laboratory Animal Administration Committee of Xi'an Jiaotong University (No. 2019–1178). All information on animals and related reagents were detailed in Supplementary Table S1.
Phenotyping of PIAS3 deficient mice
For PCR screening, tail tip samples were collected from <3 weeks old mice and genomic DNA was extracted by proteinase K digestion. Genotyping PCR primers were AAACAAGACTAAAGGAGTATGGGC (sense 1), TAGAGGAAGGGGAAGGGAACTAAG (antisense 1) and CTCAGACACTCGGAAACTCATC (antisense 2). For quantitative reverse transcription PCR (qRT-PCR) and Western blotting analyses, total RNA and proteins were extracted from aortas. The PIAS3 primers for qRT-PCR analysis were sense: ACCAAGAATGGAGCTGAGCC (sense) and TCTGGATTCCGGATCCCCTT (antisense). Antibodies against PIAS3 and β-actin were obtained from Cell Signaling Technology (Danvers, MA, United States) and TransGen Biotech Co., Ltd (Beijing, China), respectively.
Experimental AAA modeling
Experimental AAAs were induced in PIAS3−/− and PIAS3+/+ mice by transient luminal infusion of porcine pancreatic elastase (PPE, 1.5 units/mL in phosphate-buffered saline) in controlled infrarenal aortic segment as previously reported (16, 17). Briefly, 9–12 weeks old male mice were anesthetized by 2% isoflurane inhalation, and infrarenal aorta was exposed via a laparotomy. Infrarenal aorta was then infused with PPE solution via an aortotomy for 5 min under constant pressure as previously described (18). After the surgery, all mice were maintained in individual cages with free access to chow diet and water.
Measurement of abdominal aortic diameters
Prior to PPE infusion, infrarenal aorta was photographed with a digital camera. The diameter was measured using Images Plus3.0 ML (Motic Electric Group Co., Ltd, Xiamen, Fujian, China) and served the baseline level. On day 14 after PPE infusion, infrarenal aortic diameter for each mouse was measured by the same procedure prior to sacrifice and aorta was then harvested. A mouse with a more than 50% aortic dilation over the baseline was considered aneurysmal (18).
Analysis of medial elastin and SMCs
Mice were euthanized by carbon dioxide inhalation. PPE-infused aortic segment was collected, embedded in optimal cutting temperature media, and sectioned (6 μm). Frozen sections were stained with hematoxylin and eosin (H & E) and Elastic van Gieson (EVG) stains, respectively, for the assessment of general morphology and elastin integrity. Aortic media elastin degradation was graded as I (mild) to IV (severe) on EVG-stained sections as previously reported (16–20). To evaluate SMC depletion, acetone-fixed frozen sections were stained with a goat anti-SMC α-actin antibody followed a standard biotin-streptavidin peroxidase procedure. Biotinylated donkey anti-goat IgG antibody and streptavidin-peroxidase conjugate were obtained from the Jackson ImmunoResearch Laboratories Inc., West Glove, PA, United States). AEC substrate kit for color development was purchased from Vector Laboratories Inc., Burlingame, CA, United States. Aortic medial SMC depletion was graded on a scale of I (mild) to IV (severe) as reported previously (20).
Analysis of aortic leukocytes
Acetone-fixed aortic frozen sections were stained with monoclonal antibodies against CD68 (macrophages), CD4+ T cells, CD8+ T cells and B cells (B220) (18). Sections were sequentially incubated with biotinylated goat anti-rat antibody (Vector Laboratories Inc), PBS, streptavidin-peroxidase conjugate (Jackson ImmunoResearch Laboratories Inc), PBS, peroxidase substrate AEC (Vector Laboratories Inc), counterstained with hematoxylin, mounted and cover-slipped. Aortic mural macrophage accumulation was graded on a scale of I (mild) to IV (severe) (20). CD4+ T cells, CD8+ T cells and B cells were quantitated as positively stained cells per aortic cross section (ACS) (20).
Immunostaining of aneurysmal matrix metalloproteinases
Matrix metalloproteinases (MMPs), particularly MMP2 and MMP9, contribute to the pathogenesis of AAAs. MMP2 and MMP9 were assessed by immunostaining using goat anti-mouse polyclonal antibodies against MMP2 (AF1488) and MMP9 (AF909) (R & D Systems, Minneapolis, MN, United States) (Cite your JIR article). The expression levels were quantitated as the positively stained area per ACS using the image analysis software (WinRoof 6.5, Mitani Co. Ltd., Tokyo, Japan).
Analysis of mural angiogenesis
Angiogenesis was analyzed by immunostaining with rat anti-mouse CD31 monoclonal antibody (Clone 390, Biolegend Inc, San Diego, CA, United States) previously described. The neovessels number were counted under the microscope and reported as CD31-positive vessels per ACS (20, 21).
Statistical analysis
Prism 9.0 software was used for all statistical analyses. Continuous variables were expressed as mean and standard deviation (normal distribution) or media with interquartile (not normal distribution). Student's t and nonparametric Mann-Whitney tests were used for normally and nonnormally distributed data, respectively. Two-way ANOVA followed by Sidak's multiple comparisons test was used for testing statistical difference for aortic diameters among groups. Statistical significance level was set at p < 0.05.
Results
Generation of PIAS3 knockout mice
As illustrated in Figures 1A,B, PIAS3 deficient mice were successfully generated by CRISPR/Cas9 techniques. PCR genotyping demonstrated the homozygotes of PIAS3 deficiency. qRT-PCR and Western blotting analysis further confirmed the deficiency of PIAS3 at mRNA and protein levels in PIAS3−/− as compared to PIAS3+/+ mice.
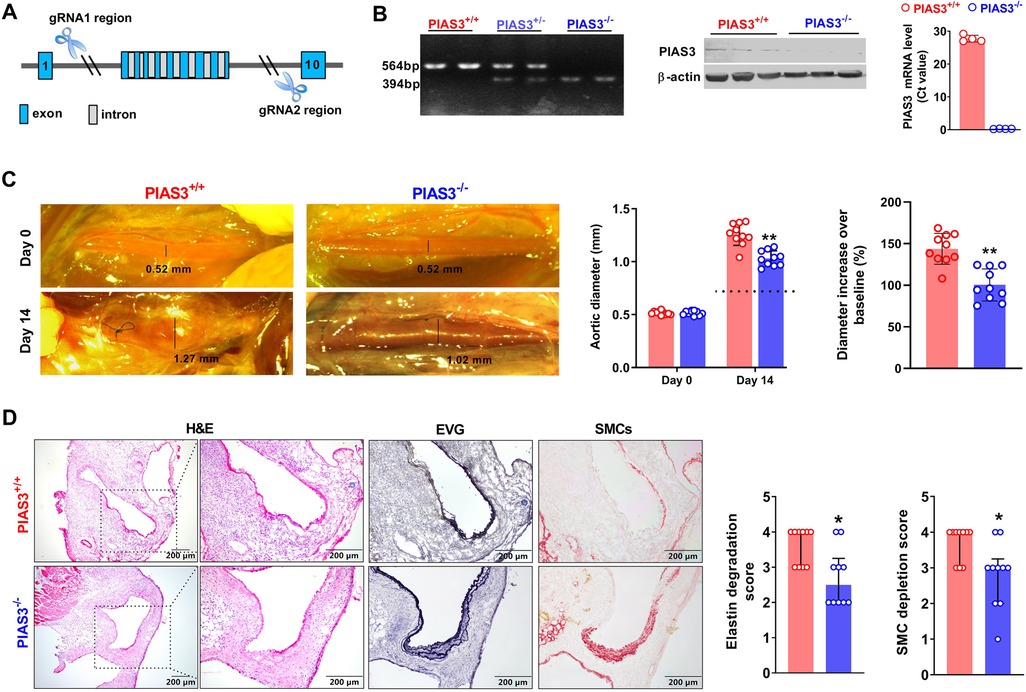
Figure 1. Genetic deficiency of PIAS3 suppresses experimental AAAs. (A): Strategy for generating PIAS3 deficient (PIAS3−/−) mice by targeting exons 2-9 via the CRISPR/Cas9 technique. (B): Characterization of PIAS3−/− mice by PCR genotyping, qRT-PCR and Western blotting analyses. (C): Representative aortic photographs and quantification of aortic diameters on day 0 and day 14 after elastase infusion. Dotted line indicates average aortic diameter after PBS infusion (approximately 0.8 mm). (D): Representative images of H&E, EVG and SMC α-actin staining as well as the semi-quantification (media and interquartile) of aneurysmal medial elastin and SMC destruction. Two-way ANOVA followed two group comparison (C) and non-parametric Mann-Whitney test (D). N = 10 for each group, *p < 0.05 and **p < 0.01 as compared to PIAS3+/+ mice.
PIAS3 deficiency suppresses PPE-induced aortic dilation in mice
Intra-infrarenal aortic PPE infusion was conducted to induce AAAs in PIAS3+/+ and PIAS3−/− mice. Experimental AAAs were successfully induced in PIAS3+/+ mice (Figure 1C). PIAS3 deficiency significantly inhibited aortic expansion as compared to PIAS3+/+ mice. Aortic diameter on day 14 after PPE infusion were 1.03 ± 0.07 mm and 1.26 ± 0.10 mm for PIAS3−/− and PIAS3+/+ mice, respectively (Figure 1C). After subtracting an average aortic dilation caused by PBS infusion (approximately 0.8 mm), PIAS3 deficiency reduced PPE-induced aortic expansion by approximately 50% (Figure 1C, middle panel). The diameter increase over the baseline was significantly less in PIAS3−/− than that in PIAS3+/+ mice (Figure 1C, right panel).
PIAS3 deficiency ameliorates medial elastin degradation and SMC depletion
Histological analysis was performed in aneurysmal aortas. In H&E staining, PPE infusion induced a remarkable aortic dilation, predominant inflammatory cell infiltration, medial elastin degradation and SMC loss (Figure 1D, left panel). However, PIAS3−/− mice were protective against the destruction of medial elastin and SMCs. In comparison with PIAS3+/+ mice, the integrity of medial elastin was relatively preserved in PIAS3−/− mice, with significantly reduced elastin degradation score (Figure 1D, middle panel). Similar was true for medial SMCs (Figure 1D, right panel).
PIAS3 deficiency inhibits aortic leukocyte accumulation
In aortic immunostaining, the score for the accumulation of macrophages, identified by CD68-positve cells, was significantly lower in PIAS3−/− than that in PIAS3+/+ mice (Figures 2A,B). Similarly, PIAS3 deficiency reduced the accumulation of CD4+ T cells, CD8+ T cells and B cells by approximately 50% (Figure 2).
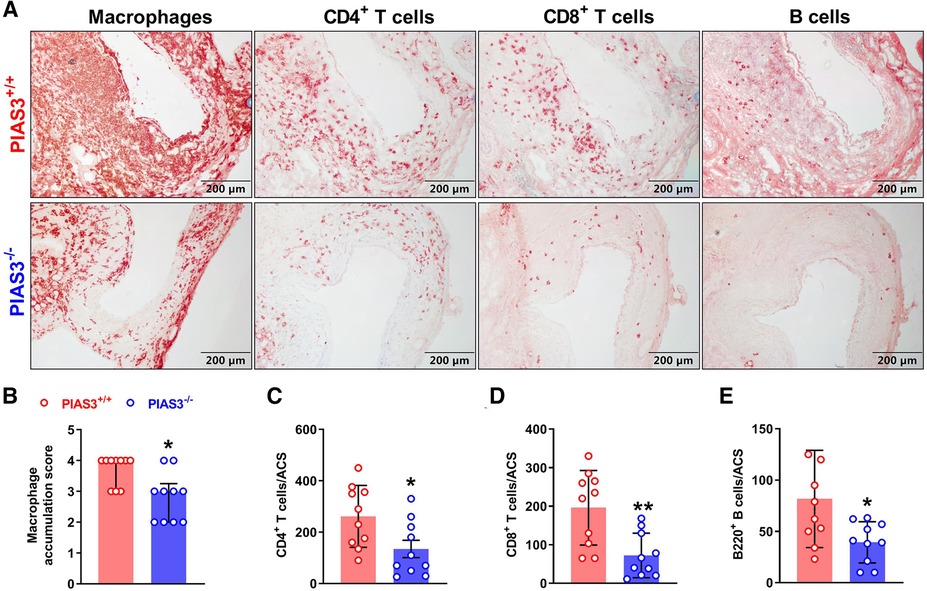
Figure 2. PIAS3 deficiency inhibits aneurysmal aortic leukocyte accumulation. (A): Representative images of immunohistochemical staining showing aneurysmal aortic leukocyte accumulation (macrophages, T cells and B cells). (B-E): Semi-quantification of aneurysmal aortic leukocyte accumulation. Aortic mural macrophage accumulation was scored from I to IV, while aortic CD4+ T cells, CD8+ T cells and B cells were quantified as positively stained cells number per aortic cross-section (ACS). Data are shown as media and interquartile (B) and mean and standard deviation (C–E). Non-parametric Mann-Whitney test (B) and Student's t test (C–E). N = 10 for each group, *p < 0.05 and **p < 0.01 as compared to wild type (PIAS3+/+) mice.
PIAS3 deficiency reduces aortic MMP2 and MMP9 expression
MMP2 and MMP9 are contributors of PPE-induced AAAs (17, 22, 23). The levels of aortic MMP2 and MMP 9 were diminished in PIAS3−/− as compared to PIAS3+/+ mice (Figure 3A). In semi-quantitative analysis, individual MMP positively stained areas were reduced by approximately 61% and 70% for MMP2 and MMP9, respectively, in PIAS3−/− as compared to PIAS3+/+ mice (Figures 3B,C).
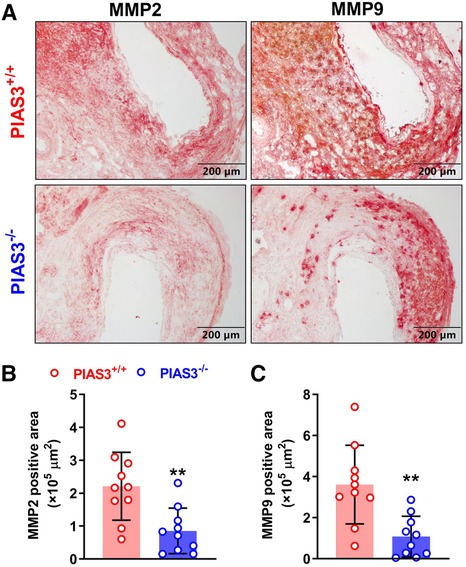
Figure 3. PIAS3 deficiency decreases the expression levels of matrix metalloproteinases 2 and 9. (A): Representative immunohistochemical staining images of MMP2 and MMP9 in aneurysmal aortas from mild type (PIAS3+/+) and PIAS3 deficient (PIAS3−/−) mice. (B): Semi-quantification of MMP2 and MMP9 expression levels in aneurysmal lesions quantitated as the positively stained area per aortic cross-section. N = 10 for each group, *p < 0.05 and **p < 0.01 as compared to PIAS3+/+ mice.
PIAS3 deficiency suppresses mural angiogenesis
Mural angiogenesis is involved in AAA pathogenesis (24–27). We thus determined whether the protective effect of PIAS3 deficiency on AAAs was associated with altered angiogenesis. In immunostaining of CD31, a maker of angiogenesis, the neovessels were significantly less in PIAS3−/− than that in PIAS3+/+ mice (Figure 4, left panel), with a 40% reduction in mural neovessels in PIAS3−/− mice (Figure 4, right panel).
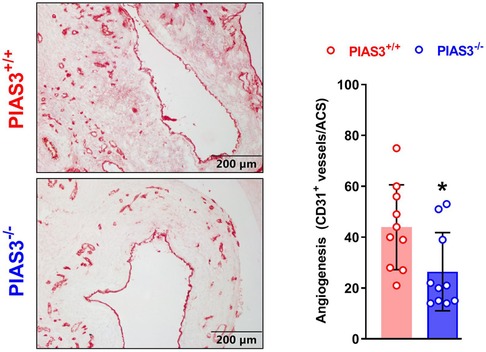
Figure 4. PIAS3 deficiency inhibits mural angiogenesis. Aortic frozen sections were immunostained with an antibody against mouse CD31. Angiogenesis was quantitated as the number of CD31-positive neovessels per aortic cross-section (ACS). N = 10 for each group, *p < 0.05 and **p < 0.01 as compared to wild type (PIAS3+/+) mice.
Discussion
Inflammation is implicated in the initiation and evolution of AAAs (28). It leads to aortic medial elastin degradation, the apoptosis and dysfunction of vascular SMC by proteolytic enzymes, free radicals, cytokines, and other inflammatory products (29). JAK2/STAT3 signaling is a main intrinsic pathway for inflammation. It is involved in the creation and sustenance of inflammatory milieus by modulating the expression of cytokines, chemokines, and other mediators (30). In this study, we found that PIAS3 deficiency attenuated experimental AAAs induced by elastase infusion. There were less aneurysmal medial elastin and SMC destruction in PIAS3−/− mice than those in PIAS3+/+ mice. Moreover, in comparison with PIAS3+/+ mice, aortic leukocyte infiltration, MMP expression and mural angiogenesis were attenuated in PIAS3−/− mice. These results demonstrate that PIAS3 deletion ameliorated experimental AAAs by preserving SMCs, inhibiting aortic leukocyte infiltration, and decreasing angiogenesis.
It has been previously shown that JAK2/STAT3 signaling activity was involved in the pathogenesis of AAAs (13, 31, 32). In human aortic tissues, JAK2/STAT3 expression levels were higher in aneurysmal than those in non-aneurysmal aortas (31). IL-6, a JAK2/STAT3 activator, was elevated in the human aneurysmal as compared to non-aneurysmal aortas (32, 33). However, previously reported influence of STAT3 pathway components on AAAs varied. Disruption of STAT3 signaling in bone marrow–derived cells aggravated whereas myeloid cell-STAT3 deletion had limited effect on experimental AAAs (34). A STAT3 inhibitor attenuated angiotensin II-induced AAA progression in mice through inhibiting vascular inflammation and maintaining autophagy (35), whereas severe angiotensin II-induced AAAs were noted in mice overexpressing SOCS3, another negative regulator of JAK2/STAT3 signaling, in T lymphocytes in association with impaired IL-17 production (34). These discrepancies of STAT3 inhibition/activation concerning AAA progression might be resulted from differential influence on different vascular structural and immune cells. In SMC, the STAT3 inhibitor suppressed inflammation related signaling activation, such as JAK2/STAT3 and NF-kB signaling, which ameliorated AAAs (35). In T cells, overexpression SOCS3 blocked STAT3 signaling activation, thereby significantly impeded Th17 development and decreased interleukin-17 production (34). Reduced IL-17 production is associated with severe vascular inflammation and enhanced susceptibility to aneurysms (36).
We previously found that PIAS3 was negatively associated with the JAK2/STAT3 activation and inflammatory responses in macrophages during atherosclerosis (14). Unexpectedly, the present study showed that PIAS3 deficiency attenuated PPE-induced AAA formation and reduced leukocyte infiltration in experimental AAA lesions. It is possible that chronic activation of STAT3 caused by PIAS3 deficiency may trigger tissue repair responses. In a recent study, SMC-specific SOCS3 deletion was protective against aortic dissection (37). Regionally activating STAT3 in the aorta may initiate host defense mechanisms thereby promoting SMC survival following vascular injury (37). Chronic activation of STAT3 evoked tissue repair responses by altering the phenotype of SMCs, macrophages, and fibroblasts, leading to enhancement of the tensile strength of the aortic wall (37). In addition, mural angiogenesis also contributes to AAA pathogenesis. In present study, PIAS3 deletion decreased aneurysmal mural angiogenesis, which might also result in the attenuation of AAA formation in PIAS3 deficient mice. PIAS3 has been shown to increase the levels of hypoxia-inducible factor (HIF)-1α, a critical angiogenic transcription factor, by stabilizing HIF-1 protein (38). HIF-1α/vascular endothelial growth factor (VEGF-A) pathway plays a vital role in the development of AAAs (39–43). Thus, angiogenesis inhibition by PIAS3 deficiency may be potentially attributed to low HIF-1 protein stability and thus reduced VEGF-A levels.
In conclusion, the present study demonstrated that genetic PIAS3 deficiency attenuated experimental AAAs in association with reduced medial elastin degradation, SMC depletion, leukocyte infiltration and aortic wall angiogenesis. Due to the limited aneurysmal tissues, the functions of MMPs and STAT3 were not determined in the present study. Further studies on the mechanism of PIAS3 regulating AAA will be conducted in the future.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary materials, further inquiries can be directed to the corresponding author/s.
Ethics statement
The animal study was reviewed and approved by the Laboratory Animal Administration Committee of Xi'an Jiaotong University (No. 2019-1178).
Author contributions
SZ, RW, BX and MM are involved in the study design; SZ, EL and RW collected and analyzed data, as well as drafted the manuscript; WF, HL, CX, PW, KT, QY, YL and RW performed experiments; EL, BX, RW and SZ critically revised manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was partly supported by grants from the Natural Science Foundation of Shaanxi Province (2023-CX-PT-17, 2021PT-056, 2023-CX-PT-01), Natural Science Project of Xi’an Jiaotong University (YXJLRH2022073) and the National Natural Science Foundation of China (82170471).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1092555/full#supplementary-material.
References
1. Tang W, Yao L, Roetker NS, Alonso A, Lutsey PL, Steenson CC, et al. Lifetime risk and risk factors for abdominal aortic aneurysm in a 24-year prospective study: the aric study (atherosclerosis risk in communities). Arterioscler, Thrombosis, and Vasc Biol. (2016) 36(12):2468–77. doi: 10.1161/ATVBAHA.116.308147
2. Takada M, Yamagishi K, Tamakoshi A, Iso H, Group JS. Body mass Index and mortality from aortic aneurysm and dissection. J Atheroscler Thromb. (2021) 28(4):338–48. doi: 10.5551/jat.57232
3. Juvonen J, Surcel HM, Satta J, Teppo AM, Bloigu A, Syrjala H, et al. Elevated circulating levels of inflammatory cytokines in patients with abdominal aortic aneurysm. Arterioscler, Thrombosis, and Vasc Biol. (1997) 17(11):2843–7. doi: 10.1161/01.atv.17.11.2843
4. Batra R, Suh MK, Carson JS, Dale MA, Meisinger TM, Fitzgerald M, et al. Il-1beta (interleukin-1beta) and tnf-alpha (tumor necrosis factor-alpha) impact abdominal aortic aneurysm formation by differential effects on macrophage polarization. Arterioscler, Thrombosis, and Vasc Biol. (2018) 38(2):457–63. doi: 10.1161/ATVBAHA.117.310333
5. Ouyang M, Wang M, Yu B. Aberrant mitochondrial dynamics: an emerging pathogenic driver of abdominal aortic aneurysm. Cardiovasc Ther. (2021) 2021:6615400. doi: 10.1155/2021/6615400
6. Xiong W, MacTaggart J, Knispel R, Worth J, Persidsky Y, Baxter BT. Blocking tnf-alpha attenuates aneurysm formation in a murine model. J Immunol. (2009) 183(4):2741–6. doi: 10.4049/jimmunol.0803164
7. Isselbacher EM. Losartan for the treatment of marfan syndrome: hope fades. J Am Coll Cardiol. (2018) 72(14):1619–21. doi: 10.1016/j.jacc.2018.07.051
8. Yu J, Morimoto K, Bao W, Yu Z, Okita Y, Okada K. Glucagon-Like peptide-1 prevented abdominal aortic aneurysm development in rats. Surgery Today. (2016) 46(9):1099–107. doi: 10.1007/s00595-015-1287-z
9. Banerjee S, Biehl A, Gadina M, Hasni S, Schwartz DM. Jak-Stat signaling as a target for inflammatory and autoimmune diseases: current and future prospects. Drugs. (2017) 77(11):1261. (Vol 77, Pg 521, 2017) doi: 10.1007/s40265-017-0772-7
10. O'Shea JJ. Targeting the jak/stat pathway for immunosuppression. Ann Rheum Dis. (2004) 63:67–71. doi: 10.1136/ard.2004.028290
11. Garbers C, Aparicio-Siegmund S, Rose-John S. The il-6/Gp130/Stat3 signaling axis: recent advances towards specific inhibition. Curr Opin Immunol. (2015) 34:75–82. doi: 10.1016/j.coi.2015.02.008
12. Kiu H, Nicholson SE. Biology and significance of the jak/stat signalling pathways. Growth Factors. (2012) 30(2):88–106. doi: 10.3109/08977194.2012.660936
13. Liao MF, Xu J, Clair AJ, Ehrman B, Graham LM, Eagleton MJ. Local and systemic alterations in signal transducers and activators of transcription (stat) associated with human abdominal aortic aneurysms. J Surg Res. (2012) 176(1):321–8. doi: 10.1016/j.jss.2011.05.041
14. Wang R, Zhang YJ, Xu LR, Lin Y, Yang XF, Bai L, et al. Protein inhibitor of activated Stat3 suppresses oxidized ldl-induced cell responses during atherosclerosis in apolipoprotein E-deficient mice. Sci Rep-Uk. (2016) 6:36790. doi: 10.1038/Srep36790
15. Okazaki Y, Furuno M, Kasukawa T, Adachi J, Bono H, Kondo S, et al. Analysis of the mouse transcriptome based on functional annotation of 60,770 full-length cdnas. Nature. (2002) 420(6915):563–73. doi: 10.1038/nature01266
16. Liu HL, Tian KL, Xia CC, Wei PP, Xu BY, Fu WL, et al. Kunming Mouse strain is less susceptible to elastase-induced abdominal aortic aneurysms. Anim Model Exp Med. (2022) 5(1):72–80. doi: 10.1002/ame2.12197
17. Liu HL, Wei PP, Fu WL, Xia CC, Li YK, Tian KL, et al. Dapagliflozin ameliorates the formation and progression of experimental abdominal aortic aneurysms by reducing aortic inflammation in mice. Oxid Med Cell Longev. (2022) 2022:8502059. doi: 10.1155/2022/8502059
18. Tian KL, Xia CC, Liu HL, Xu BY, Wei PP, Fu WL, et al. Temporal and quantitative analysis of aortic immunopathologies in elastase-induced mouse abdominal aortic aneurysms. J Immunol Res. (2021) 2021:6297332. doi: 10.1155/2021/6297332
19. Xu BH, Iida Y, Glover KJ, Ge YB, Wang Y, Xuan HJ, et al. Inhibition of vegf (vascular endothelial growth factor)-a or its receptor activity suppresses experimental aneurysm progression in the aortic elastase infusion model. Arterioscl Throm Vas. (2019) 39(8):1652–66. doi: 10.1161/Atvbaha.119.312497
20. Ikezoe T, Shoji T, Guo J, Shen F, Lu HS, Daugherty A, et al. No effect of hypercholesterolemia on elastase-induced experimental abdominal aortic aneurysm progression. Biomolecules. (2021) 11(10):1434–48. doi: 10.3390/biom11101434
21. Guo J, Shoji T, Ge Y, Zheng X, Li Y, Zhao S, et al. Treatment with the prolyl hydroxylase inhibitor jnj promotes abdominal aortic aneurysm progression in diabetic mice. Eur J Vasc Endovasc Surg. (2022) 63(3):484–94. doi: 10.1016/j.ejvs.2021.10.030
22. Longo GM, Xiong W, Greiner TC, Zhao Y, Fiotti N, Baxter BT. Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J Clin Investigation. (2002) 110(5):625–32. doi: 10.1172/JCI15334
23. Dhital S, Vyavahare NR. Nanoparticle-Based targeted delivery of pentagalloyl glucose reverses elastase-induced abdominal aortic aneurysm and restores aorta to the healthy state in mice. PloS one. (2020) 15(3):e0227165. doi: 10.1371/journal.pone.0227165
24. Zhang M, Sui W, Cheng C, Xue F, Tian Z, Cheng J, et al. Erythropoietin promotes abdominal aortic aneurysms in mice through angiogenesis and inflammatory infiltration. Science Translational Med. (2021) 13(603):eaaz4959. doi: 10.1126/scitranslmed.aaz4959
25. Lukasiewicz A, Reszec J, Kowalewski R, Chyczewski L, Lebkowska U. Assessment of inflammatory infiltration and angiogenesis in the thrombus and the wall of abdominal aortic aneurysms on the basis of histological parameters and computed tomography angiography study. Folia Histochemica et Cytobiologica. (2012) 50(4):547–53. doi: 10.5603/20323
26. Satta J, Soini Y, Mosorin M, Juvonen T. Angiogenesis is associated with mononuclear inflammatory cells in abdominal aortic aneurysms. Ann Chir Gynaecol. (1998) 87(1):40–2.9598229
27. Thompson MM, Jones L, Nasim A, Sayers RD, Bell PR. Angiogenesis in abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. (1996) 11(4):464–9. doi: 10.1016/s1078-5884(96)80183-3
28. Shi J, Guo J, Li Z, Xu B, Miyata M. Importance of Nlrp3 inflammasome in abdominal aortic aneurysms. J Atheroscler Thromb. (2021) 28(5):454–66. doi: 10.5551/jat.RV17048
29. Golledge J. Abdominal aortic aneurysm: update on pathogenesis and medical treatments. Nat Rev Cardiol. (2019) 16(4):225–42. doi: 10.1038/s41569-018-0114-9
30. Hillmer EJ, Zhang HY, Li HS, Watowich SS. Stat3 signaling in immunity. Cytokine Growth F R. (2016) 31:1–15. doi: 10.1016/j.cytogfr.2016.05.001
31. Xiao J, Wei ZJ, Chen X, Chen WQ, Zhang H, Yang CL, et al. Experimental abdominal aortic aneurysm growth is inhibited by blocking the Jak2/Stat3 pathway. Int J Cardiol. (2020) 312:100–6. doi: 10.1016/j.ijcard.2020.03.072
32. Lindeman JH, Abdul-Hussien H, Schaapherder AF, Van Bockel JH, Von der Thusen JH, Roelen DL, et al. Enhanced expression and activation of pro-inflammatory transcription factors distinguish aneurysmal from atherosclerotic aorta: il-6- and il-8-dominated inflammatory responses prevail in the human aneurysm. Clinical Science. (2008) 114(11):687–97. doi: 10.1042/CS20070352
33. Harrison SC, Smith AJP, Jones GT, Swerdlow DI, Rampuri R, Bown MJ, et al. Interleukin-6 receptor pathways in abdominal aortic aneurysm. Eur Heart J. (2013) 34(48):3707–16. doi: 10.1093/eurheartj/ehs354
34. Romain M, Taleb S, Dalloz M, Ponnuswamy P, Esposito B, Perez N, et al. Overexpression of Socs3 in T lymphocytes leads to impaired interleukin-17 production and severe aortic aneurysm formation in mice–brief report. Arteriosclerosis, Thrombosis, and Vasc Biol. (2013) 33(3):581–4. doi: 10.1161/ATVBAHA.112.300516
35. Wu QY, Cheng Z, Zhou YZ, Zhao Y, Li JM, Zhou XM, et al. A novel Stat3 inhibitor attenuates angiotensin ii-induced abdominal aortic aneurysm progression in mice through modulating vascular inflammation and autophagy. Cell Death Dis (2020) 11(2):131. doi: 10.1038/s41419-020-2326-2
36. Chandesris MO, Azarine A, Ong KT, Taleb S, Boutouyrie P, Mousseaux E, et al. Frequent and widespread vascular abnormalities in human signal transducer and activator of transcription 3 deficiency. Circ Cardiovasc Genet. (2012) 5(1):25–34. doi: 10.1161/CIRCGENETICS.111.961235
37. Hirakata S, Aoki H, Ohno-Urabe S, Nishihara M, Furusho A, Nishida N, et al. Genetic deletion of Socs3 in smooth muscle cells ameliorates aortic dissection in mice. Jacc-Basic Transl Sc. (2020) 5(2):126–44. doi: 10.1016/j.jacbts.2019.10.010
38. Nakagawa K, Kohara T, Uehata Y, Miyakawa Y, Sato-Ueshima M, Okubo N, et al. Pias3 enhances the transcriptional activity of hif-1alpha by increasing its protein stability. Biochem Biophys Res Commun. (2016) 469(3):470–6. doi: 10.1016/j.bbrc.2015.12.047
39. Yu B, Wang X, Song Y, Xie G, Jiao S, Shi L, et al. The role of hypoxia-inducible factors in cardiovascular diseases. Pharmacol Ther. (2022) 238:108186. doi: 10.1016/j.pharmthera.2022.108186
40. Kaneko H, Anzai T, Takahashi T, Kohno T, Shimoda M, Sasaki A, et al. Role of vascular endothelial growth factor-a in development of abdominal aortic aneurysm. Cardiovasc Res. (2011) 91(2):358–67. doi: 10.1093/cvr/cvr080
41. Wang W, Xu B, Xuan H, Ge Y, Wang Y, Wang L, et al. Hypoxia-Inducible factor 1 in clinical and experimental aortic aneurysm disease. J Vasc Surg. (2018) 68(5):1538–50; e2. doi: 10.1016/j.jvs.2017.09.030
42. Tedesco MM, Terashima M, Blankenberg FG, Levashova Z, Spin JM, Backer MV, et al. Analysis of in situ and ex vivo vascular endothelial growth factor receptor expression during experimental aortic aneurysm progression. Arterioscler, Thromb, Vasc Biol. (2009) 29(10):1452–7. doi: 10.1161/ATVBAHA.109.187757
Keywords: abdominal aortic aneurysm, protein inhibitor of activated STAT3, inflammation, macrophage, animal model
Citation: Fu W, Liu H, Wei P, Xia C, Yu Q, Tian K, Li Y, Liu E, Xu B, Miyata M, Wang R and Zhao S (2023) Genetic deficiency of protein inhibitor of activated STAT3 suppresses experimental abdominal aortic aneurysms. Front. Cardiovasc. Med. 10:1092555. doi: 10.3389/fcvm.2023.1092555
Received: 1 December 2022; Accepted: 27 February 2023;
Published: 15 March 2023.
Edited by:
Hiroki Aoki, Kurume University, JapanReviewed by:
Venkateswaran Subramanian, University of Missouri, United StatesXiangqian Kong, Shandong Provincial Hospital, China
© 2023 Fu, Liu, Wei, Xia, Yu, Tian, Li, Liu, Xu, Miyata, Wang and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rong Wang rongw1986@xjtu.edu.cn Sihai Zhao sihaizhao@xjtu.edu.cn
†These authors share first authorship
Specialty Section: This article was submitted to Atherosclerosis and Vascular Medicine, a section of the journal Frontiers in Cardiovascular Medicine
 Weilai Fu1,2,†
Weilai Fu1,2,†  Haole Liu
Haole Liu Enqi Liu
Enqi Liu Baohui Xu
Baohui Xu Masaaki Miyata
Masaaki Miyata Rong Wang
Rong Wang Sihai Zhao
Sihai Zhao